Abstract
Faecal egg counting techniques (FECT) form the cornerstone for the detection of gastrointestinal parasites in equines. For this purpose, several flotation, centrifugation, image- and artificial intelligence-based techniques are used, with varying levels of performance. This review aimed to critically appraise the literature on the assessment and comparison of various coprological techniques and/or modifications of these techniques used for equines and to identify the knowledge gaps and future research directions. We searched three databases for published scientific studies on the assessment and comparison of FECT in equines and included 27 studies in the final synthesis. Overall, the performance parameters of McMaster (81.5%), Mini-FLOTAC® (33.3%) and simple flotation (25.5%) techniques were assessed in most of the studies, with 77.8% of them comparing the performance of at least two or three methods. The detection of strongyle, Parascaris spp. and cestode eggs was assessed for various FECT in 70.4%, 18.5% and 18.5% studies, respectively. A sugar-based flotation solution with a specific gravity of ≥1.2 was found to be the optimal flotation solution for parasitic eggs in the majority of FECT. No uniform or standardised protocol was followed for the comparison of various FECT, and the tested sample size (i.e. equine population and faecal samples) also varied substantially across all studies. To the best of our knowledge, this is the first systematic review to evaluate studies on the comparison of FECT in equines and it highlights important knowledge gaps in the evaluation and comparison of such techniques.
Keywords: Anoplocephala, Ascarid, Comparison, Equine, Faecal egg counting technique, Strongyle
Graphical abstract

Highlights
-
•
An assessment of studies on the comparison of faecal egg counting in equine parasitology was undertaken.
-
•
A consensus on the methodology and performance parameters for faecal egg counting techniques is required.
-
•
Technical and biological sources of variability in faecal egg counts should be considered.
-
•
Minimum analytical and diagnostic performance parameters should be assessed.
1. Introduction
Helminths are common and important gastrointestinal parasites of equines as they pose a significant threat to equine health and wellbeing, particularly in foals, yearlings and geriatric horses. Strongylid (cyathostomin and strongylin) nematodes are the main internal parasites of horses, constituting more than 75% of the total parasite fauna (Tolliver et al., 1987; Bucknell et al., 1996; Lyons and Tolliver, 2004; Lichtenfels et al., 2008). Other major internal parasites found in horses include Anoplocephala spp., Parascaris spp., Habronema spp., Draschia megastoma, Oxyuris equi and Strongyloides westeri (Tolliver et al., 1987; Lyons and Tolliver, 2004; Saeed et al., 2019). Major clinical signs of parasitism in horses include unthriftiness, reduced stamina, retarded growth, abdominal distension (‘pot-belly’), diarrhoea, abdominal pain and death, especially in young and immunocompromised horses (Snyder et al., 1978; Love et al., 1999; Corning, 2009; Peregrine et al., 2014; Saeed et al., 2019).
Since the introduction of benzimidazoles in the 1960s, the control of equine internal parasites has heavily relied upon interval-based (i.e. treating yearlings and older horses regularly at 8-week intervals mostly) and/or rotational (alternating) use of various anthelmintics (Drudge and Lyons, 1966; Kaplan and Nielsen, 2010). However, the indiscriminate use of anthelmintics has resulted in the emergence of resistant nematode populations and/or shortened egg reappearance periods (an early indication of the development of resistance) for almost all currently available anthelmintics (Kaplan and Nielsen, 2010; Reinemeyer, 2012; von Samson-Himmelstjerna, 2012; Peregrine et al., 2014; Beasley et al., 2017; Martin et al., 2021). Despite the fact that the detection and enumeration of parasite eggs in faecal samples has been the mainstay of clinical and research parasitology for decades, it was only in the 1980s, when the issue of resistant worms was identified, and integrated selective treatment (targeted selected treatment) strategies based upon predetermined thresholds such as faecal egg count (FEC) data obtained from the counting of parasitic eggs within the faecal samples were proposed (Kaplan and Nielsen, 2010; Andersen et al., 2013; Nielsen et al., 2014).
Copromicroscopy, founded by C.J. Davaine in 1857 (Rinaldi, 2014), with the first faecal smear method described by Grassi, Parona and Parona in 1878 (Ballweber et al., 2014), now forms the cornerstone of parasite diagnostics and research. The simple faecal smear lacks sensitivity, and improved methods, such as simple dilution (Stoll, 1923) and a direct centrifugal flotation (DCF) technique (Lane, 1923) for humans and animals (Stoll, 1930) have subsequently been developed. Several modifications made to the Stoll method improved limits of detection and resulted in the development of some of the currently used methods such as Wisconsin (Cox and Todd, 1962) and Cornell-Wisconsin techniques (Egwang and Slocombe, 1982). Along with these centrifugation-based flotation techniques, simple or gravitation-based methods such as the McMaster (Gordon and Whitlock, 1939), the modified McMaster (Whitlock, 1948), FLOTAC® (based on the principle of the Wisconsin technique) (Cringoli, 2006; Cringoli et al., 2010), FECPAKG1® (McCoy et al., 2005; Presland et al., 2005), Mini-FLOTAC® (Barda et al., 2013; Cringoli et al., 2017) and the FECPAKG1® and FECPAKG2® (Tyson et al., 2020), were developed, and are being used in the field. More recently, artificial intelligence-based automated counting techniques have been developed for FEC (Scare et al., 2016; Slusarewicz et al., 2016; Scare et al., 2017; Cain et al., 2020; Elghryani et al., 2020; Nagamori et al., 2020; Cringoli et al., 2021). The available FEC techniques (FECT) vary in performance parameters, including estimates of sensitivity, specificity, precision and accuracy (Ballweber et al., 2014; Nielsen, 2021). Additionally, technical (e.g. loss of eggs during sample processing, type of flotation solution, eggs type and flotation capability and analyst training), and biological (e.g. egg count variation within and between samples and density-dependent fecundity of female worms) sources of variation also play a critical role in determining the performance of FECT (Nielsen, 2021). Given the substantial variation in the performance of various FECT, no single technique is fit for all purposes and the choice of a technique depends upon the intended objective and expected FEC within faecal samples (Ballweber et al., 2014). For example, for studies aimed to evaluate the egg reappearance periods for anthelmintics, the FECT should have a higher diagnostic sensitivity to be able to detect the onset of egg appearance in faeces. On the other hand, a less sensitive technique could suffice the purpose for targeted selective treatments where the objective is generally to identify animals with FEC above a certain threshold value.
To date, several studies have assessed the performance parameters of FECT for equines (Bello and Allen, 2009; Fukumoto et al., 2011; de Castro et al., 2017; Napravnikova et al., 2019). Although these studies have demonstrated varying levels of performance of different techniques, there has not been a systematic appraisal of such comparative studies in equine parasitology. Therefore, the objectives of this systematic review were to critically appraise the current knowledge on the comparison of various coprological techniques and/or modifications to assess the parasite burden in equines and to identify the knowledge gaps and future research directions in equine parasitology.
2. Materials and methods
2.1. Database searches
This systematic review was completed as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Supplementary Table S1). We searched three databases: Web of Science, Google Scholar and PubMed. The primary literature search strategy was designed for Web of Science and then customised for other databases. The literature search was completed for studies published on the comparison of various coprological techniques used for the detection of eggs of equine internal parasites until February 28th, 2021. We used various combinations of key search terms relevant to the topic to retrieve all peer-reviewed research articles, conference proceedings and postgraduate theses published in the English language. The main search terms included “gastrointestinal parasites/nematodes/helminths of equine(s)/horse(s)/donkey(s)”, “comparison/validation/assessment”, “f(a)ecal egg count(s) in equine(s)/horse(s)/donkey(s)”, “flotation/sedimentation”, “McMaster”, “Wisconsin”, “FLOTAC®”, “FECPAK®”, “Mini-FLOTAC®”, “automated counting and Parasight System®”. Additionally, we examined the reference lists of retrieved articles and reviews to identify any other articles that could be relevant to the scope of this review.
