Key Points
Question
Can a model be developed based on perioperative metabolic panel laboratory values to predict the risk of acute kidney injury (AKI) after cardiac surgery?
Findings
Risk models developed using data from 63 260 patients demonstrated good discrimination for moderate to severe AKI within 72 hours and 14 days after surgery (area under the receiver-operating characteristic curve [AUC], 0.876 and 0.854) and for AKI requiring dialysis within 72 hours and 14 days after surgery (AUC, 0.916 and 0.900), with similar performance in an external validation cohort.
Meaning
Prediction models based on perioperative basic metabolic panel laboratory values after cardiac surgery were derived and validated to predict risk of postoperative acute kidney injury within 72 hours and 14 days, although further research is needed to determine whether use of the risk prediction tool improves clinical outcomes.
Abstract
Importance
Effective treatment of acute kidney injury (AKI) is predicated on timely diagnosis; however, the lag in the increase in serum creatinine levels after kidney injury may delay therapy initiation.
Objective
To determine the derivation and validation of predictive models for AKI after cardiac surgery.
Design, Setting, and Participants
Multivariable prediction models were derived based on a retrospective observational cohort of adult patients undergoing cardiac surgery between January 2000 and December 2019 from a US academic medical center (n = 58 526) and subsequently validated on an external cohort from 3 US community hospitals (n = 4734). The date of final follow-up was January 15, 2020.
Exposures
Perioperative change in serum creatinine and postoperative blood urea nitrogen, serum sodium, potassium, bicarbonate, and albumin from the first metabolic panel after cardiac surgery.
Main Outcomes and Measures
Area under the receiver-operating characteristic curve (AUC) and calibration measures for moderate to severe AKI, per Kidney Disease: Improving Global Outcomes (KDIGO), and AKI requiring dialysis prediction models within 72 hours and 14 days following surgery.
Results
In a derivation cohort of 58 526 patients (median [IQR] age, 66 [56-74] years; 39 173 [67%] men; 51 503 [91%] White participants), the rates of moderate to severe AKI and AKIrequiring dialysis were 2674 (4.6%) and 868 (1.48%) within 72 hours and 3156 (5.4%) and 1018 (1.74%) within 14 days after surgery. The median (IQR) interval to first metabolic panel from conclusion of the surgical procedure was 10 (7-12) hours. In the derivation cohort, the metabolic panel–based models had excellent predictive discrimination for moderate to severe AKI within 72 hours (AUC, 0.876 [95% CI, 0.869-0.883]) and 14 days (AUC, 0.854 [95% CI, 0.850-0.861]) after the surgical procedure and for AKI requiring dialysis within 72 hours (AUC, 0.916 [95% CI, 0.907-0.926]) and 14 days (AUC, 0.900 [95% CI, 0.889-0.909]) after the surgical procedure. In the validation cohort of 4734 patients (median [IQR] age, 67 (60-74) years; 3361 [71%] men; 3977 [87%] White participants), the models for moderate to severe AKI after the surgical procedure showed AUCs of 0.860 (95% CI, 0.838-0.882) within 72 hours and 0.842 (95% CI, 0.820-0.865) within 14 days and the models for AKI requiring dialysis and 14 days had an AUC of 0.879 (95% CI, 0.840-0.918) within 72 hours and 0.873 (95% CI, 0.836-0.910) within 14 days after the surgical procedure. Calibration assessed by Spiegelhalter z test showed P >.05 indicating adequate calibration for both validation and derivation models.
Conclusions and Relevance
Among patients undergoing cardiac surgery, a prediction model based on perioperative basic metabolic panel laboratory values demonstrated good predictive accuracy for moderate to severe acute kidney injury within 72 hours and 14 days after the surgical procedure. Further research is needed to determine whether use of the risk prediction tool improves clinical outcomes.
This study examines the predictive accuracy of models including early changes in serum creatinine and postsurgery laboratory parameters from the basic metabolic profile to predict postoperative moderate to severe acute kidney injury and acute kidney injury requiring dialysis in patients undergoing cardiac surgery.
Introduction
Acute kidney injury (AKI) is a serious complication of cardiac surgery, and its severe form is associated with significant morbidity and mortality.1,2 Serum creatinine and blood urea nitrogen used in conjunction with urine output have been the traditional means of diagnosing and managing AKI. However, clinically notable changes often appear days after injury and preclude early therapeutic and renoprotective interventions.3 Accurate and early identification of patients at risk for AKI can facilitate targeted provision of management protocols recommended by Kidney Disease: Improving Global Outcomes (KDIGO), such as hemodynamic and volume optimization, close monitoring of kidney function, and avoidance of nephrotoxins.4,5Several AKI biomarkers that have been studied in patients undergoing cardiac surgery have demonstrated an area under the receiver-operating characteristic curve (AUC) ranging from 0.69 to 0.82. The modest discrimination capabilities of these individual markers may have contributed to their slow clinical adoption.6,7,8,9,10
The objective of this study was to explore the use of inexpensive, quantitative, and routinely collected measurements in the perioperative setting that reflect physiologic and metabolic pathways in AKI, and are not specialty specific or clinician driven, to predict AKI. This study examined the predictive accuracy of models that included early changes in serum creatinine and immediate postsurgery laboratory parameters from the basic metabolic profile to predict postoperative moderate to severe AKI and AKI requiring dialysis in a cohort of patients undergoing cardiac surgery. The models were then validated in an external cohort to determine their generalizability.
Methods
The study followed TRIPOD (transparent reporting of a multivariable prediction model for individual prognosis or diagnosis) guidance for prediction model development and validation.11 The study was approved by the institutional review board at Cleveland Clinic, and the requirement for obtaining informed consent was exempted due to retrospective with minimal risk nature of the study.
Derivation and External Validation Cohorts
The Cleveland Clinic main campus cardiovascular surgery cohort was used for predictive model derivation (2000-2019) and 3 Cleveland Clinic community hospitals were used for model validation (2009-2019). Surgical data were obtained from the Anesthesiology Institute Patient Registry and were supplemented with laboratory data retrieved from electronic medical records for all patients who underwent coronary artery bypass graft (CABG), valve, and/or aorta surgery (Table 1). Patient comorbidities and procedures were characterized using the International Classification of Diseases, Ninth Revision and the International Classification of Diseases, Tenth Revision codes. Dialysis information was extracted from the Cleveland Clinic Acute Renal Registry.
