Abstract
A next-generation anthrax vaccine candidate, AV7909, is being developed for post-exposure prophylaxis (PEP) of inhalational anthrax in combination with the recommended course of antimicrobial therapy. Clinical efficacy studies of anthrax countermeasures in humans are not ethical or feasible, therefore, licensure of AV7909 for PEP is being pursued under the US Food and Drug Administration (FDA) Animal Rule, which requires that evidence of effectiveness be demonstrated in an animal model of anthrax, where results of studies in such a model can establish reasonable likelihood of AV7909 to produce clinical benefit in humans. Initial development of a PEP model for inhalational anthrax included evaluation of post-exposure ciprofloxacin pharmacokinetics (PK), tolerability and survival in guinea pigs treated with various ciprofloxacin dosing regimens. Three times per day (TID) intraperitoneal (IP) dosing with 7.5 mg/kg of ciprofloxacin initiated 1 day following inhalational anthrax challenge and continued for 14 days was identified as a well tolerated partially curative ciprofloxacin treatment regimen. The added benefit of AV7909 vaccination was evaluated in guinea pigs given the partially curative ciprofloxacin treatment regimen. Groups of ciprofloxacin-treated guinea pigs were vaccinated 1 and 8 days post-challenge with serial dilutions of AV7909, a 1:16 dilution of AVA, or normal saline. A group of untreated guinea pigs was included as a positive control to confirm lethal B. anthracis exposure. Post-exposure vaccination with the AV7909 anthrax vaccine candidate administered in combination with the partially curative ciprofloxacin treatment significantly increased survival of guinea pigs compared to ciprofloxacin treatment alone. These results suggest that the developed model can be useful in demonstrating added value of the vaccine for PEP.
INTRODUCTION
Anthrax is considered a serious biological terrorism and military threat due to the relative ease of weaponizing Bacillus anthracis spores and highly lethal effects of inhalational exposure to the spores [1]. The virulence of B. anthracis is predicated upon encapsulation and secretion of two polypeptide-based toxins, lethal toxin (LT), consisting of protective antigen (PA) and lethal factor (LF), and edema toxin (ET), comprised of PA and edema factor (EF). PA binds to host cell receptors and forms a multimeric complex that competitively binds LF or EF and allows their translocation into the cytoplasm [2]. LF inhibits mitogen-activated protein kinase kinases, leading to macrophage apoptosis, and is considered the predominant cause of severe disease and death following exposure to anthrax spores [3, 4]. EF, a calcium-dependent adenylate cyclase, increases cyclic adenosine monophosphate levels in susceptible cells, which results in altered water homeostasis, leading to edema [3, 4, 5].
Post-exposure antimicrobial treatment can reduce the incidence or progression of disease but cannot protect against the disease resulting from germination of residual spores that may remain after cessation of the recommended antimicrobial regimen [6]. Such additional protection may be achieved by post-exposure prophylaxis (PEP) vaccination, which would be used in conjunction with antimicrobial treatment [7, 8]. BioThrax® (Anthrax Vaccine Adsorbed, AVA; Emergent BioSolutions; Lansing, MI), the anthrax vaccine currently licensed for both pre-exposure prophylaxis in persons at high risk of exposure and PEP of anthrax consists of filtered B. anthracis culture supernatant adsorbed to Alhydrogel®. The predominant means of protection provided by this vaccine is the neutralizing antibodies generated against PA.
A novel anthrax vaccine candidate, AV7909, which consists of the bulk drug substance of BioThrax vaccine and a CPG 7909 oligodeoxynucleotide (ODN) as an adjuvant, is currently being developed. Although BioThrax vaccine has been demonstrated to be safe and effective against anthrax in animal studies and clinical trials [9, 10, 11], AV7909 may offer certain advantages. In recent clinical studies, subjects vaccinated with two intramuscular (IM) doses of AV7909 exhibited higher peak titers of the neutralizing antibodies than those vaccinated with BioThrax and reached the peak titers a week earlier than BioThrax-immunized subjects [12, 13, 14]. This earlier response suggests that AV7909 has the potential to induce an enhanced immune response resulting in a more rapid protection against infection, thus making AV7909 a good candidate for use in a PEP regimen.
Because assessing efficacy of anthrax countermeasures in humans is not ethical or feasible, the ability of an anthrax vaccine to provide such added benefit must be demonstrated in an animal anthrax challenge model. Rabbits and nonhuman primates (NHPs) are the most commonly used animal models of inhalational anthrax [15]. However, the activity of CPG 7909 in rabbits has generally been observed to be significantly weaker compared to the level of activity seen in monkeys, humans, mice, and other vertebrates [16, 17]. To overcome this limitation, development of an alternative small animal model for inhalational anthrax is required. Guinea pigs have been used to study anthrax pathogenesis [18, 19] and assess immunogenicity and efficacy of anthrax vaccines [20]. Furthermore, guinea pigs have been reported to respond strongly to anthrax vaccines with CPG ODN adjuvants [21, 22]. The purpose of the studies described here was to develop a guinea pig model for the evaluation of PEP efficacy of the AV7909 vaccine candidate.
The current standard of care for PEP of inhalational anthrax is a course of antimicrobial therapy [1, 23]. For licensure of an anthrax vaccine for the PEP indication, the FDA requires demonstration of the ability of the post-exposure vaccination, administered in combination with an antimicrobial, to increase animal survival compared to antimicrobial treatment alone in an animal model that reasonably predicts response in humans. Such an animal model of vaccine efficacy requires the use of a partially curative antimicrobial regimen, designed to afford partial protection due to the truncated treatment schedule while maintaining a therapeutic dose [7, 8]. The first step in identifying such an antimicrobial regimen is an assessment of tolerability and pharmacokinetics (PK) of the antimicrobial in the selected species. In particular, it is important to understand the relationship between the minimal inhibitory concentration (MIC) of the antimicrobial for B. anthracis and the drug exposure parameters in guinea pigs. Based on this relationship, a therapeutic level of the drug can be determined, which must be maintained until the vaccine-induced protective immune response is generated.
The studies described here were aimed at evaluating tolerability and PK of ciprofloxacin in guinea pigs, followed by identifying a partially curative ciprofloxacin regimen and evaluating the ability of the co-administered AV7909 vaccine candidate to increase animal survival in a PEP scenario compared with ciprofloxacin treatment alone.
MATERIALS AND METHODS
Animals
Male and female Hartley guinea pigs (Cavia porcellus), with a target weight of 600 to 700 g were obtained from Charles River Laboratories (Wilmington, MA). All animals were in good health, free of malformations, and free of clinical signs of disease prior to placement on the study. Animals were individually housed in suspended polycarbonate bedding cages on a stainless steel rack equipped with an automatic watering system. Certified Guinea Pig Chow pellets (PMI Nutrition International, St. Louis, MO) and water were available ad libitum.
Animal studies were performed at Battelle Biomedical Research Center (West Jefferson, OH), and all animal procedures were approved by Battelle’s Institutional Animal Care and Use Committee (IACUC). The studies were conducted in compliance with the Animal Welfare Act and followed the principles of the Guide for the Care and Use of Laboratory Animals from the National Research Council.
