Abstract
The genetic variation of the human immunodeficiency virus type 1 (HIV-1) protease gene (prt) permits the classification of HIV-1 strains into five distinct protease subtypes, which follow the gag subtyping patterns. The susceptibilities of non-B-subtype strains to protease inhibitors (PIs) and other antiretroviral drugs remain largely unknown. Subtype F is the main non-B strain contributing to the Brazilian epidemic, accounting for 15 to 20% of these infections. In this work, we report the findings on 81 isolates from PI-naive Brazilian patients collected between 1993 and 1997. In addition, the relevant PI resistance mutations and their phenotypes were determined in vitro for 15 of these patients (B = 9 and F = 6). Among these, the subtype F samples evidenced high sensitivities in vitro to ritonavir and indinavir, with MICs at which 50 and 90% of the isolates are inhibited similar to those of both the Brazilian and the U.S. subtype B isolates. Analysis of the 81 Brazilian prt sequences demonstrated that the subtype F consensus sequence differs from the U.S. and Brazilian subtype B consensus in eight positions (I15V, E35D, M36I, R41K, R57K, Q61N, L63P, and L89M). The frequency of critical PI resistance substitutions (amino acid changes D30N, V82A/F/T, I84V, N88D, and L90M) among Brazilian isolates is very low (mean, 2.5%), and the associated secondary substitutions (amino acid positions 10L, 20K, 36M, 46M, 48G, 54I, 63P, 71A, and 77A) are infrequent. These observations document the relative rarity of resistance to PIs in the treatment of patients infected with HIV-1 subtype F in South America.
The human immunodeficiency virus (HIV) gag- and pol-encoded proteins are expressed as long polypeptide precursors that must be processed proteolytically, by the virus-encoded aspartyl protease, to gain biologic activity (16). Given the protease’s limited size and substrate specificity, the genetic variability of the molecule must be narrowly constrained to preserve an essential function. These properties make the protease a ready target for HIV therapeutics and have resulted in the development of a new class of synthetic drugs called protease inhibitors (PIs) (34).
The main difficulty encountered in HIV therapy is the rapid emergence of drug-resistant strains of the virus. HIV, as well as other RNA viruses, replicates as complex and dynamic distributions of mutant genomes, termed viral quasispecies (6). These quasispecies present an important obstacle to potential vaccine or drug therapy (7). A classic example of this is the development of resistance to nucleotide analogs as seen in the emergence and selection of HIV-1 variants resistant to drugs such as 3′-azido-3′-deoxythymidine (AZT) and 3′-thiacytidine during treatment (14, 36).
Resistance to PIs is associated with a complex pattern of point mutations. Eberle et al. (8) demonstrated that resistance to the potent PI saquinavir in vitro and in vivo is associated with amino acid substitutions L90M and G48V in HIV protease. While L90M is the predominant substitution in vivo, G48V is uncommon and the double substitution is rare (8). A more complex mutation pattern is involved in the resistance to ritonavir. Here, position V82 seems to be critical and the V82T, V82A, and V82F substitutions appear predominant. Although mutations at position 82 are insufficient to confer resistance alone, they appeared first in most patients under treatment. Significant phenotypic resistance requires a combination of mutations, which emerged subsequently in an ordered and stepwise fashion, including (i) M36I, I54V, and V82A/F/T; (ii) K20R, M36I, I54V, and V82A; and (iii) K20R, M36I, I54V, A71A, and V82T (20). Likewise, resistance to indinavir is related to multiple specific amino acid changes along the backbone of the protease molecule. In this case, the association of substitutions in positions V32, M46, A71, and V82 is linked to the highly resistant phenotypes (4).
All these mutations are located in the internal portion of the protease homodimer. A majority of the critical mutations are relatively conservative in nature, involving the gain or loss of a methylene group. A complex pattern of mutations is also associated with critical modifications, such as L10I, K20R, L24I, V32I, L33F, M36I, M46I, G48V, I54V, L63P, A71V/T, and V77I, which have been directly linked to resistance. Such secondary mutations are probably compensatory in order to maintain the protease structural functionality and are most often located in the external portion of the enzyme (27).
Although the global genetic diversity of HIV-1 has been considered a major obstacle to the successful development of a vaccine, its relevance in antiretroviral drug resistance remains uncertain. In total, the phylogenetic diversity within the protease gene is sufficient to segregate HIV-1 isolates into five different subtypes consistent with the gag subtype (11, 17). There are few published protease sequences from the non-B isolates, which dominate the current pandemic, and their susceptibilities to PIs are mostly unknown. In Brazil, subtype F strains are the predominant non-B variants in circulation and account for 15 to 20% of the infections (5, 11, 21, 25, 31). This subtype is found throughout South America (18), in Romania, and in Central Africa (10). In this report, we document the susceptibilities of nine Brazilian subtype B and six subtype F isolates to saquinavir and indinavir and compare the MICs at which 50 and 90% of the isolates are inhibited (MIC50 and MIC90, respectively) of these important inhibitors. Moreover, we determined the protease sequences of another 66 isolates from Brazilian PI drug-naive individuals and studied the prevalence of relevant substitutions related to PI resistance.
