Abstract
Four polyopisthocotyleans were collected from the gill filaments of carangids from off the Algerian coast, southern Mediterranean. Specimens of Gastrocotyle trachuri van Beneden & Hesse, 1863 (Gastrocotylidae) and Cemocotyle cf. trachuri Dillon & Hargis, 1965 (Heteraxinidae) from the Mediterranean horse mackerel Trachurus mediterraneus (Steindachner), Zeuxapta seriolae (Meserve, 1938) (Heteraxinidae) from the greater amberjack Seriola dumerili (Risso) and Pyragraphorus hollisae Euzet & Ktari, 1970 (Pyragraphoridae) from the pompano Trachinotus ovatus (Linnaeus) are redescribed based on newly collected specimens. Their taxonomically important morphological features (male copulatory organ and clamp sclerites) are described and illustrated, and the morphometric variation between Mediterranean and oceanic specimens is highlighted. Careful examination of the specimens of Cemocotyle Sproston, 1946 from the Mediterranean revealed that they exhibited unusual features compared with Cemocotyle trachuri Dillon & Hargis, 1965 from the Pacific, mainly the absence of the terminal lappet, thus questioning previous records of this species in the Mediterranean. New geographical locality records are provided for Z. seriolae and P. hollisae. The presence of C. cf. trachuri and Z. seriolae in the Mediterranean is noteworthy as these monogeneans were initially described in the Pacific Ocean. This study extends the geographical range of Z. seriolae to the southern Mediterranean.
Keywords: Monogenea, Polyopisthocotylea, Mediterranean, Taxonomy, Teleosts
Graphical abstract
Highlights
-
•
Gastrocotyle trachuri, Cemocotyle cf. trachuri, Zeuxapta seriolae and Pyragraphorus hollisae are redescribed.
-
•
Summarized host and distribution data of the species and comments on their taxonomy and host specificity are provided.
-
•
Algeria is a new geographical record for Z. seriolae and P. hollisae.
-
•
Z. seriolae is reported for the first time in the South-Western Mediterranean.
-
•
Unusual features of the new specimens of Cemocotyle question previous records of Cemocotyle trachuri in the Mediterranean.
1. Introduction
Monogeneans of Mediterranean fishes were among the first to have been depicted and described (Ulmer & James, 1981). Early studies on monogeneans from the North-Western Mediterranean Sea include those by Professor Louis Euzet and colleagues, who made significant contributions providing a solid ground for later studies (Euzet, 1957; Euzet & Audouin, 1959; Euzet & Razarihelisoa, 1959; Euzet & Trilles, 1961; Euzet & Ktari, 1971; Euzet & Suriano, 1973). Research efforts on monogeneans flourished as well in the South-Western Mediterranean and in the French-speaking Africa, throughout training of local researchers by Professor Euzet and parasitologists of his ‘school’ (Scholz et al., 2018), mainly Claude Combes (see e.g. Kechemir, 1978) and Jean-Lou Justine (see Chaabane et al., 2015, 2016a, b; Kheddam et al., 2016; Ayadi et al., 2017; Chaabane et al., 2017; Bouguerche et al., 2019a, b, c; Bouguerche et al., 2020a, b; Kheddam et al., 2020; Azizi et al., 2021).
In Algeria, as of the year 1995, early efforts of Dr Faiza Amine contributed significantly to the systematics of some monogeneans from off the Algerian coast (Kouider El Ouahed-Amine, 1998; Amine & Euzet, 2005; Amine et al., 2006a, b, 2007a, b). Nonetheless, regardless of the growing number of possible hosts, surveys of monogenean parasites of marine fishes from off the Algerian coast remain relatively sparse and targeted mostly and largely ecology (Kaouachi et al., 2010; Marzoug, 2012; Ramdane et al., 2013; Brahim Tazi et al., 2016; Benhamou et al., 2017; Hadjou et al., 2017; Ichalal et al., 2017; Ider et al., 2018). Moreover, these surveys focused on economically important host species, mostly sparids (Kouider El Ouahed-Amine, 1998; Kaouachi et al., 2010; Marzoug, 2012; Ramdane et al., 2013; Benhamou et al., 2017; Ider et al., 2018). In addition, most of these data were published in local scientific journals or remain unpublished in MSc and PhD theses, making them difficult to access. It is also likely that some of the monogeneans included within these studies have been misidentified.
Amongst teleost fishes frequently encountered in Algerian marine waters, carangid fishes represent suitable candidates for taxonomic surveys of parasites. They include 146 species of 32 genera (Froese & Pauly, 2016), are largely distributed worldwide, and occur in both tropical and temperate waters (Fischer et al., 1987; Smith-Vaniz, 1999). Estimation of parasite biodiversity supported by these fishes is far from being completed, as most of the implicated fish hosts are with a cosmopolitan distribution and a migratory life-style. Hence, both morphological and molecular data on their parasite fauna, along with type- and voucher specimens in recognised museum collections must be provided for each locality to assure unbiased descriptions of new species.
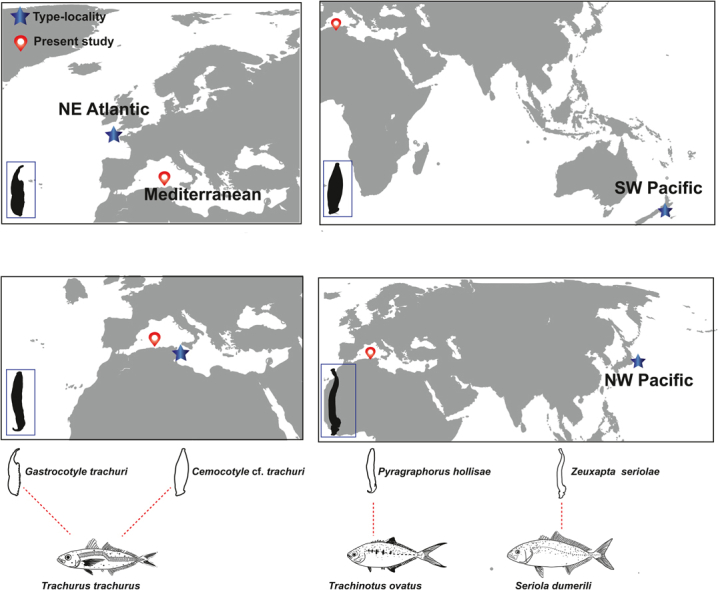
During a survey of helminth parasites of marine fishes from the South-Western (SW) Mediterranean, six monogenean species were found on the gills of carangids. The Mediterranean horse mackerel Trachurus mediterraneus (Steindachner) distributed in the North-East (NE) Atlantic and the Mediterranean was found parasitized by four species: Pseudaxine trachuri Parona & Perugia, 1890, Allogastrocotyle trachuri Nasir & Fuentes Zambrano, 1983, Cemocotyle cf. trachuri Dillon & Hargis, 1965 and Gastrocotyle trachuri van Beneden & Hesse, 1863; the pompano Trachinotus ovatus (L.) which occurs in the NE Atlantic from Africa to the North Sea, was found parasitized by the rare species Pyragraphorus hollisae Euzet & Ktari, 1970; and the cosmopolitan species Seriola dumerili (Risso) which is found in the Pacific, Atlantic and Indian oceans, and the Mediterranean, harboured a single species, Zeuxapta seriolae (Meserve, 1938). The first two monogenean species were redescribed recently (Bouguerche et al., 2019b, c). Herein, we provide detailed illustrated descriptions of the remaining four polyopisthocotylean monogeneans and discuss their taxonomic status, hosts, and distribution.
2. Materials and methods
Freshly-caught fishes collected off the Algerian coast at Cherchell (36°36′31″N, 2°11′50″E) and Bouharoun (36°37′24″N, 2°39′17″E) were purchased from local fishermen. All carangids were relatively young specimens, far from the maximum lengths reported for these species (Smith-Vaniz, 1999). Fishes were transferred to the laboratory shortly after capture and identified using keys (Fischer et al., 1987; Smith-Vaniz, 1999). Gills were removed from each fish and observed under a stereomicroscope (Carl Zeiss™ Stemi™ DV4 Stereomicroscope, Germany) for the presence of monogeneans. The following fish species were examined: Trachurus mediterraneus (total length (TL) 20–26 cm, n = 256), Trachinotus ovatus (TL 25–45 cm, n = 36), and Seriola dumerili (TL 40–42 cm, n = 2).
Monogeneans were removed alive from gills using fine dissection needles, then preserved in 70% ethanol, stained with acetic carmine, dehydrated in ethanol series (70, 96 and 100%), cleared in clove oil, and mounted in Canada balsam. Some specimens were mounted in Berleseʼs fluid to study the morphology of the clamps and the genital atrium. Drawings were made with the aid of a Leitz microscope equipped with a drawing tube (Leitz, Wetzlar, Germany). Drawings were scanned and redrawn on a computer with Adobe Illustrator (CS5). Measurements are in micrometres and are indicated as the range followed by the mean and the number of measurements in parentheses.
3. Results
3.1. Gastrocotyle trachuri van Beneden & Hesse, 1863
3.1.1. Taxonomic summary
