Abstract
The Staphylococcus aureus phosphoglucosamine mutase gene glmM was shown to be the last gene of a three-cistron operon, orf1-orf2-glmM. One transcriptional start was identified upstream of orf1, and a second start producing a monocistronic transcript was identified upstream of glmM. Disruption of glmM abolished GlmM production, decreased methicillin resistance, and resulted in teicoplanin hypersusceptibility without affecting the production of the endogenous penicillin-binding proteins and PBP 2′. Complementation of the glmM mutation by the complete glmM operon restored both methicillin resistance and normal teicoplanin susceptibility. In contrast, a highly methicillin-resistant suppressor mutant obtained by selection for growth in the presence of methicillin remained GlmM deficient and teicoplanin hypersusceptible. The suppressor mutation was not linked to the glmM operon but was correlated with decreased autolysis and increased production of a 49-kDa protein, suggesting that there is an alternative pathway for glucosamine-1-phosphate synthesis in S. aureus.
Methicillin resistance in Staphylococcus aureus is due to an additional penicillin-binding protein (PBP), PBP 2′ (PBP 2a), which has a lower affinity for methicillin than the four endogenous PBPs (26). PBP 2′ has in vitro transpeptidase activity (13), and its activity is thought to substitute for the functions of the PBPs when they are inactivated by high concentrations of β-lactams. PBP 2′ does not contribute to the peptidoglycan composition in the absence of methicillin, whereas in the presence of methicillin it seems to be able to form only muropeptide dimers (8, 9). Methicillin resistance levels are strain specific and may vary from very low to high values. Characteristic for methicillin-resistant (Mcr) S. aureus is the heterogeneous expression of the resistance with the production of a subpopulation of cells highly resistant to methicillin. This high level of resistance depends on chromosomally encoded genes (28) and can be achieved by different mechanisms, one of which has been identified as the inactivation of an autolytic activity (LytM) (12). A large number of chromosomal genes, initially called fem or aux factors (4, 10), are known to influence methicillin resistance levels. These genes are involved, directly or indirectly, in peptidoglycan metabolism. Any mutations that alter peptidoglycan precursor composition and/or precursor formation, for instance, inhibition of the lysine addition step in the stem peptide by the femF mutation (25), reduction of amidation of the stem peptide glutamate by femC inactivation (14), or shortening of the pentaglycine side chain by inactivation of the femAB operon (16, 30), reduce the level of methicillin resistance. The phosphoglucosamine mutase GlmM, initially identified as a femD mutation (4), catalyzes the conversion of glucosamine-6-phosphate to glucosamine-1-phosphate, which is an early cytoplasmic step in peptidoglycan biosynthesis (19, 32). We show here that glmM is part of a three-cistron operon and that inactivation of glmM can be overcome by a suppressor mutation that leads to high-level methicillin resistance.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
The strains and plasmids used in this study are listed in Table 1. The strains were grown at 37°C in Luria-Bertani (LB) medium (Difco, Detroit, Mich.). S. aureus was transduced with bacteriophage 80α, and transductants were selected on LB plates containing either 20 μg of erythromycin ml−1 or 10 μg of tetracycline ml−1. Escherichia coli DH10B (Gibco BRL Life Technologies, Gaithersburg, Md.) was transformed by electroporation, and transformants were selected on LB plates containing either 50 μg of ampicillin ml−1 or 10 μg of tetracycline ml−1. Antibiotic susceptibility was determined by the E test (AB BIODISK, Solna, Sweden) on Mueller-Hinton agar (Difco) after 24 h of incubation at 35°C. The antibiotic resistance profile of a culture (population analysis) was obtained by plating aliquots of an overnight culture on freshly poured LB agar plates containing increasing amounts of methicillin. The numbers of CFU were counted after 48 h of incubation at 35°C. Spontaneous autolysis of exponentially growing cells was determined in 0.05 M Tris-HCl (pH 7.5) as the loss of turbidity at an optical density of 600 nm at 37°C (15).
TABLE 1.
