Abstract
Pheochromocytomas/paragangliomas are characterized by a unique molecular landscape that allows their assignment to clusters based on underlying genetic alterations. With around 30% to 35% of Caucasian patients (a lower percentage in the Chinese population) showing germline mutations in susceptibility genes, pheochromocytomas/paragangliomas have the highest rate of heritability among all tumors. A further 35% to 40% of Caucasian patients (a higher percentage in the Chinese population) are affected by somatic driver mutations. Thus, around 70% of all patients with pheochromocytoma/paraganglioma can be assigned to 1 of 3 main molecular clusters with different phenotypes and clinical behavior. Krebs cycle/VHL/EPAS1-related cluster 1 tumors tend to a noradrenergic biochemical phenotype and require very close follow-up due to the risk of metastasis and recurrence. In contrast, kinase signaling–related cluster 2 tumors are characterized by an adrenergic phenotype and episodic symptoms, with generally a less aggressive course. The clinical correlates of patients with Wnt signaling–related cluster 3 tumors are currently poorly described, but aggressive behavior seems likely. In this review, we explore and explain why cluster-specific (personalized) management of pheochromocytoma/paraganglioma is essential to ascertain clinical behavior and prognosis, guide individual diagnostic procedures (biochemical interpretation, choice of the most sensitive imaging modalities), and provide personalized management and follow-up. Although cluster-specific therapy of inoperable/metastatic disease has not yet entered routine clinical practice, we suggest that informed personalized genetic-driven treatment should be implemented as a logical next step. This review amalgamates published guidelines and expert views within each cluster for a coherent individualized patient management plan.
Keywords: pheochromocytoma, paraganglioma, molecular cluster, diagnostics, follow-up, treatment
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS
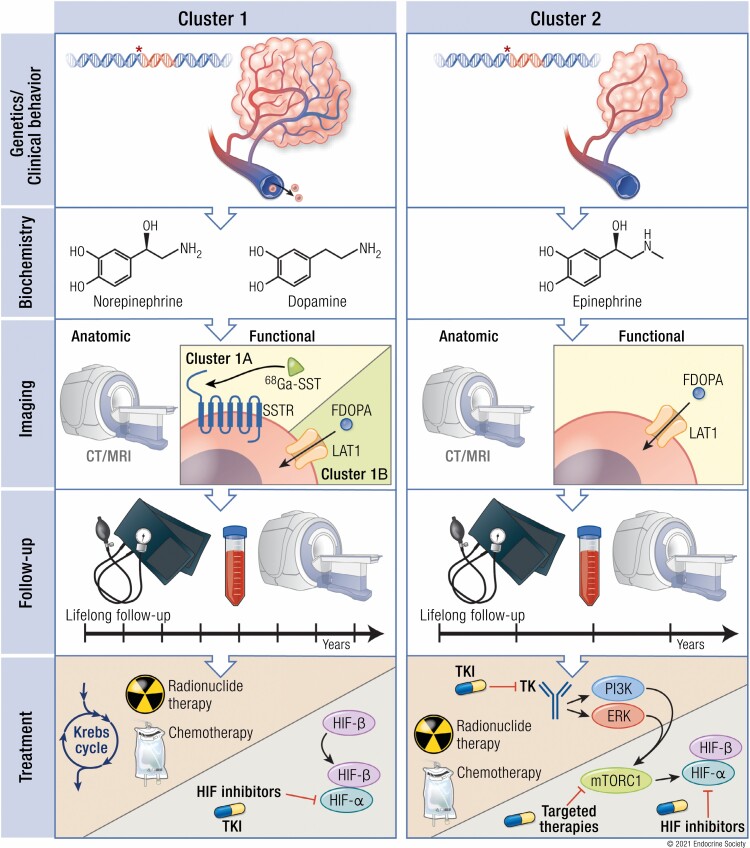
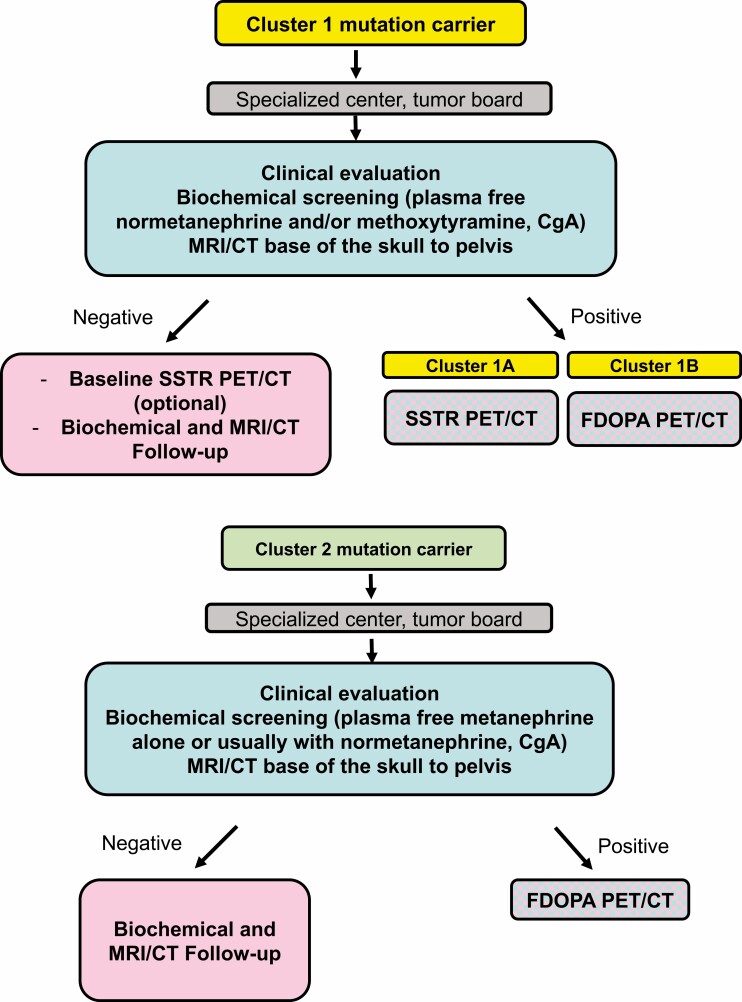
Pheochromocytomas/paragangliomas (PPGLs) are known to form 3 specific clusters based on their underlying germline or somatic mutations: pseudohypoxia-related clusters 1A and 1B; kinase signaling–related cluster 2; and Wnt signaling–related cluster 3.
These clusters also translate into clinical, biochemical, and imaging signatures which may guide follow-up and therapy, facilitating a cluster-specific (personalized) patient management plan.
Cluster 1 and probably cluster 3 show a more aggressive phenotype with a higher metastatic risk compared with cluster 2.
Cluster 1 tumors (mostly located extra-adrenally) tend to have a noradrenergic biochemical phenotype with tendency to sustained hypertension, while cluster 2 tumors (mostly located within the adrenal) tend to have an adrenergic biochemical phenotype with intermittent catecholamine secretion concomitant with episodic symptoms.
For cluster 1A, the most sensitive functional imaging modality is [68Ga]-DOTA-SSA PET/CT, while for cluster 1B and cluster 2 tumors [18F] FDOPA PET/CT is more sensitive.
All patients with a history of a PPGL and all asymptomatic mutation carriers require lifelong follow-up, individualized according to their mutation status and disease characteristics.
The therapy of choice is surgery whenever possible; for inoperable/metastatic disease, systemic therapy options include chemotherapy, radionuclide therapy, and tyrosine kinase inhibitors; however, although it is not yet established in routine clinical practice, genetically driven cluster-specific therapy should be the logical next step.
Background and Relevance
Pheochromocytomas (PCCs) and paragangliomas (PGLs) (together referred to as PPGLs) are endocrine tumors originating from neural crest–derived cells of the adrenal medulla or from the sympathetic (mostly below the diaphragm) or parasympathetic (anterior thoracic and head and neck) paraganglia. According to the most recently published guideline by the Working Group on Endocrine Hypertension of the European Society of Hypertension, the likelihood of a PPGL can be assessed by the combination of prevalence (low in patients with signs and symptoms, high in patients with an adrenal incidentaloma or susceptibility mutations or family or past history of a PPGL) and the presence of certain clinical features (1).
According to the latest World Health Organization (WHO) classification, all PPGLs are considered to have metastatic potential, replacing the previous term “malignant” (2). Since there are no reliable biological, molecular, or histological markers for predicting metastatic spread, the WHO has defined PPGL-related malignancy as the presence of distant metastases at sites where chromaffin cells are physiologically absent (eg, bone and lymph nodes) (2). Current evaluation of the metastatic potential of a PPGL is based on a multifactorial risk assessment according to tumor size (≥5 cm), extra-adrenal location, the presence of a SDHB mutation, a dopaminergic phenotype (eg, plasma methoxytyramine more than 3-fold above the upper reference limit) and high Ki-67 index (1, 3-6). Immunohistochemical staining of tumor tissue for succinate dehydrogenase subunit B (SDHB) not only provides a valuable method for identifying patients likely to have SDHB mutations, but also for determining functionality of SDHB variants of unknown significance. The method has reasonably high sensitivity but a lower specificity of around 84% (7). The combination of SDHB immunohistochemistry and metabolite profiling with machine learning algorithms considerably improves the accuracy of both methods for identifying functional SDHB mutations (8). These methods for screening for SDHx mutations are reasonable for quickly identifying patients for high metastatic risk. However, accurate genetic testing remains indispensable.
In addition to the above, the Pheochromocytoma of the Adrenal Gland Score (PASS) and Grading of Adrenal Pheochromocytoma and Paraganglioma (GAPP) score are the only globally used risk-stratification systems based on histological features (the GAPP score also includes PGLs and additionally involves the catecholamine phenotype) (9, 10). A PASS score of 4 or higher indicates potential malignant behavior with a high sensitivity of close to 100%, but with a lower specificity (75% and 76%, respectively, in 2 studies) (9, 11). Thus, although metastatic behavior cannot be reliably predicted with a PASS score of 4 or higher, the “rule-out” of malignant behavior with a PASS score of <4 or a GAPP score of <3 seems to be fairly reliable (9-13). It also seems reasonable to include all aforementioned risk factors together with the PASS/GAPP score for a more accurate risk assessment. However, until this has been proven in larger patient cohorts, regular follow-up of all patients remains mandatory (14).
Current studies based on large patient cohorts report that 10% to 15% of PCCs and a significantly higher proportion of PGLs (35%-40%) develop metastases (5, 15-21). However, currently there is no highly effective medical therapy available (4, 22). The median overall survival of patients with metastatic PPGLs has recently been reported to be 7 years (23). In a large meta-analysis, 5- and 10-year mortality rates for patients with metastatic PPGLs were 37% (7 studies, n = 738) and 29% (2 studies, n = 55), respectively. The overall mortality rate in patients with metastatic PGLs was 46%, whereas overall mortality rate in patients with metastatic PCCs was 53%. In patients with metastatic skull base and neck PGLs, the overall mortality rate ranged from 34% to 56% (24).
Uniquely, PPGLs have the highest reported degree of heritability among all tumors. When currently known germline mutations are taken into account, around 30% to 35% of patients with PPGLs are affected by germline mutations in various susceptibility genes, and a further 35% to 40% show somatic driver mutations (25-30). However, these numbers only apply to Caucasians, since among the Chinese population current evidence indicates a lower prevalence of germline mutations (21%) and a higher proportion of somatic mutations (46%) (30). In combination, germline and somatic mutations in more than 20 PPGL driver genes have been identified in around 70% of all patients with PPGLs, and these are divided into 3 main molecular clusters: pseudohypoxia cluster 1 (1A and 1B), kinase-signaling cluster 2, and Wnt signaling cluster 3. Assignment to a specific molecular cluster is associated with differences in biochemical phenotype, clinical behavior, and long-term prognosis. Aberrations in genes related to telomere maintenance (inactivation of ATRX, activation of TERT) and chromatin maintenance (SETD2) appear to additionally modify the course of disease (25, 31, 32).
Genetic testing is recommended for every patient since confirmation of the cluster affiliation has been shown to have a positive impact on PPGL management and outcomes (1, 33). Next-generation sequencing is the preferred technique to determine all relevant genetic variants in one single run (1).
The increase in genetic knowledge over the past few years has led to significant progress in personalized management. Genetic profiling has accelerated new discoveries of discrete and distinctive clinical, biochemical, and imaging signatures that allow personalized PPGL diagnosis, management, and long-term follow-up (4, 34, 35). Although cluster-specific therapy of inoperable/metastatic disease has not yet fully entered routine clinical practice, personalized genetic-driven treatment decisions based on germline and somatic mutation testing, targeted drug testing in patient-derived primary cultures, metabolomics, proteomics, profiling, and machine learning approaches, have now begun to be integrated into clinical care (8, 23, 36, 37).
In contrast to most previous publications in personalized medicine that have generally focused separately on clinical behavior, genetics, biochemistry, imaging, therapy and follow-up, this review instead focuses on specific PPGL clusters and incorporates all these modalities in a holistic assessment approach. For this approach we amalgamated all recently published guidelines and expert views on biochemistry, imaging algorithms, follow-up, and routine screening of mutation carriers and therapy; for the latter we have incorporated potential cluster-specific therapies within each cluster into a coherent and individualized patient management plan.
Personalized Management: Molecular Cluster 1
Overview: Pathophysiology and Signaling Pathways
Cluster 1 is termed the pseudohypoxic cluster since the tumors of this cluster are characterized by activation of pathways that mimic hypoxia signaling. Currently, cluster 1 is divided into 2 subclusters (cluster 1A and 1B) based on the position of the gene mutation either in the Krebs cycle (cluster 1A) or the hypoxia-signaling pathway (cluster 1B) (Fig. 1). Cluster 1A Krebs cycle-related genes (almost 100% are germline mutations, 4%-12% of sporadic PPGLs) include succinate dehydrogenase subunits (SDHx [SDHA, SDHB, SDHC, SDHD]) (germline), succinate dehydrogenase complex assembly factor-2 (SDHAF2) (germline), fumarate hydratase (FH) (germline), malate dehydrogenase 2 (MDH2) (germline), mitochondrial glutamic-oxaloacetic transaminase (GOT2) (germline), 2-oxoglutarate-malate carrier (SLC25A11) (germline), dihydrolipoamide S-succinyltransferase (DLST) (germline), and isocitrate dehydrogenase 1 (IDH1) (somatic). Cluster 1B VHL/EPAS1-related genes (about 25% are germline mutations) comprise Egl-9 prolyl hydroxylase-1 and -2 (EGLN1/2 encoding PHD1/2) (germline), von Hippel–Lindau (VHL) tumor suppressor (germline/somatic), hypoxia-inducible factor 2α (HIF2A/EPAS1) (somatic) (Fig. 1), and iron regulatory protein 1 (IRP1) (1 case report) (25-27, 29, 34, 38-42).
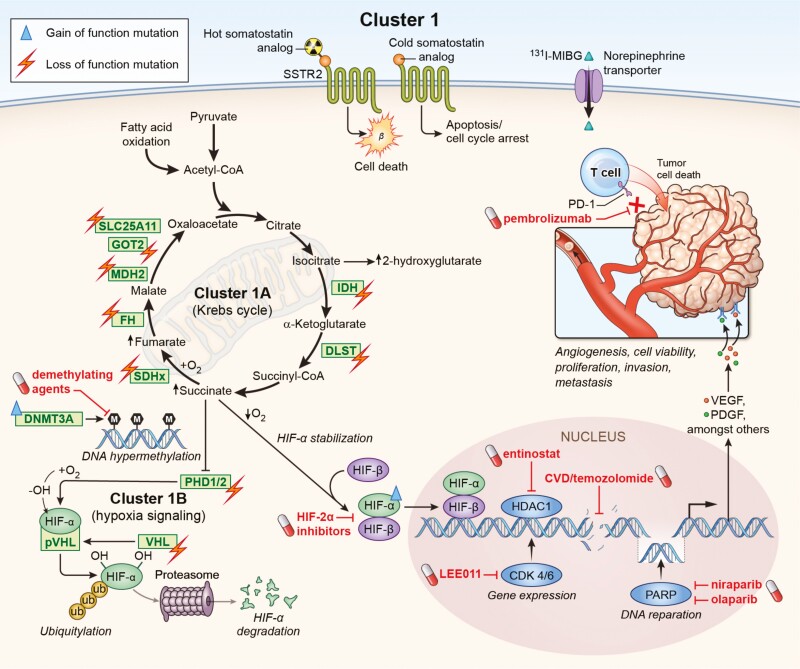
Figure 1.
Gene mutations impairing either Krebs cycle (cluster 1A) or hypoxia-signaling (cluster 1B) are associated with the development of pseudohypoxic cluster 1 PPGLs. These molecular changes offer potential targets for personalized medicine. Loss of function mutations in SDHA[AF2]/B/C/D, FH, MDH2, IDH, GOT2, SLC25A11, and DLST affect the Krebs cycle, resulting in severe impairment of mitochondrial oxidative phosphorylation and an accumulation of oncometabolites such as succinate. Accumulation of these oncometabolites as well as mutations (PDH1/2, VHL) leading to a decreased degradation of HIF-α result in an enhanced expression and stabilization of HIF-α. Moreover, gain-of-function mutation in HIF2A underlines the importance of hypoxia signaling in cluster 1 PPGLs. Highlighted in red are potential drugs that could be used to negate the molecular changes in cluster 1 PPGLs, which are in preclinical and clinical evaluation. In addition, targeting the somatostatin receptor (possibly higher expression compared to cluster 2) or the norepinephrine transporter (possibly lower expression compared to cluster 2) can be used to treat these tumors. Further approaches address immune checkpoints such as PD-1 or DNA repair mechanisms.
Cluster 1A mutations (SDHA[AF2]/B/C/D, FH, MDH2, IDH, GOT2, SLC25A11 and DLST) disrupt the Krebs cycle and result in severe impairment of mitochondrial oxidative phosphorylation (43). Consequently, ATP production is severely affected and is dependent on increased cellular glycolysis (Warburg effect) (44). Although glycolysis is less efficient in ATP production than oxidative phosphorylation, it is a fast reaction that can be increased 30-fold. This compensates for lost ATP production from cluster 1A mutation–related impairments in the electron transport chain and oxidative phosphorylation. The impairment of genes of the Krebs cycle leads to the accumulation of the oncometabolites succinate, fumarate, or 2-hydroxyglutarate. This in turn promotes DNA hypermethylation, inactivation of tumor suppressor genes (including PHD1/2), resulting in less hypoxia-inducible factor (HIF)-α hydroxylation and significantly lower HIF-α ubiquitination/degradation. This causes HIF-α stabilization, mitochondrial DNA impairment, collagen instability, and most likely an abnormal immune microenvironment (45-48).
HIF-α ubiquitination is VHL-dependent. Loss of function mutations in VHL that result in impaired binding of the VHL protein to HIF-α therefore stabilize HIF-α and lead to its accumulation. Through HIF-α stabilization, cluster 1 mutations promote angiogenesis (eg, vascular endothelial growth factor (VEGF)/PDGF transcription), tumor extravasation, migration, invasion, metastasis, and other cellular processes (49-51). In particular, increased expression and stabilization of HIF-2α are characteristic features of cluster 1 PPGLs compared with cluster 2 PPGLs (52-54).
In addition, mutations in several other genes have been identified that are directly involved in DNA hypermethylation and are also associated with PPGLs; these include histone subunit gene (H3F3A) (postzygotic) (55), DNA methyltransferase (DNMT3A) (germline) (56), and the tumor suppressor gene KIF1Bß (germline/somatic) (57, 58). A hypermethylated phenotype together with the increased activation of HIF-2α may synergistically result in the acquisition of metastatic features of SDHB-mutated PPGLs (59). Taken together, these studies provide a rationale for targeting HIF-2α and DNA methylation in cluster 1 PPGLs.
| Practical tip/synthesis: |
|---|
| • Fig. 1 summarizes the pseudohypoxia-associated cluster 1 with all its loss- and gain-of-function mutations. • Cluster 1A comprises mutations in the Krebs cycle-associated genes: SDHA[AF2]/B/C/D, FH, MDH2, IDH, GOT2, SLC25A11 and DLST. • Cluster 1B includes mutations in VHL/EPAS1-related genes: PHD1/2, VHL, HIF2A/EPAS1, IRP1. • These mutations lead to stabilization of HIF-2α and thus, among other actions, promote angiogenesis, tumor progression, migration, invasion, and metastasis. |
Penetrance, Epidemiology, and Metastatic Risk
Patients who belong to the PPGL pseudohypoxia cluster 1 group, especially those with SDHB mutations, often present at a young age (<20 years of age, some presenting at 5 years of age or less) and are predisposed to multiple and recurrent tumors with metastatic spread (60-63). SDHA/B/C/D mutations are inherited in an autosomal dominant fashion (for SDHD/AF2: penetrance with paternal inheritance and maternal imprinting). In one study, the penetrance of SDHB-related PPGLs was 21% by the age of 50 and 42% by the age of 60 (64). Another study found penetrance of SDHB-related PPGLs to be 22% by the age of 60 and 44% by the age of 80 (and for SDHD-related PPGLs, 43% by the age of 60) (65). A further study found penetrance of SDHB-related PPGLs to be 50% by the age of 85, and higher in males than in females (66). Lifetime penetrance has been shown to be <50% in SDHB mutation carriers (67). In another study, estimated lifetime penetrance for SDHB-, SDHC- and SDHA-related PPGLs was 22%, 8%, and 2%, respectively (68) (Table 1). In a cohort of 30 children with an SDHB mutation (median age of genetic testing 6.8 years) 3/30 (10%) developed PPGLs (at ages of 15, 16, and 18, respectively) during a median follow-up period of 5 years (62).
Table 1.
Penetrance of cluster 1–related PPGLs
| Penetrance | SDHB | SDHA | SDHC | SDHD | VHL |
|---|---|---|---|---|---|
| 50 years | 21% | ||||
| 60 years | 42% and 22%, respectively | 43% | |||
| 80 years | 25-65% | ||||
| Lifetime estimate | 22% | 1.7% | 8.3% | 15-20% |
The lifetime penetrance of germline VHL-related PPGLs is overall about 15% to 20% (67, 69). Penetrance among VHL mutation carriers varies considerably according to the nature of the mutation; in particular, missense mutations in type 2 VHL syndrome are more often associated with PPGL than exon deletions and truncations in type 1 VHL syndrome (70).
