Abstract
N-[4-Chloro-3-(3-methyl-2-butenyloxy)phenyl]-2-methyl-3-furancarbothioamide (UC781) is an exceptionally potent nonnucleoside inhibitor of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. We found that a 1:1 molar combination of UC781 and 3′-azido-3′-deoxythymidine (AZT) showed high-level synergy in inhibiting the replication of AZT-resistant virus, implying that UC781 can restore antiviral activity to AZT against AZT-resistant HIV-1. Neither the nevirapine plus AZT nor the 2′,5′-bis-O-(t-butyldimethylsilyl)-3′-spiro-5"-(4"-amino-1",2"-oxathiole-2",2"-dioxide plus AZT combinations had this effect. Studies with purified HIV-1 reverse transcriptase (from a wild type and an AZT-resistant mutant) showed that UC781 was a potent inhibitor of the pyrophosphorolytic cleavage of nucleotides from the 3′ end of the DNA polymerization primer, a process that we have proposed to be critical for the phenotypic expression of AZT resistance. Combinations of UC781 plus AZT did not act in synergy to inhibit the replication of either wild-type virus or UC781-resistant HIV-1. Importantly, the time to the development of viral resistance to combinations of UC781 plus AZT is significantly delayed compared to the time to the development of resistance to either drug alone.
The conversion of viral genomic RNA into double-stranded DNA is an essential step in the replication of human immunodeficiency virus type 1 (HIV-1). This conversion is a multistep process that is catalyzed entirely by the viral enzyme reverse transcriptase (RT). RT therefore provides an important target for the development of anti-HIV chemotherapeutics (13).
RT inhibitors can be grouped into two major classes. Dideoxynucleosides (ddNs) such as 3′-azido-3′-deoxythymidine (AZT) and 2′,3′-dideoxy-3′-thiacytidine are substrate-like analogs and inhibit the virus by competing with the natural deoxynucleoside triphosphate (dNTP) substrate for binding to the catalytic site of RT (minor mechanism) and, once they are incorporated into the nascent DNA chain, by terminating continued viral DNA synthesis due to the lack of a 3′ hydroxyl moiety (major mechanism) (19). The second class comprises the nonnucleoside RT inhibitors (NNRTIs), a structurally diverse assortment of compounds including nevirapine (24, 30), TIBO (34), the pyridinones (18), and the carboxanilides and thiocarboxanilides (3–7, 9, 28). NNRTIs act by binding to a site on RT distinct from the catalytic site (14, 23, 37).
Since ddNs and NNRTIs interact with different sites on RT, they may bind in a mutually nonexclusive manner; that is, both ddNs and NNRTIs may simultaneously bind to the enzyme. Accordingly, combinations of ddNs plus NNRTIs have the potential to act synergistically to inhibit HIV-1 replication. Although some investigators have reported synergy with combinations of ddNs plus NNRTIs in inhibiting wild-type (wt) HIV replication (28, 41), others have found this inhibition to be additive only (3, 4). Few studies have addressed the inhibition of antiviral agent-resistant HIV by combinations of ddNs plus NNRTIs, although in one report, a loss of synergistic response to combinations of AZT plus other drugs was noted with AZT-resistant HIV (11). These studies are often complicated by the significant differences in the inhibitory potencies of the compounds used in combination.
AZT and N-[4-chloro-3-(3-methyl-2-butenyloxy)phenyl]-2-methyl-3-furancarbothioamide (UC781) have very similar antiviral potencies (ca. 5 nM), thereby providing a useful system to test whether combinations of ddNs plus NNRTIs can synergistically inhibit the replication of both wt and drug-resistant HIV-1. Synergy of inhibition by a combination of a ddN plus an NNRTI is unlikely when the combination is used against an HIV strain resistant to one of the drugs in the combination.
In the present study, we examined the inhibitory potencies of AZT alone, UC781 alone, and a 1:1 molar combination of AZT plus UC781 against wt HIV-1, UC781-resistant HIV-1, and AZT-resistant HIV-1. We found that a 1:1 molar combination of AZT plus UC781 showed very good synergy in inhibiting the replication of an AZT-resistant clinical isolate of HIV-1, implying that UC781 “restored” the antiviral activity of AZT against this virus. We also note that the time to the development of HIV resistance to a 1:1 molar combination of AZT plus UC781 is significantly delayed compared to that for either drug alone.
MATERIALS AND METHODS
Virus strains.
The wt HIV-1 strain used in this study was the HIV-IIIB laboratory strain of HIV-1 obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (courtesy of R. C. Gallo). The AZT-resistant variant (strain 691A) was a viral clinical isolate from an AIDS patient treated with AZT monotherapy (35) and possessed the K70R and T215Y mutations. The UC781-resistant variant was developed during the present studies in our laboratories by in vitro methods that we have described previously (16, 17). The UC781-resistant virus possessed multiple mutations including K103T, V106A, and Y181C.
Other reagents.