Prior to blood collection, animals were anesthetized using 80 mg/kg of ketamine administered IP and 10 mg/kg of xylazine administered subcutaneously (SC). Blood was collected from the cranial vena cava or by cardiac puncture (for terminal collections).
Aerosol Exposure
B. anthracis Ames strain spores were prepared and characterized as described previously [23]. Aqueous suspensions of B. anthracis spores were aerosolized by a six-jet Collison nebulizer and delivered to the guinea pigs using the nose-only aerosol exposure system (CH Technologies Tower, Westwood, NJ) [25]. The inhaled dose was determined based on the bacterial exposure concentration, duration of the exposure, and the animal respiration parameters estimated using Guyton’s formula [26].
Ciprofloxacin Administration
Ciprofloxacin for injection was acquired from Hospira (Lake Forest, IL), packaged in 20-mL glass vials at a concentration of 10 mg/mL, and administered directly from the stock vial with no diluent added. Dosage was based upon the ciprofloxacin stock concentration and weight of the animal on the day prior to each treatment time point. Forty-four animals (equal number of males and females) were dosed with of 7.5 mg/kg, 10 mg/kg, 15 mg/kg, or 20 mg/kg of ciprofloxacin as a single intraperitoneal (IP) injection. Blood collections were performed by cardiac or superior (cranial) vena cava puncture at 0.5, 1, 2, 4, 8, and 12 hours. In addition, 54 animals were administered a single dose of 10 mg/kg of ciprofloxacin via the intragastric (IG) route and their blood was collected at 0.5, 1, 4, 6, 8, and 12 hours.
Ciprofloxacin Level Determination
Quantitative analysis of ciprofloxacin in guinea pig serum was performed by High Performance Liquid Chromatography (HPLC) using a Shimadzu Prominence HPLC system (Shimadzu Scientific Instruments, Columbia, MD) coupled to a tandem liquid chromatography–mass spectrometry (LC-MS/MS) AB Sciex Triple Quad 5500 System (Framingham, MA). Calibration standards and quality control samples were prepared in control serum with ciprofloxacin-d8 (Sigma-Aldrich, St. Louis, MO) used as the internal standard. The lower limit of quantitation (LLOQ) for the assay was determined to be 25.0 ng/mL and the upper limit of quantitation (ULOQ) was determined to be 10,000 ng/mL using a 50 μL serum sample size. Samples were processed using Isolute PPT+ Protein Precipitation Plates (Biotage, Charlotte, NC) along with acetonitrile. Samples were analyzed using a Zorbax Eclipse Plus C18 column (Agilent Technologies, Santa Clara, CA) along with an isocratic elution of 0.2 mL/min of formic acid in water and acetonitrile. Instrument conditions were optimized using the Turbo Ion Spray in positive multiple reaction monitoring (MRM) mode for ciprofloxacin at m/z 332.3→288.3 and for ciprofloxacin-d8 at m/z 340.2→296.2.
Ciprofloxacin Tolerability Assessment
Ciprofloxacin tolerability was assessed in animals at dose levels of 10, 15, or 20 mg/kg administered via IP injection three times daily (TID) for 14 consecutive days or at dose levels of 10, 20, or 30 mg/kg administered IG using the same schedule. The body weights were recorded daily and used for determining the dose volume throughout the 14-day treatment period. Animals were monitored three times daily with 7 to 8 hours between observations.
Partially Curative Ciprofloxacin Regiment Development
The study design for developing a partially curative ciprofloxacin regimen is summarized in Table 1. The study was conducted in 4 iterations. As a result of the excessive weight loss observed in some of the animals administered the 10 mg/kg dose of ciprofloxacin in Iterations 1 and 2, two additional iterations were performed to investigate the effects of lower doses (5 and 7.5 mg/kg).
Table 1:
Development of Partially Curative Ciprofloxacin Regimen: Study Design
| Group | Number of Animals (M/F) | Treatment | Dose (mg/kg) | Treatment Schedule | Blood Collection Schedule (Days) |
|---|---|---|---|---|---|
|
| |||||
| Iteration 1 | |||||
|
| |||||
| 1 | 4/4 | Saline | -- | 14 days TID starting 20–24 h post-challenge1 | 7, 132, 343, terminal4 |
| 2 | 10/10 | Ciprofloxacin | 10 | ||
|
| |||||
| Iteration 2 | |||||
|
| |||||
| 1 | 4/4 | Saline | -- | 14 days TID starting 20–24 h post-challenge | 7, 13, 34, terminal |
| 2 | 10/10 | Ciprofloxacin | 10 | ||
|
| |||||
| Iteration 3 | |||||
|
| |||||
| 1 | 8/7 | Ciprofloxacin | 5 | 14 days TID starting 20–24 h post-challenge | 7, 13, 34, terminal |
| 2 | 7/8 | Ciprofloxacin | 7.5 | ||
|
| |||||
| Iteration 4 | |||||
|
| |||||
| 1 | 8/8 | Ciprofloxacin | 5 | 14 days TID starting 20–24 h post-challenge | 7, 13, 34, terminal |
| 2 | 8/8 | Ciprofloxacin | 7.5 | ||
TID = three times per day; M = male; F = female
Animals were challenged on study day 0 at time 0 with a target dose of 200 LD50 of aerosolized B. anthracis spores
30 minutes (± 15 min) after the first ciprofloxacin treatment of the day
8 hours (± 1 h) after the last ciprofloxacin treatment
Terminal blood collection, if possible, on study day 34 or from any animal found dead or euthanized due to moribund condition
On the first day of each iteration of the study, animals were challenged with a target dose of aerosolized B. anthracis spores exceeding the 50% lethal dose (LD50) by 200-fold, as described previously [25]. Beginning 20 to 24 hours following a lethal anthrax spore challenge, ciprofloxacin was administered IP TID for 14 days post-challenge at dose levels of 5, 7.5, or 10 mg/kg. The animals were observed for morbidity and mortality and euthanized 21 days after the final ciprofloxacin treatment. Blood samples were collected prior to anthrax challenge, 30 minutes after the morning ciprofloxacin injection on day 8, and 8 hours after the last treatment on day 15 to evaluate peaks and troughs of plasma ciprofloxacin levels among the groups.
Assessment of PEP Efficacy of AV7909
The study design for evaluating PEP efficacy with AV7909 is summarized in Table 2. On the first day of the study, groups of 18 (equal number of males and females) animals each were challenged with targeted aerosolized anthrax spores of 200 LD50 (Table 2). Ciprofloxacin treatment (7.5 mg/kg TID for 14 days via IP injection) was initiated within 24 hours following B. anthracis challenge. At the same time, animals were vaccinated with serial dilutions of AV7909, a 1:16 dilution of BioThrax, or 0.5 mL of normal saline (ciprofloxacin-only control). Eight guinea pigs served as a positive control for lethal exposure to B. anthracis and were neither vaccinated nor treated with ciprofloxacin.