Table 1. Baseline Clinical Characteristics and Operative Information of Derivation and Validation Cohorts.
| Characteristic | No. (%) | |
|---|---|---|
| Derivation (n = 58 526) | Validation (n = 4734) | |
| Age, y | ||
| Median (IQR) | 66 (56-74) | 67 (60-74) |
| Men | 39 173 (67) | 3361 (71) |
| Women | 19 353 (33) | 1373 (29) |
| Racea | n = 56 368 | n = 4589 |
| Asian | 679 (1) | 60 (1) |
| Black | 2926 (6) | 474 (10) |
| Multiracial | 1260 (2) | 78 (2) |
| White | 51 503 (91) | 3977 (87) |
| Weight, median (IQR), kg | 82 (71-95) | 85 (74-99) |
| Comorbid diseaseb | ||
| Hypertension | 39 156 (67) | 3124 (66) |
| Diabetes | 13 726 (23) | 1373 (29) |
| Congestive heart failure | 13 671 (23) | 329 (7) |
| Coronary artery disease | 12 441 (21) | 1848 (39) |
| Pulmonary disease | 7561(13) | 565 (12) |
| Chronic kidney disease | 1776 (3) | 236 (5) |
| Preoperative laboratories, median (IQR)c | ||
| Time from blood draw to operation, d | 2 (0-5) | 2 (0-7) |
| Serum albumin, g/dL | 4.2 (3.8-4.4) | 4.2 (3.8-4.4) |
| Serum bicarbonate, mmol/L | 26 (24-28) | 26 (24-27) |
| Blood urea nitrogen, mg/dL | 18 (15-23) | 17 (14-22) |
| Serum creatinine, mg/dL | 1.0 (0.84-1.18) | 0.97 (0.82-1.15) |
| Serum potassium, mmol/L | 4.1 (3.9-4.4) | 4.2 (4-4.5) |
| Serum sodium, mmol/L | 140 (138-142) | 139 (138-141) |
| Operative procedured | ||
| Valve surgery alone | 23 314 (40) | 1041 (22) |
| CABG alone | 13 648 (23) | 2980 (63) |
| Aorta surgery | 11 521 (20) | 390 (8) |
| CABG and valve surgery | 10 043 (17) | 323 (7) |
Abbreviation: CABG, coronary artery bypass graft.
Race information was obtained based on self-identification using fixed categories retrieved from medical records. A total of 226 patients in the derivation cohort and 65 in the validation cohort declined to provide a response.
Comorbid disease was assessed using the International Classification of Diseases, Ninth Revision and International Classification of Diseases, Tenth Revision codes.
Normal laboratory ranges for preoperative laboratory were as follows: serum albumin, 3.9-4.9 mg/dL; serum bicarbonate, 22-30 mmol/L; blood urea nitrogen, 7-21 mg/dL; serum creatinine, 0.58-0.96 mg/dL for women and 0.73-1.22 mg/dL for men; serum potassium, 3.7-5.1 mmol/L; and serum sodium, 136-144 mmol/L.
Aorta surgery included root, ascending, and thoracoabdominal aortic surgery. Valve surgery included aortic, mitral, pulmonary, and tricuspid valve surgery; 96% of operations were performed using cardiopulmonary bypass.
Study Participants
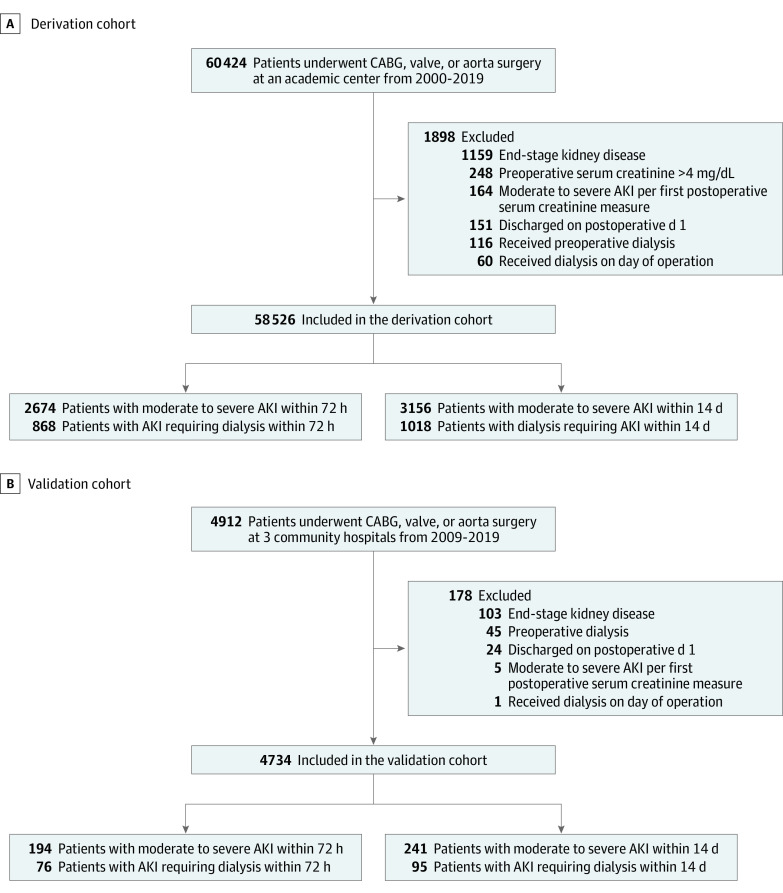
The study included all adults (18 years and older) who underwent CABG, valve, or aorta surgery (Figure 1). Patients who were receiving long-term dialysis, required preoperative dialysis (up to 6 months prior to surgery), or had preoperative serum creatinine values of 4 mg/dL or higher were excluded. Patients who developed moderate to severe AKI or initiated dialysis at or before the first postoperative metabolic panel blood draw were excluded from analysis. Self-identified race information based on fixed categories was retrieved from medical records to help describe the derivation and validation cohorts and to inform external generalizability.