The CD4+ MT-2 cell line was purchased from the American Type Culture Collection (Rockville, Md.). RPMI 1640 cell culture medium and heat-inactivated fetal bovine serum were obtained from Canadian Life Technologies/GIBCO (Toronto, Ontario, Canada). AZT was obtained from Sigma, UC781 was kindly provided by W. G. Brouwer, Uniroyal Chemical Ltd. Research Laboratories (Guelph, Ontario, Canada), nevirapine was provided by Boehr-inger-Ingelheim, and 2′,5′-bis-O-(t-butyldimethylsilyl-3′-spiro-5"-(4"-amino-1",2"-oxathiole-2",2"-dioxide (TSAO) was a gift from J. Balzarini (Rega Institute, Leuven, Belgium). Details of the construction of plasmids for the expression of both wt RT and mutant recombinant RT containing the D67N, K70R, T215F, and K219Q mutations associated with AZT resistance have been provided elsewhere (1, 20). Recombinant RT was expressed in Escherichia coli JM109, and the p66-p51 heterodimers were purified by the rapid single-step method that we have described previously (15). Both wt and mutant RTs had similar specific activities (±10%) when assayed with [3H]TTP and poly(rA)-oligo(dT)12–18.
Cell culture and virus replication.
All cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum. HIV-1 stocks were propagated by subculture in CD4+ MT-2 cells essentially as we have described previously (7). Aliquots of the cell-free culture supernatants were used as viral inocula. Generally, an inoculum equivalent to a 50% tissue culture infective dose (TCID50) of 1 × 104 to 5 × 104 was used. Cells and virus were then incubated at 37°C, and the culture medium was changed every 2 to 3 days. Virus production was assessed by measurement of viral p24 antigen levels or RT activity in the culture supernatants after various times of culture (usually 4 days postinfection). The cytopathic effects of HIV infection were analyzed by microscopic assessment of syncytium formation. The latter data were obtained by independent examination of duplicate samples by two different investigators. Stock solutions of NNRTIs were prepared in dimethyl sulfoxide (DMSO) and were stored at −20°C. Aliquots of the NNRTI stock solutions were added to culture media immediately before use. The final concentration of DMSO in these working solutions was 0.1% or less. Control experiments showed that these concentrations of DMSO had no effect either on virus infectivity or on cell viability. The 50% effective concentrations (EC50s) for drug activity were calculated from dose-response curves over a range of drug concentrations (each carried out in triplicate) as described previously (7).
In vitro development of drug resistance by HIV-1.
For the in vitro development of drug resistance by HIV-1 we used an approach similar to that which we have described previously (16, 17). Briefly, both AZT and UC781 have similar EC50s against replication of wt HIV-1. MT-2 cells (3 × 105 cells/ml) were preincubated for 30 min with the appropriate concentration of drug and were then infected with HIVIIIB (5 × 105 TCID50s). Twice weekly, one half of the cell culture medium volume was replaced with fresh medium containing the same concentration of drug(s). Once virus propagation was noted at a given drug concentration (considered as the appearance of about 70% syncytium formation), 250 μl of undiluted clarified culture supernatant obtained from the HIV-infected cells was added to 3 × 105 fresh MT-2 cells in a 1-ml final volume containing a higher drug concentration (generally twice the previous concentration). This virus propagation cycle was repeated until virus was able to readily propagate in the presence of high concentrations of the drugs. The EC50 of each drug or combination of both drugs against each resistant strain was determined as we have described previously (7) and compared to that against the wt virus. The combination index (CI) was calculated by using the program CalcuSyn (Biosoft), based on the method of Chou and Talalay (10). In general, data from three independent experiments, each of which was carried out in duplicate, were used to calculate the CI values.
Analysis of mutations in RT associated with UC781 resistance.
Cells were infected with UC781-resistant HIV in the presence of drug. Genomic DNA was purified from the infected cells (36), and HIV-1 proviral DNA was amplified by PCR with Pfu polymerase (Stratagene, La Jolla, Calif.) and 18-nucleotide (nt) primers that allowed amplification of the RT gene (5′-AAA GCA TTA GTA GAA ATT TGT ACA GAG-3′ and 5′-ATT GAA GAC ATA CAG TAA CTG TCA GGT-3′). Sequencing was performed with the T7 Sequencing kit (Pharmacia Biotech, Montreal, Quebec, Canada) and appropriate synthetic 18-nt primers corresponding to different regions of the HIV-1 RT gene. Sequencing was carried out with proviral DNA from two independent experiments.
Analysis of in vitro pyrophosphorolysis reactions.