Bacterial strains and plasmids used in this study
| S. aureus strain derived from NCTC 8325 or plasmid | Relevant genotype or insert | Relevant phenotype | Origin or reference |
|---|---|---|---|
| Strains | |||
| BB255 | Wild type | 3 | |
| BB270 | Mcr | 3 | |
| PG27 | Ω12FglmM::Tn551 | Mcs Emr; small colonies | This study; by transduction of Ω12FfemD::Tn551 from BB591(4) into BB255 |
| PG108 | mec Ω12FglmM::Tn551 | Mcs Emr | This study; by transduction of Ω12FfemD::Tn551 from BB591(4) into BB270 |
| PG100 | mec Ω12FglmM::Tn551 hmrD1 | Mcr Emr | This study; Mcr suppressor mutant of PG108 |
| PG105 | mec Ω12FglmM::Tn551 | Mcs Emr | This study; backcross of Emr from PG100 into BB270 |
| PG79 | mec Ω12FglmM::Tn551; pPG76 | Mcr Emr Tcr | This study; by transduction of PG108 |
| PG217 | mec Ω12FglmM::Tn551; pAW8 | Mcs Emr Tcr | This study; by transduction of PG108 |
| RN4220 | NCTC 8325-4 r− m+ | Restriction negative, modification positive | 21 |
| PG77 | NCTC 8325-4 r− m+; pAW8 | Tcr | This study; by electroporation of RN4220 |
| PG78 | NCTC 8325-4 r− m+; pPG76 | Tcr | This study; by electroporation of RN4220 |
| Plasmids | |||
| pAW8 | 5.1 kb, ori-pAMα1-ori-ColE1 | Tcr | E. coli-S. aureus shuttle plasmid obtained from A. Wada (17) |
| pPG76 | 5-kb PstI (argI orf1–orf2–glmM) fragment from BB270 cloned in pAW8 | Tcr | This study |
Abbreviations: Em, erythromycin; femD is a synonym for glmM; hmrD1, suppressor mutation leading to high Mcr in glmM (femD) mutants; Mc, methicillin; Tc, tetracycline; r, resistant; s, susceptible.
Cloning of glmM.
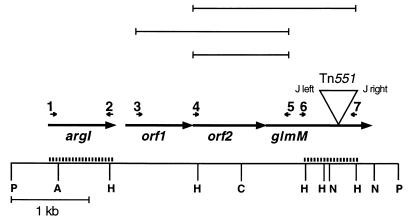
A 10.5-kb chromosomal XbaI fragment from strain PG108 containing the right junction of Tn551 was identified with a Tn551 probe, subcloned in plasmid pTZ18R (Genescribe-Z; United States Biochemical Corporation, Cleveland, Ohio), and subsequently used to probe a genomic PstI library of strain BB270 in shuttle vector pAW8 (17), yielding recombinant plasmid pPG76 which contained a 5-kb PstI fragment covering the glmM operon (Fig. 1). For complementation of S. aureus, plasmids were first electroporated into the restriction-negative, modification-positive strain S. aureus RN4220 and were then transferred by phage 80α-mediated transduction into the desired strain.
FIG. 1.
Genetic and restriction map of the region containing the glmM operon. Restriction sites are indicated on the bottom line by the following abbreviations: P, PstI; A, Asp718; H, HindII; C, ClaI; N, NsiI. Open reading frames are indicated by arrows, and the position and orientation of the Tn551 insert in glmM are shown. The Tn551 insert is not drawn to scale. The small numbered arrows show the positions and orientations of the primers used for RT-PCR. The products generated by RT-PCR are shown in the top rows. The probes used for Northern blotting are shown by heavy dashed lines.
Molecular techniques.
Standard techniques for DNA isolation, gel electrophoresis, blotting, and Southern and Northern hybridization procedures were used (23). Restriction enzymes, a randomly primed labelling kit, T4 DNA ligase, and calf intestinal alkaline phosphatase were purchased from Boehringer Mannheim (Mannheim, Germany), and T4 polynucleotide kinase was purchased from MBI Fermentas (Vilnius, Lithuania) and was used as recommended by the supplier. PCR was carried out with primers synthesized by Microsynth (Balgach, Switzerland) and AmpliTaq Gold polymerase from Perkin-Elmer (Rotkreuz, Switzerland). Primers were designed with OLIGO 4.01 primer analysis software (25a). The primers used for amplification and reverse transcription (RT)-PCR (RT-PCR) were 1 (5′ [nucleotide {nt} 107]-TGATTTAGGACCAACAGC), 2 (5′ [nt 968]-ACCTAATGAAACCGCCTG), 3 (5′ [nt 1307]-AGGGAACTAAAGCGATAC), 4 (5′ [nt 2025]-GGGGCTTGAGATTTATTG), 5 (5′ [nt 3286]-CAACTGGATTATGAGAGG), 6 (5′ [nt 3426]-TTACTTTGAAGGGGC), and 7 (5′ [nt 4116]-TTTATCTGTTACGCG).
DNA sequence analysis.
Restriction fragments of the 5-kb insert of pPG76 were subcloned in pTZ18R. The DNA was sequenced with the ALF sequenator (Pharmacia, Uppsala, Sweden) with the Thermo Sequenase fluorescence-labelled primer cycle sequencing kit with 7-deaza-dGTP from Amersham (Buckinghamshire, United Kingdom). DNA sequences were analyzed with the DNA Inspector IIe program (Textco, Inc., West Lebanon, N.H.) and the Genetics Computer Group program (University of Wisconsin Genetics Computer Group, Madison) (31a). Amino acid sequence similarities were searched for by comparison of the sequence with those in the SwissProt and TREMBL data banks.
