Key Points
Question
What is the dynamic trajectory of cognitive changes in the elderly population surviving COVID-19?
Findings
In this cohort study of 1438 COVID-19 survivors 60 years and older who were discharged from COVID-19–designated hospitals in Wuhan, China, the incidence of cognitive impairment was higher in COVID-19 survivors, especially those with severe cases, compared with uninfected participants during a 1-year follow-up period.
Meaning
The findings suggest that long-term cognitive decline is common after SARS-CoV-2 infection, indicating the necessity of evaluating the impact of the COVID-19 pandemic on the future dementia burden worldwide.
This cohort study evaluates cognitive functioning and decline in elderly COVID-19 survivors in China over a period of 12 months postinfection.
Abstract
Importance
Determining the long-term impact of COVID-19 on cognition is important to inform immediate steps in COVID-19 research and health policy.
Objective
To investigate the 1-year trajectory of cognitive changes in older COVID-19 survivors.
Design, Setting, and Participants
This cohort study recruited 3233 COVID-19 survivors 60 years and older who were discharged from 3 COVID-19–designated hospitals in Wuhan, China, from February 10 to April 10, 2020. Their uninfected spouses (N = 466) were recruited as a control population. Participants with preinfection cognitive impairment, a concomitant neurological disorder, or a family history of dementia were excluded, as well as those with severe cardiac, hepatic, or kidney disease or any kind of tumor. Follow-up monitoring cognitive functioning and decline took place at 6 and 12 months. A total of 1438 COVID-19 survivors and 438 control individuals were included in the final follow-up. COVID-19 was categorized as severe or nonsevere following the American Thoracic Society guidelines.
Main Outcomes and Measures
The main outcome was change in cognition 1 year after patient discharge. Cognitive changes during the first and second 6-month follow-up periods were assessed using the Informant Questionnaire on Cognitive Decline in the Elderly and the Telephone Interview of Cognitive Status-40, respectively. Based on the cognitive changes observed during the 2 periods, cognitive trajectories were classified into 4 categories: stable cognition, early-onset cognitive decline, late-onset cognitive decline, and progressive cognitive decline. Multinomial and conditional logistical regression models were used to identify factors associated with risk of cognitive decline.
Results
Among the 3233 COVID-19 survivors and 1317 uninfected spouses screened, 1438 participants who were treated for COVID-19 (691 male [48.05%] and 747 female [51.95%]; median [IQR] age, 69 [66-74] years) and 438 uninfected control individuals (222 male [50.68%] and 216 female [49.32%]; median [IQR] age, 67 [66-74] years) completed the 12-month follow-up. The incidence of cognitive impairment in survivors 12 months after discharge was 12.45%. Individuals with severe cases had lower Telephone Interview of Cognitive Status-40 scores than those with nonsevere cases and control individuals at 12 months (median [IQR]: severe, 22.50 [16.00-28.00]; nonsevere, 30.00 [26.00-33.00]; control, 31.00 [26.00-33.00]). Severe COVID-19 was associated with a higher risk of early-onset cognitive decline (odds ratio [OR], 4.87; 95% CI, 3.30-7.20), late-onset cognitive decline (OR, 7.58; 95% CI, 3.58-16.03), and progressive cognitive decline (OR, 19.00; 95% CI, 9.14-39.51), while nonsevere COVID-19 was associated with a higher risk of early-onset cognitive decline (OR, 1.71; 95% CI, 1.30-2.27) when adjusting for age, sex, education level, body mass index, and comorbidities.
Conclusions and Relevance
In this cohort study, COVID-19 survival was associated with an increase in risk of longitudinal cognitive decline, highlighting the importance of immediate measures to deal with this challenge.
Introduction
The COVID-19 pandemic has affected more than 418 million patients thus far, and the number is increasing.1 The long-term impact of COVID-19 on cognition has become a major public health concern.2 SARS-CoV-2 causes a variety of neurological sequelae in COVID-19 survivors, including dizziness, headache, myalgias, hypogeusia, hyposmia, polyneuropathy, myositis, cerebrovascular diseases, encephalitis, and encephalopathy.3 Such susceptibility of the central nervous system to SARS-CoV-2 has evoked great interest in neuropsychiatric investigations among COVID-19 survivors.4,5 Cognitive complaints are common in the acute6 and subacute phases of COVID-19.7 Our research, along with that of others, has demonstrated an association between SARS-CoV-2 infection and cognitive performance in older adults months after infection.8 However, the long-term trajectory of cognitive changes after SARS-CoV-2 infection remains unknown. In this study, we investigated the 1-year dynamic trajectory of cognitive changes in older COVID-19 survivors.
