Abstract
Background
It is known that inflammatory responses play an important role in the pathophysiology of COVID‐19.
Aims
In this study, we aimed to examine the role of kynurenine (KYN) metabolism on the severity of COVID‐19 disease AQ5.
Materials & Methods
Seventy COVID‐19 patients of varying severity and 30 controls were included in the study. In addition to the classical laboratory parameters, KYN, tryptophan (TRP), kynurenic acid (KYNA), 3 hydroxykynurenine (3OHKYN), quinolinic acid (QA), and picolinic acid (PA) were measured with mass spectrometry.
Results
TRP, KYN, KYN:TRP ratio, KYNA, 3OHKYN, PA, and QA results were found to be significantly different in COVID‐19 patients (p < 0.001 for all). The KYN:TRP ratio and PA of severe COVID‐19 patients was statistically higher than that of mild‐moderate COVID‐19 patients (p < 0.001 for all). When results were examined, statistically significant correlations with KYN:TRP ratio, IL‐6, ferritin, and procalcitonin were only found in COVID‐19 patients. ROC analysis indicated that highest AUC values were obtained by KYN:TRP ratio and PA (0.751 vs 0.742). In determining the severity of COVID‐19 disease, the odd ratios (and confidence intervals) of KYN:TRP ratio and PA levels that were adjusted according to age, gender, and comorbidity were determined to be 1.44 (1.1–1.87, p = 0.008) and 1.06 (1.02–1.11, p = 0.006), respectively.
Discussion & Conclusion
According to the results of this study, KYN metabolites play a role in the pathophysiology of COVID‐19, especially KYN:TRP ratio and PA could be markers for identification of severe COVID‐19 cases.
Keywords: coronavirus‐19 disease (COVID‐19), kynurenine pathway, prognosis
It has been shown that the kynurenine (KYN) pathway plays an important role in the regulation of the immune system. KYN pathway products were found to be significantly different in COVID‐19 patients. KYN:TRP ratio and picolinic acid (PA) of severe COVID‐19 patients was statistically higher than of mild‐moderate COVID‐19 patients. ROC analysis indicated that highest AUC values were obtained by KYN:TRP ratio and PA (0.751 vs 0.742). In determining the severity of COVID‐19 disease, the odd ratios (and confidence intervals) of KYN:TRP ratio and PA levels that were adjusted according to age, gender, and comorbidity were determined to be 1.44 (1.1–1.87, p = 0.008) and 1.06 (1.02–1.11, p = 0.006), respectively. According to the results of this study, KYN metabolites play a role in the pathophysiology of COVID‐19, especially KYN:TRP ratio and PA could be markers for identification of severe COVID‐19 cases.

1. INTRODUCTION
Coronavirus‐19 disease (COVID‐19), caused by severe acute respiratory syndrome–coronavirus‐2 (SARS‐CoV‐2), has caused an unprecedented public health problem worldwide. COVID‐19 may present with heterogeneous and diverse disease settings ranging from an asymptomatic or mild upper respiratory tract disease to severe cytokine storm, acute respiratory distress syndrome (ARDS), multiple organ failure, and severe viral pneumonia that can cause death. 1
The disease may present with heterogeneous and varied settings, ranging from an asymptomatic or mild upper respiratory tract disease to severe cytokine storm, acute respiratory distress syndrome (ARDS), multiple organ failure, and even severe viral pneumonia that can cause death. It is known that aggressive inflammatory responses play an important role in the pathophysiology of COVID‐19, along with the pathology that frequently occurs in the airways. Therefore, disease severity in patients is not only related to viral infection but also related to host response. 2 , 3 , 4
In many cases, patients recover following the immune response to the infection. However, in some patients, a different type of immune response is activated which mediates diffuse lung inflammation and triggers a cytokine storm. Higher levels of IL‐2, IL‐7, IL‐10, granulocyte colony stimulating factor (G‐CSF), IP‐10, MCP1, macrophage inflammatory protein 1α (MIP1α), and tumor necrosis factor (TNF) were detected, especially in severe COVID‐19 patients requiring intensive care. It has been shown that especially increasing IL‐6 levels in these patients over time are associated with mortality. 5
Tryptophan (TRP) is oxidized by tryptophan 2, 3‐dioxygenase in liver cells. The kynurenine pathway, which hosts KYN and its degradation products, is a metabolic pathway that leads to the production of nicotinamide adenine dinucleotide (NAD+), which is an important energy source. 6
In addition, kynurenine, whose conversion is catalyzed by tryptophan dioxygenase (TDO) and indoleamine 2,3‐dioxygenase (IDO), is involved in the regulation of immune system and neurological functions in many different tissues. 6 , 7
It has been shown that the KYN pathway plays an important role in the regulation of the immune system. 8 This pathway is regulated by 3 enzymes, tryptophan‐2,3‐dioxygenase (TDO), and indoleamine‐pyrrole 2,3‐dioxygenase I and 2 (IDO‐1 and IDO‐2), which synchronously control the rate limiting first step. TRP is first transformed into to N‐formylkynurenine (NFK) by IDO‐1, and then, NFK is degraded by formamidase to KYN. KYN is converted to kynurenic acid (KYNA) through transamination or to 3 hydroxykynurenine (3OHKYN) through hydroxylation. In the next stages, it nonenzymatically transforms into 3 hydroxyanthranilic acid to form quinolinic acid (QA) and picolinic acid (PA) (Figure 1). 9 , 10
FIGURE 1.