Northern blot analysis.
RNA was isolated from exponentially growing cells as described by Kullik and Giachino (22). The probes used for the Northern hybridization were obtained by PCR amplification with primers 1 and 2 or primers 6 and 7 (Fig. 1), and the probes were labelled with [α-32P]dCTP with the random primed labelling kit of Boehringer Mannheim.
RT-PCR.
RT of the mRNA was performed with reverse transcriptase H from Gibco with primer 5 or 7. The cDNA was amplified by PCR in a Perkin-Elmer Cetus DNA thermal cycler with the following primer pairs: 4 and 7, 4 and 5, and 3 and 5. Controls for detection of contaminating double-stranded DNA were made by amplifying the RNA preparations without prior RT.
Primer extension.
Primer extension analysis was carried out as described by Storz and Altuvia (29). Primers 8 [5′ [nt 1315]-TAGTTCCCTTAAAGACCGTGATGAG) and 9 (5′ [nt 3088]-TTTCACCTTTATTATGTGCTAGAAC) were used.
Membrane proteins and PBPs.
Membranes of cells grown overnight at 30°C were prepared by differential centrifugation, the PBPs were labelled with 10 μg of [phenyl-4(n)-3H]benzylpenicillin ml−1 (final concentration; 11.9 Ci mmol−1; Amersham Life Sciences) for 10 min at 30°C and were separated on sodium dodecyl sulfate-polyacrylamide gels, as described earlier (5). The proteins were stained with Coomassie brilliant blue, and the PBPs were visualized by fluorography after 4 weeks of exposure to X-ray film at −70°C.
Western blots.
Recombinant GlmM protein was obtained by cloning glmM into a pFLAG MAC vector (IBI Escherichia coli FLAG expression system; International Biotechnologies Inc., Eastman Kodak Company) (20). The recombinant GlmM containing the hydrophilic FLAG peptide Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys was purified according to the manufacturer’s recommendations and was used to prepare rabbit anti-GlmM antiserum. Western blotting was performed with cytoplasmic extracts of overnight cultures that were lysed in phosphate-buffered saline containing 1 mM phenylmethylsulfonyl fluoride and 100 μg of lysostaphin ml−1 and that were resolved on a sodium dodecyl sulfate–10% polyacrylamide gel. Rabbit anti-GlmM serum was used as a first antibody. Immunodetection was performed as described in the manual supplied with Chemoluminescence Reagent Plus (NEN Life Science Products, Boston, Mass.). Human immunoglobulin G was added at a concentration of 42 μg ml−1 to all reaction mixtures to saturate the Fc binding sites of protein A. Controls were made with the preimmune serum and with anti-FLAG monoclonal antibodies.
Nucleotide sequence accession number.
The nucleotide sequence of the 5-kb fragment can be accessed in the EMBL/GenBank under accession no. Y15477.
RESULTS
Phenotype of glmM disruption and Mcr suppressor mutants.
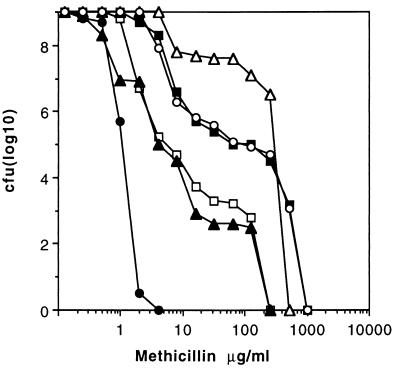
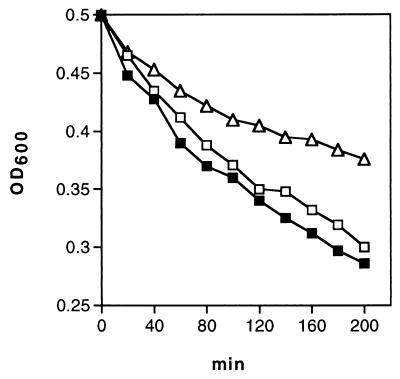
Disruption of glmM (synomymous with femD) by Tn551 reduced the level of resistance to methicillin as well as the number of subclones of strain PG108 growing in the presence of >10 μg of methicillin ml−1 compared to the growth of the Mcr parent BB270 (Fig. 2). All highly resistant subclones were still erythromycin resistant (Emr) and were thus still carrying the Tn551 insert. A representative of these subclones, strain PG100, was analyzed in more detail. It showed greatly increased resistance to different β-lactams (Table 2) and produced a larger proportion of highly Mcr subclones compared to the proportion produced by BB270 (Fig. 2). Spontaneous autolysis was found to be lower in PG100 than in BB270 and PG108 (Fig. 3). These properties were also stable after repeated