Abstract
Amphotericin B (AmB) and fluconazole (FLU) are the major antifungal drugs used in the treatment of cryptococcosis. Both drugs are believed to exert their antifungal effects through actions on cell membrane sterols. In this study we investigated whether AmB and FLU had other, more subtle effects on C. neoformans that could contribute to their therapeutic efficacy. C. neoformans cells were grown in media with subinhibitory concentrations of either AmB or FLU and analyzed for cellular charge, phagocytosis by macrophages with antibody and complement opsonins, appearance by scanning electron and light microscopies, and release of the capsular polysaccharide glucuronoxylomannan into the culture medium. Growth in the presence of either AmB or FLU resulted in major reductions in cellular charge, as measured by determination of the zeta potential. Phagocytosis studies demonstrated that exposure of C. neoformans to subinhibitory concentrations of AmB or FLU enhanced phagocytosis by macrophages. Scanning electron microscopy revealed that a large proportion of cells had an altered capsular appearance. Cells grown in medium with either AmB or FLU were smaller and released more glucuronoxylomannan into the culture medium than cells grown without antibiotics. The results suggest additional mechanisms of action for AmB and FLU that may be operative in body compartments where drug levels do not achieve the MICs. Furthermore, the results suggest mechanisms by which AmB and FLU can cooperate with humoral and cellular immune defense systems in controlling C. neoformans infections.
Cryptococcus neoformans is an opportunistic fungal pathogen that causes life-threatening meningoencephalitis in 5 to 10% of AIDS patients (11, 36, 61). Amphotericin B (AmB) and fluconazole (FLU) are the most common antifungal agents used in the treatment of infections caused by C. neoformans. Despite treatment, AIDS patients frequently relapse (54, 61), and therefore, antifungal agents are used for life-long prophylactic suppression (14). AmB is a polyene antibiotic that is thought to mediate antifungal effects by binding to cell membrane sterols and damaging the cell membrane (2). AmB may also function as an immunomodulator because it has been shown to promote nitric oxide release (38), reduce virulence (56), enhance superoxide production (57, 58), and affect cytokine secretion (59). However, levels of AmB in the tissue and the cerebrospinal fluid of humans are frequently below fungicidal levels (10, 18, 35). FLU inhibits ergosterol synthesis and is usually fungistatic for C. neoformans (51). Nevertheless, administration of either AmB or FLU can usually control cryptococcosis.
We postulated that AmB and FLU have other, nonclassical mechanisms for their activity. The relationship between cellular charge and phagocytosis in microbial pathogens is complex and poorly understood (12, 43). C. neoformans cells are negatively charged (47). Since antibiotic administration reduces bacterial cell charges (13, 37), we hypothesized that exposure of C. neoformans to AmB or FLU would decrease the magnitude of the negative charge and thus lessen the electrostatic repulsion between the yeast and phagocytic cells. Zeta potentials were calculated for cryptococcal cells with and without exposure to subinhibitory concentrations of AmB or FLU. Phagocytosis assays were performed on these cells to evaluate whether these changes in cellular charge resulted in differences in the ability of macrophages to engulf the organisms. Morphological analysis of cells grown with and without AmB or FLU was accomplished by light and scanning electron microscopies. In addition, supernatants from cultures grown with or without these drugs were tested for cryptococcal polysaccharide.
MATERIALS AND METHODS
C. neoformans strains.
C. neoformans serotype D strains ATCC 24067 and ATCC 3501 were obtained from the American Type Culture Collection (Rockville, Md.). CAP67 is an acapsular mutant (3) obtained from E. Jacobson (Richmond, Va.). Strain ATCC 24067 was selected for study because it is an extremely well characterized isolate which is used by many investigators (21). Strain ATCC 3501 is the parent of CAP67, and CAP67 can be restored to virulence and the encapsulated state by complementation with a single gene (7). Serotype D isolates are pathogenic for humans and are common in Europe (17).
The MICs of AmB (Boehringer Mannheim Inc., Mannheim, Germany) and FLU (Roerig-Pfizer, New York, N.Y.) were determined by the macrodilution method proposed by the National Committee for Clinical Laboratory Standards (45). RPMI 1640 medium (Sigma Chemical Co., St. Louis, Mo.) supplemented with l-glutamine, without bicarbonate, buffered to a pH of 7.0 with 0.165 M MOPS (morpholinepropanesulfonic acid; Sigma) was used for the assays. Polystyrene plastic tubes containing 0.1-ml aliquots of each drug at 10 times the final concentration were inoculated with approximately 2,500 cells/ml in 0.9 ml of RPMI 1640 medium. Final drug concentrations ranged from 0.03 to 128 μg/ml for FLU and 0.03 to 2 μg/ml for AmB. The cells were incubated at 35°C for 72 h, and the MICs were the lowest concentration that achieved 80% growth inhibition compared to the growth of the drug-free control for FLU and the lowest concentration at which there was absence of growth for AmB. The MICs of FLU for ATCC 24067, ATCC 3501, and CAP67 were 1, 0.5, and 1 μg/ml, respectively. The MICs of AmB for the three strains were 0.25, 0.125, and 0.25 μg/ml, respectively.
Strains were grown in Sabouraud broth (SAB; Difco Laboratories, Detroit, Mich.) or 10% fetal calf serum (FCS; Bioproducts for Science, Indianapolis, Ind.) alone and supplemented with AmB or FLU. Cultures with antifungal agents were grown in the presence of drug at concentrations that were either 0.0125, 0.25, or 0.5 the MIC for the strain. The cultures were inoculated with 3 × 103 cells/ml and were incubated at 30°C with shaking for 48 h.