The penetrance of DLST-related PPGLs is not known due to the rare nature of PPGLs with these mutations; the prevalence of DLST-related PPGLs is around 1% among PPGLs not related to other known susceptibility mutations. DLST-related PPGLs regularly present as multiple PPGLs (38).
At least 50%-60% of all patients with metastatic PPGL carry cluster 1 mutations (1, 23, 49, 71). In a retrospective study investigating 169 patients, 50% of all patients with metastatic disease had cluster 1 tumors (42% SDHB-related tumors), only 4% had cluster 2 tumors, and 46% had apparently sporadic disease (23). In a subsequent study, 60.5% of all metastatic cases were in the cluster 1 group and only 2.3 % in the cluster 2 group; the remaining patients had negative genetic test results (49). Importantly, in that latter study the higher risk of developing metastatic disease of patients with cluster 1 vs cluster 2 tumors was independent of the presence of SDHB mutations.
In a systematic literature review, the metastatic risk of patients with cluster 1 mutated tumors was assessed (37): 24.3% of patients with cluster 1 tumors showed metastases—in the subgroup of cluster 1A tumors, 40.5% were metastatic with the highest percentage of metastatic disease among the SDHB mutation carriers (46.6%); in the subgroup of cluster 1B tumors, 11% were metastatic. Of all patients with cluster 3 tumors 11.4% showed metastases, compared with only 4.1% of those with cluster 2 mutations.
Among 64 children diagnosed with an SDHB-mutated tumor at a median age of 13, 70% developed metastases at a median age of 16. After first diagnosis, metastases usually developed in the first 2 years and in years 12 to 18 after diagnosis (61). Overall, the highest metastatic risk is reported for SDHB (35%-75%), SDHA (30%-66%), and HIF2A/EPAS1 mutation carriers (>30%) (37, 40, 49, 63, 72-79). Moreover, there also seems to be an increased metastatic risk for patients with FH mutations (80). An intermediate risk (15%-29%) has been shown for SDHD mutation carriers (49, 76) and an intermediate-to-low risk for SDHC (76) and VHL (5%-8%) mutation carriers (49, 69, 81, 82) (Table 2).
Table 2.
Metastatic risk and location of cluster 1–related PPGLs
| Mutation | Metastatic risk | Location |
|---|---|---|
| SDHB | 35-75% | Sympathetic/parasympathetic PGLs, less commonly PCCs |
| SDHA | 30-66% | Sympathetic/parasympathetic PGLs, very rarely PCCs |
| SDHC | low | Sympathetic/parasympathetic PGLs, less commonly PCCs |
| SDHD | 15-29% | Sympathetic/parasympathetic (often head and neck) PGLs and PCCs |
| HIF2A/EPAS1 | >30% | Sympathetic/rarely parasympathetic PGLs and PCCs |
| VHL | 5-8% | PCCs, less commonly sympathetic PGLs, and rarely parasympathetic PGLs |
| SDHAF2 | not known | Parasympathetic (head and neck) PGLs |
Although cluster 1 is associated with the highest metastatic risk, patients with tumors of this cluster group only showed a trend to shorter overall survival in a multivariate analysis (37). Interestingly, although 70% of children with SDHB-related tumors developed metastases at a median age of 16, the estimated 5-, 10-, and 20-year overall survival rate was relatively favorable (100%, 97%, and 78%, respectively) (61). Recent studies consistently report that apart from the absence of metastases, both younger age (<40 years in 1 study) and smaller size of the primary tumor (<5 cm) at first diagnosis is associated with a better prognosis and survival (1, 3, 23, 37).
Of note, among the cluster 1 group there are some notable differences in prevalence for certain tumor locations. Cluster 1 Krebs cycle mutations are mostly associated with extra-adrenal tumors (PGLs). In particular, SDHB- and SDHC-related tumors are mainly sited in extra-adrenal locations (sympathetic/parasympathetic PGLs) and are much less commonly found as PCCs (83). SDHD-related tumors are mainly associated with head and neck PGLs, but also occur with lower prevalence at other extra-adrenal locations or within the adrenals (84). SDHA mutations are linked to sympathetic and parasympathetic PGLs (85). HIF2A/EPAS1- and FH-associated tumors can occur as PGLs and PCCs. VHL mutations most commonly lead to PCCs (50% bilateral) and less frequently to PGLs, the latter only occasionally in the head and neck (69, 81, 82). Head and neck paragangliomas (HNPGLs), which are mostly associated with SDHD and SDHAF2 mutations and less commonly with SDHB mutations, appear to be associated with less aggressive behavior and better survival compared to those at other locations (23).
| Practical tip/synthesis: |
|---|
| • Tables 1 and 2 summarize the penetrance, metastatic risk, and tumor location related to the different cluster 1 mutations. • Estimated lifetime penetrance for SDHx and VHL-related PPGLs is < 50%. • At least 50% to 60% of metastatic tumors carry cluster 1 mutations and only about 2% to 4% of metastatic tumors carry cluster 2 mutations. • Cluster 1A–related tumors confer a metastatic risk of ~40%, with the highest risk for SDHB-related (35%-75%) and SDHA-related PPGLs (30%-66%). The metastatic risk for cluster 1B HIF2A/EPAS1-related PPGLs is >30%. • SDHx-mutated tumors are mostly located at extra-adrenal locations, while VHL, FH, and HIF2A/EPAS1-related tumors are located at both intra- and extra-adrenal sites. |
Clinical Presentations
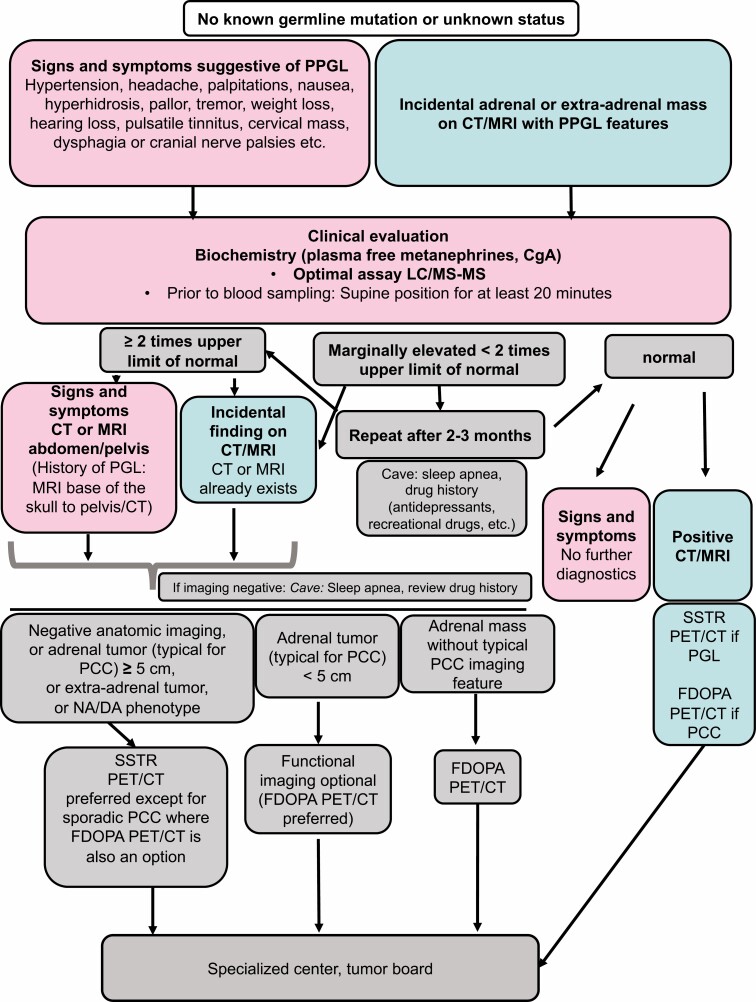
In general, testing for PPGL is usually based on one of several reasons: a known germline mutation, a previous history of a PPGL, an incidentally discovered adrenal or extra-adrenal mass compatible with a PPGL, or clinical signs and symptoms (1).
However, many clinical signs and symptoms are relatively nonspecific, such as headache or hypertension (in an increasingly obese population). Nevertheless, some signs and symptoms are more prominent in screened patients with than without PPGL; from this a score system for specific signs and symptoms has been developed to triage patients according to their likelihood of having PPGLs (−1 to +7 points) (applies to all clusters) (86): 1 point for each specific sign: pallor, hyperhidrosis, tremor (max. 3 points); 1 point for each specific symptom: palpitations, nausea (max. 2 points)—and, in addition, 1 point for a body mass index (BMI) < 25 kg/m2 and 1 point for a heart rate of 85 beats per minute (bpm) or higher, while for obesity (BMI > 30 kg/m2) 1 point is subtracted (86). A high clinical feature score (3 points or higher) indicates a 5.8-fold higher likelihood of having a PPGL compared with a lower score (86).
Moreover, postural hypotension, anxiety/panic, sense of doom, vomiting, weakness, abdominal/chest pain, constipation, weight loss, fasting hyperglycemia (up to 50%) (presenting as diabetes mellitus type II), paresthesiae, dyspnea, flushing (rarely) and visual disturbances may occur as a consequence of catecholamine secretion and subsequent adrenoceptor overstimulation. This basically applies to all clusters, although cluster-specific differences have been reported (86): Patients with cluster 1 PPGLs have lower basic symptom scores and less often suffer from tremor, anxiety/panic, and pallor (related to catecholamine excess) compared with patients with cluster 2 PCC (86). Some reports suggest that patients with cluster 1-related PPGLs may present more often with sustained hypertension due to the continuous release of norepinephrine into the circulation, while patients with cluster 2–related PPGLs more commonly present with paroxysmal symptoms due to episodic excessive tumoral epinephrine secretion (see below under “Personalized Management: Molecular Cluster 2” subsection “Clinical Presentations”) (74, 87).
PPGL-induced attacks (so-called “spells”)—less likely in cluster 1 PPGLs—may be triggered by certain medications, food, beverages (containing tyramine such as red wine and beer), surgery, anesthesia, endoscopy, severe stress, or elevated intra-abdominal pressure (palpation, defecation, pregnancy) (88, 89). Medications that have the potential to induce a catecholamine crisis include glucocorticoids, metoclopramide, droperidol, monoamine oxidase inhibitors, tricyclics (along with other antidepressants), opiates (eg, morphine, fentanyl), naloxone, glucagon, certain antibiotics (linezolid), drugs for obesity management (phentermine, sibutramine), and chemotherapy (90).
Nevertheless, some patients may be asymptomatic, especially those with small (<2 cm) tumors where there is low catecholamine production or more generally in cases where tumors produce and metabolize but do not secrete appreciable amounts of catecholamines (91-94). SDHx-mutated and other cluster 1-related PPGLs have lower catecholamine contents than other tumors; in some cases, particularly for PGL in the head and neck, the tumors may be nonfunctional (no catecholamine production, also known as “silent”). For these cases, identification based on catecholamine-related signs and symptoms or biochemical testing is not possible (3, 60, 95). Measurements of chromogranin A, a biomarker of neuroendocrine tumors, may be useful in some of these cases (96-98).
Catecholamine-related signs and symptoms of patients with metastatic PPGLs (mostly related to cluster 1) are mainly secondary to secretion of norepinephrine; in contrast, the signs and symptoms of other PPGLs (but particularly those associated with cluster 2 mutations) can reflect additional secretion of epinephrine. Despite these differences, signs and symptoms per se cannot be used to reliably distinguish metastatic from nonmetastatic patients (99).
| Practical tip/synthesis (applies to all clusters): |
|---|
| • A clinical feature score (−1 to +7 points) for signs and symptoms to triage patients according to their likelihood of PPGLs has very recently been published (86): - Pallor +1 point - Hyperhidrosis +1 point - Palpitations +1 point - Tremor +1 point - Nausea +1 point - Body mass index (BMI) < 25 kg/m2 +1 point - Heart rate of 85 bpm or higher + 1 point - Obesity (BMI > 30 kg/m2) −1 point • 5.8-fold higher probability of a PPGL with a high score of 3 or more. • Cluster 1–related PPGLs are more likely to be associated with lower basic symptom scores and sustained hypertension, compared with cluster 2-related PPGLs. • Cluster 2–related PPGLs are more likely to be associated with higher basic symptom scores, an episodic nature of symptoms, with tremor, anxiety/panic and pallor, and older age at first diagnosis, compared with cluster 1–related PPGLs. |
Biochemistry
In contrast to cluster 2, most cluster 1 PPGLs present with a noradrenergic phenotype, as assessed by elevated plasma concentrations of normetanephrine and no or relatively small increases in metanephrine (53, 92). These tumors may also be associated with or without elevations of plasma dopamine assessed by 3-methoxytyramine; large increases in plasma 3-methoxytyramine relative to normetanephrine define the dopaminergic phenotype (92). Almost all urinary dopamine is derived from renal uptake and decarboxylation of circulating L-dopa and cannot be used to determine tumoral dopamine production (100, 101).
PPGLs of the cluster 1 group are characterized by lower tumoral catecholamine contents, but higher rates of catecholamine secretion per mass of tumor tissue, compared with cluster 2 adrenergic tumors (74). This is potentially of clinical relevance since the higher rates of catecholamine secretion per mass of tumor tissue may reflect a more continuous pattern of secretion in noradrenergic than adrenergic tumors. SDHB-related PPGLs, in particular, present with lowest tumoral catecholamine contents and, outside of screening programs, large tumor size at diagnosis (see also under “Personalized Management: Molecular Cluster 1” subsection “Clinical Presentations”) (5). Possibly, large tumor size at diagnosis might reflect in part the low tumoral contents of catecholamines and often dopaminergic biochemical features that might be expected to result in an asymptomatic clinical presentation.
Increases of plasma free normetanephrine and/or 3-methoxytyramine with no or minimal increases of metanephrine (optimally measured via liquid chromatography–tandem mass spectrometry [LC-MS/MS]) point uniquely and accurately to the diagnosis of a cluster 1 PPGL (1, 6, 92, 102-105). Exceptions to this “rule” include the biochemically silent head and neck PGLs and other silent extra-adrenal tumors with mutations in SDHB, with limited amounts of catecholamines in tumor tissue and no significant increases in plasma normetanephrine or 3-methoxytyramine (60, 95). The higher risk of metastasis in noradrenergic than adrenergic PPGL (29.1% nonadrenergic vs 10.4% adrenergic) (49) most likely simply reflects the association of the former with cluster 1 mutations and the latter with cluster 2 mutations. In contrast, a dopaminergic phenotype appears to be an independent risk factor of metastatic disease (5).
The association of cluster 1 mutations with a noradrenergic or dopaminergic phenotype serves as an excellent example of how catecholamine phenotypes are linked to genetic abnormalities: tumors due to cluster 1 mutations with a noradrenergic phenotype are associated with higher expression of HIF-2α/EPAS1 than other tumors (44); they also involve mutations that lead to stabilization of HIF-2α, an important player that blocks glucocorticoid-induced expression of phenylethanolamine N-methyl transferase (PNMT), the enzyme that converts norepinephrine to epinephrine (54, 106).
In general, for the diagnosis of PPGLs, plasma free normetanephrine, metanephrine, and 3-methoxytyramine are superior to the measurement of the urinary metabolites (104). For the plasma measurements, more than a 2-fold increase above upper cutoffs of reference intervals provides a high suspicion of a PPGL; however, this is possible only with accurate measurement methods (ideally, LC-MS/MS) and appropriately applied pre-analytics (such as blood sampling after remaining in a supine position for at least 20 minutes) (1, 93, 107, 108).
At some centers, the enzyme-linked immunosorbent assay (ELISA) method has been used for measurement of plasma free metanephrines. However, as clarified in a study by Weismann et al (105), the ELISA marketed by one manufacturer measures plasma normetanephrine and metanephrine at 50% to 60% lower than by LC-MS/MS. Based on the interpretations supplied by the manufacturer according to their cutoffs, the diagnostic sensitivity of the ELISA was determined to be only 74% compared with 100% for the LC-MS/MS method. This means that with the ELISA method it can be expected that a quarter of patients with PPGLs may be missed. The ELISA method is therefore not recommended for routine use unless there is no alternative.
In an intrapatient longitudinal comparison, plasma free metanephrine, normetanephrine, and 3-methoxytyramine levels correlated with tumor burden and progress, which is relevant for the staging of metastatic cluster 1 associated PPGLs (92, 102). Aside from emergency situations, biochemical testing should almost always precede imaging (1).
| Practical tip/synthesis (cluster 1): |
|---|
| • Noradrenergic/dopaminergic phenotype (assessed by plasma free normetanephrine/3-methoxytyramine). • Low catecholamine content but constant rates of release/secretion. |
| Practical tip/synthesis (applies to all clusters): |
| • A general diagnostic flow-chart is provided by Fig. 2, while a cluster-specific diagnostic flow-chart is provided by Fig. 3. • The “gold standard” in diagnosis/screening/follow-up: plasma free metanephrines (superior to catecholamines, superior to urinary metanephrines). • Measurement optimally via LC-MS/MS. • Supine position for at least 20 minutes before taking blood. • High suspicion for a PPGL with more than a 2-fold increase above reference interval upper cutoffs. • Intrapatient longitudinal comparison: plasma free metanephrine levels correlate with tumor burden and progression. • Biochemistry should always precede imaging (exception: emergency). |
Imaging
Cluster 1 PPGLs have high metastatic risk due to (pseudo)hypoxia-induced cellular changes including the generation of oncometabolites; the tumors are characterized by low catecholamine contents, sometimes with a dopaminergic phenotype (an independent risk factor related to poor prognosis). The latter features may contribute to delayed diagnosis (due to lack of clinical signs and symptoms), therefore implying need of various PPGL-specific functional imaging modalities during screening and follow-up; this may be especially important in SDHx-related PPGLs. However, radiation exposure from anatomic and functional imaging must be carefully considered and often limited, especially for genetically predisposed and asymptomatic tumorfree carriers as well as children who will need lifelong follow-up (109).
Thus, previous and current evidence (including the European Society of Endocrinology Clinical Practice Guideline on pheochromocytoma; the European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard for radionuclide imaging of pheochromocytoma; and most recently, the Working Group on Endocrine Hypertension of the European Society of Hypertension) have recommended a specific guide for diagnosis, screening, and follow-up using biochemical evaluation and imaging modalities for cluster 1 mutation PPGLs, including mutation carriers (1, 35, 110).
In general, computed tomography (CT) imaging has a high sensitivity (around 100%) but a low specificity (50%) for the screening of adrenal tumors (PCCs). Typically, PCCs are of soft tissue attenuation and are generally more than 10 Hounsfield units (HU) and demonstrate a marked enhancement that can be heterogeneous due to cystic or degenerated regions within the lesion. On magnetic resonance imaging (MRI), a PCC is classically described as appearing “light-bulb” bright on T2-weighted imaging and is usually hypointense on T1-weighted imaging, although the presence of fat or hemorrhage could lead to high signal intensity on T1 (111).
PCCs mostly occur in association with cluster 1B VHL/EPAS1-related (see under “Personalized Management: Molecular Cluster 1” subsection “Penetrance, Epidemiology, and Metastatic Risk”) or cluster 2–related disease.
However, CT imaging is less sensitive for the screening of certain extra-adrenal tumors (eg, head and neck and sympathetic PGLs) associated with cluster 1A SDHx mutations (112, 113). Nevertheless, for head and neck PGLs, CT imaging shows better spatial resolution and fewer motion artifacts compared with MRI. CT can also precisely determine tumor extension into the temporal bone, whereas MRI provides better contrast for evaluation of extension into the surrounding soft tissue. Overall, both modalities provide complementary information for assessment of locoregional extension and determination of multiplicity.
For screening purposes of extra-adrenal tumors (head and neck and sympathetic PGLs), MRI is superior to CT imaging. MRI also shows high sensitivity for PCCs (around 95%, specificity 70%). In order to limit radiation exposure, MRI is important for initial tumor localization in children, as well as for lifelong follow-up of all patients with a history of a cluster 1-related PPGL or carrying cluster 1 mutations. The most recently published guideline suggests performing initial screening and follow-up of children with an SDHx mutation and also lifelong follow-up of adult SDHx mutation carriers with MRI (from the base of the skull to the pelvis) (see also under “Personalized Management: Molecular Cluster 1” subsection “Follow-Up”) (109). It should be considered that diffusion-weighted MRI without contrast enhancement may be sensitive enough to monitor patients on long-term surveillance after initial screening (114, 115).
For the detection of metastases, CT imaging provides a much higher sensitivity for the lung, whereas diffusion-weighted MRI is better for liver metastases. Therefore, combining both modalities may be considered in the follow-up of cluster 1 mutation carriers or in patients with a history of cluster 1-related PPGL.
The highest sensitivity and specificity for detection of cluster 1-related metastatic and multifocal PPGLs is provided by functional (ie, molecular) imaging (1, 35). Thus, functional imaging is recommended for initial screening of adult SDHx mutation carriers, for staging of metastatic/multifocal disease, for presurgery staging of PCCs ≥5 cm, and after surgery of a (sympathetic) PGL or of oligo-metastatic/multifocal disease, and it is optional in follow-up in adult SDHx mutation carriers (1, 109). Since cluster 1A SDHx-related PPGLs (mostly PGLs) strongly express the somatostatin receptor 2 (SSTR2), functional imaging with somatostatin receptor analogs (SSA) positron emission tomography-computed tomography ([68Ga]-DOTA-SSA PET/CT) is the most sensitive imaging modality in the diagnosis and screening of these tumors (35, 116-118). In contrast, cluster 1B VHL/EPAS1-related PPGLs (specifically PCCs) seem to show stronger expression of the L-type amino-acid transporter and less SSTR2 expression. Therefore, PET/CT imaging based on [18F]FDOPA is more sensitive than [68Ga]-DOTA-SSA PET/CT in VHL/EPAS1-related PPGLs (35, 79, 118-120). Although 123/131I- meta-iodobenzylguanidine (MIBG) is the most specific radiopharmaceutical (>95%), its sensitivity is decreased in small tumors and/or those associated with SDHx mutations (121).
