Abstract
Objectives
To characterize the clinical severity of covid-19 associated with the alpha, delta, and omicron SARS-CoV-2 variants among adults admitted to hospital and to compare the effectiveness of mRNA vaccines to prevent hospital admissions related to each variant.
Design
Case-control study.
Setting
21 hospitals across the United States.
Participants
11 690 adults (≥18 years) admitted to hospital: 5728 with covid-19 (cases) and 5962 without covid-19 (controls). Patients were classified into SARS-CoV-2 variant groups based on viral whole genome sequencing, and, if sequencing did not reveal a lineage, by the predominant circulating variant at the time of hospital admission: alpha (11 March to 3 July 2021), delta (4 July to 25 December 2021), and omicron (26 December 2021 to 14 January 2022).
Main outcome measures
Vaccine effectiveness calculated using a test negative design for mRNA vaccines to prevent covid-19 related hospital admissions by each variant (alpha, delta, omicron). Among patients admitted to hospital with covid-19, disease severity on the World Health Organization’s clinical progression scale was compared among variants using proportional odds regression.
Results
Effectiveness of the mRNA vaccines to prevent covid-19 associated hospital admissions was 85% (95% confidence interval 82% to 88%) for two vaccine doses against the alpha variant, 85% (83% to 87%) for two doses against the delta variant, 94% (92% to 95%) for three doses against the delta variant, 65% (51% to 75%) for two doses against the omicron variant; and 86% (77% to 91%) for three doses against the omicron variant. In-hospital mortality was 7.6% (81/1060) for alpha, 12.2% (461/3788) for delta, and 7.1% (40/565) for omicron. Among unvaccinated patients with covid-19 admitted to hospital, severity on the WHO clinical progression scale was higher for the delta versus alpha variant (adjusted proportional odds ratio 1.28, 95% confidence interval 1.11 to 1.46), and lower for the omicron versus delta variant (0.61, 0.49 to 0.77). Compared with unvaccinated patients, severity was lower for vaccinated patients for each variant, including alpha (adjusted proportional odds ratio 0.33, 0.23 to 0.49), delta (0.44, 0.37 to 0.51), and omicron (0.61, 0.44 to 0.85).
Conclusions
mRNA vaccines were found to be highly effective in preventing covid-19 associated hospital admissions related to the alpha, delta, and omicron variants, but three vaccine doses were required to achieve protection against omicron similar to the protection that two doses provided against the delta and alpha variants. Among adults admitted to hospital with covid-19, the omicron variant was associated with less severe disease than the delta variant but still resulted in substantial morbidity and mortality. Vaccinated patients admitted to hospital with covid-19 had significantly lower disease severity than unvaccinated patients for all the variants.
Introduction
The covid-19 pandemic has been defined by both the distribution of highly effective vaccines and the serial emergence of new SARS-CoV-2 genetic variants.1 Variants of concern are new genetic versions of the virus with increased transmissibility, a change in virulence or disease presentation, or a decrease in effectiveness of mitigation measures, available vaccines, or treatments.2 Five World Health Organization designated SARS-CoV-2 variants of concern are currently recognized: alpha (B.1.1.7 and descendant lineages), beta (B.1.351), gamma (P.1), delta (B.1.617.2 and AY lineages), and omicron (B.1.1.529 and BA lineages).
The highly contagious delta variant was first identified in spring 2021 and rapidly replaced other SARS-CoV-2 variants, achieving global dominance by summer 2021.3 Early studies suggested the potential for increased risk of hospital admissions for people infected with the delta variant compared with previous variants.4 5 6 The highly divergent omicron variant was identified in mid-November 2021 and quickly became the dominant variant in much of Europe and North America by late December 2021.7 The overall risk of hospital admission among those infected with the omicron variant seems to be lower than among those infected with the delta variant.8 Hospital admissions for infection due to the omicron variant do, however, occur, and disease severity and risk for progression to critical illness remain incompletely understood for this variant.
Understanding the epidemiology of SARS-CoV-2 variants and the effectiveness of existing vaccines against them are essential to guide vaccination policies and the development of new vaccines. Early studies suggested reduced vaccine effectiveness against infection and hospital admissions for omicron compared with earlier variants.9 10 11 In most prior studies, estimates of vaccine effectiveness against the omicron variant were based on infections that occurred during periods in which the omicron variant exceeded 50% in genomic surveillance. Although efficient, these approaches have the potential for misclassification of the variant and inaccurate estimates of vaccine effectiveness. Furthermore, little is known about the effectiveness of vaccines to prevent the most severe manifestations of covid-19, including respiratory failure and death, for patients with infection due to the omicron variant.
Using observational study designs, the Influenza and Other Viruses in the Acutely Ill (IVY) Network in collaboration with the US Centers for Disease Control and Prevention is studying the effectiveness of covid-19 vaccines against severe disease (see supplementary table S1).12 13 14 15 Here, we compare the clinical severity of covid-19 associated with the SARS-CoV-2 alpha, delta, and omicron variants among adults admitted to hospital in the United States, and the effectiveness of covid-19 mRNA vaccines against each of these variants.
Methods
Design and setting
We conducted a prospective observational study at 21 hospitals in the US, with enrollment of adults admitted to hospital with laboratory confirmed covid-19 (cases) and concurrent patients without covid-19 (controls). A test negative design was utilized to assess vaccine effectiveness.16 This programme was conducted by the IVY Network, which is a group consisting of geographically dispersed academic medical centers in the US, coordinated from Vanderbilt University Medical Center (Nashville, TN) and funded by CDC (see supplementary table S1). Participants enrolled in the IVY programme with hospital admission dates between 11 March 2021 and 14 January 2022 were included in this analysis.
In this analysis we compared the alpha, delta, and omicron SARS-CoV-2 variants in three ways: vaccine effectiveness of mRNA vaccines to prevent hospital admissions with each variant; disease severity among unvaccinated and vaccinated patients with each variant admitted to hospital; and vaccine effectiveness of mRNA vaccines to prevent disease progression to invasive mechanical ventilation or death after hospital admission with each variant.
