Abstract
Objective
To systematically compare the effect of direct oral anticoagulants and low molecular weight heparin for thromboprophylaxis on the benefits and harms to patients undergoing non-cardiac surgery.
Design
Systematic review and network meta-analysis of randomised controlled trials.
Data sources
Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL), up to August 2021.
Review methods
Randomised controlled trials in adults undergoing non-cardiac surgery were selected, comparing low molecular weight heparin (prophylactic (low) or higher dose) with direct oral anticoagulants or with no active treatment. Main outcomes were symptomatic venous thromboembolism, symptomatic pulmonary embolism, and major bleeding. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used for network meta-analyses. Abstracts and full texts were screened independently in duplicate. Data were abstracted on study participants, interventions, and outcomes, and risk of bias was assessed independently in duplicate. Frequentist network meta-analysis with multivariate random effects models provided odds ratios with 95% confidence intervals, and GRADE (grading of recommendations, assessment, development, and evaluation) assessments indicated the certainty of the evidence.
Results
68 randomised controlled trials were included (51 orthopaedic, 10 general, four gynaecological, two thoracic, and one urological surgery), involving 45 445 patients. Low dose (odds ratio 0.33, 95% confidence interval 0.16 to 0.67) and high dose (0.19, 0.07 to 0.54) low molecular weight heparin, and direct oral anticoagulants (0.17, 0.07 to 0.41) reduced symptomatic venous thromboembolism compared with no active treatment, with absolute risk differences of 1-100 per 1000 patients, depending on baseline risks (certainty of evidence, moderate to high). None of the active agents reduced symptomatic pulmonary embolism (certainty of evidence, low to moderate). Direct oral anticoagulants and low molecular weight heparin were associated with a 2-3-fold increase in the odds of major bleeding compared with no active treatment (certainty of evidence, moderate to high), with absolute risk differences as high as 50 per 1000 in patients at high risk. Compared with low dose low molecular weight heparin, high dose low molecular weight heparin did not reduce symptomatic venous thromboembolism (0.57, 0.26 to 1.27) but increased major bleeding (1.87, 1.06 to 3.31); direct oral anticoagulants reduced symptomatic venous thromboembolism (0.53, 0.32 to 0.89) and did not increase major bleeding (1.23, 0.89 to 1.69).
Conclusions
Direct oral anticoagulants and low molecular weight heparin reduced venous thromboembolism compared with no active treatment but probably increased major bleeding to a similar extent. Direct oral anticoagulants probably prevent symptomatic venous thromboembolism to a greater extent than prophylactic low molecular weight heparin.
Systematic review registration
PROSPERO CRD42018106181.
Introduction
Globally, more than 200 million adults undergo major non-cardiac surgery annually.1 Surgery increases the risk of venous thromboembolism, including deep vein thrombosis and pulmonary embolism.2 Antithrombotic drugs reduce the risk of postoperative venous thromboembolism but increase the risk of bleeding.3 4 5 Recommending drug treatment as thromboprophylaxis should depend on its expected net effect based on the expected risks of venous thromboembolism and bleeding.
Existing evidence based guidelines have separate recommendations for patients undergoing orthopaedic and non-orthopaedic surgery.6 7 8 9 10 Elective major orthopaedic surgery (eg, total joint arthroplasty) has been associated with a cumulative risk of developing symptomatic venous thromboembolism (deep vein thrombosis or pulmonary embolism, or both) of about 5% in the first 35 days after surgery.6 Given this substantial risk, investigators have conducted several randomised controlled trials on drug treatment for thromboprophylaxis in this surgical population, where different active agents were compared with each other and with no active treatment. This evidence pool has led guideline panels to recommend, or not to question, drug treatment as prophylaxis for total joint arthroplasty in patients who do not have an increased risk of bleeding.6 7 8 9 10 11
The risk of venous thromboembolism varies much more in other types of non-cardiac surgery.7 12 Evidence from randomised controlled trials on drug treatment for thromboprophylaxis in non-cardiac, non-orthopaedic surgeries is more limited. As a result, current guidelines on drug treatment for thromboprophylaxis in non-cardiac, non-orthopaedic surgeries emphasise the expected risk of venous thromboembolism based on the specific surgery and factors related to the patient (eg, malignancy v non-malignancy as the reason for surgery), and more often include less strong or conditional recommendations.7 13 14 15 16
Both in orthopaedic and in other non-cardiac surgeries, the best choice of drug for thromboprophylaxis is still uncertain. In orthopaedic surgery, LMWH and direct oral anticoagulants are among the most studied agents. Since their introduction to the market, most guidelines have suggested direct oral anticoagulants as an alternative to LMWH.6 10 More recently, guidelines from the American Society of Hematology recommended direct oral anticoagulants over LMWH for thromboprophylaxis based on direct comparative evidence on efficacy and safety, and on cost effectiveness, equity, acceptability, and feasibility.8 The evidence on effects, however, was rated as of moderate certainty because of the imprecision of the estimates.8 For other non-orthopaedic surgeries, current guidelines do not consider direct oral anticoagulants as an option for thromboprophylaxis because of the lack of randomised controlled trials7 13 14 15 16 and, in practice, clinicians commonly use LMWH.17 18 19 In fact, the literature has shown that most patients would choose an oral prophylactic agent, especially if blood testing for monitoring is not involved; when patients choose a parenteral agent, usually it is because of a perceived greater efficacy or more rapid effect compared with an oral medicaton.20 21 22 Based on this background evidence, we performed a systematic review and network meta-analysis of existing randomised controlled trials comparing LMWH, direct oral anticoagulants, and no active treatment for thromboprophylaxis in patients undergoing non-cardiac surgery.
Methods
The registered PROSPERO protocol (CRD42018106181) describes the a priori plan of this review. Table S1 summarises the differences between this article and the original PROSPERO protocol, and the reasons for the changes, including what we changed or added in response to comments from peer reviewers. This report complies with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for reporting of systematic reviews incorporating a network meta-analysis of healthcare interventions.23
Rationale and framework
We postulated that the relative treatment effects of LMWH and direct oral anticoagulants are similar across different surgical settings and baseline risks. Based on this assumption, we included studies evaluating these agents in different types of non-cardiac surgeries (that is, orthopaedic and non-orthopaedic), and analysed them together in our network meta-analysis. This approach allowed us to increase the evidence pool and thus the precision of our estimates for orthopaedic and non-orthopaedic surgeries. This approach also allowed us to obtain estimates of the relative efficacy and safety of LMWH and direct oral anticoagulants for non-orthopaedic surgeries, even in the absence of studies directly comparing them. We chose a network meta-analysis as the most appropriate statistical framework and tool to estimate relative treatment effects from direct and indirect evidence and to increase precision.24
In the absence or paucity of studies comparing different types of agents in the same group or class, we assumed that different direct oral anticoagulants and different LMWHs (in the same dose regimen) have similar effects. In our primary analyses, we pooled different direct oral anticoagulants and different LMWHs into the same network options.
Eligibility criteria
Eligible studies were randomised controlled trials enrolling patients aged ≥18 years undergoing major non-cardiac surgery that included major general surgery, urological and gynaecological surgery, orthopaedic surgery, and thoracic surgery, performed by open, laparoscopic, or robotic approaches. We included only randomised controlled trials because they have the most appropriate design for an intervention type of research question, and we expected to find studies implementing this design to respond to our study question.25 We a priori excluded studies conducted in vascular surgery, neurosurgery, and surgery for trauma (including fracture repair). We believe evaluating thromboprophylaxis in these surgeries requires special efficacy and safety considerations owing to the nature of these surgeries (eg, surgery involving the circulatory system is often associated with antithrombotic treatment for other reasons, or with abnormal activation and function of the coagulopathy system, such as in trauma), or the need to look at specific relevant outcomes (eg, intracranial bleeding).