According to the most recently published guideline for radionuclide (functional) imaging of PPGLs, the most sensitive imaging method for cluster 1A SDHx-related disease is [68Ga]-DOTA-SSA PET/CT with a sensitivity of 94% to 100% (35). Sensitivities of [68Ga]-DOTA-SSA PET/CT have been reported in the literature as follows: 94% for pediatric SDHx-related disease (122), 99% for metastatic SDHB-related (123) and SDHD-related (124) PPGLs, and 100% for SDHD-related head and neck PGLs (125, 126) and SDHA-related disease (85). If [68Ga]-DOTA-SSA PET/CT is not available, [18F]FDG PET/CT may be used as the second most sensitive imaging modality for SDHx-related PPGLs and [18F]FDOPA PET/CT for SDHD-related head and neck PGLs (35, 85, 122-126). In contrast, for VHL-, EPAS1(HIF2A)-, and PHD1/2-, (cluster 1B) and FH-related disease, [18F]FDOPA PET/CT is likely to be the most sensitive functional imaging method. [68Ga]-DOTA-SSA PET/CT is the second most sensitive imaging modality for VHL and [18F]FDG PET/CT is the second most sensitive imaging modality for EPAS1(HIF2A), PHD1/2 and FH mutations (35, 79, 119, 127) (Table 3). Nevertheless, one always needs to be aware of the possibility of a false positive result (128).
Table 3.
Most sensitive functional imaging modalities for cluster 1A/1B
| Functional imaging | SDHx-related (cluster 1A) | VHL-related (cluster 1B) | EPAS1(HIF2A)/PHD1/2/FH-related (cluster 1B) |
|---|---|---|---|
| First choice | [68Ga]-DOTA-SSA PET/CT | [18F]FDOPA PET/CT | [18F]FDOPA PET/CT |
| Second choice | [18F] FDG PET/CT ([18F]DOPA PET/CT for head and neck PGLs) | [68Ga]-DOTA-SSA PET/CT | [18F]FDG PET/CT |
| Practical tip/synthesis: |
|---|
| • A general diagnostic flow-chart is provided by Fig. 2; a cluster-specific diagnostic flow-chart is provided by Fig. 3. • CT imaging (native plus contrast-enhanced phase): high screening sensitivity for PCCs (native phase >10 HU) (PCCs are associated with cluster 1B and cluster 2). • MRI imaging: higher sensitivity for head and neck and sympathetic PGLs (mostly cluster 1A-related) compared with CT. • MRI overall preferable for children and long-term follow-up of children and adults. • CT superior to MRI for lung metastases, MRI superior to CT for liver metastases. • Functional imaging is recommended for staging of metastatic/multifocal disease, for presurgery staging of a PCC ≥5 cm or any PGL, after surgery in patients with oligo-metastatic/multifocal disease, and in initial screening and optional in follow-up of adult SDHx mutation carriers. • Table 3 shows the most and second most sensitive functional imaging methods for each mutation of cluster 1A/B. |
Follow-Up
In general, every patient with any of the following criteria should undergo lifelong follow-up (1, 103, 110, 129, 130): germline mutation, history of PGL, age <20 years at initial diagnosis, tumor size ≥5 cm, multiple or recurrent PPGLs, or noradrenergic/dopaminergic phenotype. It is common practice that several of these criteria apply to patients with cluster 1A SDHx-related PPGLs, which are associated with an extraordinarily high risk of recurrence, multiplicity, and metastatic disease. Accordingly, an expert consensus on follow-up of asymptomatic children and adults with cluster 1 SDHx mutations has most recently been published (109):
- Children with an initial diagnosis of SDHx mutation should undergo a clinical examination including measurements of blood pressure, biochemical testing for plasma free normetanephrine and 3-methoxytyramine (or urinary normetanephrine if the former is not available), and MRI (base of the skull to pelvis).
- After negative initial screening, a clinical follow-up and blood pressure measurement every year, biochemistry every 2 years, and an MRI (base of the skull to pelvis) every 2 to 3 years is recommended. In general, after initial screening, MRI can be performed without gadolinium enhancement (114), but preferably with diffusion-weighted imaging for maximal sensitivity (115).
- For adults, the same lifelong follow-up is suggested, apart from more frequent biochemistry every year (plasma is preferred over urine, including plasma measurements of of normetanephrine and 3-methoxytyramine and no consensus for chromogranin A). Additionally, initial screening should include functional imaging (PET/CT), but there is no consensus on alternating MRI and PET/CT during follow-up in adulthood (Table 4).
Table 4.
Follow-up of asymptomatic SDHx mutation carriers
| Follow-up of asymptomatic SDHx mutation carriers | Adults | Children |
|---|---|---|
| Initial screening | Clinical examination (including bp), biochemical testing, MRI (base of the skull to pelvis), [68Ga]-DOTA-SSA PET/CT | Clinical examination (including bp), biochemical testing, MRI (base of the skull to pelvis) (initiation: at the age of 6-10 and 10-15 years for SDHB and SDHA/C/D mutation carriers, respectively) |
| Follow-up | Every 12 months clinical examination (including bp) & biochemical testing (plasma > urine), every 24-36 months MRI (base of the skull to pelvis) (no consensus on alternating MRI and PET/CT) | Every 12 months clinical examination (including bp), every 24 months biochemical testing, every 24-36 months MRI (base of the skull to pelvis) |
Abbreviations: bp, blood pressure; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography.
The authors of the current review suggest performing an MRI (base of the skull to pelvis), alternating with a low-dose chest CT plus MRI (base of the skull, neck, abdomen, pelvis) in order to reach a higher sensitivity for lung metastases. Ideally, these conventional imaging methods could be combined with [68Ga]-DOTA-SSA-PET, where available. Screening in children should be initiated between the age of 6 to 10 years for SDHB mutation carriers and between 10 and 15 years for SDHA/C/D mutation carriers. However, genetic testing in children should only be performed if tumor screening is considered, and tumor screening should only be performed following the discovery of a mutation (109). Thus, genetic testing should be offered from the age of 6 years for potential SDHB mutation carriers and from the age of 10 years for potential SDHA/C/D mutation carriers. The 2- to 3-year imaging intervals were chosen since SDHB-related tumors can be found as early as 2 years after initial negative screening (131).
For patients with a history of an SDHA/B PPGL (highest metastatic risk), biochemistry every 6 to 12 months and imaging every 1 to 2 years is reasonable (4, 34). Since metastatic risk is similarly high for HIF2A/EPAS- and FH-mutated (limited data) tumors, follow-up of patients with a history of these tumors can be performed similarly to those with history of SDHA/B-mutated tumors (4, 34). For patients with a history of an SDHC/D/AF2- or VHL-related PPGL with a lower metastatic risk, biochemistry every 12 months and imaging intervals of 2 to 3 years seem sufficient (4, 34) (Table 5).
Table 5.
Follow-up of cluster 1A/1B mutation carriers with a history of a PPGL
| Follow-up of cluster 1 mutation carriers with a history of a PPGL | History of metastatic PPGL, history of sympathetic PGL, SDHA/B, FH HIF2A/EPAS1-related PPGLs | History of head and neck PGL, SDHC/D/AF2, VHL |
|---|---|---|
| Biochemistry | 6-12 months (for HIF2A/EPAS1 including hematocrit) | 12 months |
| Imaging (MRI base of the skull to pelvis, possibly alternating with low-dose chest CT plus MRI base of the skull, neck, abdomen, pelvis) | 12-24 months (initially 12, then 12-24 months) | 24-36 months (24 months for SDHD) VHL mutations: risk of renal cell cancer, consider abdominal MRI every 12 months; optic fundus examination every 12 months; CNS tumors, CNS MRI every 24-36 months. |
Abbreviations: CNS, central nervous system; CT, computed tomography; MRI, magnetic resonance imaging; PGL, paraganglioma; PPGL, pheochromocytoma/paraganglioma.
| Practical tip/synthesis: |
|---|
| • Table 4 summarizes the most recently published international consensus on follow-up of asymptomatic SDHx mutation carriers. • Table 5 summarizes suggestions on follow-up of patients with a history of a cluster 1-related tumor. |
Treatment
As for PPGLs related to other clusters, therapy decisions should be made in a specialized multidisciplinary (neuro)endocrine tumor board.
Surgery
For locoregional disease, surgery should always be the first-line therapy, whenever possible (1, 6, 103, 132, 133). Minimally invasive adrenalectomy is the preferred surgical standard (1). In contrast to cluster 2 PPGLs (133, 134), adrenal-sparing surgery should not be favored over total adrenalectomy in most cluster 1 tumors, since these tumors have a high risk of recurrence and metastatic spread, particularly SDHB-mutant tumors (135). Although cortical-sparing surgery is associated with development of recurrent disease in about 13% of patients with germline mutations in RET (cluster 2 PPGLs) or VHL, this is not associated with decreased survival and can be considered for less aggressive PPGLs (133, 134).
Surgical removal of a primary tumor, or tumor debulking if complete resection of a primary tumor is not possible, may be performed if metastatic disease is present in order to alleviate symptoms and signs from catecholamine excess or tumor mass effects. One extensive study indicated no beneficial effect of primary tumor removal/debulking surgery on overall survival of patients with metastatic disease (136). However, more recent studies suggest that surgical removal of a primary tumor might be associated with improved overall survival (137-139), although this remains controversial. Furthermore, studies related to overall survival of patients with only bone metastatic lesions and resection of a large primary PPGL are largely absent. Nevertheless, removing a very large primary PPGL in the presence of numerous, small metastatic lesions may improve uptake of various PPGL-specific radiopharmaceuticals in certain patients (if radionuclide therapy is planned). There are several anecdotal reports that suggest a potential beneficial effect of presumably curative surgery of the primary tumor and the metastases in oligo-metastatic disease (136, 140, 141). However, much more evidence is required for any firm recommendations.
Head and neck PGLs
For head and neck PGLs, several therapeutic options may be considered based on the patient’s chronological age, overall health and functional status, the presence of cranial nerve deficits, tumor stage, tumor multifocality, genetic background, and patient preference (84). Most patients could, at least in the short term, be simply observed. In young individuals, who are often SDHx mutation carriers, it is important to consider that most will experience tumor growth over the long term. In this setting, SDHB/C mutation carriers who usually present with a single tumor should be distinguished from SDHD patients who are at higher risk of developing multiple tumors; these patients require a step-by-step management with combination strategies (142, 143). It should also be noted that SDHB- and SDHD-related carotid body PGLs should be surgically removed when they reach a size of 1.5 or 2 cm, respectively.
The recommendation of early surgical intervention for smaller size SDHB-related PGLs is based on the higher likelihood of metastatic spread with larger sized tumors (144, 145). Some patients with large tumors resulting in brainstem compression, and/or rapidly progressive symptoms, or in rare cases of suspected malignancy or refractory catecholamine secretion, also require surgery. Other patients with the following disease characteristics are not usually candidates for surgery: advanced age, poor health status, short-life expectancy, contralateral lower cranial nerve paralysis, poor pulmonary reserve, and minimal or no symptoms. For such patients, radiotherapy (conventional external beam radiation therapy [cEBRT] or stereotactic radiosurgery [gamma knife/cyberknife]), proton beam radiation, or systemic therapy, is recommended (142, 143, 146, 147).
Alpha-adrenoceptor blockade
Current recommendations from the US Endocrine Society Practice Guideline and the Working Group on Endocrine Hypertension of the European Society of Hypertension advocate that alpha-adrenoceptor blockade should be given for 7 to 14 days before surgery (1, 103). The recommendations on preoperative preparation with alpha-adrenoceptor blockade are based on optimal care of patients, both before and during the surgery when a cardiovascular emergency and crisis may occur (148). It is advised to adequately control blood pressure and heart rate for 1 to 2 weeks before surgery and during surgery to prevent a catecholamine crisis associated with severe hypertension and other dangerous catecholamine-associated side effects (1, 6, 103).
There is no specific consensus on blood pressure and heart rate targets; however, it is recommended to reach a seated blood pressure target <130/80 mmHg (103, 148). Targets for heart rate should be 60 to 70 bpm in a seated and 70 to 80 bpm for an upright position, respectively. Nevertheless, it is often difficult to reach these targets in patients with large primary tumors, multiple catecholamine-secreting primary tumors, or metastatic disease. In addition, alpha-adrenoceptor blockade should be administered to patients with catecholamine-producing PPGLs 7 to 14 days before locoregional or systemic chemo- or radiotherapy, as well as days to 2 to 3 weeks after those therapies, in order to avoid severe catecholamine-related side effects from catecholamines that are released from therapy-induced disrupted tumor cells.
The most frequently used drugs are the nonselective and noncompetitive alpha-1/2-adrenoceptor blocker phenoxybenzamine or the selective competitive alpha-1-adrenoceptor blocker doxazosin. Other alpha-1-adrenoceptor blockers that can be used include prazosin and terazosin.
All alpha-adrenoceptor blockers can be administered 2 to 3 times daily. Phenoxybenzamine, however, has a longer half-life and noncompetitive action, so it is recommended to take an extra dose at midnight instead of the morning before surgery. There is no clear superiority of any single alpha-adrenoceptor blocker over others; however, perioperative hypertension was more effectively prevented with phenoxybenzamine, although with a higher risk of postoperative hypotension, whereas doxazosin was associated with fewer adverse effects (149, 150). Overall, based on the limited available information, there are no apparent differences in clinical outcomes of patients with either alpha-adrenoceptor blocker.
Beta-adrenoceptor blockers should never be administered before initiation of alpha-adrenoceptor blockade and in general are only required if catecholamine-induced tachyarrhythmia is present. Beta-adrenoceptor blockers should be administered 2 to 3 days after alpha-adrenoceptor blockade is initiated (88).
Currently, most experts support the view that there is little need for adrenoceptor blockade for nonfunctional PPGLs or for those that only produce dopamine. Nevertheless, there have been some case reports describing hypertensive crisis or spells during the surgery in apparently “biochemically silent pheochromocytoma” (151, 152). Since negative biochemical test results cannot be used alone to determine whether a PPGL is nonfunctional, and given the associated difficulties in defining a PPGL as biochemically silent, there is need for considerable caution when assessing whether such patients should receive adrenoceptor blockade. More evidence is required for any strong recommendations, but unlikely to become available anytime soon due to the rare nature of nonfunctional PPGLs, beyond those of the head and neck.
Tyrosine hydroxylase inhibitor metyrosine
The tyrosine hydroxylase inhibitor metyrosine, which inhibits catecholamine synthesis, can additionally help to prevent pre- and intraoperative hemodynamic instability when given in combination with phenoxybenzamine. The combination treatment reduced blood pressure fluctuations and resulted in less need for antihypertensive medication, vasoactive drugs, and fluids intra-operatively, compared with phenoxybenzamine alone (153-157). Thus, although alpha-adrenoceptor blockers should be the first choice to prevent hypertensive crisis during surgery, metyrosine may add to better hemodynamic stability pre- and intra-operatively and might be an alternative to alpha-blockers in special clinical settings, if available (157).
Systemic therapy: overview
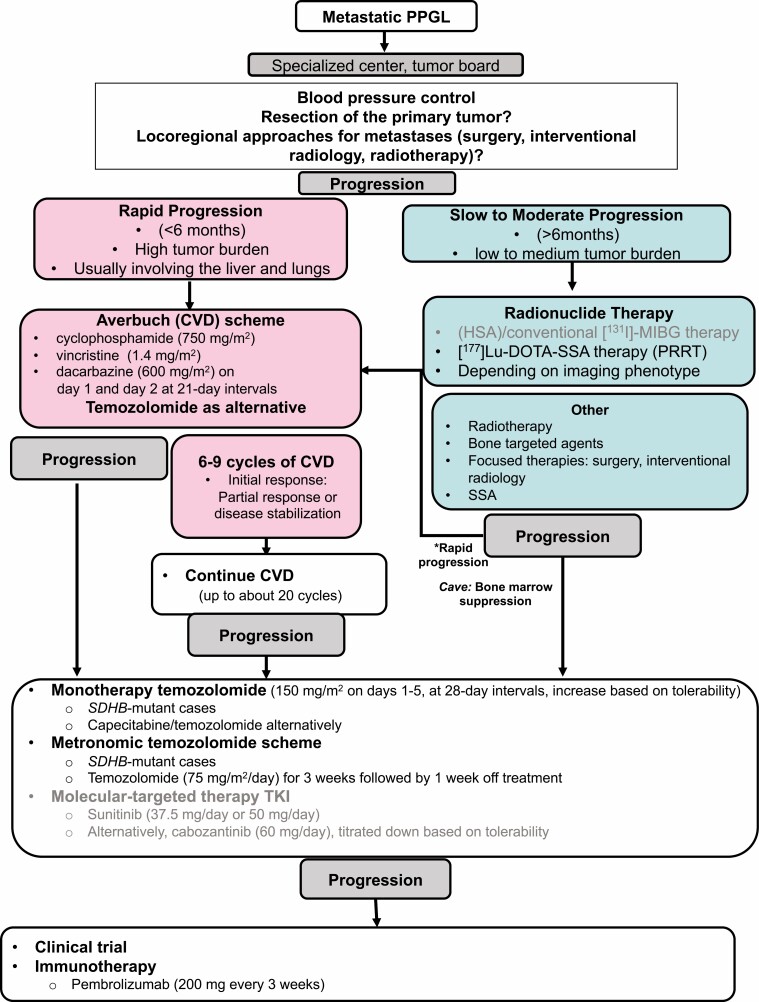
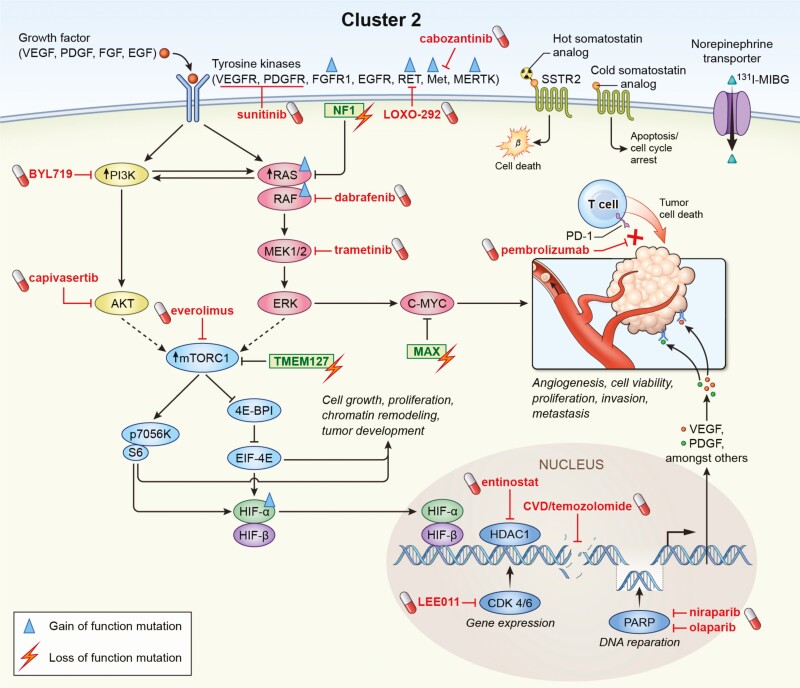
There are no generally approved systemic treatment options for metastatic PPGLs, apart from high-specific activity (HSA) [131I]-MIBG in the United States (4, 22, 158). Nevertheless, there are practiced standards of therapy for metastatic PPGLs including chemotherapy (cyclophosphamide, vincristine, and dacarbazine [CVD] scheme, or temozolomide monotherapy), radionuclide therapy ([131I]-MIBG, [177Lu]-DOTATATE), tyrosine kinase inhibitors (TKIs) (sunitinib, cabozantinib) and immunotherapy (Fig. 4) (1, 4, 22). CVD (Averbuch) chemotherapy (or some would recommend temozolomide) is recommended for rapidly progressing and radionuclide therapy for slowly to moderately growing PPGLs as first-line therapy by the most recently published guideline of the Working Group on Endocrine Hypertension of the European Society of Hypertension (1). Fig. 4 shows an overview of the therapeutic options for metastatic PPGLs, taking personalized approaches into account. These approaches include positivity on a 68Ga-DOTATATE scan for [177Lu] DOTATATE therapy (expressing SSTR2, particularly SDHx-mutated PPGLs), positivity on [123I]-MIBG scan for low or high-specific-activity [131I]-MIBG (expressing the norepinephrine transporter system, less likely positive for SDHx-mutated PPGLs), and PD-L1 status for pembrolizumab, poly(ADP-ribose)polymerase (PARP) inhibitors together with temozolomide (especially for SDHx-mutated tumors), demethylating agents (especially for SDHx-mutated tumors), and possibly HIF-2α inhibitors (particularly for cluster 1 PPGLs). The first clinical trials with HIF-2α inhibitors are currently in the initiation stage. The data from the first and only randomized placebo-controlled clinical trial (FIRST-MAPP), investigating the TKI sunitinib vs placebo in 74 PPGL patients, including those with SDHx mutations, is currently under analysis.
Figure 4.
Flow-chart for systemic therapy of metastatic disease (1, 4, 22); black letters: potentially interesting therapy for cluster 1; gray letters: potentially interesting therapy for cluster 2. Abbreviations: SSA, somatostatin analogues; TKI, tyrosine kinase inhibitor.
Completed clinical trials
However, state-of-the-art of therapy is based mostly on retrospective data, with only few prospective and no randomized clinical trials (summarized in Table 6).
Table 6.
