Abstract
Purpose:
Growth hormone (GH) has an important role in intestinal barrier function, and abnormalities in GH action have been associated with intestinal complications. Yet, the impact of altered GH on intestinal gross anatomy and morphology remains unclear.
Methods:
This study investigated the influence of GH signaling on gross anatomy, morphology, and fibrosis by characterizing the small and large intestines in male and female bovine growth hormone transgenic (bGH) mice and GH receptor gene-disrupted (GHR−/−) mice at multiple timepoints.
Results:
The length, weight, and circumference of the small and large intestines were increased in bGH mice and decreased in GHR−/− mice across all ages. Colon circumference was significantly increased in bGH mice in a sex-dependent manner while significantly decreased in male GHR−/− mice. Villus height, crypt depth, and muscle thickness of the small intestine were generally increased in bGH mice and decreased in GHR−/− mice compared to controls with age- and sex-dependent exceptions. Colonic crypt depth and muscle thickness in bGH and GHR−/− mice were significantly altered in an age- and sex-dependent manner. Fibrosis was increased in the small intestine of bGH males at 4 months of age, but no significant differences were seen between genotypes at other timepoints.
Conclusion:
This study observed notable opposing findings in the intestinal phenotype between mouse lines with GH action positively associated with intestinal gross anatomy (i.e. length, weight, and circumference). Moreover, GH action appears to alter morphology of the small and large intestines in an age- and sex-dependent manner.
Keywords: growth hormone, bGH mice, GHR−/− mice, intestinal gross anatomy, intestinal morphology
1. Introduction
Growth hormone (GH) plays an important role in intestinal health and disease. That is, studies have shown that GH fosters intestinal homeostasis and maintains the gut barrier, decreasing intestinal permeability and bacterial translocation [1,2]. In the small intestine, GH has been shown to stimulate proliferation of epithelial cells and goblet cells and differentiation of intestinal stem cells into Paneth cells and enteroendocrine stem cells [1,3]. Moreover, GH has also been shown to increase growth and inhibit apoptosis in epithelial cells of the colon [4–6]. These promotive effects on the small and large intestines happen both dependent and independent of local and endocrine insulin-like growth factor 1 (IGF-1) [5,7,8].
Abnormalities in GH action have been associated with intestinal complications. To date, there has not been a report of intestinal dysfunction in GH resistance (Laron syndrome), and individuals with GH deficiency have no change in intestinal permeability compared to controls [9]. However, inflammation in the intestines (as seen in inflammatory bowel disease [IBD; Crohn’s disease and ulcerative colitis], chronic undernutrition, celiac disease, and anorexia nervosa) has been shown to confer a secondary GH insensitivity both locally in the intestines and in the liver, and consequently, cause a decrease in endocrine IGF-1 levels [10–14]. Excess GH has been also correlated with several intestinal complications. Individuals with acromegaly have dolichocolon, megacolon, and slower gut motility [15]. Moreover, these individuals have an increased risk of developing diverticula (associated with altered collagen cross-linking), volvulus, small intestinal bacterial overgrowth, gallstones, colonic polyps, and even colorectal cancer [7,15–22].
Mouse lines with altered GH action offer a unique opportunity to more thoroughly study the longitudinal relationship between GH and intestinal phenotype. Bovine transgenic GH (bGH) mice have constitutive production of GH and thus, chronically elevated GH,IGF-1, and IGFBP3 levels. As such, these mice closely resemble the phenotype and co-morbidities experienced by individuals with acromegaly, including metabolic dysfunction, insulin resistance, type 2 diabetes, cardiovascular complications, and an increased risk of cancer [23–26]. Inversely, the GH receptor gene disrupted (GHR−/−) mouse line mirrors clinical populations of Laron syndrome, displaying GH insensitivity or resistance. That is, GHR−/− mice lack a functional GHR, which yields global GH resistance, increased circulating GH levels, and decreased IGF-1and IGFBP3 levels. Thus, unlike bGH mice, GHR−/− mice have and an overall reduced growth phenotype. Thereby, despite similar circulating GH levels, these mouse models act as complimentary models of increased and decreased GH action with opposing levels of IGF-1, IGFBP3, and overall growth and metabolic phenotype. The bGH mice also have an accelerated aging phenotype, typically dying between 13 and 15 months[27], while GHR−/− mice are extremely long-lived[28].
Previous research has demonstrated that GH-altered mice have changes in their intestinal phenotype, though this has mostly been investigated in bGH mice. That is, bGH mice have been shown to have increased mucosal growth, intestinal length and weight, and decreased apoptosis in the colonic epithelium [4,29–32]. However, the intestinal studies on bGH mice have been limited to one or two timepoints (typically early in adulthood) and to a single sex (typically males). Moreover, the only studies that have investigated the inverse relationship (i.e. decreased GH action) and the intestinal phenotype have been limited to adult growth hormone gene disrupted (GH−/−) mice early in life and an intestinal epithelial specific GHR knockout mouse line [32,33].
Thus, this study more thoroughly investigates the impact of GH on the intestinal gross anatomy and morphology in two mouse lines across multiple timepoints, especially in the context of aging/longevity. That is, this study characterizes the gross anatomy and morphology in the small and large intestines of male and female bGH mice relative to sex-matched littermate controls at 6 weeks, 3 months, 6 months, 11 months, and 13 months of age as well as an additional measurement of fibrosis at 4 months of age. For comparison, we also examine the gross anatomy and morphology in GHR−/− mice at 13 months and 26 months of age as well as fibrosis in the intestines at 8 months of age. Although the intestinal phenotypes of these two mouse lines are assessed at differing age ranges, it is important to note that the bGH and GHR−/− mice have distinctly different lifespans and aging phenotypes. Thus, the ages assessed include a mid-life timepoint comparison for each line (4 and 6 months for bGH and 8 and 13 months for GHR−/− mice) and a late-life time point comparison (13 months for bGH and 26 months for GHR−/− mice), and the 13-month time point for each line allows for a direct comparison regardless of aging progression.
2. Methods and Materials
2.1. Mouse lines
Male and female hemizygous bGH mice, homozygous GHR−/− mice, and their respective wild-type littermate controls were used. Both lines were maintained in a C57BL/6J background with the bGH line derived straight into a C57BL/6J background, while the GHR−/− line was derived in a 129/Ola-BALB/c [34]background and subsequently back crossed more than 10 generations to a C57BL/6J background. Mice were genotyped at 4 weeks after birth from tail snips using PCR primers as described previously [35].
Separate cohorts of bGH mice and respective littermate controls were used for general phenotype (i.e. body weight, length, and organ weights) and intestinal phenotype (i.e. gross anatomy and morphology) at 6 weeks of age (n = 4 per group for males; n = 2 per group for females), 3 months of age (n = 3 per group per sex), 6 months of age (n = 5 per group per sex), 11 months of age (n = 4 per group per sex), and 13 months of age (n = 3 per group per sex). Male and female GHR−/− mice and controls were used at 13 months (n = 4 per group per sex) for both intestinal gross anatomy and morphological measurements. For an older timepoint, male and female GHR−/− mice and controls at 26 months of age (n = 8 per group per sex) were used for intestinal gross anatomy and morphology (n = 4 per group per sex with the exception of n = 3 for GHR−/− females).
All mice were housed in similar conditions, in a temperature-controlled (23°C) vivarium and exposed to a 14-hour light, 10-hour dark cycle. All mice were allowed access to chow (ProLab RMH 3000; PMI Nutrition International) and water ad libitum. All procedures performed with the mice were approved by the Ohio University Institutional Animal Care and Use Committee and are in accordance with all standards set forth by federal, state, and local authorities.
2.2. General growth phenotype and tissue collection
Body weight was measured in bGH mice, GHR−/− mice, and their respective controls across multiple timepoints. GH altered mice and their respective sex- and age-matched littermate controls were sacrificed after a 12-hour fast and as previously described [23,24]. In brief, mice were sacrificed via cervical dislocation following anesthesia with CO2. Nasal-anal body length was then determined. White adipose tissue (WAT) depots (i.e. subcutaneous, mesenteric, retroperitoneal, and perigonadal), heart, kidneys, liver, and spleen were extracted, weighed, and immediately frozen in liquid nitrogen. All samples were stored at −80°C.
2.3. Intestinal gross anatomy measurements
Intestines were processed as previously described [33]. In brief, the gastrointestinal tract was removed by cutting at the pyloric-duodenal junction and the rectal-anal junction. Majority of mesenteric fat and the pancreas was removed from the intestines, and the intestines were carefully straightened. Small and large intestines were divided at the ileocecal valve. Luminal contents of the ileum, cecum, and colon were removed, and then intestines were cut longitudinally and rinsed in ice cold PBS to remove the remaining contents. The small and large intestines were straightened, and length was measured. After removal of excess PBS, intestines were weighed. A portion of the small and large intestines were then prepared for histology.
2.4. Intestinal histology
For morphological analysis, intestines of bGH, GHR−/−, and littermate control mice were prepared using the swiss-roll technique as previously described [32,36]. Six longitudinal sections (approximately 2 cm long) of the small intestines were collected (two at the beginning after the pyloric-duodenal junction for the duodenum, two in the middle for the jejunum, and two at the end before the ileocecal junction for the ileum). Four longitudinal sections for the large intestine were collected (one for the cecum and three for the colon).
After 24 hour fixation with 10% buffered neutral formalin, samples were stored in 70% ethanol until the samples were processed and embedded in paraffin. Paraffin blocks were sliced at 4 μm and consequently mounted on slides. Samples were then stained with hematoxylin and eosin for morphological assessment or picrosirius red and fast green for fibrosis measurements [37]. Slides were visualized at 100x magnification with Nikon Eclipse E60 microscope, and at least 10 pictures of non-overlapping fields were taken per slide. An average of 20 measurements for villus height, crypt depth, and muscle thickness of the duodenum, jejunum, and ileum, and crypt depth and muscle thickness of the colon were quantified using ImageJ. The two samples of each intestinal section were averaged per mouse and then for each group. To quantify fibrosis, an ImageJ macro adapted from the ImageJ tutorial was used [38].
2.5. Hydroxyproline quantification
To assess fibrosis directly, collagen content of small intestines was quantified using a hydroxyproline assay as previously described [24]. This assay used a separate cohort of mice at different ages than the histological measurements. Male bGH mice at 4 months of age (n=8) and GHR−/− mice at 8 months of age (n=8) were used, along with their respective littermate controls. The intestines were processed as described above but were flash frozen prior to the hydroxyproline measurement.
