Abstract
The mechanism of radiolabeled levofloxacin ([3H]levofloxacin) uptake by human polymorphonuclear neutrophils (PMNs) was investigated by a classical velocity centrifugation technique. PMNs were incubated with levofloxacin for 5 to 180 min under various conditions before centrifugation through an oil cushion. Radioactivity was measured in the cell pellet to determine the amount of cell-associated drug. The uptake of levofloxacin was moderate with a cellular concentration/extracellular concentration ratio of about 4 to 6. Levofloxacin accumulated in PMNs parallel to the extracellular concentration, without saturation, over the range of 2.5 to 200 mg/liter (linear regression analysis: r = 0.92; P < 0.001). The activation energy was low (36 ± 7.2 kJ/mol). Levofloxacin uptake was increased in Ca2+-depleted, EGTA-containing medium by approximately 33% (P = 0.022), while Ni2+, a Ca2+ channel inhibitor, inhibited it in a concentration-dependent manner, with the concentration that inhibited 50% of control uptake being approximately 2.65 mM. Verapamil (an l-type Ca2+ channel inhibitor) and other pharmacologic agents which modify Ca2+ homeostasis did not modify levofloxacin uptake. Interestingly, Ca2+ and Mg2+ inhibited levofloxacin uptake in a concentration-dependent manner. EGTA, Ni2+, and verapamil did not modify levofloxacin efflux; thapsigargin, a Ca2+ pool-releasing agent, modestly increased the intracellular retention of levofloxacin. In addition, contrary to other fluoroquinolones, probenecid at 1 to 10 mM did not modify either levofloxacin uptake or efflux. These data are consistent with a mechanism of passive accumulation of levofloxacin in PMNs. Extracellular Ca2+ and Mg2+ may influence the structural conformation of levofloxacin or the lipophilicity of PMN membranes, thus explaining their effect on levofloxacin uptake.
Antimicrobial agents that accumulate and remain active inside phagocytic cells are particularly useful for the treatment of infections caused by intracellular pathogens. Among those antimicrobial drugs which enter host cells, fluoroquinolones are widely acknowledged to display intracellular bioactivity against bacteria which reside and/or multiply within phagocytes (e.g., Staphylococcus aureus, Legionella pneumophila, mycobacteria, chlamydiae) (9, 10, 15, 22, 23, 30, 33).
There exists abundant literature on the intraphagocytic accumulation of fluoroquinolones in vitro (5, 14, 25–27). The intracellular accumulation of fluoroquinolones has also been recognized ex vivo (11). Levofloxacin, the levogyre isomer of d-ofloxacin, is a new fluoroquinolone (7) which demonstrates good intracellular activity against intracellular pathogens, including Chlamydia spp. (9, 23). The intraphagocytic uptake of levofloxacin has been studied previously by the fluorometric method (13, 28). In these studies, the drug was shown to be concentrated six- to eightfold within human neutrophils and to be active against phagocytized S. aureus. The mechanism of its uptake was not investigated. Since controversial data exist in support of an active process for fluoroquinolone uptake, we investigated, by a radioisotopic technique, whether levofloxacin accumulation is an active or a passive process.
(This work was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 28 September to 1 October 1997 [35], and at the 20th International Congress of Chemotherapy, Sydney, Australia, 29 June to 3 July 1997 [36].)
MATERIALS AND METHODS
Materials.
Radiolabeled levofloxacin, [3H]levofloxacin (2.45 TBq/mmol, 19 MBq/ml in ethanol-toluene [2/3; vol/vol]), was provided by Tokai Research Laboratory (Tokyo, Japan). Levofloxacin hemihydrate was supplied by Hoechst Marion Roussel (Romainville, France). The levofloxacin solution used to measure drug uptake was prepared as follows: 5 μl of [3H]levofloxacin (approximately 0.09 ng/μl) was mixed with 220 μl of Hanks balanced salt solution (HBSS; Diagnostic Pasteur, Paris, France) and 25 μl of unlabeled levofloxacin (1,000 mg/liter in HBSS). This standard solution (100 mg/liter) was further diluted to the desired final concentrations. By a similar method, standard solutions of 1,000 mg/liter were prepared to assess final concentrations of 100 and 200 mg/liter.
Nickel chloride (Ni2+), verapamil hydrochloride, probenecid [p-(dipropylsulfamoyl)benzoic acid], phorbol myristate acetate (PMA), staurosporine (star), and H7 were from Sigma; EGTA was from Merck; HBSS was from Diagnostic Pasteur; and theoretically Ca2+-deprived HBSS was from Gibco. This medium was supplemented with 1 mM magnesium chloride (Mg2+; Merck) and 1.2 mM sodium bicarbonate (Diagnostic Pasteur). Thapsigargin, ionomycin, m-cyclopiazonic acid, A23187, chlorpromazin, and W7 were from Calbiochem.
Human neutrophils.
Polymorphonuclear leukocytes (PMNs) were obtained from the venous blood of healthy volunteers by 2% dextran T-500 sedimentation followed by Ficoll-Paque centrifugation and osmotic lysis of residual erythrocytes. The viability and purity of the PMN preparation, as assessed by trypan blue exclusion, were greater than 96%.
Levofloxacin uptake.
A radiometric method was used to measure macrolide uptake (21). Briefly, 2.5 × 106 PMNs were incubated at 37°C with the radiolabeled drug (10 mg/liter under standard conditions) and were then centrifuged at 12,000 × g for 3 min at 22°C through a water-impermeable silicone-paraffin (86 and 14% [vol/vol], respectively) oil barrier. The pellet was solubilized in Hionic fluor (Packard), and the cell-associated radioactivity was quantified by liquid scintillation counting (LS-6000-S; Beckman). Standard dilution curves were used to determine the amount of cell-associated drug. The results were expressed as nanograms per 2.5 × 106 PMNs. A previously determined intracellular volume of 0.6 μl/2.5 × 106 PMNs (21) was used to determine the cellular concentration/extracellular concentration ratio (C/E). We verified that the various experimental conditions used here (pH, inhibitors, temperature) did not significantly modify this value.
