Abstract
In recent decades, tickborne disease (TBD) cases and established populations of medically important ticks have been reported over expanding geographic areas, and an increasing number of tickborne bacteria, viruses, and protozoans have been recognized as human pathogens, collectively contributing to an increasing burden of TBDs in the United States. The prevention and diagnosis of TBDs depend greatly on an accurate understanding by the public and healthcare providers of when and where persons are at risk for exposure to human-biting ticks and to the pathogens these ticks transmit. However, national maps showing the distributions of medically important ticks and the presence or prevalence of tickborne pathogens are often incomplete, outdated, or lacking entirely. Similar deficiencies exist regarding geographic variability in host-seeking tick abundance. Efforts to accurately depict acarological risk are hampered by lack of systematic and routine surveillance for medically important ticks and their associated human pathogens. In this review, we: 1) outline the public health importance of tick surveillance; 2) identify gaps in knowledge regarding the distributions and abundance of medically important ticks in the United States and the presence and prevalence of their associated pathogens; 3) describe key objectives for tick surveillance and review methods appropriate for addressing those goals; and 4) assess current capacity and barriers to implementation and sustainability of tick surveillance programs.
Keywords: tick, surveillance, United States, Ixodes, Amblyomma
The last several decades have witnessed a steady and continued rise in the number of notifiable tickborne disease (TBD) cases, which now account for more than 75% of vector-borne infections reported in the United States annually, and represent persistent and emerging threats to public health (Eisen and Eisen 2018, Rosenberg et al. 2018). Since the early 1900s, when the bacterium eventually described as Rickettsia rickettsii was identified as the first tickborne human pathogen in the United States (Ricketts 1906, 1909), 18 additional tickborne human pathogens have been recognized; remarkably, more than 40% of these agents have been described since 1980 (Paddock et al. 2016, Eisen et al. 2017) (Fig. 1). The accelerated pace of tickborne pathogen discovery can be explained, in part, by increased clinician awareness of TBDs and improved diagnostic methods, particularly molecular techniques that have revolutionized the ability and capacity to detect novel disease-causing agents (Tijsse-Klasen et al. 2014). Another key factor in the emergence and recognition of these diseases has been the rapid expansion of geographic ranges and abundance of multiple medically relevant, human-biting tick species during the last several decades (Paddock and Yabsley 2007, Teel et al. 2010, Springer et al. 2014, Paddock and Goddard 2015, Eisen et al. 2016a, Sonenshine 2018, Molaei et al. 2019). In this context, human-tick encounter rates have increased considerably the likelihood of human infections with these pathogens, particularly those infrequently or rarely found in ticks, and those distributed focally or regionally (e.g., Borrelia miyamotoi, Borrelia mayonii, Ehrlichia muris eauclairensis, Rickettsia 364D, and Heartland virus) (Gugliotta et al. 2013, Padgett et al. 2016, Pritt et al. 2016a, Pritt et al. 2016b, Brault et al. 2018). Many protozoa, bacteria, and viruses have been identified infecting ticks for which pathogenicity in humans remains unknown, suggesting that the trend of discovery of new tickborne pathogens will continue (Tijsse-Klasen et al. 2014, Bonnet et al. 2017).
Fig. 1.
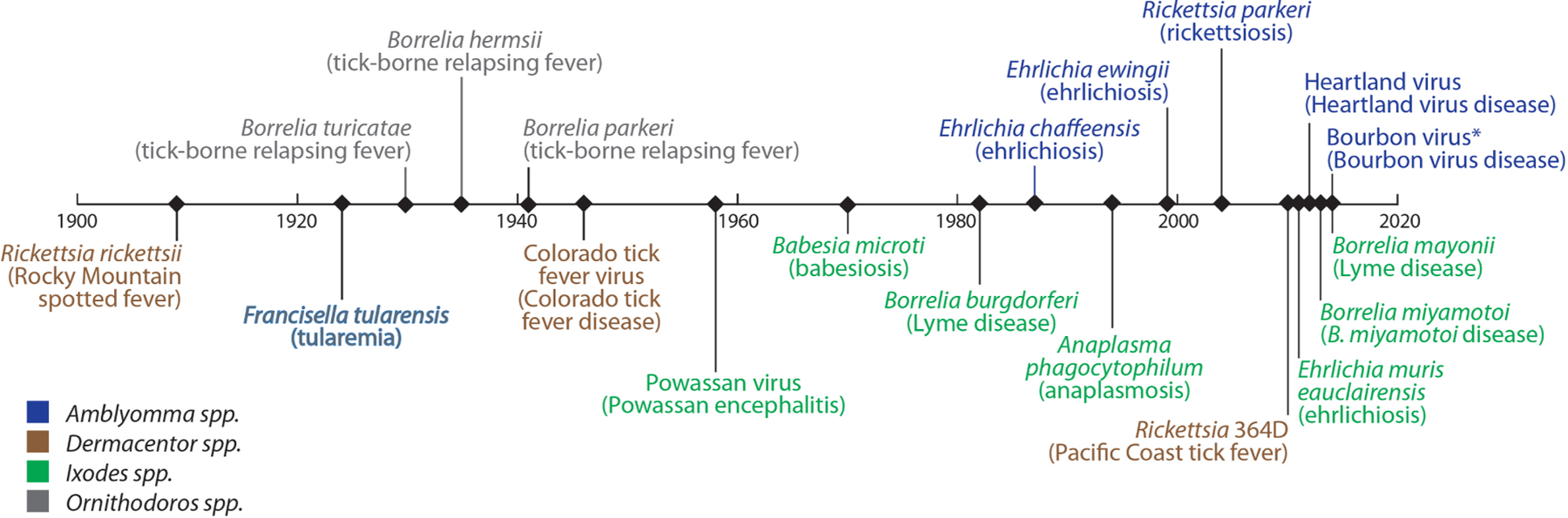
Discovery of tickborne pathogens as causes of human disease in the United States, 1900 to present.
Note: This timeline shows when tickborne pathogens were recognized as causes of human disease.
In some cases, organisms were identifed in ticks before they were associated with human disease.
In other cases, the disease was recognized before the etiological agent was found to be tickborne.
*Putative vector
The prevention and diagnosis of TBDs depend greatly on an accurate understanding by the public and healthcare providers of when and where persons are at risk for exposure to human-biting ticks and to the pathogens transmitted by these species. However, national maps showing the distributions of medically important ticks and the presence or prevalence of tickborne pathogens are often incomplete, outdated, or lacking entirely. Similar deficiencies exist regarding geographic variability in host-seeking tick abundance. In this review, we: 1) outline the public health importance of tick surveillance; 2) identify gaps in knowledge regarding distributions and abundance of medically important ticks in the United States and the presence and prevalence of their associated pathogens; 3) describe key objectives for tick surveillance and review methods appropriate for addressing those goals; and 4) assess current capacity and barriers to implementation and sustainability of tick surveillance programs. Although soft ticks (Argasidae) represent important vectors of borrelioses, particularly in the western United States (Donaldson et al. 2016, Sage et al. 2017), this review focuses exclusively on hard ticks (Acari: Ixodidae) of medical importance.
Public Health Importance of Tick Surveillance
Public health surveillance can be defined as the ongoing, systematic collection, analysis, and interpretation of health-related data essential to planning, implementation, and evaluation of public health practice. Surveillance data for TBDs provide critically important information to identify where cases have occurred but can be inaccurate when exposure occurs outside the county or state of residence. Additionally, reporting practices can vary spatially and temporally for some TBDs that are not nationally notifiable, such as B. miyamotoi disease and babesiosis. Tick surveillance complements TBD surveillance by generating representative estimates of the distribution and abundance of ticks and the presence and prevalence of their associated pathogens. These data serve several crucial public health functions, including 1) providing local information on when and where persons are at risk for exposure to ticks and tickborne pathogens as well as clarifying misperceptions of risk; 2) explaining epidemiological trends and predicting changes in risk for TBDs; and 3) informing tickborne pathogen discovery efforts.
Surveillance for ticks and tickborne pathogens bridges gaps in TBD surveillance by providing an independent data source for assessing the risk of human encounters with infected ticks. Currently, nearly all environmental interventions designed to reduce human risk of exposure to ticks and tickborne pathogens have proven inadequate to reduce the increasing rates of TBD cases (Eisen and Dolan 2016, Eisen and Gray 2016, Hinckley et al. 2016, Stafford et al. 2017, Eisen and Eisen 2018), and all strategies face inherent challenges of cost and acceptability (Gould et al. 2008, Connally et al. 2009, Hook et al. 2015, Eisen and Gray 2016, Niesobecki et al. 2019). As a result, recommendations for the prevention of TBDs focus primarily on preventing tick bites by avoiding tick habitats when ticks are active and using personal protective measures including EPA-registered repellents or permethrin-treated clothing, as well as early detection and removal of feeding ticks by daily tick checks (Piesman and Eisen 2008). In addition to clinical symptoms, diagnosis relies, in part, on assessing the probability of exposure to ticks and their associated human pathogens (Moore et al. 2016). Lack of accurate data on the distribution of ticks and pathogens impedes these prevention and diagnostic strategies. Moreover, if a vaccine for any TBD becomes commercially available, tick surveillance data could be a useful component for identifying recipient populations at high risk for TBDs. For example, when the Lymerix Lyme disease vaccine was introduced two decades ago, recommendations on who should be vaccinated were made based on assessments of a person’s likelihood of being bitten by tick vectors infected with Borrelia burgdorferi. In the absence of reliable tick surveillance data, risk maps were generated based on 1) smoothed estimates of the distributions of vector species that were based primarily on non-standardized methods for assessing tick distributions (Dennis et al. 1998), 2) predicted B. burgdorferi infection prevalence estimates based on tick-host distributions, and 3) reported cases of Lyme disease (CDC 1999).
Tick surveillance data are useful for explaining and predicting epidemiological trends. For example, although Ixodes scapularis Say, the primary vector of Lyme disease spirochetes in the United States, is broadly distributed throughout the eastern states, Lyme disease cases are reported primarily from the Northeast, Mid-Atlantic, and North-Central regions where the density of host-seeking B. burgdorferi sensu stricto-infected nymphs is significantly greater than in other parts of the tick’s range (Diuk-Wasser et al. 2012, Stromdahl and Hickling 2012, Arsnoe et al. 2015, Adams et al. 2016, Eisen et al. 2016a). Lyme disease cases have been reported among residents from all 50 states, including those where infected vector ticks have not been reported, and many of these cases have been associated with travel to high incidence areas (Forrester et al. 2015). Recognizing that the distribution of vector ticks can change substantially over time (Eisen et al. 2016a) and that the host-seeking behavior of the tick varies in ways that alter the risk of human exposure to infected ticks across the tick’s range (Diuk-Wasser et al. 2010, Diuk-Wasser et al. 2012, Stromdahl and Hickling 2012, Arsnoe et al. 2015), accurate data on the distribution of host-seeking infected ticks aids in identifying areas posing a risk of local exposures. Combining data on where Lyme disease cases have been reported with tick surveillance data might be used to predict trends in the expansion of Lyme disease-endemic areas (Bisanzio et al. 2020), but the accuracy of such models is dependent on access to high quality epidemiological and acarological surveillance data (Kugeler and Eisen 2020).