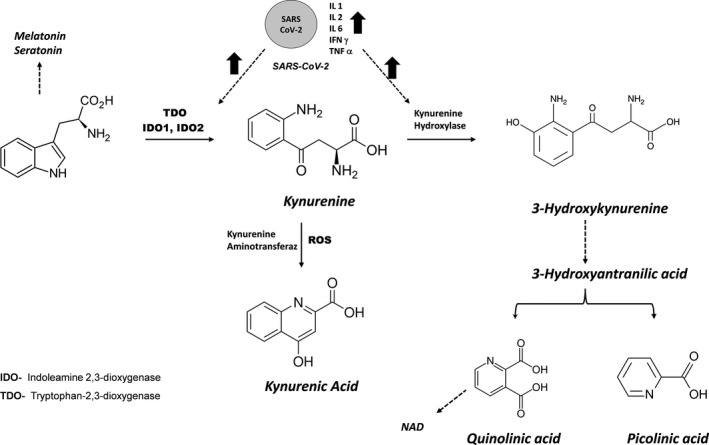
Metabolites in the KYN pathway and its possible relationship with COVID‐19
COVID‐19 or other infections lead to increases in interferon gamma (IFN‐γ), interleukin 1, 2, 6, and tumor necrosis factor‐Alpha (TNF‐α) in macrophages, resulting in increased expression of IDO and induction of the KYN pathway. 7
Activation of the KYN pathway leads to certain reactions.
KYN binds to and activates the aryl hydrocarbon receptor (AhR) in both dendritic cells and T cells. This promotes the transformation of T‐effector cells into Treg cells and also promotes IDO induction. Thus, it creates a Kyn/AhR/IDO cycle that maintains immunosuppression. 9 , 11 AhR is known to play an important role in COVID‐19 infection. 12 , 13
The decrease in TRP activates non‐depressed general control 2 (GCN2), by increasing the production of anti‐inflammatory cytokines in dendritic cells and macrophages. As a result, it may lead to differentiation and accumulation of Treg cells. 14
Changes in the amounts of KYN and its metabolites have been detected in some viral diseases such as HIV, Influenza, and HBV. 15 , 16 , 17
IDO‐1 can lead to immune tolerance independent of its enzymatic activity by acting as a signaling molecule in dendritic cells. 18 KYN pathway metabolites (e.g. KYNA, 3OHKYN, and cinnabarinic acids) exert an anti‐infective effect and may support immune tolerance. 19
In general, activation of the KYN pathway occurs in response to inflammation and can increase the severity of the disease by weakening the immune system against viruses. Similarly, in SARS‐CoV2 infection, lymphocytes in the respiratory tract cause an increase in pro‐inflammatory cytokines, which activates the KYN pathway. In recent studies, it has been stated that the KYN:TRP ratio plays a very important role in determining the inflammatory state in COVID‐19 and can be used in the determination of treatment. 7 In this study, inflammation markers (CRP, IL‐6, procalcitonin, and N/L ratio) and KYN, TRP, KYNA, 3OHKYN, QA, and PA molecules in the KYN pathway that were measured by LC MS/MS were examined in COVID‐19 patients. As a result, it is aimed to elucidate the inflammatory mechanisms and to search for new markers in determining the severity of COVID‐19 disease.
2. MATERIALS AND METHODS
A total of 70 patients diagnosed with COVID‐19 and 30 control cases in the similar age group who applied to the hospital in September 2021 were included in the study. The diagnosis of all COVID‐19 patients was made through real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) analysis of pharyngeal and nasal swab samples and radiological examination of lungs.
The sample size in the study was calculated using the G‐POWER software 3.1.9.2 (Düsseldorf, Germany) as described by Lionetti et al. 7 When alpha and power is selected as 0.05 and 0.9, respectively, the sample size was calculated to be 66 for an effect size of 0.501. Since there were three different COVID‐19 groups together with a control group, we decided to use minimum 88 patients in total. Since we thought that there might be a sample size difference between the groups, we considered that it would be appropriate to have 100 patients.
Written informed consent was obtained from all participants in the study, and all procedures were approved by the Clinical Research Ethics Committee (2021/205).
The COVID‐19 patients were divided into three main groups as mild‐to‐moderate, severe, and those hospitalized in the intensive care unit (ICU), in line with the classifications stated in the COVID‐19 guideline of the Ministry of Health (https://covid19.saglik.gov.tr/Eklenti/39061/0/covid19rehberieriskinhastatedavisipdf.pdf). Accordingly, patients with mild/moderate pneumonia symptoms, such as fever, muscle/joint pain, cough, sore throat, breath rate <30/min, and SpO2 (peripheral capillary oxygen saturation) >90 at room temperature, were classified as "Mild‐Moderate". Patients with severe pneumonia symptoms such as fever, muscle/joint pain, cough, sore throat, breath rate >30/min and require hospitalization, or clinically compatible patients with SpO2<90 at room temperature, or patients with bilateral diffuse pneumonia findings on CT scan were classified as “Severe”. The third group consisted of the COVID‐19 patients hospitalized in the ICU.
