Key Points
Cardiac, pulmonary, hepatic, and renal dysfunction were predictive of allo-HCT mortality and combined to form the SCI.
The new index stratified patients into distinct NRM risk groups and was valid in 2 cohorts.
Visual Abstract
Abstract
Individual comorbidities have distinct contributions to nonrelapse mortality (NRM) following allogeneic hematopoietic cell transplantation (allo-HCT). We studied the impact of comorbidities individually and in combination in a single-center cohort of 573 adult patients who underwent CD34-selected allo-HCT following myeloablative conditioning. Pulmonary disease, moderate to severe hepatic comorbidity, cardiac disease of any type, and renal dysfunction were associated with increased NRM in multivariable Cox regression models. A Simplified Comorbidity Index (SCI) composed of the 4 comorbidities predictive of NRM, as well as age >60 years, stratified patients into 5 groups with a stepwise increase in NRM. NRM rates ranged from 11.4% to 49.9% by stratum, with adjusted hazard ratios of 1.84, 2.59, 3.57, and 5.38. The SCI was also applicable in an external cohort of 230 patients who underwent allo-HCT with unmanipulated grafts following intermediate-intensity conditioning. The area under the receiver operating characteristic curve (AUC) of the SCI for 1-year NRM was 70.3 and 72.0 over the development and external-validation cohorts, respectively; corresponding AUCs of the Hematopoietic Cell Transplantation–specific Comorbidity Index (HCT-CI) were 61.7 and 65.7. In summary, a small set of comorbidities, aggregated into the SCI, is highly predictive of NRM. The new index stratifies patients into distinct risk groups, was validated in an external cohort, and provides higher discrimination than does the HCT-CI.
Introduction
The physiological reserve of candidates for allogeneic hematopoietic cell transplantation (allo-HCT) is assessed along 3 main axes: age, performance status, and comorbidities.1-3 Typically, comorbidities are evaluated using a standardized approach, initially codified by the Charlson Comorbidity Index4 and, later, adapted for allo-HCT with the development of the Hematopoietic Cell Transplantation–specific Comorbidity Index (HCT-CI).5 The HCT-CI aggregates the cumulative burden of 15 comorbidities. Each comorbidity is assigned an integer weight reflective of its contribution to the prediction of nonrelapse mortality (NRM).
The HCT-CI was originally developed using a heterogeneous population (N = 708) with respect to indication, conditioning, and donors who underwent allo-HCT at a single center between 1997 and 2003. Each component’s weights were drawn from the hazard ratios (HRs) for NRM of individual comorbidities, as extracted from a multivariable model.5 Performance of the HCT-CI has been studied extensively, with validity varying across centers and cohorts.3,6-15 Notably, the landscape of transplantation, supportive care, and implications of comorbidities (eg, peptic ulcer disease) have changed considerably in the past 2 decades.16 Furthermore, transplantation from alternative donors, as well as those using CD34-selected grafts, were not represented in the cohort used to develop the HCT-CI.
Ex vivo T-cell depletion with positive selection of CD34 cells in the graft is an effective and safe platform for allo-HCT.17-22 Retrospective studies have demonstrated that contemporary techniques for CD34 selection result in similar survival and relapse rates and less graft-versus-host disease (GvHD) compared with allo-HCT recipients transplanted with unmodified grafts.20,23-27 The main advantage of CD34 selection is the low risk of GvHD, obviating the need for posttransplantation immunosuppression with agents such as calcineurin inhibitors, mycophenolate mofetil, and cyclophosphamide.
Given the changes in practice over time, heterogeneity of the development set in the HCT-CI, and variations in HCT-CI performance, we sought to evaluate the influence of comorbidities on NRM in patients undergoing CD34-selected allo-HCT following myeloablative conditioning. We then aimed to develop and validate a score for NRM that would capture meaningful comorbidities. Such a score would be useful when considering the risk/benefit ratio of transplantation and could potentially impact therapeutic decisions, such as the choice of conditioning regimens.
Methods
Study design
To study the impact of individual comorbidities on NRM and develop a predictive score for NRM, we retrospectively analyzed adult patients undergoing allo-HCT for hematologic malignancies who received ex vivo CD34-selected peripheral blood stem cell allografts using the CliniMACS CD34 Reagent System (Miltenyi Biotec, Bergisch-Gladbach, Germany). Patients were treated at Memorial Sloan Kettering Cancer Center (MSKCC) between 2008 and 2018 and were conditioned with any of the following myeloablative regimens at the discretion of the treating physician: busulfan/melphalan/fludarabine, clofarabine/melphalan/thiotepa, total body irradiation (TBI; 1375 cGy)/thiotepa/cyclophosphamide, or TBI 1375 cGy/thiotepa/fludarabine.19,21,28-31 All regimens included rabbit anti-thymocyte globulin (5 mg/kg). Of 584 patients meeting inclusion criteria, 2 were excluded because of unavailable comorbidity data. Nine additional patients were excluded because their diagnosis is not in the current institutional indications at MSKCC for CD34-selected transplants (nonmalignant disease, n = 5; non-Hodgkin lymphoma, n = 4). For external validation of the score, we used an independent cohort from the Sheba Medical Center (Israel) transplanted between 2011 and 2015. Patients in the validation cohort received unmodified grafts following reduced-toxicity and reduced-intensity conditioning with fludarabine and treosulfan (30-42 g/m2) or fludarabine and melphalan (100-140 mg/m2).32,33 This study was approved by the MSKCC and Sheba Medical Center Institutional Review Boards according to the Declaration of Helsinki. All patients signed informed consent for treatment.
Definitions
Comorbidity definitions are described in supplemental Table 1.5 Adjustment of the diffusing capacity of lung for carbon monoxide (DLCo) to hemoglobin levels in the pulmonary function test was done using the Cotes and Dinkara formulas in the MSKCC and Sheba cohorts, respectively. Renal dysfunction was defined according to estimated glomerular filtration rate (eGFR; mL/min per 1.73 m2) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. This formula, based on age, creatinine, and ethnicity, is considered a more reliable marker of renal function than the Cockcroft-Gault equation34 and is well correlated with measured 24-hour creatinine clearance.35 An eGFR ≥ 90 mL/min per 1.73 m2 was considered normal, between 60 to 89.9 mL/min per 1.73 m2 was considered mildly decreased, and <60 mL/min per 1.73 m2 was considered moderately to severely decreased.36
All end points were measured from the date of graft infusion. The primary and secondary end points were NRM and overall survival (OS), respectively. Relapse and NRM were considered competing events.
