Key Points
Overall survival has improved for patients with HIV and TCLs such as AIDS-defining B-cell lymphomas in the last decade.
Low CD4 cell count predicts poor overall survival in patients with HIV and TCL, emphasizing the need for effective antiretroviral therapy.
Visual Abstract
Abstract
There are no studies comparing the prognosis for mature T-cell lymphoma (TCL) in people with HIV (PWH) to people without HIV (PWoH) and to AIDS-defining B-cell lymphomas (A-BCLs) in the modern antiretroviral therapy era. North American AIDS Cohort Collaboration on Research and Design and Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment are cohorts that enroll patients diagnosed with HIV and TCL, respectively. In our study, 52, 64, 101, 500, and 246 PWH with histologic confirmation of TCL, primary central nervous system lymphoma, Burkitt’s lymphoma, diffuse large B-cell lymphoma (DLBCL), and Hodgkin’s lymphoma (HL), respectively, and 450 TCLs without HIV were eligible for analysis. At the time of TCL diagnosis, anaplastic large-cell lymphoma (ALCL) was the most common TCL subtype within PWH. Although PWH with TCL diagnosed between 1996 and 2009 experienced a low 5-year survival probability at 0.23 (95% confidence interval [CI]: 0.13, 0.41), we observed a marked improvement in their survival when diagnosed between 2010 and 2016 (0.69; 95% CI: 0.48, 1; P = .04) in contrast to TCLs among PWoH (0.45; 95% CI: 0.41, 0.51; P = .53). Similarly, PWH with ALCLs diagnosed between 1996 and 2009 were associated with a conspicuously inferior 5-year survival probability (0.17; 95% CI: 0.07, 0.42) and consistently lagged behind A-BCL subtypes such as Burkitt’s (0.43; 95% CI:0.33, 0.57; P = .09) and DLBCL (0.17; 95% CI: 0.06, 0.46; P = .11) and behind HL (0.57; 95% CI: 0.50, 0.65; P < .0001). Despite a small number, those diagnosed between 2010 and 2016 experienced a remarkable improvement in survival (0.67; 95% CI: 0.3, 1) in comparison with PWoH (0.76; 95% CI: 0.66, 0.87; P = .58). Thus, our analysis confirms improved overall survival for aggressive B- and T-cell malignancies among PWH in the last decade.
Introduction
Mature T-cell lymphomas (TCLs) and natural killer-/T-cell lymphomas (NK/TCLs) are a group of heterogeneous neoplasms, comprising peripheral T-cell lymphomas (PTCLs) and cutaneous T-cell lymphomas (CTCLs) as its 2 major subgroups.1,2 Prior pivotal studies showed that the 5-year overall survival (OS) response of PTCL to cyclophosphamide, doxorubicin, vincristine and prednisone is suboptimal (range, 32%-43%).1-7 An exception is anaplastic lymphoma kinase (ALK)-positive (ALK+) large cell lymphoma (ALCL), which is associated with 5-year OS up to 70% after cyclophosphamide, doxorubicin, vincristine and prednisone.1,8 Overall, little is known about TCLs because they occur much less frequently than B-cell lymphomas (BCLs).
Little is known about the incidence, clinical, virologic, and immunologic features of TCLs and NK/TCLs in people with HIV (PWH) as these are less common than AIDS-defining B-cell lymphomas (A-BCLs), and currently, there are no standard guidelines for treatment of these patients. Most of the available literature for PWH and TCL is descriptive in nature, restricted to small series and/or based on compilation of cases identified through online search of published reports.9-18 The largest experience reported in 2011 was an updated retrospective multicenter review of 51 patients and demonstrated poor survival in those patients with low CD4 cell count and high viral load (VL).9 The OS was noted to be ∼12 months with no significant difference between the different subtypes of TCL. The widespread use of antiretroviral therapy (ART) has allowed for the delivery of full-dose and dose-intensive chemotherapy regimens with improved outcomes for A-BCL that could be compared with those seen in non–HIV-infected patients.19,20 In a recent retrospective multicenter international analysis of 249 patients with newly diagnosed HIV-Burkitt lymphoma (BL), contemporaneously treated in the United States and United Kingdom, no HIV-related factors influenced survival outcomes that correlated with prior reports in this subtype.21,22 Similar observations have been reported for other HIV-associated lymphomas such as diffuse large B-cell lymphoma (DLBCL), Hodgkin’s lymphoma (HL), and primary central nervous system lymphoma (PCNSL).23-25 There have been no comparative studies evaluating prognostic factors and outcomes of TCL and NK/TCL in people with and without HIV (PWoH). Furthermore, no literature exists delineating differences in clinical features and survival relative to A-BCL subtypes within PWH to inform integration of targeted therapeutics into standard frontline treatments.
We hypothesized that PWH with TCL and NK/TCL are associated with a worse survival in comparison with PWoH, but like A-BCL, their prognosis has improved in the recent years. The primary objective of this study was to compare the clinicopathologic characteristics, prognostic factors, and survival of patients with validated TCL and NK/TCL with and without HIV among those treated for lymphoma in United States and Canada. A secondary objective was to contrast the clinical features and outcomes of A-BCL and HL with TCL in PWH that would highlight similarities and differences ultimately leading to better understanding of these rare and heterogeneous lymphomas. To achieve this, we evaluated studies in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD for PWH) cohort collaboration and the Comprehensive Oncology Measures for Peripheral T-cell Lymphoma Treatment (COMPLETE for PWoH) cohort.
Methods
Study population
The study population included patients from the 2 source populations, the NA-ACCORD for PWH and COMPLETE for PWoH, both of which have been previously described.26-29 Briefly, the NA-ACCORD is a collaboration of >20 longitudinal cohorts of adults (age ≥ 18 years) with HIV in the United States and Canada. List of enrolling sites is included in the supplemental Information. Enrollment criteria include successful linkage into HIV care (defined as ≥2 HIV clinical visits within 12 months) or enrollment into a contributing interval cohort. Cohorts submit data to the Data Management Core (Washington University, Seattle, WA) using standardized submission methods, which are harmonized and undergo data quality checks before secure transfer to the Epidemiology/Biostatistics Core (Johns Hopkins University, Baltimore, MD) where data undergo additional quality checks and analytic-ready datasets are maintained. Cohorts obtain institutional review board approval for all human subject activities conducted within the NA-ACCORD.
The NA-ACCORD protocol for validation of cancer diagnoses has been previously described.30 Briefly, cancer was validated via matching to cancer registries or through submission of cancer diagnosis information (from the electronic medical record) via a web-based standardized abstraction protocol that included cancer site and histopathology. Data abstractors and reviewers were overseen by physicians and abstracted data on cancer site, diagnosis date, histopathology, grade, stage, and risk factors. Reviewers were provided detailed instructions and examples based on SEER cancer data collection instructions to determine the most accurate cancer diagnosis category and date. Further details of this process have been validated and previously described.30
The study population for this analysis included PWH receiving HIV care in 1 of 20 clinical cohorts in the NA-ACCORD who had an incident cancer diagnosis of mature TCL, NK/TCL, BL, PCNSL, DLBCL, and HL between 1 January 1996 and 31 December 2016. We analyzed only the first cancer diagnosis of any of the above HLs and non-Hodgkin’s lymphoma (NHL) subtypes if more than 1 type of cancer occurred. Individuals with previous cancer diagnoses apart from TCL, NK/TCL, BL, PCNSL, DLBCL, and HL were included in this analysis.
