Abstract
Diagnosing breast cancer (BC) in early stages increases the chances of treating this cancer in men. However, because BC is very rare in men, especially inflammatory BC (IBC), it is unlikely that screening men for BC by mammography or other tests would yield promising outcomes. The aim of this study was to report IBC in a 53-year-old man. The case was a 53-year-old man with a history of mass in the left breast and trauma to the same side as well as swelling and severe redness of the breast skin. The patient underwent neoadjuvant chemotherapy and relative responded to medical treatment. He then underwent modified mastectomy surgery and initial chest wall repair followed by radiotherapy. IBC in men is challenging due to its rarity, unknown biological behaviors, and difficulty in early diagnosis. This tumor is usually detected in advanced stages in the elderly and has a poor prognosis.
Keywords: Breast cancer, inflammatory, man
Introduction
Male breast cancer (BC) is rare, representing approximately 1% of cancers that occur in men and approximately 1% of all BCs worldwide.[1] Less than 0.2% of cancer-related deaths in men can be attributed to male BC.[2,3] Because male BC occurs at a very low incidence, BC literature, research, clinical trials, and development of new treatment options primarily focus on female BC. Although knowledge about female BC can inform male BC diagnosis and treatment, molecular and clinicopathologic features differ between male and female BC. Biologic factors, such as sex differences, hormonal regulation, and response to treatment (both tolerability and activity), must be considered when defining this disease in men and deciding upon treatment options.[3] At present, no standard of care exists for male BC, and there is an unmet need for research and therapeutic options for this disease. This review summarizes current knowledge of male BC to date with a discussion of future treatment options for the disease. Almost 1% of all BCs occur in men. In 2018, about 2550 men will be diagnosed with BC in the US, and male BC will account for 480 deaths. In comparison, it is estimated that there will be approximately 266,120 new cases of female BC and approximately 40,920 deaths due to BC in women.[4,5] According to Surveillance, Epidemiology, and End Results Program data from 2015, the incidence of invasive BC in men is 1.1:100,000 men, whereas it is 126.5:100,000 in women.[6] However, the incidence and prevalence of male BC vary by race and ethnicity, and men in certain select populations such as African Americans have higher rates of male BC in comparison to their Caucasian, Hispanic, or Asian/Pacific Islander counterparts. The median age at initial diagnosis of invasive BC is typically older in men than in women (68 vs. 62 years).[7] In both men and women, the age-adjusted rate of BC sharply increases by the fifth decade of life. Initial diagnosis of male BC often occurs at a later stage than in female BC, and male BC often exhibits more advanced disease features, such as a larger tumor size, lymph node involvement, and distant metastases at the time of diagnosis.[8] In developed countries, nearly two-thirds of invasive female BCs are localized at the time of diagnosis; in contrast, in men approximately half of the cases are localized and the other half are regional or distant disease.[6] Estimates of in situ carcinoma in men are approximately 10%; the remaining 90% can be attributed to infiltrating ductal carcinoma.[9] Infiltrating lobular carcinoma, medullary lesions, and tubular or neuroendocrine tumors are very rare in men, as is triple-negative BC (TNBC).[9,10] Male BCs usually express the estrogen receptor (ER), progesterone receptor (PR), and androgen receptor; are hormonally responsive; and most commonly present as unilateral tumors.[11] Nipple retraction or the palpation of a retroareolar mass is a common physical examination finding and may be the first clinical manifestation of male BC.[12] This report presents a case of inflammatory BC (IBC) in a 53-year-old man.