Tick surveillance can provide information to better explain trends or temporal shifts in the epidemiological characteristics of various TBDs. For example, national surveillance data for spotted fever rickettsiosis (SFR) show marked increases in incidence and decreased severity of reported cases during the last several decades, and these trends are believed to be due, in part, to infections with tickborne Rickettsia species of lower pathogenicity than R. rickettsii. During this same period, the range of Amblyomma americanum L. (the lone star tick), a species associated with spotted fever group rickettsiae of low or undetermined pathogenicity, has expanded considerably in the United States (see Amblyomma americanum section below). When incidence, hospitalization and case fatality rates of SFR during this period were regressed against the presence of A. americanum, the decade of onset of symptoms and the county of residence, an association was demonstrated between the expansion of lone star tick populations and increasing incidence and decreasing severity of reported case of SFR in the United States (Dahlgren et al. 2016).
Tick surveillance can provide objective and quantifiable data that reconcile misperceptions of risk and refocus attention to underrecognized vector species of increasing public health importance. As an example, based on a study conducted in the mid-1990s, tick surveillance in a small coastal community of Maryland with an exaggerated perception of Lyme disease risk identified a surprising predominance of A. americanum ticks, accounting for >90% of all ticks saved by residents and comprising the majority of host-seeking specimens collected from vegetation. These results were supported by a cross-sectional study that showed Lyme disease was rare in this community and disproportionally low compared to the perceived risk (Armstrong et al. 2001). Similar surveillance data can be used to quantify changing acarological risk. For example, investigators evaluated 11 yr of passive tick surveillance data from Monmouth County, NJ that included 7,722 specimens and identified a marked shift in the predominant species submitted over time. During 2006–2011, I. scapularis made up most of the submissions (49.5 ± 3.0%), followed by A. americanum (30.9 ± 3.9%). However, during the period from 2012 to 2016, lone star ticks replaced blacklegged ticks as the most frequently submitted species (45.1 ± 2.1 % for A. americanum vs. 33.9 ± 4.0% for I. scapularis), indicating increasing human encounter rates with ticks other than I. scapularis in this county (Jordan and Egizi 2019). From a public health perspective, these data can forecast increasing risk of lone star tick-associated infections such as ehrlichiosis, which is possibly underrecognized in Monmouth County by as much as two orders of magnitude, despite high reported incidence rates of Lyme disease (Egizi et al. 2017).
Tick surveillance can aid in rapidly identifying introduced species of potential public health significance. For example, Haemaphysalis longicornis Newman (the Asian longhorned tick) is native to East Asia and is an invasive agricultural pest in New Zealand, Australia and several Pacific Islands. Haemaphysalis longicornis has been implicated in the transmission of Rickettsia japonica (the cause of Japanese spotted fever) and the bunyavirus causing Severe Fever with Thromocytopenia Syndrome. Although its role in enzootic transmission of other human pathogens remains unclear, Anaplasma spp., Ehrlichia spp. and Borrelia spp. have been identified in field-collected ticks from China and Korea (Hoogstraal et al. 1968, Beard et al. 2018, Rainey et al. 2018). The Asian longhorned tick had been intercepted previously on imported animals at U.S. ports of entry, but recognition of multiple life stages of this tick infesting a sheep in Hunterdon County, NJ, in August 2017 raised significant concerns of its establishment in the United States (Rainey et al. 2018). This led to extensive coordination among federal, state, and local public health and agriculture agencies and university partners to assess the distribution of the tick in the United States. In the absence of a national tick surveillance program at that time, these efforts were largely Ad hoc but revealed that within 1 yr of initial recognition, the tick had been found in 546 counties across nine eastern states (Beard et al. 2018). Notably, retrospective review of archived specimens revealed that the tick had been present, but not detected for at least several years. Specifically, H. longicornis was collected from a deer in West Virginia in 2010 and from a dog in New Jersey in 2013 (Beard et al. 2018). Surveillance efforts continue to 1) better define the distribution of H. longicornis in the United States; 2) detect recognized or potential pathogens associated with this species; 3) determine its public health significance; and 4) assess its impact on native vector tick populations.
Effective tick surveillance programs can be leveraged to provide data that rapidly assess the distribution and prevalence of newly recognized pathogens by retrospective analysis of archived specimens. Although most tickborne human pathogens were discovered after description of the clinical syndrome, an increasing number of tickborne pathogens, including B. miyamotoi, Rickettsia parkeri, and Rickettsia 364D, were identified in ticks several decades before being associated with a TBD (Parker et al. 1939, Cory et al. 1975, Telford and Goethert 2004, Branda and Rosenberg 2013, Tijsse-Klasen et al. 2014). Knowledge gained through the use of advanced molecular detection methods that better characterize microorganisms found in ticks, coupled with laboratory vector competence studies that demonstrate which organisms are transmissible by ticks, could be used to guide pathogen (microorganisms causing illness in hosts) discovery efforts. Specifically, knowledge of the prevalence of such microorganisms in ticks could be used to define minimum sample sizes of humans per area needed to detect candidate pathogens in clinical specimens. Finally, tick surveillance efforts can assist in assessing risk for tick-associated health concerns not linked to a specific tickborne pathogen, including tick paralysis (Dworkin et al. 1999, Morshed et al. 2017) and alpha-gal (galactose-α–1,2-galactose) red meat allergy (Commins et al. 2011, Crispell et al. 2019).
Gaps in Knowledge of the Distribution and Abundance of Medically Important Ticks and the Presence and Prevalence of Their Associated Human Pathogens
Medically important ticks are present in each of the 48 states in the contiguous United States; however, their regional abundance varies considerably, and their geographic ranges can change dramatically over relatively short periods of time. The absence of ongoing and systematic sampling efforts and national tick surveillance databases hamper efforts to develop accurate depictions of the distributions and relative risks associated with these medically important ticks. In the absence of such resources, tick distribution maps have been generated based on data found through review of published peer-reviewed literature, public health agency reports, and personal communications. Many tick occurrence records have been lost because adequate data on collection methods and numbers of ticks collected by life stage often have not been included in the published literature (Gilliam et al. 2019, Lehane et al. 2020). Moreover, tick collection and pathogen detection methods differ considerably, which limits the ability to compare records over time and across sampling areas.
National data on the distribution and prevalence of tickborne pathogens in ticks and on tick abundance is sparse and often fragmentary. Although infection prevalence in ticks are described at various scales, including state, county or local levels, comprehensive distribution maps showing the presence or prevalence of tickborne pathogens found in field-collected vector ticks across the United States have not been published. Only one study, conducted almost a decade ago, used systematic sampling methods to estimate the density of host-seeking, B. burgdorferi-infected I. scapularis nymphs across the tick’s geographic range (Diuk-Wasser et al. 2010, Diuk-Wasser et al. 2012). Recent efforts to catalog tick distribution records, usually at the county level, and to develop species range maps or models, indicate that the distributions of several medically important ticks have expanded in the United States. Overall, the range of suitable habitat where ticks could become established is more widely distributed than the reported ranges of established populations, suggesting there is either potential for range expansion or that the current reported distributions under-estimate true ranges (James et al. 2015; Springer et al. 2015; Hahn et al. 2016, 2017). Below we summarize trends in the distributions of medically important ticks and prevalence of human pathogens found in them.
Ixodes scapularis
Historical changes in the geographic distribution of the blacklegged tick have been described by multiple investigators (Spielman et al. 1985, Lane et al. 1991, Eisen and Eisen 2018). As of 2016, I. scapularis was documented in 1,420 counties (45.7% of U.S. counties in the contiguous United States) from 37 states spanning from the Atlantic coastline to the eastern edge of the Great Plains. The tick was considered established (6 or more ticks or 2 or more life stages collected per county within a 12-mo period) in 842 counties distributed across 35 eastern states (Eisen et al. 2016a). This more than doubled the number of counties classified as having established populations compared with records published nearly two decades prior (Dennis et al. 1998). Models of habitat suitability for this woodland-associated tick indicated that it could become more broadly established in forested areas across the eastern United States, or may be under-reported currently (Hahn et al. 2016, 2017).
Ixodes scapularis transmits seven pathogens of humans, including Babesia microti (Spielman 1976, Piesman and Spielman 1980), B. burgdorferi sensu stricto, (Burgdorfer et al. 1982, Piesman et al. 1987), Anaplasma phagocytophilum (Telford et al. 1996), Powassan virus (Costero and Grayson 1996), E. muris eauclairensis (Karpathy et al. 2016b), B. miyamotoi (Scoles et al. 2001), and B. mayonii (Dolan et al. 2016). Data describing the prevalence of these pathogens in host-seeking ticks is incomplete, but overall indicate that pathogen prevalence varies regionally and by life stage. With the exception of B. miyamotoi and Powassan virus, which can be transmitted transovarially (Costero and Grayson 1996, Rollend et al. 2013), larvae are not infected. In the absence of zooprophylactic hosts, infection rates are usually higher in each progressive life stage as opportunities to acquire infection increase with each bloodmeal.
