Dear Editor,
Differences in effectiveness against severe disease were initially observed between COVID-19 vaccines during the period of predominance of the SARS-CoV-2 Alpha (B.1.1.7) variant, with mRNA vaccines (BNT162b2 and mRNA-1273) being more effective than the adenovirus-vectored ChAdOx1 vaccine.1, 2, 3 After the emergence of the Delta (B.1.617.2) variant, a modest decrease in protection was noted after two doses of all vaccines. However, effectiveness against mild infection was substantially attenuated in comparison to Alpha variant, especially for the ChAdOx1 vaccine.4, 5, 6 First data indicate that waning of protection against reinfection and symptomatic infection is faster for Omicron (B.1. 1.529) than for Delta infections.7 We read with interest the article by Ferré et al., showing a drastic waning of antibody response 3 months after the second dose of BNT162b2 in healthcare workers (HCWs) associated with a loss of neutralization activities against variant strains.8 Nevertheless, the assessment of T-cell responses, crucial for protection against SARS-CoV-2 infection and disease severity, has not been performed. To better understand differences of risk of COVID-19-related outcomes, we need for supportive studies that evaluate the comparative immunogenicity conferred by different vaccines and vaccination strategies and their durability.
In a prospective longitudinal cohort (COVIDIM Study) of HCWs, we analysed immune response 3 and 6 months after full vaccination against SARS-CoV-2 with either mRNA-1273, BNT162b2 and/or ChAdOx1 vaccines. SARS-CoV-2 anti-RBD IgG responses were quantified by an enzyme-linked immunosorbent assay (SARS-CoV-2 IgG II Quant assay, Abbott, Chicago, USA). Memory SARS-CoV-2-specific T-cell responses were quantitatively analyzed by an interferon-gamma (IFN-γ) release assay (IGRA) (QuantiFERON SARS-CoV-2, Qiagen, Hilden, Germany) in response to stimulation by SARS-CoV-2 spike antigens. Antibody and specific-IFN-γ levels were compared between independent groups (according to vaccine types and participants previously infected vs uninfected) with the Mann-Whitney test. All tests were two-sided, with a Type I error set at 0.05.
We enrolled 300 participants who had received one (n = 27, because of previous SARS-CoV-2 infection) or two doses (n = 273) of vaccine during February –June 2021. The mean age of HCWs was 46.4 ± 11.1 years, and 84.7% were female. We compared immune responses 3 months (96.0 ± 11.4 days) and 6 months (174.0 ± 8.4 days) after the last dose of each vaccine for the entire cohort and for previously infected vs uninfected HCWs. Previous infection was defined as anti-nucleocapsid positivity (SARS-CoV-2 IgG assay, Abbott) and/or history of positive PCR result on nasopharyngeal swab.
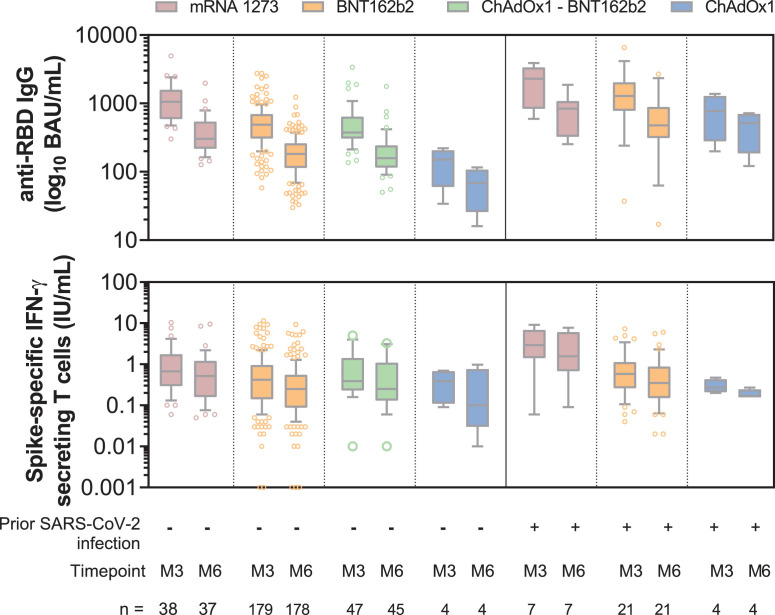