Type-host: Trachurus trachurus (L.) (syn. Caranx trachurus (L.), WoRMS, 2021), Atlantic horse mackerel [1–12, 15–22, 24, 25, 27, 29–31, 32, 34, 36–38, 40, 41, 43, 44, 47, 48; present study].
Other hosts: Trachurus mediterraneus (Steindachner) [28, 30, 33, 45, 49]; T. picturatus (Bowdich) [19, 24, 36, 38, 43, 49]; T. lathami Nichols [23, 42]; T. novaezelandiae Richardson [13]; T. indicus (Necrasov) [15]; T. capensis Castelnau [19, 24, 46]; T. trecae Cadenat [19]; Trachurus spp. [35]; Selar crumenophthalmus Bloch [14, 15]; Decapterus sp. [15]; Decapterus russelli (Rüppell) [26]; D. maruadsi (Temminck & Schlegel) [26]; Selar boops (Cuvier) [26].
Type-locality: North-East (NE) Atlantic, off France (Brest) [1].
Additional localities: Atlantic [1, 3–5, 8, 10–12, 18–25, 27, 31, 32, 36–38, 41–44, 46, 49]; Mediterranean [2, 9, 16, 17, 28, 30, 33, 34, 36–38, 40, 45, 47–49; present study]; Pacific [6, 7, 13–15, 35]; Indian Ocean [15, 26, 29].
References: [1] van Beneden & Hesse (1863); [2] Parona & Perugia (1889); [3] Nicoll (1914); [4] Baylis & Jones (1933); [5] Jones (1933); [6] Yamaguti (1938); [7] Yamaguti (1942); [8] Sproston (1946); [9] Palombi (1949); [10] Llewellyn (1956); [11] Llewellyn (1959); [12] Llewellyn (1962); [13] Lebedev (1968); [14] Yamaguti (1968); [15] Parukhin (1976); [16] Lambert (1978); [17] Orecchia & Paggi (1978); [18] Gaevskaya & Kovaleva (1979); [19] Shaw (1979); [20] Gaevskaya & Kovaleva (1980); [21] Piasecki (1982); [22] Llewellyn (1983); [23] Nasir & Fuentes Zambrano (1983); [24] Gaevskaya & Kovaleva (1985); [25] Rego (1987); [26] Parukhin (1988); [27] López-Román & De Armas Hernández (1989); [28] Radujkovic & Euzet (1989); [29] Reimer (1990); [30] Euzet et al. (1993); [31] Naidenova & Mordvinova (1997); [32] Palm et al., (1999); [33] Mollaret et al. (2000); [34] Jovelin & Justine (2001); [35] Zhang et al. (2003); [36] MacKenzie et al. (2004); [37] Campbell (2008); [38] MacKenzie et al. (2008); [39] Fernandez-Jover et al. (2010); [40] Strona et al. (2010); [41] Ângelo (2011); [42] Braicovich et al. (2012); [43] Costa et al. (2012); [44] Rahemo (2012); [45] Akmirza (2013); [46] Le Roux (2013); [47] Feki et al. (2016); [48] Ichalal et al. (2017); [49] Hamdi et al. (2019).
Descriptions: [1, 2, 5–9, 13, 14, 21, 23, 28, 41, 46; present study].
Site on host: Gills.
Voucher material: 38 voucher specimens are deposited in the collections of the Muséum National d’Histoire Naturelle, Paris, France (MNHN HEL1500–HEL1544).
3.1.2. Description
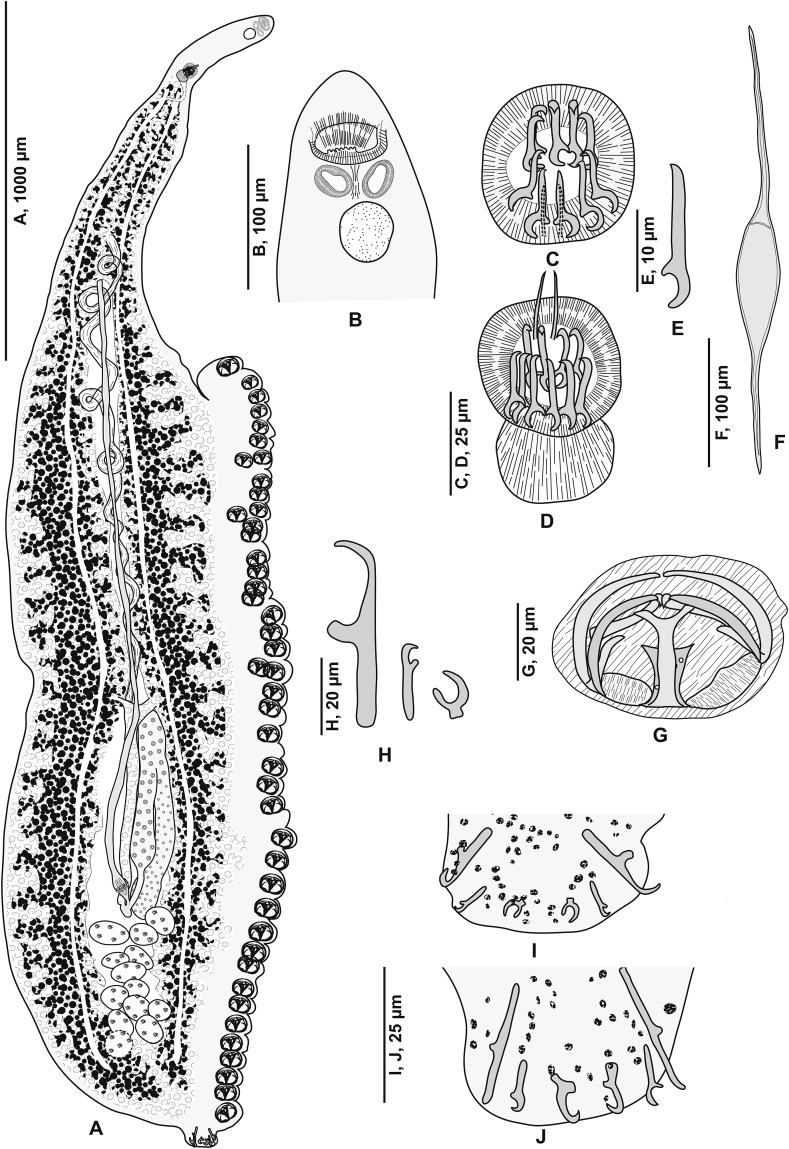
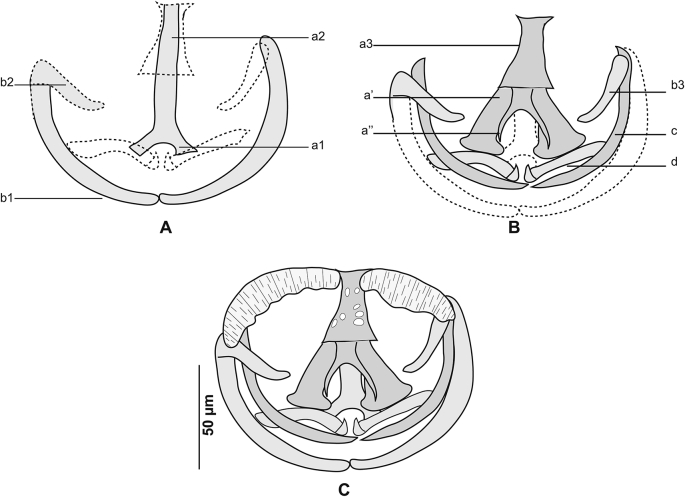
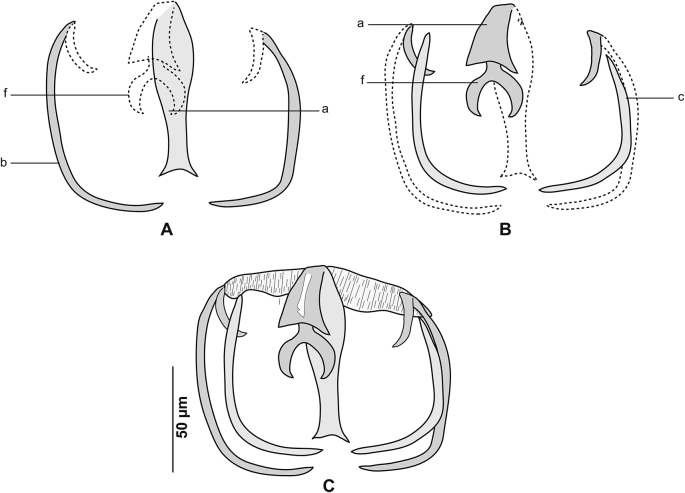
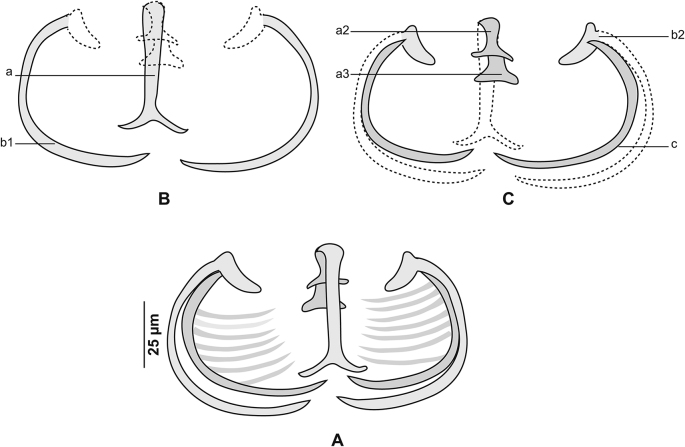
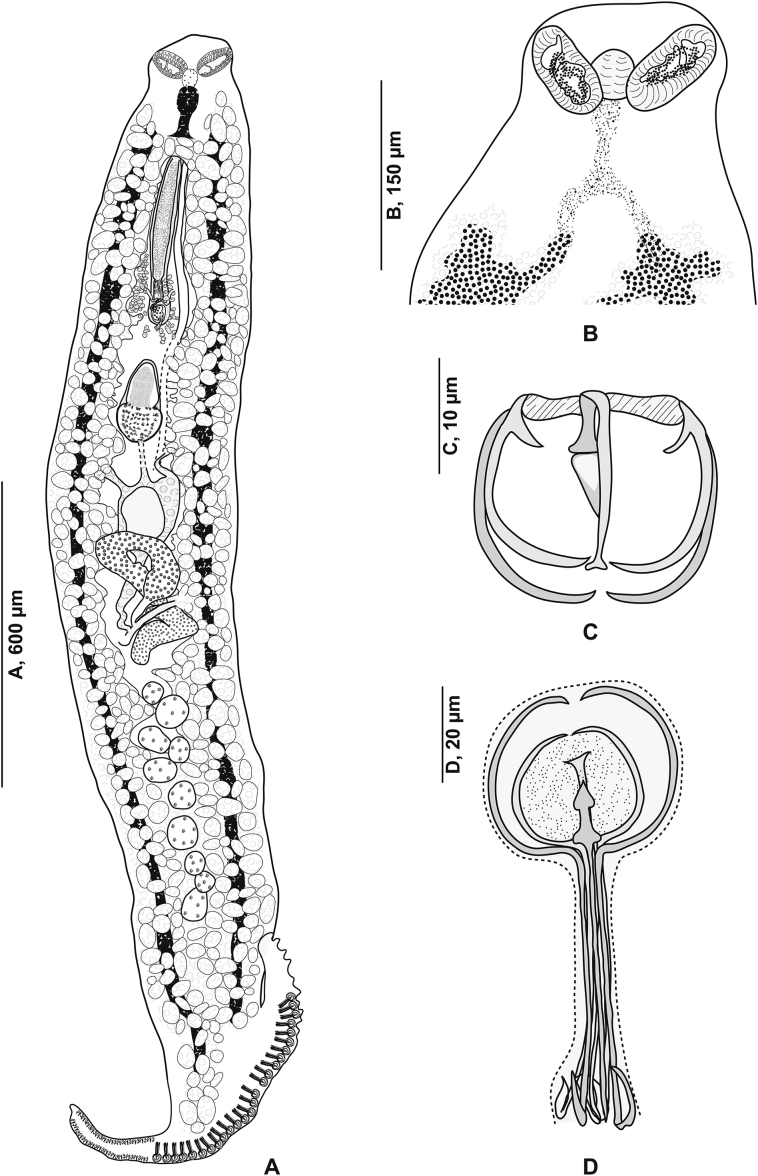
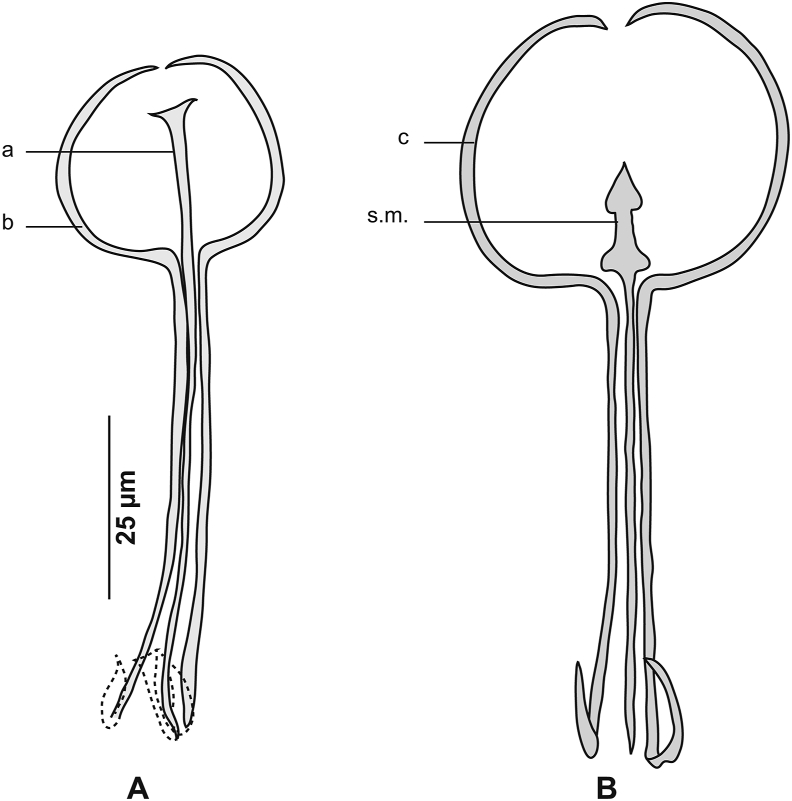
[Based on 26 specimens; Fig. 1, Fig. 2, Fig. 3; Table 1] Body elongated, narrow, symmetrical in first anterior third and considerably wider and asymmetrical posteriorly. Haptor parallel to longitudinal body axis, occupying 2/3 of total body length. Haptor with single row of clamps (Fig. 1A) and terminal lappet (Fig. 1I, J). Terminal lappet armed with 6 uncini; lateral pairs large and stout, medial pairs small (Fig. 1H). Clamps of Gastrocotyle type (Fig. 1G). Ventral arm of median spring a1 Y-shaped, long, distal part of a1 Y-shaped, with short branches of equal size, each limb abutting on short oblique sclerites. Dorsal arm of median spring a3 shorter than a1, distally broad, with 3–4 pairs of apertures arranged in 2 longitudinal symmetrical parallel rows, distal end of a1 with 2 superposed accessory skeletal pieces at its distal end: a’ represented by V-shaped process; a” (Fig. 3A). Ventral arm of ventral jaw sclerites b1, dorsal arm b2 short and curved inwards, b2 not reaching accessory skeletal piece of dorsal arm of median spring (Fig. 3B). Dorsal jaw sclerites c shorter than ventral, c reaching midline on distal side. Oblique sclerites d long, with inner ends folded inwards. Muscle connecting a2 and b2 present on proximal side (Fig. 3C).
Fig. 1.
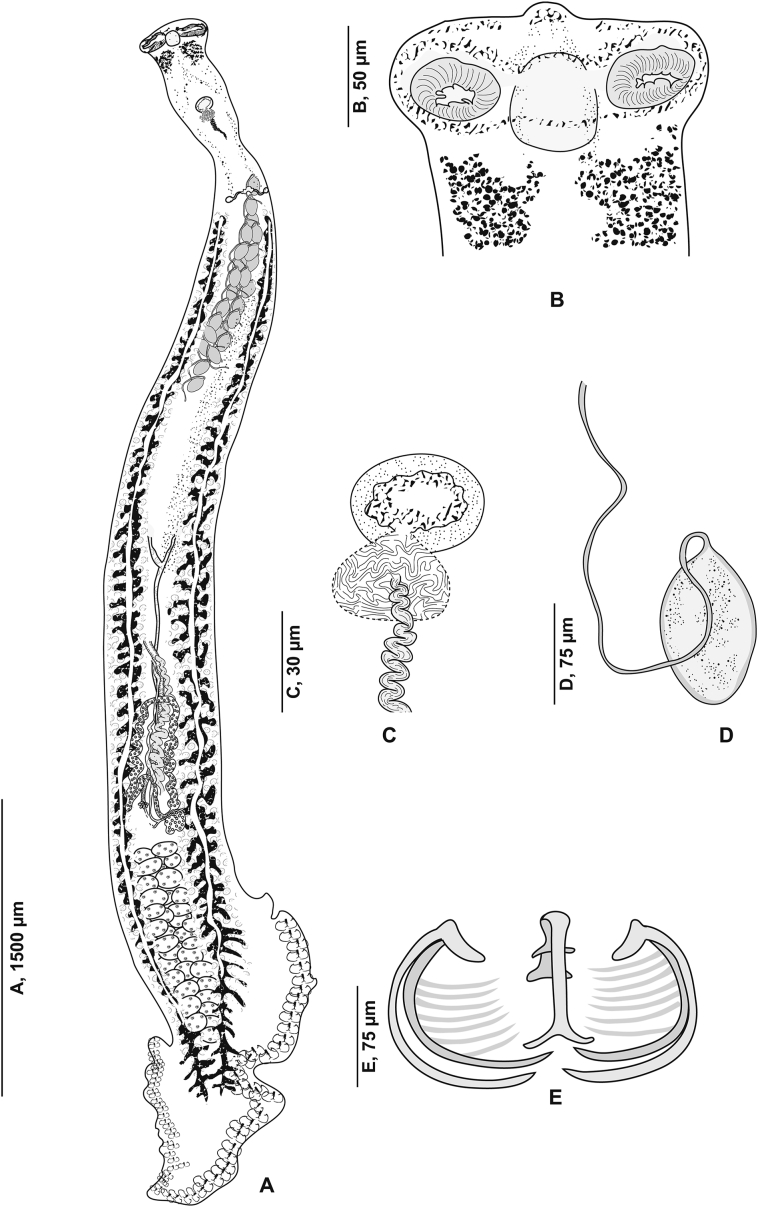
Gastrocotyle trachuri ex Trachurus mediterraneus. A Body, total view (MNHN HEL1505). B Anterior extremity showing the relative position of prohaptoral suckers, pharynx and male copulatory organ (MNHN HEL1506). C Male copulatory organ, penis invaginated (MNHN HEL1504). D Male copulatory organ, penis evaginated (MNHN HEL1506). E Atrial hook (MNHN HEL1506). F Egg (MNHN HEL1501). G Clamp, ventral view (MNHN HEL1502). H Uncini of terminal lappet (MNHN HEL1503). I, J Posterior lappet (MNHN HEL1503).
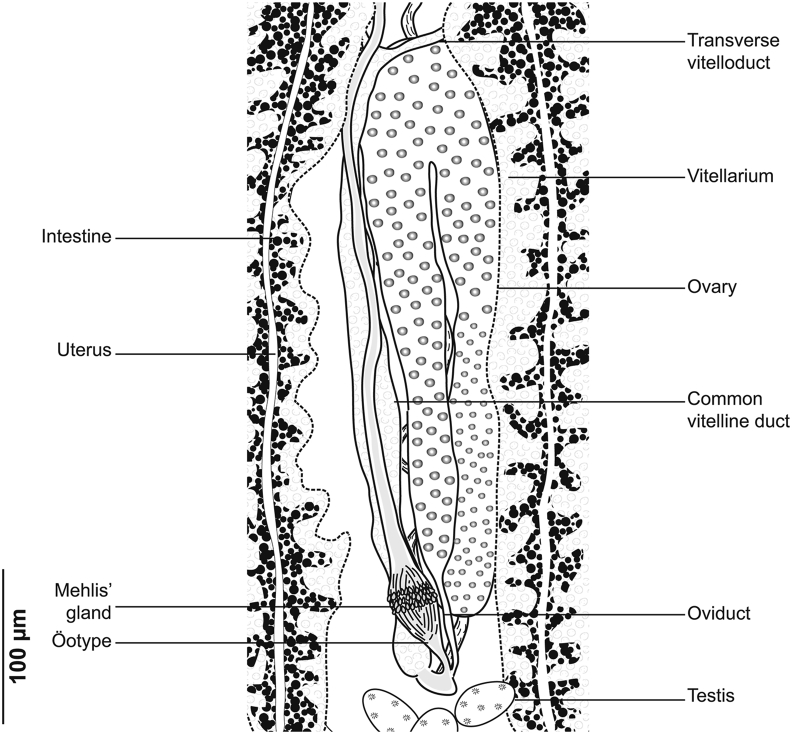
Fig. 2.
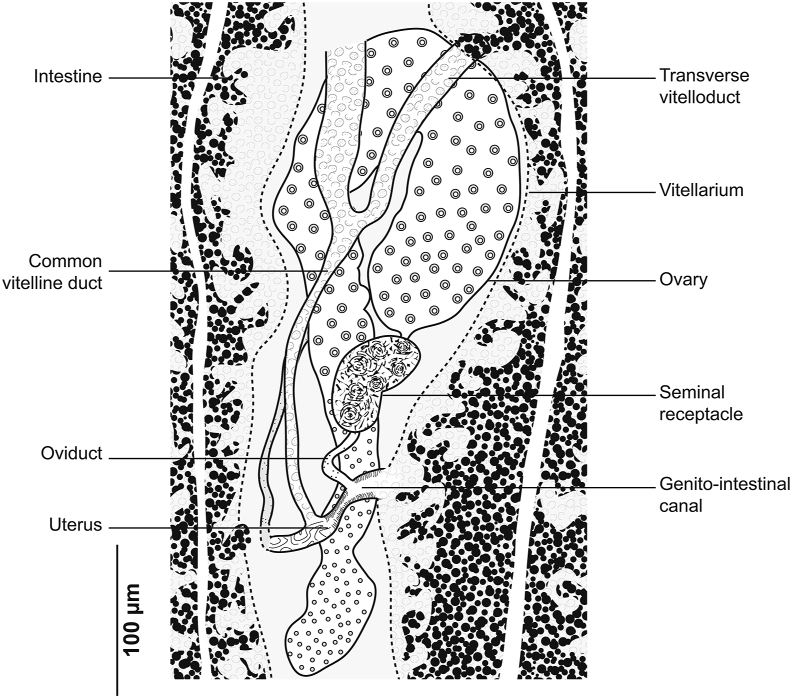
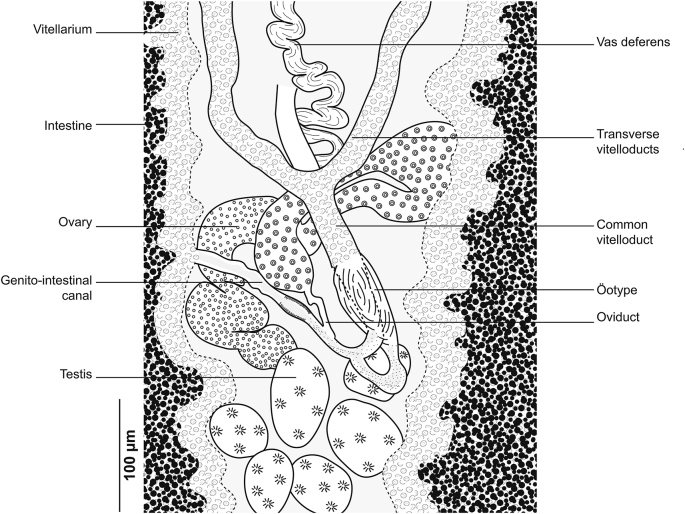
Gastrocotyle trachuri ex Trachurus mediterraneus. Detail of the reproductive organs in the region of ovary (MNHN HEL1501).
Fig. 3.
Gastrocotyle trachuri ex Trachurus mediterraneus. A Dorsal jaw. B Ventral jaw. C Clamp, dorsal view (MNHN HEL1500).
Table 1.
Measurements of Gastrocotyle trachuri van Beneden & Hesse, 1863 from various localities
| Host |
Trachurus trachurus |
T. capensis |
T. mediterraneus |
T. novaezelandiae |
Decapterus sp. |
T. trachurus |
|---|---|---|---|---|---|---|
| Locality |
SW Mediterranean, off Algeria |
SE Atlantic, off Namibia |
Central Mediterranean, off Montenegro |
SE Pacific, off Australia, the Tasman Sea |
Western Indian Ocean, off India |
NE Atlantic, off Plymouth |
| Source | Present study | Piasecki (1982) | Radujkovic & Euzet (1989) | Lebedev (1968) | Pillai (1968) | Jones (1933) |
| Body length | 2,370–3,675 (3,040; n = 13) | 2,000–3,000 | 2,690–5,030 | 2,200–3,100 | 4,700 | |
| Haptor length | 1,620–2,770 (2,140; n = 13) | |||||
| Body width | 610–1025 (775; n = 14) | 1,000 | 560–730 | 500–750 | 1,200 | |
| Clamp number | 30–40 (35; n = 16) | 33 | 25–40 | 20–24 | 22–30 | 32–40 |
| Clamp length | 60–75 (65; n = 16) | 70–100 | 50–70a | 60–80 | 45–50 | 80a |
| Clamp width | 38–58 (45; n = 14) | 55–77 | 50–60 | 60–75 | ||
| Postero-lateral hooks length | 16–20 (18; n = 15) | 25 | 10 | 17–19 | 18–20 | |
| Hamulus length | 35–52 (46; n = 9) | 58 | 50 | 42–53 | 42–45 | |
| Posterior hooks length | 20–26 (23; n = 22) | 20 | 21–24 | 22–25 | ||
| Prohaptoral sucker length | 22–33 (27; n = 10) | 23 | ||||
| Prohaptoral sucker width | 26–39 (31; n = 8) | 15 | ||||
| Pharynx length | 34–54 (43; n = 8) | 46 | ||||
| Pharynx width | 30–50 (38; n = 7) | 30 | ||||
| Distance genital atrium to anterior extremity | 185–385 (250; n = 22) | 120–250 | ||||
| Genital atrium length | 25–30 (28; n = 22) | 16a | 14–43 | 30–35 | 23a | |
| Genital atrium width | 22–33 (27; n = 22) | 10–30 | 35–35 | |||
| Number of atrial hooks | 12 (n = 26) | 12 | 12 | 16 | 12 | 12 |
| Atrial hooks length | 18–19 (18; n = 26) | 20 | 14 | 12–20 | ||
| Number of testes | 10–11 | |||||
| Egg length | 270–315 (283; n = 10) | 250 | ||||
| Egg width | 65–90 (78; n = 10) |
Abbreviations: NE, North-Eastern; SE, South-Eastern; SW, South-Western.
Diameter.
Prohaptoral suckers 2, oval, muscular, opening laterally (Fig. 1B), aseptate. Pharynx voluminous, spherical. Intestinal bifurcation posterior to genital atrium. Caeca with numerous lateral and axial diverticula.
Testes c.13 in number, small, follicular, in intercaecal field of posterior body third, often obscured by vitellarium. Vas deferens conspicuous, dorsal to uterus, running forward along body midline, expanding in its terminal part into ejaculating bulb (Fig. 1D). Male copulatory organ composed of genital atrium and stylet (Fig. 1C). Genital atrium muscular, mid-ventral, opening at short distance from anterior extremity; central conical stylet surrounded by 12 hooks arranged in circle; each hook with pointed base and curved end (Fig. 1E).