2.2. Assessment of studies and data extraction
The retrieved references were imported into EndNote X9.2 and duplicates were removed using a built-in function, and remaining references were assessed for the selection of relevant studies. The assessment criteria were based upon three main components, including (i) language: English, (ii) article type: original research articles, conference proceedings and postgraduate theses, and (iii) study topic: studies on the comparison/validation/assessment of coprological techniques for equine FEC. In the first screening step, titles and abstracts were screened for the removal of irrelevant studies. Subsequently, full-text articles/studies were retrieved for selected references through the library of the University of Melbourne and inter-library loans. The full-text studies were subjected to the second screening step using the set assessment criteria.
The relevant data were compiled from each of the selected studies into a predesigned Microsoft Excel® spreadsheet. Data were extracted for study type, publication title, year of publication, country, coprological methods used, the number of equine samples tested, information about flotation/sedimentation solutions used, any other host species included in the study, parasites detected, parameters assessed, detailed methodology, key findings and conclusions. The definitions of important epidemiological and performance-related terms are provided in Table 1, and those of analytical and diagnostic performance parameters are summarised in Table 2, Table 3.
Table 1.
Definitions of commonly used terms for the validation and comparison of diagnostic assays
| Term | Definitiona |
|---|---|
| Repeatability | The level of agreement between results of replicates of a sample both within and between runs of the same test method in a given laboratory |
| Reproducibility | The ability of a test method to provide consistent results, as determined by estimates of precision, when applied to aliquots of the same samples tested in different laboratories, preferably located in distinct or different regions or countries using the identical assay (protocol, reagents and controls) |
| Analytical specificity | The ability of the assay to distinguish the target analyte (e.g. antibody, organism or genomic sequence) from non-target analytes, including matrix components |
| Analytical sensitivity | The estimated amount of analyte in a specified matrix that would produce a positive result at least a specified percent of the time |
| Diagnostic sensitivity | The proportion of samples from known infected reference animals that test positive in an assay |
| Diagnostic specificity | The proportion of samples from known uninfected reference animals that test negative in an assay |
| Accuracy | The closeness of a test value to the expected (true) value (mean or median) for a reference standard reagent of known concentration or titre |
| Precision | The degree of dispersion (variance, standard deviation or coefficient of variation) within a series of measurements of the same sample tested under specified conditions |
Source: Jacobson and Wright (2019).
Table 2.
Principle, advantages and limitations of commonly used faecal egg counting techniques
| Method | Principle | Advantages | Limitations |
|---|---|---|---|
| Direct smear | The small amount of fresh faeces mixed with saline (or iodine) on a microscope slide | Cheap, fast processing time | Qualitative, very low accuracy, precision and sensitivity |
| Cornell-Wisconsin | Based on centrifugal flotation of eggs in a salt solution in a tube, collection onto a coverslip and counting under a microscope | Cheap, high limit of detection | Time-consuming, low accuracy and very low precision |
| McMaster | Faeces mixed in a flotation solution are loaded onto chambers of a slide and the floated eggs are counted | Cheap, medium processing time | Low sensitivity |
| FLOTAC® | Based on centrifugal-flotation of eggs in a specialised apparatus and subsequent translation of the top layer | Cheap, high sensitivity, very high accuracy and precision | Time-consuming, special equipment (centrifugation rotors) required |
| Mini-FLOTAC® | A modified version of FLOTAC without centrifugation step and reduced reading volume | High sensitivity, accuracy and precision, medium processing time | Detection of some parasites (e.g. trematodes) requires centrifugation |
| FECPAK® | Eggs are floated in a flotation solution, accumulated into a single viewing area and imaged | Does not require technical skills as eggs identified and counted remotely, digitalised images | Low accuracy, precision and medium sensitivity, time-consuming |
| Parasight System® | A faecal sample mixed in water is filtered to remove debris, eggs are labelled with a fluorescent dye, imaged and counted using an automated algorithm | High precision, does not require technical skills, fast, automated counting, digitalised images | Expensive, some results need to be confirmed visually, does not detect overlying eggs, cannot differentiate with high debris background |
Note: Sources: Cringoli et al. (2017); Sukas et al. (2019).
Table 3.
Key features and performance parameters of commonly used faecal egg counting techniques
| Method | Faeces (g) | Dilution (ml) | Reading volume (ml) | Limit of detection |
|---|---|---|---|---|
| McMaster | 4 | 56 | 0.3 | 50 |
| Modified McMaster | 4 | 56 | 1 | 15 |
| Cornell-Wisconsin | 5 | 55 | 2 | 1 |
| FLOTAC | 5 | 45 | 10 | 1 |
| Mini-FLOTAC | 5 | 45 | 2 | 5 |
| FECPAKG1 | ~20 | 230 | 1 | 25 |
| FECPAKG2 | ~20 | 210 | 0.88 | 45 |
| Parasight | 6 | 54 | 4 | 2.5 |
3. Results and discussion
3.1. Database searching, assessment, and screening
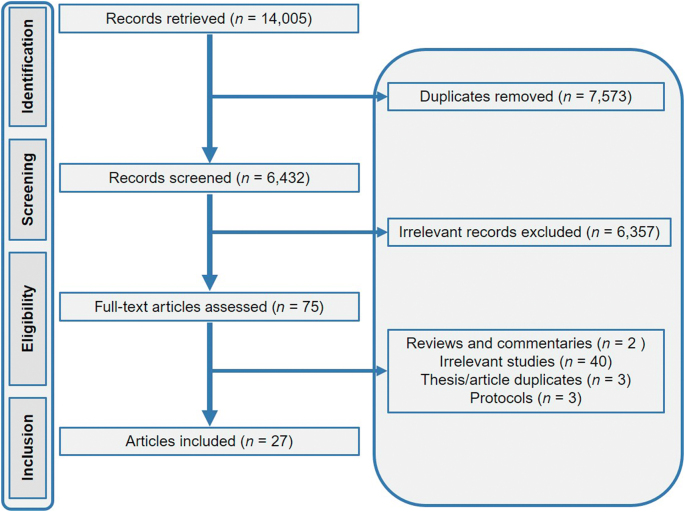
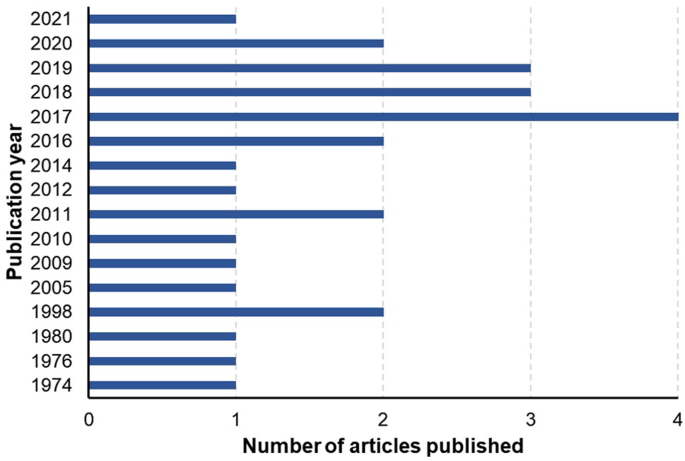
Our systematic search of the three databases for studies published on the topic produced a total of 14,005 results (Google Scholar, number of studies n = 7460; PubMed, n = 3982 and Web of Science, n = 2563). Following the removal of duplicates (n = 7573), the title/abstract screening step of 6432 references resulted in 75 studies. Subsequently, the full-text screening step yielded a total of 27 studies using our inclusion criteria (Fig. 1). These 27 studies were published between 1974 and 2021 and included 18 original research articles, five short communications, three conference proceedings/abstracts and one postgraduate thesis (Fig. 1).
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram showing the number of articles at each stage and the exclusion criteria applied in this study.