MATERIALS AND METHODS
Study population.
Blood samples from PI-naive patients included in this study were collected from persons in different Brazilian cities representing two regions of the country (southeast and north). Samples were obtained from HIV-1-seropositive individuals attending the Hemotherapy Service of the Hospital Albert Einstein, State of São Paulo, in 1993; the AIDS Clinic at the Federal University of Rio de Janeiro, in 1994; the AIDS Clinic of Evandro Chagas Hospital, State of Para, in 1996; and the AIDS Clinic of the Tropical Medicine Institute, State of Amazonas, in 1997. Of the 81 specimens, 74 were from persons who had not received any antiretroviral treatment; the remaining 7 patients were from the State of Rio de Janeiro cohort and were receiving AZT (n = 5) or dideoxyinosine (n = 2) drug therapy.
Virus isolation.
Peripheral blood mononuclear cells (PBMCs) from infected patients were cocultured with phytohemagglutinin-stimulated PBMCs from HIV-seronegative blood donors. When the p24 antigen concentration in the culture exceeded 30 ng/ml, multiple aliquots of the cell-free supernatant were harvested for drug susceptibility testing and pellets of cultured cells were saved for DNA sequencing.
PCR amplification and sequence analyses of the viral protease.
Cell pellets of the cultured and uncultured PBMCs were digested with proteinase K, and the resulting lysate was subjected to nested PCR with external primer pair DP10-DP11 and internal primer pair DP16-DP17, as previously described (11). The entire protease gene was sequenced from the resulting 297-bp product. Sequences were determined bidirectionally from the internal primer set with an ABI model 373A automated DNA sequencer and the manufacturer’s FS Dye Terminator kit (Applied Biosystems, Inc., Foster City, Calif.). The sequences were aligned with the CLUSTAL multiple sequence alignment programs and then analyzed by the maximum-likelihood (fastDNAml) and neighbor-joining distance methods included in the PHYLIP package, version 3.5c, as also previously described (11). The SIVCPZ sequences were used as an outgroup for phylogenetic comparisons. Synonymous and nonsynonymous nucleotide distances were calculated by using the Nei and Gojobori algorithm and MEGA DNA analysis software (23). The frequency of synonymous mutations (Ps) was calculated as the number of observed synonymous substitutions divided by the number of possible synonymous substitutions. Similarly, the frequency of nonsynonymous mutations (Pn) was determined as the number of observed nonsynonymous events divided by the number of possible nonsynonymous substitutions.
Drug susceptibility testing.
Fifty- to 100-fold the 50% tissue culture infectious dose of virus stock was used to infect 106 phytohemagglutinin-stimulated PBMCs in the presence or absence of increasing concentrations of saquinavir and indinavir. After 4 days, the levels of p24 antigen expressed in the cell-free supernatant were measured with the Coulter HIV-1 p24 antigen capture kit (Coulter, Hialeah, Fla.). The experiments were performed in triplicate, and a linear regression analysis was used to determine the drug concentration required to inhibit p24 antigen production by 50% (MIC50) and 90% (MIC90), compared to the drug-free controls. Drug concentrations used for saquinavir and indinavir were 1, 2, 4, 8, 16, 32, and 64 nM. Comparable results were obtained with phytohemagglutinin-stimulated primary PBMCs and the PM-1 continuous cell line.
Nucleotide sequence accession numbers.
Sequences generated in this study have been submitted to GenBank under accession no. AF079981 to AF079996.
RESULTS
Genetic analysis of the Brazilian isolates.