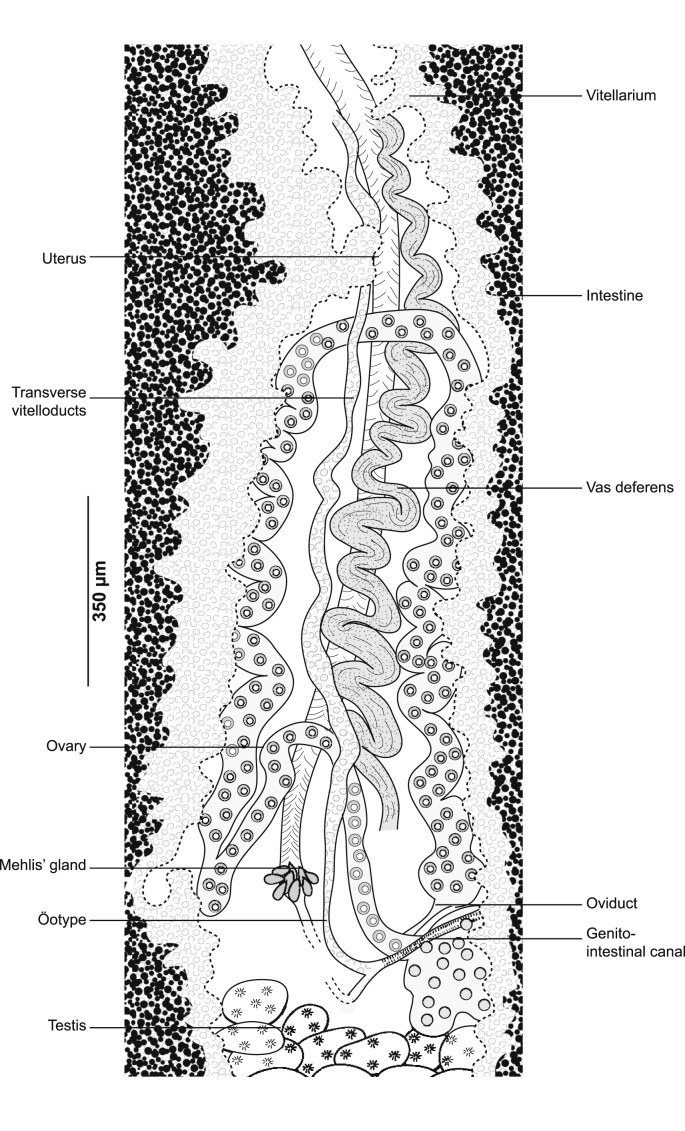
Ovary pre-testicular, longitudinally elongated, inverted U-shaped (Fig. 2). Oviduct arising from distal end of ovary and connecting common vitelline duct reservoir and oötype. Mehlis’ gland at base of oötype. Uterus originating from oötype and extending to genital atrium. Vagina not observed. Vitellarium follicular, well developed, co-extensive with caeca, extending from level of genital atrium to posterior extremity of body. Vitelloducts Y-shaped, with noticeably short branches; dorsal transverse vitelloducts fused at ovary; common vitelline duct median, fairly long. Eggs fusiform with two short polar filaments (Fig. 1F).
3.1.3. Hosts and distribution
Gastrocotyle trachuri was originally described off Brest, France (North-East Atlantic) (van Beneden & Hesse, 1863) and subsequently reported from the Atlantic, from northern and southern localities.
Currently, this gastrocotylid is widely distributed in tropical and temperate waters (see Table 2) being recorded from the North-Western Mediterranean and rarely from the South-Western Mediterranean. Records from the Central and North-Western Mediterranean are scarce and include off Italy, France, Montenegro, Spain, and Turkey. In the South-Western Mediterranean this species is known only from Tunisia and Algeria. It was frequently reported from the North Atlantic (13 records, see Table 2) and rarely from the South Atlantic (off Namibia and Angola). It occurs furthermore in North Pacific (Japan, South China Sea, East China Sea, Yellow Sea and Hawaii) and the South Pacific (Australia and Tasman Sea). In the Indian Ocean, the only records of G. trachuri are those of Lebedev and Parukhin (see Table 2). It is likely that the carangid hosts, being vastly migratory and widely distributed in Mediterranean and oceanic waters, and the monogenean being monoxenous triggered its wide dispersal.
Table 2.
Hosts and localities of Gastrocotyle trachuri van Beneden & Hesse, 1863
| Species | Locality | Reference |
|---|---|---|
| Trachurus trachurus (L.) (type-host) | NE Atlantic, off France | van Beneden & Hesse (1863) |
| NE and EC Atlantic, off the coast of Morocco to South-West Norway | MacKenzie et al. (2004, 2008) | |
| Throughout the Mediterranean | MacKenzie et al. (2004, 2008) | |
| NE Atlantic | Rego (1987); López-Román & De Armas Hernández (1989); Palm et al., (1999) | |
| NE Atlantic, Meteor Bank, off Western Sahara | Costa et al. (2012) | |
| SE Atlantic, off Namibia | Piasecki (1982) | |
| NE Atlantic, off Plymouth | Baylis & Jones (1933); Jones (1933); Sproston (1946); Llewellyn (1956, 1959, 1962, 1983); Shaw (1979); Rahemo (2012) | |
| NE Atlantic, off Portugal | Ângelo (2011) | |
| NE Atlantic, North Sea | Nicoll (1914); Naidenova & Mordvinova (1997); Campbell (2008) | |
| SE Atlantic | Gaevskaya & Kovaleva (1979) | |
| NE Atlantic, off Celtic Sea | Gaevskaya and Kovaleva, 1979, Gaevskaya and Kovaleva, 1980 | |
| NE Atlantic, off Bay of Biscay | Gaevskaya & Kovaleva (1980, 1985) | |
| NE Atlantic, off South-West Ireland | Gaevskaya & Kovaleva (1985) | |
| EC Atlantic, Strait of Gibraltar and off Western Sahara | Gaevskaya & Kovaleva (1985) | |
| SE Atlantic, off Angola | Gaevskaya & Kovaleva (1985) | |
| Mediterranean | Euzet et al. (1993); Campbell (2008) | |
| Central Mediterranean, off Italy | Parona & Perugia (1889); Palombi (1949); Orecchia & Paggi (1978); Strona et al. (2010) | |
| NW Mediterranean, off France | Lambert (1978); Jovelin & Justine (2001) | |
| SW Mediterranean, off Tunisia | Feki et al. (2016) | |
| SW Mediterranean, off Algeria | Ichalal et al. (2017); present study | |
| NW Mediterranean | MacKenzie et al., 2004, MacKenzie et al., 2008 | |
| NW Pacific, off Japan | Yamaguti (1938, 1942) | |
| NW Pacific, South China Sea | Parukhin (1976) | |
| Indian Ocean | Parukhin (1976); Reimer (1990) | |
| T. mediterraneus (Steindachner) | Mediterranean | Euzet et al. (1993) |
| Central Mediterranean, off Montenegro | Radujkovic & Euzet (1989) | |
| NW Mediterranean, off France | Mollaret et al. (2000) | |
| NE Mediterranean, off Turkey | Akmirza (2013) | |
| NW Mediterranean, off Spain | Fernandez-Jover et al. (2010) | |
| T. picturatus (Bowdich) | NE Atlantic | MacKenzie et al., 2004, MacKenzie et al., 2008; Costa et al. (2012) |
| SE Atlantic | Gaevskaya & Kovaleva (1979) | |
| NE Atlantic, off Portugal, North Sea | Gaevskaya & Kovaleva (1985) | |
| EC Atlantic, off Western Sahara | Gaevskaya & Kovaleva (1985) | |
| EC Atlantic, off Madeira | Hamdi al. (2019) | |
| SW Mediterranean, off Tunisia | Hamdi al. (2019) | |
| T. lathami Nichols | WC Atlantic, off Venezuela | Nasir & Fuentes Zambrano (1983) |
| SW Atlantic | Braicovich et al. (2012) | |
| T. novaezelandiae Richardson | SE Pacific, off Australia, Tasman Sea | Lebedev (1968) |
| T. indicus (Necrasov) | Indian Ocean | Parukhin (1976) |
| T. capensis Castelnau | SE Atlantic | Gaevskaya & Kovaleva (1979) |
| SE Atlantic, off Namibia | Gaevskaya & Kovaleva (1985) | |
| SE Atlantic, off Angola | Le Roux (2013) | |
| Trachurus trecae Cadenat | SE Atlantic | Gaevskaya & Kovaleva (1979) |
| Trachurus spp. | NE Pacific (East China Sea, Yellow Sea, South China Sea) | Zhang et al. (2003) |
| Selar crumenophtalmus Bloch | Eastern Indian Ocean, off India | Parukhin (1976) |
| Selar crumenophtalmus | EC Pacific, off Hawaii | Yamaguti (1968) |
| Decapterus sp. | Eastern Indian Ocean, off India | Parukhin (1976) |
| Decapterus russelli (Rüppell)a | SW Indian Ocean | Parukhin (1988) |
| D. maruadsi (Temminck & Schlegel) | SW Indian Ocean | Parukhin (1988) |
| Selar boops (Cuvier) | SW Indian Ocean | Parukhin (1988) |
Abbreviations: NE, North-Eastern; NW, North-Western; SE, South-Eastern; SW, South-Western; WC, Western-Central; EC, Eastern-Central.
Reported as Decapterus lajang Bleeker.
Gastrocotyle trachuri is known only from carangids. First described based on material from T. trachurus (see van Beneden & Hesse, 1863), this species has frequently been reported from seven other congeneric hosts, T. mediterraneus, T. picturatus, T. lathami, T. novaezelandiae, T. indicus, T. capensis and T. trecae, T. trachurus and other Trachurus spp. It occurs allegedly on other carangid scads, Selar crumenophtalmus and S. boops (see Parukhin, 1976, 1988), and Decapterus russelli and D. maruadsi (see Parukhin, 1988).
3.2. Cemocotyle cf. trachuri Dillon & Hargis, 1965
3.2.1. Taxonomic summary
Host: Trachurus trachurus (L.) (present study).
Locality: Off Bouharoun, Algeria, South-Western Mediterranean (present study).
Site on host: Gills.
Voucher material: 14 voucher specimens of C. cf. trachuri are deposited in the collections of the Muséum National d’Histoire Naturelle, Paris, France (MNHN HEL1545–HEL1560).
3.2.2. Description
[Based on 16 specimens; Fig. 4, Fig. 5, Fig. 6; Table 3] Body stocky, haptor considerably smaller (Fig. 4A), asymmetrical, triangular, armed with numerous clamps distributed in 2 unequal lateral rows (Fig. 4F); clamps of “muzzle” type (Fig. 4E). Ventral arm of median spring a long, enlarged in its proximal part and Y-shaped, with very short, barely visible branches. Sclerites of ventral jaw b asymmetrical; right sclerite longer than left sclerite, resulting in slight clamp asymmetry (Fig. 6A). Dorsal arm of median spring short, ending by slightly prominent T. Sclerotised piece f articulated at dorsal distal base of a. Sclerites of dorsal jaw c with the same asymmetry as anterior jaw (Fig. 6B); asymmetry induced by right sclerite longer than left sclerite c (Fig. 6C).
Fig. 4.
Cemocotyle cf. trachuri ex Trachurus mediterraneus. A Body, total view (MNHN HEL1547). B Male copulatory organ (MNHN HEL1546). C Atrial hook (MNHN HEL1546). D Anterior extremity showing the relative position of prohaptoral suckers, pharynx and male copulatory organ (MNHN HEL1546). E Clamp, ventral view (MNHN HEL1545). F, Haptor (MNHN HEL1546).
Fig. 5.
Cemocotyle cf. trachuri ex Trachurus mediterraneus. Detail of the reproductive organs in the region of ovary (MNHN HEL1547).
Fig. 6.
Cemocotyle cf. trachuri from Trachurus mediterraneus. A Ventral jaw. B Dorsal jaw. C Clamp, dorsal view (MNHN HEL1545).
Table 3.
Measurements of Cemocotyle trachuri from various localities
| Host |
T. trachurus |
T. novaezelandiae |
T. trachurus |
|---|---|---|---|
| Locality |
SW Mediterranean, off Algeria |
SW Pacific, off New Zealand |
SE Atlantic, off Namibia |
| Source | Present study | Dillon & Hargis (1965) | Piasecki (1982) |
| Body length | 1,540–3,490 (2,455; n = 14) | 2,680–3,580 (3,280) | |
| Haptor length | 340–650 (495; n = 15) | 700–790 (740) | |
| Body width | 320–850 (525; n = 16) | 400–650 (520) | |
| Clamp number | 25–34 (32; n = 13) | 28–32 (30) | 30 |
| Clamp length | 43–68 (51; n = 13) | 32–77 | |
| Clamp width | 20–46 (35; n = 13) | 33–78 | |
| Hamulus length | 29–40 (35) | 45 | |
| Posterior hook length | 22–28 (25) | ||
| Prohaptoral sucker length | 21–35 (28; n = 13) | 34–41 (37) | |
| Prohaptoral sucker width | 24–39 (31; n = 13) | 37–39 (38) | |
| Pharynx length | 20–45 (36; n = 13) | 41–48 (44) | |
| Pharynx width | 18–45 (34; n = 13) | 39–47 (42) | |
| Distance genital atrium to anterior extremity | 150–260 (220; n = 13) | ||
| Genital atrium length | 26–55 (37; n = 15) | 39–57 (48) | 33a |
| Genital atrium width | 31–52 (41; n = 15) | 44–62 (51) | |
| Long atrial spines length | 14–15 (15; n = 12) | ||
| Short atrial spines length | 8–11 (9; n = 12) | ||
| Number of testes | 11–17 | ||
| Egg length | 177–217 (199) | 260 | |
| Egg width | 72–116 (89) |
Abbreviations: SE, South-Eastern; SW, South-Western.
Diameter.
Prohaptoral suckers oval, smooth-edged, opening ventrally in anterior part of body, widely separated from each other. Pharynx muscular, subspherical. Oesophagus short. Intestinal bifurcation immediately in front of genital atrium. Caeca extend to posterior extremity of body, not confluent, do not extend into haptor.
Testes c.11 in number, follicular, post-ovarian, intercaecal; few testes obscured by vitellarium and difficult to observe and count. Vas deferens runs upwards to join ejaculatory bulb (Fig. 4D). Genital atrium armed with numerous hooks (Fig. 4C) of different sizes and shapes arranged in anterolateral part of atrium (Fig. 4B).
Ovary elongated, pretesticular, originates in mid-posterior third of body (Fig. 5), extends forward, forms a loop, and then descends to lead into oviduct. Oviduct passing dorsally to seminal receptacle and joining genito-intestinal canal. Genito-intestinal canal abutting into right intestinal branch. Uterus dorsal to ovary, extending anteriorly to genital atrium. Seminal receptacle visible, ventrally to oviduct. Vitellarium follicular, well developed, extending in 2 lateral fields from posterior level of genital atrium to haptor. Posterior extremities of vitelline fields asymmetrical; left field slightly longer. Vitelline reservoir Y-shaped; ventral transverse vitelloducts thick, fused on midline; common vitelline duct short and thick.
3.2.3. Hosts and distribution
First described off South Island, New Zealand (South-West Pacific) (Dillon & Hargis, 1965), C. trachuri was subsequently reported only from South-East Pacific off Chile and Peru (Oliva, 1999). This species has also been reported from Atlantic waters: a few records from the North-East Atlantic and a single record from the South-East Atlantic (off Namibia). In the Mediterranean, C. trachuri was reported from off France, Tunisia and Algeria (see Table 4). Note that there are no records of this species in the Indian Ocean, suggesting the presence of separated stocks of fish hosts.
Table 4.
Hosts (Trachurus spp.) and localities of Cemocotyle trachuri Dillon & Hargis, 1965
| Species | Locality | Reference |
|---|---|---|
| T. novaezelandiae Richardson (type-host) | SW Pacific, off New Zealand | Dillon & Hargis (1965) |
| T. trachurus (L.) | NE and EC Atlantic, off the coast of Morocco to south-west Norway | MacKenzie et al. (2004, 2008) |
| Throughout the Mediterranean | MacKenzie et al. (2004, 2008) | |
| SW Mediterranean, off Tunisia | Feki et al. (2016) | |
| SW Mediterranean, off Algeria | Ichalal et al. (2017); present study | |
| SE Atlantic | Gaevskaya & Kovaleva (1979) | |
| NE Atlantic, North Sea | Gaevskaya & Kovaleva (1980) | |
| EC Atlantic, Strait of Gibraltar | Gaevskaya & Kovaleva (1985) | |
| T. mediterraneus (Steindachner) | NW Mediterranean, off France | Euzet et al. (1993) |
| T. picturatus (Bowdich) | NE Atlantic, off Portugal | Costa et al. (2012) |
| EC Atlantic, off Madeira | Hamdi et al. (2019) | |
| SE Atlantic | Gaevskaa & Kovaleva (1979) | |
| NE Atlantic (off Portugal, North Sea) | Gaevskaya & Kovaleva (1985) | |
| EC Atlantic, off Western Sahara | Gaevskaya & Kovaleva (1985) | |
| SW Mediterranean, off Tunisia | Hamdi et al. (2019) | |
| T. murphyi Mann | SE Pacific, off Chile and Peru | Oliva (1999) |
| T. capensis Castelnau | SE Atlantic, off Namibia | Le Roux (2013); Piasecki (1982) |
| SE Atlantic | Gaevskaya & Kovaleva (1979) | |
| SE Atlantic, off Namibia | Gaevskaya & Kovaleva (1985) | |
| T. trecae Cadenat | SE Atlantic | Gaevskaya & Kovaleva (1979) |
| Trachurus spp. | EC and SE Atlantic, the length of the African continent to Namibia | Gaevskaya & Kovaleva (1985) |
Abbreviations: NE, North-Eastern; NW, North-Western; SE, South-Eastern; SW, South-Western; EC, Eastern-Central.
Cemocotyle trachuri is most likely a stenoxenous polyopisthocotylean, occurring only on carangids of the genus Trachurus Rafinesque (Table 4). Up to now, it was reported on seven different species: Trachurus novaezelandiae (type-host), T. trachurus, T. mediterraneus, T. picturatus, T. murphyi, T. capensis and T. trecae (see Table 4). In the present study, Cemocotyle cf. trachuri was rare and frequently observed in association with G. trachuri, which suggests a recent host switch or a potential competitive between the two species.
3.3. Zeuxapta seriolae (Meserve, 1938)
3.3.1. Taxonomic summary
Type-host: Seriola quinqueradiata Temminck & Schlegel [1, 6, 27].
Other hosts: Seriola lalandi Valenciennes (syn. Seriola dorsalis (Gill); see WoRMS, 2021) [2, 4, 8, 14, 16, 18–22, 24, 26, 27, 30, 33–38]; S. dumerili (Risso) [7, 9–11, 13, 15, 23, 29, 31, 32, 39]; Seriola hippos Günther [5]; Seriola spp. [12, 17, 25]; Caranx hippos (L.) [3, 28].
Type-locality: NW Pacific, off Japan [1].
Additional localities: Pacific [2–9, 11, 12, 14, 16–22, 24–27, 29, 30, 33–36, 38]; Atlantic [28, 37]; Mediterranean [10, 13, 15, 23, 31, 32, 39; present study].