Characteristics of levofloxacin uptake.
We first analyzed the kinetics of levofloxacin (2.5 and 10 mg/liter) uptake over a 3-h incubation period. The influences of extracellular pH, temperature, and extracellular concentrations (1.25 to 200 mg/liter) were assessed after incubation for 5 min.
Cellular location.
Levofloxacin-loaded PMNs (10 mg/liter; 15 min at 37°C) were centrifuged through the silicone-paraffin oil barrier, and the cell pellet was sonicated in the presence of 0.5% Triton X-100 (three 15-s bursts) or 0.73 M sucrose (three 5-s bursts) to protect the granules (20). After centrifugation (100,000 × g for 30 min) the amounts of marker enzymes, lactate dehydrogenase (LDH; a cytosolic marker), β-glucuronidase (a marker of azurophilic granules), and lysozyme (a marker of both azurophilic and specific granules), together with the amounts of radiolabeled levofloxacin, were determined in the pellet and the supernatant. The results were expressed as the percentage of the pellet-associated enzyme activity or radioactivity over the sum (that in the pellet plus that in the supernatant). This sum did not significantly differ from the total activity measured in a control sample of cells similarly loaded, centrifuged, and sonicated in the presence of Triton X-100 but not ultracentrifuged before enzyme activity and radioactivity determination.
Levofloxacin efflux.
Aliquots of levofloxacin-loaded PMNs were centrifuged over the silicone-paraffin oil barrier. One aliquot was used to quantify the amount of cell-associated drug (total associated drug). The other cell pellets were placed in drug-free HBSS, and at various time intervals, they were again centrifuged through the oil barrier; the radioactivity in the cell pellet and the supernatant was then measured. The sum of the radioactivity (that in the cell pellet plus that in the supernatant) did not significantly differ from the total load. Efflux of levofloxacin was expressed as the percentage of drug remaining associated with the cell pellet compared to the sum of the radioactivity (that in the pellet plus that in the supernatant).
Influence of Ca2+ and Ca2+ homeostasis in PMNs on levofloxacin uptake and efflux.
Levofloxacin uptake was measured at 5 and 15 min either in the presence of control HBSS or in theoretically Ca2+-depleted HBSS (Gibco) supplemented with 1 mM EGTA. The influence of Ni2+, an inhibitor of the Na+-Ca2+ exchanges in PMNs (20), on the uptake of levofloxacin was assessed at 5 and 15 min. The effect of 125 μM verapamil hydrochloride, an inhibitor of the l-type calcium channel, on levofloxacin uptake was also assessed after 5 and 15 min of incubation. PMNs were also pretreated for 10 min with various agents known to alter the Ca2+ homeostasis of PMNs (thapsigargin [200 nM] and cyclopiazonic acid [30 μM], which release Ca2+ from intracellular pools; ionomycin [1 μM and 100 nM] and A23187 [10−7 M], two calcium ionophores; and W7 [30 μM] and chlorpromazine [20 μM], two calmodulin antagonists) before assessment of levofloxacin uptake at 10 and 30 min. In addition, levofloxacin-loaded PMNs (15 min, 10 mg/liter) were incubated in drug-free HBSS supplemented with the various Ca2+-modifying agents before assessment of levofloxacin efflux.
Effect of probenecid on levofloxacin uptake and efflux.
PMNs were pretreated for 10 min with probenecid (1 to 10 mM) before assessment of levofloxacin uptake at 5 and 15 min. Also, levofloxacin-loaded PMNs were separated from the extracellular medium as described above and further incubated in drug-free medium supplemented with probenecid (2 mM). The percentage of drug-associated levofloxacin was measured at 10 min as described above. Since probenecid hydrochloride solution is extremely acidic, the solution was buffered with NaOH and then diluted in HBSS before use to exclude any effect due to pH on drug uptake and efflux.
Effect of PMA on levofloxacin uptake.
PMNs were pretreated for 10 or 30 min with the protein kinase C activator PMA (100, 10 ng/ml) or with two protein kinase inhibitors, H7 (200 μM) and staurosporine (star, 2 μM), or their combination, before adding [3H]levofloxacin solution; levofloxacin uptake was then measured after 10 and 30 min of incubation.
Statistical analysis.
Results are expressed as means ± standard errors of the means (SEMs) of n experiments conducted with PMNs from different volunteers. Analysis of variance (ANOVA), regression analysis, and Student’s t test for paired data were used to determine statistical significance. All tests were performed with the Statworks program, version 1.2, of Cricket software (1985).
RESULTS
Kinetics of levofloxacin uptake.
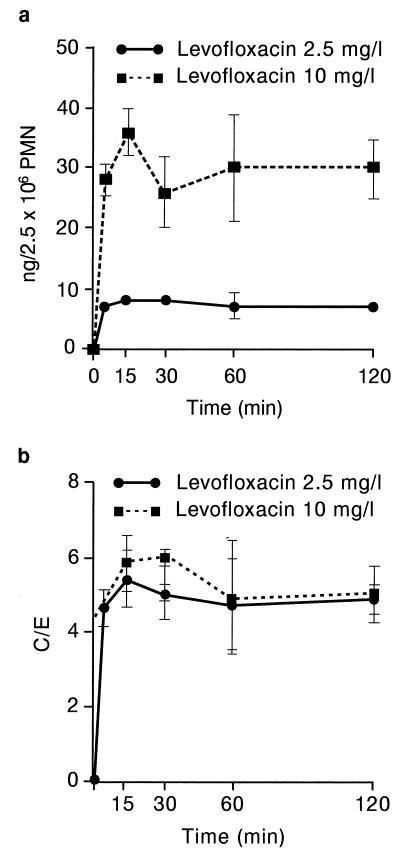
The uptake of levofloxacin was rapid (Fig. 1), reaching a maximum value as early as 5 min with either concentration (2.5 or 10 mg/liter). A prolonged incubation period did not result in increased accumulation; on the contrary, accumulation seemed to decrease, although the results did not reach statistical significance. The amount of levofloxacin was greater at an extracellular concentration of 10 mg/liter compared with that obtained with an extracellular concentration of 2.5 mg/liter (Fig. 1a). However, the C/E was in the same range for both concentrations (4.9 ± 0.32 [2.5 mg/liter] and 4.6 ± 0.44 [10 mg/liter] at 5 min [n = seven experiments]; 4 ± 0.05 [2.5 mg/liter] and 3.5 ± 0.01 [10 mg/liter] at 180 min [n = 3 experiments]). Contrary to what we previously observed with various macrolides (22, 36), there was no strong interindividual variability for levofloxacin uptake (C/E of 4.0 to 5.6 at 5 min for seven different PMN samples; C/E of 4.0 to 4.5 at 180 min for three different PMN samples).