Participants
Sites prospectively screened in-hospital adults aged 18 years or older for potential eligibility through daily review of hospital admission logs and electronic medical records. Cases with covid-19 included those admitted to hospital with a clinical syndrome consistent with acute covid-19 (≥1 of fever, cough, shortness of breath, loss of taste, loss of smell, use of respiratory support for the acute illness, or new pulmonary findings on chest imaging consistent with pneumonia) and a positive molecular or antigen test result for SARS-CoV-2 within 10 days of symptom onset. We subclassified case patients based on SARS-CoV-2 variant. Additionally, two control groups were enrolled: test negative controls, who were adults admitted to hospital with signs or symptoms consistent with acute covid-19 and tested negative for SARS-CoV-2; and syndrome negative controls, who were adults admitted to hospital without signs or symptoms consistent with acute covid-19 and tested negative for SARS-CoV-2.17 Controls were selected from lists of eligible participants admitted to hospital within two weeks of enrollment of case patients with covid-19. Sites attempted to capture all patients with covid-19 admitted to the hospital during the surveillance period and targeted a case:control ratio of about 1:1. Cases and controls were not individually matched. Respiratory samples from participants were tested for SARS-CoV-2 both locally in clinical laboratories and centrally at a research laboratory. Cases were participants who tested positive for SARS-CoV-2 at a local laboratory, the central laboratory, or both, whereas control participants were those who tested negative for SARS-CoV-2 by all tests. Supplementary appendix B provides additional details about eligibility criteria and enrollment practices.13 14
Data collection
Trained staff collected data on demographics, medical conditions, covid-19 vaccination, and hospital course through participant (or proxy) interviews and standardized review of medical records. Details of covid-19 vaccination, including dates and location of vaccination, vaccine product, and lot number, were collected through a systematic process that included participant (or proxy) interview and source verification by vaccination card, hospital records, state vaccine registries, and vaccine records requested from clinics and pharmacies.13 14
Vaccination status
Vaccine doses were classified as administered if source documentation of the dose was identified or if the participant or proxy reported a vaccine dose with a complete and plausible date and location. This analysis focused on covid-19 mRNA vaccines authorized or approved for use in the US, including BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). Participants were classified based on the number of mRNA vaccine doses received before illness onset: 0 doses (unvaccinated), one dose ≥14 days before illness (partially vaccinated), two doses ≥14 days before illness (fully vaccinated), or three doses ≥7 days before illness (boosted if immunocompetent, or with primary three dose series completed if immunocompromised). In the primary analysis, we calculated vaccine effectiveness for two vaccine doses for participants enrolled throughout the surveillance period and for three vaccine doses for participants enrolled after third doses were authorized in the US.18 19 In a secondary analysis, we calculated vaccine effectiveness for partial vaccination. Participants were excluded from this analysis if they received a covid-19 vaccine other than an mRNA vaccine (eg, Ad26.COV2 from Janssen/Johnson & Johnson), more than three vaccine doses, or a third vaccine dose before they were authorized in the US.18 19 20
Laboratory analysis
Upper respiratory specimens (nasal swabs or saliva) were collected from participants, frozen, and shipped to Vanderbilt University Medical Center, where they underwent reverse transcription quantitative polymerase chain reaction (RT-qPCR) for detection of two SARS-CoV-2 nucleocapsid gene targets (N1 and N2).21 Respiratory specimens positive for SARS-CoV-2 were shipped to the University of Michigan (Ann Arbor, MI) for viral whole genome sequencing using the ARTIC Network protocol on an Oxford Nanopore Technologies GridION instrument.22 SARS-CoV-2 lineages were assigned using the Pangolin (phylogenetic assignment of named global outbreak lineages) nomenclature.23 The WHO variant assignment was as follows: alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617.2 and AY lineages), and omicron (B.1.1.529 or BA lineages).
Severity of covid-19
We classified the severity of covid-19 based on the highest severity state reached during the index covid-19 hospital admission using a modified version of the WHO clinical progression scale (see supplementary table S2).13 24 In this analysis of patients admitted to hospital, the scale levels included: admitted to hospital without supplemental oxygen (level 4), admitted to hospital with standard supplemental oxygen (level 5), admitted to hospital with high flow nasal cannula or non-invasive ventilation (level 6), admitted to hospital with invasive mechanical ventilation (level 7), admitted to hospital with mechanical ventilation and additional organ support (eg, extracorporeal membrane oxygenation, vasopressors; level 8), and death (level 9). In addition to evaluating the full scale (levels 4-9) as an ordinal outcome, we also dichotomized the scale at level 7 to facilitate comparison between patients who experienced death or invasive mechanical ventilation (levels 7-9) compared with those who did not experience death or invasive mechanical ventilation (levels 4-6).
Statistical analysis
We classified cases of covid-19 into alpha, delta, and omicron variant categories using sequencing information for cases with lineages identified and by the predominant circulating variant at the time of hospital admission for those without a lineage identified. Periods of predominant circulation for alpha, delta, and omicron were defined based on time windows when each variant was identified in more than 50% of cases successfully sequenced in the study—alpha period: 11 March to 3 July 2021; delta period: 4 July to 25 December 2021; and omicron period: 26 December 2021 to 14 January 2022. For analyses of vaccine effectiveness to prevent hospital admissions, we evaluated cases and controls enrolled during the same period to maintain accuracy of vaccine effectiveness estimates. For analyses evaluating vaccine effectiveness against hospital admissions, we compared cases and controls in each period (alpha, delta, and omicron); cases were excluded from these analyses if they had a lineage identified by sequencing that was discordant with the period (eg, a delta lineage identified in the omicron period). For severity analyses, only cases were analyzed, and maintaining a temporal relationship with a control group was not necessary; therefore, all cases with sequencing confirmed alpha, delta, or omicron lineage were analyzed regardless of admission date; in these analyses, variant group was classified by sequencing confirmation of alpha, delta, or omicron lineage, and then by period for other cases. In sensitivity analyses, we analyzed only cases with sequencing confirmed variants.
To calculate the effectiveness of mRNA vaccines (BNT162b2 or mRNA-1273) to prevent hospital admission with covid-19, we used a test negative design in which the odds of antecedent vaccination was compared between cases and controls. We pooled participants in the test negative and syndrome negative control groups based on analyses showing highly similar vaccine coverage in the two control groups, and nearly identical estimates for vaccine effectiveness when either control group was used individually (see supplementary table S3).14 A multivariable unconditional logistic regression model was constructed with case-control status as the dependent variable, vaccination status (vaccinated v unvaccinated) as the primary independent variable, and the following covariables selected a priori as potential confounders: calendar date of admission in biweekly intervals, US Department of Health and Human Services region (10 regions), age, sex, and self-reported race and Hispanic ethnicity. Post hoc, we considered the following variables for potential inclusion as covariates, but as none of them changed the adjusted odds ratio by more than 5% they were not included in the final analysis: number of comorbidities, smoking status, living in a long term care facility before hospital admission, and working in a healthcare setting. Vaccine effectiveness to prevent hospital admission with covid-19 [Vaccine effectiveness(hospital admission)] was calculated with the adjusted odds ratio from this model as:
| Vaccine effectiveness(hospital admission)=(1−adjusted odds ratio)×100 |
Using this method, we calculated vaccine effectiveness against hospital admission with covid-19 separately for the alpha, delta, and omicron variants. Vaccine effectiveness for two vaccine doses was calculated for each period, and for three vaccine doses for the delta and omicron periods. Within each period we also calculated vaccine effectiveness for subgroups defined by immunocompromised status,14 age group (18-64 years, ≥65 years), burden of chronic medical conditions (0; ≥1 medical conditions), and vaccine product (BNT162b2, mRNA-1273), and for two vaccine doses recipients, the time between the second vaccine dose and symptom onset (14-150 days, >150 days). This threshold of 150 days was selected based on the recommendation for a third (booster) dose of mRNA vaccine after five months for immunocompetent adults.23 We report the results for subgroup analyses that had more than 150 cases and controls.
