Abstract
Over the past decade, Greece and other Mediterranean countries have witnessed the emergence and resurgence of several vector-borne diseases (VBDs), posing important public health challenges and threatening the tourist industry. An essential prerequisite for the design and execution of efficient and sustainable context-specific VBD control programmes is the establishment of integrative entomological and epidemiological surveillance systems. However, the monitoring and management of surveillance datasets (often chronologically fragmented, scattered in regional health district offices and partially accessible upon requisition), as well as their transformation into actionable information, is a complex undertaking. In light of aiding and optimizing vector control efforts in the Mediterranean Basin, we developed VectorMap-GR, an online, open access, operational management tool for entomological and complementary epidemiological monitoring data. The toolʼs key components are a set of controlled vocabularies (ontologies) running throughout the system, the systemʼs database and a map interface for data querying and display. The tool supports transformation of raw data into operationally relevant information (i.e. customized maps, charts, tables and reports) in a highly interactive fashion achieved through query filters and the ArcGIS technology embedded in the system. End-users may search for and obtain information on (i) the mosquito fauna composition, abundance and spatiotemporal dynamics; (ii) the mosquito insecticide resistance status and underlying resistance mechanisms; (iii) the occurrence of VBD pathogens and infections in vectors, animals and humans; and (iv) operationally relevant physical feature georeferenced datasets (e.g. mosquito breeding sites). VectorMap-GR was pilot implemented during 2018–2020 in a mosquito control programme in the Region of Crete (southern Greece). The programmeʼs control efforts coupled with VectorMap-GR pilot implementation phase, very likely contributed to the reduction of vector population numbers and the prevention of human VBD occurrences, recorded in this period.
Keywords: Information technology, Vector control, Surveillance, Operational management tool, Greece, Mediterranean Basin
Graphical abstract
Highlights
-
•
VectorMap-GR has a capacity for operational vector management support in the Mediterranean Basin.
-
•
The tool provides actionable entomological/epidemiological data over key GIS layers.
-
•
The output generation process is rapid, interactive and query-sensitive.
-
•
The open access, online tool displays high expandability potential.
1. Introduction
As of the late 2000s, a number of countries surrounding the Mediterranean Basin have experienced the emergence and resurgence of important vector-borne diseases (VBDs) collectively threatening human well-being (Gubler, 2010; Postigo, 2010; Eybpoosh et al., 2019).
Amidst the changing VBD scene, largely driven by climate and environmental change, global trade, transcontinental travel, unplanned urbanization, conflict, and mass population displacement (Carballo & Nerurkar, 2001; Sutherst, 2004; Semenza & Suk, 2018), Greece has faced over the last decade consecutive WNV outbreaks (NPHO, 2021) (associated with the mosquito Culex pipiens (Chaskopoulou et al., 2013)) and a series of malaria transmission events (NPHO, 2020). The current widespread distribution of the major arbovirus vector Aedes albopictus across the country (Balaska et al., 2020) further fuels the underlying potential for local VBD transmission, while phlebotomine sand fly-borne diseases (i.e. visceral and cutaneous leishmaniasis) pose additional pressure to the public and veterinary healthcare system (Kostopoulou et al., 2020; Tzani et al., 2021).
Apart from an important public health problem, VBDs in Greece also threaten the tourist industry which accounts for 20.8% of the countryʼs gross domestic product (Statista, 2020; World Bank, 2020) making VBD monitoring, prevention and control both a public health and economic priority.
In the absence of protective human vaccines against most VBDs, disease prevention and control heavily relies on vector control programmes utilizing biocides to manage vector populations (Becker, 2010). Greece and neighboring countries in the Mediterranean Basin constitute of highly heterogeneous settings (in terms of entomological, epidemiological, ecological and social contexts), often alternating and in close proximity to each other, requiring the development of local scale context-specific vector control responses (Kramer, 2011; Roiz et al., 2015; Legakis et al., 2018).
Several factors including (i) the development and selection for insecticide resistance in local disease vector populations, due to the extensive use of a limited number of active ingredients in vector and agricultural pest control (Fotakis et al., 2017; Balaska et al., 2020); (ii) the few insecticide alternative intervention options currently available; and (iii) the frequent inconsistency in financial and logistical vector control program resources in a number of high-risk regions (Kolimenakis et al., 2019; Vontas & Mavridis, 2019), increases the situational complexity furthermore necessitating the deployment of tailored responses.
A core prerequisite for developing efficient vector control programmes lies in the establishment of integrative and responsive surveillance systems. Systematic monitoring of the mosquito fauna composition, abundance, and spatiotemporal dynamics complemented by information on insecticide resistance and VBD-associated pathogen circulation, provides the basis for generating comprehensive, operationally relevant information to guide the design and execution of efficient and sustainable control programmes (Moyes et al., 2017; Fournet et al., 2018; Dacko et al., 2020).
Conventional surveillance tools and approaches for classical entomological monitoring such as overnight deployment of adult collection traps, morphological identification keys and biological assays (bioassays) for evaluating phenotypic insecticide resistance, are widely used in this respect, generating valuable surveillance information. In addition, recently introduced (or under development) novel tools and means support the generation of an ever-increasing volume of high quality, real-time surveillance data, greatly enhancing our capacity for conducting robust surveillance.
Important advancements in this direction include surveillance data harmonization initiatives (in the prism of defining surveillance protocols, guidelines, evidence-based standards and context-specific objectives) (Jourdain et al., 2019); passive, community-based surveillance programmes (building upon citizen-based mosquito specimen recognition, collection, reporting, and submission) (Kampen et al., 2015), and the development of innovative monitoring tools. State of the art tools include mobile applications and “intelligent” traps aiming at real-time mosquito identification (Minakshi et al., 2017; Kittichai et al., 2021) as well as high-throughput, multiplex molecular diagnostic tools providing rapid sample-to-answer species identification, insecticide resistance and pathogen related information (Mitsakakis et al., 2018; Mavridis et al., 2019).
However, the systematic processing of a wide range of surveillance data and its transformation into operationally relevant output is a highly challenging and complex undertaking. Established and emerging information technologies (IT) present new opportunities to improve our capacity for surveillance data management and data conversion into actionable information, providing varying levels of vector control support (Eisen & Eisen, 2011; Lozano-Fuentes et al., 2011).