Measurement of cellular charge.
The zeta potential (ζ) is a measurement of cellular charge (in millivolts) that is defined as the potential gradient that develops across the interface between a boundary liquid in contact with a solid and the mobile diffuse layer in the body of the liquid (50). It is derived from the equation ζ = (4πηm)/D, where D is the dielectric constant of the medium, η is the viscosity, and m is the electrophoretic mobility of the particle (50).
Cells grown in SAB as described above were collected and washed three times in 0.01 M sodium chloride (pH 7.0). The zeta potentials of suspensions of 106 cells/ml were measured with a Pen Kem model 501 Lazer Zee meter (24). The Lazer Zee machine determines the zeta potentials of the suspended cells by analyzing 20 to 30 cells simultaneously. This method provides an accurate measurement of the mean cellular charge for a suspension of cells (24) and has previously been used to measure cellular charge for C. neoformans (47).
Phagocytosis assays.
J774.16 is a well-characterized murine macrophage-like cell line (6) that has been extensively used to study C. neoformans-macrophage interactions. The J774.16 cells were maintained at −80°C prior to use and were prepared for the phagocytosis assays as described previously (9). C. neoformans cells were grown in SAB as described above with or without 0.5 the MIC of either AmB or FLU, collected, and washed three times in 10% heat-inactivated FCS. Cells were added to the J774.16 monolayer in a macrophage-to-yeast ratio of 1:1. The plates were incubated for 2 h at 37°C with either 20% FCS (not heat inactivated) or 10 μg of the monoclonal antibody (MAb) 18B7 per ml. MAb 18B7 binds to cryptococcal glucuronoxylomannan (GXM) (4). The monolayer was washed three times with phosphate-buffered saline (PBS; 0.137 M NaCl, 0.003 M sodium phosphate [pH 7.4]) to remove nonadherent cells, fixed with cold methanol, and stained with Giemsa (Sigma). The phagocytic index is the number of internalized yeast cells per number of macrophages per field. Internalized cells were differentiated from attached cells by their presence in a well-defined phagocytic vacuole. These measurements were determined by light microscopy (Nicon Diaphot; Nikon, Inc., Instrument Division, Garden City, N.J.) at a magnification of ×600. For each experiment three wells were examined, and the numbers of ingested cryptococcal cells and macrophages in three fields were counted, with approximately 200 macrophages per field.
Electron microscopy.
C. neoformans cells were grown in SAB or 10% FCS with or without either AmB or FLU at a concentration of 0.5 the MIC. Additionally, strains ATCC 3501 and CAP67 were grown in 10% FCS at concentrations of 0.125 and 0.25 the MIC of AmB. The cells were collected, washed three times with PBS, and then incubated in 2.5% glutaraldehyde for 1 h at room temperature. The samples were then applied to a polylysine-coated coverslip and serially dehydrated in alcohol. The samples were fixed in a critical-point drier (Samdri-790; Tousimis, Rockville, Md.), coated with gold-palladium (Desk-1; Denton Vacuum, Inc., Cherry Hill, N.J.), and viewed with a JEOL (Tokyo, Japan) JSM-6400 scanning electron microscope. Two separate sets of cultures were prepared. Cells from strains ATCC 3501 and ATCC 24067 were considered abnormal if the capsule was significantly distorted or absent. CAP67 is typically lemon shaped, and round cells were considered abnormal.
Cell and capsule measurements.
In addition to evaluation by electron microscopy, strains ATCC 3501 and ATCC 24067 that had been grown in SAB were also evaluated by light microscopy. India ink preparations were made and viewed with an Olympus AX70 (Melville, N.Y.) microscope under oil immersion at a magnification of ×1,000 with a grid with resolutions to 0.1 μm. The measurements for the capsule and the organism were averaged (n = 20). Capsule thickness was defined as the distance from the cell wall to the outer capsular border, and organism size was defined as the cell diameter inclusive of the polysaccharide capsule.
Measurement of capsular polysaccharide.
Supernatants from 48-h cultures of strains ATCC 3501 and ATCC 24067 grown in SAB or 10% FCS with and without 0.5 the MIC of AmB or FLU were analyzed by enzyme-linked immunosorbent assay for GXM. GXM is the major component of C. neoformans polysaccharide (8). The cell density for each culture was determined with a hemacytometer. Samples were spun to pellet the cells, and a 1/50 dilution of supernatant was made in Tris-buffered saline (25 mM Tris, 126 mM NaCl, 2.6 mM KCl [pH 7.2]). GXM levels in the sample supernatants were determined by capture enzyme-linked immunosorbent assay relative to the levels in the supernatant of the strain ATCC 24067 GXM standard (5). The GXM determinations were normalized by dividing the GXM concentrations by cell density.
Statistics.
Data were analyzed by using analysis of variance, independent Student’s t test, and chi-square test with Primer for Statistics, version 3.0 (McGraw-Hill, Inc., New York, N.Y.). All data are expressed as averages ± standard deviations.
RESULTS
Cellular charge.
