Abstract
Objectives
A post-lingually implanted adult typically develops hearing with an intact auditory system, followed by periods of deafness (or near deafness) and adaptation to the implant. For an early implanted child whose brain is highly plastic, the auditory system matures with consistent input from a cochlear implant. It is likely that the auditory system of early implanted cochlear implant users is fundamentally different than post-lingually implanted adults. The purpose of this study is to compare the basic psychophysical capabilities and limitations of these two populations on a spectral resolution task to determine potential effects of early deprivation and plasticity.
Design
Performance on a spectral resolution task (SMRT) was measured for 20 bilaterally implanted, prelingually deafened children (between 5 and 13 years of age) and 20 hearing children within the same age range. Additionally, 15 bilaterally implanted, post-lingually deafened adults and 10 hearing adults were tested on the same task. Cochlear implant users (adults and children) were tested bilaterally, and with each ear alone. Hearing listeners (adults and children) were tested with the unprocessed SMRT and with a vocoded version that simulates an 8-channel cochlear implant.
Results
For children with normal hearing, a positive correlation was found between age and SMRT score for both the unprocessed and vocoded versions. Older hearing children performed similarly to hearing adults in both the unprocessed and vocoded test conditions. However, for children with cochlear implants, no significant relationship was found between SMRT score and chronological age, age at implantation, or years of implant experience. Performance by children with cochlear implants was poorer than performance by cochlear implanted adults. It was also found that children implanted sequentially tended to have better scores with the first implant compared to the second implant. This difference was not observed for adults. An additional finding was that SMRT score was negatively correlated with age for adults with implants.
Conclusions
Results from this study suggest that basic psychophysical capabilities of early implanted children and post-lingually implanted adults differ when assessed in the sound field using their personal implant processors. Because spectral resolution does not improve with age for early implanted children, it seems likely that the sparse representation of the signal provided by a cochlear implant limits spectral resolution development. These results are supported by the finding that post-lingually implanted adults, whose auditory systems matured prior to the onset of hearing loss, perform significantly better than early implanted children on the spectral resolution test.
Keywords: spectral resolution, normal hearing, cochlear implant, children, auditory development
1. Introduction
Present day outcomes with cochlear implants have greatly exceeded early expectations for adults and children with severe to profound hearing loss. Originally, cochlear implants were designed with the intention of providing a useful cue to aid lip reading for post-lingually deafened individuals (e.g. Bilger and Black, 1977). Now, many cochlear implant users are able to conduct normal conversations in quiet environments and even successfully communicate on the telephone. Predicted benefits of a cochlear implant for listeners with severe to profound hearing loss are now great enough to justify implanting ears with significant residual hearing (e.g. Vermeire et al., 2008; Dorman and Gifford, 2010; Turner et al., 2010).
Advancements in sound processing strategies have been partially responsible for improvements in outcomes with implants. One early advance in cochlear implant processing was the transition from compressed analog to continuous interleaved sampling (CIS) developed by Wilson et al. (1991). Another early advance came when switching from a feature extraction algorithm (such as F0/F2, F0/F1/F2, and MPEAK) to one that encoded spectral information without feature extraction, such as SMSP and SPEAK (McKay et al., 1992; McDermott et al., 1992). More recent advances in coding include the addition of virtual channels to increase the number of sites of stimulation (e.g. Buechner et al., 2008), enhancing of temporal cues (e.g. Laneau et al., 2004; Vandali et al., 2005; Arnoldner et al., 2007), improving the algorithms for peak picking (e.g. Nogueira et al., 2005), and reducing the spread of excitation with current focusing (e.g. Landsberger et al., 2012; Srinivasan et al., 2013; Bierer and Litvak, 2016). Although all of these improvements have been developed and validated in post-lingually deafened adults, many of these improvements have been provided clinically to pre-lingually deaf children worldwide without equivalent validation. This is particularly the case in the United States, where the U.S. Food and Drug Administration (FDA) requires formal trials for new strategies. These trials are laborious and time consuming to implement with adults and virtually non-existent with children. Accordingly, many of these new strategies have not been studied formally in implanted children in the United States although many clinics provide newer sound processing strategies for children, which is designated as “off-label”.
Children born with severe to profound hearing loss may require different sound processing strategies than adults to optimize auditory and spoken language performance and development. Typically, a post-lingually implanted adult is born with an intact auditory system, followed by periods of deafness (or near deafness) and adaptation to the implant. As a result, that individual’s auditory system has had to adapt and relearn to interpret auditory input at three different stages. For an early implanted child whose brain is highly plastic, the auditory system matures with consistent input from a cochlear implant. Thus, it is possible that an individual whose entire auditory experience comes from an implant might derive greater benefit from the implant than a person born with normal hearing and later deafened. Alternatively, an auditory system that matures using the relatively sparse input from the cochlear implant may not be as robust as an intact auditory system. It is therefore likely that the auditory system of pre-lingually implanted cochlear implant users is fundamentally different than that of a post-lingually implanted adult. Furthermore, the limitations and bottlenecks for these two populations are likely to be different. Stimulation strategies and fitting procedures developed for post-lingually implanted adults may not be optimal for pre-lingually implanted users. It might be important to program cochlear implants differently for children than adults, or perhaps even develop different algorithms.
Measuring basic psychophysical properties of early implanted children and late implanted adults seems a viable approach for gaining insight to the fundamental capabilities of the different populations. Spectral resolution measures might provide particularly interesting data because psychophysical measures of spectral resolution can be made without confounds of linguistic development. Nevertheless, results on tests of spectral resolution have correlated with speech recognition scores (e.g. Henry et al., 2005; Won et al., 2007; Gifford et al., 2014; Holden et al., 2016). Despite these findings, the role of spectral resolution in the development of normal hearing or hearing loss is not well understood. Eisenberg et al. (2000) found that when degrading spectral information through a vocoder, children with normal hearing between 10 and 12 years of age perform similarly to adults on measures of speech perception, but children between 5 and 7 years of age require more spectral information to produce equivalent levels of understanding. Kirby et al. (2015) tested spectral resolution using the Spectral-temporally Modulated Ripple Test (SMRT; Aronoff and Landsberger, 2013) on children with normal hearing (aged 6–12 years) and children using hearing aids (aged 6–16 years) and found that spectral resolution performance gradually increased as a function of age for both groups. Spectral resolution outcomes for the listeners with hearing loss were lower (i.e., poorer) than for the listeners with normal hearing.
Another potential difference between early implanted children and late implanted adults may be how they use information from two ears. Cochlear implants have variable insertions (e.g. Landsberger et al., 2015) and frequently are perceived as perceptually misaligned (e.g. Aronoff et al., 2016). This misalignment might cause spectral interference between ears (e.g. Aronoff et al., 2015) and therefore, when both implants are combined, spectral resolution scores may be reduced in post-lingually deafened adults whose auditory systems developed around spectrally aligned inputs for the second implanted ear. As the auditory system of an early implanted child develops in response to the misaligned auditory inputs, he or she may have an advantage over adults in combining the misaligned spectral content.
An additional consideration is the duration between first and second implant surgeries. The auditory system will typically adapt to input from the first implant, but when a second implant is received, the auditory system will have to readapt to bilateral input. However, the two ears are rarely equivalent (e.g. different hearing losses and different neural survivals) and therefore would have unequal expected outcomes. To complicate matters, the choice of which ear to implant first is rarely random. Often, the first ear implanted is the poorer hearing ear because there is less risk associated with insertion trauma.