Case Report
The patient was a 53-year-old man with a history of mobile mass in the left breast. He had suffered trauma and hematoma in the chest wall 6 months in advance of his visit, which had partially improved. Then, about 4 months ago, he developed progressive erythema and swelling in the left anterior chest wall. There was no family history of breast or ovarian cancer. Physical examination showed extensive erythema and swelling in the left anterior chest wall that extended to the right armpit [Figure 1]. A large mass was visible in the anterior part of the chest, and a massive lymph node was evident in the left axilla, but the superclavicular lymph nodes were not palpable. All blood tests were normal [Table 1]. Ultrasonography indicated a large mass measuring approximately 100 mm × 53 mm in the subareolar left breast, which had a necrotic and cystic center in the upper section. Solid parts in the color Doppler test had blood flow and suggested tumor lesions. Several hypoechoic masses indicative of possible metastatic lymphadenopathy were observed in the left axillary, the largest of which was 20 mm × 18 mm. The analysis of mammograms taken from the left breast showed a large mass with a relatively clear margin in the subareolar left breast, which had caused edema and increased skin thickness [Figures 2 and 3]. Computed tomography (CT) of the mass revealed involvement of the axillary lymph nodes without adhesion to the chest wall and the involvement of the lung tissue and regional lymph nodes [Figure 4]. Under ultrasound guide and after local anesthesia injection, core needle biopsy and left axillary lymph nodes were obtained from fine needle aspiration ultrasound. In pathological examination, invasive ductal and Grade 2 carcinoma with lymphovascular involvement and mild lymphocytic stromal infiltration were observed. Ductal carcinoma in situ was solid and consisted of 20% cribriform. Metastatic involvement was detected in cytological examination of axillary lymph nodes. The results of immunohistochemistry showed that tumor cells were negative for ER and PR. There was no overexpression of human epidermal growth factor 2 receptor (HER2/neu) and the Ki-67 receptor was negative. Abdominal ultrasound revealed the normal liver size with regular margins and uniform echogenicity, and a 12-mm nodule was observed in the lower anterior segment of the right lobe, which was consistent with metastasis [Figure 5]. A whole-body bone scan did not show elevated absorption and was inconsistent with metastatic disease [Figure 6]. According to the American Joint Committee on Cancer's cancer staging manual (8th edition), and according to clinical criteria and inflammatory involvement, more than one-third of the skin in this patient was classified as T4dN1M1 of Stage IV anatomical and prognostic BC. The review of patient's background suggested that he had no history of hormonal drugs or chest irradiation, no family history of BC, overweight, which increases estrogen production, excessive alcohol use, which can affect liver ability to regulate the level of estrogen in the blood, liver disease, which usually leads to lower levels of androgens (male hormones) and elevated levels of estrogen (female hormones) and gynecomastia, which can increase the risk of BC. Systemic chemotherapy with bisphosphonate was conducted using 800 mg of capsaicin per day in two separate doses for a total of 6 cycles. This treatment was able to control the disease and induce desired response in terms of erythema control and shrinkage of skin involvement and lesion. Then, after 21 days of chemotherapy and restriction of the lesion, a modified mastectomy was performed on the patient's left side, and the whole breast tissue was removed using suitable lower and upper flaps. Since the pectoralis major muscle was not involved, the breast tissue was removed along with the pectoral fascia [Figure 6] and levels I and II of axillary lymph nodes were also extracted. The initial repair was conducted successfully [Figure 7]. When the wound healed, the patient underwent radiotherapy to the chest wall, armpit, and supraclavicular lymph nodes. The patient was discharged from the hospital in good general condition 24 h after the operation, and the necessary instructions for radiotherapy were given for 25 sessions and there was no problem in the follow-up after 6 months.
Figure 1.
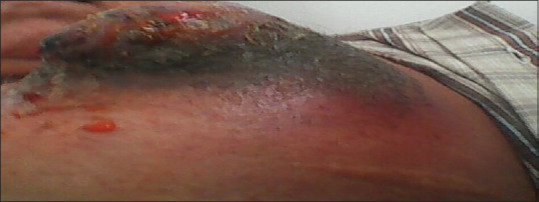
Erythema, swelling, and peripheral necrosis with ulceration in the center of the lesion in the left anterior chest wall
Table 1.
Patient tests
| HB=14.1 | PT=12 | TSH=2.78 |
| HCT=42.5 | PTT=30 | T4=88.05 |
| PLT=204 | AST=20 | T3=1.21 |
| WBC=7.8 | ALT=34 | INR=0.9 |
| RBC=4.63 | ESR=5 | CR=1.1 |
| FBS=88 | K=3.8 |
HCT: Hematocrit, HB: Hemoglobin, WBC: White blood cell, RBC: Red blood cell, FBS: Fasting blood sugar, ALT: Alanine aminotransferase, ESR: Erythrocyte sedimentation rate, AST: Aspartate transaminase, PTT: Partial thromboplastin time. PT: Prothrombin time, INR: International normalized ratio, TSH: Thyroid-stimulating hormone, CR: Creatinine, PLT: Platelets
Figure 2.
