Abstract
An immunocompetent man in his 20s came to the hospital for shortness of breath, fevers and lower back pain with unintentional 20 lbs. weight loss. Relevant history included a recent trip to Arizona 3 months prior to presentation. On arrival, he was noted to have decreased breath sounds bilaterally, and paraspinal tenderness in the lumbar area. CT scan revealed diffuse pneumonitis and an abscess with osteomyelitis in the sacrum and right iliac bone. Continued respiratory decompensation led him to the intensive care unit where he had a bronchoscopy and later sacroiliac joint fluid collection was performed. Based on his travel history, and elevated serum IgE, liposomal amphotericin B was initiated. Later his antibodies against Coccidiodes resulted elevated and fungal cultures from the bronchoalveolar lavage and abscess from the sacral vertebrae grew mould, morphologically consistent with Coccidiodes posadasii. He was transitioned to oral fluconazole and will have a close follow-up outpatient.
Keywords: mechanical ventilation, infections, bone and joint infections, pneumonia (infectious disease), adult intensive care
Background
In non-endemic areas, coccidioidomycosis is a rare disease entity and is a major cause of morbidity if not diagnosed promptly. It is a fungal infection usually caused by two genetically distinct species Coccidiodes immitis and C. posadasii.1 2 Both of them are associated with similar presentations.3 The former is found in California, commonly known as Valley fever and the latter is endemic to other parts of the USA and Mexico4–6 (figure 1). This dimorphic fungus is found in warm wet soil and can cause fungal infection through inhalation when spores are scattered through the wind, especially during earthquakes and dust storms.2 3 Person-to-person and animal-to-person disease transmission do not exist.3 After inhalation, pneumonia is often the primary infection but it may disseminate to involve the reticuloendothelial system, bones and central nervous system (CNS).3 In this case report, we diagnosed an immunocompetent young African American man with disseminated coccidioidomycosis who presented with respiratory symptoms and low back pain after returning from a trip to an endemic area 3 months prior.
Figure 1.
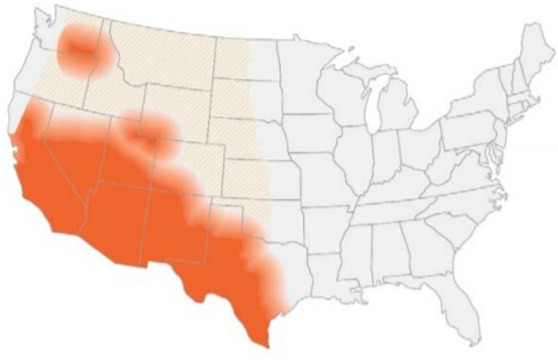
Southwestern United States, South-central Washington state and parts of Mexico with endemic coccidioidomycosis. Source: US Centres for Disease Control and Prevention. Available at https://wwwcdcgov/fungal/diseases/coccidioidomycosis/mapshtml. Public domain, no copyright permission required.
Case presentation
An African American man in his 20s with no prior medical problems was admitted to the hospital in June for shortness of breath, high-grade fevers at home and acute on chronic lower back pain with right leg radiculopathy. Back pain first began 2 months prior to presentation after a fall while roller skating. He was prescribed physical therapy which did not help with his symptoms. He travelled to Arizona 3 months prior to presentation and stayed there for 2 weeks. His social history was otherwise significant only for occasional marijuana use, working as a cook, living in Omaha, Nebraska without unusual animal exposures.
Investigations
In the emergency department, his vitals revealed a temperature of 37.5 °C, pulse rate of 110 beats per minute, blood pressure of 131/80 mm Hg and respiratory rate of 36 breaths per minute with 97% oxygen saturation on room air. His body mass index was 20.5 kg/m² and he reported an unintentional 20 lbs. weight loss within the last 30 days. On physical exam, he was noted to be tachypneic, tachycardic with bilateral decreased breath sounds, and left-sided gynaecomastia. He exhibited paraspinal tenderness in the lumbar area with decreased range of motion with a positive straight leg test on the right.