Methods
Participants
The research protocols for this study were approved by the institutional review boards of Daping Hospital and the General Hospital of the Central Theatre Command of the People’s Liberation Army, as their medical staff worked in the COVID-19–designated Huoshenshan Hospital and Tongji Taikang Hospital and were dismissed after the height of the pandemic. Because this study was conducted based on telephone interviews, the requirement for written informed consent was waived, and verbal informed consent was obtained from all participants or their legal guardians. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Participants in this study were the first group of patients hospitalized with COVID-19 who were discharged between February 10 and April 10, 2020, from 3 COVID-19–designated hospitals in Wuhan, China, including Huoshenshan Hospital, Tongji Taikang Hospital, and General Hospital of the Central Theatre Command of the People’s Liberation Army. Uninfected spouses who lived with the patients were recruited as control individuals. Inclusion and exclusion criteria were described in our previous study.9 Briefly, patients were eligible for participation if they were 60 years and older and agreed to participate. Participants were excluded if they met the following conditions: (1) did not agree to participate, did not understand the items in the questionnaires, or had communicative obstacles owing to language or hearing reasons; (2) had self-reported or diagnosed cognitive impairment preinfection; (3) had a family history of dementia in first-degree relatives; (4) had a concomitant neurological disorder potentially affecting cognitive function; or (5) had severe cardiac, hepatic, or kidney diseases or any kind of tumor.
Clinical and Cognitive Assessment
The diagnosis of COVID-19 was made based on World Health Organization interim guidance.10 COVID-19 was categorized as severe or nonsevere following the American Thoracic Society guidelines for community-acquired pneumonia.11 Accordingly, individuals with severe COVID-19 were defined as confirmed SARS-CoV-2 infection plus 1 of the following conditions: respiratory rate higher than 30 breaths per minute, severe respiratory distress, or oxygen saturation less than 90% on room air. SARS-CoV-2 infection and noninfection were confirmed by high-throughput sequencing or real-time reverse transcriptase–polymerase chain reaction assays of nasal and pharyngeal swab specimens.
The following information was collected from medical records or a knowledgeable family member for each participant: demographic characteristics, including age, sex, education level (defined by the number of years of education), body mass index (BMI), and comorbidities, including hypertension (diagnosed according to the Joint National Committee on the Detection, Evaluation, and Treatment of High Blood Pressure guidelines12); type 2 diabetes (diagnosed following the guidelines of the American Diabetes Association13); hyperlipidemia, including hypertriglyceridemia and hypercholesteremia; coronary heart disease; stroke, including ischemic and hemorrhagic stroke as verified by brain imaging; and chronic obstructive pulmonary disease (COPD) (diagnosed following the Global Strategy for the Diagnosis, Management, and Prevention of COPD14).
Cognitive Assessment
Telephone interviews were conducted to assess cognition by a group of trained raters (L.-R.W., L.J., Y.Y., X.C., Y.L., Y.C). Current cognitive status was assessed using the Chinese version of the Telephone Interview of Cognitive Status-40 (TICS-40),9,15 which includes 10 variables and has a maximum of 40 points. A score of 20 or lower was considered indicative of mild cognitive impairment (MCI), and a score of 12 or lower was considered indicative of dementia.15
Longitudinal cognitive changes were assessed as follows. For the first 6 months after patient discharge, as preinfection cognitive status was not available, cognitive changes over this period were obtained from family informants using the Chinese version of the short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE),16 which contains 16 items that rate changes in memory and other cognitive domains.17 Cognitive decline was defined as a mean item score of 3.5 or higher.16 Cognitive changes over the second 6-month period postdischarge were assessed by changes in TICS-40 scores between 6 and 12 months. A decrease of 3 or more points was defined as clinically meaningful cognitive decline.18,19 The association between IQCODE and TICS-40 scores was analyzed to ensure the consistency of the 2 cognitive assessments verified in previous studies.20,21
Longitudinal cognitive changes were classified into 4 categories. Participants with stable cognition in both the first and second half of follow-up were categorized as having stable cognitive function. Participants with cognitive decline in the first half of follow-up but stable cognition in the second half were categorized as having early-onset cognitive decline. Participants without cognitive decline in the first half of follow-up but with cognitive decline in the second half of follow-up were categorized as having late-onset cognitive decline. Participants with cognitive decline in both the first and second half of follow-up were categorized as having progressive cognitive decline.
Statistical Analysis
The demographic and clinical characteristics and cognitive outcomes of participants were presented as medians and IQRs for continuous variables and absolute values and percentages for categorical variables. For the comparison of demographic and clinical characteristics among groups, Kruskal-Wallis test, χ2 test, Fisher exact test, and Mann-Whitney U test were used as appropriate. For paired comparisons between patients and spouses, McNemar test and Wilcoxon test were used where appropriate.
Linear mixed-effects models with a random slope and intercept for each participant were used to estimate the slope of decline in TICS-40 scores during follow-up, adjusting for age, sex, education level, BMI, and each comorbidity. Adjusted logistical regression models were used to investigate factors associated with risk of cognitive impairment at 12 months, with TICS-40 score of 20 or less as the dependent variable. Multinomial adjusted logistic regression models were used to explore factors associated with risk of longitudinal cognitive decline during follow-up, with early-onset cognitive decline, late-onset cognitive decline, and progressive cognitive decline as dependent variables. The analyses were conducted in a subgroup of paired patients and spouses using adjusted conditional logistical regression models. Statistical analyses were conducted using SPSS statistical package version 25 (IBM SPSS Statistics for Windows) and R version 3.6.2 (R Foundation for Statistical Computing). Tests were 2-tailed, and significance was set at P < .05.