3.2. General characteristics of studies
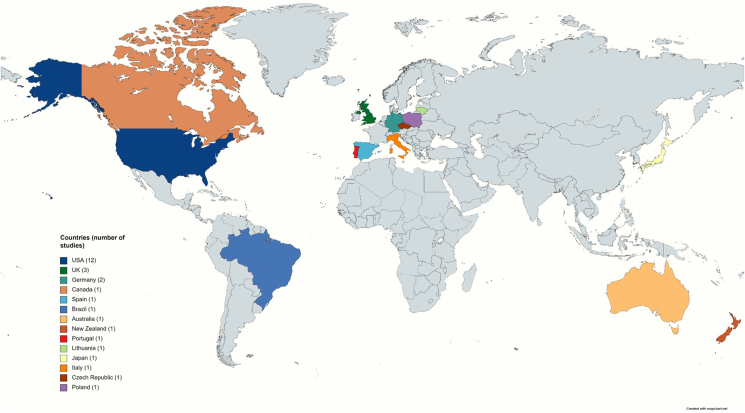
Of the 27 studies on the comparison or assessment of techniques for assessing FEC in equines, the majority originated from North America (n = 13), followed by Europe (n = 11), Australasia (n = 2), South America (n = 1) and Asia (n = 1) (one study was conducted in two regions including Europe and Australasia) (Fig. 2), and most were conducted during the last decade (2011–2021) (Fig. 3). Twenty-three studies focused on the comparison of different techniques and/or variations of the same technique, whereas the assessment of new techniques and different variables were investigated in two and five studies, respectively (Supplementary Table S2).
Fig. 2.
Geographical distribution of studies (n = 27) included in this systematic review. One study was conducted in two countries (see Supplementary Table S2); hence, 28 studies are listed in the map.
Fig. 3.
Year-wise distribution of number of published articles (n = 27) selected in this systematic review.
3.3. Techniques/methods used/tested
Most of the studies used the simple McMaster method or modifications of this technique (n = 22), followed by Mini-FLOTAC® (n = 9), flotation (n = 7), modified Wisconsin (n = 5), sedimentation (n = 4), smartphone-based/automated (n = 4), combined sedimentation-flotation (n = 3), FECPAK® (n = 2), and Parasight System® (n = 1) techniques (Supplementary Table S2). Performances of two, three and four techniques were assessed and/or compared in 11, 10 and 2 studies, respectively, whereas 6 studies investigated the effect of various variables (including specific gravity, mesh size, counting time, flotation time, flotation solution, sample weight and homogenisation, number of chambers counted and diurnal variations) on the performance of a single technique (Supplementary Table S2).
3.4. Flotation solutions and parasites studied
Solutions used for the preparation of faecal slurries and/or suspension of eggs included sodium chloride and sugar (specific gravity (sg) = 1.25–1.28, n = 8), sugar (sg = 1.2–1.3, n = 8), sodium chloride (sg = 1.2, n = 7), sodium nitrate (sg = 1.11–1.40, n = 7), zinc sulphate (sg = 1.18–1.30, n = 4), and sodium dichromate (sg = 1.35, n = 1) (see Supplementary Table S2). OʼGrady and Slocombe (1980) investigated the effect of specific gravity (1.11–1.38) of one flotation solution (sodium nitrate) on FEC and found the optimal flotation (maximum mean eggs per gram (epg) after eight minutes flotation time) of strongyle and ascarid eggs using a flotation solution with a specific gravity of 1.22–1.35. Moreover, the authors also reported that the flotation time (4–12 minutes) had no effect on FEC, using a solution of 1.27 specific gravity. In other studies, sugar solution was found to be more efficient (i.e. higher epg and lower % coefficient of variation (CV%)) compared to salt solutions for performing Mini-FLOTAC® and the McMaster technique for strongyle eggs (Silva et al., 2019), the double centrifugation technique for Anoplocephala spp. eggs (Rehbein et al., 2011) and the McMaster technique for Parascaris spp. eggs (McQueary, 1976).
Strongyles (cyathostomins and strongylins) were the most commonly investigated parasites (n = 19), followed by Parascaris spp. (n = 5), and cestodes (Anoplocephala spp., n = 5). The majority of studies investigated FEC in horses only (n = 19), whereas a few studies included other hosts, such as sheep (n = 7), cattle (n = 4), dogs (n = 4), cats (n = 2), pigs (n = 2), goats (n = 1), and llamas (n = 1) (see Supplementary Table S2).
3.5. Performance parameters
Various diagnostic parameters assessed and/or compared for different techniques included estimates of accuracy (n = 10), precision (n = 10), sensitivity (n = 6), and specificity (n = 1) (Table 4, Table 5; Supplementary Table S2). Moreover, six studies also investigated factors including mesh size, flotation time, counting time, specific gravity, the weight of the faecal sample, as well as variations arising in FEC due to technical and biological factors (Supplementary Table S2).
Table 4.
Accuracy and precision of various faecal egg counting techniques used for equine faecal samples
| Methods used | Spiked epg/flotation solution | Parasite detected | Accuracy (%) | Precision (%) | Reference |
|---|---|---|---|---|---|
| Remodified McMaster (Sheatherʼs sugar) | Not applicable | Ascarids | 6,387a | NA | McQueary (1976) |
| Strongyles | 1,403a | ||||
| Direct centrifugal flotation (Sheatherʼs sugar) | Ascarids | 90b | |||
| Strongyles | 86b | ||||
| Remodified McMaster (sodium chloride) | Ascarids | 7,413a | |||
| Strongyles | 1,456a | ||||
| Direct centrifugal flotation (sodium chloride) | Ascarids | 45b | |||
| Strongyles | 97b | ||||
| Sedimentation | 1 epg | Anoplocephala perfoliata | 0.6 | NA | Williamson et al. (1998) |
| 2 epg | 0.6 | ||||
| 10 epg | 0 | ||||
| 20 epg | 2.4 | ||||
| 100 epg | 3 | ||||
| 200 epg | 12 | ||||
| Flotation 1 | 1 epg | 0 | |||
| 2 epg | 0.18 | ||||
| 10 epg | 0.2 | ||||
| 20 epg | 0.7 | ||||
| 100 epg | 1.9 | ||||
| 200 epg | 3.9 | ||||
| Flotation 2 | 1 epg | 0.6 | |||
| 2 epg | 1 | ||||
| 10 epg | 2.2 | ||||
| 20 epg | 1.2 | ||||
| 100 epg | 10.2 | ||||
| 200 epg | 31.9 | ||||
| FECPAK and McMaster | Not applicable | Strongyles | NA | FECPAK had lower variance and thus higher precision | Presland et al. (2005) |
| Centrifugal flotation | % of eggs recovered using centrifugal flotation | Cyathostomins | 100 | NA | Bello and Allen (2009) |
| McMaster | 68–81 | ||||
| McMaster | CV associated with the level of faecal pile, faecal bolus and sample from a faecal bolus, as well as the McMaster procedure | Cyathostomins | NA | CV more dependent on individual animal and higher than faecal bolus and McMaster CV | Denwood et al. (2012) |
| Combined zinc sulfate sedimentation-flotation | 1 epg | Cyathostomins & A. perfoliata | 1.20 & 0 | NA | Becker et al. (2016) |
| 5 epg | 2.12 & 1.60 | ||||
| 10 epg | 3.46 & 1.32 | ||||
| 20 epg | 2.00 & 0.80 | ||||
| 40 epg | 4.13 & 0.90 | ||||
| 60 epg | 2.22 & 0.91 | ||||
| 80 epg | 3.51 & 1.00 | ||||
| McMaster | 1 epg | 0 & 0 | |||
| 30 epg | 22.20 & 22.20 | ||||
| 50 epg | 44.40 & 26.60 | ||||
| 80 epg | 61.00 & 30.50 | ||||
| 100 epg | 37.70 & 39.90 | ||||
| 500 epg | 75.90 & 14.90 | ||||
| 1000 epg | 40.70 & NA | ||||
| Smartphone prototype (accuracy: mean % epg; precision: 100–CV)c | 5 epg | Strongyles | 18.91 | 71.6 | Scare et al. (2017) |
| 50 epg | 57.02 | ||||
| 500 epg | 25.02 | ||||
| 1000 epg | 29.15 | ||||
| McMaster (accuracy: mean % epg; precision: 100–CV)c | 5 epg | Not applicable | 49.12 | ||
| 50 epg | 11.11 | ||||
| 500 epg | 32.22 | ||||
| 1000 epg | 21.67 | ||||
