Abstract
The cestode Dibothriocephalus nihonkaiensis (syns. Diphyllobothrium nihonkaiense and Diphyllobothrium klebanovskii), the broad fish tapeworm, is a parasitic agent of intestinal infection acquired by consumption of raw or undercooked Pacific salmon, Onchorhynchus spp. Sequencing studies conducted about a decade ago revealed the presence of two major lineages (A and B) in the broad fish tapeworm population within Asian coastal areas. However, in spite of the accumulation of sequence data on GenBank recently, no further genetic analyses of D. nihonkaiensis have been attempted. The present study assessed for the first time the global cox1 variation in D. nihonkaiensis. Novel partial cox1 sequences of 14 isolates of D. nihonkaiensis from 12 patients were generated, and a global genetic analysis was performed using the 14 novel and 79 previously published sequences for isolates from definitive and second intermediate hosts of this species was performed. A total of 48 haplotypes of three haplotype groups (Types A, B and C) were identified, and co-infections with genetically different D. nihonkaiensis were highlighted in humans and Pacific salmon.
Keywords: Dibothriocephalus nihonkaiensis, Diphyllobothriidae, Humans, Pacific salmon, Genetic diversity, Haplotype network, Japan
Graphical abstract
Highlights
-
•
First global analysis of cox1 variation in Dibothriocephalus nihonkaiensis.
-
•
First application of haplotype network analysis for a D. nihonkaiensis population.
-
•
A total of 48 haplotypes of three haplotype groups (Type A, B and C) are identified.
-
•
Co-infections with heterogenic isolates in definitive and intermediate hosts are highlighted.
1. Introduction
The broad fish tapeworm, Dibothriocephalus nihonkaiensis (Yamane, Kamo, Bylund & Wikgren, 1986) (Cestoda: Diphyllobothriidae), previously known as Diphyllobothrium nihonkaiense and Diphyllobothrium klebanovskii, is a major causative agent of intestinal parasitic diseases attributable to ingestion of second-stage larvae (plerocercoids) in raw or undercooked Pacific salmon, Onchorhynchus spp. (Waeschenbach et al., 2017; Scholz et al., 2019). Genetic diversity of D. nihonkaiensis was previously investigated in two studies conducted about a decade ago (Arizono et al., 2009a; Suzuki et al., 2010), describing two major genetic lineages (i.e. Types A and B) in phylogenetic analyses of partial cytochrome c oxidase subunit 1 (cox1) gene using about 20 isolates from humans, bears and Pacific salmon. However, despite of the accumulation of sequence data on GenBank after the two previous studies no global genetic analysis of D. nihonkaiensis has been attempted. Theoretically, humans might be infected with multiple heterogenic D. nihonkaiensis by eating Pacific salmon, but this possibility has not been confirmed because all reported cases represent single parasite infections (Yamane and Shiwaku, 2003; Arizono et al., 2009b). In addition, Pacific salmon might also be infected with multiple heterogenic D. nihonkaiensis by ingestion of the first intermediate host (copepod) harbouring procercoid larvae of this cestode, but this possibility has also not been addressed.
In the present study, we applied genetic analyses of 14 new isolates from 12 clinical cases in Japan in comparison with all isolates available in the GenBank database to assess the global genetic variability of D. nihonkaiensis. The study also confirmed co-infections with heterogenic isolates in humans and intermediate fish hosts.
2. Materials and methods
2.1. Isolates and DNA extraction
Proglottids of 14 specimens of D. nihonkaiensis were collected from 12 patients at the medical institutions in Osaka and Hyogo prefectures, Japan, between 2007 and 2020 (see patient data in Supplementary Table S1). Patients #3 and #12 excreted proglottids of two tapeworms each. The strobilae were naturally passed with faeces or were excreted after anthelminthic treatment, and most isolates (n = 13) were stored in 70% ethanol at 4–8 °C or −20 °C. One isolate (DnHs12-2) was fixed in 10% buffered formalin for about two months (July 8th to September 7th, 2020). Some proglottids of this isolate were transferred in distilled water overnight to remove the formalin solution.
DNA from a small piece of the proglottid was extracted and purified using DNeasy Blood and Tissue Kit or QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany). The isolates were identified as D. nihonkaiensis by multiplex PCR (Wicht et al., 2010b) or sequence analysis of the cytochrome c oxidase subunit 1 (cox1) (Yera et al., 2006).
