Abstract
Background
Blood plasma proteins play an important role in immune defense against pathogens, including cytokine signaling, the complement system, and the acute-phase response. Recent large-scale studies have reported genetic (i.e., protein quantitative trait loci, pQTLs) and non-genetic factors, such as age and sex, as major determinants to inter-individual variability in immune response variation. However, the contribution of blood-cell composition to plasma protein heterogeneity has not been fully characterized and may act as a mediating factor in association studies.
Methods
Here, we evaluated plasma protein levels from 400 unrelated healthy individuals of western European ancestry, who were stratified by sex and two decades of life (20–29 and 60–69 years), from the Milieu Intérieur cohort. We quantified 229 proteins by Luminex in a clinically certified laboratory and their levels of variation were analyzed together with 5.2 million single-nucleotide polymorphisms. With respect to non-genetic variables, we included 254 lifestyle and biochemical factors, as well as counts of seven circulating immune cell populations measured by hemogram and standardized flow cytometry.
Results
Collectively, we found 152 significant associations involving 49 proteins and 20 non-genetic variables. Consistent with previous studies, age and sex showed a global, pervasive impact on plasma protein heterogeneity, while body mass index and other health status variables were among the non-genetic factors with the highest number of associations. After controlling for these covariates, we identified 100 and 12 pQTLs acting in cis and trans, respectively, collectively associated with 87 plasma proteins and including 19 novel genetic associations. Genetic factors explained the largest fraction of the variability of plasma protein levels, as compared to non-genetic factors. In addition, blood-cell fractions, including leukocytes, lymphocytes, monocytes, neutrophils, eosinophils, basophils, and platelets, had a larger contribution to inter-individual variability than age and sex and appeared as confounders of specific genetic associations. Finally, we identified new genetic associations with plasma protein levels of five monogenic Mendelian disease genes including two primary immunodeficiency genes (Ficolin-3 and FAS).
Conclusions
Our study identified novel genetic and non-genetic factors associated to plasma protein levels which may inform health status and disease management.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13073-022-01032-y.
Keywords: pQTL, Plasma proteins, Immune cells, Immune variability
Background
Plasma proteins play important physiological roles in human health and disease. They participate in immune responses against pathogens (e.g., interferons, chemokines, and complement factors [1, 2]), blood clotting [3], hormone transport [4, 5], and energy metabolism regulation [6]. Plasma protein levels can reflect physiological homeostasis or pathological states including active cellular secretion [7–9], tissue leakage [10, 11], protein degradation [12], and protein excretion in urine [13]. Plasma proteins are widely used as markers of the physiological state of an individual and represent ~42% of all requested blood-based laboratory tests [10]. As of today, the US Food and Drug Agency (FDA) approved 235 plasma proteins as diagnostic, prognostic, risk predictive, or treatment response biomarkers [14, 15]) for a broad range of diseases such as cancer [16–18], pulmonary defects [19], autoimmune [20], and metabolic diseases [21]. In addition to their association with clinical outcomes, natural heterogeneity of plasma protein levels among the general population has been widely reported but is not considered in clinical applications. Recent large-scale studies performed both in healthy and disease cohorts have identified both non-genetic (e.g., age and sex) and genetic factors (i.e., protein quantitative trait loci, pQTLs) that determine variable plasma protein levels [22–24]. pQTLs are enriched in disease-susceptibility loci identified from GWAS studies [24, 25] and could have protective or modifying effects, potentially in conjunction with pathogenic mutations leading to disease due to altered expression levels, e.g., loss of homeostasis, proteotoxic stress, or insufficiency [26]. Yet, the assessment of the genetic associations reported by previous studies did not characterize the specific cell types accounting for the observed variation in plasma proteins. Thus, it remains unclear whether a fraction of the plasma protein variability initially associated with a pQTL could have been mediated by the concomitant heterogeneity in their cellular sources. This may be especially relevant for plasma proteins displaying immune-related functions, since significant variability in immune cell fractions is observed across individuals driven by both genetic and non-genetic factors [27–29].
Here, we present an in-depth characterization of heterogeneity in plasma protein levels in healthy individuals from the Milieu Intérieur study [30], with a focus on immune-related proteins. The Milieu Intérieur consortium aims at characterizing the genetic and environmental factors underlying the observed variability of the immune response in a healthy population [30]. This study was performed on 400 individuals equally distributed by sex and across two decades of life (aged 30–39 and 60–69). We evaluated the association of 229 plasma protein concentrations with a total of 254 non-genetic factors including lifestyle, environmental, physiological, and blood biochemical variables as well as with 5,201,092 common single-nucleotide polymorphisms (SNPs). To control for the natural variation in blood-cell populations, we systematically accounted for the levels of seven major blood-cell fractions, including leukocytes, lymphocytes, monocytes, neutrophils, eosinophils, basophils, and platelets. We found that together with age and sex, blood-cell fractions explain an important fraction of the inter-individual plasma protein variability. After controlling for such factors, we identified 112 pQTLs associated with 87 proteins, 19 of which are reported here for the first time. Among these, six are associated with five monogenic Mendelian disease genes (MMDGs), including 2 primary immunodeficiency (PID) genes. Such genetic variants may have potential clinical value as susceptibility or protective factors for immune-related diseases.
Methods
The Milieu Intérieur cohort
The 400 donors in this study were a subset of the 1000 healthy donors of the Milieu Intérieur cohort [27, 29–32] recruited at BioTrial (Rennes, France). The Milieur Intérieur cohort was approved by the Comité de Protection des Personnes – Ouest 6 (Committee for the protection of persons) on June 13, 2012 and by French Agence nationale de sécurité du médicament (ANSM) on June 22, 2012. The study is sponsored by Institut Pasteur (Pasteur ID-RCB Number: 2012-A00238-35) and was conducted as a single-center interventional study without an investigational product. The original protocol was registered under ClinicalTrials.gov (study # NCT01699893). The samples and data used in this study were formally established as the Milieu Interieur biocollection (NCT03905993), with approvals by the Comité de Protection des Personnes – Sud Méditerranée and the Commission nationale de l'informatique et des libertés (CNIL) on April 11, 2018. Donors included in this sub-study were stratified by sex and were between the ages of 30–39 (n = 200) or 60–69 (n = 200) years old. Participants were selected based on stringent inclusion and exclusion criteria, as detailed elsewhere [30]. To minimize the influence of population substructure on genomic analyses, the study was restricted to individuals of self-reported Metropolitan French origin for three generations (i.e., with parents and grand-parents born in continental France). Fasting whole blood samples were collected in EDTA tubes, and plasma was separated following high-speed centrifugation and stored at – 80 °C until analysis. Standard blood testing and complete hemogram was performed on fresh aliquots, while protein immunoassays were performed on frozen aliquots.
Quantification of plasma protein levels in 400 healthy individuals
The protein immunoassays and the blood tests were performed on samples taken the same day and analyzed at different times, on frozen and fresh aliquots, respectively. Blood chemical and major cell fractions were estimated through direct enumeration and standard blood panels. The concentrations of 297 plasma proteins of 400 individuals were quantified by Luminex multi-analyte immunoassays (Discovery Map v3.3 from Myriad RBM, Additional file 1: Table S1), as previously described [33]. Proteins measured included cytokines, chemokines, metabolic markers, hormones, growth factors, tissue remodeling proteins, angiogenesis markers, acute-phase reactants, cancer markers, kidney damage markers, and central nervous system (CNS) biomarkers. Protein levels were analyzed and compared with their respective lower limit of quantification (LLOQ). Among the 297 assayed proteins, 68 proteins were reported at a concentration lower than the LLOQ in at least 20% of the individuals and were filtered out. For the 229 proteins that were kept, reported concentrations lower than the LLOQ were considered as missing values (NAs), to prevent incorrect protein-environment or protein-genotype associations due to undetected or undetectable proteins. Next, for each protein, plasma-level distributions across individuals were tested for normality using the Shapiro-Wilk test on the raw and log-transformed values within the 2.5% and 97.5% percentiles. Shapiro-Wilk null hypothesis was not rejected (p-value ≤ 0.001, after multiple testing correction) for a total of 50 (22%) and 183 (80%) proteins, depending on whether raw or log-transformed values were used. These results suggested that the majority of raw protein plasma levels followed a log-normal distribution. Thus, the levels of all 229 proteins were log-transformed for downstream analyses.
Filters and tests for non-genetic variables
For each individual from the Milieu Intérieur cohort, an extensive electronic clinical record file was filled, gathering 754 lifestyle, environmental, and medical history variables as well as blood metabolite and enzyme levels from standard blood test and erythrocyte enumeration [30]. First, variables with names describing repetitive measurements over several visits after the first visit were filtered out. Second, redundant columns informative about the sex of the individual were removed. Third, mono-factorial variables, character variables, variables with missing values in 20% or more of the individuals, or varying in less than 10 individuals, variables correlated with other variables with a Spearman correlation coefficient of 1, and variables providing redundant information about the same phenotype were filtered out, for a final number of 254 variables. Then, for each of the 254 non-genetic factors, a univariate linear regression analysis was performed against the log-transformed expression levels of each of the 229 plasma proteins evaluated. Age and sex were systematically included as covariates in all such regressions, consistent with their pervasive influence shown in previous studies, as well as with their association with many of the non-genetic factors evaluated [30, 34]. Univariate linear regression was performed between each pair of proteins and non-genetic factors. In addition, to reduce the sensitivity of the linear models to outliers, the ten lowest and ten highest values of each protein were removed from the regression analysis. Significance was declared at p-value ≤ 0.05 after Bonferroni multiple testing correction accounting for the number of tests (n = 58,166). A total of 152 significant associations collectively involving 49 proteins and 20 non-genetic factors were found. In addition, seven major blood-cell fractions, i.e., leukocytes, lymphocytes, monocytes, neutrophils, eosinophils, basophils, and platelets, were assessed through hemogram on fresh aliquots, along the other blood chemicals and enzymes [30].