subcultivation under nonselective conditions and storage at −70°C. Backcrosses of Emr from PG100 into BB270 yielded methicillin-susceptible (Mcs) transductants with the same phenotype as PG108, represented here by strain PG105 (Fig. 2). This, plus the identical hybridization patterns of different chromosomal digests of PG105, PG100, and PG108 obtained when they were probed with Tn551 DNA (data not shown), confirmed that glmM was still disrupted in Mcr subclone PG100. The high level of methicillin resistance of strain PG100 was not cotransducible with Emr. A suppressor or compensatory mutation, termed here hmrD1 (standing for high-level methicillin-resistant suppressor mutant of femD), that led to the highly resistant phenotype was therefore postulated to have occurred in PG100 at a location not linked to glmM. Disruption of glmM increased the level of β-lactam susceptibility as well as the level of teicoplanin susceptibility in both Mcr BB270 and Mcs BB255, as shown by strains PG108 and PG27, respectively (Table 2); it had no effect, however, on vancomycin susceptibility. Interestingly, in the Mcr suppressor mutant PG100, the level of teicoplanin susceptibility remained lower. The hmrD1 mutation therefore suppressed only methicillin sensitivity, whereas teicoplanin hypersusceptibility seemed to be linked to the still inactivated glmM operon.
FIG. 2.
Population analysis using overnight cultures plated on increasing concentrations of methicillin. Symbols: •, BB255; ■, BB270; □, PG108; ▵, PG100; ▴, PG105; ○, PG79.
TABLE 2.
MICs of cell wall-directed antibioticsa
| Strain | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Vancomycin | Teicoplanin | Oxacillin | Methicillin | Imipenem | |
| BB255 | 1.5 | 1.0 | 0.19 | 0.75 | 0.032 |
| PG27 | 1.5 | 0.25 | 0.064 | 0.25 | 0.012 |
| BB270 | 1.5 | 1.0 | 12 | 6 | 0.25 |
| PG108 | 0.75 | 0.064 | 0.094 | 0.38 | 0.032 |
| PG100 | 1.0 | 0.064 | >256 | >256 | >32 |
| PG105 | 0.75 | 0.064 | 0.19 | 0.75 | 0.047 |
| PG79 | 1.5 | 1.0 | 16 | 12 | 0.125 |
| PG217 | 0.75 | 0.19 | 0.38 | 0.38 | 0.047 |
The MICs were determined by the E test.
FIG. 3.
Spontanous autolysis of strains BB270 (■), PG108 (□), and PG100 (▵). OD600, optical density at 600 nm.
Western blot, PBP, and membrane protein patterns in glmM mutants.
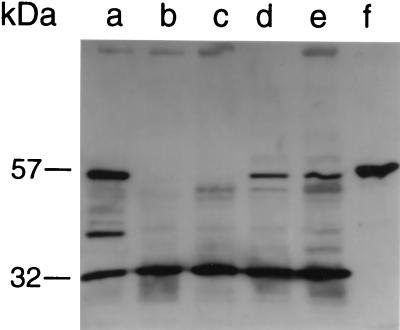
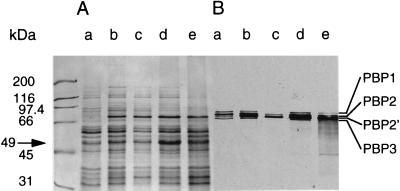
A Western blot with anti-GlmM antibodies showed, as anticipated, that the phosphoglucomutase was absent from both mutant PG108 and Mcr suppressor mutant PG100, whereas it was overproduced in complemented strain PG79 (Fig. 4). The smaller bands seen in Fig. 4 are thought to be degradation products due to GlmM overproduction. The GlmM protein migrated at a slightly higher position than expected from its calculated size. A small 32-kDa band of unknown origin and nature reacted with GlmM antibodies for all strains, irrespective of the presence of the glmM mutation. Inactivation of glmM had no influence on the production of PBP 2′ or on that of the other PBPs. The corresponding PBP bands had similar intensities in wild-type strain BB270 and mutant strain PG108 as well as in suppressor mutant strain PG100 (Fig. 5B). When strain BB270 was grown in the presence of methicillin, all PBPs except the low-affinity PBP 2′ and a fraction of PBP 2 were saturated with methicillin and could not be labelled with [3H]penicillin (Fig. 5B, lane c). In the membrane protein pattern of strain PG100 an intense 49-kDa band appeared. This band was either less prominent in or absent from the other strains (Fig. 5A), suggesting that it may result from the postulated suppressor mutation hmrD1.