Table 2:
PEP Efficacy Study Design
| Group | No. of Animals | Vaccine, Dilution | Vaccination Days Post-Challenge | Ciprofloxacin Treatment |
|---|---|---|---|---|
|
| ||||
| 1 | 18 | AV7909, 1:4 | ||
| 2 | 18 | AV7909, 1:16 | ||
| 3 | 18 | AV7909, 1:64 | ||
| 41 | 17 | AV7909, 1:128 | 1,8 | 7.5 mg/kg, TID, 14 days |
| 5 | 18 | AV7909, 1:256 | ||
| 6 | 18 | AVA, 1:16 | ||
| 7 | 18 | Normal Saline | ||
|
| ||||
| 8 | 14 | None | None | None |
One group 4 animal was removed from the study due to a non-study-related reason; as a result, group 4 had 17 animals instead of the 18 animals.
Hematology and Clinical Chemistry
Hematology assessment was performed on whole blood samples using an Advia® 120 Hematology Analyzer (Siemens, Deerfield, IL) and included the following parameters: red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), platelet count (PLT), mean platelet volume (MPV) and total and differential white blood cell parameters. For clinical chemistry assessment, serum samples were analyzed using an Advia 1200 Chemistry Analyzer (Siemens Medical Solutions Diagnostics, Tarrytown, NY) for serum proteins, liver function enzymes, kidney function parameters, electrolytes, and C-reactive protein (CRP).
Gross and Microscopic Pathology
All animals found dead or euthanized were subjected to necropsy. The tissues collected included the brain, heart, large intestine (cecum, colon, and rectum), kidneys, liver, lungs, lymph nodes (bronchial, mediastinal, mandibular, and mesenteric), mammary glands, pancreas, small intestine (duodenum, jejunum, and ileum), spleen, and stomach, as well as any other abnormal tissues or gross lesions. Tissue samples were preserved in 10% neutral buffered formalin, paraffin-embedded, and the slides were stained with hematoxylin and eosin. The microscopic findings were graded semi-quantitatively. A numerical score (Grade 1 through 4) was used to describe average severity grade for each lesion as well as the extent of bacterial invasion of the tissue.
Bacterial Burden Assessment
For the qualitative bacteremia assessment, 30 to 40 μL of whole blood was inoculated onto blood agar plates and the plates were incubated at 37°C for a minimum of 48 hours. A plate containing at least one colony with morphology consistent with B. anthracis was considered positive for B. anthracis. For the quantitative assessment, 100 μL of whole blood was plated in triplicate on tryptic soy agar (TSA). In addition, 10-fold serial dilutions were performed by transferring 100 μL of whole blood or previous dilution into 900 μL of phosphate-buffered saline (PBS). For each dilution prepared, 100 μL was plated in triplicate on TSA. Plates were incubated at 37°C for 16 to 24 hours, bacterial colonies enumerated, and the corresponding colony forming units per mL (CFU/mL) concentrations calculated.
Immune Response Assessment
Anthrax toxin-neutralizing antibody (TNA) levels in guinea pig serum samples were determined using the TNA assay, as described previously [22]. Anti-PA immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) was performed using the methodology described previously [27], optimized for the guinea pig serum matrix.
Statistical Analysis
Analysis of variance (ANOVA) was used to compare body weights, ciprofloxacin concentrations, anti-PA IgG levels, and TNA titers across groups. Pairwise two-sided Fisher’s exact tests were performed to determine if the proportion of surviving animals was significantly different among the groups as well as to compare proportions of bacteremic animals across groups on each study day. Time-to-death data were analyzed in combination with survival data to determine if there were significant differences among the groups. A Kaplan-Meier curve was plotted for each group, and pairwise log-rank tests were used to determine which groups were significantly different from each other.
RESULTS
Ciprofloxacin PK
Following IP administration, the maximum concentration of ciprofloxacin (Cmax) and the area under the concentration-time curve (AUC∞) increased with an increase in dose. The time to maximum concentration (Tmax) was similar across all doses and occurred between 30 minutes and 1 hour. The Tlast values fell at 12 hours post-treatment for all dose groups, except for the male animals in the 10 mg/kg group for which Tlast fell at 8 hours post-treatment. The elimination half-life ranged from 1.5 to 2 hours (Table 3).
Table 3:
PK Parameters for Ciprofloxacin in Guinea Pigs Following IP Administration
| Group-Target Dose (mg/kg) | Gender | Cmax (ng/mL) | Tmax (h) | Tlast (h) | Elimination Half-Life (h) | AUClast (h*ng/mL) | AUC∞ (h*ng/mL) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 7.5 | Male | 1370 | 0.667 | 6.67 | 1.32 | 3120 | 3240 |
| Female | 903 | 1.00 | 6.67 | 2.41 | 2140 | 2520 | |
| 10 | Male | 2500 | 1.00 | 8.00 | 1.52 | 6630 | 6810 |
| Female | 2770 | 0.500 | 12.0 | 1.71 | 6520 | 6570 | |
| 15 | Male | 4640 | 0.528 | 12.0 | 1.99 | 9070 | 9190 |
| Female | 3680 | 1.00 | 12.0 | 1.71 | 9180 | 9250 | |
| 20 | Male | 6240 | 0.539 | 12.0 | 1.83 | 12,400 | 12,600 |
| Female | 5300 | 0.500 | 12.0 | 1.90 | 11,900 | 12,000 | |
Cmax = peak concentration; Tmax = time to peak concentration; Tlast = time to last measurable concentration; AUC = area under the concentration-time curve; AUClast = AUC to last measurable concentration; AUC∞ = AUC to infinity
IG administration of ciprofloxacin resulted in a much lower plasma concentration of the antimicrobial at all time points, and PK analysis revealed lower Cmax and AUClast values, as well as a shorter elimination half-life, compared to those observed following IP administration (data not shown). Therefore, the IP route of administration was selected for the development of a partially curative ciprofloxacin regimen.
Ciprofloxacin Tolerability
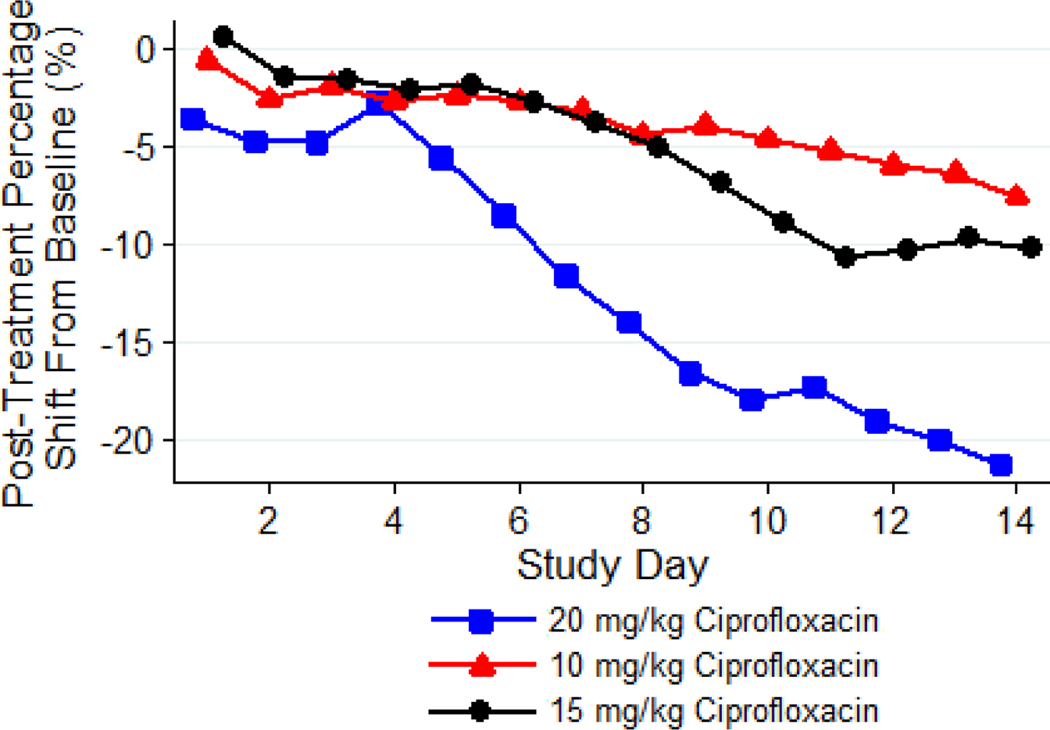
Based on the analysis of weight loss, the 10 mg/kg dose of ciprofloxacin administered IP was well tolerated by guinea pigs, while tolerability was low and moderate at the 20 and 15 mg/kg dose, respectively (Figure 1). The decrease in body weight, compared to baseline, in the group administered 20 mg/kg of ciprofloxacin was significantly greater than in groups receiving lower dose levels of the antimicrobial (P < 0.05).