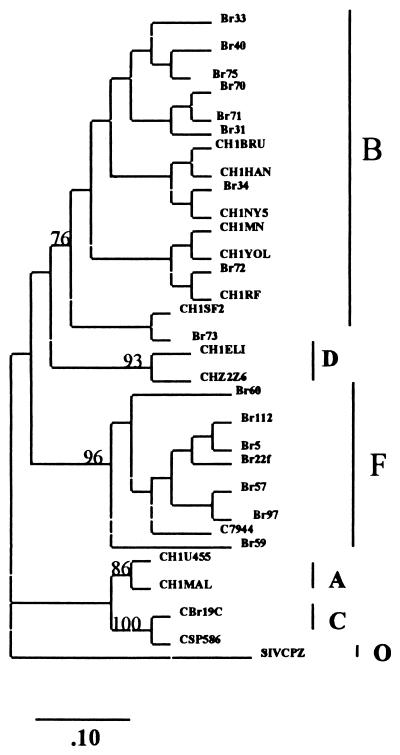
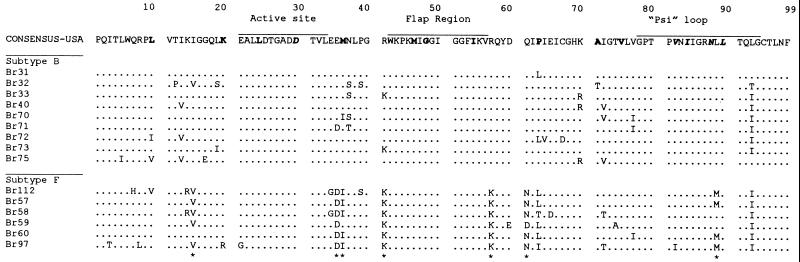
Initially, 15 samples from Brazilian PI-naive patients were cocultured with PBMCs, and their prt genes were amplified and sequenced. Phylogenetic analysis of the prt nucleotide sequence data by maximum-likelihood and neighbor-joining methods yielded essentially identical results. Figure 1 shows a neighbor-joining phylogenetic tree with these sequences. These isolates could be segregated into two distinct subtypes, B (n = 9) and F (n = 6). The intersubtype distances (Brazilian subtype F versus consensus Brazilian subtype B) were significantly greater than the intrasubtype F sequence distances (10.8 versus 6.8% for nucleic acids and 6.2 versus 3.8% for amino acids). Amino acid alignment of Brazilian subtype B and F sequences is depicted in Fig. 2. Purine-purine substitutions were most commonly seen (6%), whereas pyrimidine-pyrimidine and purine-pyrimidine substitutions accounted for the balance (up to 2% each). Most of the observed amino acid changes in the protease positions were conservative, i.e., 66% of the substitutions in the Brazilian subtype B samples were conservative and 34% were nonconservative. Alternatively, subtype F has shown 82% conservative versus 18% nonconservative amino acid changes. The Brazilian subtype B consensus sequences exhibit one amino acid difference (I93L) compared to the U.S. consensus. The Brazilian subtype F consensus sequence differed from the U.S. and Brazilian subtype B consensus sequences in eight positions (amino acid changes I15V, E35D, M36I, R41K, R57K, Q61N, L63P, and L89M). All these F-specific mutations occur infrequently as natural variants of the Brazilian subtype B consensus sequence.
FIG. 1.
Phylogenetic classification. The distinct HIV-1 subtypes are delineated, and the tree was generated by the neighbor-joining method (for details, see Materials and Methods). The scale bar shows the ratio of nucleotide substitutions for a given horizontal branch length. Vertical distances are for clarity only. The bootstrap values (mean of 100 reiterations) of all major branches of the tree are shown. The following reference sequences were included, corresponding to the subtype shown in parentheses: CHIU455 and CH1MAL (A); CH1MN, CH1NY5, CH1YOL, CH1BRU, CH1RF, and CH1SF2 (B); CSP586 and CBr19C (C); CH1ELI and CHZ2Z6 (D); and C7944 and Br22f (F). The SIVCPZ protease sequence was used as an outgroup to root the tree.
FIG. 2.
Amino acid alignment of Brazilian protease sequences: The U.S. consensus prt sequence (17) is provided for comparison (upper sequence), and Brazilian sequences are aligned beneath. The Brazilian sequences are segregated into subtypes B (upper) and F (lower). The protease functional domains (active site, flap region, and psi loop) are shown at the top of the consensus sequence. The five amino acid positions critical for PI drug resistance (amino acid positions 30D, 82V, 84I, 88N, and 90L) are depicted in boldface italics, and the secondary positions (amino acid positions 10L, 20K, 36M, 46M, 48G, 54I, 63P, 71A, and 77A) are marked in boldface only. The molecular signature sites that differentiate subtype B from subtype F are designated with asterisks.
Of note, among the five previously described amino acid changes related to PI drug resistance (D30N, V82A/F/T, I84V, N88D, and L90M), none was found in the Brazilian isolates (19). One F isolate (Br97) had the V82I substitution that has not been previously associated with resistance (V to A/F/T). Also, this mutation was not linked with other secondary changes, such as I84V, G48V, and A71V/T, that have been found in isolates highly resistant to indinavir and ritonavir. Secondary mutations implicated in the PI resistance (see Fig. 1 legend for details) could be observed in the amino acid sequences for these drug-naive patients but occurred at very low frequencies, with the exception of positions 36 and 63 (Fig. 2). In fact, these two positions are integral to the subtype F molecular signatures noted above.
There was a heterogeneous ratio of synonymous to nonsynonymous nucleotide differences between the distinct domains of the protease gene of the Brazilian B and F HIV-1 subtypes. These domains comprising the active site, flap region, and psi loop (substrate binding loop), have a higher Ps/Pn ratio in both subtypes than that of the three interspersed variable regions (Table 1 and Fig. 2). There were no significant differences in the Ps/Pn ratio among subtypes B and F throughout the entire gene sequence. However, synonymous nucleotide differences between subtypes B and F were present in several highly conserved amino acids. For example, the nucleotide triplet coding for 18Q was CAG in every subtype F isolate, compared with CAA for the majority of subtype B sequences. The Ps/Pn ratios among nucleotide sites involved in the resistance to PI (10L, 20K, 24L, D30, 32V, 33L, 36M, 46M, G48, I54, P63, A71, V77, V82, I84, N88, and L90) were analyzed. The resulting values were similar to the value generated for the entire gene, and no significant differences were noted between the ratios of Brazilian B and those of Brazilian F sequences (Table 1).