The measured potentials for ATCC 24067, ATCC 3501, and CAP67 cells grown with and without subinhibitory concentrations of AmB or FLU are listed in Table 1. The cellular charges for these strains in the absence of drug were similar to previous determinations (47). Growth of these strains in the presence of AmB or FLU resulted in cells with significantly reduced cellular charges. AmB had a more pronounced effect than FLU in reducing cellular charge for strains ATCC 3501 and CAP67. For strain ATCC 24067, the drugs acted similarly at 0.5 the MIC, but FLU at 0.25 the MIC had a greater effect than 0.25 the MIC of AmB. Hence, for all strains, growth in the presence of sub-MICs of AmB and FLU reduced the magnitude of the negative cell charge.
TABLE 1.
Cell charge of C. neoformans with or without AmB or FLU
| Strain | Drug (fold MIC)a | Zeta potentialb (mV)b | % Changea | P valued |
|---|---|---|---|---|
| ATCC 24067 | ⊘ | −25.8 ± 0.20 | ||
| FLU (0.25) | −20.8 ± 0.40 | 24 | <0.001 | |
| FLU (0.5) | −20.8 ± 0.53 | 24 | <0.001 | |
| AmB (0.25) | −22.4 ± 0.49 | 15 | <0.001 | |
| AmB (0.5) | −20.5 ± 0.26 | 26 | <0.001 | |
| ATCC 3501 | ⊘ | −25.7 ± 0.72 | ||
| FLU (0.25) | −23.9 ± 0.64 | 8 | 0.032 | |
| FLU (0.5) | −22.3 ± 0.95 | 15 | 0.008 | |
| AmB (0.25) | −21.6 ± 0.53 | 19 | 0.001 | |
| AmB (0.5) | −21.3 ± 0.50 | 21 | 0.001 | |
| CAP67 | ⊘ | −3.43 ± 0.50 | ||
| FLU (0.25) | −2.70 ± 0.35 | 27 | 0.106 | |
| FLU (0.5) | −1.90 ± 0.30 | 81 | 0.011 | |
| AmB (0.25) | −2.23 ± 0.32 | 54 | 0.025 | |
| AmB (0.5) | −1.57 ± 0.32 | 119 | 0.006 |
Absence of drug (⊘) or either AmB or FLU at concentrations of 0.25 or 0.5 the MIC for the individual strain is indicated.
The values are the means ± the standard deviations of three measurements.
The percent change is calculated by subtracting the zeta potential of cells not exposed to drug from that of cells grown in the presence of drug, dividing by the zeta potential of the drug treated cells, and multiplying by 100.
After analysis of variance determinations showed significant differences between groups, P values were calculated by Student’s t test by using measurements of zeta potential for a drug-treated strain relative to those for the same strain without drug exposure.
Phagocytosis.
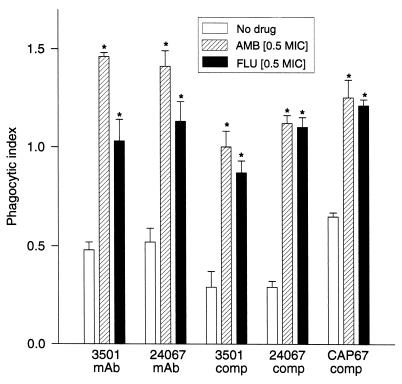
Significantly more cryptococcal cells grown in the presence of the sub-MIC of either AmB or FLU were phagocytosed compared with the numbers of phagocytosed cells grown in the absence of antifungal drugs (Fig. 1). In the presence of MAb 18B7, AmB significantly enhanced the phagocytosis of ATCC 3501 and ATCC 24067 compared to the level of phagocytosis of cells grown with FLU. However, phagocytosis of cells grown with either antifungal drug were engulfed by macrophages more frequently than control cells. With complement-derived opsonins, there was no significant difference in the level of phagocytosis of cells grown in FLU compared to the level of phagocytosis of cells grown in AmB, but more cells grown in the presence of antifungal drug than control cells were phagocytosed. Thus, for all strains, cells grown in the presence of the sub-MIC of AmB or FLU were more likely than control cells to be phagocytosed with either antibody or complement opsonins.
FIG. 1.
Phagocytic index (number of organisms engulfed divided by number of macrophages) for strains of C. neoformans grown with or without antifungal agents. MAb, MAb 18B7; comp, complement from 20% FCS (not heat inactivated). Each bar represents the results for three wells. ∗, P ≤ 0.001 for comparison of the results for either AmB- or FLU-treated cells with those for the PBS control for each group of experiments.
Scanning electron microscopy.
Scanning electron microscopy was used to analyze the shapes of the cells and the state of the polysaccharide capsule. Abnormal cells were seen in all samples of each strain, but atypical forms were significantly more common in cultures grown in SAB or 10% FCS with either FLU or AmB (Table 2). Atypical cells of encapsulated strains ATCC 24067 and ATCC 3501 consisted of organisms that were shedding or that had lost their capsules. Whereas CAP67 cells usually appear lemon shaped, atypical cells were round. Figure 2 shows representative normal and abnormal cells. For strain ATCC 3501, growth in SAB with AmB resulted in more abnormal cells than growth in SAB with FLU (P = 0.025). For strains ATCC 24067 and CAP67 there were no significant differences in the number of abnormal cells found after growth in either SAB or 10% FCS with AmB or FLU. A greater incidence of abnormal cells was seen with or without antifungal agents in the cultures grown in 10% FCS. Decreasing the concentration of AmB to 0.125 and 0.25 the MIC resulted in fewer abnormal cells (Table 3).
TABLE 2.