2.2. Amplification and sequencing
Sequences of a partial fragment of the cox1 gene were used for genetic analysis because of the numerous available sequences from the definitive and fish intermediate host species, and because of its usefulness for genetic differentiation of D. nihonkaiensis (Arizono et al., 2009a; Suzuki et al., 2010; Autier et al., 2019). A portion of the cox1 gene (approximately 710 bp) was amplified using the primer set: forward (5′-TTG ATC GTA AAT TTG GTT C-3′); reverse (5′-AAA GAA CCT ATT GAA CAA AG-3′) (Arizono et al., 2009a). PCR amplification was performed in a volume of 25 μl using TaKaRa EX Taq Hot Start Version containing 10× PCR buffer, 20 mM MgCl2, 2.5 mM of each dNTP, 5 units/μl of Takara Ex Taq HS DNA polymerase (TaKaRa Shuzo Co. Ltd., Shiga, Japan), 0.5 μM of each primer, and 2.5 μl of DNA sample. After denaturation at 94 °C for 5 min, amplification was carried out by 35 cycles consisting of denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s, and elongation at 72 °C for 1 min, followed by a final extension step at 72 °C for 7 min. Reactions were performed in a thermocycler (GeneAmp PCR System 9700 or 2720; Applied Biosystems, USA). Aliquots of the PCR products were separated by electrophoresis on a 3% agarose gel and were visualized under UV light after staining with ethidium bromide. Then the PCR products were purified using either the QIAquick Gel Extraction Kit or the QIAquick PCR Purification Kit (Qiagen Inc., Germany). DNA sequencing was performed using a BigDye Terminator v3.1 Cycle Sequencing kit with the primer sets used in the PCR on an automated sequencer (ABI3130; Applied Biosystems, USA). Sequence chromatograms from each strand were inspected using Sequencher DNA Sequence Analysis Software Version 4.1 (Gene Codes Corp., USA).
2.3. Phylogenetic and haplotype network analyses
A total of 79 cox1 sequences of D. nihonkaiensis (syns. D. nihonkaiense and D. klebanovskii; see Arizono et al., 2009a and Waeschenbach et al., 2017) available on GenBank were included in the present analyses (Table 1). Other available sequences of D. nihonkaiensis deposited on GenBank were excluded from the analyses because of their short length and the presence of ambiguous nucleotide positions or missing information for the hosts. Alignment and phylogenetic analysis were conducted using MEGA7 software (Kumar et al., 2016). The newly generated cox1 sequences were aligned with the 79 D. nihonkaiensis sequences (Table 1) registered to date in GenBank. Six sequences of related species (Dibothriocephalus latus, Dibothriocephalus dendriticus, Dibothriocephalus ditremus, Dibothriocephalus ursi, Diphyllobothrium balaenopterae and Spirometra mansoni) were used as the outgroup and for comparison of cox1 sequence divergence among different species. The final alignment was trimmed to match the shortest sequence and comprised 666 nucleotide positions with no gaps. The best-fitting model for nucleotide substitution (Hasegawa-Kishino-Yano model) was estimated with MEGA7 and applied in the maximum-likelihood analysis. Models with the lowest BIC scores (Bayesian Information Criterion) are considered to describe the substitution pattern the best. A discrete gamma distribution was used to model evolutionary rate differences among sites. The rate variation model allowed for some sites to be evolutionarily invariable. The phylogram reliability was tested with the bootstrap method using 1,000 replications. The following diversity indices for D. nihonkaiensis populations were calculated using DnaSP (ver. 5.10.01): number of haplotypes (h); haplotype diversity (Hd); nucleotide diversity (π); and Tajimaʼs D. The haplotype network of D. nihonkaiensis populations was constructed based on the median-joining method implemented in the Network software ver. 10.2.0.0 (Fluxus Technology Ltd., www.fluxus-engineering.com) using the data set prepared with DnaSP.
Table 1.