FIG. 4.
Western blot of the phosphoglucosamine mutase in the different strains. Cell extracts were probed with anti-GlmM antibodies. Lane a, PG79; lane b, PG100; lane c, PG108; lane d, BB255; lane e, BB270; and lane f, recombinant GlmM.
FIG. 5.
Membrane proteins and PBPs. (A) Coomassie blue-stained membrane proteins of strains BB255 (lane a), BB270 (lane b), BB270 (lane c), PG100 (lane d), and PG108 (lane e). All strains except strain BB270 in lane c were grown in LB medium without antibiotics. Strain BB270 in lane c was grown in the presence of 256 μg of methicillin ml−1. (B) Fluorography of the [3H]penicillin-labelled PBPs in the gel shown in panel A. The sizes of the molecular weight markers and the positions of the high-molecular-mass PBPs and of the 49-kDa band are indicated.
Cloning of glmM and complementation of a glmM mutant.
The wild-type glmM gene was cloned on a 5-kb PstI fragment from strain BB270 into shuttle vector pAW8, yielding plasmid pPG76. This plasmid restored resistance to methicillin in PG108, as seen in strain PG79, which showed the same resistance profile as BB270 (Fig. 2). In contrast to suppressor mutant PG100, PG79 showed normal teicoplanin susceptibility (Table 2). The shuttle vector pAW8 alone had no effect on β-lactam resistance or teicoplanin hypersusceptibility, as shown by the results for strain PG217 (Table 2).
DNA sequence.
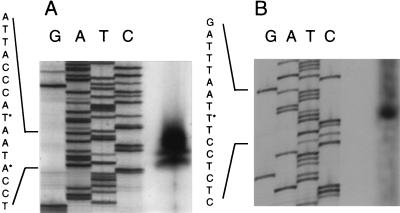
The 5-kb PstI fragment of pPG76 contained four open reading frames (ORFs), all oriented in the same direction (Fig. 1). The first ORF codes for a 302-amino acid (aa) protein with a calculated molecular mass of 33 kDa. Its sequence showed 53% identity and 73% similarity in a 300-aa overlap to the sequence of the arginase of Bacillus caldovelox (Swissprot accession no. p53608) and 52% identity and 72% similarity to that of B. subtilis (Swissprot accession no. p39138). The identity around the active site was over 70%. This ORF was therefore named argI. It was followed by a stretch of T residues with a preceding dyad symmetry characteristic for factor-independent RNA polymerase terminators (6). The second ORF, termed orf1, codes for a 269-aa protein of 29.6 kDa. Its sequence has 56% identity and 76% similarity to the sequence of a hypothetical protein encoded by ybbP in Bacillus subtilis. Two hydrophobic regions at the C-terminal region suggest that it may be membrane associated. The third ORF, orf2, separated by 4 nucleotides from orf1, encoded a 310-aa protein of 34 kDa. Its sequence had 28% identity and 53% similarity to the sequence of a hypothetical protein encoded by ybbR in B. subtilis. The fourth ORF, separated by 29 nt from orf2, is glmM coding for 451-aa protein of 49 kDa. The glmM DNA sequence is identical to that of the phosphoglucosamine mutase gene glmM in strain COL (19, 32) except for a single T-to-C difference at position 3719 which results in the change of a valine to an alanine at position 251. Tn551 truncates the terminal 99 residues of GlmM in strain PG108. The truncated GlmM still harbors the active site that contains the phosphogluco- and phosphomannomutase phosphoserine signature located between aa 91 and 95, 95 and 110, and 111 and 115. A factor-independent RNA polymerase terminator was identified downstream of glmM. The transcriptional start of orf1 and a second start preceding glmM were identified from RNA isolated from exponentially growing S. aureus containing plasmid pPG76 (Fig. 6). Two transcriptional starts separated by 3 nt were apparent for orf1. The positions of the transcriptional starts were confirmed with further primers (data not shown). Putative −35 and −10 sequences and ribosome-binding sites preceding argI, orf1, and glmM were identified. No obvious ribosome-binding site or putative −35 and −10 sequences were identifiable upstream of orf2.
FIG. 6.
Transcriptional starts. The transcriptional starts of the glmM operon were determined by primer extension. Lanes G, A, T, and C present the sequence of the corresponding region of the glmM operon. (A) Transcriptional starts of orf1. (B) Transcriptional start of glmM. Transcriptional starts are indicated with an asterisk.
Transcription of the glmM region.
