Abstract
Muscle wasting disease indications are among the most debilitating and often deadly noncommunicable disease states. As a comorbidity, muscle wasting is associated with different neuromuscular diseases and myopathies, cancer, heart failure, chronic pulmonary and renal diseases, peripheral neuropathies, inflammatory disorders, and, of course, musculoskeletal injuries. Current treatment strategies are relatively ineffective and can at best only limit the rate of muscle degeneration. This includes nutritional supplementation and appetite stimulants as well as immunosuppressants capable of exacerbating muscle loss. Arguably, the most promising treatments in development attempt to disrupt myostatin and activin receptor signaling because these circulating factors are potent inhibitors of muscle growth and regulators of muscle progenitor cell differentiation. Indeed, several studies demonstrated the clinical potential of “inhibiting the inhibitors,” increasing muscle cell protein synthesis, decreasing degradation, enhancing mitochondrial biogenesis, and preserving muscle function. Such changes can prevent muscle wasting in various disease animal models yet many drugs targeting this pathway failed during clinical trials, some from serious treatment-related adverse events and off-target interactions. More often, however, failures resulted from the inability to improve muscle function despite preserving muscle mass. Drugs still in development include antibodies and gene therapeutics, all with different targets and thus, safety, efficacy, and proposed use profiles. Each is unique in design and, if successful, could revolutionize the treatment of both acute and chronic muscle wasting. They could also be used in combination with other developing therapeutics for related muscle pathologies or even metabolic diseases.
Keywords: activin, ActRIIa, ACVR2, ActRIIb, ACVR2B, growth/differentiation factor (GDF)8, GDF11, muscle atrophy, muscle wasting, myostatin
Graphical Abstract
Graphical Abstract.
Essential Points.
Myostatin regulation of progenitor cell differentiation is nuanced as it can initiate, delay, or even inhibit differentiation depending on progenitor cell context.
Overwhelming evidence indicates that GDF11 and myostatin have nearly identical actions in striated muscle. Studies suggesting otherwise were generated with reagents now known to be invalid and possibly acting as dominant-negatives.
Myostatin and other ActRII ligands regulate muscle and nonmuscle tissues consistent with ActRII tissue distribution. Thus, actions originally defined as “off-target” for some ActRII attenuators resulted from specific rather than nonspecific drug-target interactions.
Future clinical trial successes will depend upon lessons learned from past failures, which include targeting appropriate disease indications, attenuating a redundancy of signals, and limiting drug action to skeletal and/or cardiac muscle.
Increasing muscle function and not just mass is key to clinical trial success and to regulatory approval for most muscle wasting disease indications. Accomplishing this will require novel trial designs that augment neural components of strength as, for example, with exercise.
Solely increasing muscle mass without comparable changes in muscle function may still have significant clinical potential in treating obesity, insulin resistance and type 2 diabetes.
Significant muscle atrophy or wasting satisfies every criteria for “disease” classification. It is an abnormal condition, it results from a defined pathological process and it produces characteristic and predictable outcomes consistent with anatomical change. It is also recognized by the International Classification of Diseases (M62.50) and has an estimated prevalence of 2% in the general population (1). This equates to an astonishing 156 000 000 people worldwide. If classified as a single disease, this would greatly exceed the impact of the associated primary disease indications, many of which are classified as rare and neglected by the US Food and Drug Administration (FDA).
Yet, muscle wasting is not a disease but a comorbidity and negative modifier that accelerates pathology in an incredibly large number of diverse disease indications. This profoundly complicates commercial development of muscle wasting drugs as regulations require testing and approval for each indication. This is despite strong evidence that the underlying mechanisms are highly conserved across a wide variety of disease indications (1, 2). It stands to reason, therefore, that many drugs capable of targeting these mechanisms or attenuating the activation signals could be broadly effective. They could also revolutionize the clinical treatment of many diseases.
Only 1 drug is specifically approved for treating muscle wasting, Serostim (recombinant human GH [eg, somatotropin]) for HIV-associated wasting. It effectively helps to maintain lean mass and body weight in HIV+ patients who are also GH deficient because of antiretroviral therapy (3). Serostim’s success in improving quality of life underscores the high unmet need from the severe muscle wasting that compromises patient outcomes across disparate disease indications. These include the obvious musculoskeletal injuries (MSIs) and disuse atrophy as muscle wasting is the primary impediment to rehabilitation for patients with an MSI, peripheral neuropathy, nerve injury, or even subjects exposed to prolonged spaceflight and microgravity (4-6).
At best, muscle wasting is debilitating, often limiting patient mobility, and requiring the use of assisted devises (eg, wheelchairs, braces). At worst, it predisposes to morbidity and increased mortality. Indeed, almost 80% of patients with advanced cancer develop systemic muscle wasting (cancer cachexia), which impairs mobility, compromises therapies, and is directly responsible for 30% to 50% of the resulting deaths (7, 8). Muscle wasting is inherent to the muscular dystrophies and to genetic and inflammatory myopathies, many of which are fatal. It also occurs in more than one-half of people older than 80 years who suffer from sarcopenia, the age-related progressive loss of muscle that significantly increases risk for hospitalization, disability, and again mortality (9).
Additional disease indications with muscle wasting include heart failure, chronic obstructive pulmonary disease, end-stage renal disease, chronic infection, hip fracture, malnutrition, and burns and sepsis, which is an inexhausted list (1). Common to these conditions is an elevated stress or inflammatory response where production of stress hormones (eg, cortisol), cytokines (eg, IL-1, IL-6) and myokines (eg, myostatin) either directly induce muscle wasting or contribute to it (10-12). This includes multiple members of the TGF-β superfamily that suppress muscle growth, induce muscle atrophy, and antagonize the actions of muscle growth promoters.
TGF-β Superfamily Biology
Incestuous promiscuity
The first studies describing TGF-β superfamily regulation of skeletal muscle growth and development were published more than 3 decades ago (13-16). The seminal discovery of myostatin (eg, growth/differentiating factor 8 [GDF8]) a decade later (17) and the hypermuscularized phenotype of different myostatin null (mstn-/-) vertebrates (18) ignited rapid growth of the field and, predictably, the clinical development of many myostatin attenuating therapeutics (1). Less well known is the evidence that other TGF-β superfamily members similarly suppress muscle growth via autocrine, paracrine, and endocrine means and may even contribute to the pathological wasting of muscle in various disease states (19). These include the activins (Act A, B, and AB) and GDF11, both of which signal through the ActRII receptors, ActRIIa and ActRIIb (eg, ACVR2A and ACVR2B, respectively). Moreover, these receptors are widely expressed in disparate cell types of diverse tissues and are even activated by other TGF-β superfamily ligands, namely bone morphogenic protein (BMP)-2, -7, -9, -10, and -11 (20-22). Such complexity presents unique challenges to drug development and may have been overlooked in designing the early generation (eg, first mover) myostatin/activin pathway attenuators, most of which are no longer in development.
Divergence of TGF-β superfamily ligands and receptors predates even the inaugural diversifying event in animal evolution, the parazoan (single tissue organisms) and eumetazoan (multiple distinct tissues) split (23, 24). Over the last billion years, approximately, additional gene duplications and the subsequent functional divergence generated a ligand superfamily with at least 39 known vertebrate members: TGF-β1-3; BMP1-8A,B/10/11/15/16 (16 restricted to teleost fish); GDF1/3/5-7/9/10/11/15; myostatin, inhibin (Inh) A and B, activin A/B/AB; nodal; lefty A/B; Müllerian inhibiting substance (eg, anti-Müllerian hormone); glial cell line-derived neurotrophic factor; neurturin; artemin; and persephin (23, 25). Each is generally subclassified into 4 groups: (1) TGF-βs; (2) BMPs/GDFs; (3) activin/inhibin/nodal; and (4) others. This parsing, however, is based upon nomenclature rather than true phylogenetic relationships as, for example, the BMPs and GDFs are distributed throughout multiple distinct clades (23, 26).
Further complexity arises from different families of ligands, receptors and signaling modulators (ie, Smads, see the following section) all having evolved at different rates (23) and producing a system where ligands greatly outnumber receptors and often compete for binding sites. Furthermore, ligand:receptor interactions are regulated by secreted and membrane associated binding proteins that function to antagonize receptor activation or as extracellular stores (23, 27, 28). This paradoxically increased system complexity outside the cell (ie, ligand:receptor interactions), yet simplified it inside (ie, signal transduction) as multiple related ligands often cross-react with multiple shared receptors to activate limited signaling pathways (Fig. 1). The result, functional redundancy within any particular tissue and pleiotropy across many.
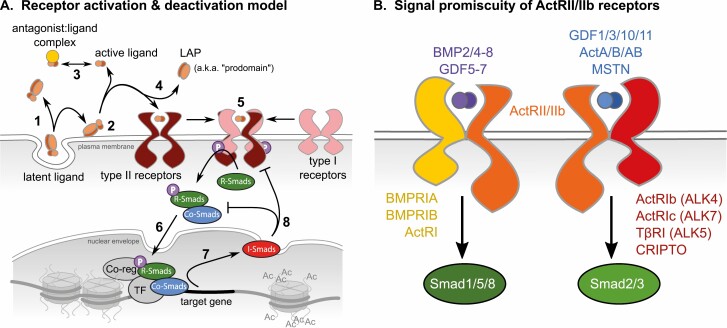
Figure 1.
Complexity of the TGF-β superfamily network. (A) General overview of the ligand-mediated signaling pathway starting with (1) secretion of the ligand:latency associated protein (LAP [prodomain]) complex and its association with binding proteins in the extracellular matrix. (2) Cytoskeletal forces and/or proteolysis release the ligand:LAP complex, often dissociating each. (3) Mature ligands are then free to associate with protein antagonists in the extracellular space or in circulation. (4) LAPs are also released with ligand binding to type II receptors followed by recruitment of type I receptors and their transphosphorylation by type II. (5) The serine kinase domain of type 1 receptors then phosphorylates receptor (R)-Smads that then bind Co-Smads allowing for (6) nuclear translocation, binding of transcription factors (TF) and coregulators and ultimately, (7) gene transactivation. (8) Inhibitory (I)-Smads are among the target genes and attenuate both receptor activation and Co-Smad complex formation. (B) Color-coding indicates the different ligands (purple and blue) that bind ActRII/IIb type II receptors (orange) that associate with different type I receptors (yellow and red) to separately activate distinct Smad signaling pathways.
Ligand secretion does not immediately result in receptor binding and activation as accessibility is limited by several high-affinity protein antagonists, some of which result from the proteolytic processing of the ligand pre-pro-proteins (27, 29, 30). Indeed, cleavage in the Golgi removes the signal peptide and divides the pro-peptide into 2 distinct forms: the amino-terminal “latent associated protein” (LAP) and the bioactive carboxyterminal region that forms the dimeric ligand. The myostatin LAP is often referred to as “prodomain” or “propeptide,” a confusing nomenclature as the actual prodomain/propeptide still contains the myostatin monomer. Thus, “LAP” is used herein to avoid confusion.
The LAP and ligand are then secreted together in an inactive form that can bind to one of many matrix-associated binding proteins (Fig. 1A, steps 1, 2). For myostatin, the primary negative regulator of skeletal muscle growth, this includes latent TGF-β binding protein-3, TGF-β binding protein-4, and decorin (31-33). This extracellular inactive pool or storage depot is analogous to intracellular secretory vesicles as ligand release is regulated, in this case by another proteolytic event that initiates the irreversible transition from latent to active state (27). It is important to note that these protein-protein associations have only been worked out for subset of ligands (eg, TGF-β, myostatin), although the conserved manner by which each ligand is processed for secretion suggests that similar associations may exist for most if not all members of the superfamily.
Additional ligand antagonists are known to competitively prevent receptor activation (Fig. 1A, step 3). For myostatin, these include follistatin, follistatin splice variants (FS288 and FS315), follistatin-like 3 (eg, follistatin-like related gene or peptide) as well as growth and differentiation factor associated serum protein-1 and -2 (eg, WAP, follistatin/Kazal, immunoglobulin, Kunitz, and netrin domain-containing, WFIKKN1 and 2). Each of these can bind to either myostatin or GDF11 through surface-exposed hydrophobic residues that lie within conserved follistatin domains and their Kazal subdomains (34). Mutagenesis and X-ray scattering studies indicate that latent myostatin complexes exist in an open conformation that resembles nonlatent or “free” activin A and BMP9 rather than the closed conformation of latent TGF-β (35). The authors suggest this could relate to the tolloid-dependent proteolytic processing that liberates myostatin from its latent complex. This conservation of structural determinants further suggests that the mechanisms of latent complex formation and release are possibly shared between myostatin, GDF11, the activins, and BMP9, all of which also bind to the same antagonists and to the ActRII receptors (36-38).
Across the superfamily, receptor activation is governed by differences in ligand binding affinities for type I and II receptors (39). These are mostly low- and high-affinity interactions, respectively, with few exceptions where the affinity roles are reversed for some BMPs. Formation of a heteromeric complex composed of the ligand dimer with 2 type I and II receptor pairs begins with the high-affinity binding interaction and is followed by recruitment of the low-affinity receptor (Fig. 1A, step 5). Crystal structure comparisons of ligand:receptor complexes reveal a nuanced process that differs between three general ligand classes: TGF-βs, activins, and BMPs. The TGF-βs bind via the “cooperative model” where type II receptors recruit the ligand and facilitate type I binding through direct interreceptor interactions (40). By contrast, activin receptor complex formation is driven by ligand flexibility as the ligand itself, not type II receptors, facilitates type I receptor recruitment. This is referred to as the “conformational selection” model. The third model, “lock and key” explains BMP receptor complex formation and is also driven by ligand:receptor rather than receptor:receptor interactions, although the contact positions differ from those in the conformational model.
Receptor signaling
The canonical receptor signaling pathways of TGF-β superfamily ligands share a generalized architecture that is comparatively simple (41). They are also very well described in the literature and can be actively interrogated using the online Reactome database (https://reactome.org/) (42). Once a receptor complex is activated, the intracellular glycine/serine-rich domain of type I receptors is transphosphorylated by the comparable domain of type II receptors (Fig. 1A, 5). This in turn activates type I receptors that subsequently phosphorylate receptor (r)-Smads on 2 C-terminal serine residues. Although all receptors in the superfamily are commonly referred to as serine/threonine kinases, at least some are actually dual-specificity kinases capable of phosphorylating tyrosine residues as well (43-45).
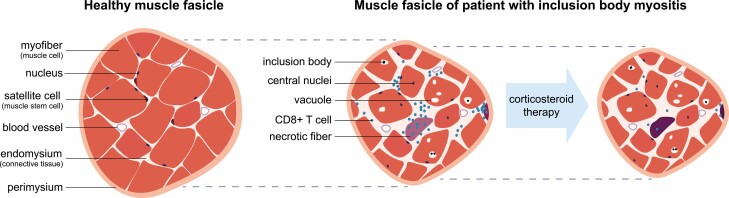
Figure 5.
Pathophysiology of IBM. Cartoon representation of muscle fascicle cross-sections from healthy subjects and from IBM patients before and after treatment with corticosteroids. Different structural components of mature muscle fascicles are labeled to the left of each panel. Dotted lines aid in representing the change in fascicle size with disease progression.
Phosphorylation of r-Smads produces a conformational change and consequently, the formation of oligomeric complexes usually composed of 2 r-Smads and a single common-mediator (co)-Smad, although heteromeric complexes of 1 or 2 r-Smads with 1 or 2 co-Smads can occur within a given context (41). This complex translocates into the nucleus where it directly associates with DNA regulatory elements and transcriptional machinery to induce gene expression (Fig. 1A, step 6). Inhibitory (i)-Smads are among the target genes expressed and ultimately function as negative feedback regulators that either prevent r-Smad:co-Smad complex formation or facilitate ubiquitin-regulated receptor degradation (Fig. 1A, steps 7, 8) (46).
Specificity of this seemingly simplistic pathway lies not in the complexity or number of steps involved, but in the composition of related molecules. This is possibly best illustrated by activin receptor signaling (Fig. 1B). Indeed, the 2 type II activin receptors (ActRIIa and ActRIIb) each associate with multiple and different type I receptors that bind different ligand dimers. This in turn activates only 2 distinct pathways that phosphorylate either Smads-1, -5, and -8 or Smads -2 and -3; pathways that are antagonized by Smad-6 and -7, respectively, the sole i-Smads (46).
Smad signaling is also known to interact with a wide variety of pathways typically involved in embryonic development (eg, Wnt, Notch, Hippo, Hedgehog), postnatal tissue growth and metabolism (eg, mitogen-activated protein kinase [MAPK], phosphoinositide 3-kinase [PI3K]) and cytokine signaling (eg, JAK/STAT, nuclear factor κB [NF-κB]) (47). The significance of such cross-talk is context-specific and only partially explains how, for example, the biological actions of a particular Smad pathway activator differs from another ligand that activates the same pathway. Specificity in action results instead from the differential expression (eg, tissue type, developmental stage, temporal timing) of receptors, binding proteins, and antagonists with subtle structural differences and to other factors (eg, proteases and transcriptional co-regulators) that influence ligand binding and Smad transcriptional activity (23, 39). Inherent to this system, however, is that multiple ligands, regardless of origin, can bind multiple receptors thereby influencing multiple tissues and physiological systems.
Skeletal muscle regulators
Most mechanistic studies investigating TGF-β superfamily regulation of muscle have focused on developmental or embryonic processes (eg, mesenchymal transitioning, myogenic determination, progenitor cell differentiation) rather than the physiological integration of postnatal muscle growth. This process especially differs from embryonic by 1 crucial aspect: the formation of new muscle, which only occurs embryologically. By contrast, postnatal growth results from muscle hypertrophy or an increase in cell size. This occurs when protein synthesis increases and/or when progenitor (eg, satellite) cells differentiate and fuse with fully formed myofibers (48).
Of these 2 processes, changes in protein synthesis and degradation generally play a more significant role in homeostatic control, although satellite cells also respond to physiological conditions and to the insults and regenerative cues that induce muscle wasting (1). When activated with exercise, injury, or chemical signals, satellite cell pools expand and some cells differentiate and fuse with existing myofibers. Donated nuclei from these cells will initially move to the center of the myofiber, a hallmark of muscle regeneration, and begin transcribing the muscle-specific genes responsible for hypertrophic growth and the myofiber phenotype (49, 50). Most of these centrally located nuclei ultimately disperse to the sarcolemma and continue transcribing genes, although long-term central nucleation can occur with significant injury (51, 52).
Different TGF-β superfamily ligands regulate these processes in both positive and negative ways (19). Indeed, BMP signaling via Smad1/5/8 stimulates hypertrophic muscle growth by increasing myofiber protein synthesis and decreasing degradation (53-55); effects that are mediated by mTOR and the ubiquitin system (eg, MUSA1, MuRF1, MAFbx), respectively. BMP signaling also helps to expand the muscle satellite/progenitor cell pool (56, 57), which similarly expands tissue regenerative capacity. The specific ligands involved include BMP7, BMP13/GDF6, and BMP14/GDF5, all of which bind a BMPRIIB:ALK3 receptor complex (19).
By contrast, the ActRII ligands have purely inhibitory actions in mature myofibers and stimulate muscle atrophy by inhibiting protein synthesis, stimulating protein degradation, and attenuating signals that enhance muscle growth (19). The latter include BMP signals as well as those activated by IGF1, the primary positive regulator of postnatal muscle growth (58, 59). Indeed, myostatin, the activins and GDF11 all bind ActRIIa/b:Alk4/5 receptor complexes activating Smad2/3 signaling and inducing muscle atrophy (21, 36, 55, 60, 61). Because Smad4 is the sole co-Smad, increased Smad2/3 signaling additionally attenuates hypertrophic Smad1/5/8 signaling via competition for Smad4. This is supported by studies with smad4-/- mice that display mild muscle atrophy and reduced strength. In addition, smad4-/-/mstn-/- double knockouts possess a wild-type muscle phenotype rather than the hypertrophic phenotype of mstn-/- mice. This indicates that development of muscle hypertrophy in mstn-/- mice results not from the absence of myostatin signaling per se, but the parallel and consequential enhancement of BMP and Smad1/5/8 signaling (54).
Physiological integration of muscle growth
Yin-yang regulation
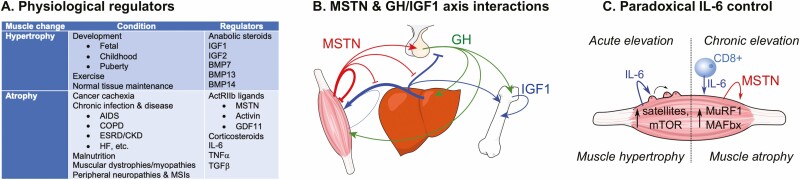
Myostatin and IGF1 are both potent regulators of muscle growth. Although their co-antagonism is well known from a cellular perspective, their relationship controlling systemic muscle growth is only now being revealed. Myostatin attenuates IGF1-induced myoblast proliferation, myotube hypertrophy and protein synthesis, suppression of the muscle ubiquitin pathway, and Akt/mTOR signaling (62-68). Some if not all of these actions appear to be shared by other Smad2/3 pathway activators including the ActRII ligands, GDF11, and activins, as well as by TGF-β (58, 69). The dualism described suggests that the homeostatic control of postnatal muscle growth, the control system that responds to different physiological and pathological conditions, is rooted in a yin-yang relationship between anabolic growth promoters and catabolic growth inhibitors. This includes not only TGF-β superfamily ligands and IGF1, but several other factors as well (Fig. 2A).
Figure 2.
Anabolic and catabolic regulation of muscle. (A) Parsing of general physiological and pathological conditions as well as the primary factors that differentially regulate skeletal muscle hypertrophy and atrophy (BMP, bone morphogenic protein; COPD, chronic obstructive pulmonary disorder; ESRD/CKD, end-stage renal disease/chronic kidney disease; GDF, growth/differentiation factor; HF, heart failure; MSTN, myostatin; MSI, musculoskeletal injury). (B) Model for MSTN interactions with the GH/IGF1 axis. Arrows represent stimulation, blocked lines inhibition. Arrow/line thickness is relative to influence. (C) Model for the paradoxical actions of IL-6 on skeletal muscle satellite cells and hypertrophy as well as on muscle protein degradation and atrophy. Colored arrows correspond to labeled factor, black arrows indicate increase (CD8+, cluster of differentiation 8 positive T-helper immune cell; MuRF1, muscle RING finger 1 [Trim63]; MAFbx, muscle atrophy F-Box [Atrogin-1]).
In addition to its autocrine/paracrine actions, recent studies suggest that myostatin also influences the systemic control of muscle growth by attenuating the GH/IGF1 axis, otherwise known as the somatomedin model of growth control (62, 70). This endocrine model is extremely well established and is based on the fact that many somatotropic effects attributed to GH are actually mediated by IGF1 produced locally (eg, in bone or muscle) or in the liver (Fig. 2B). This is particularly meaningful because, although IGF1 functions as a myokine, much if not most of its actions in muscle are mediated systemically. Circulating levels of IGF1, but not GH, are highly correlated with muscle growth (71-73), whereas GH receptors are expressed at very low levels in postnatal muscle, levels that are roughly 1/10th of those in liver (74, 75). Moreover, lean body mass and muscle function are normal in muscle-specific GH receptor knockout mice (76) but suppressed in liver-specific knockouts (77). Furthermore, muscle expression of IGF1 was elevated in the latter, indicating that local autocrine expression cannot compensate for the loss of systemic IGF1.
Muscle reliance on circulating rather than locally produced IGF1 is also supported by studies of acid labile subunit (ALS) knockout and liver IGF1-deficient mice (78, 79). In both models, circulating IGF1 levels are reduced 65% to 75%. Because IGF1 negative feedback to the pituitary is significantly suppressed, a compensatory rise in GH secretion maintains the growth of bone, but presumably not muscle as body mass was reduced. Myostatin suppression of liver-derived IGF1 would, therefore, represent a novel physiological mechanism of muscle growth antagonism.