Figure 1. Derivation and Validation Cohorts Used to Develop the Prediction Models for Acute Kidney Injury (AKI) After Cardiac Surgery.
CABG indicates coronary artery bypass.
Explanatory Variables
Laboratory data from the first postoperative metabolic panel were used to calculate change in serum creatinine from preoperative values. Serum potassium, bicarbonate, sodium, albumin, and blood urea nitrogen were also included in the model from the same postoperative panel and adjusted for the time between conclusion of the surgical procedure to the first postoperative blood draw.
Outcome Measures
The study used a modified KDIGO definition for AKI diagnosis by using the most recent preoperative serum creatinine as baseline. Stage 2 and worse AKI (per KDIGO) classification (moderate to severe AKI) and AKI requiring dialysis, within 72 hours or 14 days of surgery (or discharge/death if earlier), were the primary outcome measures (Table 2).
Table 2. Acute Kidney Injury (AKI) Classification per KDIGO Serum Creatinine Criteria After Cardiac Surgery in the Derivation Cohorta,b.
| Kidney injury classification | No. (%) | |
|---|---|---|
| 72 h (n = 58 526) | 14 d (n = 58 526) | |
| Stage 1 (mild): creatinine increase by 1.5-1.9 times baseline within 7 d or increase by ≥0.3 mg/dL within 48 h | 8899 (15) | 11 281 (19) |
| Stage 2 (moderate): creatinine increase by 2-2.9 times baseline | 1450 (2.5) | 1714 (2.9) |
| Stage 3 (severe): creatinine increase by ≥3 times baseline or increase to ≥4 mg/dL or dialysis | 1224 (2.1) | 1442 (2.5) |
The study used a modified Kidney Disease: Improving Global Outcomes (KDIGO) definition for AKI diagnosis, by using the most recent preoperative serum creatinine as baseline.
Patients who incurred moderate to severe acute kidney injury or started dialysis by the first metabolic blood panel drawn after cardiac surgery were excluded (n = 224).
Model Building
Predictor variables were restricted to those available in the routine metabolic panels that are frequently checked in the perioperative period. The most recent preoperative and first-measured postoperative serum creatinine values were used to calculate perioperative change in serum creatinine.
Four models were constructed for each end point based on (1) preoperative serum creatinine, (2) perioperative absolute change in serum creatinine, (3) models 1 and 2 combined, and (4) a final model in which, in addition to model 3, included blood urea nitrogen, potassium, bicarbonate, sodium, and albumin, adjusted for time from surgery to blood draw, as explanatory (predictor) variables (eTable 1 in the Supplement).
An example of protocol implementation at 5% risk threshold for moderate to severe AKI and AKI requiring dialysis at 72 hours was used to calculate the proportion of patients in the predicted risk category and corresponding positive and negative predictive values.
Statistical Analysis
Descriptive statistics are presented as median (IQR) for continuous variables and frequency for categorical variables. Wilcoxon rank-sum and χ2 tests were used for between-group comparisons. Multicollinearity was assessed among the predictor variables using correlation coefficients and variance inflation factor, specifically between serum creatinine and blood urea nitrogen. All variables were fitted in restricted cubic splines with 3 knots based on covariate distributions (at 10th, 50th, and 90th percentiles) to allow for flexible nonlinear associations with the primary outcome measures using multivariable logistic regression. Two clinically relevant interactions included (1) preoperative serum creatinine and perioperative change in serum creatinine and (2) perioperative serum creatinine change and timing of first metabolic blood draw from conclusion of surgical procedure. We adhered to 10 events per predictor degree of freedom (inclusive of the degrees of freedom spent due to use of cubic splines) to reduce overfitting.
The predictive discrimination of all models was assessed using concordance statistic, which also represents the AUC. Bias-corrected calibration curves were constructed to illustrate graphically any bias in predicted values, where perfectly calibrated predictions are on the 45-degree line of identity. Calibration accuracy was assessed using 2-tailed P values of the Spiegelhalter z test. The final models were internally validated and calibrated using bootstrapping of the entire derivation cohort (500 resampling iterations).12 Mean expected optimism then was calculated and subtracted from apparent index. The final model for AKI requiring dialysis within 14 days was compared with previously published Cleveland Clinic acute renal failure score applied to the current cohort at a 9.5% probability threshold using net reclassification of events and nonevents. Subgroup analyses were performed based on preoperative kidney function, year of surgical procedure, and time to first postoperative metabolic panel by testing for interaction term with model-based calculated probabilities in a logistic regression model. P <.05, and a 2-sided 95% CI that did not overlap unity, was considered statistically significant. Adjustment of type I error thresholds for multiple comparisons in secondary and subgroup analyses was not performed, so these analyses should be considered exploratory.
Multiple imputation was performed for missing values using multiple imputation based on bootstrapping and predictive mean matching, where a flexible additive model is used to predict missing values.13,14 We performed all statistical analyses and plotted graphs using the SAS Enterprise Guide, version 8.2 (SAS, Inc), and the R statistical package, version 4.0.4 (R Foundation; http://www.r-project.org). Online calculators of the regression equations representing the 4 final models using the Cleveland Clinic Risk Calculator were constructed at https://riskcalc.org/AKIpostCardiacSurgery/.
Results
Characteristics of Study Cohorts
The study consisted of 58 526 participants from the main campus hospital (derivation cohort) and 4734 patients from 3 affiliated community hospitals (external validation cohort). Figure 1 shows the patient selection process for the derivation and validation cohorts. The median (IQR) age in the derivation cohort was 66 (56-74) years; 39 173 participants (67%) were men and 51 503 (91%) were White. In the validation cohort, the median (IQR) age was 67 (56-74) years, 3361 (71%) were men, and 3977 (87%) were White. Table 1 summarizes baseline clinical characteristics and operative information for the participants. Serum albumin had the highest frequency of missing data (8.1%); 53 213 (90.9%) patients had no missing data. Overall, 14 437 patients (25%) in the derivation cohort incurred AKI stage 1 or worse per KDIGO classification within 14 days after the surgical procedure. Table 2 summarizes AKI stages per KDIGO classification and their rates in the derivation cohort. Rates of moderate to severe AKI were 4.6% (2674 patients) within 72 hours and 5.4% (3156 patients) within 14 days after the surgical procedure in the derivation cohort and 4.4% (194 patients) within 72 hours and 5.1% (241 patients) within 14 days after the surgical procedure in the validation cohort. Rates of AKI requiring dialysis were 1.5% (868 patients) within 72 hours and 1.7% (1018 patients) within 14 days in the derivation cohort and 1.6% (76 patients) within 72 hours and 2% (95 patients) within 14 days after the surgical procedure in the validation cohort.