2.6. Statistical analysis
All data are reported as mean ± SEM. Statistics were performed using R version 3.6.3. Normality and homogeneity of variance of the data were tested by plotting data on a Q-Q plot, through Shapiro-Wilks test and Levene’s homogeneity of variance. To account for the effect of genotype, sex, and the interaction between genotype and sex, two-way ANOVA and post-hoc Tukey analysis were performed on all measurements with the exception of the hydroxyproline assay. Statistical significance was set at p < 0.05. Effect size was calculated to describe the strength (or “biological significance”) of genotype, sex, and the interaction between genotype and sex on these findings through partial omega-squared (ωp2), which ranges between −1 and 1. In particular, effect size decreases as ωp2 approaches 0. The exception for this calculation was the circumference measurements of male GHR−/− mice and controls, to which effect size was calculated via Cohen’s d. Effect size also allowed for comparison of measurements between timepoints.
3. Results
3.1. Growth phenotype and absolute tissue weights in bGH mice
Body length and weight were measured in separate cohorts of bGH mice and respective controls at 6 weeks and 3, 6, 11, and 13 months of age. Body length and weight were significantly increased in male and female bGH mice compared to age-matched controls with the exception of female bGH mice at 11 months of age (Table 1).
Table 1. Growth parameters and organ weights in male and female bGH mice and controls at 6 weeks and 3, 6, 11, and 13 months of age.
Growth parameters (body length and body weight) and selected organ weights. Two-way ANOVA p values and effect sizes shown for genotype (G), sex (S), and interaction (I). Effect size was calculated by partial omega-squared (ωp2).
| ω p 2 | ω p 2 | ω p 2 | ω p 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-WT | 9.5 ± 0.10 | G: 0.001 | G: 0.91 | 23.2 ± 0.72 | G: <0.001 | G: 0.94 | 0.88 ± 0.05 | G: <0.001 | G: 0.97 | 0.11 ± 0.02 | G: <0.001 | G: 0.71 |
| M-bGH | 11.5 ± 0.04 | S: <0.001 | S: 0.98 | 31.8 ± 0.80 | S: <0.001 | S: 0.77 | 2.27 ± 0.03 | S: 0.003 | S: 0.84 | 0.27 ± 0.01 | S: 0.99 | S: −0.01 |
| F-WT | 8.9 ± 0.9 | I: 0.92 | I: −0.09 | 17.6 ± 0.52 | I: 0.28 | I: 0.03 | 0.67 ± 0.01 | I: 0.40 | I: −0.02 | 0.15 ± 0.04 | I: 0.12 | I: 0.14 |
| F-bGH | 10.9 ± 0.10 | 27.9 ± 0.2 | 1.96 ± 0.17 | 0.23 ± 0.004 | ||||||||
| M-WT | 10 ± 0.0 | G: <0.001 | G: 0.85 | 30.5 ± 0.77 | G: <0.001 | G: 0.92 | 1.00 ± 0.18 | G: <0.001 | G: 0.91 | 0.19 ± 0.05 | G: 0.04 | G: 0.21 |
| M-bGH | 11.8 ± 0.0 | S: 0.01 | S: 0.11 | 46.4 ± 0.75 | S: <0.001 | S: 0.84 | 2.58 ± 0.15 | S: 0.09 | S: 0.02 | 0.24 ± 0.04 | S: 0.15 | S: −0.02 |
| F-WT | 9.3 ± 0.06 | I: 0.78 | I: −0.005 | 23.4 ± 0.24 | I: 0.08 | I: 0.18 | 0.85 ± 0.04 | I: 0.51 | I: −0.004 | 0.15 ± 0.01 | I: 0.20 | I: 0.06 |
| F-bGH | 11 ± 0.3 | 34.8 ± 2.15 | 2.25 ± 0.10 | 0.40 ± 0.13 | ||||||||
| M-WT | 9.5 ± 0.09 | G: <0.001 | G: 0.88 | 30.1 ± 0.58 | G: <0.001 | G: 0.98 | 1.07 ± 0.02 | G: <0.001 | G: 0.98 | 0.30 ± 0.04 | G: 0.44 | G: −0.03 |
| M-bGH | 11.7 ± 0.3 | S: 0.01 | S: 0.24 | 48.2 ± 0.42 | S: <0.001 | S: 0.87 | 2.80 ± 0.03 | S: <0.001 | S: 0.47 | 0.28 ± 0.009 | S: 0.84 | S: −0.03 |
| F-WT | 9.2 ± 0.10 | I: 0.47 | I: −0.02 | 24.5 ± 0.49 | I: 0.11 | I: 0.07 | 0.84 ± 0.03 | I: 0.76 | I: −0.04 | 0.26 ± 0.04 | I: 0.49 | I: 0.02 |
| F-bGH | 11.1 ± 0.15 | 40.9 ± 0.40 | 2.53 ± 0.07 | 0.29 ± 0.03 | ||||||||
| M-WT | 9.84 ± 0.19 | G: 0.001 | G: 0.54 | 40.1 ± 3.3 | G: 0.02 | G:0.31 | 1.32 ± 0.34 | G: <0.001 | G: 0.62 | 0.77 ± 0.18 | G: 0.14 | G: 0.42 |
| M-bGH | 11.8 ± 0.18 | S: 0.04 | S: 0.24 | 51.5 ± 2.4 | S: 0.01 | S:0.39 | 3.23 ± 0.07 | S: 0.21 | S: 0.05 | 0.49 ± 0.01 | S: 0.85 | S: 0.10 |
| F-WT | 9.8 ± 0.50 | I: 0.07 | I: 0.18 | 30.0 ± 1.9 | I: 0.78 | I:−0.07 | 1.19 ± 0.05 | I: 0.39 | I: −0.02 | 0.41 ± 0.03 | I: 0.62 | I: 0.02 |
| F-bGH | 10.4 ± 0.58 | 39.4 ± 4.3 | 2.48 ± 0.49 | 0.34 ± 0.05 | ||||||||
| M-WT | 9.67 ± 0.33 | G: <0.001 | G: 0.81 | 34.5 ± 3.1 | G: <0.001 | G: 0.81 | 1.20 ± 0.15 | G: <0.001 | G: 0.96 | 0.48 ± 0.16 | G: 0.90 | G: −0.036 |
| M-bGH | 12 ± 0.28 | S: 0.04 | S: 0.29 | 48.0 ± 0.31 | S: 0.004 | S: 0.55 | 3.50 ± 0.03 | S: 0.003 | S: 0.48 | 0.32 ± 0.007 | S: 0.84 | S: 0.04 |
| F-WT | 9.33 ± 0.16 | I: 0.26 | I: 0.04 | 27.3 ± 1.6 | I: 0.91 | I: −0.09 | 1.06 ± 0.09 | I: 0.05 | I: 0.26 | 0.27 ± 0.09 | I: 0.37 | I: −0.01 |
| F-bGH | 11 ± 0.28 | 40.4 ± 1.3 | 2.86 ± 0.15 | 0.29 ± 0.06 |
At sacrifice, tissues were collected, and weights were measured. Absolute weight of mesenteric WAT showed age-dependent changes in bGH mice. At six weeks of age, mesenteric WAT was significantly increased in both male and female bGH mice compared to controls with a similar trend seen at three months of age (Table 1). By six months of age, there was no significant difference in mesenteric WAT seen between bGH mice and littermate controls. At 11 and 13 months of age, a trending decrease in mesenteric WAT was observed in male bGH mice compared to controls, but this finding was not significantly different. Age-dependent changes were also observed in other WAT depots (Supplemental Table 1). It is important to note that when normalized to body mass, relative subcutaneous WAT was decreased in bGH mice compared to controls at all timepoints and in both sexes (Supplemental Table 2). Meanwhile, relative mesenteric WAT was again age- and sex-dependent. For instance, at three months of age, relative mesenteric WAT was decreased in male bGH mice and increased in females. However, by six months of age, relative mesenteric WAT was significantly decreased in both male and female bGH mice.
Absolute weights of liver, heart, and kidneys were increased in bGH mice compared to controls across all timepoints with several age- and sex-dependent exceptions. For instance, livers of male bGH mice were significantly different relative to those of females at 6 weeks, 3 months, and 13 months (Table 1, Supplemental Figure 1). Hearts of bGH mice were overall significantly increased compared to controls with the exception of females at 11 months and males at 13 months. Kidneys of bGH mice were significantly increased with the exception of female bGH mice at 3 months. Spleens of bGH mice were also significantly increased at 3, 6, 11, and 13 months of age both in absolute weight and relative to body mass (Supplemental Figure 1, Supplemental Tables 3 and 4). It is important to note that 50% of bGH mice at 13 months of age (2 males and 1 female) had tumors in the urogenital region and liver, whereas there was no incidence of tumors in age-matched controls (data not shown). Overall, bGH mice displayed consistent changes in body length, body weight, and visceral organs across all timepoints with several age-dependent exceptions, especially in WAT depots.
3.2. Intestinal gross anatomy in bGH mice
Both intestinal length and weight were measured in male and female bGH mice at 6 weeks, 3 months, 6 months, 11 months, and 13 months of age (Figure 1). Length and weight of the small intestines (SI) were significantly explained by genotype with a moderate to large effect size across all timepoints (Figure 1). In particular, SI length was significantly increased in male bGH mice at 6 weeks and 3, 6, and 13 months of age and in female bGH mice at 6 and 13 months of age. SI weight was significantly increased in male and female bGH mice compared to controls at 6 weeks, 6 months, and 13 months of age with a significant difference only seen in male bGH mice at 11 months of age. SI circumference (specifically, the jejunum) was also selectively measured at 3 and 13 months of age in both male and female bGH mice (Supplemental Figure 3). Circumference tended to be increased in both male and female bGH mice compared to controls, albeit this finding was not statistically significant.
Figure 1. Intestinal length and weight in male and female bGH mice (red) compared to littermate controls (grey) at 6 weeks and 3, 6, 11, and 13 months of age.