References: [1] Ishii & Sawada (1938); [2] Meserve (1938); [3] Lamothe-Argumedo (1970); [4] Rohde (1978); [5] Rohde (1981 in Whittington & Chisholm, 2008); [6] Egusa (1983); [7] Ogawa & Fukudome (1994); [8] Rohde (1997); [9] Ogawa & Yokoyama (1998); [10] De Liberato et al. (2000); [11] Anshary & Ogawa (2001); [12] Ernst et al. (2002); [13] Grau et al. (2003); [14] Sharp et al. (2003); [15] Montero et al. (2004); [16] Sharp et al. (2004); [17] Chambers & Ernst (2005); [18] Mansell et al. (2005); [19] Tubbs et al. (2005); [20] Mooney et al. (2006); [21] Tubbs & Tingle (2006a, b); [22] Hutson et al. (2007a, b, c); [23] Lia et al. (2007); [24] Williams et al. (2007); [25] Whittington & Chisholm (2008); [26] Leef & Lee (2009); [27] Williams (2010); [28] Boada et al. (2012); [29] Lu et al. (2012); [30] Stuart & Drawbridge (2013); [31] Repullés-Albelda et al. (2013); [32] Öktener (2014); [33] Caraguel al. (2016); [34] Sepúlveda et al. (2016); [35] Fensham et al. (2018); [36] Vivanco-Aranda et al. (2019); [37] Camargo & Santos (2020); [38] Ingelbrecht et al. (2020); [39] Rigos et al. (2021).
Descriptions: [1–4; present study].
Site on host: Gills.
Voucher material: 8 voucher specimens are deposited in the collections of the Muséum National d’Histoire Naturelle, Paris, France (MNHN HEL1561–HEL1571).
3.3.2. Description
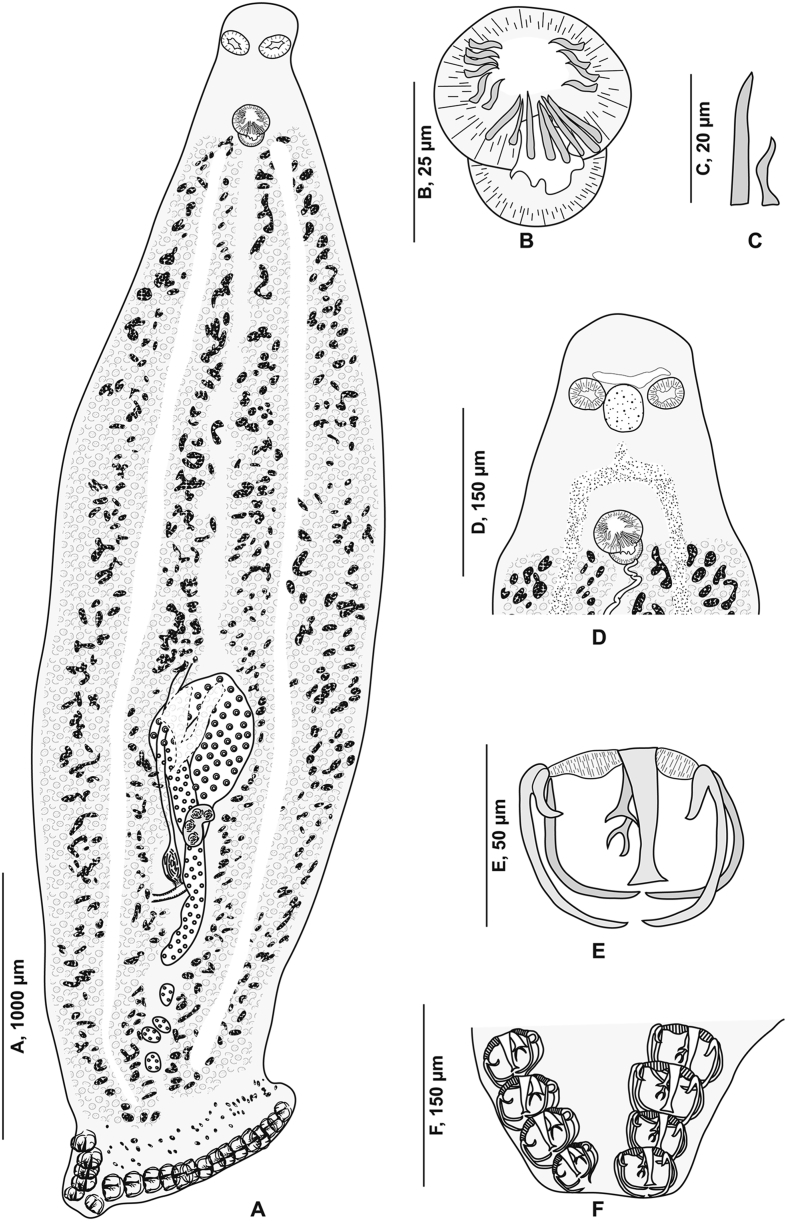
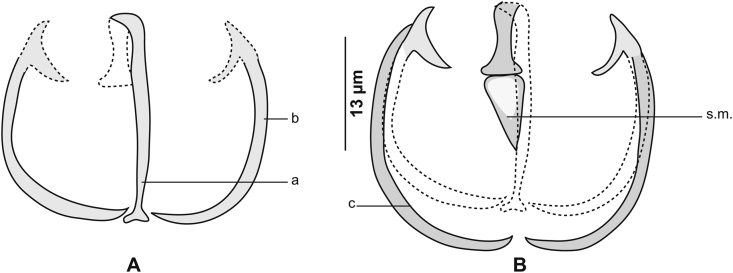
[Based on 10 specimens; Fig. 7, Fig. 8, Fig. 9, Fig. 10; Table 5] Body flat, very elongated (Fig. 7A). Haptor triangular, leaf-like and asymmetrical, armed with 2 unequal rows of clamps. Clamps of Microcotyle-type (Fig. 7E). Median spring a long, inverted Y-shaped. Sclerites b of ventral jaw slightly asymmetrical (Fig. 10A); right sclerite longer than left sclerite. Dorsal arm of median spring short, T-shaped. A small sclerite F articulated ventrally on dorsal arm of median spring (Fig. 10B). Sclerites of dorsal jaw asymmetrical (Fig. 10C).
Fig. 7.
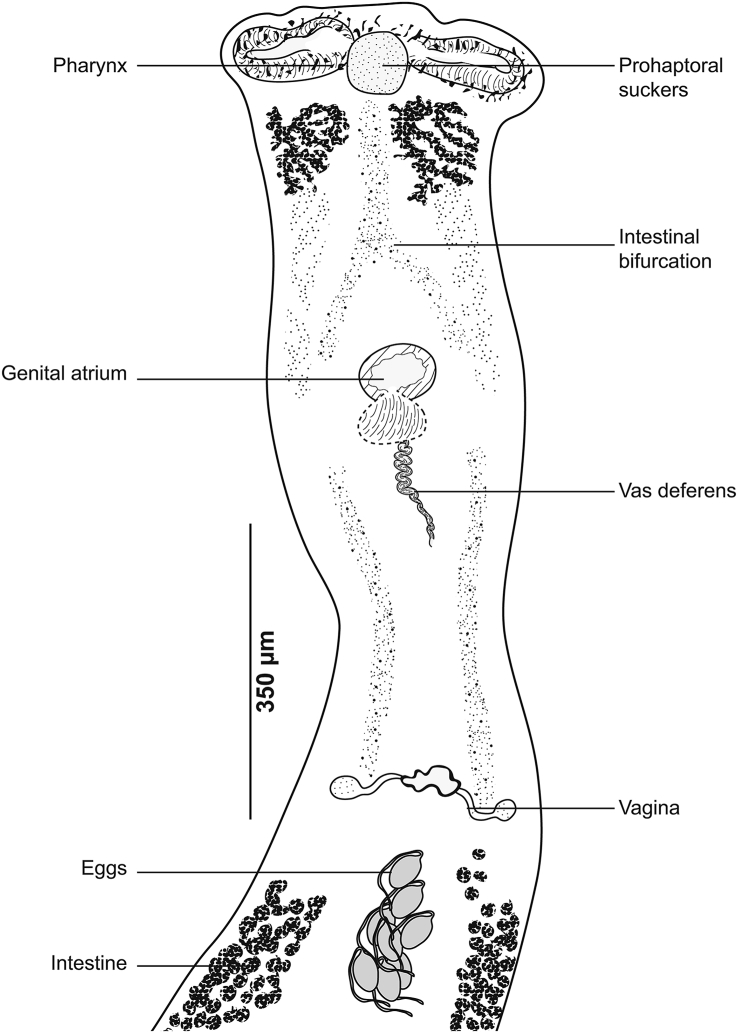
Zeuxapta seriolae ex Seriola dumerili. A Whole body (MNHN HEL1563). B Anterior extremity showing the relative position of prohaptoral suckers and pharynx (MNHN HEL1561). C Male copulatory organ (MNHN HEL1564). D Egg (MNHN HEL1564). E Clamp, ventral view (MNHN HEL1565).
Fig. 8.
Zeuxapta seriolae ex Seriola dumerili. Anterior extremity showing the relative position of prohaptoral suckers, pharynx and male copulatory organ (MNHN HEL1562).
Fig. 9.
Zeuxapta seriolae ex Seriola dumerili. Detail of the reproductive organs in the region of ovary (MNHN HEL1561).
Fig. 10.
Zeuxapta seriolae ex Seriola dumerili. A Ventral jaw. B Dorsal jaw. C Clamp, ventral view (MNHN HEL1565).
Table 5.
Measurements of Zeuxapta seriolae (Meserve, 1938) from various localities
| Host |
Seriola dumerili |
Seriola quinqueradiata |
Seriola dorsalis |
Seriola grandis |
|
|---|---|---|---|---|---|
| Locality |
SW Mediterranean, off Algeria |
NW Pacific, off Japan |
SE Pacific, off Galapagos |
Holotype |
SE Pacific, off Australia |
| Source | Present study | Ishii & Sawada (1938) | Meserve (1938) | Rohde (1978) | Rohde (1978) |
| Body length (mm) | 13.1–16.2 (14.1; n = 9) | 15–20 | 5.1–7.5 | 4.9 | 3.2–7.5 |
| Haptor length | 3,667–9,650 (4,615; n = 9) | ||||
| Body width | 870–1,550 (1,240; n = 10) | 2,000 | 1,082–1,300 | 920 | 750–1,270 |
| Clamp number on short side | 28–42 (40; n = 4) | 23–28 | 27–29 | 32 | 26–48 |
| Clamp number on long side | 30–54 (34; n = 4) | 9–10 | 38–40 | 40 | 31–55 |
| Length of clamps on short side | 115–145 (132; n = 9) | ||||
| Width of clamps on short side | 61–80 (69; n = 9) | 498–587 | 108 | 102–130a | |
| Length of clamps on long side | 145–235 (202; n = 9) | ||||
| Width of clamps on long side | 75–130 (107; n = 9) | 165 | 156–195 | ||
| Prohaptoral sucker length | 100–180 (134; n = 7) | 216–249 | 76–92 | 75–76 | 54–90 |
| Prohaptoral sucker width | 150–200 (171; n = 7) | 149–174 | 120–168 | 112–120 | 54–152 |
| Pharynx length | 50–75 (62; n = 8) | 99 | 39 | 39–47 | |
| Pharynx width | 50–75 (62; n = 8) | 66–75 | 40 | 36–43 | |
| Distance genital atrium to anterior extremity | 972–1,400 (1,155; n = 9) | 610 | 430–750 | ||
| Genital atrium length | 144–218 (172; n = 7) | ||||
| Genital atrium width | 114–207 (161; n = 8) | ||||
| Distance vagina to anterior extremity | 1,711–2,495 (2,095; n = 7) | ||||
| Number of testes | 90–130 (104; n = 4) | 93–105 | 120–140 | 95–125 | |
| Egg length | 102–145 (120; n = 10) | 149–166 | 96–136 | ||
| Egg width | 55–88 (69; n = 10) | 83–99 | 56–68 | ||
Abbreviations: SE, South-Eastern; SW, South-Western; NW, North-Western.
Diameter.
Prohaptoral suckers 2, elongated, oval and aseptate (Fig. 7B). Pharynx subspherical. Oesophagus long, diverticulated, surrounded by lateral glandular masses. Intestinal bifurcation anterior to genital atrium.
Testes numerous, c.80 in number, located in intercaecal field. Vas deferens conspicuous, running anteriorly to male copulatory organ where it swells into ejaculatory bulb (Fig. 7C). Genital atrium ventral, muscular, with muscle fibres. Genital atrium unarmed (Fig. 7C), subdivided into 2 cubicles by fine longitudinal septum. Cirrus unarmed.
Ovary large, occupying middle third of body (Fig. 9). Oviduct detaching from ovary, receiving vitelline reservoir and genito-intestinal canal. Genito-intestinal canal latter abutting into left intestinal branch. Oötype barely visible. Mehlis’ gland not observed. Uterus extending dorsally along body midline.
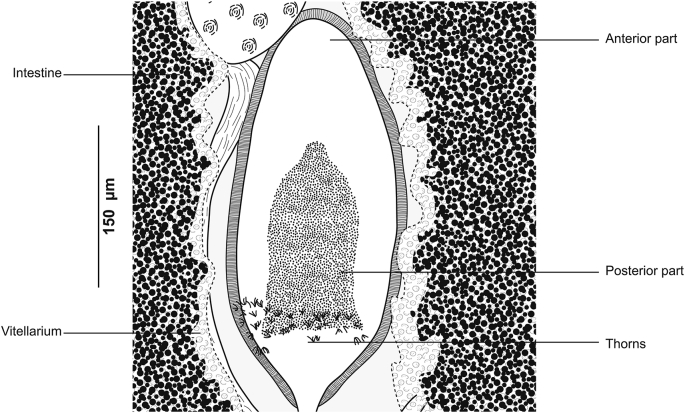
Vitellarium surrounding caeca, extending into 2 lateral fields from level of vagina to haptor. Posterior extremities of vitelline fields symmetrical, partially extending into haptor peduncle. Transverse vitelloducts not observed. Common vitelline duct exceptionally long. Vagina middorsal, at level of anterior constriction of body (Fig. 8), divided into 2 openings. Eggs subspherical, each with a long polar filament (Fig. 7D).
3.3.3. Hosts and distribution
Zeuxapta seriolae has a wide geographical distribution in both farmed and wild fish, and was reported from the Pacific and Atlantic oceans, and the Mediterranean. First described off Japan (NW Pacific), Z. seriolae was reported off Taiwan, Japan, China, California, Mexico, San Diego, Australia, Galapagos Island, Chile and New Zealand. Zeuxapta seriolae occurs also in the Atlantic (reported only off Brazil and Venezuela). From Mediterranean waters, the species was reported from off Greece, Italy, Spain and Turkey (see Table 6). This the first report of Z. seriolae off Algeria. We note that this polyopisthocotylean had never been reported in the Indian Ocean. The wide spatial distribution of this monogenean is likely linked to the host mobility (the hosts species being highly migratory and sympatric).
Table 6.
Hosts and localities of Zeuxapta seriolae (Meserve, 1938)
| Species | Locality | Reference |
|---|---|---|
| Seriola quinqueradiata Temminck & Schlegel (type-host) | NW Pacific, off Japan; SW Pacific, off Australia | Ishii & Sawada (1938); Egusa (1983); Williams (2010) |
| Seriola lalandi Valenciennesa | SE Pacific, off Galapagos | Meserve (1938) |
| SE Pacific, off Chile | Sepúlveda et al. (2016) | |
| SW Pacific, off New Zealand | Sharp et al. (2003, 2004); Mansell et al. (2005); Mooney et al. (2006); Tubbs et al. (2005); Tubbs & Tingle (2006a, b) | |
| SW Pacific, off Australia | Hutson et al. (2007a, b, c); Williams et al. (2007); Leef & Lee (2009); Williams (2010); Caraguel al. (2016); Fensham et al. (2018); Ingelbrecht et al. (2020) | |
| NE Pacific, off California | Stuart & Drawbridge (2013) | |
| EC Pacific, off Mexico | Vivanco-Aranda et al. (2019) | |
| NW Atlantic, off Brazil | Camargo & Santos (2020) | |
| NE Pacific, off San Diego | Stuart & Drawbridge (2013) | |
| Seriola lalandib | SW Pacific, off Australia | Rohde (1978, 1997) |
| Seriola dumerili Castelnau | NW Mediterranean, off Spain | Grau et al. (2003); Montero et al. (2004); Repullés-Albelda et al. (2013) |
| NE Mediterranean, off Greece | Rigos et al. (2021) | |
| NW Mediterranean, off Italy | De Liberato et al. (2000); Lia et al. (2007) | |
| Eastern Mediterranean, off Turkey | Öktener (2014) | |
| SW Mediterranean, off Algeria | Present study | |
| NW Pacific, off China | Lu et al. (2012) | |
| NW Pacific, off Japan | Anshary & Ogawa (2001) ; Ogawa & Fukudome (1994); Ogawa & Yokoyama (1998) | |
| Seriola hippos Günther | SW Pacific, off Australia | Whittington & Chisholm (2008) |
| Seriola spp. | SW Pacific, off Australia | Ernst et al. (2002); Chambers & Ernst (2005); Whittington & Chisholm (2008) |
| Caranx hippos (L.) | NW Atlantic, off Venezuela | Boada et al. (2012) |
| EC Pacific, off Mexico | Lamothe-Argumedo (1970) |
Abbreviations: NE, North-Eastern; NW, North-Western; SE, South-Eastern; SW, South-Western; EC, Eastern-Central.
Reported as S. dorsalis (Gill).
Reported as S. grandis Castelnau.
The host range of Z. seriolae is limited to two carangid genera: Seriola Cuvier and Caranx Lacépède. First described from Seriola quinqueradiata (Ishii & Sawada), this species was reported from Seriola lalandi Valenciennes (syn. S. dorsalis (Gill)), S. grandis Castelnau and S. dumerili Castelnau (see Table 6) and was also recorded on Caranx hippos (L.) (Boada et al., 2012). In the present study, this species was solely observed on its host S. dumerili and no other monogeneans were collected from this host.
3.4. Pyragraphorus hollisae Euzet & Ktari, 1970
3.4.1. Taxonomic summary
Type-host: Trachinotus ovatus L. [1; present study).
Additional hosts: Trachinotus rhodopus Gill [5]; Trachinotus blochii (Lacépède) [6]; Caranx caballus Günther [7].
Type-locality: South-Western Mediterranean, off Tunisia [1].
Additional localities: Mediterranean [2, 3; present study]; Atlantic [4]; Pacific [5, 7]; Indian Ocean [6].
References: [1] Euzet & Ktari (1970); [2] Mollaret et al. (2000); [3] Icardo-Belmonte et al. (2017); [4] Madhi & Belghyti (2006); [5] Mendoza-Garfias et al. (2017); [6] Ramadhan et al. (2019); [7] Gallegos Navarro (2020).
Descriptions: [1; present study].
Site on host: Gills.
Voucher material: 18 voucher specimens are deposited in the collections of the Muséum National d’Histoire Naturelle, Paris, France (MNHN HEL1571–HEL1584).