Heteropolymeric RNA template-primer (T-P) was prepared as described previously (1, 2, 20, 21) by using the T7 polymerase RNA transcript from AccI-linearized plasmid pHIV-PBS (2) as template and a synthetic 18-nt deoxyoligonucleotide (prPBS) that is complementary to the sequence of the tRNALys3 primer binding site. The prPBS primer was 5′ end labelled with [γ-32P]ATP and T4 polynucleotide kinase and was purified by resolution on polyacrylamide gels followed by excision and elution of the 18-nt 32P-labelled product as described previously (21). The T-P for pyrophosphorolysis was prepared by annealing 5′-[32P]prPBS (80 nM) to the pHIV-PBS RNA transcript (65 nM). The T-P was preincubated for 5 min at 37°C with RT (26 nM p66-p51 heterodimer) in 50 mM Tris-HCl (pH 7.8; 37°C) containing 60 mM KCl and 10 mM MgCl2. Reactions were initiated by the addition of each dNTP at a concentration of 50 μM (for measurement of reverse transcription) or 1 mM sodium pyrophosphate (for measurement of pyrophosphorolysis) in the absence or in the presence of UC781. After various incubation times, the reactions were stopped by the addition of an equivalent amount of sequencing gel loading buffer comprising 98% deionized formamide, 10 mM EDTA, and 1 mg each of bromophenol blue and xylene cyanol per ml. The samples were heated at 100°C for 5 min and then run on a 16% polyacrylamide–7 M urea sequencing gel. The resolved products were visualized by autoradiography.
RESULTS
Drug sensitivity of AZT-resistant and UC781-resistant HIV-1.
Both the AZT-resistant clinical variant (strain 691A) and the in vitro-selected UC781-resistant HIV-1 strains were highly resistant to the respective drugs (Table 1). However, the AZT-resistant virus was as sensitive to UC781 as wt virus, and replication of the UC781-resistant virus was inhibited by AZT equally well as replication of wt virus (Table 1); thus, cross-resistance was not observed.
TABLE 1.
Inhibition of wt and drug-resistant HIV-1 variants by UC781, AZT, and a 1:1 molar ratio combination of UC781 and AZT
| HIV-IIIB variant | EC50 (nM)a
|
||
|---|---|---|---|
| AZT | UC781 | AZT plus UC781 | |
| wt | 5.3 ± 1.25 | 10.4 ± 5 | 2.8 ± 1.4 |
| UC781 resistant | 6.6 ± 3.3 | 16,600 ± 2,300b | 11.0 ± 4.6 |
| AZT resistant | >2,500c | 13.1 ± 4 | 3.8 ± 0.8 |
EC50 was assessed by measuring p24 antigen levels in culture supernatants and by assessment of syncytium formation. The values are the means ± standard deviations from at least three separate experiments, each of which was carried out in triplicate.
UC781 showed no cytotoxic activity at concentrations up to 100 μM (the highest concentration tested in these experiments).
Concentrations of AZT higher than 2.5 μM were cytotoxic.
AZT and UC781 have similar antiviral potencies. Wild-type HIV-1 was inhibited equally well by AZT, UC781, and a 1:1 molar combination of AZT plus UC781 (Table 1). Inhibition of wt HIV by the combination of AZT plus UC781 was additive (Table 2), confirming previous observations (3, 4). Similarly, UC781-resistant HIV was inhibited by AZT equally well as wt virus was, but UC781-resistant HIV was insensitive to UC781 alone (Table 1). As expected, a 1:1 molar combination of AZT plus UC781 at any given nominal concentration was less effective at inhibiting replication of this HIV strain than AZT alone at the same nominal concentration.
TABLE 2.
CIs for inhibition of wt and drug-resistant HIV-1 variants by AZT-NNRTI combinations
| Virus | Drug combination | Ratio | CI at the following effective concna:
|
||
|---|---|---|---|---|---|
| 50% | 75% | 90% | |||
| wt | UC781-AZT | 1:1 | 1.1 ± 0.3 | 0.8 ± 0.2 | 0.7 ± 0.2 |
| UC781 resistant | UC781-AZT | 1:1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 |
| AZT resistant (691A clini-al isolate) | UC781-AZT | 1:1 | 0.3 ± 0.1 | 0.25 ± 0.1 | 0.2 ± 0.05 |
| Nevirapine-AZT | 1:1 | 1.2 ± 0.3 | 1.2 ± 0.2 | 1.4 ± 0.3 | |
| 5:1 | 1.8 | 1.5 | 1.3 | ||
| 10:1 | 0.5 | 0.5 | 0.6 | ||
| TSAO-AZT | 1:1 | 1.5 | 1.2 | 1.0 | |
| 10:1 | 0.9 | 0.95 | 1.0 | ||
CIs were calculated by the method of Chou and Talalay (10). The values reported are the means ± standard deviation from at least three independent experiments conducted with duplicate samples. Values without standard deviations are the averages from two independent experiments conducted in duplicate.