Genotyping and imputation
Each individual from the Milieu Intérieur cohort was genotyped by the HumanOmniExpress-24 BeadChip (Illumina), covering 719,665 SNPs. In total, 245,766 rare functional variants were also genotyped on a HumanExome-12 BeadChip (Illumina). After quality control, both datasets were merged, for a total of 723,341 SNPs, all mapped in GRCh37.p13 coordinates. Next, IMPUTE v.2 [35] was used to perform genotype imputation, on 1-Mb windows buffered by an additional 1 Mb. Before imputation, SNPs were phased using 500 conditioning haplotypes, 50 MCMC, 10 burn-in, and 10 pruning iterations. SNPs and allelic states were aligned to the imputation reference panel from the 1000 Genome Project Phase 1 v3 (2010/11/23). SNPs with dissimilar alleles (even after flipping) or ambiguous C/G or A/T alleles were filtered out. Imputation yielded a total of set of 37,895,612 SNPs. Removing SNPs with information metric ≤ 0.8, duplicated or monomorphic SNPs, and SNPs with missingness > 5% (SNPs with genotyping probability lower than 0.8 in an individual were considered as missing) reduced the set to 11,395,554 SNPs. Further removing non-SNP variants and filtering out variants with a MAF < 0.05 (with the --snps-only option of PLINK v1.9) in the 400 sampled individuals resulted in a final set of 5,201,092 SNPs. Following Patin et al [29], principal component analysis (PCA) of the OmniExpress array was performed on 261,827 independent SNPs with 36 reference populations from north and west Africa, Middle East, western Asia, and Europe (Human Genome Diversity Panel [36]) and the principal components (PCs) explaining more than 1 % of the total variance were kept to account for potential population stratification (PC1 = 5.42%, PC2 = 1.63%).
Genome-wide association testing of plasma proteins
To perform the pQTL mapping of plasma proteins, we chose to use a multivariate approach by incorporating, for each protein, the associated non-genetic variables as covariates, in addition to sex, age, the 7 major blood-cell fractions (leukocytes, lymphocytes, monocytes, neutrophils, eosinophils, basophils, and platelets), and the two first PCs of the genetic data. If a non-genetic variable was a redundant measure with the corresponding protein (i.e., measured by both Luminex and standard blood panel, e.g., CRP), it was not added as a covariable in the model. We used a first linear mixed model to correct the protein expression levels for their specific covariates and for kinship (mean identity by state, a.k.a. IBS = − 0.0025 ± 0.026 over each pair of individuals, with two pairs of individuals with IBS = 0.2 and IBS = 0.3, potentially indicating second-degree relatives), using per chromosome genetic relationship matrix (GRM) computed using GenAbel v1.8 [37] (leaving one chromosome out). In order to limit the correction for multiple testing while still accounting for both the number of tested SNPs and proteins, the analysis was performed separately for cis and trans QTLs, and the false discovery rate (FDR) was computed independently for cis-acting and trans-acting SNPs, following Quach et al. [38]. Cis-acting SNPs were defined as SNPs located at a maximum distance of 1 MB from the transcription start or end site of the corresponding gene, while all other SNPs are defined as trans-acting. For each protein and each kind of associations tested (cis or trans), the minimal raw p-value was reported. In addition, for each protein, 100 permutations were performed between all cis- or trans-SNPs, and the minimal p-value of each of these permutations was extracted. Next, proteins were ascendingly sorted based on their raw p-values. Then, the FDR was computed, for each protein, as the mean over the N = 100 permutations of the number of times its raw p-value is lower than the nth permutation from all proteins, divided by the rank of the corresponding protein. Protein-SNP pairs were considered as significant when the corresponding FDR was equal to or lower than 0.05, for a total of 78 cis- and 22 trans-pQTLs. To investigate the potential presence of secondary pQTLs, we performed the same analysis a second time, incorporating the genotype of the most significant SNP detected in the first round of analysis as an additional covariate. The FDR was computed independently on each analysis iteration, both considering the same number of proteins and permutations. The conditional analysis yielded 11 cis- and 1 trans-pQTLs. The significance thresholds corresponding to first-round and second-round pQTLs were respectively around 2.2e−05 and 1.5e−05 for cis and respectively around 9e−10 and 2e−10 for trans (significance thresholds were computed as the mean between the last significant and the first non-significant p-values). The genome-wide analysis yielded a total of 100 cis-pQTLs and 12 trans-pQTLs.
Contribution of non-genetic and genetic components in the variability of plasma proteins
The relative contribution of the various environmental and genetic variables was assessed using the correlation-adjusted marginal correlation score (CAR score [39]) from the care package in R. The CAR score is the shrunk estimator of the adjusted coefficient of determination (R2) of each independent variable in a linear model, which considers the marginal correlation between variables. The CAR score is determined for each independent variable within a model, representing their independent contribution to the total variability of the dependent variable. The sum of the CAR score attributed to each variable in a model is equal to the model adjusted R2. For each protein, the relative contribution of its significantly associated non-genetic variable was assessed at once. In case only a single variable was significantly associated with a protein, we used the adjusted coefficient of determination (R2) of the variable as its relative contribution in the variability of the corresponding protein levels. Then, the relative contribution of the identified pQTLs was assessed by computing their CAR score in protein-specific models incorporating age, sex, the protein-specific covariates, and the corresponding genotypes. The effect size of pQTLs was computed following a two-stage model similar to the GWAS. A first linear model was used to regress out the associated covariates (and previously identified SNP in the case of SNPs identified by the conditional analysis) from the log-transformed and non-transformed plasma protein levels, and a second linear model was used to regress the residuals of the first against the tested SNP. The Beta was extracted from this model and used as the SNP effect size.
Global impact of age and sex on plasma protein levels
In order to characterize the global impact of age and sex on plasma protein heterogeneity, while accounting for the collinearity of several plasma proteins, we performed a principal component analysis (PCA) on the expression levels of the 229 proteins across the 400 individuals. When considered independently, only PC1 and PC2 explained more than 5% of the total variability (Additional file 2: Figure S1).
Assessment of gene-gene interactions
The interactions between trans-pQTL associated proteins and candidate proteins coded by genes located within a 500-kb window centered around the associated SNP were assessed using STRING-db v11 [40] at https://string-db.org/. All protein-coding genes were queried at once through the “Multiple proteins” option and default settings (organism: “Homo sapiens”). Proteins were considered to interact when they were shown to be direct neighbors in protein-protein interaction networks, or when one of the protein was shown to be directly or indirectly involved in the regulation of the other.
Contribution of blood-cell fractions in protein level predictions
To quantify the relevance of blood-cell fractions in the prediction of plasma protein levels, we used a one-way ANOVA to compare, for each pQTL, the predictions coming from two models. A first linear model considered the genotype of the corresponding SNP, the previously defined protein-specific covariates, and age, sex, the two first PCs of the genetic data, and the 7 blood-cell fractions. A second linear model was evaluated by considering all variables used in the previous one, with the exception of the 7 blood-cell fractions. The models including pQTLs obtained from the conditional analysis additionally corrected for the SNP used for their identification. To assess the potential relevance of the different circulatory cell fractions in the different results obtained from the two pQTL mapping, the proteins were first corrected for age and sex through linear regression, and the resulting residuals regressed against each circulatory cell counts individually. Association p-values were corrected independently for each protein and are reported in Additional file 3: Table S2.
Genome-wide analysis of plasma proteins excluding blood-cell fractions
For the purpose of evaluating the contribution of blood-cell fractions in the pQTL assessment, the genome-wise association analysis was performed as previously detailed, while removing the 7 blood-cell fractions from the model (“Methods”). This approach identified 115 protein quantitative trait loci (pQTLs) collectively involving 94 proteins and 113 SNPs (FDR ≤ 0.05). Among them, 103 were defined as cis-pQTLs. In addition, 12 pQTLs were identified in trans, i.e., located further than 1 MB far from the gene boundaries, or located on another chromosome. In total, 73 proteins were associated with only one SNP, while 21 were associated with two independent SNPs. Among the 94 proteins with significant pQTLs, 83 proteins were associated exclusively with cis-pQTLs, 9 exclusively with trans-pQTLs, and 2 with both cis and trans-pQTLs. In comparison with the first analysis, 92 pQTLs were reproduced, 81 in cis, and 11 in trans. An association was considered as reproduced when the SNP, or a SNP in linkage disequilibrium with R2 ≥ 0.8, was significantly associated with the same protein at FDR ≤ 0.05. Twelve cis-pQTLs were no longer associating with the same SNPs or to SNPs in high LD (R2 ≥ 0.8) with it, but with other cis-SNPs, and 8 pQTLs (7 cis, 1 trans) were not reproduced. On the opposite, 10 additional cis- and 1 trans- pQTLs were obtained.
Co-localization of trans-pQTLs and trans-eQTLs
We assessed the co-localization between the trans-pQTLs identified in this work and previously identified trans-eQTLs associated with the same gene and reported in the GWAS Catalog V1.0.2 [41], in eQTLs from GTEx V8 or in QTLbase v1.2 [42] (http://mulinlab.org/qtlbase) through the VannoPortal [43], using a LD threshold of R2 ≥ 0.8 (computed in Europeans from the 1000 Genome Project) between each pQTL and all SNPs present in a 200-kb window centered on the pQTL.
Replication of previously identified plasma protein QTLs
We compared our significant SNP-protein pairs with four studies analyzing the genetic basis of plasma protein levels. Sun et al. [22] reported 1927 pQTLs, resulting from the analysis of 3622 plasma proteins in 3301 individuals; Suhre et al. [23] reported 539 pQTLs from the analysis of 1124 plasma proteins in 1000 individuals; Deming et al. [24] reported 56 pQTLs from the analysis of 146 plasma proteins in 818 individuals; and Zhong et al. [44] reported 144 pQTLs from the analysis of 107 plasma proteins in 101 individuals. The replication of the reported pQTLs was performed at the protein level rather than at the SNP level, due to the poor overlap in terms of sentinel SNPs reported in previous studies [22–24, 44] and our imputed set of 5,201,092 SNPs. A protein previously reported as cis-regulated (i.e., reported as significantly associated with a SNP annotated as “cis” by the authors) was considered replicated when it was significantly associated with a SNP located closer than 1 Mb around the gene extremities.
Overall, cis- and trans-pQTLs were labeled as novel when they were absent from the four reference studies used for replication, or when they—or SNPs in high LD with them (R2 ≥ 0.8, based on the reference European population from the 1000 Genome Project)—were absent from the GWAS catalog [41] and from QTLbase v1.2 [42] (http://mulinlab.org/qtlbase). The studies that previously identified the pQTLs reported in this work are referenced in Additional file 4: Table S3.
