Abstract
Hypothyroidism is a common endocrinopathy, and levothyroxine is frequently prescribed. Despite the basic tenets of initiating and adjusting levothyroxine being agreed on, there are many nuances and complexities to consistently maintaining euthyroidism. Understanding the impact of patient weight and residual thyroid function on initial levothyroxine dosage and consideration of age, comorbidities, thyrotropin goal, life stage, and quality of life as levothyroxine is adjusted can be challenging and continually evolving. Because levothyroxine is a lifelong medication, it is important to avoid risks from periods of overtreatment or undertreatment. For the subset of patients not restored to baseline health with levothyroxine, causes arising from all aspects of the patient’s life (coexistent medical conditions, stressors, lifestyle, psychosocial factors) should be broadly considered. If such factors do not appear to be contributing, and biochemical euthyroidism has been successfully maintained, there may be benefit to a trial of combination therapy with levothyroxine and liothyronine. This is not supported by the majority of randomized clinical trials, but may be supported by other studies providing lower-quality evidence and by animal studies. Given this discrepancy, it is important that any trial of combination therapy be continued only as long as a patient benefit is being enjoyed. Monitoring for adverse effects, particularly in older or frail individuals, is necessary and combination therapy should not be used during pregnancy. A sustained-release liothyronine preparation has completed phase 1 testing and may soon be available for better designed and powered studies assessing whether combination therapy provides superior therapy for hypothyroidism.
Keywords: hypothyroidism, levothyroxine, liothyronine, euthyroidism, patient-reported outcomes, quality-of-life
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
Hypothyroidism is a common condition requiring lifelong therapy with thyroid hormone replacement.
The results of levothyroxine initiation and adjustment are believed to provide patient satisfaction with therapy in the majority of cases.
Levothyroxine therapy must be continually monitored as dose requirements may change across the lifespan and with changes in a patient’s physiological and medical situation.
A subset of patients do not feel well while taking levothyroxine, despite the best attempts to optimize therapy.
For dissatisfied patients, if other potential causes of their symptoms have been fully addressed, a trial of personalized combination therapy with the addition of liothyronine can be contemplated, and can be continued as long as patient benefit and safety are maintained.
Although the majority of prior trials of combination therapy with levothyroxine and liothyronine have not shown patient benefit, these trials were largely underpowered and suffered from shortcomings such as short duration and once-daily liothyronine dosing.
Future combination therapy trials that address some of the weaknesses of prior trials, and hopefully also use a sustained release liothyronine preparation, are eagerly anticipated by physicians and patients.
Brief History of Treatment with Thyroid Hormone
The goal of treating hypothyroidism with thyroid hormone replacement remains to “provide resolution of the patient’s symptoms and hypothyroid signs,” along with normalizing the biochemical abnormalities of the hypothyroid state and avoiding undertreatment or overtreatment (1). The remarkable ability of thyroid hormone replacement to reverse the signs and symptoms of hypothyroidism has been known for centuries. The ancient Chinese are credited with using consumption of seaweeds and animal thyroids to treat thyroid conditions (2). The use of thyroid grafting and injection of thyroid gland extracts was documented in the French and English literature in the 1890s (3). Therapy quickly progressed to the oral administration of thyroid extracts. In 1893 Beadles compiled a list of 100 cases of individuals with “myxoedema and cretinism” (4), with a subsequent report of 28 similar cases by Bramwell in 1895 (5). Their respective descriptions state “… this treatment is invariably followed by an improvement…, by a rapid change in the appearance of the patient...the patient has so far recovered from the disease that it is impossible…to recognize the case as one of myxoedema” and “The effects produced in cases of myxoedema by a relatively minute quantity of thyroid extract, the rapidity with which all the characteristic symptoms disappear under the influence of the thyroid treatment, and the extraordinary improvement …. are very remarkable.” The use of desiccated thyroid extract (DTE) became the routine therapy for hypothyroidism, transitioning to the predominant use of synthetic levothyroxine (LT4) in the 1980s (6). Despite the success of such therapy, the complexities and nuances of recapitulating the euthyroid state in individuals who have developed thyroid hormone deficiency may not yet have been perfected for all patients. The optimization of therapy for all hypothyroid patients remains the goal of endocrinologists. It is possible that we may progress from the early days of “organotherapy” to the ability to be able to “cure” hypothyroidism through prevention of autoimmune damage or regeneration of functioning thyroid follicles. Until this is achieved, research continues as to how to best optimize thyroid hormone replacement therapy for as many patients as possible.
Background Regarding Hypothyroidism
Hypothalamic-Pituitary-Thyroid Axis
The hypothalamic-pituitary-thyroid axis is a classic negative feedback loop involving 4 hormones. Hypothalamic thyrotropin-releasing hormone (TRH) stimulates the release of thyrotropin hormone (TSH) from the pituitary gland. TSH supports the synthetic machinery within the thyroid gland and stimulates the resorption of thyroglobulin from within the lumen of thyroid follicles. Thyroxine (T4) and 3,5,3′-triiodothyronine (T3) are both then released from the thyroid gland into the circulation in the proportion of approximately 14:1 (1). There is a reciprocal relationship between serum concentrations of TSH and free T4 (FT4), so the concentration of FT4 increases or decreases; the response is an exponential decrease or increase in TSH concentration. An inverse relationship is therefore seen when TSH concentrations are plotted on a logarithmic scale vs FT4 concentrations on a linear scale.
Thyroid Physiology and Thyroid Hormone Action
Dietary iodine in the form of iodide or iodate is absorbed in the gastrointestinal tract and distributed in the extracellular fluid. Circulating iodide is actively transported into the thyrocyte by the sodium-iodide symporter located within its basolateral membrane. The reactive iodinium ion intermediate formed is then covalently bound to tyrosyl residues present in thyroglobulin to generate monoiodotyrosine and diiodotyrosine residues through a process known as organification. Thyroid peroxidase also catalyzes the coupling of the monoiodotyrosine and diiodotyrosine residues to generate T4 and T3 residues in thyroglobulin, which is secreted into the follicular lumen. As needed, thyroglobulin is then pinocytosed at the apical membrane, and T4 and T3 are secreted into the circulation after proteolysis of thyroglobulin. Thyroid hormones are necessary for the development and metabolic homeostasis of all the tissues and organ systems of the body. Estimates suggest that the intact thyroid gland produces approximately 85 to 100 mcg T4 per day and 5 to 6.5 mcg T3 per day (7). Type 1 and 2 deiodinases convert the prohormone T4 into T3, the active form of thyroid hormone, by outer ring deiodination, producing another 26.5 mcg of T3 daily. The type 3 deiodinase is responsible for converting T4 and T3 into their inactive forms of reverse T3 and 3,3′-diiodothyronine, respectively, via inner ring deiodination (1).
Although thyroid hormones are lipophilic, they nevertheless require specific thyroid hormone transporters to gain entry into cells (8). Three transporters from 2 transporter families have high specificity for thyroid hormones. These are monocarboxylate transporters 8 and 10 (MCT8 and MTC10) and organic anion transporting polypeptide 1C1 (OATP1C). Deiodinases and thyroid hormone transporters are differentially expressed across tissues and are thus essential for the tissue-specific actions of thyroid hormones. Once it has gained entry into the nucleus of the target cell, the active ligand T3 binds to thyroid hormone receptors, which in turn bind, mainly as heterodimers with retinoid-X receptors, to thyroid hormone response elements in target genes. Two genes, THRA and THRB, encode the thyroid hormone receptor proteins (TRα and TRβ), of which there are several splice products or isoforms. TRα1 is predominately expressed in the brain, heart, and skeletal muscle. TRβ1 is widely expressed, whereas TRβ2 is mostly expressed in the brain, pituitary, retina, and inner ear, and TRβ3 is mostly expressed in the kidney, liver, and lung. This differential expression of TRα and TRβ is also essential for the tissue-specific actions of thyroid hormones. Following the binding of the heterodimer complex to the thyroid hormone response element within the target gene, there is ensuing RNA transcription and protein synthesis in order to generate the cellular response to thyroid hormone.
Although T3 is generally accepted as the active thyroid hormone, T4 also has intrinsic genomic activity (9, 10), albeit perhaps at a higher concentration. In a recent series of studies in mice that had a triple knockout of the type 1 deiodinase (DIO1), type 2 deiodinase (DIO2), and Pax8 genes, the ability of T4 and T3 to independently regulate gene expression in the liver during the neonatal period (days 2-14) was demonstrated (11). Treatment of the knockout mice with either T4, T3, or both T4 and T3 revealed that genes could be independently or synergistically upregulated or downregulated by these hormones in a complex interplay (11). If similar findings were found in humans in other development periods, such as in adulthood, this could have theoretical implications for choosing combination therapy both with LT4 and liothyronine (LT3), rather than using LT4 or LT3 monotherapy.
Epidemiology
Throughout the world the prevalence of hypothyroidism varies from 0.25% to 4.2% (12). Prevalence rates differ for iodine-sufficient countries vs iodine-deficient countries. For example, in an iodine-deficient country (Denmark) the overall prevalence of overt hypothyroidism was 0.37%, compared with an overall prevalence of overt hypothyroidism in an iodine-sufficient area (Norway) of 0.7%. The incidence of hypothyroidism generally increases following introduction of iodine-fortification programs. Prevalence rates also differ by sex and age, with more hypothyroidism in women and older age groups. Cases of subclinical hypothyroidism (SCH) exceed those of overt hypothyroidism. For example, in a study using National Health and Nutrition Examination Survey (NHANES) III data the overall prevalence of hypothyroidism was 4.6%, with 0.3% being overt hypothyroidism and 4.3% being subclinical disease (13). With respect to overt hypothyroidism, the prevalence in the general population depends on the definition used and population studied and ranges from between 0.2% and 5.3% in Europe and 0.3% and 3.7% in the United States (12).
Clinical Manifestations
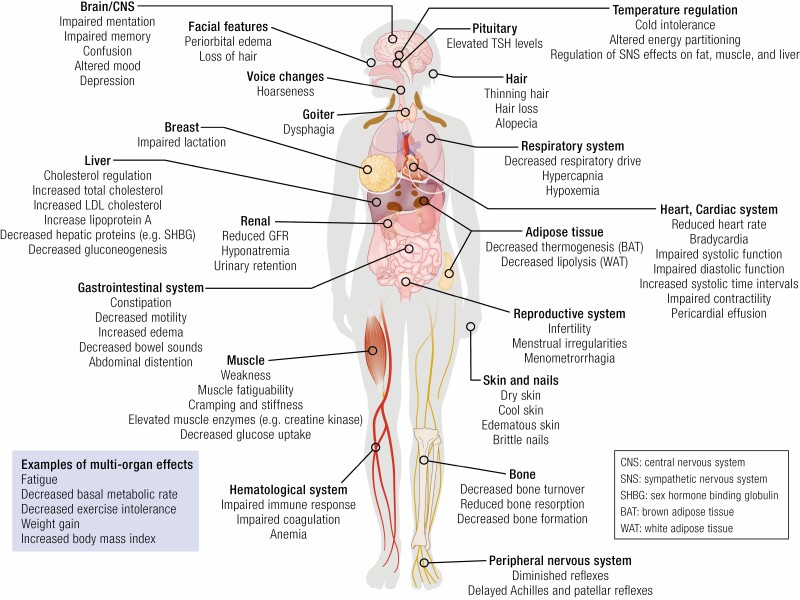
The clinical manifestations of hypothyroidism are diverse and potentially emanate from the effects of thyroid hormone deficiency in any organ system of the body (Fig. 1). Both signs and symptoms exhibit a wide spectrum of severity, ranging from subtle to profound (8).
Figure 1.
Examples of effects of hypothyroidism on organs and tissues in the adult, including signs, symptoms, and “biomarkers.”
Symptoms
The symptoms of hypothyroidism are a consequence of low levels of thyroid hormone throughout the body (see Fig. 1). Symptoms may be mild and barely perceptible or can be severe. In addition, symptoms of hypothyroidism are nonspecific and can overlap with symptoms of nonthyroid origin. For example, weight gain can be associated with untreated hypothyroidism, but can also occur in euthyroid individuals. The same statement can be made concerning the nonspecificity of fatigue, constipation, memory problems, and dry skin. By way of example, in one study, dry skin was reported in 71% of those with hypothyroidism, vs 54% of those who were euthyroid, muscle weakness was described in 21% of those with hypothyroidism, vs 21% of those who were euthyroid, and poor memory was noted in 18% of those with hypothyroidism, vs 16% of those who were euthyroid (14). This has the dual consequences that individuals with these symptoms may be more likely to be suspected of having hypothyroidism, and that individuals with these symptoms may be diagnosed with hypothyroidism because they were screened, even if hypothyroidism was not the proximate cause of their symptoms. In the same study, a change in symptoms in those with hypothyroidism, compared with those who were euthyroid (such as being colder than the previous year), had a higher likelihood ratio for hypothyroidism (14). Interestingly, these types of symptoms are prevalent in those with Hashimoto thyroiditis, even in the setting of euthyroidism. Furthermore, higher titers of thyroid peroxidase antibodies (TPO Abs) are associated with greater symptomatology (15).
Signs
Mild degrees of thyroid hormone deficiency may not result in obvious signs, except perhaps a firm goiter due to lymphocytic infiltration of the thyroid parenchyma. Over time, and with more decrement in thyroid hormone levels, cool and dry skin, coarse hair, loss of body hair, hoarse voice, coarse facial features, facial edema, generalized edema, bradycardia, and delayed relaxation phase of the deep tendon reflexes may be seen (8) (see Fig. 1). Extreme manifestations of these findings may be seen in myxedema coma. Manifestations of other autoimmune diseases such as vitiligo or hyperpigmentation may coexist with those of hypothyroidism.
Etiology
The most common cause of hypothyroidism, in which the thyroid gland itself fails, is referred to as primary hypothyroidism (8). Primary hypothyroidism is generally considered “overt” when the TSH is elevated and the FT4 is low. Milder degrees of hypothyroidism, also known as SCH, are defined by an elevated TSH, accompanied by a FT4 concentration that has not yet fallen below the normal range. Secondary or central hypothyroidism occurs when there is deficient production of TSH and is a much less common cause of hypothyroidism. Among the causes of primary hypothyroidism, autoimmune (Hashimoto) thyroiditis is the most common cause in iodine-sufficient areas. Hashimoto thyroiditis is 8 times more common in women than men, with a peak age of onset of 40 to 60 years. It occurs more frequently in White and Asian individuals than in African American individuals.
The list of medications that are associated with the development of hypothyroidism is ever expanding. There are several iatrogenic means by which the thyroid gland can be destroyed. These include surgery, radioactive iodine given as treatment for hyperthyroidism or thyroid cancer, and radiotherapy for head and neck malignancies. Infiltration of the thyroid gland associated with a spectrum of disease processes, such as sarcoidosis, hemochromatosis, hematologic malignancies, solid malignancies, and infectious agents, can impair functioning to a sufficient degree to cause hypothyroidism.
Thyroiditis can be transient and cause temporary hypothyroidism, as the second phase of a 3-phase process (hyperthyroidism, hypothyroidism, euthyroidism). Causes include granulomatous, postpartum, and silent thyroiditis. Alternatively, the thyroiditis can be more widespread and not associated with recovery, as may occur in silent thyroiditis in those who already have lymphocytic infiltration of their thyroid gland and in Riedel thyroiditis. The effect of iodine on thyroid function is complex. Not only is there a U-shaped curve with insufficient iodine causing hypothyroidism and excessive iodine causing hyperthyroidism, but also introduction of iodine supplementation programs can enhance autoimmunity and change the pattern of thyroid diseases in a country. Iodine deficiency is associated with the elevation of serum TSH levels and development of diffuse and nodular goiter as the thyroid axis attempts to maintain adequate thyroid functioning by increasing iodine uptake, accompanied also by enhanced iodine recycling. As the degree of iodine deficiency worsens, it can lead to hypothyroidism due to failure of the compensation mechanisms leading to lack of the substrate needed for thyroid hormone synthesis.
Exposure to iodine can also induce hypothyroidism in those with predisposing risk factors such as prior episodes of thyroiditis or Hashimoto thyroiditis.
Secondary hypothyroidism is characterized by insufficient TSH stimulation of a normal thyroid gland (8). This condition may be inherited because of several genetic defects for which a number of candidates genes have been identified. The acquired forms of secondary hypothyroidism include lesions in the sella turcica region (eg, pituitary adenomas, craniopharyngioma, and gliomas), pituitary damage due to surgery or radiation, head injury, vascular accidents, drugs, and infiltrative processes (lymphocytic hypophysitis, sarcoidosis, hemochromatosis, and infectious agents). In addition to pituitary dysfunction, hypothalamic dysfunction and TRH deficiency can also result in central hypothyroidism. Some of the candidates genes that are associated with TSH deficiency may also be associated with TRH deficiency.
Several rare conditions can be associated with impaired sensitivity to thyroid hormone. This was originally described as occurring with alterations in the THRA and THRB genes, but the discovery of genetic mutations and polymorphisms causing alterations in cell membrane transport (eg, MCT8) and metabolism (eg, selenocysteine insertion sequence-binding protein 2 [SECISBP2] and DIO2) of thyroid hormone have led to a broader definition of impaired sensitivity to thyroid hormone that includes many defects that could interfere with the activity of thyroid hormone. In addition to mutations causing tissue-specific hypothyroidism, an interesting and also rare cause of peripheral hypothyroidism is “consumptive hypothyroidism.” This is a paraneoplastic syndrome in which a tumor produces excessive levels of the type 3 deiodinase, leading to markedly increased degradation of T4, and resultant low T3 levels, high reverse T3 levels, and an elevated TSH.
Diagnosis
Diagnosis of thyroid “disease” is based on thyroid parameters (eg, TSH, FT4, and T3) confirmed as being outside their reference interval, combined with the signs and symptoms that would be anticipated for these “out-of-range” values. As for any other analyte, the reference interval for a thyroid analyte is derived from the 95% CIs for that particular analyte established in at least 120 normal volunteers. These reference intervals or decision limits are provided to try to aid in the interpretation of test results (16). Laboratories typically generate their own reference interval based on their local population. The statistical methods used by commercial laboratories for reference interval generation are often not described and may differ between laboratories. These reference intervals are then used by various agencies to make recommendations about categorization or decisions based on the reference intervals. Given the consequences that may occur depending on the exact limits of these reference intervals, it is critical for clinicians to appreciate the factors that can influence these reference intervals (16). The bounds of a reference interval can, for example, result in diagnoses that have minimal consequences being made, or diagnoses in which intervention would be of benefit being missed.
Serum thyrotropin
The most commonly measured thyroid analyte is the serum TSH concentration. The immunometric assays used to measure TSH have a functional sensitivity of 0.02 mIU/L or less and are able to detect the full spectrum of TSH values, ranging from the very low values seen with primary hyperthyroidism to the high values seen with primary hypothyroidism. In one study the lower limit of the TSH reference interval ranged from 0.51 to 0.63 mIU/L and the upper limit ranged from 3.60 to 4.31 mIU/L depending on the TSH assay used (17). Hypothyroidism is diagnosed when the TSH is confirmed to be above the limits of the reference interval. The effect of age on the reference interval is a particularly important issue for TSH. Serum TSH concentrations tend to rise with age so that the upper limit of the reference interval is higher in older individuals. This is a very important consideration because the benefits of thyroid hormone treatment for mild TSH elevations are not well established in older age groups (16). There are also within-person variations in serum TSH, so that individuals appear to have their own set-points (18, 19). If central hypothyroidism due to pituitary or hypothalamic dysfunction is suspected, reliance on the serum TSH values for diagnostic purposes is no longer indicated. A low or low normal TSH in the setting of a low FT4 should raise the possibility of central hypothyroidism.
Free thyroxine levels
FT4 is usually measured by immunoassay. Most clinical laboratories do not physically separate bound hormone from free hormone but use an estimation method. This means that the results of FT4 measurement are affected by protein binding and may be less accurate at extremes of binding protein concentrations, such as may occur during pregnancy. Even more than is the case with TSH, FT4 reference intervals are highly method dependent, with the lower limit of the reference interval reported as 0.61 to 0.97 ng/dL and the upper limit reported as 1.08 to 1.45 ng/dL in one study (17). FT4 levels below the limits of the reference limit are consistent with hypothyroidism. Although the gold standard for assessing thyroid status has been considered to be the serum TSH, some studies suggest that thyroid hormone levels may be important too. A recent systematic review found that clinical parameters representing the cardiac, bone, and metabolism systems were more associated with FT4 levels than TSH levels (20), suggesting that further research is needed to understand the importance of thyroid hormone levels as an indicator of thyroid status.
Log-linear thyrotropin vs free thyroxine relationship
Measurement of serum TSH is considered an ideal screening test for thyroid dysfunction because there is an inverse log-linear relationship between TSH and FT4 (8). This means that a small alteration in FT4 will produce a far larger change in serum TSH, thereby signaling even mild perturbations in FT4. Thus, even though TSH measurement is an indirect assessment of FT4, it is generally more sensitive for assessing thyroid dysfunction than measuring FT4. Reliance on TSH as an indicator of thyroid status assumes that the pituitary is functional, that the individual is not suffering from a nonthyroidal illness, and that the thyroid status is stable. When thyroid status is unstable the serum TSH concentrations may lag behind the clinical picture.
3,5,3′-Triiodothyronine levels
If measuring T3, it is generally better to measure total T3 rather than free T3 (FT3) because of the low concentrations of free hormone present and the lesser performance of immunoassays at these lower concentrations. Concentrations of total T3 are affected by binding protein abnormalities, as might occur with estrogen therapy and during pregnancy. An elevated total T3 that is above the upper limit of the reference interval is consistent with hyperthyroidism. However, total T3 that is below the lower limit of the reference interval is not necessarily sensitive or specific for hypothyroidism (8). This is because total T3 levels fall later than FT4 levels in the course of development of hypothyroidism due to enhanced T4 to T3 conversion, and also because T3 levels are lowered by illness, poor nutrition, starvation, fasting, and several drugs such as β-blockers and glucocorticoids.
Thyroid antibody testing
With Hashimoto disease, TPO antibodies and thyroglobulin Abs are generally present in the serum, precede the development of thyroid dysfunction, and signal the ongoing cellular damage to the thyroid gland that is occurring as hypothyroidism develops. Occasionally patients may have autoimmune thyroid disease with negative Ab results. TPO Abs are a risk factor for progression into hypothyroidism over time, and also for developing hypothyroidism after treatment with agents such as amiodarone, lithium, and interferon-α. Although changes in TPO Ab titers may reflect a change in disease activity, they are not useful for monitoring treatment for hypothyroidism. The presence of TPO Abs, however, is helpful for predicting the development of hypothyroidism during pregnancy and the risk of miscarriage and the failure of in vitro fertilization.