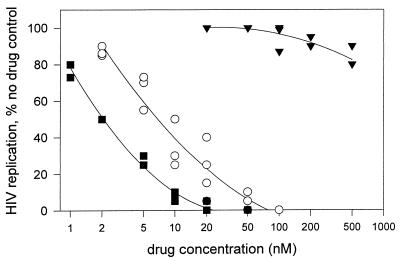
Very different results were noted with AZT-resistant viral strain 691A. This strain was highly resistant to AZT but was as sensitive to UC781 as wt HIV was (Table 1). However, the antiviral activity of a 1:1 molar combination of AZT plus UC781 against the 691A virus strain was significantly enhanced compared to that noted with similar nominal concentrations of UC781 alone (Fig. 1). The combination of AZT plus UC781 showed high-level synergy in inhibiting the replication of the AZT-resistant virus (Table 2). This implies (i) that AZT is functioning against AZT-resistant virus replication under these conditions and (ii) that UC781 therefore must restore antiviral activity to AZT against AZT-resistant HIV-1. Little or no synergy in the inhibition of AZT-resistant HIV-1 by combinations of AZT with other NNRTIs such as nevirapine and TSAO was noted (Table 2).
FIG. 1.
Concentration dependence of inhibition of AZT-resistant HIV-1 strain 691A by AZT alone (▾), UC781 alone (○), and a 1:1 molar combination of AZT plus UC781 (■). Each datum point is the average of duplicate measurements; the figure presents data from three independent experiments, each of which was carried out in duplicate.
UC781 inhibits RT-catalyzed pyrophosphorolysis.
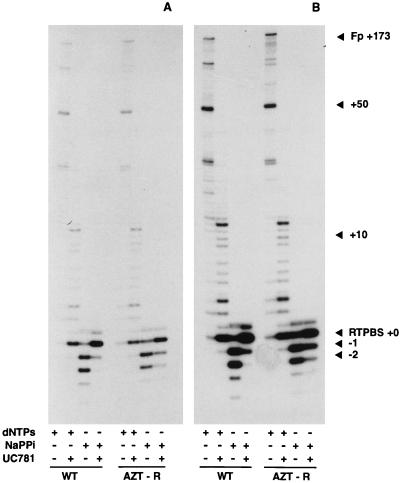
The antiviral efficacy of ddN inhibitors such as AZT is due primarily to chain termination of the nascent viral DNA (19). We have recently found that HIV-1 RT containing mutations associated with AZT resistance has an increased sensitivity to PPi (1). This results both in decreased binding of AZT triphosphate and in an increased pyrophosphorolytic cleavage of the 3′-terminal chain-terminating nucleotide. UC781 was a potent inhibitor both of DNA synthesis and of pyrophosphorolysis in vitro catalyzed by wt RT or by the RT with the D67N, K70R, T215F, and K219Q mutations (Fig. 2). The extent of inhibition of these reactions is readily discerned by the intensity of the starting 18-nt [32P]prPBS primer (denoted as RTPBS+0 in Fig. 2). This UC781-mediated inhibition of RT pyrophosphorolysis presumably allows AZT to regain chain-terminating activity against AZT-resistant virus in the presence of the nonnucleoside inhibitor and enables AZT to contribute to the overall inhibition of AZT-resistant HIV-1 exposed to combinations of AZT plus UC781.
FIG. 2.
Inhibition of HIV-1 RT-catalyzed pyrophosphorolysis by UC781. All reaction mixtures contained 65 nM pHIV-PBS RNA and 5′-[32P]prPBS T-P (prepared as described in Materials and Methods) and 26 nM p51-p66 recombinant wt or AZT-resistant RT. Other components of the individual reaction mixtures are indicated by a plus sign. The final concentrations of these components were dNTPs (dATP, dCTP, dGTP, and TTP at 50 μM each), sodium PPi (NaPPi; 1 mM), and UC781 (1 μM). RT DNA synthesis reactions are those containing dNTPs but no sodium PPi. Pyrophosphorolysis reactions are those containing sodium PPi but no dNTPs. The extent of inhibition of these reactions is readily discerned by the intensity of the starting 18-nt [32P]prPBS primer (denoted as RTPBS+0). (A and B) Two different exposures of the same gel provided to facilitate comparison of the different reactions. The exposure for panel A emphasizes pyrophosphorolytic products, while that for panel B emphasizes the forward reaction DNA polymerization products. WT, reactions catalyzed by recombinant wild-type RT; AZT-R, reactions catalyzed by recombinant RT with the D67N, K70R, T215F, and K219Q mutations; Fp, full-length DNA product (primer extended by 173 nt).
In vitro development of resistance to combinations of AZT plus UC781.
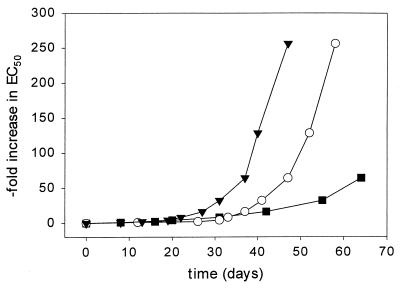
As seen in Fig. 3, HIV-1 readily develops high-level resistance to either AZT or UC781 alone. The AZT-resistant virus showed the K70R and T215Y mutations, whereas the UC781-resistant virus possessed K103T, V016A, and Y181C mutations. In contrast, resistance to 1:1 molar combinations of AZT plus UC781 develops in vitro much more slowly and to a much reduced extent than resistance to either drug alone. Sequencing of the proviral DNA produced following infection with HIV partially resistant to combinations of AZT plus UC781 revealed the following three mutations in the same clone: K103N, V118I, and Y181S. None of the regular mutations conferring AZT resistance (M41L, K70R, and T215Y) were noted in these viruses; this, however, may be due to the fact that only low-level resistance to combinations of AZT plus UC781 has so far been achieved.