Borrelia burgdorferi s.s. is the most commonly detected pathogen in host-seeking I. scapularis. Although infection prevalence can vary greatly among sampling locations, in general in the Northeast and North-Central regions, B. burgdorferi infection rates in nymphs and adults are commonly in the 15–30% and 35–60% ranges, respectively (Prusinski et al. 2014; Johnson et al. 2018; New York State Department of Health 2020a,b). Detection of A. phagocytophilum and Ba. microti, which are among the most prevalent infections in I. scapularis, appears to be more geographically focal than B. burgdorferi and local establishment of these agents in ticks typically lags temporally behind B. burgdorferi (Prusinski et al. 2014, Diuk-Wasser et al. 2016, Johnson et al. 2018). Within sites in New York and Minnesota, prevalence in host-seeking nymphs averaged from 5 to 8% for A. phagocytophilum and 3–6% for Ba. microti (Prusinski et al. 2014, Johnson et al. 2018). Borrelia miyamotoi is generally detected in I. scapularis over broad geographic areas, but often at low prevalence (typically <2% in host-seeking nymphs) (Crowder et al. 2014; Johnson et al. 2018; New York State Department of Health 2020a,b). Thus far, E. muris eauclairensis and B. mayonii have been detected only in the North-Central region and prevalence of infection in host-seeking nymphs is typically less than 2% (Johnson et al. 2018). Powassan virus was detected in less than 1% of host-seeking nymphs in Minnesota and less than 4% of host-seeking adults in New York (Aliota et al. 2014, Johnson et al. 2018). Host-seeking nymphs are seldomly encountered by drag sampling or found on people in the southeastern United States and B. burgdorferi infection rates are comparatively lower in adults relative to the Northeast and North-Central regions (Diuk-Wasser et al. 2010, Diuk-Wasser et al. 2012, Stromdahl and Hickling 2012, Arsnoe et al. 2015, Hickling et al. 2018).
Ixodes pacificus
Recent assessments of the distribution of the western blacklegged tick, I. pacificus Cooley and Kohls, which is established primarily in Pacific Coast states, showed a modest increase in the numbers of counties reporting the presence of this tick from 1995 through 2015 (Dennis et al. 1998, Eisen et al. 2016a), and models indicate that the tick is already established in most areas classified as environmentally suitable (Hahn et al. 2016). Ixodes pacificus transmits multiple pathogens, including B. burgdorferi sensu stricto (Lane et al. 1994) and A. phagocytophilum (Teglas and Foley 2006), and is a presumed vector of B. miyamotoi (Padgett et al. 2014, Krause et al. 2015). Infection prevalence varies by pathogen, geographic region, and by life stage. In California, prevalence of B. burgdorferi s.l. infection is typically higher in north-coastal counties compared with southern counties (California Department of Public Health 2019), and infection prevalence is usually higher in nymphs compared with adults, because nymphs commonly feed on lizards that clear B. burgdorferi s.s. infection during blood-feeding (Lane and Quistad 1998). In Mendocino county, an average of 5% of host-seeking nymphs were infected with B. burgdorferi, whereas within the same generational cohort, adult infection rates were often four-fold lower (Eisen et al. 2004, Eisen et al. 2010). Prevalence of A. phagocytophilum in host-seeking western blacklegged ticks in California ranged from 0.4 to 4.3% in adults to 0–0.2% in nymphs collected from three northern or central coastal counties in 2018 (California Department of Public Health 2019). Of 5,428 individually tested I. pacificus adults and 615 individually tested nymphs collected in California in 2018, 0.7 and 1.3%, respectively, were infected with B. miyamotoi (California Department of Public Health 2019).
Amblyomma americanum
Changes to the geographical range of the lone star tick during the last century provide a salient example of the distributional fluidity that can occur for medically important tick species over relatively short periods of time (Springer et al. 2014). In this respect, the boundaries for the northern limits of A. americanum have undergone remarkable changes since the beginning of the 20th century. In 1912, the northern range of the lone star tick included southern Michigan and much or all of New York, Connecticut, and Massachusetts (Hooker et al. 1912). By 1945, A. americanum was no longer recognized in any of these states, and its northern distributional limit extended from Missouri to Virginia, and along the Atlantic seaboard to southern New Jersey (Bishopp and Trembley 1945). These changes have been attributed, in part, to various anthropogenic influences, including the near extirpation of white-tailed deer (Odocoileus viginianus) during the late 19th and early 20th Century (Paddock and Yabsley 2007). However, the last several years have witnessed a steady and pronounced northward expansion of lone star tick populations that now extend into southern counties of Wisconsin and Michigan, throughout most of New York, and into several northeastern states (Springer et al. 2014, Christenson et al. 2017, Sonenshine 2018, Stafford et al. 2018). The dynamic range expansion of A. americanum also is evidenced by its westward establishment throughout most counties of Kansas and Oklahoma and parts of southeastern Nebraska (Cortinas and Spomer 2013, Barrett et al. 2015, Noden and Dubie 2017). As of 2014, A. americanum was considered established in 653 counties spanning 32 states, mainly spanning southwest from southern Iowa through southern Texas and southeast from southern Illinois through central Florida. Although A. americanum continues to expand its distribution further north (Paddock and Yabsley 2007, Springer et al. 2014, Molaei et al. 2019), its northern limit is currently further south compared with I. scapularis; nonetheless, suitability models indicate the potential for northerly range expansion (Springer et al. 2014, Springer et al. 2015). Simulation modeling to estimate minimum temperature conditions for the survival of A. americanum indicate that continued climatic changes during the 21st century could create favorable conditions for this species as far north as the densely populated areas of south-central and southeastern Canada, reinforcing the importance of systematic surveillance for lone star ticks in this region (Nelder et al. 2019, Sagurova et al. 2019).
Remarkably, the recognized public health importance of the lone star tick was relatively minimal until the early 1990s, when investigators used nascent PCR technology to implicate this species as the most likely vector of E. chaffeensis (Anderson et al. 1993). Since that time, other studies have established its role as a vector of Ehrlichia ewingii (Anziani et al. 1990), Heartland virus (Godsey et al. 2016), and has been implicated as a vector of Bourbon virus (Savage et al. 2017). Estimates of infection prevalence of adult A. americanum ticks with E. chaffeensis or E. ewingii vary considerably by region, generally ranging between 2–19% and 1–16%, respectively (Cohen et al. 2010, Maegli et al. 2016, Sayler et al. 2016, Hudman and Sargentini 2018). Data for Heartland and Bourbon virus infections are sparse, but current estimates derived from pooled adult ticks are approximately 0.1 and 0.03%, respectively (Savage et al. 2016, Savage et al. 2017). Lone star ticks also are implicated as the cause of medical conditions not yet associated with a specific pathogen, including Southern Tick-Associated Rash Illness (Masters et al. 1998) and alpha-gal allergy (Commins et al. 2011, Crispell et al. 2019). The role of A. americanum in the ecology and epidemiology of Rocky Mountain spotted fever is unclear; however, DNA of R. rickettsii occasionally is detected in specimens of this species (Berrada et al. 2011, Egizi et al. 2017), and lone star ticks can effectively transmit R. rickettsii in laboratory experiments (Parker et al. 1933, Levin et al. 2017). In a similar manner, infections with Rickettsia parkeri are detected occasionally in lone star ticks in areas where these ticks are sympatric with Amblyomma maculatum Koch (the Gulf Coast tick) (Cohen et al. 2009, Wright et al. 2015). Finally, lone star ticks commonly are infected with Rickettsia amblyommatis (Karpathy et al. 2016a), a spotted fever group Rickettsia species of undetermined importance as a pathogen of humans (Apperson et al. 2008), that nonetheless could profoundly affect human seroprevalence to spotted fever rickettsioses and dramatically impact surveillance estimates for these diseases in the United States (Straily et al. 2020).
Amblyomma maculatum
Until the middle of the 20th century, the core distribution of A. maculatum in the United States was identified as a relatively narrow band extending 100–150 miles inland from the Gulf Coast of Texas, across to Florida, and northward along the Atlantic Coast to South Carolina (Hooker et al. 1912, Bishopp and Trembley 1945, Paddock and Goddard 2015, Maestas et al. 2020, Phillips et al. 2020). Established populations of A. maculatum are now recognized in several land-locked states where few or no records of this species existed during the first half of the 20th Century, including Arkansas, Kentucky, Illinois, and Tennessee, as well as coastal regions of North Carolina, Virginia, and Delaware (Paddock and Goddard 2015, Maestas et al. 2020, Phillips et al. 2020). The largest and best-characterized inland incursion occurred in the southern Great Plains. Incidental collection records for A. maculatum in southeastern Oklahoma were first documented during the early 1940s (Bishopp and Trembley 1945). By the early 1970s, the distribution of A. maculatum had expanded considerably to include multiple counties of northeastern and south-central Oklahoma as well as parts of southeastern Kansas. During the following 30 yr, Gulf Coast ticks were collected from 65% of Oklahoma counties and 18% of Kansas counties, reflecting a contiguous inland distribution involving thousands of square miles of upland prairie (Teel et al. 2010).
Rickettsia parkeri has been detected in Gulf Coast ticks collected in almost every state included within the range of the vector. Current estimates of infection among questing adults vary from 8 to 56% and rates of 20 to 40% are not unusual (Paddock and Goddard 2015). Some inland populations of A. maculatum in the central United States are disproportionately infected with Candidatus ‘Rickettsia andeanae’, a Rickettsia species of undetermined pathogenicity that could diminish the frequency and limit distribution of R. parkeri in this region. Nonetheless, this could reflect a dynamic process and be subject to change over time. As an example, none of 122 Gulf Coast ticks collected from 9 counties in Oklahoma during 2011–2014 contained DNA of R. parkeri (Paddock et al. 2015). However, a subsequent survey conducted during 2017–2018 identified R. parkeri in approximately 3% of 172 ticks collected near Oklahoma City, indicating that persons in this metropolitan center are at now risk of acquiring this infection (Noden et al. 2020).
Dermacentor variabilis
The American dog tick, D. variabilis Say, is one of the most widely distributed ticks and is considered to be established in 42 states within the contiguous United States (Lehane et al. 2020). Suitable habitat for this tick is focused predominantly in the eastern United States, within Pacific coast states, and in western states along the Canadian border (James et al. 2015). The current range boundaries for D. variabilis could expand considerably based on climate change models for North America, indicating a northward expansion by this species throughout most of Canada during the next 50 yr (Minigan et al. 2018). Dermacentor variabilis is a vector of R. rickettsii (Levin et al. 2020) and Francisella tularensis (Reese et al. 2011). These pathogens characteristically are distributed unevenly among populations of D. variabilis and infection rates can be quite low in areas not otherwise recognized as enzootic foci. Contemporary estimates of infection among adult ticks range from <0.1 to 1.7% for R. rickettsii (Stromdahl et al. 2011, Kakumanu et al. 2018, Hecht et al. 2019) and <1 to 4.1% for F. tularensis (Goethert and Telford 2009, Whitten et al. 2019).