TABLE 1.
Proportions of synonymous and nonsynonymous nucleotide differences (Ps/Pn ratioa) between different protease gene domains and PI critical sites
| Subtype | Value for protease gene domainb
|
|||||
|---|---|---|---|---|---|---|
| AS | FR | PL | Rm | Totalc | PI CSd | |
| B | 22.2 | 11.4 | 17.4 | 3.2 | 6.5 | 6.8 |
| F | 21.3 | 10.5 | 14.4 | 3.5 | 6.3 | 6.7 |
The average numbers of synonymous substitutions per potential synonymous site and potential nonsynonymous site relative to the Brazilian consensus B or F sequence were calculated for each domain and for nonsynonymous substitutions per the critical sites for mutations related to PI resistance.
Domains: AS, active site (amino acid positions 21 to 34); FR, flap region (amino acid positions 42 to 56); PL, psi loop (amino acid positions 78 to 99); Rm, remaining regions for the molecule.
This value represents the Ps/Pn ratio with use of the whole protease gene of all samples for the calculations and comparison to the respective consensus sequence.
This value was calculated by using the critical sites at the following nucleotide positions: L10, K20, D30, V32, L33, M36, M46, I54, L63, A71, V77, V82, I84, N88, and 90L.
Susceptibilities of Brazilian subtype B and F isolates to PIs.
The susceptibilities of the 15 Brazilian isolates to saquinavir and indinavir were evaluated in vitro. Table 2 shows the risk group and clinical data for the patients as well as the susceptibility results for their viral isolates. The mean MIC50 of saquinavir was 15.78 nM (range, 5 to 35 nM) for B isolates and 6.67 nM (range, 4 to 13 nM) for F isolates. The mean MIC90 of saquinavir was 55.44 nM (range, 15 to 105 nM) for B isolates and 21.0 nM (range, 15 to 33 nM) for F isolates. The B samples could be segregated into two distinct groups with different susceptibilities to saquinavir. One group, comprising samples Br31, Br34, Br70, and Br73, had high mean MIC50 and MIC90, of 25.5 and 94.25 nM, respectively. The other group, composed of samples Br33, Br40, Br71, Br72, and Br75, had low mean MIC50 and MIC90, of 8 and 24.6 nM, respectively. When susceptibility to indinavir was evaluated, more homogeneous MIC50 and MIC90 were obtained for all samples. The mean MIC50 and MIC90 of indinavir were 6 nM (range, 3 to 10 nM) for B isolates and 17 nM (range, 11 to 22 nM) for F isolates.
TABLE 2.
Risk group and clinical and laboratory data for the patients and in vitro activities of the Brazilian subtype B and F HIV-1 isolates with saquinavir and indinavira
| Protease subtype | Epidemiological and clinical data
|
PI in vitro susceptibility (nM) (avg ± 1 SD)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Isolate | 1st serologyb | CDC stage | CD4 counts/ml | Antiviral drug | Risk group | Saquinavir
|
Indinavir
|
|||
| MIC50 | MIC90 | MIC50 | MIC90 | |||||||
| F | Br58 | Sep 94 | I | 570 | No | Het | 6 ± 0.5 | 21 ± 1.8 | 5 ± 0.45 | 17 ± 1.2 |
| Br59 | Aug 89 | I | 821 | No | Het | 5 ± 0.45 | 18 ± 0.98 | 3 ± 0.26 | 15 ± 1.0 | |
| Br60 | Jul 92 | I | 650 | No | Het | 4 ± 0.47 | 16 ± 1.7 | 8 ± 0.6 | 20 ± 1.4 | |
| Br57 | Feb 90 | II | 230 | AZT, 1 yr | Part | 7 ± 0.66 | 25 ± 1.8 | 4 ± 0.21 | 12 ± 1.2 | |
| Br97 | Jan 92 | I | 190 | No | Mult | 13 ± 0.98 | 33 ± 2.3 | 3 ± 0.23 | 11 ± 0.8 | |
| Br112 | Feb 91 | I | 614 | AZT, 1 yr | Part | 5 ± 0.34 | 15 ± 2.0 | 5 ± 0.3 | 16 ± 1.0 | |
| B | Br31 | Mar 94 | I | 468 | No | Het | 22 ± 1.8 | 95 ± 5.3 | 7 ± 0.6 | 19 ± 1.8 |
| Br33 | Jun 91 | I | 453 | No | Het | 6 ± 0.8 | 20 ± 2.3 | 10 ± 1.1 | 22 ± 2.1 | |
| Br34 | Apr 93 | II | 263 | AZT, 1 yr | Tx | 25 ± 2.1 | 92 ± 6.9 | 5 ± 0.4 | 17 ± 1.3 | |
| Br40 | Jul 85 | II | 138 | No | Hom | 8 ± 0.6 | 24 ± 1.8 | 6 ± 0.55 | 18 ± 1.4 | |
| Br70 | Apr 92 | IV | 252 | No | Het | 35 ± 2.1 | 105 ± 6.5 | 5 ± 0.43 | 13 ± 1.1 | |
| Br71 | Feb 89 | III | 286 | No | Hom | 5 ± 0.6 | 15 ± 1.2 | 9 ± 0.67 | 20 ± 1.9 | |
| Br72 | Feb 94 | IV | NA | No | Part | 12 ± 1.1 | 32 ± 2.6 | 10 ± 0.97 | 23 ± 2.2 | |
| Br73 | Apr 94 | I | 321 | No | Het | 20 ± 1.9 | 85 ± 5.9 | 9 ± 0.6 | 18 ± 1.9 | |
| Br75 | Oct 88 | II | 154 | No | Het | 9 ± 0.8 | 32 ± 3.1 | 5 ± 0.67 | 16 ± 1.4 | |
Abbreviations: Jan, January; Feb, February; Mar, March; Apr, April; Jun, June; Jul, July; Aug, August; Sep, September; Oct, October; CDC, Centers for Disease Control and Prevention; Het, heterosexual; Hom, homosexual; Part, infected sexual partner; Tx, blood transfusion; Mult, multiple factors; NA, not available.