3.4.2. Description
[Based on 21 specimens; Fig. 11, Fig. 12, Fig. 13, Fig. 14, Fig. 15, Fig. 16; Table 7] Body elongated (Fig. 11A). Haptor asymmetrical, perpendicular to body axis, armed with 2 rows of clamps of Microcotyle-type, more or less deeply transformed, in 2 groups: stalked clamps, characteristic of the genus Pyragraphorus (“muzzle”-type) (Fig. 11A), elevated on stalks and occupying oblique part of the haptor; unstalked clamps small (Microcotyle-type) (Fig. 11A), extending to apex of haptor.
Fig. 11.
Pyragraphorus hollisae ex Trachinotus ovatus. A Whole body (MNHN HEL1572). B Anterior extremity showing the relative position of prohaptoral suckers and pharynx (MNHN HEL1573). CMicrocotyle-type clamp, ventral view (MNHN HEL1574). D “Muzzle”-type clamp, dorsal view (MNHN HEL1574).
Fig. 12.
Pyragraphorus hollisae ex Trachinotus ovatus. Detail of the reproductive organs in the region of cirrus (MNHN HEL1572).
Fig. 13.
Pyragraphorus hollisae ex Trachinotus ovatus. Detail of the reproductive organs in the region of ovary (MNHN HEL1576).
Fig. 14.
Pyragraphorus hollisae ex Trachinotus ovatus. Detail of the reproductive organs in the region of vagina (MNHN HEL1576).
Fig. 15.
Pyragraphorus hollisae ex Trachinotus ovatus. “Muzzle”-type clamp. A Ventral jaw. B Dorsal jaw (MNHN HEL1575).
Fig. 16.
Pyragraphorus hollisae ex Trachinotus ovatus. Microcotyle-type clamp. A Ventral jaw. B Dorsal jaw (MNHN HEL1576).
Table 7.
Measurements of Pyragraphorus hollisae Euzet & Ktari, 1970 ex Trachinotus ovatus from South-Western Mediterranean
| Locality |
Off Algeria |
Off Tunisia |
|---|---|---|
| Source | Present study | Euzet & Ktari (1970) |
| Body length | 2,490–4,090 (3,565; n = 18) | 2,000–2,500 |
| Haptor length | 1,265–2,735 (1,960; n = 17) | |
| Body width | 366–735 (615; n = 21) | 500–600 |
| Clamp number (anterior series) | 68–80 (76; n = 12) | 70–84 |
| Clamps number (posterior series) | 35–45 (42; n = 12) | 36–44 |
| Clamp length (anterior series) | 60–85 (82) | 65–100 |
| Clamp width (anterior series) | 24–32 (30) | 25–30 |
| Clamp length (posterior series) | 30–40 (38; n = 17) | 30–37 |
| Clamp width (posterior series) | 20–32 (27; n = 17) | 22–25 |
| Prohaptoral sucker length | 40–65 (54; n = 21) | 80a |
| Prohaptoral sucker width | 70–140 (114; n = 21) | |
| Pharynx length | 36–65 (53; n = 21) | 40a |
| Pharynx width | 35–51 (47; n = 21) | |
| Cirrus length | 218–420 (323; n = 21) | |
| Distance vagina to anterior extremity | 300–1,465 (1,236; n = 20) | 800 |
| Vagina length | 220–465 (384; n = 20) | |
| Vagina width | 65–232 (166; n = 20) | |
| Number of testes | 10–25 (22; n = 12) | 8–13 |
Diameter.
Clamps of anterior series characteristic of the genus Pyragraphorus (Fig. 11D). Each clamp formed by 2 jaws of unequal size: ventral jaw (Fig. 15A) and larger posterior jaw (Fig. 15B). Ventral arm of median spring a long, T-shaped on proximal side. Lateral sclerites b of ventral jaw asymmetrical. On proximal side, sclerites b drawing semicircle giving front half of clamps circular appearance. Sclerites b straight and parallel to each other in posterior half of clamps. Circular anterior part of clamps marked by epidermal expansions. Dorsal arm of median spring a prolonged by median sclerification f; the latter ending in a spearhead in circular part of clamps. Lateral sclerites of dorsal jaw c arranged as sclerites b.
Clamps of posterior series relatively small, of Microcotyle-type (Fig. 11C). Ventral arm of median spring long, thin, ends distally in slightly prominent T (Fig. 16A). Lateral sclerites of ventral jaw b approaching midline distally. Dorsal arm of median spring inverted T-shaped (Fig. 16B). Median sclerite in “spearhead” s.m. articulated on dorsal arm of median spring. Lateral sclerites of dorsal jaw c arched and longer than b.
Pair of oval prohaptoral suckers, muscular, oval, transversely-elongated (Fig. 11B), subdivided into 2 uneven cubicles by muscle septum. Pharynx spherical, opening ventrally on midline. Oesophagus short. Intestinal bifurcation anterior to level of genital atrium. Intestinal caeca obscured by vitellarium.
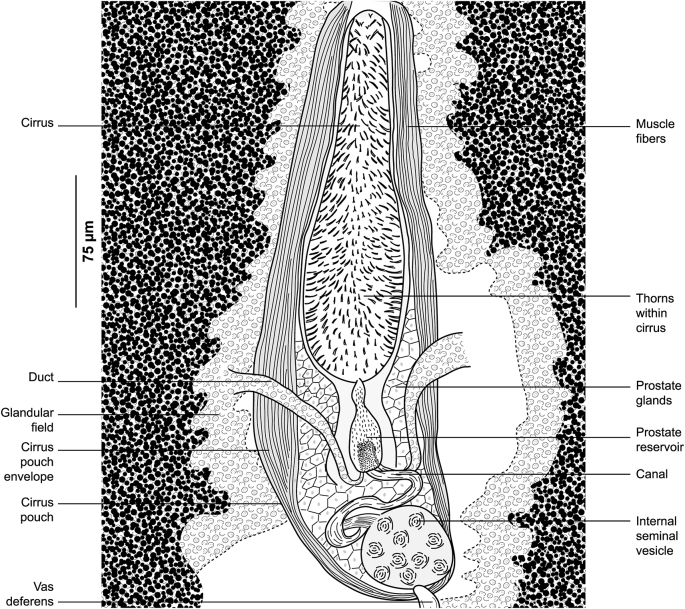
Testes c.12 in number, subcircular to oval, few and confined to post-ovarian intercaecal field, do not reach posterior junction of vitellarium. Vas deferens sinuous, arising dorsally to right of midline, extending along body midline and abutting in cirrus-pouch. Cirrus-pouch with thick wall of muscle fibres (Fig. 12), containing internal seminal vesicle, prostatic reservoir and cirrus proper. Internal seminal vesicle subspherical, connected to channel projecting forward, reaching the base of elongated and pleated chamber, corresponding to prostate reservoir. Numerous prostatic glands distributed on either side of basal third of cirrus pocket. Two ducts originating from 2 vitellarium strips, cross cirrus-pouch wall, and open at base of prostate reservoir. Prostate reservoir thins anteriorly into narrow duct, which ends in cirrus. Cirrus lateral to uterus, long, cylindrical, armed with numerous thorns (“cils” in the French nomenclature used by Euzet & Ktari (1970)).
Ovary folded, dorsal, pretesticular (Fig. 13), originates on right side, passes to left, goes up, and describes a loop to return to right where its descending branch crosses transverse branch of immature part, before throwing itself into oviduct. Genito-intestinal canal detaches from oviduct and abuts in right intestinal branch. Oviduct first receives vitelline reservoir, then forms posterior loop, which follows a swollen oötype. Mehlisʼ gland not observed. Uterus extending along body midline, dorsally, leading into genital pore. Vagina complex (Fig. 14), with smooth anterior part and posterior part forming funnel marked at its base by cilia grouped in brushes. Posteriorly, funnel continuing through narrow, short channel ending in anterior part at point of junction of transverse vitelloducts. Vitelloducts unite again at mid-length of body, at level of ovary to form ventral vitelline reservoir opening into oviduct. Vitelline follicles globular, surrounding intestinal branches.
3.4.3. Hosts and distribution
Pyragraphorus hollisae is a poorly known species. Described for the first time from off the southern Mediterranean coasts of Tunisia by Euzet & Ktari (1970), it was recorded in the Atlantic, off Morocco; and allegedly from the Pacific off Mexico and from the Indian Ocean (off Indonesia) (Table 8). In the Mediterranean, P. hollisae was also reported only off France and Spain. Algeria is a new geographical record for this monogenean.
Table 8.
Hosts and localities of Pyragraphorus hollisae Euzet & Ktari, 1970
| Species | Locality | Reference |
|---|---|---|
| Trachinotus ovatus L. (type-host) | SW Mediterranean, off Tunisia | Euzet & Ktari (1970) |
| NW Mediterranean, off France | Mollaret et al. (2000) | |
| NW Mediterranean, off Spain | Icardo-Belmonte et al. (2017) | |
| NE Eastern Central Atlantic, off Morocco | Madhi & Belghyti (2006) | |
| Trachinotus rhodopus Gill | EC Pacific, off Mexico | Mendoza-Garfias et al. (2017) |
| Trachinotus blochii (Lacépède) | Eastern Indian Ocean, off Indonesia | Ramadhan et al. (2019) |
| Caranx caballus Günther | EC Pacific, off Mexico | Gallegos Navarro (2020) |
Abbreviations: NE, North-Eastern; NW, North-Western; SW, South-Western; EC, Eastern-Central.
Pyragraphorus hollisae occurs mainly on pompanos (carangids of the genus Trachinotus) (Table 8). In addition to the type-host T. ovatus (see Euzet & Ktari, 1970), P. hollisae was reported on T. rhodopus (see Mendoza-Garfias et al., 2017), T. blochii (see Ramadhan et al., 2019) and C. caballus (see Gallegos Navarro, 2020). In the present study, P. hollisae was observed frequently in association with Gotocotyla acanthura (Parona & Perugia, 1896).
4. Discussion
To date, 42 polyopisthocotylean species have been reported from teleosts from off Algeria (Bouguerche, 2019). Unfortunately, most of these records were included in unpublished MSc and PhD theses, making them difficult to access. In addition, the monogeneans included within are often poorly and insufficiently described and these descriptions generally lack morphometric data. In the present paper, we redescribe four polyopisthocotyleans collected from the gill filaments of three carangids from off the Algerian coast, South-Western Mediterranean: Gastrocotyle trachuri (Gastrocotylidae) and Cemocotyle cf. trachuri (Heteraxinidae) from the Mediterranean horse mackerel T. mediterraneus; Zeuxapta seriolae (Heteraxinidae) from the greater amberjack S. dumerili and Pyragraphorus hollisae (Pyragraphoridae) from the pompano T. ovatus. The following sections provide accurate comparisons the morphometric variations between Mediterranean and oceanic specimens; we also briefly discuss the taxonomic status and host specificity of the four monogeneans.
4.1. Gastrocotyle trachuri van Beneden & Hesse, 1863
Among the Gastrocotylidae, the genus Gastrocotyle van Beneden & Hesse, 1863 is unique due to having only one side of the haptor developed as a marginal frill bearing a row of clamps extending along one side of the body to about halfway between the anterior margin of the ovary and the vaginal pore. Currently, Gastrocotyle includes seven species, all parasites of fishes belonging to the Carangidae Rafinesque and Scombridae Rafinesque (Table 9). The most recently described species in this genus is Gastrocotyle buckleyi Gupta & Krishna, 1980 from the malabar trevally Carangoides malabaricus (Bloch & Schneider) (syn. Caranx malabaricus) off India (Gupta & Tandon, 1980). Gastrocotyle buckleyi exhibits marked differences compared to the congeners: an elongated triangular body separated from the haptor, oblique haptor located very far from the ovary unlike in Gastrocotyle spp. in which the haptor only occupies only one side of the body and extends over more than half of its length. Gastrocotyle buckleyi should probably be removed from Gastrocotyle; however, details of the male copulatory organ of this species (unavailable in the original description) are warranted to determine its taxonomic status and we propose to consider this taxon incertae sedis pending further studies.
Table 9.
Hosts and localities of Gastrocotyle spp.
| Species | Type-host | Type-locality | Reference |
|---|---|---|---|
| Gastrocotyle trachuri van Beneden & Hesse, 1863 | Trachurus trachurus (L.) | NE Atlantic, off France | van Beneden & Hesse (1863) |
| Gastrocotyle japonica Ishii & Sawada, 1938a | Scomber japonicus Houttuyn | NW Pacific, off Japan | Ishii & Sawada (1938) |
| Gastrocotyle indica Subhapradha, 1951 | Alepes djedaba (Forsskål) | Eastern Indian Ocean, off India | Subhapradha (1951) |
| Gastrocotyle kurra Unnithan, 1968 | Decapterus russelli (Rüppell) | Eastern Indian Ocean, off India | Unnithan (1968) |
| Gastrocotyle kalla Unnithan, 1968b | Alepes djedaba (Forsskål)c | Eastern Indian Ocean, off India | Unnithan (1968) |
| Gastrocotyle mozambiquensis Lebedev & Galkina in Lebedev, 1975 | Decapterus sp. | Western Indian Ocean, off Mozambique; Eastern Indian Ocean, off India | Lebedev (1986) |
| Gastrocotyle buckleyi Gupta & Krishna, 1980 | Carangoides malabaricus (Bloch & Schneider) | Eastern Indian Ocean, off India | Gupta & Tandon (1980) |
Abbreviations: NE, North-Eastern.
Synonym of Gastrocotyle trachuri (see WoRMS, 2021).
Synonym of Gastrocotyle indica (see WoRMS, 2021).
Reported as Caranx kalla Cuvier.
Overall, the present specimens agree with the diagnosis of G. trachuri, the type-species of the genus Gastrocotyle, originally described on the Atlantic horse mackerel T. trachurus off Brest (van Beneden & Hesse, 1863). These authors provided a brief description lacking morphometric data and a poor illustration. Gastrocotyle trachuri was redescribed by Parona & Perugia (1889) and Jones (1933). The latter author pointed out that the number of hooks reported by van Beneden & Hesse (1863) is linked to the stage of development as they probably described immature specimens (Jones, 1933). We fully agree with this suggestion based on the examination of the present specimens (see Fig. 1H–J). Examination of 32 specimens of G. trachuri (Table 1) revealed that the material from off the Algerian coast agrees morphologically well with the descriptions of Jones (1933) and Radujkovic & Euzet (1989), particularly in having a terminal lappet armed with three pairs of hooks (Fig. 1H–J; also see figure 2C in Jones, 1933).
The description provided here adds several details, contributing to the diagnosis (clamp sclerites and male copulatory organ) and extends the known range for the morphometric data of G. trachuri. We provide herein for the first time, illustrated drawings of the male copulatory organ and descriptions and organisation clamp sclerites. The present specimens differ from the Atlantic specimens of G. trachuri ex T. capensis (see Piasecki, 1982) in having smaller clamps (60–75 × 38–58 vs 70–100 × 55–77 μm), smaller postero-lateral hooks (16–20 vs 25 μm), slightly reduced hamuli (35–52 vs 58 μm), and an apparently larger genital atrium (25–30 vs 16 μm).
The specimens of G. trachuri collected off Algeria differ from the Mediterranean specimens from T. mediterraneus described by Radujkovic & Euzet (1989) in having a reduced body width (610–1,025 vs 1,000 μm), larger postero-lateral hooks (16–20 vs 10 μm), and larger posterior hooks (20–26 vs 20 μm).
Unfortunately, only two measurements are available for comparison with the Atlantic specimens reported from off Portugal by Ângelo (2011) since the author provided only body length (3,000–4,000 μm) and clamp number (24–43).
The specimens of G. trachuri collected off Algeria can be distinguished from the Australian specimens (Indian Ocean) described from T. novaezelandiae by Lebedev (1968) in the smaller body length (2,370–3,675 vs 2,690–5,030 μm) and in having significantly larger clamps (60–75 × 38–58 vs 50–60 × 17–19 μm). The Australian specimens also have a reduced genital atrium width (10–30 vs 22–33 μm), while specimens from off Algeria possess fewer atrial hooks (12 vs 16) which are also longer (18–19 vs 14 μm).
The present specimens of G. trachuri can be differentiated from the Indian specimens (Indian ocean) described from Decapterus sp. by Pillai (1968) in having a greater body width (610–1,025 vs 500–750 μm), a greater number of clamps (30–40 vs 22–30) which are also larger (60–75 × 38–58 vs 45–50 × 60–75 μm), and a slightly smaller genital atrium (25–30 × 22–33 vs 30–35 × 35–35 μm).
Finally, the Mediterranean specimens of G. trachuri described here differ from the Atlantic specimens collected off Plymouth by Jones (1933) in having a shorter body (2,370–3,675 vs 4,700 μm) and a slightly shorter haptor (610–1,025 vs 1,200 μm), and in having larger prohaptoral suckers (22–33 × 26–39 vs 23 × 15 μm) and pharynx (34–54 × 30–50 vs 46 × 30 μm).
We note that only the specimens from the Indian ocean (ex T. novaezelandiae off Australia) are reported with a greater number (16) of atrial hooks (see Lebedev, 1968) whereas 12 hooks were described in all other studies (Jones, 1933; Pillai, 1968; Piasecki, 1982; Radujkovic & Euzet, 1989; Ângelo, 2011). Additionally, our careful comparison of the present morphometric data with the Mediterranean specimens described by Radujkovic & Euzet (1989) revealed a general overlap whereas both sets of Mediterranean specimens differ considerably from the oceanic specimens, from the Atlantic and Indian oceans. It is therefore possible that G. trachuri does not have a wide specificity nor geographical distribution but rather represents several G. trachuri-like species, each specific to a single host, which could not be distinguished morphologically. It is likely that future study will show that the gastrocotylid from the Mediterranean is a distinct species; this would require a detailed morphological and a molecular study of specimens from both the Mediterranean and oceanic waters.
4.2. Cemocotyle cf. trachuri Dillon & Hargis, 1965
The genus Cemocotyle Sproston, 1946 includes four species considered valid (Table 10). The type-species Cemocotyle carangis (MacCallum, 1913) was described based on material from the blue runner Caranx chrisoms (Mitchill) as Microcotyle carangis MacCallum, 1913 (see MacCallum, 1913). Based on the presence of modified clamps on one side of the haptor, Sproston (1946) erected the genus Cemocotyle to accommodate M. carangis. In the diagnosis of the genus, Sproston (1946) mentioned the presence of a terminal lappet and noted that the haptor did not contain vitellarium nor intestinal diverticula. However, the illustration of the general morphology by MacCallum (1913) clearly shows the presence of intestinal branches, vitellarium and testes penetrating the haptor.
Table 10.