Completed clinical therapy studies for metastatic PPGLs
| Author | Therapy | Patient number (n) | Complete response | Partial response | Stable disease | Median OS/PFS/TTP |
|---|---|---|---|---|---|---|
| Niemeijer et al, 2014 | CVD chemotherapy | Meta-analysis from 4 studies: n = 50 (special efficacy in SDHB mutation carriers) | 4% | 37% | 14% | PFS in 2 studies 20 and 40 months, respectively |
| Asai et al., 2017 | CVD chemotherapy | N = 23 | 4% | 22% | 22% | OS/PFS responders vs nonresponders 4.6 vs 2 years and 1.7 vs 0.3 years, respectively |
| Deutschbein et al., 2015 | CVD chemotherapy | N = 8 | 0% | 25% | 38% | PFS 5.4 months (2.5-26.8 months) |
| Tanabe et al., 2013 | CVD chemotherapy | N = 17 | 0% | 47.1%1 | 23.5% | PFS responders 40 months |
| Huang et al., 2008 | CVD chemotherapy* | N = 18 (n = 8 with SDHB orSDHD mutation) | 11% | 44% | OS responders/nonresponders 3.8/1.8 years | |
| Averbuch et al., 1988 | CVD chemotherapy* | N = 14 | 57% (complete plus partial response) | PFS 21 months (7 to >34 months) | ||
| Jawed et al., 2018 | Prolonged CVD chemotherapy (median 20.5 cycles) | N = 12 (all with SDHB mutations) | 16.7% (2/12) | 66.7% (8/12) | 0%2 | OS/PFS 3.3/2.6 years |
| Ayala-Ramirez et al., 2012 | different chemotherapy regimens | N = 54 (n = 52 evaluable) (all progressive disease at baseline) | 33%3 | OS responders/nonresponders 6.4/3.7 years | ||
| Hadoux et al., 2014 | Temozolomide monotherapy | N = 15 (n = 10 with SDHB mutations) | 0% | 33%4 | 47%4 | PFS 13.3 months |
| Tena et al., 2018 | Metronomic low-dose temozolomide plus high dose Lanreotide Autogel | N = 2 (case reports) | 0% | 0% | 100% | OS (n = 2) not reached, PFS 13 months (n = 1), PFS not reached after 27 months (n = 1) |
| Van Hulsteijn et al., 2014 | [131I]-MIBG | Meta-analysis from 17 studies: n = 243 | 3% | 27% | 52% | PFS in 2 studies 23.1 and 28.5 months, respectively |
| Loh et al., 1997 | [131I]-MIBG | Meta-analysis n = 116 | 30% (complete plus partial response) | |||
| Thorpe et al., 2020 | [131I]-MIBG | N = 125 (n = 88 evaluable) | 1% | 33% | 53% | OS responders vs nonresponders 6.3/2.4 years |
| Gonias et al., 2009 | [131I]-MIBG (phase II, prospective) | N = 50 (n = 49 evaluable) | 22% (complete plus partial response) | 43% | 5-year OS 64% | |
| Wakbayashi et al., 2019 | HSA [131I]-MIBG (phase I, prospective) | N = 20 | 10% | 65% | 6-months OS/PFS 100%/80% | |
| Noto et al., 2018 | HSA [131I]-MIBG (phase I, prospective) | N = 21 | 19% | 2-year OS 62% | ||
| Pryma et al. 2019 | HSA [131I]-MIBG (phase II, prospective) | N = 68 (evaluable n = 64) patients | 0% | 23% | 69% | OS 36.7 months: 18 months after one cycle/ 44 months after 2 cycles |
| Nastos et al., 2017 | [131I]-MIBG vs [177Lu] / [90Y] DOTATATE (PRRT) | N = 22 Patients (n = 11 MIBG, n = 9 DOTATATE, n = 2 combinations, n = 15 PGL, n = 7 PCC) (all progressive disease at baseline) | MIBG: 63% vs DOTATATE 100% | OS/PFS MIBG 41.2/20.6 months vs OS/PFS DOTATATE 60.8/38.5 months Subgroup PGLs: OS/PFS MIBG 22.8/14.4 months vs OS/PFS DOTATATE 60.8/38.5 months (P < 0.05) |
||
| Van Essen et al., 2006 | [177Lu] DOTATATE | N = 12 (n = 1 PCC, n = 5 HN, n = 6 other PGLs) | 0% | 16.7%5 | 50%5 | TTP 11 and 5 months, respectively in 2 patients, median TTP to progression not reached in PGL patients |
| Zovato et al., 2012 | [177Lu] DOTATATE | N = 4 PGLs (with SDHD mutation) (n = 2 thoracic PGLs, n = 2 HNPGLs) (all progressive disease at baseline) | 0% | 50% | 50% | - |
| Forrer et al., 2008 | [90Y] DOTATOC, 3 combined with [177Lu] DOTATATE | N = 28 (n = 9 PCC, n = 19 PGL) (all progressive disease at baseline) | 0% | 25%1 | 46.4% | TTP 3 to >42 months, median TTP 18 ± 14 (6-44) months |
| Kong et al., 2017 | [177Lu] DOTATATE, 9 combined with radiosensitizing chemotherapy | N = 20 (n = 8 PCC, n = 5 HNPGLs, n = 5 abd. PGLs, n = 2 HN plus abd. PGLs) (n = 7 SDHB, n = 1 SDHD, n = 2 no mutation, n = 10 unknown) | 0% | 36%1 | 50% | PFS 39 months, OS not reached |
| Pinato et al., 2016 | 177[Lu]-DOTATATE | N = 5 abd. PGLs (with SDHB mutation) | 0% | 20% | 60% | PFS 17 (0-78) months/mean OS 53 months (median OS not reached) |
| Puranik et al., 2015 | [90Y] DOTATOC n = 4 combined with 177[Lu]-DOTATATE* | N = 9 HN PGLs | 0% | 0% | 100% | - |
| Yadav et al., 2019 | 177[Lu]-DOTATATE | N = 25 PGLs | 0% | 28% | 56% | PFS 32 months, OS not reached |
| Imhof et al. 2011 | [90Y] DOTATOC (phase II, prospective) | N = 39 (n = 11 PCCs, n = 28 PGLs) (all progressive disease at baseline) | ns | 18% | ns | Mean OS in PCC/PGL 32/82 months |
| Vyakaranam et al., 2019 | 177[Lu]-DOTATATE | N = 22 (n = 11 sympathetic PGLs, 2 HNPGLs, n = 9 PCCs) | 0% | 9% | 91% | PFS 21.6 months |
| Zandee et al., 2019 | 177[Lu]-DOTATATE | N = 30 (n = 17 parasympathetic PGLs, n = 10 sympathetic PGLs, n = 3 PCCs) | 0% | 23% | 67% | **PFS in PGL#/PCC 13/10 months, respectively |
| Satapathy et al., 2019 | 177[Lu] /[90Y] -DOTATATE, [90Y] DOTATOC | Meta-analysis from 12 studies: n = 201 | 0% | 25%6 | 59%6 | - |
| Taieb et al., 2019 | [90Y]/ 177[Lu]-DOTATATE | Meta-analysis n = 234 | 90% (partial response plus stable disease) | |||
| Jaiswal et al., 2020 | 177[Lu]-DOTATATE | N = 15 (n = 4 PCCs, n = 4 sympathetic PGLs, n = 5 HNPGLs, n = 1 PCC + sympathetic PGL, n = 1 HNPGL + sympathetic PGL) | 0% | 7% | 73% | PFS/OS not reached after 27 months |
| Ayala-Ramirez et al., 2012 | TKI sunitinib (retrospective) | N = 17 (n = 14 evaluable) | 0% | 21% (3/14) | 36% (5/14) | PFS 4.1 months 62.5% (5/8) with stable disease or partial response SDHB mutation carriers) |
| O`Kane et al., 2019 | TKI sunitinib (phase II, prospective) | N = 25 (n = 23 evaluable) | 0% | 13% (3/23) (2/3 SDHA/B mutation) | 70% (16/23) | PFS 13.4 months (all patients with SDHx mutations partial response or stable disease) |
| NCT01967576 (completed, preliminary data) | TKI axitinib (phase II, prospective) |
N = 14 (n = 12 evaluable) | 0% | 41.7% | 41.7% | PFS 7.7 months (3.3.-16.8 months) |
| Jasim et al., 2017 | TKI pazopanib* | N = 7 (6 evaluable) | 17% | PFS/OS 6.5/14.8 months | ||
| Oh et al., 2012 | MTORC1 inhibitor everolimus (phase II, prospective) | N = 7 | 0% | 0% | 71% (5/7) | PFS 3.8 months |
| Druce et al., 2009 | MTORC1 inhibitor everolimus | N = 4 | 0% | 0% | 25% (1/4) | PFS 3 months (n = 1) |
| Naing et al., 2020 | Pembrolizumab (phase II, prospective) | N = 9 (n = 8 evaluable) | 0% | 0% | 75% | 27-weeks PFS 43% |
| Jimenez et al., 2020 | Pembrolizumab (phase II, prospective) | N = 11 | 9% | 64% | PFS 5.7 months (4.37 months-not reached) (n = 1 SDHD mutation stable disease for 24 months, n = 1 SDHB mutation tumor shrinkage >30%) | |
| NCT03165721 (completed, preliminary data) | DNA Methyltransferase inhibitor SGI-110 (guadecitabin) (phase II, prospective) | N = 1 | PFS 3.9 months |
Studies are retrospective unless indicated as prospective.
Minor response: any shrinkage of tumor which does not fulfill the criteria of partial response. If not indicated otherwise, reported minor/minimal response is included in “Stable disease.”
Black letters: potentially specifically interesting for cluster 1; gray letters: potentially specifically interesting for cluster 2.
Abbreviations: CVD, cyclophosphamide/vincristine/dacarbazine; HNPGL, head and neck paraganglioma; MIBG, meta-iodobenzylguanidine; OS, overall survival; PCC, pheochromocytoma; PFS, progression-free survival; PGL, paraganglioma; PPGL, pheochromocytoma/paraganglioma; SDHA/B/D, succinate dehydrogenase subunit A/B/D; TKI, tyrosine kinase inhibitor; TTP, time to progression.
1 Minor/minimal response included
2 Minor/minimal response excluded (2/12)
3 Overall response rate
4 According to RECIST plus PERCIST
5 Including all 12 patients of which only 11 were evaluable
6 This meta-analysis provides an overall response rate of 25% (n=179) and a disease control rate of 84% (n=151)
* prospective
**Overall PFS 30 months, parasympathetic PGL 91 months
# sympathetic
Potential cluster-specific systemic therapy: overview
Cluster-specific therapy of metastatic PPGLs has not yet entered clinical routine practice, although the distinctive molecular pathology (including signaling pathways of specific cluster-related PPGLs) suggests that some therapeutics may be more effective than others in a particular cluster (Figs 1, 2, Table 6). Correlation of therapy efficacy with mutational status has been highlighted in the most aggressive SDHB-related tumors and has been analyzed in some (retrospective) clinical studies (Table 6). Either proven or potentially effective therapeutic options for cluster 1–related PPGLs include chemotherapy (Averbuch scheme, temozolomide with or without PARP inhibitors), peptide receptor radionuclide therapy (PRRT), and HIF-2α inhibitors (Figs 1, 2, Table 6).
Figure 2.
General diagnostic flow-chart.
Chemotherapy: cyclophosphamide/vincristine/dacarbazine scheme and temozolomide
For rapidly progressive metastatic disease with a high tumor burden (often with high Ki-67), chemotherapy with the Averbuch scheme (cyclophosphamide, vincristine, dacarbazine; CVD) is the therapy of choice (1, 4, 6). To date, this is one of the most established and longest (retrospectively) studied therapies in aggressive and rapidly progressive PPGLs (159-163), and it has shown to be particularly effective in patients with cluster 1 SDHB mutations (159, 164, 165) (Table 6). Disease control rate with CVD chemotherapy was between 48% and 100%, and progression-free survival (PFS) was between 20 and 40 months (159-162, 166, 167). One retrospective study showed a significantly longer PFS and overall survival (OS) in responders vs nonresponders to CVD chemotherapy (1.7 ± 3.3 vs 0.3 ± 0.3 years, P < 0.01 and 4.6 ± 3.6 vs 2.0 ± 3.7 years, P = 0.01, respectively) (166). Another study showed a significant survival benefit for patients responding to chemotherapy compared with nonresponders (different chemotherapy schemes) (165).
After 6 to 9 cycles of CVD chemotherapy or prolonged therapy (20 cycles) with CVD (159), temozolomide monotherapy may be continued as a type of maintenance therapy, especially in SDHB-mutated cases. It may also be a reasonable first-line alternative to CVD chemotherapy with a satisfactory disease control rate of up to 80% (PFS 13.3 months) in less aggressive cases, or for patients with comorbidities (each case needs to be considered on an individual basis), specifically for those with SDHB mutations (164, 168) (Table 6). Alternatively, the combination of capecitabine plus temozolomide may be tried in analogy to the chemotherapy scheme for pancreatic neuroendocrine tumors. Where the standard dose of temozolomide is poorly tolerated, a metronomic scheme with reduced doses may be effective, particularly in patients with SDHB mutations (169). Even after progression in response to CVD chemotherapy, temozolomide monotherapy or temozolomide with capecitabine may be tried, although dacarbazine is part of CVD as a derivate of temozolomide. As a future perspective, the combination of temozolomide with PARP inhibitors may enhance efficacy in cluster 1 tumors (170). Prospective randomized clinical trials are missing and are urgently needed. A clinical phase 2 trial investigating temozolomide in combination with the PARP inhibitor olaparib is currently recruiting (NCT04394858) (Table 7).
Table 7.
Ongoing clinical therapy studies for metastatic PPGLs
| Ongoing studies | Therapy | Patient number (n) | Status |
|---|---|---|---|
| NCT04394858 | PARP inhibitor olaparib plus temozolomide (phase II, prospective) |
Recruiting | |
| NCT01850888 | [131I]-MIBG | Recruiting | |
| NCT00107289 | [131I]-MIBG (phase II, prospective) | Recruiting | |
| NCT04029428 | [177Lu] DOTATATE vs [90Y] DOTATATE vs mix each of 50% (PRRT) (phase II, prospective) | Recruiting | |
| NCT03206060 | [177Lu] DOTATATE (Lutathera) (PRRT) (phase II, prospective) | Recruiting (SDHx-related and sporadic PPGLs) | |
| NCT04276597 | 177Lu] DOTATOC (PRRT) (phase II, prospective) | Recruiting | |
| NCT04711135 | [177Lu] DOTATATE (Lutathera) (PRRT) in adolescents (phase II, prospective) | Not yet recruiting | |
| NCT03923257 | [177Lu] DOTATATE (PRRT) in children and adolescents (phase I/II, prospective) | Recruiting | |
| LAMPARA NCT03946527 |
Lanreotide (cold somatostatin analog) (phase II, prospective) |
Not yet recruiting | |
| NCT03034200 | Dopamine receptor D2 and caseinolytic protease P (ClpP) agonist ONC201(phase II, prospective) | Recruiting | |
| NCT04284774 | Farnesyltransferase inhibitor tipifarnib (RAS inactivation) (phase II, prospective) | Recruiting | |
| FIRST-MAPP Study, NCT01371201 | TKI sunitinib (phase II, prospective, first randomized placebo-controlled study) | N = 74 (closed) | Data arriving soon |
| NCT03839498 | TKI Axitinib (AG-013736) (phase II, prospective) | Recruiting | |
| NCT03008369 | TKI lenvatinib (phase II, prospective) |
Active, not recruiting | |
| NCT02302833 | TKI cabozantinib (phase II, prospective) | N = 10 | Recruiting (preliminary data from n = 10, partial response 40%, PFS 11.2) |
| NCT04400474 | Cabozantinib plus atezolizumab (CABATEN) (phase II, prospective) |
Recruiting | |
| NCT02834013 | Nivolumab plus ipilimumab (phase II, prospective) |
Recruiting | |
| NCT02721732 | Pembrolizumab (phase II, prospective) |
Recruiting | |
| NCT02923466 | VSV-IFNβ-NIS and avelumab(phase II, prospective) | Recruiting | |
| NCT04187404 | Novel Therapeutic Vaccine (EO2401) (phase I/II, prospective) | Recruiting |
Black letters: potentially specifically interesting for cluster 1; gray letters: potentially specifically interesting for cluster 2.
Abbreviations: MIBG, meta-iodobenzylguanidine; PARP, poly(ADP-ribose) polymerase; PPGL, pheochromocytoma/paraganglioma; PRRT, peptide receptor radionuclide therapy; SDHx, succinate dehydrogenase subunit x; TKI, tyrosine kinase inhibitor;
Radionuclide therapy
For patients with PPGLs that show slow to moderate progression, the best studied first-line therapeutic option is radionuclide therapy with meta-[131I]iodobenzylguanidine ([131I]-MIBG), including the novel high-specific-activity (HSA) [131I]-MIBG (Food and Drug Administration–approved in the United States) with a disease control rate of 63% to 87% (PFS, 20.6-28.5 months) for [131I]-MIBG and 92% for HSA [123I]-MIBG in a prospective phase 2 clinical study (Table 6) (1, 158, 171-178). However, there is evidence that cases of metastatic cluster 1 SDHx-related disease might be less frequently positive on a [123I]-MIBG scan, and indeed a previous study found that all metastatic noradrenergic (mainly SDHB-related) PPGLs were [123I]-MIBG-negative (121).
Therefore, somatostatin (peptide) receptor (SSTR)-based radionuclide therapy (PRRT) may be the prioritized first-line therapy option for cluster 1–related slowly to moderately progressive disease (1). PRRT is a very effective and officially approved therapy for pancreatic and midgut neuroendocrine tumors (179). Similar to neuroendocrine tumors, PPGLs (especially SDHx-associated tumors) show strong SSTR2 expression in most cases (116, 117). This is reflected by the very high sensitivity of [68Ga]-DOTA-SSA PET/CT (up to 100%) in SDHx-related disease (35). There are several small, nonrandomized retrospective clinical studies (and few prospective studies) suggesting that PRRT is one of the most effective (although not currently officially approved) clinical therapy approaches used for metastatic PPGLs, especially for PGLs associated with cluster 1 SDHx mutations (Table 6) (172, 180-191). This indicates a particularly high therapeutic potential of PRRT for SDHx-related PGLs: A prospective phase 2 PRRT study (nonrandomized) including 39 patients (all progressive at baseline) who received [90Y]DOTATOC reported an overall response rate of 18% (all PPGLs) and a remarkably long OS of 82 months for the subgroup of PGLs (n = 28) (187). Moreover, another retrospective study reported a significantly longer OS/PFS after PRRT in the subgroup of PGLs (60.8/38.5 months) compared with [131I]-MIBG therapy (22.8/14.4 months) (172). The disease control rate for PPGLs with PRRT was in most retrospective studies (10/12) ≥80% (67%-100%) and PFS was 17 to 39 months. In a recently published meta-analysis of 234 pooled PPGL patients treated with PRRT (177Lu/90Y), a high disease control rate of 90% was reported (118). However, not all patients included were progressive at baseline, which complicates data interpretation. Nevertheless, PRRT is recommended for PPGL treatment by the National Comprehensive Cancer Network (NCCN) and by the Working Group on Endocrine Hypertension of the European Society of Hypertension (1). PRRT is well tolerated with limited acute and medium-term toxicity profiles. A low rate of nephrotoxicity is observed with 177Lu. The estimated incidence for therapy-related myeloid neoplasms from neuroendocrine tumor studies ranged from 1% to 5.4%.
Alpha-particle emitting radionuclides may have advantages over conventional beta-particle emitters (192), and PRRT with an alpha-emitter (225Ac-DOTATATE) has shown promising preliminary results in gastro-enteropancreatic neuroendocrine tumor patients who are stable or refractory to 177[Lu]-DOTATATE PRRT (193).
In general, the need of therapy always has to be carefully balanced against the danger of severe bone marrow suppression, especially if radionuclide therapy is followed by chemotherapy or vice-versa. As an alternative, tyrosine kinase inhibitor therapy may be considered subsequent to radionuclide therapy (instead of chemotherapy)—this will even become more relevant if the FIRST-MAPP study as the first randomized placebo-controlled clinical trial in the field of PPGL is able to confirm efficacy of sunitinib in these patients.
Tyrosine kinase inhibitors
If there is disease progression after radionuclide therapy or chemotherapy, or if these therapeutic options are not possible or not acceptable to the patient, anti-angiogenic targeted therapy with a TKI, such as sunitinib or cabozantinib, is a therapeutic and clinically available option for PPGL patients. Sunitinib has been approved for the treatment of pancreatic neuroendocrine tumors in Europe and the United States (194), although not yet for PPGLs. Sunitinib primarily targets the kinase signaling pathways via inhibition of receptor tyrosine kinases (RTKs) (VEGFR, PDGFR, and RET) and therefore may be less effective in cluster 1 than in kinase signaling–related cluster 2 PPGLs (Figs. 2 and 3). Nevertheless, in one retrospective study investigating sunitinib in PPGLs, 5/8 (62.5%) of the patients showing stable disease or a partial response were SDHB mutation carriers. One prospective study found that all patients (n = 7) with SDHx mutations showed stable disease or a partial response, indicating potential efficacy in cluster 1–related disease, possibly through inhibition of VEGFR while VEGF is upregulated by HIF-2α under pseudohypoxic conditions (195, 196). The retrospective study and the prospective clinical phase 2 study investigating sunitinib in PPGL patients reported a disease control rate of 57% (retrospective) and 83% (prospective), respectively, and a PFS of 13.4 months in the prospective phase 2 clinical trial (195, 196). However, the data of the first and only placebo-controlled randomized phase 2 clinical study investigating sunitinib in 74 progressive PPGL patients are soon to be released (NCT01371201). As soon as these results are available, this may allow for novel randomized clinical phase 2 studies investigating the optimal sequence of therapy (for each cluster) which is not currently available. For example, comparing temozolomide vs PRRT vs sunitinib in cluster 1– and cluster 2–related disease would be of considerable importance.
Figure 3.
Cluster-specific diagnostic flow-chart.
As an alternative to sunitinib, the TKI cabozantinib is currently in clinical use (although off-label) for PPGL patients. However, there are only preliminary data from a small prospective clinical phase 2 study (n = 10, partial response 40%, PFS 11.2 months) (NCT02302833) (abstract (197)). Pazopanib, another TKI, showed moderate efficacy in a small PPGL cohort (n = 6, partial response 17%, PFS 6.5 months) (198). Similarly, the TKI axitinib showed moderate efficacy in PPGLs (n = 12, partial response 47.1%, PFS 7.7 months) (NCT0196757).
mTORC1 inhibitor everolimus
The mTORC1 inhibitor everolimus (a signaling pathway inhibitor) has already been approved for the antiproliferative treatment of midgut and pancreatic neuroendocrine tumors (199, 200). There are a few contradictory data for PPGLs from a small prospective phase 2 clinical study with a disease control rate of 71% (genetic background not assessed) and a very small retrospective study with a disease control rate 25% (no known SDHx mutation included) (201, 202). Nevertheless, there is evidence from our own translational studies in human PPGL primary cultures that these molecular-targeted drugs (alone but primarily in combination) are potentially effective in kinase signaling cluster 2–related disease (36), but they might be less effective in cluster 1–related disease. Interestingly, there is at least one patient reported in the literature (a 20-year-old woman with an SDHB-mutated tumor) who was effectively treated with a combination of the TKI sunitinib and the mTORC1 inhibitor rapamycin. After documented progression, this patient was first treated with sunitinib monotherapy (50 mg) for 1.5 years. However, due to fatigue, sunitinib was reduced to 25 mg and 4 mg of rapamycin was added 1.5 years after initiating sunitinib. This patient showed stable disease until the end of the observation period (1.5 years after addition of rapamycin, 3 years after initiating sunitinib). This suggests that combined targeted therapies, although more likely to be effective in cluster 2–mutated tumors, could also be worth studying in cluster 1–related tumors (195).