The interventions of interest were low dose LMWH (eg, enoxaparin 40 mg subcutaneously daily, or equivalent), high dose LMWH (eg, enoxaparin 30 mg subcutaneously twice daily, or equivalents), and direct oral anticoagulants, including dabigatran (220 mg orally daily), apixaban (2.5 mg orally twice a day), rivaroxaban (10 mg orally daily), and edoxaban (30 mg orally daily). We included studies evaluating these interventions compared with no active treatment (including placebo), or compared with each other. The supplementary material details our exclusion criteria, and how we classified the different interventions based on LMWH into the high dose and low dose options (table S2).
For outcomes, we considered potential benefits as reductions in symptomatic pulmonary embolism, symptomatic venous thromboembolism (any type and extension, that is, proximal or distal deep vein thrombosis, with or without pulmonary embolism, or isolated pulmonary embolism), symptomatic proximal deep vein thrombosis, and symptomatic deep vein thrombosis of any extension. Major bleeding, based on the original definition of the study, was the primary anticipated harm. We first included and extracted data on all cause death and deaths related to venous thromboembolism, but no analyses were eventually performed because of the lack of data (that is, few studies reporting these outcomes, with few events).
Data sources and searches
We searched Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL), initially up to July 2020. We built our search on the work done in the McMaster GRADE Centre (McMaster University)-American Society of Hematology collaborative project for the development of original guidelines on 10 topics on the diagnosis, prevention, and treatment of venous thromboembolism.8 26 27 28 29 To fit to our specific study question, we updated and complemented the searches done for the American Society of Hematology project (supplementary material). Searches previously done for the American Society of Hematology project were updated up to July 2020. The additional searches were done from 1965 to July 2020. We also reviewed citations of three published systematic reviews3 4 5 and one Health Technology Assessment report30 for potential eligible trials. In response to comments from peer reviewers, in August 2021 we updated our search, with the same strategy. The results of the updated search and analyses are reported separately in this article.
Study selection and data extraction
Two reviewers independently evaluated the eligibility of titles and abstracts, and full texts, with a third reviewer resolving any disagreements (IE-I, MM, MV, ST, and SY). Eight investigators (AA, FG, IE-I, MM, MV, SG, ST, and SY), working in pairs with a piloted standardised form, independently extracted information on study methods, patient characteristics, details of interventions, and definitions of outcomes and results. When necessary, a third assessor resolved discrepancies.
Risk of bias and certainty of evidence assessment
Pairs of investigators independently assessed the risk of bias of eligible randomised controlled trials with the Cochrane Collaboration’s original risk of bias tool.31 For every study, investigators made a judgment of low, unclear, or high risk of bias for each of the tool items. We also assigned each study an overall risk of bias, corresponding to the highest risk for the four items sequence generation, allocation concealment, blinding of participants and investigators, and blinding of outcome adjudicators.
We used the GRADE (grading of recommendations, assessment, development, and evaluation) working group guidance for the network meta-analysis to assess the certainty of the evidence for the direct, indirect, and network effects estimate. For each outcome and comparison, we considered risk of bias, inconsistency, indirectness, publication bias, intransitivity, incoherence, and imprecision.32 33 To judge statistical heterogeneity to assess inconsistency, we used: 0-40% to indicate might not be important heterogeneity; 30-60%, might represent moderate heterogeneity; 50-90%, might represent substantial heterogeneity; and 75-100%, considerable heterogeneity.25 We adopted a minimally contextualised GRADE approach.34 According to this approach, for each outcome and comparison, we rated the certainty of benefit or harm, with the null effect as a threshold (odds ratio of 1 or absolute risk difference of 0). If the 95% confidence interval around the point estimate crossed the decision threshold, the intervention was considered not to differ from the reference. When the point estimate was close to the null effect, however, we used clinical judgment to rate the certainty of no important effect. To make a clinical judgment, we looked at the effect size expressed as absolute risk difference, which was calculated based on the estimated odds ratios, and the baseline risks obtained from the control rates of the included studies. When we rated the certainty of no important effects, we looked at the absolute risk difference of the order of 1% for pulmonary embolism and major bleeding, 1-2% for symptomatic venous thromboembolism, and 2% for symptomatic proximal deep vein thrombosis or symptomatic deep vein thrombosis of any extension, to consider small but possibly clinically important effects. This approach means that if the entire 95% confidence interval around the absolute risk difference was within 1% or 2%, we would not rate down for imprecision. Given the variability of the baseline risks, however, we used a conservative approach and rated down for imprecision every time the 95% confidence interval crossed these thresholds, considering the full range of plausible baseline risks. Finally, if we found evidence of serious incoherence (details provided in the supplementary material), we used the evidence with the higher certainty between the direct and indirect evidence as the best estimate.33
Data synthesis and analysis
For each outcome, we first performed a frequentist meta-analysis with random effects models to estimate the treatment effects for every direct pairwise comparison. We evaluated heterogeneity by estimating the variance between studies (χ2 test and I2 statistic). We then performed a frequentist network meta-analysis based on a multivariate random effects model with restricted maximum likelihood for estimation (mvmeta command and network routine, Stata version 16.0).35 36 For each outcome, we evaluated the percentage contribution of each direct comparison to the network meta-analysis estimates (Stata netweight command).37 Based on empirical and simulation studies that have shown that frequentist and bayesians approaches for a network meta-analysis provide overlapping results in most circumstances,38 39 we chose a frequentist approach for ease of implementation and richness of tools of the Stata frequentist network routine. We also estimated ranking probabilities and the surface under the cumulative ranking curves with bayesian modelling.36 40
We reported treatment effects from direct comparison and network meta-analyses as odds ratios and corresponding 95% confidence intervals. Absolute treatment effects were presented for the network as absolute risk differences calculated for each comparison from the network odds ratio and assuming a low and a high event rate for the control group, coinciding with the lowest and highest average event rate among the studies evaluating that comparison, excluding clear outliers.
Sensitivity and subgroup analyses
The supplementary material provides details for our approach in the network meta-analysis to the assumptions of coherence (similarity of direct and indirect estimates) and transitivity (similarity across studies so that treatment options can be validly compared through indirect comparisons). We identified a priori possible sources of heterogeneity (that is, effect modifiers): type of surgery (orthopaedic or non-orthopaedic); timing of the start of thromboprophylaxis, both as a dichotomous variable (preoperatively v postoperatively) and as a continuous variable (that is, number of hours before or after surgery, with the time of surgery assigned 0); length of treatment; length of follow-up; type of funding; and study quality (that is, risk of bias). We used network meta-regressions to explore their effect modification.37 41
In response to comments from peer reviewers, we also performed the following subgroup and sensitivity analyses:
Separate network meta-analyses for the subgroups of orthopaedic and non-orthopaedic surgeries; and separate network meta-analyses, or direct pairwise meta-analyses (when only one type of comparison was represented), for specific surgical disciplines, and for specific surgical approaches (open or endoscopic) across different surgical disciplines; we performed these analyses for subgroups that included at least two studies.
A meta-regression to assess the type of direct oral anticoagulant as a possible source of heterogeneity; and separate network meta-analyses for each of the included direct oral anticoagulants.
A network meta-analysis where no treatment and placebo were included as separate network nodes and not combined into the same option of no active treatment; this sensitivity network meta-analysis had no treatment as reference and therefore also included the indirect comparison placebo versus no treatment.
Patient and public involvement
The definition of the outcomes of interest in this review was inspired by the work done in the development of the American Society of Hematology guidelines which involved a panel, including a patient representative.8 We did not evaluate whether the studies included in the review involved patients in planning, implementing, or disseminating the study.