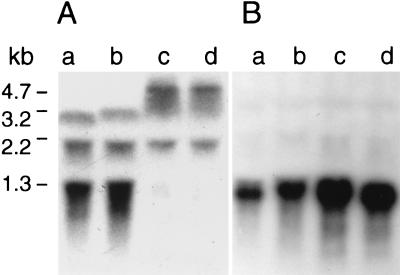
RNA extracted from exponentially growing mutant PG108, parents BB255 and BB270, and suppressor mutant PG100 were analyzed by Northern blotting with a 0.69-kb glmM-internal probe (Fig. 1) covering the Tn551 insertion site. Strains BB255 and BB270 showed three transcripts of 3.2, 2.2, and 1.3 kb with increasing intensities (Fig. 7A). As deduced from their sizes, the 3.2-kb band was thought to be a transcript covering the three ORFs, orf1-orf2-glmM, and the 2.2-kb transcript was thought to be a degradation product of that transcript. The 1.3-kb transcript was thought to originate from glmM, since it was absent from strains PG108 and PG100, which carried the Tn551-inactivated glmM, and was substituted for by a larger and weaker band of about 4.7 kb, presumably a readthrough product into Tn551.
FIG. 7.
Northern blot analysis of the glmM region. Strain BB255 (lanes a), strain BB270 (lanes b), strain PG108 (lanes c), and strain PG100 (lanes d) were probed with the internal glmM probe (A) and with the argI fragment (B). The numbers indicate the sizes of the transcripts.
Hybridization with an internal argI gene probe produced a 1.25-kb single transcript that exceeds the length of argI by about 300 nt (Fig. 7B). Its presence in all strains suggests that argI does not belong to the postulated glmM operon. Interestingly, the argI transcripts were repeatedly of stronger intensity in the glmM mutants than in BB270, although identical amounts of RNA were loaded in the lanes.
RT of the mRNA and subsequent amplification with different primer pairs yielded with primers 3 and 5 a 1,969-bp product that covers orf1 and orf2 and part of glmM from both mutant PG108 and wild-type BB270. With primers 4 and 7, a 2,091-bp fragment covering orf2-glmM was produced from strain BB270 but not from strain PG108 carrying the 5.2-kb Tn551 insert (data not shown). With primers 4 and 5, a strong 1,251-bp band could be amplified from PG108, showing the transcriptional linkage of orf2 with glmM, thus supporting the postulated operon structure orf1-orf2-glmM.
DISCUSSION
The phosphoglucosamine mutase GlmM catalyzes the conversion of glucosamine-6-phosphate to glucosamine-1-phosphate (19, 32), the initial step in the formation of the nucleotide sugar UDP-N-acetylglucosamine, which is involved in peptidoglycan biosynthesis. The final structure and composition of the peptidoglycan are only marginally affected in glmM mutants. The main changes are a 5% lower peptidoglycan cross-linking compared to that in the wild type and a reduction of a minor component of the peptidoglycan that contains an alanyl-tetraglycine instead of a pentaglycine substituent on the lysine ɛ-amino group (27). That such minor changes result in methicillin susceptibility may be explained by a lower rate of production of peptidoglycan precursors that is insufficient to support the protective action of PBP 2′ in competition with methicillin. In E. coli the glmM gene is essential (24). Since in S. aureus GlmM inactivation is tolerated and only marginally affects the peptidoglycan, there may be an alternative pathway for glucosamine-1-phosphate synthesis in S. aureus.
Since GlmM is involved in an early step of UDP-N-acetylglucosamine synthesis, not only peptidoglycan precursor formation but also wall teichoic acid substitution of the peptidoglycan may have been affected (11). However, the amount of wall teichoic acid, its composition, and N-acetylglucosaminyl substitution were indistinguishable in stationary-phase cultures of BB270 and PG108 (18), as were the plating efficiencies of phages, which depend on the N-acetylglucosaminyl modification of the wall teichoic acid (7).
The phosphoglucosamine mutase was found to be the last gene of the three-cistron operon structure orf1-orf2-glmM. A second monocistronic transcript was shown to originate from glmM. Upon Tn551-mediated glmM inactivation, the monocistronic glmM transcript disappeared and was replaced by a weaker and longer transcript. Unlike Tn551 inserts in femAB (2) or femC (14), in which Tn551-interrupted transcripts increase by 5.2 kb due to Tn551, the Tn551-interrupted glmM transcript increased by less than 5.2 kb, perhaps due to different processing of the mRNA. Although this transcript may code for a truncated GlmM product, GlmM protein production was completely abolished, as shown by Western blotting, in the mutant as well as in the suppressor mutant PG100. The nature of the 32-kDa protein that reacted with the anti-GlmM antibodies that was present in all strains analyzed needs to be studied further.