In the present experiment, we examined spectral resolution using the SMRT for children (5 to 13 years of age) and adults (18 years old and above) with normal hearing and with bilateral cochlear implants. The primary hypothesis was that pre-lingually implanted children would demonstrate poorer spectral-temporal ripple discrimination than children with normal hearing, adults with normal hearing, and post-lingually implanted adults. Children and adults with normal hearing were further evaluated with a vocoded version of the SMRT to determine the effect of reduced spectral information on an otherwise normal auditory system. This simulation was of particular interest for the children whose auditory systems developed normally but were evaluated with reduced spectral information. A secondary hypothesis was that sequentially implanted children, but not adults, would demonstrate better spectral resolution with their first implant than their second implant. A tertiary hypothesis was that children would derive greater benefit using bilateral stimulation than adults on the SMRT.
2. Methods
2.1. Subjects
Subjects were stratified by age group (adults and children) and hearing status (normal hearing and bilateral cochlear implants). A total of 24 adults and 44 children were enrolled at the University of Southern California (USC) and an additional 3 adults were enrolled at New York University (NYU). Of the 71 participants, data from 25 adults and 40 children were included in the final data set. Two adults and one child with normal hearing did not pass the audiological criterion (20 dB HL pure-tone thresholds between 250 and 8000 Hz) and were excluded. Data for two children with normal hearing (ages 9.6 years and 7.9 years) and one child with bilateral cochlear implants (age 6 years) were not included in the final analysis due to distractibility, and were considered outliers. Replacements were found for these three children. The adult subjects were comprised of 10 listeners with normal hearing (mean age 34.8 years, range 18 – 59 years) and 15 listeners with cochlear implants (mean age 59.9 years, range 31 – 75 years). The pediatric subjects consisted of 20 children with normal hearing (mean age: 9.0 years, range 6.3 – 12.6 years) and 20 bilateral cochlear implant users (mean age: 9.2 years, range 5.8 – 13.1 years). The average duration between implantations for the adult implant users was 4.13. years (range 0 – 12 years) and for the pediatric implant users was 1.95 years (range 0 – 5 years). Although the average duration between implantations was larger for adults than children, no significant difference between the delay in second implantation was detected (Mann-Whitney U = 110, nadults = 15, nchildren = 20, p = 0.185). Only bilateral cochlear implant users were recruited so that all subjects could be tested with both ears in the sound field. For comparison purposes, individual ears were also assessed for the subjects with cochlear implants.
All adult subjects were consented according to either the USC or NYU Institutional Review Board (IRB). Parents of the pediatric subjects were consented for their children. Additionally, subjects aged 7–14 years signed an assent form as recommended by the USC IRB. Children younger than 7 years did not sign an assent form. Instead, the experimenter explained the experimental procedures and had them confirm verbally that they understood. A parent or legal representative signed the consent form to authorize participation of children as subjects. Subjects were compensated for their time. Consent, hearing screening for subjects with normal hearing, and testing on the experimental protocol required less than one hour of the subject’s time. Demographic information is presented in Table 1 for the adults and children with cochlear implants.
Table 1.
Demographic information for each of the cochlear implant users is presented. Unless otherwise specified, all implant users had the same strategy and processor model on each ear. Access to the full patient map was unavailable for CA09 and as such could not verify the electrode array models for the subject.
| ID | Device | Newborn screening Pass/Fail |
Etiology | Processor | Processing Strategy |
Implant/ Electrode R |
Implant/ Electrode L |
Age at Test (yrs) |
Age CI R (yrs) |
Age CI L (yrs) |
Yrs CI R |
Yrs CI L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP01 | Cochlear | Pass | Prematurity | N5 | ACE 900 | CI24R (CA) | CI24R (CA) | 12.3 | 1.3 | 1.3 | 11 | 11 |
| CP02 | Cochlear | Fail | Likely genetic | N6 | ACE 900 | CI24RE (CA) | CI512 | 8.9 | 1.1 | 3.5 | 7.8 | 5.4 |
| CP03 | Cochlear | Fail | Likely genetic | N6 | ACE 900 | CI24RE (CA) | CI24RE (CA) | 12.4 | 2.3 | 8.8 | 10.1 | 3.6 |
| CP04 | Cochlear | Fail | Unknown | N5 | ACE 900 | CI512 | CI512 | 6.0 | 1.7 | 1.7 | 4.3 | 4.3 |
| CP05 | Cochlear | Fail | Auditory Neuropathy | N6 | ACE 900 | CI24RE (CA) | CI512 | 7.8 | 1 | 2 | 6.8 | 5.8 |
| CP06 | Cochlear | Pass | Auditory Neuropathy | N6 | ACE 900 | CI24RE (CA) | CI512 | 12.3 | 2 | 7 | 10.3 | 5.3 |
| CP07 | Cochlear | Fail | Unknown | N5 | ACE 900 | CI512 | CI512 | 6.4 | 1.3 | 1.3 | 5.1 | 5.1 |
| CP08 | Cochlear | Pass | Genetic | N6 | ACE 900 | CI512 | CI24RE (CA) | 10.8 | 5.4 | 1.3 | 5.4 | 9.5 |
| CP09 | Cochlear | Wasn’t tested | Genetic | N6 | ACE 900 | CI512 | CI24RE (CA) | 13.1 | 7.4 | 3 | 5.7 | 10.1 |
| CP10 | Cochlear | Pass | Bilateral EVA | N6 | ACE 900 | CI24RE (CA) | CI24RE (CA) | 5.8 | 5 | 5.3 | 0.8 | 0.3 |
| CP11 | Cochlear | Fail | Waardenberg | N5 | ACE 900 | CI512 | CI512 | 7.0 | 1 | 1 | 6 | 6 |
| CP12 | Cochlear | Fail | Jervel-Lange Nielsen Syndrome | N5 | ACE 900 | CI512 | CI512 | 7.1 | 1.1 | 1.1 | 6 | 6 |
| CP13 | Cochlear | Fail | Waardenberg | N6 | ACE 900 | CI24RE (CA) | CI24RE (CA) | 9.8 | 3.5 | 2.5 | 6.3 | 7.3 |
| CP14 | Cochlear | Fail | Unknown | N6 | ACE 900 | CI24RE (CA) | CI512 | 11.8 | 1.9 | 6.8 | 9.9 | 4 |