Dermacentor andersoni
As of 2006, the Rocky Mountain wood tick, D. andersoni Stiles, was reported in 87 counties and considered established in 180, for a total of 267 counties having records of the tick’s presence. The tick was found primarily in the Mountain west and Pacific Northwest. Although the records included in this survey spanned from 1903 through 2001, the majority were derived from collections from 1921 to 1940, highlighting the opportunistic nature of tick collections informing distribution maps (James et al. 2006). Dermacentor andersoni is a vector of Colorado tick fever virus (Florio et al. 1950) and R. ricketsii (Burgdorfer 1975). In the Bitterroot Valley in Montana, where Colorado tick fever virus and R. rickettsii are both established, infection rates in adults are approximately 7 and 1%, respectively. However, Rocky Mountain spotted fever cases are reported more commonly in that area, likely owing to greater severity of the disease compared with Colorado tick fever (Gage et al. 1994, Williamson et al. 2019).
Dermacentor occidentalis
The Pacific Coast tick, Dermacentor occidentalis Marx, is among the most widely distributed and frequently encountered tick species along the Pacific Coast of the United States and occurs throughout much of California, the southwestern counties of Oregon, and parts of south-central Washington. This species has been implicated as a vector of F. tularensis (Parker et al. 1929), R. rickettsii (Ricketts 1906), and Colorado tick fever virus (Kohls 1955). However, the primary public health importance of the Pacific Coast tick is its suspected role in the transmission of Rickettsia 364D, the etiological agent of Pacific Coast tick fever (Padgett et al. 2016). This pathogen is distributed sporadically and focally among California populations of D. occidentalis and may be entirely absent among hundreds of adult specimens collected from multiple locations in one county, yet present at rates of 3 to 8% in ticks collected from adjacent or nearby counties (Padgett et al. 2016, Paddock et al. 2018).
Rhipicephalus sanguineus
The brown dog tick, R. sanguineus sensu lato, is distributed broadly across the United States and its range is inextricably linked to the occurrence of domesticated dogs which serve as principal host for this species. The brown dog tick is anthropophilic and well-adapted to survive and reproduce in peridomestic settings, contributing to challenges associated with control or eradication of vector populations in settings of known transmission of a TBD (Dantas-Torres 2008). Rhipicephalus sanguineus s.l., effectively transmits R. rickettsii (Parker et al. 1933) and is responsible for outbreaks of Rocky Mountain spotted fever in the southwestern United States (Demma et al. 2005, Nicholson et al. 2006). As with other vector species of R. rickettsii, infections with this pathogen are distributed unevenly among populations of R. sanguineus s.l.; however, an infection rate of 3% has been reported from one hyperendemic focus of this disease (Demma et al. 2005).
Key Objectives for Tick Surveillance and Associated Field Methods
Tick surveillance is intended as a long-term effort that characterizes changes in the distribution and abundance of medically important ticks and the presence and prevalence of tickborne pathogens to provide actionable, evidence-based information to the public, healthcare providers and public health policy makers (CDC 2018a,b). In 2018, the Centers for Disease Control and Prevention initiated a national surveillance program focused on I. scapularis and I. pacificus and their associated pathogens. Many surveillance guidelines for these tick species are transferable to other medically important ixodid ticks in the United States, although further guidance for metastriate ticks (i.e., Amblyomma, Dermacentor, and Rhipicephalus spp.) has been developed (CDC 2020). National surveillance efforts typically focus on the county as the smallest spatial unit to align with the spatial scale at which TBD case data are reported.
Identification of those counties with reported or established populations of medically important tick species represents the most fundamental goal of tick surveillance; nonetheless, tick presence alone has limited value in assessing risk of human exposure to tickborne pathogens or for triggering interventions. For example, I. scapularis is distributed widely across the eastern United States, but detection of Lyme disease spirochetes in ticks is far more common in the Northeast, Mid-Atlantic, and North-Central states compared with the Southeast (Diuk-Wasser et al. 2012). Classifying county status for the presence of specific pathogens in ticks provides additional information needed to accurately evaluate the potential for TBD in that region following exposure to a vector species. Other goals, which require increased cost and effort to achieve, aim to better characterize the acarological risk by describing the abundance of host-seeking ticks by life stage, prevalence of pathogens within vector species, and ultimately estimate the density of host-seeking infected nymphs or adults. Density estimates of host-seeking B. burgdorferi s.s. infected nymphs (i.e., numbers of host-seeking infected ticks per area dragged) are superior predictors of the likelihood of Lyme disease occurrence than tick or pathogen presence or abundance alone (Mather et al. 1996, Pepin et al. 2012). This relationship is likely transferable to other TBDs of lower incidence. Because risks of human-tick encounters vary by season, an additional objective aims to describe the host-seeking phenology of medically important ticks by life stages. Although differences in host-seeking phenology have been described across the range of a tick species, phenology is generally consistent within geographic regions, and therefore, this measure does not need to be assessed for individual counties. For example, the life cycle of I. scapularis spans 2 to 4 yr and the timing and duration of the seasonal peaks in host-seeking activity differ depending on regional climatic conditions (Eisen et al. 2016b).
Many methods can be used to collect ticks, but some are better suited for achieving specific surveillance objectives than others. Important considerations include 1) the spatial precision of the method relative to the surveillance objective; 2) the efficiency of the method for detecting the tick species and life stage of interest; and 3) the reliability of pathogen infection rates associated with the method. In general, infection prevalence derived from host-collected or blood-fed ticks is not representative of infection rates in questing or host-seeking specimens. Therefore, methods targeting questing or host-seeking ticks should be used for objectives related to assessing pathogen prevalence or estimating the risk of infection from the bites by host-seeking infected ticks. Moreover, laboratory diagnostic methods used to detect known pathogens in ticks should be sufficiently specific to differentiate pathogenic organisms from genetically similar, but nonpathogenic organisms occurring in ticks.
Drag sampling or flagging are the most universal tick collection methods and can be used for any of the outlined surveillance objectives. Drag sampling has been identified as the single most reliable method for quantifying the density of host-seeking (infected) I. scapularis nymphs (Falco and Fish 1992). Dragging and flagging are similar methods used to collect host-seeking ticks (Daniels and Fish 1990, Carroll and Schmidtmann 1992, Falco and Fish 1992). Both methods use standard sized (typically 1 m wide by 1 m long) flannel, denim, corduroy, or other sturdy white material sufficiently textured to allow ticks to adhere to the fabric. Light-colored fabric is essential for facilitating detection of dark-colored ticks on a contrasting colored surface. Weights, such as metal washers or chains, may be sewn into the trailing edge of the fabric, or strips may be cut into the weighted edges to increase contact between the fabric and vegetation.
Although the designs of collection devices may differ slightly, the primary difference between drag sampling and flagging is in the contact between the fabric and vegetation. When drag sampling, the fabric is usually moved horizontally along the vegetation for fixed distances or times, whereas flagging more commonly uses a sweeping motion, making an arc over sampled vegetation. When using either of these methods for assessing the density of host-seeking ticks, it is critical to 1) sample a large enough area to accurately estimate abundance (≥750 m2), 2) check the fabric frequently before ticks dislodge (typically ≤15 m), 3) to sample on two or more occasions during the estimated seasonal peak of the life stage of interest. The peak of host-seeking activity can be estimated based on regular drag sampling of the same area, with sampling conducted weekly or bi-weekly, or from epidemiological data. If using case occurrence data to estimate seasonal peaks, it is important to account for disease incubation periods to discern the peak of tick activity.
Walking sampling, which entails an investigator walking through tick habitat and checking their clothing and body for crawling ticks (Carey et al. 1980, Schulze et al. 1986), is likely to be more accurate in assessing human-tick encounter rates compared with other tick collection methods, but accuracy varies among vegetation types, tick species or life stages. For example, walking sampling was similar in efficiency to flagging or dragging for adult ticks, but yielded fewer nymphs for species that generally seek hosts in the leaf litter, such as I. scapularis (Ginsberg and Ewing 1989). Therefore, walking sampling is not recommended for assessing the density of infected host-seeking nymphs of I. scapularis or I. pacificus. When using this method for density estimates, similar to dragging or flagging, light-colored clothing is recommended to increase detection of ticks, the distance walked should be standardized, consistent enough to detect ticks before they drop off, and conducted during the presumed peak of host-seeking activity of the life stage of interest.
Carbon dioxide-baited tick traps can be useful for each of the stated surveillance objectives, but their effective implementation depends largely on the particular species and surveillance goal. Similar to dragging, flagging, and walking, these traps provide high spatial precision for documenting where host-seeking ticks were collected, but their success is dependent on host-seeking behavior of the target tick species. Based on the premise that ticks have well-developed chemoreceptors and are attracted to carbon dioxide to find a host, the traps consist of a solid insulated base that holds dry ice. The base is surrounded by sticky tape to capture ticks attracted to the carbon dioxide that is released as the dry ice sublimates (Wilson et al. 1972). This method was developed originally to collect feeding stages of A. americanum, which display more aggressive and mobile host-seeking behavior compared with many other tick species. Although this method may be useful for estimating the density of host-seeking A. americanum, it is less efficient at collecting I. scapularis and therefore is not recommended for estimating the density of host-seeking I. scapularis. Given its inefficiency, narrow collection range per trap and the number of ticks required to accurately estimate infection rates in ticks, it is not recommended for estimating infection rates in I. scapularis. Nonetheless, it may be convenient for documenting infection presence in any tick species.
Deer serve as important bloodmeal hosts for several tick species, including I. scapularis, I. pacificus, and A. americanum. Inspection of hunter-killed deer is a cost-effective method of detecting changes in the distribution of ticks, particularly in areas where a tick species is emerging. It, therefore, is an acceptable method of documenting establishment of ticks in a county. However, because of the moderate home range of deer (<150 ha; Kilpatrick et al. 2001), abundance estimates obtained from deer may not correlate well with estimates of host-seeking tick densities obtained through drag sampling (French et al. 1992, Bouchard et al. 2013, Lee et al. 2013, Raizman et al. 2013). If human pathogens are detected in ticks collected from deer, this is sufficient evidence to document the presence of a pathogen in the county in which the deer was collected but is not sufficient for estimating pathogen prevalence.
Small- and medium-sized mammals, birds, and lizards often serve as hosts for immature ticks, but host preferences differ among tick species. Therefore, typical tick-host associations in particular geographic regions should be considered in determining the utility of this method for achieving surveillance goals. Trapping and inspecting these animals for ticks can provide useful information on the presence and abundance of ticks and the presence of their associated human pathogens and can be particularly useful in assessing host-seeking phenology, particularly in areas where some life stages may not host-seek openly.
Using ticks found on people or pets is increasingly being used to monitor changes in tick and pathogen distributions, particularly with the rise of citizen science (Nieto et al. 2018, Chauhan et al. 2019, Lee et al. 2019). Such methods can be useful and cost-effective. However, because people and pets often travel long distances, data derived from them should only be used for tick surveillance when travel histories are assessed, and it is found that the person or animal did not leave the county where the tick encounter occurred within 10 d of discovering the tick.