To show the severity of covid-19 by variant and vaccination status, we plotted the highest severity level on the modified WHO clinical progression scale attained for each case. We then compared severity among unvaccinated cases by variant (alpha, delta, omicron) and between unvaccinated and vaccinated cases within each variant group. In these analyses of severity, patients vaccinated with either two or three doses of an mRNA vaccine were considered fully vaccinated. These calculations were performed using a multivariable proportional odds regression model with WHO clinical progression scale as the dependent variable (levels 4-9), variant group (alpha, delta, omicron), or vaccination status (unvaccinated, vaccinated) as the primary independent variable, and the following covariables: age, sex, race and Hispanic ethnicity, and number of underlying medical conditions (0, 1, 2, 3, or ≥4 classes of chronic conditions). An adjusted proportional odds ratio >1.0 from these models indicated more severe disease for the later variant than a comparator earlier variant—for example, delta compared with alpha, and omicron compared with delta.
Next we calculated the vaccine effectiveness of mRNA vaccines to prevent disease progression to invasive mechanical ventilation or death among adults admitted to hospital with covid-19. A multivariable logistic regression model was constructed with the composite of invasive mechanical ventilation or death as the dependent variable, vaccination status (vaccinated with two or three doses v unvaccinated) as the primary independent variable, and the same covariables as included in the severity proportional odds model. Vaccine effectiveness to prevent in-hospital disease progression was calculated as:
| Vaccine effectiveness(progression)=(1−adjusted odds ratio)×100 |
Using this method, we calculated vaccine effectiveness against disease progression separately for the alpha, delta, and omicron groups.
We considered results to be statistically significant if 95% confidence intervals for odds ratios did not include the null (odds ratio=1.0), or two sided P values were <0.05. Missing values were not imputed; results were presented with denominators to indicate sample size in each analysis, and models included participants with complete data for all variables in the model. Statistical analyses were performed with Stata version 16 (College Station, TX) and SAS 9.4 (Cary, NC).
Patient and public involvement
Patients and members of the public were not directly involved in the design of this study. Throughout the study, patients were routinely engaged in the work through structured interviews conducted between research staff and patients in the hospital. These interviews included questions about patients’ perceptions and understandings of covid-19 vaccines. Results of the study will be disseminated to the public through announcements from CDC and through lay and social media.
Results
Participants and SARS-CoV-2 variants
Between 11 March 2021 and 14 January 2022, 14 128 patients were enrolled across 21 hospitals; 2438 patients were excluded from the primary analyses, most commonly for receiving more than one mRNA vaccine dose but not classifying into the two dose or three dose vaccine recipient categories (n=933), or for receiving a non-mRNA vaccine (n=682) (see supplementary figure S1). The population for analysis included 11 690 patients, comprising 5728 covid-19 cases and 5962 controls.
Results of SARS-CoV-2 genome sequencing were obtained for 2599/5728 (45.4%) cases in the analytical population. Among cases with sequencing completed, alpha was identified in 242/421 (57.5%) during the alpha period, delta in 1867/1930 (96.7%) during the delta period, and omicron in 190/248 (76.6%) during the omicron period (fig 1; also see supplementary table S4).
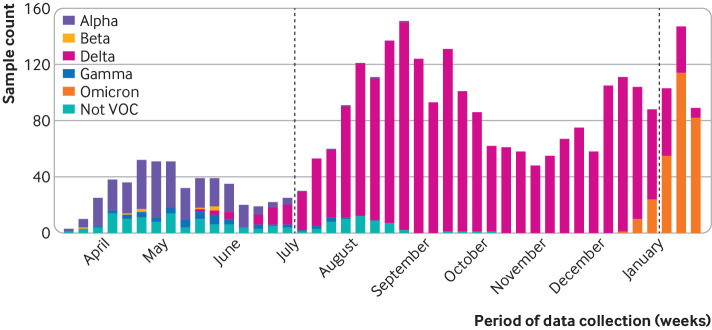
Fig 1.
Genome sequenced SARS-CoV-2 variants by week among participants with covid-19 admitted to hospital between 11 March 2021 and 14 January 2022 in 21 hospitals in the United States. Dashed lines represent start of delta variant period (4 July 2021) and start of omicron variant period (26 December 2021). The figure includes all enrolled cases with a sequencing result, without restriction to cases included in vaccine effectiveness analyses. SARS-CoV-2 variant lineages were identified for 3017 cases: alpha (n=299), beta (n=8), delta (n=2209), gamma (n=52), omicron (n=286), and lineage not designated as variant of concern (VOC, n=163)
Vaccine effectiveness to prevent hospital admission with covid-19
After excluding 146/5728 (2.5%) cases from the analysis of vaccine effectiveness against covid-19 related hospital admission who had sequence confirmed lineage discordant with the variant predominant period (eg, cases with sequencing confirmed delta variant during the alpha or omicron period), we included 5582 cases and 5962 controls in this part of the analysis. Cases included 1072 from the alpha period, 3951 from the delta period, and 559 from the omicron period. Compared with cases in the alpha and delta periods, cases in the omicron group tended to be older, have more underlying medical conditions, and be more likely to have one hospital admission or more in the past year (table 1, supplementary table S5). Consistent with increasing vaccine coverage in the US population over time, a greater proportion of cases were vaccinated (two or three doses of an mRNA vaccine) during the omicron period (291/559, 52.1%) than the alpha (119/1072, 11.1%) and delta (1080/3951, 27.3%) periods.
Table 1.