COMPLETE is a prospective, multicenter cohort study of patients with newly diagnosed mature TCL in the United States between 5 February 2010 and 5 February 2014. List of enrolling sites is included in the supplemental Information. The same inclusion/exclusion criteria for TCL and NK/TCL cases were applied to both NA-ACCORD and COMPLETE cohorts. All study participants provided written consent at enrollment, and each participating institution obtained institutional review board approval. In the COMPLETE cohort, a central review of the locked data was performed by a steering committee of experts in TCL, and queries were generated to verify data inconsistencies. A separate analysis was performed to verify the accuracy of the histologic data entered through comparison of the uploaded histologic data to the diagnostic pathology reports.27 Treatment data were available. The last follow-up date was 17 December 2018, and the database was fully locked on 28 December 2018.
For our nested study, institutional review board approval at Beth Israel Deaconess Medical Center was obtained before receipt of NA-ACCORD and COMPLETE data.
Outcomes: death
All-cause mortality at 5 years after type-specific lymphoma diagnosis was an outcome of interest. Cohorts in the NA-ACCORD have comprehensive ascertainment of deaths using active and passive methods.31 The NA-ACCORD cohorts obtain the date of death through linkages to the Social Security Death Index, the National Death Index, and/or state vital statistics registries, as well as Canadian provincial death registries; the sources change over time in some cohorts.
The COMPLETE cohort obtained the date of death through active reporting by participating site and through extensive communications with the providers at each participating site.
Further details of the methods section including description of covariates and description of the statistical tools are provided in the supplemental Information.
Results
Of the 109 167 people living with HIV and enrolled in the 20 NA-ACCORD cohorts, 1216 with a diagnosis of NHL and 246 with HL were eligible for this study. Among these eligible subjects, 52 patients with mature TCL and NK/TCL, 101 with BL, 64 with PCNSL, and 500 with DLBCL were included in the final analysis. Median follow-up for PWH in the NA-ACCORD cohort was 5.4 years (range, 2.4-9.1 years). Of the 499 patients with mature TCL and NK/TCL enrolled in the COMPLETE study, 452 patients with locked baseline records and follow-up were included for initial analysis. Two patients were further excluded from analysis who had documented evidence of HIV infection, thereby making a total of 450 patients available for the final analysis. The median follow-up was 2.2 years for PWoH in the COMPLETE cohort (range, 0.6-4.73 years).
Comparison of general patient characteristics between TCL and NK/TCL in people with and without HIV
Table 1 summarizes the baseline demographic and clinical characteristics of study subjects with and without HIV that were included in the final analysis. At the time of TCL or NK/TCL diagnosis (n = 52), PWH were significantly younger compared with PWoH (n = 452; 49 vs 60 years, respectively; P < .001). There were significantly more men (96% vs 63%; P < .001), patients of Black race (34% vs 15%; P = .006), patients with chronic kidney disease (CKD; 19% vs 2.2%; P < .001), and patients with coinfections such as hepatitis B virus (13% vs 0.9%; P < .001) and hepatitis C virus (19% vs 1.1%; P < .001) in PWH and TCL relative to PWoH. It is to be noted that all patients with TCL and NK/TCL within the COMPLETE cohort were newly diagnosed between 2010 and 2014 as opposed to only 25% of PWH in NA-ACCORD during that calendar period. Patients with a diagnosis of ALCL and PTCL-not otherwise specified (PTCL-NOS) comprised 50% and 23% of the overall cohort in PWH with TCL in comparison with 18% (P < .001) and 32% (P = .26) of these histologic subtypes in PWoH, respectively. Angioimmunoblastic T-cell lymphoma (AITL) was the third most common subtype in PWoH, accounting for 16% of overall cases in comparison with 6% in PWH with TCL (P = .07). The majority (93%) of the patients within the COMPLETE registry exhibited an Eastern Co-operative Oncology Group performance status between 0 and 1, with approximately half of the patients experiencing B symptoms and an elevated lactate dehydrogenase level as depicted in supplemental Table 1. Median International Prognostic Index was 2 (range, 1-3) for subjects in the COMPLETE cohort. This information was not available for PWH and TCL.
Table 1.
Baseline demographic and clinical characteristics for NA-ACCORD (N = 717) and COMPLETE cohorts (N = 450)
| Characteristic | PWoH with TCL and NK/TCL (n = 450 | PWH with TCL and NK/TCL (n = 52) | PWH with BL (n = 101) | PWH with PCNSL (n = 64) | PWH with DLBCL (n = 500) | P * | P † |
|---|---|---|---|---|---|---|---|
| Age at diagnosis of lymphoma (y), median (IQR) | 60 (48-70) | 49 (43-55) | 47 (39-51) | 41 (37-49) | 47 (40-55) | <.001 | .12 |
| Biological sex, no. (%) | |||||||
| Male | 283 (63) | 50 (96) | 94 (93) | 58 (91) | 437 (87) | <.001 | .11 |
| Race, no. (%) | |||||||
| White | 339/438 (77) | 30/47 (64) | 58/94 (62) | 21/58 (37) | 255/461 (55) | .006‡ | .51‡ |
| Black | 67/438 (15) | 16/47 (34) | 31/94 (33) | 34/58 (59) | 189/461 (41) | ||
| Other | 32/438 (7) | 1/47 (2) | 5/94 (5) | 3/58 (5) | 17/461 (4) | ||
| Comorbid conditions, no. (%) | |||||||
| Chronic kidney disease§ | 10 (2.2) | 10 (19) | 7 (7) | 2 (3) | 62 (12) | <.001‡ | .07‡ |
| Coinfections, no. (%) ǁ | |||||||
| HBV | 4 (0.9) | 7 (13) | 7 (7) | 7 (11) | 54/494 (11) | <.001‡ | .63 |
| HCV | 5 (1.1) | 10 (19) | 23 (23) | 11 (17) | 100 (20) | <.001‡ | >.99 |
| No of cases per calendar period, no. (%) | |||||||
| 1996-1999 2000-2009 2010-2016 | 450 (100) | 10 (19) 29 (56) 13 (25) | 13 (13) 54 (53) 34 (34) | 27 (42) 31 (48) 6 (9) | 67 (13) 292 (58) 141 (28) | .82 | |
| Alive at study exit, no. (%) | 233 (52) | 18 (35) | 53 (52) | 11 (17) | 200 (40) | .05 | .57 |
| Histologic subtypes | |||||||
| ATLL | 13 (3) | 1 (2) | >.99‡ | ||||
| AITL | 73 (16) | 3 (6) | .07 | ||||
| ALCL | 79 (18) | 26 (50) | <.001 | ||||
| CTCL | 26 (6) | 4 (8) | .54‡ | ||||
| PTCL-NOS | 143 (32) | 12 (23) | .26 | ||||
| NK/TCL | 46 (10) | 3 (6) | .44 | ||||
| T-LGL | 0 | 3 (6) | |||||
| Age at NA-ACCORD enrollment (y), median (IQR) | 44 (41-50) | 43 (35-47) | 39 (34-45) | 43 (36-50) | .08 | ||
| Time to diagnosis of lymphoma from time of NA-ACCORD enrollment (y), median (IQR) | 2.3 (0.3-5.9) | 2.8 (1.0-6.7) | 1.9 (0.6-4.3) | 3.0 (0.7-7.2) | .21 | ||
IQR, interquartile range.
P values for the comparison between patients with TCL and NK/TCL with vs without HIV infection were calculated using t tests and χ2 tests for continuous and categorical variables, respectively.
P values for the comparison between patients with TCL and NK/TCL vs with B-cell NHL (Burkitt’s, PCNSL, DLBCL combined) were calculated using t tests, Wilcoxon rank sum tests, and χ2 tests for normally distributed continuous, non-normally distributed continuous, and categorical variables, respectively.
P values based on Fisher’s exact test because of some small cell counts.