| Mini-FLOTAC (accuracy: mean % epg; precision: 100–CV)c | 5 epg | 22.22 | 64.34 | ||
| 50 epg | 75.56 | ||||
| 500 epg | 72.33 | ||||
| 1000 epg | 87.94 | ||||
| McMaster (accuracy: mean % epg; precision: 100–CV)c | 5 epg | Strongyles | 0 | 53.7 | Noel et al. (2017) |
| 50 epg | 16.67 | ||||
| 500 epg | 43.33 | ||||
| 1000 epg | 34.16 | ||||
| Mini-FLOTAC (accuracy: mean % epg; precision: 100–CV)c | 5 epg | 33.33 | 83.24 | ||
| 50 epg | 28.33 | ||||
| 500 epg | 52.33 | ||||
| 1000 epg | 56.25 | ||||
| Mini-FLOTAC (accuracy: mean % epg; precision: % CV)c | 10 epg | Strongyles | 90 | 49.6 | Bosco et al. (2018) |
| 50 epg | 90 | 10.9 | |||
| 200 epg | 96 | 8.1 | |||
| 500 epg | 82 | 3.1 | |||
| McMaster (chamber) (accuracy: mean % epg; precision: % CV)c | 10 epg | Strongyles | 70 | 135.6 | |
| 50 epg | 78 | 51.4 | |||
| 200 epg | 84 | 23.1 | |||
| 500 epg | 92 | 10.9 | |||
| McMaster (grid) (accuracy: mean % epg; precision: % CV)c | 10 epg | Strongyles | 80 | 248.6 | |
| 50 epg | 98 | 90.5 | |||
| 200 epg | 90 | 39.9 | |||
| 500 epg | 98 | 17.3 | |||
| Cornell–Wisconsin (accuracy: mean % epg; precision: % CV)c | 10 epg | Strongyles | 40 | 33.4 | |
| 50 epg | 38 | 16.6 | |||
| 200 epg | 52 | 51.8 | |||
| 500 epg | 50 | 5.2 | |||
| McMaster (precision as variance)c | including FEC < 50 epg | Cyathostomins | NA | 0.71 | Britt et al. (2017) |
| excluding FEC < 50 epg | 0.72 | ||||
| Mini-FLOTAC (precision as variance)c | including FEC < 50 epg | 0.52 | |||
| excluding FEC < 50 epg | 0.34 | ||||
| Modified-Wisconsin (precision as variability)c | Not applicable | Strongyles | NA | 0.045 | Paras et al. (2018) |
| Three-chambered McMaster (precision as variability)c | 0.311 | ||||
| Mini-FLOTAC (precision as variability)c | 0.143 | ||||
| McMaster (precision as CV)c | Not applicable | Strongylid | 3rd highest egg count | 0.45 | Went et al. (2018) |
| Mini-FLOTAC (precision as CV)c | 2nd highest egg count | 0.23 | |||
| McMaster with Fill-Flotac (precision as CV)c | highest egg count | 0.45 | |||
| Mini-FLOTAC with tongue depressor and cup (precision as CV)c | 4th highest egg count | 0.20 | |||
| Simple McMaster (accuracy: mean % epg; precision: % CV for spiked and natural infections, respectively)c | Not applicable | Ascarids | 65.53 | 62.95 & 31.20 | Napravnikova et al. (2019) |
| Strongyles | 97.53 | 44.33 & 39.53 | |||
| Concentration McMaster (accuracy: mean % epg; precision: % CV for spiked and natural infections, respectively)c | Ascarids | 83.18 | 35.71 & 17.92 | ||
| Strongyles | 88.39 | 35.64 & 25.19 | |||
| Mini-FLOTAC (accuracy: mean % epg; precision: % CV for spiked and natural infections, respectively)c | Ascarids | 90.28 | 18.95 & 14.51 | ||
| Strongyles | 74.18 | 18.25 & 8.64 | |||
| Simple flotation (precision as % CV)c | Salt | Strongyles | NA | 43.15 | Silva et al. (2019) |
| Sugar | 52.43 | ||||
| Centrifuged flotation (precision as % CV)c | Salt | 68.97 | |||
| Sugar | 86.07 | ||||
| McMaster (precision as % CV)c | Salt | 95.75 | |||
| Sugar | 53.96 | ||||
| Mini-FLOTAC (precision as % CV)c | Salt | 98.16 | |||
| Sugar | 50.23 | ||||
| FECPAKG1 | Not applicable | Strongyles | 100 | NA | Tyson et al. (2020) |
| FECPAKG2 (accuracy as a percentage of mean FECPAKG1 egg count)c | 101 |
Abbreviations: CV, coefficient of variation; epg, eggs per gram; NA, not assessed.
Mean epg.
Percent eggs recovered on four successive coverslips based on McMaster counts.
Measure of each of the corresponding parameter given in the published article.
Table 5.
Sensitivity of various faecal egg counting techniques used for equine faecal samples
| Method used | Parameter definition | Spiked epg/worm burden | Parasite detected | Sensitivity (%) | Reference |
|---|---|---|---|---|---|
| Sedimentation | Eggs detected in % samples from infected animals | Not applicable | Anoplocephala perfoliata | 22.5 | Williamson et al. (1998) |
| Flotation 1 | 25.0 | ||||
| Flotation 2 | 37.5 | ||||
| Method A (McMaster) | Detection of eggs at different worm burden levels | <100 | A. perfoliata | 11.8 | Meana et al. (1998) |
| >100 | 0 | ||||
| Method B (Modified McMaster) | <100 | 35.3 | |||
| >100 | 57.1 | ||||
| Method C (Tube and coverslip) | <100 | 35.3 | |||
| >100 | 42.9 | ||||
| FECPAK | Percentages of various spiked epg levels detected | 50 epg | Strongyles | 100 | Presland et al. (2005) |
| 100 epg | 100 | ||||
| 200 epg | 100 | ||||
| McMaster | Percentages of various spiked epg levels detected | 50 epg | 40 | ||
| 100 epg | 40 | ||||
| 200 epg | 100 | ||||
| McMaster | Detection of eggs at different worm burden levels | 5, 9, 11, 18, 23, 104, 156, 160, 162, 1700 | A. perfoliata | No eggs detected at any level of worm burden | Fukumoto et al. (2011) |
| Modified Wisconsin (sodium nitrate) | Eggs detected at worm burdens of 23, 104, 156, 160 and 1700 | ||||
| Modified Wisconsin (zinc sulfate) | Eggs detected at worm burdens of 23, 104, 156, 160 and 1700 | ||||
| Modified Wisconsin (sucrose) | Eggs detected at worm burdens of 23, 104, 156, 160, 162 and 1700 | ||||
| Flotation | No. of total tapeworms | NA | A. perfoliata | 16.7 | Tomczuk et al. (2014) |
| No. of tapeworms with gravid proglottids | 20.7 | ||||
| Sedimentation | No. of total tapeworms | 8.3 | |||
| No. of tapeworms with gravid proglottids | 10.3 | ||||
| Modified sedimentation-flotation | No. of total tapeworms | 58.3 | |||
| No. of tapeworms with gravid proglottids | 72.4 | ||||
| McMaster | No. of total tapeworms | 2.8 | |||
| No. of tapeworms with gravid proglottids | 3.4 | ||||
| Combined zinc sulfate sedimentation-flotation | Percentages of various spiked epg levels detected for cyathostomins and A. perfoliata, respectively | 1 epg | Cyathostomins & A. perfoliata | 6.7 & 0 | Becker et al. (2016) |
| 5 epg | 46.7 & 26.7 | ||||
| 10 epg | 86.7 & 35.3 | ||||
| 20 epg | 86.7 & 60.0 | ||||
| 40 epg | 100 & 80.0 | ||||
| 60 epg | 100 & 86.7 | ||||
| 80 epg | 100 & 100 | ||||
| McMaster | 1 epg | 0 & 0 | |||
| 30 epg | 13.3 & 13.3 | ||||
| 50 epg | 60 & 26.7 | ||||
| 80 epg | 60 & 60.0 | ||||
| 100 epg | 80 & 80.0 | ||||
| 500 epg | 100 & 100 | ||||
| 1000 epg | 100 & 100 | ||||
| Mini-FLOTAC | Percentages of various spiked epg levels detected | 10 epg | Strongyles | 100 | Bosco et al. (2018) |
| 50 epg | 100 | ||||
| McMaster (chamber) | 10 epg | 41.7 | |||
| 50 epg | 100 | ||||
| McMaster (grid) | 10 epg | 25.0 | |||
| 50 epg | 75.0 | ||||
| Cornell–Wisconsin | 10 epg | 100 | |||
| 50 epg | 100 | ||||
| McMaster | Percentages of various spiked epg levels detected | 0–200; 201–500; 501–1000; >1000 epg | Strongyles | 99.40 | Cain et al. (2020) |
| Wisconsin | 99.40 | ||||
| Automated egg counting technique | 98.00 |
Abbreviations: CV, coefficient of variation; epg, eggs per gram; NA, not assessed.