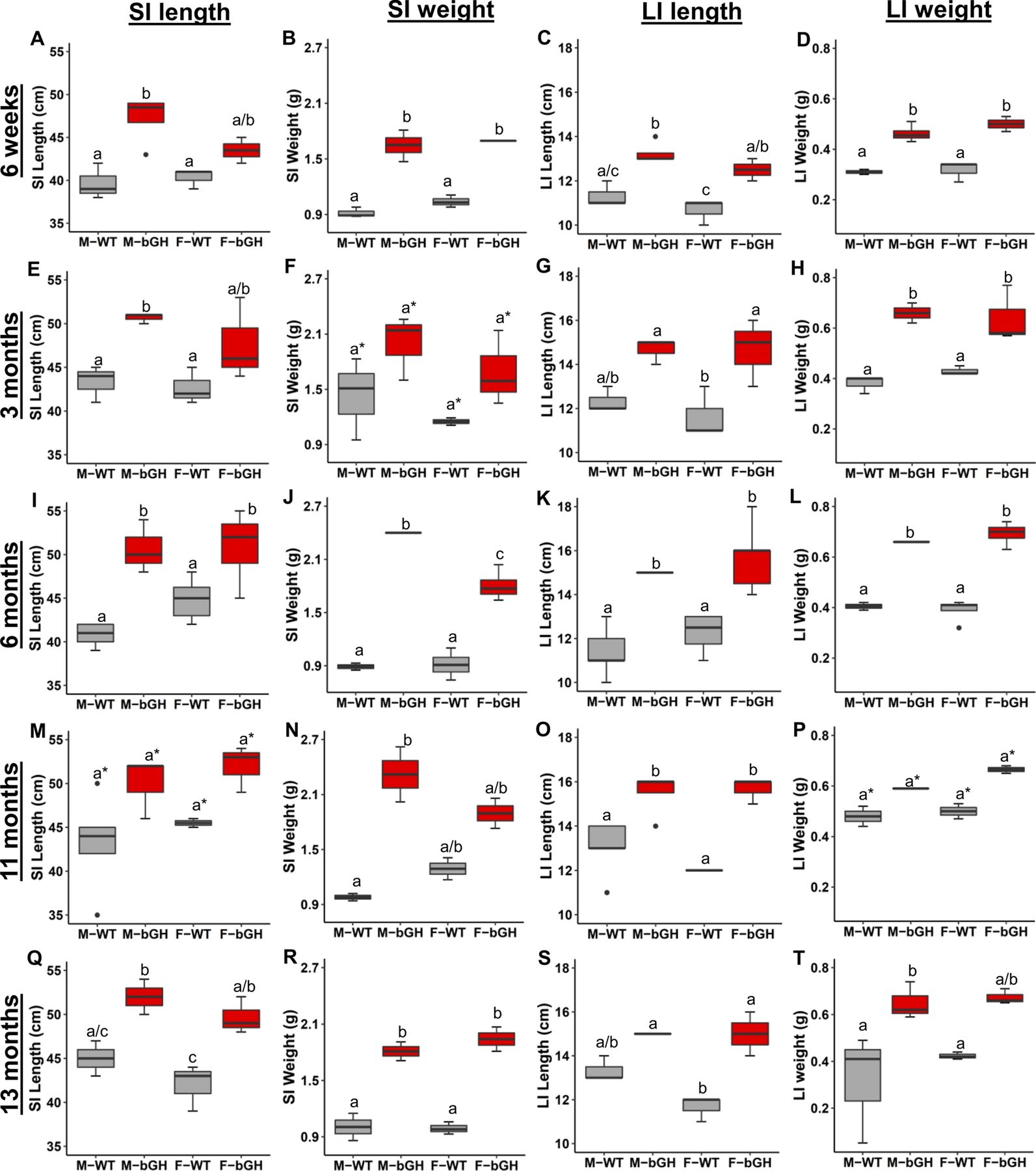
a* denotes no significant difference in the pairwise comparison but with a p < 0.05 for the genotype comparison in the two-way ANOVA. A-D: Small and large intestinal gross anatomy in bGH mice and controls at 6 weeks of age (n=4 for males and n=2 for females). A. Length of small intestines (SI). (p = 0.002 and ωp2 = 0.63 for genotype). B. Weight of the SI. (p < 0.001 and ωp2 = 0.92 for genotype). C. Length of the large intestines (LI). (p = 0.007 and ωp2 = 0.75 for genotype). D. Weight of the LI. (p < 0.001 and ωp2 = 0.86 for genotype). E-H. Intestinal gross anatomy in bGH mice and controls at 3 months of age (n=3). E. SI length (p = 0.006 and ωp2 = 0.54 for genotype). F. SI weight. (p = 0.02 and ωp2 = 0.36 for genotype). G. LI length. (p = 0.002 and ωp2 = 0.61 for genotype). H. LI weight. (p = 0.003 and ωp2 = 0.79 for genotype). I-L. Intestinal gross anatomy for bGH mice and controls at 6 months of age (n=5). I. SI length (p <0.001 and ωp2 = 0.67 for genotype and p = 0.04 and ωp2 = 0.131 for interaction.) J. SI weight. (p < 0.001 and ωp2 = 0.92 for genotype, p = 0.009 and ωp2 = 0.21 for sex, and p = 0.023 and ωp2 = 0.40 for interaction). K. LI length (p < 0.001 and ωp2 = 0.72 for genotype). L. LI weight. (p < 0.001 and ωp2 = 0.92 for genotype). M-O. Intestinal gross anatomy for bGH mice and controls at 11 months of age (n=4). M. SI length. (p = 0.009 and ωp2 = 0.42 for genotype). N. SI weight. (No significant changes). O. LI length (p = 0.002 and ωp2 = 0.67 for genotype). P. LI weight. (p = 0.03 and ωp2 = 0.74 for genotype). Q-T. Intestinal gross anatomy for bGH mice and controls at 13 months of age (n=3). Q. SI length. (p = 0.0027 and ωp2 = 0.73 for genotype). R. SI weight (p < 0.001 and ωp2 = 0.90 for genotype). S. LI length. (p < 0.001 and ωp2 = 0.79 for genotype). T. LI weight. (p = 0.039 and ωp2 = 0.56 for genotype).
Length and weight of the large intestines (LI) were significantly explained by genotype across all timepoints (Figure 1). Both male and female bGH mice had significantly increased LI length compared to respective male and female controls at 6 weeks, 3 months, 6 months, 11 months, and 13 months of age. LI weight exhibited a similar pattern with the exception of 11 months of age. Circumference of the colon also tended to be increased in bGH mice compared to controls at 3 and 13 months of age; yet this finding was sex-dependent (Supplemental Figure 3). That is, at 3 months of age, male bGH mice had significantly increased colonic circumference compared to controls, whereas female bGH mice had significantly increased circumference at 13 months of age compared to controls.
3.3. Morphological measurements in small and large intestines of bGH mice
Intestinal morphology was investigated in the small intestines (duodenum, jejunum, and ileum) and large intestines (cecum and colon) of male and female bGH mice compared to respective male and female controls. Across all timepoints, villus height, crypt depth, and muscle thickness of the jejunum tended to be increased in bGH mice compared to controls with sex- and age-dependent differences (Figure 2). At six weeks of age, the increase in villus height, crypt depth, and muscle thickness were all significantly accounted for by genotype (Figure 2A); however, neither villus height nor muscle thickness were significantly different in female bGH mice compared to controls. Villus height continued to be significantly increased in bGH mice and significantly explained by genotype at three and six months of age. Later in life (at 11 and 13 months of age), male bGH mice still tended to have increased villus height, though this finding was not significant, with a moderate effect size. Crypt depth in the jejunum was significantly explained by genotype at 3 and 13 months of age. Muscle thickness tended to exhibit more sex-dependent changes across all timepoints with a smaller difference observed between female bGH and control mice until 13 months of age. In particular, this finding was significant at 6 weeks and 3 months with trends observed at 11 and 13 months. Similar patterns were observed in the duodenum and ileum, and significance was also age- and sex-dependent (Supplemental Figure 4).
Figure 2. Intestinal morphology in the jejunum and colon of bGH mice and their littermate controls at 6 weeks, 3 months, 6 months, 11 months, and 13 months.
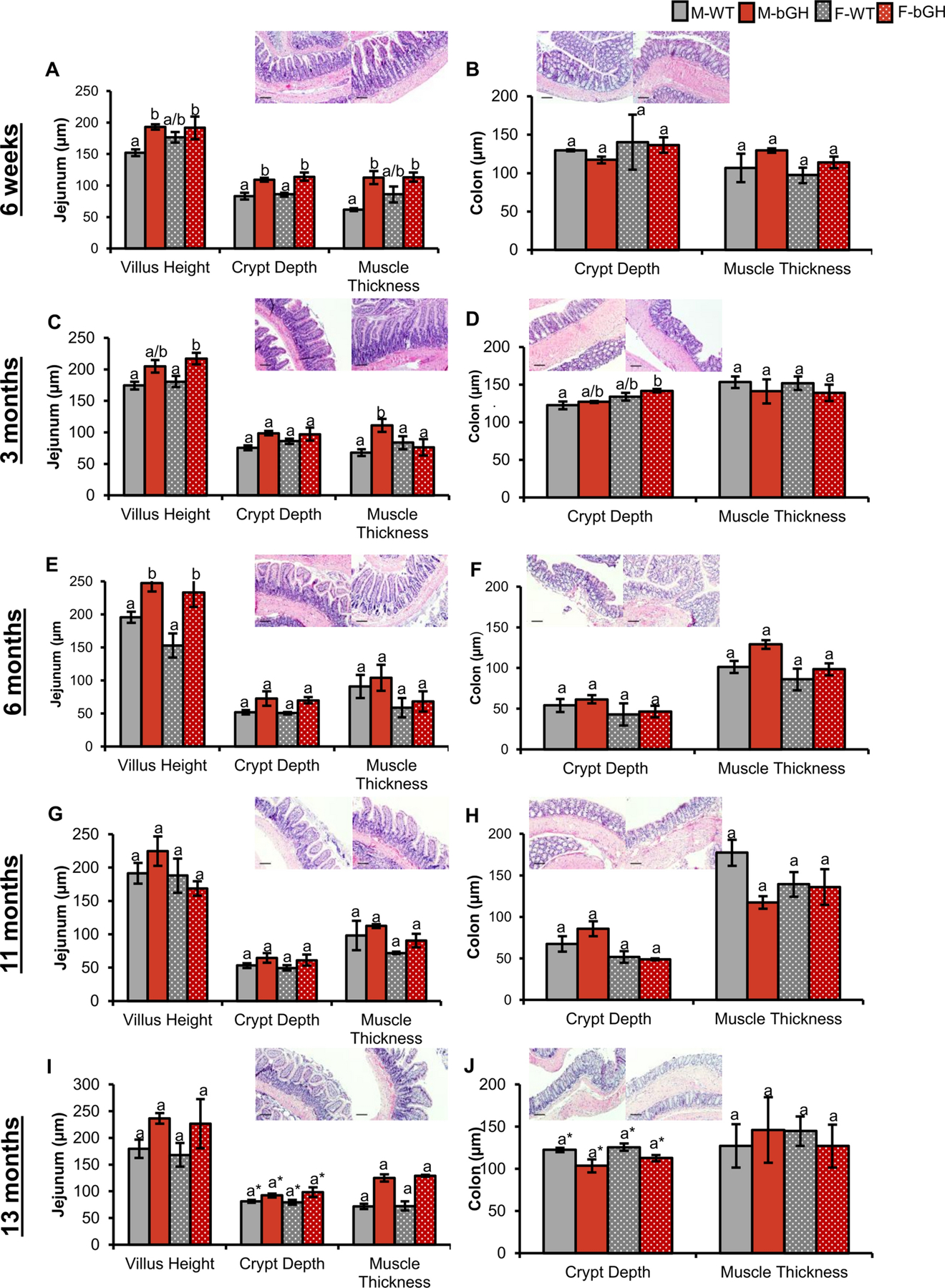
Pictures depicted are of WT mice on the left and bGH mice on the right for jejunum and colon at each respective age. Scale bars represent 100 μm. A-B: Intestinal morphology in bGH mice at 6 weeks of age (n=4 for males and n=2 for females). A. Morphology (villus height, crypt depth, and muscle thickness) of the jejunum at 6 weeks of age. Villus height, crypt depth, and muscle thickness are significantly accounted for by genotype (p = 0.004 and ωp2 = 0.51, p < 0.001 and ωp2 = 0.77, and p = 0.003 and ωp2 = 0.54, respectively). B. Colon in bGH mice and controls at 6 weeks of age. No significant difference seen in crypt depth with a very small effect size. Muscle thickness was slightly increased in bGH mice (p = 0.17 and ωp2 = 0.104 for genotype). C-D: Morphology in jejunum and colon of bGH mice and controls at 3 months of age (n=3). C. Villus height, crypt depth, muscle thickness of jejunum at 3 months of age. Villus height is significantly explained by genotype (p = 0.018 and ωp2 = 0.53). Muscle thickness is significantly explained by sex and interaction between genotype and sex (p = 0.037 and p =0.0337, respectively). D. Crypt depth and muscle thickness in colon at 3 months of age. Crypt depth in the colon is significantly explained by sex (p = 0.026 and ωp2 = 0.464). E-F: Morphology at 6 months of age (n=5). E. Villus height, crypt depth and muscle thickness in jejunum. Villus height and crypt depth are significantly accounted for by genotype (p = 0.006 and ωp2 = 0.59; and p = 0.04 and ωp2 = 0.361, respectively). F. Crypt depth and muscle thickness in colon. Muscle thickness is significantly explained by both genotype (p = 0.04 and = 0.32) and sex (p = 0.04 and = 0.49) G-H: Morphology in jejunum and colon at 11 months of age (n=4). G. Villus height, crypt depth and muscle thickness in jejunum. H. Crypt depth and muscle thickness in colon. Crypt depth was significantly explained by genotype (p = 0.03 and ωp2 = 0.68). I-J: Morphology at 13 months of age (n=3). I. Villus height, crypt depth, and muscle thickness in jejunum. Crypt depth was significantly explained by genotype (p = 0.049 and ωp2 = 0.454). J. Crypt depth and muscle thickness in the colon. Crypt depth was significantly explained by genotype (p = 0.049 and ωp2 = 0.31).