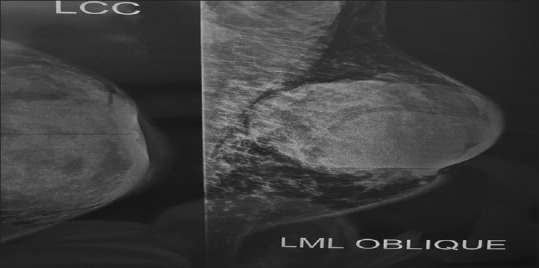
Left breast mammogram in left craniocaudal and left mediolateral oblique views. A bulky mass with a relatively clear margin was observed in the subareolar left breast, which also caused edema and increased skin thickness
Figure 3.
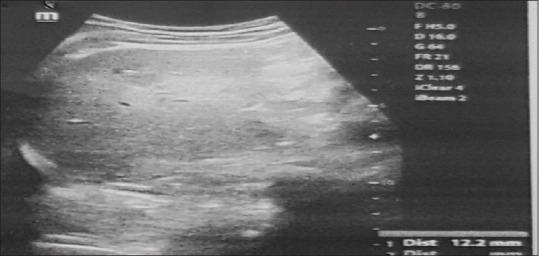
Ultrasonography of a bulky space measuring approximately 100 mm × 53 mm in the subareolar left breast
Figure 4.
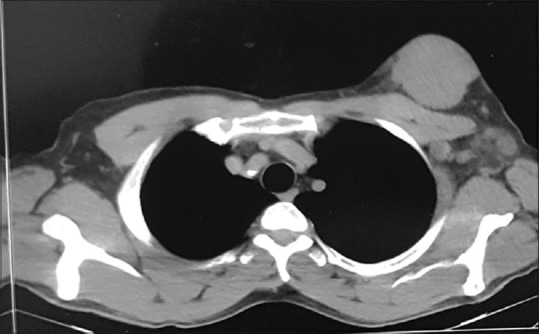
Computed tomography of the mass with involvement of the axillary lymph nodes without adhesion to the chest wall
Figure 5.
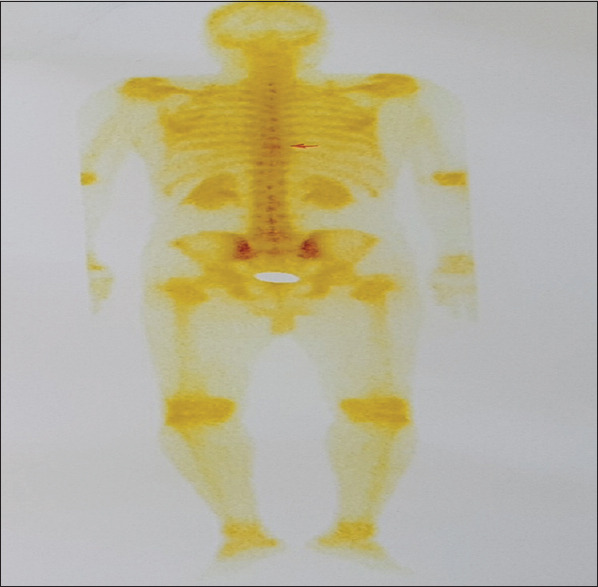
A whole-body bone scan did not show elevated absorption
Figure 6.
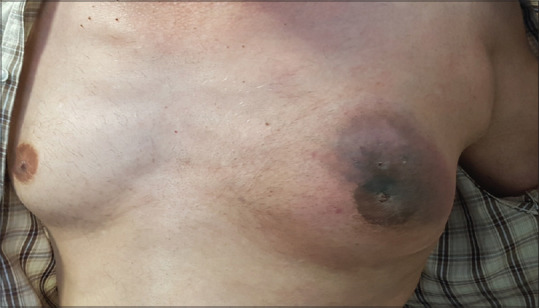
Relative improvement of erythema, swelling, and peripheral necrosis with ulceration at the center of the lesion in the left anterior chest wall following neoadjuvant chemotherapy
Figure 7.