A number of functionally diverse VBD related databases and IT tools have been developed in the recent years. Examples include (i) the IRMapper (https://www.irmapper.com/), a tool that consolidates reports of insecticide resistance in Anopheles spp. and Aedes spp. collected from several regions throughout the world and allows the visualization of results from investigations of insecticide resistance mechanisms across existing published data (Knox et al., 2014); (ii) MapVEu (https://vectorbase.org/popbio-map/web/), which searches for, analyses and describes a wide range of population data (e.g. insecticide resistance and population abundance data) including gene libraries and field collection metadata for multiple disease vectors around the globe (https://vectorbase.org/popbio-map/web/); and (c) VectorMap (http://vectormap.si.edu/), which searches for and presents collection records and distribution models for a number of arthropods (i.e. mosquitoes, sand flies, midges, fleas, mites, ticks) across the world.
However, these tools primarily dispense usable information at the research and policy levels as they do not specifically target the provision of operational feedback for the assessment and deployment of timely and context-specific local scale vector control responses. Although integrative operationally relevant IT surveillance systems, tailored to the lowest level of the control programmes are of high importance to support informed deployment of appropriate control actions (Fournet et al., 2018; Monnier et al., 2020), to date, very few such data initiatives (encompassing the vector, insecticide resistance and pathogen component in a single tool) exist.
An available tool is the Disease Data Management System (DDMS) (Eisen et al., 2011), a non-open access software customized database that has been developed to manage vector control data for disease control programmes. Yet, DDMS mainly focuses on malariometric data (including vector species distributions, malaria transmission intensities and other parameters) in sub-Saharan Africa.
VectorSurv (https://maps.vectorsurv.org/arbo) comprises an alternative integrative IT system, specifically tailored to manage arboviral disease surveillance data for mosquito control and public health agencies in several states in the USA. The system primarily centres on arboviral infection spatiotemporal trends (in mosquitoes and birds), Aedes mosquito abundances and arboviral risk index maps.
Such tools, providing operational data management support to vector control programmes are not implemented or available for Greece (or neighbouring Mediterranean Basin countries), whilst vector management (i.e. surveillance and control) programmes in the region encounter multiple data management and analysis issues upon their sole reliance on conventional data processing means (e.g. hardcopy data files, multifarious Excel spreadsheets, off-line data libraries). Frequently encountered issues include data partitioning and fragmentation amongst different agencies (resulting in data inaccessibility or partial accessibility upon requisition); delayed transformation of raw data into actionable information; and separate assessment of complementary datasets upon decision-making (evoking the mis/non-communication between VBD programme stakeholders). In addition, most generated vector/VBD surveillance maps are static (e.g. VectorNet initiative, https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/vector-net) serving as “snapshots” corresponding to specific time-points and locations, failing to capture the vector, insecticide resistance and pathogen spatiotemporal dynamics.
In light of facilitating and optimizing VBD management efforts in the Mediterranean Basin with a special focus on Greece, we designed VectorMap-GR (https://vectormap-gr.com/): an online, open access, operational management tool for entomological (and complementary epidemiological) monitoring data aiming to support the development and sustainability of tailored, evidence-based vector control programmes in the region. The tool was pilot implemented during 2018–2020 in a mosquito management programme in the Region of Crete (southern Greece).
2. Materials and methods
2.1. VectorMap-GR development
VectorMap-GR was developed through an interactive process between software developers, bioinformaticians, molecular biologists, medical entomologists and public health stakeholders. In order to attain an end product of high utility, targeted end-users were consulted during the systemʼs development process, including: research and development personnel and project managers from companies organizing and implementing vector control programmes (in Greece); members of European and national (Greek) coordinations, networks and committees addressing VBD prevention and control; public health officials from the national public health organization of Greece; central and regional public health laboratory officials (Greece); and directors, deputy heads and public health supervisors of regional health districts and regional/prefecture directorates of public health and social care (Greece).
A series of meetings were held (with the aforementioned parties) in three key phases of the systemʼs development (i.e. initiation phase: “on paper” system design; intermediate phase: systemʼs core functionalities and features in place; and pre-final phase: assessment of the system as a whole), in light of generating a positive feedback loop in terms of developing an efficacious end product for vector control support. All system functionalities were rigorously tested throughout the developmental and pilot implementation phases.
2.2. System architecture and requirements
VectorMap-GR was developed using popular and reliable open source tools. The systemʼs back-end was designed with PHP programming language and the Laravel framework (https://github.com/laravel/laravel). Extra-libraries were used for various features. Indicatively, the PHP Office library (https://github.com/php/php-src) was used for exporting data in Excel format and the Geohash library (https://github.com/lvht/geohash) for detecting the geohash (i.e. encoded geographical location) of any given map point. BFO (Basic Formal Ontology)-based ontologies were developed (following the OBO Foundry international standards) (Smith et al., 2007), through extending and adapting the MIRO ontologies designed by Dialynas et al. (2009). All input data are stored in the relational database MySql (https://github.com/mysql/mysql-server).
For the front end, jQuery (https://github.com/jquery/jquery), VueJS (https://github.com/vuejs/vue), Select2 (https://select2.org/), Highcharts (https://github.com/highcharts/highcharts) and ArcGIS (https://developers.arcgis.com/javascript/latest/) JavaScript libraries were used. The on map grouping visualization was implemented with the geohashing method. In addition, ArcGIS services were used for visualizing the different map layers including (amongst others) point mosquito breeding sites (provided by Ecodev (https://ecodev.gr/) within the framework of the vector control programme conducted under the auspices of the Region of Crete), and streams/canals, natural systems, lakes and transitional waters (obtained from https://geodata.gov.gr/en/).
VectorMap-GR is an open access tool compatible with all major web browsers (e.g. Microsoft Internet Explorer/Microsoft Edge, Mozilla Firefox, Google Chrome). The minimum server requirements are PHP version 7.3 and greater, Laravel version 8.0, and MySql version 14.4. Finally, the application is hosted on a Linux Ubuntu 18.04 server (https://ubuntu.com/).
2.3. VectorMap-GR pilot implementation
VectorMap-GR was pilot implemented in an integrated mosquito control programme in the Region of Crete, Greece, during 2018–2020. Mosquito surveillance was conducted annually from April to November and consisted of bi-weekly adult collections from fixed sites. Under the auspices of the region of Crete, VectorMap-GR was strategically positioned as a core component of the local VBD management efforts, ensuring all generated data were systematically incorporated in the system and the tool was utilized in vector control activity planning. The toolʼs broad usage (among the key stakeholders) was further promoted through tutorial presentations highlighting the systemʼs capacities and features.