Scanning electron microscopy of C. neoformans with and without exposure to AmB or FLU
| Strain | Druga | SAB
|
10% FCS
|
||
|---|---|---|---|---|---|
| No. of atypical cells/total no. of cellsb | P valuec | No. of atypical cells/total no. of cellsb | P valuec | ||
| ATCC 24067 | ⊘ | 12/80 | |||
| FLU | 34/80 | <0.001 | |||
| AmB | 47/80 | <0.001 | |||
| ATCC 3501 | ⊘ | 10/80 | 7/40 | ||
| FLU | 26/80 | 0.005 | 17/40 | 0.026 | |
| AmB | 40/80 | <0.001 | 23/40 | <0.001 | |
| CAP67 | ⊘ | 18/80 | 24/40 | ||
| FLU | 53/80 | <0.001 | 38/40 | <0.001 | |
| AmB | 57/80 | <0.001 | 40/40 | <0.001 | |
Absence of drug (⊘) or either AmB or FLU at concentrations of 0.5 the MIC for the individual strain is indicated.
The ratio represents the number of atypical cells relative to the total number of cells counted by scanning electron microscopy. For ATCC 24067 and ATCC 3501, this describes the number of cells with aberrant or absent capsules versus the total number counted. For CAP67, this represents the number of round cells compared to the total number of cells evaluated.
P values were calculated by the chi-square test by using the results of an analysis of a drug-treated strain relative to the results of an analysis of the same strain without drug exposure.
FIG. 2.
C. neoformans cells grown in the presence of subinhibitory concentrations of AmB or FLU vary morphologically. Encapsulated cryptococcal cells of strain ATCC 24076 grown in medium with 0.5 the MIC of AmB can appear normal (A) or have shed part or all of their capsules (B and C, respectively). Acapsular strain CAP67 typically appears in the shape of a lemon (D) but more commonly appears round when it is grown with 0.5 the MIC of AmB or FLU (E). Magnifications, ×2,000.
TABLE 3.
Effect of different subinhibitory concentrations of AmB on cell morphology as detected by scanning electron microscopy
| Strain | Fold AmB MICa | No. of abnormal cells/total no. of cells | P value |
|---|---|---|---|
| CAP67 | 0 | 24/40 | |
| 0.125 | 25/40 | NSb | |
| 0.25 | 33/40 | 0.004 | |
| 0.5 | 40/40 | <0.001 | |
| ATCC 3501 | 0 | 7/40 | |
| 0.125 | 9/40 | NS | |
| 0.25 | 16/40 | 0.048 | |
| 0.5 | 23/40 | <0.001 |
The concentration of AmB used in each culture (10% FCS).
NS, not significant.
Cell and capsule measurements.
Measurements of the encapsulated strains ATCC 24067 and ATCC 3501 grown with and without 0.5 the MIC of AmB or FLU were performed. For both strains, cells were larger in size, with bigger capsules when they were grown without antibiotics relative to the capsule size when they were grown with either drug (Table 4). Since the capsule has been shown to be the primary factor in determining the large negative cellular charge of C. neoformans (47), the measurements were normalized for capsule size and total organism size, and the normalized values were significantly different between cells grown without antibiotics and those grown with AmB or FLU. Capsule size was reduced to a greater extent by AmB than by FLU; however, there was no significant difference for the normalized values.
TABLE 4.
Cell measurements for strains ATCC 24067 and ATCC 3501 with and without AmB and FLU
| Strain | Druga | Cell sizeb | P valuec | Capsule sized | P value | Capsule size/total sizee | P value |
|---|---|---|---|---|---|---|---|
| ATCC 24067 | ⊘ | 5.67 ± 0.73 | 1.57 ± 0.59 | 0.21 ± 0.05 | |||
| FLU | 4.95 ± 1.11 | 0.020 | 0.91 ± 0.32 | <0.001 | 0.15 ± 0.04 | <0.001 | |
| AmB | 4.75 ± 0.79 | <0.001 | 0.86 ± 0.34 | <0.001 | 0.14 ± 0.04 | <0.001 | |
| ATCC 3501 | ⊘ | 7.25 ± 1.67 | 1.85 ± 0.67 | 0.20 ± 0.04 | |||
| FLU | 5.97 ± 1.07 | 0.007 | 0.96 ± 0.40 | <0.001 | 0.13 ± 0.03 | <0.001 | |
| AmB | 5.15 ± 1.18 | <0.001 | 0.80 ± 0.36 | <0.001 | 0.13 ± 0.04 | <0.001 |
Absence of antibiotics (⊘) or presence of either AmB or FLU at 0.5 the MIC for the cryptococcal strain is indicated.
Cell size is measured (n = 20) as the size of the cell body exclusive of the polysaccharide capsule.
P values were determined by Student’s t test by using values for a drug-treated strain compared to those for the same strain without drug exposure.
Capsule size is the distance from the cell wall to the outer capsular border.
Normalized values were determined by dividing each individual measurement of capsule size by the size of the entire organism (cell diameter inclusive of the polysaccharide capsule).
GXM shedding.
The concentration of soluble polysaccharide in the form of GXM was measured in the culture supernatants of cells grown in SAB with or without 0.5 the MIC of AmB or FLU. Supernatants from cultures grown with either drug contained higher concentrations of GXM than supernatants from cultures grown without antibiotics (Table 5). AmB had the greatest effect on capsular release. Similar results were obtained when cells were grown in 10% FCS, and the magnitude of the effect diminished with decreasing concentrations of either antifungal agent from 0.5 to 0.25 and 0.125 the MIC (data not shown).
TABLE 5.