Month and year.
Genotyping of additional Brazilian isolates.
To gain more information about the genetic variation of the protease gene, we studied 66 additional samples from four different Brazilian regions. DNA from these samples was extracted, amplified, sequenced (data not shown), subtyped, and analyzed for mutations in sites critical for PI resistance. The 66 samples could be segregated into two distinct subtypes: B (n = 44) and F (n = 17). We observed limited variability among positions critical for PI resistance (82V, 88N, and 90L), regardless of specimen subtype. These substitutions in critical positions are depicted in Table 3. Overall, this analysis showed the same pattern of variation seen in the first 15 samples, regardless of the geographic origin or subtype of the sample studied.
TABLE 3.
Mutations in the Brazilian protease gene sequences related to PI resistance
| Sequence | Amino acid position related to PI resistancea
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| U.S. consensus | 10L | 20K | 30D | 36M | 46M | 48G | 54I | 63P | 71A | 77I | 82V | 84I | 88N | 90L |
| Brazil F mutationb | I(5.8) | R(5.8) | I(76) | L(53) | M(5.8) | |||||||||
| V(5.8) | S(12) | |||||||||||||
| Total | 5.8 | 5.8 | 81.8 | 65 | 5.8 | |||||||||
| Brazil B mutationb | I(6.8) | R(2.2) | I(18) | V(2.2) | E(2.2) | L(2.2) | T(19) | T(4.5) | I(25) | A(2.2) | I(2.2) | V(4.5) | ||
| F(6.8) | M(2.2) | A(1) | V(2.2) | M(4.5) | ||||||||||
| S(1) | ||||||||||||||
| Total | 13.6 | 4.4 | 18 | 2.2 | 2.2 | 2.2 | 21 | 4.4 | 9 | |||||
Critical sites for resistance to particular drugs are as follows: nelfinavir, 30D and 88N; ritonavir and indinavir, 82V and 90L; saquinavir, 90L. Data are the amino acid changes and the percentages (in parentheses) of the isolates showing the particular mutations.
Variation in critical sites in Brazilian isolates belonging to subtypes F (n = 17) and B (n = 44).
DISCUSSION
Several factors contribute to the genetic variation of HIV-1. The viral genome is diploid and notably susceptible to recombination (28, 29). Its reverse transcriptase lacks proofreading capability, and it is estimated that one mutation occurs along the genome during each replication cycle (3, 37). The virus also has a high rate of replication in vivo (3) and is subject to many kinds of selective pressure in the infected host (3, 39).
Although the pol gene region and, particularly, the protease gene are the most conserved elements of the HIV-1 genome (32, 33), differences between the subtypes are sufficient to allow their distinction. However, the variations related to subtype assignment are not linked to known critical drug resistance substitutions. The absence of such substitutions in both subtypes suggests the selective disadvantage of drug resistance mutations. These results are consistent with a previous genotypic survey of 167 isolates from PI-naive patients within the United States (13, 38). In our analysis, the close agreement between neighbor-joining and maximum-likelihood methods supports the reliability of the phylogenetic tree of the HIV-1 protease gene (Fig. 1). The high bootstrap values (>75%) at the relevant nodes in most cases represent a greater than 96% probability that the branching of the tree is correct and support the subtype designation.
Brazilian subtype F isolates were also found in vitro to be as susceptible as subtype B isolates to indinavir and saquinavir, with the last having MIC50 and MIC90 similar to those of U.S. B isolates from drug-naive patients (MIC90, 25 to 100 nM) (5, 38). The MIC50 and MIC90 for the F isolates are more homogeneous than those for the B isolates, which may be segregated into two groups: one with high mean values (MIC50 and MIC90, 25.5 and 94.25 nM, respectively) and the other with low mean values (MIC50 and MIC90, 8 and 24.6 nM, respectively). We could not correlate this variation in the B isolates with any natural mutation, with the exception of Br70 with three different secondary mutations relevant to PI resistance.