Statistical analysis
Correlation between comorbidities was estimated with the Spearman coefficient. Comorbidities were only considered in univariable and multivariable models if their prevalence was ≥5% within the study cohort. Univariable analysis was performed by comparing the cumulative incidence of NRM in the presence or absence of the comorbidity of interest (Gray test). Multivariable models for NRM were generated for each comorbidity in a cause-specific Cox model adjusted for age, Karnofsky Performance Status, disease risk,37 recipient cytomegalovirus serostatus, HLA match, and conditioning. Interactions between comorbidities and conditioning regimen were tested and rejected at P values > .1. Acute GvHD was graded using the International Bone Marrow Transplant Registry and Glucksberg grading systems in the MSKCC and Sheba cohorts, respectively.38,39 Death and relapse were considered competing events for acute GvHD.
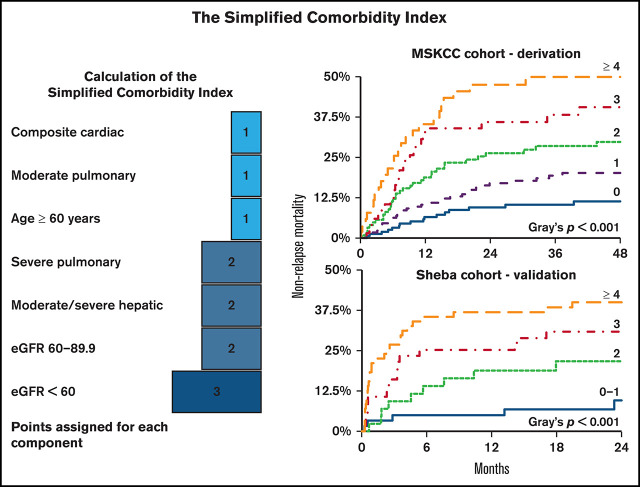
To construct the Simplified Comorbidity Index (SCI) in the MSKCC cohort, the adjusted HRs of comorbidities with a statistically significant association with NRM (P < .05), as well as recipient age, were converted to an integer score, following the example of HCT-CI. HRs of 1.3 to 2, 2.1 to 3, and 3 to 4, were assigned weights of 1, 2, and 3, respectively.5 The SCI score was the sum of these integer weights. The risk of increasing SCI values was studied in a multivariable Cox-regression model in the MSKCC and Sheba cohorts, aggregating levels 0 to 1 in the latter because of the smaller sample size. Time-dependent area under the receiver operating characteristic curve (AUC) with 95% confidence interval (CI) was calculated over both cohorts for the SCI, HCT-CI, and Comorbidity-Age Index (a composite score of age and HCT-CI) to evaluate the models’ discrimination.40 Statistical analysis was performed using R (v.4.0.0).
Results
Population characteristics
A total of 573 patients with a median age of 56 years (interquartile range [IQR], 46-64) was included in the analysis (Table 1). The majority of patients had a Karnofsky Performance Status ≥ 90 (64%). Acute leukemia was the leading transplantation indication (53%), followed by myelodysplastic syndrome (21%) and multiple myeloma (20%). HLA-matched unrelated donors were used in 49% of cases, followed by HLA-matched related donors (34%) and HLA-mismatched donors (17%). Conditioning regimens were melphalan and TBI based in 76% and 24%, respectively. The median follow-up was 4.8 years (IQR, 2.4-6.3). In the entire cohort, the probability of 4-year OS was 56.9% (95% CI, 52.7-61.5); the cumulative incidence of 4-year NRM and relapse was 24.9% (95% CI, 21.43-28.96) and 27.4% (95% CI, 23.8-31.6), respectively; and grade ≥ 2 and grade ≥ 3 day-100 acute GvHD cumulative incidence was 25% (95% CI, 19.7-31.73) and 3.43% (95% CI, 1.65-7.12), respectively.
Table 1.
Population characteristics
| MSKCC cohort | Sheba cohort | |
|---|---|---|
| Recipient age, median (IQR), y | 56 (46-64) | 61 (53-65) |
| Age <60 y | 358 (62) | 110 (48) |
| Age ≥60 y | 215 (38) | 120 (52) |
| Recipient sex | ||
| Male | 329 (57) | 144 (63) |
| Female | 244 (43) | 86 (37) |
| Karnofsky performance status | ||
| 90-100 | 365 (64) | 189 (82) |
| <90 | 207 (36) | 28 (12) |
| Missing | 1 (0.2) | 13 (6) |
| HCT-CI | ||
| 0 | 132 (23) | 37 (16) |
| 1 | 98 (17) | 47 (20) |
| 2 | 106 (18) | 38 (17) |
| 3 | 116 (20) | 40 (17) |
| ≥4 | 121 (21) | 68 (30) |
| Recipient CMV serostatus | ||
| Absent | 222 (39) | 42 (19) |
| Present | 351 (61) | 182 (81) |
| Diagnosis | ||
| Acute myeloid leukemia | 217 (38) | 53 (23) |
| Myelodysplastic syndrome | 125 (22) | 57 (25) |
| Multiple myeloma | 115 (20) | 17 (7) |
| Acute lymphoblastic leukemia | 57 (10) | 15 (7) |
| Myeloproliferative neoplasm | 31 (5) | 4 (2) |
| Chronic myeloid leukemia | 17 (3) | 3 (1) |
| Other leukemia | 9 (2) | 0 (0) |
| Lymphoma | 0 (0) | 81 (35) |
| Disease risk * | ||
| Low/intermediate | 387 (68) | 138 (60) |
| High | 140 (24) | 92 (40) |
| Unclassifiable | 46 (8) | 0 (0) |
| Time from Dx to HCT, median (IQR), mo | 7 (5-25) | 12 (4-34) |
| Donor/recipient sex | ||
| Female/male | 114 (20) | 52 (23) |
| Other | 459 (80) | 175 (77) |
| Donor type † | ||
| Matched related | 192 (34) | 100 (43) |
| Matched unrelated | 283 (49) | 98 (43) |
| HLA mismatched | 98 (17) | 32 (14) |
| HCT year, median (IQR) | 2014 (2012-2016) | 2013 (2012-2014) |
| Regimen (%) ‡ | ||
| Melphalan based | 436 (76) | NA |
| TBI based | 137 (24) | NA |
| Fludarabine-treosulfan (30 g/m2) | NA | 33 (14) |
| Fludarabine-treosulfan (36-42 g/m2) | NA | 156 (68) |
| Fludarabine-melphalan (100 mg/m2) | NA | 27 (12) |
| Fludarabine-melphalan (140 mg/m2) | NA | 14 (6) |
Unless otherwise noted, data are n (%).