Protein and gene annotations
Proteins were classified as immune-related when they were either (i) annotated as adaptive immune proteins (Table S2 from Fischer & Rausell, 2016 [45]), or innate immune proteins (Table S1 from Fischer & Rausell, 2016 [45], and Table S1 from Deschamps et al. [46]) or (ii) produced in sufficient concentrations in substantial fractions of immune cells, as described in Rausell et al. [47]. The list of primary immunodeficiency genes was obtained from Table S3 from Fischer & Rausell [45]. Gene-disease annotations were obtained from OMIM (downloaded at https://www.omim.org/downloads/ on the 2019/06/10). Entries were parsed following Caron et al. [48]. Mendelian disease genes were selected for their level of supporting evidence equal to 3 and for not having a “somatic” flag, and monogenic Mendelian disease genes (MMDGs) were further selected for not being flagged as “complex”. The list of secreted proteins was downloaded from UniProt (https://www.uniprot.org/) on the 2020/02/03, using the keywords: locations:(location:"Secreted [SL-0243]" type:component) AND organism:"Homo sapiens (Human) [9606]". The list of FDA-approved targeted proteins was downloaded on the 2020/02/05 from http://mrmassaydb.proteincentre.com/fdaassay/.
The protein classes were taken from Myriad RBM Discovery Map V3.3 table. Proteins were considered enriched or depleted in a specific class when the proportion of proteins in that class was larger than in 10,000 randomly sampled set of proteins of the same size, coming from the tested set of 229 proteins. Previous reports of pQTLs associated with monogenic Mendelian disease genes, with primary immunodeficiency genes or with genes coding for FDA-approved biomarkers were identified through QTLbase [42] (http://mulinlab.org/qtlbase).
Disease loci enrichment
To assess the potential association with diseases or other traits of the cis and trans-pQTLs reported in this work was assessed, we used hits from the NHGRI-EBI Genome Wide Association Studies (GWAS) Catalog [41], downloaded on the 2019/03/22 (file name gwas_catalog_v1.0-associations_e95_r2019-03-22.tsv). SNPs associated with a trait or a disease with a reported p-value ≤ 1e−08 and mapped to autosomes were kept. SNPs associated with traits containing “blood,” “plasma” or “serum,” and “protein” were removed. A set of pQTLs was declared as enriched when the proportion of pQTLs in a set that were GWAS SNPs or in linkage disequilibrium with GWAS SNPs (r2 ≥ 0.8) was larger in 95% of 10,000 randomly sampled set of SNPs of the same size, matched by MAF (bins of 5%). Randomly sampled SNPs were drawn from 122,757 and 384,897 SNPs, selected respectively from the 1,674,134 and 5,201,092 SNPs tested for cis and trans associations respectively (with the --indep-pairwise 100 5 0.5 function of PLINK v1.9). When several traits or diseases were associated with one locus, the most significant one was selected.
Functional annotation of pQTLs
The cis-pQTL molecular consequences were assessed using VEP v97 [49]. Each cis-pQTL was annotated based on the canonical transcript of the gene coding [50] for the regulated protein.
Results
Variation of plasma protein levels in a well-defined healthy population
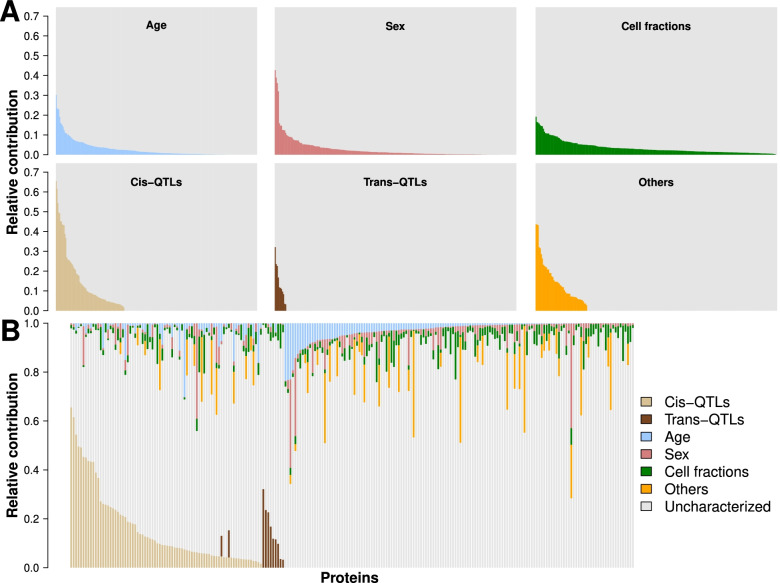
We quantified the concentrations of 297 plasma proteins in 400 healthy individuals from the Milieu Intérieur (MI) study [30] using CLIA certified assays (Additional file 1: Table S1). After quality control accounting for detection limits and missing data (“Methods”), 229 proteins were retained for downstream analyses, including 141 immune-related plasma proteins (i.e., proteins with previously identified immune functions or produced by immune cells, “Methods”). First, we evaluated the impact of individuals’ genetics and non-genetic factors on plasma protein levels. Genome-wide association tests for each of the 229 proteins were performed against a total of 5,201,092 common SNPs (minor allele frequency ≥ 0.05). Covariates that we systematically included in the analysis were age, sex, counts of 7 major blood-cell sub-populations (lymphocytes, leucocytes, neutrophils, basophils, eosinophils, monocytes, and platelets), and the first two principal components of a principal component analysis of the genetic data, representing genetic stratification in the sample (“Methods”). Additional non-genetic factors were selected among 254 lifestyle, environmental, physiological, and blood biochemical variables and added as confounders in a protein-specific manner, based on their significant association with each protein (“Methods”). The relative contribution (marginal correlation, CAR score, “Methods”) of non-genetic, genetic, and cell fraction components to the inter-individual variability of the 229 plasma proteins evaluated in this work is presented in Fig. 1 and Additional file 5: Table S4, Additional file 6: Table S5, Additional file 7: Table S6 (“Methods”).
Fig. 1.
Contribution of environmental and genetic factors to the variability of plasma protein levels. The variability explained by the associated genetic and non-genetic factors in the levels of 229 plasma protein levels, taken independently (A) or altogether (B). Each vertical bar represents the total variability of a protein, with the contribution of the considered (colored) or other and unknown (gray) factors summing up to 1. Age (blue), sex (brick-red), cis (light brown), and trans (dark brown) pQTLs correspond directly to the assessed relative importance, the cell fraction category (green) represents the cumulated relative importance of lymphocytes, leukocytes, neutrophils, eosinophils, basophils, monocytes, and platelets, and the “Others” category (orange) represent the sum of the relative importance all other non-represented variables. In A, the gray area represents 1 minus the sum of all non-considered and unknown factors. In B, the “Uncharacterized” category was computed as 1 minus the sum of all other variables or groups of variables
Consistent with previous studies [22–24, 44, 51–53], we found that age and sex had a widespread effect on plasma protein levels, each explaining on average 2.8% of the total observed variability (Fig. 1, Additional file 5: Table S4, Methods). Similar figures were observed for the subset of immune-related proteins, i.e., 2.3% and 2.1% for age and sex respectively. For specific proteins, however, the observed contribution of age and sex was particularly large, in line with previous findings. For example, age explained 30.2% of growth differentiation factor 15 variability (GDF15) [51, 54], while variability attributed to sex was 42.8% for Leptin [55], 39% for Stromelysin-1 (MMP3) [51, 56], 36.2% for FSH, and 32.1% for LH [51, 57]. Moreover, when accounting for potential covariation among the 229 proteins through a principal component analysis (PCA), both age and sex showed a strong association with the global heterogeneity of protein levels (univariate linear modeling against PC1 coordinates, p-values = 1.8e−07 and 1.4e−06, respectively; and PC2, p-values = 4.2e−24 and 3.5e−05, respectively; Additional file 2: Figure S1, “Methods”). While previous studies mostly assessed the global impact of age and sex on plasma proteins, their effects appear highly heterogeneous across proteins.
Blood-cell fractions explain a substantial part of plasma protein-level heterogeneity
To assess the potential effect of circulatory cell counts on plasma protein levels, we next quantified their relative contribution to the inter-individual variability of each protein (“Methods”). Taken together, blood-cell fractions explained on average 3.6% of the variability of the observed plasma protein levels. This contribution was comparatively higher than those of age and sex (two-sided Wilcoxon test p-value = 6.4e−11 and 2.3e−14, respectively; Fig. 1, Additional file 5: Table S4). Furthermore, blood-cell fractions explained significantly more variability for immune-related proteins than for the rest of proteins evaluated (mean explained variability 4% and 2.9% respectively, one-sided Wilcoxon test, P = 4.8e−02). Platelet counts alone explained an average of 1.6% of the variability of immune-related proteins, as compared to 0.78% for the rest of proteins (one-sided Wilcoxon test, p-value = 3.9e−04), with contributions as high as 16.3% for the Neutrophil Activating Peptide 2 and 13.3% for Thrombospondin-1. These results highlight the contribution of blood-cell fractions to the variability of plasma protein levels and support their consideration as a covariate in the assessment of genetic associations.
Plasma lipids and body mass index are important covariates of specific plasma proteins
Two other classes of non-genetic factors were found to substantially associate with plasma levels of specific proteins. First, plasma lipids such as triglycerides, HDL, LDL, and total cholesterol were associated with expression levels of 25 proteins, including various components of cholesterol particles as well as proteins involved in lipid transport (ApoA1, ApoB, ApoC1, ApoC3, ApoD, ApoE, FABP-adipocyte, SHBG), metabolism, and homeostasis (Adiponectin, Carboxypeptidase B2, C3, CFH, C-peptid, Endoglin, FGF21, IGFBP2, Leptin, Leptin Receptor, PEDF, Prostatin, PSAT, RBP4, SAP, tPA). Second, anthropometric factors such as body mass index (BMI) and abdominal circumference were associated with plasma levels of 20 proteins, most of which also associate with plasma lipids (e.g., Adiponectin, ApoD, C-peptid, FABP-adipocyte, SHBG). Blood lipids and anthropometric factors accounted on average for 11% and 8.1% of the variability of the associated plasma proteins, respectively. Yet, the highest association was observed between HDL and the Apolipoprotein A-1 (marginal correlation of 44.6%) [58] (Figs. 1, 2, Additional file 6: Table S5, “Methods”). Globally, anthropometric factors and plasma lipid levels are known to be markers of physical shape and overall health. Interestingly, the association of complement factors (C3 and CFH) with anthropometric traits may reflect the low level inflammation induced by higher body mass, both of which associate with obesity, cardiovascular diseases, and increased susceptibility to infections [59–61].