Changes to the morphology of the colon (i.e., crypt depth and muscle thickness) were also sex- and age-dependent (Figure 2). No significant difference was observed in crypt depth at 6 weeks of age, and changes in crypt depth were sex-dependent at 3 months of age. At 6 and 11 months of age, crypt depth tended to be increased, a finding significantly explained by genotype at 11 months of age. By 13 months of age, crypt depth was significantly decreased in bGH mice compared to controls. Muscle thickness tended to be more consistently increased across all timepoints with the exception of 11 months and with sex-dependent differences observed at 6 months of age.
3.4. Intestinal fibrosis in bGH mice
Fibrosis was assessed in the intestines of bGH mice using two methods: histological examination through Sirius red/Fast green staining and biochemical measurement of tissue hydroxyproline content. Sirius red staining (at 6 weeks or 3, 6, 11, or 13 months) showed no consistent changes in bGH mice compared to controls. A significant difference in SI was only explained by the interaction of genotype and sex at 11 months of age with a significant difference observed between WT males and bGH females. In LI, a significant difference observed at 6 weeks was only explained by sex while a difference at 6 months was explained by both genotype and sex with no significant differences observed between individual sub-groups (Supplemental figure 5). When hydroxyproline content was measured in the small intestine of bGH males at 4 months of age, there was a significant increase in bGH mice compared to controls (Figure 5).
Figure 5. Fibrosis.
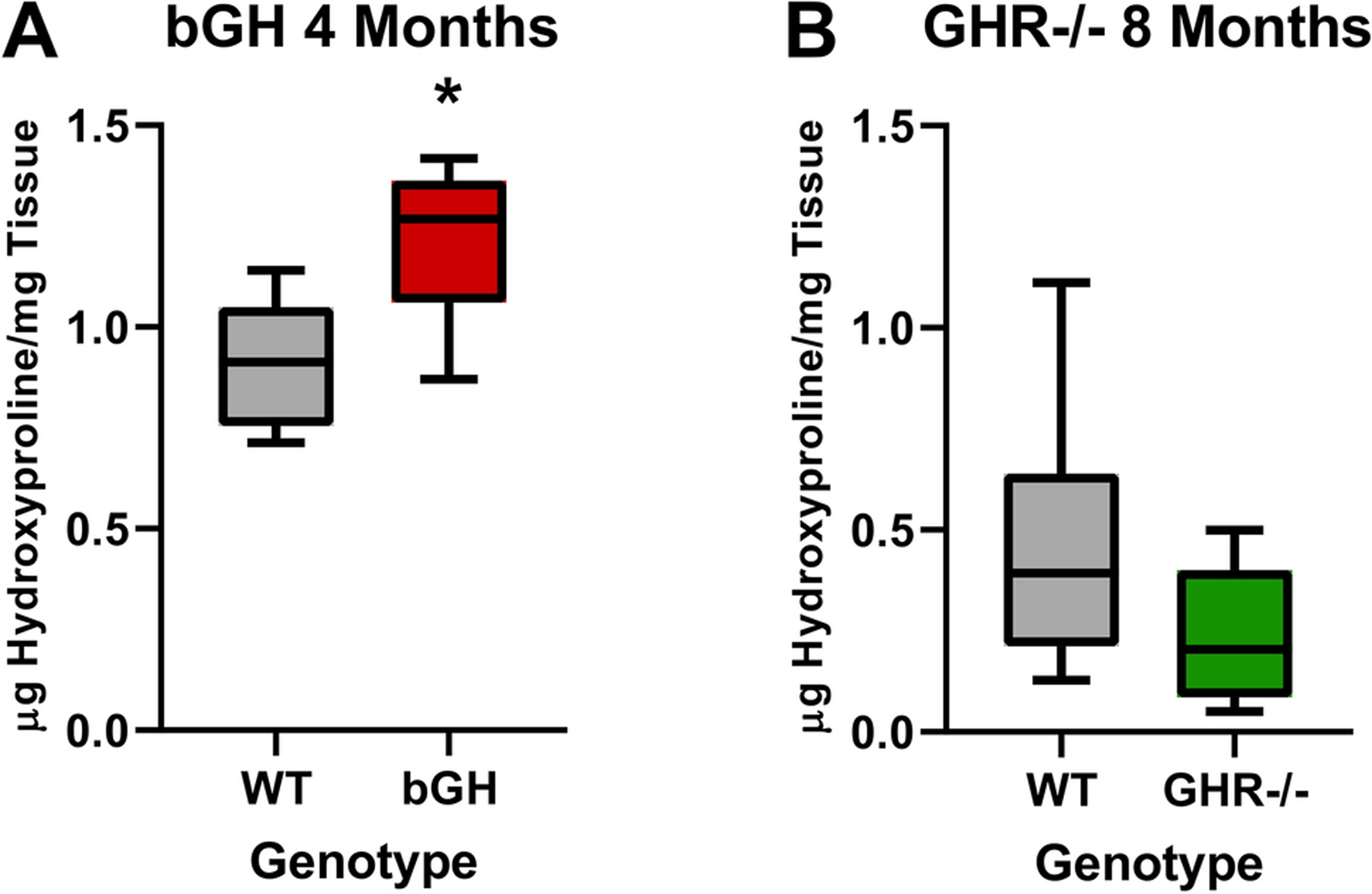
Small intestinal hydroxyproline content of male bGH and GHR−/− mice. A. Hydroxyproline content of the small intestine of bGH mice at 4 months of age (n=8). (Student t test p=0.004) B. Hydroxyproline content of the small intestine of GHR−/− mice at 8 months of age (n=8). (Student t test p=0.10). * indicates t test p<0.05
3.5. Growth parameters and absolute tissue weights in GHR−/− mice
GHR−/− mice displayed the opposite trend to bGH mice in both body length and absolute organ weights. Male and female GHR−/− mice at both 13 and 26 months had significantly decreased body length and weight compared to littermate controls (Table 2).
Table 2. Growth parameters and organ weights in male and female GHR−/− mice and controls at 13 and 26 months of age.
Growth parameters (body length and body weight) and selected organ weights. Two-way ANOVA p values and effect sizes shown for genotype (G), sex (S), and interaction (I). Effect size was calculated by partial omega-squared (ωp2).
| ω p 2 | ω p 2 | ω p 2 | ω p 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-WT | 10.4 ± 0.34 | G: < 0.001 | G: 0.83 | 42.2 ± 1.1 | G: < 0.001 | G: 0.94 | 0.97 ± 0.28 | G: 0.16 | G: 0.07 | 0.61 ± 0.08 | G: < 0.001 | G: 0.59 |
| M-GHR−/− | 7.5 ± 0.11 | S: 0.75 | S: −0.06 | 16.6 ± 2.1 | S: 0.002 | S: 0.49 | 0.79 ± 0.20 | S: 0.65 | S: −0.05 | 0.21 ± 0.07 | S: 0.08 | S: 0.14 |
| F-WT | 10.1 ± 0.1 | I: 0.52 | I: −0.04 | 32.8 ± 1.3 | I: 0.015 | I: 0.31 | 1.01 ± 0.20 | I: 0.52 | I: −0.04 | 0.41 ± 0.09 | I: 0.34 | I: −0.004 |
| F-GHR−/− | 7.6 ± 0.48 | 15.0 ± 0.46 | 0.54 ± 0.17 | 0.14 ± 0.03 | ||||||||
| M-WT | 10.00 ± 0.17 | G: < 0.001 | G: 0.94 | 37.1 ± 2.29 | G: < 0.001 | G: 0.81 | 2.08 ± 0.48 | G: < 0.001 | G: 0.50 | 0.46 ± 0.06 | G: < 0.001 | G: 0.29 |
| M-GHR−/− | 6.88 ± 0.11 | S: 0.05 | S: −0.03 | 13.0 ± 0.76 | S: 0.74 | S: −0.03 | 0.40 ± 0.02 | S: 0.66 | S: −0.02 | 0.12 ± 0.02 | S: 0.49 | S: −0.02 |
| F-WT | 9.95 ± 0.13 | I: 0.32 | I: −0.02 | 33.8 ± 2.70 | I: 0.14 | I: 0.04 | 1.80 ± 0.27 | I: 0.55 | I: −0.02 | 0.49 ± 0.14 | I: 0.76 | I: −0.03 |
| F-GHR−/− | 6.97 ± 0.11 | 15.1 ± 0.89 | 0.43 ± 0.01 | 0.20 ± 0.05 |
Absolute weights of liver, heart, kidney, and spleen were decreased in 13-month and 26-month-old GHR−/− mice compared to controls, but the finding was not significant for the livers of male and female GHR−/− mice at 13 months of age (Table 2, Supplemental Table 5). Absolute weights of subcutaneous WAT tended to be increased in GHR−/− mice at both 13 and 26 months of age. It is important to note that when normalized to body weight, subcutaneous WAT was significantly increased in GHR−/− mice compared to controls at both ages (Supplemental Table 7). Absolute weight of mesenteric WAT was significantly decreased in GHR−/− mice at both timepoints (Table 2). When normalized to body weight, relative mesenteric WAT weight was significantly decreased in GHR−/− mice only at 13 months (Supplemental table 8).