CMV, cytomegalovirus; Dx, diagnosis; HCT, hematopoietic cell transplantation; NA, not applicable (regimens specific to individual centers).
Disease risk was classified using Center for International Bone and Marrow Transplant Research criteria (MSKCC cohort) and European Society for Blood and Bone Transplantation criteria (Sheba cohort).
Matched donor was defined at an 8/8 HLA allele level for the MSKCC cohort and a 10/10 HLA level at the Sheba Medical Center. Corresponding definitions for mismatched donors were 7/8 and 9/10.
Among patients in the Sheba cohort, 173 (77%) and 51 (23%) received methotrexate-based or mycophenolate mofetil–based GvHD prophylaxis, respectively, and 143 (62%) received anti-thymocyte globulin.
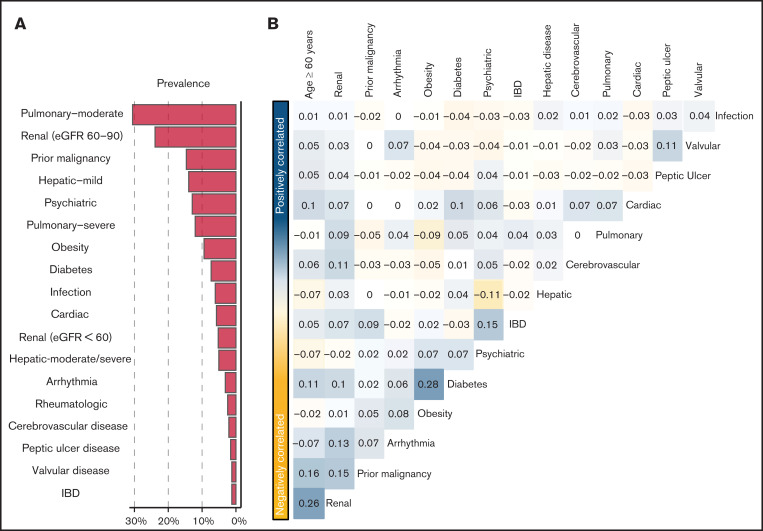
Prevalent comorbidities, determined as part of the pretransplant evaluation, included moderate (31%) and severe pulmonary (12%) disease, prior malignancy (15%), and mild hepatic disease (14%) (Figure 1A). No patient met the HCT-CI definition of moderate to severe renal comorbidity (ie, serum creatinine > 2 mg/dL). However, 24% and 5% had an eGFR of 60 to 89.9 mL/min per 1.73 m2 and <60 mL/min per 1.73 m2, respectively. The prevalence of composite cardiac disorder, defined as cardiac comorbidity meeting the HCT-CI definition, arrhythmia, or valvular disorder, was 10%. HCT-CI levels 0, 1, 2, 3, and ≥4 corresponded with 23%, 17%, 19%, 20%, and 21% of the population, respectively. Patients with an HCT-CI ≥ 4 were older (P < .001), had a lower Karnofsky Performance Status (P < .001), and were more likely to receive chemotherapy-based myeloablative conditioning (P < .001; supplemental Table 2).
Figure 1.
Comorbidities in the study population. (A) Prevalence of each of the studied comorbidities in the MSKCC. (B) Co-incidence of pairs of comorbidities across the study cohort, as measured by Spearman’s correlation coefficient. Pairs with positive coefficients are in blue, and those with negative coefficients are in yellow. Comorbidities with >1 level (ie, moderate and severe pulmonary) are studied as a single comorbidity with ordinal levels. eGFR was measured using the CKD-EPI formula. IBD, inflammatory bowel disease.
We assessed the dependency between types of comorbidities, as well as age (Figure 1B). Overall, comorbidities were not correlated, aside from weak correlations between diabetes and obesity (r = +0.28) and older age and low eGFR (r = +0.26).
Comorbidity-associated NRM
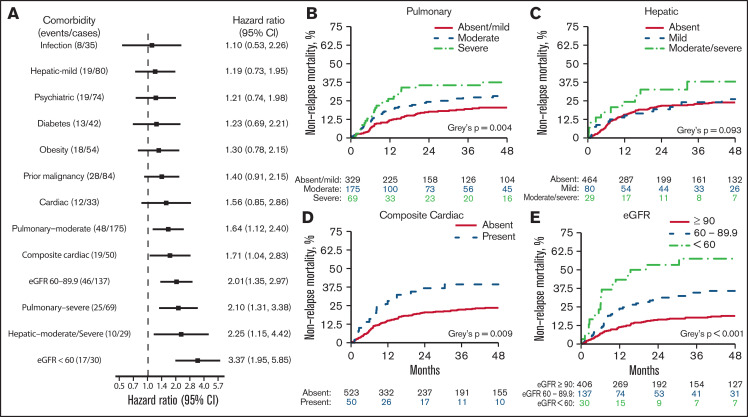
The risk of NRM varied between comorbidities when studied using multivariable Cox regression (Figure 2A). Increasing severity of pulmonary disease was associated with a greater risk for NRM. Compared with the absence of pulmonary disorder, the HR was 1.64 (95% CI, 1.12-2.40) for moderate pulmonary disease and 2.10 (95% CI, 1.31-3.38) for severe pulmonary disease. In the univariable analysis (Figure 2B), the cumulative incidence of 4-year NRM with absent, moderate, and severe pulmonary disease, was 20.4% (95% CI, 16.3-25.7), 28.2% (95% CI, 22.0-36.2), and 37.4% (95% CI, 27.4-51.2), respectively (P = .0041). Moderate to severe hepatic disease, but not mild disease, was associated with increased NRM in the multivariable analysis (HR, 2.25; 95% CI, 1.15-4.42). Cumulative NRM incidence with absent, mild, and moderate to severe hepatic disease was 23.9%, 26.1%, and 37.9%, respectively (P = .093; Figure 2C). In a multivariable analysis, no other comorbidity included in the HCT-CI was associated with increased NRM. Although cardiac comorbidity did not meet the statistical significance threshold for association with NRM (HR, 1.56; 95% CI, 0.85-2.86; P = .154), a composite of any cardiac disorder had an HR of 1.71 (95% CI, 1.04-2.83; P = .036) and a 4-year NRM of 39.3% (95% CI, 27.6-56.0) vs 23.5% (95% CI, 19.9-27.7; P = .009; Figure 2D). Compared with an eGFR ≥ 90 mL/min per 1.73 m2, increasing impairment of renal function was strongly associated with NRM; HR 2.01 for eGFR 60 to 89.9 mL/min per 1.73 m2 (95% CI, 1.35-2.97; P = .001), HR 3.37 for eGFR < 60 mL (95% CI, 1.95-5.85; P < .001). Corresponding 4-year NRM rates were 18.9% (95% CI, 15.3-23.4), 35.8% (95% CI, 28.0-45.6), and 57.5% (95% CI, 42.0-78.7; P < .001), respectively (Figure 2E).