3. Results
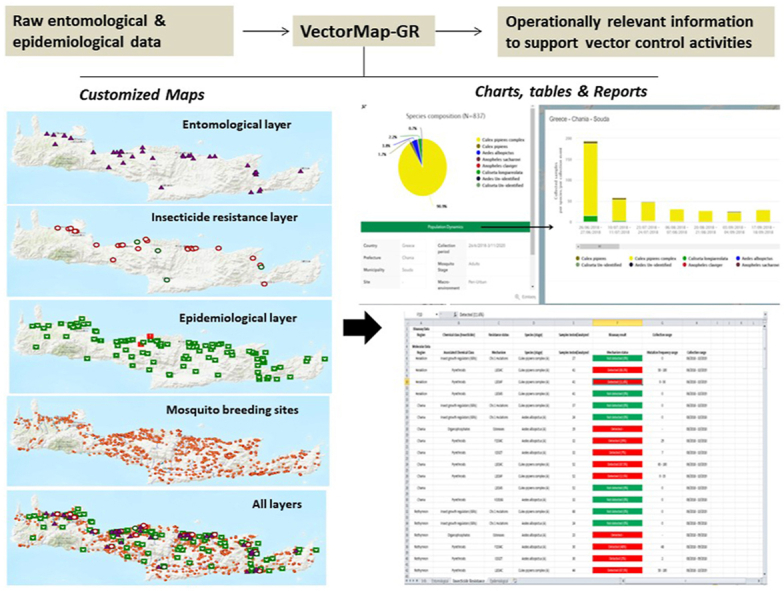
With a key goal of supporting evidence-driven vector control programmes, VectorMap-GR was designed with the capacity for: (i) entomological, insecticide resistance and complementary epidemiological data management (i.e. systematic data storage, processing and retrieval); and (ii) rapid transformation of raw data into actionable information through query-based data analysis and display on customized maps, charts and reports depicting (a) the mosquito species composition, abundance and spatiotemporal dynamics; (b) the mosquito population insecticide resistance status; (c) the occurrence of pathogens and infections in vectors, animals and humans; and (d) vector control operationally relevant physical and demographic georeferenced datasets (e.g. georeferenced mosquito breeding sites, human population density layer).
The developed system consists of a set of controlled vocabularies (ontologies) supporting platform subsystem interoperability, the system database where all the data are stored, and the map interface for data querying and display.
3.1. Controlled vocabularies
Controlled vocabularies were developed based on ontological principles describing the parameters that define the data introduced and analysed in the system (Supplementary Figure S1; Supplementary Table S1). The default controlled vocabularies (running throughout the system) are easily editable (and may be translated to any desired language), offering VectorMap-GR adaptability and expandability to new environments and local conditions. For example, new mosquito larvae habitats or resistance traits (e.g. a specific point mutation) not initially included in the default controlled vocabularies can be easily added/included. The data geolocations (coordinates and entity name(s): i.e. “Country/Prefecture/Municipality/Site”) comprise the geographical entity tree which is populated upon data entry.
3.2. System database
The system database has a capacity for fast and easy data introduction, storage, management and immediate data availability and accessibility. The database currently supports the introduction and storage of records on mosquito samples (entomological data), their insecticide resistance status and underlying mechanisms (insecticide resistance data), and records on the positive/negative detection of mosquito-borne disease infections or pathogens per se, in mosquitoes, reservoir and dead-end hosts (epidemiological data). Data may be obtained from multiple sources including published papers and unpublished datasets (upon permission).
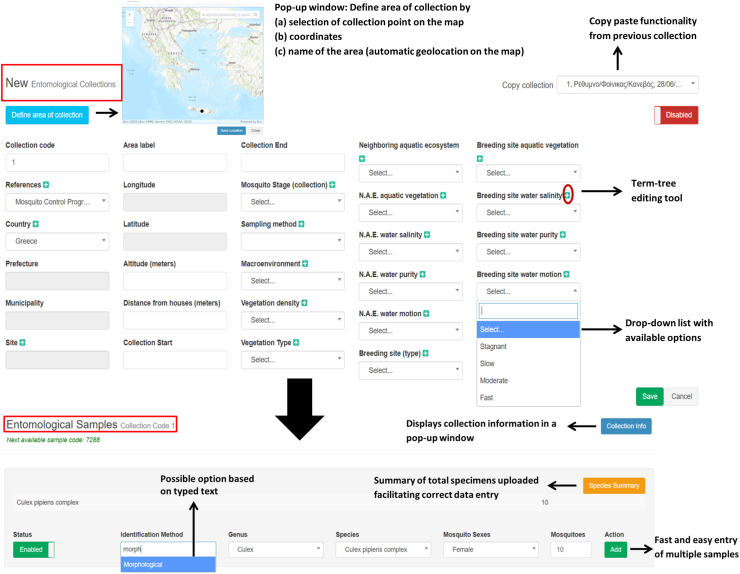
Data are uploaded on online forms and stored in respective libraries from where accredited users can search for, view, retrieve, edit or create new collections and content (Fig. 1). The online data entry forms include a number of functionalities (e.g. automated data integrity checks in the form of mandatory fields with pre-specified parameters to select from) to secure acceptable data quality upon submission and to facilitate easy and speedy data entry, search and editing.
Fig. 1.
Entomological collection (top) and entomological sample (bottom) online submission forms.
For final inclusion in the system (i.e. uploaded data acceptance/activation), data quality is evaluated by an internal curation team associated with the system developers, following specific criteria, including: published guidelines for sampling such as the ECDC guidelines for the surveillance of invasive (ECDC, 2012) and native mosquitoes (ECDC, 2014); species identification determinations (i.e. published morphological identification keys, broadly accepted molecular identification techniques and assays); established bioassay protocols, e.g. CDC and WHO bioassay guidelines (CDC, 2010; WHO, 2018); and widely used pathogen/infection detection protocols.
Importantly, in support of varying national, regional or program-specific data “openness” policies, VectorMap-GR offers the possibility for selected data accessibility (i.e. restriction) to accredited users thus, protecting on demand sensitive information (e.g. pathogen related information) and preventing unwanted access.