3.6. Gross anatomy of small and large intestines of GHR−/− mice
GHR−/− mice exhibited significantly decreased weight and length of the small and large intestines at 13 and 26 months of age relative to sex-matched controls (Figure 3). Circumferences in the jejunum, colon, and cecum were also measured in male GHR−/− mice at 24 months of age and were significantly decreased compared to the controls (p < 0.001 for all and Cohen’s d = 2.66, 2.56 and 3.56, respectively) (data not shown).
Figure 3. Intestinal length and weight in male and female GHR−/− mice (green) compared to controls (grey) at 13 and 26 months of age.
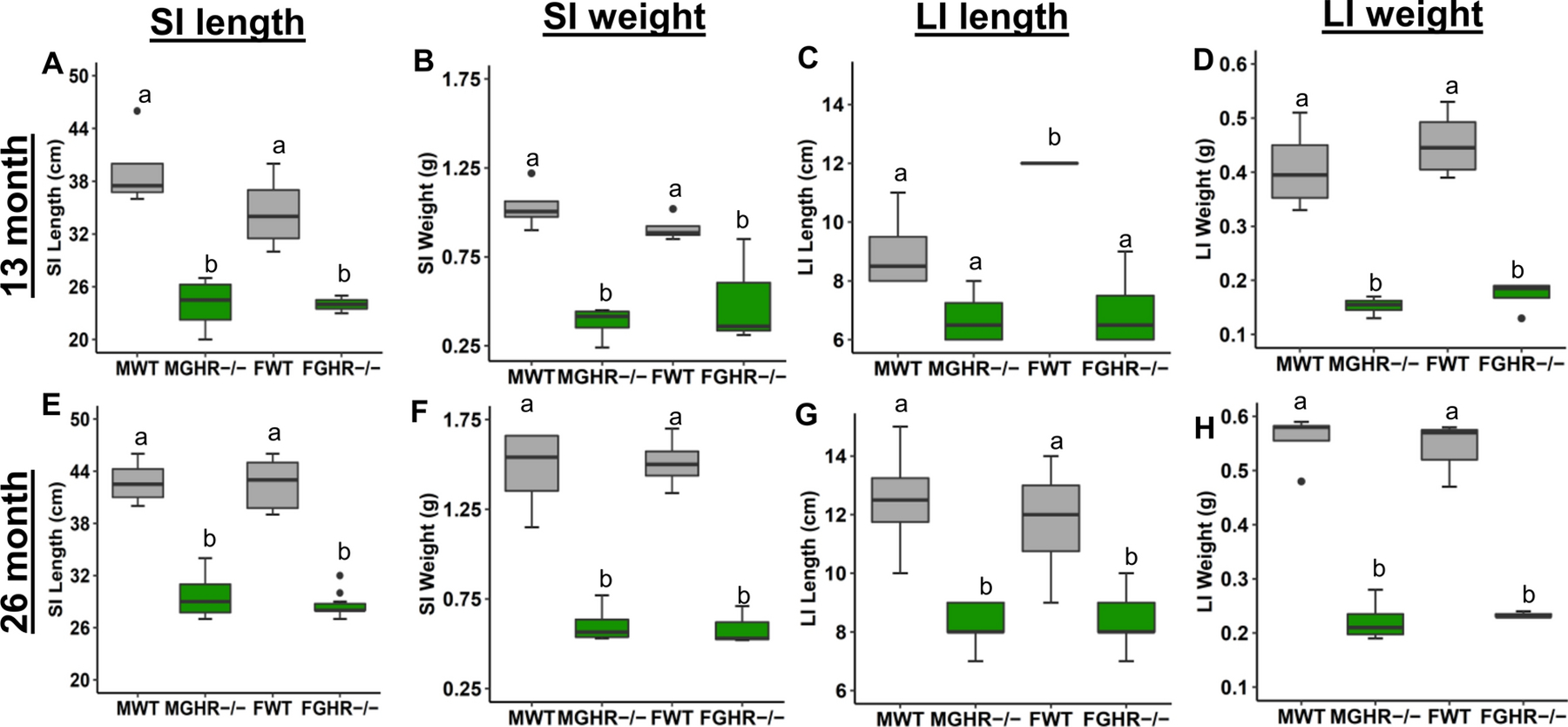
A-D. 13-month-old GHR−/− mice (n=4). A. Small intestine (SI) length. B. SI weight. C. Large intestine (LI) length. D. LI weight. E-H. 26-month-old GHR−/− mice (n=8). E. SI length. F. SI weight. G. LI length. H. LI weight. Distinct letter on the same graph represents a significant difference.
3.7. Morphology in the small and large intestines of GHR−/− mice
Morphology (i.e. villus height, crypt depth, and muscle thickness) was measured in GHR−/− mice at two ages compared to sex- and age-matched controls. At 13 months of age, GHR−/− mice displayed decreased villus height and crypt depth throughout the small intestines compared to controls with significance reached in the villus height of the duodenum and jejunum and crypt depth of the jejunum (Figure 4). Notably, villus height was significantly decreased between males, but significance was not reached in females. Crypt depth was also significantly decreased in the ileum of male GHR−/− mice compared to controls. At 26 months of age, villus height in the jejunum was significantly explained by genotype with a trending decrease observed in GHR−/− mice.
Figure 4. Morphology in jejunum and colon of GHR−/− mice (green).
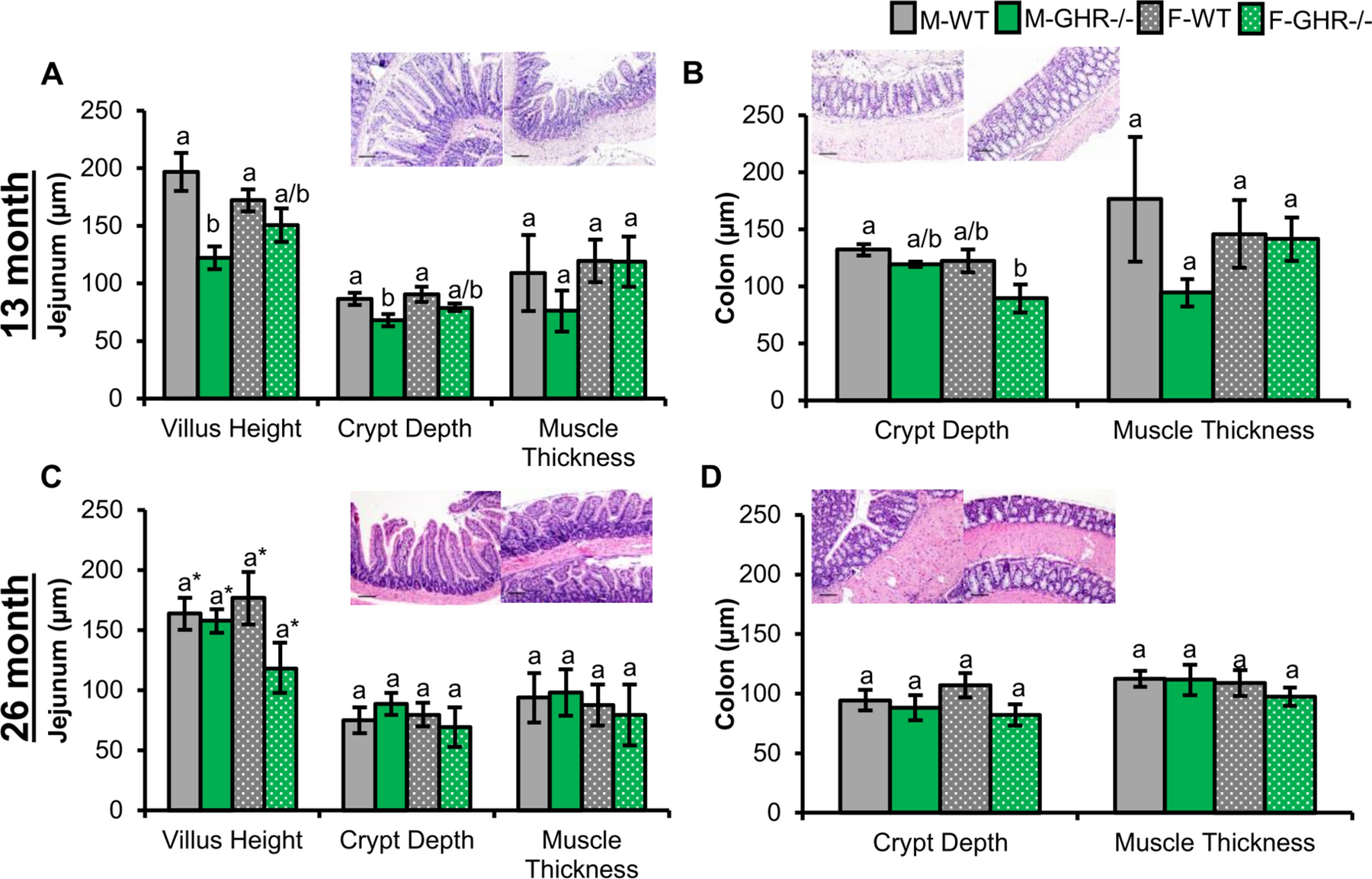
Pictures depicted in the figure are the jejunum and colon section for a respective control (left) and GHR−/− mouse (right) for each timepoint. Scale bars represent 100 μm. A-B: GHR−/− mice at 13 months of age (n=4). A. Morphology of the jejunum. Villus height significantly explained by genotype (p = 0.002 and ωp2 = 0.50). Crypt depth was significantly explained by genotype (p = 0.01 and ωp2 = 0.40). B. Crypt depth and muscle thickness in the colon (p = 0.03 and ωp2 = 0.34 for genotype for crypt depth). C-D: Morphology of the jejunum and colon in GHR−/− mice and controls at 26 months (n=3–4). C. Villus height was significantly explained by genotype (p = 0.04 and ωp2 = 0.12) with a trending decrease in GHR−/− females compared to controls (p = 0.15). D. Colonic crypt depth (p = 0.11 and ωp2 = 0.08 for genotype).