Subclinical vs overt hypothyroidism
SCH has generally been defined as an elevation in the serum TSH level above the upper limit of the reference interval, with a FT4 concentration that remains in the normal range. When making a determination of whether SCH is present, the reference interval being used clearly affects the diagnosis. In the literature upper limits of normal that have been used include TSH values of 3 mIU/L, 4 mIU/L, 5 mIU/L, and 6 mIU/L. Clearly, these chosen cutoffs are based on the reference interval for the particular TSH assay used, which is in turn defined by the laboratory providing the assay. The reference population employed by the laboratory may not be fully described, and standardized collection procedures are often not used. The statistical methods used also affect the results. The particular cutoff value for a normal TSH used in any one study will determine the prevalence of SCH that is identified (16). This choice has consequences both in terms of detection and classification of thyroid disease. Lower TSH cutoffs will have the downstream effect of identifying a greater number of individuals with SCH for whom potential treatment may be considered. An additional very important consideration before diagnosing SCH is confirming the TSH elevation. Many above-range TSH values revert to normal on follow-up, with the rate of normalization being inversely proportional to the degree of the TSH elevation above normal.
Screening
The US Preventive Services Task Force does not recommend routine screening of adults for thyroid disease (21). Other organizations differ as to whether they recommend routine screening or case finding in older asymptomatic individuals. With respect to criteria for population screening, hypothyroidism is prevalent and an important health problem. Moreover, diagnosis is simple and accurate, and treatment is efficacious, cost-effective, and safe. Confounding the issue are the possibilities that screening and early diagnosis may either detect only degrees of hypothyroidism that are mild and for which the benefits of treatment are less well documented or may detect disease for which treatment results in significant benefit. Although routine screening for thyroid dysfunction has not been shown to be of benefit for asymptomatic nonpregnant adults, mounting evidence suggests metabolic, cardiovascular, and skeletal risks in populations with TSH values above the normal range (22, 23). Although the benefits of treatment of overt hypothyroidism are undisputed, positive results from randomized controlled trials of treating SCH would bolster the case for screening (24). There are also differences of opinion regarding screening of asymptomatic pregnant adults. Although recent guidelines do not recommend universal screening, a number of risk factors such as head/neck irradiation, age older than 30 years, autoimmune disease, and iodine deficiency lead to a recommendation for screening (25).
Current Treatment of Hypothyroidism
Treatment With Levothyroxine
Since it was first commercially manufactured in 1949 (3), LT4 has become the most common preparation used to treat hypothyroidism, and is also among one of the most frequently prescribed medications in the United States and Europe (26-28). Successful use of LT4 is facilitated by multiple factors including experience with its efficacy in resolving the symptoms of hypothyroidism, long-term experience of its benefits, a favorable side effect profile, ease of administration, good intestinal absorption, a long serum half-life, the stable T3 levels that are produced, and its low cost (1).
Initiation of Levothyroxine Therapy
When initiating treatment for hypothyroidism, the dose of LT4 required to normalize a patient’s serum TSH depends largely on at least 2 factors. These are the amount of residual endogenous thyroid function retained by the patient and the patient’s weight, body mass index (BMI), or lean body mass (1, 29). In cases of mild or SCH, smaller doses of LT4 in the range of 25 to 75 mcg may be sufficient to render a patient euthyroid (30). When a patient has negligible inherent ability to produce thyroid hormone, with a patient who is athyreotic as a result of thyroidectomy representing the complete absence of such function, the LT4 dose is roughly weight based (1, 31). Numerous studies have investigated the best weight-based LT4 dose with which to initiate therapy in those with minimal thyroid function. Dose estimates between 1.4 and 1.7 mcg/kg body weight are typical (1, 31-33). If a patient’s thyroid status is at steady state before replacement, the serum TSH is indicative of the degree of residual thyroid function, with those with SCH clearly retaining some ability to produce thyroid hormone. Consequently, a patient’s serum TSH value can also be used to predict the dose of LT4 required to achieve euthyroidism (30, 34). Some, but not all, studies find that patient sex affects LT4 dosage requirement (33, 35, 36).
Various formulas or algorithms have been developed in prospective studies as a means of predicting the optimal LT4 dose that will produce euthyroidism post thyroidectomy These algorithms incorporate weight, BMI, and age, but can often be simplified to weight-based algorithms (31, 37-39). In keeping with the finding that when weight-based formulas are applied there is a tendency to over-replace those who are overweight, obese, or have a high BMI, some of these algorithms are tiered so that the weight-based dose is less for patients with higher BMIs (38, 39). Di Donna and colleagues (38) used an age- and BMI-related nomogram to predict LT4 dose prospectively and found that 68% of patients were euthyroid at first follow-up. In another prospective study a regression equation using weight, age, and a baseline LT4 dose of 125 mcg produced 72% accuracy (31). In a retrospective study of almost 600 patients who had undergone thyroidectomy, a Poisson regression model was developed and compared to other dosing regimens in the literature (35). This was one of the more complex formulas developed in that it incorporated weight, BMI, age, preoperative TSH, sex, and use of iron or multivitamins. It correctly predicted a dose of LT4 that would achieve euthyroidism in 64.8% of patients compared with 51.3% of patients using a simple weight-based formula of 1.6 mcg/kg/day. It also provided greater accuracy at higher patient BMI tertiles (59.7%) than the 38% accuracy achieved with use of 1.6 mcg/kg/day (35).
Two studies have examined whether the Thr92Ala polymorphism in the DIO2 gene affects LT4 dose requirement, with conflicting results. One study examined patients with Hashimoto hypothyroidism treated to achieve a normal TSH as well as patients with thyroid cancer treated to achieve TSH suppression (40). Regression analysis was performed to adjust LT4 doses for age, sex, BMI, and serum TSH. The presence of the Thr92Ala polymorphism was not associated with LT4 dose requirement (40). In the other study, athyreotic patients with thyroid cancer treated to achieve low TSH values (0.1-0.5 mIU/L) or suppressed TSH values (< 0.1 mIU/L) were studied. Following adjustment for age and sex, patients homozygous for the Ala allele (Ala/Ala) required a higher LT4 dose to achieve low TSH values (41). This difference, however, was not seen in the TSH-suppression group. Should an increased requirement for LT4 in Ala/Ala homozygotes be confirmed, this could be because of a decreased DIO2 enzyme velocity, which has been shown in some (42, 43) but not all studies (44).
Adjustment of Levothyroxine Therapy
Once LT4 therapy has been initiated, there is generally a need for ongoing adjustment to maintain the patient’s serum TSH stably within the desired range. Generally the desired range is within the laboratory reference range, with consideration for aiming for the age-appropriate reference range found in a relevant population without thyroid disease. For example, based on NHANES III data, for an individual in the 30- to 39-year age range the median TSH is 1.2 mIU/L, with the 2.5 and 97.5 percentiles being 0.42 to 3.56 mIU/L (13). If adjustments are made in a patient’s LT4 dosage, thyroid function should be retested after at least 6 weeks has elapsed to ensure testing is performed during steady-state conditions. TSH levels do exhibit a diurnal rhythm, including in LT4-treated patients (45). However, the magnitude of the excursion does not require testing be performed at a particular time of day. Following the initiation of LT4, ongoing adjustments can be made at 6- to 8-week intervals until the TSH is at goal. Further confirmation of euthyroidism at 3 to 6 months thereafter and then annual monitoring is appropriate for most patients.
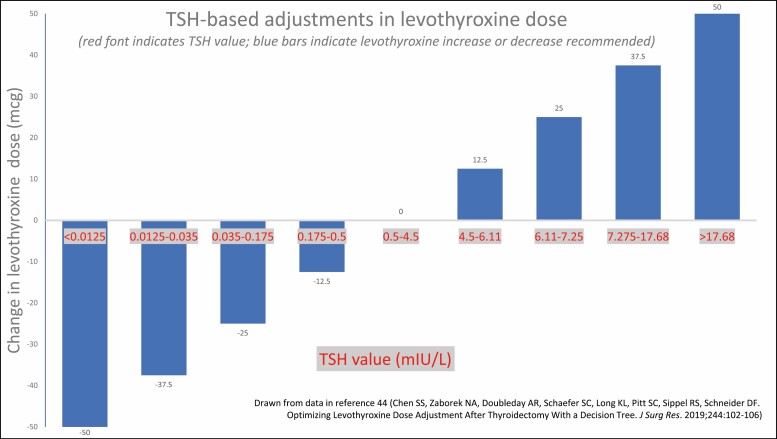
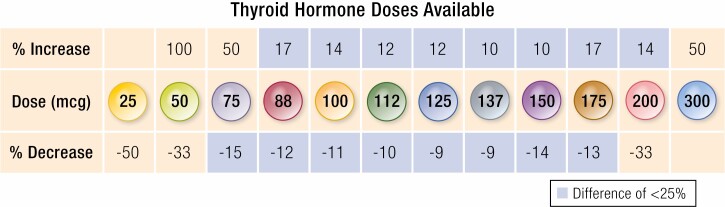
If a patient’s TSH is out of range, advancing or decreasing to the next dose increment may be pursued, with larger adjustments if the TSH is significantly deviated from the goal. In a retrospective study of thyroidectomized patients, a decision tree algorithm produced correct dosage adjustments (Fig. 2) with a similar accuracy to that achieved by experienced providers (46). Dosage strengths of the available LT4 tablets range from 25 mcg to 300 mcg. Adjustments within the dosages that include the most prescribed strengths (50 mcg-125 mcg) (47) result in percentage changes of 9% to 50% in magnitude (Fig. 3). The full range of doses available allows for fine dose titration. In addition, if necessary, smaller degrees of adjustment can be achieved by alternating 2 doses or adding half tablets on a weekly basis.
Figure 2.
Thyrotropin (TSH)-based adjustments in levothyroxine dose.
Figure 3.
Levothyroxine doses available for dosage titration.
Goals of Levothyroxine Therapy
Standard of care goals
The goals of treating hypothyroidism are to reverse the signs and symptoms of hypothyroidism and allow the patient to enjoy the improved quality of life (QOL) and health benefits associated with the euthyroid state. Concomitant with the amelioration of hypothyroid signs and symptoms, the biochemical abnormalities that signaled the development of hypothyroidism should also be reversed as TSH decreases and thyroid hormone levels rise. TSH and FT4 concentrations should be maintained within their reference ranges, as should T3 concentrations (1). Signs and symptoms of insufficient thyroid hormone emanate from all the organ systems of the body. When the hypothyroidism is severe or long-standing, these improvements can be profound; with mild degrees of hypothyroidism, the changes can be more subtle. Where there are overlapping nonthyroid causes of a particular symptom, the improvement may not be complete.
Some of the changes described by Beadles (4) as a “complete transformation” and those described by Bramwell (5) as an “extraordinary improvement” based on the early treatment of myxedema with thyroid extracts given by injection or by mouth are shown in Table 1. Interestingly, the only biochemical documentation in these cases is “The red blood corpuscles rapidly increased in one month from 2 442 000 to 4 447 000” (4) and “On 21st December the red blood corpuscles numbered 3 850 000, a gain of 1 230 000 since 28th November; and the haemoglobin equalled 68 per cent, a gain of 14 per cent since 28th November” (5).
Table 1.
Description of some of the effects of early treatment of myxedema using quotations from Beadles (4) and Bramwell (5) (some comments paraphrased)
| Organ system/body part | Prior to treatment | After treatment |
|---|---|---|
| Face | Puffiness of face, swollen eyelids, hands rough, large and swollen | Reversal of edema, skin warm and moist |
| Face | Lower lip swollen, to some extent pendulous and everted | The lips are thin |
| Face | Raises the swollen eye lid with finger so as to look at an object | Eyes were more widely opened than had ever been seen before |
| Hands | Hands swollen and spade-like | Can flex fingers into the palm in a way has not been able to do for years |
| Hair | Scalp almost destitute of hair and covered with scabs or crusts | Hair is growing splendidly and is of a dark brown, almost black color |
| Hair | Hair rough and scanty | Luxuriant crop of new hair |
| Tongue | Speech was slow and thick Feels as if words stuck in the mouth Has considerable difficulty swallowing |
Speaking quite sharply; the myxoedematous slowness and thickness have entirely disappeared Tongue does not now feel too large for mouth |
| Cardiovascular | Slowed pulse | Pulse rose |
| Cardiovascular | Pulse was slow; it varied from 40 to 50 | Pulse has risen to 71 |
| Neuromuscular | Slow movements, weakness Gait slow and clumsy |
Feeling stronger, unsteadiness of gait resolved |
| Neuromuscular | “Legs so heavy, had to drag them along” | “Kept up with a fast walker” |
| Reproductive | Menstruation had been in abeyance | Menstruation returned after commencement of thyroid treatment |
| Temperature | Suffered much from cold, temperature invariably below 97 °F (36 °C) | Feeling warmer, temperature 98.4 °F (37 °C) |
| Temperature | She always felt cold; even if she roasted herself before a hot fire, she could not get heated up | Always feels warm; perspiring naturally |
| CNS | Melancholia Restless, delusions |
Cheerful and bright Mental faculties clearer |
| CNS | Felt it an effort to think singularly stolid and unemotional | “Feel equal to do anything” |
| Weight | Edematous and increased weight | Rapid weight loss of 4 stone (56 lb) in 10 wk |
Abbreviation: CNS, central nervous system.
The health benefits anticipated from treatment of hypothyroidism could include a more favorable lipid profile, decreased progression of coronary artery disease, improved cardiac function, normalized metabolism and weight homeostasis, normalized reproductive function and fertility, improved mentation, memory, and concentration, and improved mortality. Compared with untreated patients, patients treated for their hypothyroidism have been shown to have decreased cardiovascular disease risk (myocardial infarction, stroke, atrial fibrillation, heart failure and cardiovascular death) (48) and lower all-cause mortality (49, 50).
Additional goals, including specific thyrotropin targets
Some data suggest that individual TSH set points may exist. Repeated TSH measurements in euthyroid individuals show that each individual appears to have TSH values that cluster around a specific set point (18). In addition, analysis of the relationship between TSH and FT4 in a population illustrates that rather than being a simple linear relationship, the relationship may in fact be more complex and curvilinear. The slope of the relationship appears to be affected by sex, age, genetics, and use of LT4, with each individual having TSH-FT4 set points that differ from the population as a whole (51, 52).
Many clinicians have a tendency to take age and comorbidities into account when targeting specific TSH values within the normal range for individual patients. This might include avoiding TSH values approaching the lower end of the normal range in older age groups or those with cardiovascular disease or osteoporosis, or avoiding upper normal TSH values in those who are younger or who have with lipid disorders or weight difficulties. Although it is reasonable to target specific TSH ranges for patients based on their clinical needs, generally prospective studies have not provided results that support that this approach influences QOL or clinical parameters. Examples include lack of effect of specific within-normal-range TSH goals on QOL, mood, BMI, fat mass, and cognitive function.
For example, in a study of 138 hypothyroid individuals who were randomly assigned to be treated to either 1 of 2 normal TSH targets (0.34-2.50 or 2.51-5.60 mIU/L) or a slightly elevated TSH target (5.61-12.0 mIU/L) for 6 months, there was no difference in QOL, mood, or cognition between the 3 study arms (53). In addition, there was no difference in energy expenditure (eg, resting energy expenditure, total energy expenditure, or fat oxidation) or body composition (eg, BMI, fat mass) (54). Another randomized controlled study in which 56 hypothyroid participants received each of 3 doses of LT4, with mean TSH values of 0.3, 1.0, and 2.8 mIU/L being achieved for 8-week periods, did not find any differences in well-being, hypothyroid symptoms, QOL, or cognitive function associated with the different TSH targets (55). A fourth study in which patients newly diagnosed with hypothyroidism were treated with LT4 for a year to target with a TSH in the upper half or the lower half of the normal range did not show any differences in the response to therapy in terms of changes in total cholesterol, low-density lipoprotein cholesterol, triglycerides, fat body mass, lean body mass, or bone mineral density between the 2 groups. The only difference between the groups was a greater increase in the resting energy expenditure in the group treated to achieve the lower TSH value (56).
Some epidemiologic studies appear to have different implications with respect to TSH targets. For example, an analysis of 40 studies from the literature examining the effect of variation in thyroid hormone parameters within the reference range in untreated patients on cardiovascular, bone, metabolic, pregnancy, neurological, and psychological outcomes found some effects of lower and higher TSH values within the normal range. Lower TSH levels were associated with decreased bone mineral density and increased fracture risk, whereas higher TSH levels were associated with worse cardiovascular and metabolic outcomes (22). If this study can be applied to patients being treated for hypothyroidism, it might suggest a benefit of treating subclinical thyroid disease. Another study of patients being treated for hypothyroidism examined the effect of various target TSH values on heart disease, strokes, fractures, and all-cause mortality. All TSH values within the normal range were associated with similar risk of adverse events with no gradation within the range of normal TSH values (23).
Other biomarkers of thyroid status
In addition to TSH measurements, other parameters can potentially be used to gauge tissue response to LT4. Such markers include sex hormone–binding globulin, osteocalcin, cholesterol, creatine kinase, ferritin, N-telopeptides, and enzymes such as tissue plasminogen activator, angiotensin-converting enzyme, glutathione S-transferase, and glucose 6-phosphate dehydrogenase (1). Physiological parameters include heart rate, pulse wave arrival time, echocardiographic parameters of left ventricular function, Achilles reflex time, and basal metabolic rate (1). Although serum TSH is currently believed to be the best available marker of adequate thyroid hormone replacement, some studies suggest a discordance between serum TSH and other indicators of tissue euthyroidism (1, 57, 58). Multiple cell types, mostly studied in animals, including hepatocytes, cardiac myocytes, skeletal myocytes, kidney cells, lung endothelial cells, and brain cells, express genes that are sensitive to regulation by thyroid hormone (1, 59). Measurement of thyroid hormone–responsive gene expression may provide another means of assessing the effect of thyroid hormone on various tissues. Measurement of gene expression in these tissues is understandably not routinely used for clinical assessment of thyroid hormone status, as this would require tissue biopsy. However, genes expressed in whole blood in humans respond to thyroid hormone (60), and in the future the expression of a panel of such genes might be found to be a sensitive and global indicator of thyroid status.
Avoiding iatrogenic disease
Unfortunately, despite the apparent simplicity of providing a once-daily orally active hormone replacement, many studies show that patients undergoing treatment for hypothyroidism have TSH values that fall outside the normal range. One recent study of 162 369 patients with hypothyroidism using 863 072 TSH measurements from their 23 years of follow-up showed that 11.6% of TSH values were below 0.4 mIU/L and 32.4% were above 4.0 mIU/L (23). Data from a hypothyroidism disease registry comprising 1037 patients showed that over a 5-year time span 19.8% were overtreated and 17.4% were undertreated (61). Factors associated with out-of-range TSH values included male sex being associated with undertreatment and duration of treatment being associated with overtreatment. Several earlier studies show similar rates of iatrogenic thyroid disease with rates of iatrogenic hyperthyroidism ranging from 13.7% to 38.9% and rates of iatrogenic hypothyroidism ranging from 15.9% to 26.8% (62-66). A recent study of the NHANES database suggested that iatrogenic thyroid disease (TSH > 5.6 mIU/L or TSH < 0.3 mIU/L) was more likely in Hispanic individuals than in individuals of non-Hispanic ethnicity (67). A retrospective study of a health care database showed that approximately 80% of TSH values were in range, both in patients taking LT4 and those taking DTE (68). However between visit variability in TSH concentrations was significantly greater in those taking DTE.
The potentially detrimental effect of abnormal TSH values while receiving treatment is illustrated by several studies. TSH values outside the normal range were associated with adverse events in the study by Thayakaran et al (23); the risk of heart disease and fragility fractures was increased with TSH values greater than 10 mIU/L, whereas increased mortality was associated with TSH values below 0.1 and greater than 4 mIU/L. In another registry-based cohort study, overtreatment with LT4 to achieve a TSH of less than 0.3 mIU/L was associated with increased mortality; additionally it was found that longer duration of the decreased TSH increased the mortality (50). In the same cohort undertreatment and overtreatment of hypothyroidism both were associated with increased cardiovascular risk (48). As discussed in a recent review, these findings highlight the importance of monitoring serum TSH and endeavoring to maintain it within the normal range (69).
Interestingly, there are several studies that have examined trends in LT4 prescribing (26-28, 47, 66, 70). In general, rates of prescribing LT4 have increased over time, even in older populations (47, 71), although there is some evidence that prescribing may be plateauing in certain subgroups, such as those that do not actually carry a diagnosis of hypothyroidism (28). Of concern, along with the trend for increased prescribing of LT4, there also seems to be an accompanying trend for LT4 to be prescribed for milder degrees of hypothyroidism (47, 66, 70). This raises concerns about whether some treatment is unnecessary and also exposes patients to the risk of iatrogenic thyrotoxicosis. Such concerns suggest the need for careful consideration of both the degree of TSH elevation and the patient context when considering whether treatment of an elevated TSH value, vs ongoing monitoring, is indicated (72). Older individuals, women, and those with access to routine health care are more likely to be treated for hypothyroidism (overt and SCH) as determined from an analysis of the NHANES database, whereas older individuals and those with access to routine health care are more likely to be treated for overt hypothyroidism (67).
Ensuring Effective Therapy
As LT4 therapy is generally a lifelong therapy, it is important to ensure that the prescribed regimen is not overly complex and onerous for patients. The importance of a regimen that is easy to adhere to may be inferred from studies showing the proportion of medication doses that are missed in individuals being treating for chronic conditions. For hypothyroidism approximately 66% to 68% of patients achieve adherence rates of at least 80% (73, 74), which presumably does not contribute to achieving a steady state of euthyroidism.
Simple regimens
As simple a regimen as possible that is linked to a patient’s daily routine is ideal. Although fasting regimens maximize LT4 absorption, other regimens may be acceptable or preferable if they improve adherence. If the schedule is consistently maintained, LT4 dosage increases that correct for impaired absorption can be implemented. If a more complex regimen than a single dose of LT4 once daily is employed, aids may be needed to facilitate adherence. For example, if alternating 2 doses of LT4 best keeps a patient’s TSH within the normal range, prefilled weekly pill dispensers may be helpful. In general patients can be advised to “make up” missed doses as soon as they are remembered to avoid undertreatment.