FIG. 1.
Kinetics of levofloxacin (LVFX) uptake (mean ± SEM of three to seven experiments). (a) Cellular accumulation of levofloxacin. (b) C/E.
Influence of extracellular concentrations.
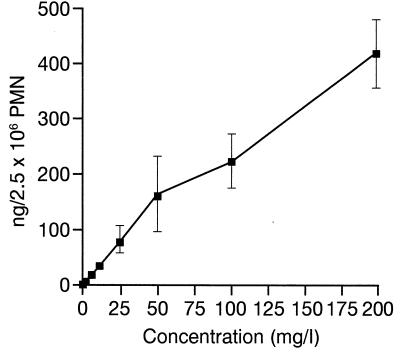
The cellular accumulation of levofloxacin increased parallel to the extracellular concentration over a wide range of concentrations (regression analysis: P < 0.001; r = 0.923; slope = 2.09) and was not saturable up to an extracellular concentration of 200 mg/liter (Fig. 2).
FIG. 2.
Effect of extracellular concentrations on levofloxacin uptake at 5 min (mean ± SEM of 4 to 12 experiments).
Effect of pH and temperature.
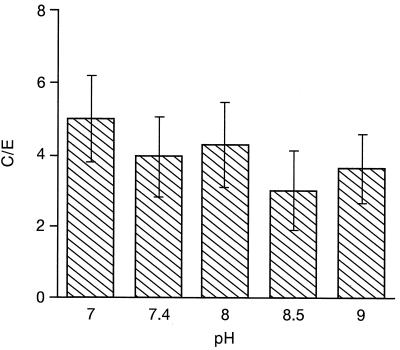
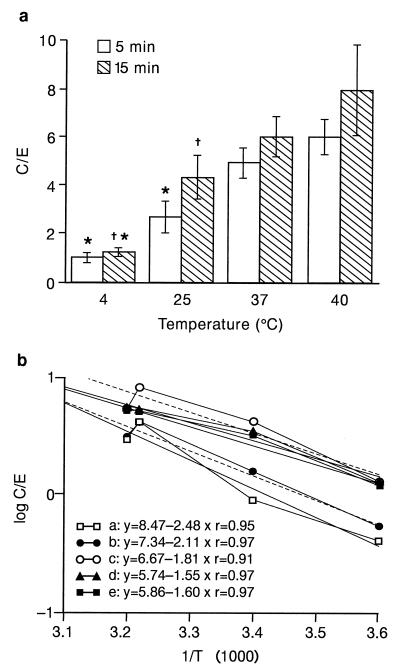
Levofloxacin uptake was not modified over the pH range of 7 to 9 (Fig. 3) (ANOVA, 0.861). Levofloxacin uptake was dependent on the temperature (Fig. 4). At 4°C, levofloxacin uptake was low (Fig. 4a) (C/E, 0.95 ± 0.20 at 5 min [n = 5 experiments]; 1.20 ± 0.17 at 15 min [n = 6 experiments] [P = 0.048 versus that at 5 min; ANOVA followed by Student’s t test]). At 25°C, levofloxacin uptake was greater than that observed at 4°C (P = 0.015 at 5 min and P = 0.009 at 15 min), and uptake was significantly greater at 15 min (4.3 ± 0.89 [n = 6 experiments] P = 0.048 versus that at 5 min]; 2.7 ± 0.62 [n = 5 experiments]). At 37°C, as indicated above, the C/Es were not different at 5 and 15 min but were significantly higher than those obtained at 4°C (4.6 ± 0.60 at 5 min; 5.7 ± 0.80 at 15 min [P < 0.001]). The C/E was also significantly higher compared to that at 25°C at 5 min (P < 0.001) but not at 15 min (P = 0.10). At 40°C, the C/Es were not significantly different from those obtained at 37°C (5.7 ± 0.7 at 5 min; 7.6 ± 1.8 at 15 min).
FIG. 3.
Effect of extracellular pH on levofloxacin uptake at 5 min (mean ± SEM of four experiments).
FIG. 4.
Effect of temperature on levofloxacin uptake. (a) Mean C/E at 5 and 15 min (mean ± SEM of five to six experiments). ∗, P < 0.05 versus that at 37°C; †, P < 0.05 versus that at 5 min of incubation. (b) Van’t Hoff plot representation of the Arrhenius equation::ΔG = −RT ln (Keq). a to e, five different experiments at temperatures of 4, 25, 37, and 40°C. Full lines, experimental values; broken lines, regression lines. See Results section for details of the calculation.
The activation energy was calculated by measuring the uptake of levofloxacin by PMNs incubated for 5 min at 4, 25, 37, and 40°C, as described previously (20), by using the Arrhenius equation: ΔG = −RT ln Keq, where ΔG is the activation energy (in calories per mole), T is the temperature (in degrees Kelvin), R is a constant (equal to 1.98), and ln Keq is the Napierian logarithm of the C/E at 5 min (when the uptake rate is maximal). ΔG can be obtained from the slope of the curve by using the Van’t Hoff plot representation of data: ln Keq = −ΔG/RT (Fig. 4b). The levofloxacin activation energy was low (36 ± 3.2 kJ/mol; range, 30 to 47 kJ/mol; r = 0.909 to 0.973 [n = 5 experiments]).
Cellular location.