Patient with CKD were defined as those who ever had an eGFR consistently <60 mL/min per 1.73 m2 for at least 3 months.
HBV, hepatitis B virus, as determined by detection of surface antigen, e antigen, or DNA quantification; HCV, hepatitis C virus as determined by detection of antibody, RNA quantification, or genotype.
Comparing outcomes of TCLs and NK/TCLs in people with and without HIV
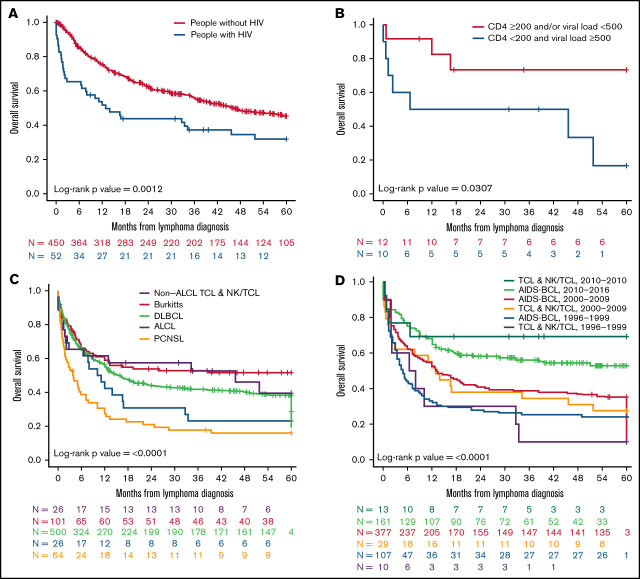
The estimated median 5-year OS was 46.2 months (range, 36.7-not reached) for PWoH with TCL and NK/TCL. This was substantially higher (P = .0012) as opposed to 12.9 months (range, 6.6-51.8 months) for PWH with TCL and NK/TCL (Figure 1A; supplemental Table 2). This statistically significant difference in survival between the 2 cohorts was observed even after adjusting for the statistically significant differences in baseline clinical characteristics such as age, race, and ALCL, using a Cox regression model (Table 2). Over a 5-year follow-up period since lymphoma diagnosis, the survival probability was 0.32 (95% confidence interval [CI]: 0.21, 0.49) and 0.45 (95% CI: 0.41, 0.51) in patients with and without HIV, respectively (supplemental Table 2). The diverse outcomes persisted through the first 5 years of lymphoma diagnosis (supplemental Table 2).
Figure 1.
OS at 5 years for patients with TCL and NK/TCL enrolled in COMPLETE relative to people with HIV with TCL and NK/TCL and A-BCL enrolled in NA-ACCORD. Kaplan-Meier analysis of (A) OS for all mature patients with TCL and NK/TCL with and without HIV, (B) OS for all patients with ALCL with and without HIV, (C) OS for all patients with TCL and NK/TCL stratified by CD4 cell count in cells/µL and viral load in copies/mL, and (D) OS for all mature patients with TCL and NK/TCL and A-BCLs stratified by subtype.
Table 2.
Cox regression analysis for baseline characteristics in NA-ACCORD (N = 717) and COMPLETE (N = 450)
| Multivariate analysis | Univariate analysis | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| HIV infection* (yes, no) | 1.92 (1.27, 2.91) | .002 | 1.80 (1.25, 2.59) | .001 |
| Age (y) | 1.02 (1.01, 1.03) | <.001 | 1.01 (1.01, 1.02) | .002 |
| Female sex (yes, no)† | 1.00 (0.77, 1.30) | .98 | ||
| Race ‡ | ||||
| Black (yes, no) | 1.65 (1.19, 2.29) | .002 | 1.72 (1.26, 2.35) | <.001 |
| Other | 1.75 (1.11, 2.77) | .02 | 1.60 (1.02, 2.52) | .04 |
| CKD§ (yes, no) | 1.35 (0.74, 2.47) | .33 | ||
| HBVǁ (yes, no) | 2.10 (1.04, 4.25) | .04 | ||
| HCVǁ (yes, no) | 1.90 (1.06, 3.39) | .03 | ||
| ATLL (yes, no) | 1.45 (0.75, 2.83) | .27 | ||
| ALCL (yes, no) | 0.55 (0.37, 0.80) | .002 | 0.59 (0.42, 0.84) | .004 |
| AITL (yes, no) | 1.12 (0.80, 1.56) | .52 | ||
| CTCL (yes, no) | 1.19 (0.74, 1.89) | .48 | ||
| NK/TCL (yes, no) | 0.89 (0.58, 1.36) | .59 | ||
| PTCL-NOS (yes, no) | 1.35 (1.04, 1.74) | .02 | ||
Reference group: non-HIV.
Reference group: male.
Reference group: White.
Patients with CKD were defined as those who ever had an eGFR consistently <60 mL/min per 1.73 m2 for at least 3 months.
HBV, hepatitis B virus, as determined by detection of surface antigen, e antigen, or DNA quantification; HCV, hepatitis C virus as determined by detection of antibody, RNA quantification, or genotype.
We next sought to compare the survival of patients with ALCL specifically, the most common histologic subtype in people with and without HIV. Patients with HIV and ALCL had an inferior median 5-year OS of 10.6 months (range, 2.1-33.4 months) contrary to those without HIV where median survival not reached (range, not reached-not reached; P ≤ .0001; Figure 1B; supplemental Table 3). The disparate outcomes for this histologic entity were observed throughout the first 5 years of lymphoma diagnosis (supplemental Table 3).
We further wanted to assess the impact of median CD4 and VL levels before the initiation of ART on outcomes after lymphoma diagnosis. Therefore, we stratified patients into 2 groups (CD4 ≥200 cells/µL and/or VL < 500 copies/mL and CD4 < 200 cells/µL and VL ≥500 copies/mL categories). As expected, patients with higher VL level and lower CD4 cell counts exhibited shorter median OS of 26.1 months (range, 1.3-not reached months) relative to the group with higher CD4 cell count and lower viral load (median OS, not reached; range, not reached-not reached; P = .03; Figure 1C; supplemental Table 4).
Comparison of general patient characteristics between TCL and NK/TCL and A-BCL among PWH
The median age at lymphoma diagnosis was comparable between TCL and NK/TCL and the DLBCL (n = 500) and BL (n = 101) subgroups of A-BCL (Table 1; supplemental Table 5). However, patients with PCNSL (n = 64) were younger than those with TCL and NK/TCL within the PWH cohort (41 vs 49 years; P < .001; supplemental Table 5). There were no statistically significant racial differences between PWH with TCL, NK/TCL, and A-BCL (Table 1; supplemental Table 5). Although there was a greater percentage of CKD in TCL and NK/TCL relative to patients with PCNSL (19% vs 3%; P = .03), the percentage of coinfections with hepatitis B virus and hepatitis C virus and the number of cases diagnosed per calendar period were not statistically different between the groups. The median age at the time of enrollment into the NA-ACCORD was 44 years (range, 41-50 years) in the TCL and NK/TCL groups, and although this was comparable to the BL and DLBCL subgroups, patients with PCNSL were younger with a median age of 39 years (range, 34-45 years; P = .01; supplemental Table 5).