FIG. 3.
Development of HIV-1 resistance in vitro to AZT alone (▾), UC781 alone (○), and a 1:1 molar combination of AZT plus UC781 (■). The datum points are averages of duplicate determinations from a representative experiment.
DISCUSSION
AZT has widespread clinical use in the treatment of HIV-infected and AIDS patients. Unfortunately, prolonged exposures to antiviral agents inevitably leads to the emergence of drug-resistant viruses (26, 29). This is particularly a problem in individuals who have received antiviral monotherapy, as was the case for AZT (12, 27, 32, 33, 38). Indeed, because of the initial treatment with AZT monotherapy, AZT-resistant HIV has become more prevalent, such that newly infected individuals may be infected with AZT-resistant strains of HIV. This, of course, presents considerable drawbacks for the continued use of AZT in antiviral therapy.
UC781 is a tightly binding inhibitor of RT (6), a property unique among the NNRTIs that have so far been described. This tight binding may indicate a somewhat different mode of interaction with the NNRTI binding pocket, consistent with the observation that UC781 has excellent activity against HIV-1 mutants resistant to other NNRTIs in vitro (4, 5, 9). The AZT-resistant virus was not cross-resistant to UC781 and vice versa. This is not surprising, because the mutations which confer resistance to each of these two drugs occur in different regions of RT (5, 9, 22, 26).
While some investigators have noted that combinations of AZT plus NNRTIs act synergistically to inhibit replication of wild-type (drug-sensitive) HIV-1 (28, 41), others have found this inhibition to be additive only (3, 4). Our data support the latter observation (Table 2). Importantly, we found that combinations of AZT plus UC781 showed high-level synergy in inhibiting the replication of AZT-resistant HIV-1 (Fig. 1; Table 2), implying that UC781 was somehow restoring the ability of AZT to act against AZT-resistant virus. To our knowledge, this is the first reported example of the restoration of AZT sensitivity to an AZT-resistant virus by use of a combination of AZT plus an NNRTI. It is important, however, that our results were obtained with an AZT-resistant clinical isolate possessing the K70R and T215Y mutations. Similar data have been obtained with recombinant HIV containing the D67N, K70R, T215F, and K219Q mutations (unpublished data). However, we have not yet tested whether UC781 is able to restore the activity of AZT against a range of AZT-resistant mutant HIV-1, such as those with only the T215Y mutation or the M41L plus T215Y mutations. These studies are in progress.
The phenotypic mechanism of resistance to ddNs such as 2′,3′-dideoxy-3′-thiacytidine, dideoxyinosine etc., generally involves a decreased ability of the RT to bind to the inhibitor (20, 40). AZT resistance is unusual in that decreased binding does not appear to be the major factor in the resistance mechanism. Indeed, RT containing mutations associated with AZT resistance is as sensitive as wt RT to inhibition by AZT triphosphate in standard in vitro enzyme assays (25, 39). However, we have recently found that AZT resistance results in large part from RT-catalyzed pyrophosphorolytic removal of chain-terminating AZT after its incorporation into the nascent DNA strand (1). While wt and AZT-resistant HIV strains may show similar rates of incorporation of chain-terminating AZT, the AZT-resistant viral RT is more effective in subsequently removing it. The pyrophosphorolytic removal of the terminal AZT allows continuation of forward viral DNA synthesis. We found that UC781 was a potent inhibitor of in vitro pyrophosphorolysis carried out by both wt and AZT-resistant RT (Fig. 2). We propose that it is the inhibition of this activity by UC781 that allows AZT to again function as a chain terminator with AZT-resistant virus.
The rapid emergence of resistant HIV mutants represents a formidable challenge to the development of anti-HIV drugs (31). The time to the development of HIV resistance in vitro to UC781 alone is significantly delayed compared to the time to the development of HIV resistance to other carboxanilide NNRTIs such as UC84 and UC38 (8). This is not due to a decreased “fitness” of UC781-resistant HIV, since this resistant virus replicates as well as wt HIV (data not shown). The delayed resistance may be due to the need for multiple mutations in RT to achieve high-level resistance to UC781 (5, 9). High-level resistance to AZT also requires multiple mutations in HIV RT (22, 26). The development of in vitro viral resistance to a 1:1 molar combination of AZT plus UC781 was significantly attenuated both in rate and in extent compared to those for either drug alone (Fig. 3).