| CP15 | Med-El | Unknown | Calcification/ossification density in oval window | Sonnet | FS4-P | Concert Flex24 | Synchrony Medium | 5.8 | 3 | 5.4 | 2.8 | 0.4 |
| CP16 | Cochlear | Fail | Unknown | R N6 L N5 | ACE 900 | CI24RE (CA) | CI422 | 5.8 | 1.5 | 3.5 | 4.3 | 2.3 |
| CP17 | Cochlear | Fail | Unknown | N6 | ACE 900 | CI24RE (CA) | C124RE (CA) | 9.5 | 3 | 2 | 6.5 | 7.5 |
| CP19 | AB | Fail | Unknown | Naida Q90 | Optima P | HR90K/ HiFocus 1J | HR90K/ HiFocus 1J | 11.9 | 4 | 5.5 | 7.9 | 6.4 |
| CP20 | Cochlear | Fail | Connexin | N6 | ACE 900 | CI512 | C124RE (CA) | 6.4 | 5.9 | 2.8 | 0.5 | 3.6 |
| CP21 | Cochlear | Fail | Unknown | N6 | ACE 2400 | CI512 | C124RE (CA) | 12.7 | 7 | 2.5 | 5.7 | 10.2 |
| ID | Lab Code |
Device | Newborn Screening Pass/Fail |
Etiology | Processor | Processing Strategy |
Implant/ Electrode R |
Implant/ Electrode L |
Age at Test (yrs) |
Age CI R (yrs) |
Age CI L (yrs) |
Yrs CI R |
Yrs CI L |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA01 | C7 | AB | Unknown | High fever/Streptomycin | R Harmony L Naida Q70 | L Optima S | C1 Radial Bipolar | HiFocus 1J | 65 | 44 | 56 | 21 | 9 |
| CA02 | C3 | AB | Unknown | Hereditary | Harmony | Optima S | HiFocus 1J | HiFocus 1J | 59 | 53 | 50 | 6 | 9 |
| CA03 | C14 | AB | Unknown | Maternal Rubella | Naida Q70 | Optima P | HiFocus 1J | HiFocus 1J | 51 | 41 | 45 | 10 | 6 |
| CA04 | N01 | Cochlear | Unknown | Unknown | N6 | ACE 1200 | CI24RE (CA) | CI24R (CS) | 70 | 59 | 55 | 11 | 15 |
| CA05 | C23 | AB | Unknown | Unknown | R Naida Q70 L Harmony | Optima S | HiFocus Helix | HiFocus 1J | 75 | 72 | 66 | 3 | 9 |
| CA06 | C24 | AB | Unknown | Unknown | Naida Q70 | Optima S | HiFocus 1J | HiFocus 1J | 61 | 58 | 59 | 3 | 2 |
| CA07 | C25 | AB | Unknown | Progressive Hearing loss | Naida Q70 | Optima S | HiFocus Mid- Scala | HiFocus Mid- Scala | 63 | 60.6 | 61.3 | 2.4 | 1.7 |
| CA08 | N02 | Cochlear | Pass | Progressive Hearing loss | N5 | ACE 900 | CI24RE | CI24RE | 31 | 22 | 26 | 9 | 5 |
| CA09 | N03 | Cochlear | Unknown | Usher Syndrome Type 3b | Freedom | ACE 900 | Unknown | Unknown | 62 | 54 | 52 | 8 | 10 |
| CA10 | C8 | AB | Unknown | Hereditary | Harmony | L Optima S | C1 Enhanced Bipolar | HiFocus 1J | 69 | 50.5 | 60.7 | 18.5 | 8.3 |
| CA11 | C105 | AB | Unknown | Progressive Hearing loss | Harmony | Optima S | HiRes90K 1J | HRes90K 1J | 52.8 | 42.8 | 42.8 | 10 | 10 |
| CA12 | C27 | AB | Unknown | Hereditary | Naida Q70 | Optima S | HiRes90K 1J | HRes90K 1J | 51 | 48 | 48 | 3.9 | 3.9 |
| CA13 | C26 | AB | Unknown | Unknown Sudden HL | Naida Q90 | Optima S | HR90K Advantage/ HiFocus MS | HR90K Advantage/ HiFocus MS | 43.5 | 42.9 | 42.9 | 0.6 | 0.6 |
| CA14 | N105 | Cochlear | Unknown | Progressive/unknown genetic | N6 | ACE | CI24RE | CI24RE | 70.5 | 68.7 | 65.2 | 1.8 | 5.3 |
| CA15 | C111 | AB | Unknown | Early Onset Progressive | Harmony | Optima S | HiRes90K 1J | HiRes90K 1J | 73.1 | 53.8 | 65 | 19.3 | 8.1 |
2.2. Stimuli
The SMRT stimuli consist of spectrally-rippled broadband noise with phase drifts that change in time at a rate of 5 Hz. The peaks and valleys of the signal are modulated in time to avoid detection of cues available from attending to a single electrode or narrow frequency bands. Subjects with normal hearing additionally were tested in a cochlear-implant simulated condition, by preprocessing the SMRT stimuli to simulate an eight-channel continuous interleaved sampling (CIS) processor (Shannon et al., 1995). The unprocessed signal is divided into eight frequency bands and low-pass filtered to extract the envelope signal. The envelope signal then modulates a noise signal. The modulated signal is band-passed with the same filters and mixed. An 8-channel vocoder was selected to simulate implant-like performance (e.g. Fishman et al., 1997; Friesen et al., 2001; Shannon et al., 2004). Vocoded stimuli were generated using AngelSim software (http://angelsim.emilyfufoundation.org/) using the “8-channel Noise Vocoded Speech” preset in which both analysis and carrier filters ranged in frequency from 200 to 7000 Hz with a filter slope of 24 dB/octave. Envelope detection for each channel was performed with a low-pass filter at 400 Hz.
The SMRT stimuli were delivered at 60 dB SPL through an audiometer and presented in the sound field from a loudspeaker at 0° azimuth. The subjects were tested in a sound-treated, double-wall booth.
2.2.1. Procedure
The SMRT is a three-alternative forced-choice (3AFC) test paradigm. The subjects were seated in front of either a touchscreen computer tablet (Dell Venue 8 Pro) at USC or a computer screen with a mouse at NYU. Conditions were randomly assigned (i.e. vocoded or unprocessed for normal hearing subjects and left ear, right ear, and bilateral conditions for cochlear implant users). During the task three boxes numbered 1, 2, and 3 appeared on the screen, lighting up red when the given stimulus was played. In a trial, subjects heard three sounds: two reference signals with 20 ripples per octave (RPO) and a target signal with a lower RPO density. They were asked to touch the box on the screen that indicated which of the three stimuli presented was perceived to be different. In the first trial, the target stimulus was presented at 0.5 RPO. The RPO density of each target stimulus varied across trials using a 1-up/1-down adaptive procedure, converging to the 50% threshold (Levitt, 1971) with a step size of 0.2 RPO. For each condition (unprocessed and vocoded for normal hearing listeners; left ear alone, right ear alone, and both ears together for cochlear implant listeners), three runs of the SMRT were performed and averaged. The order of the 6 runs for normal hearing listeners (3 repeats × 2 conditions) and 9 runs for cochlear implant listeners (3 repeats × 3 conditions) was randomized separately for each subject to minimize any biases from the testing order. Before collecting data, each subject completed a practice trial in the non-vocoded condition (listeners with normal hearing) or binaural condition (cochlear implant listeners).