Fig. 2.
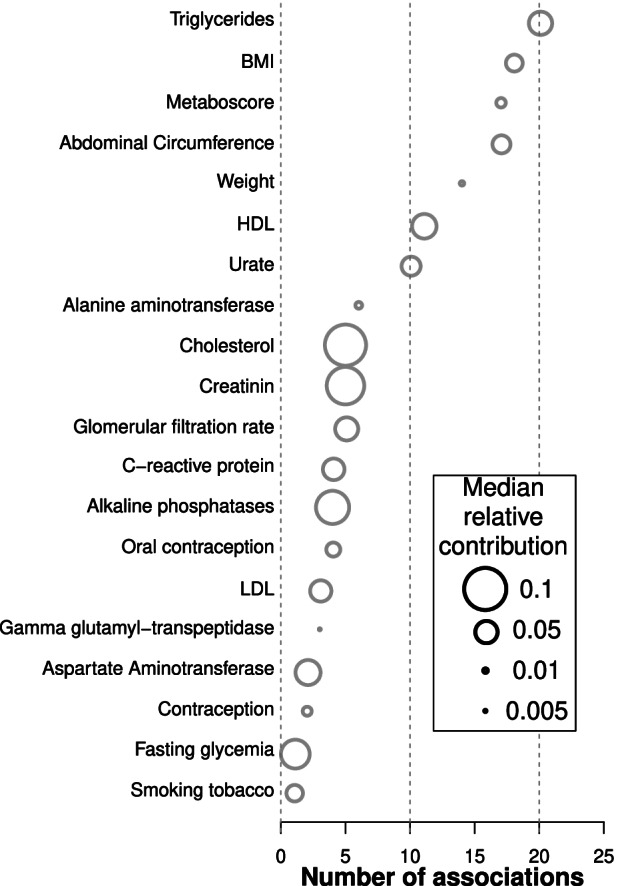
Relative contribution of selected factors in the variability of plasma protein levels. Relative contribution (CAR score [39]) of the 20 significantly associated factors to the variability of plasma protein levels. Variables are sorted depending on the number of significant associations with proteins. The number of proteins significantly associated with each variable is reported on the x-axis. The diameter of each dot represents the median CAR score of the corresponding factor in the variation of the associated plasma proteins
Contribution of human genetics on plasma protein levels
Genome-wide association testing against the 229 plasma proteins identified 112 pQTLs, including 100 cis- and 12 trans-pQTLs, and collectively involving 87 proteins and 111 SNPs (FDR ≤ 0.05; Fig. 3, Additional file 2: Figure S2, Additional file 2: Figure S3, and Additional file 7: Table S6, Methods). Among the 87 proteins, 76 were only associated with cis-SNPs, 9 with only trans-SNPs, and 2 with both. Sixty-two proteins were associated with one SNP, and 25 with two independent SNPs. Interestingly, three loci aggregated several pQTLs associated with different proteins. First, among the 12 trans-pQTLs identified, 4 (E-selectin-rs2519093; PECAM-1-rs2519093; Cadherin-1-rs635634; FASLG receptor-rs687621) were in moderate (R2 = 0.45 rs2519093 and rs687621) to high (R2 = 0.99, rs2519093 and rs635634) linkage disequilibrium (LD) and co-localized in a 18-kb region of chromosome 9, previously described as the ABO locus, and known to be associated with the expression of many plasma proteins [23, 25, 62, 63]. Second, two SNPs on chromosome 1 in high LD (R2 ≥ 0.99) at the CFHR4 locus associated, respectively, in cis (rs60642321) with CFHR1 plasma levels, and in trans (rs115094736) with TFR1 plasma levels. While the former had previously been reported in blood [64], the latter was, to the best of our knowledge, not reported before, neither as a pQTL nor as an eQTL. In addition, no physical or regulatory interactions have been reported between TFRC and any other gene or protein in a 500-kb window centered on the associated SNP (as reported in STRING-db [40], “Methods”). Last, two SNPs (rs584007 and rs3826688) in high LD (R2 ≥ 0.99), located on chromosome 19, associated in cis with the plasma levels of Apo E and Apo C1, respectively. Both SNPs are located within a known ApoE enhancer [65] and were previously described as cis-eQTLs of both genes (in blood or in other tissues), hinting at a potential co-regulation of the expression of both genes [66, 67]. Among the 12 trans-pQTLs identified therein, only two co-localized with known eQTLs for the same gene (based on VannoPortal [43], “Methods”). Last, only one gene located at the trans-pQTL locus was shown to interact with the associated gene (SORT1 and GRN, respectively; physical or regulatory interactions reported in STRING-db, Methods). Indeed, GRN encoded protein, Progranulin, was shown to bind to SORT1 encoded protein, Sortilin 1, based on co-immunoprecipitation experiments performed in various mice cell lines [68–70] and in green monkey fibroblasts, and on co-expression experiments in human breast cancer cell lines [71, 72] (Additional file 7: Table S6). Individually considered, cis-pQTLs explained a mean of 12.6% of the associated protein levels (marginal correlation interquartile range from 4.3 to 14.6%). The highest variance explained (marginal correlation, CAR score, “Methods”) by cis-pQTLs were for the rs7041 polymorphism and vitamin D-binding protein (65.6%), rs2856448 for the Tenascin-X protein (61.5%) and rs60642321 for the Complement factor H-related protein 1 (54.4%). Significant trans-pQTLs explained a mean of 12.9% of the variability of the associated protein levels (interquartile range from 8.1 to 14.6%), with a maximum contribution of 32.2% in the case of the rs115094736 SNP for the Transferrin receptor protein 1. Considering all cis-pQTLs and all trans-pQTLs, they explained on average 16.2 and 14.1% of the total variance, respectively. The per-protein global contribution of cis-QTLs to plasma-level heterogeneity were lower for immune-related proteins as compared to the rest of the evaluated proteins (13.8% and 19.5% on average, respectively, two-sided Wilcoxon test p-value = 0.10).
Fig. 3.
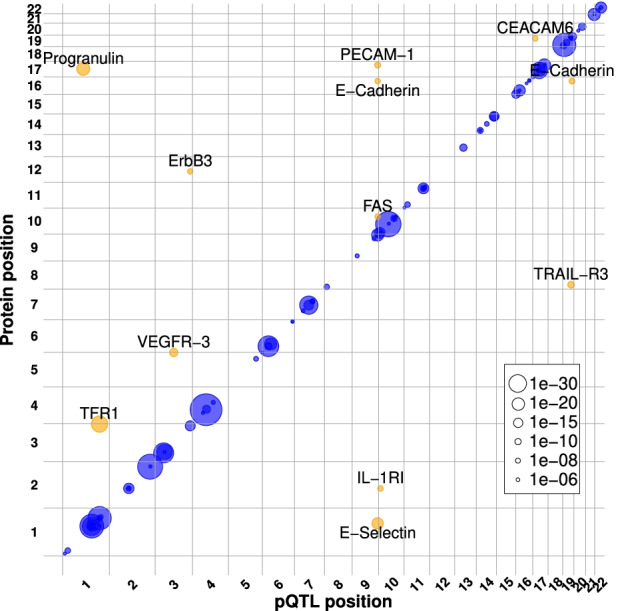
Plasma protein QTLs localization and co-regulation. The genomic positions of the cis (blue) and trans (orange) pQTLs identified in this work (x-axis) and the genomic location of the gene coding for the associated protein (y-axis). The point size is proportional to the uncorrected association p-value as reflected in the legend
Accounting for blood-cell fractions reveals new genetic associations
To evaluate the impact of considering blood-cell fractions in the evaluation of genetic associations with plasma protein levels, we tested whether a linear model accounting for cell fractions better fits protein levels than a simpler model not considering them as covariates. Out of the 112 pQTLs reported in this work, the addition of cell fractions significantly improved the linear model in 42 of the cases (one-way ANOVA, F test p-values ≤ 0.05, “Methods”). To characterize the effect of considering or not such covariate, we repeated a genome-wide pQTL assessment as previously described, while excluding blood-cell fractions from the association tests (“Methods”). Under this setting, we found 115 genetic associations with 94 proteins, as compared to the 112 associations with 87 proteins initially identified, with 84 proteins with pQTLs common to both datasets. Thus, three proteins were specific to the analysis accounting for blood-cell fractions as covariates, while 10 proteins were specific to the analysis not considering blood-cell fractions (Additional file 3: Table S2, “Methods”). Moreover, 6 of the 13 proteins showing different pQTL results between the two settings were in turn significantly associated with at least one of the seven circulatory cell fractions tested herein (CEACAM6, Hemopexin, Resistin, TARC, Thrombospondin 1, and TTR; Additional file 3: Table S2, “Methods”). Yet, the nominal p-values of genetic associations (p-value ≤ 0.05) from one analysis to the other remained significant. In addition, out of the initial 112 significant SNP-protein pairs, only 92 pairs (82%, collectively associated to 76 proteins, and including pairs involving SNPs in high LD, r2 ≥ 0.8) reached statistical significance in the second setting. Among the protein-SNP pairs not being replicated, 11 proteins (initially associated with 13 SNPs) associated with different SNPs (or with SNPs in lower LD with the previously associated SNPs, r2 < 0.8), and 7 protein-SNP pairs, involving 7 proteins, were no longer significant. Collectively considered, the p-value distribution of the 135 unique SNP-protein pairs (i.e., the 92 pairs common to both analyses, the 20 pairs specific to the first analysis and the 23 pairs specific to the second analysis) corrected for blood-cell fractions was significantly different from the p-value distribution obtained for the same protein-SNP pairs while not controlling for blood-cell fractions (two-sided paired Wilcoxon test, p-value = 1.97e−06; Additional file 2: Figure S4, Additional file 3: Table S2 and Additional file 7: Table S6). In addition, while no significant differences were observed between the effect size distributions of the two settings (two-sided paired Wilcoxon test, p-value = 0.86), controlling for cell fractions lead to a significant shift towards lower effect-size standard deviations (two-sided paired Wilcoxon test, p-value < 5.68e−23; Additional file 2: Figure S4). Overall, these results show that cell fractions are an important factor for the study of genetic and non-genetic associations with plasma protein variability across healthy individuals. However, a mediator role cannot be directly inferred from the previous associations.