3.3. Map interface
VectorMap-GR supports the rapid transformation of raw data into actionable information (i.e. customized maps, reports, charts and tables). Data are queried and presented on the map interface in a highly dynamic fashion achieved through the ArcGIS technology embedded in the system. Through the combined use of activation/configuration filters and a map, users can limit the results displayed on the map interface, thereby obtaining the desired information (customized outputs).
3.3.1. Data querying
User queries are accomplished with a set of query filters. The query filters are grouped in 4 main layer sets: (i) physical feature and demographic layers; (ii) entomological; (iii) insecticide resistance; and (iv) epidemiological. The latter (sets ii, iii and iv) include a time scale for defining the time period of interest, a geographical entity filter and basic ontologies (controlled vocabularies available through pop-up lists) for parameter selection. In addition, a panel of advanced filters is also available for stringent searches. Certain filters are interconnected with built-in parameter associations facilitating the realisation of comprehensive queries.
3.3.2. Map generation
The map generation process is interactive and query-sensitive (i.e. instant map updates). The queried data are depicted on the map as georeferenced points, with icons and symbols representing the respective datasets and a color panel illustrating the query qualitative outcomes, collectively providing a visual summary of the queried data.
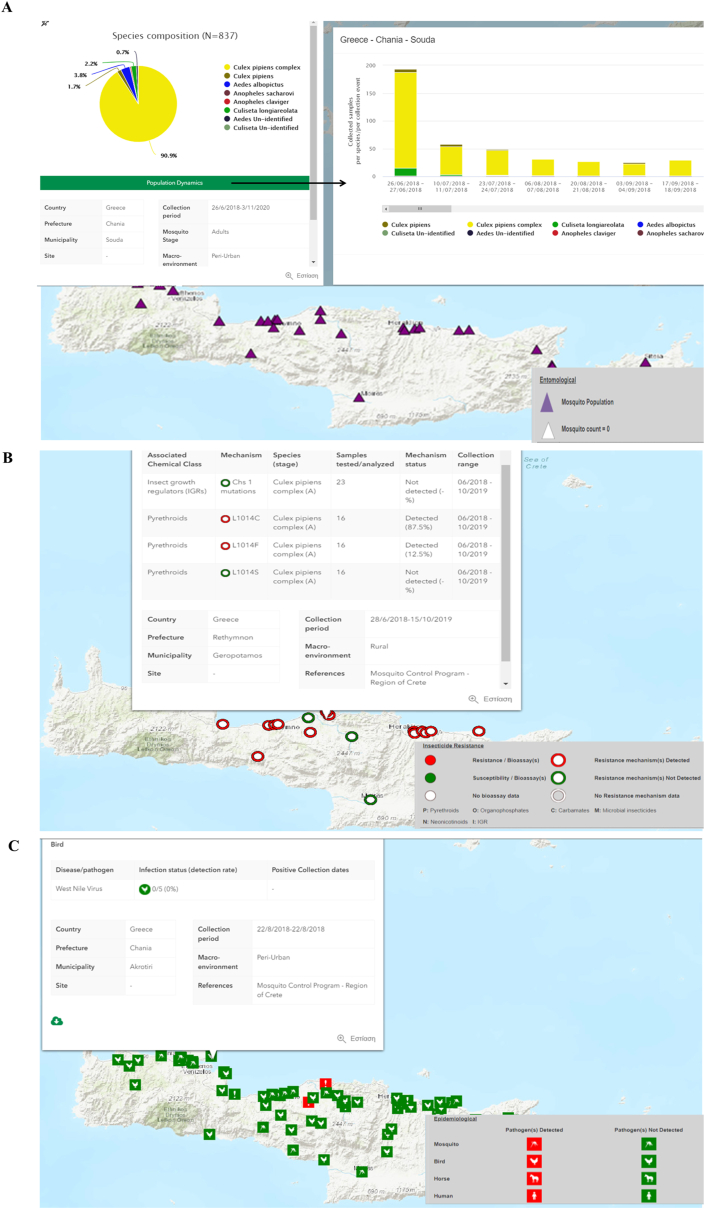
Upon map production, users can immediately visualize the known geographical distribution of mosquito populations (one or more species of interest), their insecticide resistance status (qualitative bioassay outcomes and presence/absence of resistance mechanisms), and the known distribution of VBD pathogens and infections (Fig. 2).
Fig. 2.
Mosquito population distribution and a (site-specific) entomological pop-up example displaying the mosquito species composition and temporal dynamics (A); insecticide resistance (IR) records and a (site-specific) IR pop-up example including molecular assay data (B); VBD pathogen/infection records and a (site-specific) epidemiological pop-up example displaying the West Nile virus infection rate in sampled birds (C).
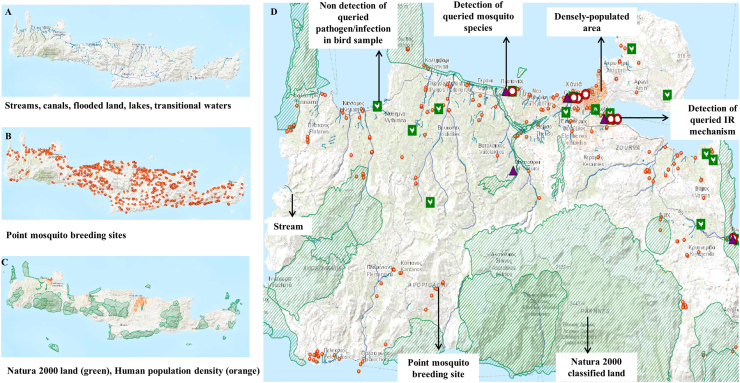
Importantly, users may also add physical feature data on the map, depicting proliferative and potential mosquito breeding sites (i.e. point breeding sites, streams, canals, flooded land, lakes, transitional waters) and Natura 2000 protected areas (i.e. networks of rare natural habitat types and core breeding/resting sites for rare and threatened species) alongside human population density data points, collectively facilitating output interpretation and decision-making (Fig. 3).
Fig. 3.
Operationally relevant GIS layers depicting proliferative and potential mosquito breeding sites (A, B); Natura 2000 land cover and human population density/distribution (C). Point mosquito breeding sites were provided by Ecodev (https://ecodev.gr/) within the framework of the vector control program conducted under the auspices of the Region of Crete. (D) Visualization of a multiple layer map (arrows and text incorporated in the figure are explanatory - not included in the generated online customized maps).