Among uninfected HCWs, anti-RBD IgG levels after 2-dose mRNA-1273 vaccine were significantly higher at M3 than those induced by 2-dose BNT162b2 vaccine (p < 0.0001), 2-dose-ChAdOx1 vaccine (p = 0.001) or the heterologous ChAdOx1 / BNT162b2 prime-boost vaccination (p < 0.0001) (Fig. 1 ). 2-dose BNT162b2 induced a higher anti-RBD IgG response than 2-dose ChAdOx1 (p = 0.003) but the heterologous ChAdOx1/BNT162b2 prime-boost vaccination achieved an antibody response comparable to that of 2-dose BNT162b2 (p = 0.26) (Fig. 1). Analysis of spike-specific T-cell response at M3 confirmed the greater immunogenic properties of 2-dose mRNA-1273 than 2-dose BNT162b2 (p = 0.02), which seems to induce a higher immune response than 2-dose ChAdOx1. However, the difference was not significant (p = 0.09) probably due to the small number of individuals in this group (Fig. 1). The heterologous ChAdOx1 / BNT162b2 vaccination achieved the same level of cellular immune response as the 2-dose mRNA-1273 (p = 0.53) or BNT162b2 (p = 0.08) vaccines (Fig. 1).
Fig. 1.
Comparative immunogenicity of mRNA-1273, BNT162b2 and ChAdOx1 COVID-19 vaccines 3 and 6 months after vaccination
Serum anti-RBD IgG antibodies and IFN-γ secreting memory T-cells via an interferon-gamma release immunoassay that uses a mix of SARS-CoV-2 spike proteins selected to activate both CD4+ and CD8+ T-cells. Box and whiskers plot indicating median and interquartile range associated with 10–90 percentile. Outliers are indicated by gray dots. One participant vaccinated with BNT162b2 withdrew during follow up, and three HCWs vaccinated with mRNA-1273 (n = 1) and ChAdOx1 / BNT162b2 (n = 2) were excluded from the M6 analysis because they developed a Delta infection between M3 and M6. BAU: binding antibody unit; IFN-γ: interferon gamma; IU: international unit; M3: 3 months after full vaccination; M6: 6 months after full vaccination; RBD: receptor-binding domain. Uninfected HCWs received two doses of vaccine. SARS-CoV-2 infected-HCWs before vaccination received one dose of vaccine.
A significant waning of antibody response induced by vaccines was observed between M3 and M6 in uninfected COVID-19 HCWs, with a median decline of anti-RBD IgG titers by 65.3% [59.3 - 72.2] with 2-dose mRNA-1273, 61.5% [54.9–68.7] with 2-dose BNT162b2, 51.2% [48.9–54.8] with 2-dose ChAdOx1 and 61.5% [55.9 - 67.0] with ChAdOx1/BNT162b2. Hence, the highest anti-RBD IgG titer 6 months after vaccination was elicited by 2-dose mRNA-1273, followed by 2-dose BNT162b2 or the heterologous ChAdOx1/BNT162b2 vaccination and finally by 2-dose ChAdOx1 (Fig. 1). Similarly, spike-specific T-cell response had a median decline over time between M3 and M6 of 26.3% [6.9–49.7] with 2-dose mRNA-1273, 40.0% [6.7 - 61.1] with 2-dose BNT162b2, 17.6% [0.0–51.5] with 2-dose ChAdOx1 and 38.8% [17.3–63.7] with the heterologous ChAdOx1/BNT162b2 vaccination. The highest specific IFN-γ response 6 months after vaccination was elicited by mRNA-1273, followed by BNT162b2 equal to ChAdOx1/BNT162b2, and finally by ChAdOx1 (Fig. 1).