Scheduling of levothyroxine
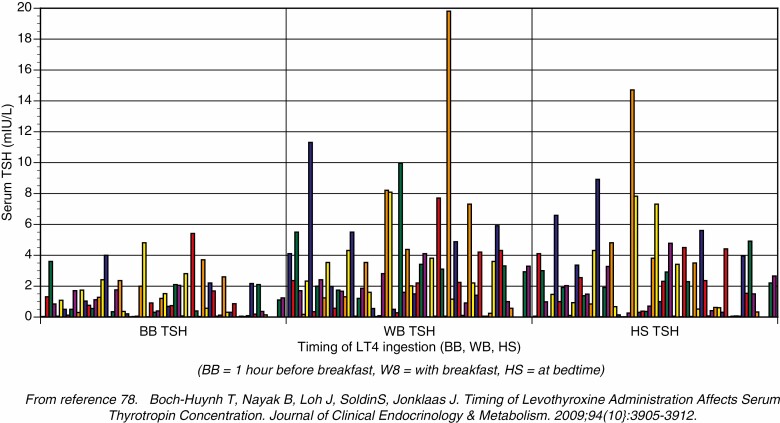
Many studies have examined the effect of timing of LT4 ingestion, and its relationship with meals, on the serum TSH. Although the specific timings examined are slightly different across the different studies and not all studies have an optimal study design, the general conclusion is that fasting regimens and bedtime regimens may both be acceptable and associated with normal TSH values (75-92) (Table 2). For studies in which one of the tested regimens resulted in a lower TSH, this is indicated by a downward arrow by the TSH value during that regimen. Clearly, it is not the TSH value that is of the most importance because this can be manipulated by altering the LT4 dose, but the major concern is the variability in the TSH value seen over time. In one of these studies, which compared 3 timing regimens (78), a with-breakfast regimen resulted in the highest and most variable TSH values, a bedtime regimen produced lower and less variable TSH values, and a regimen with a timing of 1 hour before breakfast was associated with the lowest and least variable TSH results (Fig. 4). When selecting a time for a particular patient to adopt for taking LT4, the key consideration is the regimen that is easiest for the patient to adhere to, that minimizes the number of missed doses, and maximizes the convenience for the patient.
Table 2.
Examples of studies examining the effect of levothyroxine timing on serum thyrotropin
| Study | Designa,b | Serum TSH values according to regimen | ||||
|---|---|---|---|---|---|---|
| With breakfast | 0.5 h before breakfast | 1 h before breakfast | Bedtime/2-3 h after dinner | Other | ||
| Elliot, 2001 | Nonrandomized crossover | – | – | – | 1.77 | 2.06 (1 h after breakfast) |
| Bolk, 2007 | Randomized crossover | – | 5.1 | – | 1.2↓ | – |
| Bach-Huynh, 2009 | Randomized crossover | 2.94 | – | 1.06↓ | 2.19 | – |
| Bolk 2010 | Randomized crossover | – | 2.66, 3.86 | – | 1.74, 2.36↓ | – |
| Seechurn, 2012 | Nonrandomized crossover | 12.6 | – | 3.14↓ | – | – |
| Rajput, 2011 | Randomized, parallel | – | 5.13 | – | 3.27 | – |
| Boeving, 2011 | Randomized crossover | 2.89 | - | 1.9↓ | – | – |
| Ala, 2015 | Randomized crossover | – | 2.03↓ | – | – | 3.35 (1 h before dinner) |
| Yuan, 2015 | Crossover | – | 12.10 | – | 3.9↓ | – |
| Ahmed, 2016 | Randomized trial | – | – | 6.5 | 7.7 | – |
| Cappelli, 2016a | Randomized crossover | 2.58 | – | 2.69 | - | – |
| Akın, 2018b | Open-label study | 2.6 | 2.9 | |||
| Srivastava, 2018 | Randomized crossover | – | – | 2.45, 2.14 | 2.07, 1.85 | – |
| Skelin, 2018 | Randomized crossover | 1.9 | 2.2 | 2.3 (1 h before main meal of day) | ||
| Apaydin, 2018 | Nonrandomized crossover | 2.87 | 3.62 | |||
| Chen, 2018 | Randomized trial | 2.56 | 2.16 | |||
| Navid, 2021 | Randomized, open label | 3.56 (1 h presumed) | 3.77 (1 h before dinner) | |||
↓ indicates significantly lower TSH value compared with other regimens.
Abbreviations: LT4, levothyroxine; TSH, thyrotropin.
a All trials involved the tablet form of LT4, except the Cappelli trial (82), which used liquid LT4.
b All trials involved adults, except the Akın (83) trial, which studied children.
Figure 4.
Effect of timing of levothyroxine ingestion on serum thyrotropin (TSH).
Absorption of levothyroxine
The presence of an acidic environment in the stomach seems to promote LT4 absorption, which occurs mostly in the jejunum and ileum (1). Absorption of oral LT4 is approximately 75% to 80% in the fasting state, with a decrement in absorption if the LT4 tablet is given in a nonfasting state (1). One study, for example, shows absorption as low as 59% to 68% when nonfasting, compared to 78% to 80% when fasting (93). Thus, when giving LT4 intravenously, if this is necessary in a hospitalized patient, approximately 75% of the oral outpatient dose can be used (1). Some specific foods or beverages that have been reported as being culprits in terms of reducing LT4 absorption include fiber, soybeans, coffee, and grapefruit (91, 94). The impaired absorption associated with food and beverages may be overcome by changing the time of the LT4 administration, or a higher dose of LT4 may be administered to compensate for the effect of the decreased absorption. Vitamin C, on the other hand, can improve LT4 absorption (94). There are a myriad of other drugs or conditions that can reduce the absorption of LT4, thereby necessitating a larger dose of LT4 to maintain euthyroidism. There are also other mechanisms, such as altered metabolism or altered binding to carrier proteins, by which drugs can affect the LT4 dosage.
Drugs or Conditions Affecting Levothyroxine Dose
Drugs and levothyroxine dose
Drugs can alter thyroid function in those without preexisting thyroid disease, with the effect varying from producing abnormal thyroid biochemical parameters to causing overt thyroid dysfunction. The same drugs may alter the LT4 requirement in those already being treated for hypothyroidism (94-96). The effects of drugs can be broadly divided into the following categories: i) altered transport, ii) altered metabolism, iii) altered thyroid hormone synthesis or release, iv) altered TSH secretion, and v) altered LT4 absorption. Some drugs can have more than one effect.
Altered transport of T4 can be caused by altered hepatic synthesis of thyroxine-binding globulin (TBG), with increased TBG concentrations causing increased total T4, reduced FT4, and increased TSH; whereas decreased TBG concentrations can cause decreased total T4, increased FT4, and decreased TSH (96). These changes in opposite directions are exemplified by estrogen (97) and androgens (98), with these agents potentially causing increased LT4 requirements and decreased LT4 requirements respectively (Table 3). Altered metabolism of thyroid hormone can occur through several avenues: i) increased hepatic metabolism via induction of P450, ii) decreased activation of T4 to T3 via inhibition of DIO2, and iii) increased deactivation of T4 and T3 via acceleration of type 3 deiodinase activity. Each of these changes can potentially result in the need for an increased dose of LT4 in an individual being treated for hypothyroidism (see Table 3). The list of medications that can impair LT4 absorption is extensive, with classic examples being calcium carbonate (99) and ferrous sulfate (95, 100). Other examples, including proton pump inhibitors (101) and phosphate binders (102, 103), are shown in Table 3. Agents such as calcium and iron likely adsorb to LT4 in the gastrointestinal tract and thereby reduce its absorption (1), whereas proton pump inhibitors may impair absorption by increasing gastric pH (1). With respect to altered thyroid hormone synthesis or release, drugs that may fall into this category include methimazole, propylthiouracil, checkpoint inhibitors, alemtuzumab, interferon-α, amiodarone, sunitinib, and lithium (96). The most powerful example of altered TSH secretion is bexarotene, which lowers TSH and causes profound central hypothyroidism (104). With respect to examples of drugs having more than one effect, phenytoin may be associated with altered transport and altered metabolism, and sunitinib may be associated with altered metabolism and altered thyroid hormone release (96, 105).
Table 3.
Examples of medications causing altered transport, metabolism, or absorption of levothyroxine
| Alteration in LT4 transport | Medications | Effect |
|---|---|---|
| Increased TBG | Estrogen (classic example) | Can cause increased TSH and increased LT4 requirement |
| Other examples: capecitabine, raloxifene, tamoxifen, heroin, methadone, 5-fluorouracil, mitotane, clofibrate | ||
| Decreased TBG | Androgens (classic example) | Can cause decreased TSH and decreased LT4 requirement |
| Other examples: anabolic steroids, glucocorticoids, nicotinic acid, salicylates, furosemide, heparin, NSAIDs, phenytoin, carbamezepine | ||
| Alteration in LT4 metabolism | Medications | Effect |
| Increased hepatic metabolism via induction of P450 | Phenobarbital, rifampin, phenytoin, sertraline, carbamazepine | Can increase LT4 requirement |
| Decreased activation of T4 to T3 via inhibition of type 2 deiodinase | β-blockers, steroids, radiographic contrast agents, amiodarone | Not typically associated with altered LT4 requirement |
| Increased deactivation of T4 and T3 via acceleration of type 3 deiodinase | Tyrosine kinase inhibitors (imatinib, sorafenib, motesanib, sunitinib) | Can increase LT4 requirement |
| Alteration in LT4 absorption | Medications | Effect |
| Impaired absorption | Calcium carbonate, ferrous sulfate (classic examples) | Can increase LT4 requirement |
| Phosphate binders, other calcium salts, antacids, cholestyramine, colesevelam, sucralfate, proton pump inhibitors, orlistat, chromium, ciprofloxacin |
Abbreviations: LT4, levothyroxine; NSAIDs, nonsteroidal anti-inflammatory drugs; T3, 3,5,3′-triiodothyronine; T4, thyroxine; TBG, thyroxine-binding globulin; TSH, thyrotropin.
Medical conditions and levothyroxine dose
In addition to the previously described impairment of LT4 absorption caused by food and medications, several gastrointestinal conditions may also be associated with a decrement in LT4 absorption. These include celiac disease, atrophic gastritis associated with Helicobacter pylori infection, lactose intolerance, and ulcerative colitis (94, 106). Patients with these conditions may require more than the predicted weight-based LT4 dose, with a trend toward a more typical weight-based requirement if the condition is successfully treated. For example, reversal of gastritis by treatment of H pylori is associated with improved LT4 absorption (107). It has been suggested that autoimmune chronic gastritis may also be associated with decreased LT4 absorption (108). Bariatric surgery can result both in weight loss and malabsorption, with these competing effects most commonly resulting in patients requiring a reduced LT4 dose after such surgery (109). It is possible that altered expression of ileal thyroid hormone transporters may be shown to be a cause of LT4 malabsorption (110).
Levothyroxine preparations: brands and generics
LT4 is available as a tablet preparation, a liquid preparation, and a liquid contained within a gel capsule. The various LT4 tablets available include both brand name and generic preparations. Although the tablet preparations differ with respect to their excipients such as mixers, fillers, and colorants, they are all similar in that they contain LT4 as their active ingredient. The product that is most affordable to a patient may change over time depending on the arrangements between the patient’s insurance company and relevant pharmacies. There are reports of clinically significant changes in serum TSH associated with a patient’s LT4 preparation being changed either from one brand name to another, or from a brand-name to a generic product, or from one generic to another (1, 111). The change in TSH has been attributed to different absorption of the various products associated with the different excipients. As a result of their different absorption characteristics, each of these products would also be expected to have slightly different bioequivalence.
On the other hand, a recent study of LT4 initiation showed a similar percentage of out-of-range TSH values over a 3-month period in patients treated with brand-name vs generic products (112). This may suggest that TSH values can be maintained if a particular LT4 product (either brand name or generic) can be continued. There also does not seem to be a difference in cardiovascular or skeletal outcomes in those maintained on a brand-name product compared with a generic product (113).
Bioequivalence is a measure of absorption or bioavailability that is used by the Food and Drug Administration to predict the therapeutic efficacy of drugs. The pharmacokinetics of large oral doses of LT4 is studied in volunteers who have normal endogenous thyroid function in order to determine bioequivalence (1). Pharmacokinetic parameters, including the maximum serum concentration (Cmax), time to Cmax, and area under the concentration-time curve (AUC), are then used to compare different products. If the 2 90% CIs from the natural logarithms of the AUC at 48 hours and the Cmax are both within the 80% to 125% range, the products are deemed bioequivalent by the Food and Drug Administration, and are permitted to be substituted for each other. From a clinical standpoint the concern with this approach is that measures of systemic exposure (AUC, Cmax) are used for standardization, rather than clinically relevant or biochemical end points such as serum TSH levels. Because such bioequivalence methodology does not detect LT4 doses with approximately12.5% difference from each other (eg, 100 mcg and 112 mcg tablets) (114), it is always advisable to recheck serum TSH in individuals who have been switched from one preparation to another to be sure that iatrogenic hyperthyroidism or hypothyroidism has not developed, and that the serum TSH is still in the desired target range (1). This is the rationale for advising patients to continue taking the same “identifiable” product (either brand or generic). The name of the manufacturer is on the label of the bottle, so patients can check that the manufacturer remains the same when obtaining refills.
Liquid preparations
Liquid LT4 preparations are available in which the LT4 is dissolved in glycerol and contained either within a gelatin capsule or in a solution. Liquid preparations are reported by the manufacturer to have approximately 100% absorption from the gastrointestinal tract, compared with approximately 75% absorption in the fasting state for other LT4 preparations. In addition, the dissolution of the gelatin capsule preparation appears to be relatively unaffected by pH (115). Therefore, the absorption of the liquid preparations, in theory, might be less affected by the altered pH of the gastric environment associated with food consumption, atrophic gastritis, and use of proton pump inhibitors than other LT4 products. There is a growing body of fair-quality evidence that liquid preparations may have better absorption in situations where patients are receiving proton pump inhibitors (116), tube feeds (117), or have other causes of malabsorption (118). It is also plausible that gelatin capsules may be better tolerated than tablets in individuals with allergies to the excipients contained within tablet preparations. However, at the current time, there are no data to show that clinical outcomes are improved by using LT4 liquid compared with LT4 tablets (119).
Levothyroxine Therapy in Specific Situations
LT4 is considered to be a drug with a narrow therapeutic index, and management of hypothyroidism is influenced by a number of different physiologic conditions, medical conditions, and comorbidities (120). A detailed discussion of these management issues is addressed in various clinical practice guidelines (1, 25, 121).
Pregnant patients
Preconception normalization of serum TSH is important for patients with hypothyroidism (122). Women being treated for hypothyroidism typically require a 20% to 30% increase in their LT4 dose early in the first trimester of pregnancy. This increased need is triggered by factors such as increased hepatic TBG synthesis and metabolism of thyroid hormone by the placental type 3 deiodinase (25, 122). The magnitude of the increase is greater in thyroidectomized patients than in those who retain some endogenous thyroid function. It is therefore recommended that pregnant individuals being treated for hypothyroidism be monitored regularly so that their LT4 can be increased as necessary and their TSH values can be maintained in the lower half of the trimester-specific reference range. If a pregnancy-specific reference range is not available, a TSH below 2.5 mIU/L is a reasonable goal (25). Overtreatment should be avoided, as a low TSH may be associated with adverse outcomes such as preterm delivery (123).
Pediatric population
Treatment of hypothyroidism in the pediatric population, including management of congenital hypothyroidism (124), is discussed elsewhere (125). There are special considerations for this population based on the requirement of normal thyroid function for neurocognitive development as well as growth and developmental milestones. As a reflection of their unique physiology, newborns, children, and adolescents typically require higher LT4 doses than adults. As the child advances through the pediatric age into adulthood, LT4 replacement doses decrease, with transition to the average adult dose of 1.6 mcg/kg/day once endocrine maturation is complete. The general pattern of LT4 requirements is as follows: 4 to 6 mcg/kg/day for patients aged 1 to 3 years, 3 to 5 mcg/kg/day for patients aged 3 to 10 years, 2 to 4 mcg/kg/day for patients aged 10 to 16 years, and 1.6 mcg/kg/day for patients aged 17 years or older (1, 125).
Nonadherent patients
Consistent consumption of a prescribed LT4 dose should result in serum TSH levels remaining within a fairly narrow range. If patients experience unexpected fluctuations in their serum TSH, or persistently elevated TSH concentrations despite the administration of large doses of LT4, factors affecting LT4 formulation, absorption, and metabolism should be investigated as potential causes. There are well-established protocols for determining whether LT4 absorption could be responsible, with administered LT4 test doses of between 600 mcg and 1500 mcg and normal absorption being defined as 60% or greater (126). When such factors do not appear to be responsible, variable adherence or nonadherence to LT4 therapy should be considered. Some data suggest that nonadherence rates of approximately 5% to 27% may be representative (127-129). Because LT4 is typically a lifelong medication, it is important for patients to identify a medication schedule that optimizes adherence. Patients may be taking multiple other medications that require specific administration conditions (eg, bisphosphonates) or that impair absorption. It may be necessary to choose a schedule of medication administration that is practical, even if absorption is affected, if this ensures that doses are not omitted. Dosage can then be secondarily adjusted to mitigate any impaired absorption.
If a combination of a high TSH and a high-normal or high T4 concentration is documented by laboratory testing, this pattern could be consistent with the syndrome of thyroid hormone resistance, a TSH-secreting pituitary adenoma, or recent resumption of LT4 intake before a scheduled blood test. Intuitively, patient education regarding the benefits of euthyroidism and the risks of iatrogenic thyroid disease would seem a logical approach to reducing nonadherence. However, a study providing education in the form of booklets mailed to patients’ homes did not affect serum TSH, which was used as a surrogate marker of adherence (130). If efforts to encourage regular daily consumption of LT4 are unsuccessful, options include observed therapy, including twice weekly or weekly therapy (1). Using this approach, the total weekly dose of LT4 is administered as a single once-weekly dose or divided into 2 weekly doses. In one study of individuals with elevated TSH values while taking their prescribed LT4 therapy, who were identified as not having malabsorption as a result of a T4 absorption test, achieved lower TSH values with weekly therapy, although the TSH values were not fully normalized (131). Twice or 3 times weekly LT4 administration has also been proposed as a means of improving adherence during Ramadan, when the best timing of LT4 administration may be unclear (132). A randomized crossover study compared daily vs weekly administration of LT4. Weekly therapy was associated with supratherapeutic concentrations of FT4 for about 24 hours and elevated TSH levels before the administration of the weekly dose, although FT3 levels remained within the normal range, and patients appeared not to report side effects (133). Parenteral administration of LT4 is also possible.
Central hypothyroidism
A biomarker other than serum TSH is needed to guide LT4 replacement therapy when treating patients with hypothyroidism due to TSH deficiency. Based on a randomized trial comparing 2 doses of LT4, it has been recommended that FT4 levels be kept in the upper half of the normal range (134). Slightly lower FT4 levels, perhaps in the midnormal range, have been suggested for frail or older individuals. Another opinion is that similar FT4 values should be targeted to those achieved in patients being treated for primary hypothyroidism (135).
Patients with differentiated thyroid cancer
TSH is thought to be one of several growth factors for thyroid tissue. Patients who have undergone thyroid surgery for intermediate-risk or high-risk thyroid cancer constitute a subgroup whose TSH values are intentionally kept below the normal range. Studies show either increased survival or increased relapse-free survival in patients in these risk categories with TSH suppression, but not in patients with low-risk thyroid cancer, who can be maintained with a serum TSH in the lower half of the normal range (136, 137). In higher-risk patients, the benefits of TSH suppression and the risks, such as potential bone loss and adverse cardiac effects, have to be balanced against each other on an individual patient basis.
Older patients and patients with medical conditions
For patients older than 60 to 65 years, patients who have severe, long-standing hypothyroidism, or those with coronary artery disease, a gradual approach to LT4 initiation is advised (1). In patients known to have ischemic heart disease, treatment should be initiated with lower doses of LT4 such as 25 mcg daily. In other patients at risk of coronary artery disease, but without documentation of such disease, a conservative starting dose of approximately 50 mcg per day may be advisable (1). This might be a suitable starting dose in patients who are in older age groups or who have had long-standing severe hypothyroidism. Older individuals may have other chronic medical conditions that may require altered doses of LT4 to maintain a normal TSH. For example, sick patients older than 65 years, who are taking other medications for a variety of comorbidities, in addition to LT4, require lower doses of LT4 to normalize their serum TSH than do healthy controls of a similar age who taking only LT4 (138). The difference in LT4 dose requirement persisted even after accounting for body weight. As other examples of medical conditions, patients with glomerular disease may require higher LT4 doses at times when their urinary protein losses are greatest, and those with cirrhosis may also have an increased requirement for LT4, possibly due to increased levels of TBG. “Consumptive” hypothyroidism requires treatment with large doses of LT4 until the tumor can be resected. Euthyroidism should be carefully maintained in older and frail individuals to avoid iatrogenic thyroid disease that may exacerbate the patient’s underlying medical conditions.
Hospitalized patients
When patients with hypothyroidism are hospitalized, their LT4 therapy should be continue uninterrupted. If patients are unable to take their LT4 by mouth, it should be provided by other enteral routes such as via feeding tubes or via the intravenous route. Of concern, in a review of LT4 replacement therapy in an intensive care unit, it was found that 17% of patients did not have their LT4 prescribed for more than 7 days and 21% did not have their LT4 administered when enteral feeding was instituted (139). Patients with TSH levels either above or below the reference range had median hospital length of stays that were longer (22 days) than those with normal TSH values (15 days) (139).
Coexistent adrenal insufficiency
If a patient has both untreated hypothyroidism and suspected or documented adrenal insufficiency that is as yet untreated, cortisol replacement should be started concurrently with LT4 to avoid restoration of normal renal function increasing cortisol disposal, or normalization of metabolic rate increasing the requirement for cortisol, and thus precipitating an adrenal crisis (1). If adrenal insufficiency is subsequently ruled out, cortisol replacement can be discontinued.