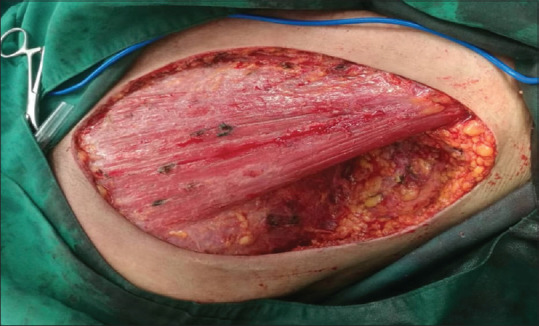
During and after modified mastectomy surgery, which initially reconstructed the chest
Discussion
Male BC is usually treated using the same strategy adopted for female BC, and the results show a prognosis comparable to that of female BC.[13] BC is often diagnosed at a more advanced stage in men than in women and there are biological differences between these two.[14,15] Compared to BC in women, it is often diagnosed by histological criteria, which are almost exclusively the subtypes of lumen (often luminal A as opposed to luminal B), though the results are contradictory.[16] In our patient, contrary to this rule, the triple type was negative. In patients with IBC, multimodal therapy, including surgery, chemotherapy, and radiotherapy, has improved local disease and long-term survival.[17] The important point is prioritization of these therapeutic measures for the best prognosis of IBC in men. In our case, due to extensive involvement, the primary surgery was not an option because it could cause significant defect in chest wall of the patient, which probably required extensive reconstruction and increased the likelihood of tumor tissue residual. Hence, it was decided to perform chemotherapy as a neoadjuvant, and if successful, local control treatments would be proposed. It induced desirable response with paclitaxel-containing protocol, as shown in Figures 1 and 6. Hence, this treatment offers a suitable solution for IBCs in men. In clinical studies, in 10 cases of IBC in men, the mean age of patients was 67.8 years. Our patient, 53 years old, was about 15 years lower than the average. Such a difference is also in keeping with the younger age of BC development in women in Iran. Main clinical symptoms included rash and swelling in the chest wall and swelling of the axillary lymph nodes. In 3 cases, breast mass was detected by mammography, in 3 cases by ultrasound and in 4 cases by CT scan.[18] In our case mass, the mass was detected in all three methods. The usefulness of dynamic contrast-enhanced MRI for the diagnosis of IBC in men has been reported in one case,[19] but it was not necessary in our research. IBC in men, including our case, was negative for ER, PR, and HER2 (known as TNBC), which may explain their poor prognosis.[20]
Conclusions
IBC in men is especially challenging due to its rarity, unknown biological behaviors, and difficulty in early diagnosis. This tumor is usually detected in the advanced stages in the elderly and its characteristics are distinct from hormone receptors and HER2, which explains its poor prognosis. Although the best line of treatment for this rare disease is still unknown, a clear and reliable treatment strategy for IBC could be identified using the treatment protocols in place for IBC treatment in women. Furthermore, a modified radical mastectomy following neoadjuvant chemotherapy is a surgical procedure for IBC in men, even if the breast tissue is greatly involved.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank the Research and Technology Deputy of Kurdistan University of Medical Sciences, Sanandaj, Iran, for accompanying us in this study.
References
- 1.Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, et al. Multidisciplinary meeting on male breast cancer: Summary and research recommendations. J Clin Oncol. 2010;28:2114–22. doi: 10.1200/JCO.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burga AM, Fadare O, Lininger RA, Tavassoli FA. Invasive carcinomas of the male breast: A morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Arch. 2006;449:507–12. doi: 10.1007/s00428-006-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darkeh MH, Azavedo E. Male breast cancer clinical features, risk factors, and current diagnostic and therapeutic approaches. Int J Clin Med. 2014;5:1068–86. [Google Scholar]
- 4.Key Statistics for Breast Cancer in Men. [Last accessed on 2018 Mar 05]. Available from: https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html .