To this end, myostatin was recently demonstrated to suppress GH-induced expression of IGF1 and ALS in primary human hepatocytes (62). It also increased expression of IGF binding protein (IGFBP)1. ALS helps to maintain the circulating IGF1 half-life (80), whereas IGFBP1 attenuates IGF action by preventing IGF1 binding to the type 1 IGF receptor (80). These results are consistent with previous studies reporting a modified circulating IGFBP profile in mstn-/- mice that would increase the bioavailable fraction of IGF1 (70). In addition, the hypermuscular phenotype of mstn-/- mice is partially suppressed in the double knockout liver IGF1-deficient (LID)-o-Mighty mouse that lacks myostatin and cannot express IGF1 in the liver (62).
All of these results together suggest that myostatin not only attenuates IGF1 action in muscle, but also IGF1 hepatic production and circulating bioavailability; actions representative of endocrine rather than autocrine function. This is an important distinction and suggests that circulating myostatin acts in tissues other than muscle. In fact, activin receptors are expressed in a wide variety of tissues including the liver and pituitary, whereas myostatin, activin, and GDF11 have all been demonstrated to influence the physiology of 1 or both of these tissues (62, 81-87). The systemic role for each ligand, however, is somewhat controversial because although several studies have quantified their circulating levels, they are inconsistently associated with muscle wasting, age, and even sex (88-91), whereas the absolute levels quantified vary with the methodology used.
Circulating myostatin levels typically range between 2 and 12 ng/mL when quantified with ELISAs and are 5- to 10-fold higher than levels of GDF11 and ActA, respectively, in human subjects (88, 89, 92-94). By contrast, studies using liquid chromatography-tandem mass spectrometry assays report similar levels of circulating myostatin, but substantially higher GDF11 levels (~3 ng/mL) (95, 96). They also suggest that myostatin levels in men are double those in women and that testosterone administration increases circulating levels of myostatin, but not those of GDF11. The testosterone data conflict with the well-documented effects of testosterone on myostatin gene and protein expression (97-99), however, and may represent a pharmacological rather than physiological response. Some of the discrepancies could result from binding protein interference with assay performance as Kalampouka et al (92) demonstrated differences in total and free myostatin levels following acid extraction with the former. Further studies are, therefore, needed to determine whether differences in circulating levels of different ActRII ligands, myostatin in particular, are either causative or consequential to pathological and age-dependent changes in muscle mass or to muscle sexual dimorphism.
Stress hormones and pro-inflammatory cytokines
Other catabolic factors that are well known to induce muscle wasting include glucocorticoids and IL-6 (11, 100) and both of these chemical messengers are mechanistically liked to myostatin and/or Smad2/3 signaling. Glucocorticoids are stress hormones that respond to a variety of stressors and, teleologically, prevent energy utilization for nonessential systems such as growth. Their atrophy-producing actions are extremely well documented and include increased protein degradation, decreased protein synthesis, metabolic dysregulation, and attenuation of GH, IGF1, and insulin signaling, all of which remarkably resembles the actions of myostatin. This is because glucocorticoids induce myostatin gene expression directly via interactions with a glucocorticoid response element in the mstn promoter (101, 102) and indirectly by stimulating expression of the transcription factors CCAAT/enhancer binding protein (C/EBP)d and nuclear factor (NF)-κB (103, 104). They also reduce expression of miR-27a and b, which compromises myostatin transcript stability (105). In turn, many of the steroids’ inhibitory actions appear to be mediated by the local production of myostatin (11, 106, 107), which also mediates the muscle antagonizing effects of some proinflammatory cytokines like IL-6.
This molecular kinship, however, is somewhat more complicated by the fact that IL-6 can paradoxically stimulate or inhibit muscle growth and because the expression and signaling of both myokines are inextricably linked (Fig. 2C). Acute upregulation of IL-6 in muscle occurs with exercise and promotes muscle regeneration and hypertrophic growth by activating satellite cells and by increasing protein synthesis (100). By contrast, chronic IL-6 stimulation, from immune cell infiltration, in tumor-responsive tissues or by tumors themselves (1, 12, 108), induces muscle wasting largely by upregulating the expression of myostatin and the E3 ubiquitin ligases MuRF1 and MAFbx (eg, atrogin-1) (2, 100, 109, 110).
Several proinflammatory cytokines like IL-6 and TNF-α activate Stat3 and/or NF-κB, respectively. These pathways in turn upregulate myostatin expression and induce muscle wasting in animal models of chronic kidney disease, severe vitamin D deficiency, cancer cachexia, cirrhosis, and likely in other disease states as well (109-114). These effects appear to be mediated in part by phospho-Stat3 induction of C/EBPd and by NF-κB (103, 104, 109, 111, 113). Conversely, myostatin is also capable of upregulating IL-6 (115), whereas Smad3 crosstalk with Stat3 or NF-κB regularly occurs in different tissues including muscle (114, 116, 117).
These results together suggest that myostatin and possibly other ActRII ligands work synergistically with proinflammatory cytokines and glucocorticoids to induce muscle wasting. This basic understanding of disease mechanism has real-world implications for drug development and particularly for disease indications where muscle wasting is primarily driven by proinflammatory cytokines. This includes the inflammatory myopathies: sporadic inclusion body myositis (IBM), dermatomyositis, polymyositis, juvenile myositis, and necrotizing autoimmune myopathy (118, 119). It also includes Duchenne muscular dystrophy (DMD) or Becker muscular dystrophy (BMD) as low-dose treatment with synthetic glucocorticoids is considered standard of care, largely because of their anti-inflammatory actions (120). These steroids, however, also induce expression of myostatin, which can exacerbate muscle wasting.
Developing alternatives include optimized dosing regimens and novel corticosteroids incapable of inducing myostatin expression and muscle wasting. In fact, the synthetic 21-aminosteroid Vamorolone (eg, VBP15) selectively activates the glucocorticoid receptor while suppressing IL-6 and TNF-α signaling (121-124). Furthermore, it suppresses muscle inflammation and necrosis, promotes muscle repair, and stabilizes the sarcolemma in animal models of DMD, the mdx mouse, while slightly improving muscle force production. It is unknown whether Vamorolone also affects myostatin expression, although its attenuation of IL-6 and TNF-α signaling suggests that it is at least incapable of indirectly inducing expression via these cytokines.
Controversy
Phenotypic fixation
Of all the muscle regulating ActRII ligands, myostatin is undeniably the most well recognized. This is likely because of the legendary myostatin null phenotypes that have been derived from genetic models and from the pharmacological attenuation of myostatin bioavailability or ActRII signaling. Some of the most notable genetic models include “Compact,” “Mighty,” and quadruple muscled mice (17, 125, 126), at least 9 breeds of “double muscled” cattle (127), racing and “bully” whippet dogs (128, 129), and “6-pack” rainbow trout (130). Several pharmacological inhibitors have replicated these phenotypes, albeit to a subtle degree, in various animal models and even in the clinic (see the following section). The general strategies used vary and include ligand sequestration with receptor-mimic ligand traps, monoclonal antibodies, and binding proteins. Monoclonal antibodies have also been used to antagonize ActRIIa and/or ActRIIb and to attenuate latent complex activation, whereas Smad7 overexpression has been used to block activin and TGF-β receptor signaling from inside the muscle cell.
Most of these technologies were developed with an incomplete understanding of myostatin or ActRII biology and it is now clear that those with extracellular targets (ie, myostatin, ActRIIa/b, the latent complex) can potentially influence nonmuscle tissues often with deleterious consequences. This should not be considered a revelation given the promiscuity and redundancy inherent to the system. For example, the wide tissue distribution of ActRII receptors suggests that targeting ActRIIa/b or their circulating ligands, even in a highly specific manner (ie, immunoneutralization with monoclonal antibodies), is expected to affect multiple if not many different tissues. This logic also applies to the use of ligand traps and binding proteins as both technologies are inherently nonspecific. Moreover, the likelihood of adverse events occurring from the specific targeting of myostatin is less possibility than probability because it shares a common ancestor with GDF11 as well as high structural homology and conserved bioactivity (18). Indeed, alignments of the 2 human amino acid sequences, starting with the RXXR furin cleavage site, are 91.7% identical and 97.9% similar. Antagonists designed to specifically target myostatin could, therefore, unintentionally also attenuate GDF11 directly, because of shared structures, or indirectly because of receptor promiscuity.
Understanding and misunderstanding myostatin
Significant confusion has arisen from conflicting reports of myostatin action. Indeed, myostatin is often described as an inhibitor of muscle precursor cell proliferation and differentiation yet rarely do factors similarly control such diametrically opposed processes. Indeed, cell cycle withdrawal is a prerequisite for differentiation making it unlikely that myostatin would inhibit proliferation without somehow advancing differentiation.
The myokine clearly inhibits muscle progenitor cell proliferation. These actions are shared by TGF-β and include basal and IGF-stimulated proliferation in cells from a variety of vertebrate models (14, 18, 64, 131-137). Despite problems arising from the use of highly selected immortalized cell lines (138), this action is incontrovertible as myostatin downregulates cyclin-dependent kinase 2 and upregulates the cyclin-dependent kinase-inhibitor p21 in primary satellite cells in vitro and in embryonic progenitors in vivo (131, 139-145). These effects together prevent phosphorylation of retinoblastoma protein, which in its hypophosphorylated state sequesters the E2F transcription factor preventing expression of genes necessary for cell-cycle progression. This in turn arrests the cell cycle in G1 and G2 and induces cellular quiescence.
Such clarity is contrasted by conflicting studies suggesting that myostatin can stimulate or inhibit muscle progenitor cell differentiation. Most studies reporting inhibition used the immortalized C2C12 myoblast cell line (146-149). These cells were derived from dystrophic dy/dy C3H mice in 1972 (150) and in the 49 intervening years, have been highly propagated under various artificial selection pressures (eg, serial passaging, intentional and unintentional clonal selection, antibiotics, contact inhibition) that could alter phenotypic expression. In fact, they no longer maintain strict myogenic programing and convert to osteoblastic or fibroblastic lineages when induced with BMP2 (151, 152) or TGF-β (144, 153, 154), respectively. They are also incapable of differentiating into mature myotubes without first being induced with contact inhibition and by removing mitotic signals (ie, serum withdrawal). Separate lines have clearly diverged as some studies report myostatin and TGF-β to stimulate rather than inhibit proliferation of these cells (138, 155-157). All of these studies together question the reliability of using C2C12 cells, or any immortalized myoblast cell line, to study muscle cell determination and differentiation.
A few studies have used primary satellite cells from different vertebrate models. Some unfortunately used the same artificial protocol to induce C2C12 differentiation (61, 67, 158, 159). This differs significantly from the physiological condition as subconfluent primary satellite cells, again from a variety of vertebrate models, spontaneously differentiate in high serum and without contact inhibition (63, 64, 160-162). In fact, studies using noninhibited primary satellite cells indicate that myostatin inhibits proliferation and either stimulates differentiation or maintains cellular quiescence (63, 64, 131, 133, 139). Additional in vivo studies with chick and mouse embryos suggest that myostatin’s myogenic influence is more nuanced and context-specific (141, 142). When overexpressed, myostatin induces p21 and MyoD expression as well as terminal differentiation, whereas when myostatin is attenuated, muscle progenitor cell pools expand and differentiation is delayed. Differentiation is also impaired in myostatin knockdown, mstn-/- and smad3-/- satellite cells, whereas transplanting mstn-/- cells into mstn+/+ muscle restores their capacity to differentiate (139, 163-165). These studies strongly suggest that myostatin functions as an initiator, not inhibitor of muscle progenitor cell differentiation.
A more complete model for myostatin action incorporates all of these studies. It suggests that when muscle progenitors are proliferating, as during embryological development or muscle regeneration, myostatin arrests the cell cycle, initiates the myogenic program, and stimulates differentiation. It also maintains quiescence and prevents terminal differentiation when satellite cells are contact-inhibited. In mature muscle, myostatin stimulates atrophy by inhibiting protein synthesis, Akt/mTOR/p70S6 signaling, and IGF1 activation of this pathway. As a complement, it also stimulates muscle protein degradation by increasing expression of at least 1 E3 ubiquitin ligase (MAFbx/atrogin-1) that drives muscle proteolysis (58, 59, 166). Other ActRII ligands have similar effects in muscle (167-169) and likely cooperate with myostatin to induce muscle wasting, although for 1 particular ligand, these actions have been obfuscated by highly questionable and irreproducible science.
GDF11
A single research group reported on the “rejuvenating” effects of GDF11 in aged mice, suggesting that the myostatin homolog can restore the aged diminished condition of skeletal muscle, heart, and cerebral vasculature to a healthy youthful state (170-172). These studies were based upon an erroneous observation that levels of GDF11, but not myostatin, decrease with age (172). Ten subsequent studies reported data conflicting with these reports, indicating that GDF11 levels are either unaffected or that they actually rise with age (61, 84, 92, 173-178), whereas myostatin levels decline (179). Reports that the reagents used in these initial studies recognized both GDF11 and myostatin (61, 179) add to the misinterpretation of the biology. Although 3 additional studies have since reported slight age-related declines in circulating GDF11 (180-182), none used validated reagents or assays that were demonstrated to not cross-react with myostatin. By contrast, target specificity in studies reporting no change in circulating GDF11 and reductions in myostatin have in fact been appropriately validated (61, 173, 174, 179).
Many, if not most, of the original rejuvenation claims have since been refuted and have been reviewed in detail (167, 183). This includes effects in muscle as several studies indicate that GDF11 can mirror the atrophy-inducing actions of myostatin (60, 61, 184-186). Specifically, that GDF11 inhibits satellite cell proliferation, induced differentiation, and myofiber size in vitro. It also impairs muscle regeneration while promoting muscle wasting and fibrosis in vivo. Claims of cardiac improvement have also been refuted because elevated GDF11 was determined to be a risk factor for frailty and cardiovascular disease (174, 187), whereas exogenous GDF11 was demonstrated to induce skeletal and cardiac muscle wasting in mice with comparable changes in function (eg, grip strength, stroke volume) (60).
Roh et al (188) has further demonstrated that FSTL-3, a known antagonist of GDF11, myostatin, and activins, improves cardiac function in an animal model of pressure overload heart failure. In both in vivo and in vitro experiments, this study also determined that recombinant GDF11 activated ActRII signaling, reduced cardiac mass and cardiomyocyte size, upregulated MuRF1 and MAFbx expression, impaired various indices of cardiac function, and induced skeletal muscle wasting. These results are a direct contradiction to the cardiac rejuvenation hypothesis (172), yet are consistent with almost every other assessment of GDF11 or ActRII ligand in striated muscle. This includes studies of myostatin or activin action in the heart (169, 189-195).
Additional concerns with the original GDF11 rejuvenation papers include experimental, data analysis and interpretation issues that have been previously reviewed (61, 167, 179, 183). Considering the wealth of studies refuting GDF11 as a rejuvenation factor, any hypothesis to the contrary should be viewed skeptically. Recent studies have even compared GDF11 and myostatin and provided a structural explanation for the former molecule’s higher binding affinity to ActRIIb, which also explains why GDF11 is slightly more potent in primary satellite cells (34, 36, 40). Ironically, these studies were performed by authors purporting the GDF11 rejuvenation hypothesis.
How could 2 nearly identical molecules that bind the same receptor with nearly identical affinities and activate identical signaling pathways have disparate action in the same cell? A possible explanation is the use of recombinant proteins generated in Escherichia coli. Bacteria lack an oxidative environment and cannot form the disulfide bridge that links the 2 GDF11 monomers into a mature dimer. Moreover, bacterial recombinants frequently form inclusion bodies that complicate purification. Producing biologically active GDF11 in E coli, therefore, requires a complicated denaturing and renaturing system before the purified protein is validated using a physiologically relevant bioassay.
The recombinant GDF11 used in the rejuvenation studies was generated in E coli (170-172). According to the vendors website, bioactivity of their recombinant GDF11 is validated “by its ability to inhibit alkaline phosphatase (ALP) activity in differentiating MC3T3/E1cells” (https://www.peprotech.com/en/recombinant-humanmurinerat-gdf-11). Note that these osteogenic cells express ALP when differentiating and that TGF-β and Smad2/3 signaling stimulates ALP expression and activity (196-198). Recombinant GDF11 should therefore increase, not inhibit ALP activity. This discrepancy questions the validity of the recombinant GDF11 used in the rejuvenation studies because it may be structurally compromised and functioning as a dominant negative. It also explains how the use of this particular recombinant GDF11 could produce results counter to those produced with biologically active peptides and with transgenic studies.
Signaling
Canonical ActRII signaling
The several ligands capable of activating ActRII signaling do so through high- or low-affinity binding interactions. Biological activity within this multiligand environment is therefore dictated by affinity/capacity dynamics where high-affinity ActRII ligands out compete low-affinity ligands (eg, BMP2/7/9) in the absence of overwhelming concentrations of the latter (20, 21, 40, 199). Because ligand dimers interact with all 4 extracellular domains of the varied type I/II receptor complexes, ordered binding interactions are ultimately dependent upon the “3 Cs”: competition, composition, and capacity.
The high-affinity ligands bind ActRIIa/b before ALK4/5, whereas the opposite occurs with the low-affinity ligands (41, 200, 201). Myostatin, a high-affinity ligand, is likely the primary driver of ActRII-mediated muscle atrophy because it circulates at levels up to 500-fold higher than any of the other ligands and has a similar receptor-binding affinity. Nevertheless, ActA and GDF11 are also high-affinity ligands that can stimulate muscle wasting and contribute to the condition in different pathophysiological states (60, 108, 167-169, 184, 202-204).
Ligand binding stabilizes ActRIIa/b-Alk4/5 receptor complexes because this enables ActRIIa/b to phosphorylate serine and threonine residues in the glycine/serine-rich domain of Alk4 or Alk5 (Fig. 3A, step 1). The resulting conformational change in Alk4/5 releases FK506 binding protein (FKBP)12, an immunophilin and peptidyl prolyl isomerase, and enables Smad7 methylation by the ActRII-bound protein arginine methyltransferase 1. The combined release of FKBP12 and methylated-Smad7 from Alk4/5 exposes substrate binding sites for Smad2/3 that are then phosphorylated by Alk4/5 on 2 C-terminal serines (41).
Figure 3.
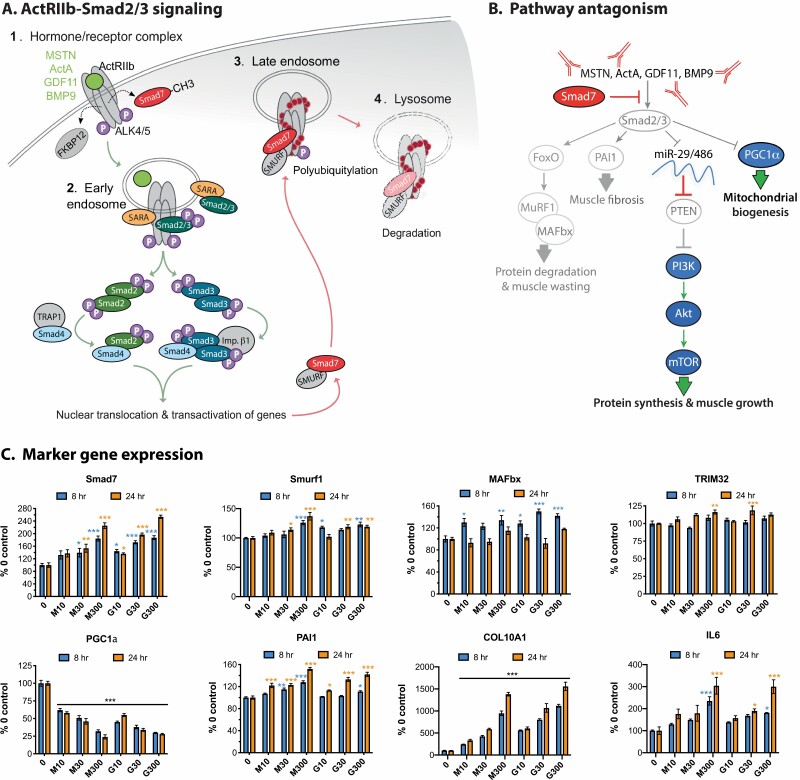
Canonical intracellular signaling pathways activated by ActRII ligands. (A) Endogenous ActRII signaling via phosphorylation (P) of Smad2 and Smad3. Includes receptor activation by myostatin (MSTN), an activin (ActA shown), growth/differentiating factor (GDF)11, or bone morphogenic protein (BMP)9. Each number represents the intracellular signaling locations and red dots represent ubiquitin. Dashed arrows represent movement of FKBP12 and methylated Smad7; green arrows direct pathway activation; red arrows direct negative feedback and signal termination. (B) Responses to pharmacological antagonism of Smad2/3 signaling in muscle. Arrows indicate activation; blocked lines inhibition. Silenced pathways are grayed, whereas blue symbols and green arrows represent pathways activated as a result of agents that attenuate ActRIIa/b activation and/or Smad2/3 signaling. These agents (red) include antibodies and ligand traps or the overexpression of Smad7, the endogenous pathway inhibitor. (C) Relative expression of the indicated genes was plotted using publicly available data from the Gene Expression Omnibus, record GSE67326 (61). This record was obtained from human skeletal muscle-derived cells (hSkMDCs) that were differentiated in vitro from primary satellite cells and then stimulated for 8 or 24 hours with 0, 10, 30, or 300 ng/mL myostatin (M) or GDF11 (G). Raw expression values were transformed to percent of 0 controls for each probe/spot. These values were then used to calculate group means (n = 4). Significant differences between means were determined using a 2-way ANOVA and Tukey’s post hoc test and are indicated by asterisks (compared with 0 controls: *P < 0.05, **0.01, ***0.001).
Canonical Smad and noncanonical signaling of TGF-β receptors is partitioned between clathrin-coated endosomes and caveolar pits, respectively, with atypical receptor tyrosine kinase signaling occurring in the latter (41). It is unknown whether ActRII signaling is also compartmentalized, although both Smad and receptor tyrosine kinase pathways can be activated in muscle depending upon the cell type and context (43, 205). Notwithstanding, canonical Smad signaling from ActRIIa/b propagates from endosomes (Fig. 3A2), like TGF-β receptors, and begins with the delivery of Smad2/3 from Smad Anchor for Receptor Activation and/or hepatic growth factor-regulated tyrosine kinase substrate; 2 FYVE finger protein traffickers (206, 207). Smad4 then transfers from either TRAP1 or another FYVE trafficker, endofin, to a phosphorylated (P)-Smad2/3 dimer (208, 209).
Most of the resulting complexes are composed of P-Smad2 or P-Smad3 dimers with a single Smad4. Heteromeric P-Smad2/3 dimers are nevertheless known to bind Smad4, which can also dimerize with either P-Smad monomer or form a trimeric complex that includes a Smad4 dimer (210). Heteromeric P-Smad2/3 trimers lacking Smad4 have also been described and adds to the diversity of signals that ultimately direct gene expression. Nuclear translocation of each complex is facilitated either by an importin or via a nuclear localization sequence within N-terminal MAD homology 1 domains that are conserved among all r-Smads (41). With the exception of Smad2, these domains also contain an 11 amino acid β-hairpin loop for binding to the Smad3/4 binding element (SBE) 5′-AGAC-3′ (or reverse complement 5′-GTCT-3′) and to other GC-rich sequences that differ from Smad1/5 sites (211-213). This loop is disrupted by an insertion in Smad2, but not in Smad2b, which prevents the predominant Smad2 from directly binding DNA (41).
Unlike many other cis regulatory elements, the SBE lacks complexity and size and the sequence itself is no doubt highly abundant throughout any vertebrate genome. Gene target specificity is, therefore, determined by the orientation, spacing and context of SBEs and other cis elements. The latter includes those for a diversity of transcription factors that associate with P-Smad2/3 complexes (47). In fact, transcription factor binding, either before or after P-Smad2/3 association, is the primary determinant of transactivation. Smad complexes also recruit histone acetyltransferases, deacetylases, methyltransferases, and even RNA binding proteins as mechanisms to regulate gene expression and RNA processing (41).