Subclinical hypothyroidism
The prevalence of SCH is strongly affected by the TSH cutoff that is used to define it. There is an ongoing debate regarding the risks and benefits of treating SCH (16). Many reviews regarding SCH conclude that there are less adverse consequences of mild SCH, compared with greater degrees of SCH, in most populations, and that this is particularly true in specific populations such as individuals aged 65 and older (140). This discussion is further complicated when consideration is given to whether TSH cutoffs for defining SCH should be guided by reference ranges that are specific for age or other characteristics of the population. Formulas for adjusting the TSH reference interval for age, ethnicity, and sex within the US population have been devised (141). Such an approach would result in a higher TSH threshold before an older patient would be categorized as having SCH. However, not all experts believe there is a need for specific reference intervals for these subpopulations.
Even when there is agreement about the existence of SCH, benefits of treating generally have not been shown, except possibly in specific age groups (142). On the other hand, there are some preliminary data that even serum TSH values that fall within the upper end of the TSH reference interval are associated with adverse outcomes (22). A meta-analysis of epidemiologic studies examined the risk of developing adverse outcomes in individuals with serum TSH values in the upper end of the normal reference range compared with the lower end of the range. Cardiovascular, metabolic, and skeletal outcomes were examined. The OR for developing adverse cardiovascular and metabolic outcomes was significant at 1.21 (95% CI, 1.15-1.27) and 1.37 (95% CI, 1.27-1.48), respectively, whereas the OR for adverse skeletal outcomes was significantly reduced at 0.55 (95% CI, 0.41-0.72) (22). Should this be confirmed, this might suggest that trials of treatment of SCH should enroll patients with even milder degrees of TSH elevation to better determine the risks and benefits of such treatment. A recent clinical practice guideline suggested that treatment of SCH should not be considered unless the TSH was above 20 mIU/L, except in certain subgroups (143). This conclusion was reached despite the fact that only 2 of the trials in their associated metanalysis included patients with TSH values above 10 mIU/L. An alternative approach is to carefully consider the clinical context when deciding whether treatment of SCH is of benefit (72).
Two recent studies have addressed the effect of LT4 on hypothyroid symptoms in older patients with SCH. Individuals older than 65 years were studied in the TRUST trial (144) and those older than 80 years were studied in the IEMO80-plus Thyroid Trial (145). Both these trials enrolled patients with persistent TSH elevations of over 4.6 mIU/L. In the TRUST trial the mean TSH values at enrollment were 6.41 and 6.38 mIU/L, with declines to 3.63 and 5.48 mIU/L in the LT4 group and the placebo group, respectively. In the study of those older than 80 years (which included some individuals from the IEMO trial and some from the TRUST trial), the TSH values at enrollment were 6.50 and 6.20 mIU/L, with reduction to 3.69 and 5.49 mIU/L in the LT4 and placebo groups, respectively. Neither of these trials showed any difference in their primary outcomes of hypothyroid symptoms or tiredness at 1 year. There was no effect of LT4 on secondary outcomes either. One could speculate that these trials could potentially have had different results if the TSH reference interval used was age-adjusted and resulted in a different definition of SCH, with a higher TSH value such as greater than 7 to 10 mIU/L being used for enrollment. Of note, a recent survey of clinicians found that 11% to 29% of those responding to the survey would treat a 80-year-old patient with tiredness and a TSH of 6.5 to 6.8 mIU/L with LT4 (146).
Myxedema coma
Untreated severe hypothyroidism can culminate in myxedema coma, a life-threatening condition characterized by hypothermia, bradycardia, hypotension, altered mental status, and multisystem organ failure. Risk factors include advanced age, poor access to health care, and other underlying major organ system diseases. Patients may present more frequently in the winter months, and most patients have severe and long-standing thyroid hormone deficiency. Treatment should include thyroxine (1.6-1.8 mcg/kg/day, with or without a 200- to 500-mcg loading dose) (1). The rationale for the loading dose is to reoccupy the empty binding sites on TBG, so that administered LT4 can contribute to raising FT4 levels. Some experts advocate coadministration of LT3 in divided doses to compensate for impaired conversion of T4 to T3. No controlled trials have been performed to evaluate the relative benefits and risks of these different approaches. Glucocorticoids should be administered in stress doses until the results of a cosyntropin stimulation test performed to check for evidence of concomitant adrenal insufficiency are available. Care should be taken to avoid exposure to potent sedative or analgesic agents that may exacerbate altered mental status. Hypothermia should be treated with external warming to reduce the risk of circulatory collapse.
Use in individuals without hypothyroidism
As previously discussed, the symptoms of hypothyroidism are nonspecific and overlap with symptoms of other conditions. Thus, even if an individual is found to have hypothyroidism following screening based on symptoms, the symptoms may or may not resolve with initiation of LT4. The symptoms may have led to the diagnosis of hypothyroidism, despite the fact that hypothyroidism was not the cause. A randomized controlled trial of LT4 vs placebo was conducted in individuals with symptoms consistent with hypothyroidism but with normal thyroid function tests (147). This therapy was unsuccessful in improving symptoms. In fact, individuals with symptoms and potentially with upper normal TSH values, slightly high TSH values, or transiently high TSH values may be prematurely or unnecessarily treated with LT4. For example, in a study of 291 individuals taking LT4 in whom there was not a confirmed diagnosis of hypothyroidism, 61% remained euthyroid off LT4 (148). In a meta-analysis of 17 studies in which LT4 therapy was discontinued, approximately one-third of patients remained euthyroid at follow-up, with euthyroidism being more likely in those whose diagnosis had been SCH (149).
Therapy Other Than Levothyroxine
It is generally agreed that LT4 is the standard-of-care therapy for hypothyroidism (1). When they are treated to consistently achieve a normal TSH, the majority of patients are believed to feel well while taking LT4, albeit more high quality studies to confirm this would be a valuable addition to our understanding. However, there is a subset of patients who do not feel fully restored to health and report reduced QOL despite being biochemically euthyroid. This has led to studies to try to understand the underpinnings of this reduced QOL and also to investigate whether alternative therapies for hypothyroidism might yield greater levels of satisfaction.
Residual Symptoms While Taking Levothyroxine
Studies that have assessed well-being or QOL have shown differences between patients treated to achieve biochemical euthyroidism and control individuals both when thyroid-specific symptoms and general symptoms have been assessed by response to questionnaires. The Thyroid Symptom Questionnaire (TSQ) identified dissatisfaction both in 397 patients receiving treatment for hypothyroidism and also in age- and sex-matched patients without hypothyroidism. Approximately 14% more patients with hypothyroidism were dissatisfied compared with the control group (150). When well-being was assessed using the General Health Questionnaire (GHQ-12), 9% more patients were dissatisfied compared with controls. The hypothyroid group did have more coexistent medical conditions but the difference between the groups remained significant even after adjusting for these. Another study, in which participants were recruited by letter and then subsequently took part in cognitive testing and completed questionnaires assessing well-being, also showed lower scores in both these areas in the euthyroid patients taking LT4, compared with standard reference values (151). A third study also showed more fatigue, higher intensity fatigue, and lower RAND-36 vitality scores based on responses to invited questionnaires in patients treated for hypothyroidism compared with control individuals (152). Another cross-sectional study used data from individuals participating in the LifeLines cohort study (153). QOL was assessed using the RAND-36 health survey. Because there were insufficient men being treated for hypothyroidism, the scores of 321 female LT4 users were compared with 1581 matched non-LT4 users. Multiple QOL domains were significantly worse in the group being treated for hypothyroidism, including physical functioning, vitality, mental health, social functioning, bodily pain, and general health (P = .003 to < .001).
The challenge, however, in interpreting these types of studies is that their findings are affected by the health of the control group and the potential that recruitment methods may capture more involvement from individuals concerned about their health or that surveys may elicit a higher response rate from individuals who are dissatisfied. With respect to use of surveys, it is also important to consider whether past or current QOL is being assessed. In a study of individuals with hyperthyroidism, QOL was assessed using a version of ThyPRO that assessed symptoms “at this moment” compared with symptoms assessed using the standard ThyPRO that retrospectively assesses symptoms over the last 4 weeks (154). These individuals recalled worse QOL when it was recalled retrospectively, compared to when it was assessed in the current moment.
In contrast to the aforementioned studies in which questionnaire participation was elicited by mail or telephone invitation, some more recent studies used email, websites, and online forums to gather data. These data, again, particularly need to be evaluated with the self-selected nature of the respondents being considered. A recent online survey of patients with hypothyroidism conducted by the American Thyroid Association evaluated the responses to questions about satisfaction with hypothyroidism therapy on a 1 to 10 scale (1 = not satisfied; 10 = very satisfied) (155). The entire group had a median score of 5 (interquartile range [IQR], 3-8). Areas identified as a source of the dissatisfaction were weight, fatigue/energy levels, mood, and memory in 69%, 77%, 48%, and 58%, respectively. In addition, those responding to the survey had a higher level of satisfaction with DTE than those receiving synthetic combination therapy with LT4 and LT3 or LT4 monotherapy (155). The reported satisfaction level was 7 (IQR, 4-8) for DTE, compared with 6 (3-8) for synthetic combination therapy, and 5 (3-7) for LT4 alone.
Poor overall patient satisfaction was also reported in a survey conducted by the British Thyroid Foundation (156). Seventy-seven percent of patients were dissatisfied with their treatment. QOL was better in those taking DTE or using synthetic LT4/LT3 combination therapy than in those using LT4. However, in multivariate analyses significant correlations with satisfaction and QOL were found only for age, sex, and various characteristics of the patient’s diagnosis and interaction with their physician. This study illustrates the complex interaction of many factors that ultimately affect the QOL in those being treated for a chronic condition such as hypothyroidism.
Potential Explanations for Residual Symptoms During Levothyroxine Treatment
There are a myriad potential explanations for why patients being treated for hypothyroidism and who are maintained with a normal serum TSH may have residual symptoms that overlap with symptoms of hypothyroidism (157, 158). As a first consideration, maintenance of a normal TSH cannot be presumed given the observation that as many as 40% of individuals receiving LT4 therapy many be either undertreated or overtreated (23, 61, 63). With respect to targeting specific TSH ranges in treated patients, most studies do not show improvement in patients’ symptoms with lower TSH values within the normal range. For example, a recent trial in which patients were randomly assigned to receive LT4 doses that resulted in 3 different ranges of TSH values showed that these different TSH targets had no effect on QOL, mood, or cognition (53). Similarly, another randomized controlled study in which hypothyroid participants received each of 3 doses of LT4 for 8-week periods with different mean TSH values of 0.3, 1.0, and 2.8 mIU/L being achieved did not find any differences in well-being, hypothyroid symptoms, QOL, or cognitive function associated with the different 8-week time periods (55).
Other factors affecting health-related quality of life
QOL is complex and affected by many factors that affect the patient’s perception of an illness and its treatment and its effect on physical, psychological, and social aspects of life (159). Other effects on QOL may include economic and cultural factors, adverse drug reactions, and patient education. Assuming a normal TSH in treated patients, other factors to be considered as potentially negatively affecting QOL may include autoimmunity, symptoms that actually stem from coexistent conditions or comorbidities, a negative effect of awareness of having a chronic condition, or “tissue hypothyroidism” (157) (Fig. 5).
Figure 5.
Potential causes of symptoms in patients with a normal thyrotropin (TSH).
The idea that TPO Abs, per se, may be associated with symptoms has been entertained. For example, comparing euthyroid individuals with TPO Ab titers greater than or less than 121 IU/mL revealed significantly greater rates of chronic fatigue (66% vs 49%), lack of concentration (32% vs 19%), and nervousness (68% vs 39%) in those with the higher TPO Abs titers (15). A cross-sectional study also found that TPO Abs levels greater than 100 IU/mL significantly increased the risk of depression (odds ratio [OR] 3.0; 95% CI, 1.3-6.8) (160). Patients with hypothyroidism do appear to have more chronic medical conditions than other populations (161), but whether this accounts for their decreased QOL is not clear. In a study of the NHANES database, in which participants taking LT4 were matched to healthy controls for age, sex, race, and serum TSH, the treated patients had higher BMIs, and were more likely to be taking β-blockers, statins, and antidepressants (161). The interaction between recognition and treatment of coexisting chronic conditions is likely to be complex. For example, in one observational study patients started on LT4 were more frequently started on statins and more frequently had their statin therapy intensified (162). A population study (163, 164) revealed that an increased risk of being diagnosed with cardiovascular disease, lung disease, diabetes, and psychiatric disease is present both before and after hypothyroidism has been diagnosed. Interestingly, even awareness of a chronic diagnosis can affect an individual’s perception of their health. In a cross-sectional population study, women had better self-reported health when they did not know of their diagnosis of hypothyroidism, and worse self-reported health when the diagnosis was known (165). The OR of an association between hypothyroidism and poor self-reported health at follow-up was 1.44 to 1.81 if the diagnosis of hypothyroidism was known at follow-up, compared with 0.51 if the diagnosis was not known at follow-up.
It is important to understand whether other causes, rather than inadequately treated hypothyroidism, are the proximate cause of patients’ symptoms (157, 166). Diagnosis of hypothyroidism may lead to an intensified search for other medical conditions and vice versa. Alternate conditions that may be amenable to treatment, or require different therapy other than thyroid hormone, may be overlooked to the patient’s detriment. It is possible that patients who take LT4 as their only medication (and their physicians) may have a tendency to attribute all a patient’s symptoms to their diagnosis of hypothyroidism; with the possibility that this attribution could either be correct or incorrect. In addition, patients and physicians may both have unrealistic expectations for their hypothyroidism therapy (156, 167). Potentially beneficial activities such as exercise, relaxation, and adequate sleep may be underemphasized. While not necessarily providing an explanation for residual symptoms, exercise has been reported by patients participating in a conference to be helpful for residual symptoms (167). In addition, exercise has been reported to improve QOL in patients with SCH (168) and to improve fatigue in patients with thyroid cancer who are undergoing TSH suppression therapy (169, 170).
Potential hypothyroidism at a tissue level
The tissue hypothyroidism hypothesis probably originated from a series of animal studies. These animal studies measured T3 concentrations at a tissue level. In a now classic study, it was determined that intravenous infusion of LT4 in thyroidectomized rats did not simultaneously normalize all the thyroid-related parameters studied by the investigators (171). Doses of LT4 that resulted in normal T3 concentrations in various tissues resulted in supraphysiologic serum concentrations of T4. For example, infusion of 2 mcg/100 gm body weight LT4 per day resulted in normal T3 levels in most tissues, but supraphysiologic serum T4 and suppressed serum TSH. Further elegant studies from the same group employing combined infusions of LT4 and LT3 in rats showed that all parameters could be normalized simultaneously with a dose of 0.9 mcg/100 gm body weight LT4 and 0.15 mcg/100 gm body weight LT3 per day (172). Deiodinase concentrations were also best normalized with this regimen. A more recent animal study also showed similar findings with regard to better normalization of thyroid hormone levels with combination therapy delivered in a “sustained release” manner (173). Continuous-release pellets of LT4 and LT3 normalized serum T4 and T3 concentrations, whereas intermittent daily doses of LT4 and LT3 did not (173). In addition, other markers of euthyroidism, such as serum cholesterol levels, mitochondrial content, and enzymatic activity within liver and skeletal muscle, better approximated values in control rats when sustained delivery both of LT4 and LT3 was employed. Likewise, the pattern of T3-responsive genes in the brain was more similar to that of control rats when combination therapy was employed. The authors postulated that tissue-specific DIO2 inactivation by ubiquitination prevented normalization of serum T3 unless combined continuous delivery was employed.
Rat and human thyroid physiology differ with respect to the molar ratio of T4:T3 secreted by the thyroid (the rat produces relatively more T3) and the proportion of thyroid hormones bound to transport proteins. On the other hand the role of the DIO2 pathway is more developed in humans. Even with species differences, the aforementioned rodent studies might suggest that local production of T3 is regulated in a tissue-specific manner, and that specific tissues may have differential dependence on circulating T3 levels based on their deiodinase activity and different patterns of deiodinase inactivation.
Altered 3,5,3′-triiodothyronine (T3) levels, elevated thyroxine to T3 ratios, and type 2 deiodinase polymorphisms
When treatment for hypothyroidism is provided as LT4 monotherapy, steady serum concentrations of T3 are seen over a 24-hour period (174). However, the T4/T3 ratio that results is higher than that associated with native thyroid functioning (167) and conversely the FT3/FT4 is lower than in the native state (175). A series of studies have examined the T3 levels achieved during LT4 monotherapy (167). These studies used a different methodology, including their selection of the comparison group. In a study that used patients as their own controls by studying the same patient before and after thyroidectomy, T3 levels were reasonably well maintained (176). However, taking available studies together shows that T3 levels are lower in a proportion of LT4-treated patients (1, 167) and may even be below the T3 reference range in some studies (175). In a small study of thyroidectomized patients, lower T3 levels during LT4 monotherapy were associated with the presence of being either homozygous or heterozygous for the Thr92Ala polymorphism of the DIO2 gene (177). If replicated in additional studies, these data could indicate that DIO2 polymorphisms may, in fact, affect circulating T3 levels in those who are athyreotic and dependent on LT4 therapy.
There are some limited data available concerning the effect of DIO2 polymorphism status on either a patient’s response to LT4 therapy or their preference for combination therapy with both LT4 and LT3 (167). One study performed an analysis of retrospective DIO2 genotyping and the QOL data from a study of combination therapy by Saravanan et al (178). Genotyping for the presence of the Thr92Ala polymorphism was performed and participant scores on the GHQ-12 were analyzed according to their polymorphism status, both at baseline and when taking their assigned LT4 or LT4/LT3. Ala/Ala homozygotes had worse GHQ scores while taking LT4 (178). In addition, they also had a better response to combination therapy compared with LT4 monotherapy based on their GHQ scores (178). Median TSH values were not different over the course of the 1-year study between patients with the same genotype receiving either combination therapy or monotherapy with LT4, thus suggesting that responses were not secondary to different TSH values. As cautioned by the study investigators, the study was underpowered, with a risk of a type 1 statistical error.
Other studies have not shown similar findings with respect to LT4 monotherapy, with 2 studies reporting there were no associations between scores of QOL and cognition in LT4-treated patients according to their Thr92Ala genotype (153, 179). Two further genotyping studies examined patients participating in the combination therapy trial conducted by Appelhof et al (180). One study examined the hypothesis that 2 DIO2 polymorphisms (ORFa-Gly3Asp and Thr92Ala) were associated with either response to LT4 monotherapy or preference for combination therapy. Neither polymorphism was associated with well-being, neurocognitive function, or preference for combination therapy (181). The other study examined the hypothesis that OATP1C1 polymorphisms were associated with response to therapy. While 2 of the polymorphisms (OATP1C1-intron3C > T and OATP1C1-C3035T) were associated with fatigue and depression that was worse in the wild types than in the homozygotes, there was no association with neurocognitive functioning or preference for combination therapy (182). On the other hand, one small study (183) that conducted retrospective genotyping of participants from a prospective combination therapy study (184) found that patients with both the Thr92Ala polymorphism and a polymorphism in one of the thyroid hormone transporters (MTC10) preferred combination therapy with both LT4 and LT3 (183). The field will be greatly advanced when the results from a prospective, adequately powered study of the effect of DIO2 and thyroid hormone transporter polymorphisms on response to therapy for hypothyroidism become available. This should answer the question of whether therapy for hypothyroidism should be informed by patient genotype.
Combination Therapy with Levothyroxine and Liothyronine
If traditional LT4 monotherapy results in a relatively lower serum T3 concentration accompanied by some patient dissatisfaction with this therapy, this might prompt consideration of combination therapy with both LT4 and LT3 (1, 157, 167). The molar ratio of T4:T3 achieved by endogenous thyroid functioning in humans is approximately 14:1 to 15:1 (1). If the therapeutic goal of thyroid hormone administration is to strive for a similar ratio, this would be best achieved using synthetic LT3, with the use of small doses and subsequent adjustments as necessary. However, there is considerable debate about what parameters should be used to make adjustments in therapy, given the short half-life of LT3 (185). Even though patient preferences for DTE have been elicited, the high T3 content of DTE makes this product difficult to use to provide physiologic ratios.
Synthetic combination therapy
Excluding one study of DTE (186), one study conducted in patients with central hypothyroidism (134), one that examined only biochemical parameters (187), and one in which patients were not individually randomly assigned (188), 14 randomized trials have been performed that examined the effect of synthetic combination therapy on patients with hypothyroidism from 1999 through 2016 (1, 167) (Table 4). The initial study in 1999 kindled interest in combination therapy (189), 12 additional studies of synthetic combination therapy were performed between 2002 and 2010 (180, 184, 190-199), and a subsequent study was completed in 2016 (200). Recently, a unique study that randomly assigned patients to 1 of 3 treatment arms, thus including both synthetic combination therapy and DTE, has been conducted (201). The design of these studies differed with respect to the outcomes studied, the use of crossover or parallel groups, blinding methodology, and the ratio of T4 to T3 employed. However, autoimmune hypothyroidism was the prevailing diagnosis in most studies. With 5 exceptions (184, 189, 193, 196, 197), these studies did not demonstrate a benefit of LT4/LT3 combination therapy. The most recent study identified a benefit of therapy containing T3 only when a subsequent subgroup analysis of the patients who remained most symptomatic while taking LT4 was conducted (201). Five meta-analyses or reviews, which incorporated the trials completed at the time of each analysis, also concluded that there is no clear advantage to combination therapy (1, 202-205). However, the heterogeneity of the trials with respect to causes of hypothyroidism, different dosing regimens, different outcome measures, different duration of treatment, and different TSH and T3 levels achieved in the combination therapy groups made it challenging to draw conclusions. Other design issues in some studies included nonvalidated outcome measures, carryover effects, overtreatment, and minimal inclusion of men and older age groups. Recent reviews and guidelines have stressed the need for larger, better-designed studies of longer duration (1, 167, 206).
Table 4.
