Dear Editor,
We read with interest the article by Chen et al.1 on the investigation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in pets, including cats and dogs, whose owners were diagnosed with SARS-CoV-2 infection during the early period of the epidemic in Wuhan, China, providing serological evidence of SARS-CoV-2 infection in companion animals. Since the emergence of SARS-CoV-2 in December 2019, it has caused a pandemic of coronavirus disease 2019 (COVID-19), which is rapidly spread by human-to-human transmission, resulting in devastating effects on human health and the economy. As of 22 February 2022, over 424 million confirmed COVID-19 cases and more than 5.8 million COVID-19-related deaths had been reported worldwide.
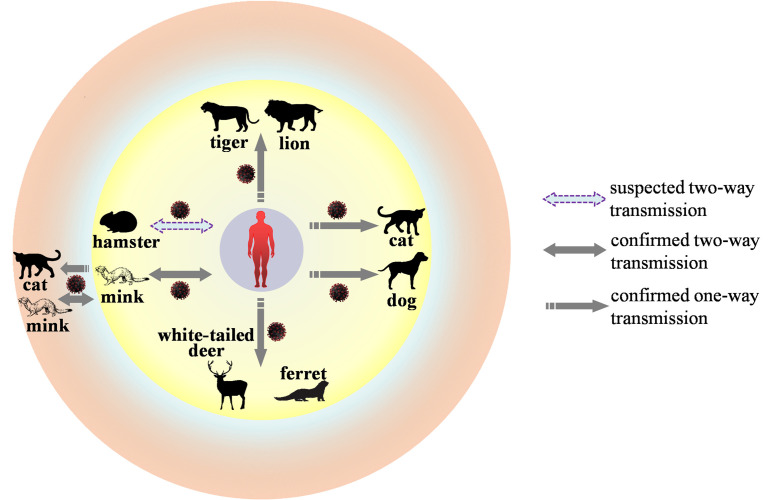
It is believed that SARS-CoV-2 probably originated in bats and was introduced into humans through an unidentified intermediate vertebrate host. Natural SARS-CoV-2 infections associated with zoonotic transmission have been identified in pets such as cats and dogs, farmed wildlife such as minks and ferrets, zoo animals such as tigers and lions, and free-ranging wildlife such as white-tailed deer.2 , 3 Notably, population-level outbreaks of SARS-CoV-2 infection in mink have been reported in Europe, including Denmark, the Netherlands, Sweden, Italy, France, Greece, Lithuania, Poland, and Spain, and North America, including USA and Canada (https://www.oie.int/en/what-we-offer/emergency-and-resilience/covid-19/#ui-id-3). SARS-CoV-2 can initiate similar clinical disease and histopathological lesions in the respiratory tracts of minks as observed in humans.4 Furthermore, accumulating evidence has emerged confirming mink-to-mink transmission, mink-to-cat transmission, and mink-to-human transmission of SARS-CoV-2 (Fig. 1 ).5 More importantly, the Y453F mutation in the S protein of the mink SARS-CoV-2 isolate can mitigate the efficacy of neutralizing antibodies.6 These findings suggest that the mink is an important host species for SARS-CoV-2. Millions of minks have been culled to prevent the spread of SARS-CoV-2. Additional animal species, such as hamsters and striped skunks are also susceptible to infection with SARS-CoV-2 in experimental settings.2 Experimental studies have further demonstrated that SARS-CoV-2 could be transmitted in minks, raccoon dogs, Megachiroptera fruit bats, and deer mice via direct contact, and in cats, ferrets, white-tailed deer, hamsters, and minks via contact and the air.7 At the beginning of 2022, a surge in the number of COVID-19 cases was reported in Hong Kong. Epidemiological monitoring indicated that imported pet hamsters might be a source of this epidemic wave, raising the possibility of hamster-to-human spillover of SARS-CoV-2, and SARS-CoV-2 variants have been identified in hamsters.8 Therefore, surveillance of SARS-CoV-2 in these susceptible animals should be strengthened, and effective measures to protect animals from possible SARS-CoV-2 infection are urgently needed.
Fig. 1.
Natural SARS-CoV-2 infection associated with zoonotic transmission.