2.1. Laboratory measurements
Counter blood cell analyses were performed on XN 9000 analyzer (Sysmex Corporation, Kobe, Japan). Serum ALT, AST, ALP, LDH, GGT, creatinine, BUN, and uric acid measurements were conducted on Roche Cobas 8000 Modular Chemistry Analyzer (Roche Diagnostics, Indianapolis IN). Procalcitonin and IL‐6 tests were conducted on Coulter Access 2 (Beckman Coulter, Inc., Brea, CA, USA). CK‐MB, troponin, and ferritin measurements were conducted on Roche Cobas e411 (Roche Diagnostics, Indianapolis IN). Fibrinogen and D‐dimer were measured on ACL TOP 700 analyzer (Diamond Diagnostics Kft. Hungary).
Measurement of KYN metabolites by LCMSMS was done by modifying the method of Tömösi et al. 20
2.1.1. Sample preparation
The 370 µl of acetone‐MeOH containing 10 µl of IS mixture (TRP, KYNA, PA, QA, and 3OHKYN) was added onto the serum sample (100 µl). The mixture was vortexed for 60 s and incubated at −20°C for 15 min. This mixture was centrifuged at 12,000 g at 4°C for 15 min, and the supernatant (400 µl) was transferred to a new tube and divided into two equal parts. After evaporation under nitrogen gas, 70 µl of derivatization reagent (n‐butanol‐acetyl chloride, 9:1, v/v) was added. It was incubated at 60°C for 1 h. The mixture was evaporated under nitrogen before reconstitution. Both parts of the sample were dissolved in 100 µl of starting eluent. The two samples were combined and used for KYN metabolite measurement. The method validation parameters, details of chromatography, and mass spectrometry settings for measuring KYN metabolites are presented in Supporting Information. 21 , 22 , 23
2.2. Statistical analyses
Analyse‐it 4.20.01 (Analyse‐it Software, Ltd. Leeds, UK) and IBM SPSS Version 26 (IBM, USA) were used for statistical analyses. Shapiro–Wilk test was used to evaluate the distribution of the data. ANOVA and post hoc Tukey's analyses were used for normally distributed data. Non‐normally distributed data were first analyzed with Kruskal–Wallis test and then corrected using Bonferroni. Chi‐squared test was applied for categorical data such as gender. Results were presented as mean ± SD or median (25%–75% values). In order to determine the independent roles of KYN metabolites in determining the severity of the disease, multivariate analyzes were performed according to age, gender and comorbidity. Receiver Operating Characteristic—ROC—analysis was performed for clinical evaluation, and results were evaluated with area under the ROC curve (AUC). A p‐value <0.05 was considered to be statistically significant.
3. RESULTS
The comorbidity data and laboratory findings of the COVID‐19 patient and control groups are presented in Table 1. It was determined that N:L ratio, BUN, troponin, D‐Dimer, fibrinogen, CRP, IL‐6, and ferritin levels, which are markers used in COVID‐19 patient follow‐up, were significantly different in mild‐moderate, severe, and COVID‐19 patients in the ICU group.
TABLE 1.
Demographic, comorbidity, and laboratory results of COVID‐19 cases and control group according to clinical data and their statistical comparisons
|
Control N = 30 (*) |
Mild‐Moderate N = 21 ($) |
Severe N = 20 (#) |
ICU N = 29 |
||
|---|---|---|---|---|---|
| Age (year) | 56.8 ± 14 | 57.8 ± 16.8 | 62.7 ± 14.16 | 63 ± 14.24 | 0.263 |
| Gender F/M | 21/15 | 11/10 | 11/9 | 13/16 | 0.107 |
| Comorbidity | |||||
| Hypertension | 5 | 8 | 8 | 13 | |
| Diabetes Mellitus | 4 | 4 | 7 | ||
| Renal diseases | 3 | 3 | 2 | ||
| COAH | 3 | 2 | 2 | ||
| Heart diseases | 3 | 3 | 7 | ||
| Others | 2 | 7 | 6 | 8 | |
| WBC (109/L) | 6.7 (5.9–7.7) | 6.76 (5.6–9.1) # | 8.94 (7.2–11.7)* | 9.24 (5.8–11.5) | 0.037 |
| Platelet (109/L) | 250 (214–293) | 211 (124–278) | 142 (102.9–257) | 337 (223–380) $ # | <0.0001 |