3. Results
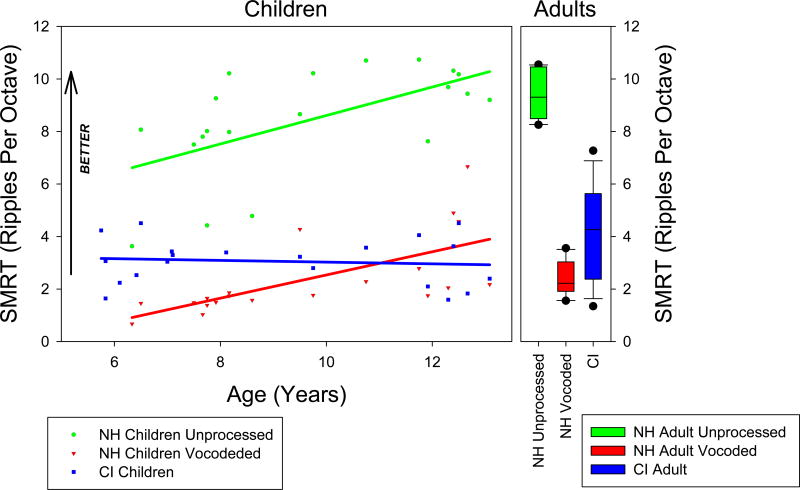
SMRT scores for the bilateral listening conditions are presented in Figure 1 as a function of age for children (left panel) or as a boxplot for adults (right panel). As indicated by the arrow, the higher the RPO the better the spectral resolution. Scores for the pediatric listeners with normal hearing are plotted with green circles, and the scores for pediatric cochlear implant users are plotted with blue squares. Results for the vocoded test for hearing children are plotted with red triangles. The best fitting regression lines for each of the three pediatric data sets are also plotted with the corresponding colors. Similar to the data presented in Kirby et al. (2015), a significant positive correlation was detected between age and SMRT scores (r = 0.597, n = 20, p = 0.0055) for the hearing children. When the SMRT was vocoded, performance decreased. Nevertheless, the positive correlation between age and SMRT remained (r = 0.663, n = 20, p = 0.00143) for the hearing children. In contrast, a weak, nonsignificant association was found between age and SMRT performance for children using bilateral implants (r = −0.102, n = 20, p = 0.667). Because the best condition for a few pediatric implant users was unilateral, the correlation was calculated between age and the best SMRT condition (bilateral or unilateral, whichever produced the higher score), yielding another weak, nonsignificant association (r = −0.0897, n = 20, p = 0.707).
Figure 1.
Bilateral performance on the SMRT is presented for children (left panel) and adults (right panel). In the left (pediatric) panel, SMRT scores (higher is better) are presented as a function of age. Green circles represent scores for normal hearing children, Red triangles represent scores for the same normal hearing children with the vocoded SMRT. Blue squares represent scores for children with cochlear implants. Adult SMRT scores are presented with boxplots (right panel) such that the green box represents the range of scores for the normal hearing adults, the red box represents the scores for normal hearing adults performing the task with vocoded stimuli, and the blue box represents the scores for adults with cochlear implants. Note that the vertical scales for the two panels match, allowing for direct comparison of results between adult and pediatric populations.
Performance by the adults with normal hearing (with and without vocoded stimuli) was similar to that of the children with normal hearing (with and without vocoded stimuli). With vocoding, the average SMRT score was 2.44 (SD: 0.67) for adults and 2.38 (SD: 1.53) for children. Without vocoding, the average SMRT score was 9.35 (SD: 0.90) for adults and 8.42 (SD: 2.08) for children. Paired t-tests failed to detect differences between adults and children with normal hearing for unprocessed (t(28) = −1.342, p = 0.19) and vocoded stimuli (t(28) = −0.117, p = 0.908). A linear regression for the pediatric normal hearing data predicts that a score of 9.35 (the adult average) on the SMRT would be reached by age 11.37 years. However, a linear fit might not be the most appropriate fit (despite the significant correlation) as presumably performance will asymptote at some age. A second order polynomial fit would predict that the children would reach average adult performance by age 9.97 years while a logarithmic fit would predict that the average adult performance would be reached at the age of 11.18 years.
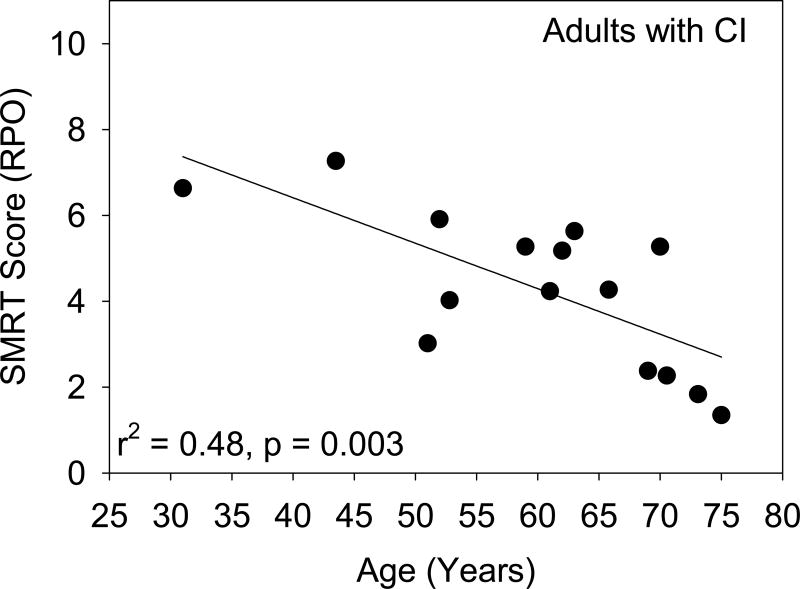
SMRT scores by cochlear implanted adults (average: 4.30 RPO) were significantly higher than those for cochlear implanted children (average: 3.06 RPO; t(33) = 2.679, p = 0.0114), but smaller than adults with normal hearing (average: 9.45 RPO; t(23) = 8.140, p < 0.0001). It is worth noting that the adults with implants were older (mean: 59.9 years) than the hearing adults (mean: 34.8 years). A basic assumption in this experiment was that implanted adults reached auditory maturity and that age would not affect the results. However, a significant correlation between age and SMRT score for CI adults was found (r = −0.701, n = 15, p = 0.003). A scatterplot of the data (Figure 2) suggests that SMRT scores may be lower for older adults. If the data are reanalyzed using only adult CI users under 65 years old such that all users have mature auditory systems but are less likely to have suffered from cognitive or auditory decline associated with aging (e.g. Lin et al., 2013), the difference between adult and pediatric CI users increases from 1.24 to 2.18 RPO and remains statistically significant (t(27)=5.197, p < 0.0001). It is worth noting that the two oldest adults (CA15 and CA05) also had the lowest RPO scores and, in fact, were performing on par with the lowest scoring children. While this finding might be suggestive of poorer spectral resolution due to aging, it may also be attributed to early progressive hearing loss of unknown magnitude as indicated for subject CA15 (See Table 1). It is therefore possible that the auditory system of this adult CI user never fully matured, thereby contributing to reduced spectral resolution as documented by Kirby et al. (2015).
Figure 2.
Bilateral performance on the SMRT is presented for adults with cochlear implants as a function of age at testing.
Similar to the pediatric population, for some adults with implants the SMRT score produced by the better unilateral condition was higher than the bilateral condition. A t-test comparing the best condition (unilateral or bilateral) for adults and children also indicated significantly better performance for adults (t(33) = −2.954, p = 0.0056). It is worth noting that the results for the hearing adults were similar to those reported by Aronoff and Landsberger (2013). A two-tailed t-test failed to detect significant differences between the hearing adult data collected for the present study and the data collected by Aronoff and Landsberger (2013) for the 8-channel vocoded stimuli (t(18)= −1.683, p=0.112) or for the unprocessed SMRT stimuli (t(18)=1.665, p=0.115).