Replication of previously reported plasma proteins presenting cis-pQTLs
We then evaluated the extent to which our study replicated previously reported plasma protein associations with proximal genetic polymorphisms (i.e., cis-pQTLs) in three recent large-scale studies [22–24, 34] (Additional file 4: Table S3). At a significance threshold based on FDR correction (“Methods”), we replicated 52.2%, 63.6%, and 77.3% of the n = 87 proteins reported with significant associations by the three studies, out of the n = 131 plasma proteins common with our study, for Sun et al. [22], Suhre et al. [23], and Deming et al. [24] respectively (Fig. 4). From a complementary perspective, 54 out of 78 (66.7%) and 5 out of 11 (45.5%) of the proteins with cis- and trans-pQTLs in our study, respectively, had been previously reported by at least one of 4 large-scale studies [22–24, 44]. Conversely, we identified 14 novel cis- and 5 novel trans-pQTLs associated with 15 plasma proteins (Additional file 7: Table S6), absent from reference repositories (i.e., the GWAS Catalog [41] and QTLbase [42], “Methods”) and large-scale plasma pQTL mapping studies [22–24, 44].
Fig. 4.
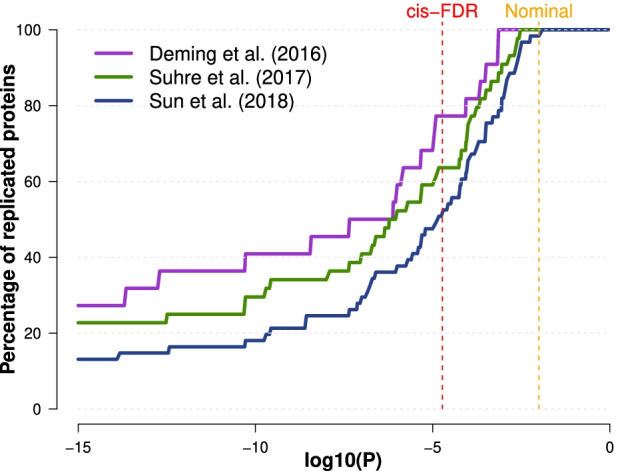
Replication of cis-pQTLs. The percentage of replication of previously reported cis-regulated proteins between our analysis and three previous studies: Sun et al. [22], Suhre et al. [23], and Deming et al. [24]. For each dataset, the percentage of replication (y-axis) as a function of the significance threshold (x-axis) was computed as the number of cis-regulated proteins reported in this work that were also reported in the corresponding dataset as cis-regulated (the “replicated” proteins) divided by the total number of proteins reported as cis-regulated in a previous study that were analyzed in our work (the “replicable” proteins). The dashed vertical lines represent the p-value significance threshold corresponding to the FDR of cis-pQTLs (red) and to the nominal replication threshold (orange)
Clinical relevance of plasma proteins and associated genetic factors
We characterized the potential medical interest of the pQTLs identified and their associated genes. Both cis- and trans-pQTLs reported in our study were significantly enriched in GWAS-based disease- or trait-associated SNPs, showing ~7 and ~7.8 times more GWAS hits, respectively, than expected (43% and 41.6% observed as compared to an expectation of 6.2% and 5%, odds ratio of 12 and 15.8 respectively, with a resampling p-value < 1e−04; “Methods,” Fig. 5, Additional file 7: Table S6). Seventeen of the 87 proteins with pQTLs are FDA-approved biomarkers, including one plasma protein not previously associated with plasma pQTLs, i.e., Cancer Antigen 15-3. In addition, among the 87 genes collectively associated to the 112 pQTLs identified, 29 genes are known monogenic Mendelian disease genes (MMDGs), including six primary immunodeficiency genes (PIDs) (“Methods”). Notably, the plasma protein levels of five out of these 29 MMDGs, including two PID genes, i.e., FCN3 and FAS—had not been previously reported to be associated with these genetic loci (pQTLs) in reference repositories (Fig. 6, “Methods”). The identification of pQTLs associated to such Mendelian disease genes may contribute to the genetic characterization of the observed incomplete penetrance or severity heterogeneity across patients suffering from primary immune deficiencies.
Fig. 5.
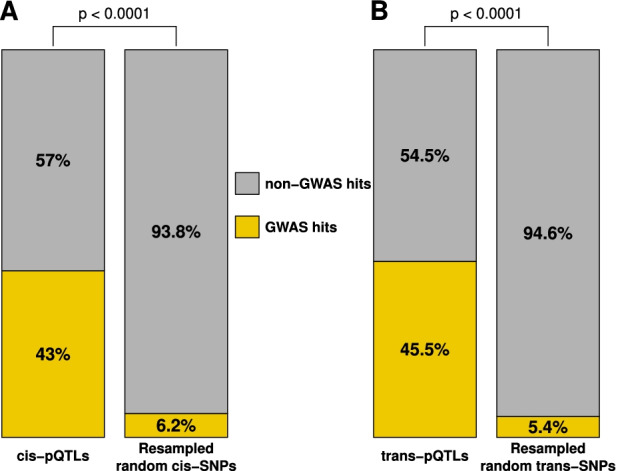
Clinical relevance of pQTLs and associated genes. Enrichment in GWAS hits (orange) of cis-pQTLs (A—left) and trans-pQTLs (B—left) in comparison with, respectively, 10,000 randomly sampled set of cis-SNPs matched by MAF (bins of 5%) (A—right) and 10,000 randomly sampled set of trans-SNPs matched by MAF (bins of 5%) (B—right). Empirical resampling p-values are shown
Fig. 6.
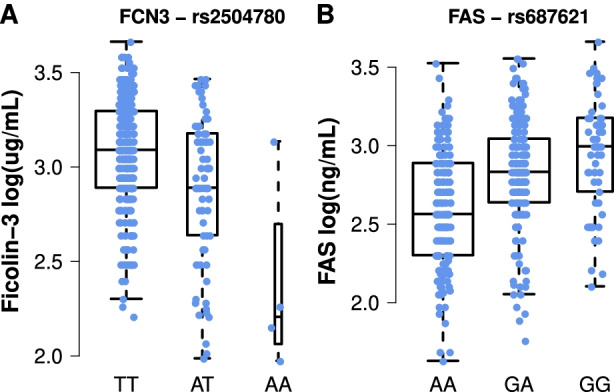
Impact of the novel pQTLs identified on the expression of the 3 target primary immunodeficiency genes. Expression levels of the two homozygous states and the heterozygous state of A Ficolin 3 × rs2504780 and B FAS × rs687621. Each dot corresponding to the non-transformed plasma levels of an individual of the corresponding genotype
Discussion
In this work, we characterized non-genetic and genetic factors explaining the natural heterogeneity of 229 plasma protein levels observed in healthy individuals. We replicated previous findings [22–24, 44, 51–53] describing that age and sex have a global impact on plasma proteins, while anthropometric variables, blood lipids, and metabolic markers are also relevant factors for specific proteins. In agreement with previous observations from this cohort [29, 31], environmental factors had a relatively low influence on protein levels in this study of well-defined healthy donors. In addition, we characterized the contribution of seven major blood-cell fractions to inter-individual heterogeneity and found that their contribution to plasma protein variability was higher than age and sex. Moreover, our results suggest that blood-cell fractions may act as important confounders of genetic associations with specific plasma protein levels. In addition to non-genetic factors, we identified 100 and 12 pQTLs acting in cis and trans, respectively, associated with 87 plasma proteins. However, the inclusion of cellular covariates in the assessment of genetic associations led to the identification of three novel proteins with pQTLs, while abrogated the signal for 10 proteins which would have otherwise led to positive hits. This could potentially be explained by the fact that all 13 proteins are expressed by specific circulating immune cell populations [73–82]. However, the interactions between these proteins, blood-cell populations, and genetic variants are less obvious to interpret, as both direct and indirect effects or co-occurring mechanisms could be involved. Some limitations of our study include the relatively modest sample size, and the focus on two age groups to identify age associations. This approach could be improved by including donors from more extreme ages of life such as neonates, pediatrics, and greater than 80 years when age may be expected to have stronger effects on physiological processes. An additional limitation is the analysis of a single homogeneous population that can be addressed through inclusion of populations from other ethnicities and backgrounds in future studies.
Although our study replicated a large number of previously reported genetic associations with plasma proteins [22–24, 44], it also identified 19 novel pQTLs associated with 15 proteins. This may stem from the well-defined healthy nature of our study population, which may reduce potential confounding lifestyle or medical factors, or from the use of quantitative values as provided by Luminex assays as compared to alternative assessment methods [83]. Interestingly, of the newly identified associations, six include proteins encoded by MMDGs, 2 of which are known to cause primary immunodeficiencies, i.e., Ficolin-3 and FAS (Fig. 6). Primary immunodeficiencies are caused by rare variants leading either to loss- or gain-of-function consequences in the affected genes [84, 85]. However, such mutations are often not fully penetrant and the associated symptoms are heterogeneous between and within families. Among possible explanations for this heterogeneity, low to mild effect common variants, such as the pQTLs identified in this work, might act as modifier of the corresponding diseases, by increasing or decreasing the expression of the corresponding proteins, and consequently mitigating or aggravating the consequences of causal variants.
Thus, the common variant associated with Ficolin-3 plasma levels identified in this work, rs2504780 (AF = 10.7%, 1:27710876, T>A), is located 9.5kb upstream of FCN3 and associated with a diminution of Ficolin-3 levels (effect size = − 3.79 μg/mL per alternative allele, Fig. 6A) of an order of magnitude comparable with heterozygous FCN3 loss-of-function variants (effect size = − 13.4 μg/mL per loss-of-function allele) [86] causing immunodeficiency 41 with lymphoproliferation and autoimmunity (OMIM 606367) [87–92]. This variant could be a risk factor for Ficolin-3 deficiency and might play a role in the observed etiology of both complete Ficolin-3 deficiency or Ficolin-3 haploinsufficiency in the response to infection and autoimmunity. Future analysis of auto-antibodies in our cohort may allow us to directly test this hypothesis. Another example is the common variant associated in trans with FAS plasma levels, rs687621 (9:136137065, A>G, AF = 36%) which increases the expression of FAS (effect size = +2.79 ng/mL per alternative allele; Fig. 6B). This variant could contribute to a protective role against the haploinsufficient forms of autoimmune lymphoproliferative syndrome (ALPS, OMIM 601859) [93–98] by increasing the expression of FAS in heterozygous loss-of-function variant carriers. However, the rs687621 polymorphism is located at the ABO locus, which is known to associate with the expression of many plasma proteins [22, 24, 99, 100]. Such an association hotspot could be explained by the glycosyltransferase activity of ABO proteins [101], which by transferring glycosyl residuals on target proteins may potentially alter its binding affinity of the associated antibody in immunoassays [102], thus constituting a technical artifact. In light of these potential caveats, the biological relevance of the FAS-associated trans-pQTL identified should be taken with caution, prior to replication in other cohorts with complementary assays. The common genetic associations identified here for plasma protein levels of PID genes could be further characterized through genetic fine-mapping and functional characterization.