Selection of any georeferenced point (on the map interface) results in a pop-up box with detailed information on the selected data point. The pop-ups provide end users with information on the mosquito species composition and population dynamics, detailed information on the insecticide resistance status of the corresponding population (bioassay records, presence and frequency of molecular resistance markers) and information on VBD pathogen circulation (including pathogen and infection detection rates, positive sample collection dates) in mosquitoes, animals and humans (Fig. 2). Depending on the zoom in/out level on the map interface, symbols and their content are grouped accordingly, enabling users to view aggregated results or individual data points.
The generated outputs can be exported in Excel (.xlsx) files including (i) the filter/search query in a search-keyword format, (ii) a snapshot figure of the output map including all selected features and corresponding map legend, and (iii) easily interpretable tables/charts depicting (the queried) location specific and prefecture level entomological, insecticide resistance and epidemiological data.
3.3.3. Pilot implementation
To date, VectorMap-GR contains a total of 1300 data collection entries (generated from the mosquito management programme in the Region of Crete), with over 9400 samples/populations (i.e. collection content) relating to mosquito population, insecticide resistance and pathogen occurrence or infection records, The systematic aggregation of the aforementioned datasets in the systemʼs database coupled with immediate data accessibility and rapid transformation into actionable information (supported by the tool) enabled the programme stakeholders to comprehensively assess and act upon the documented entomological (and complementary epidemiological) situation whilst addressing a series of operational and decision-making issues (i.e. data fragmentation and partial accessibility, slow data transformation into usable information, sole reliance on annual/seasonal reports, word of mouth information circulation).
VectorMap-GR uptake in the mosquito management programme (i) supported the rapid identification of areas (and time-points) requiring vector control activities and facilitated the design of appropriate control responses (through the provision of information on vector occurrence/composition/abundance and dynamics, breeding site locations, pathogen/infection occurrence, vector insecticide resistance status data and Natura 2000 land coverage); (ii) supported the evaluation of the vector control activities (via the comparison of entomological and epidemiological metrics pre- and post-intervention); and (iii) guided the allocation of monitoring and surveillance resources.
Overall, the regional vector control programme in Crete, coupled with VectorMap-GR pilot implementation phase, contributed to the reduction of vector population numbers between 2018 and 2020 and the prevention of human VBD occurrences (NPHO, 2020; NPHO, 2021).
4. Discussion
VectorMap-GR comprises an open access entomological data management tool supporting the production of operationally relevant surveillance outputs. The default system was specifically designed to support the development of evidence-based mosquito management programmes in the Mediterranean Basin with a special focus on Greece through embedding information relating to: (i) the countryʼs known mosquito fauna (indicatively (Patsoula et al., 2007; Beleri et al., 2017; Fotakis et al., 2017; Balaska et al., 2020; Fotakis et al., 2020a)); (ii) characteristic Mediterranean Basin breeding sites and prominent mosquito collection methods implemented in the region (ECDC, 2018); (iii) mosquito-borne diseases and pathogens reported in Greece and neighbouring countries (indicatively (Chaskopoulou et al., 2013; Gossner et al., 2018; Mavridis et al., 2018; Fotakis et al., 2019; NPHO, 2020; Tzani et al., 2021)); (iv) insecticide resistance traits, recorded in vector and nuisance species of the Mediterranean Basin (indicatively (Kioulos et al., 2014; Fotakis et al., 2017; Porretta et al., 2019; Balaska et al., 2020; Fotakis et al., 2020a,b; Guz et al., 2020)); and (v) resistance-related information against insecticides used in the region.
4.1. VectorMap-GR operational relevance
4.1.1. A platform for managing surveillance datasets
One of the key features of VectorMap-GR is the systemʼs functionality for online storing, editing, retrieving and analysing a wide range of surveillance data. Through supporting immediate data accessibility and enabling the coupling, comparison and association of different surveillance datasets the system provides a means (i.e. a technical platform) for overcoming the obstacles of data fragmentation and partial data accessibility, facilitating evidence-based mosquito management.
4.1.2. Transforming raw data into actionable information in support of local scale vector control responses
The toolʼs major asset lies in the provision of entomological and complementary epidemiological datasets in the form of coordinate specific vector control usable information (i.e. species composition and dynamics, insecticide resistance status, pathogen infection status), over fine-scale physical feature geographic information system (GIS) layers depicting known and suspected mosquito breeding sites (i.e. point mosquito breeding sites, streams, canals, lakes, flooded land and transitional waters). The latter (representing crucial vector control intervention sites) provide important information for interpreting the generated mosquito and pathogen/infection surveillance data whilst the integrative projection of the aforementioned datasets (complemented by the Natura 2000 land cover and human population density layers) may significantly facilitate (i) the deployment of chronologically and spatially targeted context-specific vector control interventions, and (ii) enhance mosquito management programme cost effectiveness (through intervention site prioritization, tailored responses and intervention evaluation).
The systemʼs capacity for local scale operational vector management support, capitalized upon the systemʼs integration and data acquisition within vector/VBD surveillance schemes, is of high potential value as most pre-existing IT systems encompassing surveillance data are confined to generating macroscale spatiotemporal information or research-oriented data, but not operational level feedback to vector management programmes.
4.1.3. Functions within a broad surveillance network
Embedded within an integrative surveillance system framework, VectorMap-GR pertains to the extending arsenal of surveillance and monitoring tools complementing their operational significance through utilizing the produced surveillance data for the generation of actionable information. In addition, the system functionality enables capitalizing on recent innovative surveillance tool advancements (e.g. molecular diagnostic tools) enhancing the accuracy and robustness of the corresponding operational feedback.
At a central level (i.e. national scale tool uptake), the provision of comparably formatted integrated surveillance data from different geographical regions and time frames (accurately delineating the VBD scene) can support the realisation of timely, evidence-based VBD policy decision-making. Notably, such has been the case with VectorSurv, which was initially applied in California and is currently being adapted by an ever-increasing number of vector control districts across the USA.
4.2. Achieving high system functionality in Greece
The default tool, which is currently implemented in a regional vector control programme in Crete (Greece), may be easily adapted to (unaccounted) specificities of other VBD settings and corresponding mosquito management programmes.