The delayed development of resistance to combinations of AZT plus UC781 may be due to the fact that high-level resistance to each of AZT and UC781 requires multiple mutations in HIV-1 RT (5, 9, 22, 26). However, it is interesting that none of the common mutations associated with AZT resistance appear in virus with partial resistance to combinations of AZT plus UC781. The V118I mutation noted in these virus is so far unreported. Site-specific mutagenesis experiments are necessary to confirm the role of this mutation in resistance to the combination of AZT plus UC781. It is possible that the numerous mutations required for resistance to the combination might have a detrimental effect on RT activity, possibly resulting in a virus with a decreased ability to replicate. Our inability to generate high-level viral resistance in vitro in the extended time frame of our experiments may be consistent with this possibility. Since resistance to AZT seems to develop more quickly in vitro than resistance to UC781 and since UC781 acts to restore the activity of AZT against AZT-resistant virus, it is possible that resistance to both drugs may not readily develop in the same virus strain without a concomitant reduction in replication capacity. We are using site-specific mutagenesis to test this hypothesis.
ACKNOWLEDGMENTS
This research was supported in part by grants (to M.A.P.) from the Medical Research Council of Canada (grants GR-13918 and UI-14280) and from the International Research Scholar’s Program of the Howard Hughes Medical Institute. M.A.P. is an MRC/NHRDP Senior Scientist (HIV/AIDS) and an International Research Scholar of the Howard Hughes Medical Institute.
REFERENCES
- 1.Arion D, Kaushik N, McCormick S, Borkow G, Parniak M A. Phenotypic mechanism of HIV-1 resistance to 3′-azido, 3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry. 1998;37:15908–15917. doi: 10.1021/bi981200e. [DOI] [PubMed] [Google Scholar]
- 2.Arts E J, Li X, Gu Z, Kleiman L, Parniak M A, Wainberg M A. Comparison of deoxyoligonucleotide and tRNALys3 as primers in an endogenous human immunodeficiency virus-1 in vitro reverse transcription/template-switching reaction. J Biol Chem. 1994;269:14672–14680. [PubMed] [Google Scholar]
- 3.Balzarini J, Perez-Perez M J, Velazquez S, San-Felix A, Camarasa M-J, De Clercq E, Karlsson A. Suppression of the breakthrough of human immunodeficiency virus type 1 (HIV-1) in cell culture by thiocarboxanilide derivatives when used individually or in combination with other HIV-1-specific inhibitors (i.e., TSAO derivatives) Proc Natl Acad Sci USA. 1995;92:5470–5474. doi: 10.1073/pnas.92.12.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzarini J, Brouwer W G, Dao D C, Osika E M, De Clercq E. Identification of novel thiocarboxanilide derivatives that suppress a variety of drug-resistant mutant human immunodeficiency virus type 1 strains at a potency similar to that for wild-type virus. Antimicrob Agents Chemother. 1996;40:1454–1466. doi: 10.1128/aac.40.6.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini J, Pelemans H, Aquaro S, Perno C F, Witvrouw M, Schols D, De Clercq E, Karlsson A. Highly favourable antiviral activity and resistance profile of the novel thiocarboxanilide pentenyloxy ether derivatives UC781 and UC82 as inhibitors of human immunodeficiency virus type 1 (HIV-1) replication. Mol Pharmacol. 1996;50:394–401. [PubMed] [Google Scholar]
- 6.Barnard J, Borkow G, Parniak M A. The thiocarboxanilide UC781 is a tight-binding nonnucleoside inhibitor of HIV-1 reverse transcriptase. Biochemistry. 1997;36:7786–7792. doi: 10.1021/bi970140u. [DOI] [PubMed] [Google Scholar]
- 7.Borkow G, Barnard J, Nguyen T M, Belmonte A, Wainberg M A, Parniak M A. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J Virol. 1997;71:3023–3030. doi: 10.1128/jvi.71.4.3023-3030.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borkow, G., D. Arion, N. Kaushik, and M. A. Parniak. Unpublished data.