Current Capacity and Barriers to Implementation and Sustainability of Tick Surveillance Programs
Shortly before CDC issued guidance for the surveillance of I. scapularis and I. pacificus and their associated pathogens (CDC 2018a,b), a survey was administered to 140 vector-borne disease professionals across the United States to assess tick surveillance practices at state and sub-state level jurisdictions (Mader et al. 2020). Overall, the survey revealed that approximately two-thirds of respondents were engaged in passive tick surveillance, but fewer than half reported that their jurisdiction was engaged in routine, active surveillance. Most of those conducting some form of tick surveillance were focused on the most basic surveillance goal of identifying tick presence. Considerably fewer jurisdictions assessed pathogen presence or prevalence or quantified acarological risk based on densities of infected host-seeking ticks. Among those aiming to detect pathogens in ticks, the majority were focused on identifying pathogens associated with Ixodes spp., or Rickettsia spp. in metastriate ticks. Although respondents expressed a desire to expand their capacity to conduct tick surveillance, stated barriers to doing so included a lack of consistent funding, limited infrastructure and training, and a need for guidance on best practices.
In their 2018 report to Congress, the national Tickborne Diseases Working Group recommended funding studies and activities on tick biology and TBD ecology, including systematic tick surveillance efforts, particularly in regions beyond the Northeast and Upper Midwest (Tickborne Diseases Working Group 2018). Beginning in 2018, the Centers for Disease Control and Prevention increased funding to state health departments, including those beyond the Northeast and Upper Midwest, to support tick surveillance efforts and issued guidance on surveillance for medically important ticks and their associated human pathogens. Improving the efficiency and cost-effectiveness of such programs is paramount to their sustainability. Given resource limitations, it is not surprising that many jurisdictions focus on the most basic objective of documenting tick presence by county. Efforts aimed at quantifying densities of host-seeking ticks and infection rates in ticks add considerable cost and effort, but addressing these surveillance objectives also provides more robust estimates of TBD risk.
Additional research is needed to provide data-driven recommendations for reducing the cost of surveillance programs while maximizing the collection of actionable public health data. Some obvious operational research questions include the following. Once a tick or tickborne pathogen becomes established in a given location, how much variation is typically observed in tick densities or infection prevalence from year to year? Is there a predictable period between introduction of a tick species and its associated pathogen(s) before a stable infection prevalence or tick density is observed to allow scaled-back surveillance following establishment? What level of variability in tick densities or pathogen prevalence is acceptable and are there thresholds that would engage public health mitigation? How frequently should sites be sampled to obtain accurate information on risk of TBDs while minimizing costs for maintaining a surveillance program? How many sites should be sampled per county to accurately assess TBD risk and how does environmental variability in the county impact those estimates? How does passive surveillance data (e.g., tick submissions from the public or from veterinarians) compare with active surveillance (e.g., tick drag sampling) for estimating TBD occurrence or incidence? Can pathogen detection assays or testing strategies be improved to reduce cost while retaining sensitivity and specificity?
Conclusions
Current and accurate information on when and where persons are at risk for TBDs aids in prevention and diagnosis. In the absence of a national tick surveillance program, data from published studies and health departments has been used to develop tick distribution maps, revealing remarkable geographic expansion of several medically important ticks in recent decades. However, such maps are of limited value because collection efforts are not uniform across the country, resulting in maps that likely under-represent the actual distribution of ticks. National maps showing abundance of host-seeking ticks provide improved data for assessing the likelihood of human encounters with medically important ticks; however, depending on the tick species, such maps are either not current or non-existent. Likewise, national maps showing the distribution of TBD agents found in host-seeking ticks are lacking. Being bitten by an infected vector tick is a primary risk factor for TBDs. National tick and tickborne pathogen surveillance efforts can improve our understanding of geographic variation in risk factors for TBDs, and efforts to build such a program have increased in recent years. Nonetheless, sustainability of tick surveillance programs is dependent on improving their fiscal support, efficiency, coordination, and cost-effectiveness.
References Cited
- Adams DA, Thomas KR, Jajosky R, Sharp P, Onweh D, Schley A, Anderson W, Faulkner A, and Kugeler K. 2016. Summary of notifiable infectious disease conditions - United States, 2014. Morbid. Mortal. Wkly. Rep 63: 1–52. [DOI] [PubMed] [Google Scholar]
- Aliota MT, Dupuis AP 2nd, Wilczek MP, Peters RJ, Ostfeld RS, and Kramer LD. 2014. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis. 14: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BE, Sims KG, Olson JG, Childs JE, Piesman JF, Happ CM, Maupin GO, and Johnson BJ. 1993. Amblyomma americanum: a potential vector of human ehrlichiosis. Am. J. Trop. Med. Hyg 49: 239–244. [DOI] [PubMed] [Google Scholar]
- Anziani OS, Ewing SA, and Barker RW. 1990. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am. J. Vet. Res 51: 929–931. [PubMed] [Google Scholar]
- Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, and Watson DW. 2008. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 8: 597–606. [DOI] [PubMed] [Google Scholar]
- Armstrong PM, Brunet LR, Spielman A, and Telford SR 3rd. 2001. Risk of Lyme disease: perceptions of residents of a Lone Star tick-infested community. Bull. World Health Organ 79: 916–925. [PMC free article] [PubMed] [Google Scholar]
- Arsnoe IM, Hickling GJ, Ginsberg HS, McElreath R, and Tsao JI. 2015. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PLoS One 10: e0127450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AW, Noden BH, Gruntmeir JM, Holland T, Mitcham JR, Martin JE, Johnson EM, and Little SE. 2015. County scale distribution of Amblyomma americanum (Ixodida: Ixodidae) in Oklahoma: addressing local deficits in tick maps based on passive reporting. J. Med. Entomol 52: 269–273. [DOI] [PubMed] [Google Scholar]
- Beard CB, Occi J, Bonilla DL, Egizi AM, Fonseca DM, Mertins JW, Backenson BP, Bajwa WI, Barbarin AM, Bertone MA, et al. 2018. Multistate Infestation with the exotic disease-vector tick Haemaphysalis longicornis—United States, August 2017-September 2018. MMWR. Morb. Mortal. Wkly. Rep 67: 1310–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrada ZL, Goethert HK, Cunningham J, and Telford SR 3rd. 2011. Rickettsia rickettsii (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from Kansas. J. Med. Entomol 48: 461–467. [DOI] [PubMed] [Google Scholar]
- Bisanzio D, Fernández MP, Martello E, Reithinger R, and Diuk-Wasser MA. 2020. Current and future spatiotemporal patterns of Lyme disease reporting in the Northeastern United States. JAMA Netw. Open 3: e200319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopp FC, and Trembley HL. 1945. Distribution and hosts of certain North American ticks. J. Parasitol 31: 1–54. [Google Scholar]
- Bonnet SI, Binetruy F, Hernández-Jarguín AM, and Duron O. 2017. The Tick Microbiome: why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front. Cell. Infect. Microbiol 7: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Leighton PA, Beauchamp G, Nguon S, Trudel L, Milord F, Lindsay LR, Bélanger D, and Ogden NH. 2013. Harvested white-tailed deer as sentinel hosts for early establishing Ixodes scapularis populations and risk from vector-borne zoonoses in southeastern Canada. J. Med. Entomol 50: 384–393. [DOI] [PubMed] [Google Scholar]
- Branda JA, and Rosenberg ES. 2013. Borrelia miyamotoi: a lesson in disease discovery. Ann. Intern. Med 159: 61–62. [DOI] [PubMed] [Google Scholar]
- Brault AC, Savage HM, Duggal NK, Eisen RJ, and Staples JE. 2018. Heartland virus epidemiology, vector association, and disease potential. Viruses 10: E498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W 1975. A review of rocky mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J. Med. Entomol 12: 269–278. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, and Davis JP. 1982. Lyme disease-a tick-borne spirochetosis? Science. 216: 1317–1319. [DOI] [PubMed] [Google Scholar]
- California Department of Public Health. 2019. Vector-borne disease section annual report 2018, pp. 7–12. In Kjemtrup AM, Kramer V (eds.), Infectious Diseases Branch. California Department of Health, Sacramento, California. https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/VBDSAnnualReport18.pdf. [Google Scholar]
- Carey AB, Krinsky WL, and Main AJ. 1980. Ixodes dammini (Acari: Ixodidae) and associated ixodid ticks in South-central Connecticut, USA. J. Med. Entomol 17: 89–99. [DOI] [PubMed] [Google Scholar]
- Carroll JF, and Schmidtmann ET. 1992. Tick sweep: modification of the tick drag-flag method for sampling nymphs of the deer tick (Acari: Ixodidae). J. Med. Entomol 29: 352–355. [DOI] [PubMed] [Google Scholar]
- CDC. 1999. Recommendations for the use of Lyme disease vaccing: recommendations of the Advisory Committee on immunization practices (ACIP). Morbid. Mortal. Wkly. Rep 48 No. RR-748: 1–17. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr4807a1.htm. [PubMed] [Google Scholar]
- CDC. 2018a. Surveillance for Ixodes scapularis and pathogens found in this tick species in the United States, pp. 1–17. https://www.cdc.gov/ticks/surveillance/index.html.
- CDC. 2018b. Surveillance for Ixodes pacificus and pathogens found in this tick species in the United States, pp. 1–17, https://www.cdc.gov/ticks/surveillance/index.html.
- CDC. 2020. Guide to the surveillance of metastriate ticks (Acari: Ixodidae) and their associated pathogens in the United States, pp. 1–17. https://www.cdc.gov/ticks/surveillance/index.html.