Comparison of HIV-related characteristics between TCL and NK/TCL and A-BCL among PWH
The median time to lymphoma diagnosis from the time of NA-ACCORD enrollment was 2.3 years (range, 0.3-5.9 years) for the TCL and NK/TCL subsets, which was equivalent to the 3 A-BCL subgroups (Table 1). Although the pattern and type of antiretroviral regimens within PWH changed over the course of cohort enrollment period as expected, most of the patients (92%-96%) within the TCL and NK/TCL group and A-BCL subgroups were on at least 1 ART class during their cohort enrollment period without statistically significant differences among the subtypes (P = .61; Table 3). Median duration of time in the cohort and median duration of ART were similar among the TCL, NK/TCL, and A-BCL subgroups. However, within the A-BCL group, patients with PCNSL had significantly shorter duration of cohort enrollment and ART relative to the BL and DLBCL subgroups. Similar results were observed for the median duration of ART therapy preceding and after the diagnosis of lymphoma (Table 3). Among risk behaviors and exposures, men who have sex with men was the dominant risk factor for HIV transmission among patients with BL and DLBCL (supplemental Table 6). Pneumocystis jirovecii, Candida, and cytomegalovirus were the most common AIDS-defining infections, albeit the frequency varied across the 4 groups. Hypertension and diabetes were the most common comorbid medical conditions within PWH. Although an equal distribution in the prevalence of diabetes was seen among the 4 groups, a lower prevalence of hypertension was noted in patients with PCNSL compared with the other 3 groups (supplemental Table 6).
Table 3.
Antiretroviral therapy (ART) characteristics for PWH with T-cell, NK/T-cell, and AIDS-defining B-cell lymphomas in NA-ACCORD (N = 717)
| Features | TCL and NK/TCL (n = 52) | BL (n = 101) | PCNSL (n = 64) | DLBCL (n = 500) | P * |
|---|---|---|---|---|---|
| ART therapy† | |||||
| Yes, no. (%) | 48 (92) | 96 (95) | 61 (95) | 479 (96) | .61‡ |
| Different categories of ART, no. (%) | |||||
| Standard (NNRTI, PI, and NRTI)§ | 14 (27) | 12 (12) | 20 (31) | 124 (25) | .02ǁ |
| Standard (3-4 NRTIs)§ | 4 (8) | 2 (2) | 2 (3) | 29 (6) | .29 |
| Other (2 or fewer drugs)¶ | 42 (81) | 86 (85) | 56 (90) | 449 (90) | .18 |
| INSTI-based# | 13 (25) | 35 (35) | 6 (9) | 142 (28) | .004ǁ |
| Unknown | 0 | 0 | 0 | 1 (<1) | |
| Median length of time since NA-ACCORD enrollment (y) | 5.2 (2.2-8.8) | 5.9 (2.8-9.6) | 3.3 (1.5-5.5) | 5.6 (2.5-9.5) | <.001ǁ |
| Duration of ART (y), median (IQR) | 6.5 (3.3-9.2) | 7.6 (2.5-12.8) | 3.9 (2.1-8.2) | 7.9 (3.6-13.8) | <.001ǁ |
| Duration of ART before lymphoma diagnosis (y), median (IQR) | 3.9 (1.3-6.5) | 2.9 (0.7-8.0) | 2.5 (0.9-5.5) | 4.8 (1.2-9.4) | .01ǁ |
| Duration of ART after lymphoma diagnosis (y), median (IQR) | 1.4 (0.2-4.8) | 3.2 (0.3-7.3) | 0.3 (0.1-1.2) | 1.3 (0.3-6.1) | <.001ǁ |
P values for the comparison between patients in different lymphoma groups (Burkitt’s, PCNSL, DLBCL, and TCL and NK/TCLs) were calculated using Kruskal-Wallis and χ2 tests for non-normally distributed continuous variables and categorical variables, respectively.
ART therapy here is defined as patients being on at least 1 class of antiretroviral medications during their cohort enrollment period.
P values based on Fisher’s exact test because of some small cell counts.
Patient was considered on standard ART if they fell into 1 of the following 2 categories on 1 occasion or more during their cohort enrollment period: (1) were on at least 1 NNRTI, 1 PI, and 1 NRTI triple combination or (2) were on at least 3 NRTIs.
Bonferroni adjusted P values for pairwise comparison: PI-based ART: Burkitt’s vs TCL and NK/TCL, PCNSL, DLBCL: .03, .008, <.001, NNRTI-based ART: PCNSL vs Burkitt’s, DLBCL: .02, .003, Non-standard regimen ART: Burkitt’s vs PCNSL, DLBCL: .02, .046, Median duration of time in NA-ACCORD cohort: PCNSL vs Burkitt’s, DLBCL: <.001, <.001, Median duration of ART: PCNSL vs Burkitt’s, DLBCL: .048, <.001, Duration of ART before lymphoma diagnosis: PCNSL vs DLBCL: .02, duration of ART after lymphoma diagnosis: PCNSL vs Burkitt’s, DLBCL: <.001, <.001.
Patients were considered on other ART if they were on 2 or less drugs during their cohort enrollment period.
#Patients were considered on INSTI-based ART if 1 of the ART drugs was an INSTI during their cohort enrollment period. It is to be noted that, for example, if a patient was on 1 INSTI and 1 PI, they were included in both INSTI- and PI-based categories. During the cohort enrollment period, if a patient was on an INSTI, PI, or NNRTI drug more than once after a disruption, this was captured more than once in the categories specified, resulting in percentage greater than 100% when combined.
We hypothesized that HIV RNA level (VL) and CD4 cell count before initiation of ART could predict survival based on previous reports20; hence, patients with TCL and NK/TCL and A-BCL were stratified into 2 groups: CD4 ≥ 200 cells/µL and/or VL < 500 copies/mL and CD4 < 200 cells/µL and VL ≥ 500 copies/mL before or at ART initiation (Table 4). Although median viral loads and CD4 cell counts for PWH at or before commencement of ART varied considerably within the four subtypes of lymphomas, it was broadly comparable across patients with TCL, NK/TCL, and A-BCL. More than half of the patients with BL demonstrated CD4 ≥ 200 cells/µL and/or VL < 500 copies/mL like patients with TCL and NK/TCL, in contrast to approximately 40% for patients with DLBCL and 23% for patients with PCNSL (Table 4).
Table 4.
CD4 cell count and HIV VL stratified by lymphoma subtype before or at ART start date, NA-ACCORD (N = 717)
| Features | Number of patients | TCL and NK/TCL | BL | PCNSL | DLBCL | P * |
|---|---|---|---|---|---|---|
| CD4 (cells/µL) | Total (n) | 25 | 64 | 29 | 257 | |
| <200 (n) | 12 | 25 | 23 | 150 | .002† | |
| >200 (n) | 13 | 39 | 6 | 107 | ||
| Median (IQR) | 214 (47-377) | 236 (145-416) | 56 (14-179) | 148 (55-316) | <.001† | |
| VL (copies/mL) | Total (n) | 24 | 64 | 23 | 241 | |
| <500 (n) | 4 | 5 | 1 | 12 | .12‡ | |
| ≥500 (n) | 20 | 59 | 22 | 229 | ||
| Median (IQR) | 56 163 (12 758-164 204) | 55 469 (15 047-144 419) | 118 000 (62 668-260 849) | 89 678 (21 828-318 419) | .03 | |
| Total (n) | 22 | 62 | 22 | 234 | ||
| CD4 ≥200/µL and/or VL <500 copies/mL (n) | 12 | 40 | 5 | 93 | <.001† | |
| CD4 <200/µL and VL ≥500 copies/mL (n) | 10 | 22 | 17 | 141 | ||
The CD4 cell count and VL exhibited here represent values measured closest to before or at ART start date.
P values for the comparison between patients in different lymphoma groups (Burkitt’s, PCNSL, DLBCL, and TCL and NK/TCLs) were calculated using Kruskal-Wallis and χ2 tests for non-normally distributed continuous variables and categorical variables, respectively.
Bonferroni-adjusted P values for pairwise comparison: CD4 cell count <200 vs >200: Burkitt’s vs PCNSL: .004, median CD4 cell count: Burkitt’s vs PCNSL, DLBCL: <.001, .006, Median VL: PCNSL vs DLBCL: .02, CD4 ≥200 and/or VL <500 vs CD4 <200 and VL ≥500: Burkitt’s vs PCNSL, DLBCL: .01, .005.