| Neutrophil (109/L) | 3.9 (3.4–4.7) | 5.4 (3.2–6.7) | 8.14 (4.4–10.5) * | 6.7 (4.2–9.5) * | <0.0001 |
| Lymphocyte (109/L) | 2.2(1.7–2.5) | 1.1 (0.80–1.5) * | 0.69 (0.55–1.23) * | 0.95 (0.68–1.16) * | <0.0001 |
| N:LRatio | 1.8 (1.6–2.4) | 4.7 (2.8–6.5) * | 13.1 (5.6–17.1) * | 5.4 (4.3–12.1) * $ | <0.0001 |
| ALT (U/L) | 20 (14–24.5) | 15.6 (12–23) | 36 (15–49.275) | 55 (22–74) *$ | <0.0001 |
| ALP (U/L) | 57 (44–72) | 65.1 (57–88) | 68 (53.5–117) | 60 (51–78) | 0.072 |
| AST (U/L) | 19 (14–22) | 25 (20–34) | 47 (26–64) * | 43 (30–70) *$ | <0.0001 |
| LDH (U/L) | 154 (136–173) | 227 (159–271) | 337 (226–474) *$ | 398 (325–470) *$ | <0.0001 |
| GGT (U/L) | 17 (13–21) | 18.9 (16–41) | 66.5 (38–98) *$ | 60 (34–101) *$ | <0.0001 |
| Creatinine (mg/dl) | 0.76 (0.66–0.87) | 1.58 (0.63–2.23) * | 1.05 (0.71–1.32) | 0.75 (0.68–0.87) | 0.029 |
| BUN (mg/dl) | 12 (10.75–15) | 23 (12–51) * | 20 (14–23) * | 22 (19–27) * | <0.0001 |
| Uric Acid (mg/dl) | 4.55 (4–5.4) | 5.4 (4.55–6.70) | 3.9 (2.78–5.07) $ | 3.9 (3.28–5.82) | 0.017 |
| Procalcitonin (ng/ml) | 0.18 (0.14–0.23) | 0.34 (0.22–0.65) * | 0.15 (0.08–0.54) | 0.74 (0.66–0.87) *# | <0.0001 |
| CK‐MB (mg/L) | 1.03 (0.78–1.30) | 1.34 (0.82–2.32) | 1.19 (0.75–2.23) | 1.42 (0.92–1.96) | 0.069 |
| Troponin (ng/ml) | 3.0 (2.0–4.0) | 11 (6.8–16.5) * | 11.64 (8.9–15.8) * | 9.1 (4.7–17.4) * | <0.0001 |
| D‐Dimer (mg/L) | 66 (44–90) | 515 (233–1011) * | 667.5 (417–871) * | 500 (261–1194) * | <0.0001 |
| Fibrinogen (mg/dl) | 1.68 (1.38–2.33) | 4.43 (2.82–5.64) * | 5.28 (3.8–6.31) * | 5.2 (4.15–6.03) * | <0.0001 |
| CRP (mg/dl) | 1.05 (0.69–2.32) | 48.3 (21.10–99.7) * | 105 (77–143) * | 56 (34–90) * | <0.0001 |
| IL‐6 (pg/ml) | 2.01 (1.66–3.15) | 29 (11.8–69.2) * | 45.91 (36.4–68.0) * | 34.5 (7.9–110) * | <0.0001 |
| Ferritin | 68 (53–120) | 277 (97–598) * | 523 (304–877) * | 675 (388–1327) * | <0.0001 |
| TRP (ng/ml) | 11927 (10840–12731) | 9540 (7039–11991) * | 9483 (6380–12302) * | 9592 (8392–11477) * | 0.004 |
| KYN (ng/ml) | 367 (330–416) | 409 (261–493) | 689 (413–1004) * | 678 (482–951) * $ | <0.0001 |
| KYN:TRP ratio | 0.031 (0.026–0.037) | 0.038 (0.029–0.069)* | 0.062 (0.048–0.107) *$ | 0.072 (0.044–0.106) *$ | <0.0001 |
| KYNA (ng/ml) | 21.2 (13.1–28.9) | 52.5 (28.2–110) * | 57.5 (41.9–200) * | 60.1 (48.2–104) * | <0.0001 |
| 3OHKYN (ng/ml) | 36.04 (25.4–42.1) | 38 (17.4–78) | 50.1 (29.9–80) | 48.14 (26–110) * | 0.042 |
| QA (ng/ml) | 17.3 (12.4–24.18) | 59 (40.5–101) * | 86.6 (59.5–189) * | 97.5 (58.8–195) * $ | <0.0001 |
| PA (ng/ml) | 5.1 (2.9–12.1) | 21 (13.9–30) * | 34.1 (18.6–55.7) *$ | 34.5 (22.0–53.2) *$ | <0.0001 |
Abbreviations: 3OHKYN, 3 Hydroxykynurenine; ALP, Alkaline Phosphatase; ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; BUN, Blood Urea Nitrogen; CRP, C‐reactive protein; GGT, Gamma‐Glutamyl Transferase; ICU, Intensive Care Unit; IL‐6, Interleukin‐6; KYN, Kynurenine; KYNA, Kynurenic Acid; LDH, Lactate Dehydrogenase; N:L Ratio Neutrophil–Lymphocyte ratio; PA, Picolinic acid; QA, Quinolinic acid; TRP, Tryptophan; WBC, White blood cell.
(*) Statistical difference with the control group p < 0.05, ($) Statistical difference with the mild‐moderate COVID‐19 group p < 0.05, (#) statistical difference with the severe COVID‐19 group p < 0.05.
Bold indicates statistically significant value (p < 0.05).
Among them, only N:L ratio and LDH levels were found to be statistically significantly higher in ICU cases compared to mild‐moderate patient group (Table 1).
When KYN metabolite levels of the COVID‐19 patient groups were compared with their control group counterparts, all KYN metabolite values (TRP, KYN, KYN:TRP ratio, KYNA, 3OHKYN, PA, and QA) were found to be statistically different between the groups (Table 1 and Figure 2).
FIGURE 2.