Immunotherapy (checkpoint inhibitors)
There are also 2 small, recent prospective phase 2 clinical studies suggesting that immunotherapy might be an option in selected cases with no other remaining therapeutic options (203, 204). While disease control rates were similar to other therapeutic options (75% and 73%, respectively), the PFS was unfortunately rather low (<6 months) (203, 204). Although 1 patient with a SDHD mutation showed a stable disease for 24 months and 1 SDHB-mutated patient experienced a tumor shrinkage of >30% (followed by severe hepatotoxicity) (203), these numbers are too low to assume a unique benefit for cluster 1 SDHB/D mutation carriers. This requires further investigation but highlights the need for germline testing of each patient and/or somatic testing of each tumor in order to define such correlations between cluster affiliation (mutation) and therapeutic response.
Cold SSTR2 analogs
For midgut and pancreatic neuroendocrine tumors, with a Ki-67 <10%, “cold” somatostatin analogs (biotherapy)–octreotide LAR and lanreotide autogel—have been approved for anti-proliferative therapy in Europe and the United States (205, 206). PPGLs with strong SSTR2 expression, particularly cluster 1 SDHx-related PGLs, which also show best responses to PRRT, might be treated by analogy. However, data from prospective studies are lacking and are urgently needed.
Ongoing clinical trials
Table 7 shows all current and ongoing clinical therapy studies for metastatic PPGLs.
Locoregional therapy
Additionally, antiresorptive therapies, such as bisphosphonates and denosumab, are regularly administered in the case of larger (usually numerous) bone metastases, by analogy with other neuroendocrine tumor bone metastases (207). Conventional external beam radiation therapy (cEBRT) or stereotactic radiosurgery are also well-established approaches for the therapy of single (often more-rapidly growing) bone and other metastases in oligo-metastatic disease in order to attenuate tumor growth or alleviate mass effects, and for symptomatic relief (147, 207, 208). Locoregional therapy approaches, including radiofrequency ablation or cryoablation, may also be reasonable approaches for single metastases such as liver metastases in oligo-metastatic disease (209). The use of cEBRT in a surgical tumor bed is currently unproven.
| Practical tip/synthesis (applies to all clusters): |
|---|
| • Whenever possible, curative surgery should be the therapy of choice. • At least 7-14 days before surgery, other medical procedures (such as endoscopy), or systemic therapies, alpha-adrenoceptor blockade should be initiated in biochemically positive cases, unless this is purely elevated dopamine/3-methoxytyramine. • Surgery of a primary tumor in a patient with metastatic disease may be considered if there is a mass effect or reason to decrease high catecholamine levels to alleviate their organ-related damage/dysfunction. • Figure 4 summarizes a therapy flow-chart which suggests a potential sequence of therapy for a practicing physician, including dosing. • For rapidly progressive PPGLs, the recommended first-line therapy is chemotherapy (CVD scheme, or some would recommend temozolomide), and for slowly to moderately progressing PPGLs the recommended first-line therapy is radionuclide therapy—either with [131I]-MIBG or PRRT—depending on tumor uptake and location (1). • In the case of stable disease after 6 to 9 cycles of CVD chemotherapy, previously rapidly progressing PPGLs may be treated with prolonged chemotherapy (up to about 20 cycles) or de-escalated to temozolomide maintenance therapy. • In the case of progression to CVD chemotherapy, temozolomide monotherapy or temozolomide in combination with capecitabine (or a PARP inhibitor) may be chosen. In cases of poor tolerability, a metronomic scheme of temozolomide is possible. Alternatively, a TKI may be chosen. • In the case of progression of moderately growing PPGL following radionuclide therapy, if there is a high tumor burden then CVD chemotherapy should be considered. However, where the tumor burden is only moderate, temozolomide alone or in combination with capecitabine, or a TKI, may be more appropriate. • In the case of progression in response to temozolomide (+/- capecitabine) or a TKI, the alternative treatment can then be used. Following progression with both approaches, inclusion into a clinical trial or immunotherapy with pembrolizumab may be an option. • In some cases, active surveillance may be most appropriate if disease progression is slow. |
Personalized Management: Molecular Cluster 2
Overview: Pathophysiology and Signaling Pathways
Molecular cluster 2 includes mutations involving tyrosine kinase (TK)-linked signaling pathways (Fig. 5) (25, 26, 34, 129, 210-212). Cluster 2 comprises mutations in the rearranged-during-transfection (RET) proto-oncogene (encoding a receptor TK) (germline/somatic) and genes encoding for the neurofibromin 1 (NF1) tumor suppressor (germline/somatic), HRAS (somatic), transmembrane protein 127 (TMEM127) (germline), Myc-associated factor X (MAX) (germline/somatic) and fibroblast growth factor receptor 1 (FGFR1) (somatic) (25, 30, 213). Moreover, rare cases with mutations in genes encoding the receptor TKs Met (germline/somatic) and MERTK (germline), encoding B-Raf (somatic) and the nerve growth factor receptor (NGFR, not yet supported by other studies) are described (25, 55). These mutations are linked to overactivation of the phosphatidylinositol-3-kinase (PI3K)/AKT, mechanistic target of rapamycin (mTORC1)/p70S6 kinase (p70S6K), and RAS/RAF/ERK signaling pathways; the resulting cellular impacts include promotion of sustained cell growth, survival, proliferation, chromatin remodeling, angiogenesis, and a metabolic switch to glycolysis and glutaminolysis (25, 214). As mentioned earlier, most patients with these tumors show a better clinical outcome compared to patients with cluster 1–related tumors.
Figure 5.
Gene mutations leading to an activation of kinase signaling pathways (cluster 2) and derived molecular targets for a personalized therapy. Mutations in RET, NF1, HRAS, TMEM127, MAX, FGFR1, Met, MERTK, BRAF and NGFR activate phosphatidylinositol-3-kinase (PI3K)/AKT, mammalian target of rapamycin (mTORC1)/p70S6 kinase (p70S6K), and RAS/RAF/ERK signaling pathways. Highlighted in red are potential drugs that address the molecular changes in cluster 2 PPGLs and are in preclinical and clinical evaluation. Molecular-targeted signaling pathway inhibitors (alone and in combination) might be specifically effective in these tumors. Moreover, similar to cluster 1, cluster 2 PPGLs provide the somatostatin receptor (possibly lower expression compared to cluster 1) and the norepinephrine transporter (possibly higher expression compared to cluster 1) as potential targets for the treatment of these tumors. Immune checkpoint inhibitors and drugs addressing DNA repair and synthesis mechanisms are furthermore under evaluation.
| Practical tip/synthesis: |
|---|
| • Figure 5 summarizes the kinase signaling pathway–associated cluster 2 with all its loss- and gain-of-function mutations. • Cluster 2 comprises mutations in the following genes associated with tyrosine kinase signaling: RET, NF1, HRAS, TMEM127, MAX, FGFR1, and rare cases with Met, MERTK, BRAF, and NGFR. • These mutations lead to activation of tyrosine kinase-associated signaling pathways, such as PI3K/AKT, RAS/RAF/ERK, and mTORC1, and finally as a common intersection point with cluster 1 to increased synthesis of HIF-α and, among other changes, to enhanced cell growth, cell survival, and tumor formation. |
Penetrance, Epidemiology, and Metastatic Risk
The penetrance of RET- (multiple endocrine neoplasia 2, MEN2-) related PCCs (autosomal dominantly inherited) is relatively high (around 50%), with frequent bilateral/multiple occurrence (in 50%-80%) (215, 216). MEN2-related PCCs may possibly result from initial adrenal hyperplasia, and MEN2 is also associated with a high prevalence of medullary thyroid carcinoma and less often (in MEN2A) primary hyperparathyroidism (215). Although the peak age of PCC manifestation is 40 years, the tumors can present in childhood by the age of 11 (216). RET mutations also occur as somatic mutations.
The penetrance of NF1-related PCCs (autosomal dominantly inherited) is lower (around 5%-8%) and less frequently associated with bilateral disease (around 12%) compared with RET-related PCCs (217, 218). NF1-related PCCs rarely occur in children, and more regularly present around the age of 50 years. NF1 mutations also occur as somatic mutations and, as such, represent one of the more common drivers of sporadic PCC. However, there appears to be some ethnic diversity for this since the overall frequency of NF1 mutations in a European cohort (15.9%) was higher than in a Chinese cohort (6.6%) (30).
The penetrance of TMEM127 mutations is unknown due to the rare nature of PCCs with mutations in this gene. Accordingly, the prevalence of TMEM127-related tumors seems to be very low (not more than 2% of patients without other known susceptibility mutations); among the few reported cases, TMEM127-related tumors present as solitary nonmetastatic PCC in patients older than 40 years (219, 220).
The penetrance of MAX-related PCCs is not well established, but recent evidence in 2 families who underwent screening suggests a high penetrance (221). The prevalence of MAX-related PCCs seems to be very low in groups of patients without other known susceptibility mutations (around 1%); these tumors are often bilateral (67%) (222).
HRAS and FGFR1 mutations, which seem to be exclusively somatic in nature, are more common in the Chinese (HRAS: 16.5%; FGFR1: 9.8%) than European populations (HRAS: 9.8%; FGFR1: 2.2%) (30). Interestingly, in the European cohort, tumors with HRAS mutations are predominantly PCCs, which is consistent with other cluster 2 tumors, whereas in the Chinese cohort both HRAS and FGFR1 mutations are relatively common in PGLs. As described earlier (see “Personalized Management: Molecular Cluster 1” subsection “Penetrance, Epidemiology, and Metastatic Risk”), in the European cohort, PGLs are mostly restricted to cluster 1 and show a noradrenergic or occasionally a dopaminergic phenotype; in the Chinese cohort, a large proportion of epinephrine-producing PGLs was identified due to mutations in HRAS and FGFR1 (30).
Metastatic risk of cluster 2–related PCCs is low and RET, NF1, TMEM127 and MAX mutations are almost exclusively associated with PCCs (217, 218, 220) (Table 8). In one study, only 2.3 % of all metastatic cases belonged to cluster 2 (49). RET-(MEN2A-) and TMEM127-related PCCs are almost exclusively nonmetastatic (metastatic risk <5%) (46, 215, 220). MEN2B is associated with a higher metastatic risk compared with MEN2A (216). The metastatic risk of NF1-related PCCs is also low (2%-12%) (49, 217). The estimated metastatic risk of MAX-related PPGLs is around 10% (222). Nevertheless, metastatic spread remains possible for any tumor of the cluster 2 group.
Table 8.
Penetrance/prevalence, metastatic risk, location of cluster 2–related PCCs
| RET | NF1 | TMEM127 | MAX | |
|---|---|---|---|---|
| Penetrance | Around 50% (50%-80% multiple) | Around 7%-8% (12% multiple) | Penetrance unknown, prevalence around 2% (single tumors) | Penetrance unknown, prevalence around 1% (67% multiple) |
| Metastatic risk | <5% (3.5%) | Around 2%-12% | Mostly benign | Around 10% |
| Location | adrenal |
| Practical tip/synthesis: |
|---|
| • Table 8 summarizes the penetrance, metastatic risk, and location of cluster 2–related PPGLs. • RET-related penetrance of PCCs depends on the specific RET mutation and is high (around 50%, multiple/bilateral 50%-80%), while the penetrance of NF1, TMEM127, and MAX is much lower. • Of all metastatic PPGLs, only around 2%-4% belong to cluster 2 (see under “Personalized Management: Molecular Cluster 1” subsection “Penetrance, Epidemiology, and Metastatic Risk”). • The metastatic risk of cluster 2–related PPGLs is low (around 4%-5%) (see under “Personalized Management: Molecular Cluster 1” subsection “Penetrance, Epidemiology, and Metastatic Risk”). • Cluster 2–related tumors are almost exclusively located adrenally (PCCs). |
Clinical Presentations
In cluster 2–related PPGLs, there is suggestive evidence that signs and symptoms are mainly of an episodic nature associated with paroxysmal excessive secretory activity. This presentation appears related to high tumoral catecholamine contents, low rates of constant catecholamine secretion (per mass of tumor tissue), and a well-developed regulatory control of secretion that nevertheless can respond to provocative stimuli; this contrasts with cluster 1 tumors, which show low catecholamine contents but higher rates of continuous secretion and less developed secretory control (sustained hypertension) (74, 87, 102).
All potential PPGL-related specific and nonspecific signs and symptoms and possible stimuli of the so-called “spells”—especially in cluster 2–related PPGLs—are described in detail under “Personalized Management: Molecular Cluster 1” subsection “Clinical Presentations.” Patients with cluster 2–related PCCs commonly show a more pronounced “signs and symptoms score”—described in detail under “Personalized Management: Molecular Cluster 1” subsection “Clinical Presentations”— and more likely suffer from pallor, tremor, and anxiety/panic, compared with those with cluster 1–related disease (86). This may be partly explained by the—although episodic—excessive catecholamine secretion during a so-called spell.
For RET-related PCCs the predominant stimulation of beta-adrenoceptors by epinephrine is presumably responsible for the presentation of episodic tachycardia/palpitations and paroxysmal hypertension rather than sustained hypertension (223). However, only around 50% of patients with RET-related PCCs present with signs and symptoms, which may reflect negligible or low rates of catecholamine secretion as well as discovery as part of screening programs. Similarly, patients with NF1-related PCCs can often be asymptomatic and normotensive (218).
| Practical tip/synthesis (applies to all clusters): |
|---|
| • The recently established signs and symptoms score (−1 to +7 points) to triage patients according to their likelihood of PPGLs is provided under “Personalized Management: Molecular Cluster 1” subsection “Clinical Presentations” (“Synthesis”). • Signs and symptoms of cluster 2–related PPGLs are mainly of an episodic nature associated with paroxysmal excessive catecholamine secretion (triggered by stimuli) due to a high catecholamine content, but low rates of constant secretion and a well-developed regulatory control, in contrast to cluster 1 tumors. • Patients with cluster 2–related PPGLs have higher signs and symptoms scores and more often suffer from pallor, tremor, and anxiety/panic, compared with those with cluster 1–related PPGLs. |
Biochemistry
In contrast to cluster 1, cluster 2-related PCCs are characterized by an adrenergic phenotype, likely reflecting origins of the tumors from fully differentiated chromaffin cells (49). The exception to this involves PCC due to MAX mutations, in which lack of MAX prevents induction of phenylethanolamine-N-methyltransferase by glucocorticoids (54).
The adrenergic phenotype is defined by a tumor content of epinephrine that exceeds 5% of the contents of all catecholamines; this can be assessed by measurements of plasma metanephrine relative to normetanephrine, the respective metabolites of epinephrine and norepinephrine (73, 87, 102). Although elevations of plasma or urinary metanephrines can indicate that the tumor produces epinephrine, this is not always reflected by secretion of the parent catecholamine. Vice-versa, negative results for measurements of catecholamines themselves (ie, not their metabolites) do not necessarily mean that the tumor is nonfunctional; such tumors may episodically release epinephrine when provoked by stimuli (see under “Personalized Management: Molecular Cluster 1” subsection “Clinical Presentations”) (87, 102). This provides one reason why measurement of the metabolites (metanephrine, normetanephrine, 3-methoxytyramine) provides a more sensitive diagnostic test than the parent catecholamines (see under “Personalized Management: Molecular Cluster 1” subsection “Biochemistry”). Adrenergic tumors invariably show additional increases in plasma or urinary normetanephrine; only rarely do these tumors show exclusive increases in metanephrine (73, 92, 102).
Accordingly, patients with RET-related PCCs invariably show elevations in plasma concentrations or urinary outputs of metanephrine (the metabolite of epinephrine) (87, 224). The same applies to NF1-associated PCCs (92). TMEM127-related PCCs also show an adrenergic phenotype (73, 102). HRAS and FGFR1 mutations, which are relatively common in the Chinese population, are also associated with an adrenergic phenotype (30).
| Practical tip/synthesis (cluster 2): |
|---|
| • A general diagnostic flow-chart is provided by Fig. 2; a cluster-specific diagnostic flow-chart is provided by Fig. 3. • With the exception of MAX-related PCCs (mixed or noradrenergic phenotype), cluster 2–related tumors have an adrenergic phenotype (assessed by elevated plasma or urinary metanephrine). • This adrenergic signature indicates a more mature phenotype. • Adrenergic tumors are characterized by high catecholamine content and high rates of production of metanephrines, but overall low rates of catecholamine secretion and well-developed secretory control. |
Imaging
Since cluster 2–related tumors are usually located intra-adrenally (exceptions, HRAS- and FGFR1-related PGLs in the Chinese population), anatomic abdominal imaging with CT or MRI is usually sufficient for tumor localization (see also under “Personalized Management: Molecular Cluster 1” subsection “Imaging”). For tumors ≥5 cm, an additional contrast-enhanced CT of the chest, or functional imaging, is reasonable to exclude metastases.
Cluster 2 kinase signaling–related tumors exhibit high [18F]FDOPA uptake with low uptake of the remaining adrenal gland (35, 225). Thus, if there are inconclusive results on anatomic imaging (eg, distorted anatomy, very small tumors, multifocality), or for staging purposes of metastatic disease or PCCs ≥5 cm, the most sensitive functional imaging method for all cluster 2–related PCCs (>1 cm) seems to be [18F]FDOPA PET/CT (35). However, there is only clear evidence for this in a small series (n = 5) of MAX-related PCCs in a head-to-head comparison of [18F]FDOPA PET/CT with [68Ga]-DOTA-SSA PET/CT and [18F]FDG PET/CT (35, 225). The second most sensitive functional imaging modality for cluster 2–related PCCs is probably [68Ga]-DOTA-SSA PET/CT (35, 225). A specific advantage of [18F]FDOPA over [68Ga]-DOTA-SSA relates to its low uptake by healthy adrenal tissue compared with PCC, which allows for the detection of multiple PCCs within the same gland.
| Practical tip/synthesis: |
|---|
| • A general diagnostic flow-chart is provided by Fig. 2; a cluster-specific diagnostic flow-chart is provided by Fig. 3. • High screening sensitivity for cluster 2–related PCCs: abdominal CT imaging (native phase >10 HU plus contrast-enhanced phase) or contrast-enhanced abdominal MRI. • MRI overall preferable for children and long-term follow-up of children and adults. • CT superior to MRI for lung metastases, MRI superior to CT for liver metastases. • For PCCs ≥5 cm: Additional presurgery contrast-enhanced thoracic CT or functional imaging to exclude metastases. • For inconclusive results on anatomic imaging or staging of metastatic/multifocal disease, the most sensitive functional imaging method for cluster 2–related PCCs is [18F]FDOPA PET/CT (the second most sensitive one is most likely [68Ga]-DOTA-SSA PET/CT). |
Follow-Up
For asymptomatic RET mutation carriers, yearly follow-up for PCCs including clinical investigation and biochemical testing should start between 11 and 16 years of age—depending on the specific RET mutation (high or moderate risk for PCCs) (caveat: always consider the risk of medullary thyroid carcinoma and primary hyperparathyroidism) (216) (Table 9). Patients with a history of a RET-related PCC should undergo a lifelong follow-up including yearly clinical investigation and biochemical testing, at least initially; for patients with high and moderate risk for PCCs (depending on the specific RET mutation), follow-up may include abdominal/pelvic MRI every 5 years (1, 4, 34, 216) (Table 10). This is particularly important for centers where measurements of plasma free or urinary fractionated metanephrines are not available.
Table 9.
Follow-up of asymptomatic cluster 2-mutation carriers
| Follow-up of asymptomatic mutation carriers | RET | NF1 |
|---|---|---|
| Clinical and biochemical evaluation | Initial screening by the age of 11-16 years depending on the specific mutation, then every 12 months (higher penetrance) Risk of primary hyperparathyroidism and medullary thyroid carcinoma (every 12 months calcitonin, calcium, PTH if applicable) |
Initial screening by the age of 10-14 years, then every 36 months (lower penetrance) |
Table 10.
Follow-up of cluster 2 mutation carriers with a history of a PCC
| Follow-up of cluster 2 mutation carriers with a history of a PCC | RET (high/moderate risk for PCC), NF1, TMEM127, MAX | RET(low risk for PCC) |
|---|---|---|
| Clinical and biochemical evaluation | 12 months | 12 months |
| Imaging (abdominal/pelvic MRI) | At least every 5 years | optional |
Despite a rather low metastatic risk of NF1-related PCCs, most recently published guidelines nevertheless recommend the initiation of a biochemical screening of asymptomatic NF1 mutation carriers every 3 years from the age of 10 to 14 years (Table 9) (226). This is supported by the high proportion (>80%) of asymptomatic patients with an NF1-related PCC (218). As there is a high number of asymptomatic patients, it also seems reasonable to include patients with a history of an NF1-related PCC into a structured lifelong follow-up, with yearly clinical and biochemical evaluation and abdominal/pelvic MRI every 5 years (Table 10). By analogy to RET- and NF1-mutation carriers with a history of a PCC, the authors suggest a similar follow-up for patients with a history of a TMEM127- or MAX-related PCC, with yearly clinical and biochemical evaluation and abdominal/pelvic MRI once every 5 years (Table 10).