Hosts and localities of Cemocotyle spp.
| Species | Reported as | Type-host | Type-locality | Reference |
|---|---|---|---|---|
| C. carangis (MacCallum, 1913) Sproston, 1946 | Microcotyle carangis MacCallum, 1913a | Caranx chrysos (Mitchill) (Carangidae) | NW Atlantic, off Massachusetts | MacCallum (1913); Sproston (1946) |
| C. borinquensis Price, 1962 | Caranx caballus Günther (Carangidae)b | WC Atlantic, off Puerto Rico | Price (1962) | |
| C. noveboracensis Price, 1962 | Axine carangis MacCallum, 1919a; Axine (Heteraxine) carangis (MacCallum, 1919) Yamaguti, 1938a,c | Caranx hippos (L.); Caranx ruber (Bloch) (Carangidae) | NW Atlantic, off New York | Price (1962) |
| C. trachuri Dillon & Hargis, 1965 | Trachurus novaezelandiae Richardson (Carangidae) | SW Pacific, off New Zealand | Dillon & Hargis (1965) |
Abbreviations: NW, North-Western; SW, South-Western; WC, Western-Central.
Junior synonym.
Reported as Paratractus caballus.
According to Yamaguti (1938); this species is placed in the genus Axine, subgenus Heteraxine (Yamaguti, 1938).
Price (1962) indicated the presence of a terminal lappet in his description of Cemocotyle borinquensis Price, 1962 from the green jack Caranx caballus Günther (syn. Paratractus caballus (Risso)) off Puerto Rico, USA. After re-examination of specimens of C. carangis from the collection of MacCallum, Price (1962) suggested Cemocotyle noveboracensis (MacCallum, 1919) Price, 1962to group specimens collected on Caranx hippos (L.) and Caranx ruber (Bloch) (see Price, 1962); this author also considered that the presence of C. carangis in Trachinotus carolinus (L.) is unusual and due to an accidental parasitism. However, we remark that a Cemocotyle sp. has already been reported on Trachinotus goodei Jordan & Evermann, another carangid host of the same genus (Luque & Cézar, 2004).
In addition, the presence of a terminal lappet in Cemocotyle spp. has been indicated in three species: C. carangis, C. borinquensis (see Price, 1962; Kritsky et al., 2011) and C. noveboracensis (see Price, 1962). However, Kritsky et al. (2011) after examining the type-specimens and 52 other specimens of C. noveboracensis from the USA, confirmed the lack of a terminal lappet. Similarly, these authors studied several specimens of C. carangis from Caranx hippos off Puerto Rico from Museum collections and confirmed the lack of a terminal lappet and attributed these specimens to C. noveboracensis (see Kritsky et al., 2011). However, these authors emphasized that C. noveboracensis differs from all congeners in the lack of a terminal lappet and the organization of the male copulatory organ.
Cemocotyle trachuri was reported on various Trachurus spp. (see Table 4). None of the previous records included descriptions, illustrations or morphometric data. These host records are probably based on insufficient evidence and unjustifiable. Although Piasecki (1982) provided a very brief description, he included no details of internal anatomy and morphology and listed only a few measurements. Hence, an illustrated redescription along with morphometric data seemed necessary considering the dubious reports of this species and the uncertainty of its occurrence on the hosts in previous records.
Specimens of Cemocotyle collected on T. trachurus off Algeria, differ from the Pacific specimens of C. trachuri from T. novaezelandiae (see Dillon & Hargis, 1965) in having a shorter haptor (340–650 vs 700–790 μm), smaller prohaptoral suckers (21–35 × 24–39 vs 34–41 × 37–39 μm), a clearly smaller pharynx (20–45 × 18–45 vs 41–48 × 39–47 μm), and a smaller genital atrium (26–55 × 31–52 vs 39–57 × 44–62 μm). Unfortunately, clamp dimensions were not given for the Pacific specimens.
The Mediterranean specimens of Cemocotyle can be distinguished from C. trachuri (reported on the same host, T. trachurus) off Namibia (Piasecki, 1982) in having slightly reduced clamp width (25–34 vs 30 μm). However, only a few measurements for the Atlantic specimens are available for comparison (Table 3).
Details of morphology and anatomy of the specimens collected from T. trachurus in the present study were carefully considered and we conclude that these specimens are similar to C. trachuri in general morphology and internal anatomy, except with regard to the terminal lappet, which is lacking in the present material. Our specimens are clearly smaller than specimens of C. trachuri from T. novaezelandiae, but differences were not obvious; they have different hosts (T. trachurus vs T. novaezelandiae) and the localities are very distant (Mediterranean vs Pacific Ocean). However, the proposal of a new species based on the absence of terminal lappet is not desirable as it would cause additional instability to the already confused composition of the genus (Kritsky et al., 2011). Hence, pending comparison of molecular sequences from both hosts and localities, we use Cemocotyle cf. trachuri to designate the heteraxinid collected from T. trachurus from the South-Western Mediterranean.
We emphasize the simultaneous occurrence of four polyopisthocotylean species, i.e. Gastrocotyle trachuri, Cemocotyle cf. trachuri, Pseudaxine trachuri and Allogastrocotyle trachuri in the same fish host specimen. The latter two species were recently described and illustrated (Bouguerche et al., 2019b, c).
4.3. Zeuxapta seriolae(Meserve, 1938)
At present, the genus Zeuxapta includes three species considered valid (Table 11): Z. kahala, Z. seriolae and Z. taylori. The first two species parasitize carangids of the genus Seriola Cuvier and the third parasitizes the scombrid Thunnus albacares (Bonnaterre). Only Z. seriolae occurs in the Mediterranean.
Table 11.
Hosts and localities of Zeuxapta spp.
| Species | Reported as | Type-host | Type locality | Reference |
|---|---|---|---|---|
| Z. seriolae (Meserve, 1938) Price, 1962 | Zeuxapta japonica Yamaguti, 1961a; Zeuxapta zyxivaginata Unnithan, 1957a | Seriola lalandi Valenciennes (Carangidae)b | SE Pacific, off Galapagos | Meserve (1938); Price (1962); Unnithan (1957) |
| Z. kahala (Yamaguti, 1968) Ogawa & Egusa, 1980 | Aspinatrium kahala (Yamaguti, 1968)a | Seriola dumerili (Risso) (Carangidae) | NE Pacific, off Hawaii | Ogawa & Egusa (1980); Yamaguti (1968) |
| Z. taylori Payne, 1990 | Thunnus albacares (Bonnaterre) (Scombridae) | NE Pacific, off California | Payne (1990) |
Abbreviations: NE, North-Eastern; SE, South-Eastern.
Junior synonym.
Reported as Seriola dorsalis.
By the possession of an asymmetric haptor, the organization of the genital atrium and the vagina, our specimens collected on S. dumerili are placed within the Heteraxinidae Unnithan, 1957 and are members of the genus Zeuxapta Unnithan, 1957 (see Mamaev, 1990; Yamaguti, 1963). By their morpho-anatomical characters, our specimens of Z. seriolae are similar to those described in different regions.
However, comparison of morphometric data of the Mediterranean specimens of Z. seriolae from S. dumerili revealed that they can be differentiated from specimens collected from S. quinqueradiata in the Pacific (off Japan; see Ishii & Sawada, 1938) in having a slightly shorter body (13.07–16.2 vs 15–20 mm) and a shorter haptor (870–1,550 vs 2,000 μm), more clamps on short and on long side, somewhat smaller prohaptoral suckers (100–180 vs 216–249 μm), and a smaller pharynx (50–75 vs 99 μm). The Pacific specimens also have larger eggs (102–145 × 55–88 vs 149–166 × 83–99 μm).
The Mediterranean specimens of Z. seriolae differ from those collected from S. dorsalis off the Galapagos (Meserve, 1938) in having a significantly greater body length (13.07–16.2 mm vs 5,110–7,540 μm), more clamps on short and on long side, and an apparently larger prohaptoral sucker (100–180 vs 76–92 μm). Dimensions of clamps, pharynx, and genital atrium are not available for specimens off Galapagos (Table 5).
The Mediterranean specimens of Z. seriolae can be distinguished from the specimens from S. grandis collected in the Pacific (off Australia; see Rohde, 1978) in having an apparently greater body length (13.07–16.20 mm vs 3,200–7,500 μm) and body width (870–1,550 vs 750–1,270 μm), larger prohaptoral suckers (100–180 × 150–200 vs 54–90 × 54–152 μm) and larger pharynx (50–75 × 50–75 vs 39–47 × 36–43 μm). Specimens from Australia have fewer clamps (Table 5) but the number of clamps (and egg size) of specimens from both localities (Algeria and Australia) show overlapping ranges. Dimensions of clamps, pharynx and genital atrium were not given for the Australian specimens. We note however, that the two populations differ in the distance between the genital atrium and anterior extremity (972–1,400 vs 430–750 μm).
In light of the available data, we could not draw any conclusion regarding differences between the Mediterranean and Pacific specimens, as specimens from off Algeria are smaller than Pacific specimens from off Japan (Ishii & Sawada, 1938) but larger than those from off Galapagos (Meserve, 1938) and off Australia (Rohde, 1978). Probably the size of Z. seriolae depends on host size and some specimens from previous studies were collected from larger host individuals, as shown for another polyopisthocotylean occurring on the same host (Price, 1962; Montero et al., 2003). Similarly, we stress the importance of including data on host size in taxonomic studies of monogeneans (Montero et al., 2003). Large variation in morphometric data of Z. seriolae was already demonstrated by Rohde (1978). In addition, Montero et al. (2003) listed an additional species of the Heteraxininae, Pseudoallencotyla pricey (Kritsky, Noble & Moser, 1978), occurring on more than one host species and from a broad geographical area (Montero et al., 2003). It is likely that the long-distance migrations of the host allowed the establishment of the monogenean Z. seriolae in different waters. Nevertheless, it would be interesting to obtain cox1 sequences of these heteraxinids from the various fish host species; this would possibly lead to detection of the presence of several cryptic species.
Algeria in a new locality record for Z. seriolae and this finding extends the geographical range of this species to the South-Western Mediterranean. As descriptions and illustrations of clamps sclerites were not included in previous descriptions, this redescription extends the knowledge of some important taxonomic features of this monogenean.
4.4. Pyragraphorus hollisae Euzet & Ktari, 1970
Currently, Pyragraphorus includes only two valid species: Pyragraphorus pyragraphorus (MacCallum & MacCallum, 1913) and P. hollisae Euzet & Ktari, 1970 (WoRMS, 2021) (Table 12). All other species previously included in this genus, i.e. Pyragraphorus incomparabilis (MacCallum, 1916), Pyragraphorus hippos Hargis, 1956 and Pyragraphorus caballeroiZerecero, 1960 are currently included in the genus Allopyragraphorus Yamaguti, 1963 (see Yamaguti, 1963). Note that all the previously mentioned species are known only from species of the Carangidae, belonging to the genera Trachinotus and Caranx (see MacCallum, 1913; 1916; Sproston, 1946; Hargis, 1956; Zerecero, 1960; Yamaguti, 1963; Euzet & Ktari, 1970).
Table 12.
Hosts and localities of Pyragraphorus spp.
| Species | Reported as | Type-host | Type-locality | Reference |
|---|---|---|---|---|
| Pyragraphorus pyragraphorus (MacCallum & MacCallum, 1913) | Microcotyle pyragraphorus MacCallum & MacCallum, 1913a | Trachinotus carolinus (L.) (Carangidae) | Probably from NW Atlantic (Sproston, 1946; Hargis, 1956) | MacCallum & MacCallum (1913); Sproston (1946) |
| Pyragraphorus hollisae Euzet & Ktari, 1970 | Trachinotus ovatus (L.) (Carangidae) | SW Mediterranean, off Tunisia | Euzet & Ktari (1970) |
Abbreviations: NW, North-Western; SW, South-Western.
Junior synonym.
Pyragraphorus spp. are characterized by having a horizontally oriented haptor with a fish-tail appearance and armed with modified clamps; the distal half bears two rows of normal clamps and the proximal half bears two rows of modified clamps (Sproston, 1946).
The present specimens agree with the diagnosis of P. hollisae, originally described from the pompano T. ovatus collected off Tunisia, South-Western Mediterranean (Euzet & Ktari 1970) and comparison of the morphometric data for the present material with the redescription of this species provided by Euzet & Ktari (1970) did reveal a few differences (Table 7). The specimens from off Algeria differ from the specimens from off Tunisia in having a greater body length (2,490–4,090 vs 2,000–2,500 μm) and a slightly wider body (366–735 vs 500–600 μm); a wider range of variation of the distance between the vagina and anterior extremity (300–1,465 vs 800 μm) and a greater upper limit for the number of testes (10–25 vs 8–13). However, these differences are subtle and should be considered to represent intraspecific variation.
Funding
The research leading to the results presented in this publication was partly carried out with infrastructure funded by the Direction Générale de la Recherche Scientifique et du Développement Technologique(DGRSDT) and by the Laboratoire de Biodiversité et Environnement: Interactions – Génomes (LBEIG), Université des Sciences et de la Technologie Houari Boumediene (USTHB), Algiers, Algeria. This study was also supported by the Institut de Systématique, Évolution, Biodiversité (ISYEB), Muséum national dʼHistoire naturelle (MNHN) Paris, France), and a framework agreement project of the DeepBlue Project: Distance Crossborder Traineeship Programme co-financed by the European Maritime and Fisheries Fund (EMFF) for the analysis, interpretation of data and the writing of the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CRediT author statement
Chahinez Bouguerche: Methodology, Writing - Original Draft, Conceptualisation, Writing, Funding acquisition. Fadila Tazerouti: Methodology, Project administration, Review. Jean-Lou Justine: Methodology, Writing - Original Draft, Conceptualisation, Writing - Review & Editing, Supervision, Project administration, Funding acquisition. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no competing interests.
Acknowledgements
Our thanks are due to fishermen from Algiers, especially Bourouba Mohamed, Boumerahe Malak and Ammi Said.