Several studies have identified gene targets of ActRII signaling, most notably those regulated by myostatin and GDF11 in vitro (61, 214) or in muscle from mstn-/- or ActRII-attenuated animals (215-219). Such targets are predictably involved in muscle cell growth and development, structure, and protein homeostasis, but also glucose metabolism. Studies with primary human satellite cells differentiated into mature myotubes identified identical targets for myostatin and GDF11, which is further indicative of functional conservation rather than divergence (61). Smad7 is among these genes and is upregulated as a form of intracellular negative feedback (Fig. 3B,C). Its effects include direct interference of Smad2/3 nuclear transactivation, blocking cytosolic Smad2/3/4 complex formation, attenuating ALK4/5 substrate binding, and directing the proteasomal degradation of ActRIIa/b-Alk4/5 receptors (46, 220). The MAD homology 2 domain plays a key role in all of these effects because it contains motifs necessary for binding to Smads2/3 and to ALK4/5 (46). Receptor degradation is mediated by E3 ubiquitin ligases, Smad ubiquitination regulatory factors (SMURF)1 or 2 (Fig. 3A, step 3), which in turn recruit the E2 ubiquitin conjugating enzymes that ultimately lead to receptor polyubiquitylation and lysosome fusion with the endosome (Fig. 3A, step 4) (220). Additional evidence suggests that both iSmads may potentially suppress rSmad-induced gene expression by associating with inhibitory cis elements, corepressors, or histone deacetylases (41), although these actions have yet to be documented in muscle. Nevertheless, Smad7 very clearly prevents constitutive ActRII activation and obstructs active Smad2/3 signaling independent of tissue or activating ligand.
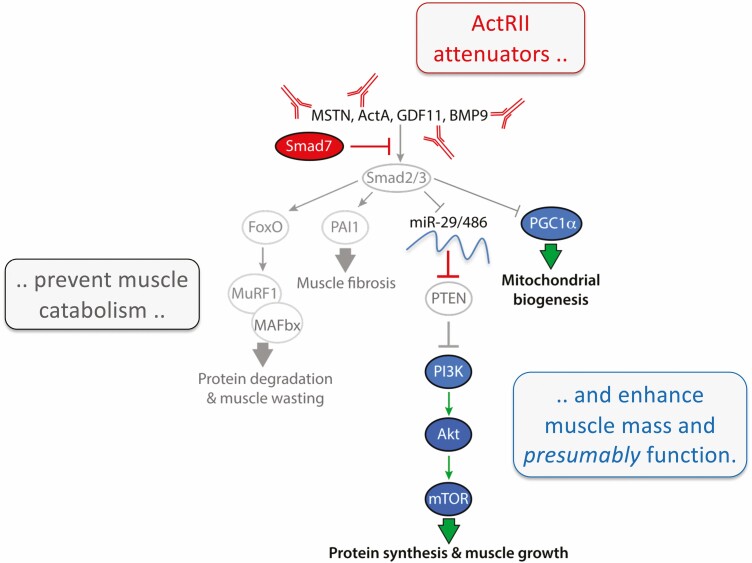
Pharmacological approaches to attenuating ActRII signaling have primarily focused on preventing receptor activation (Fig. 3B). They include the immunoneutralization of myostatin, the myostatin-LAP latent complex, or ActRIIa/b. Soluble receptor ligand traps that recognize multiple ActRII ligands and binding proteins with limited specificity for a subset of ligands have also been developed (1). Contrary to these extracellular approaches, Smad7’s pleiotropic antagonism has been exploited as a gene therapeutic (169, 191). Each approach has merit (discussed in the following section) and, although they may differ in specificity and efficacy, they all seek to prevent Smad2/3 activation of muscle wasting pathways. More specifically, those leading to altered protein synthesis:degradation rates, muscle fibrosis and reduced mitochondrial biogenesis (Fig. 3B).
Indeed, Smad2/3 signaling induces MuRF1 and MAFbx expression via FOXO1/3a-dependent and independent means and this stimulates muscle proteolysis (2). This is complemented by reduced expression of 2 microRNAs, miR29 and miR486, that inhibit translation of phosphatase and tensin homolog, which converts PIP3 to PIP2 and thereby suppresses Akt activation, induction of mTOR signaling and protein synthesis (166, 221). Smad2/3 signaling also induces plasminogen activator inhibitor (PAI)1 gene expression (166, 222), driving fibrosis, and suppresses peroxisome proliferator-activated receptor-γ coactivator (PGC)1α expression (223, 224), which compromises mitochondria biogenesis. Successfully attenuating ActRII-Smad2/3 signaling by any means would presumably increase muscle mass, prevent muscle wasting, and improve muscle function.
ActRII-induced marker gene expression
Many aspects of the ActRII signaling pathway, as well as its biological implications, are evident in the transcriptional changes induced by myostatin and GDF11. Moreover, such changes are remarkably similar for both ligands (61), indicating once again that they share similar if not identical roles in muscle. In mature human muscle cells, both myokines increase Smad7 and Smurf1 expression (Fig. 3C) and to a lesser degree that of Smurf2 (data not shown). The induction of Smad7 is particularly noteworthy as this iconic change serves as a positive control for recombinant viability and ensures that neither peptide is functioning as a dominant-negative (see previous). Myostatin and GDF11 also increase expression of MAFbx and to a lesser degree, TRIM32, another E3 ubiquitin ligase. These changes occurred in a temporal fashion matching the ligases known early and late activity, respectively (1). MAFbx not only directs ActRII degradation, but also the loss of eukaryotic translation initiation factor 3 subunit f (225, 226) whereas in parallel, TRIM32 targets desmin, α-actinin, filamentous actin, and plakoglobin, a desmosomal component required for PI3K activity (227, 228). These events together culminate in Z-line and sarcomere destruction as well as the suppressed protein synthesis that underscores muscle atrophy.
Both myostatin and GDF11 substantially downregulated PGC1α expression even at the lowest doses (Fig. 3C). These data contribute to the mounting evidence that ActRII ligands and Smad2/3 signaling suppress PGC1α and in turn, mitochondrial numbers and function (166, 224, 229-233). This occurs in both muscle and fat cells, whereas attenuating these signals can sometimes do the opposite. Fatty acid oxidation and expression of the controlling mitochondrial genes are elevated in tissues from mstn-/- mice and following treatment with a soluble ActRIIb ligand trap (233). Other markers of mitochondria function including NAD+ are restored when cachetic mice are similarly treated (232), although conflicting reports suggest that mitochondria numbers and function are compromised or unaffected in the absence of pathological insult (234, 235). Manfredi et al (231) recently demonstrated myostatin to suppress muscle mitochondria numbers and function via downregulation of G-protein receptor kinase 2. Thus, mitochondrial enhancement with ActRII attenuation is likely to be optimal under conditions and tissues where ActRII activation is elevated and pro-mitochondrial signaling (ie, PGC1α, G-protein receptor kinase 2) is suppressed.
In contrast to the PGC1α data is the substantial upregulation of PAI1, which is again linked to Smad2/3 signaling but not directly to myostatin or GDF11 (166). This key regulator of extracellular matrix remodeling is key to the development of muscle fibrosis and its upregulation is complemented by an even more dramatic upregulation of collagen type X α1. This highlights an important point: muscle cells as well as fibroblasts and fibro/adipogenic progenitor cells all contribute to muscle fibrosis. It further suggests that although ActRII ligands are well known to stimulate the latter cells directly (205, 222, 236, 237), attenuating ActRII signaling in all cell types has the potential to prevent muscle fibrosis with disease.
Cytokine signaling was also examined because it is an established driver of muscle atrophy and is known to induce myostatin expression (109-111, 116). Furthermore, attenuating these pathways, specifically Stat3, can reduce myostatin expression and prevent muscle wasting. Both myostatin and GDF11 were found to similarly increase IL-6 expression in reciprocal fashion (Fig. 3C), whereas Smad2/3 and Stat3 signaling are known to regularly crosstalk in different tissues (116). Thus, the 2 pathways likely cooperate to induce muscle wasting.
Temporal regulation of downstream effectors is evident as MAFbx appears to be an early-stage marker with differences detected at 8 hours but not at 24. Late-stage markers include the Smurfs, TRIM32, and IL-6, in which significant differences were primarily detected at 24 hours. Smad7, PAI1, and especially collagen type X α1 as well as the loss of PGC1α are likely excellent markers independent of time as the changes noted occurred early and were sustained. Further interrogation of this Gene Expression Omnibus record is warranted and could reveal additional markers of enhanced ActRII signaling or conversely, of signal attenuation. Indeed, such markers could prove invaluable in advancing preclinical drug development or even evaluating patient responses during clinical trials.
ActRII noncanonical signaling
Crosstalk between canonical TGF-β receptor signaling (ie, Smad) with non-Smad signaling pathways occurs in a context-specific manner (41, 47). Implicated pathways include those for Wnt, Notch, Hippo, Hedgehog, growth factors (ie, MAPKs and PI3K/Akt) and inflammatory cytokines (ie, NF-κB and Stats). Smad3-Stat3 interactions are archetypical for the entire system because they can be antagonistic or cooperative depending on the cell and tissue type as well as differentiation status (116). Although ActRII signaling has yet to be implicated in such a diverse array of pathways, context-dependent interactions in muscle include those with Stat3, the MAPKs, PI3K/Akt (discussed previously), and possibly other pathways (eg, NF-κB) that are beginning to be interrogated (114).
The cooperativity between Smad3 and Stat3 that occurs with TGF-β stimulation of nonmuscle cells has yet to be described for any ActRII ligand, although it is plausible considering the redundancy of action (eg, MuRF1 expression, muscle wasting) for both pathways. Muscle ActRII signaling and Stat3 are nevertheless interrelated through the induction of myostatin expression, which is mediated by Stat3 upregulation of the transcription factor C/EBPδ (97, 104, 109-111, 238, 239). By contrast, ActRIIa/b-Smad2/3 crosstalk with MAPK signaling is very well documented and involves all 3 of the serine/threonine kinases: extracellular signal-regulated kinase (Erk)1/2, p38, and c-Jun N-terminal kinase (Jnk).
In C2C12 cells, myostatin stimulates Erk1/2 phosphorylation via Ras activation. This suppresses myotube formation in differentiating cells (43) and inhibits Pax7 expression in proliferating cells (240). This latter effect is a requirement for differentiation (241-243) because myostatin downregulation of Pax7 attenuates self-renewal and, conversely, attenuating myostatin enriches the population of cells incapable of forming multinucleated myotubes, those that are Pax7+ and MyoD- (240). Studies with proliferating primary satellite cells from mice and sheep further establish the ActRIIa/b-Erk1/2-Pax7 link as disrupting ActRII ligand availability with Gasp-1 overexpression or reducing myostatin production with siRNA similarly increase Erk1/2 phosphorylation and reduce Pax7 expression (164, 244). These studies additionally suggest that p38 and possibly other aspects of growth factor signaling are also involved in Pax7 suppression.
The link between p38 activation, Pax7 downregulation, and muscle cell differentiation is very well-established in satellite cells and myoblast cell lines where it is initiated by the nonreceptor Src tyrosine kinase (245-249). In fact, different p38 isoforms are involved in every aspect of myogenesis with p38a (MAPK14) and p38b (MAPK11) initiating progenitor cell differentiation and fusion and phosphorylating substrate (eg, MAPKAPK2, EZH2, p18Hamlet, MSK1, MEF2, E47, BAF60c) that drives myogenic programming (250). That myostatin and activin both activate p38 in muscle progenitors (239, 251, 252) and fibroblasts (205) suggests that ActRII-p38 signaling is intrinsic to different cell types and more importantly, that myostatin, activin, and GDF11 stimulate muscle progenitor cell differentiation. This conflicts with the common misconception that ActRII signaling inhibits differentiation. The p38 inhibitor SB202190 suppresses activin-induced C/EBPb phosphorylation and expression of MAFbx, UBR2 (another E3 ubiquitin ligase), and LC3-II (an autophagosomal marker) (239). Furthermore, it prevents activin-induced muscle atrophy in vitro and in vivo, suggesting that p38 is not only fundamental to the principal actions of ActRII ligands in muscle, but that it is more canonical than non.
Myostatin has also been demonstrated to activate Jnk in proliferating and differentiating C2C12 cells (253, 254). SiRNA studies suggest that MAP3K7 (eg, TGF-β-activated kinase, TAK1) and MKK4 are involved and that the TAK1-MKK4-Jnk cascade is required for myostatin-induction of p21 and cell-cycle arrest. Myostatin additionally activates Jnk in fibroblasts (255) and human hepatic stellate cells (87), where it promotes a fibrotic phenotype. These studies clearly suggest that Jnk is potentially involved in ActRII signaling, although additional studies with primary satellite cells are needed to determine whether this cascade is uniquely activated in immortalized myoblasts or ubiquitous to muscle progenitors. They are also needed to identify the downstream targets and myogenic processes.
Disease Indications Targeted by ActRII Attenuators
General overview
As discussed previously, significant muscle wasting occurs in a number of diverse primary indications and diseases (1, 2), many of which are not always obvious. It is inherent to neuromuscular disease and occurs in most chronic disease states, yet it can also be disguised in obesity-related disorders. These include sarcopenic obesity and type 2 diabetes mellitus, where elevated serum levels of myostatin, TNF-α, IL-6, and other inflammatory cytokines drive muscle loss in the presence of heightened adiposity (256-262). Often commercially overlooked is muscle wasting with MSIs and in subjects exposed to prolonged spaceflight/microgravity because of reduced use or gravitational load (4, 263, 264). The military is particularly interested in addressing this problem as the muscle wasting caused by MSIs, not denervation, is the primary medical problem compromising military readiness (265-269). In fact, 90% of denervated muscles become reinnervated within a year, yet only 10% of muscle strength is ever permanently restored (270).
To date, 19 ActRII-attenuating drugs have been commercially developed for a muscle wasting disease indication (Table 1). This excludes drugs targeting non-muscle wasting conditions as, for example, Regeneron’s Garetosmab (REGN2477), an activin-A antibody for treating fibrodysplasia ossificans progressiva (271). Although a cursory review suggests the targeted indications reflect a diversity of diseases and conditions, a closer examination reveals a bias for neuromuscular diseases. Of the 45 clinical trials performed to date, 32 targeted a neuromuscular disease or were performed on healthy subjects in support of such programs (Table 2). This is contrasted by only 13 trials performed on other diseases/conditions. These include end-stage renal disease/chronic kidney disease, sarcopenia, cancer cachexia, chronic obstructive pulmonary disease, hip fracture/arthroplasty, and obesity/type 2 diabetes mellitus. Furthermore, only 2 drugs exclusively target a nonneuromuscular disease.
Table 1.
Myostatin/ActRII-attenuating drugs currently or previously in development
| Company | Drug name | Drug class | Mechanism of action |
|---|---|---|---|
| Acceleron Pharma | ACE-031/536/2494 | Ligand traps, ActRIIb- or follistatin-based | Sequester ActRII ligands in circulation or in extracellular environment |
| ACE-083 | |||
| FST288-Fc | |||
| ActRIIb:ALK4-Fc | |||
| ActRIIA-Fc | |||
| Atara Biotherapeutics | PINTA 745 (AMG 745) | α-myostatin peptibody | Sequester myostatin in circulation |
| AAVogen | AVGN7 (AAV6:SMAD7) | Smad7 gene therapy | Attenuate ActRIIa/b-Smad2/3 signaling regardless of ligand |
| Biogen (acquired 2 drugs from AliveGen) | BIIB101 (formerly ALG-801; plus -802) | ActRIIb ligand traps | Sequester ActRII ligands in circulation |
| Eli Lilly | Landogrozumab (LY2495655) | α-myostatin mAb | Sequester myostatin in circulation |
| Nationwide Children’s Hospital/Milo Biotechnology | AAV1:FS344 | Follistatin gene therapy | Sequester myostatin and activins in muscle extracellular space and in circulation |
| Novartis | Bimagrumab (BYM338) | α-ActRIIa/b mAb | Prevent binding of different ligands to ActRII receptors |
| Pfizer | Domagrozumab (PF-06252616) | α-myostatin mAb | Sequester myostatin in circulation |
| Regeneron | Trevogrumab (REGN1033, SAR391786) | α-myostatin mAb | Sequester myostatin in circulation |
| Roche | RO7239361 (RG6206, BMS-986089) | α-myostatin monobody/adnectin | Sequester myostatin in circulation |
| Scholar Rock | SRK-015 | α-latent myostatin mAb | Prevent latent myostatin from being activated |
| Wyeth (acquired by Pfizer) | Stamulumab (MYO-029) | α-myostatin mAb | Sequester myostatin in circulation |
α, anti; Act, activin; mAb, monoclonal antibody.
Table 2.
Status of myostatin/ActRII-attenuating drugs
| Drug, status | Identification | Stage | Participants, age, n | Results summary |
|---|---|---|---|---|
| ACE-031, suspended | NCT00755638 | P1 | Healthy, 45-75, 48 | Completed, no results posted |
| NCT00952887 | P1 | Healthy, 45-75, 70 | ||
| NCT01099761 | P2 | DMD, 4+, 35 | 39% TAEs, terminated | |
| ACE-083, suspended | NCT02257489 | P1 | Healthy, 45-75, 58 | No TAEs |
| NCT02927080 | P2 | FSHD, 18+, 58 | Muscle function unaffected | |
| NCT03943290 | P2 | FSHD and CMT, 18+, 150 | Terminated | |
| NCT03124459 | P2 | CMT, 18+, 42 | Terminated | |
| ACE-2494, suspended | NCT03478319 | P1 | Healthy, 45-75, 48 | Completed, no results |
| AAV1:FS344 | NCT01519349 | P1/2a | BMD, 18+, 6 | BMD: larger myofibers, possibly improved function (6MWT) |
| IBM, 18+, 9 | IBM: larger myofibers, improved function (6MWT) | |||
| NCT02354781 | P1/2 | DMD, 7, 3 | Completed, no DLTs. | |
| AVGN7 | PC | IBM | PoC complete, GLP/tox ongoing | |
| BIIB110 | No entry | ? | ||
| Bimagrumab (BYM338), unclear | NCT01423110 | P2 | IBM, 40-80, 14 | Same frequency of SAEs in treatment and placebo groups. Enhance LBM. No or minor effect on muscle function (6MWT, sIFA) |
| NCT02250443 | P2/3 | IBM, 40-75, 10 | ||
| NCT01925209 | P2/3 | IBM, 36-85, 251 | ||
| NCT02573467 | P3 | IBM, 36+, 211 | ||
| NCT01601600 | P2 | Sarcopenia, 65+, 40 | Increased measures of muscle mass, no or minor improvement of muscle function (eg, SPPB, 6MWT) | |
| NCT02333331 | P2 | Sarcopenia, 70+, 217 | ||
| NCT02468674 | P2 | Sarcopenia, 70+, 160 | ||
| NCT01669174 | P2 | COPD, 40-80, 67 | ||
| NCT01433263 | P2 | Cancer cachexia, 18+, 57 | Muscle mass and function unaffected | |
| NCT02152761 | P2 | Hip fracture, 60+, 251 | Increased LBM, function unaffected | |
| NCT03005288 | P2 | Obesity/T2DM, 18-75, 77 | Increased LBM, decreased fat mass, and improved insulin sensitivity | |
| Domagrozumab (PF-06252616), unclear | NCT01616277 | P1 | Healthy, 18-64, 86 | Completed, no results posted. Published (405) |
| NCT02841267 | P1/2 | LGMD2I, 18-99, 19 | No SAEs, no effect on multiple muscle function tests | |
| NCT02310763 | P2 | DMD, 6-15, 121 | No SAEs. Function unaffected | |
| NCT02907619 | P2 | DMD, 6-18, 59 | No efficacy. Terminated | |
| Landogrozumab (LY2495655), unclear | NCT01341470 | P1 | Healthy, 24-85, 47 | No SAEs |
| NCT01604408 | P2 | Older fallers, 75+, 201 | LBM preserved; muscle function improved (358) | |
| NCT01369511 | P2 | Hip arthroplasty, 50+, 400 | Small increase in LBM, muscle function unaffected (360) | |
| NCT01524224 | P1 | Advanced cancer, 18+, 29 | SAEs in 25%-67% of subjects in 5 highest dose groups (8 total) | |
| NCT01505530 | P2 | Pancreatic cancer, 18+, 125 | Muscle volume and function unaffected, survival concerns (359). Terminated | |
| PINTA 745 (AMG 745), suspended | No entry | P1 | Pancreatic cancer | Small LBM increase, no SAEs (364) |
| NCT01958970 | P1/2 | ESRD, 18-85, 51 | LBM unaffected | |
| NCT00975104 | P2 | Sarcopenia | Withdrawn | |
| RO7239361 (BMS-986089), suspended | NCT03100630 | P1 | Healthy | Completed, no results posted |
| NCT02515669 | P1/2 | DMD, 5-10, 43 | Failed preplanned futility analysis for efficacy. Terminated | |
| NCT03039686 | P2/3 | DMD, 6-11, 166 | Completed; no effect on muscle mass or strength | |
| SRK-015 | NCT03921528 | P2 | SMA, 2-21, 55 | Active, not recruiting |
| Stamulumab (MYO-029), suspended | NCT00563810 | P1 | Healthy, 18-80, 72 | Completed, no results posted |
| NCT00104078 | P1/2 | BMD/FSHD/LGMD, 18+, 108 | Completed, no results posted. Published (357) = SAEs same in treatment and placebo; no effect on muscle mass or strength | |
| Trevogrumab (REGN1033, SAR391786), unclear | NCT01507402 | P1 | Healthy, 18-85, 76 | Completed, no results posted |
| NCT01720576 | P1 | Healthy, 60+, 60 | ||
| NCT01910220 | P1 | Healthy, 60+, 125 | ||
| NCT02741739 | P1 | Healthy, 18-65, 28 | ||
| NCT02943239 | P1 | Healthy, 35-70, 82 | ||
| NCT01963598 | P2 | Sarcopenia, 70+, 253 | ||
| NCT03710941 | P2 | IBM | Withdrawn |
Data and results obtained from clinicaltrials.gov unless otherwise referenced.
Abbreviations: 6MWT, 6-minute walk test; BMD, Becker muscular dystrophy; CMT, Charcot-Marie Tooth disease; COPD, chronic obstructive pulmonary disease; DMD, Duchenne muscular dystrophy; ESRD, end-stage renal disease; FSHD, facioscapulohumeral muscular dystrophy; GLP/tox, good laboratory practices/toxicology; IBM, inclusion body myositis; LBM, lean body mass; LGMD, limb-girdle muscular dystrophy; P#, clinical trial phase; PC, preclinical; PoC, proof of concept; SAE, ; sIFA, sIBM physical functional assessment; SMA, spinal muscular atrophy; SPPB, short physical performance battery; SAE, serious adverse event; TAE, treatment-related adverse event; “suspended,” formally announced; T2DM, type 2 diabetes mellitus; “unclear”, not listed on corporate website’s pipeline
The most common disease indications targeted are DMD (and/or the less severe BMD) and IBM with 7 and 6 clinical trials, respectively (Table 2). In fact, 4 different drug programs have been developed for each of these indications compared with 6 for facioscapulohumeral muscular dystrophy, limb girdle muscular dystrophy, spinal muscular atrophy (SMA), and Charcot-Marie Tooth disease combined. Clinical testing, regardless of indication, has yielded inconsistent results (see the following section) with most, but not all, drugs passing safety requirements and meeting their preestablished anatomical endpoints, but not functional endpoints. Although several notable failures have been reported, the underlying causes have not been thoroughly explored and may have little to do with drug action, but with the choice of disease indication and the related pathologies. It is helpful, therefore, to examine the mechanisms of disease pathogenesis in the most commonly targeted indications: the muscular dystrophies and IBM.