The lower intrasubtype diversity among the Brazilian F subtype variants than among the subtype B strains circulating in Brazil probably reflects a recent introduction of these strains into the country. Extraordinary high ratios of synonymous to nonsynonymous nucleotide differences were found between functional domains in the protease gene. There is a clear selective pressure, as signified by high Ps/Pn ratios in functional domains of the enzyme such as the active site, flap region, and psi loop. Alternatively, the interspersed variable regions experience more variation, with sixfold-lower Ps/Pn ratios. High rates of Pn are also observed in the variable parts of the HIV env gene, a characteristic that has been attributed to adaptive evolution in response to immune pressure (1, 30). For the protease gene, the evolutionary pressure driving this phenomenon is not known. Similarly, high rates of Pn have been observed in the reverse transcriptase genes for patients receiving antiretroviral therapy with reverse transcriptase inhibitors (3, 12, 26). However, the Ps/Pn ratio of the critical sites for PI resistance acts similarly to a variable region, indicating selective equality among PI-sensitive alleles. The absence of the critical resistance substitutions in a high proportion of the drug-naive population suggests that these mutations are not advantageous to viral physiology and that their fixation is possible only under intense selective pressure, i.e., drug therapy.
The PIs are important components of the potent multidrug combinations currently used to treat persons with HIV infection. Almost all the clinical and laboratory data related to treatment with PIs are based on studies of HIV isolates belonging to subtype B. Since the beginning of the 1990s, surveys conducted in Brazil have identified subtype F as the main non-B isolate in that country (21, 25). Moreover, non-B-subtype infections are becoming more common in Europe and North America (2, 9, 15). The Brazilian government is currently sponsoring large HIV treatment programs with drug combinations that include the main commercially available PIs. Information about the drug susceptibilities of non-B-subtype isolates is critically important to this program. Our results clearly show a low frequency of substitutions related to PI resistance as well as similar MIC50 and MIC90 of saquinavir and indinavir for Brazilian viral isolates. These in vitro observations predict the efficacy of these PIs in the treatment of patients infected with subtype F in South America.
REFERENCES
- 1.Balfe P, Simmonds P, Ludlam C A, Bishop J O, Brown A J. Concurrent evolution of human immunodeficiency virus type 1 in patients infected from the same source: rate of sequence change and low frequency of inactivating mutations. J Virol. 1990;64:6221–6233. doi: 10.1128/jvi.64.12.6221-6233.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodine S K, Mascola J R, Weiss P J, Ito S I, Porter K R, Artenstein A W, Garland F C, McCutchan F E, Burke D S. Detection of diverse HIV-1 genetic subtypes in the USA. Lancet. 1995;346:1198–1199. doi: 10.1016/s0140-6736(95)92901-0. [DOI] [PubMed] [Google Scholar]
- 3.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1996;271:670–671. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 4.Condra J H, Holder D J, Schleif W A, Blahy O M, Danovich R M, Gabryelski L J, Graham D J, Laird D, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, Yang T, Chodakewitz J A, Deutsch P J, Leavitt R Y, Massari F E, Mellors J W, Squires K E, Steigbigel R T, Teppler H, Emini E A. Genetic correlates of in vivo viral resistance to indinavir, a human immunodeficiency virus type 1 protease inhibitor. J Virol. 1996;70:8270–8276. doi: 10.1128/jvi.70.12.8270-8276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Costa S M, Schechter M, Shindo N, Vicente A C, Oliveira E F, Pinto M E, Tanuri A. Sequence and phylogenetic analysis of glycoprotein 120 of an HIV type 1 variant (GWGR) prevalent in Brazil. AIDS Res Hum Retroviruses. 1995;11:1143–1145. doi: 10.1089/aid.1995.11.1143. [DOI] [PubMed] [Google Scholar]
- 6.Domingo E. Genetic variation and quasi-species. Curr Opin Genet Dev. 1992;2:61–63. doi: 10.1016/s0959-437x(05)80323-5. [DOI] [PubMed] [Google Scholar]