References
- Akmirza A. Monogeneans of fish near Gökçeada, Turkey. Turk. J. Zool. 2013;37:441–448. [Google Scholar]
- Amine F., Euzet L. Deux espèces nouvelles du genre Lamellodiscus Johnston & Tiegs, 1922 (Monogenea: Diplectanidae) parasites de Sparidae (Teleostei) des côtes de lʼAlgérie. Syst. Parasitol. 2005;60:187–196. doi: 10.1007/s11230-004-6346-6. [DOI] [PubMed] [Google Scholar]
- Amine F., Euzet L., Kechemir-Issad N. Description de deux nouvelles espèces de Lamellodiscus Johnston & Tiegs, 1922 (Monogenea: Diplectanidae) du groupe morphologique “ignoratus”, parasites de Diplodus sargus et D. vulgaris (Teleostei: Sparidae) Syst. Parasitol. 2006;64:37–45. doi: 10.1007/s11230-005-9016-4. [DOI] [PubMed] [Google Scholar]
- Amine F., Neifar L., Euzet L. Lamellodiscus sanfilippoi n. sp. (Monogenea, Diplectanidae) parasite branchial de Diplodus sargus (Teleostei, Sparidae) en Méditerranée. Parasite. 2006;13:45–49. doi: 10.1051/parasite/2006131045. [DOI] [PubMed] [Google Scholar]
- Amine F., Euzet L., Kechemir-Issad N. Description de Lamellodiscus confusus n. sp. (Monogenea: Diplectanidae), parasite de Sarpa salpa (Teleostei: Sparidae) Parasite. 2007;14:281–285. doi: 10.1051/parasite/2007144281. [DOI] [PubMed] [Google Scholar]
- Amine F., Euzet L., Kechemir-Issad N. Lamellodiscus theroni sp. nov. (Monogenea, Diplectanidae), a gill parasite from Diplodus puntazzo (Teleostei, Sparidae) from the Mediterranean Sea. Acta Parasitol. 2007;52:305–309. doi: 10.2478/s11686-007-0052-x. [DOI] [Google Scholar]
- Ângelo Â.C.F.P. The School of Agriculture of Coimbra; Coimbra, Portugal: 2011. Contribution to the parasitic study of wild fish from the Atlantic coast: the case of mackerel and horse mackerel. (Thesis) [Google Scholar]
- Anshary H., Ogawa K. Microhabitats and mode of attachment of Neoheterobothrium hirame, a monogenean parasite of Japanese flounder. Fish Pathol. 2001;36:21–26. [Google Scholar]
- Ayadi Z.E.M., Gey D., Justine J.-L., Tazerouti F. A new species of Microcotyle (Monogenea: Microcotylidae) from Scorpaena notata (Teleostei: Scorpaenidae) in the Mediterranean Sea. Parasitol. Int. 2017;66:37–42. doi: 10.1016/j.parint.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Azizi R., Bouguerche C., Santoro M., Gey D., Tazerouti F., Justine J.-L., Bahri S. Redescription and molecular characterization of two species of Pauciconfibula (Monogenea, Microcotylidae) from trachinid fishes in the Mediterranean Sea. Parasitol. Res. 2021 doi: 10.1007/s00436-021-07097-9. (in press) [DOI] [PubMed] [Google Scholar]
- Baylis H., Jones E.I. Some records of parasitic worms from marine fishes at Plymouth. J. Mar. Biol. Assoc. U. K. 1933;18:627–634. [Google Scholar]
- Benhamou F., Marzoug D., Boutiba Z., Kostadinova A., Pérez-del-Olmo A. Parasite communities in two sparid fishes from the western Mediterranean: a comparative analysis based on samples from three localities off the Algerian coast. Helminthologia. 2017;54:26–35. doi: 10.1515/helm-2017-0003. [DOI] [Google Scholar]
- Boada M., Bashirullah A., Marcano J., Alió J., Vizcaíno G. Estructura comunitaria de ectoparásitos en branquias del jurel Caranx hippos (Linnaeus, 1776) en Santa Cruz y Carúpano, estado Sucre, Venezuela. Rev. Científica (Maracaibo) 2012;22:259–272. [Google Scholar]
- Bouguerche C. Université des Sciences et Technologie Houari Boumedienne (USTHB); Algiers, Algeria: 2019. Étude taxinomique des Polyopisthocotylea Odhner, 1912 (Monogenea, Plathelminthes) parasites de quelques Téléostéens de la côte algérienne. (Thesis) [Google Scholar]
- Bouguerche C., Gey D., Justine J.-L., Tazerouti F. Microcotyle visa n. sp. (Monogenea: Microcotylidae), a gill parasite of Pagrus caeruleostictus (Valenciennes) (Teleostei: Sparidae) off the Algerian coast, Western Mediterranean. Syst. Parasitol. 2019;96:131–147. doi: 10.1007/s11230-019-09842-2. [DOI] [PubMed] [Google Scholar]
- Bouguerche C., Gey D., Justine J.-L., Tazerouti F. Towards the resolution of the Microcotyle erythrini species complex: description of Microcotyle isyebi n. sp. (Monogenea, Microcotylidae) from Boops boops (Teleostei, Sparidae) off the Algerian coast. Parasitol. Res. 2019;118:1417–1428. doi: 10.1007/s00436-019-06293-y. [DOI] [PubMed] [Google Scholar]
- Bouguerche C., Tazerouti F., Gey D., Justine J.-L. Redescription and molecular characterisation of Allogastrocotyle bivaginalis Nasir & Fuentes Zambrano, 1983 (Monogenea: Gastrocotylidae) from Trachurus picturatus (Bowdich) (Perciformes: Carangidae) off the Algerian coast, Mediterranean Sea. Syst. Parasitol. 2019;96:681–694. doi: 10.1007/s11230-019-09883-7. [DOI] [PubMed] [Google Scholar]
- Bouguerche C., Justine J.-L., Tazerouti F. Redescription of Flexophora ophidii Prost & Euzet, 1962 (Monogenea: Diclidophoridae) from Ophidion barbatum (Ophidiidae) off the Algerian coast, Mediterranean Sea. Syst. Parasitol. 2020;97:827–833. doi: 10.1007/s11230-020-09948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouguerche C., Tazerouti F., Gey D., Justine J.-L. No vagina, one vagina, or multiple vaginae? An integrative study of Pseudaxine trachuri (Monogenea, Gastrocotylidae) leads to a better understanding of the systematics of Pseudaxine and related genera. Parasite. 2020;27:50. doi: 10.1051/parasite/2020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahim Tazi A.N., Meddour A., Nadjadi Z., Boutiba Z. First records of helminth parasites of Dicentrarchus labrax in the Western Coast of Algeria. J. Appl. Environ. Biol. Sci. 2016;6:46–51. [Google Scholar]
- Braicovich P.E., Luque J.L., Timi J.T. Geographical patterns of parasite infracommunities in the rough scad, Trachurus lathami Nichols, in the southwestern Atlantic Ocean. J. Parasitol. 2012;98:768–777. doi: 10.1645/GE-2950.1. [DOI] [PubMed] [Google Scholar]
- Camargo A., Santos C. Morphological and molecular analyses of Pseudomazocraes sulamericana n. sp., Pseudomazocraes selene Hargis, 1957, Cemocotyle carangis (MacCallum, 1913) and Zeuxapta seriolae (Meserve, 1938) (Monogenea: Mazocraeidea) from carangid fishes in the south-western Atlantic Ocean. J. Helminthol. 2020;94:E28. doi: 10.1017/S0022149X18000949. [DOI] [PubMed] [Google Scholar]
- Campbell N. University of Aberdeen; Aberdeen, Scotland: 2008. Population studies of horse mackerel Trachurus trachurus (L.) and herring Clupea harengus L. using parasites as biological tags. (Thesis) [Google Scholar]
- Caraguel C., Fensham J., Landos M., Bubner E., DʼAntignana T. The direction of sampling bias when hooking fish from a sea cage: case study of fluke monitoring in Australian farmed yellowtail kingfish (Seriola lalandi) Front. Vet. Sci. Conference Abstract: AquaEpi I. 2016:2016. doi: 10.3389/conf.FVETS.2016.02.00041. [DOI] [Google Scholar]
- Chaabane A., Neifar L., Justine J.-L. Pseudorhabdosynochus regius n. sp. (Monogenea, Diplectanidae) from the mottled grouper Mycteroperca rubra (Teleostei) in the Mediterranean Sea and Eastern Atlantic. Parasite. 2015;22:9. doi: 10.1051/parasite/2015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaabane A., Justine J.-L., Gey D., Bakenhaster M.D., Neifar L. Pseudorhabdosynochus sulamericanus (Monogenea, Diplectanidae), a parasite of deep-sea groupers (Serranidae) occurs transatlantically on three congeneric hosts (Hyporthodus spp.), one from the Mediterranean Sea and two from the western Atlantic. PeerJ. 2016;4 doi: 10.7717/Peerj.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaabane A., Neifar L., Gey D., Justine J.-L. Species of Pseudorhabdosynochus (Monogenea, Diplectanidae) from groupers (Mycteroperca spp., Epinephelidae) in the Mediterranean and Eastern Atlantic Ocean, with special reference to the “beverleyburtonae group” and description of two new species. PloS One. 2016;11 doi: 10.1371/journal.pone.0159886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaabane A., Neifar L., Justine J.-L. Diplectanids from Mycteroperca spp. (Epinephelidae) in the Mediterranean Sea: Redescriptions of six species from material collected off Tunisia and Libya, proposal for the 'Pseudorhabdosynochus riouxi group’, and a taxonomic key. PloS One. 2017;12 doi: 10.1371/journal.pone.0171392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.B., Ernst I. Dispersal of the skin fluke Benedenia seriolae (Monogenea: Capsalidae) by tidal currents and implications for sea-cage farming of Seriola spp. Aquaculture. 2005;250:60–69. [Google Scholar]
- Costa G., Melo-Moreira E., de Carvalho M.P. Helminth parasites of the oceanic horse mackerel Trachurus picturatus Bowdich, 1825 (Pisces: Carangidae) from Madeira Island, Atlantic Ocean, Portugal. J. Helminthol. 2012;86:368–372. doi: 10.1017/S0022149X11000502. [DOI] [PubMed] [Google Scholar]
- De Liberato C., Di Cave D., Berrilli F., Orecchia P. Fish parasites and related problems in Italian mariculture facilities. Parassitologia. 2000;42:166. [Google Scholar]
- Dillon W.A., Hargis W.J. In: Llano G.A., editor. vol. 5. Antarctic Research Series. John Wiley & Sons, Inc.; Hoboken, USA: 1965. Monogenetic trematodes from the southern Pacific Ocean. 2. Polyopisthocotyleids from New Zealand fishes: the families Discocotylidae, Microcotylidae, Axinidae and Gastrocotylidae; pp. 251–280. (Biology of the Antarctic Seas II). [Google Scholar]
- Egusa S. Disease problems in Japanese yellowtail, Seriola quinqueradiata, culture: a review. Rapp. P-V. Reun-Cons. Int. Explor. Mer. 1983;182:10–18. [Google Scholar]
- Ernst I., Whittington I., Corneillie S., Talbot C. Monogenean parasites in sea-cage aquaculture. Austasia Aquacult. 2002;16:46–48. [Google Scholar]
- Euzet L. Recherches sur les Monogenoidea parasites de Poissons marins. Ann. Parasitol. Hum. Comp. 1957;32:469–481. [PubMed] [Google Scholar]
- Euzet L., Audouin J. Sur un genre nouveau de Monogenoidea parasite de la dorade Chrysophrys aurata. L. Rev. Trav. Inst. Pêches. Marit. 1959;23:317–322. [Google Scholar]
- Euzet L., Ktari M. Pyragraphorus hollisae n. sp. (Monogenea) parasite of Lichia glauca (L., 1758) (Carangidae) in the Mediterranean. Inst. Biol. Univ. Nac. Autón. Méx., Ser. Zool. 1970;41:61–67. [Google Scholar]
- Euzet L., Ktari M. Aspinatrium gallieni n. sp. (Monogenea, Polyopisthocotylea) parasite de Strongylura acus Lacépède, 1803 en Mediterranée. Bull. Soc. Zool. Fr. Evol. Zool. 1971;96:509–517. [Google Scholar]
- Euzet L., Razarihelisoa M. Sur quelques monogènes de Sphyraena commersonii (Teleostei: Sphyraenidae) Bull. Soc. Zool. Fr. 1959;84:77–85. [Google Scholar]
- Euzet L., Suriano D.M. Plectanocotyloides obscurum n. g., n. sp. (Monogenea) parasite branchial d’Aspitrigla obscura (Téléostéen) en Méditerranée. Bull. Mus. Natl. Hist. Nat, 3e série, Zoologie. 1973;137:807–813. [Google Scholar]
- Euzet L., Trilles J.-P. Sur l’anatomie et la biologie de Cyclocotyla bellones (Otto, 1821) (Monogenea-Polyopisthocotylea) Rev. Suisse Zool. 1961;68:182–193. [Google Scholar]
- Euzet L., Combes C., Caro C.A. Second International Symposium on Monogenea, Montpellier/Sète. 1993. Check list of Monogenea of Mediterranean fish. [Google Scholar]
- Feki M., Châari M., Neifar L., Boudaya L. Spatial variability of helminth parasites to recognize the discrimination of juvenile and young adult areas of horse mackerel, Trachurus trachurus (Linnaeus, 1758) off the coast of Tunisia. Fish. Res. 2016;183:318–325. [Google Scholar]
- Fensham J., Bubner E., DʼAntignana T., Landos M., Caraguel C.G. Random and systematic sampling error when hooking fish to monitor skin fluke (Benedenia seriolae) and gill fluke (Zeuxapta seriolae) burden in Australian farmed yellowtail kingfish (Seriola lalandi) Prev. Vet. Med. 2018;153:7–14. doi: 10.1016/j.prevetmed.2018.02.012. [DOI] [PubMed] [Google Scholar]
- Fernandez-Jover D., Faliex E., Sanchez-Jerez P., Sasal P., Bayle-Sempere J.T. Coastal fish farming does not affect the total parasite communities of wild fish in SW Mediterranean. Aquaculture. 2010;300:10–16. [Google Scholar]
- Fischer W., Bauchot M.-L., Schneider M. vol. 2. FAO; Rome: 1987. pp. 761–1530. (Fiches FAO d’identification des espèces pour les besoins de la pêche. (Révision 1). Méditerranée et mer Noire. Zone de pêche 37. Volume II. Vertébrés. Publication préparée par la FAO, résultat d’un accord entre la FAO et la Commission des Communautés Européennes (Projet GCP/INT/422/EEC) financée conjointement par ces deux organisations). [Google Scholar]
- Froese R., Pauly D., editors. FishBase. World Wide Web electronic publication; 2016. www.fishbase.org [Google Scholar]
- Gaevskaya A.V., Kovaleva A.A. Monogenean parasites of Atlantic horsemackerels of the genus Trachurus. Zool. Zh. 1979;58:1110–1116. (In Russian) [Google Scholar]
- Gaevskaya A.V., Kovaleva A.A. Reasons for the similarities and differences between the parasite faunas of two subspecies of Trachurus trachurus in the Atlantic Ocean. Biol. Nauki (Moscow) 1980;198:52–56. [Google Scholar]
- Gaevskaya A.V., Kovaleva A.A. The parasite fauna of the oceanic horse mackerel Trachurus picturatus picturatus and eco-geographical characteristics of its formation. Ehkol. Morya. 1985;20:80–84. (In Russian) [Google Scholar]
- Gallegos Navarro Y. Universidad Autónoma de Guerrero; Guerrero, Mexico: 2020. Influencia de los factores ambientales en la estructuración de comunidades de parásitos y su utilidad como biomarcadores poblacionales, de 4 especies de carángidos. (Thesis) [Google Scholar]
- Grau A., Crespo S., Pastor E., Gonzalez P., Carbonell E. High infection by Zeuxapta seriolae (Monogenea: Heteraxinidae) associated with mass mortalities of amberjack Seriola dumerili Risso reared in sea cages in the Balearic Islands (Western Mediterranean) Bull. Eur. Assoc. Fish Pathol. 2003;23:139–142. [Google Scholar]
- Gupta S., Tandon V. On some digenetic trematodes from marine fishes of Puri, Orissa. Indian J. Helminthol. 1980;35:112–136. [Google Scholar]
- Hadjou Z., Ramdane Z., Tazi N., Bellal A., Charane M. Effect of parasitism on the length/weight relationship and the condition index in two groups of Pagellus acarne (Risso, 1826) (Perciformes Sparidae), parasitized and unparasitized specimens, from the eastern Coast of Algeria. Biodiversity J. 2017;8:889–894. [Google Scholar]
- Hamdi I., Hermida M., Tlig S.Z., Benmansour B. Distribution of two monogenean of Trachurus picturatus from Tunisia, Mediterranean Sea and Madeira Island, Atlantic Ocean. Paper presented at the Rapp. Comm. Int. Mer. Médit. 2019;42:23. http://ciesm.org/online/archives/abstracts/pdf/42/CIESM_Congress_2019_Cascais_article_0023.pdf [Google Scholar]
- Hargis W.J. Monogenetic trematodes of Gulf of Mexico fishes. Part X. The family Microcotylidae Taschenberg, 1879. Trans. Am. Microsc. Soc. 1956;75:436–453. [Google Scholar]
- Hutson K.S., Ernst I., Mooney A.J., Whittington I.D. Metazoan parasite assemblages of wild Seriola lalandi (Carangidae) from eastern and southern Australia. Parasitol. Int. 2007;56:95–105. doi: 10.1016/j.parint.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Hutson K.S., Ernst I., Whittington I.D. Risk assessment for metazoan parasites of yellowtail kingfish Seriola lalandi (Perciformes: Carangidae) in South Australian sea-cage aquaculture. Aquaculture. 2007;271:85–99. doi: 10.1016/j.aquaculture.2007.03.020. [DOI] [Google Scholar]
- Hutson K.S., Smith B.P., Godfrey R.T., Whittington I.D., Chambers C.B., Ernst I., Gillanders B.M. A tagging study on yellowtail kingfish (Seriola lalandi) and Samson fish (S. hippos) in south Australian waters. Trans. Proc. Roy. Soc. S. Aust. 2007;131:128–134. [Google Scholar]
- Icardo-Belmonte S., Taura-Pardo C., Montero F.E., Ahuir-Baraja A.E. 8th International Symposium on Monogenea. 6–11 August 2017. Masaryk University, Faculty of Science; Brno, Czech Republic: 2017. Pyragraphorus hollisae Euzet & Ktari, 1970 infecting pompano, Trachinotus ovatus (L.) in captivity. [Google Scholar]
- Ichalal K., Chikoune A., Ramdane Z., Iguer-Ouada M., Kacher M. The parasite fauna of Trachurus trachurus (Linnaeus, 1758) (Teleostei: Carangidae) from the eastern coast of Algeria. Bull. Soc. Zool. Fr. 2017;142:29–45. [Google Scholar]
- Ider D., Ramdane Z., Trilles J.-P., Amara R. Metazoan parasites of Boops boops (Linnaeus, 1758) from the Algerian coast. Cah. Biol. Mar. 2018;59:225–233. [Google Scholar]
- Ingelbrecht J., Miller T.L., Lymbery A.J., Maita M., Torikai S., Partridge G. Anthelmintic herbal extracts as potential prophylactics or treatments for monogenean infections in cultured yellowtail kingfish (Seriola lalandi) Aquaculture. 2020;520:734776. [Google Scholar]
- Ishii N., Sawada T. Livro jubilar do Professor Lauro Travassos. Rio de Janeiro. 1938. Studies on the ectoparasitic trematodes; pp. 231–243. [DOI] [Google Scholar]
- Jones E.I. Studies on the Monogenea of Plymouth. Gastrocotyle trachuri v. Ben. and Hesse, 1863. J. Mar. Biol. Assoc. U. K. 1933;19:227–232. [Google Scholar]
- Jovelin R., Justine J.-L. Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) inferred from partial 28S rDNA sequences. Int. J. Parasitol. 2001;31:393–401. doi: 10.1016/S0020-7519(01)00114-X. [DOI] [PubMed] [Google Scholar]
- Kaouachi N., Boualleg C., Bensouilah M., Marchand B. Monogenean parasites in sparid fish (Pagellus genus) in eastern Algeria coastline. Afr. J. Microbiol. Res. 2010;4:989–993. [Google Scholar]
- Kechemir N. Évolution ultrastructurale du tégument dʼHalipegus ovocaudatus Vulpian, 1858 au cours de son cycle biologique. Z. Parasitenkd. 1978;57:17–33. doi: 10.1007/BF00927626. [DOI] [Google Scholar]
- Kheddam H., Justine J.-L., Tazerouti F. Hexabothriid monogeneans from the gills of deep-sea sharks off Algeria, with the description of Squalonchocotyle euzeti n. sp. (Hexabothriidae) from the kitefin shark Dalatias licha (Euselachii, Dalatiidae) Helminthologia. 2016;53:354–362. doi: 10.1515/helmin-2016-0034. [DOI] [Google Scholar]
- Kheddam H., Chisholm L.A., Tazerouti F. Septitrema lichae n. g., n. sp. (Monogenea: Monocotylidae) from the nasal tissues of the deep-sea kitefin shark, Dalatias licha (Bonnaterre) (Squaliformes: Dalatiidae), off Algeria. Syst. Parasitol. 2020;97:553–559. doi: 10.1007/s11230-020-09942-4. [DOI] [PubMed] [Google Scholar]