Characteristics of patients admitted to hospital without covid-19 (controls) and with covid-19 (cases) during periods of dominant SARS-CoV-2 variant: alpha (11 March to 3 July 2021), delta (4 July to 25 December 2021), and omicron (26 December 2021 to 14 January 2022). Values are numbers (percentages) unless stated otherwise
| Characteristics | Controls (n=5962) | Cases by variant period | ||
|---|---|---|---|---|
| Alpha (n=1072) | Delta (n=3951) | Omicron (n=559) | ||
| Median (IQR) age (years) | 63 (50-72) | 56 (43-65.5) | 57 (43-69) | 62 (49-73) |
| Women | 2975 (49.9) | 519 (48.4) | 1803 (45.6) | 264 (47.2) |
| Race and ethnicity: | ||||
| Non-Hispanic white | 3611 (60.6) | 484 (45.1) | 2183 (55.3) | 279 (49.9) |
| Non-Hispanic black | 1240 (20.8) | 285 (26.6) | 820 (20.8) | 127 (22.7) |
| Hispanic, any race | 772 (12.9) | 220 (20.5) | 695 (17.6) | 111 (19.9) |
| Non-Hispanic, other | 253 (4.2) | 63 (5.9) | 179 (4.5) | 33 (5.9) |
| Unknown | 86 (1.4) | 20 (1.9) | 74 (1.9) | 9 (1.6) |
| US Census region: | ||||
| North east | 885 (14.8) | 158 (14.7) | 686 (17.4) | 159 (28.4) |
| South | 2371 (39.8) | 395 (36.8) | 1544 (39.1) | 196 (35.1) |
| Midwest | 1374 (23.0) | 248 (23.1) | 978 (24.8) | 104 (18.6) |
| West | 1332 (22.3) | 271 (25.3) | 743 (18.8) | 100 (17.9) |
| Resident of long term care facility | 321/5778 (5.6) | 25/1039 (2.4) | 120/3795 (3.2) | 30/534 (5.6) |
| ≥1 hospital admission in past year | 3031/5537 (54.7) | 282/956 (29.5) | 1015/3682 (27.6) | 226/535 (42.2) |
| Current tobacco use | 1016/5302 (19.2) | 103/887 (11.6) | 366/3451 (10.6) | 59/470 (12.6) |
| Median (IQR) No of chronic conditions* | 2 (1-3) | 1 (1-3) | 1 (0-3) | 2 (1-3) |
| Categories of medical conditions*: | ||||
| Chronic cardiovascular disease | 4158 (69.7) | 589 (54.9) | 2141 (54.2) | 359 (64.2) |
| Chronic pulmonary disease | 1973 (33.1) | 231 (21.5) | 827 (20.9) | 151 (27.0) |
| Diabetes mellitus | 1962 (32.9) | 316 (29.5) | 1135 (28.7) | 164 (29.3) |
| Immunocompromising condition* | 1458 (24.5) | 172 (16.0) | 659 (16.7) | 138 (24.7) |
| Obesity | 2391/5900 (40.5) | 616/1056 (58.3) | 2099/3909 (53.7) | 260/556 (46.8) |
| Vaccination status: | ||||
| Unvaccinated | 2054 (34.5) | 953 (88.9) | 2871 (72.7) | 268 (47.9) |
| 2 doses (<150 days) | 2029 (34.0) | 119 (11.1) | 352 (8.9) | 34 (6.1) |
| 2 doses (≥150 days) | 1411 (23.7) | 0 (0) | 667 (16.9) | 177 (31.7) |
| 3 doses | 468 (7.8) | 0 (0) | 61 (1.5) | 80 (14.3) |
| Vaccine product received: | n=3908 | n=119 | n=1080 | n=291 |
| BNT162b2 (Pfizer-BioNTech) | 2269 (58.1) | 81 (68.1) | 708 (65.6) | 203 (69.8) |
| mRNA-1273 (Moderna) | 1615 (41.3) | 37 (31.1) | 368 (34.1) | 84 (28.9) |
| Mixed | 24 (0.6) | 1 (0.8) | 4 (0.4) | 4 (1.4) |
| Median (IQR) days since dose 3 | 41 (23-64) | — | 38 (23-65) | 69.5 (41.5-97) |
IQR=interquartile range.
See supplementary table S5 for baseline characteristics of cases limited to those with a sequencing confirmed variant.
Obtained using structured medical chart review and defined as one or more of the following: active solid organ cancer (active cancer defined as treatment for the cancer or newly diagnosed cancer in past six months), active hematologic cancer, HIV infection without AIDS, AIDS, congenital immunodeficiency syndrome, previous splenectomy, previous solid organ transplant, immunosuppressive drugs, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, scleroderma, or inflammatory bowel disease, including Crohn’s disease and ulcerative colitis.
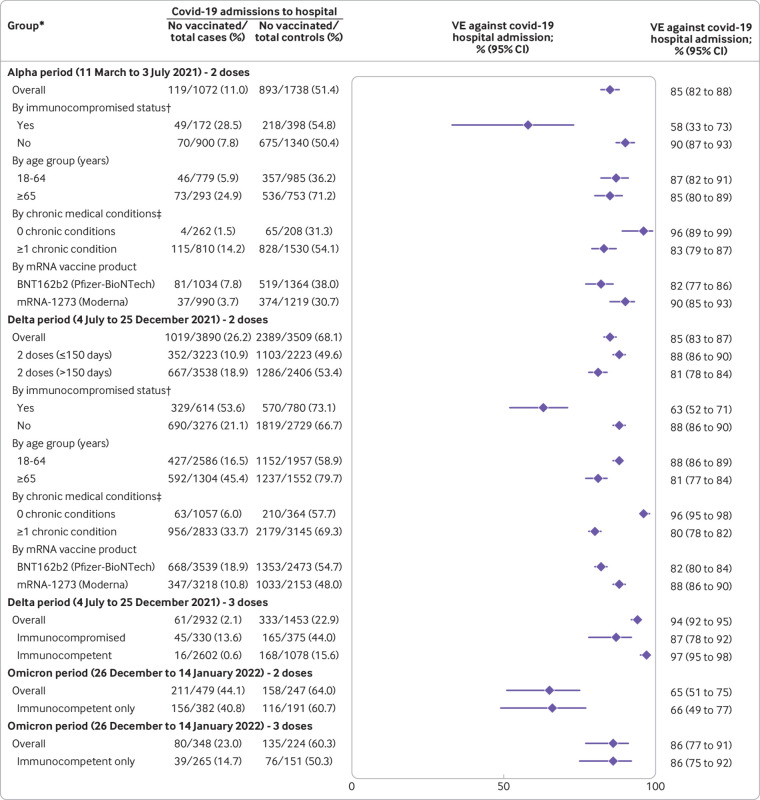
Vaccine effectiveness for two doses of mRNA vaccine to prevent hospital admission with covid-19 was 85% (95% confidence interval 82% to 88%) in the alpha period, 85% (83% to 87%) in the delta period, and 65% (51% to 75%) in the omicron period (fig 2). Vaccine effectiveness for three mRNA vaccine doses in the omicron period was 86% (77% to 91%), which was similar to the effectiveness of two doses during the alpha and delta periods. Within the delta period, vaccine effectiveness for two vaccine doses was lower when the second vaccine dose was more than 150 days before illness onset (81%, 78% to 84%) than 14-150 days (88%, 86% to 90%). Within the delta period, vaccine effectiveness of three vaccine doses (94%, 92% to 95%) was higher than two doses, with high vaccine effectiveness observed after a third dose in both immunocompetent (97%, 95% to 98%) and immunocompromised (87%, 78% to 92%) patients (fig 2). Within each period, vaccine effectiveness was lower for immunocompromised compared with immunocompetent patients and lower for the BNT162b2 vaccine than for the mRNA-1273 vaccine (fig 2). Results of sensitivity analyses limited to cases with sequence confirmed variants were consistent with those of primary analyses (see supplementary table S6). Supplementary appendix C presents the vaccine effectiveness results for partial vaccination (either one dose of an mRNA vaccine or two doses, with the second dose received <14 days before illness onset).
Fig 2.
Vaccine effectiveness (VE) of mRNA vaccines to prevent hospital admissions with covid-19 by variant group, including alpha, delta, and omicron. *Groups with ≥150 cases and controls were reported. Groups with <150 cases or <150 controls were not reported owing to low precision of VE estimates with these sample sizes. †Obtained using structured medical chart review, and defined as one or more of the following: active solid organ cancer (active cancer defined as treatment for the cancer or newly diagnosed cancer in past six months), active hematologic cancer, HIV infection without AIDS, AIDS, congenital immunodeficiency syndrome, previous splenectomy, previous solid organ transplant, immunosuppressive drugs, systemic lupus erythematosus, rheumatoid arthritis, psoriasis, scleroderma, or inflammatory bowel disease, including Crohn’s disease or ulcerative colitis. ‡Obtained using structured medical chart review, and defined as conditions within one or more of the following categories: cardiovascular disease, neurologic disease, pulmonary disease, gastrointestinal disease, endocrine disease, renal disease, hematologic disease, malignancy, immunosuppression not captured in other categories, autoimmune condition, or other condition (sarcoidosis, amyloidosis, or unintentional weight loss ≥10 pounds (4.5 kg) in past 90 days)
Severity of covid-19
The analysis of disease severity included data collected to 31 January 2022. Of 5728 case patients in the study, 5413 (94.5%) had complete data on clinical outcomes and were included in the severity analysis, comprising 1060 in the alpha group, 3788 in the delta group, and 565 in the omicron group (table 2). Including both vaccinated and unvaccinated patients, 582/5413 (11.7%) patients with covid-19 died within 28 days during the index hospital admission, including 81/1060 (7.6%) in the alpha group, 461/3788 (12.2%) in the delta group, and 40/565 (7.1%) in the omicron group.