The intracellular location of levofloxacin was studied after 15 min of incubation. In the presence of 0.5% Triton X-100, sonication resulted in the breakage of all cytoplasmic and granular membranes, as indicated by the release of more than 98% of lysozyme, β-glucuronidase, and LDH in the supernatant. Pellet (granule and cytoplasmic membrane)-associated levofloxacin was about 6.4% ± 0.61% of the total amount of cell-associated drug (n = 3 experiments). In the pellet obtained in the presence of 0.73 M sucrose (which contained preserved granules and cytoplasmic membranes, as indicated by the presence of about 96% β-glucuronidase and lysozyme and less than 5% LDH), the amount of levofloxacin was moderate, about 20.7% ± 5.55% of the total amount of cell-associated drug. These data suggest that levofloxacin is located mainly in the cytoplasms of PMNs.
Levofloxacin efflux.
Levofloxacin rapidly egressed from drug-loaded PMNs placed into drug-free medium. At 5 min, about 94% of the total load was recovered from the supernatant (cell-associated levofloxacin, 6.2% ± 1.6% of the total amount [n = 3 experiments]). After 15 and 30 min of incubation, levofloxacin was almost totally released from the cells: the cell-associated amounts of levofloxacin were 1.3% ± 0.5% and 1.4% ± 0.8% of total load at 15 and 30 min of incubation, respectively (n = 3 experiments).
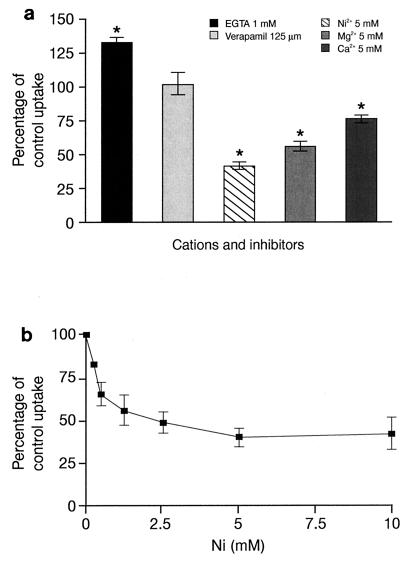
Effect of Ca2+ chelators and Ca2+ channel inhibitors on levofloxacin uptake.
We have previously reported that extracellular calcium is a cation important for macrolide uptake (20, 34). Therefore, we investigated whether this cation is also involved in the accumulation of levofloxacin by PMNs. In Ca2+-deprived medium (Ca2+-free HBSS plus 1 mM EGTA) levofloxacin uptake was significantly increased at 5 min (133% ± 7.5% of control uptake) (Fig. 5a), but this effect was not detected after 15 min of incubation (levofloxacin uptake, 104% ± 3.03% of control uptake). When the Ca2+-deprived medium was supplemented with increasing concentrations of either Ca2+ (1 to 10 mM) or Mg2+ (1 to 5 mM), levofloxacin uptake was progressively impaired (Fig. 5a). By regression analysis, the concentration of Ca2+ which inhibited 50% of the control levofloxacin uptake (IC50) was 15.2 mM (P < 0.001; r = 0.857; slope = −3.4), and the IC50 of Mg2+ was 8.8 mM (P < 0.001; r = 0.904; slope = −5.8). Ni2+, a blocker of the Na+-Ca2+ exchanger, also inhibited levofloxacin uptake at 5 min in a concentration-dependent manner (Fig. 5a and b). By logarithmic regression analysis, the IC50 of Ni2+ was determined to be 2.65 mM (P = 0.001; r = 0.637; slope = −23.3). After 15 min of incubation the effect of Ni2+ was less impressive, with an IC50 of 4.97 mM (linear regression analysis: P = 0.011; r = 0.759; slope = −7.95). The l-type Ca2+ channel inhibitor verapamil (125 μM) did not modify levofloxacin uptake over a 30-min incubation period (102% ± 16.0%, 83% ± 6.0%, and 91% ± 13.6% of control uptake at 5, 15, and 30 min, respectively).
FIG. 5.
Effects of Ca2+, Mg2+, EGTA, and Ca2+ channel inhibitors on levofloxacin uptake. (a) Effect of cations and inhibitors on levofloxacin uptake at 5 min (mean ± SEM of three to six experiments). ∗, P < 0.05. (b) Concentration-dependent effect of Ni2+ on levofloxacin uptake at 5 min (mean ± SEM of five to six experiments).
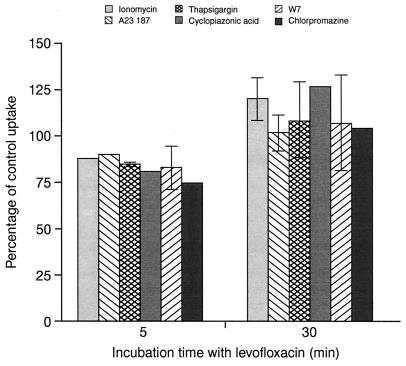
We further assessed whether other agents known to alter Ca2+ homeostasis of PMNs interfered with drug uptake (Fig. 6). Neither Ca2+ ionophores, Ca2+ storage release-inducing agents, nor calmodulin antagonists modified the levels of levofloxacin accumulation at 5 and 30 min.
FIG. 6.
Effect of pharmacological agents which alter Ca2+ homeostasis in PMNs. PMNs were pretreated for 10 min with the following agents: ionomycin at 1 μM, A23187 at 10−7 M, thapsigargin at 200 nM, cyclopiazonic acid at 30 μM, W7 at 30 μM, and chlorpromazine at 20 μM. Levofloxacin was then added and uptake was measured at 5 or 30 min (mean ± SEM for three experiments or mean only for two experiments).