For each patient with first diagnosis of a cluster 2-related PCC ≥5 cm, a chest CT would be reasonable to rule out metastatic disease; however, this is unnecessary in the routine follow-up of these mutation carriers due to their low metastatic risk and almost exclusively adrenal location (PCCs) of cluster 2–related disease.
| Practical tip/synthesis: |
|---|
| • Table 9 summarizes the recommended follow-up of asymptomatic cluster 2 mutation carriers. • Table 10 summarizes the recommended follow-up of patients with a history of a cluster 2–related tumor. |
Treatment
We have previously discussed potential differences in therapeutic management between cluster 1- and cluster 2-related disease in “Personalized Management: Molecular Cluster 1” subsection “Treatment.” In terms of therapy (surgery, alpha-adrenoceptor blockade, and therapy of metastatic disease) the same state-of-the-art recommendations currently apply to cluster 2 as for cluster 1–related PPGLs. This includes mostly adrenal-sparing surgery for cluster 2–related local disease, CVD chemotherapy for rapidly growing tumors, and radionuclide therapy (specifically [131I]MIBG or PRRT) as first-line therapy for slowly to moderately growing metastatic disease (1) (Fig. 4 “Therapy of metastatic disease”). Therapy is not yet cluster-specific; however, systemic therapy is only infrequently necessary in cluster 2–related disease since only around 2% to 4% of metastatic PPGLs bear cluster 2 mutations (23, 37). Nevertheless, we suggest potential individual cluster 2–specific current and future systemic therapy approaches, such as: [131I]MIBG therapy; kinase signaling pathway–related TKIs (sunitinib, cabozantinib, LOXO-292, lenvatinib, axitinib among others); and other specific targeted signaling pathway inhibitors alone and in combination (PI3K/AKT/mTORC1 inhibitors and RAF/MEK/ERK inhibitors) (gray letters in Fig. 4 and footnote 2 in Table 6, Fig. 5) (36, 227, 228).
| Practical tip/synthesis (applies to all clusters): |
|---|
| • Whenever possible, surgery is the therapy of choice. • Details in terms of surgery, surgery in (oligo-)metastatic disease and alpha-adrenoceptor blockade are described under “Personalized Management: Molecular Cluster 1” subsection “Treatment.” • Figure 4 summarizes a therapy flow-chart which suggests a potential sequence of therapy for the practicing physician, including dosing. • All suggestions in terms of therapy and the sequencing of therapy are described in detail under “Personalized Management: Molecular Cluster 1” subsection “Treatment” and apply to all clusters. • For rapidly progressive PPGLs, the recommended first-line therapy is chemotherapy (CVD scheme) and for slowly to moderately progressive PPGLs, the recommended first-line therapy is radionuclide therapy—either with [131I]-MIBG or PRRT—depending on tumor uptake and location (1). • Systemic therapy approaches which might be specifically effective in kinase signaling cluster 2–related PCCs are [131I]MIBG therapy, kinase signaling pathway inhibitors such as TKIs (cabozantinib, sunitinib, LOXO-292, lenvatinib, axitinib, etc.), and PI3K/AKT/mTORC1 and RAS/RAF/MEK/ERK inhibitors (Figs. 2, 3). |
Personalized Management: Molecular Cluster 3
Overview: Pathophysiology and Signaling Pathways
In contrast to clusters 1 and 2, cluster 3 is still mostly unexplored, but appears to involve Wnt signaling (Fig. 6).
Figure 6.
Gene mutations leading to an activation of Wnt signaling (cluster 3) and derived molecular targets for a personalized therapy. Mutations in MAML3 and CSDE1 activate Wnt/ß-catenin signaling. Highlighted in red are potential drugs that address the molecular changes in cluster 3 PPGLs and are in preclinical evaluation.
Wnt signaling–related cluster 3 comprises the “mastermind-like” transcriptional coactivator 3 (MAML3) fusion gene (gain-of-function event) and somatic driver mutations (0% germline mutations) in the cold shock domain-containing E1 (CSDE1) gene (Fig. 6) (25). MAML3 fusion genes lead to overactivation of Wnt/Hedgehog signaling. A gain-of-function mutation in CSDE1 leads to overactivation of ß-catenin, a target of Wnt signaling. Both events, in turn, lead to increased angiogenesis, cell proliferation, survival, invasion, metastasis, and deregulation of metabolism.
| Practical tip/synthesis: |
|---|
| • Figure 6 summarizes the Wnt signaling-associated cluster 3 with its gain-of-function mutations/events. • Cluster 3 comprises the MAML3 fusion gene and somatic mutations in CSDE1 associated with overactivation of Wnt- and ß-catenin signaling leading, among others, to angiogenesis, proliferation, survival, invasion, metastasis, and deregulation of metabolism. |
Epidemiology, Clinical Presentations, and Metastatic Risk
For cluster 3–related tumors, only somatic mutations have been identified to date. Wnt-altered PPGLs with MAML3 fusion genes were all associated with metastatic disease and showed poor aggressive-disease-free survival (eg, a short time until the occurrence of either distant metastases, local recurrence, or positive regional lymph nodes), indicating an aggressive phenotype with high risk of multiplicity, recurrence, and metastases (25, 229). Moreover, Ki-67 expression analyzed by immunohistochemistry and correlating with a high metastatic risk was highest in one tumor with a MAML3 fusion gene in the analysis by Fishbein et al (25). Wnt signaling–related tumors seem to be mainly located within the adrenals (PCCs) (25).
Demonstrating the putative role of MAML3 in oncogenesis, different neuroendocrine tumor cell lines transiently transfected with MAML3 (FL) or exon 1-deleted MAML3 (dEx1, mimicking the fusion) showed increased invasion and colony formation in vitro, probably through activation of Wnt signaling (229).
| Practical tip/synthesis: |
|---|
| • Cluster 3-related tumors are identified by somatic mutations and are associated with a high risk of recurrence, multiplicity, and metastases. • Adrenal location. |
Biochemistry
Wnt signaling–related PPGLs showed the highest chromogranin A overexpression among all clusters (25). The catecholamine phenotype is at present unknown.
| Practical tip/synthesis: |
|---|
| • Highest chromogranin A overexpression among all clusters. • Catecholamine phenotype unknown. |
Imaging
For anatomic imaging, the same applies as for the other clusters; however, the most sensitive functional imaging modality is unknown.
| Practical Tip/Synthesis: |
|---|
| • For anatomic imaging see “Imaging” under “Personalized Management: Molecular Cluster 1” and “Personalized Management: Molecular Cluster 2.” • Most sensitive functional imaging modality unknown. |
Follow-Up
Optimal follow-up is unknown for these rare tumors. However, according to a reported high risk of recurrence, multiplicity, and metastases, follow-up should be performed by analogy with cluster 1A-related PPGLs.
| Practical tip/synthesis: |
|---|
| • Follow-up with history of an aggressive cluster 3–related PCC analogous to cluster 1A until further information is obtained. |
Treatment
In terms of therapy (surgery, alpha-adrenoceptor blockade, and therapy of metastatic disease) the same state-of-the-art currently applies for all clusters (see “Treatment” under “Personalized Management: Molecular Cluster 1” and “Personalized Management: Molecular Cluster 2” Fig. 4).
Regarding molecular-targeted therapies, targeting Wnt signaling seems reasonable for cluster 3–related tumors (Fig. 6). In different neuroendocrine tumor cell lines (also overexpressing chromogranin A), the research group of Nölting and Auernhammer et al showed good efficacy of the PORCN inhibitor WNT974, which inhibits Wnt signaling, and of the ß-catenin inhibitor PRI-724 (230).
| Practical tip/synthesis (applies to all clusters): |
|---|
| • Whenever possible, surgery is the therapy of choice. • Details in terms of surgery, surgery in (oligo-)metastatic disease and alpha-adrenoceptor blockade are described in “Treatment” under “Personalized Management: Molecular Cluster 1.” • Figure 4 summarizes a therapy flow-chart which suggests a potential sequence of therapy for a practicing physician, including dosing. • All suggestions in terms of therapy and sequence of therapy are described in detail in “Treatment” under “Personalized Management: Molecular Cluster 1” and apply to all clusters. • Future systemic therapy approaches that might be specifically effective in Wnt signaling cluster 3-related PCCs are specific inhibitors of Wnt signaling (Fig. 6). |
Conclusion/ Individual Patient Management
Table 11 summarizes the special features of each cluster for an individualized patient management plan.
Table 11.
Individualized management plan depending on the cluster affiliation
| Cluster | Cluster 1A (Krebs cycle-related): SDHx (SDHA, B, C, D, F2), FH, MDH2 (10%-15% of PPGL) | Cluster 1B (VHL/EPAS1-related): VHL, EPAS1(HIF2A), (15%-20% of PPGL) | Cluster 2 (kinase signaling–related): RET, NF1, MAX, TMEM127, HRAS (50%-60% of PCC/PGL) | Cluster 3 (Wnt signaling–related): CSDE1, MAML3 (5%-10% of PCC/PGL) |
|---|---|---|---|---|
| Percentage of germline mutations | Almost 100% germline | 25% germline (0%EPAS1(HIF2A)) | 20% germline | 0% germline |
| Signaling pathways | Pseudohypoxia, Krebs cycle-related, HIF-2α stabilization | Pseudohypoxia, VHL/EPAS1-related, HIF-2α stabilization | Kinase signaling: PI3K/AKT, RAS/RAF/ERK, mTORC1/p70S6K | Wnt signaling |
| Biochemistry | Noradrenergic/dopaminergic (low catecholamine content, constant release) | Noradrenergic (low catecholamine content, constant release) | Adrenergic with additional elevation of normetanephrine (high catecholamine content, better secretory control, episodic secretion) | Unknown, highest CgA overexpression |
| Symptoms | More likely constant hypertension and tachycardia/-arrhythmia | More likely constant hypertension and tachycardia/-arrhythmia | More likely episodic “spells”, higher sign/symptom scores, more likely tremor, anxiety/panic, pallor | Unknown |
| Imaging | [68Ga]-DOTA-SSA PET/CT (except for FH) | [18F]FDOPA PET/CT (also for FH) | [18F]FDOPA PET/CT | Unknown |
| Tumor location | Mostly extra-adrenal | Adrenal, extra-adrenal | Adrenal | Adrenal |
| Metastatic risk | High-intermediate | Intermediate-low | Low | High-intermediate |
| Age of presentation | Early (20-30 years old, earliest 5 years old) | Early, some during childhood | Late (40-50 years old), some can present early (earliest 10 years old) | Unknown |
| Therapy | Surgery, systemic: CVD, temozolomide, SSTR2-based radionuclide therapy (PRRT), (HSA) [131I]-MIBG, TKIs | Surgery, systemic: CVD, temozolomide, SSTR2-based radionuclide therapy (PRRT), (HSA) [131I]-MIBG, TKIs | Surgery, systemic in rare cases: (HSA) [131I]-MIBG, TKIs, SSTR2-based radionuclide therapy (PRRT), CVD, temozolomide | Surgery, systemic: CVD, temozolomide, SSTR2-based radionuclide therapy (PRRT), (HSA) [131I]-MIBG, TKIs |
Black letters: potentially specifically interesting for cluster 1; gray letters: potentially specifically interesting for cluster 2
Abbreviations: CgA, chromogranin A; CVD, cyclophosphamide/vincristine/dacarbazine; HSA, high-specific activity; MIBG, meta-iodobenzylguanidine; PCC, pheochromocytoma; PET/CT, positron emission tomography/computed tomography; PGL, paraganglioma; PPGL, pheochromocytoma/paraganglioma; PRRT, peptide receptor radionuclide therapy; SDHA/x, succinate dehydrogenase subunit A/x; SSTR, somatostatin receptor; TKI, tyrosine kinase inhibitor; VHL, von Hippel–Lindau.
Table 12 summarizes the follow-up suggestions for patients with a history of a PPGL depending on the underlying mutation status and disease characteristics for an individualized patient follow-up guide.
Table 12.
Individualized follow-up of patients with a history of a PPGL depending on the underlying mutation status and disease characteristics
| Follow-up (history of a PPGL) | High risk | Intermediate risk | Low risk |
|---|---|---|---|
| History of metastatic PPGL, history of sympathetic PGL, SDHA, SDHB, and FH (FH limited data), HIF2A/EPAS1 | History of head and neck PGL, history of high-risk PCC (noradrenergic, ≥5 cm, recurrent, multiple) SDHAF2, SDHC, SDHD, VHL, NF1, MAX, TMEM127, RET with high/moderate risk for PCC | History of low-risk PCC (adrenergic, <5 cm), RET with low risk for PCC | |
| Clinical, biochemistry | 6-12 months (for HIF2A/EPAS1including hematocrit) | 12 months (6 months for high-risk PCC) | 12 months |
| Imaging (MRI base of the skull to pelvis/ MRI base of the skull, neck, abdomen, pelvis plus low-dose contrast-enhanced chest CT, alternating, for cluster 1; MRI abdomen/pelvis for cluster 2) | 12-24 months (with history of disease initially 12, then 12-24 months) 6-12 months for history of very large primary PPGLs or those with large necrosis, high Ki67, and vascular and lymphatic invasion |
24-36 months for SDHAF2, SDHC, SDHD (24 months for SDHD),VHL At least every 5 years for NF1, MAX, TMEM127, RET (only abdominal/pelvic MRI) |
Optional |
| Special cases | For HIF2A/EPAS1: optic fundus examination every 12 months; PCC ≥5 cm: preoperative staging with additional contrast-enhanced chest CT or functional imaging History of nonfunctioning PPGL: Alternating, MRI (base of the skull to pelvis)/MRI base of the skull/neck/abdomen/pelvis plus low-dose contrast-enhanced chest CT every 24 months History of metastatic PPGL/ sympathetic PGL: functional imaging 3-6 months postsurgery, afterwards, alternating, yearly MRI (base of the skull to pelvis)/MRI base of the skull/neck/abdomen/pelvis plus low-dose chest CT, possibly functional imaging every 24-36 months |
VHL mutations: risk of renal cell cancer, consider abdominal MRI every 12 months; optic fundus examination every 12 months; CNS tumors, CNS MRI every 24-36 months; RET mutations: risk of primary hyperparathyroidism and medullary thyroid carcinoma (every 12 months calcitonin, calcium, PTH if applicable) |
|
| Postsurgery | Clinical and biochemical follow-up 3-6 weeks after surgery (after recovery) |
Vision and Outlook
We predict that machine learning algorithms using artificial intelligence combined with well-established clinical and biochemical determinants will assist in identifying patients at risk for developing metastases with very high accuracy. This would aid the choice of optimal therapy and follow-up and may eventually foster cure of disease and prevention of metastatic spread due to early diagnosis. Related to this, a moderately high proportion of the aggressive cluster 1A tumors occur in children. Thus, new guidelines covering PPGL management in children are important.
In terms of therapy, the data of the first randomized placebo-controlled clinical trial in PPGLs investigating sunitinib (FIRST-MAPP) are awaited and may open up new horizons for clinical studies. As a necessary next step, studies to predict the optimal sequencing of systemic therapy for inoperable/metastatic PPGLs should be planned—for example, comparing temozolomide vs radionuclide therapy vs sunitinib/cabozantinib.
Cluster-specific management regarding patient education, diagnostics (biochemistry, imaging) and follow-up are already widely acknowledged. Nevertheless, cluster-specific, genetically driven therapy requiring next-generation sequencing of individual tumors (with possibly single cell sequencing) will very likely be an essential part of the management of these tumors in the future. Indeed, in some centers this approach is currently underway utilizing complementary models, including multiple drug testing in patient primary cultures (36), and evaluating individual markers for response to specific targeted therapies.
Ongoing clinical trials are currently underway for the investigation of multiple targeted therapies; these include radionuclide therapy with SSTR2 agonists/antagonists and alpha-emitters, combination strategies with radiosensitizers, MIBG therapy, cold SSTR2 analogs (octreotide/lanreotide), PARP inhibitors plus temozolomide, demethylating agents, HIF-2α inhibitors (Belzutifan [PT2977]), immunotherapy (checkpoint inhibitors), different TKIs, TKIs in combination with immunotherapy, farnesyltransferase inhibitors, and therapeutic vaccines.
The ongoing PROSPHEO registry trial (NCT03344016) might be able to precisely answer the question as to the optimal follow-up for PPGL patients (together with novel artificial intelligence approaches) and aid in achieving the goal of preventing metastatic spread and death from PPGLs—and ultimately our vision of precision disease prevention.
Acknowledgments
Figure 6 was created by Luke Harrison with Biorender.com.
Financial Support: This work has been supported by the German Research Foundation [Deutsche Forschungsgemeinschaft (DFG)] within the CRC/Transregio 205/2, “The Adrenal: Central Relay in Health and Disease” to Svenja Nölting, Nicole Bechmann, Felix Beuschlein, Martin Fassnacht, Matthias Kroiss and Graeme Eisenhofer.
Glossary
Abbreviations
- BMI
body mass index
- bpm
beats per minute
- CT
computed tomography
- CSDE1
cold shock domain-containing E1
- CVD
cyclophosphamide/vincristine/dacarbazine
- ELISA
enzyme-linked immunosorbent assay
- FGFR1
fibroblast growth factor receptor 1
- GAPP
Grading of Adrenal Pheochromocytoma and Paraganglioma
- HIF
hypoxia-inducible factor
- HNPGL
head and neck paraganglioma
- HSA
high-specific activity
- HU
Hounsfield units
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- MAML3
“mastermind-like” transcriptional coactivator 3
- MAX
Myc-associated factor X
- MEN2
multiple endocrine neoplasia type 2
- MIBG
meta-iodobenzylguanidine
- MRI
magnetic resonance imaging
- mTOR
mechanistic target of rapamycin
- NF1
neurofibromin 1
- NGFR
nerve growth factor receptor
- OS
overall survival
- PARP
poly(ADP-ribose) polymerase
- PASS
Pheochromocytoma of the Adrenal Gland Score
- PCC
pheochromocytoma
- PET/CT
positron emission tomography/computed tomography
- PFS
progression-free survival
- PGL
paraganglioma
- PI3K
phosphoinositide-3-kinase
- PPGL
pheochromocytoma/paraganglioma
- PRRT
peptide receptor radionuclide therapy
- RTK
receptor tyrosine kinase
- SDHB
succinate dehydrogenase subunit B
- SSA
somatostatin receptor analogue
- SSTR2
somatostatin receptor 2
- TK
tyrosine kinase
- TKI
tyrosine kinase inhibitor
- TMEM127
transmembrane protein 127
- VEGF
vascular endothelial growth factor
- VHL
von Hippel–Lindau
Additional Information
Disclosures: Neither the corresponding author nor her co-authors have a conflict of interest that is relevant to the subject matter or materials included in this work.