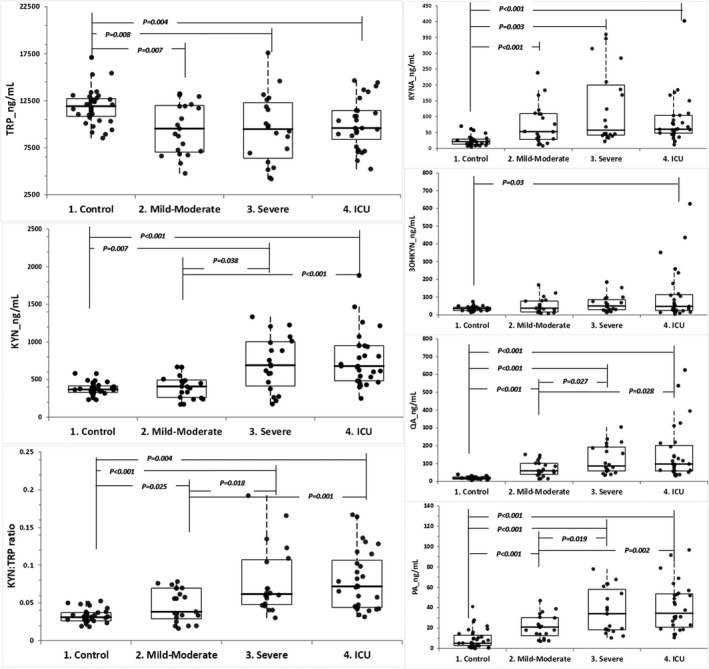
Comparison of COVID‐19 patient groups with different severity levels and healthy control groups with respect
When the features that will distinguish severe and ICU cases from mild‐moderate cases were examined, it was observed that KYN, QA, and PA levels and KYN:TRP ratio of Mild/Moderate cases were statistically significantly different from their counterparts in ICU and severe cases (Table 1 and Figure 2).
According to the Spearman correlation analysis results, when the total cases were examined, it was determined that there were statistically significant correlations between KYN metabolites and especially inflammatory markers. As expected, statistically significant positive correlations were found between IL‐6 and KYN:TRP ratio (Table 2).
TABLE 2.
Relationship of KYN metabolites with other inflammatory parameters in COVID‐19 patient and control groups and total cases
| Group | TRP | KYN | KYN:TRP ratio | KYNA | 3OHKYN | QA | PA |
|---|---|---|---|---|---|---|---|
| N:L Ratio | |||||||
| Total | −0.339* | 0.271* | 0.412* | 0.570* | 0.393* | 0.609* | 0.521* |
| COVID‐19 | −0.184 | 0.074 | 0.129 | 0.373* | 0.384* | 0.374* | 0.185 |
| Control | 0.083 | 0.026 | −0.018 | 0.023 | 0.303 | 0.116 | 0.211 |
| LDH | |||||||
| Total | −0.069 | 0.470* | 0.442* | 0.510* | 0.127 | 0.546* | 0.567* |
| COVID‐19 | 0.260* | 0.271* | 0.029 | 0.034 | 0.016 | 0.009 | 0.250* |
| Control | 0.007 | 0.068 | 0.165 | 0.116 | 0.044 | 0.027 | −0.067 |
| Procalcitonin | |||||||
| Total | −0.210* | 0.316* | 0.418* | 0.368* | 0.226* | 0.446* | 0.358* |
| COVID−19 | −0.090 | 0.230 | 0.322* | 0.274* | 0.239* | 0.324* | 0.144 |
| Control | −0.079 | −0.110 | 0.089 | −0.058 | 0.031 | 0.061 | 0.220 |
| Troponin | |||||||
| Total | −0.290* | 0.351* | 0.447* | 0.590* | 0.237* | 0.664* | 0.431* |
| COVID‐19 | −0.105 | 0.027 | 0.083 | 0.344* | 0.244* | 0.283* | −0.103 |
| Control | −0.087 | 0.056 | 0.097 | −0.136 | −0.140 | 0.082 | 0.247 |
| D‐Dimer | |||||||
| Total | −0.218* | 0.334* | 0.400* | 0.536* | 0.065 | 0.606* | 0.573* |
| COVID‐19 | 0.118 | −0.037 | −0.182 | 0.069 | −0.153 | −0.013 | 0.104 |
| Control | −0.181 | −0.132 | 0.089 | −0.101 | −0.046 | −0.100 | 0.086 |
| Fibrinogen | |||||||
| Total | −0.156 | 0.240* | 0.322* | 0.469* | 0.225* | 0.602* | 0.507* |
| COVID‐19 | 0.103 | −0.139 | −0.176 | 0.018 | 0.169 | 0.159 | 0.110 |
| Control | 0.242 | −0.110 | −0.208 | 0.132 | 0.057 | −0.086 | −0.113 |
| CRP | |||||||
| Total | −0.291* | 0.343* | 0.475* | 0.626* | 0.215* | 0.750* | 0.530* |
| COVID‐19 | −0.081 | −0.089 | −0.067 | 0.216 | 0.200 | 0.336 | −0.004 |
| Control | 0.079 | 0.268 | 0.231 | 0.220 | −0.152 | 0.259 | −0.125 |
| IL‐6 | |||||||
| Total | −0.384* | 0.442* | 0.599* | 0.498* | 0.295* | 0.682* | 0.524* |
| COVID‐19 | −0.158 | 0.295* | 0.376* | 0.144 | 0.268* | 0.303* | 0.089 |
| Control | −0.160 | 0.033 | 0.234 | −0.211 | −0.018 | 0.047 | 0.126 |
| Ferritin | |||||||
| Total | −0.185 | 0.503* | 0.517* | 0.630* | 0.212* | 0.655* | 0.584* |
| COVID‐19 | 0.067 | 0.392* | 0.305* | 0.370* | 0.216 | 0.288* | 0.285* |