Plasma protein levels can be considered as end-of-chain signal integrators, and their levels are influenced by several molecular mechanisms (e.g., mRNA transcription, Kozak sequence affinity and other translation initiation mechanisms, codon usage, translation rate, post-transcriptional modifications [103–107]). A combination of targeted genome and transcriptome sequencing, ribosome occupancy assay, and intracellular protein assays in the cell type or tissue of interest would allow the identification of the causal variants and the molecular mechanisms mediating the observed associations. Finally, the phenotypic consequences of pQTLs associated to plasma levels coded by PID genes should be further characterized both in healthy and PID patients, where protective or modifier roles could be further established.
Conclusions
Our study showed that, together with age and sex, circulatory blood-cell fractions are a major factor explaining the inter-individual heterogeneity of plasma protein levels in the general population. In addition, 100 cis- and 12 trans-pQTLs were identified, which explained the largest fraction of the variability of plasma protein levels as compared to non-genetic factors. Such pQTLs were significantly enriched in GWAS-based disease- or trait-associated SNPs. Furthermore, among 19 previously unreported genetic associations, 6 involved 5 known monogenic Mendelian disease genes, including 2 primary immunodeficiency genes. The potential use of the identified genetic and non-genetic factors associated to plasma protein levels as biomarkers to inform health and disease status would require further investigation.
Supplementary Information
Additional file 1: Table S1. List of the 297 plasma proteins assayed.
Additional file 2: Figure S1. Principal component analysis of age and sex effects. Figure S2. Manhattan plots and allelic expression of levels of cis-pQTLs. Figure S3. Manhattan plots and allelic expression of levels of trans-pQTLs. Figure S4. Impact of blood-cell fractions on associated protein-SNP pairs statistics.
Additional file 3: Table S2. Proteins with analysis-specific significant genetic association.
Additional file 4: Table S3. Summary of previous plasma pQTL study design.
Additional file 5: Table S4. Description of the association between plasma protein levels, age, sex and blood-cell fractions.
Additional file 6: Table S5. Description of the significant protein-environmental variable pairs and variable contribution the variability of the protein expression levels.
Additional file 7: Table S6. Annotations of cis and trans pQTLs.
Acknowledgements
We would like to thank all the donors for their contribution to the study. We also thank the members of The Milieu Intérieur Consortium for their insightful comments.
Author: The Milieu Intérieur Consortium†.
† The Milieu Intérieur Consortium is composed of the following team leaders: Laurent Abel (Hôpital Necker), Andres Alcover (Institut Pasteur), Hugues Aschard (Institut Pasteur), Philippe Bousso (Institut Pasteur), Nollaig Bourke (Trinity College Dublin), Petter Brodin (Karolinska Institutet), Pierre Bruhns (Institut Pasteur), Nadine Cerf-Bensussan (INSERM UMR 1163 – Institut Imagine), Ana Cumano (Institut Pasteur), Caroline Demangel (Institut Pasteur), Christophe d’Enfert (Institut Pasteur), Ludovic Deriano (Institut Pasteur), Marie-Agnès Dillies (Institut Pasteur), James Di Santo (Institut Pasteur), Françoise Dromer (Institut Pasteur), Gérard Eberl (Institut Pasteur), Jost Enninga (Institut Pasteur), Jacques Fellay (EPFL, Lausanne), Ivo Gomperts-Boneca (Institut Pasteur), Milena Hasan (Institut Pasteur), Magnus Fontes (Institut Roche), Gunilla Karlsson Hedestam (Karolinska Institutet), Serge Hercberg (Université Paris 13), Molly A Ingersoll (Institut Pasteur), Rose Anne Kenny (Trinity College Dublin), Olivier Lantz (Institut Curie), Frédérique Michel (Institut Pasteur), Hugo Mouquet (Institut Pasteur), Cliona O'Farrelly (Trinity College Dublin), Etienne Patin (Institut Pasteur), Sandra Pellegrini (Institut Pasteur), Stanislas Pol (Hôpital Côchin), Antonio Rausell (INSERM UMR 1163 – Institut Imagine), Frédéric Rieux-Laucat (INSERM UMR 1163 – Institut Imagine), Lars Rogge (Institut Pasteur), Anavaj Sakuntabhai (Institut Pasteur), Olivier Schwartz (Institut Pasteur), Benno Schwikowski (Institut Pasteur), Spencer Shorte (Institut Pasteur), Frédéric Tangy (Institut Pasteur), Antoine Toubert (Hôpital Saint-Louis), Mathilde Touvier (Université Paris 13), Marie-Noëlle Ungeheuer (Institut Pasteur), Christophe Zimmer (Institut Pasteur), Matthew L. Albert (Insitro)§, Darragh Duffy (Institut Pasteur)§, Lluis Quintana-Murci (Institut Pasteur)§.
§ co-coordinators of the Milieu Intérieur Consortium.
Additional information can be found at: www.milieuinterieur.fr.
Authors’ contributions
Conceptualization, BCa, MLA, LQC, DD, AR; methodology, BCa, EP, MR, BCh, MLA, LQC, DD, AR; software, BCa; formal analysis, BCa, EP, MR, MLA, LQC, DD, AR; investigation, BCa, EP, MR, BCh, MLA, LQC, DD, AR; data curation, BCa, EP, BCh; writing (original draft), BCa, LQC, DD, AR; writing (review and editing), BCa, DD, AR; supervision, LQC, DD, AR; project administration, LQC, DD; and funding acquisition, MLA, LAC, DD, AR. LQM and DD are co-coordinators of the Milieu Intérieur Consortium (more information available at http://www.milieuinterieur.fr/en). All authors read and approved the final manuscript.
Funding
The study is sponsored by Institut Pasteur (Pasteur ID-RCB Number: 2012-A00238-35) and was conducted as a single-center interventional study without an investigational product. This work benefited from support of the French government’s Invest in the Future program. This program is managed by the Agence Nationale de la Recherche, reference ANR-10-LABX-69-01. The Clinical Bioinformatics Laboratory of the Imagine Institute was partly supported by the French National Research Agency (ANR) “Investissements d’Avenir” Program (Grant ANR-10-IAHU-01).
Availability of data and materials
The SNP array data that support the findings of this study have been deposited in the European Genome-Phenome Archive (EGA) with the accession code EGAS00001002460 [27, 29–32] (https://ega-archive.org/studies/EGAS00001002460). Access to individuals’ genotype, biological readouts (such as plasma protein levels), and phenotype data is provided for research use only after review and approval by the Milieu Intérieur data access committee, in line with patient privacy and confidentiality agreements. Requests can be sent to milieuinterieurdac@pasteur.fr.
The 110 summary statistics files corresponding to the 87 proteins with identified pQTLs, containing positional and statistical information for all 5,201,092 evaluated SNPs, are openly available at the GWAS catalog [41] (https://www.ebi.ac.uk/gwas/), through single variant or gene query or through bulk download, with accession numbers ranging from GCST90085705 to GCST90085814.
All other data are available in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
The clinical study was approved by the Comité de Protection des Personnes - Ouest 6 on June 13, 2012 and by the French Agence Nationale de Sécurité du Médicament on June 22, 2012, and has been performed in accordance with the Declaration of Helsinki. The protocol is registered under ClinicalTrials.gov (study# NCT01699893). Informed consent was obtained from participants after the nature and possible consequences of the studies were explained.
Consent for publication
Not applicable.
Competing interests
M.L.A. is an employee of HIBIO, South San Francisco, California CA, 94080. The remaining authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lluis Quintana-Murci, Darragh Duffy and Antonio Rausell contributed equally to this work.
Contributor Information
Darragh Duffy, Email: darragh.duffy@pasteur.fr.
Antonio Rausell, Email: antonio.rausell@institutimagine.org.
the Milieu Intérieur Consortium:
Laurent Abel, Andres Alcover, Hugues Aschard, Philippe Bousso, Nollaig Bourke, Petter Brodin, Pierre Bruhns, Nadine Cerf-Bensussan, Ana Cumano, Caroline Demangel, Christophe d’Enfert, Ludovic Deriano, Marie-Agnès Dillies, James Di Santo, Françoise Dromer, Gérard Eberl, Jost Enninga, Jacques Fellay, Ivo Gomperts-Boneca, Milena Hasan, Magnus Fontes, Gunilla Karlsson Hedestam, Serge Hercberg, Molly A. Ingersoll, Rose Anne Kenny, Olivier Lantz, Frédérique Michel, Hugo Mouquet, Cliona O’Farrelly, Etienne Patin, Sandra Pellegrini, Stanislas Pol, Antonio Rausell, Frédéric Rieux-Laucat, Lars Rogge, Anavaj Sakuntabhai, Olivier Schwartz, Benno Schwikowski, Spencer Shorte, Frédéric Tangy, Antoine Toubert, Mathilde Touvier, Marie-Noëlle Ungeheuer, Christophe Zimmer, Matthew L. Albert, Darragh Duffy, and Lluis Quintana-Murci
References
- 1.Belardelli F. Role of interferons and other cytokines in the regulation of the immune response. APMIS. 1995;103:161–179. doi: 10.1111/j.1699-0463.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 2.Ray S, Patel SK, Kumar V, Damahe J, Srivastava S. Differential expression of serum/plasma proteins in various infectious diseases: Specific or nonspecific signatures. Prot Clin Appl. 2014;8:53–72. doi: 10.1002/prca.201300074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davie EW. Introduction to the blood coagulation cascade and cloning of blood coagulation factors. J Protein Chem. 1986;5:247–253. [Google Scholar]
- 4.Pardridge WM. Plasma protein-mediated transport of steroid and thyroid hormones. Am J Physiol Endocrinol Metab. 1987;252:E157–E164. doi: 10.1152/ajpendo.1987.252.2.E157. [DOI] [PubMed] [Google Scholar]
- 5.Pardridge WM. In: Targeted delivery of hormones to tissues by plasma proteins. in Comprehensive Physiology. Terjung R, editor. Wiley; 2011. p. cp070114. [Google Scholar]
- 6.Qaid MM, Abdelrahman MM. Role of insulin and other related hormones in energy metabolism - A review. Cogent Food Agric. 2016;2.