- 9.Buckheit R W, Snow M J, Fliakas-Boltz V, Kinjerski T L, Russell J D, Pallansch L A, Brouwer W G, Yang S S. Highly potent oxathiin carboxanilide derivatives with efficacy against nonnucleoside reverse transcriptase inhibitor-resistant human immunodeficiency virus isolates. Antimicrob Agents Chemother. 1997;41:831–837. doi: 10.1128/aac.41.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 11.Cox S W, Albert J, Wahlberg J, Uhlen M, Wahren B. Loss of synergistic response to combinations containing AZT in AZT-resistant HIV-1. AIDS Res Hum Retroviruses. 1992;8:1229–1234. doi: 10.1089/aid.1992.8.1229. [DOI] [PubMed] [Google Scholar]
- 12.D’Aquila R T, Johnson V A, Welles S L, Japour A J, Kuritzkes D R, DeGruttola V, Reichelderfer P S, Coombs R W, Crumpacker C S, Kahn J O. Zidovudine resistance and HIV-1 disease progression during antiretroviral therapy. Ann Intern Med. 1995;122:401–408. doi: 10.7326/0003-4819-122-6-199503150-00001. [DOI] [PubMed] [Google Scholar]
- 13.De Clercq E. HIV-1-specific RT inhibitors: highly selective inhibitors of human immunodeficiency virus type 1 that are specifically targeted at the viral reverse transcriptase. Med Res Rev. 1993;13:229–258. doi: 10.1002/med.2610130303. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Das K, Moereels H, Koymans L, Andries K, Janssen P A, Hughes S H, Arnold E. Structure of HIV-1 RT/TIBO R 86183 complex reveals similarity in the binding of diverse nonnucleoside inhibitors. Nat Struct Biol. 1995;2:407–415. doi: 10.1038/nsb0595-407. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher R S, Holleschak G, Nagy E, Arion D, Borkow G, Gu Z, Wainberg M A, Parniak M A. Single-step purification of recombinant wild-type and mutant HIV-1 reverse transcriptase. Prot Expression Purif. 1996;7:27–32. doi: 10.1006/prep.1996.0004. [DOI] [PubMed] [Google Scholar]
- 16.Gao Q, Gu Z, Parniak M A, Li X, Wainberg M A. In vitro selection of variants of human immunodeficiency virus type 1 resistant to 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine. J Virol. 1992;66:12–19. doi: 10.1128/jvi.66.1.12-19.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Q, Gu Z, Salomon H, Nagai K, Parniak M A, Wainberg M A. Generation of multiple drug resistance by sequential in vitro passage of the human immunodeficiency virus type 1. Arch Virol. 1994;136:111–122. doi: 10.1007/BF01538821. [DOI] [PubMed] [Google Scholar]
- 18.Goldman M E, Nunberg J H, O’Brien J A, Quintero J C, Schlief J M, Freund K F, Gaul S L, Saari W S, Wai J S, Hoffman J M, Anderson P S, Hupe D J, Emini E A, Stern A M. Pyridinone derivatives: specific human immunodeficiency virus type 1 reverse transcriptase inhibitors with antiviral activity. Proc Natl Acad Sci USA. 1991;88:6863–6867. doi: 10.1073/pnas.88.15.6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goody R S, Muller B, Restle T. Factors contributing to the inhibition of HIV reverse transcriptase by chain-terminating nucleotides in vitro and in vivo. FEBS Lett. 1991;291:1–5. doi: 10.1016/0014-5793(91)81089-q. [DOI] [PubMed] [Google Scholar]
- 20.Gu Z, Fletcher R S, Arts E J, Wainberg M A, Parniak M A. The K65R mutant reverse transcriptase associated with HIV-1 resistance to 2′,3′-dideoxycytidine, 2′,3′-dideoxy-3′-thiacytidine and 2′,3′-dideoxyinosine shows reduced sensitivity to specific dideoxynucleoside triphosphate inhibitors in vitro. J Biol Chem. 1994;269:28118–28122. [PubMed] [Google Scholar]
- 21.Gu Z, Arts E J, Parniak M A, Wainberg M A. Mutated K65R recombinant HIV-1 reverse transcriptase shows diminished chain termination in the presence of 2′,3′-dideoxycytidine-5′-triphosphate and other drugs. Proc Natl Acad Sci USA. 1995;92:2760–2764. doi: 10.1073/pnas.92.7.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellam P, Boucher C A B, Larder B A. Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contributes to the development of high-level resistance to zidovudine. Proc Natl Acad Sci USA. 1992;89:1934–1938. doi: 10.1073/pnas.89.5.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohlstaedt L A, Wang J, Friedman J M, Rice P A, Steitz T A. Crystal structure at 3.5 Å resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 24.Kopp E B, Miglietta J J, Shrutkowski A G, Shih C-K, Grob P M, Skoog M T. Steady state kinetics and inhibition of HIV-1 reverse transcriptase by a non-nucleoside dipyridodiazepinone, BI-RG-587, using a heteropolymeric template. Nucleic Acids Res. 1991;19:3035–3039. doi: 10.1093/nar/19.11.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacey S F, Reardon J E, Furfine E S, Kunkel T A, Bebenek K, Eckert K A, Kemp S D, Larder B A. Biochemical studies on the reverse transcriptase and RNase H activities from HIV strains resistant to 3′-azido-3′-deoxythymidine. J Biol Chem. 1992;267:15789–15794. [PubMed] [Google Scholar]