Preoperative serum creatinine was measured within 24 hours before the surgical procedure in 42% of patients and within 7 days before the surgical procedure in 84% of patients; 35% of patients had serum creatine measured during the index hospitalization. The median (IQR) time from the conclusion of the cardiac surgical procedure and the first-available metabolic panel was 10 (7-12) hours in the derivation cohort and 6 (2-11) hours in the validation cohort. In the derivation cohort, perioperative median (IQR) serum creatinine increased by 0.20 mg/dL (0-0.40) within 72 hours and 0.15 mg/dL (0-0.39) within 14 days in patients with moderate to severe AKI and by 0.13 mg/dL (−0.07 to 0.39) within 72 hours and 0.1 mg/dL (−0.10 to 0.35) within 14 days in patients with AKI requiring dialysis. eFigure 1 in the Supplement shows the duration to AKI diagnosis, moderate to severe AKI classification, and dialysis initiation.
Higher incidence of AKI was observed in patients with elevated preoperative serum creatinine and postoperative rise in serum creatinine (Figure 2) in the final models (P < .001). However, of note, perioperative change in serum creatinine showed a U-shaped relationship with AKI in unadjusted models, where lower postoperative serum creatinine levels (compared with baseline) were also associated with increased AKI incidence (eFigure 2 in the Supplement). Moreover, preoperative serum creatinine level modified the association of perioperative rise in serum creatinine and AKI in the final models (P < .001 for interaction; eFigure 3 in the Supplement).
Figure 2. Adjusted Odds Ratios for Moderate to Severe Acute Kidney Injury (AKI) and AKI Requiring Dialysis Within 72 Hours of Surgery in the Derivation Cohort (n = 58 526) .
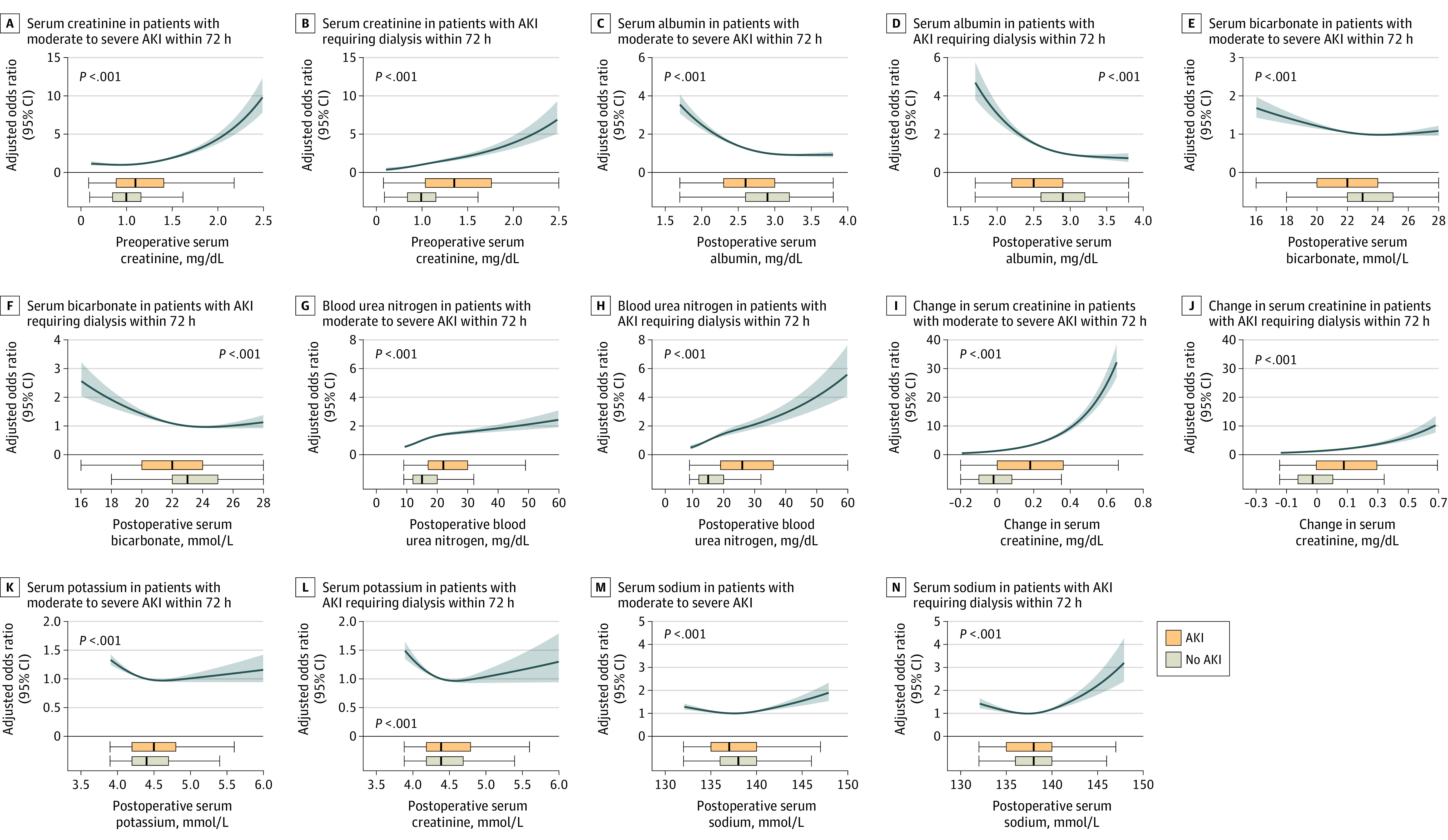
The shaded area represents the 95% CI from the restricted cubic spline models. All plots were centered at median values for the respective variables. The odds ratio for each variable was adjusted for the median values of other variables in the model: preoperative serum creatinine at 1 mg/dL, serum albumin at 2.9 mg/dL, serum bicarbonate at 23 mmol/L, blood urea nitrogen at 15 mg/dL, change in serum creatinine at −0.06 mg/dL, serum potassium at 4.4 mmol/L, serum sodium at 138 mmol/L, and time from end of the operation to metabolic panel blood draw at 10 hours. Boxplots display the laboratory parameter distribution with or without AKI along the shared x-axis of each plot; boxes show the IQR, with the line representing the median point, box edges representing first and third quartiles, and whiskers extending to the value closest to 1.5 times the IQR (more extreme values not plotted).