| Control | 0.117 | −0.090 | −0.253 | 0.156 | −0.239 | −0.001 | −0.183 |
| TRP | |||||||
| Total | −0.024 | −0.528* | −0.229* | −0.303* | −0.433* | −0.179 | |
| COVID‐19 | 0.187 | −0.388* | −0.172 | −0.285* | −0.317* | 0.138 | |
| Control | 0.047 | −0.611* | 0.492* | −0.019 | −0.106 | −0.126 | |
| KYN | |||||||
| Total | 0.832* | 0.615* | 0.382* | 0.631* | 0.533* | ||
| COVID‐19 | 0.800* | 0.503* | 0.319* | 0.481* | 0.441* | ||
| Control | 0.691* | 0.357* | 0.371* | 0.566* | 0.168 | ||
| KYN:TRP ratio | |||||||
| Total | 0.604* | 0.432* | 0.765* | 0.545* | |||
| COVID‐19 | 0.512* | 0.441* | 0.608* | 0.303* | |||
| Control | −0.075 | 0.295* | 0.514* | 0.212 | |||
| KYNA | |||||||
| Total | 0.485* | 0.764* | 0.518* | ||||
| COVID‐19 | 0.631* | 0.655* | 0.250* | ||||
| Control | −0.036 | 0.070 | −0.017 | ||||
| 3OHKYN | |||||||
| Total | 0.475* | 0.264* | |||||
| COVID‐19 | 0.592* | 0.140 | |||||
| Control | 0.242 | 0.279 | |||||
| QA | |||||||
| Total | 0.575* | ||||||
| COVID‐19 | 0.147 | ||||||
| Control | 0.159 | ||||||
Bold indicates statistically significant value (p < 0.05).
When only the correlation results in COVID‐19 patients were examined, significant correlations were found between KYN:TRP ratio and IL‐6, ferritin, and procalcitonin. However, no significant correlation was found between KYN metabolites and other laboratory parameters in the healthy control group (Table 2).
When the correlation among the metabolites in the KYN pathway was examined, it was found that there was a statistically significant correlation between some KYN pathway metabolites in both healthy controls and COVID‐19 patients and the total group (Table 2).
When COVID‐19 patient group was compared with the healthy control, the ROC analysis results showed that the distinctive feature of “classical laboratory parameters and KYN metabolites” was high. However, considering the main purpose of the present study, evaluation of the severity of COVID‐19 disease, it has been determined that KYN:TRP ratio and PA values are among the KYN metabolite parameters having most substantial differences in severe COVID‐19 patients.
It has been evaluated that these two parameters can be used more effectively than other parameters in differentiating severe patients and clarifying the pathophysiology (Figure 3).
FIGURE 3.
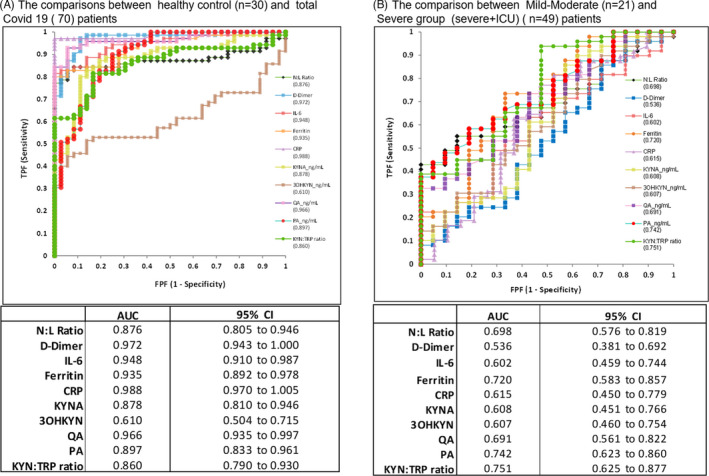
ROC analysis results of the comparison of COVID‐19 patients with the control group and mild‐moderate COVID‐19 patients with severe COVID‐19‐ICU patients with respect to inflammatory markers and KYN metabolites
KYN:TRP ratio and PA levels, which were considered to be significant in determining the severity of the disease, were adjusted according to age, gender, and comorbidity using multivariate regression analysis. Accordingly, the odd ratio and confidence intervals of KYN:TRP ratio and PA were determined as 1.44 (1.1–1.87, p = 0.008) and 1.06 (1.02–1.11, p = 0.006), respectively. Based on these results, it was concluded that KYN:TRP ratio and PA levels have a role in determining the severity of COVID‐19 patients independently from comorbidity, age, and gender.