P values based on Fisher’s exact test because of some small cell counts.
We also interrogated the median CD4 cell counts and VL at the time of lymphoma diagnosis or within 3 years leading up to it. Despite remarkable heterogeneity within each of the 4 groups of patients, once again patients with PCNSL manifested considerably lower CD4 counts and higher VL in comparison with the other 3 subgroups (Table 5).
Table 5.
CD4 cell count and HIV VL stratified by lymphoma subtype at lymphoma diagnosis, NA-ACCORD (N = 717)
| TCL and NK/TCL (n = 52) | BL (n = 101) | PCNSL (n = 64) | DLBCL (n = 500) | P * | |
|---|---|---|---|---|---|
| Patients with any CD4 measurement, no. (%) | 45 (87) | 96 (95) | 51 (80) | 467 (93) | .001†‡ |
| Patients with CD4 measurement within 3 y before cancer diagnosis§, no. (%) | 40 (77) | 92 (91) | 45 (70) | 424 (85) | .002† |
| CD4 (cells/µL), median (IQR) | 142 (83-469) | 309 (170-491) | 45 (10-128) | 182 (72-372) | <.001† |
| Patients with any VL measurement, no. (%) | 44 (85) | 96 (95) | 50 (78) | 467 (93) | <.001†‡ |
| Patients with VL measurement within 3 y before cancer diagnosis§, no. (%) | 38 (73) | 92 (91) | 44 (69) | 427 (85) | <.001† |
| VL (copies/mL), median (IQR) | 400 (48-38 140) | 1738 (50-23 422) | 72 550 (2941-266 305) | 6510 (75-110 362) | <.001† |
The CD4 cell count and VL exhibited here represent values measured either at time of cancer diagnosis or up to 3 years before cancer diagnosis.
P values for the comparison between patients in different lymphoma groups (Burkitt’s, PCNSL, DLBCL, and TCL and NK/TCLs) were calculated using Kruskal-Wallis and χ2 tests for non-normally distributed continuous variables and categorical variables, respectively.
Bonferroni adjusted P values for pairwise comparison: patients with any CD4 measurement: PCNSL vs Burkitt’s, DLBCL: .02, .005, patients with CD4 measurement within 3 years before cancer diagnosis: PCNSL vs Burkitt’s, DLBCL: .007, .04, median CD4 count: TCL and NK/TCL vs PCNSL: .001, Burkitt’s vs PCNSL, DLBCL: <.001, <.001, DLBCL vs PCNSL <.001, patients with any viral load measurement: PCNSL vs Burkitt’s, DLBCL: .01, .002, patients with viral load measurement within 3 years before cancer diagnosis: Burkitt’s vs TCL and NK/TCL, PCNSL: .04, .003, PCNSL vs DLBCL: .008, median viral load: PCNSL vs TCL and NK/TCL, Burkitt’s, DLBCL: .002, <.001, .02.
P values based on Fisher’s exact test because of some small cell counts.
The vast majority of patients had at least 1 HIV RNA level and 1 CD4 level measured within 3 months before the diagnosis of lymphoma.
Comparing outcomes of TCL and NK/TCL with A-BCL in people with HIV
We investigated variations in the OS of PWH with A-BCL compared with TCL and NK/TCL. Within the A-BCL, patients with PCNSL had worse survival, with a 5-year median OS of 3.8 months (range, 2.0-7.2 months), followed by DLBCL (15.6 months; range, 12.7-22.2 months) and BL (median OS, not reached; range, 13.1-not reached months), which was statistically significant (P < .0001; Figure 1D; supplemental Table 7). Within TCLs and NK/TCLs, patients with ALCL had a lower median OS of 10.6 months (range, 2.1-33.4 months) in comparison with the other TCLs (45.6 months; range, 2.8-not reached months). Overall, when contrasting outcomes of A-BCL with TCL and NK/TCL at 5 years since lymphoma diagnosis, patients with PCNSL were associated with the worst survival, followed by ALCL, DLBCL, non-ALCL TCL, and BL subsets.
To assess the impact of potentially uncaptured mortality because of loss to follow-up, we performed a sensitivity analysis where we set more stringent criteria on the censoring time as mentioned above. The results of the sensitivity analysis were no different to the main analysis, thereby resulting in similar conclusions (supplemental Table 8).
Comparison of general patient characteristics and outcomes between TCL and NK/TCL and HL among PWH
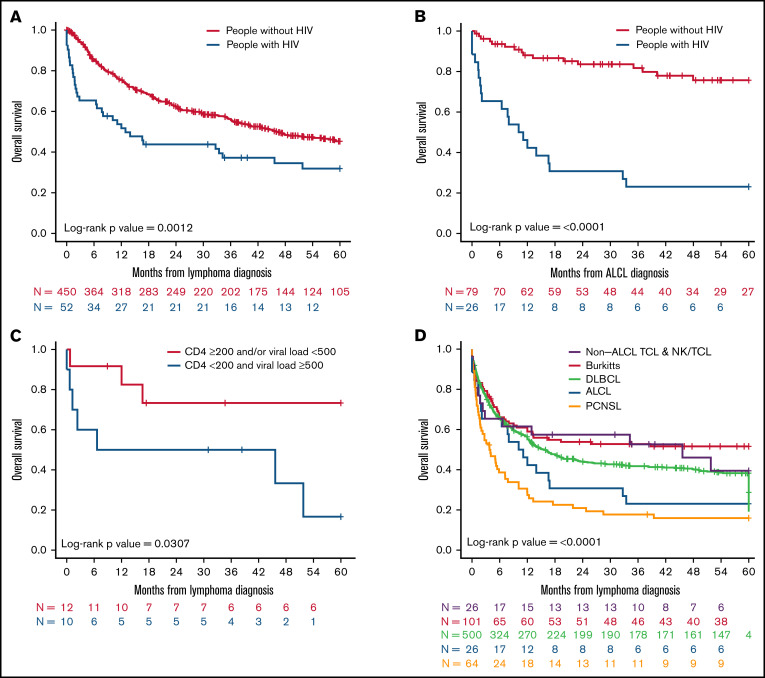
We also investigated the differences between PWH with TCL and NK/TCL with a non–AIDS-defining illness such as HL (supplemental Table 9). Except for a greater percentage of people of White race in the TCL group, there were no statistically relevant variations between the 2 groups with respect to clinical features such as age at diagnosis of lymphoma, sex, CKD, number of cases per calendar period, and coinfections with hepatitis B and C virus. Median time to diagnosis of lymphoma from time of cohort enrollment was significantly longer for PWH with HL at 3.9 years (range, 1.9-7.5 years) as opposed to PWH with TCL and NK/TCL at 2.3 years (range, 0.3-5.9 years; P = .004). PWH with HL had superior 5-year median OS (median OS, not reached; range, not reached-not reached) relative to the TCL and NK/TCL groups (12.9 months; range, 6.6-51.8 months), which was statistically significant (P < .0001; supplemental Table 2; supplemental Figure 1A). Survival for both groups of patients improved over time, and PWH with HL continued to demonstrate greater 5-year median OS when stratified per calendar period of diagnosis (supplemental Figure 1B).