- Kouider El Ouahed-Amine F. Université des Sciences et Technologie Houari Boumedienne (USTHB); Algiers, Algeria: 1998. Contribution à lʼétude des Monogènes parasites des poissons Sparidae (Téléostéens) du littoral Algérois. Approche taxonomique et écologique. (Thesis) [Google Scholar]
- Kritsky D.C., McAleese W.J., Bakenhaster M.D. Heteronchoineans (Monogenoidea) from the gills of crevalle jack, Caranx hippos (Perciformes, Carangidae), from Everglades National Park, Florida, with a redescription of Protomicrocotyle mirabilis (Gastrocotylinea, Protomicrocotylidae) Comp. Parasitol. 2011;78:265–274. doi: 10.1654/4505.1. [DOI] [Google Scholar]
- Lambert A. Recherches sur les stades larvaires des Monogènes de Poissons. Ann. Parasitol. Hum. Comp. 1978;53:551–559. doi: 10.1051/parasite/1978536551. [DOI] [PubMed] [Google Scholar]
- Lamothe-Argumedo R. Monogéneos de peces II. Reporte de tres especies de Monogenea parásitas de las branquias de Caranx hippos del Pacífico mexicano y redescripción de Zeuxapta seriolae (Meserve, 1938) Price, 1962. Rev. Biol. Trop. 1970;16:153–169. [Google Scholar]
- Le Roux J.L. University of Cape Town; South Africa: 2013. Parasite Assemblages of Cape horse mackerel (Trachurus capensis Castelnau, 1861) from the northern and southern Benguela.https://open.uct.ac.za/bitstream/handle/11427/6673/thesis_sci_le_roux_j.pdf?sequence=1&isAllowed=y (MSc Thesis) [Google Scholar]
- Lebedev B.I. In: Helminths of the Pacific Ocean. Skrjabin K.I., Mamaev Y.L., editors. Nauka; Moscow: 1968. Monogenea from fishes of the New Zealand-Australian shelf and the south China Sea (Monogenoidea: Gastrocotylidae, Gastrocotylinae) (In Russian) [Google Scholar]
- Lebedev B.I. Nauka; Moscow: 1986. Monogenea: Suborder Gastrocotylinea. (In Russian) [Google Scholar]
- Leef M.J., Lee P.S. Preliminary investigation into the killing effect of kingfish (Seriola lalandi) serum and mucus against the monogenean parasites Benedenia seriolae and Zeuxapta seriolae. Aquacult. Int. 2009;17:607. [Google Scholar]
- Lia R., Zizzo N., Tinelli A., Lionetti A., Cantacessi C., Otranto D. Mass mortality in wild greater amberjack (Seriola dumerili) infected by Zeuxapta seriolae (Monogenea: Heteraxinidae) in the Ionian Sea. Bull. Eur. Assoc. Fish Pathol. 2007;27:108. [Google Scholar]
- Llewellyn J. The host-specificity, micro-ecology, adhesive attitudes, and comparative morphology of some trematode gill parasites. J. Mar. Biol. Assoc. U. K. 1956;35:113–127. [Google Scholar]
- Llewellyn J. The larval development of two species of gastrocotylid trematode parasites from the gills of Trachurus trachurus. J. Mar. Biol. Assoc. U. K. 1959;38:461–467. [Google Scholar]
- Llewellyn J. The life histories and population dynamics of monogenean gill parasites of Trachurus trachurus (L.) J. Mar. Biol. Assoc. U. K. 1962;42:587–600. [Google Scholar]
- Llewellyn J. Sperm transfer in the monogenean gill parasite Gastrocotyle trachuri. Proc. R. Soc. Lond. B Biol. Sci. 1983;219:439–446. [Google Scholar]
- López-Román R., De Armas Hernández F. Investigation in Parasitology: Collection of Papers. Dalʼnevostochnoe otdelenie AN SSSR; Vladivostok: 1989. Monogeneans in sea fish of Canary Archipelago; pp. 24–31. [Google Scholar]
- Lu C.-H., Ku C.-C., Wen C.-M., Chen S.-N. Effects of the gill parasite Zeuxapta seriolae (Monogenea: Heteraxinidae) on the sea cage-cultured amberjack Seriola dumerili (Risso, 1810) at Penghu Island (Pescadores), Taiwan. J. Fish. Soc. Taiwan. 2012;39:107–114. [Google Scholar]
- Luque J.L., Cézar D. Metazoan ectoparasites of the pompano Trachinotus goodei Jordan & Evermann, 1896 (Osteichthyes: Carangidae) from the coastal zone of Rio de Janeiro State, Brazil. Acta Sci. Biol Sci. 2004;26:19–24. [Google Scholar]
- MacCallum G.A. Further notes on the genus Microcotyle. Zool. Jahrb. 1913;35:389–402. [Google Scholar]
- MacCallum G.A. Some new species of parasitic trematodes of marine fishes. Zoopathologica I. 1916;1:5–34. [Google Scholar]
- MacCallum G.A., MacCallum W.G. Four species of Microcotyle, M. pyragraphorus, macroura, eueides and acanthophallus. Zool. Jahrb. Abt. Syst. 1913;34:223–244. https://www.biodiversitylibrary.org/item/38162 [Google Scholar]
- MacKenzie K., Campbell N., Mattiucci S., Ramos P., Pereira A., Abaunza P. A checklist of the protozoan and metazoan parasites reported from the Atlantic horse mackerel, Trachurus trachurus (L.) Bull. Eur. Assoc. Fish Pathol. 2004;24:180–184. [Google Scholar]
- MacKenzie K., Campbell N., Mattiucci S., Ramos P., Pinto A., Abaunza P. Parasites as biological tags for stock identification of Atlantic horse mackerel Trachurus trachurus L. Fish. Res. 2008;89:136–145. [Google Scholar]
- Madhi Y., Belghyti D. Répartition branchiale des monogènes Gotocotyla acanthura et Pyragraphorus hollisae parasite du pompano, Trachinotus ovatus (Pisces; Carangidae) de la côte de Mehdia (Maroc) Agron. Afr. 2006;18:117–124. [Google Scholar]
- Mamaev Y.L. The systematical composition of the family Heteraxinidae and other allied families of Monogenea. Folia Parasitol. 1990;37:225–230. [PubMed] [Google Scholar]
- Mansell B., Powell M., Ernst I., Nowak B.F. Effects of the gill monogenean Zeuxapta seriolae (Meserve, 1938) and treatment with hydrogen peroxide on pathophysiology of kingfish, Seriola lalandi Valenciennes, 1833. J. Fish. Dis. 2005;28:253–262. doi: 10.1111/j.1365-2761.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- Marzoug D. Université dʼOran; Oran, Algeria: 2012. Biodiversity and structure of parasite communities in two commercial fish species from Western Mediterranean coasts of Algeria. (PhD Thesis) [Google Scholar]
- Mendoza-Garfias B., García-Prieto L., León G.P.-P.D. Checklist of the Monogenea (Platyhelminthes) parasitic in Mexican aquatic vertebrates. Zoosystema. 2017;39:501–598. doi: 10.5252/z2017n4a5. [DOI] [Google Scholar]
- Meserve F.G. vol. 2. The University of Southern California Press; Los Angeles: 1938. Some monogenetic trematodes from the Galapagos Islands and the neighboring Pacific; pp. 31–75. (Allan Hancock Pacific Expeditions). [Google Scholar]
- Mollaret I., Jamieson B.G.M., Justine J.-L. Phylogeny of the Monopisthocotylea and Polyopisthocotylea (Platyhelminthes) inferred from 28S rDNA sequences. Int. J. Parasitol. 2000;30:171–185. doi: 10.1016/s0020-7519(99)00197-6. [DOI] [PubMed] [Google Scholar]
- Montero F.E., Aznar F.J., Fernández M., Raga J.A. Redescription of Allencotyla mcintoshi (Monogenea), with an emended diagnosis of Allencotyla. J. Parasitol. 2003;89:133–136. doi: 10.1645/0022-3395(2003)089[0133:ROAMPM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Montero F.E., Crespo S., Padros F., De la Gandara F., Garcia A., Raga J.A. Effects of the gill parasite Zeuxapta seriolae (Monogenea: Heteraxinidae) on the amberjack Seriola dumerili Risso (Teleostei: Carangidae) Aquaculture. 2004;232:153–163. doi: 10.1016/s0044-8486(03)00536-2. [DOI] [Google Scholar]
- Mooney A.J., Ernst I., Whittington I.D. An egg-laying rhythm in Zeuxapta seriolae (Monogenea: Heteraxinidae), a gill parasite of yellowtail kingfish (Seriola lalandi) Aquaculture. 2006;253:10–16. doi: 10.1016/j.aquaculture.2004.11.029. [DOI] [Google Scholar]
- Naidenova N., Mordvinova T. Helminth fauna of Mediterranean sea fish upon the data of the IBSS’s expeditions (1959–1973) Ekol. Morya. 1997;46:69–74. [Google Scholar]
- Nasir P., Fuentes Zambrano J. Algunos tremátodos monogenéticos Venezolanos. Riv. Parassitol. 1983;44:203–408. [Google Scholar]
- Nicoll W. The trematode parasites of fishes from the English Channel. J. Mar. Biol. Assoc. U. K. 1914;10:466–505. [Google Scholar]
- Ogawa K., Egusa S. Two species of microcotylid monogeneans collected from black sea bream, Acanthopagrus schlegeli (Bleeker) (Teleostei: Sparidae) Jpn. J. Parasitol. 1980;29:455–462. [Google Scholar]
- Ogawa K., Fukudome M. Mass mortality caused by blood fluke (Paradeontacylix) among amberjack (Seriola dumerili) imported to Japan. Fish Pathol. 1994;29:265–269. [Google Scholar]
- Ogawa K., Yokoyama H. Parasitic diseases of cultured marine fish in Japan. Fish Pathol. 1998;33:303–309. [Google Scholar]
- Öktener A. An updated checklist of parasitic helminths of marine fish from Turkey. Transylv. Rev. Syst. Ecol. Res. 2014;16:55–96. [Google Scholar]
- Oliva M. Metazoan parasites of the jack mackerel Trachurus murphyi (Teleostei, Carangidae) in a latitudinal gradient from South America (Chile and Peru) Parasite. 1999;6:223–230. doi: 10.1051/parasite/1999063223. [DOI] [PubMed] [Google Scholar]
- Orecchia P., Paggi L. Aspetti di sistematica e di ecologia degli elminti parassiti di pesci marini studiati presso l'Istituto di Parassitologia dell’Universita di Roma. Parassitologia. 1978;20:2. [PubMed] [Google Scholar]
- Palm H.W., Klimpel S., Bucher C. Checklist of metazoan fish parasites of German coastal waters. Berichte aus dem Institut fur Meereskunde an der Christain-Alberechts-Universitat Kiel. 1999;307:148. [Google Scholar]
- Palombi A. I trematodi d’Italia. Parte I. Trematodi Monogenetici. Arch. Zool. Ital. 1949;34:203–408. [Google Scholar]
- Parona A., Perugia C. Di alcuni trematodi ectoparassiti di pesci marini. Nota preventiva (Res Ligusticae, 8) Ann. Mus. Civ. Stor. Nat. Genova. 1889;2:740–747. [Google Scholar]
- Parukhin A.M. Naukova Dumka; Kiev: 1976. Parasitic Worms of Food Fishes of the Southern Seas. (In Russian) [Google Scholar]
- Parukhin A. Helminth fauna of commercial fishes from the Saya-de-Malya Bank (Indian Ocean) Nauchnye Doki. Vyss. Shkoly. Biol. Nauki. 1988;8:34–37. [PubMed] [Google Scholar]
- Payne R.R. Four new Monogenea (Axinidae and Heteraxinidae) from eastern Pacific Ocean fishes. J. Helminthol. Soc. Wash. 1990;57:93–103. [Google Scholar]
- Piasecki W. Parasitofauna of Cape horse mackerel, Trachurus trachurus capensis Castelnau, 1861. Acta Ichthyol. Piscatoria. 1982;12:43–56. [Google Scholar]
- Pillai S. University of Kerala; Thiruvananthapuram, India: 1968. Trematode parasites of fish monogenea from Kerala coastal fishes. (Thesis) [Google Scholar]
- Price E.W. North American monogenetic trematodes. XI. The family Heteraxinidae. J. Parasitol. 1962;48:402–418. [PubMed] [Google Scholar]
- Radujkovic B.M., Euzet L. Parasites des Poissons marins du Monténégro: Monogènes. Acta Adriat. 1989;30:51–135. [Google Scholar]
- Rahemo Z.I. Distribution of the nervous elements in the haptor of four peculiarly-clamped monogenean fish parasites to match with its morphology. Trends. Parasitol. Res. 2012;1:10–14. [Google Scholar]
- Ramadhan F.N., Subekti S., Koesdarto S. 9th International Fisheries Symposium. 18-21 November 2019, Malaysia. 2019. First report of Pyragraphorus hollisae on silver pompano Trachinotus blochii by using scanning electron microscope (SEM) in Indonesia. [Google Scholar]
- Ramdane Z., Trilles J.-P., Mahe K., Amara R. Metazoan ectoparasites of two teleost fish, Boops boops (L.) and Mullus barbatus barbatus L. from Algerian coast: diversity, parasitological index and impact of parasitism. Cybium. 2013;37:59–66. [Google Scholar]
- Rego A. Identificaçao de helmintes de peixes da costa continental portuguesa. Garcia De Orta Ser. Zool. 1987;12:113–116. [Google Scholar]
- Reimer L. Monogenea von Fischen der Küsten von Namibia und Mocambique. Wissenschaftliche Zeitschrift der Pädagogischen Hochschule ‘Liselotte Herrman’ Güstrow. 1990;1:27–38. [Google Scholar]
- Repullés-Albelda A., Kostadinova A., Raga J.A., Montero F.E. Seasonal population dynamics of Zeuxapta seriolae (Monogenea: Heteraxinidae) parasitising Seriola dumerili (Carangidae) in the Western Mediterranean. Vet. Parasitol. 2013;193:163–171. doi: 10.1016/j.vetpar.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Rigos G., Katharios P., Kogiannou D., Cascarano C.M. Infectious diseases and treatment solutions of farmed greater amberjack Seriola dumerili with particular emphasis in Mediterranean region. Rev. Aquacult. 2021;13:301–323. [Google Scholar]
- Rohde K. Monogenean gill parasites of the kingfish Seriola grandis Castlenau (Carangidae) from the Great Barrier Reef. Publ. Seto Mar. Biol. Lab. Spec. Publ. Ser. 1978;24:369–376. [Google Scholar]
- Rohde K. Ultrastructure of the protonephridial system of the oncomiracidium of Zeuxapta seriolae (Meserve, 1938) (Monogenea, Polyopisthocotylea, Axinidae) Acta Parasitol. 1997;42:127–131. doi: 10.1007/s004360050375. [DOI] [PubMed] [Google Scholar]
- Scholz T., Vanhove M., Smit N., Jayasundera Z., Gelnar M. (Eds.) A guide to the parasites of African freshwater fishes. Abc Taxa. 2018;18:1–425. [Google Scholar]
- Sepúlveda F., Torres J., Infante C., González M. Potential role of ectoparasites (Zeuxapta seriolae and Caligus lalandei) in the transmission of pathogenic bacteria in yellowtail kingfish Seriola lalandi, inferred from cultivable microbiota and molecular analyses. J. Fish. Dis. 2016;40:979–985. doi: 10.1111/jfd.12582. [DOI] [PubMed] [Google Scholar]
- Sharp N., Poortenaar C., Diggles B., Willis T.J. Metazoan parasites of yellowtail kingfish, Seriola lalandi lalandi, in New Zealand: prevalence, intensity, and site preference. N. Z. J. Mar. Freshw. Res. 2003;37:273–282. [Google Scholar]
- Sharp N.J., Diggles B., Poortenaar C., Willis T.J. Efficacy of Aqui-S, formalin and praziquantel against the monogeneans, Benedenia seriolae and Zeuxapta seriolae, infecting yellowtail kingfish Seriola lalandi lalandi in New Zealand. Aquaculture. 2004;236:67–83. [Google Scholar]
- Shaw M.K. The ultrastructure of the clamp wall of the monogenean gill parasite Gastrocotyle trachuri. Z. Parasitenkd. 1979;58:243–258. [Google Scholar]
- Smith-Vaniz W.F. In: FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific, Vol. 4 Bony Fishes Part 2 (Mugilidae to Carangidae) Carpenter K.E., Niem V.H., editors. FAO; Rome: 1999. Carangidae; pp. 2659–2756. [Google Scholar]
- Sproston N. A synopsis of the monogenetic trematodes. Trans. Zool. Soc. Lond. 1946;25:185–600. [Google Scholar]
- Strona G., Stefani F., Galli P. Monogenoidean parasites of Italian marine fish: an updated checklist. Ital. J. Zool. 2010;77:419–437. [Google Scholar]
- Stuart K.R., Drawbridge M.A. Captive spawning and larval rearing of California yellowtail (Seriola lalandi) Aquacult. Res. 2013;44:728–737. [Google Scholar]
- Subhapradha C. Vallisiopsis contorta n. g. and n. sp. and Gastrocotyle indica n. sp., monogenetic trematodes from marine fishes of the Madras Coast. Parasitology. 1951;41:162–165. doi: 10.1017/s0031182000084006. [DOI] [PubMed] [Google Scholar]
- Tubbs L., Tingle M. Bioavailability and pharmacokinetics of a praziquantel bolus in kingfish Seriola lalandi. Dis. Aquat. Org. 2006;69:233–238. doi: 10.3354/dao069233. [DOI] [PubMed] [Google Scholar]
- Tubbs L., Tingle M. Effect of dose escalation on multiple dose pharmacokinetics of orally administered praziquantel in kingfish Seriola lalandi. Aquaculture. 2006;261:1168–1174. [Google Scholar]
- Tubbs L.A., Poortenaar C.W., Sewell M.A., Diggles B.K. Effects of temperature on fecundity in vitro, egg hatching and reproductive development of Benedenia seriolae and Zeuxapta seriolae (Monogenea) parasitic on yellowtail kingfish Seriola lalandi. Int. J. Parasitol. 2005;35:315–327. doi: 10.1016/j.ijpara.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Ulmer M.J., James H.A. Monogeneans of marine fishes from the Bay of Naples. Trans. Am. Microsc. Soc. 1981;100:392–409. doi: 10.2307/3226153. [DOI] [Google Scholar]
- Unnithan R.V. On the functional morphology of a new fauna of Monogenea on fishes from Trivandrum and environs. Part I. Axinidae fam. nov. Bull. Cent. Res. Inst. Univ. Kerala. Ser. C. 1957;5:27–122. [Google Scholar]
- Unnithan R.V. On six species of monogenetic trematodes, parasitic on the gills of marine fishes from the Indian seas. Treubia. 1968;27:141–164. [Google Scholar]
- van Beneden P.J., Hesse C.E. Recherches sur les Bdellodes (Hirudinées) et les Trématodes marins. Mem. Acad. R. Sci. Lett. Belg. 1863;34:1–150. doi: 10.5962/bhl.title.11767. [DOI] [Google Scholar]
- Vivanco-Aranda M., Lechuga-Sandoval C., Río-Zaragoza O.B.D., Viana M.T. Mixed parasitism induced experimentally in yellowtail, Seriola dorsalis reared in RAS: intensity and spatial distribution on the skin and gills. Lat. Am. J. Aquat. Res. 2019;47:156–163. [Google Scholar]
- Whittington I.D., Chisholm L.A. Diseases caused by Monogenea. J. Fish. Dis. 2008;2:683–816. [Google Scholar]
- Williams R.E. The University of Adelaide; Adelaide, Australia: 2010. Oral treatments for monogenean parasites of farmed yellowtails, Seriola spp. (Carangidae)http://hdl.handle.net/2440/60980 (PhD Thesis) [Google Scholar]
- Williams R.E., Ernst I., Chambers C.B., Whittington I.D. Efficacy of orally administered praziquantel against Zeuxapta seriolae and Benedenia seriolae (Monogenea) in yellowtail kingfish Seriola lalandi. Dis. Aquat. Org. 2007;77:199–205. doi: 10.3354/dao01824. [DOI] [PubMed] [Google Scholar]
- WoRMS Editorial Board World Register of Marine Species. 2021. https://www.marinespecies.org Available from. at VLIZ.
- Yamaguti S. Studies on the helminth fauna of Japan. Part 24. Trematodes of fishes, V. Jpn. J. Zool. 1938;8:15–74. [Google Scholar]
- Yamaguti S. Studies on the helminth fauna of Japan. Part 37. Trematodes of fishes, VIII. Jpn. J. Med. Sci. VI Bacteriol. Parasit. 1942;2:105–129. [Google Scholar]
- Yamaguti S. John Wiley & Sons, Inc.; Hoboken, USA: 1963. Systema Helminthum. Volume IV: Monogenea and Aspidocotylea. [Google Scholar]
- Yamaguti S. University of Hawaii Press; Honolulu: 1968. Monogenetic Trematodes of Hawaiian Fishes. [Google Scholar]
- Zerecero C. In: Libro Homenaje al Dr Eduardo Caballero y Caballero. Bravo-Hollis M., Zerecero M.C., Flores-Barroeta L., Hidalgo-Escalante E., Winter H.A., editors. Instituto Politecnico Nacional; Mexico City: 1960. Pyragraphorus caballeroi n. sp., trematodo de la subclase Monogenea (Carus, 1863) en peces marinos del Oceano Pacifico del Norte; pp. 345–351. [Google Scholar]
- Zhang J., Yang T., Liu L., Ding X. A list of monogeneans from Chinese marine fishes. Syst. Parasitol. 2003;54:111–130. doi: 10.1023/a:1022581523683. [DOI] [PubMed] [Google Scholar]