Figure 2.
NRM associated with individual comorbidities. (A) Forest plot of HR for NRM associated with the presence of individual comorbidities; HRs are extracted from a multivariable model. (B-E) Cumulative incidence of NRM. P values were from a multivariable cause-specific Cox model. eGFR was measured using the CKD-EPI formula.
The SCI
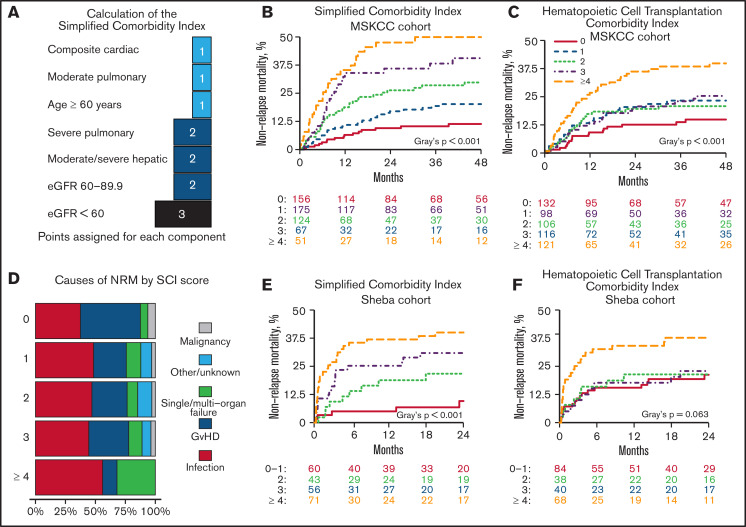
To develop the SCI, we included comorbidities associated with a significant increase in NRM (Figures 2A,3A). Age with a cutoff of 60 years was also included because it reflects physiological reserve to some extent and is related to increased NRM risk (HR, 1.64; 95% CI, 1.23-2.19; P = .001). HRs of the components were transformed into score weights, using the same cutoffs as in the HCT-CI.5 Definitions and weights of the SCI’s components are listed in Table 2. The SCI’s scale ranges from 0 to 9; 27%, 31%, 22%, 12%, or 9% of patients had a score of 0, 1, 2, 3, or ≥4, respectively. Corresponding rates for 4-year NRM (Figure 3B) were 11.4% (95% CI, 7.1-18.1), 20.2% (95% CI, 14.7-27.6), 29.8% (95% CI, 22.4-39.7), 40.6% (95% CI, 29.7-55.3), and 49.9% (95% CI, 37.8-66.0; P < .001); corresponding rates for OS were 74.5% (95% CI, 67.4-82.4), 61.2% (95% CI, 53.8-69.7), 43.9% (95% CI, 35.3-54.6), 43.6% (95% CI, 32.3-58.9), and 38.1% (95% CI, 26.7-54.4; P < .001), respectively. In a multivariable model (Table 3) adjusted for performance status, disease risk, cytomegalovirus (CMV) serostatus, donor type, and conditioning regimen, the simplified score was the strongest predictor of NRM and OS. Scores of 3 and ≥4 had an HR for NRM of 5.03 (95% CI, 2.64-9.60) and 7.04 (95% CI, 3.61-13.73), respectively. SCI remained an independent predictor of NRM when considering grade ≥ 2 acute GvHD in a multivariable time-dependent Cox regression model (supplemental Table 3). Patients with an HCT-CI score of 1, 2, or 3 had a similar cumulative incidence of NRM (23.3%; 95% CI, 15.9-34.1), 20.8% (95% CI, 14.2-30.5), or 25.4% (95% CI, 18.2-35.3), respectively; Figure 3C). The SCI’s AUC for NRM was higher than the HCT-CI and the Comorbidity-Age Index at all time points (Table 4), with the largest difference at the 1-year mark (70.3, 95% CI, 64.4-76.1; 61.74, 95% CI, 54.6-67.1; and 61.0, 95% CI, 54.0-68.1, respectively). Notably, age ≥ 60 years as a component of the SCI contributed to its discrimination, because the performance of the SCI without age was lower than the version with age (supplemental Table 4). Causes of NRM by SCI level are shown in Figure 3D.
Figure 3.
The SCI. (A) Schematic diagram showing the points added for each component of the score. NRM cumulative incidence by the SCI and HCT-CI scores over the MSKCC (B-C) and Sheba (E-F) cohorts. (D) Cause of death stratified by SCI score. eGFR was measured using the CKD-EPI formula.
Table 2.
SCI definitions
| SCI feature | SCI definition | SCI assigned score |
|---|---|---|
| Composite cardiac disease/dysfunction | Coronary artery disease, congestive heart failure, history of myocardial infarction, or left ventricular ejection fraction ≤ 50% OR atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias OR valvular heart disease (except mitral valve prolapse) | 1 |
| Moderate pulmonary dysfunction | DLCo* or FEV1 66-80% or dyspnea on slight activity | 1 |
| Age ≥ 60 y | 1 | |
| Severe pulmonary dysfunction | DLCo* or FEV1 < 66 or dyspnea at rest/oxygen dependent | 2 |
| Moderate to severe hepatic dysfunction | Serum bilirubin > 1.5 times ULN; ALT or AST > 2.5 times ULN, or chronic hepatitis | 2 |
| Mild renal dysfunction | eGFR† between 60 and 89.9 mL/min per 1.73 m2 | 2 |
| Moderate to severe renal dysfunction | eGFR < 60 mL/min per 1.73 m2 | 3 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DLCo, diffusion capacity of the lung for CO; FEV1, forced expiratory volume in 1 second; ULN, upper limit of normal.
DLCo was corrected for hemoglobin using the formula of Cotes et al.49
eGFR was calculated using the CKD-EPI equation of Levey et al.34
Table 3.