Results
Demographic Characteristics of Participants
A total of 1438 COVID-19 survivors (691 male [48.05%] and 747 female [51.95%]; median [IQR] age, 69 [66-74] years) and 438 uninfected control individuals (222 male [50.68%] and 216 female [49.32%]; median [IQR] age, 67 [66-74] years) completed the 6-month and 12-month visits (Figure 1). Survivors included in this study had a higher proportion who received antiviral therapy (76.98% vs 73.43%; P = .02) and lower proportion who received ribavirin (0.63% vs 2.01%; P < .001) compared with those excluded from this study. No intergroup difference was found in other characteristics between survivors included and not included in this study (eTable 1 in the Supplement). Furthermore, no differences were found in the demographic characteristics between the 438 spouses who participated in this study and the 287 spouses who did not, indicating that the participants were representative of the whole cohort (eTable 2 in the Supplement).
Figure 1. Screening Flowchart.
Longitudinal cognitive decline was assessed using the Chinese version of the short form of the Informant Questionnaire on Cognitive Decline in the Elderly, current cognitive impairment using the Informant Questionnaire on Cognitive Decline in the Elderly and the Telephone Interview of Cognitive Status-40.
Among the participants who completed the 12-month follow-up, COVID-19 survivors were not different from control individuals in age, sex distribution, education level, BMI, and frequency of comorbidities, including hypertension, diabetes, hyperlipidemia, stroke, coronary heart disease, and COPD (Table 1). In the paired subgroup of 438 COVID-19 survivors and their uninfected spouses, survivors were older (median [IQR] age, 68 [65-78] vs 67 [66-74]; P < .001) and had a higher frequency of hypertension (47.03% vs 34.47%; P < .001) than their spouses (eTable 3 in the Supplement).
Table 1. Demographic and Baseline Information of Participants.
| Variable | COVID-19 survivors | Uninfected control individuals (n = 438) | P value survivors vs control individuals | P value severe vs nonsevere cases | ||
|---|---|---|---|---|---|---|
| Total group (n = 1438) | Severe cases (n = 260) | Nonsevere cases (n = 1178) | ||||
| Age, median (IQR), y | 69 (66-74) | 71 (67-79) | 68 (66-73) | 67 (66-74) | .30a | <.001a |
| Female, No. (%) | 747 (51.95) | 127 (48.85) | 621 (52.72) | 216 (49.32) | .35b | .27b |
| Male, No. (%) | 691 (48.05) | 133 (51.15) | 557 (47.28) | 222 (50.68) | .35b | .27b |
| Education, median (IQR), y | 12 (9-12) | 12 (6-12) | 12 (9-12) | 12 (9-12) | >.99a | .05a |
| BMI, median (IQR) | 23.99 (22.54-25.38) | 24.38 (22.90-25.64) | 23.93 (22.44-25.33) | 24.19 (22.51-25.69) | >.99a | .009a |
| Comorbidities, No. (%) | ||||||
| Hypertension | 561 (39.01) | 133 (51.15) | 426 (36.16) | 151 (34.47) | .09b | <.001b |
| Diabetes | 274 (19.05) | 65 (25.00) | 208 (17.66) | 81 (18.49) | .84b | .01b |
| Hyperlipidemia | 142 (9.87) | 31 (11.92) | 111 (9.42) | 39 (8.90) | .58b | .25b |
| Stroke history | 79 (5.49) | 42 (16.15) | 37 (3.14) | 30 (6.85) | .29b | <.001b |
| Coronary heart disease | 193 (13.42) | 71 (27.31) | 121 (10.27) | 61 (13.93) | .81b | <.001b |
| COPD | 142 (9.87) | 43 (16.38) | 99 (8.40) | 41 (9.36) | .78b | <.001b |
| ICU admission, No. (%) | 72 (5.01) | 72 (27.69) | 0 | NA | NA | <.001b |
| Mechanical ventilation, No. (%) | 83 (5.77) | 83 (31.92) | 0 | NA | NA | <.001b |
| High-flow oxygen therapy, No. (%) | 290 (20.17) | 106 (40.77) | 184 (15.62) | NA | NA | <.001b |
| Delirium, No. (%) | 92 (6.40) | 82 (31.54) | 10 (0.85) | NA | NA | <.001b |
| Length of hospital stay (IQR), d | 20 (15-25) | 28 (22-34) | 19 (14-23) | NA | NA | <.001a |
| Antiviral therapy, No. (%) | 1107 (76.98) | 209 (80.38) | 898 (76.23) | NA | NA | .17b |
| Lianhua qingwen | 703 (48.89) | 136 (52.31) | 567 (48.13) | NA | NA | .24b |
| Arbidol | 530 (36.86) | 106 (40.77) | 424 (35.99) | NA | NA | .16b |
| Kaletra | 125 (8.69) | 28 (10.77) | 97 (8.23) | NA | NA | .18b |
| Oseltamivir | 52 (3.62) | 10 (3.85) | 42 (3.57) | NA | NA | .85b |
| Ribavirin | 9 (0.63) | 2 (0.77) | 7 (0.59) | NA | NA | .67b |
| Other antiviral drugsc | 20 (1.39) | 3 (1.15) | 17 (1.44) | NA | NA | >.99b |
| Antibacterial therapy, No. (%) | 274 (19.05) | 143 (55.00) | 131 (11.12) | NA | NA | <.001b |
| IVIg treatment, No. (%) | 165 (11.47) | 143 (55.00) | 22 (1.87) | NA | NA | <.001b |
| Glucocorticoid, No. (%) | 296 (20.58) | 144 (55.38) | 152 (12.90) | NA | NA | <.001b |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IVIg, intravenous immunoglobulin; NA, not applicable.