Comparing outcomes of TCL, NK/TCL, A-BCL, and HL in people with HIV over time
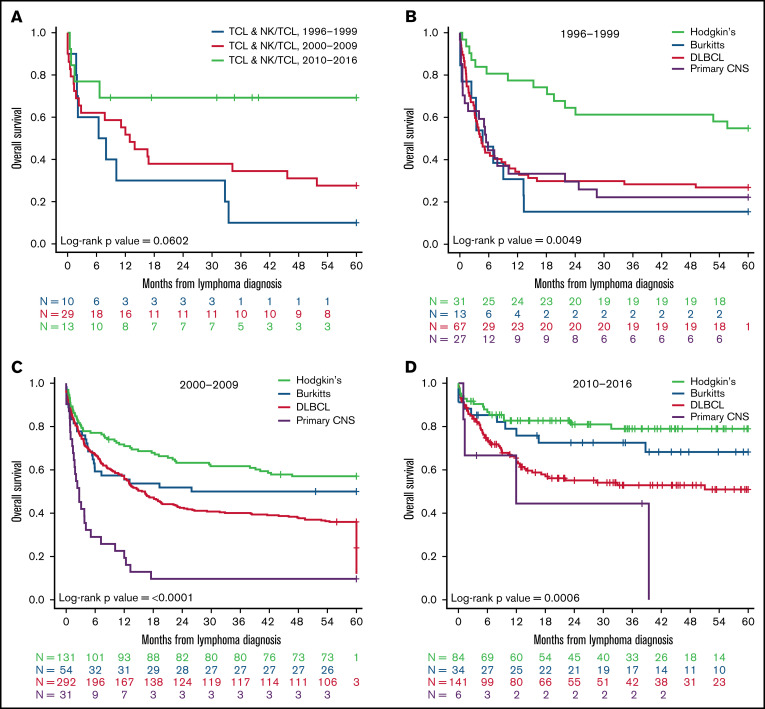
Next, to determine the impact of changes related to clinical management of patients over time on outcomes since lymphoma diagnosis, we stratified patients into 3 calendar periods based on time of their lymphoma diagnosis (1996-1999, 2000-2009, and 2010-2016). Except for PCNSL, we observed marked improvement in OS for all other subtypes of NHL with passage of time. The highest survival was observed for patients diagnosed between 2010 and 2016 (Figure 2A-D). Patients with PCNSL, however, continued to manifest worse outcomes in comparison with other A-BCL counterparts such as BL and DLBCL without appreciable progress in the last 2 decades. Of particular interest was a marked enhancement in the median OS of patients with ALCL, which started to mirror current outcomes for PWoH with ALCL.8
Figure 2.
OS at 5 years for people with HIV diagnosed with A-BCL and TCL and NK/TCL in NA-ACCORD per calendar period. Kaplan-Meier analysis of (A) OS for patients with TCL and NK/TCL stratified by period of diagnosis from 1996 to 2016, (B) OS of PWH with A-BCL such as BL, PCNSL, DLBCL, and HL diagnosed from 1996 to 1999, (C) OS of PWH A-BCL such as BL, PCNSL, DLBCL, and HL diagnosed from 2000 to 2009, and (D) OS of PWH with A-BCL such as Burkitt’s lymphoma, PCNSL, DLBCL, and HL diagnosed from 2010 to 2016.
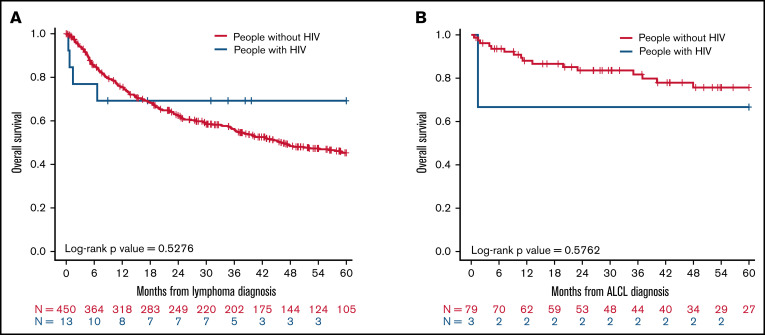
When we performed additional sensitivity analysis to compare survival of TCL and NK/TCL patients with (n = 13) and without HIV (n = 450) diagnosed in the same study years (2010-2014), we observed no difference in the 5-year median OS between the 2 populations (P = .53; Figure 3A). Similar results were observed for patients with the ALCL subtype diagnosed between 2010 and 2016 with HIV (n = 3) and without HIV (n = 79; P = .58; Figure 3B).
Figure 3.
OS at 5 years for patients with TCL and NK/TCL enrolled in COMPLETE relative to people with HIV with TCL and NK/TCL enrolled in NA-ACCORD between 2010 and 2016. Kaplan-Meier analysis of (A) OS for all mature patients with TCL and NK/TCL with and without HIV, (B) OS for all patients with ALCL with and without HIV.
Discussion
To our knowledge, this is the first study to compare clinical characteristics and outcomes of patients with mature TCLs and NK/TCLs among patients with and without HIV given the rarity of this neoplasm. Our analysis, based on 2 of the largest and most diverse cohort collaborations of PWH and patients with TCL in United States, found significantly inferior survival for patients with TCLs and NK/TCLs among PWH that were diagnosed between 1996 and 2009. However, this improved substantially for patients diagnosed in 2010 and onward. Even after adjusting for several statistically significant variations in baseline demographics such as age, sex, race, and histologic subtype in a Cox multivariate stepwise analysis, we found that PWH with TCL and NK/TCL were at a higher risk for death when juxtaposed with similar patients without HIV. Thus, we were able to confirm our initial hypothesis that HIV infection was an independent predictor of increased death for patients with TCL and NKTCL at least for patients diagnosed between 1996 and 2009. Although the sample size was small, no difference in survival for patients with TCL and NK/TCL with and without HIV was noted when diagnosed in the same study period of 2010 to 2016, underlining potential contributions of efficacious ART, earlier diagnosis of HIV, and directed treatment. The large number of well-characterized NHLs observed in NA-ACCORD also enabled us to examine multiple factors simultaneously, including, known NHL risk factors such as hepatitis viral coinfections, to define the independent association between HIV-specific factors and prognosis for patients with TCL and NK/TCL.
Furthermore, we found that, unlike PWoH, ALCL is the most common histologic subtype of TCL within PWH and conspicuously exhibited a worse survival compared with its non-HIV counterpart between 1996 and 2009 with eminent improvement from 2010 onward, despite a small dataset. Our results reaffirm and expand findings from a MEDLINE review–based study of 37 cases of HIV-associated ALCLs that reported a median OS of just 5 months.18 Furthermore, patients with CD4 count < 200 cells/µL before administration of ART manifested a significantly lower OS as opposed to those with CD4 count ≥ 200 cells/µL, underscoring the need to evaluate biological relationships between immune constitution and T-cell lymphomagenesis and the need for early, sustained, and effective ART.
Our study is the first to determine differences within PWH with mature TCLs and NK/TCLs and A-BCLs. Although the survival of patients with ALCL consistently lagged behind the BK and DLBCL subgroups from 1996 to 2009, it has remarkably improved in the last decade, now paralleling outcomes of these A-BCL subtypes. Whether this benefit was driven by changes in ART or lymphoma-related management or because of the combination remains unclear and could not be addressed in our study. ART-related factors that could have contributed to augmentation of survival include modifications in first-line and subsequent antiretroviral regimens from 1996 to 2016 such as drug switches, drug substitutions, optimal adherence to ART, and progressive improvement in supportive care. Lack of knowledge of lymphoma-directed treatment details for NA-ACCORD participants limited our ability to ascertain that this was not a confounding variable when elucidating causes for the distinct outcomes of subjects with TCL and NK/TCL in our study. However, we believe that the expansion in survival for all lymphoma subgroups included in this analysis among PWH supports the notion that temporal changes in potent ART contributed at least partially toward favorable outcomes for this population.