Muscular dystrophies
The muscular dystrophies are genetic disorders arising from mutations in a variety of genes encoding striated muscle proteins. Many of these proteins stabilize muscle cell structures or the extracellular matrix (272) and include sarcolemmal and basement membrane proteins as well as their posttranslational modifying enzymes. Other implicated genes include those for nuclear membrane and endoplasmic reticulum proteins and not surprisingly, myofibrillar proteins. DMD is the most common form and results from point mutations, indels, and/or duplications within the largest protein-coding gene in the genome, dmd. Other disorders include Emery-Dreifuss muscular dystrophy, facioscapulohumeral muscular dystrophy, myotonic dystrophy, oculopharyngeal muscular dystrophy, 6 congenital muscular dystrophies, and 34 limb-girdle muscular dystrophies.
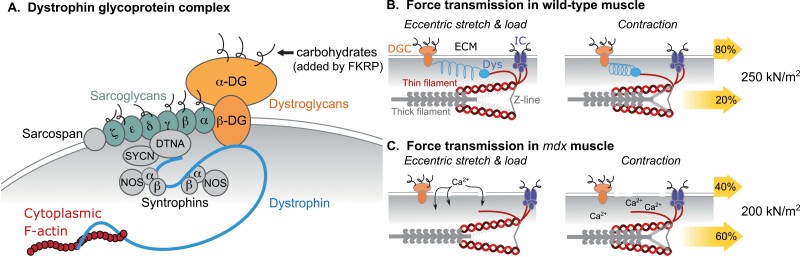
The dmd gene codes for the dystrophin protein, which links the myofiber contractile machinery to lateral components of the sarcolemma known as the costamere (Fig. 4A). This occurs primarily via noncovalent binding of the dystrophin amino terminal domain to F-actin and the carboxy terminal to proteins within the dystrophin-associated glycoprotein complex (DGC) (273, 274). In turn, F-actin is anchored to the extracellular matrix through the Z-line/integrin complex, whereas the DGC binds the matrix directly (Fig. 4B). The dystrophin interior contains 4 hinge domains and 24 spectrin-like repeats that together expand and compress with myofiber stretch and contraction, respectively, providing a means of force transfer within and between muscle fibers. The DGC also localizes ion channels and enzymes that regulate mechano-elicited Ca2+ release and the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS).
Figure 4.
Dystrophin/costamere functional relationship. The costamere is composed of 2 protein complexes: the dystrophin-associated glycoprotein complex (DGC) and the integrin complex (IC). (A) Structural components of the DGC using color-coded labels for individual proteins or protein classes (DG, dystroglycan; DTNA, dystrobrevin-a; FKRP, Fukutin-related protein; NOS, nitric oxide synthase; SYCN, syncoilin). Dystrophin binds filamentous (F)-actin that in turn binds Z-line components of sarcomeres, physically linking the contractile machinery to the costamere and the extracellular matrix. Anchoring the costamere to the basement membrane depends upon proper glycosylation of different proteins including α-dystroglycan and the sarcoglycans. (B, C) Model for longitudinal and lateral force transmission based on contractile studies of healthy and dystrophic (mdx) mice. Color-coded labels in panel B apply to all panels representing the eccentric (lengthening) contraction cycle (Dys, dystrophin; ECM, extracellular matrix). The percentage of total specific force (numbers on right) transmitted laterally or longitudinally are represented by upper and lower yellow arrows, respectively, in each panel.
Because the DGC and integrin complex anchor the sarcolemma to the extracellular matrix, the loss of dystrophin or its association with F-actin or the DGC impairs force transfer across the sarcolemma (Fig. 4B,C) (275, 276). The loss of dystrophin’s signaling role results in excess Ca2+ entry and dysregulated ROS and RNS, whereas repeated eccentric or lengthening contractions can further damage the sarcolemma and compromise excitation-contraction coupling. Thus, muscle dysfunction with DMD, in fact with many other muscular dystrophies as well, has many causes that are in fact inherent to the muscle cell itself. These include problems in total force generation, lateral force transfer, Ca2+ handling, and the production of ROS and RNS. Weakness and muscle loss is further exacerbated by the muscle fibrosis and necrosis that accumulate with chronic injury.
Inclusion body myositis
This rare disease is 1 of 5 inflammatory myopathies that also include dermatomyositis, polymyositis, necrotizing autoimmune myositis, and the most recently identified, anti-synthetase syndrome-overlap myositis (277). The entire group shares the common generalized features of endomysial inflammation that, from a pathogenic perspective, is both symptomatic and causative as well as elevated serum creatine kinase levels. Each myositis is distinguished, however, by unique patterns of muscle weakness, histological features, and by autoimmune markers that include different autoantibodies and immune cell infiltrates.
Although muscle wasting is common to all types of myositis, sporadic IBM should not be confused with the hereditary inclusion body myopathies, which are nonimmune disorders resulting from mutations in GNE, VCP, or MYH2 (278). Disease progression with IBM is slow yet constant and symptoms typically first occur in subjects 45 to 70 years old. (118). Unlike DMD, muscle wasting and physical impairment in IBM patients progressively develops in distinct muscle groups, most notably the finger and wrist flexors, knee extensors and ankle dorsiflexors. This suggests that locally administered therapeutics could significantly benefit IBM patients, although a systemic approach is still preferred as long-term systemic muscle wasting is common.
The earliest signs of impairment are usually associated with walking as IBM patients have difficulty climbing stairs and experience knee buckling because of knee extensor weakness and wasting of the quadriceps (1, 118, 279). Ankle dorsiflexion weakness also develops and can cause foot-drop, which increases the risk of fall-related injuries (eg, hip fracture) that further exacerbate muscle wasting (280, 281). Another early and iconic sign of IBM is impaired grip strength due to finger flexor weakness while muscles of the upper arm and shoulder as well as hip abductors and flexors also weaken with time. Dysphagia develops in the most serious cases and can cause mortality from nutritional deficits, systemic muscle wasting, and aspiration pneumonia (282-284).
The iconic namesake feature of the disease, inclusion bodies, is derived histologically (Fig. 5). These heterogenous protein aggregates are often contained within rimmed vacuoles that are sometimes absent in patients (~20%) and are not always present in every biopsy. The diagnostic criteria, therefore, are multifaceted and minimally require finger flexor or quadricep weakness, lymphocytic invasion (primarily CD8+ T cells) of nonnecrotic fibers and rimmed vacuoles (118, 279). T-cell invasion is mechanistically linked to muscle wasting as these and other immune cells secrete inflammatory cytokines that directly induce atrophy signals as well as myostatin expression (see previous). Central nuclei and fibrosis are also present, although not to the extent as that seen in biopsies of dystrophic muscle. Corticosteroids were traditionally administered to suppress the inflammatory response, but are no longer a recommended standard-of-care treatment because they also induce atrophy signals and myostatin expression, exacerbating the condition.
Rationale and challenges of targeting DMD, IBM, and other indications
Despite the high unmet clinical need in the muscular dystrophies, it is surprising that so many ActRII attenuators have been developed toward their treatment as abnormalities in muscle protein structure/function, not muscle wasting per se, underly the disease pathology. In this context, increasing muscle mass without addressing the genetic basis for disease pathology may not yield durable benefit. Indeed, several studies demonstrated the potential of ActRII attenuators to increase muscle mass in dystrophin-deficient animals (285-290), yet the persistent structural pathologies limited improvements in muscle function. Similar results were obtained in clinical trials that were largely unsuccessful (see previous section) with no improvements in muscle function or marginal improvements unlikely to satisfy FDA benchmarks. These results are not entirely discouraging, however, because they establish a proof of concept for the clinical approach. In fact, the advent of gene replacement, gene editing, or exon-skipping technologies to stabilize muscle, stop degeneration, and improve muscle quality—in essence to address the underlying functional pathology—provide a context for ActRII attenuators to fully rescue the accumulated loss of mass and strength.
The need for the “combinatorial approach” is epitomized by the fact that dystrophin-corrective or dystrophin-replacement therapy in animal models is only partially effective in restoring muscle function to normal wild-type levels (291, 292), whereas combinatorial approaches are clearly superior (293-296). Note that most assessments of these therapeutics were performed using young animals with relatively mild pathology. The few studies using older animals with advanced pathologies report only partial improvement as muscle function, regardless of metric, remains well below that of age-matched healthy controls (297-300). This also appears true for other muscular dystrophies (301) and suggests that ancillary approaches (eg, ActRII attenuators) are needed to enhance muscle mass and strength to overcome the functional deficit caused by years of muscle degeneration (eg, fibrosis, necrosis). Clinical trials of gene replacement and exon skipping technologies have consistently shown little to no improvement in functional outcomes (302, 303). Failing to reach threshold levels of dystrophin may or may not explain these results as Sarepta recently reported their microdystrophin gene therapeutic (SRP-9001) to restore dystrophin immunoreactivity to 55.4% of healthy control levels and that 70.5% of fibers were dystrophin-positive (304). These levels greatly exceed the predicted threshold of 20% to 30% expression and 50% of fibers (305), which begs the question, “Why not try a combinatorial?”
In contrast to DMD, where dystrophin deficiency impairs muscle force generation and destabilizes the sarcolemma, the myofibers of IBM patients are structurally intact and lack inherent functional abnormalities. Thus, there is no obvious reason why an ActRII-attenuating drug should not work in IBM patients. The indication is not without challenge, however, which is to counter the persistent inflammatory response that drives muscle wasting. Corticosteroids are potent anti-inflammatories, but they activate muscle catabolism (11) and for this reason are no longer used with IBM patients. By contrast, several studies suggest that attenuating ActRIIa/b, their ligands or their signaling can prevent muscle wasting in conditions with persistent inflammatory signaling like IBM (306), cancer cachexia (169, 307-312), or with direct cytokine challenge (169). This likely explains why so many ActRII attenuators have been developed to treat IBM. Such drugs include monoclonal antibodies, circulating ligand traps, and 2 gene therapeutics. The former 2 approaches would presumably need to be administered frequently as stopping treatment would restore inflammatory insult. The latter, by contrast, are durable by nature and appear optimally suited for treating chronic conditions like IBM. Overcoming the redundancy of signals from multiple ActRII ligands and from TGF-β activation of Smad2/3 signaling is an additional challenge, one that cannot be addressed by targeting single ligands. Thus, the most successful approaches would either need to attenuate multiple ligands, possibly non-ActRII ligands as well, or the shared intracellular signals.
Regulatory challenges present additional hurdles to development especially for nonneuromuscular disease indications. Sarcopenia, for example, lacks a universally recognized definition and method for diagnosing and, although these have been recently defined for cancer cachexia (313, 314), it is still considered a comorbidity of secondary importance. Drug approval by the FDA is granted for prolonging life, improving quality of life, or having a “clinically meaningful” outcome measure. Demonstrating an increase in overall survival is exceedingly difficult when treating comorbidities, so most muscle wasting drug programs pursue the latter 2 ambiguously defined criteria. Improvements in lean mass satisfy the quality-of-life criteria, but they must reflect comparable improvements in muscle function to be considered clinically meaningful. Furthermore, a functional scale must be validated in the specific disease indication and, for some, this information may not exist.
Drug Development Status
Drug classes
Nineteen ActRII-attenuating drugs have or are currently being developed (Table 1). These include 9 ligand traps, 6 monoclonal antibodies, 2 pepti-/monobodies, and 2 gene therapeutics. The most common mechanism of action is ligand sequestration, although both specific and nonspecific approaches are used to target myostatin or multiple ActRII ligands, respectively. All of these drugs act in the circulation and/or within the extracellular environment, providing opportunities for off-target interactions whose biological consequences depend upon ligand capacity and drug affinity. This includes a gene therapeutic that introduces the cDNA for a secreted follistatin isoform capable of binding the activins, myostatin, and, based on sequence conservation, GDF11.
Such multiplicity of targets is not inherently problematic because any of the multiple ActRII ligands are capable of stimulating muscle wasting. However, and this is potentially concerning, all of these ligands additionally act outside muscle and their antagonism, if not in a muscle-specific fashion, would presumably affect nonmuscle tissues even if the drug-ligand interaction is highly specific. The same logic applies to the lone α-ActRIIb monoclonal as the receptor is widely expressed in visceral organs, the central nervous system, reproductive tissues, striated muscle, and some endocrine glands (see www.proteinatlas.org, (315)). The only drug that does not attempt to disrupt ligand availability or ligand-receptor interactions is a muscle-specific Smad7 gene therapeutic designed to suppress ActRII signaling independent of the activating ligand. This drug’s seeming advantage is not without limits, however, as each attenuating approach has both strengths and weaknesses that could limit drug use to specific disease indications or general disease classes.
Ligand traps
Several circulating ligand traps have been developed by industry and academic programs (316-325). This approach accounts for ligand promiscuity by targeting multiple ActRII ligands and has proven very successful in stimulating muscle hypertrophy with local or systemic administration and in many different animal models of muscle wasting or neuromuscular disease. The approach is also a double-edged sword, however, because the lack of specificity produced serious safety concerns in an early clinical trial. This in turn inspired development of revised ligand traps with limited targets.
Acceleron has led the field with at least 4 ligand traps being tested in clinical trials (Table 2). ACE-083 is a modified 291 amino acid follistatin lacking 24 C-terminal residues (FS291) and is linked to the human IgG2 Fc domain (326, 327). Another similar drug, FST288-Fc, has also been tested in animals (328), although its developmental status is not described on the corporate website. All of the other traps are IgG chimeras with the extracellular domains (ECDs) of wild-type or modified ActRIIb, ActRIIa, or the ActRIIb:ALK4 complex (Table 1) and are based upon wild-type receptor ECD isoforms with differing affinities for different ligands (21). Mouse equivalents were generated for some of these molecules and are named with “RAP” instead of “ACE.”
The first receptor ligand trap was a soluble wild-type mouse ActRIIb ECD-Fc fusion that was recently demonstrated to prevent muscle and bone loss with prolonged spaceflight (319, 329), although the recombinant human and mouse equivalents, ACE-031 and RAP-031, have likely received more attention than any other drug in the class. They effectively prevent wasting and improve muscle function/strength in healthy animals (325, 330) and in models of DMD (331), amyotrophic lateral sclerosis (332), and hypoxia (333), while additionally increasing lean mass and insulin responsiveness in a model of diet-induced obesity (334). In clinical trials, ACE-031 increased lean mass and thigh muscle volume while also improving bone and fat metabolism in healthy middle age and elderly volunteers (335). However, a phase 2 trial with DMD boys was terminated prematurely (336) because of signs often seen in patients with hereditary hemorrhagic telangiectasia, a disease caused by mutations in endoglin or ALK-1 that impair TGF-β receptor signaling in endothelial cells (337).
Authors suggest that BMP9 attenuation was likely the cause as BMP9 maintains endothelial cells, stimulates angiogenesis, and signals via ALK-1, although BMP10 and even GDF11 have similar actions (338, 339). In fact, Acceleron’s most recent trap, ActRIIA-Fc, attenuates GDF11, myostatin, and activin action in endothelial cells (340). It also blocks arteriolar remodeling while stimulating vascular apoptosis in animal models of pulmonary hypertension. These surprising results suggest that GDF11, myostatin, and activin participate in blood vessel maintenance and in the pathogenesis of pulmonary hypertension. They also raise safety concerns for any ActRII attenuator that functions via ligand sequestration, particularly in the circulation. Such concerns may be misplaced with acute treatment protocols, but not with those requiring multiple treatments over the long term as, for example, when treating a chronic condition.
Acceleron has since conducted clinical trials of 3 ligand traps with vastly reduced or nonexistent affinities for BMP9. These include 2 modified ActRIIb ECDs, ACE-2494 and ACE-536 (Luspatercept), as well as ACE-083. Although a detailed description of ACE-2494 could not be found in the literature, ACE-536 combines the IgG1 Fc domain with ActRIIb residues 24 through 131 (322, 341). This ECD lacks 4 amino acids from the N-terminal, 3 from the C-terminal, and contains an L79D substitution, all of which limits preferential binding to myostatin, GDF11, and activin B, although it still binds BMP6 and BMP10. Structural modifications in ACE-083 increase its affinity for the extracellular matrix over wild-type follistatin and, hypothetically, this should retain ACE-083 in the locally administered environment where it is less likely to influence blood vessel integrity in nonmuscle tissues.
ACE-536 is being developed to treat chronic anemia (eg, thalassemia, myelodysplastic syndrome, myelofibrosis), not muscle wasting disease, and has already obtained an FDA approval on strong preclinical and clinical trial results (342, 343). By contrast, clinical development of both ACE-083 and ACE-2494 has been suspended, the former for not significantly enhancing metrics of muscle function or quality of life (326) and the latter because of antidrug antibodies among study participants. Such results are discouraging, especially to the neuromuscular disease community as ACE-083 studies with mdx and Trembler-J mice, models for DMD and Charcot-Marie Tooth disease, respectively, produced highly promising results (327). ACE-2492 and other ActRIIb ligand traps proved similarly beneficial in murine models of osteogenesis imperfecta, a disease characterized by fragile bones and muscle wasting, as they consistently increased several metrics of muscle and bone mass as well as the force generating capacity of different muscles (316, 324, 344, 345). In fact, ActRII ligands are well known to control osteogenesis and bone mineralization in addition to their effects on muscle, which explains the recent interest in using ActRII attenuators in treating bone disease (346, 347).
Immunoneutralizers
A popular alternative to sequestering multiple ActRII ligands with a trap is the specific immunoneutralization of myostatin, its LAP, or ActRIIa/b. Drugs in this class include 4 antimyostatin monoclonals that were tested in animal models of different muscular dystrophies, sarcopenia, and cancer cachexia (311, 348-351); a peptibody and monobody/adnectin tested in models of stroke, androgen deficiency, and chronic kidney disease (115, 352); an anti-ActRIIa/b monoclonal tested in models of bone healing, glucocorticoid excess, cancer cachexia, and cisplatin toxicity (309, 353, 354); as well as a myostatin LAP monoclonal tested in models of SMA and glucocorticoid excess (355, 356). This laundry list likely underestimates the actual composite of disease models tested in industrial laboratories; notwithstanding, each drug has been reported to increase muscle mass, prevent wasting to varying degrees, and enhance or partially restore some aspect of muscle function. These results, although impressive, have not yet translated to clinical success because only 1 immunoneutralizer (SRK-015) is still clearly in development (Table 2).
The first myostatin monoclonal tested in clinical trials, MYO-029/Stamulumab, originated with Wyeth and was acquired by Pfizer. It was tested in 2 clinical trials with healthy and dystrophic subjects and was well tolerated with no treatment-related serious adverse events. However, muscle mass and a multitude of function assessments were all unaffected by treatment (357) and drug development was officially suspended.
Similar results were obtained with other myostatin monoclonals despite being tested in a diversity of indications including disuse atrophy and cancer cachexia (Landogrozumab, Eli Lilly) (358-360) as well as DMD and limb girdle muscular dystrophy 2l (Domagrozumab) (361, 362). Each drug was also extensively tested in healthy subjects 18 to 85 years old where safety was not a concern, although muscle or lean body mass were at best minimally improved in trials with nonhealthy subjects, whereas metrics of muscle function were mostly unaffected (Table 2). Safety was also an issue for Landogrozumab because of a high frequency of serious adverse events and survival concerns in subjects with pancreatic cancer (359). Similar concerns were not evident in trials with older fallers (358) or subjects with hip arthroplasty (360), and deaths in subjects with pancreatic cancer were mostly disease-related (359).
The most extensively tested myostatin antibody is Trevogrumab (REGN1033, Regeneron), a fully humanized monoclonal with an IgG4 Fc domain (351). Antibodies in this class are divalent yet function as monovalents with often greater specificity and higher affinities, but lower inflammatory risk profiles and longer half-lives (363). In fact, Trevogrumab’s affinity for GDF11 is undetectable using surface plasmon resonance (351). The drug has been clinically tested with healthy subjects as a monotherapy and as a combinatorial with Garetosmab (REGN2477), an Act-A monoclonal, and was originally being developed for treating sarcopenia and IBM (Table 2). Regeneron announced in 2019, however, that a trial testing Trevogrumab alone and as the combinatorial in IBM subjects would be withdrawn. The sarcopenia trial has since been completed and although no results have been posted to clinicaltrial.gov, Trevogrumab is no longer listed as a pipeline drug and its development status is unclear.
The other 2 myostatin-targeted drugs in this class are antibody fusions. AMG 745 was originally developed by Amgen and was licensed to Atara Biotherapeutics, which changed the name to Pinta 745. It fuses a “myostatin-neutralizing” peptide to an N-terminal human IgG Fc domain and has a long circulating half-life (364). The proprietary peptide is not described in the literature and its exact nature of antagonism is not publicly known. It is also unknown whether it similarly neutralizes GDF11, although it does not affect Act-A (115). Separate clinical trials for androgen deprived pancreatic cancer and end-stage renal disease failed to demonstrate efficacy because although lean body mass was slightly elevated with treatment, this was likely attributed to a comparable drop in fat mass (364). These disappointing results likely contributed to a third trial for sarcopenia being withdrawn as well as suspension of the program (Table 2). This fate was mirrored by the other fusion, initially developed by Bristol-Meyer Squibb (BMS-986089), and licensed to Roche (R07239361 or RG6206). This adnectin combines the Fab domain of an antimyostatin monobody with a fibronectin type III domain (365) and binds both myostatin and GDF11 with sub-nM affinity (meeting abstract, (366)). Despite this potential advantage, a clinical trial failed to demonstrate efficacy with DMD boys, whereas a separate trial was terminated.
By far, the most extensively tested ActRIIa/b attenuator is Bimagrumab (BYM338, Novartis) with 1355 subjects participating in 11 clinical trials that include 3 phase 3 trials for IBM (Table 2). This fully humanized monoclonal neutralizes the ligand binding domains of both ActRIIa and ActRIIb, albeit with 50-fold higher affinity for the latter (367). It has also been tested in a wide variety of disease animal models where it enhanced muscle mass, prohibited muscle wasting, activated mTOR, and attenuated myostatin- and activin-induced Smad2/3 signaling while suppressing MuRF1/MAFbx expression (309, 353, 354). These studies primarily focused on changes in muscle mass with little attention to function. Indeed, only 1 of these studies assessed function (tetanic force in tibialis anterior muscles), and it was only marginally improved in a model of glucocorticoid excess, but not compared with the IgG control group (353).
The apparent prioritization of mass over functional metrics may have been a costly oversight as clinical testing of Bimagrumab consistently demonstrated an ability to improve muscle mass, but not function (Table 2). This was most evident in the 2 largest clinical trials to date for any ActRII attenuator, both of which included 251 subjects with either IBM (368-370) or hip fracture. The latter trial has yet to be published (see clinicaltrials.gov for results); nevertheless, Bimagrumab failed to improve objective measures of muscle function in the IBM trial that included 6-minute walk distance, isometric quadricep strength, and short physical performance battery tests (eg, gait speed, maintain balance, chair rise time, etc.). A significant difference in a self-reporting measure specifically designed for IBM subjects (sIFA, sporadic IBM physical functioning assessment) was detected in the highest dose group and as with short physical performance battery, a dose-treatment trend was reflected in a negative correlation between dose and P level. Such minimal efficacy was apparently insufficient to support further development as although Bimagrumab was later demonstrated to lower fat mass, improve insulin sensitivity, and restore glucose homeostasis in trials with obese insulin-resistant subjects or those with type 2 diabetes (329, 371, 372), it failed to improve muscle function and the drug is no longer listed on the corporate pipeline.