Selected characteristics of clinical trials of combination therapy
| Authors | LT3 Dosing frequency | Approximate mcg ratio of T4:T3 | Serum T3 or FT3 higher in T4/T3 arm? | Trial duration | Neurocognitive measures | QOL, mood, measures | Genotyping done to assess effect of Thr92Ala? |
|---|---|---|---|---|---|---|---|
| Appelhof (10:1), 2005 | Twice daily | 10:1 | Yes | 15 wk | No diff | No diff | No |
| Appelhof (5:1), 2005 | Twice daily | 5:1 | Yes | 15 wk | No diff | No diff | No |
| Bunevicius, 1999 | Once daily | 10:1 | Yes | 5 wk | ↑ combo | ↑ combo | No |
| Bunevicius, 2002 | Once daily | 6.5:1 | No | 5 wk | No diff | No diff (tendency combo) | No |
| Clyde, 2003 | Twice daily | 6.5:1 | Yes | 4 mo | No diff | No diff | No |
| Escobar-Morreale, 2005 | Once daily | 15:1 or 12:1 | No | 8 wk | ↑ combo (some parameters only) | No diff | No |
| Fadeyev, 2010 | Once daily | 6:1 | NA | 6 mo | NA | NA | No |
| Hoang, 2013 | Once daily | 4:1 | Yes | 16 wk | No diff | No diff | No |
| Kaminski, 2016 | Once daily | 5:1 | No | 8 wk | NA | No diff | No |
| Nygaard, 2009 | Once daily | 4:1 | Yes (FT3 index) | 12 wk | NA | ↑ combo | Retrospective, yes |
| Rodriguez, 2005 | Once daily | 7:1 | Yes | 6 wk | No diff | No diff (no diff for fatigue) | No |
| Saravanan, 2005 | Once daily | 8:1 | No | 12 mo | NA | ↑ combo (3 mo only) (minority of measures) | Retrospective, yes |
| Sawka, 2003 | Twice daily | 3.5:1 | Yes | 15 wk | NA | No diff | No |
| Shakir, 2021 | Once daily | 9.5:1 | Yes | 22 wk | No diff (except in one domain in subgroup analysis of those with worst scores on LT4) | No diff (except in subgroup analysis of those with worst scores on LT4) | Prospective, no |
| Shakir, 2021 | Once daily | 4:1 | Yes | 22 wk | No diff (except in one domain in subgroup analysis of those with worst scores on LT4) | No diff (except in subgroup analysis of those with worst scores on LT4) | Prospective, no |
| Siegmund, 2004 | Once daily | 19:1 | No | 12 wk | No diff | No diff | No |
| Valizadeh, 2009 | Twice daily | 4:1 | Yes | 4 mo | NA | ↑ combo (minority of measures) | No |
| Walsh, 2003 | Once daily | 8.5:1 | No | 10 wk | No diff | No diff | No |
Italics indicate desiccated thyroid extract.
Abbreviations: diff, difference; FT3, free 3,5,3′-triiodothyronine; LT4, levothyroxine; NA, not assessed; QOL, quality of life; T3, 3,5,3′-triiodothyronine; T4, thyroxine.
Focusing on specific outcomes from these trials, health-related QOL or mood was studied in 14 trials, with heterogeneous results (1, 167, 201). The superiority of combination therapy on multiple measures was demonstrated in 2 trials, one conducted by Bunevicius and colleagues (189) and one conducted by Nygaard et al (184). These 2 trials included patients with thyroid cancer and low TSH values (189), and used a large single dose of liothyronine (20 mcg) (184) respectively. The superiority of combination therapy on a minority of the measures that were assessed was seen in 2 further trials (196, 197). In 1 of these trials the benefit was seen at 3 months but was no longer seen at 12 months (196). Of note, the latter is the only combination therapy study of 12 months’ duration, with the next longest trials being 4 months and 6 months in duration. The remaining 10 trials did not show a superiority of combination therapy with respect to improving QOL for the group studied as a whole. With the most recent trial (201), when a subgroup analysis was performed for those most symptomatic while taking LT4, there was significant improvement in QOL. Examining those who scored worst on LT4 as assessed by the 36-point TSQ-36, 12-point QOL GHQ-12, and the Beck Depression Inventory, these individuals had significant improvement in these indices when switched to LT4/LT3.
Neurocognitive functioning was studied in 11 trials, again with heterogeneous results (1, 167, 201). Combination therapy was judged to be superior based on multiple measures in one trial (189). Combination therapy was also found to be superior based on a minority of measures in another trial (193). The remaining 9 trials did not show a superior effect of combination therapy on neurocognitive functioning (1, 167). An example of a negative trial with respect to both QOL and cognitive functioning is a 4-month study by Clyde et al conducted in 2003 (191). In this study, LT4 alone was compared with combination therapy by replacing 50 mcg of LT4 with 7.5 mcg of LT3. No beneficial changes was seen in body weight, serum lipid levels, hypothyroid symptoms as measured by a health-related QOL questionnaire, or neurocognitive functioning assessed using standard measures. The recent trial reported in 2021 showed significant improvement in one domain of the Wechsler memory scale-version IV (Visual Memory Index) with combination therapy, but only for the subgroup of participants who scored worst on this test while taking LT4 (201).
Patient preference during combination therapy compared with monotherapy has been studied in 5 blinded, 2-arm crossover design trials and 2 blinded, parallel-design trials (1, 167). With respect to the crossover design trials, the combination therapy was preferred in 4 trials that, when combined, included 128 patients (184, 189, 190, 193). Another trial of 101 patients did not demonstrate a preference for combination therapy (194). Of the 2 parallel-design trials, patients reported a preference for combination therapy in 1 trial of 130 patients (180), with no preference for combination therapy reported in another trial of 573 patients (196). The former trial was characterized by some degree of overtreatment and preference for combination therapy was associated with weight loss (180). The latter trial was the largest and longest duration trial of combination therapy conducted thus far (196). In a recently conducted meta-analysis of preference data from the combination therapy trials, Akirov and colleagues (207) examined preference as a binomial distribution of choices (the patient preferred combination therapy vs no preference for combination therapy). The preference rate was found to be no different from chance at 46.2%, although preference did seem to be associated with the magnitude of the LT3 dose (207). For the study by Shakir et al (201), across the 3 study arms, DTE was ranked first by 45% of participants, LT4/LT3 was ranked first by 32% of participants, and LT4 was ranked first by 23% of participants.
Some factors to consider if combination therapy is being contemplated are the risks of therapy. Risks have not been fully investigated because most studies of combination therapy have been of relatively short duration. The risks include T3 thyrotoxicosis, cardiac arrhythmias, and decreased bone mineral density. Participants in the Cardiovascular Health Study who were aged 65 years and older and were taking thyroid hormone replacement had their thyroid function tests studied (63). Of those taking LT4, 37.5% had low TSH values (27.8% SCH; 9.7% overt hypothyroidism), compared with those taking combination therapy, of whom 42.5% had low TSH values (40% SCH; 2.5% overt hyperthyroidism). As most studies have recruited predominately relatively healthy middle-aged women, the risk in men, those with comorbidities, and older or frail populations have not been studied. It is generally agreed that combination therapy should not be undertaken in pregnant women for fear of insufficient thyroid hormone reaching the fetus due to degradation of T3 by the placenta (208). There are some data about potential risk of LT3 described in observational studies. One 17-year observational study, comparing 400 LT3 users with those who used only LT4 (n = 33 995), found no increase in atrial fibrillation or fractures with LT3 use, a trend toward increased use of antidepressants, and a trend toward an increased hazard ratio for breast cancer (209). This same study did find an increased use of antipsychotic medications associated with LT3 use, with the adjusted hazard ratio for being prescribed an antipsychotic medication if LT3 had ever been used being 2.26 (CI, 1.64-3.11) (209). The trend for breast cancer risk was not confirmed by a registry study from Sweden. LT3 users (n = 11 147) were compared with LT4 users (n = 575 461) over an 8.1-year period and no increased incidence or mortality from breast cancer or other cancers was identified (210). The financial costs associated with LT3 prescriptions are variable and have increased in some countries (211-213), with the result that the number of LT3 prescriptions have decreased (211-213). Should LT3 be the preferred therapy for some subgroups of hypothyroid patients, this increased prescription cost could affect patient access to LT3.
Combination therapy with desiccated thyroid extract
Combination therapy can also be provided by use of DTE. Unlike synthetic combination therapy, the ratio of T4:T3 cannot be adjusted, unless LT4 therapy is added to lower the relative amount of T3. The T4:T3 ratio of DTE is approximately 4:1. One trial of DTE randomly assigned patients to either LT4 or DTE and then switched participants to the other therapy after 16 weeks, for a further 16 weeks of therapy (186). During the DTE treatment arm, patients had significantly higher serum levels of T3 and lower levels of FT4. Multiple different parameters of QOL were assessed during the trial, and these parameters did not differ between the 2 groups. However, 49% of patients preferred the DTE, compared with 19% preferring LT4 and 33% having no preference (186). Preference for DTE was found to be associated with weight loss. In this study, there was also no routine documentation of the daily excursions in T3 concentrations that were associated with DTE use. Only 2 patients had serum T3 levels measured on 1 occasion 3 hours after taking the thyroid extract (186). The T3 concentration in these patients rose from 129 to 175 ng/dL and from 138 to 169 ng/dL, thus not providing data to counter the older studies (214-216) showing hypertriiodothyroninemia 2 to 5 hours after thyroid extract use. The clinical consequences of such serum T3 excursions are unknown. These high T3 levels may be of particular concern in patients receiving suppressive therapy for thyroid cancer using a thyroid extract, or in frail or older populations. With the trial by Shakir et al (201), similar to the situation seen with synthetic combination therapy, DTE resulted in improvement over LT4 when examining those who scored worst on assessments made while they were taking LT4. Significant improvements were seen in the TSQ-36, GHQ-12, Beck Depression Inventory, and Visual Memory Index.
Despite the earlier negative randomized, controlled trial, and perhaps in keeping with the subgroup analysis in the later trial, there is considerable patient interest in “natural” combination therapy in the form of DTE. A study examining the online posts of patients regarding treatment with DTE was conducted. Eighty-one percent of patients described DTE as moderately effective and 77% described it as more effective than the previous therapy. The most frequently described benefits associated with DTE use were an improvement in symptoms (56%) and a change in overall well-being (34%). Side effects of DTE were described by one-fifth of patients (217). The previously mentioned online survey of patients with hypothyroidism conducted by the American Thyroid Association found that those responding to the survey had a higher level of satisfaction with DTE than those receiving other therapies (155). Satisfaction level on a scale of 1 to 10 was 7 (IQR, 4-8) for DTE, compared with 6 (3, 8) for synthetic combination therapy with LT4 and LT3, and 5 (3, 7) for LT4 alone.
Monitoring of Combination Therapy
If combination therapy is chosen for a patient, it could potentially include synthetic LT4 and LT3, with 2 decisions to be made. These are selection of the ratio of T4 to T3, and choice of the frequency of the LT3 administration (daily, twice daily, 3 times daily). Alternatively, albeit not recommended by any clinical practice guideline (1, 158, 218), thyroid extracts could be considered to be a fixed-dose combination therapy with a T4/T3 of approximately 4.2:1 with T3 being given once daily. A pharmacodynamic study has shown that the LT4/LT3 equivalence ratio is approximately 3:1. For example, approximately 150-mcg LT4 is equivalent to 50-mcg LT3 (219). Therefore, if a patient were being converted from LT4 monotherapy to combination therapy, with maintenance of the same dose, there would need to be a reduction in the LT4 dose depending on the LT3 dose being added, according to the 3:1 conversion factor. Recommendations for converting a patient who is not doing well while taking LT4 from LT4 monotherapy to combination therapy have been published (218). There are other important considerations if rational combination therapy is to be safely undertaken. Endogenous fluctuations in FT3 are small and were on the order of an excursion of 0.6 pmol/L (0.39 pg/mL) during the day in one study in which 24-hour blood sampling was conducted (220). If steady serum levels of T3 are both physiologic and desirable, either multiple small doses of LT3 (eg, 2 mcg 4 times daily or 2-3 mcg 3 times a day) would be needed. These examples employ small doses of LT3 that are not available as single tablets. Currently LT3 is available as 5-mcg, 25-mcg, and 50-mcg tablets. In addition, there is agreement that it is not realistic to expect patients to be able to adhere to dosing of LT3 given more than twice daily (167, 218). Alternatively, a sustained-release T3 preparation would be needed.
An important parameter that could be targeted during combination therapy is patient symptoms. This is important because unresolved symptoms are what typically lead a patient to request combination therapy. The attendant question is which symptoms or QOL measures would best reflect successful combination therapy. Symptoms can be assessed using validated QOL questionnaires. Since changes in thyroid symptoms might not be as apparent in general QOL questionnaires, it is important that standardized and/or validated thyroid-related QOL questionnaires be used. Examples of such questionnaires include the ThyPRO, Chronic Thyroid Questionnaire, and Underactive-Thyroid-Dependent Quality of Life Questionnaire (221-223) (reviewed in [1, 167]). However, such questionnaires have not typically been employed in a routine clinical setting but have been used primarily in the research setting, so this is an untested and potentially time-consuming approach.
The optimal biochemical parameters for monitoring of patients receiving combination therapy has not been elucidated. It is not clear whether serum TSH is as helpful a marker of euthyroidism in a patient receiving combination therapy, compared with in patients receiving traditional monotherapy (167). Potential targets, in addition to serum TSH, include FT4, T3, FT3, and the FT4/FT3 ratio (167). The concept of considering serum T3 as a therapeutic target has been quite controversial (1, 224). An additional concern exists if serum FT3 is a potential target. Assays for FT3 may be less accurate, in part because of the low concentrations of hormone being measured (225-227). The FT3/FT4 ratio has been proposed as a simple estimate of deiodinase activity during LT4 monotherapy (228). It is possible that an FT3/FT4 ratio would reflect a combination of peripheral conversion plus the exogenous LT3 being supplied during combination therapy. However, even if the FT4/FT3 or FT3/FT4 ratio is a meaningful target, the desired target value has not been defined (167). It is likely that any monitoring strategy that includes a combination of several laboratory analytes would necessitate additional costs for the patient. These costs would be in addition to the extra costs of the LT3 therapy itself.
In addition to selecting the best thyroid analytes to use for monitoring combination therapy, another consideration is the timing of testing and whether a peak or trough thyroid hormone level is being sampled. High serum T3 levels may be encountered, depending on the timing of phlebotomy. Only 2 trials of synthetic combination therapy assessed postdosing T3 concentrations, and did so in only a small subset of 10 to 12 patients (174, 192). In one study patients were taking 10-mcg LT3 once daily combined with LT4. A 42% increase in serum FT3 concentration was seen within 4 hours after the administration of 10-mcg LT3 (174). Three of the 10 patients had serum T3 levels that were above the upper limit of the laboratory reference range for part of the day. In the other study, in which 5% of the patient’s dose of LT4 was replaced by a single dose of LT3 that was prepared in house, there was a 54% increase in FT3 concentrations approximately 2 hours after LT3 administration (192).
Attention to the timing of phlebotomy is particularly important if LT3 is given only once daily. Serum T3 and FT3 levels peak approximately 2.5 hours after dose administration (174, 192, 229, 230). A trough serum T3 level is clearly both lower and more predictable than a postdose T3 level, as illustrated in 3 studies of once-daily LT3 dosing (174, 192, 229). The 24-hour profile of serum TSH concentrations following once-daily LT4/LT3 administration appears to show more fluctuation than serum TSH levels following daily LT4 administration (174). In patients receiving combination therapy, the TSH nadir was at 6 hours following LT4/LT3 dosing, before returning to the predosing value about 10 hours later (174). If a trough T3 concentration, and its associated TSH level, was being targeted because of the predictability of analyte values before the next LT3 dosing, phlebotomy in the early morning before any of that day’s LT3 administration might be particularly useful for monitoring therapy.
Monotherapy with liothyronine
Monotherapy with LT3 has rarely been used for long-term therapy, in part because of the need to take LT3 multiple times a day to maintain a reasonably stable serum TSH. Preference for LT3 alone compared with LT4 alone has not been studied in a controlled fashion. LT3 is well absorbed in the gastrointestinal tract and is available in 3 dosages of 5, 25, and 50 mcg. The major drawback of LT3 therapy is that, because of its short half-life of slight less than 24 hours (230, 231), its use is associated with peaks and troughs in its serum concentration (174, 229) unless it is administered multiple times daily (219). This is in contrast to the steady T3 concentrations that are achieved with LT4 therapy (174). Peak serum levels, which are attained within 4 hours of administration, can be associated with hyperthyroid symptoms such as anxiety, tremors, and palpitations. A clinical trial compared serum TSH concentrations achieved with either LT4 or LT3 therapy each delivered 3 times daily (219). Relatively stable T4 and T3 concentrations and similar TSH concentrations could be achieved, but a lengthy adjustment period, with a mean duration of 32 weeks, was required to achieve the rigorous TSH goal of 0.5 to 1.5 mIU/L, perhaps suggesting that adherence to a thrice-daily regimen was difficult. This study demonstrated that 40-mcg T3 or 115-mcg T4 (1:3 ratio) produced similar mean TSH concentrations of 1.4 and 1.2 mIU/L, respectively. LT3 monotherapy administered for 30 days resulted in a small decrease in weight (3%) and a significant decrease in total cholesterol, low-density lipoprotein cholesterol, and apolipoprotein B, compared with LT4 monotherapy (232). The effect of LT3 monotherapy to reduce lipid parameters suggests an effect at the level of the liver. There was a nonsignificant increase in sex hormone–binding globulin that was also documented, which would support the concept of a hepatic site of action. Currently LT3 therapy is not recommended because of uncertainty about any benefits, difficulty with titration, lack of experience regarding parameters to monitor therapy, and concern about T3 thyrotoxicosis (185).
Physician prescribing of T3-containing products
Despite the lack of conclusive data showing the superiority of T3-containing therapies, physicians do prescribe combination therapy, and willingness to do so is supported by surveys of physicians. A survey of members of the American Thyroid Association described an index patient who was biochemically and clinical euthyroid and queried the 363 participants regarding their choice of therapy (233). For the index patient, 98% of physicians continued current LT4 therapy. However, as the patient scenario incorporated other patient characteristics, physicians opted to increase the LT4 dose or prescribe LT4/LT3 therapy. The tendency to prescribe LT4/LT3 was dramatically increased by patient symptoms (OR 25.6; 95% CI, 9-73; P < .0001) (233). All other characteristics included (low serum T3 levels, presence of a polymorphism, a request for LT3, or a patient-stated preference for LT3) significantly increased the likelihood that alternative therapies would be prescribed in multivariate analyses (P < .0001, all cases). Athyreotic status, patient sex, and BMI did not increase the likelihood that LT4/LT3 would be prescribed. However, older age and the presence of a comorbidity reduced the likelihood that LT4/LT3 was prescribed (P = .0002 and < .0001, respectively). In addition, physicians practicing in North America were more likely to prescribe T3-containing therapies than physicians practicing outside North America (234), and employing survey administration at 2 different time points, there was a trend for prescribing of T3 products to increase over time (235). The willingness of physicians to prescribe combination therapy for patients with symptoms presumably stems from the desire to improve the QOL of these symptomatic individuals. Although such an improvement is not demonstrated by the completed randomized trials that included hypothyroid patients broadly, there is a possibility that different results might be obtained if symptomatic patients were specifically recruited. An open-label trial without a control group in which patients with persistent symptoms on LT4 were all switched to LT4/LT3 showed improvement in their ThyPRO-39 composite score from 54 (IQR, 34-74) to 15 (11, 28) at 3 months and 20 (14, 26) at 12 months (236).
In a survey of practices surrounding use of thyroid hormone for patients with both hypothyroidism and euthyroidism administered to Polish endocrinologists, it was ascertained that physicians were willing to prescribe thyroid hormone for patients without a diagnosis of hypothyroidism. This included prescribing not only for patients with infertility and thyroid autoantibodies, but also for patients with goiter, fatigue, obesity, hypercholesterolemia, and depression (237). About a third of physicians expressed a willingness to prescribe combination therapy for hypothyroid patients with unresolved symptoms despite a normal TSH. Nevertheless, the physicians attributed the symptoms to the burden of chronic disease, psychosocial factors, comorbidities, and unrealistic expectations, among other causes (237). Given that current physician practice patterns may incorporate the use of therapies other than LT4, there is a critical need for more research into the benefits and risks of these therapies. Not only is there a need not to withhold beneficial therapies if a subset of patients may respond favorably, but there is also a corresponding obligation to be cognizant of the potential risks of these therapies, especially if there is no benefit.
Consensus Statement on Combination Therapy Trials
Recently a group of investigators with expertise both in basic/translational and clinical thyroid research used the evidence presented during a conference discussing combination therapy to reach a consensus regarding whether future trials of combination therapy were justified, considering the large number of trials that had already been conducted. The group opinion was that there was equipoise for future trials, based on the shortcomings of the trials that had been completed (167). These shortcomings related to study end points, participant selection, incorporation of polymorphisms and trial design. Examples of shortcomings include inadequate incorporation of patient-reported outcomes (PROs) as primary outcomes; lack of formal assessment of patient preference; recruitment of a general population of hypothyroid patients, rather than targeting those who have unresolved symptoms; inadequate power to study the effect of therapy for the expected effect sizes and response rates; and short duration trials.
Study outcome
There was consensus that a PRO with content and validity with respect to thyroid-related QOL that also had responsiveness to change should be used. ThyPRO 39 was favored as a primary end point, and furthermore patient preference was favored as a secondary trial outcome (167). It was felt that future studies should be powered based on an effect size of at least 0.5, and preferably 0.3. Based on these criteria applied to prior studies, the only 2 studies powered for an effect size of 0.3 were the crossover design study by Walsh and colleagues (number of patients = 100; number required = 107) (194) and the parallel design study by Saravanan et al (number of patients = 697; number required = 352) (196).
Participant inclusion
With regard to patient recruitment to future trials, there was agreement that a thyroid-related QOL survey should be used to assess baseline satisfaction for the purposes of participant inclusion in the trial (167). There was also agreement that participants should have a normal TSH while being treated with at least 1.2 mcg/kg/day LT4. This was thought to be a key inclusion criterion to capture those who were substantially dependent on LT4 and to maximize that possibility that symptoms were associated with LT4 replacement therapy. A computer modeling study found that studies of combination therapy conducted thus far included patients who had different degrees of residual thyroid function (238). Residual thyroid function in turn affected the doses of LT4 and LT3 that would be predicted to result in midnormal TSH, FT4, and T3/FT3 levels. Incorporating a 1.2-mcg/kg/day requirement would mean that trials would not study individuals with SCH, who could potentially be studied in different trials. There was only 75% agreement that “following exclusion of other causes of symptoms, patients who do not report relief of their symptoms with LT4 therapy should specifically be recruited for combination therapy trials” (167). Certainly, if only patients with remaining symptoms, and who were dissatisfied at baseline, were recruited it would be anticipated to increase the expected response rate. Examples of potential inclusion criteria, if this approach were taken, are i) a score greater than 4 in the 12-question TSQ, a validated instrument used to assess hypothyroid symptomatology, or ii) an overall health-related QOL score using the ThyPRO-39 composite score of more than 32 (167). Supporting this approach is the recent finding that there was only a response to T3-containing therapy in a subgroup analysis of those who were most symptomatic while taking LT4 (201).