Out of 15 studies that investigated the accuracy and precision (expressed as percentage precision and coefficient of variation) of FECT, five studies included both measures of diagnostic test performance (Noel et al., 2017; Scare et al., 2017; Bosco et al., 2018; Went et al., 2018; Napravnikova et al., 2019) whereas the remaining studies included one only (Table 4). The accuracy and precision of the McMaster technique and Mini-FLOTAC® were compared in five and eight studies, respectively, and reported variable results, with accuracy being higher for the McMaster method in one study (Went et al., 2018) but lower in three studies (Noel et al., 2017; Scare et al., 2017; Bosco et al., 2018). One study reported greater accuracy of the McMaster technique for strongyles but lower accuracy for Parascaris spp. eggs when compared with Mini-FLOTAC® (Napravnikova et al., 2019). Conversely, Mini-FLOTAC® was found to be more precise than the McMaster technique for strongyles in seven studies (Britt et al., 2017; Noel et al., 2017; Scare et al., 2017; Bosco et al., 2018; Paras et al., 2018; Went et al., 2018; Napravnikova et al., 2019) whereas one study reported similar precision for both techniques (Silva et al., 2019) (Table 4).
The sensitivity of the McMaster technique was compared with other techniques such as FECPAK®, Wisconsin, flotation, sedimentation, combined sedimentation-flotation, Mini-FLOTAC® and automated egg counting in six studies: five of these studies (Presland et al., 2005; Fukumoto et al., 2011; Tomczuk et al., 2014; Becker et al., 2016; Bosco et al., 2018) reported lower sensitivity of the McMaster technique, while one study (Cain et al., 2020) reported similar sensitivity levels for McMaster, Wisconsin and an automated egg counting technique (Table 5).
Among studies on other important factors affecting the performance of FECT (Supplementary Table S2), one study reported the effect of using salt and sugar-based flotation solutions and found that Sheatherʼs sugar solution provided better flotation of equine Parascaris spp. eggs (McQueary, 1976). In another study conducted by OʼGrady and Slocombe (1980), the specific gravity range 1.22–1.35 was found to optimise the recovery of strongyle and ascarid eggs, with no effect of flotation time using a flotation solution of 1.27 specific gravity. Moreover, the same study reported the recovery of the greatest number of strongyle eggs using a mesh size of 500 μm2, whereas the recovery of Parascaris spp. eggs appeared to not be influenced by the use of mesh sizes between 200 and 500 μm2 (OʼGrady and Slocombe, 1980). Similarly, Rehbein et al. (2011) investigated the effect of flotation solution type and the weight of the faecal sample on the recovery of tapeworm eggs and found that the use of larger sample sizes in sugar-based flotation solutions provided greater FEC. Slusarewicz et al. (2019) investigated the effect of duration of egg counting (at-leisure, two-minutes and one-minute) on FEC of the McMaster technique compared to Parasight system® and reported a substantial decrease in the precision (one-third, assessed by the coefficient of variation) and FEC (50–60% decrease) for the former method following one-minute counting instead of at-leisure counting. Another study used a Fill-FLOTAC device to investigate the effect of sample homogenisation on FEC and reported that homogenisation resulted in improved accuracy with no effect on precision (Went et al., 2018). The diagnostic specificity was compared in a single study which reported higher values for the McMaster method than that of Mini-FLOTAC (Noel et al., 2017) (Supplementary Table S2).
To the best of our knowledge, this is the first systematic review to critically appraise studies that assessed and/or compared the performance of FECT in horses. The findings of this review highlight the potential opportunities for improvements in future studies aiming to validate and/or compare various FECT for horses and other animal species. It is clear from the results presented above that there is no consensus on the methodology and assessed parameters (and their interpretations) in FECT comparison studies. Although the McMaster technique has been used as a reference standard (a test of known and high accuracy) by most researchers when assessing and comparing other techniques, it lacks diagnostic sensitivity for samples with lower egg counts. Additionally, the analytical and diagnostic capabilities of the McMaster technique are affected by a number of factors (e.g. the amount and type of faecal samples, volume and specific gravity of flotation solutions) (Roepstorff and Nansen, 1998). More recently, some studies have used FLOTAC® (Cringoli, 2006) and Mini-FLOTAC® (Cringoli et al., 2017) as reference standards because of reportedly higher sensitivities in comparison to the McMaster technique. However, there is also considerable variation in the performance of these methods and other similar techniques due to a variety of factors that need to be thoroughly investigated for intended species in order to use them as reference standards (the best test available at a time for comparison) (Duggan, 1992; Claassen, 2005).
The choice of flotation solution is one of the key factors that may affect the FEC of a sample, but it often receives less consideration. Several studies have reported that the sucrose-based solutions provide more accurate FEC in horses and other animals than other flotation solutions (McQueary, 1976; Cringoli et al., 2004; Rehbein et al., 2011; Silva et al., 2019). However, no single flotation solution is ideal for all FECT and/or different egg types in faeces. For example, Cringoli et al. (2017) reported that for Mini-FLOTAC®, sodium chloride with a specific gravity of 1.20 and glucose-salt solutions with a specific gravity of 1.24–1.26 were the most efficient flotation solutions for equine strongyles, whereas zinc sulphate with a specific gravity of 1.35 and glucose-salt solutions with the specific gravity of 1.24–1.26 were better for Parascaris spp. eggs.
Another important factor to consider for FECT is the specific gravity used for a flotation solution. Previously, OʼGrady and Slocombe (1980) demonstrated that the best flotation of parasite eggs was achieved with solutions with a specific gravity between 1.22 and 1.35. Currently, the majority of FEC methods utilise flotation solutions with specific gravity ≥ 1.2. This value is also in agreement with the estimated mean specific gravities calculated for strongylid, Parascaris spp. and Anoplocephala spp. eggs by Norris et al. (2018) as 1.0453, 1.0903 and 1.0636, respectively. The only other evidence available is about the specific gravity of Parascaris equorum eggs (1.0969) (David and Lindquist, 1982). Exceeding the upper limit of specific gravity (1.35) usually results in the flotation of faecal debris and rapid crystallisation of the flotation solution (OʼGrady and Slocombe, 1980). Additionally, Denwood et al. (2012) investigated the sources of variability in FEC results (both within and between animals) and reported that within animal variation (unequal distribution of eggs between faecal piles) was the most important factor responsible for variation in FEC. They also reported that no diurnal variation was found in FEC and these findings were in agreement with those of Carstensen et al. (2013). Recently, Wilkes et al. (2019) reported significant variability in strongyle and ascarid FEC in individual foals between different portions of a faecal pile, different faecal piles, and samples collected across different days. Given the significance of these important factors for FEC, studies aiming to validate and compare various FECT should take into account differences arising from using various flotation solutions, the size and the number of faecal (sub)samples used for testing.
The reported higher performance (accuracy and precision) of Mini-FLOTAC® compared to the McMaster technique is probably because the former technique requires and analyses a larger, uniformly homogenised (using a Fill FLOTAC device) faecal sample; thereby, it has a lower multiplication factor than that of the McMaster method. The only study which reported higher accuracy for McMaster was probably due to the use of Fill-FLOTAC in combination with the McMaster technique, resulting in a better homogenisation of eggs within faecal samples (Went et al., 2018). This study also reported that the Fill-FLOTAC affected accuracy, but not precision, while the counting chamber (Mini-FLOTAC vs McMaster) affected precision, but not accuracy (Went et al., 2018). However, further testing would be required to support these preliminary findings. Similarly, the sensitivity and efficiency of different FECT are directly related to faecal quantity, dilution factor and the volume of suspension analysed (Lester and Matthews, 2014; Daş et al., 2020).