Multivariable HRs for NRM and OS
| NRM | OS | ||||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| SCI score | |||||
| 0 | Reference | ||||
| 1 | 2.04 (1.11-3.74) | .021 | 1.71 (1.13-2.59) | .0105 | |
| 2 | 3.09 (1.66-5.73) | .000 | 2.6 (1.7-3.97) | <.001 | |
| 3 | 5.03 (2.64-9.6) | .000 | 3.15 (1.95-5.07) | <.001 | |
| ≥ 4 | 7.04 (3.61-13.73) | .000 | 4.08 (2.46-6.77) | 0 | |
| Regimen | |||||
| Melphalan-based | Reference | ||||
| TBI-based | 0.89 (0.53-1.5) | .670 | 1.03 (0.71-1.49) | .888 | |
| Karnofsky performance status | |||||
| ≤ 80 | Reference | ||||
| 90 − 100 | 1.53 (1.08-2.17) | .017 | 1.53 (1.17-2) | .002 | |
| CIBMTR disease risk | |||||
| Low/Intermediate | Reference | ||||
| High | 1.05 (0.7-1.58) | .818 | 1.31 (0.97-1.76) | .078 | |
| Unclassifiable | 0.57 (0.27-1.18) | .130 | 0.53 (0.28-0.98) | .042 | |
| Recipient CMV serostatus | |||||
| Negative | Reference | ||||
| Positive | 1.13 (0.8-1.61) | .491 | 1.19 (0.91-1.57) | .197 | |
| Donor type | |||||
| Matched related | Reference | ||||
| Matched unrelated | 1.1 (0.73-1.65) | .662 | 0.92 (0.68-1.24) | .590 | |
| Mismatched | 2.4 (1.51-3.82) | .000 | 1.7 (1.19-2.42) | .003 | |
CIBMTR, Center for International Blood and Marrow Transplant Research; CMV, cytomegalovirus.
Table 4.
Models’ NRM discrimination by the AUC (95% CI)
| SCI | HCT-CI | Comorbidity-Age[40]* Index | Comorbidity-Age[6o]* Index | |
|---|---|---|---|---|
| MSKCC cohort | ||||
| Years | ||||
| 1 | 70.3 (64.4-76.1) | 61.7 (54.6-67.1) | 61.0 (54.0-68.1) | 58.8 (55.1-67.0) |
| 2 | 68.9 (63.3-74.4) | 63.1 (55.8-67.2) | 62.5 (55.8-69.2) | 59.4 (56.9-68.1) |
| 3 | 68.7 (63.1-74.4) | 63.0 (55.6-66.9) | 62.7 (55. 8-69.6) | 59.6 (56.9-68.5) |
| 4 | 67.5 (61.6-73.5) | 63.3 (56.0-67.4) | 62.5 (55.4-69.7) | 59.9 (56.5-68.6) |
| Sheba cohort | ||||
| Years | ||||
| 1 | 72.0 (62.0-76.5) | 65.7 (56.7-74.6) | 63.7 (55.2-72.2) | 64.4 (56.0-72.8) |
| 2 | 67.6 (57.4-77.9) | 62.8 (53.3-72.2) | 61.1 (52.0-70.3) | 57.1 (52.4-70.6) |
| 3 | 64.9 (54.1-75.7) | 63.6 (53.1-74.1) | 63.5 (53.0-74.1) | 62.4 (51.9-73.0) |
| 4 | 61.8 (48.0-75.5) | 64.4 (52.7-76.1) | 61.9 (49.8-74.0) | 59.2 (46.6-71.8) |
The Comorbidity-Age Index was developed with age ≥ 40 years as a threshold to add a point to the HCT-CI. Because the impact of age has likely changed over time, we tested the index with the original age threshold (Comorbidity-Age[40] Index) and with age ≥ 60 years as a threshold (Comorbidity-Age[60] Index).
An online calculator for the SCI is available at https://jfdemo.shinyapps.io/sciapp/.
External validation
Population characteristics of the Sheba cohort are listed in Table 1. The leading indications for transplant were lymphoma (35%) and acute leukemia (30%). Donors were primarily HLA-matched siblings (43%), followed by HLA-matched unrelated donors (43%). Patients received fludarabine and treosulfan (82%) or fludarabine and melphalan (18%). The median follow-up was 2.8 years (IQR, 1.8-4.3). In the entire cohort, the probability of 2-year OS was 46.9% (95% CI, 40.2-53.6); the cumulative incidence of 2-year NRM and relapse was 26.4% (95% CI, 20.6-32.2) and 28.0% (95% CI, 22.1-33.9), respectively; and the cumulative incidence of grade ≥ 2 and grade ≥ 3 day-100 acute GvHD was 20.1% (95% CI, 14.9-25.3) and 7.4% (95% CI, 4.0-10.8), respectively.
In a multivariable Cox regression model, increasing SCI levels were associated with a greater risk for NRM (HR, 1.94 [95 CI%, 0.60-6.25]; HR, 2.90 [95% CI, 1.02-8.20]; HR, 4.07 [95% CI, 1.45-11.45]; supplemental Table 5). The impact of SCI on NRM remained consistent in a time-dependent Cox-regression model, adjusted for acute GVHD (supplemental Table 6). SCI scores of 0-1, 2, 3, and ≥4 corresponded with 2-year NRM of 9.6% (95% CI, 1.4-17.8), 21.7% (95% CI, 9.1-34.3), 30.9% (95% CI, 18.7-43.1), and 40.0% (95% CI, 28.5-51.5; P = .001) and 2-year OS of 60.7% (95% CI, 47.2-74.3), 56.4% (95% CI, 41.0-71.7), 42.9% (95% CI, 29.6-56.3), and 31.9% (95% CI, 20.8-43.0; P < .001). The same score groups, but using HCT-CI, had 2-year NRM of 21.2, 21.4, 22.9, and 37.7 (95% CIs of 12.1-30.3, 8.2-34.6, 9.8-36.0, and 25.9-49.4, respectively; P = .063). Similar to the MSKCC cohort, AUC for NRM at 1 year was higher with the SCI (72.0; 95% CI, 62.0-76.5) compared with the HCT-CI (65.7; 95% CI, 56.7-74.6) and the Comorbidity-Age Index (63.7; 95% CI, 55.2-72.2; Table 4).
Discussion
Comorbidities are a critical component in evaluating candidates’ suitability for allo-HCT and individualizing protocols to patients’ features. In this retrospective analysis, we studied the relationship between common comorbidities and NRM among patients undergoing CD34-selected allogeneic transplantation following myeloablative conditioning. Of the comorbidities with a prevalence ≥5% and included in the original HCT-CI, only pulmonary and moderate-to-severe hepatic dysfunction were associated with a significant increase in NRM. A composite of cardiac comorbidity consisting of cardiac comorbidity, arrhythmia, and valvular disorders was also related to excess NRM. As measured by the eGFR, the degree of renal dysfunction was among the strongest predictors of NRM. A score combining these selected comorbidities and age (the SCI) distinguished between low- and high-risk patients. It was validated in an external cohort of patients undergoing allo-HCT with unmodified grafts and reduced-intensity/toxicity conditioning.