Possibly the most unique immunoneutralizing approach is that of SRK-015, which targets the myostatin latent complex bound to the muscle extracellular matrix (373). The monoclonal recognizes a LAP/“prodomain” epitope and induces a conformational change that decreases solvent accessibility, hinders protease cleavage, and prevents the release/activation of myostatin. In studies with murine models of SMA, SRK-015 had a modest yet significant effect on muscle mass and partially restored plantarflexor function (355). Significance was lost when torque measures were normalized to muscle mass, which from a practical perspective means little as the muscles were nevertheless stronger. Moreover, measures of cortical and trabecular bone growth were also enhanced. This effect likely resulted from increased physical bone load due to muscle hypertrophy as myostatin is not produced in bone. It is also highly encouraging as SMA is distinguished from many other muscle wasting diseases by a brittle bone phenotype because and mineral density (374).
Gene therapeutics
The 2 ActRII-attenuating gene therapeutics use very different approaches. The first was a serotype 1 capsid adeno-associated virus carrying a cDNA expression cassette for a follistatin isoform (AAV1:FS344) (375) and was developed at Nationwide Children’s Hospital before being out-licensed to Milo Biotechnology. Recombinant AAVs are nonpathogenic, nonreplicative, and do not generally activate a significant immune response (376). They also very rarely integrate payload genes into host cell genomes and are related to adenoviruses by name only. The AAV1 capsid is ubiquitously trophic to many tissues, whereas the promoter (cytomegalovirus [CMV]) has a similarly diverse activity profile (377). This combination could limit drug use to only local routes of administration because the combination of systemic delivery with the circulating profile of the secreted follistatin isoform raises concerns of off-target effects.
The FS344 “payload gene” codes for a particular isoform that is transcriptionally processed to produce FS315, which contains a 27 amino acid C-terminal extension compared with FS288. The latter, as well as Acceleron’s FST288-Fc, strongly bind heparin and heparan sulfate, thereby associating with the extracellular matrix (378, 379). The extension in FS315, however, interferes with this binding and has been reported to also influence activin binding, although the degree is somewhat controversial.
Initial studies comparing 6 different follistatin isoforms, including FS288 and FS315, used radioligand binding assays and reported nearly identical affinities of 540 to 680 pM (379, 380). The same group later used surface plasmon resonance and reported affinities of 46 and 432 pM for FS288 and FS315, respectively (381). This 10-fold difference is likely an overestimate and is inconsistent with their reporting a 2-fold difference in luciferase reporter and radioreceptor crosslinking assays. It also may be due to steric hindrance of activin binding sites because activin was immobilized to the chip via its N-terminus and crystal structure studies of Act-A bound to FS288 or FS315 indicate this region is important to complex formation (382).
Because FS315 does not associate with proteoglycans, it enters the circulation even when AAV1:FS344 is administered locally (375). Thus, its reduced affinity for ActRII ligands other than myostatin appears to prevent nonspecific off-target effects. This was demonstrated in clinical and preclinical studies by quantifying circulating levels FS315, which were elevated, whereas those of gonadotropins and reproductive steroids were unaffected (306, 383, 384). Preclinical studies with nonhuman primates (cynomolgus macaques) and intramuscular delivery of AAV1:FS344 further reported increases in muscle mass (3% in legs of controls vs 21% in treated) and quadricep force (26% twitch and 12% tetanic forces over contralateral control legs, n = 1) (384). This drug was then tested in clinical trials with BMD and IBM patients where metrics of muscle mass and structure improved with treatment (eg, increased fiber size distribution, less fibrosis) as did performance in the 6-minute walk test (306, 383).
Although these results are encouraging, the trials were understandably underpowered as the primary outcomes were safety and not efficacy. Thus, some data were expressed qualitatively or were highly variable. These and other concerns over study design, blinding, and data normalization in the IBM study were raised in a letter to the editor (385) and have been addressed by the study director (386). Whether these concerns have influenced the status of future trials is unclear as none to date has been announced.
The second gene therapeutic in the space is a serotype 6 AAV carrying a Smad7 cDNA expression construct (AAV6:Smad7 or AVGN7). The original drug incorporated the ubiquitously active CMV promoter, although the current derivative incorporates a muscle-specific enhancer constructed from the creatine kinase promoter. AVGN7 was developed through a collaboration between faculty at the Washington State University and the Baker Heart and Diabetes Institute. The inventors include one of the authors herein (B.D.R.), who is also the founder and chief executive officer of AAVogen as well as Paul Gregorevic, who is now affiliated with the University of Melbourne.
AVGN7 was designed to specifically address the shortcomings and concerns of previously developed ActRII attenuators; namely the lack of muscle specificity, the potential for off-target effects, and the redundancy of signals. The AAV6 vector itself has high tropism for striated muscle, can be titered to limit transduction to these tissues, and displays rapid transgene expression kinetics (377). It is also superior to the other “muscle trophic” AAVs (ie, serotypes 8 and 9) in primate hearts (387). The use of a muscle-specific promoter provides further control and is the second level of protection against off-target effects. The third is the payload gene itself, Smad7, which has only intracellular targets. In contrast to ligand-specific approaches that include antibodies and traps, AVGN7 attenuates ActRII signaling regardless of the activating ligand and can potentially antagonize other catabolic ligands that cross-talk with Smad2/3. This approach would presumably address the challenge of signal redundancy as well or better than ligand traps or ActRII immunoneutralization yet without the comparable off-target risks.
Two preclinical studies with AVGN7 suggest it is broadly effective in stimulating skeletal muscle hypertrophy, increasing muscle function, and enhancing exercise capacity (169, 191). Dose-dependent efficacy was demonstrated with local and systemic administration while changes in strength (twitch force) were proportional to those in mass and were correlated to an increase in the number and size of type II fibers. Most importantly, muscle wasting was prevented in different models of cancer cachexia and even in cytokine challenge models resembling chronic inflammation. This includes mice overexpressing myostatin or Act-A or in the cancer models, conditions with greatly elevated levels of circulating IL-6 and muscle signaling. These data suggest that AVGN7 has the potential to treat a variety of muscle wasting disease states, especially those where muscle inflammation is the primary insult.
Blood vessel integrity was normal in all tissues sampled and Smad7 was only overexpressed in striated muscle. Muscle specificity was further demonstrated by quantifying expression of Smad2/3 target genes that again were only affected in striated muscle. Mechanistically, AVGN7 prevented Smad3 phosphorylation and down-regulated FOXO1/3, MuRF1 and MAFbx. This increased protein synthesis rates while inhibiting protein degradation, actions fundamental to the prevention of muscle wasting.
AVGN7 also increased heart mass, slightly but significantly, in healthy mice and prevented the cardiac cachexia that commonly occurs with cancer (169, 191). This effect has also been documented with an ActRIIb ligand trap (312) and is particularly noteworthy as cardiac cachexia/atrophy occurs with many muscle wasting diseases (388, 389), DMD, and heart failure, where cardiac and respiratory depression is the primary cause for mortality. Simultaneously addressing cardiac and skeletal muscle wasting, therefore, is critically important.
Cardiac hypertrophy develops from physiological and pathological conditions that have distinctly different consequences and regulatory signals (390). “Physiological hypertrophy” is a highly adaptive response to exercise and is unquestionably good. It is also driven by IGF1 and receptor tyrosine kinase signaling. “Pathological hypertrophy,” by contrast, is highly maladaptive, occurs with congestive heart failure, myocardial infarction, and uncontrolled hypertension, for example, and is driven by G-protein-coupled receptor/stress signaling. Evidence strongly suggests that attenuating myostatin induces beneficial physiological cardiac hypertrophy as cardiac function, cardiomyocyte contractility, cardiac progenitor cell pools, Ca2+ handling, excitation-contraction coupling, and respiratory performance during high-intensity exercise are all enhanced in myostatin null mice and/or when treating with AVGN7 or other ActRII attenuators (189-192, 391, 392). ActRIIb ligand traps additionally preserve cardiac function and prevent development of pathological hypertrophy in models of myocardial infarction with ischemic injury, age-related heart failure, and left ventricular overload (188, 393, 394).
These data strongly suggest that attenuating ActRII signaling in the heart, with AVGN7 or any other ActRII attenuator, could enhance cardiac function by producing physiological hypertrophy. This in turn has the potential to treat cardiac cachexia and cardiovascular disease separately or to simultaneously treat the wasting of both skeletal and cardiac muscle. AVGN7 is being developed to treat IBM, other inflammatory myopathies, and DMD, all chronic diseases with cardiac involvement (395) that could benefit from the durability of a gene therapy approach like AVGN7.
Reason for Optimism ... Really!
The proverbial glass is indeed half full. Despite the failure of most clinical trials to meet functional endpoints, nearly all successfully met their anatomical endpoints of enhanced muscle mass or attenuated markers of degeneration. This alone has clear clinical benefits especially in treating obesity-related disorders (371, 372, 396). The generally good safety record as well as the success of some trials, however limited, in meeting functional endpoints is indeed encouraging and together beg the question, “What if?”
What if disease indications were more carefully chosen?
The majority of trials were performed with dystrophic subjects, those with underlying functional abnormalities inherent to muscle. Enhancing muscle mass per se was not expected to correct such defects, but to partially compensate by adding mass to dystrophic muscle: a dubious concept at best. In fact, trials with nondystrophic subjects or those with mild dystrophic phenotypes were more apt to meet functional endpoints. These include trials with AAV1:FS344 and BMD or IBM subjects, Bimagrumab with IBM or sarcopenic subjects, and Landogrozumab with old fallers (Table 2). In addition, most trials were performed for chronic disease indications, yet tested drugs requiring highly frequent administrations. Such mismatch reflects a misunderstanding of muscle’s “use it or lose it” nature as gains in mass disappear when stimulants are removed. Testing durable drugs (eg, gene therapeutics) with chronic disease indications (eg, muscular dystrophies, IBM) and transient drugs (eg, immunoneutralizers) with acute indications (eg, cancer cachexia, musculoskeletal injuries) could potentially yield more successes.
What if dose or treatment regimens could be optimized to compensate for pharmacokinetic challenges or off-target effects?
Many of these drugs bind multiple ActRII ligands either by design or from sequence conservation among ligands. Increasing doses beyond those already tested could saturate intended targets and begin cross-reacting with unintended targets, which risks producing off-target effects. By contrast, optimizing dose frequency or duration could potentially augment efficacy without undue risk. This approach may not work with drugs that are rapidly cleared (397), but is particularly attractive for those having potentially long half-lives (351, 364).
What if clinical trials were designed to equate functional with mass improvements?
Simultaneous changes in muscle mass and function more readily occur when load is applied to the muscle. This occurs with exercise, for example, which builds both muscle and neural components of strength. Exercise increases motor unit recruitment, synchronization, and rate coding while inhibiting peripheral feedback inhibition (398). It was also critical to the clinical development of the only approved antimuscle wasting drug, Serostim (399, 400), to maximizing anabolic steroid use (401) and to the AAV1:FS344 trials that met functional endpoints (306, 383, 402). Thus, it is extremely surprising that so few clinical trials of ActRII attenuators incorporated exercise components especially as exercise has the added benefit of stimulating muscle amino acid uptake and protein synthesis. Many of these trials, in fact many clinical trials for muscle diseases in general, used grip strength as an outcome measure. This particular metric is difficult to control and, in elderly patients, may better reflect peripheral neuromotor function rather than muscle function (403) and should probably be avoided in trials with aging subjects.
What if new drug development programs learned from the experiences of “first-mover” drugs?
First movers to any market have several advantages, but frequently succumb to technological challenges. This fundamental rule of business perfectly applies to the ActRII attenuator market as technological challenges are arguably responsible for the clinical failures. Ligand trap designs, for example, can produce off-target effects by attenuating multiple targets in multiple tissues. Circulating immunoneutralizers either cannot compensate for the redundancy of signals, due to target specificity, or they again risk producing tissue-wide off-target effects because of the ubiquitous distribution of ActRII receptors. The more recently developed drugs, however, appear to be designed to address these challenges. SRK-015 is largely muscle specific by the fact that myostatin is primarily produced in muscle and because it targets a unique LAP epitope. AVGN7 was designed for even more muscle specificity and to attenuate multiple signals, thus addressing the issue of signal redundancy as well. Although AAV1:FS344 technically lacks muscle specificity, it primarily targets locally produced myostatin when administered locally.
Answering these “what if” questions and addressing the challenges of first-mover drugs will no doubt benefit development of SRK-015, AAV1:FS344, and AVGN7. These answers could also help refine programs currently on hold or even repurpose drugs for different indications, a point perfectly illustrated by Bimagrumab’s success with obese and diabetic subjects (371, 372). Resolving the most significant challenges of tissue specificity and ligand redundancy is likely key to overall field success, although opportunities arising from the parallel and emerging markets with neuromuscular disease drugs should not be overlooked. Indeed, combinatorial approaches have a greater potential to restore muscle function in dystrophic animals (293-296) and more recently, in SMA mice (404). This latter study combined an antisense morpholino to restore spinal motor neuron 1 expression and a gene therapeutic to express myostatin’s LAP domain. This approach not only enhanced motor function beyond that achieved by morpholino treatment alone, but also the neural circuits that influence strength development (eg, innervation, neuromuscular junctions, synapse formation, sensory neuron size). Thus, a combinatorial approach featuring a gene replacement, correcting or editing therapeutic, an ActRII attenuator, and an exercise program to stabilize muscle and prevent degeneration, enhance muscle mass, and improve muscle function, respectively, could ultimately and finally fill the glass full.
Glossary
Abbreviations
- Act
activin
- ALP
alkaline phosphatase
- ALS
acid labile subunit
- BMD
Becker muscular dystrophy
- BMP
bone morphogenic protein
- C/EBP
CCAAT/enhancer binding protein
- DGC
dystrophin-associated glycoprotein complex
- DMD
Duchenne muscular dystrophy
- ECD
extracellular domain
- Erk
extracellular signal-regulated kinase
- FDA
US Food and Drug Administration
- FKBP
FK506 binding protein
- GDF
growth/differentiating factor
- IBM
inclusion body myositis
- IGFBP
IGF binding protein
- Jnk
c-Jun N-terminal kinase
- LAP
latent associated protein
- MAPK
mitogen-activated protein kinase
- MSI
musculoskeletal injury
- NF-κB
nuclear factor κB
- PAI
plasminogen activator inhibitor
- PGC
proliferator-activated receptor-γ coactivator
- PI3K
phosphoinositide 3-kinas
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SBE
Smad3/4 binding element
- SMA
spinal muscular atrophy
- SMURF
Smad ubiquitination regulatory factor
Acknowledgments
Financial Support: Writing of this review was supported by National Institutes of Health grants from the National Cancer Institute (R44CA221539 to B.D.R.) and the National Institute for Arthritis and Musculoskeletal and Skin diseases (R43AR075438 to B.D.R.; R01AR071618 to C.W.W.).
Author Contributions: B.D.R. and C.W.W. are solely responsible for the preparation and writing of this review.
Additional Information
Disclosures: B.D.R. is the founder, chief executive officer, and majority stock owner of AAVogen, Inc, which develops AVGN7 as discussed in this review.
References
- 1. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14(1):58-74. [DOI] [PubMed] [Google Scholar]
- 2. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307(6):E469-E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rochira V, Guaraldi G. Growth hormone deficiency and human immunodeficiency virus. Best Pract Res Clin Endocrinol Metab. 2017;31(1):91-111. [DOI] [PubMed] [Google Scholar]
- 4. Bettis T, Kim BJ, Hamrick MW. Impact of muscle atrophy on bone metabolism and bone strength: implications for muscle-bone crosstalk with aging and disuse. Osteoporos Int. 2018;29(8):1713-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howard EE, Pasiakos SM, Fussell MA, Rodriguez NR. Skeletal muscle disuse atrophy and the rehabilitative role of protein in recovery from musculoskeletal injury. Adv Nutr. 2020;11(4):989-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedrich O, Reid MB, Van den Berghe G, et al. The sick and the weak: neuropathies/myopathies in the critically Ill. Physiol Rev. 2015;95(3):1025-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fearon KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44(8):1124-1132. [DOI] [PubMed] [Google Scholar]
- 8. Fearon K, Arends J, Baracos V. Understanding the mechanisms and treatment options in cancer cachexia. Nat Rev Clin Oncol. 2013;10(2):90-99. [DOI] [PubMed] [Google Scholar]
- 9. Cruz-Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13(1):1-7. [DOI] [PubMed] [Google Scholar]
- 10. Zhang P, Chen X, Fan M. Signaling mechanisms involved in disuse muscle atrophy. Med Hypotheses. 2007;69(2):310-321. [DOI] [PubMed] [Google Scholar]
- 11. Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013;45(10):2163-2172. [DOI] [PubMed] [Google Scholar]
- 12. Zhou J, Liu B, Liang C, Li Y, Song YH. Cytokine signaling in skeletal muscle wasting. Trends Endocrinol Metab. 2016;27(5):335-347. [DOI] [PubMed] [Google Scholar]
- 13. Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol. 1987;133(3):567-572. [DOI] [PubMed] [Google Scholar]
- 14. Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138(2):311-315. [DOI] [PubMed] [Google Scholar]
- 15. Cheifetz S, Like B, Massagué J. Cellular distribution of type I and type II receptors for transforming growth factor-beta. J Biol Chem. 1986;261(21):9972-9978. [PubMed] [Google Scholar]
- 16. Massagué J, Cheifetz S, Endo T, Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986;83(21):8206-8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83-90. [DOI] [PubMed] [Google Scholar]
- 18. Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev. 2008;29(5):513-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sartori R, Gregorevic P, Sandri M. TGFβ and BMP signaling in skeletal muscle: potential significance for muscle-related disease. Trends Endocrinol Metab. 2014;25(9):464-471. [DOI] [PubMed] [Google Scholar]
- 20. Aykul S, Martinez-Hackert E. Transforming growth factor-β family ligands can function as antagonists by competing for type II receptor binding. J Biol Chem. 2016;291(20):10792-10804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sako D, Grinberg AV, Liu J, et al. Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIb. J Biol Chem. 2010;285(27):21037-21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Souza TA, Chen X, Guo Y, et al. Proteomic identification and functional validation of activins and bone morphogenetic protein 11 as candidate novel muscle mass regulators. Mol Endocrinol. 2008;22(12):2689-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinck AP, Mueller TD, Springer TA. Structural biology and evolution of the TGF-beta family. Cold Spring Harb Perspect Biol. 2016;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suga H, Ono K, Miyata T. Multiple TGF-beta receptor related genes in sponge and ancient gene duplications before the parazoan-eumetazoan split. FEBS Lett. 1999;453(3):346-350. [DOI] [PubMed] [Google Scholar]
- 25. Feiner N, Begemann G, Renz AJ, Meyer A, Kuraku S. The origin of bmp16, a novel Bmp2/4 relative, retained in teleost fish genomes. BMC Evol Biol. 2009;9:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mueller TD, Nickel J. Promiscuity and specificity in BMP receptor activation. FEBS Lett. 2012;586(14):1846-1859. [DOI] [PubMed] [Google Scholar]
- 27. Rifkin DB, Rifkin WJ, Zilberberg L. LTBPs in biology and medicine: LTBP diseases. Matrix Biol. 2018;71-72:90-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee SJ. Extracellular regulation of myostatin: a molecular rheostat for muscle mass. Immunol Endocr Metab Agents Med Chem. 2010;10:183-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suh J, Lee YS. Myostatin inhibitors: panacea or predicament for musculoskeletal disorders? J Bone Metab. 2020;27(3):151-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cotton TR, Fischer G, Wang X, et al. Structure of the human myostatin precursor and determinants of growth factor latency. Embo J. 2018;37(3):367-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson SB, Goldberg AL, Whitman M. Identification of a novel pool of extracellular pro-myostatin in skeletal muscle. J Biol Chem. 2008;283(11):7027-7035. [DOI] [PubMed] [Google Scholar]
- 32. Miura T, Kishioka Y, Wakamatsu J, et al. Decorin binds myostatin and modulates its activity to muscle cells. Biochem Biophys Res Commun. 2006;340(2):675-680. [DOI] [PubMed] [Google Scholar]
- 33. Lamar KM, Bogdanovich S, Gardner BB, et al. Overexpression of latent TGFβ binding protein 4 in muscle ameliorates muscular dystrophy through myostatin and TGFβ. Plos Genet. 2016;12(5):e1006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McCoy JC, Walker RG, Murray NH, Thompson TB. Crystal structure of the WFIKKN2 follistatin domain reveals insight into how it inhibits growth differentiation factor 8 (GDF8) and GDF11. J Biol Chem. 2019;294(16):6333-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walker RG, McCoy JC, Czepnik M, et al. Molecular characterization of latent GDF8 reveals mechanisms of activation. Proc Natl Acad Sci U S A. 2018;115(5):E866-E875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walker RG, Czepnik M, Goebel EJ, et al. Structural basis for potency differences between GDF8 and GDF11. BMC Biol. 2017;15(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sakamoto K, Kanematsu-Yamaki Y, Kamada Y, et al. Identification of ligand-selective peptidic ActRIIB-antagonists using phage display technology. Biochem Biophys Rep. 2017;11:33-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Townson SA, Martinez-Hackert E, Greppi C, et al. Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J Biol Chem. 2012;287(33):27313-27325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martinez-Hackert E, Sundan A, Holien T. Receptor binding competition: a paradigm for regulating TGF-beta family action. Cytokine Growth Factor Rev. 2021;57:39-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goebel EJ, Corpina RA, Hinck CS, et al. Structural characterization of an activin class ternary receptor complex reveals a third paradigm for receptor specificity. Proc Natl Acad Sci U S A. 2019;116(31):15505-15513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Derynck R, Budi EH. Specificity, versatility, and control of TGF-beta family signaling. Sci Signal. 2019;12(570): 1-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fabregat A, Sidiropoulos K, Viteri G, et al. Reactome diagram viewer: data structures and strategies to boost performance. Bioinformatics. 2018;34(7):1208-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang W, Chen Y, Zhang Y, Wang X, Yang N, Zhu D. Extracellular signal-regulated kinase ½ mitogen-activated protein kinase pathway is involved in myostatin-regulated differentiation repression. Cancer Res. 2006;66(3):1320-1326. [DOI] [PubMed] [Google Scholar]
- 44. Carlson CJ, Booth FW, Gordon SE. Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol. 1999;277(2 Pt 2):R601-R606. [DOI] [PubMed] [Google Scholar]
- 45. Lee MK, Pardoux C, Hall MC, et al. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. Embo J. 2007;26(17):3957-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miyazawa K, Miyazono K. Regulation of TGF-beta family signaling by inhibitory smads. Cold Spring Harb Perspect Biol. 2017;9(3):a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luo K. Signaling cross talk between TGF-beta/smad and other signaling pathways. Cold Spring Harb Perspect Biol. 2017;9(1):a022137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goodman CA. Role of mTORC1 in mechanically induced increases in translation and skeletal muscle mass. J Appl Physiol (1985). 2019;127(2):581-590. [DOI] [PubMed] [Google Scholar]