We also assessed the effects of EGTA and Ni2+ on levofloxacin efflux. Levofloxacin-loaded PMNs were separated from the extracellular medium by centrifugation and were placed into drug-free HBSS (control), drug-free Ca2+-deprived and 1 mM EGTA-supplemented HBSS (Ca2+ chelation), or drug-free HBSS containing 5 mM Ni2+. Efflux was measured, as described in the Materials and Methods section, at 5, 15, and 30 min. The percentage of cell-associated levofloxacin was similar in all three efflux media: 6.2% ± 1.60%, 7.2% ± 2.17%, and 7.2% ± 0.80% at 5 min; 1.3% ± 0.50%, 1.2% ± 0.25%, and 2.8% ± 1.4% at 15 min; and 1.4% ± 0.80%, 1.4% ± 0.91%, and 1.6% ± 0.0% at 30 min for control, Ca2+-deprived, and Ni2+-containing media, respectively (n = 3 experiments). We further assessed the influence of agents which increase the intracellular Ca2+ concentration (ionomycin at 100 nM and 1 μM, A23187 at 10−5 and 10−6 M, and thapsigargin at 200 nM) on levofloxacin efflux at 5 min to avoid the consequence of a Ca2+ concentration increase on PMN degranulation. Only thapsigargin, but to a lesser extent ionomycin at 100 nM, modestly but nonsignificantly increased the level of levofloxacin retention in PMNs (12% ± 0.5% and 10% ± 1.0% for thapsigargin and ionomycin, respectively).
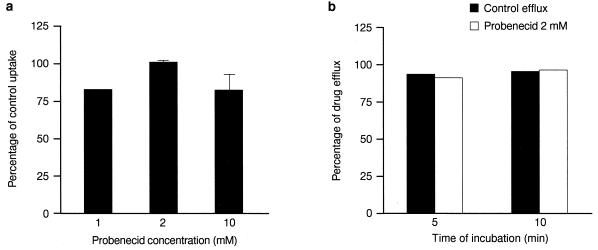
Effect of probenecid on levofloxacin uptake and efflux.
Various investigators have suggested that fluoroquinolone (e.g., ciprofloxacin) efflux is mediated by an organic anion transporter which is inhibitable by probenecid (4, 31). We therefore investigated whether probenecid modifies levofloxacin accumulation by PMNs. In contrast to the data reported from experiments with ciprofloxacin, probenecid (up to 10 mM) did not increase levofloxacin uptake (Fig. 7a). Probenecid (2 mM) also did not modify the efflux of this drug (Fig. 7b).
FIG. 7.
Effect of probenecid on levofloxacin uptake and efflux. (a) Effect of probenecid on uptake. PMNs were pretreated with probenecid at 1 to 10 mM for 30 min before the addition of levofloxacin for 5 min (mean ± SEM of three experiments or mean only for two experiments). (b) Effect of probenecid on levofloxacin efflux. Levofloxacin-loaded PMNs were placed into drug-free medium containing probenecid at 2 mM. At 5 and 10 min, samples were centrifuged, as described in the Materials and Methods section, and the percentage of the total load of levofloxacin released in the supernatant was measured (mean of two experiments).
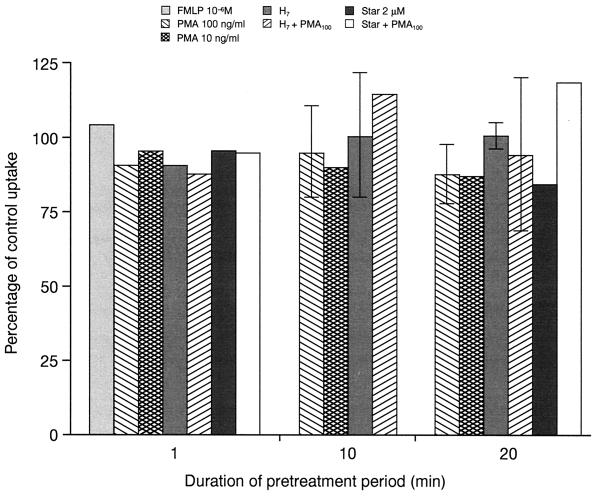
Effect of protein kinase activators and inhibitors on levofloxacin uptake.
Recently, some investigators have reported that ciprofloxacin uptake is mediated by an active transport which is strongly enhanced by protein kinase C activation (17). PMNs were pretreated for 1, 10, or 20 min with PMA at 100 and 10 ng/ml or control HBSS before incubation with levofloxacin at 10 mg/liter. Levofloxacin uptake was measured after 10 min of incubation. H7 (200 μM) or staurosporine (2 μM) was added 10 min before or during PMA activation of PMNs. Activation of PMNs was also induced with a 1-min pretreatment of the cells with formyl-methionyl-leucyl-phenylalanine (FMLP) at 10−6 M. None of the treatments modified levofloxacin uptake at 10 min (Fig. 8) or at 30 and 60 min (data not shown).
FIG. 8.
Effect of protein kinase activators and inhibitors on levofloxacin uptake. PMNs were pretreated for the indicated period of time (1 to 20 min) with the following agents: FMLP at 10−6 M, PMA at 100 ng/ml, PMA at 10 ng/ml, H7 at 200 μM, H7 plus PMA at 100 ng/ml, staurosporine (Star) at 2 μM, and staurosporine plus PMA at 100 ng/ml. Levofloxacin was then added for 5 min and uptake was measured as described in the Materials and Methods section (mean ± SEM for three experiments or mean only for two experiments).
DISCUSSION
Since the first quinolone derivative (nalidixic acid) synthesis in 1962, there has been an upsurge of interest in this class of drug. After the apparently fortuitous discovery that the combination of a 7-piperazinyl ring and a 6-fluorine atom on the quinoline or 1,8-naphthyridine nucleus dramatically enhanced the antibacterial activities of the so-called fluoroquinolones, rapid progress has been made in 4-quinolone research, with more than 7,000 analogues documented in the literature, and research is ongoing (2, 3).
Among the potential targets of these drugs, intracellular pathogens remain a major challenge. Accordingly, the cellular accumulation of fluoroquinolones is a parameter of major importance. Fluoroquinolone uptake by phagocytes is widely studied in vitro (5, 13, 17–19, 24–28, 37). For most fluoroquinolone derivatives, uptake is moderate (about 4- to 7-fold; that for grepafloxacin, however, is about 60-fold) (32) and rapid (within the first 5 min). This is followed by a plateau. Uptake is not saturable at extracellular concentrations of up to 25 to 50 mg/liter, and few modifications in the uptake pattern are induced by metabolic inhibitors or competitive inhibitors of the various active transport systems that exist in phagocytes such as amino acid, hexose, or nucleoside carrier systems (5, 12, 24, 32, 37). The efflux of fluoroquinolones except for that of NM-394 (24) from drug-loaded phagocytes is very rapid, and the cellular location of these drugs has been suggested to be mainly the cytosolic compartment (5).
