Abstract
Background
Extracellular vesicles (EVs) released by blood cells have proinflammation and procoagulant action. Patients with systemic lupus erythematosus (SLE) present high vascular inflammation and are prone to develop cardiovascular diseases. Therefore, we postulated that the EV populations found in blood, including platelet EVs (PEVs) and red blood cell EVs (REVs), are associated with SLE disease activity and SLE-associated cardiovascular accidents.
Method
We assessed autotaxin (ATX) plasma levels by ELISA, the platelet activation markers PAC1 and CD62P, ATX bound to platelets and the amounts of plasma PEVs and REVs by flow cytometry in a cohort of 102 patients with SLE, including 29 incident cases of SLE and 30 controls. Correlation analyses explored the associations with the clinical parameters.
Result
Platelet activation markers were increased in patients with SLE compared with healthy control, with the marker CD62P associated with the SLE disease activity index (SLEDAI). The incident cases show additional associations between platelet markers (CD62P/ATX and PAC1/CD62P) and the SLEDAI. Compared with controls, patients with SLE presented higher levels of PEVs, phosphatidylserine positive (PS+) PEVs, REVs and PS+ REVs, but there is no association with disease activity. When stratified according to the plasma level of PS+ REVs, the group of patients with SLE with a high level of PS+ REVs presented a higher number of past thrombosis events and higher ATX levels.
Conclusion
Incident and prevalent forms of SLE cases present similar levels of platelet activation markers, with CD62P correlating with disease activity. Though EVs are not associated with disease activity, the incidence of past thrombotic events is higher in patients with a high level of PS+ REVs.
Keywords: lupus erythematosus, systemic; lipids; autoimmunity
Key messages.
What is already known about this subject?
Patients with systemic lupus erythematosus (SLE) show high levels of platelet-derived vesicles, and the platelet activation marker CD62P is positively associated with disease activity in SLE.
What does this study add?
Patients with SLE show high levels of erythrocyte-derived vesicles.
A high level of phosphatidylserine positive (PS+) erythrocyte-derived vesicles correlates with elevated levels of platelet-derived and erythrocyte-derived vesicles.
Plasma autotaxin is higher in patients with SLE with a high level of PS+ extracellular vesicles (EVs), and past thrombotic events are positively associated with a high level of PS+ erythrocyte-derived vesicles.
How might this impact on clinical practice or future developments?
The data suggest that a high plasma level of PS+ red blood cell EVs is a potential biomarker for managing the risk of thrombotic events in individuals with SLE.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune rheumatic disease (SARD). Patients with SLE present a wide range of clinical phenotypes. One characteristic of SLE is a high vascular inflammation associated with damages in multiple organs.1 Patients with SLE have a higher risk of dying from cardiovascular diseases.2 The vascular inflammation associated with SLE development relates to an immune response to autoantigens3 4 and a production of type I interferon.5–7
Platelet activation also induces the liberation of extracellular vesicles (EVs).8 EVs are small vesicles liberated by activated cells. The EVs include exosomes and microvesicles. The fusion of multivesicular bodies with the plasma membrane releases the exosomes. Plasma membrane budding generates the microvesicles, which often occurs after cell loss of the membrane phospholipid asymmetry. Some EVs formed after the loss of the plasma membrane asymmetry present the phosphatidylserine (PS) at their surface. EVs from platelets (PEVs) and red blood cells (REVs) can, through the exposition of PS, recruit mediators of the coagulation cascade and initiate the formation of a blood clot.9–11 Patients with SLE present higher levels of platelet activation markers and platelets are a source of autoantigen, notably by the release of free mitochondria.8 12 Patients with SLE have increased circulating levels of EVs, notably from platelet origin,13 14 that serve as a source for interferon-α production and binding sites for immune complexes.15–17 The exosomes are also associated with enhanced proinflammatory cytokine and chemokine production notably type I interferon in SLE.15 16 18
PEVs are the largest EV population in the blood, which are found in higher amounts in the plasma of patients with SLE. However, besides that PEVs are a source of autoantigens and bind immune complexes, there is little knowledge on their role in SLE.14 19 In some studies, there was an association between PEVs and the SLE disease activity index (SLEDAI),14 but no association was reported in others.20 However, PEVs were associated with the progression of atherosclerosis in patients with SLE through the thickening of the vascular wall.19
Platelet activation also releases autotaxin (ATX), a phospholipase with proinflammatory properties. ATX is associated with the pathophysiology of rheumatoid arthritis and cardiovascular diseases.21–23 ATX catalyses the production of lysophosphatidic acid (LPA), one of the few known activators of red blood cells which induce the liberation of REVs.24 25 Patients with SLE are more at risk to suffer from cardiovascular diseases, a leading cause of death for patients with SLE. EVs and platelet activation are factors in the development of cardiovascular diseases in SLE and other autoimmune diseases. PEVs and REVs have proinflammatory and procoagulant activities.9 10 26–28 Since patients with SLE have a high level of plasma EVs, we focused our study on PEVs and REVs, the two most abundant EV populations found in the blood, and the associated vascular events.
Material and methods
Patients with SLE and healthy donors
SARD-BDB (Systemic Autoimmune Rheumatic Disease biobank and database repository of the CHU de Québec-Université Laval) recruited prevalent patients with SLE with a disease duration superior to 15 months and incident patients with SLE with a disease duration equal or under 15 months. The inclusion of patients with SLE into the biobank was according to the 1997 revised American College of Rheumatology (ACR) classification criteria.29 30 In total, we included 102 patients with SLE in our study based on the patients with SLE available at the date of February 2019. We did not apply additional criteria for selecting patients. A control group formed by 30 healthy donors, under no medication and without known illness, was also recruited by SARD-BDB (mean age 50±8 years, female 63.33%).
SARD-BDB protocol
The SARD-BDB provided the plasma and platelet-free plasma (PFP) from patients with SLE. The PFP was processed using previously described standardised protocols.12 Aliquots of the PFP samples were prepared and stored at −80°C to limit thaw-freezing cycles. Patients included in the study gave informed written consent according to the declaration of Helsinki. Patients with SLE had to meet the ACR classification criteria for SLE revised in 1997.29 30 Antiphospholipid syndrome (APS) was diagnosed according to the 2006 revised Sapporo criteria.31 Variables were collected at the time of the first visit to the SARD-BDB including, sociodemographic variables, disease characteristics, clinical variables, common haematology tests, cardiovascular and thrombosis risk factors and current use of medication. The following cut-off for positivity were used for anticardiolipin and anti-β2 glycoprotein I antibodies. For IGM and IGG against cardiolipin, patients were considered normal with value under 10 U/mL, abnormal when they were higher than 40 U/mL and equivocal for other values. For IGM and IGG against β2 Glycoprotein I, patients were considered normal with value under 7 U/mL, abnormal when higher than 10 U/mL, and equivocal for other values.