- 7.Domingo E, Menendez-Arias L, Quinones-Mateu M E, Holguin A, Gutierrez-Rivas M, Martinez M A, Quer J, Novella I S, Holland J J. Viral quasispecies and the problem of vaccine-escape and drug-resistant mutants. Prog Drug Res. 1997;48:99–128. doi: 10.1007/978-3-0348-8861-5_4. [DOI] [PubMed] [Google Scholar]
- 8.Eberle J, Bechowsky B, Rose D, Hanser V, Von Der Helm K, Gurtter L, Nitschki H. Resistance of HIV type 1 to proteinase inhibitor RO 31-8959. AIDS Res Hum Retroviruses. 1995;11:671–676. doi: 10.1089/aid.1995.11.671. [DOI] [PubMed] [Google Scholar]
- 9.Fransen K, Buve A, Nkengasong J N, Laga M, van der Groen G. Longstanding presence in Belgians of multiple non-B HIV-1 subtypes. Lancet. 1996;347:1403. doi: 10.1016/s0140-6736(96)91042-9. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 10.Hu D J, Dondero T J, Rayfield M A, George J R, Schochetman G, Jaffe H W, Luo C C, Kalish M L, Weniger B G, Pau C P, Schable C A, Curran J W. The emerging genetic diversity of HIV. The importance of global surveillance for diagnostics, research, and prevention. JAMA. 1996;275:210–216. [PubMed] [Google Scholar]
- 11.Janini L M, Pieniazek D, Peralta J M, Schechter M, Tanuri A, Vicente A C, de la Torre N, Pieniazek N J, Luo C C, Kalish M L, Schochetman G, Rayfield M A. Identification of single and dual infections with distinct subtypes of human immunodeficiency virus type 1 by using restriction fragment length polymorphism analysis. Virus Genes. 1996;13:69–81. doi: 10.1007/BF00576981. [DOI] [PubMed] [Google Scholar]
- 12.Keulen W, Boucher C, Berkhout B. Nucleotide substitution patterns can predict the requirements for drug-resistance of HIV-1 proteins. Antivir Res. 1996;31:45–57. doi: 10.1016/0166-3542(96)00944-8. [DOI] [PubMed] [Google Scholar]
- 13.Kozal M J, Shah N, Shen N, Yang R, Fucini R, Merigan T C, Richman D D, Morris D, Hubbell E, Chee M, Gingeras T R. Extensive polymorphisms observed in HIV-1 clade B protease gene using high-density oligonucleotide arrays. Nat Med. 1996;2:753–759. doi: 10.1038/nm0796-753. [DOI] [PubMed] [Google Scholar]
- 14.Larder B A, Kemp S D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT) Science. 1989;246:1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- 15.Lasky M, Perret J L, Peeters M, Bibollet-Ruche F, Liegeois F, Patrel D, Molinier S, Gras C, Delaporte E. Presence of multiple non-B subtypes and divergent subtype B strains of HIV-1 in individuals infected after overseas deployment. AIDS. 1997;11:43–51. doi: 10.1097/00002030-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Lillehoj E P, Salazar F H R, Mervis R J, Raum M G, Chan H W, Ahmad N, Venkatesan S. Purification and structural characterization of the putative gag-pol protease of human immunodeficiency virus. J Virol. 1988;62:3053–3058. doi: 10.1128/jvi.62.8.3053-3058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louwagie J, McCutchan F E, Peeters M, Brennan T P, Sanders-Buell E, Eddy A, van der Groen G, Fransen K, Gershy-Damet G M, Deleys R, Burke D S. Phylogenetic analysis of gag genes from 70 international HIV-1 isolates provides evidence for multiple genotypes. AIDS. 1993;7:769–780. doi: 10.1097/00002030-199306000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Marquina S, Leitner T, Rabinovich R D, Benetucci J, Libonatti O, Albert J. Coexistence of subtypes B, F, and as B/F env recombinant of HIV type 1 in Buenos Aires, Argentina. AIDS Res Hum Retroviruses. 1996;12:1651–1654. doi: 10.1089/aid.1996.12.1651. [DOI] [PubMed] [Google Scholar]
- 19.Mellors J W, Schinazi R F, Larder B A. Mutations in retroviral genes associated with drug resistance. In: Mayers G, editor. Human retroviruses and AIDS: a compilation and analysis of nucleic acid and and amino acid sequences. Los Alamos, N.Mex: Theoretical Biology and Biophysics, Los Alamos National Laboratory; 1996. pp. 215–235. [Google Scholar]
- 20.Molla A, Korneyeva M, Gao Q, Vasavanonda S, Schipper P J, Mo H M, Markowitz M, Chernyavskiy T, Niu P, Lyons N, Hsu A, Granneman G R, Ho D D, Boucher C A, Leonard J M, Norbeck D W, Kempf D J. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 1996;2:760–766. doi: 10.1038/nm0796-760. [DOI] [PubMed] [Google Scholar]
- 21.Morgado M G, Sabino E C, Shpaer E G, Bongertz V, Brigido L, Guimaraes M D, Castilho E A, Galvao-Castro B, Mullins J I, Hendry R M, Mayer A. V3 region polymorphisms in HIV-1 from Brazil: prevalence of subtype B strains divergent from North American/European prototype and detection of subtype F. AIDS Res Hum Retroviruses. 1994;10:569–576. doi: 10.1089/aid.1994.10.569. [DOI] [PubMed] [Google Scholar]