- Chauhan G, McClure J, Hekman J, Marsh PW, Bailey JA, Daniels RF, Genereux DP, and Karlsson EK. 2019. Combining citizen science and genomics to investigate tick, pathogen, and commensal microbiome at single-tick resolution. Front. Genet 10: 1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christenson M, Lee X, Larson S, Johnson DH, Jensen J, Meller M, and Paskewitz S. 2017. Occurrence of Amblyomma americanum (Acari: Ixodidae) and human infection with Ehrlichia chaffeensis in Wisconsin, 2008–2015. J. Med. Entomol 54: 752–756. [DOI] [PubMed] [Google Scholar]
- Cohen SB, Yabsley MJ, Garrison LE, Freye JD, Dunlap BG, Dunn JR, Mead DG, Jones TF, and Moncayo AC. 2009. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA. Emerg. Infect. Dis 15: 1471–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SB, Yabsley MJ, Freye JD, Dunlap BG, Rowland ME, Huang J, Dunn JR, Jones TF, and Moncayo AC. 2010. Prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in ticks from Tennessee. Vector Borne Zoonotic Dis. 10: 435–440. [DOI] [PubMed] [Google Scholar]
- Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, Kocan KM, Fahy JV, Nganga LW, Ronmark E, et al. 2011. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α−1,3-galactose. J. Allergy Clin. Immunol 127: 1286–1293.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connally NP, Durante AJ, Yousey-Hindes KM, Meek JI, Nelson RS, and Heimer R. 2009. Peridomestic Lyme disease prevention: results of a population-based case-control study. Am. J. Prev. Med 37: 201–206. [DOI] [PubMed] [Google Scholar]
- Cortinas R, and Spomer S. 2013. Lone star tick (Acari: Ixodidae) occurrence in Nebraska: historical and current perspectives. J. Med. Entomol 50: 244–251. [DOI] [PubMed] [Google Scholar]
- Cory J, Yunker CE, Howarth JA, Hokama Y, Hughes LE, Thomas LA, and Clifford CM. 1975. Isolation of spotted fever group and Wolbachia-like agents from field-collected materials by means of plaque formation in mammalian and mosquito cells. Acta Virol. 19: 443–445. [PubMed] [Google Scholar]
- Costero A, and Grayson MA. 1996. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari: Ixodidae). Am. J. Trop. Med. Hyg 55: 536–546. [DOI] [PubMed] [Google Scholar]
- Crispell G, Commins SP, Archer-Hartman SA, Choudhary S, Dharmarajan G, Azadi P, and Karim S. 2019. Discovery of alpha-gal-containing antigens in North American tick species believed to induce red meat allergy. Front. Immunol 10: 1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder CD, Carolan HE, Rounds MA, Honig V, Mothes B, Haag H, Nolte O, Luft BJ, Grubhoffer L, Ecker DJ, et al. 2014. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg. Infect. Dis 20: 1678–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, and Behravesh CB. 2016. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickttsiosis in the United States. Am. J. Trop. Med. Hyg 94: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels TJ, and Fish D. 1990. Spatial distribution and dispersal of unfed larval Ixodes dammini (Acari: Ixodidae) in southern New York. Environ. Entomol 19: 1029–1033. [Google Scholar]
- Dantas-Torres F 2008. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet. Parasitol 152: 173–185. [DOI] [PubMed] [Google Scholar]
- Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J Jr, Zaki SR, et al. 2005. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N. Engl. J. Med 353: 587–594. [DOI] [PubMed] [Google Scholar]
- Dennis DT, Nekomoto TS, Victor JC, Paul WS, and Piesman J. 1998. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol 35: 629–638. [DOI] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vannier E, and Krause PJ. 2016. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 32: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vourc’h G, Cislo P, Hoen AG, Melton F, Hamer S, Rowland M, Cortinas R, Hickling GJ, Tsao JI, et al. 2010. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Global Ecol. Biogeogr 19: 504–514. [Google Scholar]
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc’h G, Melton F, Hickling GJ, et al. 2012. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am. J. Trop. Med. Hyg 86: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan MC, Hojgaard A, Hoxmeier JC, Replogle AJ, Respicio-Kingry LB, Sexton C, Williams MA, Pritt BS, Schriefer ME, and Eisen L. 2016. Vector competence of the blacklegged tick, Ixodes scapularis, for the recently recognized Lyme borreliosis spirochete Candidatus Borrelia mayonii. Ticks Tick. Borne. Dis 7: 665–669. [DOI] [PubMed] [Google Scholar]
- Donaldson TG, Pèrez de León AA, Li AY, Li AI, Castro-Arellano I, Wozniak E, Boyle WK, Hargrove R, Wilder HK, Kim HJ, et al. 2016. Assessment of the geographic distribution of Ornithodoros turicata (Argasidae): climate variation and host diversity. Plos Negl. Trop. Dis 10: e0004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin MS, Shoemaker PC, and Anderson DE. 1999. Tick paralysis: 33 human cases in Washington State, 1946–1996. Clin. Infect. Dis 29: 1435–1439. [DOI] [PubMed] [Google Scholar]
- Egizi A, Fefferman NH, and Jordan RA. 2017. Relative risk for ehrlichiosis and Lyme disease in an area where vectors for both are sympatric, New Jersey, USA. Emerg. Infect. Dis 23: 939–945. [DOI] [PubMed] [Google Scholar]
- Eisen L, and Dolan MC. 2016. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol 53: 1063–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, and Eisen L. 2018. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends Parasitol. 34: 295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L, and Gray J. 2016. Lyme borreliosis prevention strategies: United States versus Europe, pp. 429–450. In Braks MAH, van Wierner SE, Takken W, and Sprong H (eds.), Ecology and prevention of Lyme borreliosis. Wageningen Academic Publishers, Wageningen, The Netherlands. [Google Scholar]
- Eisen RJ, Mun J, Eisen L, and Lane RS. 2004. Life stage-related differences in density of questing ticks and infection with Borrelia burgdorferi sensu lato within a single cohort of Ixodes pacificus (Acari: Ixodidae). J. Med. Entomol 41: 768–773. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, and Beard CB. 2016a. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J. Med. Entomol 53: 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Ogden NH, and Beard CB. 2016b. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J. Med. Entomol 53: 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Kugeler KJ, Eisen L, Beard CB, and Paddock CD. 2017. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 58: 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Girard YA, Fedorova N, Mun J, Slikas B, Leonhard S, Kitron U, and Lane RS. 2010. A spatially-explicit model of acarological risk of exposure to Borrelia burgdorferi-infected Ixodes pacificus nymphs in northwestern California based on woodland type, temperature, and water vapor. Ticks Tick. Borne. Dis 1: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco RC, and Fish D. 1992. A comparison of methods for sampling the deer tick, Ixodes dammini, in a Lyme disease endemic area. Exp. Appl. Acarol 14: 165–173. [DOI] [PubMed] [Google Scholar]
- Florio L, Miller MS, and Mugrage ER. 1950. Colorado tick fever; isolations of virus from Dermacentar variabilis obtained from Long Island, New York, with immunological comparisons between Eastern and Western strains. J. Immunol 64: 265–272. [PubMed] [Google Scholar]
- Forrester JD, Brett M, Matthias J, Stanek D, Springs CB, Marsden-Haug N, Oltean H, Baker JS, Kugeler KJ, Mead PS, et al. 2015. Epidemiology of Lyme disease in low-incidence states. Ticks Tick. Borne. Dis 6: 721–723. [DOI] [PubMed] [Google Scholar]
- French JB Jr, Schell WL, Kazmierczak JJ, and Davis JP. 1992. Changes in population density and distribution of Ixodes dammini (Acari: Ixodidae) in Wisconsin during the 1980s. J. Med. Entomol 29: 723–728. [DOI] [PubMed] [Google Scholar]
- Gage KL, Schrumpf ME, Karstens RH, Burgdorfer W, and Schwan TG. 1994. DNA typing of rickettsiae in naturally infected ticks using a polymerase chain reaction/restriction fragment length polymorphism system. Am. J. Trop. Med. Hyg 50: 247–260. [DOI] [PubMed] [Google Scholar]
- Gilliam B, Gronemeyer P, Chakraborty S, Winata F, Lyons LA, Miller-Hunt C, Tuten HC, Debosik S, Freeman D, O’Hara-Ruiz M, et al. 2020. Impact of unexplored data sources on the historical distribution of three vector tick species in Illinois. J. Med. Entomol 57: 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS, and Ewing CP. 1989. Comparison of flagging, walking, trapping, and collecting from hosts as sampling methods for northern deer ticks, Ixodes dammini, and lone-star ticks, Amblyomma americanum (Acari:Ixodidae). Exp. Appl. Acarol 7: 313–322. [DOI] [PubMed] [Google Scholar]
- Godsey MS, Savage HM, Burkhalter KL, Bosco-Lauth AM, and Delorey MJ. 2016. Transmission of Heartland Virus (Bunyaviridae: Phlebovirus) by experimentally infected Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol 53: 1226–1233. [DOI] [PubMed] [Google Scholar]