Flow cytometry
Detection of platelet activation
Platelet-rich plasma was prepared immediately following blood sample collection and used for monitoring platelet activation markers. Five µL of platelet-rich plasma were incubated 30 min at room temperature in the dark with 3 µL of anti-CD41-V450 (BD Bioscience Canada, Mississauga, Ontario, Canada), a marker of platelets, 15 µL of anti-CD62P-APC and 15 µL of anti-PAC1-FITC (BD Bioscience Canada, Mississauga, Ontario, Canada) and anti-ATX (BD Bioscience Canada, Mississauga, Ontario, Canada) in 100 µL of phosphate buffered saline(PBS). The samples were mixed with 400 µL of PBS to stop labelling and analysed using a high sensitivity flow cytometer BD Canto II Special Order Research Product with the gating strategy described in online supplemental figure 1A. The flow cytometer settings were as follows: FSC at 300 V, SSC at 335 V, Pacific blue at 500 V, FITC at 500 V, PE at 500 V and APC at 500 V.
lupus-2021-000605supp002.pdf (627.1KB, pdf)
Detection of plasma EVs
Five µL of PFP were incubated for 30 min at room temperature and in the dark with 3 µL anti-CD41-V450 (BD Bioscience Canada, Mississauga, Ontario, Canada), a marker for platelet-derived EVs, and with 3 µL anti-CD235a-PECy7 (BD Bioscience Canada, Mississauga, Ontario, Canada) to label the REVs. To detect PS exposed on the outer membrane leaflet, we added 3 µL Annexin V FITC (BD Bioscience Canada, Mississauga, Ontario, Canada) to the plasma samples in 100 µL final Annexin V binding buffer (BD Bioscience Canada, Mississauga, Ontario, Canada). The samples were mixed with Annexin V binding buffer (200 µL) to stop labelling and processed under 90 min on a high sensitivity flow cytometer BD Canto II Special Order Research Product with a small particle option as previously described.32 Online supplemental figure 1B shows the gating strategy for the detection of plasma PEVs and REVs. Silica particles of 100, 500 and 1000 nm (Kisker Biotech GmbH, Steinfurt, Germany) allowed to set up a gate differentiating the events of size between 100 and 1000 nm (online supplemental figure 1C). The flow cytometer settings were as follows: FSC at 300 V, SSC at 300 V, PECy7 at 500 V, FITC at 500 V and APC at 500 V. To determine the absolute amounts of REVs and PEVs in the samples, we added known concentrations of 2 µm APC polystyrene beads (BD Bioscience Canada, Mississauga, Ontario, Canada) or 3 µm polystyrene beads (Polysciences, Pennsylvania, USA).
Specificity of EV detection was validated by destroying EVs from the sample by Triton X-100 treatment or pelleting EVs by a 100 000 g ultracentrifugation (online supplemental figure 1D). Labelling in EDTA-supplemented buffer and absence of annexin V buffer was used to validate the specificity of Annexin V labelling (online supplemental figure 1E). Finally, a coincidence test validated that our measurements of PEVs and REVs were quantitative (online supplemental figure 1F, G).
Every day before monitoring platelets activation markers and plasma EVs, a test of performance tracking of high sensitivity flow cytometry was done using BD cytometer setup and tracking beads (BD Bioscience Canada, Mississauga, Ontario, Canada).
Autotaxin measurement
Plasma concentrations of ATX (ng/mL) were quantified using a Human ENPP-2/Autotaxin Quantikine ELISA Kit (R&D Systems, Minneapolis, Minneapolis, USA) and following the manufacturer instructions.
Analysis and statistics
Flow cytometry data analysis used the FlowJo V10 software (FlowJo, Oregon, USA) and the statistical analysis used the GraphPad Prism 7.0 software (GraphPad Software, San Diego, USA) and SAS V.9.4 (SAS Institute, Cary, North Carolina, USA). Comparisons between groups used the Kruskal-Wallis tests with Dunn’s multiple comparison post-test or the Wilcoxon Mann Whitney test for continuous variables, depending on the number of groups. The exact Pearson χ² test was used to compare groups for categorical variables. Spearman’s correlation coefficient (rs) determined the association between continuous variables. Only variables monitored at the first visit (baseline) were considered for the analyses.
Results
Patient characteristics
The characteristics at baseline of the 102 patients with SLE included in the cohort are presented in table 1. For 29 of 102 patients, the SLE diagnosis was 15 months or less before their inclusion in the cohort. These patients that started their treatments recently and had not their SLE under control were considered incident cases. The characteristics of the prevalent and incident cases are detailed in online supplemental table 1). SLE prevalent cases were the patients diagnosed for more than 15 months. Women represented 83% of all SLE cases. About a quarter of the patients had thrombocytopaenia (23.5%). Patients with SLE with renal disorders and APS represented 24.5% and 15.7% of the SLE cohort, respectively. The prevalence of past thrombotic events in the SLE cohort was 11.8%. Furthermore, 30 patients had atherosclerosis plaques, and 42 had no plaques (table 1). The anticoagulant drugs such as aspirin and warfarin were used by 26.47% and 9.80% of patients with SLE, respectively. Neither the use of anticoagulant drugs nor of antimalarials was associated with platelet and EV readouts.
Table 1.
Characteristics for patients with SLE (n=102) included in the study at baseline
| Demographics | Mean±SD or N (%) |
| Age, years | 49.98±14.58 |
| Gender, female | 85 (83.33) |
| BMI | 25.49±4.66 |
| Disease duration, years, n=98 | 10.63±12.07 |
| Onset | |
| Incident | 29 (28.43) |
| Prevalent | 73 (71.57) |
| APS | 16 (15.69) |
| Diabetes | 3 (2.94) |
| Dyslipidaemia | 8 (7.84) |
| ACR criteria n=99 | n (%) |
| Arthritis | 79 (77.45) |
| Thrombocytopaenia | 24 (23.53) |
| Malar rash | 26 (25.49) |
| Discoid rash | 18 (17.65) |
| Haemolytic anaemia | 4 (3.92) |
| Renal disorder | 25 (24.51) |
| Medication | n (%) |
| NSAID/Cox-II inhibitors | 25 (24.51) |
| Antimalarials | 77 (75.49) |
| Immunomodulators | 86 (84.31) |
| Methotrexate | 16 (15.69) |
| Biologic agents | 7 (6.86) |
| Steroids | 22 (21.57) |
| Other DMARD | 28 (27.45) |
| Prednisone | 21 (20.59) |
| Clinical characteristics | Mean±SD or n (%) |
| SLEDAI, n=99 | 3.07±3.70 |
| Platelet, 109 /L, n=101 | 227.20±74.91 |
| MPV, fL, n=101 | 8.92±6.10 |
| Haemoglobin, g/L, n=101 | 129.34±12.29 |
| CRP, mg/L, n=82 | 4.47±7.25 |
| ESR, mm/hour, n=94 | 13.55±16.55 |
| Lupus anticoagulant, n=89 | 10.78 (11) |
| Anticardiolipin IGG, n=87 | |
| Equivocal | 2 (2.30) |
| Abnormal | 13 (14.94) |
| Anticardiolipin IGM, n=87 | |
| Equivocal | 1 (1.15) |
| Abnormal | 12 (13.79) |
| Anti-β2GPI IGG, n=97 | |
| Abnormal | 8 (9.20) |
| Anti-β2GPI IGM, n=87 | |
| Abnormal | 15 (17.24) |
| Cardiovascular damages | Mean±SD or n (%) |
| History of thrombosis* | |
| Venous | 7 (6.86) |
| Arterial | 4 (3.92) |
| Microcirculation | 1 (0.98) |
| Plaque, n=72 | 30 (29.41) |
| CIMT, mm, n=35 | 0.62±0.12 |
Antimalarials include hydroxychloroquine and chloroquine
*The numbers refer to the number of patients, not to the number of past cardiovascular events.