Morphology of the colon differed in the GHR−/− mice compared to controls in a sex-dependent manner (Figure 4). Crypt depth was significantly different in GHR−/− mice at 13 months of age with a significant difference observed between male controls and female GHR−/− mice. A similar trend in decreased colonic crypt depth was observed at 26 months. Muscle thickness was not significantly different between GHR−/− mice and controls, although there was trending decrease in GHR−/− males at 13 months of age.
3.8. Intestinal fibrosis in GHR−/− mice
Fibrosis was assessed in the intestines of GHR−/− mice using the methods described above. There were no significant changes in fibrosis of GHR−/− mice compared to controls at either age or between sexes using either assay (Figure 5 and Supplemental Figure 6).
4. Discussion
This is the first study to characterize the intestinal gross anatomy and morphology in small and large intestines of two mouse lines with altered GH action at multiple timepoints. As expected, male and female bGH mice consistently had increased body weight, body length, and absolute weights of visceral organs (i.e. liver, heart, and kidneys) with age-dependent changes observed in WAT. Meanwhile, GHR−/− mice had significantly decreased body weight, body length, and absolute weights of visceral organs with a tendency toward increased weight in subcutaneous WAT. Notably, we observed opposing findings in intestinal gross anatomy and morphology of the small intestine between bGH mice and GHR−/− mice. Overall, bGH mice had significantly increased length and weight of the small and large intestines. Circumference of the small and large intestines also tended to be increased in bGH mice compared to controls, albeit this finding was not significant in the jejunum and was sex-dependent in the colon. Inversely, length and weight of the small and large intestines were significantly decreased in both male and female GHR−/− mice compared to controls. Villus height, crypt depth, and muscle thickness tended to be increased in the small intestines of bGH mice, albeit again with sex- and age-dependent exceptions. Meanwhile, villus height and crypt depth tended to be decreased in GHR−/− mice compared to controls. Crypt depth and muscle thickness of the colon were altered in an age-dependent manner in bGH mice relative to controls and was minimally altered in GHR−/− mice. Collagen content of bGH intestines was increased compared to controls at one time point, while GHR−/− intestines were unchanged compared to controls. Collectively, these findings suggest that GH action is positively associated with various measures of intestinal gross anatomy and morphology in an age- and sex-dependent manner.
The general phenotype of bGH and GHR−/− mice, including growth, body composition, adipose tissue, and visceral organ weights, and lifespan, has been well-characterized in numerous previous studies [23,25,26,35,39]. Similar to our findings, bGH mice consistently have increased body weight and length throughout their life [23,26]. Moreover, at 12–13 months of age, bGH mice have increased relative and absolute weights of visceral organs (i.e. liver, heart, kidneys, and spleens) and decreased relative and absolute weights of WAT depots [23,26]. GHR−/− mice have significantly decreased body length and weight and absolute weights of visceral organs with increased absolute and relative weights of subcutaneous WAT [35,39]. Thus, the findings reported for most tissue weights are as expected and maintained over the timepoints measured.
GH action influences the gross anatomy of small and large intestines. Excess GH appears to increase length, weight, and circumference of small and large intestines consistently throughout the lifespan of bGH mice relative to controls. Moreover, GHR−/− mice had decreased length, weight, and circumference of small and large intestines at both mid-life and later in life. Overall, GH alteration appears to have caused a similar response in the small and large intestines within each genotype, albeit with age- and sex-dependent differences. Although this is the first study to track changes in gross anatomy of both male and female bGH and GHR−/− mice at multiple timepoints, other studies have assessed intestinal gross anatomy due to alterations in GH action. Overall, previous studies that have investigated the relationship between GH and the intestinal gross anatomy fall into three categories: 1) those that examine the effect of GH administration on intestinal dysfunction (i.e. short bowel syndrome, IBD, and intestinal obstruction) in rodents or clinical cohorts [40–44], 2) those that observe the intestinal complications in patients with acromegaly [4,7,15–19,45–47], or 3) those that characterize the intestinal phenotype in mouse lines with altered GH or IGF-1 signaling [8,29,31,33,48–50]. As for the third category, young (approximately 2 months old) and adult (6 months old) bGH mice have increased length and weight of the small intestine and colon [29,31,32]. Inversely, mouse lines with decreased global or local GH/IGF-1 signaling tend to have decreased length and weight [32,33]. Similarly, intestinal epithelial cell-specific IGF-1 knockout mice have decreased small intestinal length [49], and intestine epithelial-specific GH receptor knockout (IntGHRKO) mice have decreased large intestinal weight [33].
Notably, bGH mice appear to mirror the intestinal phenotype seen in individuals with acromegaly. Several studies have reported that individuals with acromegaly present with dolichocolon (abnormally long large intestine), megacolon (abnormally wide large intestine), increased surface area, and increased loop complexity [15,16,45,46]. Interestingly, increased intestinal surface area and length have been associated with the slower gut transit time/decreased intestinal motility and consequent increased risk in small intestinal bacterial overgrowth, volvulus, and gallstone formation; all of which have been observed in individuals with acromegaly [15,18,46,51].
Our observations in these mouse lines suggest that GH action influences the size of the morphology in small intestines. This finding also emulates what has been previously reported in both clinical and animal studies. In terms of mouse lines with altered GH action, increased villus height has been observed previously in both young and adult (6 months) bGH mice [30,32,52]. Villus height also corresponds well with the increased surface area or circumference observed in bGH mice seen in both this study and previously in male bGH mice at 6 months of age [32]. Increased crypt depth has also been observed in male bGH mice at 6 months of age [32]. Moreover, GH has been shown to stimulate proliferation and differentiation of intestinal stem cells into Paneth cells [3,53]; both of which reside in the crypt and could contribute toward increased crypt depth in the small intestines of bGH mice. The increase in muscle thickness seen in our bGH mice correlates well with intestinal fibrosis and subsequent changes in gut motility. In particular, individuals with acromegaly have been shown to have decreased gut motility and increased collagen cross-linking in the intestines, which has been associated with an increased prevalence of diverticulosis [16]. The significant increase in hydroxyproline in the small intestines of young bGH mice in this study demonstrates an altered collagen state in the intestines that further supports the correspondence between bGH mice and individuals with acromegaly.
This is also one of the few studies to examine the intestinal phenotype in both male and female bGH and GHR−/− mice. Most studies on mouse lines have exclusively focused on one sex or have combined results between sexes [29,31,32,49]. Yet, as seen in a previous study on IntGHRKO mice, there are sex-dependent alterations in the intestines, including gross anatomy, intestinal permeability, intestinal fat absorption, and glucose tolerance [33]. In general, male GH altered mice exhibited a more significant change in gross anatomy (length, weight, and circumference) and morphology with relatively few exceptions (e.g. villus height of the duodenum in young bGH mice and LI circumference at 13 months of age). This finding is not surprising as females have different GH pulsatility patterns, different levels of GH regulatory hormones such as GHRH, ghrelin, and somatostatin, and different levels of GH intracellular signaling [54–57]. Likewise, many studies have shown sex-specific findings in GH altered mice, including body composition, glucose tolerance, insulin sensitivity, gene expression, and even longevity [39,58–61], including fibrosis in this study when both sexes were examined.
Another important distinction of this study are the age-dependent findings. In terms of gross anatomy, we observed an overall increase of length and weight with age and a more significant differentiation in the intestinal gross anatomy between mouse groups with advancing age. Moreover, morphology of the colon displayed interesting age-dependent findings. For instance, crypt depth tended to decrease with age in bGH mice and normalize relative to controls with age in the GHR−/− mice, at least in males. Yet, other research has shown that GH has a protective effect on the colon. That is, chemically-induced colitis in young (two-month-old) bGH mice results in less inflammation and crypt damage than controls with colitis [31]. Apoptosis has also been shown to be reduced in colonic epithelial cells in both bGH mice at 3 and 9 months of age and individuals with acromegaly [4,62]. Similarly, we observed relatively few changes – or an overall increase - in the morphology of the colon of young bGH mice. At the same time, GH excess has also been associated with hyperplastic polyps and shown to suppress p53/p21 in the colonic mucosa, induce DNA damage in colonocytes, and increase colonic cell survival, epithelial-mesenchymal transition factors, and cell motility [5,6,31,62]. The age-dependent differences in crypt depth and muscle thickness of our GH altered mice may represent the pleotropic effect of excess GH on the colon and the importance of age when assessing GH on colon function.
Although this is the first study to characterize the intestinal gross anatomy and morphology in male and female bGH and GHR−/−mice across multiple timepoints, there are several potential limitations and questions that remain. First and foremost, a potential limitation of this study is the small sample size used at several timepoints (such as bGH mice at 6 weeks, 3 months, and 13 months and GHR−/− mice at 26 months). Several trends that were observed at those timepoints had a larger effect size but were not statistically significant, potentially due to the small sample size. Another important limitation to highlight is the lack of overlap in ages between the two mouse lines. with only one true shared timepoint (at 13 months) regardless of age progression. Due to the different aging phenotypes of the two mouse lines, the timepoints in this study, however, did allow, for a mid-life and later-life age comparison. However, a comparison between bGH mice and GHR−/− mice at an earlier timepoint (i.e. 3 months or 6 months) would be important to conduct in a subsequential study. Moreover, this study was descriptive and limited to intestinal gross anatomy and morphology. This choice to focus on these intestinal measurements discounts important metrics of intestinal functionality, including intestinal fat and macronutrient absorption, intestinal motility, permeability, and immune function – to name a few. These experiments would be particularly important to conduct in future studies due to the significant differences observed in intestinal function in individuals with acromegaly and since GH has been shown to influence different cell types involved in these functions (like Paneth cells and enteroendocrine cells). Since we observed intestinal gross anatomy and morphology in two mouse lines with extremes in GH signaling, our findings support a role of GH on the intestinal function, which needs to be addressed in future studies. Still, it is important to note that IGF-1 is also altered in both mouse lines (decreased in GHR−/− and increased in bGH); thus, this study does not dissect the specific roles of GH or IGF-1 on the intestinal phenotype.