Results
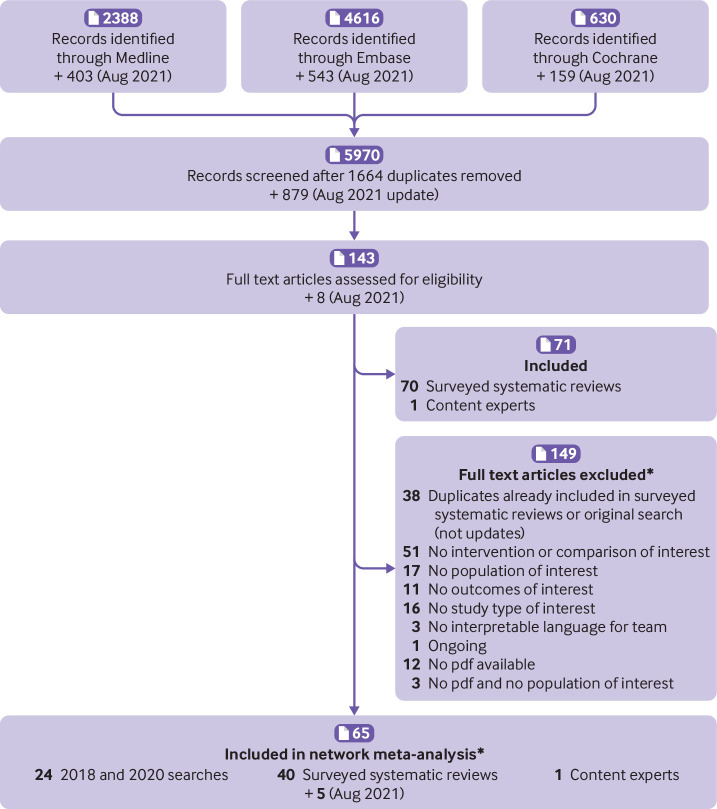
Of the 5970 non-duplicate citations screened, 78 randomised controlled trials, included in 75 reports and involving 60 068 patients, were eligible for inclusion in our study (fig 1). Of these, 68 studies (included in 65 references), involving 45 445 patients, provided data on at least one of our prespecified outcomes and were included in our network meta-analysis. The supplementary material lists the excluded studies with reasons for exclusion. Table S18 reports the main characteristics of the eligible studies.
Fig 1.
Study flow diagram for evidence source and selection. *Supplementary material lists excluded and included studies after screening of full text
Of the 68 studies included in the network meta-analysis, 51 (75%) enrolled 36 852 patients undergoing orthopaedic surgery; the remaining 17 enrolled 8593 patients undergoing general (10), gynaecological (four), urological (one), and thoracic (two) surgery. All randomised controlled trials used parallel group designs; 67 with two arms and one with three arms. The most frequent comparisons in the included trials were low dose LMWH versus no active treatment, and direct oral anticoagulants versus low dose LMWH (fig 2). Most trials in non-orthopaedic surgeries (14 of 17) compared low dose LMWH with placebo or no treatment; only one of the non-orthopaedic studies evaluated direct oral anticoagulants compared with low dose LMWH.42 Figure 3 represents the network plots for each of the analysed outcomes, and summarises the risk of bias of the original studies contributing to each direct comparison. Overall, the most frequent bias in the original studies where the risk of bias was judged to be high was lack of blinding of participants and staff (fig S1). For all of the analysed outcomes, the direct comparison between direct oral anticoagulants and low dose LMWH provided the greatest contribution to the network estimates (fig S2).
Fig 2.
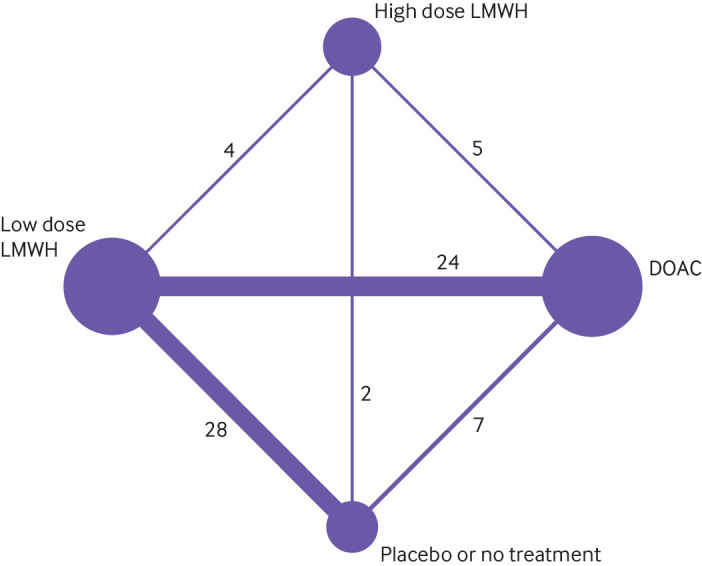
Network plot of studies included in network meta-analysis. LMWH=low molecular weight heparin; DOAC=direct oral anticoagulants. Each node indicates a treatment modality and is sized proportionally to the number of patients who received the treatment modality. Each line connecting two nodes indicates a direct comparison between two modalities, and the thickness of each line is proportional to the number of trials directly comparing the two modalities. The number of trials directly comparing the two modalities is shown
Fig 3.
Network plot of studies included in network meta-analysis by study outcome, with risk of bias representation. LMWH=low molecular weight heparin; DOAC=direct oral anticoagulants; VTE=venous thromboembolism; PE=pulmonary embolism; DVT=deep vein thrombosis. Node size is proportional to number of patients in included studies receiving that option; edge width is proportional to number of studies including that comparison. Edge colours are red (when the most frequent (mode) highest risk of bias was high in studies evaluating that comparison, for the bias items sequence generation, allocation concealment, blinding of participants and investigators, and blinding of outcome adjudicators); yellow (when the most frequent highest risk of bias was unclear); and green (when the most frequent highest risk of bias was low)
Benefits
In 25 trials involving 30 230 patients, 235 patients (0.78%) had symptomatic venous thromboembolism; in 61 studies involving 40 588 patients, 100 patients (0.25%) had a symptomatic pulmonary embolism; in 13 studies involving 4343 patients, 42 patients (0.95%) had symptomatic proximal deep vein thrombosis; and in 38 studies involving 32 338 patients, 173 (0.53%) had symptomatic deep vein thrombosis of any extension. Table 1 and tables S3-S5 show the results of the network meta-analysis on symptomatic venous thromboembolism, symptomatic pulmonary embolism, and symptomatic proximal and deep vein thrombosis of any extension, for all comparisons. The certainty of the evidence was high that all of the active treatments probably reduced symptomatic venous thromboembolism compared with no active treatment (table 1, fig S4). Direct oral anticoagulants were associated with a significant (P=0.02) reduction in symptomatic venous thromboembolism compared with low dose LMWH (odds ratio 0.53, 95% confidence interval 0.32 to 0.89), but not compared with high dose LMWH (0.93, 0.51 to 1.71); the certainty of the evidence was moderate. We found no significant difference between the network options on symptomatic pulmonary embolism; the certainty of the evidence was low to moderate (table S3). Direct oral anticoagulants, but not LMWH at low or high doses, was superior to no active treatment on symptomatic proximal deep vein thrombosis and deep vein thrombosis of any extension; the certainty of the evidence was low to high, depending on the comparison (tables S4-S5).
Table 1.