ACR, American college of rheumatology; APS, antiphospholipid syndrome; BMI, body mass index; CIMT, carotid intima-media thickness; CRP, C reactive protein; DMARD, disease modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; MPV, mean platelet volume; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation; SLEDAI, SLE disease activity index.
lupus-2021-000605supp001.pdf (77.4KB, pdf)
Patients with SLE present higher platelet activation and plasma EV levels at baseline
When all the prevalent and the incident cases were considered at baseline, patients with SLE present a higher percentage of platelet with the activation marker PAC1 and CD62P by comparison to healthy controls (table 2). In addition, a higher number of platelets were positive for the phospholipase ATX (table 2). ATX is known to be secreted by and bind the integrins of activated platelets.33–35 However, the total ATX plasma level was not different between the SLE and the healthy group (table 2). The plasma of patients with SLE shows high levels of platelet-derived and RBC-derived EVs, including those exposing PS at their surface (table 2). Furthermore, 79.3% of the plasma PEVs and 23.6% of the plasma REVs were PS+ in the control group, and this proportion increased respectively to 87.8% and 45.4% in the SLE cohort.
Table 2.
High platelet activation and EV levels are found in patients with SLE at baseline
| Healthy | SLE | Pvalue | |
| (n=30) | (n=98) | ||
| ATX, ng/mL | 257.8 (219.9; 340.1) | 251.4 (199.1; 333.9) | 0.2442 |
| Platelet, % | (n=30) | (n=40) | |
| PAC1+ | 3.65 (1.10; 8.50) | 7.85 (2.88; 15.75) | 0.0140 |
| PAC1+ ATX+ | 0.60 (0.20; 1.00) | 1.90 (0.60; 4.30) | 0.0042 |
| CD62P+ | 2.35 (1.70; 4.30) | 5.55 (2.33; 9.53) | 0.0059 |
| CD62P+ ATX+ | 0.70 (0.30; 0.80) | 1.35 (0.60; 4.35) | 0.0046 |
| PAC1+ CD62P+ | 0.70 (0.40; 1.40) | 2.00 (0.90; 4.98) | 0.0006 |
| PAC1+ CD62P+ ATX+ | 0.28 (0.15; 0.63) | 0.85 (0.379; 3,24) | 0.0020 |
| EVs, EVs/µL | (n=30) | (n=102) | |
| PEVs | 479 (275; 1074) | 3569 (1752; 7144) | <0.0001 |
| PS+ PEVs | 377 (221; 816) | 3228 (1601; 6260) | <0.0001 |
| REVs | 424 (296; 603) | 1936 (851; 4495) | <0.0001 |
| PS+ REVs | 91 (55; 144) | 1064 (171; 2294) | <0.0001 |
Results are presented as median (IQR), pvalue based on Wilcoxon Mann Whitney test. Significant pvalues are in bold.
ATX, autotaxin; EV, extracellular vesicle; IQR, interquartile range; PEV, platelet EV; PS+, phosphatidylserine positive; REV, red blood cell EV; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index.
Prevalent and incident patients with SLE show similar levels of plasma EVs and platelet activation
We investigated the difference between recently diagnosed and established cases of SLE by comparing the incident and prevalent cases (n=29 and n=73, respectively). We monitored three platelet activation markers (PAC1, CD62P and platelet-bound ATX), as well as plasma ATX levels and the amounts of platelet-derived or RBC-derived EVs including those exposing PS.
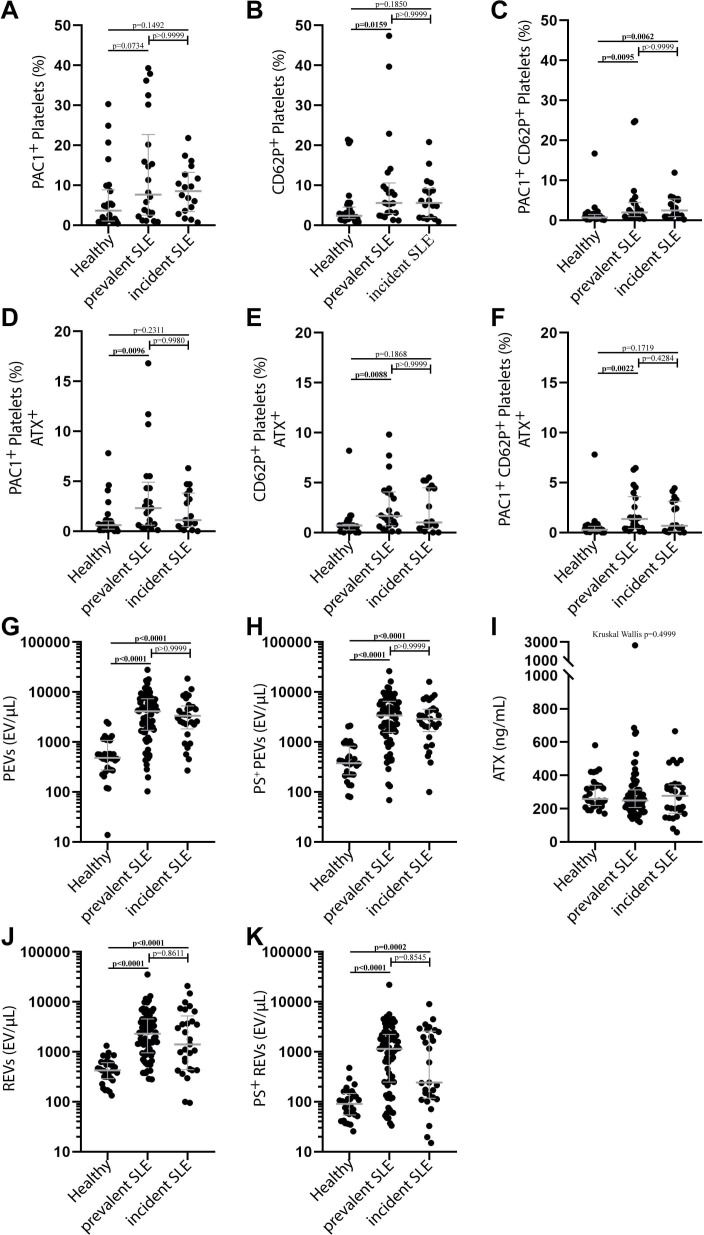
The incident SLE cases show no significant difference for the platelet activation marker PAC1 or CD62P compared with healthy control (figure 1A, B). In comparison to healthy patients, we report a significantly higher percentage of platelets exposing both the activation markers PAC1 and CD62P (figure 1C). The prevalent and the incident SLE cases did not show a significant difference for the platelet activation marker PAC1 compared with healthy controls (figure 1A). In contrast, the amounts of CD62P positive for prevalent patients (figure 1B) and PAC1-CD62P double-positive platelets for both prevalent and incident patients (figure 1C) were significantly different from the control group. Compared with healthy controls, the levels of double-positive PAC1-ATX (figure 1D), double-positive CD62P-ATX (figure 1E) and triple-positive PAC1-CD62P-ATX platelets (figure 1F) were significantly different from those of prevalent but not those of incident SLE cases. ATX plasma concentration in healthy controls, prevalent and incident SLE cases are similar (figure 1I). However, plasma EV levels in prevalent and incident cases were significantly different from those of the control group (figure 1G, H). Nevertheless, the levels of platelet activation markers and plasma EVs of the prevalent SLE were not significantly different from those of the incident SLE cases (figure 1A–F). Platelet activation is associated with the SLEDAI score in incident cases of SLE.