Laboratory evaluation showed a white blood cell count of 25.2 k/µL (reference range, 4.0–12.0 k/µL), platelet count 515 k/µL (reference range, 140–440 k/µL) and a serum sodium level of 131 mmol/L (reference range, 135–145 mmol/L). Manual differential was significant for neutrophils at 79% (reference range, 43%–77%), lymphocytes 8% (reference range, 13%–44%) and eosinophils 6% (reference range, 0%–5%). Other initial tests showed a serum C reactive protein of 229 mg/L (reference range, ≤9 mg/L), procalcitonin 1.69 ng/mL (reference range, ≤0.05 ng/mL). CT angiogram of his chest revealed mediastinal and hilar lymphadenopathy, diffuse pneumonitis with a differential of hypersensitivity disease, atypical infectious aetiology or organising pneumonia (figure 2). CT scan of the abdomen showed an abscess of 8.2 cm with osteomyelitis in the sacrum and right iliac bone crossing the sacroiliac joint (figure 3). Based on his travel history and involvement of multiple organs, serum Coccidiodes antibodies were ordered and later in his hospitalisation returned elevated—IgM 6.0 (reference range, <1.0) and IgG 8.8 (reference range <1.0). Additionally, his serum 1,3 beta-d-glucan was 249 (reference range, <80) and IgE was 4500 IU/mL (reference range, 0–158 IU/mL).
Figure 2.
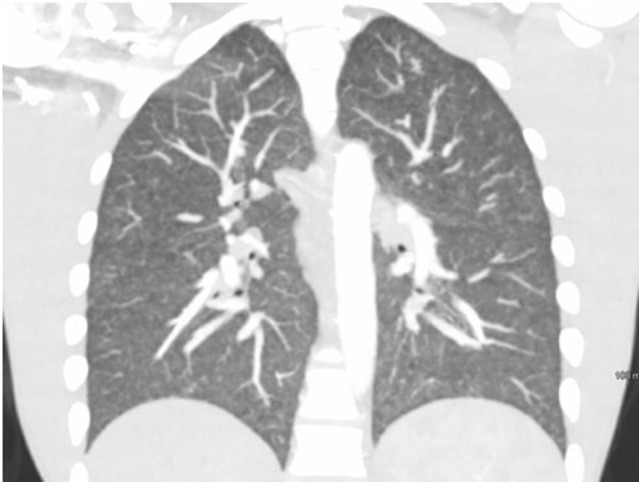
CT angiogram chest showing mediastinal, hilar lymphadenopathy with diffuse pneumonitis.
Figure 3.
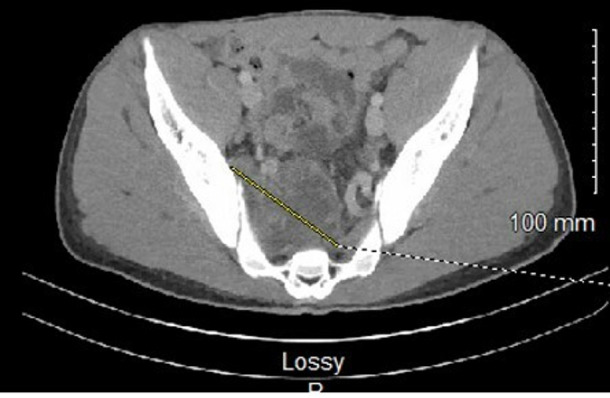
CT abdomen showing abscess of 8.2 cm with osteomyelitis in the sacrum and right iliac bone.
Due to his atypical presentation, antibiotics were withheld, and he underwent CT-guided right sacroiliac joint fluid collection with removal of 100 mL purulent fluid. He continued to have worsening respiratory status, dyspnoea, cough with a requirement of 5 litres of oxygen through nasal cannula. He was transferred to intensive care unit where bronchoscopy was performed showing total nucleated cells of 178 with predominant neutrophils and no eosinophils.