- Goethert HK, and Telford SR 3rd. 2009. Nonrandom distribution of vector ticks (Dermacentor variabilis) infected by Francisella tularensis. PLoS Pathog. 5: e1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould LH, Nelson RS, Griffith KS, Hayes EB, Piesman J, Mead PS, and Cartter ML. 2008. Knowledge, attitudes, and behaviors regarding Lyme disease prevention among Connecticut residents, 1999–2004. Vector Borne Zoonotic Dis. 8: 769–776. [DOI] [PubMed] [Google Scholar]
- Gugliotta JL, Goethert HK, Berardi VP, and Telford SR 3rd. 2013. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med 368: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Jarnevich CS, Monaghan AJ, and Eisen RJ. 2016. Modeling the geographic distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J. Med. Entomol 53: 1176–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MB, Jarnevich CS, Monaghan AJ, and Eisen RJ. 2017. Response: the geographic distribution of Ixodes scapularis (Acari: Ixodidae) revisited: the importance of assumptions about error balance. J. Med. Entomol 54: 1104–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht JA, Allerdice MEJ, Dykstra EA, Mastel L, Eisen RJ, Johnson TL, Gaff HD, Varela-Stokes AS, Goddard J, Pagac BB, et al. 2019. Multistate survey of American dog ticks (Dermacentor variabilis) for rickettsia species. Vector Borne Zoonotic Dis. 19: 652–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling GJ, Kelly JR, Auckland LD, and Hamer SA. 2018. Increasing prevalence of Borrelia burgdorferi sensu stricto-infected blacklegged ticks in Tennessee Valley, Tennessee, USA. Emerg. Infect. Dis 24: 1713–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley AF, Meek JI, Ray JA, Niesobecki SA, Connally NP, Feldman KA, Jones EH, Backenson PB, White JL, Lukacik G, et al. 2016. Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J. Infect. Dis 214: 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogstraal H, Roberts FH, Kohls GM, and Tipton VJ. 1968. Review of Haemaphysalis (kaiseriana) longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and Northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J. Parasitol 54: 1197–1213. [PubMed] [Google Scholar]
- Hook SA, Nelson CA, and Mead PS. 2015. U.S. public’s experience with ticks and tick-borne diseases: results from national healthstyles surveys. Ticks Tick. Borne. Dis 6: 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker WA, Bishopp FC, and Wood HP. 1912. The life history and bionomics of some North American ticks. U.S. Dept. Agric. Bureau Entomol. Bull 106: 239p. [Google Scholar]
- Hudman DA, and Sargentini NJ. 2018. Prevalence of tick-borne pathogens in northeast Missouri. Mo. Med 115: 162–168. [PMC free article] [PubMed] [Google Scholar]
- James AM, Freier JE, Keirans JE, Durden LA, Mertins JW, and Schlater JL. 2006. Distribution, seasonality, and hosts of the Rocky Mountain wood tick in the United States. J. Med. Entomol 43: 17–24. [PubMed] [Google Scholar]
- James AM, Burdett C, McCool MJ, Fox A, and Riggs P. 2015. The geographic distribution and ecological preferences of the American dog tick, Dermacentor variabilis (Say), in the U.S.A. Med. Vet. Entomol 29: 178–188. [DOI] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Maes SE, Hojgaard A, Fleshman A, Boegler KA, Delory MJ, Slater KS, Karpathy SE, Bjork JK, et al. 2018. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick. Borne. Dis 9: 1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan RA, and Egizi A. 2019. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006 – 2016. PLoS One 14: e0211778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakumanu ML, Ponnusamy L, Sutton H, Meshnick SR, Nicholson WL, and Apperson CS. 2018. Prevalence of Rickettsia Species (Rickettsiales: Rickettsiaceae) in Dermacentor variabilis Ticks (Acari: Ixodidae) in North Carolina. J. Med. Entomol 55: 1284–1291. [DOI] [PubMed] [Google Scholar]
- Karpathy SE, Slater KS, Goldsmith CS, Nicholson WL, and Paddock CD. 2016a. Rickettsia amblyommatis sp. nov., a spotted fever group Rickettsia associated with multiple species of Amblyomma ticks in North, Central and South America. Int. J. Syst. Evol. Microbiol 66: 5236–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpathy SE, Allerdice ME, Sheth M, Dasch GA, and Levin ML. 2016b. Co-Feeding transmission of the Ehrlichia muris-like agent to mice (Mus musculus). Vector Borne Zoonotic Dis. 16: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick HJ, Spohr SM, and Lima KK. 2001. Effects of population reduction on home ranges of female white-tailed deer at high densities. Can. J. Zool 79: 949–954. [Google Scholar]
- Kohls GM 1955. Colorado tick fever discovered in California. Calif. Vector Views 2: 17. [Google Scholar]
- Krause PJ, Fish D, Narasimhan S, and Barbour AG. 2015. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect 21: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugeler KJ, and Eisen RJ. 2020. Challenges in Predicting Lyme Disease Risk. JAMA Netw. Open 3: e200328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, and Quistad GB. 1998. Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis). J. Parasitol 84: 29–34. [PubMed] [Google Scholar]
- Lane RS, Piesman J, and Burgdorfer W. 1991. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol 36: 587–609. [DOI] [PubMed] [Google Scholar]
- Lane RS, Brown RN, Piesman J, and Peavey CA. 1994. Vector competence of Ixodes pacificus and Dermacentor occidentalis (Acari: Ixodidae) for various isolates of Lyme disease spirochetes. J. Med. Entomol 31: 417–424. [DOI] [PubMed] [Google Scholar]
- Lee X, Hardy K, Johnson DH, and Paskewitz SM. 2013. Hunter-killed deer surveillance to assess changes in the prevalence and distribution of Ixodes scapularis (Acari: Ixodidae) in Wisconsin. J. Med. Entomol 50: 632–639. [DOI] [PubMed] [Google Scholar]
- Lee X, Murphy DS, Hoang D, and Paskewitz SM. 2019. Passive animal surveillance to identify ticks in Wisconsin, 2011–2017. Insects 10: e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehane A, Parise C, Evans C, Beati L, Nicholson WL, and Eisen RJ. 2020. Reported county-level distribution of the American dog tick (Acari: Ixodidae) in the Contiguous United States. J. Med. Entomol 57: 131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Zemtsova GE, Killmaster LF, Snellgrove A, and Schumacher LBM. 2017. Vector competence of Amblyomma americanum (Acari: Ixodidae) for Rickettsia rickettsii. Ticks Tick. Borne. Dis 8: 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ML, Ford SL, Hartzer K, Krapiunaya L, Stanley H, and Snellgrove AN. 2020. Minimal duration of tick attachment sufficient for transmission of infectious Rickettsia rickettsii (Rickettsiales: Rickettsiaceae) by its primary vector Dermacentor variabilis (Acari: Ixodidae): duration of Rickettsial reactivation in the vector revisited. J. Med. Entomol 57: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader EM, Ganser C, Geiger A, Harrington LC, Foley JE, Smith RL, Mateus-Pinilla N, Teel PD, and Eisen RJ. 2021. A survey of tick surveillance and control practices in the United States. J. Med. Entomol 58: 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegli A, Loy JD, and Cortinas R. 2016. Note on Ehrlichia chaffeensis, Ehrlichia ewingii, and “Borrelia lonestari” infection in lone star ticks (Acari: Ixodidae), Nebraska, USA. Ticks Tick. Borne. Dis 7: 154–158. [DOI] [PubMed] [Google Scholar]
- Maestas LP, Reeser SR, McGay PJ, and Buoni MH. 2020. Surveillance for Amblyomma maculatum (Acari: Ixodidae) and Rickettsia parkeri (Rickettsiales: Rickettsiaceae) in the state of Delaware, and their public health implications. J. Med. Entomol 57: 979–983. [DOI] [PubMed] [Google Scholar]
- Masters E, Granter S, Duray P, and Cordes P. 1998. Physician-diagnosed erythema migrans and erythema migrans-like rashes following lone star tick bites. Arch. Dermatol 134: 955–960. [DOI] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, and Matyas BT. 1996. Entomologic index for human risk of Lyme disease. Am. J. Epidemiol 144: 1066–1069. [DOI] [PubMed] [Google Scholar]
- Minigan JN, Hager HA, Peregrine AS, and Newman JA. 2018. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick. Borne. Dis 9: 354–362. [DOI] [PubMed] [Google Scholar]
- Molaei G, Little EAH, Williams SC, and Stafford KC. 2019. Bracing for the worst - range expansion of the lone star tick in the Northeastern United States. N. Engl. J. Med 381: 2189–2192. [DOI] [PubMed] [Google Scholar]
- Moore A, Nelson C, Molins C, Mead P, and Schriefer M. 2016. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme Disease, United States. Emerg. Infect. Dis 22: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshed M, Li L, Lee MK, Fernando K, Lo T, and Wong Q. 2017. A Retrospective cohort study of tick paralysis in British Columbia. Vector Borne Zoonotic Dis. 17: 821–824. [DOI] [PubMed] [Google Scholar]
- Nelder MP, Russell CB, Clow KM, Johnson S, Weese JS, Cronin K, Ralevski F, Jardine CM, and Patel SN. 2019. Occurrence and distribution of Ambylomma americanum as determined by passive surveillance in Ontario, Canada (1999–2016). Ticks Tick. Borne. Dis 10: 146–155. [DOI] [PubMed] [Google Scholar]
- New York State Department of Health. 2020a. https://health.data.ny.gov/Health/Deer-Tick-Surveillance-Adults-Oct-to-Dec-excluding/vzbp-i2d4.
- New York State Department of Health. 2020b. https://health.data.ny.gov/Health/Deer-Tick-Surveillance-Nymphs-May-to-Sept-excludin/kibp-u2ip.