4. DISCUSSION
Previous studies found significant changes in the KYN pathway in COVID‐19 patients. 7 , 24 , 25 , 26 High KYN:TRP ratio in the studies was interpreted in 2 different ways. According to the first point of view, the kynurenine pathway is considered to be activated due to inflammation, indicating an excessive immune reactivity. According to the second point of view, immune tolerance resulting from activation of the KYN pathway is thought to weaken the immune response to the virus, delaying virus clearance, and leading to the development of acute respiratory distress syndrome and multiple organ failure. 4 , 25 , 26
It has been suggested that the KYN:TRP ratio can be used in the selection of drugs such as glucocorticoids, JAK inhibitors, or anti‐IL‐6. 7
In their study which demonstrated that KYN:TRP ratio was related to the severity of the infection and the prevalence of lymphopenia, Lionetto et al suggested that individual examination of KYN metabolites would provide valuable information. 7 In their study where they have analyzed metabolites of COVID‐19 patients, Thomas et al. found decreases in TRP, serotonin, indole pyruvate, 3OHKYN, and anthranilic acid and increases in KYN, KYNA, nicotinic acid, and PA levels. The authors demonstrated a correlation between Kyn metabolites and IL‐6 levels. 24 However, in this study, 3OHKYN values were not found to be low. We think that this difference is due to the derivatized method used in this study which is known to be a more sensitive method. In parallel with the previous literature, which reported high levels of cytokines in patients hospitalized in ICUs, high levels were detected especially for IL‐6 in COVID‐19 patients in the present study. 5
In this study, significant correlations were found between N:L ratio, procalcitonin, CRP, IL‐6, and ferritin and some KYN pathway metabolites, especially in the total and COVID‐19 patient groups, but no significant correlations were found in the healthy group (Table 2). Correlations, especially in the COVID‐19 patient group, may be important in determining the severity of the disease. While Mangge et al. suggested KYN level as a potential marker in determining the fatality of COVID‐19 patients, fatality was not evaluated in our study. 27 Danlos et al., 28 on the contrary, suggested an inhibitory effect of KYN or anthranilic acid on IDO and TPO, having an immunosuppressive effect. Particularly, according to the results of correlation analysis in patient and total groups, KYN pathway metabolites (especially KYN:TRP ratio, PA, and QA) may be markers of the inflammatory response.
Some kynurenine metabolites (especially KYN) are neuroactive and have a potent effect on excitatory synaptic transmission by activating or inhibiting various classes of glutamate receptors. 10 It is stated that these metabolites may be associated with the neurological symptoms that can be seen in SARS‐CoV2 infection. 7 It is possible that, through IDO, KYN metabolism may influence the clinical course of COVID‐19 pulmonary disease. 29
In addition, TRP and especially 3OHKYN may affect oxidative stress, superoxide, and NO production. Both SARS‐CoV‐2 pneumonia and SARS‐CoV‐2 cytokine storm cause severe oxidative stress. Activated IDO‐1 may represent a local antioxidant defense against this oxidative stress. 30
In the present study, parallel results were obtained with the previous literature on the KYN:TRP ratio. In addition to the KYN:TRP ratio, especially KYNA, 3‐OHKYN, PA, and QA measurements were made, and the correlation between the severity of COVID patients and the rate of admission to the intensive care unit was examined. In the present study, a derivatization method was used which enables the measurement of molecules present in low concentrations such as QA, PA, and 3‐OHKYN. One of the important findings of this study is that, in addition to the KYN:TRP ratio, PA is also an important marker in determining the severity of the disease. When the age, gender, and comorbidity‐adjusted odd ratio values were examined, both PA and KYN:TRP ratios were found to be statistically significant. The present study is novel in demonstrating the importance of PA in the assessment of the severity of the COVID‐19 disease.
According to the results of the ROC analysis performed to determine the clinical severity of COVID‐19, PA and KYN:TRP ratio values were found to have the highest AUC values in determining the severity (0.742 and 0.751, respectively). ROC analysis was not performed in the study by Lionetto et al. In their study, Mannge et al found the AUC value as 0.83 for the KYN level in the ROC analysis performed for the evaluation of fatality. Fatality was not evaluated in the present study.
Especially the limited sample size, the low number of mild‐moderate COVID‐19 patients and the absence of asymptomatic cases are among the important limitations of the present study. In addition, while the sampling times of COVID‐19 cases differed considerably, samples of ICU cases were especially taken on the 3–4th day. Furthermore, it should be considered that drugs (anti‐inflammatory, steroid, etc.) used in COVID‐19 patients may have significant effects on these pathways. Since the number of severe/ICU cases who lost their lives during the study was only 3, no specific evaluation was performed about them.
In the literature, there are very few and limited studies on the KYN pathway in the pathophysiology of COVID‐19. The present study is unique both in terms of measuring additional metabolites such as KYNA, QA, PA, 3OHKYN, and comparing COVID −19 patient groups of different severity levels. Results of the present study suggest that especially PA and KYN:TRP ratio may be potential markers for the detection of severe COVID‐19 cases and KYN metabolites may have a role in the pathophysiology of COVID‐19.
CONFLICT OF INTEREST
The authors declare no competing interests.