Figure 1: Mean Post-treatment Percentage Shift in Body Weights from Baseline over Time, by Group.
Guinea pigs (n = 8 per group) were treated with 10, 15, or 20 mg/kg of ciprofloxacin administered via IP injection TID for 14 days. The body weights were recorded daily. Based on the analysis of the weight loss, the 10 mg/kg dose of ciprofloxacin was well tolerated by guinea pigs, while tolerability was low and moderate at the 20 and 15 mg/kg. The decrease in body weight, compared to baseline, in the group administered 20 mg/kg of ciprofloxacin was significantly greater than in groups receiving lower dose levels of the antimicrobial (P < 0.05).
Clinical observations of animals in the 10 mg/kg dose group demonstrated mild abnormal clinical signs throughout the treatment period ranging from a small amount of stool to a few animals exhibiting a rough hair coat and swelling at the site of injection. In contrast, all animals in the 15 mg/kg dose group showed a small amount of stool or no stool and rough hair coats. Three out of eight animals in this group were euthanized prior to the completion of the 14-day treatment regimen due to losing greater than 20% of their weight. The incidence and severity of these abnormal clinical signs in the animals receiving 20 mg/kg of ciprofloxacin were much higher compared to those receiving lower dose levels and, with the exception of one animal that was found dead, all of the animals in this group were euthanized prior to scheduled termination.
The most consistent histopathology findings in animals that received 20 mg/kg of ciprofloxacin included tubular nephritis in the kidneys and necrosis within the abdominal wall, which appeared to be related to the acidic pH (3.5 to 4.6) of the injected solution rather than to a direct effect of the ciprofloxacin itself (data not shown). Changes in complete blood count (CBC) and clinical chemistry parameters were minor and had no clinical significance (data not shown).
Development of Partially Curative Regimen of Ciprofloxacin
The average inhaled dose of aerosolized B. anthracis spores was 270 ± 142 LD50, or 13.5 × 106 CFU based on a previously determined LD50 of 5.0 × 104 CFU [25]. None of the ciprofloxacin-treated animals were bacteremic during the treatment regimen. After completion of 14 days of ciprofloxacin treatment, most non-survivors became bacteremic at the time of death or euthanasia, including all of the animals in the 5 or 7.5 mg/kg dose groups and 77% of the animals treated with 10 mg/kg. Gross lesions typical of B. anthracis infection were present in all animals that died or were euthanized due to moribund condition. There were no gross lesions in any animal that survived to the scheduled termination on day 34.
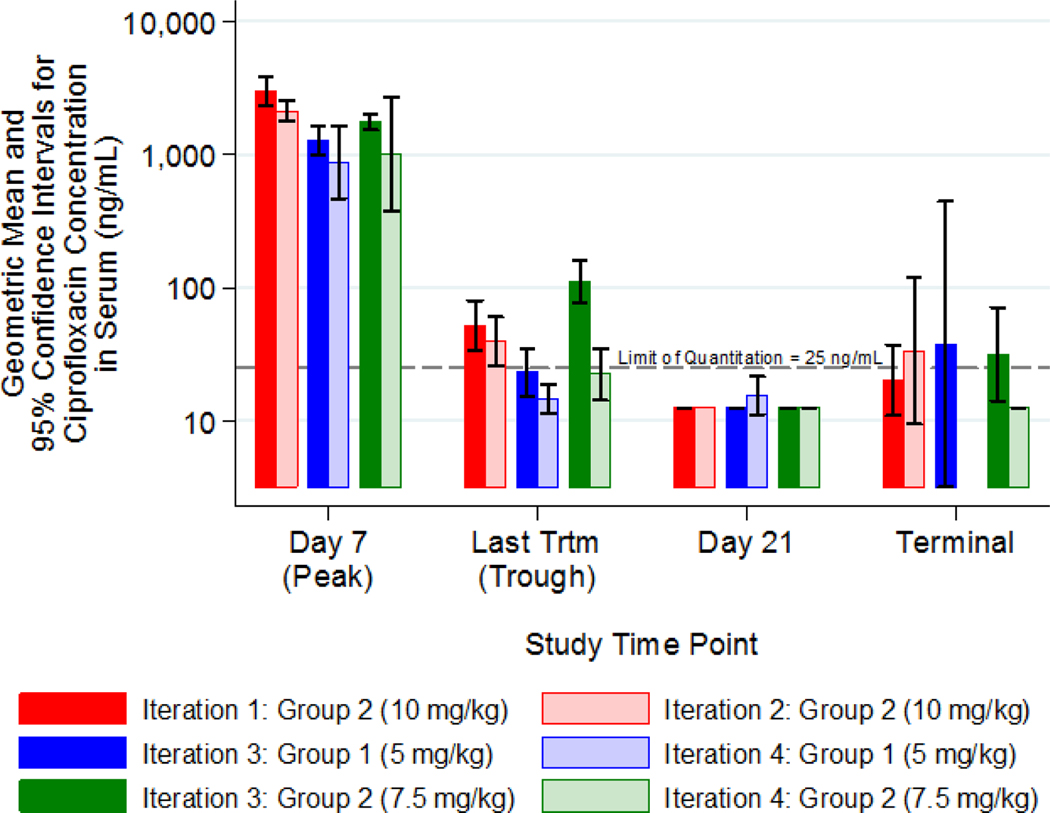
Peak ciprofloxacin concentrations were within the range of the Cmax determined previously in the course of the PK study [2497.05 ng/mL with 95% confidence interval of (1796.30, 3471.18) ng/mL]. The trough concentrations dropped slightly below the ciprofloxacin B. anthracis MIC50 of 64 ng/mL [27] at all dose levels (Figure 2).