Mann-Whitney U test.
Pearson χ2 test.
Other antiviral drugs included chloroquine phosphate, hydroxychloroquine, and ritonavir.
Compared with individuals with nonsevere cases, individuals with severe cases were older and had a lower education level; higher BMI; a greater number of comorbidities, including hypertension (51.15% vs 47.28%; P < .001), diabetes (25.00% vs 17.66%; P = .01), stroke (16.15% vs 3.14%; P < .001), coronary heart disease (27.31% vs 10.27%; P < .001), and COPD (16.38% vs 8.40%; P < .001); higher frequencies of intensive care unit admission (27.69% vs 0%; P < .001), mechanical ventilation (31.92% vs 0%; P < .001), high-flow oxygen therapy (40.77% vs 15.62%; P < .001), and delirium during hospitalization (31.54% vs 0.85%; P < .001); and a longer length of hospital stay (median [IQR] stay, 28 [22-34] days vs 19 [14-23] days; P < .001). Severe cases were more frequent than nonsevere cases in individuals receiving antibacterial therapy (55.00% vs 11.12%; P < .001), intravenous immunoglobulin treatment (55.00% vs 1.87%; P < .001), and glucocorticoid treatment (55.38% vs 12.90%; P < .001) (Table 1).
Cognitive Impairment 12 Months After Discharge
COVID-19 survivors had lower TICS-40 scores than control individuals at both 6 months (median [IQR], 29 [24-32] vs 30 [26-33]; P < .001) and 12 months (median [IQR], 29 [24-32] vs 31 [26-33]; P < .001) after patient discharge. Individuals with severe cases had lower TICS-40 scores (indicating worse cognition) than those with nonsevere cases (median [IQR], 24.00 [18.25-29.00] vs 30.00 [26.00-33.00]; P < .001) and control individuals (median [IQR], 24.00 [18.25-29.00] vs 30.00 [26.00-33.25]; P < .001) at 6 months. Individuals with severe cases also had lower TICS-40 scores than those with nonsevere cases (median [IQR] severe, 22.50 [16.00-28.00] vs nonsevere, 30.00 [26.00-33.00]; P < .001) and control individuals (median [IQR] severe, 22.50 [16.00-28.00] vs control, 31.00 [26.00-33.00]; P < .001) at 12 months. Individuals with nonsevere cases and control individuals differed in IQCODE scores but not in TICS-40 scores during follow-up (Figure 2A, B, and C). The overall incidence of cognitive impairment in survivors 12 months after discharge was 12.45%. Among individuals with severe cases, 26 (10.00%) had dementia and 69 (26.54%) had MCI at 6 months. The numbers increased to 39 (15.00%) for dementia and remained at 68 (26.15%) for MCI at 12 months, which were higher than in those with nonsevere cases (dementia, 9 [0.76%], P < .001 and MCI, 63 [5.35%]; P < .001) and control individuals (dementia, 3 [0.68%]; P < .001; MCI, 22 [5.02%]; P < .001). Survivors of nonsevere COVID-19 and control individuals had comparable frequencies of dementia and MCI at both 6 and 12 months (Figure 2D and E).
Figure 2. Cognitive Trajectory of Patients With Severe and Nonsevere COVID-19 and Control Individuals During 1-Year Follow-up.

Comparison of Telephone Interview of Cognitive Status-40 (TICS-40) scores among severe COVID-19 survivors, nonsevere COVID-19 survivors, and uninfected control individuals at 6 and 12 months was calculated using Wilcoxon (Mann-Whitney U) test. Proportions of participants with different cognitive statuses at 6 and 12 months were calculated using χ2 test. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) scores ≥3.5 were considered indicative of cognitive decline. A decrease of ≥3 points on the TICS-40 from baseline during follow-up was considered indicative of clinically meaningful cognitive decline. H, Values adjusted for age, sex, education level, body mass index, and each comorbidity (Table 1) using linear mixed-effects models. Normalization of data was performed using minimum-maximum normalization.
aDifference in mild cognitive impairment.
bDifference in dementia.
Severe COVID-19 (OR, 9.10; 95% CI, 5.61-14.75), but not nonsevere COVID-19 (OR, 1.10; 95% CI, 0.69-1.76), was associated with a higher risk of cognitive impairment at 12 months, adjusting for age, sex, education level, BMI, and comorbidities (Figure 3A). In the paired subgroup of patients and their spouses, both nonsevere (OR, 1.81; 95% CI, 1.09-3.00) and severe COVID-19 (OR, 5.91; 95% CI, 3.57-9.80) were associated with a higher risk of cognitive impairment at 12 months, adjusting for age, sex, education level, BMI, and comorbidities (eFigure in the Supplement).
Figure 3. Factors Associated With Risk of Longitudinal Cognitive Decline in the Total Cohort.