VBD control in Greece and neighbouring countries largely relies on regional and prefecture level vector management programmes (of varying budget sizes, workforce, and available equipment, e.g. trap types, means of insecticidal applications). As the respective programmes (facing different entomological, epidemiological and ecological settings and challenges) are designed, implemented and evaluated by the corresponding local public health departments and collaborating private companies, system deployment at this administrative level will likely result in: (i) increased VBD control efficiency through providing regional/prefecture-context-specific, operational information to the immediate stakeholders; and (ii) in system sustainability (qualitatively and technically supported by the tool curators and developers), ensuring long-term high quality operational support provided by the tool.
4.3. System reliance on the input data
Whether solely implemented within local VBD management programmes or as an integral component of a central VBD policy scheme, the operational potential of VectorMap-GR in terms of increased VBD control efficiency largely relies on the quality, quantity, completeness and speed of entry of the raw surveillance data. VBD management programme strategic planning, ensuring robust and high-quality data generation, inclusive and fast data entry in the system (e.g. through assigned accredited users immediately linked to the control programmes), is essential in succeeding full system capacity and ensuring long-term sustainability. Key entomological surveillance aspects in this direction include appropriate trap selection, use of different trap type combinations, and their systematic deployment at continual time intervals and in fixed locations (ECDC, 2012). Regarding insecticide resistance tracking, pre- and post-control programme execution of biological, biochemical and molecular diagnostic assays measuring the levels of phenotypic resistance, enzyme activities and frequency of resistance markers respectively, is critical (Dusfour et al., 2019). Finally, comprehensive pathogen surveillance, including the implementation of active and passive pathogen/infection reporting in multiple hosts (reservoir, dead-end hosts) and vectors, tracking infection acquisition sites and appropriate sample preservation is of high importance (Jourdain et al., 2019) (Supplementary Table S2).
4.4. System expandability and future directions
VectorMap-GR displays high expandability potential achievable through its flexible structure and compatibility with existing IT systems. Following small coding adjustments, other arthropod vectors such as sand flies and ticks can be included in the system. Also, the toolʼs decision-making support functionality may be readily advanced by incorporating meteorological data, biocide intervention land coverage data, VBD risk assessment models for the generation of real time VBD risk maps and automated notification services including alerts when vector populations and pathogen detection rates reach certain thresholds. Last but not least, the toolʼs compatibility with other IT systems enables their potential integration advancing upon the different multi-system strengths and functionalities.
Funding
This work was partially sponsored by the Region of Crete under the programme “Integrated mosquito control program in the region of Crete”. MO, TK, PS and ZT were supported by the project “Advanced Research Activities in Biomedical and Agro alimentary Technologies” (MIS 5002469) which is implemented under the “Action for the Strategic Development on the Research and Technological Sector”, funded by the Operational Programme “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) and co-financed by Greece and the European Union (European Regional Development Fund). MF was supported by the General Secretariat for Research and Technology (GSRT) and the Hellenic Foundation for Research and Innovation (HFRI) in the context of the action “1st Proclamation of Scholarships from ELIDEK for Ph.D. Candidates”. KM was supported by Greece and the European Union (European Social Fund - ESF) through the Operational Programme “Human Resources Development, Education and Lifelong Learning” in the context of the project “Reinforcement of Postdoctoral Researchers - 2nd Cycle” (MIS-5033021), implemented by the State Scholarships Foundation (ΙΚΥ).
Ethical approval
Not applicable.
CRediT author statement
Emmanouil A. Fotakis: Conceptualization, Methodology, Validation, Supervision, Writing - Original Draft, Writing - Review & Editing. Manolis Orfanos: Software, Writing - Review & Editing. Thodoris Kouleris: Software, Writing - Review & Editing. Panagiotis Stamatelopoulos: Software. Zisis Tsiropoulos: Software. Anastasia Kampouraki: Validation. Ilias Kioulos: Validation. Konstantinos Mavridis: Validation. Alexandra Chaskopoulou: Validation, Writing - Review & Editing. George Koliopoulos: Methodology, Validation. John Vontas: Conceptualization, Validation, Funding acquisition, Supervision, Writing - Review & Editing.
Declaration of competing interests
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Special thanks to Ecodev for providing, within the framework of the vector control program conducted under the auspices of the Region of Crete, the GIS layer data depicting the point mosquito breeding sites in Crete.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2021.100053.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
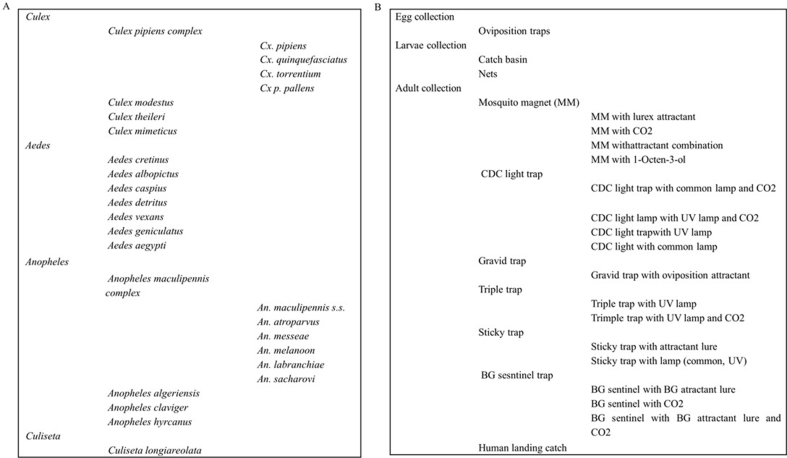
FigS1.
Supplementary Figure S1. Semi-schematic listing of Mosquito ID term tree (left) and Sampling Method term tree (right). Not all classes are listed.
Supplementary Table S1. Controlled vocabularies.
Supplementary Table S2. Key surveillance aspects for optimal VectorMap-GR operational performance.