References
- 1. Lenders JWM, Kerstens MN, Amar L, et al. . Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens. 2020;38(8):1443-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lam AK. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol. 2017;28(3):213-227. [DOI] [PubMed] [Google Scholar]
- 3. Schovanek J, Martucci V, Wesley R, et al. . The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC Cancer. 2014;14:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nölting S, Ullrich M, Pietzsch J, et al. . Current management of pheochromocytoma/paraganglioma: a guide for the practicing clinician in the era of precision medicine. Cancers (Basel). 2019;11(10). doi: 10.3390/cancers11101505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisenhofer G, Lenders JW, Siegert G, et al. . Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48(11):1739-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fassnacht M, Assie G, Baudin E, et al. . Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1476-1490. [DOI] [PubMed] [Google Scholar]
- 7. Van Nederveen FH, Gaal J, Favier J, et al. . An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10(8):764-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallace PW, Conrad C, Brückmann S, et al. . Metabolomics, machine learning and immunohistochemistry to predict succinate dehydrogenase mutational status in phaeochromocytomas and paragangliomas. J Pathol. 2020;251(4):378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26(5):551-566. [DOI] [PubMed] [Google Scholar]
- 10. Kimura N, Takayanagi R, Takizawa N, et al. ; Phaeochromocytoma Study Group in Japan . Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21(3):405-414. [DOI] [PubMed] [Google Scholar]
- 11. Strong VE, Kennedy T, Al-Ahmadie H, et al. . Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery. 2008;143(6):759-768. [DOI] [PubMed] [Google Scholar]
- 12. Kimura N, Takekoshi K, Naruse M. Risk stratification on pheochromocytoma and paraganglioma from laboratory and clinical medicine. J Clin Med. 2018;7(9). doi: 10.3390/jcm7090242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stenman A, Zedenius J, Juhlin CC. The value of histological algorithms to predict the malignancy potential of pheochromocytomas and abdominal paragangliomas-a meta-analysis and systematic review of the literature. Cancers (Basel). 2019;11(2). doi: 10.3390/cancers11020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenhofer G, Tischler AS. Neuroendocrine cancer. Closing the GAPP on predicting metastases. Nat Rev Endocrinol. 2014;10(6):315-316. [DOI] [PubMed] [Google Scholar]
- 15. Patel D, Phay JE, Yen TWF, et al. . Update on pheochromocytoma and paraganglioma from the SSO endocrine/head and neck disease-site work group. Part 1 of 2: advances in pathogenesis and diagnosis of pheochromocytoma and paraganglioma. Ann Surg Oncol. 2020;27(5):1329-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Remine WH, Chong GC, Van Heerden JA, Sheps SG, Harrison EG Jr. Current management of pheochromocytoma. Ann Surg. 1974;179(5):740-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Proye CA, Vix M, Jansson S, Tisell LE, Dralle H, Hiller W. “The” pheochromocytoma: a benign, intra-adrenal, hypertensive, sporadic unilateral tumor. Does it exist? World J Surg. 1994;18(4):467-472. [DOI] [PubMed] [Google Scholar]
- 18. Goldstein RE, O’Neill JA Jr, Holcomb GW 3rd, et al. . Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229(6):755-64; discussion 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mannelli M, Ianni L, Cilotti A, Conti A. Pheochromocytoma in Italy: a multicentric retrospective study. Eur J Endocrinol. 1999;141(6):619-624. [DOI] [PubMed] [Google Scholar]
- 20. Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005;90(4):2110-2116. [DOI] [PubMed] [Google Scholar]
- 21. Edström Elder E, Hjelm Skog AL, Höög A, Hamberger B. The management of benign and malignant pheochromocytoma and abdominal paraganglioma. Eur J Surg Oncol. 2003;29(3):278-283. [DOI] [PubMed] [Google Scholar]
- 22. Nölting S, Grossman A, Pacak K. Metastatic phaeochromocytoma: spinning towards more promising treatment options. Exp Clin Endocrinol Diabetes. 2018;127(2-03):117-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hescot S, Curras-Freixes M, Deutschbein T, et al. ; European Network for the Study of Adrenal Tumors (ENS@T) . Prognosis of Malignant Pheochromocytoma and Paraganglioma (MAPP-Prono Study): A European Network for the Study of Adrenal Tumors Retrospective Study. J Clin Endocrinol Metab. 2019;104(6):2367-2374. [DOI] [PubMed] [Google Scholar]
- 24. Hamidi O, Young WF Jr, Gruber L, et al. . Outcomes of patients with metastatic phaeochromocytoma and paraganglioma: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2017;87(5):440-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fishbein L, Leshchiner I, Walter V, et al. ; Cancer Genome Atlas Research Network . Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31(2):181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jochmanova I, Pacak K. Genomic landscape of pheochromocytoma and paraganglioma. Trends Cancer. 2018;4(1):6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burnichon N, Vescovo L, Amar L, et al. . Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20(20):3974-3974. [DOI] [PubMed] [Google Scholar]
- 28. Luchetti A, Walsh D, Rodger F, et al. . Profiling of somatic mutations in phaeochromocytoma and paraganglioma by targeted next generation sequencing analysis. Int J Endocrinol. 2015;2015:138573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gieldon L, William D, Hackmann K, et al. . Optimizing genetic workup in pheochromocytoma and paraganglioma by integrating diagnostic and research approaches. Cancers (Basel). 2019;11(6). doi: 10.3390/cancers11060809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang J, Zhang J, Pang Y, et al. . Sino-European differences in the genetic landscape and clinical presentation of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2020;105(10):3295-3307. [DOI] [PubMed] [Google Scholar]
- 31. Fishbein L, Khare S, Wubbenhorst B, et al. . Whole-exome sequencing identifies somatic ATRX mutations in pheochromocytomas and paragangliomas. Nat Commun. 2015;6:6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Job S, Draskovic I, Burnichon N, et al. . Telomerase activation and ATRX mutations are independent risk factors for metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2019;25(2):760-770. [DOI] [PubMed] [Google Scholar]
- 33. Buffet A, Ben Aim L, Leboulleux S, et al. ; French Group of Endocrine Tumors (GTE) and COMETE Network . Positive impact of genetic test on the management and outcome of patients with paraganglioma and/or pheochromocytoma. J Clin Endocrinol Metab. 2019;104(4):1109-1118. [DOI] [PubMed] [Google Scholar]
- 34. Crona J, Taïeb D, Pacak K. New perspectives on pheochromocytoma and paraganglioma: toward a molecular classification. Endocr Rev. 2017;38(6):489-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taïeb D, Hicks RJ, Hindié E, et al. . European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2019;46(10):2112-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fankhauser M, Bechmann N, Lauseker M, et al. . synergistic highly potent targeted drug combinations in different pheochromocytoma models including human tumor cultures. Endocrinology. 2019;160(11):2600-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma: a systematic review and individual patient meta-analysis. Endocr Relat Cancer. 2019;26(5):539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Remacha L, Pirman D, Mahoney CE, et al. . Recurrent germline DLST mutations in individuals with multiple pheochromocytomas and paragangliomas. Am J Hum Genet. 2019;104(4):651-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pang Y, Gupta G, Yang C, et al. . A novel splicing site IRP1 somatic mutation in a patient with pheochromocytoma and JAK2V617F positive polycythemia vera: a case report. BMC Cancer. 2018;18(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Letouzé E, Martinelli C, Loriot C, et al. . SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23(6):739-752. [DOI] [PubMed] [Google Scholar]
- 41. Remacha L, Comino-Méndez I, Richter S, et al. . Targeted exome sequencing of Krebs cycle genes reveals candidate cancer-predisposing mutations in pheochromocytomas and paragangliomas. Clin Cancer Res. 2017;23(20):6315-6324. [DOI] [PubMed] [Google Scholar]
- 42. Buffet A, Morin A, Castro-Vega LJ, et al. . Germline mutations in the mitochondrial 2-oxoglutarate/malate carrier SLC25A11 gene confer a predisposition to metastatic paragangliomas. Cancer Res. 2018;78(8):1914-1922. [DOI] [PubMed] [Google Scholar]
- 43. Bezawork-Geleta A, Rohlena J, Dong L, Pacak K, Neuzil J. Mitochondrial complex II: at the crossroads. Trends Biochem Sci. 2017;42(4):312-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bancos I, Bida JP, Tian D, et al. . High-throughput screening for growth inhibitors using a yeast model of familial paraganglioma. PLoS One. 2013;8(2):e56827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jochmanova I, Pacak K. Pheochromocytoma: the first metabolic endocrine cancer. Clin Cancer Res. 2016;22(20):5001-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Selak MA, Armour SM, MacKenzie ED, et al. . Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77-85. [DOI] [PubMed] [Google Scholar]
- 47. Crooks DR, Maio N, Lang M, et al. . Mitochondrial DNA alterations underlie an irreversible shift to aerobic glycolysis in fumarate hydratase-deficient renal cancer. Sci Signal. 2021;14(664). doi: 10.1126/scisignal.abc4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bailis W, Shyer JA, Zhao J, et al. . Distinct modes of mitochondrial metabolism uncouple T cell differentiation and function. Nature. 2019;571(7765):403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bechmann N, Moskopp ML, Ullrich M, et al. . HIF2α supports pro-metastatic behavior in pheochromocytomas/paragangliomas. Endocr Relat Cancer. 2020;27(11):625-640. [DOI] [PubMed] [Google Scholar]
- 50. Jochmanová I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105(17):1270-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12(1):9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Favier J, Plouin PF, Corvol P, Gasc JM. Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am J Pathol. 2002;161(4):1235-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eisenhofer G, Huynh TT, Pacak K, et al. . Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer. 2004;11(4):897-911. [DOI] [PubMed] [Google Scholar]
- 54. Qin N, de Cubas AA, Garcia-Martin R, et al. . Opposing effects of HIF1α and HIF2α on chromaffin cell phenotypic features and tumor cell proliferation: Insights from MYC-associated factor X. Int J Cancer. 2014;135(9):2054-2064. [DOI] [PubMed] [Google Scholar]
- 55. Toledo RA, Qin Y, Cheng ZM, et al. . Recurrent mutations of chromatin-remodeling genes and kinase receptors in pheochromocytomas and paragangliomas. Clin Cancer Res. 2016;22(9):2301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Remacha L, Curras-Freixes M, Torres-Ruiz R, et al. . Gain-of-function mutations in DNMT3A in patients with paraganglioma. Genet Med. 2018;20(12):1644-1651. [DOI] [PubMed] [Google Scholar]
- 57. Schlisio S, Kenchappa RS, Vredeveld LC, et al. . The kinesin KIF1Bbeta acts downstream from EglN3 to induce apoptosis and is a potential 1p36 tumor suppressor. Genes Dev. 2008;22(7):884-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Welander J, Andreasson A, Juhlin CC, et al. . Rare germline mutations identified by targeted next-generation sequencing of susceptibility genes in pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2014;99(7):E1352-E1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morin A, Goncalves J, Moog S, et al. . TET-mediated hypermethylation primes SDH-Deficient cells for HIF2α-driven mesenchymal transition. Cell Rep. 2020;30(13):4551-4566.e7. [DOI] [PubMed] [Google Scholar]
- 60. Timmers HJ, Kozupa A, Eisenhofer G, et al. . Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92(3):779-786. [DOI] [PubMed] [Google Scholar]
- 61. Jochmanova I, Abcede AMT, Guerrero RJS, et al. . Clinical characteristics and outcomes of SDHB-related pheochromocytoma and paraganglioma in children and adolescents. J Cancer Res Clin Oncol. 2020;146(4):1051-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tufton N, Shapiro L, Sahdev A, et al. . An analysis of surveillance screening for SDHB-related disease in childhood and adolescence. Endocr Connect. 2019;8(3):162-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Srirangalingam U, Walker L, Khoo B, et al. . Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin Endocrinol (Oxf). 2008;69(4):587-596. [DOI] [PubMed] [Google Scholar]
- 64. Rijken JA, Niemeijer ND, Jonker MA, et al. . The penetrance of paraganglioma and pheochromocytoma in SDHB germline mutation carriers. Clin Genet. 2018;93(1):60-66. [DOI] [PubMed] [Google Scholar]
- 65. Andrews KA, Ascher DB, Pires DEV, et al. . Tumour risks and genotype-phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD. J Med Genet. 2018;55(6):384-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jochmanova I, Wolf KI, King KS, et al. . SDHB-related pheochromocytoma and paraganglioma penetrance and genotype-phenotype correlations. J Cancer Res Clin Oncol. 2017;143(8):1421-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jafri M, Whitworth J, Rattenberry E, et al. . Evaluation of SDHB, SDHD and VHL gene susceptibility testing in the assessment of individuals with non-syndromic phaeochromocytoma, paraganglioma and head and neck paraganglioma. Clin Endocrinol (Oxf). 2013;78(6):898-906. [DOI] [PubMed] [Google Scholar]
- 68. Benn DE, Zhu Y, Andrews KA, et al. . Bayesian approach to determining penetrance of pathogenic SDH variants. J Med Genet. 2018;55(11):729-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rednam SP, Erez A, Druker H, et al. . Von Hippel-Lindau and hereditary pheochromocytoma/paraganglioma syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23(12):e68-e75. [DOI] [PubMed] [Google Scholar]
- 70. McNeill A, Rattenberry E, Barber R, Killick P, MacDonald F, Maher ER. Genotype-phenotype correlations in VHL exon deletions. Am J Med Genet A. 2009;149A(10):2147-2151. [DOI] [PubMed] [Google Scholar]
- 71. Dahia PLM, Clifton-Bligh R, Gimenez-Roqueplo AP, Robledo M, Jimenez C. HEREDITARY ENDOCRINE TUMOURS: CURRENT STATE-OF-THE-ART AND RESEARCH OPPORTUNITIES: Metastatic pheochromocytomas and paragangliomas: proceedings of the MEN2019 workshop. Endocr Relat Cancer. 2020;27(8):T41-T52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tufton N, Sahdev A, Drake WM, Akker SA. Can subunit-specific phenotypes guide surveillance imaging decisions in asymptomatic SDH mutation carriers? Clin Endocrinol (Oxf). 2019;90(1):31-46. [DOI] [PubMed] [Google Scholar]
- 73. Eisenhofer G, Lenders JW, Timmers H, et al. . Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem. 2011;57(3):411-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Eisenhofer G, Pacak K, Huynh TT, et al. . Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18(1):97-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Turkova H, Prodanov T, Maly M, et al. . Characteristics and outcomes of metastatic SDHB and sporadic pheochromocytoma/paraganglioma: an national institutes of health study. Endocr Pract. 2016;22(3):302-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee H, Jeong S, Yu Y, et al. . Risk of metastatic pheochromocytoma and paraganglioma in SDHx mutation carriers: a systematic review and updated meta-analysis. J Med Genet. 2020;57(4):217-225. [DOI] [PubMed] [Google Scholar]
- 77. Zhuang Z, Yang C, Lorenzo F, et al. . Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367(10):922-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pacak K, Jochmanova I, Prodanov T, et al. . New syndrome of paraganglioma and somatostatinoma associated with polycythemia. J Clin Oncol. 2013;31(13):1690-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Därr R, Nambuba J, Del Rivero J, et al. . Novel insights into the polycythemia-paraganglioma-somatostatinoma syndrome. Endocr Relat Cancer. 2016;23(12):899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Castro-Vega LJ, Buffet A, De Cubas AA, et al. . Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23(9):2440-2446. [DOI] [PubMed] [Google Scholar]
- 81. Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med. 1999;340(24):1872-1879. [DOI] [PubMed] [Google Scholar]
- 82. Nielsen SM, Rhodes L, Blanco I, et al. . Von Hippel-Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol. 2016;34(18):2172-2181. [DOI] [PubMed] [Google Scholar]
- 83. Else T, Marvin ML, Everett JN, et al. . The clinical phenotype of SDHC-associated hereditary paraganglioma syndrome (PGL3). J Clin Endocrinol Metab. 2014;99(8):E1482-E1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Taïeb D, Kaliski A, Boedeker CC, et al. . Current approaches and recent developments in the management of head and neck paragangliomas. Endocr Rev. 2014;35(5):795-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jha A, de Luna K, Balili CA, et al. . Clinical, diagnostic, and treatment characteristics of SDHA-related metastatic pheochromocytoma and paraganglioma. Front Oncol. 2019;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Geroula A, Deutschbein T, Langton K, et al. . Pheochromocytoma and paraganglioma: clinical feature-based disease probability in relation to catecholamine biochemistry and reason for disease suspicion. Eur J Endocrinol. 2019;181(4):409-420. [DOI] [PubMed] [Google Scholar]
- 87. Eisenhofer G, Huynh TT, Elkahloun A, et al. . Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. Am J Physiol Endocrinol Metab. 2008;295(5):E1223-E1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab. 2007;92(11):4069-4079. [DOI] [PubMed] [Google Scholar]
- 89. Berends AMA, Kerstens MN, Lenders JWM, Timmers H. Approach to the patient: perioperative management of the patient with pheochromocytoma or sympathetic paraganglioma. J Clin Endocrinol Metab. 2020;105(9):3088-3103. [DOI] [PubMed] [Google Scholar]
- 90. Eisenhofer G, Rivers G, Rosas AL, Quezado Z, Manger WM, Pacak K. Adverse drug reactions in patients with phaeochromocytoma: incidence, prevention and management. Drug Saf. 2007;30(11):1031-1062. [DOI] [PubMed] [Google Scholar]
- 91. Eisenhofer G, Lenders JW, Goldstein DS, et al. . Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines. Clin Chem. 2005;51(4):735-744. [DOI] [PubMed] [Google Scholar]
- 92. Eisenhofer G, Deutschbein T, Constantinescu G, et al. . Plasma metanephrines and prospective prediction of tumor location, size and mutation type in patients with pheochromocytoma and paraganglioma. Clin Chem Lab Med. 2020;59(2):353-363. [DOI] [PubMed] [Google Scholar]
- 93. Lenders JW, Pacak K, Walther MM, et al. . Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287(11):1427-1434. [DOI] [PubMed] [Google Scholar]
- 94. Crout JR, Sjoerdsma A. Turnover and metabolism of catecholamines in patients with pheochromocytoma. J Clin Invest. 1964;43:94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Timmers HJ, Pacak K, Huynh TT, et al. . Biochemically silent abdominal paragangliomas in patients with mutations in the succinate dehydrogenase subunit B gene. J Clin Endocrinol Metab. 2008;93(12):4826-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zuber S, Wesley R, Prodanov T, Eisenhofer G, Pacak K, Kantorovich V. Clinical utility of chromogranin A in SDHx-related paragangliomas. Eur J Clin Invest. 2014;44(4):365-371. [DOI] [PubMed] [Google Scholar]
- 97. Hsiao RJ, Parmer RJ, Takiyyuddin MA, O’Connor DT. Chromogranin A storage and secretion: sensitivity and specificity for the diagnosis of pheochromocytoma. Medicine (Baltimore). 1991;70(1):33-45. [PubMed] [Google Scholar]
- 98. Bilek R, Vlcek P, Safarik L, et al. . Chromogranin A in the laboratory diagnosis of pheochromocytoma and paraganglioma. Cancers (Basel). 2019;11(4):586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li M, Pamporaki C, Fliedner S, et al. . Metastatic pheochromocytoma and paraganglioma: signs and symptoms related to catecholamine secretion. Discov Oncol. 2021;12:9. doi: 10.1007/s12672-021-00404-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brown MJ, Allison DJ. Renal conversion of plasma DOPA to urine dopamine. Br J Clin Pharmacol. 1981;12(2):251-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zimlichman R, Levinson PD, Kelly G, Stull R, Keiser HR, Goldstein DS. Derivation of urinary dopamine from plasma dopa. Clin Sci (Lond). 1988;75(5):515-520. [DOI] [PubMed] [Google Scholar]
- 102. Eisenhofer G, Klink B, Richter S, Lenders JW, Robledo M. Metabologenomics of Phaeochromocytoma and Paraganglioma: an integrated approach for personalised biochemical and genetic testing. Clin Biochem Rev. 2017;38(2):69-100. [PMC free article] [PubMed] [Google Scholar]
- 103. Lenders JW, Duh QY, Eisenhofer G, et al. ; Endocrine Society . Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915-1942. [DOI] [PubMed] [Google Scholar]
- 104. Eisenhofer G, Prejbisz A, Peitzsch M, et al. . Biochemical diagnosis of chromaffin cell tumors in patients at high and low risk of disease: plasma versus urinary free or Deconjugated O-Methylated catecholamine metabolites. Clin Chem. 2018;64(11):1646-1656. [DOI] [PubMed] [Google Scholar]
- 105. Weismann D, Peitzsch M, Raida A, et al. . Measurements of plasma metanephrines by immunoassay vs liquid chromatography with tandem mass spectrometry for diagnosis of pheochromocytoma. Eur J Endocrinol. 2015;172(3):251-260. [DOI] [PubMed] [Google Scholar]
- 106. Funahashi H, Imai T, Tanaka Y, et al. . Discrepancy between PNMT presence and relative lack of adrenaline production in extra-adrenal pheochromocytoma. J Surg Oncol. 1994;57(3):196-200. [DOI] [PubMed] [Google Scholar]
- 107. Därr R, Pamporaki C, Peitzsch M, et al. . Biochemical diagnosis of phaeochromocytoma using plasma-free normetanephrine, metanephrine and methoxytyramine: importance of supine sampling under fasting conditions. Clin Endocrinol (Oxf). 2014;80(4):478-486. [DOI] [PubMed] [Google Scholar]
- 108. Boyd J, Leung AA, Sadrzadeh H, et al. . A high rate of modestly elevated plasma normetanephrine in a population referred for suspected PPGL when measured in a seated position. Eur J Endocrinol. 2019;181(3):301-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Amar L, Pacak K, Steichen O, et al. . International consensus on initial screening and follow-up of asymptomatic SDHx mutation carriers. Nat Rev Endocrinol. 2021;17(7):435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Plouin PF, Amar L, Dekkers OM, et al. ; Guideline Working Group . European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur J Endocrinol. 2016;174(5):G1-G10. [DOI] [PubMed] [Google Scholar]
- 111. Blake MA, Cronin CG, Boland GW. Adrenal imaging. AJR Am J Roentgenol. 2010;194(6):1450-1460. [DOI] [PubMed] [Google Scholar]
- 112. Buitenwerf E, Berends AMA, van Asselt ADI, et al. . Diagnostic accuracy of computed tomography to exclude pheochromocytoma: a systematic review, meta-analysis, and cost analysis. Mayo Clin Proc. 2019;94(10):2040-2052. [DOI] [PubMed] [Google Scholar]
- 113. Buitenwerf E, Korteweg T, Visser A, et al. . Unenhanced CT imaging is highly sensitive to exclude pheochromocytoma: a multicenter study. Eur J Endocrinol. 2018;178(5):431-437. [DOI] [PubMed] [Google Scholar]
- 114. Daniel E, Jones R, Bull M, Newell-Price J. Rapid-sequence MRI for long-term surveillance for paraganglioma and phaeochromocytoma in patients with succinate dehydrogenase mutations. Eur J Endocrinol. 2016;175(6):561-570. [DOI] [PubMed] [Google Scholar]
- 115. Tufton N, White G, Drake WM, Sahdev A, Akker SA. Diffusion-weighted imaging (DWI) highlights SDHB-related tumours: A pilot study. Clin Endocrinol (Oxf). 2019;91(1):104-109. [DOI] [PubMed] [Google Scholar]
- 116. Ziegler CG, Brown JW, Schally AV, et al. . Expression of neuropeptide hormone receptors in human adrenal tumors and cell lines: antiproliferative effects of peptide analogues. Proc Natl Acad Sci U S A. 2009;106(37):15879-15884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Van Essen M, Krenning EP, De Jong M, Valkema R, Kwekkeboom DJ. Peptide Receptor Radionuclide Therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol. 2007;46(6):723-734. [DOI] [PubMed] [Google Scholar]