Prediction of Moderate to Severe AKI and AKI Requiring Dialysis in the Derivation Cohort
Six variables from the first-available postoperative metabolic panel (serum creatinine, blood urea nitrogen, sodium, bicarbonate, albumin, and potassium) were used in the bootstrapped model of the derivation cohort and adjusted for preoperative serum creatinine and time from end of surgical procedure to first metabolic panel. The final models had better predictive performance compared with creatinine-only–based models both in discrimination and calibration (eTable 1 in the Supplement). See eTable 2 in the Supplement for a full description of the final models for individual risk calculation.
The final models for moderate to severe AKI were well calibrated across the range of all probabilities, whereas final models for AKI requiring dialysis tended to overestimate events at higher probabilities (eFigure 4 in the Supplement). The AUC was 0.876 (95% CI, 0.869-0.883) for moderate to severe AKI within 72 hours and 0.854 (95% CI, 0.850-0.861) for moderate to severe AKI within 14 days after the surgical procedure and 0.916 (95% CI, 0.907-0.926) for AKI requiring dialysis within 72 hours and 0.900 (95% CI, 0.890-0.910) for AKI requiring dialysis within 14 days after the surgical procedure. eTable 3 in the Supplement shows the distribution of patients and events within predicted risk categories for the final models. Results of prespecified subgroup analyses based on preoperative serum creatinine, year of surgery, and timing of postoperative metabolic panel are presented in eTable 4 in the Supplement.
Model Performance in Validation Cohort
All 4 final models performed well in the external validation cohort. The AUC was 0.860 (95% CI, 0.838-0.882) for moderate to severe AKI within 72 hours and 0.842 (95% CI, 0.820-0.865) within 14 days after the surgical procedure and 0.879 (95% CI, 0.840-0.918) for AKI requiring dialysis within 72 hours and 0.873 (95% CI, 0.836-0.910) for AKI requiring dialysis within 14 days of the surgical procedure (Figure 3). All models were well calibrated (eFigure 4 in the Supplement), with Spiegelhalter z statistic P values greater than .05.
Figure 3. Receiver-Operating Characteristic Curves for Moderate to Severe AKI and AKI Requiring Dialysis in the Derivation and Validation Cohorts.
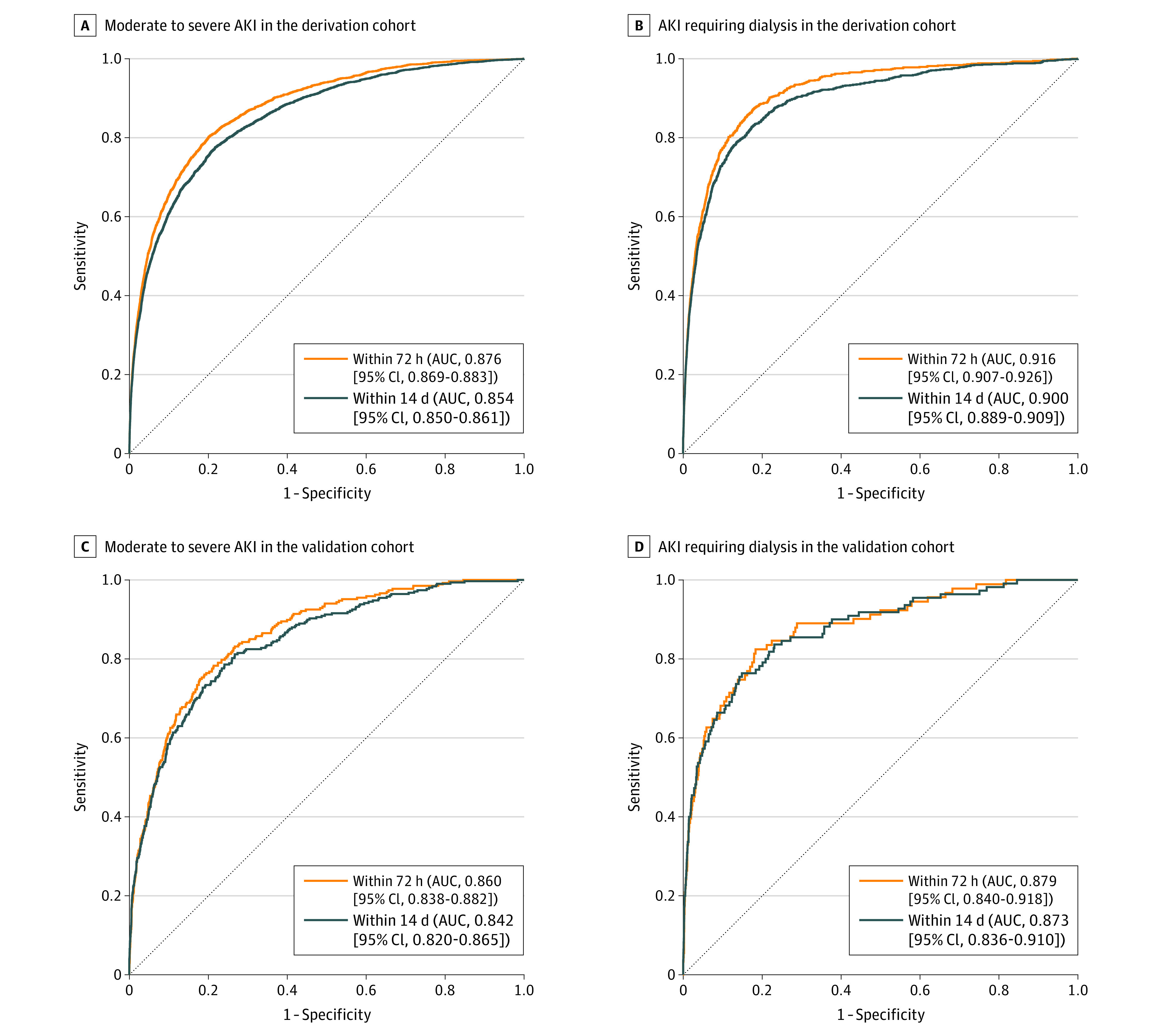
The models include preoperative serum creatinine, perioperative change in serum creatinine, and postoperative serum albumin, bicarbonate, urea, potassium, sodium, time from end of the operation to panel blood draw, and 2 interaction terms.