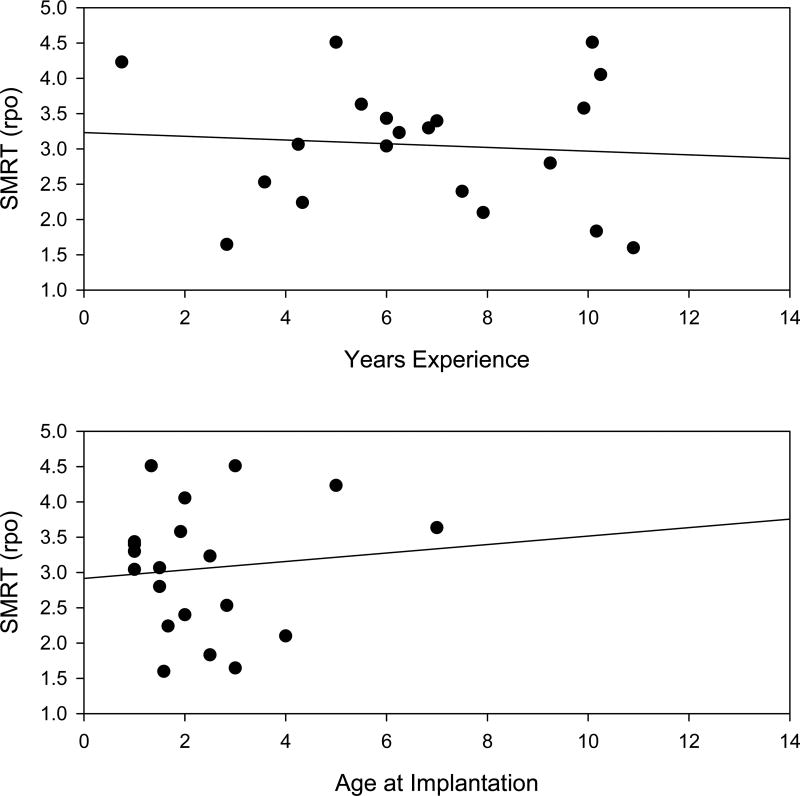
In Figure 1, as well as the corresponding analysis, children with cochlear implants were evaluated as a function of their chronological age. It is worth considering that performance on the SMRT may depend less either on chronological age than on years of experience with a cochlear implant (defined as time between evaluation and activation of their first implant), or the age when receiving their first implant. Figure 3 presents SMRT scores for cochlear implanted children as a function of years of cochlear implant experience (top panel), and age at implantation (bottom panel). No significant associations were found between SMRT scores and years of experience (r = −0.0813, n = 20, p=0.733) or age at implantation (r = 0.101, n = 20, p = 0.673).
Figure 3.
Bilateral SMRT performance for children with cochlear implants as a function of years of experience with a cochlear implant (top panel) and age at implantation (bottom panel). The correlations explain no more than 1% of the variability and none are significant.
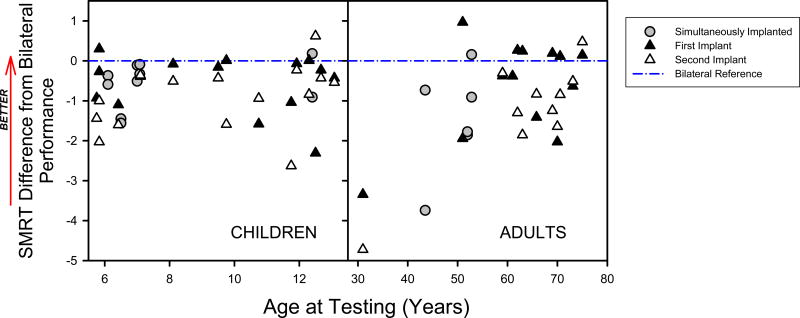
Comparisons were also conducted between individual ear and bilateral SMRT scores for the cochlear implant users. Figure 4 displays the individual ear SMRT scores in reference to the bilateral data (designated as the dashed blue line on the graph) for the children (left panel) and adults (right panel). Sequential (triangles) versus simultaneous (circles) implantation is also shown on the figure. For sequential implanted users, the first implant is represented by a solid triangle whereas the second implant is represented by a hollow triangle. All of the adult cochlear implant users were implanted sequentially. Notably, one adult subject (CA06) was unable to complete the SMRT task with the left ear (second implanted ear). For sequentially implanted subjects, SMRT scores were found to be better with the first implant for children (Z = −1.988, p = 0.048) but not for adults (Z = 1.245, p = 0.240). The Wilcoxon Signed Rank Test was used for this analysis because the assumption of normality was violated for the children when analyzed with the t-test (Shapiro-Wilk Normality Test). The time difference between implantation of the first and second ear and the SMRT between-ear difference score were not significantly correlated for either the pediatric (r = 0.433, n = 20, p = 0.0567) or adult (r = −0.342, n = 11, p = 0.303) data sets. Additionally, there were no significant associations between duration of time with the second implant and with SMRT scores for the second implant (children: r = 0.258, n = 15, p = 0.354; adults: r =0.072, n = 11, p = 0.838) or with bilateral implants (children: r = −0.119, n = 15, p = 0.672; adults: r = 0.132, n = 11, p = 0.698).
Figure 4.
Performance on the SMRT task with a single cochlear implant relative to bilateral performance are plotted as a function of age for children (left panel) and adults (right panel). Each plotted point represents the deviation between the bilateral SMRT and one of the unilateral SMRT conditions. Baseline performance (i.e. the bilateral SMRT score for a given user) is set to 0 and is represented by the dashed blue line. Positive values indicate a unilateral SMRT condition that provides a higher score than the bilateral baseline. Negative values indicate a unilateral SMRT condition that provides a lower score than the bilateral baseline. For sequentially implanted users, the first implant is represented by a solid triangle and the second implant is represented by a hollow triangle. For simultaneously implanted users, scores with each implant alone are represented by a grey circle. Note that for one subject (CA06), no SMRT score was measurable for the second implant and therefore no second data implant data point is presented for this subject.
For 75% of children with cochlear implants, bilateral listening produced higher SMRT scores than listening with their better ear (defined as the ear providing the higher average SMRT score), although CP01, CP03, CP06, CP13, and CP16 had improved performance with their better ear than in the bilateral condition. The average improvement in the bilateral condition relative to the best ear for children (0.33 RPO, SD: 0.522) was significant (t(19) = −2.792, p=0.0116). The percentage of adult cochlear implant listeners for whom the better ear alone yielded higher SMRT scores than in the bilateral condition was 47%. Specifically, these listeners were CA03, CA05, CA07, CA09, CA10, CA11, and CA14). One subject (CA05) performed better on the SMRT with either ear alone than with both implants together, while another subject (CA08) was able to discriminate by at least by 3 RPO better in the bilateral condition than in the best ear alone. Although the average improvement in the bilateral condition relative to the best ear for adults (0.49 RPO, SD:1.094) was larger than the average improvement observed for children, it was not found to be significant (t(14)=1.679, p = 0.115) for adults.
4. Discussion
Results from adults and children with normal hearing suggest that performance on a spectral resolution task is age dependent. It appears that spectral maturation occurs between the ages of 9.5 and 11.5 years of age depending on the curve used to fit the data. However, the data are sparse around this age and therefore the estimate is approximate. These results are consistent with the SMRT results reported by Kirby et al. (2015). When the SMRT is measured in children under vocoded conditions, absolute performance drops but the significant correlation between age and SMRT score is maintained. However, no significant correlations were found for cochlear implanted children between SMRT scores and the child’s age, age at implantation, or experience with the implant. These findings suggest that development of spectral resolution may be hindered in early deafened children with cochlear implants.