In previously SARS-CoV-2-infected vaccinees with a single dose, antibody responses at M3 exceeded those found in uninfected HCWs fully vaccinated with mRNA-1273 (p = 0.03), BNT162b2 (p < 0.0001), and ChAdOx1 (p = 0.03) vaccines. At M6, we observed similar drop values to those measured in uninfected HCWs, and anti-RBD IgG titers remained higher at M6 in previously SARS-CoV-2-infected than in uninfected HCWs for each vaccine (mRNA-1273 (p = 0.07); BNT162b2 (p < 0.001); and ChAdOx1 (p = 0.01)) (Fig. 1). Analysis of spike-specific T-cell response confirmed the greater immunogenic properties of the mRNA-1273 vaccine at M3 and M6 than those of the others vaccines (Fig. 1). Specific IFN-γ response induced by a single vaccine dose of mRNA-1273 in previously infected HCWs exceeded at M3 and M6 that induced by 2-dose mRNA-1273 in uninfected HCWs (p = 0.02 and p = 0.03, respectively). In contrast, no difference was found between the two groups for BNT162b2 or ChAdOx1 vaccines.
We report the variable immunogenicity elicited by four different strategies of COVID-19 vaccines 3 months and 6 months after a primary course of vaccination. Our results confirm differences previously observed in side-to-side comparisons of vaccines early after vaccination and show that these differences last over time. Six months after vaccination, HCWs in both groups (uninfected and infected) vaccinated with mRNA-1273 had higher antibody titers and anti-spike T-cell response than those vaccinated with BNT162b2. However, the difference in immunogenicity according to previous infection was greater than the difference between the 2 mRNA vaccines. These results are consistent with those reported 3 to 10 weeks after vaccination.9 , 10 Previous studies indicated that a heterologous vaccination schedule with a first dose of ChAdOx1 followed by a second dose of mRNA vaccine provided higher antibody response one month after the boost than 2-dose ChAdOx1.11 Our results indicate that the greater effectiveness of ChAdOx1/BNT162b2 prime-boost vaccination was maintained for up to 6 months, and conferred humoral and cellular response similar to that of 2-dose BNT162b2. The clinical effectiveness of vaccines requires on-going (re) evaluation, particularly in the context of the emergence of variants of concern. Further long-term studies on the effect of booster vaccines (recommended since the late 2021) should help us to assess the dynamics of immunogenicity according to the vaccination regimens used over time and to validate the most effective vaccination strategies.
Funding
The COVIDIM study is funded by the Clinical Research and Innovation Direction of the Clermont-Ferrand University hospital.
Ethical approval
Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by the Ile-de-France VIII ethics committee of France and registered on ClinicalTrials.gov (NCT04896788).
Informed consent
Written informed consent was obtained from all individuals included in this study.
Author contributions
C.H., B.E. and B.P. planned the research; B.B, C.A., A.B., J.C., F.D., M.J., A.M., C.R., M.V., B.E. and C.H. performed the enrollment of participants in the COVIDIM study; A.O. managed the study; B.B and H.C. analyzed the data; C.L. and B.B realized the statistic tests; B.B., B.E and C.H wrote the paper. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declaration of Competing Interest
Authors state no conflict of interest.
Acknowledgments
We thank all the healthcare workers enrolled in this study, and Jeffrey Watts for revision of the English manuscript.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiruvengadam R., Awasthi A., Medigeshi G., Bhattacharya S., Mani S., Sivasubbu S., et al. Effectiveness of ChAdOx1 nCoV-19 vaccine against SARS-CoV-2 infection during the delta (B.1.617.2) variant surge in India: a test-negative, case-control study and a mechanistic study of post-vaccination immune responses. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00680-0. Available at https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00680-0/fulltext, In press. Accessed 20 December 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruxvoort K.J., Sy L.S., Qian L., Ackerson B.K., Luo Y., Lee G.S., et al. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SARS-CoV-2 variants of concern and variants under investigation. UK Health Security Agency 2021;43.
- 8.Ferré V.M., Lebourgeois S., Menidjel R., Collin G., Chenane H.R., Guindi M.O., et al. Decreasing humoral response among healthcare workers up to 4 months after two doses of BNT162b2 vaccine. J Infect. 2022;84:248–288. doi: 10.1016/j.jinf.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naranbhai V., Garcia-Beltran W.F., Chang C.C., Mairena C.B., Thierauf J.C., Kirkpatrick G., et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2 and Ad26.COV2.S COVID-19 vaccines. J Infect Dis. 2021:jiab593. doi: 10.1093/infdis/jiab593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326:1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X., Shaw R.H., Stuart A.S.V., Greenland M., Aley P.K., Andrews N.J., et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.