6. Conclusions
We observed several notable and opposing differences in intestinal gross anatomy and morphology between mouse lines with GH excess and decreased GH action. That is, male and female bGH mice had increased length, weight, and circumference of the small and large intestines. Villus height, crypt depth, and muscle thickness tended to be increased in bGH mice. Inversely, GHR−/− mice had significantly decreased intestinal gross anatomical measures with decreased villus height and crypt depth in the small intestine. Together, these findings suggest that GH promotes overall gross anatomy of the small and large intestines across multiple timepoints from early to late adulthood. This is the first study to thoroughly describe the morphology of the intestines in both male and female bGH and GHR−/− mice across multiple timepoints, highlighting minute, age-dependent and sex-dependent changes seen in both the small and large intestines, especially in muscle thickness. Future research is needed to delve into how these intestinal changes may impact the local intestinal environment and function and overall, metabolism, growth, and health of the individual.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Julie Buckley and the Ohio University Histology Core for assistance in preparing and staining intestinal sections. We would also like to thank Alison Brittain for tissue samples at 6 weeks of age, Stephen Bell for his assistance in the dissection and adipose tissue extraction of the bGH mice and controls at all timepoints, Silvana Duran for her assistance toward tissue and body weight measurements for GHR−/− mice at 13 months of age, and Zachary Jackson for his assistance in preparing intestinal samples at 6 and 11 months of age.
Funding:
This work was supported in part by NIH grant #AG059779, Ohio University Heritage College of Osteopathic Medicine, The Diabetes Institute at Ohio University, and the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll. This study was partially funded by the John J. Kopchick Molecular and Cellular Biology/Translational Biomedical Sciences Research Fellowship and a fellowship from Osteopathic Heritage Foundations at Ohio University Heritage College of Osteopathic Medicine.
ABBREVIATIONS
- bGH
bovine growth hormone transgenic mice
- GHR−/−
growth hormone receptor gene disrupted mice
- SI
small intestines
- LI
large intestines
- IBD
inflammatory bowel diseases
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Conflicts of interest: The authors have no conflict of interest.
Code availability: Upon request
Ethics approval: All animal procedures were approved by the Ohio University Institutional Animal Care and Use Committee and complied with federal, state, and local laws.
Availability of data and material:
Upon request
REFERENCES
- 1.Kaymakci A, Guven S, Ciftci I, Akillioglu I, Aktan M, Eker HH, Sutcu A, Abasiyanik A: Protective effects of growth hormone on bacterial translocation and intestinal damage in rats with partial intestinal obstruction. Bratislava Medical Journal 115(07), 395–399 (2014). doi: 10.4149/bll_2014_078 [DOI] [PubMed] [Google Scholar]
- 2.Scopa CD, Koureleas S, Tsamandas AC, Spiliopoulou I, Alexandrides T, Filos KS, Vagianos CE: Beneficial effects of growth hormone and insulin-like growth factor I on intestinal bacterial translocation, endotoxemia, and apoptosis in experimentally jaundiced rats. J Am Coll Surg 190(4), 423–431 (2000). doi: 10.1016/s1072-7515(99)00285-9 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Tseng SH, Yao CL, Li C, Tsai YH: Distinct Effects of Growth Hormone and Glutamine on Activation of Intestinal Stem Cells. JPEN J Parenter Enteral Nutr 42(3), 642–651 (2018). doi: 10.1177/0148607117709435 [DOI] [PubMed] [Google Scholar]
- 4.Bogazzi F, Ultimieri F, Raggi F, Russo D, Lombardi M, Cosci C, Brogioni S, Gasperi M, Bartalena L, Martino E: Reduced colonic apoptosis in mice overexpressing bovine growth hormone occurs through changes in several kinase pathways. Growth Hormone & IGF Research 19(5), 432–441 (2009). doi: 10.1016/j.ghir.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 5.Chesnokova V, Zonis S, Barrett RJ, Gleeson JP, Melmed S: Growth Hormone Induces Colon DNA Damage Independent of IGF-1. Endocrinology 160(6), 1439–1447 (2019). doi: 10.1210/en.2019-00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesnokova V, Zonis S, Zhou C, Recouvreux MV, Ben-Shlomo A, Araki T, Barrett R, Workman M, Wawrowsky K, Ljubimov VA, Uhart M, Melmed S: Growth hormone is permissive for neoplastic colon growth. Proc Natl Acad Sci U S A 113(23), E3250–3259 (2016). doi: 10.1073/pnas.1600561113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cats A, Dullaart RP, Kleibeuker JH, Kuipers F, Sluiter WJ, Hardonk MJ, de Vries EG: Increased epithelial cell proliferation in the colon of patients with acromegaly. Cancer Res 56(3), 523–526 (1996). [PubMed] [Google Scholar]
- 8.Pereira-Fantini PM, Thomas SL, Taylor RG, Nagy E, Sourial M, Fuller PJ, Bines JE: Colostrum Supplementation Restores Insulin-like Growth Factor −1 Levels and Alters Muscle Morphology Following Massive Small Bowel Resection. JPEN J Parenter Enteral Nutr 32(3), 266–275 (2008). doi: 10.1177/0148607108316197 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Arnes J, Sierra C, Tinahones F, Monzon A, Lopez MJ, Mazuecos N, Soriguer F, Valverde E: Intestinal permeability in adult patients with growth hormone deficiency. J Endocrinol Invest 24(2), 78–82 (2001). doi: 10.1007/BF03343817 [DOI] [PubMed] [Google Scholar]
- 10.Soendergaard C, Kvist PH, Thygesen P, Reslow M, Nielsen OH, Kopchick JJ, Holm TL: Characterization of Growth Hormone Resistance in Experimental and Ulcerative Colitis. International Journal of Molecular Sciences 18(10), 2046 (2017). doi: 10.3390/ijms18102046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR: Colitis induced bone loss is gender dependent and associated with increased inflammation. Inflamm Bowel Dis 19(8), 1586–1597 (2013). doi: 10.1097/MIB.0b013e318289e17b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahad A, Weiss B: Current therapy of pediatric Crohn’s disease. World J Gastrointest Pathophysiol 6(2), 33–42 (2015). doi: 10.4291/wjgp.v6.i2.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han X, Ren X, Jurickova I, Groschwitz K, Pasternak BA, Xu H, Wilson TA, Hogan SP, Denson LA: Regulation of intestinal barrier function by signal transducer and activator of transcription 5b. Gut 58(1), 49–58 (2009). doi: 10.1136/gut.2007.145094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker MD, Zylberberg HM, Green PHR, Katz MS: Endocrine complications of celiac disease: a case report and review of the literature. Endocr Res 44(1–2), 27–45 (2019). doi: 10.1080/07435800.2018.1509868 [DOI] [PubMed] [Google Scholar]
- 15.Resmini E, Parodi A, Savarino V, Greco A, Rebora A, Minuto F, Ferone D: Evidence of Prolonged Orocecal Transit Time and Small Intestinal Bacterial Overgrowth in Acromegalic Patients. J Clin Endocrinol Metab 92(6), 2119–2124 (2007). doi: 10.1210/jc.2006-2509 [DOI] [PubMed] [Google Scholar]
- 16.Wassenaar MJ, Cazemier M, Biermasz NR, Pereira AM, Roelfsema F, Smit JW, Hommes DW, Felt-Bersma RJ, Romijn JA: Acromegaly is associated with an increased prevalence of colonic diverticula: a case-control study. J Clin Endocrinol Metab 95(5), 2073–2079 (2010). doi: 10.1210/jc.2009-1714 [DOI] [PubMed] [Google Scholar]
- 17.Colao A, Balzano A, Ferone D, Panza N, Grande G, Marzullo P, Bove A, Iodice G, Merola B, Lombardi G: Increased prevalence of colonic polyps and altered lymphocyte subset pattern in the colonic lamina propria in acromegaly. Clin Endocrinol (Oxf) 47(1), 23–28 (1997). doi: 10.1046/j.1365-2265.1997.00253.x [DOI] [PubMed] [Google Scholar]
- 18.Thomas LA, Veysey MJ, Murphy GM, Russell-Jones D, French GL, Wass JA, Dowling RH: Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut 54(5), 630–635 (2005). doi: 10.1136/gut.2003.028431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon MB, Nakhle S, Ludlam WH: Patients with Acromegaly Presenting with Colon Cancer: A Case Series. Case Rep Endocrinol 2016 (2016). doi: 10.1155/2016/5156295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McFadden JP, Corrall RJ: Sigmoid volvulus in acromegaly. Cmaj 136(10), 1060 (1987). [PMC free article] [PubMed] [Google Scholar]
- 21.Simsek Z, Uskudar O, Deveci M, Aktas B: Acute colonic pseudo-obstruction in acromegalic patient with dolicho-megacolon mimicking colonic volvulus. The Turkish journal of gastroenterology : the official journal of Turkish Society of Gastroenterology 23(3), 307–308 (2012). [PubMed] [Google Scholar]
- 22.Hancerliogullari O, Senocak R, Kaymak S, Lapsekili E, Sinan H: An uncommon cause of acute abdomen in an acromegalic patient: colonic volvulus. Ann Ital Chir 89, 572–576 (2018). [PubMed] [Google Scholar]
- 23.Jara A, Benner CM, Sim D, Liu X, List EO, Householder LA, Berryman DE, Kopchick JJ: Elevated Systolic Blood Pressure in Male GH Transgenic Mice Is Age Dependent. Endocrinology 155(3), 975–986 (2014). doi: 10.1210/en.2013-1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Householder LA, Comisford R, Duran-Ortiz S, Lee K, Troike K, Wilson C, Jara A, Harberson M, List EO, Kopchick JJ, Berryman DE: Increased fibrosis: A novel means by which GH influences white adipose tissue function. Growth Horm IGF Res 39, 45–53 (2018). doi: 10.1016/j.ghir.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pendergrass WR, Li Y, Jiang D, Wolf NS: Decrease in cellular replicative potential in “giant” mice transfected with the bovine growth hormone gene correlates to shortened life span. J Cell Physiol 156(1), 96–103 (1993). doi: 10.1002/jcp.1041560114 [DOI] [PubMed] [Google Scholar]