- Nicholson WL, Paddock CD, Demma L, Traeger M, Johnson B, Dickson J, McQuiston J, and Swerdlow D. 2006. Rocky Mountain spotted fever in Arizona: documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Ann. N. Y. Acad. Sci 1078: 338–341. [DOI] [PubMed] [Google Scholar]
- Niesobecki S, Hansen A, Rutz H, Mehta S, Feldman K, Meek J, Niccolai L, Hook S, and Hinckley A. 2019. Knowledge, attitudes, and behaviors regarding tick-borne disease prevention in endemic areas. Ticks Tick. Borne. Dis 10: 101264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto NC, Porter WT, Wachara JC, Lowrey TJ, Martin L, Motyka PJ, and Salkeld DJ. 2018. Using citizen science to describe the prevalence and distribution of tick bite and exposure to tick-borne diseases in the United States. PLoS One 13: e0199644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden BH, and Dubie T. 2017. Involvement of invasive eastern red cedar (Juniperus virginiana) in the expansion of Amblyomma americanum in Oklahoma. J. Vector Ecol 42: 178–183. [DOI] [PubMed] [Google Scholar]
- Noden BH, Roselli MA, and Loss SR. 2020. Rickettsia parkeri and Candidatus Rickettsia andeanae in Amblyomma maculatum Group Ticks. Emerg. Infect. Dis 26: 371–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, and Yabsley MJ. 2007. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr. Top. Microbiol. Immunol 315: 289–324. [DOI] [PubMed] [Google Scholar]
- Paddock CD, and Goddard J. 2015. The evolving medical and veterinary importance of the gulf coast tick (Acari: Ixodidae). J. Med. Entomol 52: 230–252. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Denison AM, Dryden MW, Noden BH, Lash RR, Abdelghani SS, Evans AE, Kelly AR, Hecht JA, Karpathy SE, et al. 2015. High prevalence of “Candidatus Rickettsia andeanae” and apparent exclusion of Rickettsia parkeri in adult Amblyomma maculatum (Acari: Ixodidae) from Kansas and Oklahoma. Ticks Tick. Borne. Dis 6: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Lane RS, Staples JE, and Labruna MB. 2016. Changing paradigms for tick-borne diseases in the Americas, pp. 221–257. In Mack A (ed.), Global health impacts of vector-borne diseases: workshop summary. National Academies Press, Washington, D.C. [PubMed] [Google Scholar]
- Paddock CD, Yoshimizu MH, Zambrano ML, Lane RS, Ryan BM, Espinosa A, Hacker JK, Karpathy SE, and Padgett KA. 2018. Rickettsia Species Isolated from Dermacentor occidentalis (Acari: Ixodidae) from California. J. Med. Entomol 55: 1555–1560. [DOI] [PubMed] [Google Scholar]
- Padgett K, Bonilla D, Kjemtrup A, Vilcins IM, Yoshimizu MH, Hui L, Sola M, Quintana M, and Kramer V. 2014. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. PLoS One 9: e110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett KA, Bonilla D, Eremeeva ME, Glaser C, Lane RS, Porse CC, Castro MB, Messenger S, Espinosa A, Hacker J, et al. 2016. The eco-epidemiology of pacific coast tick fever in California. Plos Negl. Trop. Dis 10: e0005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RR, Brooks CS, and Marsh H. 1929. The occurrence of Bacterium tularense in the wood tick, Dermacentor occidentalis, in California. Public Health Rep. 44: 1299–1300.19315193 [Google Scholar]
- Parker RR, Philip CB, and Jellison WL. 1933. Rocky Mountain spotted fever: potentialities of tick transmission in relation to geographical occurrence in the United States. Am. J. Trop. Med 13: 341–379. [Google Scholar]
- Parker RR, Kohls GM, Cox GW, and Davis GE. 1939. Observations on an infectious agent from Amblyomma maculatum. Public Hlth. Rep 54: 1482–1484. [Google Scholar]
- Pepin KM, Eisen RJ, Mead PS, Piesman J, Fish D, Hoen AG, Barbour AG, Hamer S, and Diuk-Wasser MA. 2012. Geographic variation in the relationship between human Lyme disease incidence and density of infected host-seeking Ixodes scapularis nymphs in the Eastern United States. Am. J. Trop. Med. Hyg 86: 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips VC, Zieman EA, Kim CH, Stone CM, Tuten HC, and Jiménez FA. 2020. Documentation of the expansion of the gulf coast tick (Amblyomma maculatum) and Rickettsia parkeri: first report in Illinois. J. Parasitol 106: 9–13. [PubMed] [Google Scholar]
- Piesman J, and Eisen L. 2008. Prevention of tick-borne diseases. Annu. Rev. Entomol 53: 323–343. [DOI] [PubMed] [Google Scholar]
- Piesman J, and Spielman A. 1980. Human babesiosis on Nantucket Island: prevalence of Babesia microti in ticks. Am. J. Trop. Med. Hyg 29: 742–746. [DOI] [PubMed] [Google Scholar]
- Piesman J, Mather TN, Sinsky RJ, and Spielman A. 1987. Duration of tick attachment and Borrelia burgdorferi transmission. J. Clin. Microbiol 25: 557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Allerdice MEJ, Sloan LM, Paddock CD, Munderloh UG, Rikihisa Y, Tajima T, Paskewitz SM, Neitzel DF, Hoang Johnson DK, et al. 2016a. Proposal to rename Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans in the upper midwestern United States. Int. J. Sys. Evol. Bacteriol 67: 2121–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritt BS, Respicio-Kingry LB, Sloan LM, Schriefer ME, Replogle AJ, Bjork J, Liu G, Kingry LC, Mead PS, Neitzel DF, et al. 2016b. Borrelia mayonii sp. nov., a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int. J. Syst. Evol. Microbiol 66: 4878–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee J, and Backenson PB. 2014. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis (Acari: Ixodidae) collected from recreational lands in the Hudson Valley Region, New York State. J. Med. Entomol 51: 226–236. [DOI] [PubMed] [Google Scholar]
- Rainey T, Occi JL, Robbins RG, and Egizi A. 2018. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol 55: 757–759. [DOI] [PubMed] [Google Scholar]
- Raizman EA, Holland JD, and Shukle JT. 2013. White-tailed deer (Odocoileus virginianus) as a potential sentinel for human Lyme disease in Indiana. Zoonoses Public Health 60: 227–233. [DOI] [PubMed] [Google Scholar]
- Reese SM, Petersen JM, Sheldon SW, Dolan MC, Dietrich G, Piesman J, and Eisen RJ. 2011. Transmission efficiency of Francisella tularensis by adult American dog ticks (Acari: Ixodidae). J. Med. Entomol 48: 884–890. [DOI] [PubMed] [Google Scholar]
- Ricketts HR 1906. The transmission of Rocky Mountain spotted fever by the bite of the wood tick (Dermacentor occidentalis). J. Am Med. Assoc 47: 358. [Google Scholar]
- Ricketts HR 1909. A microorganism which apparently has a specific relationship to Rocky Mountain spotted fever. A preliminary report. JAMA 52: 379–384. [Google Scholar]
- Rollend L, Fish D, and Childs JE. 2013. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick. Borne. Dis 4: 46–51. [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, et al. 2018. Vital Signs: Trends in reported vectorborne disease cases—United States and territories, 2004–2016. Morb. Mortal. Wkly. Rep 67: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage KM, Johnson TL, Teglas MB, Nieto NC, and Schwan TG. 2017. Ecological niche modeling and distribution of Ornithodoros hermsi associated with tick-borne relapsing fever in western North America. Plos Negl. Trop. Dis 11: e0006047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagurova I, Ludwig A, Ogden NH, Pelcat Y, Dueymes G, and Gachon P. 2019. Predicted Northward expansion of the geographic range of the tick vector Amblyomma americanum in North America under future climate conditions. Environ. Health Perspect 127: 107014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage HM, Godsey MS Jr, Panella NA, Burkhalter KL, Ashley DC, Lash RR, Ramsay B, Patterson T, and Nicholson WL. 2016. Surveillance for heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol 53: 607–612. [DOI] [PubMed] [Google Scholar]
- Savage HM, Burkhalter KL, Godsey MS Jr, Panella NA, Ashley DC, Nicholson WL, and Lambert AJ. 2017. Bourbon virus in field-collected ticks, Missouri, USA. Emerg. Infect. Dis 23: 2017–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayler KA, Loftis AD, Beatty SK, Boyce CL, Garrison E, Clemons B, Cunningham M, Alleman AR, and Barbet AF. 2016. Prevalence of tick-borne pathogens in host-seeking Amblyomma americanum (Acari: Ixodidae) and Odocoileus virginianus (Artiodactyla: Cervidae) in Florida. J. Med. Entomol 53: 949–956. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Bowen GS, Lakat MF, Parkin WE, and Shisler JK. 1986. Seasonal abundance and hosts of Ixodes dammini (Acari: Ixodidae) and other ixodid ticks from an endemic Lyme disease focus in New Jersey, USA. J. Med. Entomol 23: 105–109. [DOI] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, and Fish D. 2001. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1: 21–34. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE 2018. Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 15: e478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A 1976. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am. J. Trop. Med. Hyg 25: 784–787. [DOI] [PubMed] [Google Scholar]
- Spielman A, Wilson ML, Levine JF, and Piesman J. 1985. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu. Rev. Entomol 30: 439–460. [DOI] [PubMed] [Google Scholar]
- Springer YP, Eisen L, Beati L, James AM, and Eisen RJ. 2014. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J. Med. Entomol 51: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer YP, Jarnevich CS, Barnett DT, Monaghan AJ, and Eisen RJ. 2015. Modeling the present and future geographic distribution of the lone star tick, Amblyomma americanum (Ixodida: Ixodidae), in the continental United States. Am. J. Trop. Med. Hyg 93: 875–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford KC 3rd, Williams SC, and Molaei G. 2017. Integrated pest management in controlling ticks and tick-associated diseases. J. Integr. Pest Manage 8: 28. [Google Scholar]
- Stafford KC 3rd, Molaei G, Little EAH, Paddock CD, Karpathy SE, and Labonte AM. 2018. Distribution and establishment of the lone star tick in Connecticut and implications for range expansion and public health. J. Med. Entomol 55: 1561–1568. [DOI] [PubMed] [Google Scholar]
- Straily A, Stuck S, Singleton J, Brennan S, Marcum S, Condit M, Lee C, Kato C, Tonnetti L, Stramer SL, et al. 2020. Antibody titers reactive with Rickettsia rickettsii in blood donors and implications for surveillance of spotted fever rickettsiosis in the United States. J. Infect. Dis 221: 1371–1378. [DOI] [PubMed] [Google Scholar]
- Stromdahl EY, and Hickling GJ. 2012. Beyond Lyme: aetiology of tick-borne human diseases with emphasis on the south-eastern United States. Zoonoses Public Health 59(suppl 2): 48–64. [DOI] [PubMed] [Google Scholar]
- Stromdahl EY, Jiang J, Vince M, and Richards AL. 2011. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group Rickettsiae in the United States. Vector Borne Zoonotic Dis. 11: 969–977. [DOI] [PubMed] [Google Scholar]
- Teel PD, Ketchum HR, Mock DE, Wright RE, and Strey OF. 2010. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol 47: 707–722. [DOI] [PubMed] [Google Scholar]
- Teglas MB, and Foley J. 2006. Differences in the transmissibility of two Anaplasma phagocytophilum strains by the North American tick vector species, Ixodes pacificus and Ixodes scapularis (Acari: Ixodidae). Exp. Appl. Acarol 38: 47–58. [DOI] [PubMed] [Google Scholar]
- Telford SR 3rd, and Goethert HK. 2004. Emerging tick-borne infections: rediscovered and better characterized, or truly ‘new’? Parasitology. 129(Suppl): S301–S327. [DOI] [PubMed] [Google Scholar]
- Telford SR 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, and Persing DH. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. U. S. A 93: 6209–6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tickborne Diseases Working Group. 2018. Tickborne disease working group 2018 report to congress. https://www.hhs.gov/sites/default/files/tbdwg-report-to-congress-2018.pdf.
- Tijsse-Klasen E, Koopmans MP, and Sprong H. 2014. Tick-borne pathogen—reversed and conventional discovery of disease. Front. Public Health 2: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten T, Demontigny C, Bjork J, Foss M, Peterson M, Scheftel J, Neitzel D, Sullivan M, and Smith K. 2019. Prevalence of Francisella tularensis in Dermacentor variabilis ticks, Minnesota, 2017. Vector Borne Zoonotic Dis. 19: 596–603. [DOI] [PubMed] [Google Scholar]
- Williamson BN, Fischer RJ, Lopez JE, Ebihara H, and Schwan TG. 2019. Prevalence and strains of colorado tick fever virus in rocky mountain wood ticks in the bitterroot valley, Montana. Vector Borne Zoonotic Dis. 19: 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JG, Kinzer DR, Sauer JR, and Hair JA. 1972. Chemo-attraction in the lone star tick (Acarina: Ixodidae). I. Response of different developmental stages to carbon dioxide administered via traps. J. Med. Entomol 9: 245–252. [DOI] [PubMed] [Google Scholar]
- Wright CL, Sonenshine DE, Gaff HD, and Hynes WL. 2015. Rickettsia parkeri transmission to Amblyomma americanum by cofeeding with Amblyomma maculatum (Acari: Ixodidae) and potential for spillover. J. Med. Entomol 52: 1090–1095. [DOI] [PubMed] [Google Scholar]