Although these data argue for a purely passive accumulation process, some investigators have proposed that additional active mechanisms are also involved in the entry or efflux of these drugs. In particular, this has been advocated recently for pefloxacin (18, 19) and ciprofloxacin (17). In the case of pefloxacin, the investigators first observed that at high temperatures (42°C) there appeared an active process that was not visible at lower temperatures but that this process disappeared in the presence of energetic metabolism inhibitors (18). In addition, the same group (19) also demonstrated that at 37°C, Ca2+ (5 mM) briefly increased pefloxacin uptake by human monocytes and that a pretreatment with the l-type Ca2+ channel inhibitor verapamil impaired pefloxacin uptake in a concentration-dependent manner. However, since phagocytes do not seem to possess active l-type Ca2+ channels, a characteristic of electrically excitable cells, verapamil could play a role in drug uptake by a mechanism other than impairment of Ca2+ uptake, and in particular, this agent is also known to alter membrane fluidity. The investigators suggested that pefloxacin transport into monocytes was at least partly related to a Ca2+-dependent mechanism. This mechanism is likely to be peculiar to pefloxacin since neither ofloxacin nor norfloxacin competed with pefloxacin for uptake. In the case of ciprofloxacin, Loo et al. (17) reported that PMA, a protein kinase C activator, strongly enhanced ciprofloxacin uptake in a concentration- and time-dependent manner by up to 59-fold. This effect was suppressed in the presence of protein kinase inhibitors, particularly protein kinase C and MAP kinase inhibitors. The investigators suggested that a phosphorylation-dependent process was involved in ciprofloxacin uptake. However, it must be noted that PMA stimulation of neutrophils may result in a strong disturbance of the membrane structure. A moderate (about 50%) increase in levofloxacin and ofloxacin uptake at 20 min by PMA has also been reported by Pascual et al. (28).
The data from studies with levofloxacin reported here are in agreement with those found in the literature (28, 32) and are consistent with a purely passive accumulation mechanism. First, the uptake of this drug is moderate (five- to sixfold the external concentration) and parallels the extracellular concentration without saturation (Fig. 2). Second, although drug uptake is strongly decreased at 4°C, alteration of lipid membrane structure at this low temperature may also explain the decreased accumulation of levofloxacin. In addition, there does not seem to be a trapping mechanism for this drug or firm binding to cellular components since it is located mainly in the cytosol and freely and rapidly egresses from loaded cells placed into drug-free medium. A rapidly reversible binding to some cellular structures may, however, explain the four- to sixfold accumulation of this drug when an excess of free drug is present in the extracellular medium, as was the case under our experimental conditions. An interesting phenomenon was that the deprivation of extracellular Ca2+, moderately (+33%) but significantly increased levofloxacin uptake at 5 min, but this effect was no longer evidenced at longer incubation times (Fig. 5a). By contrast, Mg2+ or Ca2+ impaired levofloxacin uptake in a concentration-dependent manner. Also, Ni2+, a blocker of Ca2+ channels and of the Na+-Ca2+ exchanger, which in resting neutrophils regulates the entry of Ca2+, impaired levofloxacin uptake in a concentration-dependent manner (Fig. 5b). Other agents which modify Ca2+ homeostasis in neutrophils (e.g., calcium ionophores, Ca2+ pool-releasing drugs, and calmodulin antagonists) did not alter levofloxacin uptake (Fig. 6). In addition, neither Ni2+ nor EGTA impaired levofloxacin efflux. These data suggest that levofloxacin may chelate Ca2+ or Mg2+, as has already been reported with other fluoroquinolones for Mg2+ (1), and in this form it is less likely to diffuse freely through lipidic membranes. In Ca2+-deprived, EGTA-supplemented medium, less levofloxacin-Ca2+ complex would be formed, resulting in increased drug uptake. By impairing Ca2+ entry into the neutrophils, Ni2+ would artifactually increase the external Ca2+ concentration, and so the complexation of Ca2+ with levofloxacin which would result in an impairment of drug uptake. A confirmatory experiment should be one in which one finds an increase in the intracellular concentration of Ca2+ that results in an intracellular complexation of levofloxacin, thereby decreasing its efflux. The ionophores used in this study did not modify levofloxacin efflux. Only the Ca2+ pool-releasing agent thapsigargin and ionomycin at 100 nM (which at this concentration mainly behaves like thapsigargin and not like an ionophore) modestly increased the levofloxacin retention. These data do not argue against our hypothesis because, first, although levofloxacin is located mainly (80%) in the cytosol, a possible compartmentalization of the drug inside the cells cannot be excluded, and second, also, the intracellular Ca2+ rise stimulated by ionophores or Ca2+ pool-releasing agents should not be uniformly distributed in the cytosol. Lastly, due to the high IC50 of Ca2+ (about 15 mM), it is unlikely that such a large increase can be induced by Ca2+-modifying agents.
Other possibilities are that Ni2+ itself (better than Mg2+) combines with levofloxacin and results in inhibition of its uptake or that levofloxacin, a zwitterionic compound, uses some cation channel to enter the cellules and that cations competitively inhibit its uptake. It must be noted that in this context, levofloxacin differs considerably from pefloxacin, whose uptake is modestly and briefly (5 min) increased in the presence of Ca2+ (19).