- 7.Dimou E, Nickel W. Unconventional mechanisms of eukaryotic protein secretion. Curr Biol. 2018;28:R406–R410. doi: 10.1016/j.cub.2017.11.074. [DOI] [PubMed] [Google Scholar]
- 8.Rabouille C. Pathways of unconventional protein secretion. Trends Cell Biol. 2017;27:230–240. doi: 10.1016/j.tcb.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Zhao K-W, Murray EJB, Murray SS. HK2 proximal tubule epithelial cells synthesize and secrete plasma proteins predominantly through the apical surface. J Cell Biochem. 2017;118:924–933. doi: 10.1002/jcb.25786. [DOI] [PubMed] [Google Scholar]
- 10.Geyer PE, Holdt LM, Teupser D, Mann M. Revisiting biomarker discovery by plasma proteomics. Mol Syst Biol. 2017;13:942. doi: 10.15252/msb.20156297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson NL. The clinical plasma proteome: a survey of clinical assays for proteins in plasma and serum. Clin Chem. 2010;56:177–185. doi: 10.1373/clinchem.2009.126706. [DOI] [PubMed] [Google Scholar]
- 12.Heutinck KM, ten Berge IJM, Hack CE, Hamann J, Rowshani AT. Serine proteases of the human immune system in health and disease. Mol Immunol. 2010;47:1943–1955. doi: 10.1016/j.molimm.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Jia L, et al. An attempt to understand kidney’s protein handling function by comparing plasma and urine proteomes. PLoS One. 2009;4:e5146. doi: 10.1371/journal.pone.0005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA approved biomarkers. (http://mrmassaydb.proteincentre.com/fdaassay/).
- 15.Amur S, LaVange L, Zineh I, Buckman-Garner S, Woodcock J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin Pharmacol Ther. 2015;98:34–46. doi: 10.1002/cpt.136. [DOI] [PubMed] [Google Scholar]
- 16.Leth-Larsen R, Lund RR, Ditzel HJ. Plasma membrane proteomics and its application in clinical cancer biomarker discovery. Mol Cell Proteomics. 2010;9:1369–1382. doi: 10.1074/mcp.R900006-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enroth S, et al. High throughput proteomics identifies a high-accuracy 11 plasma protein biomarker signature for ovarian cancer. Commun Biol. 2019;2:221. doi: 10.1038/s42003-019-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rho J, Lampe P. High-throughput analysis of plasma hybrid markers for early detection of cancers. Proteomes. 2014;2:1–17. doi: 10.3390/proteomes2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu AC, et al. Current status and future opportunities in lung precision medicine research with a focus on biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute Research Statement. Am J Respir Crit Care Med. 2018;198:e116–e136. doi: 10.1164/rccm.201810-1895ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goudy K, et al. Human IL2RA null mutation mediates immunodeficiency with lymphoproliferation and autoimmunity. Clin Immunol. 2013;146:248–261. doi: 10.1016/j.clim.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ojha A, Ojha U, Mohammed R, Chandrashekar A, Ojha H. Current perspective on the role of insulin and glucagon in the pathogenesis and treatment of type 2 diabetes mellitus. CPAA. 2019;11:57–65. doi: 10.2147/CPAA.S202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun BB, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558:73–79. doi: 10.1038/s41586-018-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suhre K, et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun. 2017;8:14357. doi: 10.1038/ncomms14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alzheimer’s Disease Neuroimaging Initiative (ADNI) et al. Genetic studies of plasma analytes identify novel potential biomarkers for several complex traits. Sci Rep. 2016;6:18092. doi: 10.1038/srep18092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao C, et al. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat Commun. 2018;9:3268. doi: 10.1038/s41467-018-05512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriya H. Quantitative nature of overexpression experiments. MBoC. 2015;26:3932–3939. doi: 10.1091/mbc.E15-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piasecka B, et al. Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc Natl Acad Sci USA. 2018;115:E488–E497. doi: 10.1073/pnas.1714765115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Astle WJ, et al. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell. 2016;167:1415–1429.e19. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patin E, et al. Natural variation in the parameters of innate immune cells is preferentially driven by genetic factors. Nat Immunol. 2018;19:302–314. doi: 10.1038/s41590-018-0049-7. [DOI] [PubMed] [Google Scholar]
- 30.Thomas S, et al. The Milieu Intérieur study — an integrative approach for study of human immunological variance. Clin Immunol. 2015;157:277–293. doi: 10.1016/j.clim.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 31.For The Milieu Intérieur Consortium et al. Human genetic variants and age are the strongest predictors of humoral immune responses to common pathogens and vaccines. Genome Med. 2018;10:59. doi: 10.1186/s13073-018-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scepanovic P, et al. A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome. 2019;7:130. doi: 10.1186/s40168-019-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duffy D, et al. The ABCs of viral hepatitis that define biomarker signatures of acute viral hepatitis: DUFFY ET AL. Hepatology. 2014;59:1273–1282. doi: 10.1002/hep.26901. [DOI] [PubMed] [Google Scholar]
- 34.Landi F, et al. Body mass index is strongly associated with hypertension: results from the longevity check-up 7+ study. Nutrients. 2018;10:1976. doi: 10.3390/nu10121976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fairley S, Lowy-Gallego E, Perry E, Flicek P. The International Genome Sample Resource (IGSR) collection of open human genomic variation resources. Nucleic Acids Res. 2020;48:D941–D947. doi: 10.1093/nar/gkz836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- 38.Quach H, et al. Genetic adaptation and Neandertal admixture shaped the immune system of human populations. Cell. 2016;167:643–656.e17. doi: 10.1016/j.cell.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuber V, Strimmer K. High-dimensional regression and variable selection using CAR scores. Stat Appl Genet Mol Biol. 2011;10.
- 40.Szklarczyk D, et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buniello A, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–D1012. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng Z, et al. QTLbase: an integrative resource for quantitative trait loci across multiple human molecular phenotypes. Nucleic Acids Res. 2020;48:D983–D991. doi: 10.1093/nar/gkz888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang D, et al. VannoPortal: multiscale functional annotation of human genetic variants for interrogating molecular mechanism of traits and diseases. Nucleic Acids Res. 2021:gkab853. 10.1093/nar/gkab853. [DOI] [PMC free article] [PubMed]
- 44.Zhong W, et al. Whole-genome sequence association analysis of blood proteins in a longitudinal wellness cohort. Genome Med. 2020;12:53. doi: 10.1186/s13073-020-00755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fischer A, Rausell A. Primary immunodeficiencies suggest redundancy within the human immune system. Sci Immunol. 2016;1:eaah5861. doi: 10.1126/sciimmunol.aah5861. [DOI] [PubMed] [Google Scholar]
- 46.Deschamps M, et al. Genomic signatures of selective pressures and introgression from archaic hominins at human innate immunity genes. Am J Hum Genet. 2016;98:5–21. doi: 10.1016/j.ajhg.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rausell A, et al. Common homozygosity for predicted loss-of-function variants reveals both redundant and advantageous effects of dispensable human genes. Proc Natl Acad Sci USA. 2020;117:13626–13636. doi: 10.1073/pnas.1917993117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caron B, Luo Y, Rausell A. NCBoost classifies pathogenic non-coding variants in Mendelian diseases through supervised learning on purifying selection signals in humans. Genome Biol. 2019;20:32. doi: 10.1186/s13059-019-1634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McLaren W, et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinsella RJ, et al. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database. 2011;2011:bar030. doi: 10.1093/database/bar030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehallier B, et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat Med. 2019;25:1843–1850. doi: 10.1038/s41591-019-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bjelosevic S, et al. Quantitative age-specific variability of plasma proteins in healthy neonates, children and adults. Mol Cell Proteomics. 2017;16:924–935. doi: 10.1074/mcp.M116.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Enroth S, Johansson Å, Enroth SB, Gyllensten U. Strong effects of genetic and lifestyle factors on biomarker variation and use of personalized cutoffs. Nat Commun. 2014;5:4684. doi: 10.1038/ncomms5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doerstling S, Hedberg P, Öhrvik J, Leppert J, Henriksen E. Growth differentiation factor 15 in a community-based sample: age-dependent reference limits and prognostic impact. Upsala J Med Sci. 2018;123:86–93. doi: 10.1080/03009734.2018.1460427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manicourt D-H, Fujimoto N, Obata K, Thonar EJ-MA. Serum levels of collagenase, stromelysin-1, and timp-1. Arthritis Rheum. 1994;37:1774–1783. doi: 10.1002/art.1780371211. [DOI] [PubMed] [Google Scholar]
- 57.Svechnikov K, Söder O. Ontogeny of gonadal sex steroids. Best Pract Res Clin Endocrinol Metab. 2008;22:95–106. doi: 10.1016/j.beem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Kontush A, et al. In: Structure of HDL: particle subclasses and molecular components. in High Density Lipoproteins. von Eckardstein A, Kardassis D, et al., editors. Springer International Publishing; 2015. pp. 3–51. [DOI] [PubMed] [Google Scholar]
- 59.Zewinger S, et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat Immunol. 2020;21:30–41. doi: 10.1038/s41590-019-0548-1. [DOI] [PubMed] [Google Scholar]
- 60.Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes. 2013;37:333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 61.Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 62.The UK10K Consortium The UK10K project identifies rare variants in health and disease. Nature. 2015;526:82–90. doi: 10.1038/nature14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan Z, et al. Primate transcript and protein expression levels evolve under compensatory selection pressures. Science. 2013;342:1100–1104. doi: 10.1126/science.1242379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emilsson V, et al. Co-regulatory networks of human serum proteins link genetics to disease. Science. 2018;361:769–773. doi: 10.1126/science.aaq1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shih S-J, et al. Duplicated downstream enhancers control expression of the human apolipoprotein E gene in macrophages and adipose tissue. J Biol Chem. 2000;275:31567–31572. doi: 10.1074/jbc.M005468200. [DOI] [PubMed] [Google Scholar]