The original HCT-CI was developed using allo-HCT performed 2 decades ago.5 The development cohort included a heterogeneous group of patients receiving myeloablative and reduced-intensity conditioning and a mixture of GvHD regimens. Importantly, the transplantation field has changed dramatically in the past 20 years16,41; indications for transplantation, patient and disease profile, transplantation techniques, and supportive care have shifted. These changes provided the impetus to revisit the weights of the HCT-CI components. Furthermore, in the original HCT-CI, transplants with CD34-selected grafts were not included. Acknowledging the limitation of a small sample size and excluding comorbidities with a low burden (ie, <5%), several comorbidities stand out. First, there was a stepwise increase in NRM with a greater degree of pulmonary dysfunction; NRM cumulative incidence with severe pulmonary disease, defined based on aberrant pulmonary function tests, was 37.4% (95% CI, 27.4-51.2) at 2 years. Indeed, pretransplant pulmonary dysfunction, as well as a decline in pulmonary function tests posttransplant, has repeatedly been shown to be a determinant of NRM.10,42-44 Thresholds for pulmonary comorbidities are derived from DLCo and forced expiratory volume in 1 second. These measures were selected because they are not correlated with one another and were predictive of mortality and respiratory failure in earlier studies. Overall, pulmonary dysfunction is likely the most robust element from the comorbidities considered in the HCT-CI. In our cohort, moderate-to-severe, but not mild, hepatic dysfunction was associated with increased NRM. The relationship between a pretransplantation elevation in transaminases and posttransplantation early mortality, sinusoidal obstruction syndrome, and liver injury was demonstrated in studies performed in the late 1990s. Since then, transplantation practice has changed, with better supportive care and the introduction of ursodiol and defibrotide to prevent and treat sinusoidal obstruction syndrome. These changes may explain why we and other investigators find that only a severe distortion of hepatic biomarkers resulted in elevated mortality.10 Cardiac comorbidity, as defined by in the HCT-CI (ie, coronary artery disease, congestive heart failure, myocardial infarction, or ejection fraction <50%), was present in only 6% of the MSKCC population. Valvular disorders and arrhythmia were also infrequent and were grouped with cardiac comorbidity to form a composite cardiac comorbidity that was associated with excess NRM. Ideally, it would be best to study elements of cardiac dysfunction in hematopoietic cell transplantation recipients, but, individually, these are rare.
Renal dysfunction is emerging as an important predictor of NRM following allogeneic transplantation.10,45,46 A creatinine threshold > 2 mg/dL, as used in the HCT-CI, would disqualify many patients from allogeneic transplantation, especially for myeloablative conditioning. Using eGFR, which is more sensitive to impaired renal function, we could identify patients at risk for NRM who were not captured using the HCT-CI criteria. Although none of the patients had creatinine > 2 mg/dL, ∼30% had an eGFR < 90 mL/min per 1.73 m2, corresponding with stage ≥2 renal dysfunction. Adopting this more sensitive threshold, we find renal function to be one of the key determinants of hematopoietic cell transplantation outcomes. Patients with an eGFR of 60 to 89.9 mL/min per 1.73 m2 and <60 mL/min per 1.73 m2 had markedly high rates of NRM (35.8% and 57.5%, respectively). One of the primary advantages of CD34-selected allografts is the freedom from systemic immunosuppression, including calcineurin inhibitors posttransplantation. Given these agents’ nephrotoxic potential, we hypothesized that patients with underlying renal dysfunction would be good candidates for CD34-selected transplantation; however, these data would suggest otherwise. Nonmyeloablative or reduced-intensity conditioning platforms with posttransplantation cyclophosphamide, minimizing calcineurin inhibitor exposure, may be a better option in patients with baseline renal comorbidity; however, this requires further exploration.
Selection of comorbidities in the original HCT-CI score was driven by the NRM HR for each comorbidity in a multivariable model, irrespective of the 95% CI, P value, or comorbidity prevalence. Notably, 11 of the 17 comorbidities in the HCT-CI occurred in <5% of the cohort, with some being as low as 1%. In the current analysis, we used a more conservative approach. First, we excluded rare comorbidities. Then, we adopted a P value < .05 for determining whether there is an association with NRM. Using these criteria, we did not find an association between NRM and diabetes mellitus, psychiatric comorbidity, infection requiring continuation of antimicrobial treatment after day 0, obesity, or prior malignancy. Importantly, “absence of evidence is not evidence of absence,” and the study may have been underpowered to detect these effects. However, because comorbidities guide therapeutic decisions, we exercised caution. The Comorbidity-Age Index is a modified version of the HCT-CI that assigns points for age ≥ 40 years.47 Although the addition of age improved the SCI's discrimination, the Comorbidity-Age Index did not provide significant benefit over the HCT-CI (Table 4; supplemental Table 4). With current practice, selecting a restrictive age threshold of 60 years in SCI may be more informative of NRM and could explain, in part, the better discrimination.
The SCI is a simple tool to implement. Nevertheless, its levels distinguish well between risk groups, identifying patients at low and high risk for mortality. There is greater than one third NRM with scores ≥ 3, suggesting that these patients should be considered for other forms of transplantation or therapy. Segregation was not as clear with the HCT-CI in the MSKCC and Sheba cohorts, as reflected by the overlapping NRM rates between risk groups and lower AUC. There appears to be a higher proportion of organ failure in the SCI development cohort in patients with an SCI of 4, implying that baseline organ dysfunction directly contributed to death; however, establishing a direct link is challenging. Patients with a high SCI are likely to be less resilient to allo-HCT’s typical complications, such as infections and GvHD.
The HCT-CI remains an established tool for the standardized assessment of comorbidities. Nevertheless, it is expected that there will be considerable variation in its performance across centers and studies.3,6-15 Grouping of risk levels, as is often done in prognostic scores, differences in the population characteristics, and the accuracy of comorbidity data all contribute to this heterogeneity. We strongly recommend that centers using the HCT-CI for decision making validate its utility and calibration in their populations. Adding to the complexity of applying comorbidity scores, specific comorbidities may interact with the type of regimen used rather than its overall intensity.10 Therefore, an alternative strategy to optimize the allo-HCT outcomes may be guided by considering individual comorbidities, rather than a composite score, when selecting the conditioning regimen. In the current analysis, we did not identify any interactions between the conditioning regimen and comorbidities; however, the study may have been underpowered to detect such interactions.