Our study has several other limitations including small sample size of PWH and TCL in comparison with PWoH. Lack of knowledge of ALK expression within the NA-ACCORD cohort makes it hard to distinguish if a particular subtype of ALCL (ALK+ or ALK−) is more common among PWH and at a greater survival disadvantage than the other. Known risk factors such as human T-cell lymphotropic virus type 1 and Epstein-Barr virus infections and International Prognostic Index–related risk factors were not captured in NA-ACCORD, and hence, its effect on outcomes for PWH could not be estimated. In addition, we acknowledge the missing data for several patient and disease characteristics including cause of death, treatment details, and International Prognostic Index score for the NA-ACCORD study subjects as another major weakness of the study. The time from enrollment into the cohort to diagnosis of lymphoma was 2.3 years for patients with TCL and NK/TCL and did not differ from the A-BCL subgroups. It is difficult to ascertain the latency time with more precision from the time of HIV infection to the diagnosis of lymphoma in our cohort because many NA-ACCORD participants were diagnosed with HIV for variable duration before cohort enrollment. It is likely that comprehensive assessments of recent, past, cumulative, and nadir or peak measures of CD4 count and VL using demographics-adjusted, cohort-stratified Cox models with novel statistical models are needed for this. The duration of ART before developing lymphoma did not vary prominently between TCL, NK/TCL, and DLBCL, the most common A-BCL, but was substantially shorter for patients with PCNSL. This can at least partly provide rationale for the dismal prognosis of patients with PCNSL, albeit no definite conclusions can be made outside of prospective randomized study.19 It is also important to mention that the dissimilar enrollment and follow-up period for PWH (1996-2016) relative to PWoH (2010-2018) could contribute to the observed difference in survival for patients with TCL and NK/TCL, which was accounted for by our sensitivity analysis. Finally, the COMPLETE cohort enrolled newly diagnosed patients with TCL and NK/TCL with and without HIV as opposed to NA-ACCORD, which only enrolled people with HIV, thereby raising the possibility of confounding results. We controlled for this by carefully excluding both patients with HIV in the COMPLETE cohort for our study.
However, we argue that, despite these drawbacks, prospective cohort studies such as ours provide a unique perspective of real-world disease outcomes and prognostic factors for patients with TCL and NK/TCL with and without HIV in the contemporary ART era that can be compromised by the scrutiny of clinical trials and/or registrational studies, making it more clinically relevant. In contrast to previous studies conducted in single health care systems, the diversity of our cohorts regarding geographic, demographic, and clinical characteristics including the full spectrum of HIV disease severity and comorbidities makes our findings more broadly applicable to PWH in settings where treatment with ART is readily available.
In conclusion, by focusing our analysis on TCL and NK/TCL and comparing outcomes among patients with and without HIV, within 2 large, diverse, and well-characterized cohorts, we have shown with broad generalizability that HIV infection is independently associated with inferior survival and provided robust estimates of the risk associated with HIV-specific factors. Our results suggest that viral suppression and boosting of CD4 count by commencing early and uninterrupted use of ART can contribute toward vastly improved prognosis for this rare subtype of NHL, like A-BCL. Further investigation into impact of immunosuppression on T-cell lymphomagenesis, better understanding incorporation of targeted strategies such as brentuximab vedotin and ALK inhibitors such as crizotinib into treatment paradigms for TCL subtypes, and access to novel therapeutic approaches should be prioritized for PWH with TCL to maximize their outcomes.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by National Institutes of Health grants U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01AG053100, R24AI067039, R34DA045592, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA036297, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24 AA020794, U54GM133807, UL1RR024131, UL1TR000004, UL1 TR000083, UL1TR002378, Z01CP010214, and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention; contract 90047713 from the Agency for Healthcare Research and Quality; contract 90051652 from the Health Resources and Services Administration; the Grady Health System; grants CBR-86906, CBR-94036, HCP-97105, and TGF-96118 from the Canadian Institutes of Health Research; Ontario Ministry of Health and Long Term Care; and the Government of Alberta, Canada. Additional support was provided by the National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Heart, Lung, and Blood Institute, Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Human Genome Research Institute, National Institute for Mental Health, National Institute on Drug Abuse, National Institute on Aging, National Institute of Dental & Craniofacial Research, National Institute of Neurological Disorders and Stroke, National Institute of Nursing Research, National Institute on Alcohol Abuse and Alcoholism, National Institute on Deafness and Other Communication Disorders, and National Institute of Diabetes and Digestive and Kidney Diseases.
These data were collected by cancer registries participating in the National Program of Cancer Registries of the Centers for Disease Control and Prevention. This work was also supported by Acrotech Biopharma, LLC. S.J. is supported by National Cancer Institute K08 career development award K08CA230498.
NA-ACCORD Collaborating Cohorts and Representatives: AIDS Clinical Trials Group Longitudinal Linked Randomized Trials, Constance A. Benson and Ronald J. Bosch; AIDS Link to the IntraVenous Experience: Gregory D. Kirk; Emory-Grady HIV Clinical Cohort, Vincent Marconi and Jonathan Colasanti; Fenway Health HIV Cohort, Kenneth H. Mayer and Chris Grasso; HAART Observational Medical Evaluation and Research, Robert S. Hogg, Viviane Lima, P. Richard Harrigan, Julio SG Montaner, Benita Yip, Julia Zhu, and Kate Salters; HIV Outpatient Study, Kate Buchacz and Jun Li; HIV Research Network, Kelly A. Gebo and Richard D. Moore; Johns Hopkins HIV Clinical Cohort, Richard D. Moore; John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University, Jeffrey Jacobson; Kaiser Permanente Mid-Atlantic States, Michael A. Horberg; Kaiser Permanente Northern California, Michael J. Silverberg; Longitudinal Study of Ocular Complications of AIDS, Jennifer E. Thorne; MACS/WIHS Combined Cohort Study, Todd Brown, Phyllis Tien, and Gypsyamber D’Souza; Maple Leaf Medical Clinic, Graham Smith, Mona Loutfy, and Meenakshi Gupta; The McGill University Health Centre, Chronic Viral Illness Service Cohort, Marina B. Klein; Multicenter Hemophilia Cohort Study–II, Charles Rabkin; Ontario HIV Treatment Network Cohort Study, Abigail Kroch, Ann Burchell, Adrian Betts, and Joanne Lindsay; Parkland/UT Southwestern Cohort, Ank Nijhawan; Retrovirus Research Center, Universidad Central del Caribe, Bayamon Puerto Rico, Angel M. Mayor; Southern Alberta Clinic Cohort, M. John Gill; Study of the Consequences of the Protease Inhibitor Era, Jeffrey N. Martin; Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy, Jun Li and John T. Brooks; University of Alabama at Birmingham 1917 Clinic Cohort, Michael S. Saag, Michael J. Mugavero, and James Willig; University of California at San Diego, Laura Bamford and Maile Karris; University of North Carolina at Chapel Hill HIV Clinic Cohort, Joseph J. Eron and Sonia Napravnik; University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane; Vanderbilt Comprehensive Care Clinic HIV Cohort, Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner; Veterans Aging Cohort Study, Kathleen McGinnis and Amy Justice.
NA-ACCORD Study Administration: Executive Committee, Richard D. Moore, Keri N. Althoff, Stephen J. Gange, Mari M. Kitahata, Jennifer S. Lee, Michael S. Saag, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman; Administrative Core, Richard D. Moore, Keri N. Althoff, and Aimee M. Freeman; Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Liz Morton, Justin McReynolds, and William B. Lober; Epidemiology and Biostatistics Core, Stephen J. Gange, Jennifer S. Lee, Brenna Hogan, Elizabeth Humes, Raynell Lang, Sally Coburn, Lucas Gerace, and Alyssa Furukawa.