The data collected in this experiment does not directly address the reasons for why the cochlear implant prevents older children from performing better on a spectral resolution task after a period of time and experience with the device. However, one possible explanation is that the sparser signal provided by a cochlear implant not only increases the difficulty of the SMRT task for these children, but also adversely affects the development of spectral resolution. In contrast, children born with normal hearing demonstrate positive associations between age and SMRT score, even when the SMRT stimuli are spectrally reduced through an 8-channel vocoder. From these collective findings, we hypothesize that reduced spectral coding alone may not constrain the development of spectral processing, but that early deafness in combination with cochlear implant stimulation likely contributes to these findings. This idea is further compatible with the finding that post-lingually deafened adults with cochlear implants, whose auditory system originally developed in response to normal acoustic input, perform better on the SMRT than cochlear implanted children. However, to test this hypothesis, a longitudinal study would be required to track the developmental time course of spectral resolution in young children with normal hearing and with cochlear implants. Such a study most likely would require a specialized procedure designed for assessing spectral resolution in toddlers and perhaps even infants (e.g. Horn et al., 2016).
An alternative explanation for the adult-child differences may relate to their implant devices. The majority of children in the study used the Cochlear device (18/20), whereas the majority of adults were Advanced Bionics users (11/15). Although it was originally assumed that there would be no device differences, it is possible that the Advanced Bionics device provides better spectral resolution than the Cochlear device. All of the Advanced Bionics users (adult and pediatric) in the study had been fitted with the Optima strategy, a current-steering algorithm that provides up to a possible 135 stimulation locations from 16 electrode sites in an attempt to improve spectral coding. All of the Cochlear device users (adult and pediatric) had been fitted with the ACE strategy, in which only a subset of the 22 electrodes provides stimulation in a sweep of the electrode array, with no stimulation provided to the other electrodes. As a result, the ACE processing strategy might in fact benefit spectral resolution by enhancing spectral contrasts.
Kirby et al. (2015) measured SMRT in children with normal hearing and with moderate hearing loss (using hearing aids) aged 6 years and older. They reported that SMRT scores improved with age for both groups. This finding suggests that whatever the bottleneck is that curtails the development of spectral resolution with cochlear implanted children is not present for children who perceive the stimuli through acoustic amplification. Notably, performance by the children with hearing loss was typically worse than that of the children with normal hearing in both the Kirby et al. and current studies, but was better than the cochlear implanted children evaluated in the present study. A similar experiment was conducted by Sheffield et al. (2016) in which spectral resolution (using a spectral modulation detection task) was measured for children using both unprocessed and vocoded stimuli. Consistent with our findings, they reported a significant relationship between age and spectral resolution results for hearing children with unprocessed and vocoded stimuli.
Allen and Wightman (1992) measured the ability of children (aged 4–9 years) and adults with normal hearing to perform spectral pattern discriminations using stimuli that were similar to a spectral ripple (e.g. Henry and Turner, 2003; Won et al., 2007). Although the task was different than the SMRT (and therefore results cannot be directly compared to Kirby et al. (2015) or the present study), they also found that performance on a spectral task for older children (approximately 9 years of age) was similar to that of adults, but that the younger children (approximately 5 years of age) performed significantly worse on the task. Investigating a spectral ripple discrimination task in infants with normal hearing, Horn et al. (2016) found that spectral resolution was worse for infants than for adults.
In a related study, Eisenberg et al. (2000) manipulated the number of spectral channels in a vocoder to investigate its effect on speech recognition as a function of age in adults and children with normal hearing. They found that older children (aged 10–12 years) were similarly affected by spectral distortions as the adults but that younger children (aged 5–7 years) required a greater number of spectral channels to achieve similar results. These results are compatible with the present findings.
Another interesting finding emerged from this study in terms of bilateral versus unilateral performance. For the sequentially implanted children, SMRT scores were significantly better for the earlier implanted ear. These results are consistent with findings from other studies showing that sequentially implanted children tend to perform better with their first implanted ear. Gordon and Papsin (2009) found monosyllabic word recognition in quiet to be worse in the second implanted ear than the first implanted ear if the duration between implants was 2 years or larger. Fitzgerald et al. (2013) found a significant negative correlation between the time between implant surgeries and performance on a monosyllabic word test in quiet. Illg et al., (2013) reported that the average monosyllabic recognition in quiet with the second implanted ear was 26 percentage points lower than the average monosyllabic score with the first implant when the time between implant surgeries was 5–7 years, which were the shortest inter-implantation duration evaluated. When the duration between surgeries was more than 9 years, the average difference between the two ears increased to 54 percentage points on a monosyllabic test in quiet. Such results also support early simultaneous implantation (e.g. Summerfield et al., 2002; Ramsden et al., 2009; Henkin et al., 2014; Lopez-Torrijo et al., 2015). In the present data, the interval between sequential implantation did not have an effect on spectral resolution for children. The correlation between the inter-implant interval and differences in SMRT scores for the two ears approached (p = 0.0567) but failed to reach significance. It has been reported that a period of time may be required before the child derives benefit from the second implant (e.g. Litovsky et al., 2006; Peters et al., 2007). However, the present data do not support this finding; there was no significant relationship between years of experience with the second implant and spectral resolution performance in either the bilateral or second-ear alone conditions.
Although no correlation between age and measure of spectral resolution was observed for children with implants, a negative correlation was observed for age and spectral resolution for adults with implants. As far as we are aware, this is the first study to suggest that older adults have poorer spectral resolution than younger adults. Further studies might be conducted to specifically examine spectral resolution as a function of age in which a more appropriate range of ages are recruited to verify this finding. Nevertheless, these results should not be surprising as there are several published studies which suggest that auditory performance decreases in older adults even when hearing ability (as defined by an audiogram) is controlled. For example, Vermeire et al. (2016) demonstrated that older adults with age-appropriate hearing thresholds had poorer temporal resolution (as measured with a gap detection task) than younger audiometrically normal hearing adults. Similarly, Schvartz-Leyzac and Chatterjee (2015) demonstrated that older adults with normal hearing had larger fundamental frequency difference limens than younger adults with normal hearing.
In contrast to the results on children, no differences were detected between first and second implants for adults. It is possible that the time delay between first and second implants is less relevant for adults with post-lingual hearing loss than it is for children with pre-lingual loss. It is worth noting that the duration between implantations was shorter for the sequentially implanted children than for the adults; however, this difference was not found to be statistically significant.
Despite potential misalignments between ears for the two electrode arrays, the implanted children as a group performed significantly better when listening with both ears together than with the better ear alone. This finding suggests the possibility of bilateral enhancement for spectral processing by early implanted children despite the likely mismatches in insertions for the two electrode arrays (e.g. Landsberger et al., 2015). These results also are consistent with other benefits observed for bilaterally implanted children (e.g. Godar and Litovsky, 2010; Grieco-Calub and Litovsky, 2010; Litovsky and Gordon, 2016). However, this bilateral advantage was not shown for the adult implant subjects in this study. Although this adult-child discrepancy is not easily explainable, it is possible that the smaller number of adults tested compared to children failed to produce this bilateral advantage due to insufficient power. Nevertheless, the benefit seems to be more variable for adults. One adult subject’s (CA08) bilateral SMRT score was more than 3 RPO better than their better ear SMRT score, while another adult subject (CA05) had worse bilateral scores compared to scores with either the first or second implant. It may be the case that adults vary in the ways they fuse the signal from the two implants despite possible mismatches in electrode insertions. Some adults may overcome the bilateral misalignments and show a bilateral improvement. Others may be unable to overcome the bilateral misalignments and show a bilateral decrement relative to their better ear alone. A third potential group are adults who may learn to ignore or tune out the poorer-performing ear and rely primarily on the better ear during bilateral listening tasks.