Results of network meta-analysis on symptomatic venous thromboembolism for all comparisons
| Comparisons* | No of direct comparisons | No of events/ participants in direct comparisons | Direct odds ratio (95% CI) |
Network odds ratio (95% CI) | Network absolute risk difference (events per 1000 patients (95% CI)) | Network certainty of evidence | |
|---|---|---|---|---|---|---|---|
| Estimated risk with reference (%)† | Absolute risk difference (95% CI) | ||||||
| Low dose LMWH v no treatment or placebo | 10 | 76/3544 | 0.25 (0.16 to 0.41) | 0.33 (0.16 to 0.67) | Low, 0.1 | 2 fewer (from 5 fewer to 1 fewer) | High |
| High, 3.2‡ | 21 fewer (from 27 fewer to 11 fewer) | ||||||
| High dose LMWH v no treatment or placebo | — | — | — | 0.19 (0.07 to 0.54) | Low, 0.1 | 4 fewer (from 14 fewer to 1 fewer) | High |
| High, 1.6 | 13 more (from 15 fewer to 7 fewer) | ||||||
| Direct oral anticoagulants v no treatment or placebo | 1 | 3/253 | 0.50 (0.05 to 4.80) | 0.17 (0.07 to 0.41) | Low, 0.1§ | 5 fewer (from 12 fewer to 1 fewer) | High |
| High, 1.6§ | 13 more (from 15 fewer to 9 fewer) | ||||||
| High dose v low dose LMWH | — | — | — | 0.57 (0.26 to 1.27) | Low, 0.1 | 1 fewer (from 1 fewer to 1 more) | Moderate |
| High, 2.2 | 9 fewer (from 16 fewer to 6 more) | ||||||
| Direct oral anticoagulants v low dose LMWH | 11 | 94/18 479 | 0.49 (0.32 to 0.73) | 0.53 (0.32 to 0.89) | Low, 0.1 | 1 fewer (from <1 fewer to >1 fewer) | Moderate |
| High, 2.2 | 10 fewer (from 15 fewer to 3 fewer) | ||||||
| Direct oral anticoagulants v high dose LMWH | 3 | 72/7954 | 0.94 (0.59 to 1.50) | 0.93 (0.51 to 1.71) | Low, 0.7 | 1 fewer (from 3 fewer to 5 more) | Moderate |
| High, 1.2 | 1 fewer (from 6 fewer to 8 more) | ||||||
LMWH= low molecular weight heparin.
For comparisons between active agents, results are shown with low dose LMWH as reference, the option most commonly represented in the included studies.
Low and high risk with reference coinciding with the lowest and highest event rate in the studies included in the network meta-analysis evaluating that comparison (excluding clear outliers). For studies with no events, a low risk with reference of 0.1% was used. For comparisons with no studies with direct comparisons, but a network odds ratio could be estimated, and for comparisons evaluated only in one study, the lowest and highest rates from other relevant direct comparisons were used as an example.
Three studies had a much higher rate but were considered outliers: one study in orthopaedics (29.4%, publication year 2004) and two studies in general surgery (13.8%, year 2018; 7.4%, year 2012). With a baseline rate of 30%, 14%, or 7%, the point estimate for the corresponding absolute risk reduction would be as high as 194, 94, or 47 fewer events, respectively.
Actual event rate in the reference group (no treatment or placebo) in the only study included was 1.6.
Harms
The definition of major bleeding varied across trials but in most instances included fatal bleeding, bleeding in critical organs, bleeding leading to a specific decrease in haemoglobin concentration, and bleeding leading to reoperation. In 55 studies involving 41 023 patients, 345 patients (0.84%) had major bleeding. All agents were associated with a significant increase in major bleeding compared with no active treatment (P=0.002 for low dose LMWH, from direct comparison; P=0.006 for high dose LMWH; P=0.04 for direct oral anticoagulants; certainty of evidence moderate to high; table 2 and fig S5). High dose LMWH had the largest effect size (odds ratio 3.07, 95% confidence interval 1.39 to 6.77). High dose LMWH was associated with a significant (P=0.04) increase in major bleeding compared with low dose LMWH (1.87, 1.06 to 3.31; certainty of evidence moderate), but not compared with direct oral anticoagulants (table 2).
Table 2.
Results of network meta-analysis on major bleeding for all comparisons
| Comparisons* | No of direct comparisons | No of events/ participants in direct comparisons | Direct odds ratio (95% CI) |
Network odds ratio (95% CI) | Network absolute risk difference (events per 1000 patients (95% CI)) | Network certainty of evidence | |
|---|---|---|---|---|---|---|---|
| Estimated risk with reference (%)† | Absolute risk difference (95% CI) | ||||||
| Low dose LMWH v no treatment or placebo | 22 | 60/5953 | 2.04 (1.28 to 3.22) | 2.04 (1.28 to 3.22)‡ | Low, 0.1 | 1 more (from <1 more to 2 more) | Moderate |
| High, 1.5§ | 16 more (from 4 more to 33 more) | ||||||
| High dose LMWH v no treatment or placebo | 1 | 3/100 | 0.51 (0.05 to 5.00) | 3.07 (1.39 to 6.77) | Low, 0.1¶ | 2 more (from <1 more to 6 more) | Moderate |
| High, 1.2¶ | 25 more (from 5 more to 69 more) | ||||||
| Direct oral anticoagulants v no treatment or placebo | 4 | 5/755 | 2.08 (0.52 to 8.33) | 2.01 (1.08 to 3.73) | Low, 0.1 | 1 more (from <1 more to 3 more) | High |
| High, 0.8 | 8 more (from 1 fewer to 22 more) | ||||||
| High dose v low dose LMWH | 3 | 20/952 | 2.51 (1.07 to 5.87) | 1.87 (1.06 to 3.31) | Low, 0.1 | 1 more (from <1 more to 2 more) | Moderate |
| High, 2.0 | 17 more (from 1 more to 46 more) | ||||||
| Direct oral anticoagulants v low dose LMWH | 20 | 191/24811 | 1.22 (0.92 to 1.60) | 1.23 (0.89 to 1.69) | Low, 0.1 | < <1 more (from 1 fewer to <1 more) | Moderate |
| High, 4.4 | 10 more (from 5 fewer to 30 more) | ||||||
| Direct oral anticoagulants v high dose LMWH | 5 | 66/8452 | 0.66 (0.41 to 1.07) | 0.66 (0.38 to 1.18) | Low, 0.1 | < <1 fewer (from <1 fewer to <1 more) | Moderate |
| High, 1.9 | 6 fewer (from 12 fewer to 3 more) | ||||||
LMWH= low molecular weight heparin.
For comparisons between active agents, results are shown with low dose LMWH as reference, the option most commonly represented in the included studies.
Low and high risk with reference coinciding with the lowest and highest event rate in the studies included in the network meta-analysis evaluating that comparison (excluding clear outliers). For studies with no events, a low risk with reference of 0.1% was used. For comparisons with no studies with direct comparisons, but a network odds ratio could be estimated, and for comparisons evaluated only in one study, the lowest and highest rates from other relevant direct comparisons were used as an example.
Estimate of odds ratio based on direct comparisons because of high (even if non-significant) loop specific incoherence, with direct comparisons contributing mostly to network estimates. Network odds ratio was 1.64 (95% confidence interval 0.94 to 2.88).
Highest rate was 4.5%, found in two of 22 studies: one study in thoracic surgery (publication year 1989) and one in orthopaedic surgery (year 2008), which were considered outliers here. If 4.5% was used as the highest baseline risk, the corresponding absolute risk reduction would be 47 more (95% confidence interval from 13 more to 100 more).
The 1.2% rate was chosen for consistency with other comparisons with no active treatment. In the only study comparing high dose LMWH with no active treatment (publication year 1986), the actual event rate in the control group was 4%, which would lead to a network absolute risk reduction of 62 more (95% confidence interval from 7 more to 182 more).
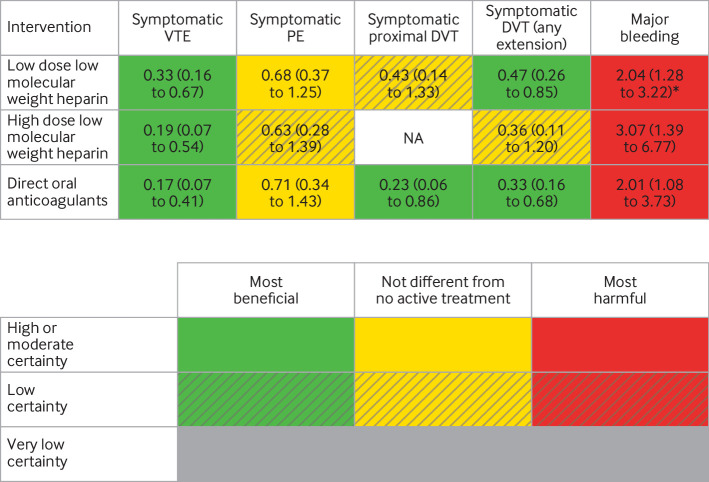
Tables S6-S10 show details of the GRADE assessment for each outcome. Figure 4 summarises the network treatment effects for every active treatment compared with no active treatment on benefit and harm outcomes, combining effect sizes with the certainty of evidence. Tables S11 and S12 report the ranking probabilities, mean ranks, and surface under the cumulative ranking curve values for the effects on any symptomatic venous thromboembolism and major bleeding. With symptomatic venous thromboembolism as an example, table S13 shows how the network odds ratios can be combined with baseline risks from the literature specific to the type of surgery or procedure, to obtain absolute treatment effects specific to the surgery or procedure.