- 5.How common is Breast Cancer? [Last accessed on 2018 Mar 05]. Available form: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breastcancer.html .
- 6.Surveillance Research Program NCI. SEER* Explorer: An interactive website for SEER cancer statistics [Google Scholar]
- 7.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. Bethesda, MD: National Cancer Institute; 2017. SEER Cancer Statistics Review, 1975–2014. [Google Scholar]
- 8.Giotta F, Acito L, Candeloro G, Del Medico P, Gadaleta-Caldarola G, Giordano G, et al. Eribulin in male patients with breast cancer: The first report of clinical outcomes. Oncologist. 2016;21:1298–305. doi: 10.1634/theoncologist.2016-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardoso F, Bartlett JM, Slaets L, Van Deurzen CH, van Leeuwen-Stok E, Porter P, Linderholm B, Hedenfalk I, Schröder C, Martens J, Bayani J. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncology. 2018;29:405–17. doi: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso F, Bartlett JM, Slaets L, van Deurzen CH, van Leeuwen-Stok E, Porter P, et al. Characterization of male breast cancer: Results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer program. Ann Oncol. 2018;29:405–17. doi: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruddy KJ, Winer EP. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Annals of oncology. 2013;24:1434–43. doi: 10.1093/annonc/mdt025. [DOI] [PubMed] [Google Scholar]
- 12.Cutuli B. Strategies in treating male breast cancer. Expert Opin Pharmacother. 2007;8:193–202. doi: 10.1517/14656566.8.2.193. [DOI] [PubMed] [Google Scholar]
- 13.Mills GC, Alperin JB, Trimmer KB. Studies on variant glucose-6-phosphate dehydrogenases: G6PD Fort Worth. Biochem Med. 1975;13:264–75. doi: 10.1016/0006-2944(75)90084-8. [DOI] [PubMed] [Google Scholar]
- 14.Lautrup MD, Thorup SS, Jensen V, Bokmand S, Haugaard K, Hoejris I, et al. Male breast cancer: A nation-wide population-based comparison with female breast cancer. Acta Oncol. 2018;57:613–21. doi: 10.1080/0284186X.2017.1418088. [DOI] [PubMed] [Google Scholar]
- 15.Weir J, Zhao YD, Herman T, Algan Ö. Clinicopathologic features and radiation therapy utilization in patients with male breast cancer: A national cancer database study. Breast Cancer (Auckl) 2018;12:1–7. doi: 10.1177/1178223418770687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornegoor R, Verschuur-Maes AH, Buerger H, Hogenes MC, de Bruin PC, Oudejans JJ, et al. Molecular subtyping of male breast cancer by immunohistochemistry. Mod Pathol. 2012;25:398–404. doi: 10.1038/modpathol.2011.174. [DOI] [PubMed] [Google Scholar]
- 17.Perez CA, Fields JN, Fracasso PM, Philpott G, Soares RL, Jr, Taylor ME, et al. Management of locally advanced carcinoma of the breast. II. Inflammatory carcinoma. Cancer. 1994;74(Suppl 1):466–76. doi: 10.1002/cncr.2820741336. [DOI] [PubMed] [Google Scholar]
- 18.Borgen PI, Woung GY, Vlamis V, Potter C, Hoffmann B, Kinne DW, et al. Current management of male breast cancer. A review of 104 cases. Ann Surg. 1992;215:451–7. doi: 10.1097/00000658-199205000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morita Y, Syoji T, Goto H, Nakajima T, Hukasawa T, Taniwaka K. A case report of male inflammatory breast cancer. Nihon Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association) 2005;66:36–40. [Google Scholar]
- 20.Sutherland S, Ashley S, Walsh G, Smith IE, Johnston SR. Inflammatory breast cancer—The Royal Marsden Hospital experience: a review of 155 patients treated from 1990 to 2007. Cancer. 2010;116:2815–20. doi: 10.1002/cncr.25178. [DOI] [PubMed] [Google Scholar]