Figure 1.
High platelet activation and EV levels are found in incident and prevalent patients with SLE at baseline. Percentage of platelets expressing activation markers PAC1 (A), CD62P (B) and the combination of PAC1 and CD62P (C) for the healthy (n=30), prevalent (n=22) and incident SLE (n=18) group. Percentage of platelets expressing ATX in combination with activation markers PAC1 (D), CD62P (E) or with both PAC1 and CD62P (F) for the healthy (n=30), prevalent (n=22) and incident SLE (n=18) group. (G) Number of plasma PEVs (G) and PS+ PEVs (H) for the healthy (n=30), prevalent (n=73) and incident SLE (n=29) group. Plasma concentration of ATX for the healthy (n=30), prevalent (n=70) and incident SLE (n=28) (I). Amounts of plasma REVs (J) and PS+ REVs (K) for the healthy (n=30), prevalent (n=73) and incident SLE (n=29) group. Data are reported as median with IQR, Kruskal-Wallis test with Dunnett post-test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. ATX, autotaxin; EV, extracellular vesicle; IQR, interquartile range; PEV, platelet EV; REV, red blood cell EV; SLE, systemic lupus erythematosus.
Despite significant increases in the incident and prevalent SLE groups, there was no association between the EV populations and the SLEDAI score (table 3). Only CD62P, a marker of platelet activation, was consistently associated with a higher SLEDAI score for the incident and prevalent cases, or when we considered the whole SLE cohort patients (table 3). However, incident SLE cases show additional associations with platelet activation markers as the levels of platelets highly positive for both CD62P and ATX or CD62P and PAC1 are significantly associated with the SLEDAI score (table 3).
Table 3.
Spearman correlation between the platelet activation markers, EV levels and the SLEDAI score
| All SLE | Prevalent SLE | Incident SLE | |||||||
| n | rs | Pvalue | n | rs | Pvalue | n | rs | Pvalue | |
| Plasma ATX | 95 | −0.17 | 0.0998 | 69 | −0.17 | 0.1695 | 26 | −0.18 | 0.3717 |
| Platelet activation | 39 | 22 | 17 | ||||||
| PAC1+ | 0.09 | 0.5859 | −0.08 | 0.7141 | 0.46 | 0.0646 | |||
| PAC1+ ATX+ | 0.02 | 0.9032 | −0.18 | 0.4273 | 0.41 | 0.1040 | |||
| CD62P+ | 0.48 | 0.0021 | 0.43 | 0.0479 | 0.59 | 0.0127 | |||
| CD62P+ ATX+ | 0.19 | 0.2492 | 0.03 | 0.8818 | 0.56 | 0.0207 | |||
| PAC1+ CD62P+ | 0.27 | 0.0927 | 0.01 | 0.9790 | 0.65 | 0.0045 | |||
| PAC1+ CD62P+ ATX+ | 0.12 | 0.4565 | −0.04 | 0.8600 | 0.43 | 0.0817 | |||
| PEVs | 99 | 72 | 27 | ||||||
| Total | 0.06 | 0.5377 | 0.01 | 0.9093 | 0.28 | 0.1574 | |||
| PS+ | 0.05 | 0.6389 | 0.00 | 0.9842 | 0.25 | 0.2122 | |||
| REVs | 99 | 72 | 27 | ||||||
| Total | −0.07 | 0.4806 | −0.09 | 0.4623 | 0.04 | 0.8275 | |||
| PS+ | −0.08 | 0.4274 | −0.12 | 0.3166 | 0.16 | 0.4325 | |||
Significant pvalues are in bold.
ATX, autotaxin; EV, extracellular vesicle; PEV, platelet EV; PS+, phosphatidylserine positive; REV, red blood cell EV; SLE, systemic lupus erythematosus.
Higher PS+ REVs are associated with vascular damages in patients with SLE
The percentage of PS+ REVs increased from 23.6% in the control group to 45.7% for patients with SLE. In addition, we perceived a double distribution of PS+ REVs in patients with SLE (figure 1K). Next, we investigated if high PS+ REV levels in patients with SLE had clinical significance. Incident and prevalent patients with SLE did not present differences in the plasma levels of ATX, platelet activation markers and for both RBC and platelet EVs (figure 1A–K). Therefore, we choose not to distinguish between the incident and prevalent SLE cases while analysing the potential clinical implications of high plasma levels of PS+ REVs. Based on the levels of PS+ REVs, we divided the SLE cohort into two distinct groups (figure 1K). We applied a cut-off at 1000 EV/µL to distinguish the patients with a level of plasma PS+ REVs within the range of the healthy controls from those with EV concentrations superior to the cut-off. The latter were considered high plasma levels of PS+ REVs. Fifty-two patients with SLE (51%) had a plasma PS+ REV number over the cut-off level. The 50 patients with SLE with a concentration of plasma PS+ REVs lower than the cut-off were considered “low” for this biomarker.