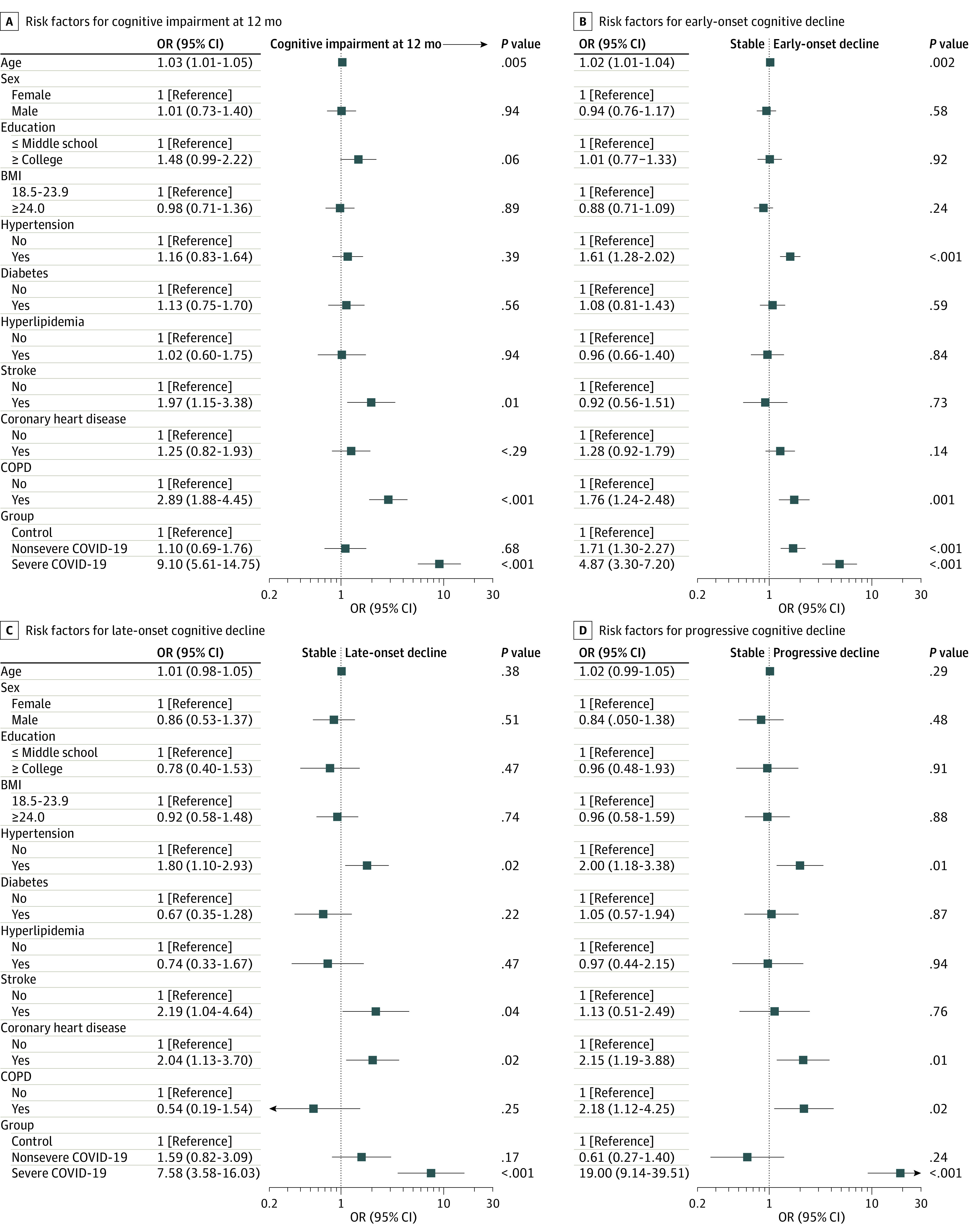
A, Cognitive impairment was defined by a Telephone Interview of Cognitive Status-40 score ≤20. In the investigation of risk factors for cognitive impairment at 12 months after patient discharge, ordinary logistic regression models were used. In the investigation of risk factors for longitudinal cognitive decline, multinomial regression models were used. All factors (including age, sex, education level, body mass index [BMI], and each comorbidity) different from the one examined were adjusted in the regression models. Each comorbidity was adjusted as an independent variable without accumulation. COPD indicates chronic obstructive pulmonary disease.
Longitudinal Cognitive Change During Follow-up
The IQCODE score (higher score indicates larger longitudinal cognitive decline) at 6 months’ follow-up was higher in individuals with severe cases than in those with nonsevere cases (median [IQR], 3.63 [3.15-4.36] vs 3.18 [3.00-3.56]; P < .001) and control individuals (median [IQR], 3.63 [3.15-4.36] vs 3.06 [3.00-3.38]; P < .001). Moreover, individuals with nonsevere cases also had higher IQCODE scores than control individuals (median [IQR], 3.18 [3.00-3.56] vs 3.06 [3.00-3.38]; P < .001) (Figure 2C). Specifically, 158 individuals with severe cases, 340 individuals with nonsevere cases, and 92 control participants reported cognitive decline within the first 6 months (as reflected by an IQCODE score of 3.5 or higher). The proportion of participants with longitudinal cognitive decline during the first 6 months was higher among those with severe cases than those with nonsevere cases (60.77% vs 28.86%; P < .001) and control individuals (60.77% vs 21.00%; P < .001). Individuals with nonsevere cases also had a higher proportion of participants with cognitive decline than control individuals during the first 6 months (28.86% vs 21.00%; P < .001) (Figure 2F).