Figure 2: Development of Partially Curative Antimicrobial Regimen: Peak and Trough Ciprofloxacin Concentrations (Geometric Means and 95% Confidence Intervals).
Animals were challenged with a target dose of 200 LD50 of aerosolized B. anthracis spores.
Ciprofloxacin was administered via the IP route TID for 14 days post-challenge, starting 20 to 24 hours post-challenge, at dose levels of 5, 7.5, or 10 mg/kg. The animals were observed for morbidity and mortality and euthanized 21 days after the final ciprofloxacin treatment. Blood samples were collected prior to the anthrax challenge, 30 minutes after the morning ciprofloxacin injection on day 8 and 8 hours after the last treatment on day 15, to evaluate peaks and troughs of plasma ciprofloxacin levels among the groups. Peak ciprofloxacin concentrations were dose-dependent. The trough concentrations dropped slightly below the MIC50 of 64 ng/mL at all dose levels.
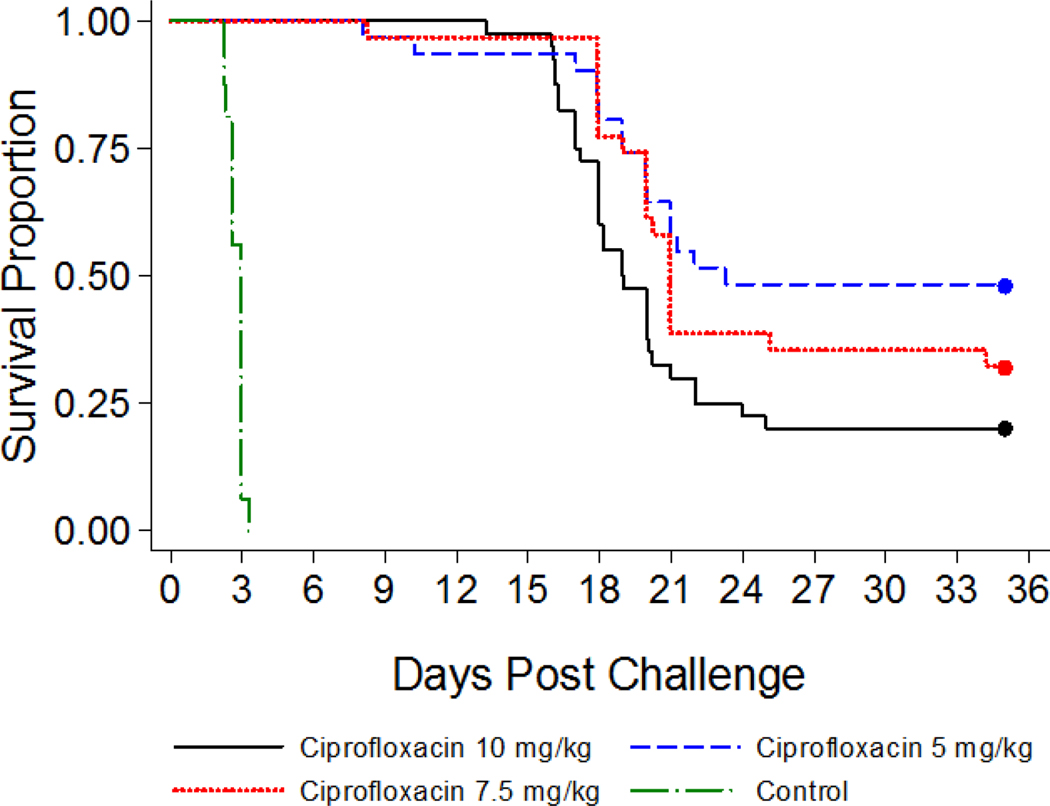
All saline-treated control animals succumbed to anthrax infection within three days of challenge, while most of the ciprofloxacin-treated guinea pigs survived during the treatment phase of the study (Figure 3). After discontinuation of the antimicrobial treatment, 48% of the animals treated with 5 mg/kg of ciprofloxacin survived, while 32% and 20% survival was observed among animals in the 7.5 mg/kg and 10 mg/kg dose group, respectively. The lower survival in the 10 mg/kg treatment group was likely due to ciprofloxacin tolerability issues. In the 5 mg/kg treatment group, the average percent survival was higher but not consistent across two iterations (27 and 69%, respectively). Therefore, the 7.5 mg/kg ciprofloxacin dose was selected for the PEP model based on the consistent low (~30 percent) survival and tolerance of ciprofloxacin at this dose level.
Figure 3: Development of Partially Curative Antimicrobial Regimen: Survival Time to Death.
Time is relative to the end of lethal inhalational challenge to B. anthracis. Animals were challenged with a target dose of 200 LD50 of aerosolized B. anthracis spores and ciprofloxacin was administered IP TID for 14 days post-challenge, starting 20 to 24 hours post-challenge, at dose levels of 5, 7.5, or 10 mg/kg. The animals were observed for morbidity and mortality and euthanized 21 days after the final ciprofloxacin treatment.
PEP Efficacy of AV7909
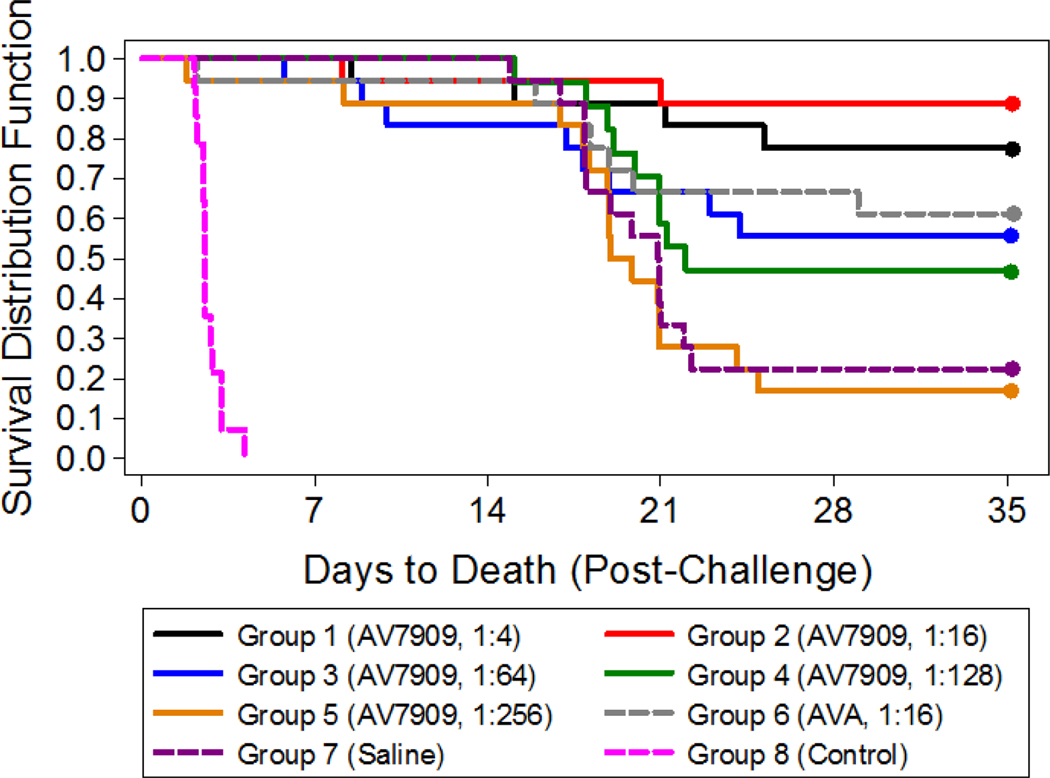
The average inhaled dose of aerosolized B. anthracis Ames strain spores was 385 ± 71 LD50. All animals in the control group succumbed to anthrax disease, while the groups vaccinated with the serial dilutions of AV7909 were protected against challenge in a dose-dependent manner (Figure 4). Importantly, the proportions of surviving animals in the groups vaccinated with 1:4 and 1:16 dilutions of AV7909 were significantly greater (P = 0.0132 and P = 0.0007, respectively) than that in the group that received ciprofloxacin only, indicating that the vaccine afforded additional protection in comparison to ciprofloxacin treatment alone.