Fig 4.
Network meta-analysis results (network odds ratio (95% confidence interval)) based on GRADE (grading of recommendations, assessment, development, and evaluation) assessment of certainty of evidence, and treatment benefit and harm, with no active treatment as reference. PE=pulmonary embolism; VTE=venous thromboembolism; DVT=deep vein thrombosis; NA=not available. *Based on direct comparison
Sensitivity and subgroup analyses
Type of surgery
Tables S14 and S15 summarise how the different direct comparisons between the network options, and direct comparisons of different direct oral anticoagulants, were represented in the included studies across surgery subgroups (orthopaedic, non-orthopaedic, general, gynaecological, open, and endoscopic surgeries). Overall, when performed separately for studies in orthopaedic and non-orthopaedic surgery, the network meta-analysis provided overlapping estimates for relative treatment effects (table 3, figs S6 and S7). We found greater efficacy of low dose LMWH on any symptomatic venous thromboembolism versus no active treatment in trials in non-orthopaedic surgery (odds ratio 0.10, 95% confidence interval 0.03 to 0.37) compared with trials in orthopaedic surgery (0.53, 0.23 to 1.24), with the meta-regression showing a significant interaction (P=0.04).
Table 3.
Results of network meta-analyses on treatment benefit and harm for comparisons between active agents and no active treatment, by surgery category
| Intervention | Network odds ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Any symptomatic venous thromboembolism | Major bleeding | ||||||
| All surgeries | Orthopaedic surgeries | Non-orthopaedic surgeries | All surgeries | Orthopaedic surgeries | Non-orthopaedic surgeries | ||
| No of direct comparison (No of events/No of patients) | 25 (235/30 230) | 19 (193/28 293) | 6 (42/1937) | 55 (345/41 023) | 42 (296/37 506) | 13 (49/3517) | |
| Low dose LMWH | 0.33 (0.16 to 0.67) | 0.53 (0.23 to 1.24) | 0.10 (0.03 to 0.37) | 2.04 (1.28 to 3.22)* | 1.03 (0.47 to 2.26) | 1.93 (0.78 to 4.82) | |
| High dose LMWH | 0.19 (0.07 to 0.54) | 0.29 (0.09 to 0.91) | NA | 3.07 (1.39 to 6.77) | 2.03 (0.84 to 4.92) | 1.90 (0.03 to 129.92) | |
| Direct oral anticoagulants | 0.17 (0.07 to 0.41)† | 0.27 (0.10 to 0.71)‡ | 0.07 (0.01 to 0.60)§ | 2.01 (1.08 to 3.73) | 1.25 (0.56 to 2.77) | 1.86 (0.08 to 44.40) | |
LMWH= low molecular weight heparin; NA=not available.
Based on direct comparison.
Network odds ratio for direct oral anticoagulants versus low dose LMWH on symptomatic venous thromboembolism is 0.53 (95% confidence interval 0.32 to 0.89).
Network odds ratio for direct oral anticoagulants versus low dose LMWH on symptomatic venous thromboembolism is 0.51 (0.31 to 0.85).
Network odds ratio for direct oral anticoagulants versus low dose LMWH on symptomatic venous thromboembolism is 0.64 (0.11 to 3.85).
Figures S8-S11 present forest plots with results of the exploratory network or pairwise meta-analyses in the subgroups of studies in open versus endoscopic surgeries, and separately in general and gynaecologic surgeries. Network meta-regressions showed no significant interaction or could not be performed because some subgroups of studies evaluated only one type of comparison (that is, only low dose molecular weight heparin v no active treatment).
Type of direct oral anticoagulant
Figures S12-S15 show forest plots with results of the exploratory network meta-analyses, where for direct comparisons of direct oral anticoagulants, we included studies evaluating each oral anticoagulant separately (that is, dabigatran, rivaroxaban, apixaban, and edoxaban). Estimates of relative treatment effects on any symptomatic venous thromboembolism and major bleeding overlapped across subgroups, with the overall meta-regression not showing any interaction between effects and type of direct oral anticoagulant.
Placebo and no treatment as two separate network options
Among the 25 studies included in the network meta-analysis on any symptomatic venous thromboembolism, 11 evaluated a comparison including no active treatment; of these, nine used no treatment and two used placebo. Among the 55 studies included in the network meta-analysis on major bleeding, 27 evaluated a comparison including no active treatment; of these, 17 used no treatment and 10 used placebo. In the sensitivity analyses where no treatment and placebo were included as separate options, all of the active treatments tended to be associated with a greater reduction of any symptomatic venous thromboembolism, and a greater increase of major bleeding, when compared with no treatment rather than when compared with placebo (fig S16). Estimates for comparisons involving placebo were generally less precise, however. Compared with no treatment, placebo was associated with a lower risk of symptomatic venous thromboembolism that was not significant (odds ratio 0.30, 95% confidence interval 0.03 to 2.92) and with a significant (P=0.02) higher risk of major bleeding (3.38, 1.20 to 9.49).
Exploratory analyses on other possible sources of heterogeneity
The supplementary material shows detailed results for the distribution across studies of other prespecified possible sources of heterogeneity (including timing of the start of thromboprophylaxis, length of treatment, length of follow-up, type of funding, and study quality) and the related meta-regression analyses. Even when a trend was noted in the subgroup analyses, no significant interaction between the variable and treatment effects was found in the meta-regression analyses. We found a significant interaction (P=0.02) between length of follow-up and effects on major bleeding, which did not have a plausible clinical rationale; because of the lack of power and no adjustment for other possible sources of heterogeneity, this finding was thought to be caused by chance or residual confounding.
Results of updated search
Our updated search found 879 potentially eligible references published between July 2020 and August 2021 (fig 1). After screening the full text of the articles, we identified five studies meeting our inclusion criteria, involving 50-400 patients who were randomised, with four of the studies in major orthopaedic surgery (table S16). None of the five studies provided outcome data that could be included in our network meta-analysis on the any symptomatic venous thromboembolism outcome; all provided data on major bleeding. When we updated our network meta-analysis on major bleeding with data from the new studies, we obtained results similar to the main study (table 2 and table S17).
Discussion
Principal findings
In our systematic review and network meta-analysis, involving more than 45 000 patients undergoing non-cardiac surgery across 68 randomised controlled trials, we found evidence of moderate-to-high certainty that LMWH and direct oral anticoagulants reduce venous thromboembolic events of any extension associated with symptoms compared with no active treatment, with point estimates for odds ratios between 0.17 and 0.33. Direct oral anticoagulants probably reduce symptomatic venous thromboembolism more than LMWH given at the most standard prophylactic dose (odds ratio 0.53, 95% confidence interval 0.32 to 0.89). We found no difference in efficacy between LMWH at a standard prophylactic dose and LMWH at higher (intermediate) doses. We could not find a significant relative treatment effect on symptomatic pulmonary embolism. The certainty of the evidence was moderate to high that all of the active agents increase major bleeding compared with no active treatment, with point estimates for odds ratios between 2.01 and 3.07. LMWH at higher than prophylactic doses probably increases the risk compared with low dose LMWH (1.87, 1.06 to 3.31).