In summary, we show that a small number of comorbidities are highly informative of NRM risk following allogeneic transplantation with CD34-selected grafts and myeloablative conditioning. Grouping these comorbidities, as well as the patient age, yielded a simplified score that could discriminate well between patients at low and high risk for NRM. Although the score was developed on a relatively selected and homogenous cohort of patients, it was applicable in an external cohort of patients receiving unmanipulated grafts following reduced-intensity and reduced-toxicity conditioning. Nevertheless, external validation in larger cohorts and across a variety of transplantation strategies are warranted for broader implementation. The score could be used to estimate the NRM risk associated with transplantation. It can supplement decision support tools, such as geriatric assessment,48 to obtain a comprehensive approximation of the risk for NRM. Based on the calculated NRM probability, physicians and investigators may design interventions to mitigate risk or consider alternative therapeutic strategies.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported in part by National Institutes of Health/National Cancer Institute grant P01 CA23766 and National Institutes of Health/National Cancer Institute Cancer Center Support grant P30 CA008748. A.A.T. is supported by a grant from the Alfonso Martin Escudero Foundation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: R.S., S.D., and M.-A.P. designed the study; R.S., J.A.F., and S.D. performed analyses; R.S., A.S., and M.-A.P. supervised the study; J.R. served as the data manager; R.S., A.A.T., M.S.-E., N.C.F., and L.Y.S.S. coded clinical data into the database; R.S. and J.A.F. wrote the manuscript; and C.C., S.T.A., A.A.T., M.S.-E., N.C.F., L.Y.S.S., J.N.B., P.D., S.A.G., A.I.G., B.G., A.A.J., R.J.L., R.J.O., E.B.P., I.P., D.M.P., C.S.S., M.S., B.S., G.L.S., J.P.S., R.T., M.R.M.v.d.B., J.W.Y., A.N., S.D., A.S., and M.-A.P. critically reviewed the manuscript and made substantial contributions to the interpretation of the data and the final version of the manuscript.
Conflict-of-interest disclosure: P.D. has served on the advisory board for Kite (Gilead). S.A.G. has received research funding from Amgen, Actinuum, Celgene, Johnson & Johnson, Miltenyi Biotec, Takeda, and Omeros and has served on advisory boards for Amgen, Actinuum, Celgene, Johnson & Johnson, Janssen, Jazz Pharmaceutical, Takeda, Novartis, Kite, and Spectrum Pharma. C.S.S. has served as a paid consultant on advisory boards for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite/a Gilead Company, Celgene, Gamida Cell, Karyopharm Therapeutics, and GlaxoSmithKline and has received research funds for clinical trials from Juno Therapeutics, Celgene, Bristol Myers Squibb, Precision Biosciences, and Sanofi-Genzyme. M.-A.P. has received personal fees from AbbVie, Bellicum, Bristol Myers Squibb, Celgene, Cidara Therapeutics, Incyte, Kite/Gilead, Medigene, Miltenyi Biotec, MolMed, Nektar Therapeutics, NexImmune, Novartis, Omeros, Merck, Servier, and Takeda; serves on the Data Safety and Monitoring Board for Cidara Therapeutics, Medigene, and Servier; and has received clinical trial support from Incyte, Kite/Gilead, and Miltenyi Biotec. B.G. has received research funding from Actinium. R.J.O. has received consulting fees, research funding, and royalties from Atara Biotherapeutics. I.P. has received research funding from Merck and serves on the Data Safety and Monitoring Board (uncompensated) for ExcellThera. D.M.P. has received research funding from Takeda and serves on advisory boards for Kadmon, Generon, and Ceramedix. M.S. has received consulting fees from McKinsey & Company and Omeros Corporation; has received consulting fees and research funding from Angiocrine Bioscience; serves on the advisory board for Kite (Gilead); and has served as a speaker for i3 Health. G.L.S. has received research funding from Amgen and Janssen Pharmaceuticals. M.R.M.v.d.B. has received research support and stock options from Seres Therapeutics; has received royalties from Wolters Kluwer; has consulted for, received honorarium from, or participated in advisory boards for Seres Therapeutics, Jazz Pharmaceuticals, Rheos, Therakos, WindMIL Therapeutics, Amgen, Merck & Co., Inc., Magenta Therapeutics and DKMS Medical Council (board), Forty Seven, Inc. (spouse), Pharmacyclics (spouse), and Kite Pharmaceuticals (spouse); and has an intellectual property licensing agreement with Seres Therapeutics and Juno Therapeutics. S.T.A. has received honoraria for presenting a project in partnership with Abbott Laboratories. The remaining authors declare no competing financial interests.
Correspondence: Roni Shouval, Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 298, New York, NY 10065; e-mail: shouvalr@mskcc.org; and Miguel-Angel Perales, Adult Bone Marrow Transplantation Service, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 298, New York, NY 10065; email: peralesm@mskcc.org.