References
- Balaska S., Fotakis E.A., Kioulos I., Grigoraki L., Mpellou S., Chaskopoulou A., Vontas J. Bioassay and molecular monitoring of insecticide resistance status in Aedes albopictus populations from Greece, to support evidence-based vector control. Parasit. Vectors. 2020;13 doi: 10.1186/s13071-020-04204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker N., editor. Mosquitoes and their control. 2nd ed. Springer; Boston: 2010. [Google Scholar]
- Beleri S., Chatzinikolaou S., Nearchou A., Patsoula E. Entomological study of the mosquito fauna in the regional unit of drama, region of East Macedonia-Thrace, Greece (2015 to 2016) Vector Borne Zoonotic Dis. 2017;17:665–671. doi: 10.1089/vbz.2017.2113. [DOI] [PubMed] [Google Scholar]
- Carballo M., Nerurkar A. Migration, refugees, and health risks. Emerg. Infect. Dis. 2001;7:556–560. doi: 10.3201/eid.0707.017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Centers for Disease Control and Prevention; Atlanta: 2010. Guideline for evaluating insecticide resistance in vectors using the CDC bottle bioassay.https://www.cdc.gov/malaria/resources/pdf/fsp/ir_manual/ir_cdc_bioassay_en.pdf [Google Scholar]
- Chaskopoulou A., Dovas C.I., Chaintoutis S.C., Kashefi J., Koehler P., Papanastassopoulou M. Detection and early warning of West Nile virus circulation in Central Macedonia, Greece, using sentinel chickens and mosquitoes. Vector Borne Zoonotic Dis. 2013;13:723–732. doi: 10.1089/vbz.2012.1176. [DOI] [PubMed] [Google Scholar]
- Dacko N.M., Nava M.R., Vitek C., Debboun M. Mosquitoes, communities, and public health in Texas. Elsevier; Amsterdam: 2020. Mosquito surveillance; pp. 221–247. [DOI] [Google Scholar]
- Dialynas E., Topalis P., Vontas J., Louis C. MIRO and IRbase: IT tools for the epidemiological monitoring of insecticide resistance in mosquito disease vectors. PLoS Negl. Trop. Dis. 2009;3:e465. doi: 10.1371/journal.pntd.0000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusfour I., Vontas J., David J.-P., Weetman D., Fonseca D.M., Corbel V., Raghavendra K., Coulibaly M.B., Martins A.J., Kasai S., Chandre F. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC . European Centre for Disease Prevention and Control; Stockholm: 2012. Guidelines for the surveillance of invasive mosquitoes in Europe.https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/TER-Mosquito-surveillance-guidelines.pdf [PubMed] [Google Scholar]
- ECDC . European Centre for Disease Prevention and Control; Stockholm: 2014. Guidelines for the surveillance of native mosquitoes in Europe.https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/surveillance-of%20native-mosquitoes%20-guidelines.pdf [Google Scholar]
- ECDC . European Centre for Disease Prevention and Control; Stockholm: 2018. Field sampling methods for mosquitoes, sandflies, biting midges and ticks.https://www.ecdc.europa.eu/sites/default/files/documents/Vector-sampling-field-protocol-2018.pdf [Google Scholar]
- Eisen L., Coleman M., Lozano-Fuentes S., McEachen N., Orlans M., Coleman M. Multi-disease data management system platform for vector-borne diseases. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen L., Eisen R.J. Using geographic information systems and decision support systems for the prediction, prevention, and control of vector-borne diseases. Annu. Rev. Entomol. 2011;56:41–61. doi: 10.1146/annurev-ento-120709-144847. [DOI] [PubMed] [Google Scholar]
- Eybpoosh S., Fazlalipour M., Baniasadi V., Pouriayevali M.H., Sadeghi F., Ahmadi Vasmehjani A., et al. Epidemiology of West Nile virus in the Eastern Mediterranean region: A systematic review. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotakis E.A., Chaskopoulou A., Grigoraki L., Tsiamantas A., Kounadi S., Georgiou L., Vontas J. Analysis of population structure and insecticide resistance in mosquitoes of the genus Culex, Anopheles and Aedes from different environments of Greece with a history of mosquito borne disease transmission. Acta Trop. 2017;174:29–37. doi: 10.1016/j.actatropica.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Fotakis E.A., Giantsis I.A., Avgerinou A., Kourtidis S., Agathaggelidou E., Kapoula C., et al. Identification of Leishmania species in naturally infected sand flies from refugee camps, Greece. Emerg. Infect. Dis. 2019;25:361–364. doi: 10.3201/eid.2502.181359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotakis E.A., Giantsis I.A., Castells Sierra J., Tanti F., Balaska S., Mavridis K., et al. Population dynamics, pathogen detection and insecticide resistance of mosquito and sand fly in refugee camps, Greece. Inf. Dis. Poverty. 2020;9 doi: 10.1186/s40249-020-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotakis E.A., Mastrantonio V., Grigoraki L., Porretta D., Puggioli A., Chaskopoulou A., et al. Identification and detection of a novel point mutation in the chitin synthase gene of Culex pipiens associated with diflubenzuron resistance. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet F., Jourdain F., Bonnet E., Degroote S., Ridde V. Effective surveillance systems for vector-borne diseases in urban settings and translation of the data into action: A scoping review. Inf. Dis. Poverty. 2018;7 doi: 10.1186/s40249-018-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossner C.M., Ducheyne E., Schaffner F. Increased risk for autochthonous vector-borne infections transmitted by Aedes albopictus in continental Europe. EuroSurveill. 2018;23:1800268. doi: 10.2807/1560-7917.ES.2018.23.24.1800268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler D.J. In: Vector biology, ecology and control. Atkinson P.W., editor. Springer; Netherlands: 2010. The global threat of emergent/re-emergent vector-borne diseases; pp. 39–62. [DOI] [Google Scholar]
- Guz N., Cagatay N.S., Fotakis E.A., Durmusoglu E., Vontas J. Detection of diflubenzuron and pyrethroid resistance mutations in Culex pipiens from Muğla, Turkey. Acta Trop. 2020;203:105294. doi: 10.1016/j.actatropica.2019.105294. [DOI] [PubMed] [Google Scholar]
- Jourdain F., Samy A.M., Hamidi A., Bouattour A., Alten B., Faraj C., et al. Towards harmonisation of entomological surveillance in the Mediterranean area. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampen H., Medlock J.M., Vaux A., Koenraadt C., van Vliet A., Bartumeus F., et al. Approaches to passive mosquito surveillance in the EU. Parasit. Vectors. 2015;8:9. doi: 10.1186/s13071-014-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioulos I., Kampouraki A., Morou E., Skavdis G., Vontas J. Insecticide resistance status in the major West Nile virus vector Culex pipiens from Greece: Resistance status of Culex pipiens from Greece. Pest Manag. Sci. 2014;70:623–627. doi: 10.1002/ps.3595. [DOI] [PubMed] [Google Scholar]