Authorship
Contribution: S.J. and C.S. designed the research; Min Jung Koh, M.H.M., Min Ji Koh, R.S., C.D.A., C.S., and S.J. performed the research; Min Jung Koh, M.H.M., Min Ji Koh, R.S., C.D.A., C.S., and S.J. analyzed data; F.M.F., A.M.M., J.G., M.E., E.C., J.E.T., M.J.S., M.A.H., K.N.A., A.E.N., K.A.M., J.S.L., C.S.R., S.N., J.L., J.L.C., C.S., and S.J. reviewed the manuscript; and Min Jung Koh, M.H.M., and S.J. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Salvia Jain, Jon and Jo Ann Hagler Center for Lymphoma, Massachusetts General Hospital Cancer Center, Harvard Medical School, 55 Fruit St, Boston, MA 02114-2696; e-mail: salvia.jain@mgh.harvard.edu.
References
- 1.Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project . International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130. [DOI] [PubMed] [Google Scholar]
- 2.Hsi ED, Horwitz SM, Carson KR, et al. Analysis of peripheral T-cell lymphoma diagnostic workup in the United States. Clin Lymphoma Myeloma Leuk. 2017;17(4):193-200. [DOI] [PubMed] [Google Scholar]
- 3.Vose JM. Peripheral T-cell non-Hodgkin’s lymphoma. Hematol Oncol Clin North Am. 2008;22(5):997-1005, x. [DOI] [PubMed] [Google Scholar]
- 4.Lansigan F, Horwitz SM, Pinter-Brown LC, et al. Outcomes for relapsed and refractory peripheral T-cell lymphoma patients after front-line therapy from the COMPLETE Registry. Acta Haematol. 2020;143(1):40-50. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, Federico M, Caballero D, et al. Therapeutic options in relapsed or refractory peripheral T-cell lymphoma. Cancer Treat Rev. 2014;40(9):1080-1088. [DOI] [PubMed] [Google Scholar]
- 6.Moskowitz AJ, Lunning MA, Horwitz SM. How I treat the peripheral T-cell lymphomas. Blood. 2014;123(17):2636-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carson KR, Horwitz SM, Pinter-Brown LC, et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer. 2017;123(7):1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz S, O’Connor OA, Pro B, et al. ; ECHELON-2 Study Group . Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo JJ, Beltran BE, Bibas M, et al. Prognostic factors in patients with HIV-associated peripheral T-cell lymphoma: a multicenter study. Am J Hematol. 2011;86(3):256-261. [DOI] [PubMed] [Google Scholar]
- 10.Arzoo KK, Bu X, Espina BM, Seneviratne L, Nathwani B, Levine AM. T-cell lymphoma in HIV-infected patients. J Acquir Immune Defic Syndr. 2004;36(5):1020-1027. [DOI] [PubMed] [Google Scholar]
- 11.Chadburn A, Cesarman E, Jagirdar J, Subar M, Mir RN, Knowles DM. CD30 (Ki-1) positive anaplastic large cell lymphomas in individuals infected with the human immunodeficiency virus. Cancer. 1993;72(10):3078-3090. [DOI] [PubMed] [Google Scholar]
- 12.Gold JE, Ghali V, Gold S, Brown JC, Zalusky R. Angiocentric immunoproliferative lesion/T-cell non-Hodgkin’s lymphoma and the acquired immune deficiency syndrome: a case report and review of the literature. Cancer. 1990;66(11):2407-2413. [DOI] [PubMed] [Google Scholar]
- 13.González-Clemente JM, Ribera JM, Campo E, Bosch X, Montserrat E, Grau JM. Ki-1+ anaplastic large-cell lymphoma of T-cell origin in an HIV-infected patient. AIDS. 1991;5(6):751-755. [DOI] [PubMed] [Google Scholar]
- 14.Berger TG, Kerschmann RL, Roth R, Schulze K, Zackheim HS. Sézary’s syndrome and human immunodeficiency virus infection. Arch Dermatol. 1995;131(6):739-741. [DOI] [PubMed] [Google Scholar]
- 15.Nava VE, Cohen P, Kalan M, Ozdemirli M. HIV-associated anaplastic large cell lymphoma: a report of three cases. AIDS. 2008;22(14):1892-1894. [DOI] [PubMed] [Google Scholar]
- 16.Proca DM, De Renne L, Marsh WL Jr, Keyhani-Rofagha S. Anaplastic large cell lymphoma in a human immunodeficiency virus-positive patient with cytologic findings in bladder wash: a case report. Acta Cytol. 2008;52(1):83-86. [DOI] [PubMed] [Google Scholar]
- 17.Shibata D, Brynes RK, Rabinowitz A, et al. Human T-cell lymphotropic virus type I (HTLV-I)-associated adult T-cell leukemia-lymphoma in a patient infected with human immunodeficiency virus type 1 (HIV-1). Ann Intern Med. 1989;111(11):871-875. [DOI] [PubMed] [Google Scholar]
- 18.Perez K, Castillo J, Dezube BJ, Pantanowitz L. Human immunodeficiency virus-associated anaplastic large cell lymphoma. Leuk Lymphoma. 2010;51(3):430-438. [DOI] [PubMed] [Google Scholar]
- 19.Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV. 2017;4(11):e495-e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernández-Ramírez RU, Qin L, Lin H, et al. ; North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS . Association of immunosuppression and HIV viraemia with non-Hodgkin lymphoma risk overall and by subtype in people living with HIV in Canada and the USA: a multicentre cohort study. Lancet HIV. 2019;6(4):e240-e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alderuccio JP, Olszewski AJ, Evens AM, et al. HIV-associated Burkitt lymphoma: outcomes from a US-UK collaborative analysis. Blood Adv. 2021;5(14):2852-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evens AM, Danilov A, Jagadeesh D, et al. Burkitt lymphoma in the modern era: real-world outcomes and prognostication across 30 US cancer centers. Blood. 2021;137(3):374-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro JT, Lloveras N, Ribera JM, Oriol A, Mate JL, Feliu E. The prognosis of HIV-infected patients with diffuse large B-cell lymphoma treated with chemotherapy and highly active antiretroviral therapy is similar to that of HIV-negative patients receiving chemotherapy. Haematologica. 2005;90 (5):704-706. [PubMed] [Google Scholar]
- 24.Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012;30(33):4111-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olszewski AJ, Fallah J, Castillo JJ. Human immunodeficiency virus-associated lymphomas in the antiretroviral therapy era: analysis of the National Cancer Data Base. Cancer. 2016;122(17):2689-2697. [DOI] [PubMed] [Google Scholar]
- 26.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol. 2007;36(2):294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carson KR, Horwitz SM, Pinter-Brown LC, et al. A prospective cohort study of patients with peripheral T-cell lymphoma in the United States. Cancer. 2017;123(7):1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SI, Horwitz SM, Foss FM, et al. ; COMPLETE Investigators . The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: report from COMPLETE, a prospective, multicenter cohort study [correction published in Cancer. 2019;125(21):3893]. Cancer. 2019;125(9):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuver RN, Khan N, Schwartz M, et al. Single agents vs combination chemotherapy in relapsed and refractory peripheral T-cell lymphoma: results from the comprehensive oncology measures for peripheral T-cell lymphoma treatment (COMPLETE) registry. Am J Hematol. 2019;94(6):641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverberg MJ, Lau B, Achenbach CJ, et al. ; North American AIDS Cohort Collaboration on Research and Design of the International Epidemiologic Databases to Evaluate AIDS . persons with HIV in North America: a cohort study. Ann Intern Med. 2015;163(7):507-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samji H, Cescon A, Hogg RS, et al. ; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA . Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.