There was discussion as to whether patients with low serum T3 levels while taking LT4 monotherapy should be specifically recruited to any new trials. The stratification of results according baseline T3 levels or trough T3 levels achieved during combination therapy was also discussed. Only 50% of the statement task force thought this approach had merit (167). Of note, during the synthetic combination trials completed by 2016, approximately 50% of them achieved the same T3/FT3 levels both in the monotherapy and combination therapy arms, and approximately 50% achieved higher T3/FT3 levels in the combination therapy arm (1, 167). One very small open-label study examined baseline and follow-up T3 levels in patients who commenced combination therapy and then elected to continue it. There was no correlation with either T3 level and the desire to continue combination therapy (239).
Although there was universal agreement (100% consensus) that future trials should be adequately powered to study the effects of polymorphisms in the DIO2 and thyroid hormone transporters (MCT8, MCT 10, OATP1C1) (167), this statement is unlikely to be realized without enormous expense and large collaborative consortia. Almost all studies thus far have reported retrospectively on the effect of genotype. Unless future prospective trials are large and well-funded, they are likely to remain underpowered.
Trial design
The trial design favored by the writing group was a randomized, placebo-controlled, double-blind, parallel-design trial (167). Although most felt that a trial of a year’s duration was ideal (80% agreement), there was not universal agreement, as there was concern about participant retention and the potential for participants to discontinue the trial to request LT3 from their health care provider. The longer trial was felt to be beneficial for assessing the durability of the response (with interim outcome assessments at 3 and 6 months) and the effects on cardiac and skeletal health, but the costs that would be incurred were recognized. Most agreed (90%) that the trial should be “pragmatic” and include participants with comorbidities as long as they were managed to achieve stability. This was felt to be important to ensure that trial results were representative and could be generalized to the hypothyroid population as a whole. The topic of DTE engendered much discussion. A proposal that a future combination therapy trial should include 3 arms (LT4, LT4/LT3, DTE) garnered only 67% consensus. Those favoring the inclusion of DTE felt this was important because of the considerable patient interest in this product, whereas those reluctant to support this design were concerned about patient safety, and trial complexity and costs. Since the publication of the consensus statement, the 3-arm crossover trial that included both DTE and LT4/LT3 has been reported (201).
T3-product administration
Of the completed synthetic combination therapy trials, none of them used LT3 administered 3 times daily, as was achieved in the LT3 monotherapy trials (219, 232). Of the 14 trials already described reported through 2016, 10 used once-daily administration of LT3 and 4 used twice-daily LT3 (1, 167). The working group for the consensus statement (167) reached 100% agreement that a sustained release T3 preparation was the preferred product for future trials, should it become available. Otherwise, there was also 100% agreement that twice-daily administration of LT3 should be used in a physiologic ratio.
The goal of a sustained-release T3 preparation that would avoid fluctuating serum T3 levels has been pursued for some years. Various pharmaceutical companies have aspired to such a product, but without bringing it to fruition. It is possible to obtain various thyroid hormone preparations from compounding pharmacies. For example, a compounding pharmacy may mix T3 with methylcellulose and dispense tablets designed to have a sustained-release profile. Compounding pharmacies are state regulated, but the issue of whether they are also subject to federal jurisdiction has been debated (240). Such pharmacies should be used with caution as historically there have been problems with rapid loss of potency of compounded LT4 products (241). More recent reports of compounded LT3 preparations have not yet demonstrated a sustained-release profile in vivo (242).
Use of LT4/T3 combination therapy that employed a custom-made sustained-release T3 preparation was described in a 2004 publication (243), but then not reported on further. Participants had similar TSH values during LT4 therapy and during combination therapy, and the combination therapy including the sustained-release T3 achieved steady concentrations of T3 over a 10-hour period. T3 sulfate is a metabolite of T4 and T3 that does not itself have biological activity (244) but has been shown to be converted to T3 and to lower TSH levels in thyroidectomized rats (245). T3 sulfate has also been reported to have a sustained-release profile, as initially demonstrated in patients who were withdrawn from thyroid hormone in preparation for radioiodine treatment. In such hypothyroid patients, administration of a single dose of T3 sulfate resulted in steady serum T3 levels for about 48 hours (246). Subsequently, in a phase 2 study (247) the same investigators replaced 25-mcg LT4 with 40 mcg of T3 sulfate in thyroidectomized patients and found that this therapy lowered serum TSH and FT4 and produced a ratio of FT4/FT3 that was similar to the ratio seen in individuals with native euthyroidism (175, 176, 248).
Efforts have been pursued (249) to identify new delivery strategies for T3 that delay absorption from the gastrointestinal tract or other depots. Most recently, a zinc-coordinated form of LT3 (poly-zinc-liothyronine) in capsules was administered to hypothyroid rats (250). Compared with LT3, the poly-zinc-liothyronine produced a lower serum T3 peak and exhibited a longer time period over which the T3 concentrations remained at a plateau in pharmacokinetic studies in treated rats. Chronic poly-zinc-liothyronine administration lowered serum TSH, lowered body weight, decreased cholesterol levels, and stimulated T3-response genes in a similar manner to LT3 (250). Recently a phase 1 single-dose, double-blind, placebo-controlled study of poly-zinc-liothyronine was completed in 12 healthy volunteers. It has been reported in medRxiv (a preprint server for health sciences) (251) but has not yet been peer reviewed. The poly-zinc-liothyronine reached a lower maximum serum T3 concentration than the LT3 and exhibited a 6-hour and 12-hour plateau. The area under the curve was greater for the poly-zinc-liothyronine at the 12- to 24-hour and 24- to 48-hour periods. Future studies will determine whether this profile will translate into improved stability of serum T3 levels and more satisfactory therapy for patients.
Reduction of thyroid peroxidase antibodies and preventing thyroid autoimmunity
It has been hypothesized that TPO Abs themselves may affect patient QOL, even though the TSH is normal. This has been considered both in those who are receiving LT4 therapy and in those not initiated on LT4. One trial addressed the former concept. Patients with continued symptoms while receiving LT4, who also had significantly elevated TPO Ab titers of greater than 1000 IU/mL (median 2232 IU/mL), were randomly assigned to thyroidectomy or continued medical management. In those who underwent thyroidectomy, TPO Abs declined to a median of 152 IU/mL. Health-related QOL and fatigue improved in the thyroidectomized patients and were sustained at 12 to 18 months (252). There was no group that underwent sham thyroidectomy. An earlier nonrandomized study of thyroidectomy for benign thyroid disease examined hypothyroid symptoms before and after thyroidectomy in a group of individuals of whom only 25% were taking LT4 prethyroidectomy. There was no improvement in QOL in the group overall, although those with higher TPO Abs were more likely to have an improvement in emotional role and bodily pain concepts of the SF-36 (253). Such treatment measures need to be carefully further evaluated because thyroidectomy can be associated with decreased QOL, albeit in the setting of thyroid cancer with its additional associated treatments and complications (254, 255).
Interventions to prevent the evolution of Hashimoto disease have not yet been realized. The pathogenesis of Hashimoto disease is complex and likely involves intertwining genetic susceptibility, environmental factors, sex, and potentially microchimerism (256). There has been interest in the possibility of reducing TPO Abs and preventing the onset of Hashimoto hypothyroidism. Trials of selenium supplementation have been associated with reduction of TPO Abs (257), but disease progression or QOL outcomes have not been sufficiently studied (258). Differences in the gut microbiome have been shown in patients with Hashimoto thyroiditis compared with control patients and compared with patients with Graves disease (259-262). Patients with celiac disease with and without Hashimoto disease also appear to have distinct gut microbiomes (263). Thus, it has been hypothesized that alterations in the gut microbiome may be associated with the development and progression of Hashimoto hypothyroidism. Other therapies tested as a means of reducing TPO Abs such as specific diets have not been sufficiently or rigorously studied (264). Studies in individuals with both celiac disease and Hashimoto thyroiditis have shown varying effects of a diet that is low in gluten on thyroid status. In a prospective study of cases with celiac disease compared with controls without celiac disease, there was no effect of a low-gluten diet on TPO Abs but there was a decreased thyroid volume that occurred only in the patients with celiac disease (265). A reduction in TPO Abs was seen after a low-gluten diet in those with both TPO Abs and transglutaminase Abs, compared with a group with a similar Ab status who consumed a gluten-containing diet (266).
Summary and Areas of Future Research
Despite the significant advances that have been made in our understanding of thyroid hormone metabolism and action, some of these insights have not been easy to translate into practical applications. For example, although we recognize that LT4 does not recapitulate all aspects of normal thyroid physiology, perhaps as best shown in animal studies, we still do not have a “physiologic” replacement therapy. In contrast, some of the challenges that continue to face physicians treating patients with hypothyroidism concern the most fundamental aspects of therapy. With respect to this former issue, a significant number of patients with hypothyroidism are either undertreated or overtreated. This is an intricate problem, as out-of-range TSH values may be due to multiple factors as diverse as confounding medications, interfering conditions, or intrinsic patient variables. The patient variables may be as basic as adhering to prescribed therapy. Such problems, with multiple etiologies, may require creative and sustained efforts to partner with patients and other members of the health care team for them to be addressed.
These challenges are also accompanied by exciting opportunities for gaining understanding that may enable us to fully reverse the consequences of hypothyroidism. Advances in our understanding of deiodinase and thyroid hormone transporter polymorphisms may ultimately enable us to provide therapy tailored to individual patients. In addition to understanding the genetic underpinning of thyroid hormone delivery to tissues, associated future goals include developing more physiologic, sustained-release combination thyroid hormone therapies incorporating T3, and facilitating achievement of adequate thyroid hormone levels in all tissues. It is exciting with regard to T3 preparations that a product that begins to recapitulate some of the profile of a sustained-release preparation has completed phase 1 trials in the United States. Another hope for the future is the regeneration of functional thyroid follicles from embryonic or pluripotent stem cells, although so far this has been achieved only in animal models. Such cells, once differentiated into thyroid follicular cells, have been shown to be capable of forming 3-dimensional follicles, expressing thyroid-specific genes, responding to TSH stimulation, actively transporting iodine, and expressing thyroglobulin (267, 268). Stem cells also seem to be involved in regenerating thyroid cells after experimentally induced thyroid damage in mouse models (269). Should such successes be extended to humans, this would not only provide the opportunity for a spectrum of studies to further our understanding of thyroid physiology, pathophysiology, and disease, but could also pave the way for regenerating the full hormonal profile of a normally functioning thyroid gland and thereby restoring euthyroidism in its entirety for those affected by this lifelong disease (270).
Acknowledgments
Financial Support: This work is supported by the National Institutes of Health (grant Nos. R01DE025822 and UL1TR001409 to J.J. and Dr Joseph Verbalis respectively).
Glossary
Abbreviations
- AUC
area under the concentration-time curve
- BMI
body mass index
- Cmax
maximum serum concentration
- DIO2
type 2 deiodinase
- DTE
desiccated thyroid extract
- FT3
free 3,5,3′-triiodothyronine
- FT4
free thyroxine
- GHQ-12
General Health Questionnaire
- IQR
interquartile range
- LT3
liothyronine
- LT4
levothyroxine
- MCT
monocarboxylate transporter
- NHANES
National Health and Nutrition Examination Survey
- OATP1C
organic anion transporting polypeptide 1C1
- OR
odds ratio
- PRO
patient-reported outcome
- QOL
quality of life
- SCH
subclinical hypothyroidism
- T3
3,5,3′-triiodothyronine
- T4
thyroxine
- TBG
thyroxine-binding globulin
- TPO Abs
thyroid peroxidase antibodies
- TRH
thyrotropin-releasing hormone
- TSH
thyrotropin
- TSQ
Thyroid Symptom Questionnaire
Additional Information
Disclosures: The author has no relevant disclosures.
References
- 1. Jonklaas J, Bianco AC, Bauer AJ, et al. ; American Thyroid Association Task Force on Thyroid Hormone Replacement . Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24(12):1670-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slater S. The discovery of thyroid replacement therapy. Part 1: in the beginning. J R Soc Med. 2011;104(1):15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slater S. The discovery of thyroid replacement therapy. Part 3: a complete transformation. J R Soc Med. 2011;104(3):100-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beadles C. The treatment of myxoedema and cretinism, being a review of the treatment of these diseases with the thyroid gland, with a table of 100 published cases. J Mental Science. 1893;39:509-536. [Google Scholar]
- 5. Bramwell B. The thyroid treatment of myxoedema and sporadic cretinism, with notes of twenty-three cases of myxoedema and five cases of sporadic cretinism, treated by thyroid extract. Edinburgh Hosp Rep. 1895;3:116-249. [Google Scholar]
- 6. Kaufman SC, Gross TP, Kennedy DL. Thyroid hormone use: trends in the United States from 1960 through 1988. Thyroid. 1991;1(4):285-291. [DOI] [PubMed] [Google Scholar]
- 7. Pilo A, Iervasi G, Vitek F, Ferdeghini M, Cazzuola F, Bianchi R. Thyroidal and peripheral production of 3,5,3’-triiodothyronine in humans by multicompartmental analysis. Am J Physiol. 1990;258(4 Pt 1):E715-E726. [DOI] [PubMed] [Google Scholar]
- 8. Jonklaas J. Hypothyroidism. In: Robertson PR, ed. De Groot’s Endocrinology. Basic Science and Clinical Practice. 8th ed. Elsevier; 2021. [Google Scholar]
- 9. Gil-Ibáñez P, Belinchón MM, Morte B, Obregón MJ, Bernal J. Is the intrinsic genomic activity of thyroxine relevant in vivo? Effects on gene expression in primary cerebrocortical and neuroblastoma cells. Thyroid. 2017;27(8):1092-1098. [DOI] [PubMed] [Google Scholar]
- 10. Bogazzi F, Bartalena L, Brogioni S, et al. L-thyroxine directly affects expression of thyroid hormone-sensitive genes: regulatory effect of RXRbeta. Mol Cell Endocrinol. 1997;134(1):23-31. [DOI] [PubMed] [Google Scholar]
- 11. Galton VA, Martinez ME, Dragon JA, St Germain DL, Hernandez A. The intrinsic activity of thyroxine is critical for survival and growth and regulates gene expression in neonatal liver. Thyroid. 2021;31(3):528-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301-316. [DOI] [PubMed] [Google Scholar]
- 13. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. [DOI] [PubMed] [Google Scholar]
- 14. Canaris GJ, Steiner JF, Ridgway EC. Do traditional symptoms of hypothyroidism correlate with biochemical disease? J Gen Intern Med. 1997;12(9):544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ott J, Promberger R, Kober F, et al. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid. 2011;21(2):161-167. [DOI] [PubMed] [Google Scholar]
- 16. Jonklaas J, Razvi S. Reference intervals in the diagnosis of thyroid dysfunction: treating patients not numbers. Lancet Diabetes Endocrinol. 2019;7(6):473-483. [DOI] [PubMed] [Google Scholar]
- 17. Barth JH, Luvai A, Jassam N, et al. Comparison of method-related reference intervals for thyroid hormones: studies from a prospective reference population and a literature review. Ann Clin Biochem. 2018;55(1):107-112. [DOI] [PubMed] [Google Scholar]
- 18. Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002;87(3):1068-1072. [DOI] [PubMed] [Google Scholar]
- 19. van der Spoel E, Roelfsema F, van Heemst D. Within-person variation in serum thyrotropin concentrations: main sources, potential underlying biological mechanisms, and clinical implications. Front Endocrinol (Lausanne). 2021;12:619568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fitzgerald SP, Bean NG, Falhammar H, Tuke J. Clinical parameters are more likely to be associated with thyroid hormone levels than with thyrotropin levels: a systematic review and meta-analysis. Thyroid. 2020;30(12):1695-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. LeFevre ML; US Preventive Services Task Force . Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162(9):641-650. [DOI] [PubMed] [Google Scholar]
- 22. Taylor PN, Razvi S, Pearce SH, Dayan CM. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab. 2013;98(9):3562-3571. [DOI] [PubMed] [Google Scholar]
- 23. Thayakaran R, Adderley NJ, Sainsbury C, et al. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ. 2019;366:l4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cappola AR, Cooper DS. Screening and treating subclinical thyroid disease: getting past the impasse. Ann Intern Med. 2015;162(9):664-665. [DOI] [PubMed] [Google Scholar]
- 25. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315-389. [DOI] [PubMed] [Google Scholar]
- 26. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA. 2015;314(17):1818-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell AL, Hickey B, Hickey JL, Pearce SH. Trends in thyroid hormone prescribing and consumption in the UK. BMC Public Health. 2009;9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jonklaas J, DeSale S. Levothyroxine prescriptions trends may indicate a downtrend in prescribing. Ther Adv Endocrinol Metab. 2020;11:2042018820920551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santini F, Pinchera A, Marsili A, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab. 2005;90(1):124-127. [DOI] [PubMed] [Google Scholar]
- 30. Kabadi UM, Jackson T. Serum thyrotropin in primary hypothyroidism. A possible predictor of optimal daily levothyroxine dose in primary hypothyroidism. Arch Intern Med. 1995;155(10):1046-1048. [PubMed] [Google Scholar]
- 31. Mistry D, Atkin S, Atkinson H, et al. Predicting thyroxine requirements following total thyroidectomy. Clin Endocrinol (Oxf). 2011;74(3):384-387. [DOI] [PubMed] [Google Scholar]
- 32. Devdhar M, Drooger R, Pehlivanova M, Singh G, Jonklaas J. Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid. 2011;21(8):821-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jonklaas J. Sex and age differences in levothyroxine dosage requirement. Endocr Pract. 2010;16(1):71-79. [DOI] [PubMed] [Google Scholar]
- 34. Kabadi UM. Optimal daily levothyroxine dose in primary hypothyroidism. Its relation to pretreatment thyroid hormone indexes. Arch Intern Med. 1989;149(10):2209-2212. [PubMed] [Google Scholar]
- 35. Zaborek NA, Cheng A, Imbus JR, et al. The optimal dosing scheme for levothyroxine after thyroidectomy: a comprehensive comparison and evaluation. Surgery. 2019;165(1):92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cunningham JJ, Barzel US. Lean body mass is a predictor of the daily requirement for thyroid hormone in older men and women. J Am Geriatr Soc. 1984;32(3):204-207. [DOI] [PubMed] [Google Scholar]
- 37. Olubowale O, Chadwick DR. Optimization of thyroxine replacement therapy after total or near-total thyroidectomy for benign thyroid disease. Br J Surg. 2006;93(1):57-60. [DOI] [PubMed] [Google Scholar]
- 38. Di Donna V, Santoro MG, de Waure C, et al. A new strategy to estimate levothyroxine requirement after total thyroidectomy for benign thyroid disease. Thyroid. 2014;24(12):1759-1764. [DOI] [PubMed] [Google Scholar]
- 39. Elfenbein DM, Schaefer S, Shumway C, Chen H, Sippel RS, Schneider DF. Prospective intervention of a novel levothyroxine dosing protocol based on body mass index after thyroidectomy. J Am Coll Surg. 2016;222(1):83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heemstra KA, Hoftijzer HC, van der Deure WM, et al. Thr92Ala polymorphism in the type 2 deiodinase is not associated with T4 dose in athyroid patients or patients with Hashimoto thyroiditis. Clin Endocrinol (Oxf). 2009;71(2):279-283. [DOI] [PubMed] [Google Scholar]
- 41. Torlontano M, Durante C, Torrente I, et al. Type 2 deiodinase polymorphism (threonine 92 alanine) predicts L-thyroxine dose to achieve target thyrotropin levels in thyroidectomized patients. J Clin Endocrinol Metab. 2008;93(3):910-913. [DOI] [PubMed] [Google Scholar]
- 42. Jo S, Fonseca TL, Bocco BMLC, et al. Type 2 deiodinase polymorphism causes ER stress and hypothyroidism in the brain. J Clin Invest. 2019;129(1):230-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Canani LH, Capp C, Dora JM, et al. The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2005;90(6):3472-3478. [DOI] [PubMed] [Google Scholar]
- 44. Peeters RP, van den Beld AW, Attalki H, et al. A new polymorphism in the type II deiodinase gene is associated with circulating thyroid hormone parameters. Am J Physiol Endocrinol Metab. 2005;289(1):E75-E81. [DOI] [PubMed] [Google Scholar]
- 45. Sturgess I, Thomas SH, Pennell DJ, Mitchell D, Croft DN. Diurnal variation in TSH and free thyroid hormones in patients on thyroxine replacement. Acta Endocrinol (Copenh). 1989;121(5):674-676. [DOI] [PubMed] [Google Scholar]