In the second 6 months, individuals with severe cases had a higher proportion of participants with cognitive decline than individuals with nonsevere cases (80 [30.77%] vs 56 [4.75%]; P < .001) and control individuals (80 [30.77%] vs 23 [5.25%]; P < .001) (Figure 2G). Moreover, individuals with severe cases had a higher speed of cognitive decline than those with nonsevere cases (slope, −0.039; 95% CI, −0.047 to −0.032 vs slope, −0.0003; 95% CI, −0.004 to 0.003; P < .001) and control individuals (slope, −0.039; 95% CI, −0.047 to −0.032 vs slope, 0.002; 95% CI, −0.004 to 0.007; P < .001); however, no difference in speed of cognitive decline was found between individuals with nonsevere cases and control individuals (slope, −0.0003; 95% CI, −0.004 to −0.003 vs slope, 0.002; 95% CI, −0.004 to 0.007; P = .09) (Figure 2H).
Compared with individuals with nonsevere cases and control individuals, individuals with severe cases more frequently experienced early-onset cognitive decline (severe: 39.62%, nonsevere: 27.67%, control: 18.49%), late-onset cognitive decline (severe: 9.62%, nonsevere: 3.57%, control: 2.74%), and progressive cognitive decline (severe: 21.15%, nonsevere: 1.19%, control: 2.28%), while individuals with nonsevere cases more frequently experienced early-onset cognitive decline than control individuals (27.67% vs 18.49%). Noninfected control individuals more frequently experienced stable cognitive function than participants with nonsevere cases (76.48% vs 67.57%) and those with severe cases (76.48% vs 29.62%) (Table 2).
Table 2. Cognitive Trajectory of COVID-19 Survivors and Uninfected Control Individuals.
| Category | Changes in cognitiona | No. (%) of participants | P valueb | |||||
|---|---|---|---|---|---|---|---|---|
| 0-6 mo | 6-12 mo | Severe | Nonsevere | Control | Severe vs nonsevere | Severe vs control | Nonsevere vs control | |
| Stable cognitive function | Stable | Stable | 77 (29.62) | 796 (67.57) | 335 (76.48) | <.001 | <.001 | <.001 |
| Early-onset cognitive decline | Declined | Stable | 103 (39.62) | 326 (27.67) | 81 (18.49) | <.001 | <.001 | <.001 |
| Late-onset cognitive decline | Stable | Declined | 25 (9.62) | 42 (3.57) | 12 (2.74) | <.001 | <.001 | .53 |
| Progressive cognitive decline | Declined | Declined | 55 (21.15) | 14 (1.19) | 10 (2.28) | <.001 | <.001 | .11 |
Change in cognition from SARS-CoV-2 infection to 6 months of recovery was determined by the Chinese version of the short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE). An IQCODE score ≥ 3.5 indicates decreased cognition. An IQCODE score <3.5 indicates stable or improved cognition. Change in cognition from 6 months to 12 months of recovery was determined by the IQCODE and the Telephone Interview of Cognitive Status-40 (TICS-40). A decreased TICS-40 score indicates decreased cognition. An unchanged or increased TICS-40 score indicates stable and improved cognition, respectively.
Comparisons between groups: Pearson χ2 test.
In the total cohort, nonsevere COVID-19 was associated with a higher risk of early-onset cognitive decline (OR, 1.71; 95% CI, 1.30-2.27), while severe COVID-19 was associated with a higher risk of early-onset cognitive decline (OR, 4.87; 95% CI, 3.30-7.20), late-onset cognitive decline (OR, 7.58; 95% CI, 3.58-16.03), and progressive cognitive decline (OR, 19.00; 95% CI, 9.14-39.51), adjusting for age, sex, education level, BMI, and comorbidities (Figure 3B-D).
In the paired subgroup, nonsevere COVID-19 was associated with an increase in risk of both early-onset cognitive decline (OR, 1.41; 95% CI, 1.04-1.90) and late-onset cognitive decline (OR, 3.40; 95% CI, 1.71-6.73), while severe COVID-19 was associated with an increase in risk of early-onset cognitive decline (OR, 2.23; 95% CI, 1.53-3.24), late-onset cognitive decline (OR, 4.70; 95% CI, 2.17-10.20), and progressive cognitive decline (OR, 4.87; 95% CI, 2.10-11.29), adjusting for age, sex, education, BMI, and comorbidities (eFigure in the Supplement).
Discussion
Postinfection cognitive outcomes following COVID-19 have been reported but the long-term dynamic trajectory of cognitive changes in COVID-19 survivors remains unclear. Earlier pandemics have provided evidence showing the adverse effects of severe respiratory diseases on cognitive functions. Approximately 15% of patients infected with severe acute respiratory syndrome or Middle East respiratory syndrome showed long-term cognitive deficits, such as memory and attention impairment.22 With the increasing number of patients who survive COVID-19, the cognitive sequelae of this disease have attracted much attention.23 Recent studies found that COVID-19 was associated with an increase in risk of being diagnosed with dementia within 6 months after infection.4,24 Consistent with this, we found that approximately 3.3% of COVID-19 survivors had dementia and 9.1% had MCI at 12 months after discharge; in particular, the incidences of dementia and MCI were 15.00% and 26.15% in individuals with severe cases, respectively. The incidence of dementia or MCI was not different between individuals with nonsevere cases and uninfected control individuals. These findings suggest that COVID-19, especially severe COVID-19, may be associated with long-term cognitive impairment.