Although better spectral resolution is assumed to be desirable for children, the clinical ramifications are unclear. Holden et al. (2016) reported significant correlations for implanted adults between SMRT score and CNC words in quiet, AzBio sentences in quiet, AzBio sentences in noise, and HINT sentences in R-Space noise. Similar correlations have been found for adult implant users on other measures of spectral resolution (e.g. Henry et al., 2005; Won et al., 2007; Gifford et al., 2014; Drennen et al., 2014). It is entirely possible that an early implanted child may reach a similar level of speech recognition to that of post-lingually deafened adult implant users, despite discrepancies in spectral resolution scores. As the child’s auditory system develops with a cochlear implant, it is possible that other acoustic cues become more salient in the absence of robust spectral information. Furthermore, while the manuscript reports statistically significant differences between various subject groups on the SMRT, it remains unclear how large of difference on the SMRT is clinically relevant. For context, a linear regression of data extracted from Holden et al. (2016) suggests that at least for adult CI users, an improvement of 1 RPO on the SMRT would correspond to understanding 11.2 percentage points more words on AzBio sentences with an 8dB SNR. Similarly, a linear regression of data extracted from Zhou (2017) suggest that an improvement of 1 RPO corresponds to a 2.63 dB SRT improvement for CUNY sentences with a 10 Hz modulated white noise.
The two important findings from this study are that (1) pediatric spectral resolution of pre-lingually implanted children is poorer than that of post-lingually implanted adults, and (2) spectral resolution performance does not improve as a function of age in these children. Based on these findings, a processing strategy that is designed specifically for young children to improve spectral resolution could be particularly beneficial. Spectral resolution might be improved by changing the number of channels (e.g. Buechner et al., 2008; Zhou and Pfingst, 2012) or reducing the interaction between channels (e.g. Srinivasan et al., 2013; Bierer and Litvak, 2016). It is also possible, however, that the auditory system has limited capacity to develop spectral resolution in response to a cochlear implant and that pediatric processing strategies should focus on enhancing other cues, such as temporal coding. Temporal coding might be enhanced by increasing modulation depths to make temporal modulations more salient (e.g. Vandali et al., 2005), explicitly encoding temporal information by changes in stimulation rate (e.g. Arnoldner et al., 2007), or using analog stimulation to encode the temporal waveforms (e.g. Nogueira and Buechner, 2012).
Acknowledgments
We are grateful to John Niparko for his help in providing the opportunity for the collaboration between New York University and the University of Southern California. We are further grateful to Natalia Stupak for collecting the data for three of the adult CI users tested at NYU. We thank Justin Aronoff for providing raw data from Aronoff and Landsberger (2013) for comparison with the data presented in the current manuscript. Qian-Jie Fu and the Emily Fu Foundation provided the free download of AngelSIM which was used to vocode stimuli. Stacey Ochoa provided clinical demographic data for the pediatric cochlear implant subjects. Support for this research was provided by the NIH/NIDCD (R01 DC012152).
References
- Allen P, Wightman F. Spectral pattern discrimination by children. J Speech Hear Res. 1992;35:222–233. doi: 10.1044/jshr.3501.222. [DOI] [PubMed] [Google Scholar]
- Arnoldner C, Riss D, Brunner M, et al. Speech and music perception with the new fine structure speech coding strategy: preliminary results. Acta Otolaryngol. 2007;127:1298–1303. doi: 10.1080/00016480701275261. [DOI] [PubMed] [Google Scholar]
- Aronoff JM, Landsberger DM. The development of a modified spectral ripple test. J Acoust Soc Am. 2013;134:EL217–EL222. doi: 10.1121/1.4813802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff JM, Padilla M, Fu Q-J, et al. Contralateral Masking in Bilateral Cochlear Implant Patients: A Model of Medial Olivocochlear Function Loss. PLoS One. 2015;10:e0121591. doi: 10.1371/journal.pone.0121591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff JM, Padilla M, Stelmach J, et al. Clinically Paired Electrodes Are Often Not Perceived as Pitch Matched. Trends in Hearing. 2016;20 doi: 10.1177/2331216516668302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer JA, Litvak L. Reducing Channel Interaction Through Cochlear Implant Programming May Improve Speech Perception: Current Focusing and Channel Deactivation. Trends Hear. 2016;20 doi: 10.1177/2331216516653389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger RC, Black FO. Auditory prostheses in perspective. Ann Otol Rhinol Laryngol Suppl. 1977;86:3–10. doi: 10.1177/00034894770860s301. [DOI] [PubMed] [Google Scholar]
- Buechner A, Brendel M, Krueger B, et al. Current steering and results from novel speech coding strategies. Otol Neurotol. 2008;29:203–207. doi: 10.1097/mao.0b013e318163746. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Gifford RH. Combining acoustic and electric stimulation in the service of speech recognition. Int J Audiol. 2010;49:912–919. doi: 10.3109/14992027.2010.509113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan WR, Anderson ES, Won JH, et al. Validation of a clinical assessment of spectral-ripple resolution for cochlear implant users. Ear Hear. 2014;35:e92–e98. doi: 10.1097/AUD.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg LS, Shannon RV, Martinez AS, et al. Speech recognition with reduced spectral cues as a function of age. J Acoust Soc Am. 2000;107:2704–2710. doi: 10.1121/1.428656. [DOI] [PubMed] [Google Scholar]
- Fishman KE, Shannon RV, Slattery WH. Speech recognition as a function of the number of electrodes used in the SPEAK cochlear implant speech processor. J Speech Lang Hear Res. 1997;40:1201–1215. doi: 10.1044/jslhr.4005.1201. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MB, Green JE, Fang Y, et al. Factors influencing consistent device use in pediatric recipients of bilateral cochlear implants. Cochlear Implants Int. 2013;14:257–265. doi: 10.1179/1754762812Y.0000000026. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, et al. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Gifford RH, Hedley-Williams A, Spahr AJ. Clinical assessment of spectral modulation detection for adult cochlear implant recipients: a non-language based measure of performance outcomes. Int J Audiol. 2014;53:159–164. doi: 10.3109/14992027.2013.851800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SP, Litovsky RY. Experience with bilateral cochlear implants improves sound localization acuity in children. Otol Neurotol. 2010;31:1287–1292. doi: 10.1097/MAO.0b013e3181e75784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KA, Papsin BC. Benefits of short interimplant delays in children receiving bilateral cochlear implants. Otol Neurotol. 2009;30:319–331. doi: 10.1097/MAO.0b013e31819a8f4c. [DOI] [PubMed] [Google Scholar]
- Grieco-Calub TM, Litovsky RY. Sound localization skills in children who use bilateral cochlear implants and in children with normal acoustic hearing. Ear Hear. 2010;31:645–656. doi: 10.1097/AUD.0b013e3181e50a1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin Y, Swead RT, Roth DA, et al. Evidence for a right cochlear implant advantage in simultaneous bilateral cochlear implantation. Laryngoscope. 2014;124:1937–1941. doi: 10.1002/lary.24635. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW. The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners. J Acoust Soc Am. 2003;113:2861–2873. doi: 10.1121/1.1561900. [DOI] [PubMed] [Google Scholar]