A gene organization similar to that of the glmM operon is found in the B. subtilis genome (ybbP-ybbR-ybbT), where the sequences of YbbP, YbbR, and YbbT have identities of 56, 28, and 67% to those of ORF1, ORF2, and GlmM, respectively. The upstream argI transcript was clearly independent from the glmM operon, although argI transcription was increased in glmM-inactivated strains, which may point to a complex interaction between the glmM operon and arginase transcription.
Interestingly, the suppressor mutation hmrD1 in PG100 restored only methicillin resistance but did not alter teicoplanin hypersusceptibility. The glycopeptide teicoplanin inhibits both transglycosylation and transpeptidation by binding to the terminal d-Ala-d-Ala of the peptidoglycan precursor, enhancing its activity by its membrane anchor (1). This suggests that, besides GlmM activity, a membrane component may be affected in the glmM mutants since the hydropathy profile predicts that ORF1 is a membrane-associated protein. This may be the reason for the teicoplanin hypersusceptibility.
The decreased spontaneous autolysis, plus the higher level of production of a 49-kDa membrane protein, may be due to the suppressor mutation in PG100. A compensatory mutation leading to high-level Mcr also occurs in femC mutants (31). The nature of these compensatory mutations is unknown. There is evidence that several different mechanisms may be able to confer high-level methicillin resistance (31). One of these mechanisms was shown to be by inactivation of an autolytic activity (12). A similar mechanism may act here since PG100 showed decreased autolysis as well.
ACKNOWLEDGMENTS
This work was supported by Swiss National Science Foundation grant 31-42182.94 and Jubiläumsspende der Universität Zürich.
We thank K. Shaw for fruitful discussions.
REFERENCES
- 1.Beauregard D A, Williams D H, Gwynn M N, Knowles D J C. Dimerization and membrane anchors in extracellular targeting of vancomycin group antibiotics. Antimicrob Agents Chemother. 1995;39:781–785. doi: 10.1128/AAC.39.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger-Bächi B, Barberis-Maino L, Strässle A, Kayser F H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989;219:263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- 3.Berger-Bächi B, Kohler M L. A novel site on the chromosome of Staphylococcus aureus influencing the level of methicillin resistance: genetic mapping. FEMS Microbiol Lett. 1983;20:305–309. [Google Scholar]
- 4.Berger-Bächi B, Strässle A, Gustafson J E, Kayser F H. Mapping and characterization of multiple chromosomal factors involved in methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1367–1373. doi: 10.1128/aac.36.7.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger-Bächi B, Strässle A, Kayser F H. Characterization of an isogenic set of methicillin-resistant and -susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986;5:697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- 6.Brendel V, Trifonov E N. A computer algorithm for testing potential prokaryotic terminators. Nucleic Acids Res. 1984;12:4411–4427. doi: 10.1093/nar/12.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coyette J, Ghuysen J M. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. IX. Teichoic acid and phage adsorption. Biochemistry. 1968;7:2385–2389. doi: 10.1021/bi00846a048. [DOI] [PubMed] [Google Scholar]
- 8.de Jonge B L M, Chang Y S, Gage D, Tomasz A. Peptidoglycan composition in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J Biol Chem. 1992;267:11255–11259. [PubMed] [Google Scholar]
- 9.de Jonge B L M, Tomasz A. Abnormal peptidoglycan produced in a methicillin-resistant strain of Staphylococcus aureus grown in the presence of methicillin: functional role for penicillin-binding protein 2A in cell wall synthesis. Antimicrob Agents Chemother. 1993;37:342–346. doi: 10.1128/aac.37.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lencastre H, Tomasz A. Reassessment of the number of auxiliary genes essential for expression of high-level methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2590–2598. doi: 10.1128/aac.38.11.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer W. Bacterial phosphoglycolipids and lipoteichoic acids. In: Kates M, editor. Glycolipids, phosphoglycolipids, and sulfoglycolipids. New York, N.Y: Plenum Press; 1990. pp. 123–234. [Google Scholar]