Table 2.
In-hospital clinical outcomes among adults admitted with covid-19 by variant group (alpha, delta, omicron). Values are numbers (percentages) unless stated otherwise
| Outcome | Alpha group (n=1060) | Delta group (n=3788) | Omicron group (n=565) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated (n=116) | Unvaccinated (n=944) | P value* | Vaccinated (n=1045) | Unvaccinated (n=2743) | P value* | Vaccinated (n=293) | Unvaccinated (n=272) | P value* | |||
| Death | 5 (4.3) | 76 (8.1) | 0.15 | 138 (13.2) | 323 (11.8) | 0.23 | 15 (5.1) | 25 (9.2) | 0.059 | ||
| Invasive mechanical ventilation | 7 (6.0) | 201 (21.3) | <0.001 | 152 (14.5) | 681 (24.8) | <0.001 | 35 (11.9) | 49 (18.0) | 0.043 | ||
| Composite of death or invasive mechanical ventilation | 10 (8.6) | 218 (23.1) | <0.001 | 210 (20.1) | 748 (27.3) | <0.001 | 42 (14.3) | 54 (19.9) | 0.08 | ||
| Admitted to intensive care unit | 24 (20.7) | 353 (37.4) | <0.001 | 321 (30.7) | 1179/2742 (43.0) | <0.001 | 66 (22.5) | 89/271 (32.8) | 0.006 | ||
| Non-invasive ventilation | 15 (12.9) | 167 (17.7) | 0.20 | 151 (14.4) | 470 (17.1) | 0.046 | 39 (13.3) | 43 (15.8) | 0.40 | ||
| High flow oxygen | 15 (12.9) | 324 (34.3) | <0.001 | 289 (27.7) | 1148 (41.9) | <0.001 | 59 (20.1) | 84 (30.9) | 0.003 | ||
| Vasopressors | 6 (5.2) | 191 (20.2) | <0.001 | 155 (14.8) | 647 (23.6) | <0.001 | 36 (12.3) | 46 (16.9) | 0.12 | ||
| New renal replacement therapy | 5 (4.3) | 43 (4.6) | 0.91 | 49 (4.7) | 159 (5.8) | 0.18 | 14 (4.8) | 12 (4.4) | 0.84 | ||
| Median (IQR) hospital length of stay among survivors | 5 (3-8) | 5 (3-9) | 0.56 | 5 (3-9) | 6 (3-10) | <0.001 | 5 (3-9) | 6 (3-11) | 0.050 | ||
| Venous thromboembolic event | 7 (6.0) | 55 (5.8) | 0.93 | 46 (4.4) | 250 (9.1) | <0.001 | 15 (5.1) | 22 (8.1) | 0.15 | ||
| Stroke | 0 (0) | 18 (1.9) | 0.13 | 9 (0.9) | 44 (1.6) | 0.08 | 3 (1.0) | 4 (1.5) | 0.63 | ||
| Myocardial infarction | 1 (0.9) | 19 (2.0) | 0.39 | 29 (2.8) | 58 (2.1) | 0.23 | 5 (1.7) | 5 (1.8) | 0.91 | ||
IQR=interquartile range.
Obtained using χ2 testing, not adjusting for other factors.
Among unvaccinated cases, severity of covid-19 on the WHO clinical progression scale was highest for the delta group (delta v alpha adjusted proportional odds ratio 1.28, 95% confidence interval 1.11 to 1.46) and lowest for the omicron group (omicron v alpha 0.79, 0.62 to 1.01; omicron v delta 0.61, 0.49 to 0.77) (fig 3). Among unvaccinated cases, in-hospital death occurred in 76/944 (8.1%) in the alpha group, 323/2743 (11.8%) in the delta group, and 25/272 (9.2%) in the omicron group (table 2). Severity of covid-19 on the WHO clinical progression scale was substantially lower for vaccinated cases than for unvaccinated cases in each variant group: alpha (adjusted proportional odds ratio 0.33, 0.23 to 0.49), delta (0.44, 0.37 to 0.51), and omicron (0.61, 0.44 to 0.85).
Fig 3.
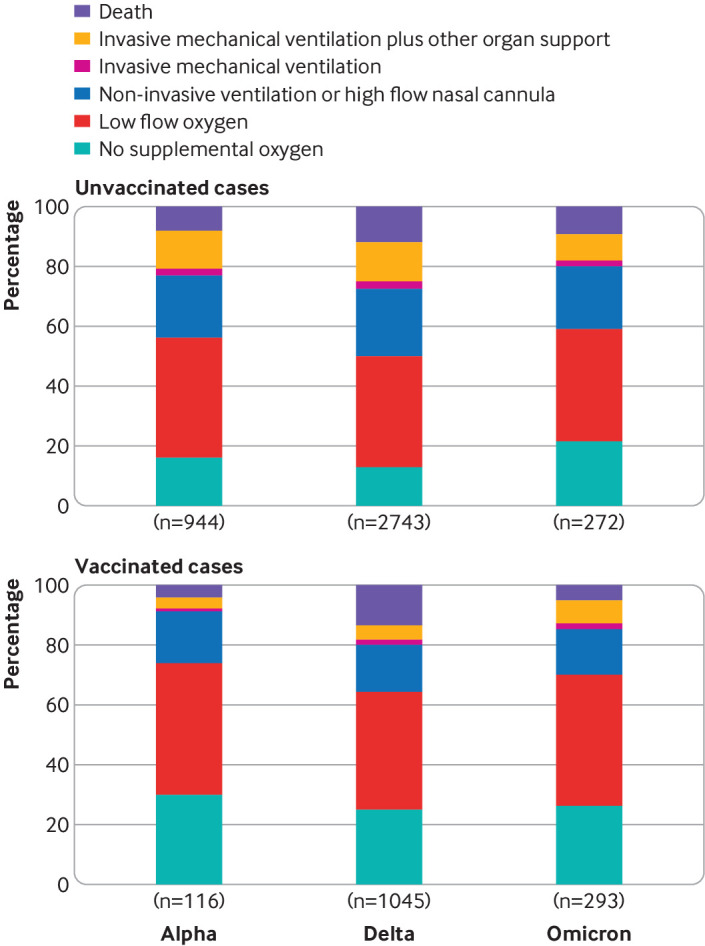
Severity of covid-19 during index hospital admission among adults with covid-19, by SARS-CoV-2 variant for unvaccinated and vaccinated patients. Disease severity was classified based on the highest severity level reached on the World Health Organization clinical progression scale, which ranged from hospital admission without supplemental oxygen (lowest level) to death (highest level). Among unvaccinated patients, severity was higher for the delta than alpha variant (adjusted proportional odds ratio 1.28, 95% confidence interval 1.11 to 1.46) and lower for the omicron than delta variant (0.61, 0.49 to 0.77). For each variant, severity was lower for vaccinated patients (two or three doses of an mRNA vaccine) than unvaccinated patients, including for alpha (adjusted proportional odds ratio 0.33, 0.23 to 0.49), delta (0.44, 0.37 to 0.51), and omicron (0.61, 0.44 to 0.85)
Across all variants, vaccinated patients with covid-19 who died tended to be older and had multiple medical conditions or immunocompromising conditions. Compared with the 424 unvaccinated patients with covid-19 who died, 158 vaccinated patients who died were older (median 72 v 61 years; P<0.001), were more likely to be immunocompromised (41% v 13%; P<0.001), had more categories of chronic medical conditions (median 3 v 2; P<0.001), and had more prescribed drugs before hospital admission (median 10 v 5; P<0.001).