Figure 4: PEP Study with AV7909 and Ciprofloxacin: Survival and Time to Death.
Time is relative to the end of lethal inhalational challenge to B. anthracis. Animals (n = 18 in groups 1–3 and 5–7; n = 17 in group 4) were vaccinated 1 and 8 days post-challenge with serial dilutions of AV7909 (groups 1–5), a 1:16 dilution of AVA (group 6), or 0.5 mL of normal saline (group 7). Group 8 (n = 14) served as a positive control for lethal exposures to B. anthracis and were neither vaccinated nor treated with ciprofloxacin. Ciprofloxacin treatment started 1 day post-challenge and continued through 14 days post-challenge (groups 1–7); animals in group 8 (untreated control) were administered normal saline under the same regimen. The proportions of surviving animals in the groups vaccinated with 1:4 and 1:16 dilutions of AV7909 (groups 1 and 2) were significantly greater
(P = 0.0132 and P = 0.0007, respectively) than that in the group that received ciprofloxacin only (group 7).
Mean peak ciprofloxacin concentrations ranged from 1121.9 to 1750.6 ng/mL across all groups, while mean trough concentrations ranged from <LLOQ to 35.764 ng/mL. The ranges of peak and trough levels were similar to those observed previously at this dose level in unvaccinated guinea pigs, indicating that AV7909 vaccination did not affect ciprofloxacin levels.
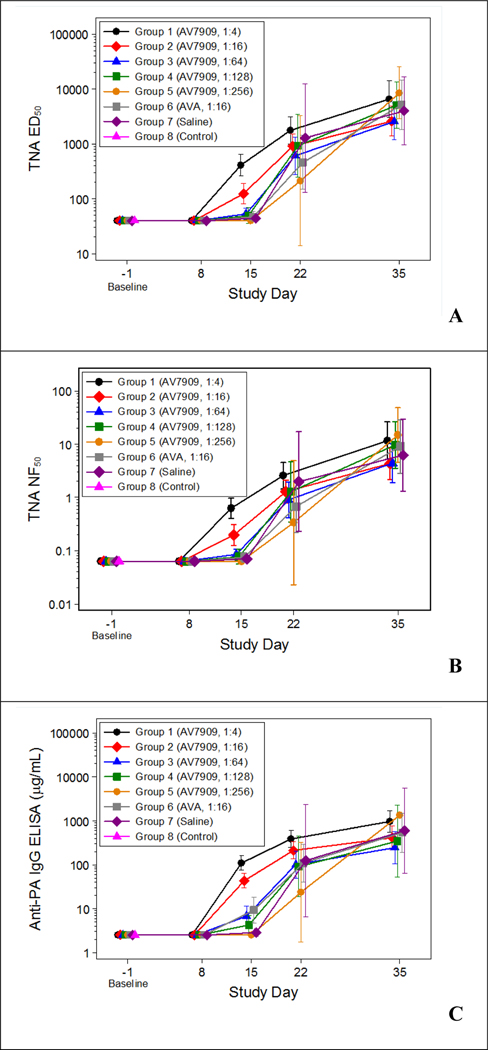
All antibody levels were below the LLOQ prior study initiation and on study day 8. There were significant differences (P < 0.05) among the group geometric means on study day 15 as measured by the TNA assay and anti-PA IgG ELISA (Figure 5). On study day 15, the groups vaccinated with 1:4 and 1:16 dilutions of AV7909 (groups 1 and 2, respectively) had geometric mean TNA 50% effective dilution (ED50), TNA 50% neutralization factor (NF50), and anti-PA IgG ELISA values that were significantly greater than those in groups 3 through 7. Furthermore, the group vaccinated with a 1:4 dilution of AV7909 (group 1) had geometric mean antibody responses that were significantly greater than those in the group vaccinated with a 1:16 dilution of AV7909 (group 2). Also on study day 15, the geometric mean anti-PA IgG ELISA values in the group vaccinated with a 1:64 dilution of AV7909 (group 3) and in the group vaccinated with a 1:16 dilution of AVA (group 6) were significantly greater than that in the group vaccinated with a 1:256 dilution of AV7909 (group 5). Finally, the geometric mean anti-PA IgG ELISA value in the group vaccinated with a 1:16 dilution of AVA (group 6) was significantly greater on study day 15 than that in the antimicrobial-only group (group 7). These data indicate a vaccine dose-dependent immune response to PEP vaccination with AV7909. There were also significant differences among the group geometric means on study day 22 for anti-PA IgG ELISA, with the groups vaccinated with 1:4 and 1:16 dilutions of AV7909 (groups 1 and 2, respectively) having a significantly greater geometric mean than that in the group vaccinated with a 1:256 dilution of AV7909 (group 5). By day 35, differences across vaccine dose groups waned, with all surviving animals exhibiting high antibody levels.
Figure 5: Guinea Pig Immune Response against Anthrax: TNA ED50 (A), TNA NF50 (B), and anti-PA IgG (C) Group Geometric Means with 95 Percent Confidence Intervals by Study Day.
Animals were vaccinated 1 and 8 days post-challenge with serial dilutions of AV7909 (Groups 1–5), a 1:16 dilution of AVA (Group 6), or 0.5 mL of normal saline (Group 7). Group 8 served as a positive control for lethal exposures to B. anthracis and were neither vaccinated nor treated with ciprofloxacin. Immune response analyses were performed as described previously [22, 23]. All animals in the control group (Group 8) died prior to Study Day 8; therefore, Group 8 was not included in the ANOVA models fitted on the post-challenge study days.
DISCUSSION
The purpose of using medical countermeasures for PEP is to prevent onset of disease following a known or suspected exposure to a pathogen. Different forms of PEP may be employed, including active immunization with vaccines, passive immunization with immune globulins, preventive administration of antimicrobial drugs, or a combination of multiple countermeasures. PEP can be an effective method in preventing development of the disease and reducing the risk of secondary transmission of the pathogenic agent. PEP has been recommended for various infectious diseases, including rabies, Varicella zoster, human immunodeficiency virus (HIV), tetanus, viral hepatitis, influenza, group A streptococcal infection, meningococcal infection, pertussis, tuberculosis, anthrax, and others [29, 30].