Model Performance Compared to Previously Published Cleveland Clinic Acute Renal Failure Score
In the derivation cohort, the previously reported Cleveland Clinic Score for AKI after cardiac surgery15,16 had an AUC of 0.812 (95% CI, 0.798-0.827) for AKI requiring dialysis within 14 days. At the probability threshold of 9.5% for AKI requiring dialysis within 14 days, the net reclassification of events was 14% (95% CI, 3%-25%) and the net reclassification for nonevents was 63% (95% CI, 47%-77%), in favor of the current model.
Application of the Predictive Model in Postoperative Management
The predictive models applied at a median of 10 hours after the surgical procedure predicted moderate to severe AKI classification occurrence at a median (IQR) of 3 (2-5) days and AKI requiring dialysis at a median (IQR) of 4 (2-8) days after surgery (eFigure 1 in the Supplement). The clinical application of these models will identify high-risk patients based on a range of predicted risk thresholds for AKI (eTable 5 in the Supplement). For instance, at a risk threshold of 5% or higher, 20% of patients were categorized as high risk for moderate to severe AKI and 6% were categorized as high risk for AKI requiring dialysis within 72 hours. The corresponding positive predictive values were 30% (95% CI, 29%-32%) for moderate to severe AKI and 14% (95% CI, 13%-15%) for AKI requiring dialysis; the negative predictive values were 97% (95% CI, 97%-97%) for moderate to severe AKI and 99% (95% CI, 99%-100%) for AKI requiring dialysis (eTable 5 in the Supplement).
Discussion
Using data from a routinely collected postoperative basic metabolic panel, discriminative predictive models for moderate to severe AKI associated with cardiac surgery were developed and validated. The final models included preoperative serum creatinine, perioperative change in serum creatinine, and other elements from the postoperative metabolic panel profile and demonstrated high levels of predictive discrimination in the derivation and validation cohorts.
Small changes in serum creatinine after cardiac surgery have been associated with worse outcome.17,18,19,20,21 However, serum creatinine is considered a poor marker of glomerular filtration rate early in AKI due to the lag period required to reach steady state (constant generation rate). The more severe and abrupt the decline in kidney function is, the larger the discrepancy. In the final models used in this study, both perioperative absolute change in serum creatinine and the duration from surgical procedure to blood draw of the first postoperative metabolic panel were accounted for. Indeed, the rate of change in serum creatinine by factoring in the duration from the surgical procedure (by including an interaction term) outperformed the absolute change in serum creatinine even within the relatively small range of creatinine values reported.
Moreover, intraoperative dilution of serum creatinine due to intravenous fluid administration is yet another important and often overlooked factor that may lead to delay in AKI diagnosis.22 In the current cohort, perioperative serum creatinine change had a U-shaped association with AKI when unadjusted for other variables. In other words, in addition to the anticipated association of serum creatinine rise in the postoperative period with AKI, a decrease in serum creatinine was also associated with AKI in a graded fashion. However, the association of lower postoperative serum creatinine with AKI did not persist in the final models. Indeed, the inclusion of other elements from the metabolic panel, such as potassium, sodium, and bicarbonate, independently contributed to the predictive capacity of the model because they capture clinically relevant downstream complications of kidney injury in a multidimensional approach (electrolyte perturbations and acid-base derangements). Furthermore, the inclusion of serum albumin in the model may help adjust for intravascular volume changes that typically occur during surgery, which account not only for the volume change but also influence the rate and magnitude of change of all elements in the metabolic panel.
Previous predictive models for AKI after cardiac surgery have traditionally focused on preoperative variables and rarely incorporated intraoperative risk factors.15,23,24 In addition, many included clinician-driven risk factors (eg, use of intra-aortic balloon pump, blood transfusions), which may vary over time and from one center to another and thereby limit their external validity and performance over time.
In the past few decades, several structural biomarkers of kidney tubular injury have been evaluated as potential biomarkers that may facilitate timely diagnosis and early intervention for AKI.25,26,27,28,29 An adequately powered multicenter study, which simultaneously examined several biomarkers (urine interleukin 18, urine and plasma neutrophil gelatinase-associated lipocalin) reported AUC ranging from 0.73 to 0.76 (calibration was not reported).7 Prognostic biomarkers, such as G1 cell cycle arrest proteins, have not been extensively studied in cardiac surgery. Only 2 studies to date have investigated the role of tissue inhibitor of metalloproteinase-2 and insulin growth factor-binding protein 7 in predicting severe moderate to severe AKI and collectively only 17 patients reached the end point.9,30,31 A 2021 study investigated the feasibility of implementing the KDIGO-derived treatment bundle in high-risk patients identified by elevated tissue inhibitor of metalloproteinase-2 and insulin growth factor-binding protein 7levels after cardiac surgery.5 Of note, the latter study was comprised of participants with a higher incidence of moderate to severe AKI (24%) and AKI requiring dialysis (6.3%) than the current study, and implementation of such a protocol would likely have diminishing returns in settings with lower incidence of AKI in which kidney-protective practices are already in use. Nevertheless, a stepped approach of identifying high-risk patients using the current investigation models followed by confirmation with tissue inhibitor of metalloproteinase-2 and insulin growth factor-binding protein 7 warrants further investigation.