In addition to several cross-sectional studies showing that SARS-CoV-2 infection is associated with an increase in risk of cognitive impairment,8,23,25,26 our study added novel information about the dynamic change in the cognition of COVID-19 survivors. In our cohort, severe COVID-19 was associated with an increase in risk of early-onset, late-onset, and progressive cognitive decline, while nonsevere COVID-19 was associated with an increase in risk of early-onset cognitive decline, with adjustment for age and comorbidities, which are well-recognized risk factors for cognitive impairment,27,28,29 suggesting that SARS-CoV-2 infection may be associated with further risk of longitudinal cognitive decline beyond these confounding factors. It is worth noting that 21% of individuals with severe cases in this cohort experienced progressive cognitive decline, suggesting that COVID-19 may cause long-lasting damage to cognition. These findings imply that the pandemic may substantially contribute to the world dementia burden in the future.
The mechanisms of the long-term effects of COVID-19 on cognition are multifaceted. First, neurovascular elements might be involved in the development of postinfection cognitive decline in COVID-19 survivors,30,31 as reinforced by our findings that vascular risk factors, such as stroke, coronary heart disease, and hypertension, were associated with longitudinal cognitive decline. In this study, nonsevere COVID-19 was associated with cognitive impairment at 12 months and late-onset cognitive decline in the paired cohort, but not in the whole cohort. This discrepancy might be attributed to higher frequencies of hypertension and stroke history in survivors in the paired cohort in comparison with other survivors. Second, long-lasting hypoxia may also contribute substantially to postinfection cognitive decline, as neurons are sensitive to hypoxic injury, and individuals with severe cases may be under a more severe hypoxic status after infection than those with nonsevere cases.32,33 This hypothesis is supported by our finding that COPD was associated with an increase in risk of longitudinal cognitive decline. Third, inflammatory factors have been shown to not return to normal status months after recovery, especially in individuals with severe COVID-19.34 Chronic systemic inflammation after SARS-CoV-2 infection exacerbates neurodegeneration, thus potentially leading to long-term cognitive deficits.35 This notion is supported by the finding that neurodegenerative biomarkers were increased in COVID-19 survivors.36,37 COVID-19–associated microglia and astrocyte subpopulations share features with pathological cell states seen in neurodegenerative disease.38,39 It is also possible that the virus can directly invade the brain and damage neurons.40
Limitations
This study has some limitations. First, owing to the emerging infection risk, telephone questionnaires were used to follow up on the cognitive functions of participants. This method of follow-up may not be as accurate as face-to-face interviews, although telephone-based questionnaires have been validated.9,15,17 Second, the lack of cognitive information before SARS-CoV-2 infection is an inherent limitation of this study that may lead to an overestimation of the impact of COVID-19 on postinfection cognitive decline. As cognitive decline might be affected by both preexisting cognitive impairment and COVID-19, we excluded participants with known preexisting cognitive impairment and a family history of dementia. However, this reduced the generalizability of the findings. Furthermore, this study lacks information about biomarkers of neuronal injury; thus, the etiology of cognitive decline could not be determined. In addition, the mismatch of sample sizes between survivors and control individuals and the relatively high rate of loss-to-follow-up weakened the power of the findings.
Conclusions
In this cohort study of COVID-19 survivors 60 years and older who were discharged from COVID-19–designated hospitals in Wuhan, China, SARS-CoV-2 infection, especially severe infection, was associated with an increase in risk of longitudinal cognitive decline. The findings highlight the importance of immediate measures to deal with this challenge.
eTable 1. Demographic data of subjects who completed and did not complete the 12-month follow-up
eTable 2. Demographic data of spouses who participated and did not participate in this study
eTable 3. Pairwise comparison of clinical characteristics of COVID-19 patients and uninfected spouses
eFigure. Risk factors associated with longitudinal cognitive decline in COVID-19 survivors and spouses
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Accessed February 21, 2022. https://covid19.who.int/
- 2.Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. doi: 10.1016/j.lanepe.2021.100122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akbarialiabad H, Taghrir MH, Abdollahi A, et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49(6):1163-1186. doi: 10.1007/s15010-021-01666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8(2):130-140. doi: 10.1016/S2215-0366(20)30462-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531-542. doi: 10.1016/j.bbi.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi: 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144(4):1263-1276. doi: 10.1093/brain/awab009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampshire A, Trender W, Chamberlain SR, et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine. 2021;39:101044. doi: 10.1016/j.eclinm.2021.101044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YH, Wang YR, Wang QH, et al. Post-infection cognitive impairments in a cohort of elderly patients with COVID-19. Mol Neurodegener. 2021;16(1):48. doi: 10.1186/s13024-021-00469-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance, 28 January 2020. Accessed February 1, 2020. https://apps.who.int/iris/handle/10665/330893
- 11.Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association . Classification and diagnosis of diabetes. Diabetes Care. 2016;39(suppl 1):S13-S22. doi: 10.2337/dc16-S005 [DOI] [PubMed] [Google Scholar]