Vaccine effectiveness to prevent covid-19 progression after hospital admission
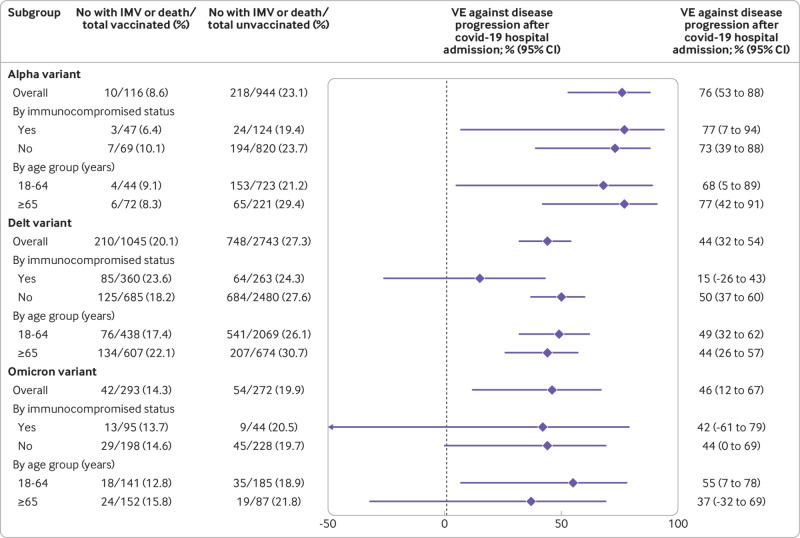
Among patients admitted to hospital with covid-19, vaccine effectiveness of mRNA vaccination (two or three doses) to prevent progression to invasive mechanical ventilation or death was 76% (95% confidence interval 53% to 88%) for the alpha variant, 44% (32% to 54%) for the delta variant, and 46% (12% to 67%) for the omicron variant. Vaccine effectiveness to prevent disease progression was observed in immunocompetent patients for all three variants but not observed in immunocompromised patients for the delta or omicron variant (fig 4). Results were similar when cases were limited to those with a sequence confirmed variant (see supplementary table S6).
Fig 4.
Effectiveness of two or three doses of mRNA vaccines among adults admitted to hospital with covid-19 to prevent disease progression to invasive mechanical ventilation (IMV) or death, by SARS-CoV-2 variant. VE=vaccine effectiveness
Discussion
The predominant circulating SARS-CoV-2 variant in the US changed from alpha to delta in July 2021 and then to omicron in December 2021. Understanding the severity of covid-19 with each variant and the effectiveness of available vaccines against them is essential for guiding vaccination policies and directing future vaccine development. The mRNA covid-19 vaccines that were authorized for use in the US in 2020 (BNT162b2 and mRNA-1273) were highly effective at preventing hospital admissions for all three variants during the subsequent year. However, three doses of an mRNA vaccine were necessary to achieve similar effectiveness against omicron in the winter of 2021-22 as two doses achieved for alpha and delta variants earlier in the year. Furthermore, although hospital admissions with covid-19 did occur among vaccinated patients, vaccination was associated with reduced risk of progression to invasive mechanical ventilation or death for all three variants.
Among unvaccinated adults admitted to hospital with covid-19, the delta variant was associated with the most severe disease, followed by the alpha variant and then the omicron variant. Among unvaccinated patients admitted to hospital, covid-19 due to the omicron variant was about 79% and 61% as severe as covid-19 due to the alpha and delta variants, respectively. The omicron variant was, however, associated with substantial critical illness and death, with 15% of patients admitted to hospital with the omicron variant (vaccinated and unvaccinated) progressing to invasive mechanical ventilation, and 7% dying in hospital.
Strengths and limitations of this study
This work has several strengths. The vaccine effectiveness analyses applied a test negative design to a large population of patients admitted to hospital with symptomatic, laboratory confirmed covid-19 along with concurrent controls, which enabled control for healthcare seeking behavior, robust subgroup analyses, and evaluation of outcomes beyond hospital admission, including level of respiratory support and mortality. Ascertainment of vaccination status was robust, with trained staff conducting patient interviews and searching multiple sources of vaccination records on a patient-by-patient basis. Respiratory samples collected in the study underwent centralized RT-qPCR testing and viral whole genome sequencing, which enabled precise characterization of time periods dominated by different variants.
The study also has limitations. First, use of in-patient controls might lead to biased estimates if control patients had different characteristics from people in the general community; however, vaccine coverage in the control population within this study tracked closely with that in the adult population in the US.25 Second, this study only evaluated patients admitted to hospital and thus does not inform vaccine effectiveness against mild covid-19 or differences in disease severity among people with SARS-CoV-2 variants in the outpatient setting. Third, the study only evaluated mRNA vaccines, not other types of covid-19 vaccines. Fourth, the analyses of in-hospital severity did not account for potential differences in clinical management during the periods when the alpha, delta, and omicron variants predominated that may have impacted outcomes. Fifth, although the test negative design is the preferred method for evaluating vaccine effectiveness with observational data,16 it has known potential limitations, including collider bias 26 27; the risk of collider bias was minimized in the current study by evaluating only severely ill patients.16 Sixth, sequencing did not identify a variant for some cases—typically those with low viral loads in tested respiratory samples. Variant classification for cases without a sequencing confirmed variant was based on the predominant circulating variant at the time; variant misclassification was possible for these cases, but sensitivity analyses limited to sequencing confirmed cases produced results similar to those in the primary analysis.
Comparison with other studies
Earlier studies from England5 and Scotland4 found an increased risk of hospital admission with the delta variant compared with alpha variant. More recent studies have suggested that people with a diagnosis of covid-19 due to the omicron variant are less likely to be admitted to hospital than those with the delta variant.8 This study adds robust measurements of disease severity after hospital admission and shows that the delta variant was associated with more severe covid-19 than the alpha and omicron variants, driven largely by higher rates of advanced respiratory support.