The large size of the current cohort made it feasible to study clinically relevant end points, namely moderate to severe AKI, in different time intervals following cardiac surgery. The models were also externally validated with only marginal decrease in discrimination and preserved calibration. Moreover, by using routinely measured, standardized, readily available, objective parameters, the models could be readily implemented elsewhere. However, these models may require further validation in other populations; for example, the model intercept may need to be updated if there is a substantial difference in baseline AKI risk. Additionally, further research is needed to understand whether the use of this risk prediction tool improves clinical outcomes.
Limitations
This study has several limitations. First, although the models showed excellent discrimination, they overestimated predicted risk for AKI requiring dialysis in higher-risk categories. The latter is likely attributed to distribution of risk in the population where there was a lower density of patients in higher-risk categories. However, in practice, a distinction between 40% vs 80% risk is not crucial considering the actual event rate is less than 2%.32 Moreover, there is inevitable trade-off between discrimination and calibration, and a model cannot perform perfectly in both.33 Second, the current models are fairly complex to use with regard to individual risk calculation. However, as illustrated in the present study, the parameters used in our models follow nonlinear associations, and the use of cubic spline functions is an efficient, albeit computationally heavy, method that avoids introducing biased results. It may be possible for the models to be integrated into electronic medical records because they solely rely on discrete digitized data points. Third, competing events (such as mortality) were not accounted for in the analyses. However, mortality in the early postoperative period is typically in the setting of shock and multisystem organ failure where dialysis is often initiated, thus incurring study end points even if creatinine-based criteria are not met. Fourth, AKI classification in the current study was based on creatinine and did not include urine output criteria.
Conclusions
Among patients undergoing cardiac surgery, a prediction model based on perioperative basic metabolic panel laboratory values demonstrated good predictive accuracy for moderate to severe acute kidney injury within 72 hours and 14 days after surgery. Further research is needed to determine whether use of the risk prediction tool improves clinical outcomes.
eTable 1. Performance of Acute Kidney Injury Models in the Derivation Cohort
eTable 2. Regression Equations for the Four Final Models
eTable 3. Distribution of Patients and Events within Pre-specified Predicted Risk Categories in the Derivation Cohort
eTable 4. Model Performance for Moderate to Severe Acute Kidney Injury and Dialysis Requiring Acute Kidney Injury in the Validation Cohort, Stratified by Preoperative Serum Creatinine, Chronological Year, and Duration in Hours to Metabolic Panel Post-surgery
eTable 5. Proportion of Patients at Risk for AKI2/3 and AKID Who Would be Targeted for Intervention, by Applying a Range of Probability Thresholds, and Corresponding Sensitivity, Specificity, Positive and Negative Predictive Values
eFigure 1. Timing of (a) AKI Diagnosis, (b) Moderate to Severe Acute Kidney InjuryClassification, and (c) Dialysis Initiation Post-surgery in the Derivation Cohort
eFigure 2. Unadjusted Odds Ratios of Preoperative Serum Creatinine and Acute kidney Injury in the Derivation Cohort
eFigure 3. Adjusted Odds Ratios of Acute Kidney Injury According to Preoperative Serum Creatinine and Perioperative Change in Serum Creatinine Interaction in the Derivation Cohort
eFigure 4a. Bias Adjusted High Resolution Calibration Plot ofModerate to Severe Acute Kidney Injury Models in the (i) Derivation Cohort, and (ii) Validation Cohort. Vertical Axis Represents Observed Incidence (actual probability), and Horizontal Axis, Predicted Probability; red lines denote outcomes within 72 hours, and black lines within 14 days
eFigure 4b. Bias Adjusted High Resolution Calibration Plot of Dialysis Requiring Acute Kidney Injury Models in the (i) Derivation Cohort, and (ii) Validation Cohort. Vertical axis represents observed incidence (actual probability), and horizontal axis, predicted probability; red lines denote outcomes within 72 hours, and black lines within 14 days
References
- 1.Lange HW, Aeppli DM, Brown DC. Survival of patients with acute renal failure requiring dialysis after open heart surgery: early prognostic indicators. Am Heart J. 1987;113(5):1138-1143. doi: 10.1016/0002-8703(87)90925-2 [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104(4):343-348. doi: 10.1016/S0002-9343(98)00058-8 [DOI] [PubMed] [Google Scholar]
- 3.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19-32. doi: 10.2215/CJN.00240605 [DOI] [PubMed] [Google Scholar]
- 4.Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):649-672. doi: 10.1053/j.ajkd.2013.02.349 [DOI] [PubMed] [Google Scholar]
- 5.Zarbock A, Küllmar M, Ostermann M, et al. Prevention of cardiac surgery-associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: the PrevAKI-multicenter randomized controlled trial. Anesth Analg. 2021;133(2):292-302. doi: 10.1213/ANE.0000000000005458 [DOI] [PubMed] [Google Scholar]
- 6.Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107(9):1145-1152. doi: 10.1172/JCI12089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh CR, Coca SG, Thiessen-Philbrook H, et al. ; TRIBE-AKI Consortium . Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22(9):1748-1757. doi: 10.1681/ASN.2010121302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis. 2015;66(6):993-1005. doi: 10.1053/j.ajkd.2015.06.018 [DOI] [PubMed] [Google Scholar]