- 46. Chen SS, Zaborek NA, Doubleday AR, et al. Optimizing levothyroxine dose adjustment after thyroidectomy with a decision tree. J Surg Res. 2019;244:102-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jonklaas J, DeSale S. The ages and TSH values of patients being prescribed levothyroxine. Ther Adv Endocrinol Metab. 2020;11:2042018820937896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Duration of over- and under-treatment of hypothyroidism is associated with increased cardiovascular risk. Eur J Endocrinol. 2019;180(6):407-416. [DOI] [PubMed] [Google Scholar]
- 49. Huang HK, Wang JH, Kao SL. Association of hypothyroidism with all-cause mortality: a cohort study in an older adult population. J Clin Endocrinol Metab. 2018;103(9):3310-3318. [DOI] [PubMed] [Google Scholar]
- 50. Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid. 2018;28(5):566-574. [DOI] [PubMed] [Google Scholar]
- 51. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Functional and symptomatic individuality in the response to levothyroxine treatment. Front Endocrinol (Lausanne). 2019;10:664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Recent advances in thyroid hormone regulation: toward a new paradigm for optimal diagnosis and treatment. Front Endocrinol (Lausanne). 2017;8:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Samuels MH, Kolobova I, Niederhausen M, Janowsky JS, Schuff KG. Effects of altering levothyroxine (L-T4) doses on quality of life, mood, and cognition in L-T4 treated subjects. J Clin Endocrinol Metab. 2018;103(5):1997-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Samuels MH, Kolobova I, Niederhausen M, Purnell JQ, Schuff KG. Effects of altering levothyroxine dose on energy expenditure and body composition in subjects treated with LT4. J Clin Endocrinol Metab. 2018;103(11):4163-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab. 2006;91(7):2624-2630. [DOI] [PubMed] [Google Scholar]
- 56. Boeving A, Paz-Filho G, Radominski RB, Graf H, Amaral de Carvalho G. Low-normal or high-normal thyrotropin target levels during treatment of hypothyroidism: a prospective, comparative study. Thyroid. 2011;21(4):355-360. [DOI] [PubMed] [Google Scholar]
- 57. Ridgway EC, Cooper DS, Walker H, et al. Therapy of primary hypothyroidism with L-triiodothyronine: discordant cardiac and pituitary responses. Clin Endocrinol (Oxf). 1980;13(5):479-488. [DOI] [PubMed] [Google Scholar]
- 58. Meier C, Trittibach P, Guglielmetti M, Staub JJ, Müller B. Serum thyroid stimulating hormone in assessment of severity of tissue hypothyroidism in patients with overt primary thyroid failure: cross sectional survey. BMJ. 2003;326(7384):311-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Feng X, Jiang Y, Meltzer P, Yen PM. Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol Endocrinol. 2000;14(7):947-955. [DOI] [PubMed] [Google Scholar]
- 60. Massolt ET, Meima ME, Swagemakers SMA, et al. Thyroid state regulates gene expression in human whole blood. J Clin Endocrinol Metab. 2018;103(1):169-178. [DOI] [PubMed] [Google Scholar]
- 61. Okosieme OE, Belludi G, Spittle K, Kadiyala R, Richards J. Adequacy of thyroid hormone replacement in a general population. QJM. 2011;104(5):395-401. [DOI] [PubMed] [Google Scholar]
- 62. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med. 2000;160(4):526-534. [DOI] [PubMed] [Google Scholar]
- 63. Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. High frequency of and factors associated with thyroid hormone over-replacement and under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab. 2009;94(4):1342-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Parle JV, Franklyn JA, Cross KW, Jones SR, Sheppard MC. Thyroxine prescription in the community: serum thyroid stimulating hormone level assays as an indicator of undertreatment or overtreatment. Br J Gen Pract. 1993;43(368):107-109. [PMC free article] [PubMed] [Google Scholar]
- 65. Ross DS, Daniels GH, Gouveia D. The use and limitations of a chemiluminescent thyrotropin assay as a single thyroid function test in an out-patient endocrine clinic. J Clin Endocrinol Metab. 1990;71(3):764-769. [DOI] [PubMed] [Google Scholar]
- 66. Taylor PN, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks: evidence from a large community-based study. JAMA Intern Med. 2014;174(1):32-39. [DOI] [PubMed] [Google Scholar]
- 67. Ettleson MD, Bianco AC, Zhu M, Laiteerapong N. Sociodemographic disparities in the treatment of hypothyroidism: NHANES 2007-2012. J Endocr Soc. 2021;5(7):bvab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kuye R, Riggs C, King J, Heilmann R, Kurz D, Milchak J. Thyroid stimulating hormone stability in patients prescribed synthetic or desiccated thyroid products: a retrospective study. Ann Fam Med. 2020;18(5):452-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Perros P, Nirantharakumar K, Hegedüs L. Recent evidence sets therapeutic targets for levothyroxine-treated patients with primary hypothyroidism based on risk of death. Eur J Endocrinol. 2021;184(2):C1-C3. [DOI] [PubMed] [Google Scholar]
- 70. Brito JP, Ross JS, El Kawkgi OM, et al. Levothyroxine use in the United States, 2008-2018. JAMA Intern Med. Published online June 21, 2021. doi: 10.1001/jamainternmed.2021.2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Somwaru LL, Arnold AM, Cappola AR. Predictors of thyroid hormone initiation in older adults: results from the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2011;66(7):809-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sawka AM, Cappola AR, Peeters RP, Kopp PA, Bianco AC, Jonklaas J. Patient context and thyrotropin levels are important when considering treatment of subclinical hypothyroidism. Thyroid. 2019;29(10):1359-1363. [DOI] [PubMed] [Google Scholar]
- 73. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28(4):437-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Medication adherence and use of generic drug therapies. Am J Manag Care. 2009;15(7):450-456. [PMC free article] [PubMed] [Google Scholar]
- 75. Elliott DP. Effect of levothyroxine administration time on serum TSH in elderly patients. Ann Pharmacother. 2001;35(5):529-532. [DOI] [PubMed] [Google Scholar]
- 76. Bolk N, Visser TJ, Nijman J, Jongste IJ, Tijssen JG, Berghout A. Effects of evening vs morning levothyroxine intake: a randomized double-blind crossover trial. Arch Intern Med. 2010;170(22):1996-2003. [DOI] [PubMed] [Google Scholar]
- 77. Bolk N, Visser TJ, Kalsbeek A, van Domburg RT, Berghout A. Effects of evening vs morning thyroxine ingestion on serum thyroid hormone profiles in hypothyroid patients. Clin Endocrinol (Oxf). 2007;66(1):43-48. [DOI] [PubMed] [Google Scholar]
- 78. Bach-Huynh TG, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab. 2009;94(10):3905-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Seechurn S, Sharma S, Samsom O. Administration of levothyroxine 45-60 minutes before breakfast improves biochemical availability as evidenced by reduced thyrotropin levels. Open J Endocr Metab Dis. 2012;2:36-39. [Google Scholar]
- 80. Perez CL, Araki FS, Graf H, de Carvalho GA. Serum thyrotropin levels following levothyroxine administration at breakfast. Thyroid. 2013;23(7):779-784. [DOI] [PubMed] [Google Scholar]
- 81. Ala S, Akha O, Kashi Z, et al. Changes in serum TSH and T4 levels after switching the levothyroxine administration time from before breakfast to before dinner. Int J Endocrinol. 2015;2015:156375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cappelli C, Pirola I, Daffini L, et al. A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: results of the TICO study. Thyroid. 2016;26(2):197-202. [DOI] [PubMed] [Google Scholar]
- 83. Akın O. Morning vs. bedtime levothyroxine administration: what is the ideal choice for children? J Pediatr Endocrinol Metab. 2018;31(11):1249-1255. [DOI] [PubMed] [Google Scholar]
- 84. Skelin M, Lucijanić T, Liberati-Čizmek AM, et al. Effect of timing of levothyroxine administration on the treatment of hypothyroidism: a three-period crossover randomized study. Endocrine. 2018;62(2):432-439. [DOI] [PubMed] [Google Scholar]
- 85. Chen S, Zhang F, Peng W. Evaluation of the efficacy and safety of oral levothyroxine at different times in the treatment of hypothyroidism. Chin J Mod Drug Appl. 2018;12:71-73. [Google Scholar]
- 86. Apaydin M, Beysel S, Kizilgul M, et al. The effect of bedtime or morning intake of levothyroxine in patients with hypothyroidism. Glob J Endocrinol Metab. 2018;1:1-3. [Google Scholar]
- 87. Ahmed J, AL-Emara Z, All Mansour A. Timing of levothyroxine in the treatment of primary hypothyroidism. Br J Med Res. 2016;12:1-6. [Google Scholar]
- 88. Srivastava S, Sharma G, Rathore M, et al. A crossover study evaluating effect of timing of levothyroxine on thyroid hormone status in patients of hypothyroidism. J Assoc Physicians India. 2018;66(9):37-40. [PubMed] [Google Scholar]
- 89. Rajput R, Chatterjee S, Rajput M. Can levothyroxine be taken as evening dose? Comparative evaluation of morning versus evening dose of levothyroxine in treatment of hypothyroidism. J Thyroid Res. 2011;2011:505239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yuan G, Fan Y, Yan Q. Effect of taking L-T4 in bedtime vs morning on changes of serum thyroid hormone concentration in patients with hypothyroidism. Chinese Journal of Nuclear Medicine and Molecular Imaging. 2015;35(4):265-267. [Google Scholar]
- 91. Wiesner A, Gajewska D, Paśko P. Levothyroxine interactions with food and dietary supplements—a systematic review. Pharmaceuticals (Basel). 2021;14(3):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Navid A, Dayal D, Kaur H, Gupta A, Attri SV. Comparative efficacy of early morning versus bedtime administration of levothyroxine in children with hypothyroidism: a prospective, open label, randomized, case-control study. Pediatr Endocrinol Diabetes Metab. 2021;27(3):178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wenzel KW, Kirschsieper HE. Aspects of the absorption of oral L-thyroxine in normal man. Metabolism. 1977;26(1):1-8. [DOI] [PubMed] [Google Scholar]
- 94. Skelin M, Lucijanić T, Amidžić Klarić D, et al. Factors affecting gastrointestinal absorption of levothyroxine: a review. Clin Ther. 2017;39(2):378-403. [DOI] [PubMed] [Google Scholar]
- 95. Irving SA, Vadiveloo T, Leese GP. Drugs that interact with levothyroxine: an observational study from the Thyroid Epidemiology, Audit and Research Study (TEARS). Clin Endocrinol (Oxf). 2015;82(1):136-141. [DOI] [PubMed] [Google Scholar]
- 96. Burch HB. Drug effects on the thyroid. N Engl J Med. 2019;381(8):749-761. [DOI] [PubMed] [Google Scholar]
- 97. Arafah BM. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N Engl J Med. 2001;344(23):1743-1749. [DOI] [PubMed] [Google Scholar]
- 98. Arafah BM. Decreased levothyroxine requirement in women with hypothyroidism during androgen therapy for breast cancer. Ann Intern Med. 1994;121(4):247-251. [DOI] [PubMed] [Google Scholar]
- 99. Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283(21):2822-2825. [DOI] [PubMed] [Google Scholar]
- 100. Campbell NR, Hasinoff BB, Stalts H, Rao B, Wong NC. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117(12):1010-1013. [DOI] [PubMed] [Google Scholar]
- 101. Sachmechi I, Reich DM, Aninyei M, Wibowo F, Gupta G, Kim PJ. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13(4):345-349. [DOI] [PubMed] [Google Scholar]
- 102. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Effect of phosphate binders upon TSH and L-thyroxine dose in patients on thyroid replacement. Int Urol Nephrol. 2007;39(2):599-602. [DOI] [PubMed] [Google Scholar]
- 103. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17(8):763-765. [DOI] [PubMed] [Google Scholar]
- 104. Sherman SI, Gopal J, Haugen BR, et al. Central hypothyroidism associated with retinoid X receptor-selective ligands. N Engl J Med. 1999;340(14):1075-1079. [DOI] [PubMed] [Google Scholar]
- 105. Torino F, Barnabei A, Paragliola R, Baldelli R, Appetecchia M, Corsello SM. Thyroid dysfunction as an unintended side effect of anticancer drugs. Thyroid. 2013;23(11):1345-1366. [DOI] [PubMed] [Google Scholar]
- 106. Virili C, Stramazzo I, Santaguida MG, et al. Ulcerative colitis as a novel cause of increased need for levothyroxine. Front Endocrinol (Lausanne). 2019;10:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354(17):1787-1795. [DOI] [PubMed] [Google Scholar]
- 108. Cellini M, Santaguida MG, Virili C, et al. Hashimoto’s thyroiditis and autoimmune gastritis. Front Endocrinol (Lausanne). 2017;8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gadiraju S, Lee CJ, Cooper DS. Levothyroxine dosing following bariatric surgery. Obes Surg. 2016;26(10):2538-2542. [DOI] [PubMed] [Google Scholar]
- 110. Chung CW, Mo EY, Jung GS, et al. Decreased expression of ileal thyroid hormone transporters in a hypothyroid patient: a case report. Front Endocrinol (Lausanne). 2021;12:664839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Flinterman LE, Kuiper JG, Korevaar JC, et al. Impact of a forced dose-equivalent levothyroxine brand switch on plasma thyrotropin: a cohort study. Thyroid. 2020;30(6):821-828. [DOI] [PubMed] [Google Scholar]
- 112. Brito JP, Ross JS, Sangaralingham L, et al. Comparative effectiveness of generic vs brand-name levothyroxine in achieving normal thyrotropin levels. JAMA Netw Open. 2020;3(9):e2017645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Brito JP, Ross JS, Deng Y, et al. Cardiovascular outcomes and rates of fractures and falls among patients with brand-name versus generic L-thyroxine use. Endocrine. Published online June 5, 2021. doi: 10.1007/s12020-021-02779-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Blakesley V, Awni W, Locke C, Ludden T, Granneman GR, Braverman LE. Are bioequivalence studies of levothyroxine sodium formulations in euthyroid volunteers reliable? Thyroid. 2004;14(3):191-200. [DOI] [PubMed] [Google Scholar]
- 115. Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm. 2009;72(1):105-110. [DOI] [PubMed] [Google Scholar]
- 116. Vita R, Saraceno G, Trimarchi F, Benvenga S. Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J Clin Endocrinol Metab. 2014;99(12):4481-4486. [DOI] [PubMed] [Google Scholar]
- 117. Pirola I, Daffini L, Gandossi E, et al. Comparison between liquid and tablet levothyroxine formulations in patients treated through enteral feeding tube. J Endocrinol Invest. 2014;37(6):583-587. [DOI] [PubMed] [Google Scholar]
- 118. Pirola I, Formenti AM, Gandossi E, et al. Oral liquid L-thyroxine (L-t4) may be better absorbed compared to L-T4 tablets following bariatric surgery. Obes Surg. 2013;23(9):1493-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nagy EV, Perros P, Papini E, Katko M, Hegedüs L. New formulations of levothyroxine in the treatment of hypothyroidism: trick or treat? Thyroid. 2021;31(2):193-201. [DOI] [PubMed] [Google Scholar]
- 120. Duntas LH, Jonklaas J. Levothyroxine dose adjustment to optimise therapy throughout a patient’s lifetime. Adv Ther. 2019;36(Suppl 2):30-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA guideline: management of subclinical hypothyroidism. Eur Thyroid J. 2013;2(4):215-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Okosieme OE, Khan I, Taylor PN. Preconception management of thyroid dysfunction. Clin Endocrinol (Oxf). 2018;89(3):269-279. [DOI] [PubMed] [Google Scholar]
- 123. Lemieux P, Yamamoto JM, Nerenberg KA, et al. Thyroid laboratory testing and management in women on thyroid replacement before pregnancy and associated pregnancy outcomes. Thyroid. 2021;31(5):841-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. van Trotsenburg P, Stoupa A, Léger J, et al. Congenital hypothyroidism: a 2020-2021 consensus guidelines update—an ENDO-European Reference Network initiative endorsed by the European Society for Pediatric Endocrinology and the European Society for Endocrinology. Thyroid. 2021;31(3):387-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hanley P, Lord K, Bauer AJ. Thyroid disorders in children and adolescents: a review. JAMA Pediatr. 2016;170(10):1008-1019. [DOI] [PubMed] [Google Scholar]
- 126. Gonzales KM, Stan MN, Morris JC III, Bernet V, Castro MR. The levothyroxine absorption test: a four-year experience (2015-2018) at the Mayo Clinic. Thyroid. 2019;29(12):1734-1742. [DOI] [PubMed] [Google Scholar]
- 127. Kumar R, Shaukat F. Adherence to levothyroxine tablet in patients with hypothyroidism. Cureus. 2019;11(5):e4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Machado-Alba JE, Machado-Duque ME. Adherence to levothyroxine prescription in patients with hypothyroidism. Rev Med Chil. 2020;148(6):740-745. [DOI] [PubMed] [Google Scholar]
- 129. Crilly M. Thyroxine adherence in primary hypothyroidism. Lancet. 2004;363(9420):1558. [DOI] [PubMed] [Google Scholar]
- 130. Crilly M, Esmail A. Randomised controlled trial of a hypothyroid educational booklet to improve thyroxine adherence. Br J Gen Pract. 2005;55(514):362-368. [PMC free article] [PubMed] [Google Scholar]
- 131. Walker JN, Shillo P, Ibbotson V, et al. A thyroxine absorption test followed by weekly thyroxine administration: a method to assess non-adherence to treatment. Eur J Endocrinol. 2013;168(6):913-917. [DOI] [PubMed] [Google Scholar]
- 132. Dabbous Z, Alowainati B, Darwish S, et al. A prospective study comparing two-time points of thyroid hormone replacement during the holy month of Ramadan. Int J Endocrinol. 2019;2019:9843961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Grebe SK, Cooke RR, Ford HC, et al. Treatment of hypothyroidism with once weekly thyroxine. J Clin Endocrinol Metab. 1997;82(3):870-875. [DOI] [PubMed] [Google Scholar]
- 134. Slawik M, Klawitter B, Meiser E, et al. Thyroid hormone replacement for central hypothyroidism: a randomized controlled trial comparing two doses of thyroxine (T4) with a combination of T4 and triiodothyronine. J Clin Endocrinol Metab. 2007;92(11):4115-4122. [DOI] [PubMed] [Google Scholar]
- 135. Koulouri O, Auldin MA, Agarwal R, et al. Diagnosis and treatment of hypothyroidism in TSH deficiency compared to primary thyroid disease: pituitary patients are at risk of under-replacement with levothyroxine. Clin Endocrinol (Oxf). 2011;74(6):744-749. [DOI] [PubMed] [Google Scholar]
- 136. Jonklaas J, Sarlis NJ, Litofsky D, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16(12):1229-1242. [DOI] [PubMed] [Google Scholar]
- 137. Carhill AA, Litofsky DR, Ross DS, et al. Long-term outcomes following therapy in differentiated thyroid carcinoma: NTCTCS registry analysis 1987-2012. J Clin Endocrinol Metab. 2015;100(9):3270-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kabadi UM. Variability of L-thyroxine replacement dose in elderly patients with primary hypothyroidism. J Fam Pract. 1987;24(5):473-477. [PubMed] [Google Scholar]
- 139. Barrett NA, Jones A, Whiteley C, Yassin S, McKenzie CA. Management of long-term hypothyroidism: a potential marker of quality of medicines reconciliation in the intensive care unit. Int J Pharm Pract. 2012;20(5):303-306. [DOI] [PubMed] [Google Scholar]
- 140. Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly. 2014;144:w14058. [DOI] [PubMed] [Google Scholar]
- 141. Boucai L, Hollowell JG, Surks MI. An approach for development of age-, gender-, and ethnicity-specific thyrotropin reference limits. Thyroid. 2011;21(1):5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Razvi S, Weaver JU, Butler TJ, Pearce SH. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172(10):811-817. [DOI] [PubMed] [Google Scholar]
- 143. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. [DOI] [PubMed] [Google Scholar]
- 144. Stott DJ, Rodondi N, Bauer DC; TRUST Study Group . Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;377(14):e20. [DOI] [PubMed] [Google Scholar]
- 145. Mooijaart SP, Du Puy RS, Stott DJ, et al. Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. JAMA. 2019;322(20):1977-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Razvi S, Arnott B, Teare D, Hiu S, O’Brien N, Pearce S. Multinational survey of treatment practices of clinicians managing subclinical hypothyroidism in older people in 2019. Eur Thyroid J. 2021;10(4):330-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pollock MA, Sturrock A, Marshall K, et al. Thyroxine treatment in patients with symptoms of hypothyroidism but thyroid function tests within the reference range: randomised double blind placebo controlled crossover trial. BMJ. 2001;323(7318):891-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Livadas S, Bothou C, Androulakis I, Boniakos A, Angelopoulos N, Duntas L. Levothyroxine replacement therapy and overuse: a timely diagnostic approach. Thyroid. 2018;28(12):1580-1586. [DOI] [PubMed] [Google Scholar]
- 149. Burgos N, Toloza FJK, Singh Ospina NM, et al. Clinical outcomes after discontinuation of thyroid hormone replacement: a systematic review and meta-analysis. Thyroid. 2021;31(5):740-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Saravanan P, Chau WF, Roberts N, Vedhara K, Greenwood R, Dayan CM. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. Clin Endocrinol (Oxf). 2002;57(5):577-585. [DOI] [PubMed] [Google Scholar]
- 151. Wekking EM, Appelhof BC, Fliers E, et al. Cognitive functioning and well-being in euthyroid patients on thyroxine replacement therapy for primary hypothyroidism. Eur J Endocrinol. 2005;153(6):747-753. [DOI] [PubMed] [Google Scholar]
- 152. van de Ven AC, Netea-Maier RT, de Vegt F, et al. Is there a relationship between fatigue perception and the serum levels of thyrotropin and free thyroxine in euthyroid subjects? Thyroid. 2012;22(12):1236-1243. [DOI] [PubMed] [Google Scholar]
- 153. Wouters HJ, van Loon HC, van der Klauw MM, et al. No effect of the Thr92Ala polymorphism of deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid. 2017;27(2):147-155. [DOI] [PubMed] [Google Scholar]
- 154. Boesen VB, Feldt-Rasmussen U, Bjorner JB, et al. How should thyroid-related quality of life be assessed? Recalled patient-reported outcomes compared to here-and-now measures. Thyroid. 2018;28(12):1561-1570. [DOI] [PubMed] [Google Scholar]
- 155. Peterson SJ, Cappola AR, Castro MR, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. 2018;28(6):707-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Mitchell AL, Hegedüs L, Žarković M, Hickey JL, Perros P. Patient satisfaction and quality of life in hypothyroidism: an online survey by the British Thyroid Foundation. Clin Endocrinol (Oxf). 2021;94(3):513-520. [DOI] [PubMed] [Google Scholar]
- 157. Jonklaas J. Persistent hypothyroid symptoms in a patient with a normal thyroid stimulating hormone level. Curr Opin Endocrinol Diabetes Obes. 2017;24(5):356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84(6):799-808. [DOI] [PubMed] [Google Scholar]
- 159. Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2(4):137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Pop VJ, Maartens LH, Leusink G, et al. Are autoimmune thyroid dysfunction and depression related? J Clin Endocrinol Metab. 1998;83(9):3194-3197. [DOI] [PubMed] [Google Scholar]