Determinations of polysaccharide shedding for cryptococcal strains ATCC 24067 and ATCC 3501
| Strain | Druga | GXM/organism densityb |
|---|---|---|
| ATCC 24067 | ⊘ | 9.2 × 10−6 |
| FLU | 8.5 × 10−5 | |
| AmB | 1.1 × 10−4 | |
| ATCC 3501 | ⊘ | 5.3 × 10−6 |
| FLU | 2.4 × 10−5 | |
| AmB | 7.3 × 10−5 |
Absence of antibiotics (⊘) or presence of either AmB or FLU at 0.5 the MIC is indicated.
Normalized value of GXM (in micrograms per milliliter) divided by the number of organisms per milliliter in the culture from which the supernatant was collected.
DISCUSSION
The major antifungal effects of AmB and FLU are considered to be mediated via binding of cell membrane sterols and alteration of sterol formation, respectively. However, there is also considerable evidence that AmB can function as an immunomodulator (28, 38, 56–59). In this study, we demonstrated that growth of C. neoformans in the presence of subinhibitory levels of either AmB or FLU had a wide range of effects on the organism, including (i) reduced negative cell charge, (ii) decreased antiphagocytic properties, (iii) altered cell morphology with a reduction in cell and capsule size, and (iv) increased levels of release of capsular GXM into culture supernatants.
Antibiotic administration has been shown to significantly and rapidly alter bacterial cell surface charges (13, 37), and these changes can enhance bacterial uptake by phagocytes (42, 49). Subinhibitory concentrations of macrolides have been shown to diminish the production of extracellular polysaccharide in Staphylococcus aureus (48). In C. neoformans, the reduction of cell charge and the more avid phagocytosis of cells grown in the presence of sub-MICs of AmB and FLU are probably related, at least in part, to the observation that these cells have smaller polysaccharide capsules. The C. neoformans capsule is antiphagocytic (31) and confers a large negative charge on the cell (33, 47). An inverse association between capsule size and cell phagocytosis has been documented for several cryptococcal strains (1, 19, 26, 53, 60). Furthermore, poorly encapsulated and acapsular cells are rapidly phagocytosed and are nonpathogenic (3, 7, 22, 34). The increased concentration of GXM in the supernatants of cells grown with subinhibitory concentrations of AmB or FLU suggests that the drugs promote capsule shedding. Thus, the diminution in cell and capsule sizes observed for cells grown with AmB or FLU correlates with the alteration in cell charge measured for strains ATCC 24067 and ATCC 3501. However, despite the lack of a polysaccharide capsule, strain CAP67 was also noted to have a profound change in cellular charge after growth in the presence of these drugs. This indicates that additional alterations in other cellular structures are occurring, resulting in a net reduction in cellular charge.
Although the antiphagocytic properties of the C. neoformans capsule are probably related to its high negative charge, the relationship between cell charge and phagocytosis is not well understood for microorganisms. Studies with polysytene and polyacrolein microspheres have demonstrated a dependence of macrophage phagocytosis on the charge, size, and hydrophobicity of the microspheres (27, 29, 55). However, phagocytosis of bioactive microspheres is also highly dependent on the milieu in which the interaction occurs (25). In our study, both encapsulated and nonencapsulated cryptococcal organisms grown in medium with sub-MICs of AmB or FLU were more avidly phagocytosed by macrophages than were cells from the same strain grown in medium without drug. The effect was observed with both complement-derived and antibody opsonins. Presumably, the decreased magnitude of cell charge observed in the cells grown with antibiotic facilitated increased interactions with macrophages. For Candida albicans, growth of the yeast with antifungal drugs, including AmB, has been shown to diminish the cellular charge and increase the adherence of the cells to acrylic surfaces (37). Another factor in the efficiency of phagocytosis is cell size. Large cryptococcal cells are difficult for macrophages to ingest. Hence, the reduction in cell size observed for cells grown with AmB or FLU may have increased the level of ingestion independently of the effects of cellular charge.
The observation that complement-derived and antibody opsonins were significantly more effective in promoting phagocytosis of cells grown in the presence of AmB or FLU suggests a mechanism by which these drugs could cooperate with the immune system to promote containment of the fungal infection. The combination of antibody and either drug has been shown to be more effective than either agent alone in vivo and in vitro (16, 40, 41). Our observations suggest that drug administration may result in fungal cell changes that enhance the efficacy of antibody in promoting the clearance of infection through phagocytic cells. Human serum can inhibit multiplication of C. neoformans and can enhance the activity of FLU (44). In addition, exposure of C. neoformans to human serum results in the deposition of C3 fragments via activation of the alternative pathway (30). The increased incidence of cells that appear to be abnormal in the cultures grown in 10% FCS compared to their incidence in the SAB cultures suggests that normal components of serum can affect cell characteristics and may augment the effects of AmB and FLU.
We previously demonstrated that both antibody and AmB can enhance nitrogen oxide production in macrophages (38, 39). Thus, the effect of AmB on antibody-mediated phagocytosis may be additive or synergistic. Furthermore, the observation that MAb 18B7 promoted phagocytosis, despite a reduction in capsule size, indicates that the epitopes recognized by this antibody are expressed in cells exposed to these drugs. This finding is important given that MAb 18B7 is planned to be used as an adjunct to antifungal therapy in patients with cryptococcosis (4).