- 26.Larder B A, Kemp S D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT) Science. 1989;246:1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- 27.Larder B A, Darby G, Richman D D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 28.McMahon J B, Buckheit R W, Jr, Gulakowski R J, Currens M J, Vistica D T, Shoemaker R H, Stinson S F, Russell J D, Bader J P, Narayanan V L, Schultz R J, Brouwer W G, Felauer E E, Boyd M R. Biological and biochemical anti-human immunodeficiency virus activity of UC 38, a new non-nucleoside reverse transcriptase inhibitor. J Pharmacol Exp Ther. 1996;276:298–305. [PubMed] [Google Scholar]
- 29.Mellors J W, Dutchman G E, Im G J, Tramontano E, Winkler S R, Cheng Y C. In vitro selection and molecular characterization of human immunodeficiency virus-1 resistant to non-nucleoside inhibitors of reverse transcriptase. Mol Pharmacol. 1992;41:446–451. [PubMed] [Google Scholar]
- 30.Merluzzi V J, Hargrave K D, Labadia M, Grozinger K, Skoog M T, Wu J C, Shih C-K, Eckner K, Hattox S, Adams J, Rosehthal A S, Faanes R, Eckner R J, Koup R A, Sullivan J L. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science. 1990;250:1411–1413. doi: 10.1126/science.1701568. [DOI] [PubMed] [Google Scholar]
- 31.Mitsuya H, Yarchoan R, Broder S. Molecular targets for AIDS therapy. Science. 1990;249:1533–1544. doi: 10.1126/science.1699273. [DOI] [PubMed] [Google Scholar]
- 32.Montaner J S, Singer J, Schechter M T, Raboud J M, Tsoukas C, O’Shaughnessy M, Ruedy J, Nagai K, Salomon H, Spira B, Wainberg M A. Clinical correlates of in vitro HIV-1 resistance to zidovudine. Results of the Multicentre Canadian AZT Trial. AIDS. 1993;7:189–196. doi: 10.1097/00002030-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Montaner J S, Schechter M T, Rachlis A, Gill J, Beaulieu R, Tsoukas C, Raboud J, Cameron B, Salomon H, Dunkle L, Wainberg M A. Didanosine compared with continued zidovudine therapy for HIV-infected patients with 200 to 500 CD4 cells/mm3. A double-blind, randomized, controlled trial. Canadian HIV Trials Network Protocol 002 Study Group. Ann Intern Med. 1995;123:561–571. doi: 10.7326/0003-4819-123-8-199510150-00001. [DOI] [PubMed] [Google Scholar]
- 34.Pauwels R, Andries K, Desmyter J, Schols D, Kukla M J, Breslin H J, Raeymaeckers A, Van Gelder J, Woestenborghs R, Heykants J, Schellekens K, Janssen M A, De Clercq E, Janssen P A J. Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature. 1990;343:470–474. doi: 10.1038/343470a0. [DOI] [PubMed] [Google Scholar]
- 35.Rooke R, Tremblay M, Soudeyns H, DeStephano L, Yao X-J, Fanning M, Montaner J S, O’Shaughnessy M, Gelmon K, Tsoukas C, Wainberg M A. Isolation of drug-resistant variants of HIV-1 from patients on long-term zidovudine therapy. AIDS. 1989;3:411–415. doi: 10.1097/00002030-198907000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Smerdon S J, Jäger J, Kohlstaedt L A, Chirino A J, Friedman J M, Rice P A, Steitz T A. Structure of the binding site for nonnucleoside inhibitors of the reverse transcriptase of human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1994;91:3911–3915. doi: 10.1073/pnas.91.9.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tudor-Williams G, St. Clair M H, McKinney R E, Maha M, Walter E, Santacroce S, Mintz M, O’Donnell K, Rudoll T, Vavro C L. HIV-1 sensitivity to zidovudine and clinical outcome in children. Lancet. 1992;339:15–19. doi: 10.1016/0140-6736(92)90140-x. [DOI] [PubMed] [Google Scholar]
- 39.Wainberg M A, Tremblay M, Rooke R, Blain N, Soudeyns H, Parniak M A, Yao X-J, Li X, Fanning M, Montaner J S G, O’Shaughnessy M, Tsoukas C, Falutz J, Stern M, Belleau B, Ruedy J. Characterization of reverse transcriptase activity and susceptibility to other nucleosides of AZT-resistant variants of HIV-1. Results from the Canadian AZT Multicentre Study. Ann N Y Acad Sci. 1991;616:346–355. doi: 10.1111/j.1749-6632.1990.tb17855.x. [DOI] [PubMed] [Google Scholar]
- 40.Wainberg M A, Drosopoulos W C, Salomon H, Hsu M, Borkow G, Parniak M A, Gu Z, Song Q, Manne J, Islam S, Castriota G, Prasad V R. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science. 1996;271:1282–1285. doi: 10.1126/science.271.5253.1282. [DOI] [PubMed] [Google Scholar]
- 41.Yang S S, Fliakas-Boltz V, Bader J P, Buckheit R W., Jr Characteristics of a group of nonnucleoside reverse transcriptase inhibitors with structural diversity and potent anti-human immunodeficiency virus activity. Leukemia. 1995;9:S75–S85. [PubMed] [Google Scholar]
- 42.Yao X-J, Wainberg M A, Parniak M A. Mechanism of inhibition of HIV-1 infection in vitro by purified extract of Prunella vulgaris. Virology. 1992;187:56–62. doi: 10.1016/0042-6822(92)90294-y. [DOI] [PubMed] [Google Scholar]