Emerging vaccine effectiveness estimates globally suggest reduced effectiveness against the omicron variant compared with previous variants,28 29 30 including an estimate of 70% vaccine effectiveness for two doses of the BNT162b2 vaccine to prevent hospital admissions due to the omicron variant in South Africa in November-December 2021.9 Using electronic health record data from sites across the US, the VISION Network recently estimated mRNA vaccine effectiveness against hospital admissions due to the omicron variant to be to 52% for two vaccine doses, with the second dose received within 180 days before illness onset, 38% for two doses received more than 180 days before illness onset, and 82% for three vaccine doses.11 Previous studies largely relied on estimating the predominant circulating SARS-CoV-2 variants from external data. This study adds vaccine effectiveness results against severe disease using sequencing data from within the study and found strong protection against the omicron variant with three mRNA vaccine doses in the first several months after receipt of a third dose.
Policy implications
These data indicate that the omicron variant is associated with serious covid-19 among those admitted to hospital, and preventive measures are needed. Vaccination with existing mRNA vaccine formulations is an effective preventive measure against the omicron variant, both for the prevention of hospital admissions and for the prevention of progression to critical illness and death among those admitted to hospital. Third doses of mRNA vaccines are needed to achieve high levels of protection. These findings support recent recommendations in the US for a third mRNA vaccine dose for both immunocompetent19 and immunocompromised18 adults as a key approach to protecting populations against the omicron variant.
The serial emergence of new SARS-CoV-2 variants, including delta and omicron, has challenged public health agencies to develop vaccine policies that counter the impact of waning immunity (the decline in protection of vaccine doses over time against the same variant) and viral immune evasion (new viral variants that are less susceptible to existing vaccines). Vaccine booster doses of the same vaccine formulation used in the primary vaccine series are designed to counter waning immunity. Substantial viral immune evasion would require new vaccine formulations targeting new variants to maintain protection. Boosters were implemented in several countries in response to covid-19 spikes with emergence of the delta variant. This study suggests that these booster doses were largely effective in preventing severe disease with both the delta variant and subsequently with the omicron variant. As the covid-19 pandemic continues to evolve, routine monitoring of vaccine effectiveness, especially against severe disease, and surveillance programmes to identify viral variants will be essential to inform decisions about booster vaccine policies and vaccine strain updates.
Conclusions
In this large, multistate study of adults in the US, mRNA vaccines were associated with strong protection against hospital admissions with covid-19 due to the alpha, delta, and omicron variants. Furthermore, for each of the variants, vaccination was associated with a reduced risk of covid-19 progressing to critical illness or death. Although disease severity for in-hospital patients was somewhat lower for the omicron variant than alpha and delta variants, patients admitted to hospital with covid-19 due to the omicron variant still had a substantial risk of critical illness and death. These findings suggest that vaccination against covid-19, including a third dose of an mRNA vaccine, is critical for protecting populations against covid-19-associated morbidity and mortality.
What is already known on this topic
Two doses of mRNA vaccines were highly effective for preventing hospital admissions with covid-19 due to the alpha and delta variants during the first year of the covid-19 vaccination programme in the United States
Authorized third doses of mRNA covid-19 vaccines were administered from August 2021 in an effort to maintain vaccine effectiveness over time
What this study adds
Effectiveness of two doses of an mRNA vaccine to prevent hospital admission with covid-19 was found to be lower for the omicron variant than alpha and delta variants (65%, 85%, and 85%, respectively), whereas three vaccine doses were found to achieve effectiveness against the omicron variant (86%) similar to two doses against the alpha and delta variants
The severity of disease among patients admitted to hospital with covid-19 was found to be lower with the omicron versus delta variant
Patients with the omicron variant were, however, found to be at risk of critical illness and death, with 15% of patients with the omicron variant in this study progressing to invasive mechanical ventilation and 7% to death
Acknowledgments
See supplementary appendix A for full list of investigators and collaborators in the Influenza and other Viruses in the Acutely Ill (IVY) Network.
Web extra.
Extra material supplied by authors
Supplementary information: supplementary appendix A-C, tables S1-S6, and figure S1
Contributors: ASL, MWT, and JDC contributed equally to this work as lead authors. WHS (protocol and data integrity and participant enrollment), MWT (statistical analysis), ASL (viral sequencing laboratory methods), and JDC (reverse-transcription polymerase chain reaction laboratory methods) are the guarantors for this work. WHS was responsible for the decision to submit the manuscript and takes responsibility for the work overall. ASL, MWT, JDC, MMP, and WHS wrote the initial manuscript draft (the authors alone wrote the manuscript without outside assistance). ASL, MWT, JDC, HKT, CJL, CGG, SJS, MK, JRV, MMP, and WHS conceptualized the study methods. ASL, JDC, MG, AAG, TMcN, SG, DJD, HKT, JDC, NMM, AZ, NIS, KWG, DCF, DNH, AS, MEP, HLE, MCE, MNG, AM, NJJ, VS, JSS, IDP, SMB, ETM, ASM, AK, CLH, LWB, CCtL, AD, JGW, AJG, NQ, SYC, CM, CR, HMB, JHK, NH, CGG, TWR, WBS, AB, KNW, JPR, and WHS collected the data. MWT, CJL, KWH, YZ, KA, and SMO were responsible for statistical analysis and data management. WHS acquired the funding. ASL, MWT, JDC, MG, AAG, TMcN, SG, DJD, HKT, JDC, NMM, AZ, NIS, KWG, DCF, DNH, AS, MEP, HLE, MCE, MNG, AM, NJJ, VS, JSS, IDP, SMB, ETM, ASM, AK, CLH, LWB, CCtL, AD, JGW, AJG, NQ, SYC, CM, CR, HMB, JHK, NH, CGG, TWR, WBS, AB, KNW, JPR, ASL, KWH, YZ, KA, SJS, SMO, MK, JRV, MMP, and WHS critically reviewed the manuscript for important intellectual content. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was funded by the US Centers for Disease Control and Prevention (CDC, award 75D30121F00002 to WHS). Scientists from CDC participated in all aspects of this study, including its design, analysis, interpretation of data, writing of the report, and the decision to submit the article for publication. The REDCap data tool used in this study was supported by a Clinical and Translational Science Award (UL1 TR002243) from the National Center for Advancing Translational Sciences, National Institutes of Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of CDC or the Agency for Toxic Substances and Disease Registry.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare the following. This study was funded by the US Centers for Disease Control and Prevention (CDC). ASL reports consultant fees from Sanofi and fees from Roche for membership on a trial steering committee. JDC reports grant support from CDC and National Institutes of Health (NIH). MG reports grant support from CDC. AAG reports grant support from CDC, NIH, Department of Defense (DoD), and an investigator initiated grant support from AbbVie and Faron Pharmaceuticals. HKT reports a grant from CDC. JDC reports a grant from the NIH (K23HL153584). DCF reports consultant fees from Cytovale and membership on a Medpace data safety monitoring board (DSMB). DNH reports a contract from CDC (via subcontract with Vanderbilt University Medical Center) and salary support from Incyte, EMPACT Precision Medicine, and the Marcus Foundation. MCE reports talks on nutrition in covid-19 pneumonia at the Nutritional Science and Practice Conference sponsored by Abbott Laboratories. MNG reports grant support from CDC, funding from the National Heart, Lung, and Blood Institute, and fees for participating on a DSMB for Regeneron. IDP reports grants from CDC, NIH, Intermountain Research and Medical Foundation, and Janssen Pharmaceuticals, institutional fees from Asahi Kasei Pharma and from Regeneron. SMB reports grants from CDC, Sedana, Janssen, NIH, and DoD; fees from Hamilton for chairing a DSMB; institutional fees from Faron; book royalties from Oxford University and Brigham Young University; and personal fees from New York University for service on a DSMB. ETM reports a grant from Merck for unrelated work. AK reports grants from Gilead, Ely Lily, United Therapeutics, Johnson and Johnson (Actelion), Liquidia Pharmaceuticals, and 4D Medical. SYC was a speaker for La Jolla Pharmaceuticals and a consultant for PureTech Health. JHK reports grant support from NIH/National Institute of Allergy and Infectious Diseases (1K23 AI137321-01A1). NH reports grants from CDC, Sanofi, and Quidel. CGG reports consultant fees from Pfizer, Merck, and Sanofi-Pasteur and grants from Campbell Alliance/Syneos Health, CDC, NIH, Food and Drug Administration, Agency for Healthcare Research and Quality, and Sanofi. TR reports grant support from CDC. CJL reports grants from CDC, NIH, DoD, and the Marcus Foundation; organizational contract fees from bioMerieux, Endpoint, and Entegrion; and a patent issued to Cincinnati Children’s Hospital Medical Center for risk stratification in sepsis and septic shock. WHS reports grant funding from CDC for this work, grants and consultant fees from Merck outside this work, and consultant fees from Aerpio Pharmaceuticals outside this work.