On average, across the included studies, the absolute event rates for symptomatic venous thromboembolism outcomes and major bleeding were low (<1%); the rate of symptomatic pulmonary embolism was very low (<0.3%). This finding resulted in small absolute differences (table 1 and table 2), for active agents compared with no active treatment, and between different agents. Overall, this result highlights the need for careful evaluation of the benefit of thromboprophylaxis against the possible harms, looking at relative treatment effects and also how they translate into absolute rate differences.
Strengths and limitations of this review
The main strength of our study is that we obtained estimates of relative treatment effects with high precision, although we looked at only symptomatic venous thromboembolism events. These events are more relevant to patients but are expected to be less frequent than venous thromboembolism found through systematic screening and counted as outcomes regardless of the presence of symptoms. Also, we estimated effects for comparisons that were not directly evaluated in the existing studies (eg, comparisons including direct oral anticoagulants in non-orthopaedic surgeries). Table 3 proves that both the non-orthopaedic and orthopaedic surgery settings benefited from our approach in terms of gain in precision, for efficacy and safety estimates.
Our study had limitations. We based our analyses on the assumption of transitivity. Transitivity in network meta-analyses assumes that indirect comparisons (AC and BC) validly estimate the unobserved head-to-head comparison (AB).43 This assumption also implies that the studies are sufficiently similar in their distribution of possible effect modifiers so that indirect comparisons can be used as a valid method to compare two treatment options. We undertook several approaches to minimise and test this assumption, and looked for possible sources of heterogeneity or effect modifiers. Our subgroup and meta-regression analyses had an expected limited power, however, and could only be exploratory. In particular, for the type of surgical setting as a possible effect modifier, as expected, we had a small number of studies in non-orthopaedic surgery (only six of 25 for the symptomatic venous thromboembolism outcome), and only one recent study on direct oral anticoagulants in this setting.
Table 3 shows that estimates of relative treatment effects for all studies, and for the orthopaedic and non-orthopaedic subgroups separately, mostly overlapped and differed mainly only in precision. We found a significant (quantitative) interaction (P=0.04) for the effect of low dose LMWH versus no active treatment, with a greater and more precise effect in non-orthopaedic than orthopaedic surgeries. These analyses were driven by the results of two small, single centre, randomised controlled trials in patients undergoing general surgery (mostly cancer resections), however, that found a high rate of symptomatic venous thromboembolism in the control group (7.4% and 13.8%) and extreme relative risk reductions of these events with prophylactic LMWH (odds ratios >10).44 45 This finding emphasises how these exploratory analyses based on a small number of studies might be particularly sensitive to the outstanding results of one or two studies. Overall, these results do not refute our initial assumption that any possible difference in the relative effects of LMWH or direct oral anticoagulants between orthopaedic and non-orthopaedic surgeries would be small. Moreover, these differences would be less substantial than possible differences in baseline risks, based on patient and surgery risk factors, which determines different absolute treatment effects even assuming constant relative treatment effects. In this context, to look for an average estimate for the relative treatment effect, of increased precision, is a reasonable approach to support decision making.
To highlight the importance of looking at absolute treatment effects in a risk-benefit assessment, in table 1 and table 2 we calculated ranges of absolute treatment effects with control event rates in the included trials, and rated the certainty of evidence (precision) based on these absolute treatment effects, rather than on the network odds ratios. Even if clinically more appropriate, this approach assigns an important role to the choice of what control risk estimates to adopt. We used the highest and lowest baseline risks in the included studies to look at a plausible range, after removing a few studies (some in orthopaedic and some in non-orthopaedic surgery), where the event rate in the control group was exceptionally higher than the average. We considered these studies outliers, attributing the differences mainly to methodological or contextual factors (these studies were small and conducted in only one centre). Should these higher rates be true, however, they would translate into higher absolute treatment effects and different considerations of benefits and risk-benefits. Another possible limitation of the use of baseline risks from the included studies is that our review covered a wide timeframe, and when studies were conducted might play a role. In fact, when clear outliers were excluded, we noted a trend for higher event rates in the older trials. We did not find a significant trend, and the use of estimates from observational studies (cohort studies, registries), rather than randomised controlled trials, would be more appropriate to confirm this trend. However, it is plausible that the rate of venous thromboembolism events might be affected by recently recommended early postoperative mobilisation in orthopaedic and non-orthopaedic surgery in enhanced recovery protocols.46 47 48
Another limitation could be that we focused on LMWH and direct oral anticoagulants, and did not consider other drug treatment options for thromboprophylaxis, such as unfractionated heparin, fondaparinux, vitamin K antagonists, and aspirin. We decided, however, to take a pragmatic approach and look at agents that have been more extensively studied for thromboprophylaxis, and that could be more appealing options to evaluate in any future trial that our work could inform.
Comparison with other studies
Several published systematic reviews have evaluated randomised controlled trials on drug treatment for prophylaxis in surgical populations, and some have used a network meta-analysis approach.3 49 50 51 52 53 54 55 All of the systematic reviews, however, included studies in major elective orthopaedic surgery only (total hip or knee replacement). To our knowledge, ours is the first systematic review and network meta-analysis of the literature on drug treatment for thromboprophylaxis in patients undergoing surgery, looking at non-cardiac surgery as a whole. Moreover, the existing systematic reviews and network meta-analyses do not distinguish or analyse separately symptomatic events versus events detected by systematic imaging screening but not associated with symptoms.3 49 55
Although our work was based on some of the background work to the development of the latest guidelines from the American Society of Hematology, our work was not included in these guidelines, and differs from them in intent (obtaining reliable estimates of effects that can be used in decision making venues v making recommendations); in framework (approach to non-cardiac surgery v different types of surgeries); and in actual research questions (eg, comparison between drug treatment for thromboprophylaxis and no thromboprophylaxis in major joint surgery was not prioritised by the guidelines panel).8
Finally, our work provides a comprehensive appraisal of the existing evidence on thromboprophylaxis in non-cardiac surgery, which is instrumental to the identification of gaps and opportunities for further research. We quantified important differences in the evidence related to orthopaedic and non-orthopaedic surgeries, in terms of quantity and evaluated comparisons (with direct oral anticoagulants still under investigated in non-orthopaedic surgery), and methodological quality. Generally, orthopaedic surgery trials were of higher quality and were often sponsored by industry (supplementary material). By focusing on symptomatic venous thromboembolism events, we could also assess how often the original studies did not focus on or report these outcomes. Finally, with our search update, we showed that in the past year, new studies in the published relevant literature had similar limitations (mainly in major joint surgery, and mostly small, and with no report of symptomatic events).
Study implications for research and practice
Our work has identified the limitations of the existing evidence. Notwithstanding the gain in precision, some of our estimates were based on evidence of moderate or low certainty, partly because of the methodological limitations of the included studies. In particular, our sensitivity analysis with placebo and no treatment as two separate network options found that active agents tended to be more effective and less safe compared with no treatment than when compared with placebo. This finding suggests that lack of blinding might bias outcome ascertainment and adjudication. Only a minority of trials including a comparison with no active thromboprophylaxis used placebo, especially in non-orthopaedic surgeries. Our review identified the need for large trials, especially in those non-cardiac surgeries where the risk-benefit of thromboprophylaxis might be more controversial. There is the need for trials that adopt high methodological standards (including blinding) and outcome definitions for venous thromboembolism and bleeding that are clinically relevant and objective. The ARTS (Avoiding Risks of Thrombosis and Bleeding in Surgery) trial will compare thromboprophylaxis with a direct oral anticoagulant (apixaban) versus no anticoagulation (placebo) in patients undergoing intra-abdominal, gynaecological, or urological surgery on symptomatic venous thromboembolism and major bleeding.56 ARTS will help fill the gaps that our review identified.