As anticipated, the patients with SLE with a higher level of PS+ REVs also show a higher number of REVs and PEVs (total and PS+) compared with those with ‘low’ concentrations of PS+ REVs (table 4). Furthermore, the high PS+ REV levels are associated with a lower platelet count and a high level of plasma ATX (table 4). The high PS+ REV counts were not associated with the disease duration but were associated with past thrombotic events (table 4). Only two patients (4%) with low plasma PS+ REV levels had a history of venous thrombosis. Among patients with high PS+ REVs, 5 (10%) presented a history of venous thrombosis, 4 (8%) of arterial thrombosis and 1 (2%) of microcirculation thrombosis. Since patients with APS are more at risk to develop thrombosis, we performed the analyses without considering patients with SLE with APS. The incidence of past thrombotic events in the group with high PS+ REVs was still statistically higher when we excluded the patients with APS from the analyses (online supplemental table 2). In this group of patients, the plasma levels of PS+ REVs were not significantly associated with the presence of autoantibodies (table 4). Surprisingly, patients with high amounts of PS+ REVs tend to have a lower SLEDAI score, and APS incidence tended to be higher than for patients with low PS+ REV levels (table 4).
Table 4.
Comparison of patients with SLE with low and high PS+ REVs
| Demographic characteristics | PS+ REV | |||
| Low | High | Pvalue | ||
| Gender | Female | 44 (88) | 41 (79) | 0.2897 |
| Age, years | 52.00 (41.00; 62.00) | 50.50 (37.50; 59.00) | 0.5592 | |
| BMI | 25.35 (22.43; 29.04) | 23.59 (21.46; 28.50) | 0.1062 | |
| Disease duration, years | 7.93 (0.76; 21.64) | 3.58 (1.41; 15.87) | 0.1426 | |
| Onset | Incident | 16 (32) | 13 (25) | 0.5124 |
| Prevalent | 34 (68) | 39 (75) | ||
| APS | 4 (8) | 12 (23) | 0.0551 | |
| Clinical characteristics | ||||
| SLEDAI | 2.00 (0.00; 6.00) | 1.50 (0.00; 4.00) | 0.0776 | |
| Platelet, 109 /L | 242.00 (195.00; 291.00) | 202.00 (176.50; 246.00) | 0.0262 | |
| MPV, fL | 8.70 (8.00; 10.00) | 8.90 (8.05; 9.55) | 0.6992 | |
| Haemoglobin, g/L | 129.00 (121.00; 137.00) | 131.00 (124.00; 138.00) | 0.3264 | |
| CRP, mg/L | 2.90 (1.21; 3.42) | 2.00 (1.00; 5.00) | 0.7683 | |
| ESR, mm/hour | 8.00 (4.00; 20.00) | 6.00 (3.00; 14.00) | 0.1674 | |
| Lupus anticoagulant | Presence | 4 (10) | 7 (15) | 0.5374 |
| Anticardiolipin IGG | Abnormal | 3 (8) | 10 (20) | 0.0674 |
| Equivocal | 2 (5) | 0 (0) | ||
| Anticardiolipin IGM | Abnormal | 3 (8) | 9 (18) | 0.1633 |
| Equivocal | 1 (3) | 0 (0) | ||
| Anti-β2GPI IGG | Abnormal | 1 (3) | 7 (14) | 0.1306 |
| Anti-β2GPI IGM | Abnormal | 4 (11) | 11 (22) | 0.2521 |
| Laboratory measurements | ||||
| ATX, ng/mL | 227.75 (183.10; 295.30) | 274.50 (207.83; 408.73) | 0.0318 | |
| PEVs, EVs/µL | 2419 (1012; 4566) | 4853 (2532; 8230) | 0.0006 | |
| PS+ PEVs, EVs/µL | 2042 (866; 4051) | 4384 (2238; 7696) | 0.0006 | |
| REVs, EVs/µL | 842 (426; 1387) | 4436 (3064; 7166) | <0.0001 | |
| ACR criteria | ||||
| Arthritis | 37 (76) | 42 (84) | 0.3262 | |
| Thrombocytopaenia | 9 (18) | 15 (30) | 0.2414 | |
| Malar rash | 17 (35) | 9 (18) | 0.0705 | |
| Discoid rash | 11 (22) | 7 (14) | 0.3080 | |
| Haemolytic anaemia | 3 (6) | 1 (2) | 0.3622 | |
| Renal disorder | 12 (24) | 13 (26) | 1.0000 | |
| Cardiovascular damages | ||||
| History of thrombosis | 2 (4) | 10 (19) | 0.0283 | |
| Medication | ||||
| NSAID/ Cox-II Inhibitors | 14 (28) | 11 (21) | 0.4931 | |
| Prednisone | 8 (16) | 13 (25) | 0.3299 | |
| Immunomodulators | 42 (86) | 44 (88) | 0.7742 | |
Continuous variables are presented as median (IQR), pvalue based on Wilcoxon Mann Whitney test. Categorical variables are presented as n (%), pvalue based on exact Pearson χ² test. Significant pvalues are in bold.
ACR, American College of Rheumatology; APS, antiphospholipid syndrome; ATX, autotaxin; BMI, body mass index; CIMT, carotid intima-media thickness; CRP, C reactive protein; DMARD, disease modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; EV, extracellular vesicle; MPV, mean platelet volume; NSAID, non-steroidal anti-inflammatory drug; PEV, platelet EV; PS+, phosphatidylserine positive; REV, red blood cell EV; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index.
We verified if the history of past thrombotic events and high plasma PS+ PEV levels were positively associated. For these analyses, we divided the SLE cohort based on the levels of PS+ PEVs. Since PS+ PEVs show a continuous distribution, contrary to PS+ REVs, we considered all patients with quantities higher than the maximum of controls as ‘high’ and the rest as ‘low’. There is no statistical difference in the incidence of past thrombotic events between the two groups. Besides, plasma ATX concentration was significantly higher in the group with a high level of PS+ PEVs (online supplemental table 3). In addition, the plasma PS+ PEV levels correlated with disease duration. The group with a high plasma PS+ PEV levels correlated with a shorter disease duration (online supplemental table 3). We further determined with Spearman rank correlation that plasma ATX concentrations correlated in patients with SLE with the quantities of PEVs and REVs (respectively, r2=0.350, pvalue=0.0004 and r2=0.272, pvalue=0.0066).
Discussion
In this study, we report on a high plasma level of REVs in patients with SLE. Furthermore, the percentage of PS+ REVs in the plasma of patients with SLE almost doubled compared with the healthy group. The patients with a high level of plasma REVs also have elevated plasma PEVs. There was no correlation between the EV levels, including those that are PS+, and the SLEDAI. About half of the patients with SLE had PS+ REV levels like the healthy controls. However, a high level of PS+ REVs was positively associated with a lower platelet concentration and a higher incidence of past thrombotic events.