- Henry BA, Turner CW, Behrens A. Spectral peak resolution and speech recognition in quiet: normal hearing, hearing impaired, and cochlear implant listeners. J Acoust Soc Am. 2005;118:1111–1121. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Holden LK, Firszt JB, Reeder RM, et al. Factors Affecting Outcomes in Cochlear Implant Recipients Implanted With a Perimodiolar Electrode Array Located in Scala Tympani. Otol Neurotol. 2016;37:1662–1668. doi: 10.1097/MAO.0000000000001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn DL, Won JH, Rubinstein JT, et al. Spectral Ripple Discrimination in Normal-Hearing Infants. Ear and Hearing. 2016 doi: 10.1097/AUD.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illg A, Giourgas A, Kral A, et al. Speech comprehension in children and adolescents after sequential bilateral cochlear implantation with long interimplant interval. Otol Neurotol. 2013;34:682–689. doi: 10.1097/MAO.0b013e31828bb75e. [DOI] [PubMed] [Google Scholar]
- Kirby BJ, Browning JM, Brennan MA, et al. Spectro-temporal modulation detection in children. The Journal of the Acoustical Society of America. 2015;138:EL465–EL468. doi: 10.1121/1.4935081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger DM, Padilla M, Srinivasan AG. Reducing current spread using current focusing in cochlear implant users. Hear Res. 2012;284:16–24. doi: 10.1016/j.heares.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger DM, Svrakic M, Roland JT, Jr, et al. The Relationship between Insertion Angles, Default Frequency Allocations, and Spiral Ganglion Place Pitch in Cochlear Implants. Ear and Hearing. 2015 doi: 10.1097/AUD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneau J, Wouters J, Moonen M. Relative contributions of temporal and place pitch cues to fundamental frequency discrimination in cochlear implantees. J Acoust Soc Am. 2004;116:3606–3619. doi: 10.1121/1.1823311. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49(Suppl 2):467+. [PubMed] [Google Scholar]
- Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Gordon K. Bilateral cochlear implants in children: Effects of auditory experience and deprivation on auditory perception. Hear Res. 2016;338:76–87. doi: 10.1016/j.heares.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky RY, Johnstone PM, Godar S, et al. Bilateral cochlear implants in children: localization acuity measured with minimum audible angle. Ear Hear. 2006;27:43–59. doi: 10.1097/01.aud.0000194515.28023.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Torrijo M, Mengual-Andres S, Estelles-Ferrer R. Clinical and logopaedic results of simultaneous and sequential bilateral implants in children with severe and/or profound bilateral sensorineural hearing loss: A literature review. Int J Pediatr Otorhinolaryngol. 2015;79:786–792. doi: 10.1016/j.ijporl.2015.03.030. [DOI] [PubMed] [Google Scholar]
- McDermott HJ, McKay CM, Vandali AE. A new portable sound processor for the University of Melbourne/Nucleus Limited multielectrode cochlear implant. J Acoust Soc Am. 1992;91:3367–3371. doi: 10.1121/1.402826. [DOI] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ, Vandali AE, et al. A comparison of speech perception of cochlear implantees using the Spectral Maxima Sound Processor (SMSP) and the MSP (MULTIPEAK) processor. Acta Otolaryngol. 1992;112:752–761. doi: 10.3109/00016489209137470. [DOI] [PubMed] [Google Scholar]
- Nogueira W, Büchner A, Lenarz T, et al. A Psychoacoustic "NofM"-Type Speech Coding Strategy for Cochlear Implants. EURASIP Journal on Advances in Signal Processing. 2005;2005:1–16. [Google Scholar]
- Nogueira W, Buechner A. Conveying low frequency information through analog electrical stimulation in cochlear implants; 2012 Proceedings of the 20th European Signal Processing Conference (EUSIPCO); 2012. pp. 509–513. [Google Scholar]
- Peters BR, Litovsky R, Parkinson A, et al. Importance of age and postimplantation experience on speech perception measures in children with sequential bilateral cochlear implants. Otol Neurotol. 2007;28:649–657. doi: 10.1097/01.mao.0000281807.89938.60. [DOI] [PubMed] [Google Scholar]
- Ramsden JD, Papsin BC, Leung R, et al. Bilateral simultaneous cochlear implantation in children: our first 50 cases. Laryngoscope. 2009;119:2444–2448. doi: 10.1002/lary.20630. [DOI] [PubMed] [Google Scholar]
- Schvartz-Leyzac KC, Chatterjee M. Fundamental-frequency discrimination using noise-band-vocoded harmonic complexes in older listeners with normal hearing. J Acoust Soc Am. 2015;138:1687–1695. doi: 10.1121/1.4929938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV, Fu QJ, Galvin J., 3rd The number of spectral channels required for speech recognition depends on the difficulty of the listening situation. Acta Otolaryngol. 2004;(Suppl):50–54. doi: 10.1080/03655230410017562. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, et al. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Sheffield SW, Simha M, Jahn KN, et al. The Effects of Acoustic Bandwidth on Simulated Bimodal Benefit in Children and Adults with Normal Hearing. Ear Hear. 2016;37:282–288. doi: 10.1097/AUD.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan AG, Padilla M, Shannon RV, et al. Improving speech perception in noise with current focusing in cochlear implant users. Hear Res. 2013;299C:29–36. doi: 10.1016/j.heares.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield AQ, Marshall DH, Barton GR, et al. A cost-utility scenario analysis of bilateral cochlear implantation. Arch Otolaryngol Head Neck Surg. 2002;128:1255–1262. doi: 10.1001/archotol.128.11.1255. [DOI] [PubMed] [Google Scholar]
- Turner CW, Gantz BJ, Karsten S, et al. Impact of hair cell preservation in cochlear implantation: combined electric and acoustic hearing. Otol Neurotol. 2010;31:1227–1232. doi: 10.1097/MAO.0b013e3181f24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandali AE, Sucher C, Tsang DJ, et al. Pitch ranking ability of cochlear implant recipients: a comparison of sound-processing strategies. J Acoust Soc Am. 2005;117:3126–3138. doi: 10.1121/1.1874632. [DOI] [PubMed] [Google Scholar]
- Vermeire K, Anderson I, Flynn M, et al. The influence of different speech processor and hearing aid settings on speech perception outcomes in electric acoustic stimulation patients. Ear Hear. 2008;29:76–86. doi: 10.1097/AUD.0b013e31815d6326. [DOI] [PubMed] [Google Scholar]
- Vermeire K, Knoop A, Boel C, et al. Speech Recognition in Noise by Younger and Older Adults. Annals of Otology, Rhinology & Laryngology. 2016;125:297–302. doi: 10.1177/0003489415611424. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, et al. Better speech recognition with cochlear implants. Nature. 1991;352:236–238. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- Won JH, Drennan WR, Rubinstein JT. Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. J Assoc Res Otolaryngol. 2007;8:384–392. doi: 10.1007/s10162-007-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N. Deactivating stimulation sites based on low-rate thresholds improves spectral ripple and speech reception thresholds in cochlear implant users. The Journal of the Acoustical Society of America. 2017;141:EL243–EL248. doi: 10.1121/1.4977235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Pfingst BE. Psychophysically based site selection coupled with dichotic stimulation improves speech recognition in noise with bilateral cochlear implants. J Acoust Soc Am. 2012;132:994–1008. doi: 10.1121/1.4730907. [DOI] [PMC free article] [PubMed] [Google Scholar]