- 49. Egner IM, Bruusgaard JC, Gundersen K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development. 2016;143(16):2898-2906. [DOI] [PubMed] [Google Scholar]
- 50. Conceição MS, Vechin FC, Lixandrão M, et al. Muscle fiber hypertrophy and myonuclei addition: a systematic review and meta-analysis. Med Sci Sports Exerc. 2018;50(7):1385-1393. [DOI] [PubMed] [Google Scholar]
- 51. Blaveri K, Heslop L, Yu DS, et al. Patterns of repair of dystrophic mouse muscle: studies on isolated fibers. Dev Dyn. 1999;216(3):244-256. [DOI] [PubMed] [Google Scholar]
- 52. Folker ES, Baylies MK. Nuclear positioning in muscle development and disease. Front Physiol. 2013;4:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Winbanks CE, Chen JL, Qian H, et al. The bone morphogenetic protein axis is a positive regulator of skeletal muscle mass. J Cell Biol. 2013;203(2):345-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sartori R, Schirwis E, Blaauw B, et al. BMP signaling controls muscle mass. Nat Genet. 2013;45(11):1309-1318. [DOI] [PubMed] [Google Scholar]
- 55. Chen JL, Walton KL, Hagg A, et al. Specific targeting of TGF-β family ligands demonstrates distinct roles in the regulation of muscle mass in health and disease. Proc Natl Acad Sci U S A. 2017;114(26):E5266-E5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ono Y, Calhabeu F, Morgan JE, Katagiri T, Amthor H, Zammit PS. BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Differ. 2011;18(2):222-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aoyama K, Yamane A, Suga T, Suzuki E, Fukui T, Nakamura Y. Bone morphogenetic protein-2 functions as a negative regulator in the differentiation of myoblasts, but not as an inducer for the formations of cartilage and bone in mouse embryonic tongue. BMC Dev Biol. 2011;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol. 2010;346:267-278. [DOI] [PubMed] [Google Scholar]
- 59. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. Febs J. 2013;280(17):4294-4314. [DOI] [PubMed] [Google Scholar]
- 60. Zimmers TA, Jiang Y, Wang M, et al. Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic Res Cardiol. 2017;112(4):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Egerman MA, Cadena SM, Gilbert JA, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22(1):164-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Czaja W, Nakamura YK, Li N, et al. Myostatin regulates pituitary development and hepatic IGF1. Am J Physiol Endocrinol Metab. 2019;316(6):E1036-E1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garikipati DK, Rodgers BD. Myostatin stimulates myosatellite cell differentiation in a novel model system: evidence for gene subfunctionalization. Am J Physiol Regul Integr Comp Physiol. 2012;302(9):R1059-R1066. [DOI] [PubMed] [Google Scholar]
- 64. Garikipati DK, Rodgers BD. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation: evidence for endocrine-regulated transcript processing. J Endocrinol. 2012;215(1):177-187. [DOI] [PubMed] [Google Scholar]
- 65. Lokireddy S, Mouly V, Butler-Browne G, et al. Myostatin promotes the wasting of human myoblast cultures through promoting ubiquitin-proteasome pathway-mediated loss of sarcomeric proteins. Am J Physiol Cell Physiol. 2011;301(6):C1316-C1324. [DOI] [PubMed] [Google Scholar]
- 66. McFarlane C, Plummer E, Thomas M, et al. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol. 2006;209(2):501-514. [DOI] [PubMed] [Google Scholar]
- 67. Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296(6):C1258-C1270. [DOI] [PubMed] [Google Scholar]
- 68. Morissette MR, Cook SA, Buranasombati C, Rosenberg MA, Rosenzweig A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am J Physiol Cell Physiol. 2009;297(5):C1124-C1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224(1):7-16. [DOI] [PubMed] [Google Scholar]
- 70. Williams NG, Interlichia JP, Jackson MF, Hwang D, Cohen P, Rodgers BD. Endocrine actions of myostatin: systemic regulation of the IGF and IGF binding protein axis. Endocrinology. 2011;152(1):172-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. 2008;154(3):557-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Climent V, Marín F, Picó A. Pharmacologic therapy in growth hormone disorders and the heart. Curr Med Chem. 2007;14(13):1399-1407. [DOI] [PubMed] [Google Scholar]
- 73. Colao A. The GH-IGF-I axis and the cardiovascular system: clinical implications. Clin Endocrinol (Oxf). 2008;69(3):347-358. [DOI] [PubMed] [Google Scholar]
- 74. Hill DJ, Freemark M, Strain AJ, Handwerger S, Milner RD. Placental lactogen and growth hormone receptors in human fetal tissues: relationship to fetal plasma human placental lactogen concentrations and fetal growth. J Clin Endocrinol Metab. 1988;66(6):1283-1290. [DOI] [PubMed] [Google Scholar]
- 75. Frick GP, Tai LR, Baumbach WR, Goodman HM. Tissue distribution, turnover, and glycosylation of the long and short growth hormone receptor isoforms in rat tissues. Endocrinology. 1998;139(6):2824-2830. [DOI] [PubMed] [Google Scholar]
- 76. Vijayakumar A, Buffin NJ, Gallagher EJ, et al. Deletion of growth hormone receptors in postnatal skeletal muscle of male mice does not alter muscle mass and response to pathological injury. Endocrinology. 2013;154(10):3776-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. List EO, Berryman DE, Funk K, et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yakar S, Rosen CJ, Beamer WG, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110(6):771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu JL, Yakar S, LeRoith D. Mice deficient in liver production of insulin-like growth factor I display sexual dimorphism in growth hormone-stimulated postnatal growth. Endocrinology. 2000;141(12):4436-4441. [DOI] [PubMed] [Google Scholar]
- 80. Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol. 2010;167(3):344-351. [DOI] [PubMed] [Google Scholar]
- 81. Coerver KA, Woodruff TK, Finegold MJ, Mather J, Bradley A, Matzuk MM. Activin signaling through activin receptor type II causes the cachexia-like symptoms in inhibin-deficient mice. Mol Endocrinol. 1996;10(5):534-543. [DOI] [PubMed] [Google Scholar]
- 82. Brown CW, Li L, Houston-Hawkins DE, Matzuk MM. Activins are critical modulators of growth and survival. Mol Endocrinol. 2003;17(12):2404-2417. [DOI] [PubMed] [Google Scholar]
- 83. Guo W, Wong S, Bhasin S. AAV-mediated administration of myostatin pro-peptide mutant in adult Ldlr null mice reduces diet-induced hepatosteatosis and arteriosclerosis. Plos One. 2013;8(8):e71017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu A, Dong W, Peng J, et al. Growth differentiation factor 11 worsens hepatocellular injury and liver regeneration after liver ischemia reperfusion injury. Faseb J. 2018;32(9):5186-5198. [DOI] [PubMed] [Google Scholar]
- 85. Bloise E, Ciarmela P, Dela Cruz C, Luisi S, Petraglia F, Reis FM. Activin A in mammalian physiology. Physiol Rev. 2019;99(1):739-780. [DOI] [PubMed] [Google Scholar]
- 86. Wang W, Yang X, Yang J, et al. GDF11 impairs liver regeneration in mice after partial hepatectomy. Clin Sci (Lond). 2019;133(20):2069-2084. [DOI] [PubMed] [Google Scholar]
- 87. Delogu W, Caligiuri A, Provenzano A, et al. Myostatin regulates the fibrogenic phenotype of hepatic stellate cells via c-jun N-terminal kinase activation. Dig Liver Dis. 2019;51(10):1400-1408. [DOI] [PubMed] [Google Scholar]
- 88. Furihata T, Kinugawa S, Fukushima A, et al. Serum myostatin levels are independently associated with skeletal muscle wasting in patients with heart failure. Int J Cardiol. 2016;220:483-487. [DOI] [PubMed] [Google Scholar]
- 89. Ju CR, Chen RC. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir Med. 2012;106(1):102-108. [DOI] [PubMed] [Google Scholar]
- 90. Peng LN, Lee WJ, Liu LK, Lin MH, Chen LK. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2018;9(4):635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Anaya-Segura MA, García-Martínez FA, Montes-Almanza LA, et al. Non-invasive biomarkers for Duchenne muscular dystrophy and carrier detection. Molecules. 2015;20(6):11154-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kalampouka I, van Bekhoven A, Elliott BT. Differing effects of younger and older human plasma on C2C12 myocytes in vitro. Front Physiol. 2018;9:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lakshman KM, Bhasin S, Corcoran C, et al. Measurement of myostatin concentrations in human serum: Circulating concentrations in young and older men and effects of testosterone administration. Mol Cell Endocrinol. 2009;302(1):26-32. [DOI] [PubMed] [Google Scholar]
- 94. Hofmann M, Halper B, Oesen S, et al. Serum concentrations of insulin-like growth factor-1, members of the TGF-beta superfamily and follistatin do not reflect different stages of dynapenia and sarcopenia in elderly women. Exp Gerontol. 2015;64:35-45. [DOI] [PubMed] [Google Scholar]
- 95. Bergen HR 3rd, Farr JN, Vanderboom PM, et al. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay. Skelet Muscle. 2015;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Peng L, Gagliano-Juca T, Pencina KM, et al. Age trends in growth and differentiation factor-11 and myostatin levels in healthy men, measured using liquid chromatography tandem mass spectrometry: differential response to testosterone. J Gerontol A Biol Sci Med Sci. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Son BK, Eto M, Oura M, et al. Low-intensity exercise suppresses CCAAT/enhancer-binding protein δ/myostatin pathway through androgen receptor in muscle cells. Gerontology. 2019;65(4):397-406. [DOI] [PubMed] [Google Scholar]
- 98. Kovacheva EL, Hikim AP, Shen R, Sinha I, Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010;151(2):628-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dandona P, Dhindsa S, Ghanim H, Saad F. Mechanisms underlying the metabolic actions of testosterone in humans: a narrative review. Diabetes Obes Metab. 2021;23(1):18-28. [DOI] [PubMed] [Google Scholar]
- 100. Belizário JE, Fontes-Oliveira CC, Borges JP, Kashiabara JA, Vannier E. Skeletal muscle wasting and renewal: a pivotal role of myokine IL-6. Springerplus. 2016;5:619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ma K, Mallidis C, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S. Characterization of 5’-regulatory region of human myostatin gene: regulation by dexamethasone in vitro. Am J Physiol Endocrinol Metab. 2001;281(6):E1128-E1136. [DOI] [PubMed] [Google Scholar]
- 102. Qin J, Du R, Yang YQ, et al. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res Vet Sci. 2013;94(1):84-89. [DOI] [PubMed] [Google Scholar]
- 103. Fry CS, Nayeem SZ, Dillon EL, et al. Glucocorticoids increase skeletal muscle NF-kappaB inducing kinase (NIK): links to muscle atrophy. Physiol Rep. 2016;4(21):e13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Allen DL, Cleary AS, Hanson AM, Lindsay SF, Reed JM. CCAAT/enhancer binding protein-delta expression is increased in fast skeletal muscle by food deprivation and regulates myostatin transcription in vitro. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1592-R1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Allen DL, Loh AS. Posttranscriptional mechanisms involving microRNA-27a and b contribute to fast-specific and glucocorticoid-mediated myostatin expression in skeletal muscle. Am J Physiol Cell Physiol. 2011;300(1):C124-C137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schakman O, Gilson H, Kalista S, Thissen JP. Mechanisms of muscle atrophy induced by glucocorticoids. Horm Res. 2009;72 Suppl 1:36-41. [DOI] [PubMed] [Google Scholar]
- 107. Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197(1):1-10. [DOI] [PubMed] [Google Scholar]
- 108. Pettersen K, Andersen S, van der Veen A, et al. Autocrine activin A signalling in ovarian cancer cells regulates secretion of interleukin 6, autophagy, and cachexia. J Cachexia Sarcopenia Muscle. 2020;11(1):195-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang L, Pan J, Dong Y, et al. Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metab. 2013;18(3):368-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gopinath SD. Inhibition of Stat3 signaling ameliorates atrophy of the soleus muscles in mice lacking the vitamin D receptor. Skelet Muscle. 2017;7(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Silva KA, Dong J, Dong Y, et al. Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin-proteasome system, leading to preservation of muscle mass in cancer cachexia. J Biol Chem. 2015;290(17):11177-11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Seto DN, Kandarian SC, Jackman RW. A key role for leukemia inhibitory factor in C26 cancer cachexia. J Biol Chem. 2015;290(32):19976-19986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Qiu J, Thapaliya S, Runkana A, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110(45):18162-18167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sriram S, Subramanian S, Sathiakumar D, et al. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging Cell. 2011;10(6):931-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang L, Rajan V, Lin E, et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. Faseb J. 2011;25(5):1653-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Itoh Y, Saitoh M, Miyazawa K. Smad3-STAT3 crosstalk in pathophysiological contexts. Acta Biochim Biophys Sin (Shanghai). 2018;50(1):82-90. [DOI] [PubMed] [Google Scholar]
- 117. Chen JL, Walton KL, Qian H, et al. Differential effects of IL6 and activin A in the development of cancer-associated cachexia. Cancer Res. 2016;76(18):5372-5382. [DOI] [PubMed] [Google Scholar]
- 118. Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol. 2019;15(5):257-272. [DOI] [PubMed] [Google Scholar]
- 119. Georgantas RW, Streicher K, Greenberg SA, et al. Inhibition of myogenic microRNAs 1, 133, and 206 by inflammatory cytokines links inflammation and muscle degeneration in adult inflammatory myopathies. Arthritis Rheumatol. 2014;66(4):1022-1033. [DOI] [PubMed] [Google Scholar]
- 120. Quattrocelli M, Zelikovich AS, Salamone IM, Fischer JA, McNally EM. Mechanisms and clinical applications of glucocorticoid steroids in muscular dystrophy. J Neuromuscul Dis. 2021;8(1):39-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fiorillo AA, Tully CB, Damsker JM, Nagaraju K, Hoffman EP, Heier CR. Muscle miRNAome shows suppression of chronic inflammatory miRNAs with both prednisone and vamorolone. Physiol Genomics. 2018;50(9):735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Heier CR, Yu Q, Fiorillo AA, et al. Vamorolone targets dual nuclear receptors to treat inflammation and dystrophic cardiomyopathy. Life Sci Alliance. 2019;2(1):e201800186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Heier CR, Damsker JM, Yu Q, et al. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol Med. 2013;5(10):1569-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Reeves EKM, Hoffman EP, Nagaraju K, Damsker JM, McCall JM. VBP15: preclinical characterization of a novel anti-inflammatory delta 9,11 steroid. Bioorg Med Chem. 2013;21(8):2241-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Szabó G, Dallmann G, Müller G, Patthy L, Soller M, Varga L. A deletion in the myostatin gene causes the compact (Cmpt) hypermuscular mutation in mice. Mamm Genome. 1998;9(8):671-672. [DOI] [PubMed] [Google Scholar]
- 126. Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. Plos One. 2007;2(8):e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Aiello D, Patel K, Lasagna E. The myostatin gene: an overview of mechanisms of action and its relevance to livestock animals. Anim Genet. 2018;49(6):505-519. [DOI] [PubMed] [Google Scholar]
- 128. Mosher DS, Quignon P, Bustamante CD, et al. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. Plos Genet. 2007;3(5):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shelton GD, Engvall E. Gross muscle hypertrophy in whippet dogs is caused by a mutation in the myostatin gene. Neuromuscul Disord. 2007;17(9-10):721-722. [DOI] [PubMed] [Google Scholar]
- 130. Medeiros EF, Phelps MP, Fuentes FD, Bradley TM. Overexpression of follistatin in trout stimulates increased muscling. Am J Physiol Regul Integr Comp Physiol. 2009;297(1):R235-R242. [DOI] [PubMed] [Google Scholar]
- 131. Salabi F, Nazari M, Chen Q, Nimal J, Tong J, Cao WG. Myostatin knockout using zinc-finger nucleases promotes proliferation of ovine primary satellite cells in vitro. J Biotechnol. 2014;192 Pt A:268-280. [DOI] [PubMed] [Google Scholar]
- 132. Greene EA, Allen RE. Growth factor regulation of bovine satellite cell growth in vitro. J Anim Sci. 1991;69(1):146-152. [DOI] [PubMed] [Google Scholar]
- 133. Seiliez I, Sabin N, Gabillard JC. Myostatin inhibits proliferation but not differentiation of trout myoblasts. Mol Cell Endocrinol. 2012;351(2):220-226. [DOI] [PubMed] [Google Scholar]
- 134. Hathaway MR, Hembree JR, Pampusch MS, Dayton WR. Effect of transforming growth factor beta-1 on ovine satellite cell proliferation and fusion. J Cell Physiol. 1991;146(3):435-441. [DOI] [PubMed] [Google Scholar]
- 135. McFarland DC, Velleman SG, Pesall JE, Liu C. The role of myostatin in chicken (Gallus domesticus) myogenic satellite cell proliferation and differentiation. Gen Comp Endocrinol. 2007;151(3):351-357. [DOI] [PubMed] [Google Scholar]
- 136. Kamanga-Sollo E, Pampusch MS, White ME, Hathaway MR, Dayton WR. Insulin-like growth factor binding protein (IGFBP)-3 and IGFBP-5 mediate TGF-beta- and myostatin-induced suppression of proliferation in porcine embryonic myogenic cell cultures. Exp Cell Res. 2005;311(1):167-176. [DOI] [PubMed] [Google Scholar]
- 137. Kamanga-Sollo E, Pampusch MS, White ME, Dayton WR. Role of insulin-like growth factor binding protein (IGFBP)-3 in TGF-beta- and GDF-8 (myostatin)-induced suppression of proliferation in porcine embryonic myogenic cell cultures. J Cell Physiol. 2003;197(2):225-231. [DOI] [PubMed] [Google Scholar]
- 138. Rodgers BD, Wiedeback BD, Hoversten KE, Jackson MF, Walker RG, Thompson TB. Myostatin stimulates, not inhibits, C2C12 myoblast proliferation. Endocrinology. 2014;155(3):670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162(6):1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. McFarlane C, Hui GZ, Amanda WZ, et al. Human myostatin negatively regulates human myoblast growth and differentiation. Am J Physiol Cell Physiol. 2011;301(1):C195-C203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sato F, Kurokawa M, Yamauchi N, Hattori MA. Gene silencing of myostatin in differentiation of chicken embryonic myoblasts by small interfering RNA. Am J Physiol Cell Physiol. 2006;291(3):C538-C545. [DOI] [PubMed] [Google Scholar]
- 142. Manceau M, Gros J, Savage K, et al. Myostatin promotes the terminal differentiation of embryonic muscle progenitors. Genes Dev. 2008;22(5):668-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yamada M, Tatsumi R, Yamanouchi K, et al. High concentrations of HGF inhibit skeletal muscle satellite cell proliferation in vitro by inducing expression of myostatin: a possible mechanism for reestablishing satellite cell quiescence in vivo. Am J Physiol Cell Physiol. 2010;298(3):C465-C476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Dong Y, Lakhia R, Thomas SS, et al. Interactions between p-Akt and Smad3 in injured muscles initiate myogenesis or fibrogenesis. Am J Physiol Endocrinol Metab. 2013;305(3):E367-E375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. George RM, Biressi S, Beres BJ, et al. Numb-deficient satellite cells have regeneration and proliferation defects. Proc Natl Acad Sci U S A. 2013;110(46):18549-18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277(51):49831-49840. [DOI] [PubMed] [Google Scholar]
- 147. Thomas M, Langley B, Berry C, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275(51):40235-40243. [DOI] [PubMed] [Google Scholar]
- 148. Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res. 2003;286(2):263-275. [DOI] [PubMed] [Google Scholar]
- 149. Yuzawa H, Koinuma D, Maeda S, Yamamoto K, Miyazawa K, Imamura T. Arkadia represses the expression of myoblast differentiation markers through degradation of Ski and the Ski-bound Smad complex in C2C12 myoblasts. Bone. 2009;44(1):53-60. [DOI] [PubMed] [Google Scholar]
- 150. Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270(5639):725-727. [DOI] [PubMed] [Google Scholar]
- 151. Katagiri T, Yamaguchi A, Komaki M, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127(6 Pt 1):1755-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yang Q, Jian J, Abramson SB, Huang X. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J Bone Miner Res. 2011;26(6):1188-1196. [DOI] [PubMed] [Google Scholar]
- 153. Zhu J, Li Y, Shen W, et al. Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem. 2007;282(35):25852-25863. [DOI] [PubMed] [Google Scholar]
- 154. Li Y, Foster W, Deasy BM, et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164(3):1007-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Schabort EJ, van der Merwe M, Loos B, Moore FP, Niesler CU. TGF-beta’s delay skeletal muscle progenitor cell differentiation in an isoform-independent manner. Exp Cell Res. 2009;315(3):373-384. [DOI] [PubMed] [Google Scholar]
- 156. Haugk KL, Roeder RA, Garber MJ, Schelling GT. Regulation of muscle cell proliferation by extracts from crushed muscle. J Anim Sci. 1995;73(7):1972-1981. [DOI] [PubMed] [Google Scholar]
- 157. Quinn LS, Steinmetz B, Maas A, Ong L, Kaleko M. Type-1 insulin-like growth factor receptor overexpression produces dual effects on myoblast proliferation and differentiation. J Cell Physiol. 1994;159(3):387-398. [DOI] [PubMed] [Google Scholar]
- 158. McFarland DC, Velleman SG, Pesall JE, Liu C. Effect of myostatin on turkey myogenic satellite cells and embryonic myoblasts. Comp Biochem Physiol A Mol Integr Physiol. 2006;144(4):501-508. [DOI] [PubMed] [Google Scholar]
- 159. Zhang Y, Wang Y, Yulin B, et al. CRISPR/Cas9-mediated sheep MSTN gene knockout and promote sSMSCs differentiation. J Cell Biochem. 2018;120(2):1794-1806. [DOI] [PubMed] [Google Scholar]
- 160. Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125(6):1275-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Thomas D, Lehmann S, Kuchler S, Marschal P, Zanetta JP. Differential expression of an endogenous mannose-binding protein R1 during muscle development and regeneration delineating its role in myoblast fusion. Glycobiology. 1994;4(1):23-38. [DOI] [PubMed] [Google Scholar]
- 162. Beermann ML, Ardelt M, Girgenrath M, Miller JB. Prdm1 (Blimp-1) and the expression of fast and slow myosin heavy chain isoforms during avian myogenesis in vitro. Plos One. 2010;5(4):e9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ge X, McFarlane C, Vajjala A, et al. Smad3 signaling is required for satellite cell function and myogenic differentiation of myoblasts. Cell Res. 2011;21(11):1591-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wei C, Ren H, Xu L, et al. Signals of Ezh2, Src, and Akt Involve in myostatin-Pax7 pathways regulating the myogenic fate determination during the sheep myoblast proliferation and differentiation. Plos One. 2015;10(3):e0120956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tsao J, Vernet DA, Gelfand R, et al. Myostatin genetic inactivation inhibits myogenesis by muscle-derived stem cells in vitro but not when implanted in the mdx mouse muscle. Stem Cell Res Ther. 2013;4(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Goodman CA, McNally RM, Hoffmann FM, Hornberger TA. Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol Endocrinol. 2013;27(11):1946-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Egerman MA, Glass DJ. The role of GDF11 in aging and skeletal muscle, cardiac and bone homeostasis. Crit Rev Biochem Mol Biol. 2019;54(2):174-183. [DOI] [PubMed] [Google Scholar]
- 168. Walton KL, Chen JL, Arnold Q, et al. Activin A-induced cachectic wasting is attenuated by systemic delivery of its cognate propeptide in male mice. Endocrinology. 2019;160(10):2417-2426. [DOI] [PubMed] [Google Scholar]
- 169. Winbanks CE, Murphy KT, Bernardo BC, et al. Smad7 gene delivery prevents muscle wasting associated with cancer cachexia in mice. Sci Transl Med. 2016;8(348):348ra98. [DOI] [PubMed] [Google Scholar]
- 170. Sinha M, Jang YC, Oh J, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344(6184):649-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Katsimpardi L, Litterman NK, Schein PA, et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344(6184):630-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Loffredo FS, Steinhauser ML, Jay SM, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153(4):828-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Semba RD, Gonzalez-Freire M, Moaddel R, et al. Altered plasma amino acids and lipids associated with abnormal glucose metabolism and insulin resistance in older adults. J Clin Endocrinol Metab. 2018;103(9):3331-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Schafer MJ, Atkinson EJ, Vanderboom PM, et al. Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23(6):1207-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Yang R, Fu S, Zhao L, et al. Quantitation of circulating GDF-11 and β2-MG in aged patients with age-related impairment in cognitive function. Clin Sci (Lond). 2017;131(15):1895-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Chen Y, Guo Q, Zhang M, et al. Relationship of serum GDF11 levels with bone mineral density and bone turnover markers in postmenopausal Chinese women. Bone Res. 2016;4:16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bueno JL, Ynigo M, de Miguel C, et al. Growth differentiation factor 11 (GDF11) - a promising anti-ageing factor - is highly concentrated in platelets. Vox Sang. 2016;111(4):434-436. [DOI] [PubMed] [Google Scholar]
- 178. Ahn ST, Suh SI, Moon H, Hyun C. Evaluation of growth differentiation factor 11 (GDF11) levels in dogs with chronic mitral valve insufficiency. Can J Vet Res. 2016;80(1):90-92. [PMC free article] [PubMed] [Google Scholar]