The study guarantors (WHS, MWT, ASL, and JDC) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: Results will be disseminated to relevant communities through public health announcements from the US Centers for Disease Control and Prevention, through press releases in the lay press, and public presentations by the investigators.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This programme was approved as a public health surveillance activity with waiver of informed consent by institutional review boards at the US Centers for Disease Control and Prevention, the programme’s coordinating center at Vanderbilt University Medical Center, and each participating site.
Data availability statement
No additional data available.
References
- 1. Lauring AS, Hodcroft EB. Genetic Variants of SARS-CoV-2-What Do They Mean? JAMA 2021;325:529-31. 10.1001/jama.2020.27124. [DOI] [PubMed] [Google Scholar]
- 2.Tracking SARS-CoV-2 variants. https://www.who.int/health-topics/typhoid/tracking-SARS-CoV-2-variants (accessed 26 Oct 2021).
- 3.GISAID - hCov19 Variants. https://www.gisaid.org/hcov19-variants/ (accessed 26 Oct 2021).
- 4. Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health Scotland and the EAVE II Collaborators . SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021;397:2461-2. 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Twohig KA, Nyberg T, Zaidi A, et al. COVID-19 Genomics UK (COG-UK) consortium . Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis 2022;22:35-42. 10.1016/S1473-3099(21)00475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taylor CA, Patel K, Pham H, et al. COVID-NET Surveillance Team . Severity of Disease Among Adults Hospitalized with Laboratory-Confirmed COVID-19 Before and During the Period of SARS-CoV-2 B.1.617.2 (Delta) Predominance - COVID-NET, 14 States, January-August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1513-9. 10.15585/mmwr.mm7043e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viana R, Moyo S, Amoako DG, et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022;7. 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewnard JA, Hong VX, Patel MM, et al. Clinical outcomes among patients infected with Omicron (B.1.1.529) SARS-CoV-2 variant in southern California. medRxiv 2022; 01.11.22269045. 10.1101/2022.01.11.22269045. [DOI] [Google Scholar]
- 9. Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med 2022;386:494-6. 10.1056/NEJMc2119270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson AG, Amin AB, Ali AR, et al. MSHI . COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence - 25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep 2022;71:132-8. 10.15585/mmwr.mm7104e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a Third Dose of mRNA Vaccines Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults During Periods of Delta and Omicron Variant Predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022;71:139-45. 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tenforde MW, Self WH, Naioti EA, et al. IVY Network Investigators. IVY Network . Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults - United States, March-July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1156-62. 10.15585/mmwr.mm7034e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tenforde MW, Self WH, Adams K, et al. Influenza and Other Viruses in the Acutely Ill (IVY) Network . Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021;326:2043-54. 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tenforde MW, Patel MM, Ginde AA, et al. Tenforde MW, Patel MM, Ginde AA, et al. Influenza and Other Viruses in the Acutely Ill (IVY) Network . Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States. Clin Infect Dis 2021;ciab687. 10.1093/cid/ciab687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Self WH, Tenforde MW, Rhoads JP, et al. IVY Network . Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions - United States, March-August 2021. MMWR Morb Mortal Wkly Rep 2021;70:1337-43. 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel MK, Bergeri I, Bresee JS, et al. Evaluation of post-introduction COVID-19 vaccine effectiveness: Summary of interim guidance of the World Health Organization. Vaccine 2021;39:4013-24. 10.1016/j.vaccine.2021.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewnard JA, Patel MM, Jewell NP, et al. Theoretical Framework for Retrospective Studies of the Effectiveness of SARS-CoV-2 Vaccines. Epidemiology 2021;32:508-17. 10.1097/EDE.0000000000001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. FDA. 2021. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised (accessed 31 Jan 2022).
- 19.Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Expands Eligibility for COVID-19 Vaccine Boosters. FDA. 2021. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-expands-eligibility-covid-19-vaccine-boosters (accessed 31 Jan 2022).
- 20. Tenforde MW, Patel MM, Gaglani M, et al. IVY Network . Effectiveness of a Third Dose of Pfizer-BioNTech and Moderna Vaccines in Preventing COVID-19 Hospitalization Among Immunocompetent and Immunocompromised Adults - United States, August-December 2021. MMWR Morb Mortal Wkly Rep 2022;71:118-24. 10.15585/mmwr.mm7104a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel. 2021. www.fda.gov/media/134922/download (accessed 26 May 2021).
- 22.Quick J. nCoV-2019 sequencing protocol v3 (LoCost). protocols.io. 2020. www.protocols.io/view/ncov-2019-sequencing-protocol-v3-locost-bh42j8ye (accessed 26 May 2021).
- 23. Rambaut A, Holmes EC, O’Toole Á, et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol 2020;5:1403-7. 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marshall JC, Murthy S, Diaz J, et al. WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020;20:e192-7. 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. COVID Data Tracker. 2022. https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed 31 Jan 2022).
- 26. Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun 2020;11:5749. 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan SG, Tchetgen Tchetgen EJ, Cowling BJ. Theoretical Basis of the Test-Negative Study Design for Assessment of Influenza Vaccine Effectiveness. Am J Epidemiol 2016;184:345-53. 10.1093/aje/kww064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rufino J, Baquero C, Frey D, et al. Using Survey Data to Estimate the Impact of the Omicron Variant on Vaccine Efficacy against COVID-19 Infection. Public and Global Health. medRxiv 2022. 10.1101/2022.01.21.22269636 [DOI] [PMC free article] [PubMed]
- 29. Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med 2022; published online 21 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Young-Xu Y. Effectiveness of mRNA COVID-19 Vaccines against Omicron among Veterans. medRxiv 2022; 10.1101/2022.01.15.22269360. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: supplementary appendix A-C, tables S1-S6, and figure S1
Data Availability Statement
No additional data available.