- 12.Fujimura T, Murakami K. Increase of methicillin resistance in Staphylococcus aureus caused by deletion of a gene whose product is homologous to lytic enzymes. J Bacteriol. 1997;179:6294–6301. doi: 10.1128/jb.179.20.6294-6301.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaisford W C, Reynolds P E. Methicillin resistance in Staphylococcus epidermidis. Relationship between the additional penicillin-binding protein and an attachment transpeptidase. Eur J Biochem. 1989;185:211–218. doi: 10.1111/j.1432-1033.1989.tb15104.x. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson J, Strässle A, Hächler H, Kayser F H, Berger-Bächi B. The femC locus of Staphylococcus aureus required for methicillin resistance includes the glutamine synthetase operon. J Bacteriol. 1994;176:1460–1467. doi: 10.1128/jb.176.5.1460-1467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafson J E, Berger-Bächi B, Strässle A, Wilkinson B J. Autolysis of methicillin-resistant and -susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:566–572. doi: 10.1128/aac.36.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henze U, Sidow T, Wecke J, Labischinski H, Berger-Bächi B. Influence of femB on methicillin resistance and peptidoglycan metabolism in Staphylococcus aureus. J Bacteriol. 1993;175:1612–1620. doi: 10.1128/jb.175.6.1612-1620.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiwa H, Tsuchida N. New shuttle vectors for Escherichia coli and Bacillus subtilis. I. Construction and characterization of plasmid pHY460 with twelve unique cloning sites. Gene. 1984;32:129–134. doi: 10.1016/0378-1119(84)90040-4. [DOI] [PubMed] [Google Scholar]
- 18.Jenni R, Berger-Bächi B. Teichoic acid content in different lineages of Staphylococcus aureus NCTC8325. Arch Microbiol. 1998;170:171–178. doi: 10.1007/s002030050630. [DOI] [PubMed] [Google Scholar]
- 19.Jolly L, Wu S W, Van Heijenoort J, De Lencastre H, Mengin Lecreuix D, Tomasz A. The femR315 gene from Staphylococcus aureus, the interruption of which results in reduced methicillin resistance, encodes a phosphoglucosamine mutase. J Bacteriol. 1997;179:5321–5325. doi: 10.1128/jb.179.17.5321-5325.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komatsuzawa, H., K. Ohta, M. Sugai, T. Fujiwara, J. Suzuki, B. Berger-Bächi, and H. Suginaka. Tn551-insertional inactivation of fmtB gene renders methicillin-resistant Staphylococcus aureus susceptible to oxacillin and Triton X-100. Submitted for publication.
- 21.Kreiswirth B N, Löfdahl S, Bentley M J, O’Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:680–685. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 22.Kullik I, Giachino P. The alternative sigma factor sigma(b) in Staphylococcus aureus—regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 23.Maniatis T, Fritsch E F, Sambrook J E. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 24.Mengin-Lecreulx D, van Heijenoort J. Characterization of the essential gene glmM encoding phosphoglucosamine mutase in Escherichia coli. J Biol Chem. 1996;271:32–39. doi: 10.1074/jbc.271.1.32. [DOI] [PubMed] [Google Scholar]
- 25.Ornelas-Soares A, de Lencastre H, de Jonge B L M, Tomasz A. Reduced methicillin resistance in a new Staphylococcus aureus transposon mutant that incorporates muramyl dipeptides into the cell wall peptidoglycan. J Biol Chem. 1994;269:27246–27250. [PubMed] [Google Scholar]
- 25a.Rejchlik W. OLIGO X.01 primer analysis software. Plymouth, Minn: National Biosciences Inc; 1992. [Google Scholar]
- 26.Reynolds P E, Brown D F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985;192:28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- 27.Roos M H H. Das Murein von Staphylococcus aureus. Untersuchung des Einflusses von Antibiotikaresistenzdeterminanten auf die Feinstruktur des Peptidoglykan von Staphylococcus aureus. Ph.D. thesis. Berlin, Germany: Freie Universität Berlin; 1995. [Google Scholar]
- 28.Ryffel C, Strässle A, Kayser F H, Berger-Bächi B. Mechanisms of heteroresistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:724–728. doi: 10.1128/aac.38.4.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Storz G, Altuvia S. OxyR regulon. Methods Enzymol. 1994;234:217–223. doi: 10.1016/0076-6879(94)34088-9. [DOI] [PubMed] [Google Scholar]
- 30.Strandén A M, Ehlert K, Labischinski H, Berger-Bächi B. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997;179:9–16. doi: 10.1128/jb.179.1.9-16.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strandén A M, Roos M, Berger-Bächi B. Glutamine synthetase and heteroresistance in methicillin-resistant Staphylococcus aureus. Microb Drug Resist. 1996;2:201–207. doi: 10.1089/mdr.1996.2.201. [DOI] [PubMed] [Google Scholar]
- 31a.University of Wisconsin Genetics Computer Group. Program manual for the Wisconsin package, version 8. Madison: University of Wisconsin Genetics Computer Group; 1994. [Google Scholar]
- 32.Wu S W, De Lencastre H, Sali A, Tomasz A. A phosphoglucomutase-like gene essential for the optimal expression of methicillin resistance in Staphylococcus aureus: molecular cloning and DNA sequencing. Microb Drug Resist. 1996;2:277–286. doi: 10.1089/mdr.1996.2.277. [DOI] [PubMed] [Google Scholar]