4. Future implications
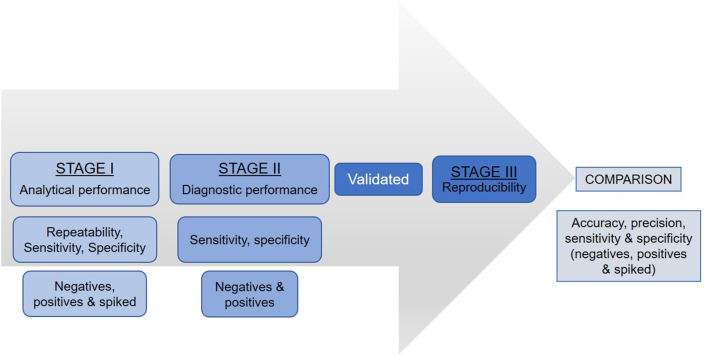
With the growing concerns of anthelmintic resistance and, importantly, the lack of new anthelmintics available to control equine parasites, there is an increased interest amongst veterinary professionals and animal-health officials in the development and validation of veterinary assays for accurate diagnosis, surveillance and monitoring of parasitic infections (Nielsen, 2021). It is therefore important to properly validate a FEC method prior to its comparison with existing standard techniques. For an assay to be considered validated for an intended purpose, there are certain critical requirements that must be met, as outlined in guidelines for the development and validation of diagnostic assays by the World Organisation for Animal Health (Office International des Epizooties: OIE) (Jacobson and Wright, 2019). The developmental stage of a diagnostic test involves the description of the intended purpose, standardisation and optimisation of the assay. Test validation is critical and is often not completed properly for veterinary diagnostic tests in general and FECT in particular. This stage includes measuring analytical parameters (repeatability, analytical sensitivity and specificity), diagnostic parameters (diagnostic sensitivity and specificity) and reproducibility (Jacobson and Wright, 2019). Once an assay completes both of these stages, it is considered validated and can then be used in the target population for its intended purpose (Jacobson and Wright, 2019). For calculating the required number of samples to be tested through each stage of the assay development and validation, prior consultation with a (bio)statistician should be made as per the recommendations of the OIE. After the test is validated, its comparison with existing FEC methods can be made, and the important parameters which need to be compared include accuracy, precision, repeatability, and diagnostic sensitivity and specificity (Fig. 4). Additionally, the FECT comparison studies should also pay attention to other important sources of variation (both technical and biological as outlined in this article) in FEC and their potential impact on the performance of FECT in designing and conducting such studies. It is anticipated that this proposed assessment method of developing and validating a new diagnostic method and comparing a new test with existing test(s) would help the parasitological community standardise methods for parasite diagnostics as per the OIE guidelines.
Fig. 4.
Flowchart diagram of steps involved in validation and comparison of a diagnostic assay.
5. Conclusions
In conclusion, this systematic review has clearly demonstrated that there is a lack of consensus on the methodology used, assessed performance parameters, and interpretation of results for studies on the assessment and comparison of FECT. Most studies used the McMaster technique to compare the performance of FECT and reported higher estimates of accuracy, precision and sensitivities for other techniques. Sugar-based solutions were reported to perform better for egg recovery in most of the techniques. This systematic review highlights the need for thorough validation studies which characterise the analytical and diagnostic parameters of existing and new FECT as outlined by the OIE standards. For example, for future studies aiming to validate or compare a new technique, analytical and diagnostic parameters should be tested experimentally.
Funding
The financial assistance for this project was provided by the AgriFutures Australia, Thoroughbred Breeders Australia and Boehringer Ingelheim Animal Health Australia Pty. Ltd. Boehringer Ingelheim did not have any role in the design or content of this manuscript.
CRediT author statement
Abdul Ghafar, Abdul Jabbar: Conceptualization, Data curation & Writing - Original Draft. Abdul Jabbar: Funding and Supervision; Ghazanfar Abbas, Justine King, Caroline Jacobson, Kristopher J. Hughes, Charles El-Hage, Anne Beasley, Jenni Bauquier, Edwina J.A. Wilkes, John Hurley, Lucy Cudmore, Peter Carrigan, Brett Tennent-Brown, Martin K. Nielsen, Charles G. Gauci, Ian Beveridge: Writing - Reviewing & Editing. All authors read and approved the final manuscript.
Data availability
All data reported in this paper are either included the manuscript or presented as a supplementary material.
Declaration of competing interests
The authors are members of the Australian Equine Parasitology Advisory Panel supported by AgriFutures Australia and Boehringer Ingelheim Animal Health Australia. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Given his role as a Co-Editor, Abdul Jabbar had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Luis Cardoso and Aneta Kostadinova.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2021.100046.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Table S1. PRISMA checklist.
Supplementary Table S1. Studies on the comparison/assessment of faecal egg counting techniques for equine gastrointestinal parasites.
References
- Andersen U., Howe D., Olsen S., Nielsen M. Recent advances in diagnosing pathogenic equine gastrointestinal helminths: the challenge of prepatent detection. Vet. Parasitol. 2013;192:1–9. doi: 10.1016/j.vetpar.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Ballweber L., Beugnet F., Marchiondo A., Payne P. American Association of Veterinary Parasitologists' review of veterinary fecal flotation methods and factors influencing their accuracy and use - is there really one best technique? Vet. Parasitol. 2014;204:73–80. doi: 10.1016/j.vetpar.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Barda B.D., Rinaldi L., Ianniello D., Zepherine H., Salvo F., Sadutshang T., et al. Mini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: experience from the field. PLoS Negl. Trop. Dis. 2013;7:e2344. doi: 10.1371/journal.pntd.0002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley A., Kotze A., Allen K., Coleman G. A survey of macrocyclic lactone efficacy in Australian cyathostomin populations. Vet. Parasitol. Reg. Stud. 2017;8:127–132. doi: 10.1016/j.vprsr.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Becker A.-C., Kraemer A., Epe C., Strube C. Sensitivity and efficiency of selected coproscopical methods - sedimentation, combined zinc sulfate sedimentation-flotation, and McMaster method. Parasitol. Res. 2016;115:2581–2587. doi: 10.1007/s00436-016-5003-8. [DOI] [PubMed] [Google Scholar]
- Bello T.R., Allen T.M. Comparison of two fecal egg recovery techniques and larval culture for cyathostomins in horses. Am. J. Vet. Res. 2009;70:571–573. doi: 10.2460/ajvr.70.5.571. [DOI] [PubMed] [Google Scholar]
- Bosco A., Maurelli M.P., Ianniello D., Morgoglione M.E., Amadesi A., Coles G.C., et al. The recovery of added nematode eggs from horse and sheep faeces by three methods. BMC Vet. Res. 2018;14 doi: 10.1186/s12917-017-1326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt A., Kaplan R., Paras K., Turner K., Abrams A., Duberstein K. A comparison of McMasters versus mini-FLOTAC techniques in quantifying small strongyles in equine fecal egg assessments. J. Equine Vet. Sci. 2017;100:97. [Google Scholar]
- Bucknell D., Hoste H., Gasser R., Beveridge I. The structure of the community of strongyloid nematodes of domestic equids. J. Helminthol. 1996;70:185–192. doi: 10.1017/s0022149x0001539x. [DOI] [PubMed] [Google Scholar]