The mechanisms by which growth in AmB or FLU alters cell and capsule size and cell shape are unknown. Exposure of cells to subinhibitory concentrations of AmB or FLU has been shown to alter their lipid profiles (20, 23), and these changes may affect the shape of the yeast cells. Regulation of cell shape is a complex process influenced by factors such as osmotic pressure, temperature, and nutrition. Specific genes have been shown to regulate cell shape in some types of yeast (15, 52). Exposure of cryptococcal cells to either AmB or FLU may constitute a stress that induces changes in gene expression that consequently results in alteration in cell size. The mechanism by which these drugs increased the level of polysaccharide shedding is also not understood. Little is known about the attachment of the polysaccharide capsule to the cell wall except for the fact that it is reversible (32). Like the changes in cell size, the increased release of GXM may reflect alterations in the cell wall and/or changes in gene regulation as a consequence of cell stress.
Two previous studies in the literature are relevant to the findings reported here. In 1974, Borowski et al. (1) reported that growth of C. neoformans in the presence of low concentrations of AmB could reduce the size of the capsule and promote serum-mediated phagocytosis by murine macrophages. To our knowledge, that report was never followed up by further studies. In 1991, Dromer and Charreire (16) did investigate the binding of an anticapsular MAb to C. neoformans cells exposed to AmB and showed that the average fluorescence intensity increased and that AmB-treated cells were more avidly phagocytosed by murine macrophages. Although the mechanism of action of this effect was not investigated, the investigators suggested that AmB might alter the capsular structure. Our observations are consistent with these prior reports and also extend these findings to FLU. Furthermore, we demonstrated that the differences in capsule size are accompanied by changes in cellular charge, cell size, and the amount of polysaccharide that is shed.
Our results suggest a potential explanation for the common clinical observation that antifungal agents can be effective when their concentrations in tissue are below the MIC for C. neoformans (10, 35, 46). Our results indicate that exposure to a sub-MIC of AmB or FLU can alter C. neoformans cells in a manner that could reduce their virulence and/or increase their susceptibility to host immune mechanisms. The changes in C. neoformans resulting from exposure to drug at levels below the MIC may represent additional mechanisms of drug action that contribute to the effectiveness of these medications. Studies of the effects of drugs at subinhibitory levels may be a fertile area for investigation into the mechanism of drug action.
ACKNOWLEDGMENTS
This work was supported by grants from NIH (grants RO1-AI22774, AI13342, and HL59842 [to A.C.] and grant K08-AI01489 [to J.D.N.]) the Burroughs Wellcome Trust (to A.C.), a minority student supplement to NIH grant RO1-AI33774 (to W.C.), and the Infectious Diseases Society of America (to J.D.N.).
REFERENCES
- 1.Borowski J, Jakoniuk P, Jablonska W, Jakubicz W, Szpak A. The influence of antifungal antibiotics on some determinants of virulence in Candida albicans and Cryptococcus neoformans cells. Experientia. 1974;30:1210–1211. doi: 10.1007/BF01923696. [DOI] [PubMed] [Google Scholar]
- 2.Brajtburg J, Powderly W G, Kobayashi G, Medoff G. Amphotericin B: current understanding of mechanism of action. Antimicrob Agents Chemother. 1990;34:183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulmer G S, Sans M D, Gunn C M. Cryptococcus neoformans. I. Nonencapsulated mutants. J Bacteriol. 1976;94:1475–1479. doi: 10.1128/jb.94.5.1475-1479.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman D L, Kozel T R, Lendvai N, Mukhergee J, Pirofski L-A, Rivera J, Rosas A L, Scharff M D, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall A, Mukherjee J, Scharff M D. Monoclonal antibody based ELISA for cryptococcal polysaccharide. J Immunol Methods. 1992;154:27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- 6.Chan J, Xing Y, Magliozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Y C, Kwon-Chung K J. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherniak M R, Sundstrom J B. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507–1512. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleare W, Casadevall A. The different binding patterns of two immunoglobulin M monoclonal antibodies to Cryptococcus neoformans serotype A and D strains correlate with serotype classification and differences in functional assays. Clin Diagn Lab Immunol. 1998;5:125–129. doi: 10.1128/cdli.5.2.125-129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collette N, Van der Auwera P, Meunier F, Lambert C, Sculier J P, Coune A. Tissue distribution and bioactivity of amphotericin B administered in liposomes to cancer patients. J Antimicrob Chemother. 1991;27:535–548. doi: 10.1093/jac/27.4.535. [DOI] [PubMed] [Google Scholar]
- 11.Currie B P, Casadevall A. Estimation of the prevalence of cryptococcal infection among HIV infected individuals in New York City. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 12.Curtis A S G. Cell adhesion. Prog Biophys Mol Biol. 1973;27:317–386. [Google Scholar]
- 13.Dealler S F. Antibiotics induce rapid bacterial zeta potential changes. J Antimicrob Chemother. 1991;3:470–473. doi: 10.1093/jac/28.3.470. [DOI] [PubMed] [Google Scholar]