A high level of platelet activation markers and plasma PEVs were reported previously in patients with SLE.36 CD62P (P-selectin) is only present on the activated platelet following the fusion of the α-granules with the plasma membrane.37 The platelet activation marker CD62P (P-selectin) is elevated in patients with SLE and is positively associated with the SLEDAI score.36 This study confirms the positive association between the number of CD62P+ platelets and disease activity. The stratification of the incident and prevalent cases of SLE did not highlight differences regarding the levels of plasma EVs, PS+ EVs and platelet activation markers. In incident SLE cases, the percentages of platelets positive for CD62P, CD62P/ATX or PAC1/ATX double-positive, and PAC1/CD32P/ATX triple-positive, were not statistically different from the healthy group. It may be due to the small number of patients with newly diagnosed SLE included in the present study. However, in the incident and prevalent cases, the SLEDAI score correlated with platelet activation as monitored by the cell surface exposure of CD62P. In incident SLE cases, we found other associations between the platelet activation markers and the SLEDAI score. Those include the CD62P/ATX and CD62P/PAC1 double-positive platelets. PAC1 monitor the activation of αIIbβ3 integrin complex in platelets38 39 Of note, the ATX stored in α-granules and released on platelet activation can bind the platelet αIIbβ3 integrin.33–35 It would suggest that the liberation of α-granule and the activated form of platelet integrins contributes to the early phase of SLE disease progression. While, at later stages, when the disease becomes chronic, only the liberation of the content of α-granule is of importance.
It was possible to divide the patients with SLE based on the levels of plasma PS+ REVs. About half of the patients had plasma levels of PS+ REVs below the threshold of 1000 REVs/µL found in healthy controls. The other half were patients with SLE with high to very high levels of PS+ REVs. The results suggest that a high amount of plasma PS+ REVs is associated with a higher incidence of past cardiovascular events. Similar analyses based on the levels of PS+ PEVs yield no significant association between a high level of PS+ PEVs and the incidence of past thrombotic events. Thus, a high amount of PS+ REVs is specifically associated with history of past cardiovascular events. A longitudinal study on incident cases with no antecedent thrombosis or cardiovascular diseases would confirm if patients with SLE with a high level of plasma PS+ REVs are more at risk of future thrombosis. REVs exposing PS+ can recruit different actors of the coagulation cascade and be a source for the generation of large quantities of thrombin.10 40 REVs are also a source of the von Willebrand factor.41 Patients with SLE with a high plasma level of PS+ REVs also show high amounts of plasma ATX. ATX is the enzyme that produces LPA.42 The binding of ATX to αIIbβ3 integrin of activated platelets enhance its catalytic activity.33 34 Though we did not monitor the plasma LPA levels, a role for LPA in RBC activation and production of PS+ REVs cannot be excluded.24 43
Recent studies highlighted a possible role for phosphatidylserine-specific phospholipase A1 in SLE physiopathogenesis.44 45 Serum levels of phosphatidylserine-specific phospholipase A1 are significantly higher in patients with SLE with high disease activity. Besides, patient treatment with immunosuppressive therapies lowered the amount of serum phosphatidylserine-specific phospholipase A.44 Serum phosphatidylserine-specific phospholipase A1 and ATX are also higher in patients with lupus nephritis.45 However, there was an inverse correlation between the levels of serum ATX and disease activity.45 Of note, we observed that patients with low plasma PS+ REV level tend to have a higher SLEDAI and lower levels of plasma ATX. The role of ATX and phosphatidylserine-specific phospholipase A1 in SLE pathophysiology is not known. Increased expression of phosphatidylserine-specific phospholipase A1 is associated with many pathological conditions, including autoimmune and cardiovascular diseases.46 Phosphatidylserine-specific phospholipase A1 can hydrolyse PS into lysoPS, and ATX can hydrolyse the lysoPS into LPA.46 On one side, long-chain lysoPS may contribute to immune cell activation, including macrophages.47 On the other side, stimulation of red blood cells with LPA induces the liberation of REVs.24 25 LPA induces the production of REVs by red blood cells in a concentration-dependent and LPA species-dependent manner through activation of LPAR3.48 Low concentrations of LPA induce the production of PS- REVs while the production of PS+ REVs by red blood cells requires concentrations of LPA ≥5 µM.48 Further studies should determine if the patients with SLE with a high plasma level of PS+ REVs and ATX also show elevated amounts of phosphatidylserine-specific phospholipase A1.
Atherosclerosis is a lead cause of cardiovascular incidents and is a known comorbidity factor in SLE.49–53 PEVs exposing PS were associated with accelerated thickening of the intima-media in patients with SLE.19 In addition, several EV populations promote the development of atherosclerosis through the recruitment of immune cells in the vascular wall and the production of cytokines.54–56 Besides, through the uptake of EVs, vascular wall infiltrated macrophages accumulate lipids and transform into foam cells.57–59 Macrophages phagocyte the PS+ EVs at a higher rate.60 Therefore, even if PS+ REVs are not associated with SLE progression, they still could be implicated in the progression of atherosclerosis and thrombotic events associated with SLE. We did not dispose of sufficient measurements to investigate a potential link between the levels of PS+ REVs and the carotid intima-media thickness test. The overtime impacts of PS+ REVs and other blood cell-derived PS+ EVs on the thickening of carotid intima-media of patients with SLE would be worth investigating in patients with SLE.
In summary, patients with SLE show a high number of plasma PEVs and REVs and high surface exposure of the platelet activation markers PAC1 and CD62P compared with controls. There are no significant differences between the incident and prevalent cases of SLE. The levels of EVs do not correlate with the SLEDAI score. However, the analyses of CD62P exposure on the platelet surface show an association with SLEDAI, consistent with the documented association between this platelet activation marker and disease activity. We divided patients into groups with low and high PS+ REV levels. The analyses show a lower platelet concentration and a higher incidence of past thrombotic events in patients with SLE with a high level of PS+ REVs. There was no association between the history of thrombosis and elevated plasma PS+ PEV levels. Further studies are required to determine if the plasma level of PS+ REVs is a potential biomarker for managing the cardiovascular risk of individuals with SLE.
Acknowledgments
We are grateful to the patients who participated in this study. We thank the Fonds Pierre Borgeat sur l'Arthrite et les Maladies Rhumatismales from Laval University for a doctoral recruitment scholarship to Stephan Hasse.
Footnotes
Contributors: SGB conceived and supervised the experiments. SGB and SH designed the experiments. PRF managed the recruitment of patients with SLE, the clinical data and the collection of blood samples. SH, A-CD and CZ performed the experiments. SH and A-SJ performed the statistical analyses. SH drafted the manuscript. SGB, A-SJ and SH reviewed and edited the final manuscript. SGB, EB and PRF applied for funding. All authors have read and agreed to the published version of the manuscript. SGB is responsible for overall content and acts as guarantor.
Funding: This project was supported by a research grant from the Canadian Institutes for Health Research (MOP-142210).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The ethics review board of the CHU de Québec-Université Laval reviewed and validated the study (Project # 2016-2558). Participants gave informed consent to participate in the study before taking part.