We believe our work summarises the current knowledge and can inform decision making, in developing guidelines and in clinical practice. Decision makers can use our network relative treatment effects and combine them with trustful (and ideally up-to-date) baseline risk estimates specific to their population and surgery, to obtain absolute treatment effects for benefits and harms. In the supplementary material (table S13), we have provided examples of these calculations of baseline risks for symptomatic venous thromboembolism specific to the surgery or procedure; most were obtained from an ongoing systematic review of observational studies.57
Conclusions
We showed that direct oral anticoagulants and LMWH probably reduce symptomatic venous thromboembolism in major non-cardiac surgery, and that direct oral anticoagulants probably have a relatively greater efficacy than LMWH at the standard prophylactic dose. All of the drug treatment options probably increase bleeding. With the network meta-analysis approach and focusing on symptomatic events, our study provided comprehensive evidence on relative treatment effects. Many of our estimates were based on evidence of moderate-to-high certainty and can inform decision making. We showed that pulmonary embolism was consistently rare after non-cardiac surgery and that overall reported rates for symptomatic thrombotic events and major bleeding events are rare (<1%), but with possible differences across surgical populations and centres. Therefore, we emphasise the need to use our study findings for net effect evaluation of perioperative thromboprophylaxis that accounts for these additional factors.
What is already known of this topic
Direct oral anticoagulants might be reasonable alternatives to low molecular weight heparin for thromboprophylaxis in non-cardiac surgery
Direct oral anticoagulants have been extensively evaluated only in orthopaedic surgery, and often in asymptomatic venous thromboembolism
Based on the assumption that relative treatment effects are similar across surgical settings and varying baseline risks, a network meta-analysis combines all of the available direct and indirect evidence to estimate relative treatment effects with increased precision
What this study adds
This systematic review and network meta-analysis found that low molecular weight heparin (at standard prophylactic and higher doses) and direct oral anticoagulants were associated with a significant decrease in symptomatic venous thromboembolism of any extension, and an increase in major bleeding, compared with no active treatment.
Direct oral anticoagulants were more effective than prophylactic low molecular weight heparin in symptomatic venous thromboembolism but no agent was effective in preventing pulmonary embolism
Knowing the baseline risks for venous thromboembolism and bleeding specific to patients and surgeries, these findings could inform decisions on the net benefit of thromboprophylaxis in non-cardiac surgery
Acknowledgments
We thank Juan Jose Yepes-Nuñez and Wojtek Wiercioch for their help with the design of the search strategy and data extraction for the systematic review, and Behnam Sadeghirad for help with the statistical analysis.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: MM acted as principal investigator; she had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. MM, GHG, HJS, and PDV were responsible for the study concept and design, and supervised the study. All of the authors contributed to the acquisition, analysis, or interpretation of the data; and critical revision of the manuscript for important intellectual content. MM and IE-I drafted the manuscript. MM and MW conducted the statistical analysis. MM, IE-I, and HJS provided administrative, technical, and material support. MM and PDV are the guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research was not funded by a specific grant from any funding agency in the public, commercial, or not-for-profit sectors. PDV received support from the Canadian Urological Association Scholarship Foundation for his work relevant to this study. MM is supported by a McMaster University Department of Medicine Career Research Award and a PSI Foundation Mid-Career Clinical Research Award. LIL and KAOT are supported by the Academy of Finland (309387, 340957), Sigrid Jusélius Foundation, and Competitive Research Funding of the Helsinki University Hospital (TYH2019321, TYH2020248).
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no specific support for the submitted work; PDV received support from the Canadian Urological Association Scholarship Foundation for his work relevant to this study; FG’s institution received research funding from F Hoffmann-La Roche, Novo Nordisk, Takeda, Pfizer, and Bayer, all outside the submitted work; PJD reports grants from Abbott Diagnostics, Boehringer-Ingelheim, Roche Diagnostics, and Siemens and non-financial support from Philips Healthcare outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: Dissemination of the results of this work to relevant stakeholders includes presentation of the current work in scientific or lay format in conferences and workshops; dissemination to clinicians and researchers through the Reducing Global Perioperative Risk web based, multimedia resource centre (http://perioperative-risk.amjmed.com/); and posting of plain language summaries on social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This study did not require ethics approval or patient consent.
Data availability statement
No additional data available.
References
- 1. Weiser TG, Haynes AB, Molina G, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015;385(Suppl 2):S11. 10.1016/S0140-6736(15)60806-6. [DOI] [PubMed] [Google Scholar]
- 2. Gordon RJ, Lombard FW. Perioperative venous thromboembolism: a review. Anesth Analg 2017;125:403-12. 10.1213/ANE.0000000000002183. [DOI] [PubMed] [Google Scholar]
- 3. Kapoor A, Ellis A, Shaffer N, et al. Comparative effectiveness of venous thromboembolism prophylaxis options for the patient undergoing total hip and knee replacement: a network meta-analysis. J Thromb Haemost 2017;15:284-94. 10.1111/jth.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mismetti P, Laporte S, Darmon JY, Buchmüller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg 2001;88:913-30. 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 5. Rahn DD, Mamik MM, Sanses TVD, et al. Society of Gynecologic Surgeons Systematic Review Group . Venous thromboembolism prophylaxis in gynecologic surgery: a systematic review. Obstet Gynecol 2011;118:1111-25. 10.1097/AOG.0b013e318232a394. [DOI] [PubMed] [Google Scholar]
- 6. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(Suppl):e278S-325S. 10.1378/chest.11-2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(Suppl):e227S-77S. 10.1378/chest.11-2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson DR, Morgano GP, Bennett C, et al. American Society of Hematology 2019 guidelines for management of venous thromboembolism: prevention of venous thromboembolism in surgical hospitalized patients. Blood Adv 2019;3:3898-944. 10.1182/bloodadvances.2019000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kozek-Langenecker S, Fenger-Eriksen C, Thienpont E, Barauskas G, ESA VTE Guidelines Task Force . European guidelines on perioperative venous thromboembolism prophylaxis: surgery in the elderly. Eur J Anaesthesiol 2018;35:116-22. 10.1097/EJA.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence. Guidelines. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. 2019. https://www.nice.org.uk/guidance/ng89. [PubMed]
- 11. Mont MA, Jacobs JJ. AAOS clinical practice guideline: preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg 2011;19:777-8. 10.5435/00124635-201112000-00008. [DOI] [PubMed] [Google Scholar]
- 12. Tikkinen KAO, Craigie S, Agarwal A, et al. Procedure-specific risks of thrombosis and bleeding in urological cancer surgery: systematic review and meta-analysis. Eur Urol 2018;73:242-51. 10.1016/j.eururo.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 13. Mandalà M, Falanga A, Roila F, ESMO Guidelines Working Group . Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22(Suppl 6):vi85-92. 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 14. Lyman GH, Bohlke K, Khorana AA, et al. American Society of Clinical Oncology . Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol 2015;33:654-6. 10.1200/JCO.2014.59.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Violette PD, Lavallée LT, Kassouf W, Gross PL, Shayegan B. Canadian Urological Association guideline: Perioperative thromboprophylaxis and management of anticoagulation. Can Urol Assoc J 2019;13:105-14. 10.5489/cuaj.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tikkinen KAO, Cartwright R, Gould MK, et al. Thromboprophylaxis in urological surgery. European Association of Urology Guidelines Office 2017. https://uroweb.org/guideline/thromboprophylaxis/