- 14.Dismukes, W. E. 1993. Management of cryptococcosis. Clin. Infect. Dis. 17(Suppl. 2):507–512. [DOI] [PubMed]
- 15.Drgonova J, Drgon T, Tanaka K, Kollar R, Chen G-C, Ford R A, Chan C S M, Takai Y, Cabib E. Rho 1p, a yeast protein at the interface between cell polarization and morphogenesis. Science. 1996;272:277–279. doi: 10.1126/science.272.5259.277. [DOI] [PubMed] [Google Scholar]
- 16.Dromer F, Charreire J. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococcosis and mechanisms of action. J Infect Dis. 1991;163:1114–1120. doi: 10.1093/infdis/163.5.1114. [DOI] [PubMed] [Google Scholar]
- 17.Dromer F, Mathoulin S, Dupont B, Letenneur L, Ronin O. Individual and environmental factors associated with infection due to Cryptococcus neoformans serotype D. French Cryptococcosis Study Group. Clin Infect Dis. 1996;23:91–96. doi: 10.1093/clinids/23.1.91. [DOI] [PubMed] [Google Scholar]
- 18.Dugoni B M, Guglielmo B J, Hollander H. Amphotericin B concentrations in cerebral spinal fluid in patients with AIDS and cryptococcal meningitis. Clin Pharmacol. 1989;8:220–221. [PubMed] [Google Scholar]
- 19.Dykstra M A, Friedman L, Murphy J W. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect Immun. 1977;16:129–135. doi: 10.1128/iai.16.1.129-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franzot S P, Hamdan J S. Effect of amphotericin B on the lipids of five different strains of Cryptococcus neoformans. Mycopathologia. 1994;128:85–89. doi: 10.1007/BF01103014. [DOI] [PubMed] [Google Scholar]
- 21.Franzot S P, Mukherjee J, Cherniak R, Chen L C, Hamdan J S, Casadevall A. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun. 1998;66:89–97. doi: 10.1128/iai.66.1.89-97.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fromtling R A, Shadomy H J, Jacobson E S. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia. 1982;79:23–29. doi: 10.1007/BF00636177. [DOI] [PubMed] [Google Scholar]
- 23.Ghannoum M A, Spellberg B J, Ibrahim A S, Ritchie J A, Currie B, Spitzer E D, Edwards J E J, Casadevall A. Sterol composition of Cryptococcus neoformans in the presence and absence of fluconazole. Antimicrob Agents Chemother. 1994;38:2029–2033. doi: 10.1128/aac.38.9.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetz P J, Penniman J G., Jr A new technique for microelectrophoretic measurements. Am Lab. 1976;1976:212–230. [Google Scholar]
- 25.Ikada Y, Tabata Y. Phagocytosis of bioactive microspheres. J Bioactive Compatible Polymers. 1986;1:32–46. [Google Scholar]
- 26.Ikeda R, Shinoda T, Kagaya K, Fukazawa Y. Role of serum factors in the phagocytosis of weakly or heavily encapsulated Cryptococcus neoformans strains by guinea pig peripheral blood leukocytes. Microbiol Immunol. 1984;28:51–61. doi: 10.1111/j.1348-0421.1984.tb02946.x. [DOI] [PubMed] [Google Scholar]
- 27.Illum L, Jacobson L O, Muller R H, Mak E, Davis S S. Surface characteristics and the interaction of colloidal particles with mouse peritoneal macrophages. Biomaterials. 1987;8:113–117. doi: 10.1016/0142-9612(87)90099-8. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson E S, Tinnell S B. Antioxidant function of fungal melanin. J Bacteriol. 1993;175:7102–7104. doi: 10.1128/jb.175.21.7102-7104.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaguchi H, Koiwai N, Ohtsuka Y, Miyamoto N, Sasakawa S. Phagocytosis of laxex particles by leukocytes. I. Dependence of phagocytosis on the size and surface potentials of particles. Biomaterials. 1986;7:61–66. doi: 10.1016/0142-9612(86)90091-8. [DOI] [PubMed] [Google Scholar]
- 30.Kozel T R, deJong B C, Grinsell M M, MacGill R S, Wall K K. Characterization of anticapsular monoclonal antibodies that regulate activation of the complement system by the Cryptococcus neoformans capsule. Infect Immun. 1998;66:1538–1546. doi: 10.1128/iai.66.4.1538-1546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozel T R, Gotschlich E C. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- 32.Kozel T R, Hermerath C A. Binding of cryptococcal polysaccharide to Cryptococcus neoformans. Infect Immun. 1984;43:879–886. doi: 10.1128/iai.43.3.879-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozel T R, Reiss E, Cherniak R. Concomitant but not causal association between surface charge and inhibition of phagocytosis by cryptococcal polysaccharide. Infect Immun. 1980;29:295–300. doi: 10.1128/iai.29.2.295-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon-Chung K J, Rhodes J C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986;51:218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louria D B. Some aspects of the absorption, distribution, and excretion of amphotericin B in man. Antibiot Med Clin Ther. 1958;5:295–301. [PubMed] [Google Scholar]
- 36.Mitchell T G, Perfect J R. Cryptococcus in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyake Y, Tsunoda T, Minagi S, Akagawa Y, Tsuru H, Suginaka H. Antifungal drugs affect adherence of Candida albicans to acrylic surfaces by changing the zeta-potential of fungal cells. FEMS Microbiol Lett. 1990;69:211–214. doi: 10.1016/0378-1097(90)90067-z. [DOI] [PubMed] [Google Scholar]