References
- 1.Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016;2:16039. 10.1038/nrdp.2016.39 [DOI] [PubMed] [Google Scholar]
- 2.Yurkovich M, Vostretsova K, Chen W, et al. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res 2014;66:608–16. 10.1002/acr.22173 [DOI] [PubMed] [Google Scholar]
- 3.Zhu H, Luo H, Yan M, et al. Autoantigen microarray for high-throughput autoantibody profiling in systemic lupus erythematosus. Genomics Proteomics Bioinformatics 2015;13:210–8. 10.1016/j.gpb.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaniv G, Twig G, Shor DB-A, et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev 2015;14:75–9. 10.1016/j.autrev.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 5.Crow MK, Olferiev M, Kirou KA. Targeting of type I interferon in systemic autoimmune diseases. Transl Res 2015;165:296–305. 10.1016/j.trsl.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stetson DB. Endogenous retroelements and autoimmune disease. Curr Opin Immunol 2012;24:692–7. 10.1016/j.coi.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lövgren T, Eloranta M-L, Båve U, et al. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum 2004;50:1861–72. 10.1002/art.20254 [DOI] [PubMed] [Google Scholar]
- 8.Lood C, Tydén H, Gullstrand B, et al. Decreased platelet size is associated with platelet activation and anti-phospholipid syndrome in systemic lupus erythematosus. Rheumatology 2017;56:408–16. 10.1093/rheumatology/kew437 [DOI] [PubMed] [Google Scholar]
- 9.Kim Y, Xia BT, Jung AD, et al. Microparticles from stored red blood cells promote a hypercoagulable state in a murine model of transfusion. Surgery 2018;163:423–9. 10.1016/j.surg.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipets EN, Antonova OA, Shustova ON, et al. Use of Thrombodynamics for revealing the participation of platelet, erythrocyte, endothelial, and monocyte microparticles in coagulation activation and propagation. PLoS One 2020;15:e0227932. 10.1371/journal.pone.0227932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tripisciano C, Weiss R, Eichhorn T, et al. Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep 2017;7:6522. 10.1038/s41598-017-03262-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melki I, Allaeys I, Tessandier N, et al. Platelets release mitochondrial antigens in systemic lupus erythematosus. Sci Transl Med 2021;13. 10.1126/scitranslmed.aav5928. [Epub ahead of print: 17 02 2021]. [DOI] [PubMed] [Google Scholar]
- 13.Sellam J, Proulle V, Jüngel A, et al. Increased levels of circulating microparticles in primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther 2009;11:R156. 10.1186/ar2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López P, Rodríguez-Carrio J, Martínez-Zapico A, et al. Circulating microparticle subpopulations in systemic lupus erythematosus are affected by disease activity. Int J Cardiol 2017;236:138–44. 10.1016/j.ijcard.2017.02.107 [DOI] [PubMed] [Google Scholar]
- 15.Salvi V, Gianello V, Busatto S, et al. Exosome-delivered microRNAs promote IFN-α secretion by human plasmacytoid DCs via TLR7. JCI Insight 2018;3. 10.1172/jci.insight.98204. [Epub ahead of print: 17 05 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato Y, Park J, Takamatsu H, et al. Apoptosis-derived membrane vesicles drive the cGAS-STING pathway and enhance type I IFN production in systemic lupus erythematosus. Ann Rheum Dis 2018;77:1507–15. 10.1136/annrheumdis-2018-212988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominguez-Gutierrez PR, Ceribelli A, Satoh M, et al. Positive correlation of STAT1 and miR-146a with anemia in patients with systemic lupus erythematosus. J Clin Immunol 2014;34:171–80. 10.1007/s10875-013-9973-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong C, Zhou Q, Fu T, et al. Circulating exosomes Derived-miR-146a from systemic lupus erythematosus patients regulates senescence of mesenchymal stem cells. Biomed Res Int 2019;2019:6071308. 10.1155/2019/6071308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fortin PR, Cloutier N, Bissonnette V, et al. Distinct subtypes of Microparticle-containing immune complexes are associated with disease activity, damage, and carotid intima-media thickness in systemic lupus erythematosus. J Rheumatol 2016;43:2019–25. 10.3899/jrheum.160050 [DOI] [PubMed] [Google Scholar]
- 20.Pereira J, Alfaro G, Goycoolea M, et al. Circulating platelet-derived microparticles in systemic lupus erythematosus. association with increased thrombin generation and procoagulant state. Thromb Haemost 2006;95:94–9. [PubMed] [Google Scholar]
- 21.Miyabe Y, Miyabe C, Iwai Y, et al. Activation of fibroblast-like synoviocytes derived from rheumatoid arthritis via lysophosphatidic acid-lysophosphatidic acid receptor 1 cascade. Arthritis Res Ther 2014;16:461. 10.1186/s13075-014-0461-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyabe Y, Miyabe C, Iwai Y, et al. Necessity of lysophosphatidic acid receptor 1 for development of arthritis. Arthritis Rheum 2013;65:2037–47. 10.1002/art.37991 [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Kraemer M, Fang XF, et al. Lpa receptor 4 deficiency attenuates experimental atherosclerosis. J Lipid Res 2019;60:972–80. 10.1194/jlr.M091066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen DB, Ly TBT, Wesseling MC, et al. Characterization of microvesicles released from human red blood cells. Cell Physiol Biochem 2016;38:1085–99. 10.1159/000443059 [DOI] [PubMed] [Google Scholar]
- 25.Khandoga AL, Pandey D, Welsch U, et al. GPR92/LPA₅ lysophosphatidate receptor mediates megakaryocytic cell shape change induced by human atherosclerotic plaques. Cardiovasc Res 2011;90:157–64. 10.1093/cvr/cvq369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melki I, Tessandier N, Zufferey A, et al. Platelet microvesicles in health and disease. Platelets 2017;28:214–21. 10.1080/09537104.2016.1265924 [DOI] [PubMed] [Google Scholar]
- 27.Zecher D, Cumpelik A, Schifferli JA. Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arterioscler Thromb Vasc Biol 2014;34:313–20. 10.1161/ATVBAHA.113.302378 [DOI] [PubMed] [Google Scholar]
- 28.Fischer D, Büssow J, Meybohm P, et al. Microparticles from stored red blood cells enhance procoagulant and proinflammatory activity. Transfusion 2017;57:2701–11. 10.1111/trf.14268 [DOI] [PubMed] [Google Scholar]
- 29.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis & Rheumatism 1982;25:1271–7. 10.1002/art.1780251101 [DOI] [PubMed] [Google Scholar]