- 118. Taïeb D, Jha A, Treglia G, Pacak K. Molecular imaging and radionuclide therapy of pheochromocytoma and paraganglioma in the era of genomic characterization of disease subgroups. Endocr Relat Cancer. 2019;26(11):R627-R652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Janssen I, Chen CC, Zhuang Z, et al. . Functional imaging signature of patients presenting with polycythemia/paraganglioma syndromes. J Nucl Med. 2017;58(8):1236-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gild ML, Naik N, Hoang J, et al. . Role of DOTATATE-PET/CT in preoperative assessment of phaeochromocytoma and paragangliomas. Clin Endocrinol (Oxf). 2018;89(2):139-147. [DOI] [PubMed] [Google Scholar]
- 121. Fonte JS, Robles JF, Chen CC, et al. . False-negative ¹²³I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocr Relat Cancer. 2012;19(1):83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jha A, Ling A, Millo C, et al. . Superiority of 68Ga-DOTATATE over 18F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx)-related pheochromocytoma and paraganglioma in the pediatric population. Eur J Nucl Med Mol Imaging. 2018;45(5):787-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Janssen I, Blanchet EM, Adams K, et al. . Superiority of [68Ga]-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clin Cancer Res. 2015;21(17):3888-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jha A, Ling A, Millo C, et al. . Superiority of 68Ga-DOTATATE PET/CT to other functional and anatomic imaging modalities in the detection of SDHD-related pheochromocytoma and paraganglioma–a comparative prospective study. J Nucl Med. 2018;59(supplement 1):46. [Google Scholar]
- 125. Janssen I, Chen CC, Taieb D, et al. . 68Ga-DOTATATE PET/CT in the localization of head and neck Paragangliomas compared with other functional imaging modalities and CT/MRI. J Nucl Med. 2016;57(2):186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Archier A, Varoquaux A, Garrigue P, et al. . Prospective comparison of (68)Ga-DOTATATE and (18)F-FDOPA PET/CT in patients with various pheochromocytomas and paragangliomas with emphasis on sporadic cases. Eur J Nucl Med Mol Imaging. 2016;43(7):1248-1257. [DOI] [PubMed] [Google Scholar]
- 127. Nambuba J, Därr R, Janssen I, et al. . Functional imaging experience in a germline fumarate hydratase mutation–positive patient with pheochromocytoma and paraganglioma. AACE Clin Case Rep. 2016;2(3):176-181. [Google Scholar]
- 128. Moffat D, Richards P, Kurzawinski TR, Khan S, Khoo B, Grossman A. Misleading 68 GALLIUM-dotatate PET scan in a patient with a history of a phaeochromocytoma: Unsuspected uptake in papillary thyroid carcinoma metastases. J Neuroendocrinol. 2021;33(5):e12964. [DOI] [PubMed] [Google Scholar]
- 129. Favier J, Amar L, Gimenez-Roqueplo AP. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101-111. [DOI] [PubMed] [Google Scholar]
- 130. Bausch B, Wellner U, Bausch D, et al. . Long-term prognosis of patients with pediatric pheochromocytoma. Endocr Relat Cancer. 2014;21(1):17-25. [DOI] [PubMed] [Google Scholar]
- 131. Tufton N, Shapiro L, Srirangalingam U, et al. . Outcomes of annual surveillance imaging in an adult and paediatric cohort of succinate dehydrogenase B mutation carriers. Clin Endocrinol (Oxf). 2017;86(2):286-296. [DOI] [PubMed] [Google Scholar]
- 132. Walz MK, Alesina PF, Wenger FA, et al. . Posterior retroperitoneoscopic adrenalectomy–results of 560 procedures in 520 patients. Surgery. 2006;140(6):943-948; discussion 948. [DOI] [PubMed] [Google Scholar]
- 133. Castinetti F, Qi XP, Walz MK, et al. . Outcomes of adrenal-sparing surgery or total adrenalectomy in phaeochromocytoma associated with multiple endocrine neoplasia type 2: an international retrospective population-based study. Lancet Oncol. 2014;15(6):648-655. [DOI] [PubMed] [Google Scholar]
- 134. Neumann HPH, Tsoy U, Bancos I, et al. ; International Bilateral-Pheochromocytoma-Registry Group . Comparison of pheochromocytoma-specific morbidity and mortality among adults with bilateral pheochromocytomas undergoing total adrenalectomy vs cortical-sparing adrenalectomy. JAMA Netw Open. 2019;2(8):e198898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nockel P, El Lakis M, Gaitanidis A, et al. . Preoperative genetic testing in pheochromocytomas and paragangliomas influences the surgical approach and the extent of adrenal surgery. Surgery. 2018;163(1):191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ellis RJ, Patel D, Prodanov T, et al. . Response after surgical resection of metastatic pheochromocytoma and paraganglioma: can postoperative biochemical remission be predicted? J Am Coll Surg. 2013;217(3):489-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Roman-Gonzalez A, Zhou S, Ayala-Ramirez M, et al. . Impact of surgical resection of the primary tumor on overall survival in patients with metastatic pheochromocytoma or sympathetic paraganglioma. Ann Surg. 2018;268(1):172-178. [DOI] [PubMed] [Google Scholar]
- 138. Strajina V, Dy BM, Farley DR, et al. . Surgical treatment of malignant pheochromocytoma and paraganglioma: retrospective case series. Ann Surg Oncol. 2017;24(6):1546-1550. [DOI] [PubMed] [Google Scholar]
- 139. Hamidi O, Young WF Jr, Iñiguez-Ariza NM, et al. . Malignant pheochromocytoma and paraganglioma: 272 patients over 55 years. J Clin Endocrinol Metab. 2017;102(9):3296-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wei S, Wu D, Yue J. Surgical resection of multiple liver metastasis of functional malignant pheochromocytoma: a case report and literature review. J Cancer Res Ther. 2013;9(Suppl): S183-S185. [DOI] [PubMed] [Google Scholar]
- 141. Arnas-Leon C, Sánchez V, Santana Suárez AD, Quintana Arroyo S, Acosta C, Martinez Martin FJ. Complete remission in metastatic pheochromocytoma treated with extensive surgery. Cureus. 2016;8(1):e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Capatina C, Ntali G, Karavitaki N, Grossman AB. The management of head-and-neck paragangliomas. Endocr Relat Cancer. 2013;20(5):R291-R305. [DOI] [PubMed] [Google Scholar]
- 143. Moore MG, Netterville JL, Mendenhall WM, Isaacson B, Nussenbaum B. Head and neck paragangliomas: an update on evaluation and management. Otolaryngol Head Neck Surg. 2016;154(4):597-605. [DOI] [PubMed] [Google Scholar]
- 144. Assadipour Y, Sadowski SM, Alimchandani M, et al. . SDHB mutation status and tumor size but not tumor grade are important predictors of clinical outcome in pheochromocytoma and abdominal paraganglioma. Surgery. 2017;161(1):230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ellis RJ, Patel D, Prodanov T, Nilubol N, Pacak K, Kebebew E. The presence of SDHB mutations should modify surgical indications for carotid body paragangliomas. Ann Surg. 2014;260(1):158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Dupin C, Lang P, Dessard-Diana B, et al. . Treatment of head and neck paragangliomas with external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89(2):353-359. [DOI] [PubMed] [Google Scholar]
- 147. Vogel J, Atanacio AS, Prodanov T, et al. . External beam radiation therapy in treatment of malignant pheochromocytoma and paraganglioma. Front Oncol. 2014;4:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pacak K, Taïeb D, Lenders J, et al. . Pheochromocytoma. DeGroot’s Endocrinology. 8th ed. Elsevier; 2021. [Google Scholar]
- 149. Buitenwerf E, Osinga TE, Timmers H, et al. . Efficacy of alpha-blockers on hemodynamic control during pheochromocytoma resection: a randomized controlled trial. J Clin Endocrinol Metab. 2020;105(7):2381-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. van der Zee PA, de Boer A. Pheochromocytoma: a review on preoperative treatment with phenoxybenzamine or doxazosin. Neth J Med. 2014;72(4):190-201. [PubMed] [Google Scholar]
- 151. Kota SK, Kota SK, Panda S, Modi KD. Pheochromocytoma: an uncommon presentation of an asymptomatic and biochemically silent adrenal incidentaloma. Malays J Med Sci. 2012;19(2):86-91. [PMC free article] [PubMed] [Google Scholar]
- 152. El-Doueihi RZ, Salti I, Maroun-Aouad M, El Hajj A. Bilateral biochemically silent pheochromocytoma, not silent after all. Urol Case Rep. 2019;24:100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Steinsapir J, Carr AA, Prisant LM, Bransome ED Jr. Metyrosine and pheochromocytoma. Arch Intern Med. 1997;157(8):901-906. [PubMed] [Google Scholar]
- 154. Bholah R, Bunchman TE. Review of pediatric pheochromocytoma and paraganglioma. Front Pediatr. 2017;5:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ludwig AD, Feig DI, Brandt ML, Hicks MJ, Fitch ME, Cass DL. Recent advances in the diagnosis and treatment of pheochromocytoma in children. Am J Surg. 2007;194(6):792-796; discussion 796. [DOI] [PubMed] [Google Scholar]
- 156. Perry RR, Keiser HR, Norton JA, et al. . Surgical management of pheochromocytoma with the use of metyrosine. Ann Surg. 1990;212(5):621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Naruse M, Satoh F, Tanabe A, et al. . Efficacy and safety of metyrosine in pheochromocytoma/paraganglioma: a multi-center trial in Japan. Endocr J. 2018;65(3):359-371. [DOI] [PubMed] [Google Scholar]
- 158. Pryma DA, Chin BB, Noto RB, et al. . Efficacy and safety of high-specific-activity 131I-MIBG therapy in patients with advanced pheochromocytoma or paraganglioma. J Nucl Med. 2019;60(5):623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Jawed I, Velarde M, Darr R, et al. . Continued tumor reduction of metastatic pheochromocytoma/paraganglioma harboring succinate dehydrogenase subunit b mutations with cyclical chemotherapy. Cell Mol Neurobiol. 2018;38(5):1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EP. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2014;81(5):642-651. [DOI] [PubMed] [Google Scholar]
- 161. Averbuch SD, Steakley CS, Young RC, et al. . Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann Intern Med. 1988;109(4):267-273. [DOI] [PubMed] [Google Scholar]
- 162. Huang H, Abraham J, Hung E, et al. . Treatment of malignant pheochromocytoma/paraganglioma with cyclophosphamide, vincristine, and dacarbazine: recommendation from a 22-year follow-up of 18 patients. Cancer. 2008;113(8):2020-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Deutschbein T, Fassnacht M, Weismann D, Reincke M, Mann K, Petersenn S. Treatment of malignant phaeochromocytoma with a combination of cyclophosphamide, vincristine and dacarbazine: own experience and overview of the contemporary literature. Clin Endocrinol (Oxf). 2015;82(1):84-90. [DOI] [PubMed] [Google Scholar]
- 164. Hadoux J, Favier J, Scoazec JY, et al. . SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma. Int J Cancer. 2014;135(11):2711-2720. [DOI] [PubMed] [Google Scholar]
- 165. Ayala-Ramirez M, Feng L, Habra MA, et al. . Clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic extra-adrenal paragangliomas: insights from the largest single-institutional experience. Cancer. 2012;118(11):2804-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Asai S, Katabami T, Tsuiki M, Tanaka Y, Naruse M. Controlling tumor progression with cyclophosphamide, vincristine, and dacarbazine treatment improves survival in patients with metastatic and unresectable malignant pheochromocytomas/paragangliomas. Horm Cancer. 2017;8(2):108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Tanabe A, Naruse M, Nomura K, Tsuiki M, Tsumagari A, Ichihara A. Combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine in patients with malignant pheochromocytoma and paraganglioma. Horm Cancer. 2013;4(2):103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bravo EL, Kalmadi SR, Gill I. Clinical utility of temozolomide in the treatment of malignant paraganglioma: a preliminary report. Horm Metab Res. 2009;41(9):703-706. [DOI] [PubMed] [Google Scholar]
- 169. Tena I, Gupta G, Tajahuerce M, et al. . Successful second-line metronomic temozolomide in metastatic paraganglioma: case reports and review of the literature. Clin Med Insights Oncol. 2018;12:1179554918763367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Pang Y, Lu Y, Caisova V, et al. . Targeting NAD+/PARP DNA repair pathway as a novel therapeutic approach to SDHB-mutated cluster I pheochromocytoma and paraganglioma. Clin Cancer Res. 2018;24(14):3423-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Noto RB, Pryma DA, Jensen J, et al. . Phase 1 study of high-specific-activity i-131 MIBG for metastatic and/or recurrent pheochromocytoma or paraganglioma. J Clin Endocrinol Metab. 2018;103(1):213-220. [DOI] [PubMed] [Google Scholar]
- 172. van Hulsteijn LT, Niemeijer ND, Dekkers OM, Corssmit EP. (131)I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2014;80(4):487-501. [DOI] [PubMed] [Google Scholar]
- 173. Nastos K, Cheung VTF, Toumpanakis C, et al. . Peptide receptor radionuclide treatment and (131)I-MIBG in the management of patients with metastatic/progressive phaeochromocytomas and paragangliomas. J Surg Oncol. 2017;115(4):425-434. [DOI] [PubMed] [Google Scholar]
- 174. Castellani MR, Seghezzi S, Chiesa C, et al. . (131)I-MIBG treatment of pheochromocytoma: low versus intermediate activity regimens of therapy. Q J Nucl Med Mol Imaging. 2010;54(1):100-113. [PubMed] [Google Scholar]
- 175. Loh KC, Fitzgerald PA, Matthay KK, Yeo PP, Price DC. The treatment of malignant pheochromocytoma with iodine-131 metaiodobenzylguanidine (131I-MIBG): a comprehensive review of 116 reported patients. J Endocrinol Invest. 1997;20(11):648-658. [DOI] [PubMed] [Google Scholar]
- 176. Gonias S, Goldsby R, Matthay KK, et al. . Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol. 2009;27(25):4162-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Thorpe MP, Kane A, Zhu J, Morse MA, Wong T, Borges-Neto S. Long-term outcomes of 125 patients with metastatic pheochromocytoma or paraganglioma treated with 131-I MIBG. J Clin Endocrinol Metab. 2020;105(3):e494-e501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wakabayashi H, Inaki A, Yoshimura K, et al. . A phase I clinical trial for [131I]meta-iodobenzylguanidine therapy in patients with refractory pheochromocytoma and paraganglioma. Sci Rep. 2019;9(1):7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Strosberg J, El-Haddad G, Wolin E, et al. ; NETTER-1 Trial Investigators . Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine Tumors. N Engl J Med. 2017;376(2):125-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. van Essen M, Krenning EP, Kooij PP, et al. . Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. 2006;47(10):1599-1606. [PubMed] [Google Scholar]
- 181. Zovato S, Kumanova A, Demattè S, et al. . Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL). Horm Metab Res. 2012;44(5):411-414. [DOI] [PubMed] [Google Scholar]
- 182. Forrer F, Riedweg I, Maecke HR, Mueller-Brand J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q J Nucl Med Mol Imaging. 2008;52(4):334-340. [PubMed] [Google Scholar]
- 183. Kong G, Grozinsky-Glasberg S, Hofman MS, et al. . Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma. J Clin Endocrinol Metab. 2017;102(9):3278-3287. [DOI] [PubMed] [Google Scholar]
- 184. Pinato DJ, Black JR, Ramaswami R, Tan TM, Adjogatse D, Sharma R. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med Oncol. 2016;33(5):47. [DOI] [PubMed] [Google Scholar]
- 185. Puranik AD, Kulkarni HR, Singh A, Baum RP. Peptide receptor radionuclide therapy with (90)Y/ (177)Lu-labelled peptides for inoperable head and neck paragangliomas (glomus tumours). Eur J Nucl Med Mol Imaging. 2015;42(8):1223-1230. [DOI] [PubMed] [Google Scholar]
- 186. Yadav MP, Ballal S, Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res. 2019;9(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Imhof A, Brunner P, Marincek N, et al. . Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29(17):2416-2423. [DOI] [PubMed] [Google Scholar]
- 188. Vyakaranam AR, Crona J, Norlen O, et al. . Favorable outcome in patients with pheochromocytoma and paraganglioma treated with (177)Lu-DOTATATE. Cancers (Basel). 2019;11(7):909. doi: 10.3390/cancers11070909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Zandee WT, Feelders RA, Smit Duijzentkunst DA, et al. . Treatment of inoperable or metastatic paragangliomas and pheochromocytomas with peptide receptor radionuclide therapy using 177Lu-DOTATATE. Eur J Endocrinol. 2019;181(1):45-53. [DOI] [PubMed] [Google Scholar]
- 190. Satapathy S, Mittal BR, Bhansali A. ‘Peptide receptor radionuclide therapy in the management of advanced pheochromocytoma and paraganglioma: A systematic review and meta-analysis’. Clin Endocrinol (Oxf). 2019;91(6):718-727. [DOI] [PubMed] [Google Scholar]
- 191. Jaiswal SK, Sarathi V, Memon SS, et al. . 177Lu-DOTATATE therapy in metastatic/inoperable pheochromocytoma-paraganglioma. Endocr Connect. 2020;9(9):864-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Navalkissoor S, Grossman A. Targeted alpha particle therapy for neuroendocrine tumours: the next generation of peptide receptor radionuclide therapy. Neuroendocrinology. 2019;108(3):256-264. [DOI] [PubMed] [Google Scholar]
- 193. Ballal S, Yadav MP, Bal C, Sahoo RK, Tripathi M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging. 2020;47(4):934-946. [DOI] [PubMed] [Google Scholar]
- 194. Raymond E, Dahan L, Raoul JL, et al. . Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501-513. [DOI] [PubMed] [Google Scholar]
- 195. Ayala-Ramirez M, Chougnet CN, Habra MA, et al. . Treatment with sunitinib for patients with progressive metastatic pheochromocytomas and sympathetic paragangliomas. J Clin Endocrinol Metab. 2012;97(11):4040-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. O’Kane GM, Ezzat S, Joshua AM, et al. . A phase 2 trial of sunitinib in patients with progressive paraganglioma or pheochromocytoma: the SNIPP trial. Br J Cancer. 2019;120(12):1113-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Jimenez C. PM, Busaidy N, Habra MA, Waguespack S, Jessop A. A phase 2 study to evaluate the effects of cabozantinib in patients with unresectable metastatic pheochromocytomas and paragangliomas. International Symposium on Pheochromocytoma and Paraganglioma Sydney, Australia.2017.
- 198. Jasim S, Suman VJ, Jimenez C, et al. . Phase II trial of pazopanib in advanced/progressive malignant pheochromocytoma and paraganglioma. Endocrine. 2017;57(2):220-225. [DOI] [PubMed] [Google Scholar]
- 199. Yao JC, Shah MH, Ito T, et al. ; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group . Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Yao JC, Fazio N, Singh S, et al. ; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group . Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Oh DY, Kim TW, Park YS, et al. . Phase 2 study of everolimus monotherapy in patients with nonfunctioning neuroendocrine tumors or pheochromocytomas/paragangliomas. Cancer. 2012;118(24):6162-6170. [DOI] [PubMed] [Google Scholar]
- 202. Druce MR, Kaltsas GA, Fraenkel M, Gross DJ, Grossman AB. Novel and evolving therapies in the treatment of malignant phaeochromocytoma: experience with the mTOR inhibitor everolimus (RAD001). Horm Metab Res. 2009;41(9):697-702. [DOI] [PubMed] [Google Scholar]
- 203. Jimenez C, Subbiah V, Stephen B, et al. . Phase II clinical trial of pembrolizumab in patients with progressive metastatic pheochromocytomas and paragangliomas. Cancers (Basel). 2020;12(8). doi: 10.3390/cancers12082307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Naing A, Meric-Bernstam F, Stephen B, et al. . Phase 2 study of pembrolizumab in patients with advanced rare cancers. J Immunother Cancer. 2020;8(1). doi: 10.1136/jitc-2019-000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Rinke A, Müller HH, Schade-Brittinger C, et al. ; PROMID Study Group . Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656-4663. [DOI] [PubMed] [Google Scholar]
- 206. Caplin ME, Pavel M, Ćwikła JB, et al. ; CLARINET Investigators . Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224-233. [DOI] [PubMed] [Google Scholar]
- 207. Ayala-Ramirez M, Palmer JL, Hofmann MC, et al. . Bone metastases and skeletal-related events in patients with malignant pheochromocytoma and sympathetic paraganglioma. J Clin Endocrinol Metab. 2013;98(4):1492-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Breen W, Bancos I, Young WF Jr, et al. . External beam radiation therapy for advanced/unresectable malignant paraganglioma and pheochromocytoma. Adv Radiat Oncol. 2018;3(1):25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Kohlenberg J, Welch B, Hamidi O, et al. . Efficacy and safety of ablative therapy in the treatment of patients with metastatic pheochromocytoma and paraganglioma. Cancers (Basel). 2019;11(2). doi: 10.3390/cancers11020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44(5):328-333. [DOI] [PubMed] [Google Scholar]
- 211. Nölting S, Grossman AB. Signaling pathways in pheochromocytomas and paragangliomas: prospects for future therapies. Endocr Pathol. 2012;23(1):21-33. [DOI] [PubMed] [Google Scholar]
- 212. Buffet A, Burnichon N, Favier J, Gimenez-Roqueplo AP. An overview of 20 years of genetic studies in pheochromocytoma and paraganglioma. Best Pract Res Clin Endocrinol Metab. 2020;34(2):101416. [DOI] [PubMed] [Google Scholar]
- 213. Welander J, Łysiak M, Brauckhoff M, Brunaud L, Söderkvist P, Gimm O. Activating FGFR1 Mutations in Sporadic Pheochromocytomas. World J Surg. 2018;42(2):482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Icard P, Lincet H. A global view of the biochemical pathways involved in the regulation of the metabolism of cancer cells. Biochim Biophys Acta. 2012;1826(2):423-433. [DOI] [PubMed] [Google Scholar]
- 215. Grubbs EG, Halperin DM, Waguespack SG, Gagel RF. History of the multiple endocrine neoplasia workshops and overview of MEN2019. Endocr Relat Cancer. 2020;27(8). doi: 10.1530/ERC-20-0201 [DOI] [PubMed] [Google Scholar]
- 216. Wells SA Jr, Asa SL, Dralle H, et al. ; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma . Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Al-Sharefi A, Javaid U, Perros P, et al. . Clinical presentation and outcomes of phaeochromocytomas/paragangliomas in neurofibromatosis type 1. Eur Endocrinol. 2019;15(2):95-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Képénékian L, Mognetti T, Lifante JC, et al. . Interest of systematic screening of pheochromocytoma in patients with neurofibromatosis type 1. Eur J Endocrinol. 2016;175(4):335-344. [DOI] [PubMed] [Google Scholar]
- 219. Qin Y, Yao L, King EE, et al. . Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42(3):229-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Yao L, Schiavi F, Cascon A, et al. . Spectrum and prevalence of FP/TMEM127 gene mutations in pheochromocytomas and paragangliomas. JAMA. 2010;304(23):2611-2619. [DOI] [PubMed] [Google Scholar]
- 221. Seabrook AJ, Harris JE, Velosa SB, et al. . Multiple endocrine tumors associated with germline MAX mutations: multiple endocrine neoplasia Type 5? J Clin Endocrinol Metab. 2021;106(4):1163-1182. [DOI] [PubMed] [Google Scholar]
- 222. Burnichon N, Cascón A, Schiavi F, et al. . MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clin Cancer Res. 2012;18(10):2828-2837. [DOI] [PubMed] [Google Scholar]
- 223. Eisenhofer G, Walther MM, Huynh TT, et al. . Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab. 2001;86(5):1999-2008. [DOI] [PubMed] [Google Scholar]
- 224. Pacak K, Ilias I, Adams KT, Eisenhofer G. Biochemical diagnosis, localization and management of pheochromocytoma: focus on multiple endocrine neoplasia type 2 in relation to other hereditary syndromes and sporadic forms of the tumour. J Intern Med. 2005;257(1):60-68. [DOI] [PubMed] [Google Scholar]
- 225. Taïeb D, Jha A, Guerin C, et al. . 18F-FDOPA PET/CT imaging of MAX-related pheochromocytoma. J Clin Endocrinol Metab. 2018;103(4):1574-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Geurts JL, Strong EA, Wang TS, Evans DB, Clarke CN. Screening guidelines and recommendations for patients at high risk of developing endocrine cancers. J Surg Oncol. 2020;121(6):975-983. [DOI] [PubMed] [Google Scholar]
- 227. Nölting S, Giubellino A, Tayem Y, et al. . Combination of 13-Cis retinoic acid and lovastatin: marked anti-tumor potential in vivo in a pheochromocytoma allograft model in female athymic nude mice. Endocrinology. 2014;155(7):2377-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Nölting S, Garcia E, Alusi G, et al. . Combined blockade of signalling pathways shows marked anti-tumour potential in phaeochromocytoma cell lines. J Mol Endocrinol. 2012;49(2):79-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Alzofon N, Koc K, Panwell K, et al. . Mastermind Like Transcriptional Coactivator 3 (MAML3) drives neuroendocrine tumor progression. Mol Cancer Res. Published online ahead of print June 15, 2021. doi: 10.1158/1541-7786.MCR-20-0992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Jin XF, Spoettl G, Maurer J, Nolting S, Auernhammer CJ. Inhibition of Wnt/beta-Catenin signaling in neuroendocrine tumors in vitro: antitumoral effects. Cancers (Basel). 2020;12(2). doi: 10.3390/cancers12020345 [DOI] [PMC free article] [PubMed] [Google Scholar]