- 179. Rodgers BD, Eldridge JA. Reduced circulating GDF11 is unlikely responsible for age-dependent changes in mouse heart, muscle, and brain. Endocrinology. 2015;156(11):3885-3888. [DOI] [PubMed] [Google Scholar]
- 180. Tian J, Lei XX, Xuan L, Tang JB, Cheng B. The effects of aging, diabetes mellitus, and antiplatelet drugs on growth factors and anti-aging proteins in platelet-rich plasma. Platelets. 2019;30(6):773-792. [DOI] [PubMed] [Google Scholar]
- 181. Añón-Hidalgo J, Catalán V, Rodríguez A, et al. Circulating GDF11 levels are decreased with age but are unchanged with obesity and type 2 diabetes. Aging (Albany NY). 2019;11(6):1733-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zhang Y, Shao J, Wang Z, et al. Growth differentiation factor 11 is a protective factor for osteoblastogenesis by targeting PPARgamma. Gene. 2015;557(2):209-214. [DOI] [PubMed] [Google Scholar]
- 183. Rodgers BD. The immateriality of circulating GDF11. Circ Res. 2016;118(10):1472-1474. [DOI] [PubMed] [Google Scholar]
- 184. Hammers DW, Merscham-Banda M, Hsiao JY, Engst S, Hartman JJ, Sweeney HL. Supraphysiological levels of GDF11 induce striated muscle atrophy. EMBO Mol Med. 2017;9(4):531-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Zhou Y, Sharma N, Dukes D, et al. GDF11 treatment attenuates the recovery of skeletal muscle function after injury in older rats. Aaps J. 2017;19(2):431-437. [DOI] [PubMed] [Google Scholar]
- 186. Hinken AC, Powers JM, Luo G, Holt JA, Billin AN, Russell AJ. Lack of evidence for GDF11 as a rejuvenator of aged skeletal muscle satellite cells. Aging Cell. 2016;15(3):582-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Glass DJ. Elevated GDF11 is a risk factor for age-related frailty and disease in humans. Cell Metab. 2016;24(1):7-8. [DOI] [PubMed] [Google Scholar]
- 188. Roh JD, Hobson R, Chaudhari V, et al. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11(482):eaau8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Jackson MF, Li N, Rodgers BD. Myostatin regulates tissue potency and cardiac calcium-handling proteins. Endocrinology. 2014;155(5):1771-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jackson MF, Luong D, Vang DD, et al. The aging myostatin null phenotype: reduced adiposity, cardiac hypertrophy, enhanced cardiac stress response, and sexual dimorphism. J Endocrinol. 2012;213(3):263-275. [DOI] [PubMed] [Google Scholar]
- 191. Maricelli JW, Bishaw YM, Wang B, Du M, Rodgers BD. Systemic SMAD7 gene therapy increases striated muscle mass and enhances exercise capacity in a dose-dependent manner. Hum Gene Ther. 2018;29(3):390-399. [DOI] [PubMed] [Google Scholar]
- 192. Rodgers BD, Interlichia JP, Garikipati DK, et al. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. J Physiol. 2009;587(Pt 20):4873-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Chen JL, Walton KL, Winbanks CE, et al. Elevated expression of activins promotes muscle wasting and cachexia. Faseb J. 2014;28(4):1711-1723. [DOI] [PubMed] [Google Scholar]
- 194. Han HQ, Zhou X, Mitch WE, Goldberg AL. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol. 2013;45(10):2333-2347. [DOI] [PubMed] [Google Scholar]
- 195. Smith SC, Zhang X, Zhang X, et al. GDF11 does not rescue aging-related pathological hypertrophy. Circ Res. 2015;117(11):926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Gao L, Li SL, Li YK. Liraglutide promotes the osteogenic differentiation in MC3T3-E1 cells via regulating the expression of Smad2/3 through PI3K/Akt and Wnt/β-catenin pathways. DNA Cell Biol. 2018;37(12):1031-1043. [DOI] [PubMed] [Google Scholar]
- 197. Song NJ, Kwon SM, Kim S, et al. Sulfuretin induces osteoblast differentiation through activation of TGF-β signaling. Mol Cell Biochem. 2015;410(1-2):55-63. [DOI] [PubMed] [Google Scholar]
- 198. Sowa H, Kaji H, Yamaguchi T, Sugimoto T, Chihara K. Smad3 promotes alkaline phosphatase activity and mineralization of osteoblastic MC3T3-E1 cells. J Bone Miner Res. 2002;17(7):1190-1199. [DOI] [PubMed] [Google Scholar]
- 199. Olsen OE, Wader KF, Hella H, et al. Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun Signal. 2015;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Ehrlich M, Gutman O, Knaus P, Henis YI. Oligomeric interactions of TGF-β and BMP receptors. FEBS Lett. 2012;586(14):1885-1896. [DOI] [PubMed] [Google Scholar]
- 201. Heldin CH, Moustakas A. Signaling receptors for TGF-beta family members. Cold Spring Harb Perspect Biol. 2016;8(8):a022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Loumaye A, de Barsy M, Nachit M, et al. Circulating Activin A predicts survival in cancer patients. J Cachexia Sarcopenia Muscle. 2017;8(5):768-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Loumaye A, de Barsy M, Nachit M, et al. Role of Activin A and myostatin in human cancer cachexia. J Clin Endocrinol Metab. 2015;100(5):jc20144318. [DOI] [PubMed] [Google Scholar]
- 204. Jones JE, Cadena SM, Gong C, et al. Supraphysiologic administration of GDF11 induces cachexia in part by upregulating GDF15. Cell Rep. 2018;22(6):1522-1530. [DOI] [PubMed] [Google Scholar]
- 205. Li ZB, Kollias HD, Wagner KR. Myostatin directly regulates skeletal muscle fibrosis. J Biol Chem. 2008;283(28):19371-19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Miura S, Takeshita T, Asao H, et al. Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol Cell Biol. 2000;20(24):9346-9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell. 1998;95(6):779-791. [DOI] [PubMed] [Google Scholar]
- 208. Wurthner JU, Frank DB, Felici A, et al. Transforming growth factor-beta receptor-associated protein 1 is a Smad4 chaperone. J Biol Chem. 2001;276(22):19495-19502. [DOI] [PubMed] [Google Scholar]
- 209. Chen YG, Wang Z, Ma J, Zhang L, Lu Z. Endofin, a FYVE domain protein, interacts with Smad4 and facilitates transforming growth factor-beta signaling. J Biol Chem. 2007;282(13):9688-9695. [DOI] [PubMed] [Google Scholar]
- 210. Lucarelli P, Schilling M, Kreutz C, et al. Resolving the combinatorial complexity of smad protein complex formation and its link to gene expression. Cell Syst. 2018;6(1):75-89.e11. [DOI] [PubMed] [Google Scholar]
- 211. Morikawa M, Koinuma D, Miyazono K, Heldin CH. Genome-wide mechanisms of Smad binding. Oncogene. 2013;32(13):1609-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Morikawa M, Koinuma D, Tsutsumi S, et al. ChIP-seq reveals cell type-specific binding patterns of BMP-specific Smads and a novel binding motif. Nucleic Acids Res. 2011;39(20):8712-8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Johnson K, Kirkpatrick H, Comer A, Hoffmann FM, Laughon A. Interaction of Smad complexes with tripartite DNA-binding sites. J Biol Chem. 1999;274(29):20709-20716. [DOI] [PubMed] [Google Scholar]
- 214. Deng B, Zhang F, Wen J, et al. The transcriptomes from two adipocyte progenitor cell types provide insight into the differential functions of MSTN. Genomics. 2020;112(5):3826-3836. [DOI] [PubMed] [Google Scholar]
- 215. Barbé C, Bray F, Gueugneau M, et al. Comparative proteomic and transcriptomic analysis of follistatin-induced skeletal muscle hypertrophy. J Proteome Res. 2017;16(10):3477-3490. [DOI] [PubMed] [Google Scholar]
- 216. Javed R, Jing L, Yang J, Li X, Cao J, Zhao S. miRNA transcriptome of hypertrophic skeletal muscle with overexpressed myostatin propeptide. Biomed Res Int. 2014;2014:328935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Cassar-Malek I, Passelaigue F, Bernard C, Léger J, Hocquette JF. Target genes of myostatin loss-of-function in muscles of late bovine fetuses. BMC Genomics. 2007;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Steelman CA, Recknor JC, Nettleton D, Reecy JM. Transcriptional profiling of myostatin-knockout mice implicates Wnt signaling in postnatal skeletal muscle growth and hypertrophy. Faseb J. 2006;20(3):580-582. [DOI] [PubMed] [Google Scholar]
- 219. Li X, Xie S, Qian L, Cai C, Bi H, Cui W. Identification of genes related to skeletal muscle growth and development by integrated analysis of transcriptome and proteome in myostatin-edited Meishan pigs. J Proteomics. 2020;213:103628. [DOI] [PubMed] [Google Scholar]
- 220. de Ceuninck van Capelle C, Spit M, Ten Dijke P. Current perspectives on inhibitory SMAD7 in health and disease. Crit Rev Biochem Mol Biol. 2020;55(6):691-715. [DOI] [PubMed] [Google Scholar]
- 221. Hitachi K, Nakatani M, Tsuchida K. Myostatin signaling regulates Akt activity via the regulation of miR-486 expression. Int J Biochem Cell Biol. 2014;47:93-103. [DOI] [PubMed] [Google Scholar]
- 222. Artaza JN, Singh R, Ferrini MG, Braga M, Tsao J, Gonzalez-Cadavid NF. Myostatin promotes a fibrotic phenotypic switch in multipotent C3H 10T1/2 cells without affecting their differentiation into myofibroblasts. J Endocrinol. 2008;196(2):235-249. [DOI] [PubMed] [Google Scholar]
- 223. Fournier B, Murray B, Gutzwiller S, et al. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol Cell Biol. 2012;32(14):2871-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Tiano JP, Springer DA, Rane SG. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor γ coactivator 1-α (PGC-1α) during exercise. J Biol Chem. 2015;290(12):7671-7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Csibi A, Leibovitch MP, Cornille K, Tintignac LA, Leibovitch SA. MAFbx/Atrogin-1 controls the activity of the initiation factor eIF3-f in skeletal muscle atrophy by targeting multiple C-terminal lysines. J Biol Chem. 2009;284(7):4413-4421. [DOI] [PubMed] [Google Scholar]
- 226. Lagirand-Cantaloube J, Offner N, Csibi A, et al. The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. Embo J. 2008;27(8):1266-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Cohen S, Lee D, Zhai B, Gygi SP, Goldberg AL. Trim32 reduces PI3K-Akt-FoxO signaling in muscle atrophy by promoting plakoglobin-PI3K dissociation. J Cell Biol. 2014;204(5):747-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Cohen S, Zhai B, Gygi SP, Goldberg AL. Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy. J Cell Biol. 2012;198(4):575-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. Faseb J. 2013;27(5):1981-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Braga M, Pervin S, Norris K, Bhasin S, Singh R. Inhibition of in vitro and in vivo brown fat differentiation program by myostatin. Obesity (Silver Spring). 2013;21(6):1180-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Manfredi LH, Ang J, Peker N, Dagda RK, McFarlane C. G protein-coupled receptor kinase 2 regulates mitochondrial bioenergetics and impairs myostatin-mediated autophagy in muscle cells. Am J Physiol Cell Physiol. 2019;317(4):C674-C686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Hulmi JJ, Penna F, Pöllänen N, et al. Muscle NAD+ depletion and Serpina3n as molecular determinants of murine cancer cachexia-the effects of blocking myostatin and activins. Mol Metab. 2020;41:101046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Zhang C, McFarlane C, Lokireddy S, et al. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia. 2012;55(1):183-193. [DOI] [PubMed] [Google Scholar]
- 234. Béchir N, Pecchi É, Relizani K, et al. Mitochondrial impairment induced by postnatal ActRIIB blockade does not alter function and energy status in exercising mouse glycolytic muscle in vivo. Am J Physiol Endocrinol Metab. 2016;310(7):E539-E549. [DOI] [PubMed] [Google Scholar]
- 235. Ploquin C, Chabi B, Fouret G, et al. Lack of myostatin alters intermyofibrillar mitochondria activity, unbalances redox status, and impairs tolerance to chronic repetitive contractions in muscle. Am J Physiol Endocrinol Metab. 2012;302(8):E1000-E1008. [DOI] [PubMed] [Google Scholar]
- 236. Pons M, Koniaris LG, Moe SM, Gutierrez JC, Esquela-Kerscher A, Zimmers TA. GDF11 induces kidney fibrosis, renal cell epithelial-to-mesenchymal transition, and kidney dysfunction and failure. Surgery. 2018;164(2):262-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Dong J, Dong Y, Chen Z, Mitch WE, Zhang L. The pathway to muscle fibrosis depends on myostatin stimulating the differentiation of fibro/adipogenic progenitor cells in chronic kidney disease. Kidney Int. 2017;91(1):119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Kobayashi M, Kasamatsu S, Shinozaki S, Yasuhara S, Kaneki M. Myostatin deficiency not only prevents muscle wasting but also improves survival in septic mice. Am J Physiol Endocrinol Metab. 2021;320(1):E150-E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Ding H, Zhang G, Sin KW, et al. Activin A induces skeletal muscle catabolism via p38β mitogen-activated protein kinase. J Cachexia Sarcopenia Muscle. 2017;8(2):202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. McFarlane C, Hennebry A, Thomas M, et al. Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res. 2008;314(2):317-329. [DOI] [PubMed] [Google Scholar]
- 241. Chen JF, Tao Y, Li J, et al. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010;190(5):867-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Dey BK, Gagan J, Dutta A. miR-206 and -486 induce myoblast differentiation by downregulating Pax7. Mol Cell Biol. 2011;31(1):203-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Olguin HC, Yang Z, Tapscott SJ, Olwin BB. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol. 2007;177(5):769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Brun C, Périé L, Baraige F, Vernus B, Bonnieu A, Blanquet V. Absence of hyperplasia in Gasp-1 overexpressing mice is dependent on myostatin up-regulation. Cell Physiol Biochem. 2014;34(4):1241-1259. [DOI] [PubMed] [Google Scholar]
- 245. Lu H, Shah P, Ennis D, et al. The differentiation of skeletal muscle cells involves a protein-tyrosine phosphatase-alpha-mediated C-Src signaling pathway. J Biol Chem. 2002;277(48):46687-46695. [DOI] [PubMed] [Google Scholar]
- 246. Mozzetta C, Consalvi S, Saccone V, Forcales SV, Puri PL, Palacios D. Selective control of Pax7 expression by TNF-activated p38α/polycomb repressive complex 2 (PRC2) signaling during muscle satellite cell differentiation. Cell Cycle. 2011;10(2):191-198. [DOI] [PubMed] [Google Scholar]
- 247. Niu A, Wen Y, Liu H, Zhan M, Jin B, Li YP. Src mediates the mechanical activation of myogenesis by activating TNFα-converting enzyme. J Cell Sci. 2013;126(Pt 19):4349-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Palacios D, Mozzetta C, Consalvi S, et al. TNF/p38α/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7(4):455-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ronda AC, Buitrago C, Boland R. Role of estrogen receptors, PKC and Src in ERK2 and p38 MAPK signaling triggered by 17β-estradiol in skeletal muscle cells. J Steroid Biochem Mol Biol. 2010;122(4):287-294. [DOI] [PubMed] [Google Scholar]
- 250. Segalés J, Perdiguero E, Muñoz-Cánoves P. Regulation of muscle stem cell functions: a focus on the p38 MAPK signaling pathway. Front Cell Dev Biol. 2016;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, Bonetto A. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget. 2016;7(28):43442-43460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Sriram S, Subramanian S, Juvvuna PK, et al. Myostatin augments muscle-specific ring finger protein-1 expression through an NF-kB independent mechanism in SMAD3 null muscle. Mol Endocrinol. 2014;28(3):317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Huang Z, Zhang K, Chen X, Meng J, Chen D. Effect of siRNA targeted against MKK4 on myostatin-induced downregulation of differentiation marker gene expression. Mol Cell Biochem. 2008;310(1-2):241-244. [DOI] [PubMed] [Google Scholar]
- 254. Huang Z, Chen D, Zhang K, Yu B, Chen X, Meng J. Regulation of myostatin signaling by c-Jun N-terminal kinase in C2C12 cells. Cell Signal. 2007;19(11):2286-2295. [DOI] [PubMed] [Google Scholar]
- 255. Hu SL, Chang AC, Huang CC, Tsai CH, Lin CC, Tang CH. Myostatin promotes interleukin-1β expression in rheumatoid arthritis synovial fibroblasts through inhibition of miR-21-5p. Front Immunol. 2017;8:1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013;2013:204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Biolo G, Cederholm T, Muscaritoli M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: from sarcopenic obesity to cachexia. Clin Nutr. 2014;33(5):737-748. [DOI] [PubMed] [Google Scholar]
- 258. Workeneh B, Bajaj M. The regulation of muscle protein turnover in diabetes. Int J Biochem Cell Biol. 2013;45(10):2239-2244. [DOI] [PubMed] [Google Scholar]
- 259. Wang F, Liao Y, Li X, Ren C, Cheng C, Ren Y. Increased circulating myostatin in patients with type 2 diabetes mellitus. J Huazhong Univ Sci Technolog Med Sci. 2012;32(4):534-539. [DOI] [PubMed] [Google Scholar]
- 260. Amor M, Itariu BK, Moreno-Viedma V, et al. Serum myostatin is upregulated in obesity and correlates with insulin resistance in humans. Exp Clin Endocrinol Diabetes. 2019;127(8):550-556. [DOI] [PubMed] [Google Scholar]
- 261. Chung JO, Park SY, Chung DJ, Chung MY. Serum myostatin levels are positively associated with diabetic retinopathy in individuals with type 2 diabetes mellitus. J Diabetes Complications. 2020;34(7):107592. [DOI] [PubMed] [Google Scholar]
- 262. Dial AG, Monaco CMF, Grafham GK, et al. Muscle and serum myostatin expression in type 1 diabetes. Physiol Rep. 2020;8(13):e14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Demontis GC, Germani MM, Caiani EG, Barravecchia I, Passino C, Angeloni D. Human pathophysiological adaptations to the space environment. Front Physiol. 2017;8:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Tanaka K, Nishimura N, Kawai Y. Adaptation to microgravity, deconditioning, and countermeasures. J Physiol Sci. 2017;67(2):271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Zambraski EJ, Yancosek KE. Prevention and rehabilitation of musculoskeletal injuries during military operations and training. J Strength Cond Res. 2012;26 Suppl 2:S101-S106. [DOI] [PubMed] [Google Scholar]
- 266. Bell NS, Schwartz CE, Harford T, Hollander IE, Amoroso PJ. The changing profile of disability in the U.S. Army: 1981-2005. Disabil Health J. 2008;1(1):14-24. [DOI] [PubMed] [Google Scholar]
- 267. Corona BT, Rivera JC, Owens JG, Wenke JC, Rathbone CR. Volumetric muscle loss leads to permanent disability following extremity trauma. J Rehabil Res Dev. 2015;52(7):785-792. [DOI] [PubMed] [Google Scholar]
- 268. Garg K, Ward CL, Hurtgen BJ, et al. Volumetric muscle loss: persistent functional deficits beyond frank loss of tissue. J Orthop Res. 2015;33(1):40-46. [DOI] [PubMed] [Google Scholar]
- 269. Rivera JC, Corona BT. Muscle-related disability following combat injury increases with time. US Army Med Dep J. 2016:30-34. [PubMed] [Google Scholar]
- 270. Stefancic M, Vidmar G, Blagus R. Long-term recovery of muscle strength after denervation in the fibular division of the sciatic nerve. Muscle Nerve. 2016;54(4):702-708. [DOI] [PubMed] [Google Scholar]
- 271. Vanhoutte F, Liang S, Ruddy M, et al. Pharmacokinetics and pharmacodynamics of garetosmab (Anti-Activin A): results from a first-in-human phase 1 study. J Clin Pharmacol. 2020;60(11):1424-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Mercuri E, Bönnemann CG, Muntoni F. Muscular dystrophies. Lancet. 2019;394(10213):2025-2038. [DOI] [PubMed] [Google Scholar]
- 273. Le Rumeur E, Winder SJ, Hubert JF. Dystrophin: more than just the sum of its parts. Biochim Biophys Acta. 2010;1804(9):1713-1722. [DOI] [PubMed] [Google Scholar]
- 274. Murphy S, Ohlendieck K. The biochemical and mass spectrometric profiling of the dystrophin complexome from skeletal muscle. Comput Struct Biotechnol J. 2016;14:20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Avila G. Disturbed Ca2+ homeostasis in muscle-wasting disorders. Adv Exp Med Biol. 2018;1088:307-326. [DOI] [PubMed] [Google Scholar]
- 276. Allen DG, Whitehead NP, Froehner SC. Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol Rev. 2016;96(1):253-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Dalakas MC. Inflammatory myopathies: update on diagnosis, pathogenesis and therapies, and COVID-19-related implications. Acta Myol. 2020;39(4):289-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Broccolini A, Mirabella M. Hereditary inclusion-body myopathies. Biochim Biophys Acta. 2015;1852(4):644-650. [DOI] [PubMed] [Google Scholar]
- 279. Lloyd TE, Mammen AL, Amato AA, Weiss MD, Needham M, Greenberg SA. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology. 2014;83(5):426-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Hiscock A, Dewar L, Parton M, Machado P, Hanna M, Ramdharry G. Frequency and circumstances of falls in people with inclusion body myositis: a questionnaire survey to explore falls management and physiotherapy provision. Physiotherapy. 2014;100(1):61-65. [DOI] [PubMed] [Google Scholar]
- 281. Milte R, Crotty M. Musculoskeletal health, frailty and functional decline. Best Pract Res Clin Rheumatol. 2014;28(3):395-410. [DOI] [PubMed] [Google Scholar]
- 282. Cox FM, Titulaer MJ, Sont JK, Wintzen AR, Verschuuren JJ, Badrising UA. A 12-year follow-up in sporadic inclusion body myositis: an end stage with major disabilities. Brain. 2011;134(Pt 11):3167-3175. [DOI] [PubMed] [Google Scholar]
- 283. Oh TH, Brumfield KA, Hoskin TL, Kasperbauer JL, Basford JR. Dysphagia in inclusion body myositis: clinical features, management, and clinical outcome. Am J Phys Med Rehabil. 2008;87(11):883-889. [DOI] [PubMed] [Google Scholar]
- 284. Price MA, Barghout V, Benveniste O, et al. Mortality and causes of death in patients with sporadic inclusion body myositis: survey study based on the clinical experience of specialists in Australia, Europe and the USA. J Neuromuscul Dis. 2016;3(1):67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Benabdallah BF, Bouchentouf M, Rousseau J, et al. Inhibiting myostatin with follistatin improves the success of myoblast transplantation in dystrophic mice. Cell Transplant. 2008;17(3):337-350. [DOI] [PubMed] [Google Scholar]
- 286. Benabdallah BF, Bouchentouf M, Tremblay JP. Improved success of myoblast transplantation in mdx mice by blocking the myostatin signal. Transplantation. 2005;79(12):1696-1702. [DOI] [PubMed] [Google Scholar]
- 287. Byron CD, Hamrick MW, Wingard CJ. Alterations of temporalis muscle contractile force and histological content from the myostatin and Mdx deficient mouse. Arch Oral Biol. 2006;51(5):396-405. [DOI] [PubMed] [Google Scholar]
- 288. Hulmi JJ, Oliveira BM, Silvennoinen M, et al. Muscle protein synthesis, mTORC1/MAPK/Hippo signaling, and capillary density are altered by blocking of myostatin and activins. Am J Physiol Endocrinol Metab. 2013;304(1):E41-E50. [DOI] [PubMed] [Google Scholar]
- 289. Morine KJ, Bish LT, Selsby JT, et al. Activin IIB receptor blockade attenuates dystrophic pathology in a mouse model of Duchenne muscular dystrophy. Muscle Nerve. 2010;42(5):722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol. 2002;52(6):832-836. [DOI] [PubMed] [Google Scholar]
- 291. Rodgers BD, Bishaw Y, Kagel D, Ramos JN, Maricelli JW. Micro-dystrophin gene therapy partially enhances exercise capacity in older adult mdx mice. Mol Ther Methods Clin Dev. 2020;17:122-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Hamm SE, Fathalikhani DD, Bukovec KE, et al. Voluntary wheel running complements microdystrophin gene therapy to improve muscle function in mdx mice. Mol Ther Methods Clin Dev. 2021;21:144-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Abmayr S, Gregorevic P, Allen JM, Chamberlain JS. Phenotypic improvement of dystrophic muscles by rAAV/microdystrophin vectors is augmented by Igf1 codelivery. Mol Ther. 2005;12(3):441-450. [DOI] [PubMed] [Google Scholar]
- 294. Gregorevic P, Plant DR, Leeding KS, Bach LA, Lynch GS. Improved contractile function of the mdx dystrophic mouse diaphragm muscle after insulin-like growth factor-I administration. Am J Pathol. 2002;161(6):2263-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Rodino-Klapac LR, Janssen PM, Shontz KM, et al. Micro-dystrophin and follistatin co-delivery restores muscle function in aged DMD model. Hum Mol Genet. 2013;22(24):4929-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Xin C, Chu X, Wei W, et al. Combined gene therapy via VEGF and mini-dystrophin synergistically improves pathologies in temporalis muscle of dystrophin/utrophin double knockout mice. Hum Mol Genet. 2021;30(14):1349-1359. [DOI] [PubMed] [Google Scholar]
- 297. Gregorevic P, Blankinship MJ, Allen JM, Chamberlain JS. Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol Ther. 2008;16(4):657-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Bostick B, Shin JH, Yue Y, Duan D. AAV-microdystrophin therapy improves cardiac performance in aged female mdx mice. Mol Ther. 2011;19(10):1826-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Bostick B, Shin JH, Yue Y, Wasala NB, Lai Y, Duan D. AAV micro-dystrophin gene therapy alleviates stress-induced cardiac death but not myocardial fibrosis in >21-m-old mdx mice, an end-stage model of Duchenne muscular dystrophy cardiomyopathy. J Mol Cell Cardiol. 2012;53(2):217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Bostick B, Yue Y, Long C, et al. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged Mdx mice. Mol Ther. 2009;17(2):253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Vannoy CH, Xiao W, Lu P, Xiao X, Lu QL. Efficacy of gene therapy is dependent on disease progression in dystrophic mice with mutations in the FKRP gene. Mol Ther Methods Clin Dev. 2017;5:31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Sheikh O, Yokota T. Developing DMD therapeutics: a review of the effectiveness of small molecules, stop-codon readthrough, dystrophin gene replacement, and exon-skipping therapies. Expert Opin Investig Drugs. 2021;30(2):167-176. [DOI] [PubMed] [Google Scholar]
- 303. Sarepta Therapeutics I. Sarepta Therapeutics Announces Top-line Results for Part 1 of Study 102 Evaluating SRP-9001, its Investigational Gene Therapy for the Treatment of Duchenne Muscular Dystrophy. GlobeNewswire; 2021. https://www.globenewswire.com/news-release/2021/01/07/2155237/0/en/Sarepta-Therapeutics-Announces-Top-line-Results-for-Part-1-of-Study-102-Evaluating-SRP-9001-its-Investigational-Gene-Therapy-for-the-Treatment-of-Duchenne-Muscular-Dystrophy.html [Google Scholar]
- 304. Ingram D, Rodino-Klapac L. Clinical update: study SRP-9001-103: 12-week expression and safety data using commercially representative material.2021. https://investorrelations.sarepta.com/static-files/09358a6f-98f9-4e11-a59e-db6b0d0f584f.