- Cain J.L., Slusarewicz P., Rutledge M.H., McVey M.R., Wielgus K.M., Zynda H.M., et al. Diagnostic performance of McMaster, Wisconsin, and automated egg counting techniques for enumeration of equine strongyle eggs in fecal samples. Vet. Parasitol. 2020;284:109199. doi: 10.1016/j.vetpar.2020.109199. [DOI] [PubMed] [Google Scholar]
- Carstensen H., Larsen L., Ritz C., Nielsen M.K. Daily variability of strongyle fecal egg counts in horses. J. Equine Vet. Sci. 2013;33:161–164. [Google Scholar]
- Claassen J.A. The gold standard: not a golden standard. BMJ. 2005;330:1121. [Google Scholar]
- Corning S. Equine cyathostomins: a review of biology, clinical significance and therapy. Parasit. Vectors. 2009;2(Suppl. 2):S1. doi: 10.1186/1756-3305-2-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox P., Todd A. Survey of gastrointestinal parasitism in Wisconsin dairy cattle. J. Am. Vet. Med. Assoc. 1962;141:706–709. [PubMed] [Google Scholar]
- Cringoli G. FLOTAC, a novel apparatus for a multivalent faecal egg count technique. Parassitologia. 2006;48:381–384. [PubMed] [Google Scholar]
- Cringoli G., Amadesi A., Maurelli M.P., Celano B., Piantadosi G., Bosco A., et al. The Kubic FLOTAC microscope (KFM): a new compact digital microscope for helminth egg counts. Parasitology. 2021;48:427–434. doi: 10.1017/S003118202000219X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cringoli G., Maurelli M.P., Levecke B., Bosco A., Vercruysse J., Utzinger J., Rinaldi L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 2017;12:1723–1732. doi: 10.1038/nprot.2017.067. [DOI] [PubMed] [Google Scholar]
- Cringoli G., Rinaldi L., Maurelli M.P., Utzinger J. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat. Protoc. 2010;5:503–515. doi: 10.1038/nprot.2009.235. [DOI] [PubMed] [Google Scholar]
- Cringoli G., Rinaldi L., Veneziano V., Capelli G., Scala A. The influence of flotation solution, sample dilution and the choice of McMaster slide area (volume) on the reliability of the McMaster technique in estimating the faecal egg counts of gastrointestinal strongyles and Dicrocoelium dendriticum in sheep. Vet. Parasitol. 2004;123:121–131. doi: 10.1016/j.vetpar.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Daş G., Klauser S., Stehr M., Tuchscherer A., Metges C.C. Accuracy and precision of McMaster and Mini-FLOTAC egg counting techniques using egg-spiked faeces of chickens and two different flotation fluids. Vet. Parasitol. 2020;283:109158. doi: 10.1016/j.vetpar.2020.109158. [DOI] [PubMed] [Google Scholar]
- David E.D., Lindquist W.D. Determination of the specific gravity of certain helminth eggs using sucrose density gradient centrifugation. J. Parasitol. 1982;68:916–919. [PubMed] [Google Scholar]
- de Castro L.L.D., Abrahão C.L., Buzatti A., Molento M.B., Bastianetto E., Rodrigues D.S., et al. Comparison of McMaster and mini-FLOTAC fecal egg counting techniques in cattle and horses. Vet. Parasitol. 2017;10:132–135. doi: 10.1016/j.vprsr.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Denwood M., Love S., Innocent G., Matthews L., McKendrick I., Hillary N., et al. Quantifying the sources of variability in equine faecal egg counts: implications for improving the utility of the method. Vet. Parasitol. 2012;188:120–126. doi: 10.1016/j.vetpar.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Drudge J., Lyons E. Control of internal parasites of the horse. J. Am. Vet. Med. Assoc. 1966;148:378–383. [PubMed] [Google Scholar]
- Duggan P.F. Time to abolish “gold standard”. BMJ. 1992;304:1121. [Google Scholar]
- Egwang T., Slocombe J. Evaluation of the Cornell-Wisconsin centrifugal flotation technique for recovering trichostrongylid eggs from bovine feces. Can. J. Comp. Med. 1982;46:133–137. [PMC free article] [PubMed] [Google Scholar]
- Elghryani N., Crispell J., Ebrahimi R., Krivoruchko M., Lobaskin V., McOwan T., et al. Preliminary evaluation of a novel, fully automated, telenostic device for rapid field-diagnosis of cattle parasites. Parasitology. 2020;147:1249–1253. doi: 10.1017/S0031182020001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S., Sato R., Nagata M., Arakaki H., Goto T., Mochizukio R., et al. Comparison of four floating methods of fecal examination for equine cestode eggs. Jpn. J. Anim. Hyg. 2011;36:131–135. [Google Scholar]
- Gordon H.M., Whitlock H. A new technique for counting nematode eggs in sheep faeces. J. Counc. Sci. Ind. Res. 1939;12:50–52. [Google Scholar]
- Jacobson R., Wright P. Manual of diagnostic tests and vaccines for terrestrial animals. OIE; 2019. Principles and methods of validation of diagnostic assays for infectious diseases; pp. 72–87.https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/1.01.06_VALIDATION.pdf [Google Scholar]
- Kaplan R., Nielsen M. An evidence-based approach to equine parasite control: it ainʼt the 60s anymore. Equine Vet. Educ. 2010;22:306–316. [Google Scholar]
- Lane C. The mass diagnosis of ankylostome infestation. Parts II to VII. Trans. R. Soc. Trop. Med. Hyg. 1923;17:407–436. [Google Scholar]
- Lester H., Matthews J. Faecal worm egg count analysis for targeting anthelmintic treatment in horses: points to consider. Equine Vet. J. 2014;46:139–145. doi: 10.1111/evj.12199. [DOI] [PubMed] [Google Scholar]
- Lichtenfels J.R., Kharchenko V.A., Dvojnos G.M. Illustrated identification keys to strongylid parasites (Strongylidae: Nematoda) of horses, zebras and asses (Equidae) Vet. Parasitol. 2008;156:4–161. doi: 10.1016/j.vetpar.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Love S., Murphy D., Mellor D. Pathogenicity of cyathostome infection. Vet. Parasitol. 1999;85:113–122. doi: 10.1016/s0304-4017(99)00092-8. [DOI] [PubMed] [Google Scholar]
- Lyons E., Tolliver S. Prevalence of parasite eggs (Strongyloides westeri, Parascaris equorum, and strongyles) and oocysts (Emeria leuckarti) in the feces of Thoroughbred foals on 14 farms in central Kentucky in 2003. Parasitol. Res. 2004;92:400–404. doi: 10.1007/s00436-003-1068-2. [DOI] [PubMed] [Google Scholar]
- Martin F., Svansson V., Eydal M., Oddsdóttir C., Ernback M., Persson I., Tydén E. First report of resistance to ivermectin in Parascaris univalens in Iceland. J. Parasitol. 2021;107:16–22. doi: 10.1645/20-91. [DOI] [PubMed] [Google Scholar]
- McCoy M., Edgar H., Kenny J., Gordon A., Dawson L., Carson A. Evaluation of on-farm faecal worm egg counting in sheep. Vet. Rec. 2005;156:21–23. doi: 10.1136/vr.156.1.21. [DOI] [PubMed] [Google Scholar]
- McQueary C.A. College of Agriculture, Montana State University-Bozeman; Montana, USA: 1976. Prevalence of equine gastrointestinal parasites in Montana, correlation of Parascaris equorum egg per gram counts and worm burdens, and comparison of two parasite egg counting techniques for equine feces.https://scholarworks.montana.edu/xmlui/bitstream/handle/1/5702/31762100148434.pdf?sequence=1 MSc Thesis, [Google Scholar]
- Meana A., Luzon M., Corchero J., Gómez-Bautista M. Reliability of coprological diagnosis of Anoplocephala perfoliata infection. Vet. Parasitol. 1998;74:79–83. doi: 10.1016/s0304-4017(97)00145-3. [DOI] [PubMed] [Google Scholar]
- Nagamori Y., Sedlak R.H., DeRosa A., Pullins A., Cree T., Loenser M., et al. Evaluation of the VETSCAN IMAGYST: an in-clinic canine and feline fecal parasite detection system integrated with a deep learning algorithm. Parasit. Vectors. 2020;13:346. doi: 10.1186/s13071-020-04215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napravnikova J., Petrtyl M., Stupka R., Vadlejch J. Reliability of three common fecal egg counting techniques for detecting strongylid and ascarid infections in horses. Vet. Parasitol. 2019;272:53–57. doi: 10.1016/j.vetpar.2019.07.001. [DOI] [PubMed] [Google Scholar]