Other data that argue against an active transport process for levofloxacin uptake are those obtained after pretreatment of neutrophils with various agents which either stimulate (PMA, FMLP) or impair (H7, staurosporine) various protein phosphorylation processes in the neutrophils. Pascual et al. (28) have reported that PMA slightly increased ofloxacin and levofloxacin uptake at 20 min, and Loo et al. (17) have observed a similar, even considerably stronger, effect for ciprofloxacin. Here we did not observe any significant effect of a 1- to 20-min pretreatment of PMA on levofloxacin uptake at 10 min, a time when the rate of uptake is optimal and should depend on an active process (if such a process exists). At the present time there is no clear explanation for this discrepancy; it is possible that the reactive oxygen species produced by PMA-activated neutrophils alter the fluoroquinolone structure and the associated fluorescence of these molecules, because both groups of investigators used this technique to measure drug uptake, or that PMA, by altering membrane fluidity, also favors drug uptake at longer incubation times.
Other controversial data arise from the use of probenecid to block fluoroquinolone efflux. Some investigators have observed that probenecid, by inhibiting an organic anion transporter active in the J774 macrophage-like cell line, enhanced the accumulation of norfloxacin, thereby increasing the intracellular bioactivity of this drug (as well as that of ciprofloxacin) against Listeria monocytogenes (4, 31). Here we observed no effect of probenecid on levofloxacin accumulation or efflux (Fig. 7). Similar results have been obtained with pefloxacin and monocytes (19). Whether levofloxacin and pefloxacin do not use this transporter to egress from human phagocytic cells or this transporter is more active in immortalized cell lines such as J774 cells remains to be determined.
Another interesting hypothesis regarding the mechanism of the transmembrane transport of levofloxacin was the possible involvement of the multidrug transporter P glycoprotein (P-gP), a 170-kDa membrane glycoprotein which mediates the transport of a variety of lipophilic substrates including fluoroquinolones (6, 14, 29). However, the importance of this transporter for quinolones has been demonstrated in epithelial cells and cell lines only. Although a P-gP-like protein has been identified inside PMNs (16), a role of P-gP in the uptake of levofloxacin by PMNs is unlikely because the P-gP-reversing agent verapamil did not modify either the accumulation or the efflux of levofloxacin.
In conclusion, all the data reported here strongly support a passive accumulation mechanism of levofloxacin in human neutrophils. This moderate (four- to sixfold) but rapid accumulation may be of use in the eradication of intracellular susceptible pathogens, particularly those located in the cytosolic compartment.
REFERENCES
- 1.Berridge M J, Irvine R F. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 2.Bryskier A. Novelties in the field of fluoroquinolones. Exp Opin Invest Drugs. 1997;6:1227–1245. doi: 10.1517/13543784.6.9.1227. [DOI] [PubMed] [Google Scholar]
- 3.Bryskier, A., and J. F. Chantot. 1995. Classification and structure-activity relationships of fluoroquinolones. Drugs 49(Suppl. 2):16–28. [DOI] [PubMed]
- 4.Cao C X, Silverstein S C, Neu H C, Steinberg T H. J774 macrophages secrete antibiotics via organic anion transporters. J Infect Dis. 1992;165:322–328. doi: 10.1093/infdis/165.2.322. [DOI] [PubMed] [Google Scholar]
- 5.Carlier, M.-B., B. Scorneaux, A. Zenebergh, J.-F. Desnottes, and P. M. Tulkens. 1990. Cellular uptake, localization and activity of fluoroquinolones in uninfected and infected macrophages. J. Antimicrob. Chemother. 26(Suppl. B):27–39. [DOI] [PubMed]
- 6.Cornet-Boyaka E, Huneau J-F, Mordrelle A, Boyaka P N, Carbon C, Rubinstein E, Tomé D. Secretion of sparfloxacin from the human intestinal Caco-2 cell line is altered by P-glycoprotein inhibitors. Antimicrob Agents Chemother. 1998;42:2607–2611. doi: 10.1128/aac.42.10.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis R, Bryson H M. Levofloxacin. A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 1994;47:677–700. doi: 10.2165/00003495-199447040-00008. [DOI] [PubMed] [Google Scholar]
- 8.Della Bianca V, Greskowiak M, De Togni P, Cassatella M, Rossi F. Inhibition by verapamil of neutrophil responses to formyl methionyl leucyl phenylalanine and phorbol myristate acetate. Mechanisms involving Ca2+ changes, cyclic AMP and protein kinase. Biochem Biophys Acta. 1985;845:223–236. doi: 10.1016/0167-4889(85)90180-6. [DOI] [PubMed] [Google Scholar]
- 9.Donati M, Rumpianesi F, Marchetti F, Sambri V, Cevenini R. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Comparative in vitro activity of levofloxacin against Chlamydia pneumoniae, C. psittaci and C. trachomatis, abstr. E154; p. 141. [Google Scholar]
- 10.Fu K P, Hilliard J, Isaacson D, Tobia A J, Rosenthale M E, McGuire J L. In-vivo evaluation of ofloxacin in Salmonella typhimurium infection in mice. J Antimicrob Chemother. 1990;25:263–268. doi: 10.1093/jac/25.2.263. [DOI] [PubMed] [Google Scholar]
- 11.Garaffo R, Jambou D, Chichmanian R M, Ravoine S, Lapalus P. In vitro and in vivo ciprofloxacin pharmacokinetics in human neutrophils. Antimicrob Agents Chemother. 1991;35:2215–2218. doi: 10.1128/aac.35.11.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia I, Pascual A, Guzman M C, Perea E J. Uptake and intracellular activity of sparfloxacin in human polymorphonuclear leukocytes and tissue culture cells. Antimicrob Agents Chemother. 1992;36:1053–1056. doi: 10.1128/aac.36.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia I, Pascual A, Salvador J, Conejo M C, Perea E J. Effect of paclitaxel alone or in combination on the intracellular penetration and activity of quinolones in human neutrophils. J Antimicrob Chemother. 1996;38:859–863. doi: 10.1093/jac/38.5.859. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths N M, Hirst B H, Simmons N L. Active secretion of the fluoroquinolone ciprofloxacin by human intestinal epithelial Caco-2 cell layers. Br J Pharmacol. 1993;108:575–576. doi: 10.1111/j.1476-5381.1993.tb12844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havlichek D, Saravolatz L, Pohlod D. Effect of quinolones and other antimicrobial agents on cell-associated Legionella pneumophila. Antimicrob Agents Chemother. 1987;31:1529–1534. doi: 10.1128/aac.31.10.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klimecki W T, Futscher B W, Grogan T M, Dalton W S. P-glycoprotein expression and function in circulating blood cells from normal volunteers. Blood. 1994;83:2451–2458. [PubMed] [Google Scholar]