- 22.Myers G, Korber B, Wain-Hobson S, Smith R, Pavalaskis G N. Human retroviruses and AIDS: a compilation and analysis of nucleic acid and and amino acid sequences. Los Alamos, N.Mex: Theoretical Biology and Biophysics, Los Alamos National Laboratory; 1995. [Google Scholar]
- 23.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3:418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels R, Andries K, Desmyter J, Schols D, Kukla M J, Breslin H J, Raeymaeckers A, Van Gelder J, Woestenborghs R, Heykants J, Schellekans K, Janssen M, de Clercq E, Janssen P. Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature. 1990;343:470–474. doi: 10.1038/343470a0. [DOI] [PubMed] [Google Scholar]
- 25.Potts K E, Kalish M L, Lott T, Orloff G, Luo C C, Bernard M A, Alves C B, Badaro R, Suleiman J, Ferreira O, Schochetman G, Johnson W D, Ou C-Y, Ho J L the Brazilian Collaborative AIDS Research Group. Genetic heterogeneity of the V3 region of the HIV-1 envelope glycoprotein in Brazil. AIDS. 1993;7:1191–1197. doi: 10.1097/00002030-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Quinones-Mateu M E, Holguin A, Dopazo J, Najera I, Domingo E. Point mutant frequencies in the pol gene of human immunodeficiency virus type 1 are two- to threefold lower than those of env. AIDS Res Hum Retroviruses. 1996;12:1117–1128. doi: 10.1089/aid.1996.12.1117. [DOI] [PubMed] [Google Scholar]
- 27.Ridky T, Leis J. Development of drug resistance to HIV-1 protease inhibitors. J Biol Chem. 1995;270:29621–29623. doi: 10.1074/jbc.270.50.29621. [DOI] [PubMed] [Google Scholar]
- 28.Robertson D L, Hahn B H, Sharp P M. Recombination in AIDS viruses. J Mol Evol. 1995;40:249–259. doi: 10.1007/BF00163230. [DOI] [PubMed] [Google Scholar]
- 29.Robertson D L, Sharp P M, McCutchan F E, Hahn B H. Recombination in HIV-1. Nature. 1995;374:124–126. doi: 10.1038/374124b0. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigo A G, Mullins J I. Human immunodeficiency virus type 1 molecular evolution and the measure of selection. AIDS Res Hum Retroviruses. 1996;12:1681–1685. doi: 10.1089/aid.1996.12.1681. [DOI] [PubMed] [Google Scholar]
- 31.Sabino E C, Diaz R S, Brigido L F, Learn G H, Mullins J I, Reingold A L, Duarte A J, Mayer A, Busch M P. Distribution of HIV-1 subtypes seen in an AIDS clinic in Sao Paulo City, Brazil. AIDS. 1996;10:1579–1584. doi: 10.1097/00002030-199611000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Seibert S A, Howell C Y, Hughes M K, Hughes A L. Natural selection on the gag, pol, and env genes of human immunodeficiency virus 1 (HIV-1) Mol Biol Evol. 1995;12:803–813. doi: 10.1093/oxfordjournals.molbev.a040257. [DOI] [PubMed] [Google Scholar]
- 33.Seillier-Moiseiwitsch F, Margolin B H, Swanstrom R. Genetic variability of the human immunodeficiency virus: statistical and biological issues. Annu Rev Genet. 1994;28:559–596. doi: 10.1146/annurev.ge.28.120194.003015. [DOI] [PubMed] [Google Scholar]
- 34.Sham H L, Zhao C, Stewart K D, Betebenner D A, Lin S, Park C H, Kong X P, Rosenbrook W, Jr, Herrin T, Madigan D, Vasavanonda S, Lyons N, Molla A, Saldivar A, Marsh K C, McDonald E, Wideburg N E, Denissen J F, Robins T, Kempf D J, Plattner J J, Norbeck D W. A novel, picomolar inhibitor of human immunodeficiency virus type 1 protease. J Med Chem. 1995;39:392–397. doi: 10.1021/jm9507183. [DOI] [PubMed] [Google Scholar]
- 35.Subbaro, S., and G. Schochetman. 1996. Genetic variability of HIV-1. AIDS 10(Suppl. A):S13–S23. [DOI] [PubMed]
- 36.Tisdale M, Kemp S D, Parry N R, Larder B A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams K J, Loeb L A. Retroviral reverse transcriptases: error frequencies and mutagenesis. Curr Top Microbiol Immunol. 1992;176:165–180. doi: 10.1007/978-3-642-77011-1_11. [DOI] [PubMed] [Google Scholar]
- 38.Winslow D L, Stack S, King R, Scarnati H, Bincsik A, Otto M J. Limited sequence diversity of the HIV type 1 protease gene from clinical isolates and in vitro susceptibility to HIV protease inhibitors. AIDS Res Hum Retroviruses. 1995;11:107–113. doi: 10.1089/aid.1995.11.107. [DOI] [PubMed] [Google Scholar]
- 39.Wolinsky S M, Korber B T, Neumann A U, Daniels M, Kunstman K J, Whetsell A J, Furtado M R, Cao Y, Ho D D, Safrit J T, Koup R A. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science. 1996;272:537–542. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]