- 66.LifeLines Cohort Study et al. Single-cell RNA sequencing identifies celltype-specific cis-eQTLs and co-expression QTLs. Nat Genet. 2018;50:493–497. doi: 10.1038/s41588-018-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillies CE, et al. An eQTL Landscape of Kidney Tissue in Human Nephrotic Syndrome. Am J Hum Genet. 2018;103:232–244. doi: 10.1016/j.ajhg.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gass J, et al. Progranulin regulates neuronal outgrowth independent of Sortilin. Mol Neurodegeneration. 2012;7:33. doi: 10.1186/1750-1326-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prudencio M, et al. Misregulation of human sortilin splicing leads to the generation of a nonfunctional progranulin receptor. Proc Natl Acad Sci U S A. 2012;109:21510–21515. doi: 10.1073/pnas.1211577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jian J, et al. Progranulin directly binds to the CRD2 and CRD3 of TNFR extracellular domains. FEBS Lett. 2013;587:3428–3436. doi: 10.1016/j.febslet.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rhost S, et al. Sortilin inhibition limits secretion-induced progranulin-dependent breast cancer progression and cancer stem cell expansion. Breast Cancer Res. 2018;20:137. doi: 10.1186/s13058-018-1060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee WC, et al. Targeted manipulation of the sortilin–progranulin axis rescues progranulin haploinsufficiency. Hum Mol Genet. 2014;23:1467–1478. doi: 10.1093/hmg/ddt534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nichols WL, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14:171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 74.Bonnefoy A, et al. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107:955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel L, et al. Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochem Biophys Res Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 76.Buckley AR. Prolactin, a lymphocyte growth and survival factor. Lupus. 2001;10:684–690. doi: 10.1191/096120301717164912. [DOI] [PubMed] [Google Scholar]
- 77.Montgomery DW. Prolactin production by immune cells. Lupus. 2001;10:665–675. doi: 10.1191/096120301717164895. [DOI] [PubMed] [Google Scholar]
- 78.Katakura T, Miyazaki M, Kobayashi M, Herndon DN, Suzuki F. CCL17 and IL-10 as effectors that enable alternatively activated macrophages to inhibit the generation of classically activated macrophages. J Immunol. 2004;172:1407–1413. doi: 10.4049/jimmunol.172.3.1407. [DOI] [PubMed] [Google Scholar]
- 79.Achuthan A, et al. Granulocyte macrophage colony-stimulating factor induces CCL17 production via IRF4 to mediate inflammation. J Clin Investig. 2016;126:3453–3466. doi: 10.1172/JCI87828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hosomi S, et al. CEACAM1 on activated NK cells inhibits NKG2D-mediated cytolytic function and signaling: Innate immunity. Eur J Immunol. 2013;43:2473–2483. doi: 10.1002/eji.201242676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarantis H, Gray-Owen SD. Defining the roles of human carcinoembryonic antigen-related cellular adhesion molecules during neutrophil responses to Neisseria gonorrhoeae. Infect Immun. 2012;80:345–358. doi: 10.1128/IAI.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kolla V, et al. Carcinoembryonic cell adhesion molecule 6 in human lung: regulated expression of a multifunctional type II cell protein. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1019–L1030. doi: 10.1152/ajplung.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raffield LM, et al. Comparison of proteomic assessment methods in multiple cohort studies. Proteomics. 2020;20:1900278. doi: 10.1002/pmic.201900278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischer A, Rausell A. What do primary immunodeficiencies tell us about the essentiality/redundancy of immune responses? Semin Immunol. 2018;36:13–16. doi: 10.1016/j.smim.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 85.Casanova J-L, Abel L. Human genetics of infectious diseases: unique insights into immunological redundancy. Semin Immunol. 2018;36:1–12. doi: 10.1016/j.smim.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munthe-Fog L, et al. Immunodeficiency associated with FCN3 mutation and Ficolin-3 deficiency. N Engl J Med. 2009;360:2637–2644. doi: 10.1056/NEJMoa0900381. [DOI] [PubMed] [Google Scholar]
- 87.Michalski M, et al. H-ficolin (ficolin-3) concentrations and FCN3 gene polymorphism in neonates. Immunobiology. 2012;217:730–737. doi: 10.1016/j.imbio.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Michalski M, et al. Primary Ficolin-3 deficiency – is it associated with increased susceptibility to infections? Immunobiology. 2015;220:711–713. doi: 10.1016/j.imbio.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Endo Y, Matsushita M, Fujita T. Role of ficolin in innate immunity and its molecular basis. Immunobiology. 2007;212:371–379. doi: 10.1016/j.imbio.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 90.Schlapbach LJ, et al. Congenital H-ficolin deficiency in premature infants with severe necrotising enterocolitis. Gut. 2011;60:1438–1439. doi: 10.1136/gut.2010.226027. [DOI] [PubMed] [Google Scholar]
- 91.Hein E, et al. Functional analysis of ficolin-3 mediated complement activation. PLoS One. 2010;5:e15443. doi: 10.1371/journal.pone.0015443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barkai LJ, et al. Decreased ficolin-3-mediated complement lectin pathway activation and alternative pathway amplification during bacterial infections in patients with type 2 diabetes mellitus. Front Immunol. 2019;10:509. doi: 10.3389/fimmu.2019.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamada A, Arakaki R, Saito M, Kudo Y, Ishimaru N. Dual role of Fas/FasL-mediated signal in peripheral immune tolerance. Front Immunol. 2017;8. [DOI] [PMC free article] [PubMed]
- 94.Rieux-Laucat F, Magérus-Chatinet A, Neven B. The autoimmune lymphoproliferative syndrome with defective FAS or FAS-ligand functions. J Clin Immunol. 2018;38:558–568. doi: 10.1007/s10875-018-0523-x. [DOI] [PubMed] [Google Scholar]
- 95.Magerus-Chatinet A, et al. FAS-L, IL-10, and double-negative CD4−CD8− TCR α/β+ T cells are reliable markers of autoimmune lymphoproliferative syndrome (ALPS) associated with FAS loss of function. Blood. 2009;113:3027–3030. doi: 10.1182/blood-2008-09-179630. [DOI] [PubMed] [Google Scholar]
- 96.Magerus-Chatinet A, et al. Onset of autoimmune lymphoproliferative syndrome (ALPS) in humans as a consequence of genetic defect accumulation. J Clin Invest. 2011;121:106–112. doi: 10.1172/JCI43752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuehn HS, et al. FAS haploinsufficiency is a common disease mechanism in the human autoimmune lymphoproliferative syndrome. J.I. 2011;186:6035–6043. doi: 10.4049/jimmunol.1100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Le Deist F, et al. Clinical, immunological, and pathological consequences of Fas-deficient conditions. Lancet. 1996;348:719–723. doi: 10.1016/S0140-6736(96)02293-3. [DOI] [PubMed] [Google Scholar]
- 99.de Vries PS, et al. Whole-genome sequencing study of serum peptide levels: the atherosclerosis risk in communities study. Hum Mol Genet. 2017;26:3442–3450. doi: 10.1093/hmg/ddx266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ruffieux H, et al. A fully joint Bayesian quantitative trait locus mapping of human protein abundance in plasma; 2019. http://biorxiv.org/lookup/doi/10.1101/524405. https://doi.org/10.1101/524405. [DOI] [PMC free article] [PubMed]
- 101.Qasim AN, Reilly MP. Emery and Rimoin’s Principles and Practice of Medical Genetics. Elsevier; 2013. Genetics of atherosclerotic cardiovascular disease; pp. 1–37. [Google Scholar]
- 102.Suhre K, McCarthy MI, Schwenk JM. Genetics meets proteomics: perspectives for large population-based studies. Nat Rev Genet. 2021;22:19–37. doi: 10.1038/s41576-020-0268-2. [DOI] [PubMed] [Google Scholar]
- 103.Acevedo JM, Hoermann B, Schlimbach T, Teleman AA. Changes in global translation elongation or initiation rates shape the proteome via the Kozak sequence. Sci Rep. 2018;8:4018. doi: 10.1038/s41598-018-22330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hanson G, Coller J. Codon optimality, bias and usage in translation and mRNA decay. Nat Rev Mol Cell Biol. 2018;19:20–30. doi: 10.1038/nrm.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Collart MA, Weiss B. Ribosome pausing, a dangerous necessity for co-translational events. Nucleic Acids Res. 2020;48:1043–1055. doi: 10.1093/nar/gkz763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nedialkova DD, Leidel SA. Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell. 2015;161:1606–1618. doi: 10.1016/j.cell.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nachtergaele S, He C. The emerging biology of RNA post-transcriptional modifications. RNA Biol. 2017;14:156–163. doi: 10.1080/15476286.2016.1267096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. List of the 297 plasma proteins assayed.
Additional file 2: Figure S1. Principal component analysis of age and sex effects. Figure S2. Manhattan plots and allelic expression of levels of cis-pQTLs. Figure S3. Manhattan plots and allelic expression of levels of trans-pQTLs. Figure S4. Impact of blood-cell fractions on associated protein-SNP pairs statistics.
Additional file 3: Table S2. Proteins with analysis-specific significant genetic association.
Additional file 4: Table S3. Summary of previous plasma pQTL study design.
Additional file 5: Table S4. Description of the association between plasma protein levels, age, sex and blood-cell fractions.
Additional file 6: Table S5. Description of the significant protein-environmental variable pairs and variable contribution the variability of the protein expression levels.
Additional file 7: Table S6. Annotations of cis and trans pQTLs.
Data Availability Statement
The SNP array data that support the findings of this study have been deposited in the European Genome-Phenome Archive (EGA) with the accession code EGAS00001002460 [27, 29–32] (https://ega-archive.org/studies/EGAS00001002460). Access to individuals’ genotype, biological readouts (such as plasma protein levels), and phenotype data is provided for research use only after review and approval by the Milieu Intérieur data access committee, in line with patient privacy and confidentiality agreements. Requests can be sent to milieuinterieurdac@pasteur.fr.
The 110 summary statistics files corresponding to the 87 proteins with identified pQTLs, containing positional and statistical information for all 5,201,092 evaluated SNPs, are openly available at the GWAS catalog [41] (https://www.ebi.ac.uk/gwas/), through single variant or gene query or through bulk download, with accession numbers ranging from GCST90085705 to GCST90085814.
All other data are available in this published article and its supplementary information files.