- Nielsen M., Pfister K., von Samson-Himmelstjerna G. Selective therapy in equine parasite control - application and limitations. Vet. Parasitol. 2014;202:95–103. doi: 10.1016/j.vetpar.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K. What makes a good fecal egg count technique? Vet. Parasitol. 2021:109509. doi: 10.1016/j.vetpar.2021.109509. [DOI] [PubMed] [Google Scholar]
- Noel M.L., Scare J.A., Bellaw J.L., Nielsen M.K. Accuracy and precision of mini-FLOTAC and McMaster techniques for determining equine strongyle egg counts. J. Equine Vet. Sci. 2017;48:182–187. [Google Scholar]
- Norris J.K., Steuer A.E., Gravatte H.S., Slusarewicz P., Bellaw J.L., Scare J.A., Nielsen M.K. Determination of the specific gravity of eggs of equine strongylids, Parascaris spp., and Anoplocephala perfoliata. Vet. Parasitol. 2018;260:45–48. doi: 10.1016/j.vetpar.2018.08.004. [DOI] [PubMed] [Google Scholar]
- OʼGrady M.R., Slocombe J.O. An investigation of variables in a fecal flotation technique. Can. J. Comp. Med. 1980;44:148–157. [PMC free article] [PubMed] [Google Scholar]
- Paras K.L., George M.M., Vidyashankar A.N., Kaplan R.M. Comparison of fecal egg counting methods in four livestock species. Vet. Parasitol. 2018;257:21–27. doi: 10.1016/j.vetpar.2018.05.015. [DOI] [PubMed] [Google Scholar]
- Peregrine A.S., Molento M.B., Kaplan R.M., Nielsen M.K. Anthelmintic resistance in important parasites of horses: does it really matter? Vet. Parasitol. 2014;201:1–8. doi: 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Presland S., Morgan E., Coles G. Counting nematode eggs in equine faecal samples. Vet. Rec. 2005;156:208–210. doi: 10.1136/vr.156.7.208. [DOI] [PubMed] [Google Scholar]
- Rehbein S., Lindner T., Visser M., Winter R. Evaluation of a double centrifugation technique for the detection of Anoplocephala eggs in horse faeces. J. Helminthol. 2011;85:409–414. doi: 10.1017/S0022149X10000751. [DOI] [PubMed] [Google Scholar]
- Reinemeyer C.R. Anthelmintic resistance in non-strongylid parasites of horses. Vet. Parasitol. 2012;185:9–15. doi: 10.1016/j.vetpar.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Rinaldi L. Ghent University, Merelbeke, Belgium & Università degli Studi di Napoli Federico II; Naples, Italy: 2014. The coprological diagnosis of gastrointestinal nematode infections in small ruminants.https://biblio.ugent.be/publication/5645001 PhD Thesis, [Google Scholar]
- Roepstorff A., Nansen P. FAO; Rome, Italy: 1998. Epidemiology, diagnosis and control of helminth parasites of swine.http://www.fao.org/3/x0520e/x0520e.pdf [Google Scholar]
- Saeed M.A., Beveridge I., Abbas G., Beasley A., Bauquier J., Wilkes E., et al. Systematic review of gastrointestinal nematodes of horses from Australia. Parasit. Vectors. 2019;12:188. doi: 10.1186/s13071-019-3445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scare J., Slusarewicz P., Mills C., Pagano S., Hauck E., Nielsen M. Comparison of a smart-phone based automated parasite egg count system to the McMaster & mini-FLOTAC methods. J. Equine Vet. Sci. 2016;39:S49. [Google Scholar]
- Scare J.A., Slusarewicz P., Noel M.L., Wielgus K.M., Nielsen M.K. Evaluation of accuracy and precision of a smartphone based automated parasite egg counting system in comparison to the McMaster and Mini-FLOTAC methods. Vet. Parasitol. 2017;247:85–92. doi: 10.1016/j.vetpar.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Silva P., Luz A., Rolim A., Carvalho R., Gomes L., Neto I., et al. 18th International Conference on Life Sciences for Sustainable Development University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania. 2019. Comparison study between four coprological methods and two flotation solutions in Sorraia horse (Equus ferus caballus) - preliminary results.https://www.researchgate.net/publication/336497526_Comparison_study_between_four_coprological_methods_and_two_flotation_solutions_in_Sorraia_horse_Equus_ferus_caballus_-Preliminary_results [Google Scholar]
- Slusarewicz M., Slusarewicz P., Nielsen M.K. The effect of counting duration on quantitative fecal egg count test performance. Vet. Parasitol. 2019;2:100020. doi: 10.1016/j.vpoa.2019.100020. [DOI] [PubMed] [Google Scholar]
- Slusarewicz P., Pagano S., Mills C., Popa G., Chow K.M., Mendenhall M., et al. Automated parasite faecal egg counting using fluorescence labelling, smartphone image capture and computational image analysis. Int. J. Parasitol. 2016;46:485–493. doi: 10.1016/j.ijpara.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Snyder S., England J., McChesney A. Cryptosporidiosis in immunodeficient Arabian foals. Vet. Pathol. 1978;15:12–17. doi: 10.1177/030098587801500102. [DOI] [PubMed] [Google Scholar]
- Stoll N.R. Investigations on the control of hookworm disease. XV. An effective method of counting hookworm eggs in feces. Am. J. Epidemiol. 1923;3:59–70. [Google Scholar]
- Stoll N.R. On methods of counting nematode ova in sheep dung. Parasitology. 1930;22:116–136. [Google Scholar]
- Sukas S., Van Dorst B., Kryj A., Lagatie O., De Malsche W., Stuyver L.J. Development of a lab-on-a-disk platform with digital imaging for identification and counting of parasite eggs in human and animal stool. Micromachines. 2019;10:852. doi: 10.3390/mi10120852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver S., Lyons E., Drudge J. Prevalence of internal parasites in horses in critical tests of activity of parasiticides over a 28-year period (1956–1983) in Kentucky. Vet. Parasitol. 1987;23:273–284. doi: 10.1016/0304-4017(87)90013-6. [DOI] [PubMed] [Google Scholar]
- Tomczuk K., Kostro K., Szczepaniak K.O., Grzybek M., Studzinska M., Demkowska-Kutrzepa M., Roczen-Karczmarz M. Comparison of the sensitivity of coprological methods in detecting Anoplocephala perfoliata invasions. Parasitol. Res. 2014;113:2401–2406. doi: 10.1007/s00436-014-3919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson F., Dalesman S., Brophy P.M., Morphew R.M. Novel equine faecal egg diagnostics: validation of the FECPAKG2. Animals. 2020;10:1254. doi: 10.3390/ani10081254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Samson-Himmelstjerna G. Anthelmintic resistance in equine parasites - detection, potential clinical relevance and implications for control. Vet. Parasitol. 2012;185:2–8. doi: 10.1016/j.vetpar.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Went H.A., Scare J.A., Steuer A.E., Nielsen M.K. Effects of homogenizing methods on accuracy and precision of equine strongylid egg counts. Vet. Parasitol. 2018;261:91–95. doi: 10.1016/j.vetpar.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Whitlock H. Some modifications of the McMaster helminth egg-counting technique and apparatus. J. Counc. Sci. Ind. Res. 1948;21:177–180. [Google Scholar]
- Wilkes E.J.A., Woodgate R.G., Raidal S.L., Hughes K.J. The application of faecal egg count results and statistical inference for clinical decision making in foals. Vet. Parasitol. 2019;270:7–12. doi: 10.1016/j.vetpar.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Williamson R., Beveridge I., Gasser R. Coprological methods for the diagnosis of Anoplocephala perfoliata infection of the horse. Aust. Vet. J. 1998;76:618–621. doi: 10.1111/j.1751-0813.1998.tb10242.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. PRISMA checklist.
Supplementary Table S1. Studies on the comparison/assessment of faecal egg counting techniques for equine gastrointestinal parasites.
Data Availability Statement
All data reported in this paper are either included the manuscript or presented as a supplementary material.