Fungal cultures from the bronchoalveolar lavage and abscess from the sacral vertebrae grew mould, morphologically consistent with C. posadasii (figure 4). He continued to have headaches which started after the admission. Given evidence of disseminated disease, he underwent lumbar puncture on day 9. Cerebrospinal fluid (CSF) analysis showed white cell count of 2/µL (reference range, 0–5/µL), CSF protein of 34 mg/dL (reference range, 12–60 mg/dL), CSF glucose of 44 mg/dL (reference range, 4–75 mg/dL) with negative culture which ruled out meningeal involvement.
Figure 4.
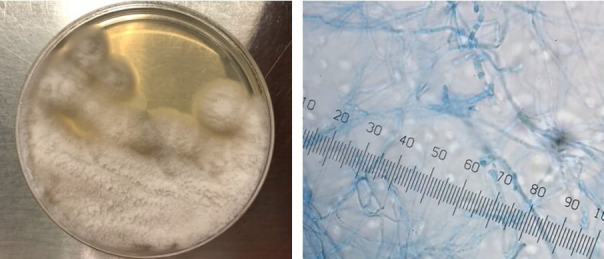
Sabouraud dextrose agar culture and arthroconidia of Coccidiodes posadasii.
Differential diagnosis
Our patient presented with diffuse pneumonitis, osteomyelitis and eventually progressed to acute respiratory failure. Common causes of community-acquired pleuropulmonary infections are bacterial pathogens including Staphylococcus aureus, Salmonella species, Streptococcus pneumoniae, but to have concomitant disseminated bone involvement is rare. If it occurs, it can be haematogenous which was not evident in this patient since blood cultures did not isolate any organisms. Other fungal infections such as histoplasmosis, blastomycosis can also present in a similar fashion but social history and exposure in the endemic area can help narrow down differentials. Nocardia should be kept in differentials if dealing with immunocompromised patients. Uncontrolled HIV disease can predispose a person to opportunistic fungal infections and similarly coccidioidomycosis which we ruled out with a non-reactive fourth generation HIV screening test. Travelling outside the USA should also raise a suspicion of miliary tuberculosis and pott’s disease which needs prompt isolation. Contact with infected cattle can cause Brucellosis. Chronic symptoms of weight loss, fever and malaise can also be secondary to malignancy or lymphoma which must be ruled out by flow cytometry or pathology. Additionally, a combination of serum eosinophilia along with elevated IgE levels is associated with allergic bronchopulmonary aspergillosis when patients present with reactive airway disease.
Treatment
Based on his travel history, involvement of multiple organs, and elevated serum IgE, liposomal amphotericin B (5 mg/kg/day) was initiated. Later his antibodies against Coccidiodes resulted elevated and fungal cultures isolated C. posadasii. Given the involvement of sacroiliac joint, he was evaluated by the orthopaedic neurosurgery team who recommended conservative management since his symptoms improved with medical and physical therapy.
Outcome and follow-up
After 1 week of intravenous liposomal amphotericin B, he was started on high-dose oral fluconazole 800 mg daily. Intravenous liposomal amphotericin B was continued for another week after discharge. He will have a close follow-up with neurosurgery team for repeat imaging of the spine and infectious disease specialist for a continuation of oral azole for a minimum of 2 years.