- 30.Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 31.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006;4:295–306. 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 32.Marcoux G, Duchez A-C, Cloutier N, et al. Revealing the diversity of extracellular vesicles using high-dimensional flow cytometry analyses. Sci Rep 2016;6:35928. 10.1038/srep35928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fulkerson Z, Wu T, Sunkara M, et al. Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J Biol Chem 2011;286:34654–63. 10.1074/jbc.M111.276725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pamuklar Z, Federico L, Liu S, et al. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. Journal of Biological Chemistry 2009;284:7385–94. 10.1074/jbc.M807820200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leblanc R, Lee S-C, David M, et al. Interaction of platelet-derived autotaxin with tumor integrin αvβ3 controls metastasis of breast cancer cells to bone. Blood 2014;124:3141–50. 10.1182/blood-2014-04-568683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boilard E, Blanco P, Nigrovic PA. Platelets: active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol 2012;8:534–42. 10.1038/nrrheum.2012.118 [DOI] [PubMed] [Google Scholar]
- 37.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev 2009;23:177–89. 10.1016/j.blre.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shattil SJ, Hoxie JA, Cunningham M, et al. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem 1985;260:11107–14. 10.1016/S0021-9258(17)39154-8 [DOI] [PubMed] [Google Scholar]
- 39.Lu Q, Malinauskas RA. Comparison of two platelet activation markers using flow cytometry after in vitro shear stress exposure of whole human blood. Artif Organs 2011;35:137–44. 10.1111/j.1525-1594.2010.01051.x [DOI] [PubMed] [Google Scholar]
- 40.Van Der Meijden PEJ, Van Schilfgaarde M, Van Oerle R, et al. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost 2012;10:1355–62. 10.1111/j.1538-7836.2012.04758.x [DOI] [PubMed] [Google Scholar]
- 41.Straat M, van Hezel ME, Böing A, et al. Monocyte-Mediated activation of endothelial cells occurs only after binding to extracellular vesicles from red blood cell products, a process mediated by β-integrin. Transfusion 2016;56:3012–20. 10.1111/trf.13851 [DOI] [PubMed] [Google Scholar]
- 42.Tanaka M, Okudaira S, Kishi Y, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem 2006;281:25822–30. 10.1074/jbc.M605142200 [DOI] [PubMed] [Google Scholar]
- 43.Chung S-M, Bae O-N, Lim K-M, et al. Lysophosphatidic acid induces thrombogenic activity through phosphatidylserine exposure and procoagulant microvesicle generation in human erythrocytes. Arterioscler Thromb Vasc Biol 2007;27:414–21. 10.1161/01.ATV.0000252898.48084.6a [DOI] [PubMed] [Google Scholar]
- 44.Sawada T, Kurano M, Shirai H, et al. Serum phosphatidylserine-specific phospholipase A1 as a novel biomarker for monitoring systemic lupus erythematosus disease activity. Int J Rheum Dis 2019;22:2059–66. 10.1111/1756-185X.13689 [DOI] [PubMed] [Google Scholar]
- 45.Iwata Y, Kitajima S, Yamahana J, et al. Higher serum levels of autotaxin and phosphatidylserine-specific phospholipase A1 in patients with lupus nephritis. Int J Rheum Dis 2021;24:231–9. 10.1111/1756-185X.14031 [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Hasse S, Bourgoin SG. Phosphatidylserine-Specific phospholipase A1: a friend or the devil in disguise. Prog Lipid Res 2021;83:101112. 10.1016/j.plipres.2021.101112 [DOI] [PubMed] [Google Scholar]
- 47.Khandelwal N, Shaikh M, Mhetre A, et al. Fatty acid chain length drives lysophosphatidylserine-dependent immunological outputs. Cell Chem Biol 2021;28:1169–79. 10.1016/j.chembiol.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasse S, Duchez A-C, Fortin P, et al. Interplay between LPA2 and LPA3 in LPA-mediated phosphatidylserine cell surface exposure and extracellular vesicles release by erythrocytes. Biochem Pharmacol 2021;192:114667. 10.1016/j.bcp.2021.114667 [DOI] [PubMed] [Google Scholar]
- 49.Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers 2019;5:56. 10.1038/s41572-019-0106-z [DOI] [PubMed] [Google Scholar]
- 50.Gustafsson JT, Simard JF, Gunnarsson I, et al. Risk factors for cardiovascular mortality in patients with systemic lupus erythematosus, a prospective cohort study. Arthritis Res Ther 2012;14:R46. 10.1186/ar3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan MJ. Premature vascular damage in systemic lupus erythematosus. Autoimmunity 2009;42:580–6. 10.1080/08916930903002479 [DOI] [PubMed] [Google Scholar]
- 52.Urowitz MB, Ibañez D, Gladman DD. Atherosclerotic vascular events in a single large lupus cohort: prevalence and risk factors. J Rheumatol 2007;34:70–5. [PubMed] [Google Scholar]
- 53.Organization WH . Cardiovascular diseases (CVDs) fact sheet. 2017, 2017. Available: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 54.Fu Z, Zhou E, Wang X, et al. Oxidized low-density lipoprotein-induced microparticles promote endothelial monocyte adhesion via intercellular adhesion molecule 1. Am J Physiol Cell Physiol 2017;313:C567–74. 10.1152/ajpcell.00158.2016 [DOI] [PubMed] [Google Scholar]
- 55.Li C, Li S, Zhang F, et al. Endothelial microparticles-mediated transfer of microRNA-19b promotes atherosclerosis via activating perivascular adipose tissue inflammation in apoE−/− mice. Biochem Biophys Res Commun 2018;495:1922–9. 10.1016/j.bbrc.2017.11.195 [DOI] [PubMed] [Google Scholar]
- 56.Gao W, Liu H, Yuan J, et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF ‐α mediated NF ‐κB pathway. J Cell Mol Med 2016;20:2318–27. 10.1111/jcmm.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nguyen M-A, Karunakaran D, Geoffrion M, et al. Extracellular vesicles secreted by atherogenic macrophages transfer microRNA to inhibit cell migration. Arterioscler Thromb Vasc Biol 2018;38:49–63. 10.1161/ATVBAHA.117.309795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barberio MD, Kasselman LJ, Playford MP, et al. Cholesterol efflux alterations in adolescent obesity: role of adipose-derived extracellular vesical microRNAs. J Transl Med 2019;17:232. 10.1186/s12967-019-1980-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keyel PA, Tkacheva OA, Larregina AT, et al. Coordinate stimulation of macrophages by microparticles and TLR ligands induces foam cell formation. J.i. 2012;189:4621–9. 10.4049/jimmunol.1200828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsumoto A, Takahashi Y, Ogata K, et al. Phosphatidylserine-deficient small extracellular vesicle is a major somatic cell-derived sEV subpopulation in blood. iScience 2021;24:102839. 10.1016/j.isci.2021.102839 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
lupus-2021-000605supp002.pdf (627.1KB, pdf)
lupus-2021-000605supp001.pdf (77.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.