- Kittichai V., Pengsakul T., Chumchuen K., Samung Y., Sriwichai P., Phatthamolrat N., et al. Deep learning approaches for challenging species and gender identification of mosquito vectors. Sci. Rep. 2021;11:4838. doi: 10.1038/s41598-021-84219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox T.B., Juma E.O., Ochomo E.O., Pates Jamet H., Ndungo L., Chege P., et al. An online tool for mapping insecticide resistance in major Anopheles vectors of human malaria parasites and review of resistance status for the Afrotropical region. Parasit. Vectors. 2014;7:76. doi: 10.1186/1756-3305-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolimenakis A., Bithas K., Latinopoulos D., Richardson C. On lifestyle trends, health and mosquitoes: Formulating welfare levels for control of the Asian tiger mosquito in Greece. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulou D., Gizzarelli M., Ligda P., Foglia Manzillo V., Saratsi K., Montagnaro S., et al. Mapping the canine vector-borne disease risk in a Mediterranean area. Parasit. Vectors. 2020;13:282. doi: 10.1186/s13071-020-04153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E. Policy Department Structural and Cohesion Policies European Parliament; 2011. Economic, social and territorial situation of Greece.https://www.europarl.europa.eu/RegData/etudes/note/join/2011/460052/IPOL-REGI_NT(2011)460052_EN.pdf [Google Scholar]
- Legakis A., Constantinidis T., Petrakis P.V. In: Global biodiversity. 1st ed. Pullaiah T., editor. Apple Academic Press; Boca Raton: 2018. Biodiversity in Greece; pp. 71–113. [DOI] [Google Scholar]
- Lozano-Fuentes S., Barker C.M., Coleman M., Coleman M., Park B., Reisen W.K., Eisen L. In: Efficient decision support systems - practice and challenges in biomedical related domain. Jao C., editor. InTech; London: 2011. Emerging information technologies to provide improved decision support for surveillance, prevention, and control of vector-borne diseases. [Google Scholar]
- Mavridis K., Fotakis E.A., Kioulos I., Mpellou S., Konstantas S., Varela E., et al. Detection of West Nile virus – lineage 2 in Culexpipiens mosquitoes, associated with disease outbreak in Greece, 2017. Acta Trop. 2018;182:64–68. doi: 10.1016/j.actatropica.2018.02.024. [DOI] [PubMed] [Google Scholar]
- Mavridis K., Wipf N., Medves S., Erquiaga I., Müller P., Vontas J. Rapid multiplex gene expression assays for monitoring metabolic resistance in the major malaria vector Anopheles gambiae. Parasit. Vectors. 2019;12:9. doi: 10.1186/s13071-018-3253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakshi M., Bharti P., Chellappan S. 2017 European Conference on Networks and Communications (EuCNC) EuCNC; 2017. Identifying mosquito species using smart-phone cameras; pp. 1–6. [Google Scholar]
- Mitsakakis K., Hin S., Müller P., Wipf N., Thomsen E., Coleman M., et al. Converging human and malaria vector diagnostics with data management towards an integrated holistic One Health approach. Int. J. Environ. Res. Publ. Health. 2018;15:259. doi: 10.3390/ijerph15020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier N., Barth-Jaeggi T., Knopp S., Steinmann P. Core components, concepts and strategies for parasitic and vector-borne disease elimination with a focus on schistosomiasis: A landscape analysis. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes C.L., Vontas J., Martins A.J., Ng L.C., Koou S.Y., Dusfour I., et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NPHO . National Public Health Organization; Athens, Greece: 2020. Annual epidemiological surveillance report: Malaria in Greece, 2020.https://eody.gov.gr/wp-content/uploads/2019/01/MALARIA_ANNUAL_REPORT_2020_ENG.pdf [Google Scholar]
- NPHO . National Public Health Organization; Athens, Greece: 2021. West Nile virus.https://eody.gov.gr/en/disease/west-nile-virus/ [Google Scholar]
- Patsoula E., Samanidou-Voyadjoglou A., Spanakos G., Kremastinou J., Nasioulas G., Vakalis N.C. Molecular characterization of the Anophelesmaculipennis complex during surveillance for the 2004 Olympic Games in Athens. Med. Vet. Entomol. 2007;21:36–43. doi: 10.1111/j.1365-2915.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- Porretta D., Fotakis E.A., Mastrantonio V., Chaskopoulou A., Michaelakis A., Kioulos I., et al. Focal distribution of diflubenzuron resistance mutations in Culex pipiens mosquitoes from Northern Italy. Acta Trop. 2019;193:106–112. doi: 10.1016/j.actatropica.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Postigo J.A.R. Leishmaniasis in the World Health Organization Eastern Mediterranean region. Int. J. Antimicrob. Agents. 2010;36(Suppl. 1):S62–S65. doi: 10.1016/j.ijantimicag.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Roiz D., Ruiz S., Soriguer R., Figuerola J. Landscape effects on the presence, abundance and diversity of mosquitoes in Mediterranean wetlands. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza J.C., Suk J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018;365 doi: 10.1093/femsle/fnx244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B., Ashburner M., Rosse C., Bard J., Bug W., Ceusters W., et al. The OBO Foundry: coordinated evolution of ontologies to support biomedical data integration. Nat. Biotechnol. 2007;25:1251–1255. doi: 10.1038/nbt1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statista . Statista; Hamburg, Germany: 2020. Total contribution of travel and tourism to GDP in Greece from 2012 to 2028.https://www.statista.com/statistics/644573/travel-tourism-total-gdp-contribution-greece/ [Google Scholar]
- Sutherst R.W. Global change and human vulnerability to vector-borne diseases. Clin. Microbiol. Rev. 2004;17:136–173. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzani M., Barrasa A., Vakali A., Georgakopoulou T., Mellou K., Pervanidou D. Surveillance data for human leishmaniasis indicate the need for a sustainable action plan for its management and control, Greece, 2004 to 2018. EuroSurveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.18.2000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vontas J., Mavridis K. Vector population monitoring tools for insecticide resistance management: Myth or fact? Pestic. Biochem. Physiol. 2019;161:54–60. doi: 10.1016/j.pestbp.2019.08.005. [DOI] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2018. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes.https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf?sequence=1 [Google Scholar]
- World Bank . The World Bank; Washington, D.C: 2020. International tourism, expenditures (current US$) - Greece.https://data.worldbank.org/indicator/ST.INT.XPND.CD?locations=GR [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Controlled vocabularies.
Supplementary Table S2. Key surveillance aspects for optimal VectorMap-GR operational performance.