Discussion
Coccidiodes was first isolated by Alejandro Posadas in 1888 from the skin lesions of an infected person.1 In the laboratory setting, this fungus takes over a week to grow, forming white-coloured cottony mycelia at room temperate. The colony turns brown as it ages.1 Under the microscope, arthroconidia are seen as septated hyphae. Inside human tissues, endospores containing thick-walled spherules can be seen. When suspected, laboratory staff should be made aware as biosafety level II practices are required.1 3 Most commonly, serology tests are used for diagnosis and can be monitored to see treatment response.2 IgM antibodies develop between the first and third week after exposure while IgG antibodies are present between weeks 2 and 28.2 6 According to Cox et al, multiple organ system involvement and disseminated disease were associated with IgE formation; high IgE levels along with eosinophilia was linked to a poor prognosis.7 8 Based on the literature, sensitivity of the culture was 80% and histology was 90%.9 In addition to serology, cultures and microscopy, newer molecular tests including PCR can be used for diagnosis.3
Based on disease severity, coccidioidomycosis is divided into three classifications: primary pulmonary, chronic pulmonary and disseminated disease. After exposure to Coccidiodes, the incubation period ranges from 1 to 4 weeks and rarely can be up to 20 years.2 9 Fortunately, only 1 out of 3 patients develops symptomatic disease, usually with pulmonary signs due to inhalational exposure.4 10 The most noted symptom is an influenza-like presentation.3 4 Sixty per cent of the infected patients do not feel any symptoms and mild cases usually recover without intervention.6 In high-prevalence areas, it frequently presents as community-acquired pneumonia. After recovery of primary infection, pulmonary nodules can remain present lifelong which may not calcify and do not require treatment.2 If symptoms persist more than 3 months, it advances to chronic progressive coccidioidomycosis. In less than 1% of cases, with risk factors such as underling immunocompromised state, pregnancy, poorly controlled diabetes, interleukin-12-interferon-γ axis defect and use of exogenous immunomodulatory drugs, haematogenous dissemination can occur which can be fatal with central nervous system involvement.6 8 10 11 However, there are a few case reports of disseminated coccidioidomycosis in apparently immunocompetent patients.4 10 12 It is hypothesised that such a patient population should be tested to rule out pulmonary immune deficiencies such as IL-12 and STAT3-mediated immunity.8 12 Disseminated disease is more commonly seen in men, Hispanics and African Americans.2 13
After the lungs, the skin is the second most commonly involved organ.1 During dissemination, bone and joint involvement is reported in less than half of cases and patients can present with osteomyelitis and lytic lesions, mostly of the thoracic and lumbar region with or without pulmonary manifestations.4 6 9 MRI is more sensitive than CT scan; however, PET/CT scan does show increased fluorodeoxyglucose activity in disseminated cases.8 14 To confirm the diagnosis, a biopsy is necessary to exclude other aetiologies such as malignancy, lymphoma or tuberculosis.6 14
Musculoskeletal manifestations are predominantly seen in patients of Asian and African American descent, especially those who have symptomatic pulmonary disease.6 However, suspicion should be high for vertebral coccidioidomycosis in patients with back pain and travel to an endemic area even in the absence of respiratory signs.13 Surgical intervention is crucial along with antifungals when abscess formation causes instability of the spine, neurological deficits or symptoms of cord compression.4 9 The most commonly performed surgical interventions include debridement, drainage of the abscess and spinal fusion.9 If vertebral fusion is needed, titanium-based hardware help prevents biofilm formation and is associated with a lesser probability of disease relapse.6 Bony disease can extend to involve the central nervous system leading to meningitis. In such cases symptoms can widely vary from nausea and vomiting to encephalopathy and seizures.5
Antifungals are recommended when pulmonary symptoms are severe, in cases of chronic disease, or with dissemination.2 Amphotericin B was the first treatment reported in 1959 for coccidioidomycosis. Later, azoles acquired the primary role in management during the 1980s due to better side effect profile and tolerability.1 6 Fluconazole is the most commonly prescribed antifungal for coccidioidomycosis, although voriconazole has shown better results in the treatment of disseminated disease.9 For musculoskeletal involvement, itraconazole and posaconazole may be preferred due to better bone penetration.2 10 Amphotericin B and posaconazole are reserved for refractory disseminated disease.15 Ruling out CNS disease is crucial, which requires either intrathecal amphotericin B or azoles followed by lifelong treatment.2 15 After initial treatment of 12–18 months, lifelong suppression is also recommended in immunocompromised patients.8 9 13 Relapse of disseminated disease after stopping antifungals may occur up to 30%, necessitating long-term follow.2 13
Learning points.