- 17.Loo K C, Cario A C, Zhang F, Walters J D. Regulation of ciprofloxacin uptake in human promyelocytic leukemia cells and polymorphonuclear leukocytes. J Leukocyte Biol. 1997;61:619–623. doi: 10.1002/jlb.61.5.619. [DOI] [PubMed] [Google Scholar]
- 18.Memin E, Panteix G, Revol A. Is the uptake of pefloxacin in human blood monocytes a simple diffusion process? J Antimicrob Chemother. 1996;38:789–798. doi: 10.1093/jac/38.5.787. [DOI] [PubMed] [Google Scholar]
- 19.Memin E, Panteix G, Revol A. Carrier-mediated system for pefloxacin uptake in human monocytes. J Antimicrob Chemother. 1997;40:263–268. doi: 10.1093/jac/40.2.263. [DOI] [PubMed] [Google Scholar]
- 20.Mtairag E M, Abdelghaffar H, Douhet C, Labro M T. Role of extracellular calcium in in vitro uptake and intraphagocytic location of macrolides. Antimicrob Agents Chemother. 1995;39:1676–1682. doi: 10.1128/aac.39.8.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mtairag E M, Abdelghaffar H, Labro M T. Investigation of dirithromycin and erythromycylamine uptake by human neutrophils in vitro. J Antimicrob Chemother. 1994;33:523–536. doi: 10.1093/jac/33.3.523. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen S L, Obel N, Storgaard M, Andersen P L. The effect of quinolones on the intracellular killing of Staphylococcus aureus in neutrophil granulocytes. J Antimicrob Chemother. 1997;39:617–622. doi: 10.1093/jac/39.5.617. [DOI] [PubMed] [Google Scholar]
- 23.Orfila J, Haider F, Bryskier A. Program and abstracts of the 20th International Congress of Chemotherapy, Sydney, Australia. International Society of Chemotherapy; 1997. Levofloxacin: comparative in vitro activity against Chlamydia psittaci, abstr. 3273; p. 93. [Google Scholar]
- 24.Ozaki M, Komori K, Matsuda M, Yamaguchi R, Honmura T, Tomii Y, Nishimura I, Nishino T. Uptake and intracellular activity of NM394, a new quinolone, in human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1996;40:739–742. doi: 10.1128/aac.40.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pascual A, Garcia I, Ballesta S, Perea E J. Uptake and intracellular activity of trovafloxacin in human phagocytes and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1997;41:274–277. doi: 10.1128/aac.41.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual A, Garcia I, Conejo M C, Perea E J. Fluorometric and high performance liquid chromatographic measurement of quinolone uptake by human neutrophils. Eur J Clin Microbiol Infect Dis. 1991;10:969–971. doi: 10.1007/BF02005456. [DOI] [PubMed] [Google Scholar]
- 27.Pascual A, Garcia I, Perea E J. Fluorometric measurement of ofloxacin uptake by human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1989;33:653–656. doi: 10.1128/aac.33.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pascual A, Garcia I, Perea E J. Uptake and intracellular activity of an optically active ofloxacin isomer in human neutrophils and tissue culture cells. Antimicrob Agents Chemother. 1990;34:277–280. doi: 10.1128/aac.34.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabbaa L, Dautrey S, Colas-Linhart N, Carbon C, Farinotti R. Intestinal elimination of ofloxacin enantiomers in the rat: evidence of a carrier-mediated process. Antimicrob Agents Chemother. 1996;40:2126–2130. doi: 10.1128/aac.40.9.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastogi N, Blom-Potan M C. Intracellular bactericidal activity of ciprofloxacin and ofloxacin against Mycobacterium tuberculosis multiplying in the J-774 macrophage cell line. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1990;273:195–199. doi: 10.1016/s0934-8840(11)80249-5. [DOI] [PubMed] [Google Scholar]
- 31.Rudin D E, Gao P X, Cao C X, Neu H C, Silverstein S C. Gemfibrozil enhances the listeriacidal effects of fluoroquinolone antibiotics in J774 macrophages. J Exp Med. 1992;176:1439–1447. doi: 10.1084/jem.176.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taira K, Koga H, Kohno S. Accumulation of a newly developed fluoroquinolone, OPC-17116, by human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1993;37:1877–1881. doi: 10.1128/aac.37.9.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Rensburg C E J, Joone G, Anderson R. Interactions of the oxygen-dependent antimicrobial system of the human neutrophil with difloxacin, ciprofloxacin, pefloxacin and fleroxacin in the intraphagocytic eradication of Staphylococcus aureus. J Med Microbiol. 1990;32:15–17. doi: 10.1099/00222615-32-1-15. [DOI] [PubMed] [Google Scholar]
- 34.Vazifeh D, Abdelghaffar H, Labro M T. Cellular accumulation of the new ketolide RU 64004 by human neutrophils: comparison with that of azithromycin and roxithromycin. Antimicrob Agents Chemother. 1997;41:2099–2107. doi: 10.1128/aac.41.10.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazifeh D, Bryskier A, Labro M T. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Investigation of the mechanism underlying levofloxacin uptake by human neutrophils, abstr. A74; p. 15. [Google Scholar]
- 36.Vazifeh D, Bryskier A, Labro M T. Program and abstracts of the 20th International Congress of Chemotherapy, Sydney, Australia. International Society of Chemotherapy; 1997. Levofloxacin uptake by human polymorphonuclear neutrophils in vitro, abstr. 3295; p. 97. [Google Scholar]
- 37.Yamamoto T, Kusajima H, Hosaka M, Fukuda H, Oomori Y, Shinoda H. Uptake and intracellular activity of AM-11155 in phagocytic cells. Antimicrob Agents Chemother. 1996;40:2756–2759. doi: 10.1128/aac.40.12.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]