- 161. Peterson SJ, McAninch EA, Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab. 2016;101(12):4964-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Idrees T, Prieto WH, Casula S, et al. Use of statins among patients taking levothyroxine: an observational drug utilization study across sites. J Endocr Soc. 2021;5(7):bvab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedüs L. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid. 2014;24(5):802-808. [DOI] [PubMed] [Google Scholar]
- 164. Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedüs L. Type and extent of somatic morbidity before and after the diagnosis of hypothyroidism. a nationwide register study. PLoS One. 2013;8(9):e75789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jørgensen P, Langhammer A, Krokstad S, Forsmo S. Diagnostic labelling influences self-rated health. A prospective cohort study: the HUNT study, Norway. Fam Pract. 2015;32(5):492-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Perros P, Van Der Feltz-Cornelis C, Papini E, Nagy EV, Weetman AP, Hegedüs L. The enigma of persistent symptoms in hypothyroid patients treated with levothyroxine: a narrative review. Clin Endocrinol (Oxf). Published online March 30, 2021. doi: 10.1111/cen.14473 [DOI] [PubMed] [Google Scholar]
- 167. Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Thyroid. 2021;31(2):156-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Werneck FZ, Coelho EF, Almas SP, et al. Exercise training improves quality of life in women with subclinical hypothyroidism: a randomized clinical trial. Arch Endocrinol Metab. 2018;62(5):530-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. dos Santos Vigário P, Chachamovitz DS, Cordeiro MF, et al. Effects of physical activity on body composition and fatigue perception in patients on thyrotropin-suppressive therapy for differentiated thyroid carcinoma. Thyroid. 2011;21(7):695-700. [DOI] [PubMed] [Google Scholar]
- 170. dos Santos Vigário P, Chachamovitz DS, Teixeira PeF, Rocque MeL, Santos ML, Vaisman M. Exercise is associated with better quality of life in patients on TSH-suppressive therapy with levothyroxine for differentiated thyroid carcinoma. Arq Bras Endocrinol Metabol. 2014;58(3):274-281. [DOI] [PubMed] [Google Scholar]
- 171. Escobar-Morreale HF, Obregón MJ, Escobar del Rey F, Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96(6):2828-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Escobar-Morreale HF, del Rey FE, Obregón MJ, de Escobar GM. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137(6):2490-2502. [DOI] [PubMed] [Google Scholar]
- 173. Werneck de Castro JP, Fonseca TL, Ueta CB, et al. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest. 2015;125(2):769-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Saravanan P, Siddique H, Simmons DJ, Greenwood R, Dayan CM. Twenty-four hour hormone profiles of TSH, free T3 and free T4 in hypothyroid patients on combined T3/T4 therapy. Exp Clin Endocrinol Diabetes. 2007;115(4):261-267. [DOI] [PubMed] [Google Scholar]
- 175. Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One. 2011;6(8):e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA. 2008;299(7):769-777. [DOI] [PubMed] [Google Scholar]
- 177. Castagna MG, Dentice M, Cantara S, et al. DIO2 Thr92Ala reduces deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab. 2017;102(5):1623-1630. [DOI] [PubMed] [Google Scholar]
- 178. Panicker V, Saravanan P, Vaidya B, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009;94(5):1623-1629. [DOI] [PubMed] [Google Scholar]
- 179. Young Cho Y, Jeong Kim H, Won Jang H, et al. The relationship of 19 functional polymorphisms in iodothyronine deiodinase and psychological well-being in hypothyroid patients. Endocrine. 2017;57(1):115-124. [DOI] [PubMed] [Google Scholar]
- 180. Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005;90(5):2666-2674. [DOI] [PubMed] [Google Scholar]
- 181. Appelhof BC, Peeters RP, Wiersinga WM, et al. Polymorphisms in type 2 deiodinase are not associated with well-being, neurocognitive functioning, and preference for combined thyroxine/3,5,3’-triiodothyronine therapy. J Clin Endocrinol Metab. 2005;90(11):6296-6299. [DOI] [PubMed] [Google Scholar]
- 182. van der Deure WM, Appelhof BC, Peeters RP, et al. Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin Endocrinol (Oxf). 2008;69(5):804-811. [DOI] [PubMed] [Google Scholar]
- 183. Carlé A, Faber J, Steffensen R, Laurberg P, Nygaard B. Hypothyroid patients encoding combined MCT10 and DIO2 gene polymorphisms may prefer L-T3 + L-T4 combination treatment—data using a blind, randomized, clinical study. Eur Thyroid J. 2017;6(3):143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Nygaard B, Jensen EW, Kvetny J, Jarløv A, Faber J. Effect of combination therapy with thyroxine (T4) and 3,5,3’-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol. 2009;161(6):895-902. [DOI] [PubMed] [Google Scholar]
- 185. Jonklaas J. Risks and safety of combination therapy for hypothyroidism. Expert Rev Clin Pharmacol. 2016;9(8):1057-1067. [DOI] [PubMed] [Google Scholar]
- 186. Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MK. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98(5):1982-1990. [DOI] [PubMed] [Google Scholar]
- 187. Fadeyev VV, Morgunova TB, Sytch JP, Melnichenko GA. TSH and thyroid hormones concentrations in patients with hypothyroidism receiving replacement therapy with L-thyroxine alone or in combination with L-triiodothyronine. Hormones (Athens). 2005;4(2):101-107. [PubMed] [Google Scholar]
- 188. Krysiak R, Szkróbka W, Okopień B. Sexual function and depressive symptoms in young women with hypothyroidism receiving levothyroxine/liothyronine combination therapy: a pilot study. Curr Med Res Opin. 2018;34(9):1579-1586. [DOI] [PubMed] [Google Scholar]
- 189. Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ Jr. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Engl J Med. 1999;340(6):424-429. [DOI] [PubMed] [Google Scholar]
- 190. Bunevicius R, Jakuboniene N, Jakubonien N, et al. Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves’ disease. Endocrine. 2002;18(2):129-133. [DOI] [PubMed] [Google Scholar]
- 191. Clyde PW, Harari AE, Getka EJ, Shakir KM. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA. 2003;290(22):2952-2958. [DOI] [PubMed] [Google Scholar]
- 192. Siegmund W, Spieker K, Weike AI, et al. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14:1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. Clin Endocrinol (Oxf). 2004;60(6):750-757. [DOI] [PubMed] [Google Scholar]
- 193. Escobar-Morreale HF, Botella-Carretero JI, Gómez-Bueno M, Galán JM, Barrios V, Sancho J. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005;142(6):412-424. [DOI] [PubMed] [Google Scholar]
- 194. Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab. 2003;88(10):4543-4550. [DOI] [PubMed] [Google Scholar]
- 195. Fadeyev VV, Morgunova TB, Melnichenko GA, Dedov II. Combined therapy with L-thyroxine and L-triiodothyronine compared to L-thyroxine alone in the treatment of primary hypothyroidism. Hormones (Athens). 2010;9(3):245-252. [DOI] [PubMed] [Google Scholar]
- 196. Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab. 2005;90(2):805-812. [DOI] [PubMed] [Google Scholar]
- 197. Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, Momtazi S, Musavinasab N, Hayatbakhsh MR. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009;34(3):80-89. [DOI] [PubMed] [Google Scholar]
- 198. Rodriguez T, Lavis VR, Meininger JC, Kapadia AS, Stafford LF. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. Endocr Pract. 2005;11(4):223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Sawka AM, Gerstein HC, Marriott MJ, MacQueen GM, Joffe RT. Does a combination regimen of thyroxine (T4) and 3,5,3’-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. J Clin Endocrinol Metab. 2003;88(10):4551-4555. [DOI] [PubMed] [Google Scholar]
- 200. Kaminski J, Miasaki FY, Paz-Filho G, Graf H, Carvalho GA. Treatment of hypothyroidism with levothyroxine plus liothyronine: a randomized, double-blind, crossover study. Arch Endocrinol Metab. 2016;60(6):562-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine + liothyronine in hypothyroidism. J Clin Endocrinol Metab. Published online June 29, 2021. doi: 10.1210/clinem/dgab478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Ma C, Xie J, Huang X, et al. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism. Nucl Med Commun. 2009;30(8):586-593. [DOI] [PubMed] [Google Scholar]
- 203. Escobar-Morreale HF, Botella-Carretero JI, Morreale de Escobar G. Treatment of hypothyroidism with levothyroxine or a combination of levothyroxine plus L-triiodothyronine. Best Pract Res Clin Endocrinol Metab. 2015;29(1):57-75. [DOI] [PubMed] [Google Scholar]
- 204. Joffe RT, Brimacombe M, Levitt AJ, Stagnaro-Green A. Treatment of clinical hypothyroidism with thyroxine and triiodothyronine: a literature review and metaanalysis. Psychosomatics. 2007;48(5):379-384. [DOI] [PubMed] [Google Scholar]
- 205. Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006;91(7):2592-2599. [DOI] [PubMed] [Google Scholar]
- 206. Jonklaas J, Cappola AR, Celi FS. Editorial: combination therapy for hypothyroidism: the journey from bench to bedside. Front Endocrinol (Lausanne). 2020;11:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Akirov A, Fazelzad R, Ezzat S, Thabane L, Sawka AM. A systematic review and meta-analysis of patient preferences for combination thyroid hormone treatment for hypothyroidism. Front Endocrinol (Lausanne). 2019;10:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Landers K, Richard K. Traversing barriers—how thyroid hormones pass placental, blood-brain and blood-cerebrospinal fluid barriers. Mol Cell Endocrinol. 2017;458:22-28. [DOI] [PubMed] [Google Scholar]
- 209. Leese GP, Soto-Pedre E, Donnelly LA. Liothyronine use in a 17 year observational population-based study—the TEARS study. Clin Endocrinol (Oxf). 2016;85(6):918-925. [DOI] [PubMed] [Google Scholar]
- 210. Planck T, Hedberg F, Calissendorff J, Nilsson A. Liothyronine use in hypothyroidism and its effects on cancer and mortality. Thyroid. 2021;31(5):732-739. [DOI] [PubMed] [Google Scholar]
- 211. Stedman M, Taylor P, Premawardhana L, Okosieme O, Dayan C, Heald AH. Trends in costs and prescribing for liothyronine and levothyroxine in England and Wales 2011-2020. Clin Endocrinol (Oxf). 2021;94(6):980-989. [DOI] [PubMed] [Google Scholar]
- 212. Stedman M, Taylor P, Premawardhana L, Okosieme O, Dayan C, Heald AH. Liothyronine and levothyroxine prescribing in England: a comprehensive survey and evaluation. Int J Clin Pract. 2021;75(9):e14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Taylor PN, Razvi S, Muller I, et al. Liothyronine cost and prescriptions in England. Lancet Diabetes Endocrinol. 2019;7(1):11-12. [DOI] [PubMed] [Google Scholar]
- 214. LeBoff MS, Kaplan MM, Silva JE, Larsen PR. Bioavailability of thyroid hormones from oral replacement preparations. Metabolism. 1982;31(9):900-905. [DOI] [PubMed] [Google Scholar]
- 215. Jackson IM, Cobb WE. Why does anyone still use desiccated thyroid USP? Am J Med. 1978;64(2):284-288. [DOI] [PubMed] [Google Scholar]
- 216. Penny R, Frasier SD. Elevated serum concentrations of triiodothyronine in hypothyroid patients. Values for patients receiving USP thyroid. Am J Dis Child. 1980;134(1):16-18. [DOI] [PubMed] [Google Scholar]
- 217. Toloza FJK, Espinoza Suarez NR, El Kawkgi O, et al. Patient experiences and perceptions associated with the use of desiccated thyroid extract. Medicina (Kaunas). 2020;56(4):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. Eur Thyroid J. 2012;1(2):55-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Celi FS, Zemskova M, Linderman JD, et al. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol (Oxf). 2010;72(5):709-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Russell W, Harrison RF, Smith N, et al. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab. 2008;93(6):2300-2306. [DOI] [PubMed] [Google Scholar]
- 221. Jaeschke R, Guyatt G, Cook D, Harper S, Gerstein HC. Spectrum of quality of life impairment in hypothyroidism. Qual Life Res. 1994;3(5):323-327. [DOI] [PubMed] [Google Scholar]
- 222. Watt T, Hegedüs L, Groenvold M, et al. Validity and reliability of the novel thyroid-specific quality of life questionnaire, ThyPRO. Eur J Endocrinol. 2010;162(1):161-167. [DOI] [PubMed] [Google Scholar]
- 223. McMillan CV, Bradley C, Woodcock A, Razvi S, Weaver JU. Design of new questionnaires to measure quality of life and treatment satisfaction in hypothyroidism. Thyroid. 2004;14(11):916-925. [DOI] [PubMed] [Google Scholar]
- 224. Abdalla SM, Bianco AC. Defending plasma T3 is a biological priority. Clin Endocrinol (Oxf). 2014;81(5):633-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Baloch Z, Carayon P, Conte-Devolx B, et al. ; Guidelines Committee, National Academy of Clinical Biochemistry . Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13(1):3-126. [DOI] [PubMed] [Google Scholar]
- 226. Araque KA, Klubo-Gwiezdzinska J, Nieman LK, Welsh K, Soldin SJ. Assessment of thyroid function tests and harmonization: opinion on thyroid hormone harmonization. Ther Adv Endocrinol Metab. 2019;10:2042018819897049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Soldin OP, Soldin SJ. Thyroid hormone testing by tandem mass spectrometry. Clin Biochem. 2011;44(1):89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Hoermann R, Midgley JE, Larisch R, Dietrich JW. Integration of peripheral and glandular regulation of triiodothyronine production by thyrotropin in untreated and thyroxine-treated subjects. Horm Metab Res. 2015;47(9):674-680. [DOI] [PubMed] [Google Scholar]
- 229. Jonklaas J, Burman KD. Daily administration of short-acting liothyronine is associated with significant triiodothyronine excursions and fails to alter thyroid-responsive parameters. Thyroid. 2016;26(6):770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Jonklaas J, Burman KD, Wang H, Latham KR. Single-dose T3 administration: kinetics and effects on biochemical and physiological parameters. Ther Drug Monit. 2015;37(1):110-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Van Tassell B, Wohlford GF IV, Linderman JD, et al. Pharmacokinetics of L-triiodothyronine in patients undergoing thyroid hormone therapy withdrawal. Thyroid. 2019;29(10):1371-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Celi FS, Zemskova M, Linderman JD, et al. Metabolic effects of liothyronine therapy in hypothyroidism: a randomized, double-blind, crossover trial of liothyronine versus levothyroxine. J Clin Endocrinol Metab. 2011;96(11):3466-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Jonklaas J, Tefera E, Shara N. Physician choice of hypothyroidism therapy: influence of patient characteristics. Thyroid. 2018;28(11):1416-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Jonklaas J, Tefera E, Shara N. Prescribing therapy for hypothyroidism: influence of physician characteristics. Thyroid. 2019;29(1):44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Jonklaas J, Tefera E, Shara N. Short-term time trends in prescribing therapy for hypothyroidism: results of a survey of American Thyroid Association members. Front Endocrinol (Lausanne). 2019;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Michaelsson LF, la Cour JL, Medici BB, Watt T, Faber J, Nygaard B. Levothyroxine/liothyronine combination therapy and quality of life: is it all about weight loss? Eur Thyroid J. 2018;7(5):243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Bednarczuk T, Attanasio R, Hegedüs L, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS* questionnaire survey of Polish physicians. *THESIS: Treatment of Hypothyroidism in Europe by Specialists: an International Survey. Endokrynol Pol. 2021;72(4):357-365. [DOI] [PubMed] [Google Scholar]
- 238. DiStefano J III, Jonklaas J. Predicting optimal combination LT4 + LT3 therapy for hypothyroidism based on residual thyroid function. Front Endocrinol (Lausanne). 2019;10:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Medici BB, la Cour JL, Michaelsson LF, Faber JO, Nygaard B. Neither baseline nor changes in serum triiodothyronine during levothyroxine/liothyronine combination therapy predict a positive response to this treatment modality in hypothyroid patients with persistent symptoms. Eur Thyroid J. 2017;6(2):89-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Mullarkey T. Pharmacy compounding of high-risk level products and patient safety. Am J Health Syst Pharm. 2009;66(17 Suppl 5):S4-S13. [DOI] [PubMed] [Google Scholar]
- 241. Boulton DW, Fawcett JP, Woods DJ. Stability of an extemporaneously compounded levothyroxine sodium oral liquid. Am J Health Syst Pharm. 1996;53(10):1157-1161. [DOI] [PubMed] [Google Scholar]
- 242. Bakhteyar H, Cassone C, Kohan HG, Sani SN. Kinetic analysis of drug release from compounded slow-release capsules of liothyronine sodium (T3). Int J Pharm Compd. 2017;21(5):418-425. [PubMed] [Google Scholar]
- 243. Hennemann G, Docter R, Visser TJ, Postema PT, Krenning EP. Thyroxine plus low-dose, slow-release triiodothyronine replacement in hypothyroidism: proof of principle. Thyroid. 2004;14(4):271-275. [DOI] [PubMed] [Google Scholar]
- 244. Spaulding SW, Smith TJ, Hinkle PM, Davis FB, Kung MP, Roth JA. Studies on the biological activity of triiodothyronine sulfate. J Clin Endocrinol Metab. 1992;74(5):1062-1067. [DOI] [PubMed] [Google Scholar]
- 245. Santini F, Hurd RE, Lee B, Chopra IJ. Thyromimetic effects of 3,5,3’-triiodothyronine sulfate in hypothyroid rats. Endocrinology. 1993;133(1):105-110. [DOI] [PubMed] [Google Scholar]
- 246. Santini F, Giannetti M, Ricco I, et al. Steady-state serum T3 concentrations for 48 hours following the oral administration of a single dose of 3,5,3’-triiodothyronine sulfate (T3S). Endocr Pract. 2014;20(7):680-689. [DOI] [PubMed] [Google Scholar]
- 247. Santini F, Ceccarini G, Pelosini C, et al. Treatment of hypothyroid patients with L-thyroxine (L-T4) plus triiodothyronine sulfate (T3S). a phase II, open-label, single center, parallel groups study on therapeutic efficacy and tolerability. Front Endocrinol (Lausanne). 2019;10:826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Taylor PN, Eligar V, Muller I, Scholz A, Dayan C, Okosieme O. Combination thyroid hormone replacement; knowns and unknowns. Front Endocrinol (Lausanne). 2019;10:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Idrees T, Price JD, Piccariello T, Bianco AC. Sustained release T3 therapy: animal models and translational applications. Front Endocrinol (Lausanne). 2019;10:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Da Conceição RR, Fernandes GW, Fonseca TL, Bocco BMLC, Bianco AC. Metal coordinated poly-zinc-liothyronine provides stable circulating triiodothyronine levels in hypothyroid rats. Thyroid. 2018;28(11):1425-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Dumitrescu AM, Hanlon EC, Arosemena M, et al. Extended absorption of liothyronine from poly-zinc-liothyronine (PZL): results from a phase 1, double-blind, randomized, and controlled study in humans. Thyroid. 2021. doi: 10.1089/thy.2021.0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Guldvog I, Reitsma LC, Johnsen L, et al. Thyroidectomy versus medical management for euthyroid patients with Hashimoto disease and persisting symptoms: a randomized trial. Ann Intern Med. 2019;170(7):453-464. [DOI] [PubMed] [Google Scholar]
- 253. Promberger R, Hermann M, Pallikunnel SJ, Seemann R, Meusel M, Ott J. Quality of life after thyroid surgery in women with benign euthyroid goiter: influencing factors including Hashimoto’s thyroiditis. Am J Surg. 2014;207(6):974-979. [DOI] [PubMed] [Google Scholar]
- 254. Goswami S, Peipert BJ, Mongelli MN, et al. Clinical factors associated with worse quality-of-life scores in United States thyroid cancer survivors. Surgery. 2019;166(1):69-74. [DOI] [PubMed] [Google Scholar]
- 255. Husson O, Haak HR, Oranje WA, Mols F, Reemst PH, van de Poll-Franse LV. Health-related quality of life among thyroid cancer survivors: a systematic review. Clin Endocrinol (Oxf). 2011;75(4):544-554. [DOI] [PubMed] [Google Scholar]
- 256. Ajjan RA, Weetman AP. The pathogenesis of Hashimoto’s thyroiditis: further developments in our understanding. Horm Metab Res. 2015;47(10):702-710. [DOI] [PubMed] [Google Scholar]
- 257. Wichman J, Winther KH, Bonnema SJ, Hegedüs L. Selenium supplementation significantly reduces thyroid autoantibody levels in patients with chronic autoimmune thyroiditis: a systematic review and meta-analysis. Thyroid. 2016;26(12):1681-1692. [DOI] [PubMed] [Google Scholar]
- 258. Winther KH, Wichman JE, Bonnema SJ, Hegedüs L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017;55(2):376-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. de Freitas Cayres LC, Vilela de Salis LV, Rodrigues GSP, et al. Detection of alterations in the gut microbiota and intestinal permeability in patients with Hashimoto thyroiditis. Front Immunol. 2021;12:579140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Liu S, An Y, Cao B, Sun R, Ke J, Zhao D. The composition of gut microbiota in patients bearing Hashimoto’s thyroiditis with euthyroidism and hypothyroidism. Int J Endocrinol. 2020;2020:5036959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Jiang W, Yu X, Kosik RO, et al. Gut microbiota may play a significant role in the pathogenesis of Graves’ disease. Thyroid. 2021;31(5):810-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Zhao F, Feng J, Li J, et al. Alterations of the gut microbiota in Hashimoto’s thyroiditis patients. Thyroid. 2018;28(2):175-186. [DOI] [PubMed] [Google Scholar]
- 263. Bibbò S, Abbondio M, Sau R, et al. Fecal microbiota signatures in celiac disease patients with poly-autoimmunity. Front Cell Infect Microbiol. 2020;10:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Liontiris MI, Mazokopakis EE. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Points that need more investigation. Hell J Nucl Med. 2017;20(1):51-56. [DOI] [PubMed] [Google Scholar]
- 265. Metso S, Hyytiä-Ilmonen H, Kaukinen K, et al. Gluten-free diet and autoimmune thyroiditis in patients with celiac disease. A prospective controlled study. Scand J Gastroenterol. 2012;47(1):43-48. [DOI] [PubMed] [Google Scholar]
- 266. Krysiak R, Szkróbka W, Okopień B. The effect of gluten-free diet on thyroid autoimmunity in drug-naïve women with Hashimoto’s thyroiditis: a pilot study. Exp Clin Endocrinol Diabetes. 2019;127(7):417-422. [DOI] [PubMed] [Google Scholar]
- 267. Ma R, Morshed SA, Latif R, Davies TF. Thyroid cell differentiation from murine induced pluripotent stem cells. Front Endocrinol (Lausanne). 2015;6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Antonica F, Kasprzyk DF, Opitz R, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491(7422):66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Ma R, Morshed SA, Latif R, Davies TF. A stem cell surge during thyroid regeneration. Front Endocrinol (Lausanne). 2020;11:606269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Hollenberg AN, Choi J, Serra M, Kotton DN. Regenerative therapy for hypothyroidism: mechanisms and possibilities. Mol Cell Endocrinol. 2017;445:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]