- 9.Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, et al. Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care. 2015;5(1):50. doi: 10.1186/s13613-015-0076-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings JJ, Shaw AD, Shi J, Lopez MG, O’Neal JB, Billings FT IV. Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg. 2019;157(4):1545-1553.e5. doi: 10.1016/j.jtcvs.2018.08.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. J Clin Epidemiol. 2015;68(2):134-143. doi: 10.1016/j.jclinepi.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 12.Efron B, Gong G. A leisurely look at the bootstrap, the jackknife, and cross-validation. Am Stat. 1983;37(1):36-48. doi: 10.2307/2685844 [DOI] [Google Scholar]
- 13.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361-387. doi: [DOI] [PubMed] [Google Scholar]
- 14.Harrell FE. Regression Modeling Strategies. Springer; 2001. doi: 10.1007/978-1-4757-3462-1 [DOI] [Google Scholar]
- 15.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16(1):162-168. doi: 10.1681/ASN.2004040331 [DOI] [PubMed] [Google Scholar]
- 16.Kiers HD, van den Boogaard M, Schoenmakers MC, et al. Comparison and clinical suitability of eight prediction models for cardiac surgery-related acute kidney injury. Nephrol Dial Transplant. 2013;28(2):345-351. doi: 10.1093/ndt/gfs518 [DOI] [PubMed] [Google Scholar]
- 17.Anderson RJ, O’Brien M, MaWhinney S, et al. Mild renal failure is associated with adverse outcome after cardiac valve surgery. Am J Kidney Dis. 2000;35(6):1127-1134. doi: 10.1016/S0272-6386(00)70050-3 [DOI] [PubMed] [Google Scholar]
- 18.Ryckwaert F, Boccara G, Frappier JM, Colson PH. Incidence, risk factors, and prognosis of a moderate increase in plasma creatinine early after cardiac surgery. Crit Care Med. 2002;30(7):1495-1498. doi: 10.1097/00003246-200207000-00016 [DOI] [PubMed] [Google Scholar]
- 19.Ryckwaert F, Alric P, Picot MC, Djoufelkit K, Colson P. Incidence and circumstances of serum creatinine increase after abdominal aortic surgery. Intensive Care Med. 2003;29(10):1821-1824. doi: 10.1007/s00134-003-1958-x [DOI] [PubMed] [Google Scholar]
- 20.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597-1605. doi: 10.1097/01.ASN.0000130340.93930.DD [DOI] [PubMed] [Google Scholar]
- 21.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226-233. doi: 10.1001/archinternmed.2010.514 [DOI] [PubMed] [Google Scholar]
- 22.Macedo E, Bouchard J, Soroko SH, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14(3):R82. doi: 10.1186/cc9004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijeysundera DN, Karkouti K, Dupuis JY, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297(16):1801-1809. doi: 10.1001/jama.297.16.1801 [DOI] [PubMed] [Google Scholar]
- 24.Demirjian S, Schold JD, Navia J, et al. Predictive models for acute kidney injury following cardiac surgery. Am J Kidney Dis. 2012;59(3):382-389. doi: 10.1053/j.ajkd.2011.10.046 [DOI] [PubMed] [Google Scholar]
- 25.Wellwood JM, Ellis BG, Price RG, Hammond K, Thompson AE, Jones NF. Urinary N-acetyl- beta-D-glucosaminidase activities in patients with renal disease. Br Med J. 1975;3(5980):408-411. doi: 10.1136/bmj.3.5980.408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237-244. doi: 10.1046/j.1523-1755.2002.00433.x [DOI] [PubMed] [Google Scholar]
- 27.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14(10):2534-2543. doi: 10.1097/01.ASN.0000088027.54400.C6 [DOI] [PubMed] [Google Scholar]
- 28.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43(3):405-414. doi: 10.1053/j.ajkd.2003.10.040 [DOI] [PubMed] [Google Scholar]
- 29.Herget-Rosenthal S, Marggraf G, Hüsing J, et al. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004;66(3):1115-1122. doi: 10.1111/j.1523-1755.2004.00861.x [DOI] [PubMed] [Google Scholar]
- 30.Dusse F, Edayadiyil-Dudásova M, Thielmann M, et al. Early prediction of acute kidney injury after transapical and transaortic aortic valve implantation with urinary G1 cell cycle arrest biomarkers. BMC Anesthesiol. 2016;16(1):76. doi: 10.1186/s12871-016-0244-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Zou Z, Jin J, et al. Urinary TIMP-2 and IGFBP7 for the prediction of acute kidney injury following cardiac surgery. BMC Nephrol. 2017;18(1):177. doi: 10.1186/s12882-017-0592-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gail MH, Pfeiffer RM. On criteria for evaluating models of absolute risk. Biostatistics. 2005;6(2):227-239. doi: 10.1093/biostatistics/kxi005 [DOI] [PubMed] [Google Scholar]
- 33.Diamond GA. What price perfection? calibration and discrimination of clinical prediction models. J Clin Epidemiol. 1992;45(1):85-89. doi: 10.1016/0895-4356(92)90192-P [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Performance of Acute Kidney Injury Models in the Derivation Cohort
eTable 2. Regression Equations for the Four Final Models
eTable 3. Distribution of Patients and Events within Pre-specified Predicted Risk Categories in the Derivation Cohort
eTable 4. Model Performance for Moderate to Severe Acute Kidney Injury and Dialysis Requiring Acute Kidney Injury in the Validation Cohort, Stratified by Preoperative Serum Creatinine, Chronological Year, and Duration in Hours to Metabolic Panel Post-surgery
eTable 5. Proportion of Patients at Risk for AKI2/3 and AKID Who Would be Targeted for Intervention, by Applying a Range of Probability Thresholds, and Corresponding Sensitivity, Specificity, Positive and Negative Predictive Values
eFigure 1. Timing of (a) AKI Diagnosis, (b) Moderate to Severe Acute Kidney InjuryClassification, and (c) Dialysis Initiation Post-surgery in the Derivation Cohort
eFigure 2. Unadjusted Odds Ratios of Preoperative Serum Creatinine and Acute kidney Injury in the Derivation Cohort
eFigure 3. Adjusted Odds Ratios of Acute Kidney Injury According to Preoperative Serum Creatinine and Perioperative Change in Serum Creatinine Interaction in the Derivation Cohort
eFigure 4a. Bias Adjusted High Resolution Calibration Plot ofModerate to Severe Acute Kidney Injury Models in the (i) Derivation Cohort, and (ii) Validation Cohort. Vertical Axis Represents Observed Incidence (actual probability), and Horizontal Axis, Predicted Probability; red lines denote outcomes within 72 hours, and black lines within 14 days
eFigure 4b. Bias Adjusted High Resolution Calibration Plot of Dialysis Requiring Acute Kidney Injury Models in the (i) Derivation Cohort, and (ii) Validation Cohort. Vertical axis represents observed incidence (actual probability), and horizontal axis, predicted probability; red lines denote outcomes within 72 hours, and black lines within 14 days