- 17. Al Rawahi B, Le Gal G, Auer R, Carrier M. A survey of thrombosis experts evaluating practices and opinions regarding venous thromboprophylaxis in patients post major abdominal surgery. Thromb J 2017;15:2. 10.1186/s12959-016-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nelson DW, Simianu VV, Bastawrous AL, et al. Colorectal Writing Group for Surgical Care and Outcomes Assessment Program-Comparative Effectiveness Research Translation Network (SCOAP-CERTAIN) Collaborative . Thromboembolic complications and prophylaxis patterns in colorectal surgery. JAMA Surg 2015;150:712-20. 10.1001/jamasurg.2015.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lund L, Nisen H, Järvinen P, et al. Use of venous-thrombotic-embolic prophylaxis in patients undergoing surgery for renal tumors: a questionnaire survey in the Nordic countries (The NORENCA-2 study). Res Rep Urol 2018;10:181-7. 10.2147/RRU.S177774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong A, Kraus PS, Lau BD, et al. Patient preferences regarding pharmacologic venous thromboembolism prophylaxis. J Hosp Med 2015;10:108-11. 10.1002/jhm.2282. [DOI] [PubMed] [Google Scholar]
- 21. Cimminiello C, Anderson FA, Jr. Physician and patient perceptions of the route of administration of venous thromboembolism prophylaxis: results from an international survey. Thromb Res 2012;129:139-45. 10.1016/j.thromres.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 22. Lanéelle D, Le Brun C, Mauger C, et al. SFMV VTE Study Group . Patient characteristics and preferences regarding anticoagulant treatment in venous thromboembolic disease. Front Cardiovasc Med 2021;8:675969. 10.3389/fcvm.2021.675969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 24. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thomas J, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. Updated February 2021. https://training.cochrane.org/handbook/current
- 26. Lim W, Le Gal G, Bates SM, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv 2018;2:3226-56. 10.1182/bloodadvances.2018024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv 2018;2:3198-225. 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv 2018;2:3292-316. 10.1182/bloodadvances.2018024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wiercioch W, Nieuwlaat R, Akl EA, et al. Methodology for the American Society of Hematology VTE guidelines: current best practice, innovations, and experiences. Blood Adv 2020;4:2351-65. 10.1182/bloodadvances.2020001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sterne JA, Bodalia PN, Bryden PA, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess 2017;21:1-386. 10.3310/hta21090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puhan MA, Schünemann HJ, Murad MH, et al. GRADE Working Group . A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 33. Brignardello-Petersen R, Bonner A, Alexander PE, et al. GRADE Working Group . Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol 2018;93:36-44. 10.1016/j.jclinepi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 34. Brignardello-Petersen R, Florez ID, Izcovich A, et al. GRADE working group . GRADE approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. BMJ 2020;371:m3900. 10.1136/bmj.m3900. [DOI] [PubMed] [Google Scholar]
- 35. White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 2012;3:111-25. 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White IR. Multivariate random-effects meta-regression: Updates to mvmeta. Stata J 2011;11:255-70. 10.1177/1536867X1101100206. [DOI] [Google Scholar]
- 37. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seide SE, Jensen K, Kieser M. A comparison of bayesian and frequentist methods in random-effects network meta-analysis of binary data. Res Synth Methods 2020;11:363-78. 10.1002/jrsm.1397. [DOI] [PubMed] [Google Scholar]
- 39. Sadeghirad B, Brignardello-Petersen R, Johnston BC, Guyatt GH, Beyene J. Comparing bayesian and frequentist approaches for network meta-analysis: an empirical study. Abstracts of the Global Evidence Summit, Cape Town, South Africa. Cochrane Database Syst Rev 2017;(9 Suppl 1). 10.1002/14651858.CD201702. [DOI] [Google Scholar]
- 40. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 41. Salanti G, Marinho V, Higgins JP. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. J Clin Epidemiol 2009;62:857-64. 10.1016/j.jclinepi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 42. Guntupalli SR, Brennecke A, Behbakht K, et al. Safety and efficacy of apixaban vs enoxaparin for preventing postoperative venous thromboembolism in women undergoing surgery for gynecologic malignant neoplasm: a randomized clinical trial. JAMA Netw Open 2020;3:e207410. 10.1001/jamanetworkopen.2020.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riley RD, Jackson D, Salanti G, et al. Multivariate and network meta-analysis of multiple outcomes and multiple treatments: rationale, concepts, and examples. BMJ 2017;358:j3932. 10.1136/bmj.j3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dar TI, Wani KA, Ashraf M, et al. Low molecular weight heparin in prophylaxis of deep vein thrombosis in Asian general surgical patients: A Kashmir experience. Indian J Crit Care Med 2012;16:71-4. 10.4103/0972-5229.99107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang YH, Qiu H, He XL, Sun XW. Prevention of venous thromboembolism after resection of primary liver cancer with low molecular weight heparin and its association with P-selectin, lysosomal granule glycoprotein, platelet activating factor and plasma D-dimer. Eur Rev Med Pharmacol Sci 2018;22:4657-62. 10.26355/eurrev_201807_15525. [DOI] [PubMed] [Google Scholar]
- 46. Wainwright TW, Gill M, McDonald DA, et al. Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Acta Orthop 2020;91:3-19. 10.1080/17453674.2019.1683790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gustafsson UO, Scott MJ, Schwenk W, et al. Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care. European Society for Clinical Nutrition and Metabolism (ESPEN) International Association for Surgical Metabolism and Nutrition (IASMEN) . Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg 2013;37:259-84. 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 48. Mortensen K, Nilsson M, Slim K, et al. Enhanced Recovery After Surgery (ERAS®) Group . Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Br J Surg 2014;101:1209-29. 10.1002/bjs.9582. [DOI] [PubMed] [Google Scholar]
- 49. Lewis S, Glen J, Dawoud D, et al. Venous thromboembolism prophylaxis strategies for people undergoing elective total knee replacement: a systematic review and network meta-analysis. Lancet Haematol 2019;6:e530-9. 10.1016/S2352-3026(19)30155-3. [DOI] [PubMed] [Google Scholar]
- 50. Cohen A, Drost P, Marchant N, et al. The efficacy and safety of pharmacological prophylaxis of venous thromboembolism following elective knee or hip replacement: systematic review and network meta-analysis. Clin Appl Thromb Hemost 2012;18:611-27. 10.1177/1076029612437579. [DOI] [PubMed] [Google Scholar]
- 51. Neumann I, Rada G, Claro JC, et al. Oral direct factor Xa inhibitors versus low-molecular-weight heparin to prevent venous thromboembolism in patients undergoing total hip or knee replacement: a systematic review and meta-analysis. Ann Intern Med 2012;156:710-9. 10.7326/0003-4819-156-10-201205150-00421. [DOI] [PubMed] [Google Scholar]
- 52. Sobieraj DM, Coleman CI, Tongbram V, et al. Comparative effectiveness of low-molecular-weight heparins versus other anticoagulants in major orthopedic surgery: a systematic review and meta-analysis. Pharmacotherapy 2012;32:799-808. 10.1002/j.1875-9114.2012.01106.x. [DOI] [PubMed] [Google Scholar]
- 53. Lu X, Lin J. Low molecular weight heparin versus other anti-thrombotic agents for prevention of venous thromboembolic events after total hip or total knee replacement surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord 2018;19:322. 10.1186/s12891-018-2215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sun G, Wu J, Wang Q, et al. Factor Xa inhibitors and direct thrombin inhibitors versus low-molecular-weight heparin for thromboprophylaxis after total hip or total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2019;34:789-800.e6. 10.1016/j.arth.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 55. Feng W, Wang X, Huang D, Lu A. Ranking the efficacy of anticoagulants for the prevention of venous thromboembolism after total hip or knee arthroplasty: A systematic review and a network meta-analysis. Pharmacol Res 2021;166:105438. 10.1016/j.phrs.2021.105438. [DOI] [PubMed] [Google Scholar]
- 56. Violette PD, Cartwright R, Devereaux PJ, et al. ARTS: A Large, International Trial of Thromboprophylaxis in Intra-abdominal, Gynecologic, and Urologic Surgery. Eur Urol Focus 2021;7:1222-5. 10.1016/j.euf.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 57. Lavikainen LI, Guyatt GH, Lee Y, et al. Systematic reviews of observational studies of Risk of Thrombosis and Bleeding in General and Gynecologic Surgery (ROTBIGGS): introduction and methodology. Syst Rev 2021;10:264. 10.1186/s13643-021-01814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material
Data Availability Statement
No additional data available.