Supporting information
Supplementary Material
Cihan M, Doğan Ö, Ceran Serdar C, Altunçekiç Yıldırım A, Kurt C, Serdar MA. Kynurenine pathway in Coronavirus disease (COVID‐19): Potential role in prognosis. J Clin Lab Anal. 2022;36:e24257. doi: 10.1002/jcla.24257
DATA AVAILABILITY STATEMENT
All datasets used during the present study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Gustine JN, Jones D. Immunopathology of hyperinflammation in COVID‐19. Am J Pathol. 2021;191:4‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong CK, Lam CWK, Wu AKL, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanai M, Funakoshi H, Takahashi H, et al. Tryptophan 2,3‐dioxygenase is a key modulator of physiological neurogenesis and anxiety‐related behavior in mice. Molecular Brain. 2009;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lionetto L, Ulivieri M, Capi M, et al. Increased kynurenine‐to‐tryptophan ratio in the serum of patients infected with SARS‐CoV2: An observational cohort study. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zou MH. Tryptophan‐kynurenine pathway is dysregulated in inflammation and immune activation. Front Biosci. 2015;20:4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grohmann U, Puccetti P. The coevolution of IDO1 and AhR in the emergence of regulatory T‐cells in mammals. Front Immunol. 2015;6:58. doi: 10.3389/fimmu.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwarcz R. Kynurenines and glutamate: multiple links and therapeutic implications. Adv Pharmacol. 2016;76:13‐37. doi: 10.1016/bs.apha.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mondanelli G, Coletti A, Greco FA, et al. Positive allosteric modulation of indoleamine 2,3‐dioxygenase 1 restrains neuroinflammation. Proc Natl Acad Sci USA. 2020;117:3848‐3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anderson G, Carbone A, Mazzoccoli G. Aryl hydrocarbon receptor role in co‐ordinating SARS‐CoV‐2 entry and symptomatology: linking cytotoxicity changes in COVID‐19 and cancers; modulation by racial discrimination stress. Biology. 2020;9:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turski WA, Wnorowski A, Turski GN, Turski CA, Turski L. AhR and IDO1 in pathogenesis of Covid‐19 and the “Systemic AhR Activation Syndrome:” a translational review and therapeutic perspectives. Restor Neurol Neurosci. 2020;38:343‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eleftheriadis T, Pissas G, Antoniadi G, et al. Indoleamine 2,3‐dioxygenase downregulates T‐cell receptor complex ζ‐chain and c‐Myc, and reduces proliferation, lactate dehydrogenase levels and mitochondrial glutaminase in human T‐cells. Mol Med Rep. 2016;13:925‐932. [DOI] [PubMed] [Google Scholar]
- 15. Yap SH, Abdullah NK, McStea M, et al. HIV/Human herpesvirus co‐infections: Impact on tryptophan‐kynurenine pathway and immune reconstitution. PLoS One. 2017;12:e0186000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pizzini A, Kurz K, Santifaller J, et al. Assessment of neopterin and indoleamine 2,3‐dioxygenase activity in patients with seasonal influenza: a pilot study. Influenza Other Respir Viruses. 2019;13:603‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma L, Xu B, Wang W, Deng W, Ding M. Analysis of tryptophan catabolism in HBV patients by HPLC with programmed wavelength ultraviolet detection. Clin Chim Acta. 2009;405:94‐96. [DOI] [PubMed] [Google Scholar]
- 18. Mellor AL, Munn DH. Ido expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762‐774. [DOI] [PubMed] [Google Scholar]
- 19. Fazio F, Zappulla C, Notartomaso S, et al. Cinnabarinic acid, an endogenous agonist of type‐4 metabotropic glutamate receptor, suppresses experimental autoimmune encephalomyelitis in mice. Neuropharmacology. 2014;81:237‐243. [DOI] [PubMed] [Google Scholar]
- 20. Tömösi F, Kecskeméti G, Cseh EK, et al. A validated UHPLC‐MS method for tryptophan metabolites: application in the diagnosis of multiple sclerosis. J Pharm Biomed Anal. 2020;185:113246. [DOI] [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute (CLSI) . Mass spectrometry in the clinical laboratory: general principles and guidance; proposed guideline, CLSI C50‐P; 2007.
- 22. Clinical and Laboratory Standards Institute (CLSI) . Liquid chromatography‐mass spectrometry methods; approved guideline, CLSI C62‐A; 2014.
- 23. Bioanalytical Method Validation. Rockville, MD: Food and Drug Administration (FDA); 2018. Available from: https://www.fda.gov/files/drugs/published/Bioanalytical‐Method‐Validation‐Guidance‐for‐Industry.pdf. (Accessed September 9, 2021) [Google Scholar]
- 24. Thomas T, Stefanoni D, Reisz JA, et al. COVID‐19 infection alters kynurenine and fatty acid metabolism, correlating with IL‐6 levels and renal status. JCI Insight. 2020;5(15):e140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimhofer T, Lodge S, Whiley L, et al. Integrative modeling of quantitative plasma lipoprotein, metabolic, and amino acid data reveals a multiorgan pathological signature of SARS‐CoV‐2 infection. J Proteome Res. 2020;19:4442‐4454. [DOI] [PubMed] [Google Scholar]
- 26. Lawler NG, Gray N, Kimhofer T, et al. Systemic perturbations in amine and kynurenine metabolism associated with acute SARS‐CoV‐2 infection and inflammatory cytokine responses. J Proteome Res. 2021;20:2796‐2811. [DOI] [PubMed] [Google Scholar]
- 27. Mangge H, Herrmann M, Meinitzer A, et al. Increased kynurenine indicates a fatal course of COVID‐19. Antioxidants. 2021;1:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Danlos F‐X, Grajeda‐Iglesias C, Durand S, et al. Metabolomic analyses of COVID‐19 patients unravel stage‐dependent and prognostic biomarkers. Cell Death Dis. 2021;12(3):258. doi: 10.1038/s41419-021-03540-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aldajani WA, Salazar F, Sewell HF, Knox A, Ghaemmaghami AM. Expression and regulation of immune‐modulatory enzyme indoleamine 2,3‐dioxygenase (IDO) by human airway epithelial cells and its effect on T cell activation. Oncotarget. 2016;7(36):57606‐57617. doi: 10.18632/oncotarget.11586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci USA. 1990;87(7):2506‐2510. doi: 10.1073/pnas.87.7.2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
All datasets used during the present study are available from the corresponding author on reasonable request.