- 38.Mozaffarian N, Berman J W, Casadevall A. Enhancement of nitric oxide synthesis by macrophages represents an additional mechanism of action for amphotericin B. Antimicrob Agents Chemother. 1997;41:1825–1829. doi: 10.1128/aac.41.8.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mozaffarian N, Berman J W, Casadevall A. Immune complexes increase nitric oxide production by interferon-gamma-stimulated murine macrophage-like J774.16 cells. J Leukoc Biol. 1995;57:657–662. doi: 10.1002/jlb.57.4.657. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee J, Feldmesser M, Scharff M D, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan enhance fluconazole efficacy. Antimicrob Agents Chemother. 1995;39:1398–1405. doi: 10.1128/aac.39.7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukherjee J, Zuckier L S, Scharff M D, Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994;38:580–587. doi: 10.1128/aac.38.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muratsugu M, Miyake Y, Ishida N, Hyodo A, Terayama K. Decrease in surface charge density of Klebsiella pneumoniae treated with cefodizime and enhancement of phagocytic function of polymorphonuclear lymphocytes stimulated by the drug-treated bacteria. Biol Pharm Bull. 1995;18:1259–1263. doi: 10.1248/bpb.18.1259. [DOI] [PubMed] [Google Scholar]
- 43.Nagura H, Asai J, Katsumata Y, Kojima K. Role of electric surface charge of cell membrane in phagocytosis. Acta Pathol Jpn. 1973;23:279–290. doi: 10.1111/j.1440-1827.1973.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 44.Nassar F, Brummer E, Stevens D A. Different components in human serum inhibit multiplication of Cryptococcus neoformans and enhance fluconazole activity. Antimicrob Agents Chemother. 1995;39:2490–2493. doi: 10.1128/aac.39.11.2490. . (Erratum, 40:1330, 1996.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing for yeasts. Proposed standard M27-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1992. [Google Scholar]
- 46.Nguyen M H, Clancy C J, Yu V L, Yu V C, Morris A J, Snydman D R, Sutton D A, Rinaldi M G. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J Infect Dis. 1998;177:425–430. doi: 10.1086/514193. [DOI] [PubMed] [Google Scholar]
- 47.Nosanchuk J, Casadevall A. Cellular charge of Cryptococcus neoformans: contributions from the capsular polysaccharide, melanin, and monoclonal antibody binding. Infect Immun. 1997;65:1836–1841. doi: 10.1128/iai.65.5.1836-1841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohtomo, T., Y. Ohshima, Y. Usui, Y. Ichiman, N. Yanagisawa, E. Yokota, and J. Shimada. 1995. Effect of sub-inhibitory concentrations of clarithromycin and erythromycin on the production of Staphylococcus aureus capsules. J. Chemother. 7(Suppl. 4):9–11. [PubMed]
- 49.Ramadan M A, Tawfik A F, Shibl A M, Gemmell C G. Postantibiotic effect of azithromycin and erythromycin on streptococcal susceptibility to phagocytosis. J Med Microbiol. 1995;42:362–366. doi: 10.1099/00222615-42-5-362. [DOI] [PubMed] [Google Scholar]
- 50.Richmond D V, Fisher D J. The electrophoretic mobility of microorganisms. Adv Microb Physiol. 1973;9:1–29. doi: 10.1016/s0065-2911(08)60375-6. [DOI] [PubMed] [Google Scholar]
- 51.Saag M S, Dismukes W E. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother. 1988;32:1–8. doi: 10.1128/aac.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sengar A S, Markley N A, Marini N J, Young D. Mkh1, a MEK kinase required for cell wall integrity and proper response to osmotic and temperature stress in Schizosaccharomyces pombe. Mol Cell Biol. 1997;17:3508–3519. doi: 10.1128/mcb.17.7.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Small J M, Mitchell T G. Strain variation in antiphagocytic activity of capsular polysaccharides from Cryptococcus neoformans serotype A. Infect Immun. 1989;57:3751–3756. doi: 10.1128/iai.57.12.3751-3756.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spitzer E D, Spitzer S G, Freundlich L F, Casadevall A. Persistence of the initial infection in recurrent cryptococcal meningitis. Lancet. 1993;341:595–596. doi: 10.1016/0140-6736(93)90354-j. [DOI] [PubMed] [Google Scholar]
- 55.Tabata Y, Ikada Y. Effect of size and surface charge of polymer microspheres on their phagocytosis by macrophages. Biomaterials. 1988;9:356–361. doi: 10.1016/0142-9612(88)90033-6. [DOI] [PubMed] [Google Scholar]
- 56.Tohyama M, Kawakami K, Saito A. Anticryptococcal effects of amphotericin B is mediated through macrophage production of nitric oxide. Antimicrob Agents Chemother. 1996;40:1919–1923. doi: 10.1128/aac.40.8.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson E, Thorson L, Speert D P. Enhancement of macrophage superoxide anion production by amphotericin B. Antimicrob Agents Chemother. 1991;35:796–800. doi: 10.1128/aac.35.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf J E, Massof S E. In vivo activation of macrophage oxidative burst activity by cytokines and amphotericin B. Infect Immun. 1990;58:1296–1300. doi: 10.1128/iai.58.5.1296-1300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamaguchi H, Abe S, Tokuda Y. Immunomodulating activity of antifungal drugs. Ann N Y Acad Sci. 1993;685:447–457. doi: 10.1111/j.1749-6632.1993.tb35905.x. [DOI] [PubMed] [Google Scholar]
- 60.Yasuoka A, Kohno S, Yamada H, Kaku M, Koga H. Influence of molecular sizes of Cryptococcus neoformans capsular polysaccharide on phagocytosis. Microbiol Immunol. 1994;38:851–856. doi: 10.1111/j.1348-0421.1994.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 61.Zuger A, Louie E, Holzman R S, Simberkoff M S, Rahal J J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome: diagnostic features and outcome of treatment. Ann Intern Med. 1986;104:234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]