- 305. Duan D. Systemic AAV micro-dystrophin gene therapy for Duchenne muscular dystrophy. Mol Ther. 2018;26(10):2337-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Mendell JR, Sahenk Z, Al-Zaidy S, et al. Follistatin gene therapy for sporadic inclusion body myositis improves functional outcomes. Mol Ther. 2017;25(4):870-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Busquets S, Toledo M, Orpí M, et al. Myostatin blockage using actRIIB antagonism in mice bearing the Lewis lung carcinoma results in the improvement of muscle wasting and physical performance. J Cachexia Sarcopenia Muscle. 2012;3(1):37-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Guo W, Pencina KM, O’Connell K, et al. Administration of an activin receptor IIB ligand trap protects male juvenile rhesus macaques from simian immunodeficiency virus-associated bone loss. Bone. 2017;97:209-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Hatakeyama S, Summermatter S, Jourdain M, Melly S, Minetti GC, Lach-Trifilieff E. ActRII blockade protects mice from cancer cachexia and prolongs survival in the presence of anti-cancer treatments. Skelet Muscle. 2016;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Murphy KT, Chee A, Gleeson BG, et al. Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R716-R726. [DOI] [PubMed] [Google Scholar]
- 311. Smith RC, Cramer MS, Mitchell PJ, et al. Myostatin neutralization results in preservation of muscle mass and strength in preclinical models of tumor-induced muscle wasting. Mol Cancer Ther. 2015;14(7):1661-1670. [DOI] [PubMed] [Google Scholar]
- 312. Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531-543. [DOI] [PubMed] [Google Scholar]
- 313. Administration FaD. The Voice of the Patient: Sarcopenia. In: (CDER) CfDEaR, ed. 2017. https://www.fda.gov/media/108220/download [Google Scholar]
- 314. Meza-Valderrama D, Marco E, Davalos-Yerovi V, et al. Sarcopenia, malnutrition, and cachexia: adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients. 2021;13(3):761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 316. DiGirolamo DJ, Singhal V, Chang X, Lee SJ, Germain-Lee EL. Administration of soluble activin receptor 2B increases bone and muscle mass in a mouse model of osteogenesis imperfecta. Bone Res. 2015;3:14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Goh BC, Singhal V, Herrera AJ, et al. Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J Biol Chem. 2017;292(33):13809-13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Guo W, Pencina KM, Gagliano-Jucá T, et al. Effects of an ActRIIB.Fc ligand trap on cardiac function in simian immunodeficiency virus-infected male rhesus macaques. J Endocr Soc. 2018;2(8):817-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Lee SJ, Lehar A, Meir JU, et al. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc Natl Acad Sci U S A. 2020;117(38):23942-23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Nielsen C, Potter RM, Borowy C, Jacinto K, Kumar R, Carlson CG. Postnatal hyperplasic effects of ActRIIB blockade in a severely dystrophic muscle. J Cell Physiol. 2017;232(7):1774-1793. [DOI] [PubMed] [Google Scholar]
- 321. O’Connell KE, Guo W, Serra C, et al. The effects of an ActRIIb receptor Fc fusion protein ligand trap in juvenile simian immunodeficiency virus-infected rhesus macaques. Faseb J. 2015;29(4):1165-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Suragani RN, Cadena SM, Cawley SM, et al. Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med. 2014;20(4):408-414. [DOI] [PubMed] [Google Scholar]
- 323. Suragani RN, Cawley SM, Li R, et al. Modified activin receptor IIB ligand trap mitigates ineffective erythropoiesis and disease complications in murine β-thalassemia. Blood. 2014;123(25):3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Tauer JT, Rauch F. Novel ActRIIB ligand trap increases muscle mass and improves bone geometry in a mouse model of severe osteogenesis imperfecta. Bone. 2019;128:115036. [DOI] [PubMed] [Google Scholar]
- 325. Cadena SM, Tomkinson KN, Monnell TE, et al. Administration of a soluble activin type IIB receptor promotes skeletal muscle growth independent of fiber type. J Appl Physiol (1985). 2010;109(3):635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Glasser CE, Gartner MR, Wilson D, Miller B, Sherman ML, Attie KM. Locally acting ACE-083 increases muscle volume in healthy volunteers. Muscle Nerve. 2018;57(6):921-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Pearsall RS, Davies MV, Cannell M, et al. Follistatin-based ligand trap ACE-083 induces localized hypertrophy of skeletal muscle with functional improvement in models of neuromuscular disease. Sci Rep. 2019;9(1):11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Castonguay R, Lachey J, Wallner S, et al. Follistatin-288-Fc fusion protein promotes localized growth of skeletal muscle. J Pharmacol Exp Ther. 2019;368(3):435-445. [DOI] [PubMed] [Google Scholar]
- 329. Lee SJ, Reed LA, Davies MV, et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc Natl Acad Sci U S A. 2005;102(50):18117-18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Koncarevic A, Cornwall-Brady M, Pullen A, et al. A soluble activin receptor type IIb prevents the effects of androgen deprivation on body composition and bone health. Endocrinology. 2010;151(9):4289-4300. [DOI] [PubMed] [Google Scholar]
- 331. Pistilli EE, Bogdanovich S, Goncalves MD, et al. Targeting the activin type IIB receptor to improve muscle mass and function in the mdx mouse model of Duchenne muscular dystrophy. Am J Pathol. 2011;178(3):1287-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Morrison BM, Lachey JL, Warsing LC, et al. A soluble activin type IIB receptor improves function in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2009;217(2):258-268. [DOI] [PubMed] [Google Scholar]
- 333. Pistilli EE, Bogdanovich S, Mosqueira M, Lachey J, Seehra J, Khurana TS. Pretreatment with a soluble activin type IIB receptor/Fc fusion protein improves hypoxia-induced muscle dysfunction. Am J Physiol Regul Integr Comp Physiol. 2010;298(1):R96-R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Akpan I, Goncalves MD, Dhir R, et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes (Lond). 2009;33(11):1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Attie KM, Borgstein NG, Yang Y, et al. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve. 2013;47(3):416-423. [DOI] [PubMed] [Google Scholar]
- 336. Campbell C, McMillan HJ, Mah JK, et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle Nerve. 2017;55(4):458-464. [DOI] [PubMed] [Google Scholar]
- 337. Snodgrass RO, Chico TJA, Arthur HM. Hereditary haemorrhagic telangiectasia, an inherited vascular disorder in need of improved evidence-based pharmaceutical interventions. Genes (Basel). 2021;12(2):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Tillet E, Bailly S. Emerging roles of BMP9 and BMP10 in hereditary hemorrhagic telangiectasia. Front Genet. 2014;5:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Zhang C, Lin Y, Liu Q, et al. Growth differentiation factor 11 promotes differentiation of MSCs into endothelial-like cells for angiogenesis. J Cell Mol Med. 2020;24(15):8703-8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Yung LM, Yang P, Joshi S, et al. ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci Transl Med. 2020;12(543):eaaz5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Verma A, Suragani RN, Aluri S, et al. Biological basis for efficacy of activin receptor ligand traps in myelodysplastic syndromes. J Clin Invest. 2020;130(2):582-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Kubasch AS, Fenaux P, Platzbecker U. Development of luspatercept to treat ineffective erythropoiesis. Blood Adv. 2021;5(5):1565-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Cappellini MD, Taher AT. The use of luspatercept for thalassemia in adults. Blood Adv. 2021;5(1):326-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Jeong Y, Daghlas SA, Xie Y, et al. Skeletal response to soluble activin receptor type IIB in mouse models of osteogenesis imperfecta. J Bone Miner Res. 2018;33(10):1760-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Jeong Y, Daghlas SA, Kahveci AS, et al. Soluble activin receptor type IIB decoy receptor differentially impacts murine osteogenesis imperfecta muscle function. Muscle Nerve. 2018;57(2):294-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Cui Y, Yi Q, Sun W, et al. Molecular basis and therapeutic potential of myostatin on bone formation and metabolism in orthopedic disease. Biofactors. 2020;1-11. 10.1002/biof.1675. [DOI] [PubMed] [Google Scholar]
- 347. Dankbar B, Fennen M, Brunert D, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med. 2015;21(9):1085-1090. [DOI] [PubMed] [Google Scholar]
- 348. Bogdanovich S, Krag TO, Barton ER, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420(6914):418-421. [DOI] [PubMed] [Google Scholar]
- 349. Bogdanovich S, McNally EM, Khurana TS. Myostatin blockade improves function but not histopathology in a murine model of limb-girdle muscular dystrophy 2C. Muscle Nerve. 2008;37(3):308-316. [DOI] [PubMed] [Google Scholar]
- 350. St Andre M, Johnson M, Bansal PN, et al. A mouse anti-myostatin antibody increases muscle mass and improves muscle strength and contractility in the mdx mouse model of Duchenne muscular dystrophy and its humanized equivalent, domagrozumab (PF-06252616), increases muscle volume in cynomolgus monkeys. Skelet Muscle. 2017;7(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Latres E, Pangilinan J, Miloscio L, et al. Myostatin blockade with a fully human monoclonal antibody induces muscle hypertrophy and reverses muscle atrophy in young and aged mice. Skelet Muscle. 2015;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Desgeorges MM, Devillard X, Toutain J, et al. Pharmacological inhibition of myostatin improves skeletal muscle mass and function in a mouse model of stroke. Sci Rep. 2017;7(1):14000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Lach-Trifilieff E, Minetti GC, Sheppard K, et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol. 2014;34(4):606-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Tankó LB, Goldhahn J, Varela A, et al. Does activin receptor blockade by bimagrumab (BYM338) pose detrimental effects on bone healing in a rat fibula osteotomy model? Calcif Tissue Int. 2016;99(3):310-321. [DOI] [PubMed] [Google Scholar]
- 355. Long KK, O’Shea KM, Khairallah RJ, et al. Specific inhibition of myostatin activation is beneficial in mouse models of SMA therapy. Hum Mol Genet. 2019;28(7):1076-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Pirruccello-Straub M, Jackson J, Wawersik S, et al. Blocking extracellular activation of myostatin as a strategy for treating muscle wasting. Sci Rep. 2018;8(1):2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Wagner KR, Fleckenstein JL, Amato AA, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63(5):561-571. [DOI] [PubMed] [Google Scholar]
- 358. Becker C, Lord SR, Studenski SA, et al. ; STEADY Group . Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015;3(12):948-957. [DOI] [PubMed] [Google Scholar]
- 359. Golan T, Geva R, Richards D, et al. LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J Cachexia Sarcopenia Muscle. 2018;9(5):871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Woodhouse L, Gandhi R, Warden SJ, et al. ; STUDY INVESTIGATORS . A phase 2 randomized study investigating the efficacy and safety of myostatin antibody LY2495655 versus placebo in patients undergoing elective total hip arthroplasty. J Frailty Aging. 2016;5(1):62-70. [DOI] [PubMed] [Google Scholar]
- 361. Wagner KR, Abdel-Hamid HZ, Mah JK, et al. Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy. Neuromuscul Disord. 2020;30(6):492-502. [DOI] [PubMed] [Google Scholar]
- 362. Wagner KR, Abdel-Hamid HZ, Mah JK, et al. Corrigendum to “Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy” [Neuromuscular Disorders, Vol. 30 (6) 2020, 492-502]. Neuromuscul Disord. 2021;31(2):167-168. [DOI] [PubMed] [Google Scholar]
- 363. Davies AM, Sutton BJ. Human IgG4: a structural perspective. Immunol Rev. 2015;268(1):139-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Padhi D, Higano CS, Shore ND, Sieber P, Rasmussen E, Smith MR. Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2014;99(10):E1967-E1975. [DOI] [PubMed] [Google Scholar]
- 365. Zhu Y, D’Arienzo C, Lou Z, et al. LC-MS/MS multiplexed assay for the quantitation of a therapeutic protein BMS-986089 and the target protein Myostatin. Bioanalysis. 2016;8(3):193-204. [DOI] [PubMed] [Google Scholar]
- 366. Madireddi M, Malone H, Kukral D, et al. BMS-986089 is a high affinity anti-myostatin adnectin that increases muscle volume in three preclinical species. Neuromuscul Disord. 2016;26:S94-S95. [Google Scholar]
- 367. Morvan F, Rondeau JM, Zou C, et al. Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy. Proc Natl Acad Sci U S A. 2017;114(47):12448-12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Amato AA, Hanna MG, Machado PM, et al. ; RESILIENT Study Extension Group . Efficacy and safety of bimagrumab in sporadic inclusion body myositis: long-term extension of RESILIENT. Neurology. 2021;96(12):e1595-e1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Hanna MG, Badrising UA, Benveniste O, et al. ; RESILIENT Study Group . Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial. Lancet Neurol. 2019;18(9):834-844. [DOI] [PubMed] [Google Scholar]
- 370. Mori-Yoshimura M, Yamashita S, Suzuki N, et al. [Late phase II/III study of BYM338 in patients with sporadic inclusion body myositis (RESILIENT): Japanese cohort data]. Rinsho Shinkeigaku. 2019;59(12):806-813. [DOI] [PubMed] [Google Scholar]
- 371. Garito T, Roubenoff R, Hompesch M, et al. Bimagrumab improves body composition and insulin sensitivity in insulin-resistant individuals. Diabetes Obes Metab. 2018;20(1):94-102. [DOI] [PubMed] [Google Scholar]
- 372. Heymsfield SB, Coleman LA, Miller R, et al. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA Netw Open. 2021;4(1):e2033457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Dagbay KB, Treece E, Streich FC Jr, et al. Structural basis of specific inhibition of extracellular activation of pro- or latent myostatin by the monoclonal antibody SRK-015. J Biol Chem. 2020;295(16):5404-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Nasomyont N, Hornung LN, Wasserman H. Intravenous bisphosphonate therapy in children with spinal muscular atrophy. Osteoporos Int. 2020;31(5):995-1000. [DOI] [PubMed] [Google Scholar]
- 375. Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 2009;39(3):283-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Hastie E, Samulski RJ. Adeno-associated virus at 50: a golden anniversary of discovery, research, and gene therapy success–a personal perspective. Hum Gene Ther. 2015;26(5):257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073-1080. [DOI] [PubMed] [Google Scholar]
- 378. Nakamura T, Sugino K, Titani K, Sugino H. Follistatin, an activin-binding protein, associates with heparan sulfate chains of proteoglycans on follicular granulosa cells. J Biol Chem. 1991;266(29):19432-19437. [PubMed] [Google Scholar]
- 379. Sugino K, Kurosawa N, Nakamura T, et al. Molecular heterogeneity of follistatin, an activin-binding protein. Higher affinity of the carboxyl-terminal truncated forms for heparan sulfate proteoglycans on the ovarian granulosa cell. J Biol Chem. 1993;268(21):15579-15587. [PubMed] [Google Scholar]
- 380. Sugino H, Sugino K, Hashimoto O, Shoji H, Nakamura T. Follistatin and its role as an activin-binding protein. J Med Invest. 1997;44(1-2):1-14. [PubMed] [Google Scholar]
- 381. Hashimoto O, Kawasaki N, Tsuchida K, Shimasaki S, Hayakawa T, Sugino H. Difference between follistatin isoforms in the inhibition of activin signalling: activin neutralizing activity of follistatin isoforms is dependent on their affinity for activin. Cell Signal. 2000;12(8):565-571. [DOI] [PubMed] [Google Scholar]
- 382. Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS. The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell. 2005;9(4):535-543. [DOI] [PubMed] [Google Scholar]
- 383. Mendell JR, Sahenk Z, Malik V, et al. A phase 1/2a follistatin gene therapy trial for becker muscular dystrophy. Mol Ther. 2015;23(1):192-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Kota J, Handy CR, Haidet AM, et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. 2009;1(6):6ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Greenberg SA. Unfounded claims of improved functional outcomes attributed to follistatin gene therapy in inclusion body myositis. Mol Ther. 2017;25(10):2235-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Mendell JR. Reply to letter to the editor. Mol Ther. 2017;25(10):2238-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Gao G, Bish LT, Sleeper MM, et al. Transendocardial delivery of AAV6 results in highly efficient and global cardiac gene transfer in rhesus macaques. Hum Gene Ther. 2011;22(8):979-984. [DOI] [PubMed] [Google Scholar]
- 388. Krysztofiak H, Wleklik M, Migaj J, et al. Cardiac cachexia: a well-known but challenging complication of heart failure. Clin Interv Aging. 2020;15:2041-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Rausch V, Sala V, Penna F, Porporato PE, Ghigo A. Understanding the common mechanisms of heart and skeletal muscle wasting in cancer cachexia. Oncogenesis. 2021;10(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15(7):387-407. [DOI] [PubMed] [Google Scholar]
- 391. Morissette MR, Stricker JC, Rosenberg MA, et al. Effects of myostatin deletion in aging mice. Aging Cell. 2009;8(5):573-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Butcher JT, Ali MI, Ma MW, et al. Effect of myostatin deletion on cardiac and microvascular function. Physiol Rep. 2017;5(23):e13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Castillero E, Akashi H, Najjar M, et al. Activin type II receptor ligand signaling inhibition after experimental ischemic heart failure attenuates cardiac remodeling and prevents fibrosis. Am J Physiol Heart Circ Physiol. 2020;318(2):H378-H390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Lim S, McMahon CD, Matthews KG, Devlin GP, Elston MS, Conaglen JV. Absence of myostatin improves cardiac function following myocardial infarction. Heart Lung Circ. 2018;27(6):693-701. [DOI] [PubMed] [Google Scholar]
- 395. Jayakumar D, Zhang R, Wasserman A, Ash J. Cardiac manifestations in idiopathic inflammatory myopathies: an overview. Cardiol Rev. 2019;27(3):131-137. [DOI] [PubMed] [Google Scholar]
- 396. Allen DL, Hittel DS, McPherron AC. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc. 2011;43(10):1828-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Singh P, Rong H, Gordi T, Bosley J, Bhattacharya I. Translational pharmacokinetic/pharmacodynamic analysis of MYO-029 antibody for muscular dystrophy. Clin Transl Sci. 2016;9(6):302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Suchomel TJ, Nimphius S, Bellon CR, Stone MH. The importance of muscular strength: training considerations. Sports Med. 2018;48(4):765-785. [DOI] [PubMed] [Google Scholar]
- 399. Esposito JG, Thomas SG, Kingdon L, Ezzat S. Growth hormone treatment improves peripheral muscle oxygen extraction-utilization during exercise in patients with human immunodeficiency virus-associated wasting: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89(10):5124-5131. [DOI] [PubMed] [Google Scholar]
- 400. Moyle GJ, Daar ES, Gertner JM, et al. ; Serono 9037 Study Team . Growth hormone improves lean body mass, physical performance, and quality of life in subjects with HIV-associated weight loss or wasting on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2004;35(4):367-375. [DOI] [PubMed] [Google Scholar]
- 401. Andrews MA, Magee CD, Combest TM, Allard RJ, Douglas KM. Physical effects of anabolic-androgenic steroids in healthy exercising adults: a systematic review and meta-analysis. Curr Sports Med Rep. 2018;17(7):232-241. [DOI] [PubMed] [Google Scholar]
- 402. Al-Zaidy SA, Sahenk Z, Rodino-Klapac LR, Kaspar B, Mendell JR. Follistatin gene therapy improves ambulation in Becker muscular dystrophy. J Neuromuscul Dis. 2015;2(3):185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189-222. [DOI] [PubMed] [Google Scholar]
- 404. Zhou H, Meng J, Malerba A, et al. Myostatin inhibition in combination with antisense oligonucleotide therapy improves outcomes in spinal muscular atrophy. J Cachexia Sarcopenia Muscle. 2020;11(3):768-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Bhattacharya I, Pawlak S, Marraffino S, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of domagrozumab (PF-06252616), an antimyostatin monoclonal antibody, in healthy subjects. Clin Pharmacol Drug Dev. 2018;7(5):484-497. [DOI] [PubMed] [Google Scholar]