Development and licensure of PEP countermeasures against life-threatening infectious disease under the FDA Animal Rule, when clinical efficacy studies are not ethical or feasible, require the use of well-characterized animal models, which can demonstrate both the fidelity to the clinical disease scenario and the ability to predict the human response to the PEP intervention [31]. In the case of anthrax, the current PEP animal model paradigm requires that the vaccine is administered concurrently with antimicrobial treatment, following anthrax challenge [32]. As such, development of a PEP animal model for anthrax presents a greater technical challenge than development of a pre-exposure prophylaxis model, in large part because of the very narrow window that must be met for the antimicrobial dose and regimen. Specifically, the key element to the success of such a model is to determine the exact dose and duration of the antimicrobial treatment that allows a sufficient number of animals to survive long enough for a vaccine-induced protective immune response to develop, without rescuing all of the animals thereby obscuring any added protective effect of the vaccine. Indeed, while the National Institute of Allergy and Infectious Diseases (NIAID) successfully developed a rabbit PEP model for anthrax, successful development of a reproducible NHP PEP model proved to be technically challenging because of an inability to find a reproducible, partially protective antimicrobial regimen, despite intense effort and dedication of significant resources [32].
Our approach followed the general strategy employed by NIAID for the development of the rabbit PEP model. Specifically, we targeted a subcurative antibacterial treatment resulting in a consistent 30 to 50% animal survival rate, which would allow demonstration of the added value of the vaccine if survival outcomes significantly increased in vaccinated animals compared to those subjected to antimicrobial treatment only. In rabbit studies, dosing animals once a day (SID) for seven days with levofloxacin at 50 mg/kg via oral gavage, with the first dose administered within 6 to 12 hours after lethal anthrax challenge, resulted in a survival rate that varies between 23% [6] and 56% [8].
In the guinea pig model, treatment with ciprofloxacin, tetracycline, erythromycin, cefazolin, and trimethoprim-sulfamethoxazole initiated 24 hours post-infection TID for a period of 14 days was evaluated [33]. In these studies, the 10 mg/kg dose of ciprofloxacin was found to be well tolerated and allowed 55% of animals to survive, while higher doses were not well tolerated. Our results confirmed these findings, in that ciprofloxacin doses above 10 mg/kg resulted in major adverse effects associated with weight loss.
We were able to achieve consistent survival of 32% of guinea pigs, when ciprofloxacin was administered IP TID for 14 days at the dose level 7.5 mg/kg, starting 20 to 24 hours post-challenge. As observed with the rabbit PEP model, 100% of the animals survived during the antimicrobial treatment and death was only observed after antimicrobial discontinuation. This finding was consistent with the ability of this antimicrobial regimen to maintain ciprofloxacin plasma concentrations above the MIC during most of the treatment period.
Having demonstrated suitability of the developed guinea pig model for the assessment of the PEP efficacy of an anthrax vaccine, we were able to apply the model to assessing the efficacy of an investigational next-generation anthrax vaccine candidate, AV7909, in a PEP scenario. When administered after lethal anthrax aerosol anthrax exposure, AV7909 significantly improved protection of the animals as compared to antimicrobial treatment alone. Protection of animals after discontinuation of ciprofloxacin treatment was likely the result of a robust anthraxtoxin neutralizing antibody response observed in the study.
In summary, the guinea pig model described here appears suitable for the evaluation of PEP efficacy of anthrax vaccines in support of licensure of these medical countermeasures under the FDA Animal Rule. Furthermore, evaluation of AV7909 efficacy in the developed animal model following lethal challenge with aerosolized B. anthracis spores demonstrated that vaccination increased the guinea pig survival over that observed with post-exposure antimicrobial treatment alone. Importantly, vaccination of guinea pigs with a range of doses of AV7909 resulted in a dose-dependent immune response on days 15 and 22, as measured by TNA and anti-PA IgG ELISA.
It should be noted that the developed guinea pig PEP model is not designed to identify the protective neutralizing antibody level predictive of clinical benefit in humans. Rather, its purpose is to model the clinical PEP scenario and demonstrate the added survival benefit conferred by the vaccine compared to the antibiotic treatment alone. As such, the model can provide supportive evidence of AV7909 effectiveness for PEP.
ACKNOWLEDGMENTS
The authors thank Greg Stark, Mike Anderson, and Nancy Niemuth for statistical analysis of the data. This project was funded, in whole or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract HHSN272200800051C.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT Author Statement
Mark R. Perry: Investigation, Data Curation, Writing - Original Draft
Boris Ionin: Methodology, Writing - Review and Editing
Roy E. Barnewall: Investigation, Data Curation
Michelle L. Vassar: Investigation, Data Curation
Joshua J. Reece: Conceptualization, Project administration
Sukjoon Park: Conceptualization, Project administration
Laurence Lemiale: Project administration
Mario H. Skiadopoulos: Supervision
Jeffry D. Shearer: Supervision, Writing - Review and Editing
Vladimir Savransky: Conceptualization, Methodology, Writing - Review and Editing
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Anthrax as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 1999; 281(18):1735–1745. Review. Erratum in: JAMA 2000; 283(15):1963. [DOI] [PubMed] [Google Scholar]
- 2.Abrami L, Lindsay M, Parton RG, Leppla SH, van der Goot FG. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J Cell Biol. 2004; 166(5):645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brossier F, Mock M. Toxins of Bacillus anthracis. Toxicon. 2001; 39(11):1747–1755. [DOI] [PubMed] [Google Scholar]
- 4.Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003; 19:45–70. [DOI] [PubMed] [Google Scholar]
- 5.Bann JG, Hultgren SJ. Structural biology: anthrax hijacks host receptor. Nature. 2004; 430(7002):843–844. [DOI] [PubMed] [Google Scholar]