References
- 1.Kanate AS, Perales MA, Hamadani M. Eligibility criteria for patients undergoing allogeneic hematopoietic cell transplantation. J Natl Compr Canc Netw. 2020;18(5):635-643. [DOI] [PubMed] [Google Scholar]
- 2.Tay J, Daly A, Jamani K, et al. Patient eligibility for hematopoietic stem cell transplantation: a review of patient-associated variables. Bone Marrow Transplant. 2019;54(3):368-382. [DOI] [PubMed] [Google Scholar]
- 3.Potdar R, Varadi G, Fein J, Labopin M, Nagler A, Shouval R. Prognostic scoring systems in allogeneic hematopoietic stem cell transplantation: where do we stand? Biol Blood Marrow Transplant. 2017;23(11):1839-1846. [DOI] [PubMed] [Google Scholar]
- 4.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 5.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bokhari SW, Watson L, Nagra S, et al. Role of HCT-comorbidity index, age and disease status at transplantation in predicting survival and non-relapse mortality in patients with myelodysplasia and leukemia undergoing reduced-intensity-conditioning hemopoeitic progenitor cell transplantation. Bone Marrow Transplant. 2012;47(4):528-534. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Liu DH, Liu KY, et al. Impact of pretransplantation risk factors on post transplantation outcome of patients with acute myeloid leukemia in remission after haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(2):283-290. [DOI] [PubMed] [Google Scholar]
- 8.Nakaya A, Mori T, Tanaka M, et al. Does the hematopoietic cell transplantation specific comorbidity index (HCT-CI) predict transplantation outcomes? A prospective multicenter validation study of the Kanto Study Group for Cell Therapy. Biol Blood Marrow Transplant. 2014;20(10):1553-1559. [DOI] [PubMed] [Google Scholar]
- 9.Törlén J, Remberger M, Le Blanc K, Ljungman P, Mattsson J. Impact of pretransplantation indices in hematopoietic stem cell transplantation: knowledge of center-specific outcome data is pivotal before making index-based decisions. Biol Blood Marrow Transplant. 2017;23(4):677-683. [DOI] [PubMed] [Google Scholar]
- 10.Fein JA, Shimoni A, Labopin M, et al. The impact of individual comorbidities on non-relapse mortality following allogeneic hematopoietic stem cell transplantation. Leukemia. 2018;32(8):1787-1794. [DOI] [PubMed] [Google Scholar]
- 11.Shouval R, Fein JA, Shouval A, et al. External validation and comparison of multiple prognostic scores in allogeneic hematopoietic stem cell transplantation. Blood Adv. 2019;3(12):1881-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110(13):4606-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25(27):4246-4254. [DOI] [PubMed] [Google Scholar]
- 14.Lim ZY, Ingram W, Brand R, et al. Impact of pretransplant comorbidities on alemtuzumab-based reduced-intensity conditioning allogeneic hematopoietic SCT for patients with high-risk myelodysplastic syndrome and AML. Bone Marrow Transplant. 2010;45(4):633-639. [DOI] [PubMed] [Google Scholar]
- 15.ElSawy M, Storer BE, Pulsipher MA, et al. Multi-centre validation of the prognostic value of the haematopoietic cell transplantation- specific comorbidity index among recipient of allogeneic haematopoietic cell transplantation. Br J Haematol. 2015;170(4):574-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shouval R, Fein JA, Labopin M, et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019;6(11):e573-e584. [DOI] [PubMed] [Google Scholar]
- 17.Cho C, Hsu M, Barba P, et al. Long-term prognosis for 1-year relapse-free survivors of CD34+ cell-selected allogeneic hematopoietic stem cell transplantation: a landmark analysis. Bone Marrow Transplant. 2017;52(12):1629-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamari R, Chung SS, Papadopoulos EB, et al. CD34-selected hematopoietic stem cell transplants conditioned with myeloablative regimens and antithymocyte globulin for advanced myelodysplastic syndrome: limited graft-versus-host disease without increased relapse. Biol Blood Marrow Transplant. 2015;21(12):2106-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roldan E, Perales MA, Barba P. Allogeneic stem cell transplantation with CD34+ cell selection. Clin Hematol Int. 2019;1(3):154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30(26):3194-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17(9):1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg JD, Zheng J, Ratan R, et al. Early recovery of T-cell function predicts improved survival after T-cell depleted allogeneic transplant. Leuk Lymphoma. 2017;58(8):1859-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malard F, Labopin M, Cho C, et al. Ex vivo and in vivo T cell-depleted allogeneic stem cell transplantation in patients with acute myeloid leukemia in first complete remission resulted in similar overall survival: on behalf of the ALWP of the EBMT and the MSKCC. J Hematol Oncol. 2018;11(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barba P, Hilden P, Devlin SM, et al. Ex vivo CD34+-selected T cell-depleted peripheral blood stem cell grafts for allogeneic hematopoietic stem cell transplantation in acute leukemia and myelodysplastic syndrome is associated with low incidence of acute and chronic graft-versus-host disease and high treatment response. Biol Blood Marrow Transplant. 2017;23(3):452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19(6):898-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barba P, Ratan R, Cho C, et al. Hematopoietic cell transplantation comorbidity index predicts outcomes in patients with acute myeloid leukemia and myelodysplastic syndromes receiving CD34+ selected grafts for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23(1):67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015;50(4):493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91(3):1083-1090. [PubMed] [Google Scholar]
- 29.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467-480. [PubMed] [Google Scholar]
- 30.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008;14(4):458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scordo M, Shah GL, Kosuri S, et al. Effects of late toxicities on outcomes in long-term survivors of ex-vivo CD34+-selected allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2018;24(1):133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spyridonidis A, Labopin M, Savani BN, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients [published correction appears in Bone Marrow Transplant. 2020;55(6):1213]. Bone Marrow Transplant. 2020;55(6):1114-1125. [DOI] [PubMed] [Google Scholar]
- 33.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar BV, Mohan T. Retrospective comparison of estimated GFR using 2006 MDRD, 2009 CKD-EPI and Cockcroft-Gault with 24 hour urine creatinine clearance. J Clin Diagn Res. 2017;11(5):BC09-BC12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 suppl 1):S1-S266. [PubMed] [Google Scholar]
- 37.ASBMT RFI 2017 - Disease Classifications Corresponding to CIBMTR Classifications. https://higherlogicdownload.s3.amazonaws.com/ASBMT/43a1f41f-55cb-4c97-9e78-c03e867db505/UploadedImages/ASBMT_RFI_2018B_CIBMTR_Disease_Classifications.pdf. Accessed 01 August 2021.
- 38.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295-304. [DOI] [PubMed] [Google Scholar]
- 39.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97(4):855-864. [DOI] [PubMed] [Google Scholar]
- 40.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32(30):5381-5397. [DOI] [PubMed] [Google Scholar]
- 41.McDonald GB, Sandmaier BM, Mielcarek M, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003–2007 versus 2013–2017 cohorts. Ann Intern Med. 2020;172(4):229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parimon T, Madtes DK, Au DH, Clark JG, Chien JW. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. Am J Respir Crit Care Med. 2005;172(3):384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savani BN, Montero A, Srinivasan R, et al. Chronic GVHD and pretransplantation abnormalities in pulmonary function are the main determinants predicting worsening pulmonary function in long-term survivors after stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(12):1261-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldberg SL, Klumpp TR, Magdalinski AJ, Mangan KF. Value of the pretransplant evaluation in predicting toxic day-100 mortality among blood stem-cell and bone marrow transplant recipients. J Clin Oncol. 1998;16(12):3796-3802. [DOI] [PubMed] [Google Scholar]
- 45.Shouval R, de Jong CN, Fein J, et al. Baseline renal function and albumin are powerful predictors for allogeneic transplantation-related mortality. Biol Blood Marrow Transplant. 2018;24(8):1685-1691. [DOI] [PubMed] [Google Scholar]
- 46.Au BK, Gooley TA, Armand P, et al. Reevaluation of the pretransplant assessment of mortality score after allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2015;21(5):848-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-Age Index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin RJ, Elko TA, Devlin SM, et al. Impact of geriatric vulnerabilities on allogeneic hematopoietic cell transplantation outcomes in older patients with hematologic malignancies. Bone Marrow Transplant. 2020;55(1):157-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cotes JE, Dabbs JM, Elwood PC, et al. Iron-deficiency anaemia: its effect on transfer factor for the lung (diffusing capacity) and ventilation and cardiac frequency during sub-maximal exercise. Clin Sci. 1972;42(3):325-335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.