- 26.Palmer AJ, Chung MY, List EO, Walker J, Okada S, Kopchick JJ, Berryman DE: Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology 150(3), 1353–1360 (2009). doi: 10.1210/en.2008-1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf E, Kahnt E, Ehrlein J, Hermanns W, Brem G, Wanke R: Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev 68(1–3), 71–87 (1993). doi: 10.1016/0047-6374(93)90141-d [DOI] [PubMed] [Google Scholar]
- 28.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ: Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144(9), 3799–3810 (2003). doi: 10.1210/en.2003-0374 [DOI] [PubMed] [Google Scholar]
- 29.Michaylira CZ, Ramocki NM, Simmons JG, Tanner CK, McNaughton KK, Woosley JT, Greenhalgh CJ, Lund PK: Haplotype Insufficiency for Suppressor of Cytokine Signaling-2 Enhances Intestinal Growth and Promotes Polyp Formation in Growth Hormone-Transgenic Mice. Endocrinology 147(4), 1632–1641 (2006). doi: 10.1210/en.2005-1241 [DOI] [PubMed] [Google Scholar]
- 30.Ohneda K, Ulshen MH, Fuller CR, D’Ercole AJ, Lund PK: Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology 112(2), 444–454 (1997). doi: 10.1053/gast.1997.v112.pm9024298 [DOI] [PubMed] [Google Scholar]
- 31.Williams KL, Fuller CR, Dieleman LA, DaCosta CM, Haldeman KM, Sartor RB, Lund PK: Enhanced survival and mucosal repair after dextran sodium sulfate–induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology 120(4), 925–937 (2001). doi: 10.1053/gast.2001.22470 [DOI] [PubMed] [Google Scholar]
- 32.Jensen EA, Young JA, Jackson Z, Busken J, List EO, Carroll RK, Kopchick JJ, Murphy ER, Berryman DE: Growth hormone deficiency and excess alter the gut microbiome in adult male mice. Endocrinology (2020). doi: 10.1210/endocr/bqaa026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young JA, Jensen EA, Stevens A, Duran-Ortiz S, List EO, Berryman DE, Kopchick JJ: Characterization of an intestine-specific GH receptor knockout (IntGHRKO) mouse. Growth Horm IGF Res 46–47, 5–15 (2019). doi: 10.1016/j.ghir.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ: A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proceedings of the National Academy of Sciences of the United States of America 94(24), 13215–13220 (1997). doi: 10.1073/pnas.94.24.13215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ: Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Hormone & IGF Research 14(4), 309–318 (2004). doi: 10.1016/j.ghir.2004.02.005 [DOI] [PubMed] [Google Scholar]
- 36.Bialkowska AB, Ghaleb AM, Nandan MO, Yang VW: Improved Swiss-rolling Technique for Intestinal Tissue Preparation for Immunohistochemical and Immunofluorescent Analyses. J Vis Exp(113) (2016). doi: 10.3791/54161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedossa P, Lemaigre G, Bacci J, Martin E: Quantitative estimation of the collagen content in normal and pathologic pancreas tissue. Digestion 44(1), 7–13 (1989). doi: 10.1159/000199886 [DOI] [PubMed] [Google Scholar]
- 38.Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW: ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18(1), 529 (2017). doi: 10.1186/s12859-017-1934-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E, O’Connor P, Okada S, Kopchick JJ: Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci 65(1), 31–40 (2010). doi: 10.1093/gerona/glp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo M, Li Y, Li J: Effect of growth hormone, glutamine, and enteral nutrition on intestinal adaptation in patients with short bowel syndrome. Turk J Gastroenterol 24(6), 463–468 (2013). doi: 10.4318/tjg.2013.0555 [DOI] [PubMed] [Google Scholar]
- 41.Goulet O, Dabbas-Tyan M, Talbotec C, Kapel N, Rosilio M, Souberbielle JC, Corriol O, Ricour C, Colomb V: Effect of recombinant human growth hormone on intestinal absorption and body composition in children with short bowel syndrome. JPEN J Parenter Enteral Nutr 34(5), 513–520 (2010). doi: 10.1177/0148607110362585 [DOI] [PubMed] [Google Scholar]
- 42.Byrne TA, Persinger RL, Young LS, Ziegler TR, Wilmore DW: A new treatment for patients with short-bowel syndrome. Growth hormone, glutamine, and a modified diet. Ann Surg 222(3), 243–255 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slonim AE, Bulone L, Damore MB, Goldberg T, Wingertzahn MA, McKinley MJ: A Preliminary Study of Growth Hormone Therapy for Crohn’s Disease. New England Journal of Medicine 342(22), 1633–1637 (2000). doi: 10.1056/nejm200006013422203 [DOI] [PubMed] [Google Scholar]
- 44.Vortia E, Kay M, Wyllie R: The role of growth hormone and insulin-like growth factor-1 in Crohn’s disease: implications for therapeutic use of human growth hormone in pediatric patients. Curr Opin Pediatr 23(5), 545–551 (2011). doi: 10.1097/MOP.0b013e32834a7810 [DOI] [PubMed] [Google Scholar]
- 45.Renehan AG, Painter JE, Bell GD, Rowland RS, O’Dwyer ST, Shalet SM: Determination of large bowel length and loop complexity in patients with acromegaly undergoing screening colonoscopy. Clin Endocrinol (Oxf) 62(3), 323–330 (2005). doi: 10.1111/j.1365-2265.2005.02217.x [DOI] [PubMed] [Google Scholar]
- 46.Veysey MJ, Thomas LA, Mallet AI, Jenkins PJ, Besser GM, Wass JA, Murphy GM, Dowling RH: Prolonged large bowel transit increases serum deoxycholic acid: a risk factor for octreotide induced gallstones. Gut 44(5), 675–681 (1999). doi: 10.1136/gut.44.5.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dworakowska D, Gueorguiev M, Kelly P, Monson JP, Besser GM, Chew SL, Akker SA, Drake WM, Fairclough PD, Grossman AB, Jenkins PJ: Repeated colonoscopic screening of patients with acromegaly: 15-year experience identifies those at risk of new colonic neoplasia and allows for effective screening guidelines. Eur J Endocrinol 163(1), 21–28 (2010). doi: 10.1530/eje-09-1080 [DOI] [PubMed] [Google Scholar]
- 48.Kopchick JJ, List EO, Kelder B, Gosney ES, Berryman DE: Evaluation of growth hormone (GH) action in mice: discovery of GH receptor antagonists and clinical indications. Mol Cell Endocrinol 386(0), 34–45 (2014). doi: 10.1016/j.mce.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Y, Song Y, Han Q, Liu W, Xu J, Yu Z, Zhang R, Li N: Intestinal epithelial cell-specific IGF1 promotes the expansion of intestinal stem cells during epithelial regeneration and functions on the intestinal immune homeostasis. Am J Physiol Endocrinol Metab 315(4), E638–E649 (2018). doi: 10.1152/ajpendo.00022.2018 [DOI] [PubMed] [Google Scholar]
- 50.Chen T, Zheng F, Tao J, Tan S, Zeng L, Peng X, Wu B: Insulin-Like Growth Factor-1 Contributes to Mucosal Repair by β-Arrestin2–Mediated Extracellular Signal-Related Kinase Signaling in Experimental Colitis. The American Journal of Pathology 185(9), 2441–2453 (2015). doi: 10.1016/j.ajpath.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 51.Veysey MJ, Thomas LA, Mallet AI, Jenkins PJ, Besser GM, Murphy GM, Dowling RH: Colonic transit influences deoxycholic acid kinetics. Gastroenterology 121(4), 812–822 (2001). doi: 10.1053/gast.2001.28015 [DOI] [PubMed] [Google Scholar]
- 52.Ulshen MH, Dowling RH, Fuller CR, Zimmermann EM, Lund PK: Enhanced growth of small bowel in transgenic mice overexpressing bovine growth hormone. Gastroenterology 104(4), 973–980 (1993). doi: 10.1016/0016-5085(93)90263-c [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Tsai YH, Tseng BJ, Tseng SH: Influence of Growth Hormone and Glutamine on Intestinal Stem Cells: A Narrative Review. Nutrients 11(8) (2019). doi: 10.3390/nu11081941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barkan AL, Dimaraki EV, Jessup SK, Symons KV, Ermolenko M, Jaffe CA: Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. J Clin Endocrinol Metab 88(5), 2180–2184 (2003). doi: 10.1210/jc.2002-021169 [DOI] [PubMed] [Google Scholar]
- 55.Jessup SK, Dimaraki EV, Symons KV, Barkan AL: Sexual dimorphism of growth hormone (GH) regulation in humans: endogenous GH-releasing hormone maintains basal GH in women but not in men. J Clin Endocrinol Metab 88(10), 4776–4780 (2003). doi: 10.1210/jc.2003-030246 [DOI] [PubMed] [Google Scholar]
- 56.Hindmarsh PC, Dennison E, Pincus SM, Cooper C, Fall CH, Matthews DR, Pringle PJ, Brook CG: A sexually dimorphic pattern of growth hormone secretion in the elderly. J Clin Endocrinol Metab 84(8), 2679–2685 (1999). doi: 10.1210/jcem.84.8.5915 [DOI] [PubMed] [Google Scholar]
- 57.Leung KC, Johannsson G, Leong GM, Ho KK: Estrogen regulation of growth hormone action. Endocr Rev 25(5), 693–721 (2004). doi: 10.1210/er.2003-0035 [DOI] [PubMed] [Google Scholar]
- 58.Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ: The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol 9(6), 366–376 (2013). doi: 10.1038/nrendo.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Outeirino-Blanco E, Garcia-Buela J, Sangiao-Alvarellos S, Brandon I, Pena L, Pertega-Diaz S, Martinez T, Cordido F: Sexual dimorphism on growth hormone secretion after oral glucose administration. Horm Metab Res 44(7), 533–538 (2012). doi: 10.1055/s-0032-1304578 [DOI] [PubMed] [Google Scholar]
- 60.Liu Z, Mohan S, Yakar S: Does the GH/IGF-1 axis contribute to skeletal sexual dimorphism? Evidence from mouse studies. Growth Horm IGF Res 27, 7–17 (2016). doi: 10.1016/j.ghir.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hao P, Waxman DJ: Functional Roles of Sex-Biased, Growth Hormone-Regulated MicroRNAs miR-1948 and miR-802 in Young Adult Mouse Liver. Endocrinology 159(3), 1377–1392 (2018). doi: 10.1210/en.2017-03109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bogazzi F, Ultimieri F, Raggi F, Russo D, Costa A, Marciano E, Bartalena L, Martino E: Changes in the expression of suppressor of cytokine signalling (SOCS) 2 in the colonic mucosa of acromegalic patients are associated with hyperplastic polyps. Clin Endocrinol (Oxf) 70(6), 898–906 (2009). doi: 10.1111/j.1365-2265.2008.03431.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon request