- 14.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557-582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 15.Fong TG, Fearing MA, Jones RN, et al. Telephone interview for cognitive status: creating a crosswalk with the mini-mental state examination. Alzheimers Dement. 2009;5(6):492-497. doi: 10.1016/j.jalz.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuh JL, Teng EL, Lin KN, et al. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening tool for dementia for a predominantly illiterate Chinese population. Neurology. 1995;45(1):92-96. doi: 10.1212/WNL.45.1.92 [DOI] [PubMed] [Google Scholar]
- 17.Mok VC, Wong A, Lam WW, et al. Cognitive impairment and functional outcome after stroke associated with small vessel disease. J Neurol Neurosurg Psychiatry. 2004;75(4):560-566. doi: 10.1136/jnnp.2003.015107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson C, Teo K, Gao P, et al. ; ONTARGET and TRANSCEND Investigators . Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10(1):43-53. doi: 10.1016/S1474-4422(10)70250-7 [DOI] [PubMed] [Google Scholar]
- 19.Diener HC, Sacco RL, Yusuf S, et al. ; Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) study group . Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PROFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7(10):875-884. doi: 10.1016/S1474-4422(08)70198-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton L, Tyson SF. Screening for cognitive impairment after stroke: a systematic review of psychometric properties and clinical utility. J Rehabil Med. 2015;47(3):193-203. doi: 10.2340/16501977-1930 [DOI] [PubMed] [Google Scholar]
- 21.Pratt LA, Weeks JD, Goulding MR. Measures of cognitive functioning in the 1994-2000 second longitudinal study of aging. Natl Health Stat Report. 2008;(2):1-15. [PubMed] [Google Scholar]
- 22.Rabinovitz B, Jaywant A, Fridman CB. Neuropsychological functioning in severe acute respiratory disorders caused by the coronavirus: implications for the current COVID-19 pandemic. Clin Neuropsychol. 2020;34(7-8):1453-1479. doi: 10.1080/13854046.2020.1803408 [DOI] [PubMed] [Google Scholar]
- 23.Miskowiak KW, Johnsen S, Sattler SM, et al. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39-48. doi: 10.1016/j.euroneuro.2021.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416-427. doi: 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattioli F, Stampatori C, Righetti F, Sala E, Tomasi C, De Palma G. Neurological and cognitive sequelae of COVID-19: a four month follow-up. J Neurol. 2021;268(12):4422-4428. doi: 10.1007/s00415-021-10579-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hugon J, Msika EF, Queneau M, Farid K, Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. 2022;269(1):44-46. doi: 10.1007/s00415-021-10655-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang YL, Fennema-Notestine C, Holland D, et al. ; Alzheimer’s Disease Neuroimaging Initiative . APOE interacts with age to modify rate of decline in cognitive and brain changes in Alzheimer’s disease. Alzheimers Dement. 2014;10(3):336-348. doi: 10.1016/j.jalz.2013.05.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh B, Mielke MM, Parsaik AK, et al. A prospective study of chronic obstructive pulmonary disease and the risk for mild cognitive impairment. JAMA Neurol. 2014;71(5):581-588. doi: 10.1001/jamaneurol.2014.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zlokovic BV, Gottesman RF, Bernstein KE, et al. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 2020;16(12):1714-1733. doi: 10.1002/alz.12157 [DOI] [PubMed] [Google Scholar]
- 30.Qin Y, Wu J, Chen T, et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021;131(8):147329. doi: 10.1172/JCI147329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kas A, Soret M, Pyatigoskaya N, et al. ; on the behalf of CoCo-Neurosciences study group and COVID SMIT PSL study group . The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur J Nucl Med Mol Imaging. 2021;48(8):2543-2557. doi: 10.1007/s00259-020-05178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon JJ, Heyman B, Ko JP, Condos R, Lynch DA. CT of postacute lung complications of COVID-19. Radiology. 2021;301(2):211396. doi: 10.1148/radiol.2021211396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747-758. doi: 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou M, Yin Z, Xu J, et al. Inflammatory profiles and clinical features of coronavirus 2019 survivors 3 months after discharge in Wuhan, China. J Infect Dis. 2021;224(9):1473-1488. doi: 10.1093/infdis/jiab181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77(11):1-7. doi: 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prudencio M, Erben Y, Marquez CP, et al. Serum neurofilament light protein correlates with unfavorable clinical outcomes in hospitalized patients with COVID-19. Sci Transl Med. 2021;13(602):eabi7643. doi: 10.1126/scitranslmed.abi7643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Virhammar J, Nääs A, Fällmar D, et al. Biomarkers for central nervous system injury in cerebrospinal fluid are elevated in COVID-19 and associated with neurological symptoms and disease severity. Eur J Neurol. 2021;28(10):3324-3331. doi: 10.1111/ene.14703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang AC, Kern F, Losada PM, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595(7868):565-571. doi: 10.1038/s41586-021-03710-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keren-Shaul H, Spinrad A, Weiner A, et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169(7):1276-1290.e17. doi: 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 40.Song E, Zhang C, Israelow B, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218(3):e20202135. doi: 10.1084/jem.20202135 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Demographic data of subjects who completed and did not complete the 12-month follow-up
eTable 2. Demographic data of spouses who participated and did not participate in this study
eTable 3. Pairwise comparison of clinical characteristics of COVID-19 patients and uninfected spouses
eFigure. Risk factors associated with longitudinal cognitive decline in COVID-19 survivors and spouses