- 6.Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J Hyg (Lond). 1956; 54(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ionin B, Hopkins RJ, Pleune B, Sivko GS, Reid FM, Clement KH, Rudge TL Jr, Stark GV, Innes A, Sari S, Guina T, Howard C, Smith J, Swoboda ML, Vert-Wong E, Johnson V, Nabors GS, Skiadopoulos MH. Evaluation of immunogenicity and efficacy of anthrax vaccine adsorbed for postexposure prophylaxis. Clin Vaccine Immunol. 2013; 20(7):1016–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leffel EK, Bourdage JS, Williamson ED, Duchars M, Fuerst TR, Fusco PC. Recombinant protective antigen anthrax vaccine improves survival when administered as a postexposure prophylaxis countermeasure with antibiotic in the New Zealand white rabbit model of inhalation anthrax. Clin Vaccine Immunol. 2012; 19(8):1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brachman PS, Gold H, Plotkin SA, Fekety FR, Werrin M, Ingraham NR. Field Evaluation of a Human Anthrax Vaccine. Am J Public Health Nations Health. 1962; 52(4):632–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fellows PF, Linscott MK, Ivins BE, Pitt ML, Rossi CA, Gibbs PH, Friedlander AM. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine. 2001; 19(23–24):3241–3247. Erratum in: Vaccine 2001; 20(3–4):635. [DOI] [PubMed] [Google Scholar]
- 11.Quinn CP, Sabourin CL, Niemuth NA, Li H, Semenova VA, Rudge TL, Mayfield HJ, Schiffer J, Mittler RS, Ibegbu CC, Wrammert J, Ahmed R, Brys AM, Hunt RE, Levesque D, Estep JE, Barnewall RE, Robinson DM, Plikaytis BD, Marano N; AVRP Laboratory Working Group. A three-dose intramuscular injection schedule of anthrax vaccine adsorbed generates sustained humoral and cellular immune responses to protective antigen and provides long-term protection against inhalation anthrax in rhesus macaques. Clin Vaccine Immunol. 2012; 19(11):1730–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rynkiewicz D, Rathkopf M, Sim I, Waytes AT, Hopkins RJ, Giri L, DeMuria D, Ransom J, Quinn J, Nabors GS, Nielsen CJ. Marked enhancement of the immune response to BioThrax® (Anthrax Vaccine Adsorbed) by the TLR9 agonist CPG 7909 in healthy volunteers. Vaccine. 2011; 29(37):6313–6320. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins RJ, Daczkowski NF, Kaptur PE, Muse D, Sheldon E, LaForce C, Sari S, Rudge TL, Bernton E. Randomized, double-blind, placebo-controlled, safety and immunogenicity study of 4 formulations of Anthrax Vaccine Adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers. Vaccine. 2013; 31(30):3051–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hopkins RJ, Kalsi G, Montalvo-Lugo VM, Sharma M, Wu Y, Muse DD, Sheldon EA, Hampel FC, Lemiale L. Randomized, double-blind, active-controlled study evaluating the safety and immunogenicity of three vaccination schedules and two dose levels of AV7909 vaccine for anthrax post-exposure prophylaxis in healthy adults. Vaccine. 2016; 34(18):2096–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CBER. 23 April 2002. Anthrax vaccines: efficacy testing and surrogate markers of immunity. U.S. Department of Health and Human Services, Food and Drug Administration. http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/TranscriptsMinutes/UCM054606.pdf. [Google Scholar]
- 16.Rankin R, Pontarollo R, Ioannou X, Krieg AM, Hecker R, Babiuk LA, van Drunen Littel-van den Hurk S. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev. 2001; 11(5):333–340. [DOI] [PubMed] [Google Scholar]
- 17.Ionin B. Development of a Guinea Pig Model for Post-Exposure Prophylaxis of Inhalation Anthrax. 8th Annual Biodefense Vaccines & Therapeutics Conference, Animal Model Forum, June 15, 2010. [Google Scholar]
- 18.Ross JM. On the histopathology of experimental anthrax in the guinea-pig. Br J Exp Pathol. 1955; 36(3):336–339. [PMC free article] [PubMed] [Google Scholar]
- 19.Ross JM. The pathogenesis of anthrax following the administration of spores by the respiratory route. J. Pathol. Bacteriol. 1957; 73:485–494. [Google Scholar]
- 20.Ivins BE, Ezzell JW Jr, Jemski J, Hedlund KW, Ristroph JD, Leppla SH. Immunization studies with attenuated strains of Bacillus anthracis. Infect Immun. 1986; 52(2):454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu M, Hine PM, James Jackson W, Giri L, Nabors GS. Increased potency of BioThrax anthrax vaccine with the addition of the C-class CpG oligonucleotide adjuvant CPG 10109. Vaccine. 2007; 25(3):526–534. [DOI] [PubMed] [Google Scholar]
- 22.Savransky V, Shearer JD, Gainey MR, Sanford DC, Sivko GS, Stark GV, Li N, Ionin B, Lacy MJ, Skiadopoulos MH. Correlation between anthrax lethal toxin-neutralizing antibody levels and survival in guinea pigs and nonhuman primates vaccinated with the AV7909 anthrax vaccine candidate. Vaccine. 2017; 35(37):4952–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semenova VA, Schiffer J, Steward-Clark E, Soroka S, Schmidt DS, Brawner MM, Lyde F, Thompson R, Brown N, Foster L, Fox S, Patel N, Freeman AE, Quinn CP. Validation and long-term performance characteristics of a quantitative enzyme linked immunosorbent assay (ELISA) for human anti-PA IgG. J Immunol Methods. 2012; 376(1–2):97–107. [DOI] [PubMed] [Google Scholar]
- 24.Kao LM, Bush K, Barnewall R, Estep J, Thalacker FW, Olson PH, Drusano GL, Minton N, Chien S, Hemeryck A, Kelley MF. Pharmacokinetic considerations and efficacy of levofloxacin in an inhalational anthrax (postexposure) rhesus monkey model. Antimicrob Agents Chemother. 2006; 50(11):3535–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savransky V, Sanford DC, Syar E, Austin JL, Tordoff KP, Anderson MS, Stark GV, Barnewall RE, Briscoe CM, Lemiale-Biérinx L, Park S, Ionin B, Skiadopoulos MH. Pathology and pathophysiology of inhalational anthrax in a guinea pig model. Infect Immun. 2013; 81(4):1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyton AC. Measurement of the respiratory volumes of laboratory animals. Am J Physiol. 1947; 150(1):70–7. [DOI] [PubMed] [Google Scholar]
- 27.Turnbull PC, Sirianni NM, LeBron CI, Samaan MN, Sutton FN, Reyes AE, Peruski LF Jr. MICs of selected antibiotics for Bacillus anthracis, Bacillus cereus, Bacillus thuringiensis, and Bacillus mycoides from a range of clinical and environmental sources as determined by the Etest. J Clin Microbiol. 2004. Aug;42(8):3626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inglesby TV, O’Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K; Working Group on Civilian Biodefense. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA. 2002; 287(17):2236–2252. Erratum in: JAMA 2002; 288(15):1849. [DOI] [PubMed] [Google Scholar]
- 29.Bader MS, McKinsey DS. Postexposure prophylaxis for common infectious diseases. Am Fam Physician. 2013; 88(1):25–32. Review. Erratum in: Am Fam Physician. 2013; 88(10):636. [PubMed] [Google Scholar]
- 30.Bader MS, Brooks A, Kelly DV, Srigley JA. Postexposure management of infectious diseases. Cleve Clin J Med. 2017; 84(1):65–80. [DOI] [PubMed] [Google Scholar]
- 31.CDER and CBER. Product Development Under the Animal Rule: Guidance for Industry. U.S. Department of Health and Human Services, Food and Drug Administration. October 2015. https://www.fda.gov/media/88625/download. [Google Scholar]
- 32.CBER. Pathway to Licensure for Protective Antigen-based Anthrax Vaccines for a Post-exposure Prophylaxis Indication Using the Animal Rule. Briefing Document for the Vaccines and Related Biological Products Advisory Committee Meeting, November 16, 2010. https://wayback.archive-it.org/7993/20170113080506/http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM232400.pdf. [Google Scholar]
- 33.Altboum Z, Gozes Y, Barnea A, Pass A, White M, Kobiler D. Postexposure prophylaxis against anthrax: evaluation of various treatment regimens in intranasally infected guinea pigs. Infect Immun. 2002; 70(11):6231–6241. [DOI] [PMC free article] [PubMed] [Google Scholar]