Vaccines are one of the most effective weapons for preventing and controlling infectious diseases, such as COVID-19. Since the explosion of COVID-19, researchers from various countries have developed safe and effective SARS-CoV-2 vaccines. Several vaccines including the mRNA vaccine, inactivated vaccines, recombinant protein vaccine, and adenovirus vector vaccine have been approved for emergency use authorization worldwide (https://www.who.int/news-room/questions-and-answers/ item/coronavirus-disease-(covid-19)-vaccines). These vaccines have been confirmed to be safe and effective in restricting the spread of COVID-19 and reducing the risk of severe outcomes resulting from SARS-CoV-2.9 The rates of infection and disease severity among individuals receiving booster shots for COVID-19 are substantially lower than those of unvaccinated individuals.9 To date, more than 10 billion doses of COVID-19 vaccine have been administered. However, the stark disparities in vaccine distribution are profound, and the majority of the population in low-income affected countries in Asia, Africa, and the Americas have not received COVID-19 vaccines. Hence, inequities in vaccine coverage could have negative repercussions on our efforts to curb this disease worldwide. The first COVID-19 vaccine for animals (Carnivac-Cov) developed by Russia have been shown to elicit robust responses in animals vulnerable to SARS-CoV-2 infection such as dogs, cats, foxes, and minks (https://www.rbth.com/science-and-tech/333615-worlds-first-vaccine-animals). The strategy of vaccination in susceptible animal species, especially pets which have frequent contact with humans, has not yet attracted sufficient attention. A previous study has shown that vaccination of poultry with the H5/H7 influenza virus vaccine not only successfully prevented influenza in poultry, but also prevented human infection with H7N9 virus in China,10 which provides a new insight for controlling the COVID-19 pandemic.
In summary, vaccination of susceptible animals could protect them against SARS-CoV-2, break the chain of animal-to-animal and even animal-to-human transmission, and eliminate the risk of emergence of novel SARS-CoV-2 variants with increased virulence in humans. Thus, one health strategy should be considered to control the circulation of SARS-CoV-2 in all possible susceptible animals and humans via immunization.
Declaration of Competing Interest
None
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 31902251 and 32002289), the Science and Technology Project of Jiangxi Province (Nos. 2018ACB21027, 20212ACB205004, and 20203BBF63020), and the Scientific and Technological Research Project of Foshan (2020001000151).
References
- 1.Chen J., Huang C., Zhang Y., Zhang S., Jin M. Severe acute respiratory syndrome coronavirus 2-specific antibodies in pets in Wuhan, China. J Infect. 2020;81(3):e68–ee9. doi: 10.1016/j.jinf.2020.06.045. SepPubMed PMID: 32579982. Pubmed Central PMCID: PMC7306203. Epub 2020/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hobbs E.C., Reid T.J. Animals and SARS-CoV-2: species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transbound Emerg Dis. 2021;68(4):1850–1867. doi: 10.1111/tbed.13885. JulPubMed PMID: 33091230. Pubmed Central PMCID: PMC8359434. Epub 2020/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hale V.L., Dennis P.M., McBride D.S., et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602(7897):481–486. doi: 10.1038/s41586-021-04353-x. FebPubMed PMID: 34942632. Pubmed Central PMCID: PMC8857059. Epub 2021/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuai L., Zhong G., Yuan Q., et al. Replication, pathogenicity, and transmission of SARS-CoV-2 in minks. Natl Sci Rev. 2021;8(3):nwaa291. doi: 10.1093/nsr/nwaa291. MarPubMed PMID: 34676095. Pubmed Central PMCID: PMC7798852. Epub 2021/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharun K., Tiwari R., Natesan S., Dhama K. SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the one health approach during the ongoing COVID-19 pandemic. Vet Q. 2021;41(1):50–60. doi: 10.1080/01652176.2020.1867776. Jan 1PubMed PMID: 33349165. Pubmed Central PMCID: PMC7833041. Epub 2020/12/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M., Zhang L., Kruger N., et al. SARS-CoV-2 mutations acquired in mink reduce antibody-mediated neutralization. Cell Rep. 2021;35(3) doi: 10.1016/j.celrep.2021.109017. Apr 20PubMed PMID: 33857422. Pubmed Central PMCID: PMC8018833. Epub 2021/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meekins D.A., Gaudreault N.N., Richt J.A. Natural and experimental SARS-CoV-2 infection in domestic and wild animals. Viruses. 2021 Oct 4;13(10) doi: 10.3390/v13101993. PubMed PMID: 34696423. Pubmed Central PMCID: PMC8540328. Epub 2021/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kok K.H., Wong S.C., Chan W.M., et al. Cocirculation of two SARS-CoV-2 variant strains within imported pet hamsters in Hong Kong. Emerg Microbes Infect. 2022:1–39. doi: 10.1080/22221751.2022.2040922. Feb 9PubMed PMID: 35135441. Epub 2022/02/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattiuzzi C., Lippi G. COVID-19 vaccines efficacy in preventing or limiting SARS-CoV-2 infections. J Infect. 2022 doi: 10.1016/j.jinf.2022.01.033. Jan 31PubMed PMID: 35108600. Pubmed Central PMCID: PMC8802165. Epub 2022/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J., Deng G., Ma S., et al. Rapid evolution of H7N9 highly pathogenic viruses that emerged in China in 2017. Cell Host Microbe. 2018;24(4):558–568. doi: 10.1016/j.chom.2018.08.006. Oct 10e7PubMed PMID: 30269969. Pubmed Central PMCID: PMC6310233. Epub 2018/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]