Disseminated and extrapulmonary coccidioidomycosis, more common in immunodeficient patients, can also be seen in the immunocompetent.
Systemic antifungal therapy should be initiated promptly to limit the dissemination if clinical suspicion is high with relevant geographic history.
Like our case, elevated serum IgE and eosinophilia can hint towards the diagnosis while serology tests and cultures are pending.
Medical therapy coupled with surgical intervention for vertebral osteomyelitis has been associated with improved outcomes.
Footnotes
Contributors: AA wrote the manuscript with input from other authors. DQ helped in getting cultures slides and radiology images. BK supervised the project. JH contributed to the final version and helped in obtaining the consent form.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Map disclaimer: The depiction of boundaries on this map does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. This map is provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Welsh O, Vera-Cabrera L, Rendon A, et al. Coccidioidomycosis. Clin Dermatol 2012;30:573–91. 10.1016/j.clindermatol.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 2.Gabe LM, Malo J, Knox KS. Diagnosis and management of coccidioidomycosis. Clin Chest Med 2017;38:417–33. 10.1016/j.ccm.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 3.Diaz JH. Travel-related risk factors for coccidioidomycosis. J Travel Med 2018;25. 10.1093/jtm/tay027. [Epub ahead of print: 01 01 2018]. [DOI] [PubMed] [Google Scholar]
- 4.Nakhla SG. Complications and management of a rare case of disseminated coccidioidomycosis to the vertebral spine. Case Rep Infect Dis 2018;2018:1–3. 10.1155/2018/8954016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C-Y, Jerng J-S, Ko J-C, et al. Disseminated coccidioidomycosis. Emerg Infect Dis 2005;11:177–9. 10.3201/eid1101.040613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramanathan D, Sahasrabudhe N, Kim E. Disseminated coccidioidomycosis to the Spine-Case series and review of literature. Brain Sci 2019;9. 10.3390/brainsci9070160. [Epub ahead of print: 07 07 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox RA, Baker BS, Stevens DA. Specificity of immunoglobulin E in coccidioidomycosis and correlation with disease involvement. Infect Immun 1982;37:609–16. 10.1128/iai.37.2.609-616.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai M, Thauland TJ, Huang AY, et al. Disseminated coccidioidomycosis treated with interferon-γ and Dupilumab. N Engl J Med Overseas Ed 2020;382:2337–43. 10.1056/NEJMoa2000024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Del-Campo E, Kalb S, Rangel-Castilla L, et al. Spinal coccidioidomycosis: a current review of diagnosis and management. World Neurosurg 2017;108:69–75. 10.1016/j.wneu.2017.08.103 [DOI] [PubMed] [Google Scholar]
- 10.Reach P, Paugam A, Kahan A, et al. Coccidioidomycosis of the spine in an immunocompetent patient. Joint Bone Spine 2010;77:611–3. 10.1016/j.jbspin.2010.02.041 [DOI] [PubMed] [Google Scholar]
- 11.Odio CD, Marciano BE, Galgiani JN, et al. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis 2017;23:308–11. 10.3201/eid2302.160505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz J, Herrera R, Gulati S, et al. Disseminated coccidioidomycosis in an immunocompetent Asian male. Chest 2020;158:A506. 10.1016/j.chest.2020.08.482 [DOI] [Google Scholar]
- 13.Szeyko LA, Taljanovic MS, Dzioba RB, et al. Vertebral coccidioidomycosis: presentation and multidisciplinary management. Am J Med 2012;125:304–14. 10.1016/j.amjmed.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 14.Manning MA, Kuo PH, Yeager AM. Disseminated coccidioidomycosis masquerading as recurrent lymphoma. BMJ Case Rep 2018;165. doi: 10.1136/bcr-2018-224965. [Epub ahead of print: 26 May 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galgiani JN, Ampel NM, Blair JE, et al. 2016 infectious diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis 2016;63:e112–46. 10.1093/cid/ciw360 [DOI] [PubMed] [Google Scholar]