Data for the isolates of Dibothriocephalus nihonkaiensis analyzed in this study
| Host | Locality | Isolate | GenBank ID | Haplotype | Genetic lineage | Reference |
|---|---|---|---|---|---|---|
| Human (n = 54) | Japan: Kyoto | Dn1 | AB288371 | H_4 | A | Arizono et al. (2009a) |
| Dn2 | AB288372 | H_2 | A | |||
| Dn3 | AB288373 | H_4 | A | |||
| Dn4 | AB374999 | H_37 | A | |||
| Dn5 | AB375000 | H_4 | A | |||
| Dn6 | AB375001 | H_38 | A | |||
| Dn7 | AB375002 | H_39 | A | |||
| Dn8 | AB375003 | H_40 | B | |||
| Dn9 | AB375004 | H_41 | B | |||
| Japan: Tokyo | Dnh1 | AB521692 | H_32 | A | Suzuki et al. (2010) | |
| Dnh2 | AB521693 | H_32 | A | |||
| Dnh3 | AB521694 | H_4 | A | |||
| Dnh4 | AB521695 | H_33 | A | |||
| Japan: Nara | 2009-40 | AB573407 | H_4 | A | Nishiohuku et al. (unpublished) | |
| 2009-18 | AB573405 | H_19 | A | |||
| 2009-19 | AB573406 | H_22 | A | |||
| 2009-41 | AB573408 | H_23 | A | |||
| 2010-6 | AB573409 | H_3 | A | |||
| Japan | 2013-16 | AB821272 | H_4 | A | Yamasaki and Ashida (unpublished) | |
| Japan: Tochigi | 2017-024 | LC312466 | H_7 | B | Yamasaki and Shimada (unpublished) | |
| China: Shanghai | CHN-005 | AB684623 | H_4 | A | Chen et al. (2014) | |
| CHN-002 | AB684621 | H_15 | A | |||
| CHN-003 | AB684622 | H_16 | A | |||
| South Korea | PCH | DQ768189 | H_45 | A | Yera et al. (2008) | |
| CSS | DQ768190 | H_46 | A | |||
| KCH | DQ768188 | H_44 | A | |||
| China: Heilongjiang | Sample 2 | LC070677 | H_9 | B | Cai et al. (2017) | |
| South Korea | Not named 1 | EF420138 | H_42 | A | Kim et al. (2007) | |
| Japan: Hokkaido | Not named 2 | AB268585 | H_18 | A | Nakao et al. (2007) | |
| Japan: Tochigi | Not named 3 | AB508838 | H_4 | A | Yanagida et al. (2010) | |
| Japan: Tokyo | Not named 4 | AB610797 | H_4 | A | Yamasaki and Nakamura (unpublished) | |
| Japan | Not named 5 | AB781787 | H_17 | A | Ishida and Yamasaki (unpublished) | |
| Japan: Nagasaki | Not named 6 | AB544064 | H_7 | B | Ishida and Yamasaki (unpublished) | |
| Japan: Saitama | Not named 7 | AB597273 | H_1 | A | Ikeda et al. (2012) | |
| Not named 8 | AB597274 | H_24 | A | |||
| Switzerland | Not named 9 | AM412559 | H_43 | A | Wicht et al. (2007) | |
| Not named 10 | AM412560 | H_7 | B | |||
| Japan: Hamamatsu | Not named 11 | AB636314 | H_18 | A | Ohta et al. (2011) | |
| Japan: Asahikawa | Not named 12 | AB364645 | H_7 | B | Yamasaki and Kuramochi (2009) | |
| Japan: Tokyo | Not named 13 | AB015755 | H_6 | A | Miyadera et al. (2001) | |
| Japan: Osaka | DnHs1 | LC589648 | H_4 | A | This study | |
| DnHs2 | LC589649 | H_1 | A | |||
| DnHs3-1a | LC589650 | H_2 | A | |||
| DnHs3-2 | LC589651 | H_3 | A | |||
| DnHs4 | LC589652 | H_4 | A | |||
| DnHs5 | LC589653 | H_4 | A | |||
| DnHs6 | LC589654 | H_5 | A | |||
| DnHs7 | LC589655 | H_4 | A | |||
| DnHs8 | LC589656 | H_47 | A | |||
| DnHs9 | LC589657 | H_21 | A | |||
| DnHs10 | LC589658 | H_21 | A | |||
| DnHs11 | LC589659 | H_48 | A | |||
| Japan: Hyogo | DnHs12–1 | LC589660 | H_4 | A | ||
| DnHs12–2 | LC589661 | H_29 | B | |||
| Brown bear (n = 3) | Russia: Kamchatka | RB1 | AB375660 | H_7 | B | Arizono et al. (2009a) |
| RB2 | AB375661 | H_34 | B | |||
| RB3 | AB375662 | H_35 | A | |||
| Pink salmon (n = 1) | USA: Alaska | US361b | KY000483 | H_8 | A | Kuchta et al. (2017) |
| Chum salmon (n = 19) | Russia: Okhotsk | Ok1 | AB375672 | H_4 | A | Arizono et al. (2009a) |
| Russia: Kamchatka | Ok2 | AB375673 | H_36 | A | ||
| Japan: Aomori | Dnk1 | AB521674 | H_4 | A | Suzuki et al. (2010) | |
| Japan: Hokkaido | Dnk2 | AB521675 | H_26 | A | ||
| Japan: Hokkaido | Dnk3 | AB521676 | H_27 | A | ||
| Japan: Hokkaido | Dnk4 | AB521677 | H_28 | C | ||
| Japan: Hokkaido | Dnk5 | AB521678 | H_29 | B | ||
| Japan: Miyagi | Dnk6 | AB521679 | H_4 | A | ||
| Japan: Miyagi | Dnk7 | AB521680 | H_30 | A | ||
| Japan: Iwate | Dnk8 | AB521681 | H_30 | A | ||
| Japan: Iwate | Dnk9 | AB521682 | H_30 | A | ||
| Japan: Hokkaido | Dnk10 | AB521683 | H_4 | A | ||
| Japan: Hokkaido | Dnk11 | AB521684 | H_4 | A | ||
| Japan: Hokkaido | Dnk12 | AB521685 | H_4 | A | ||
| Japan: Hokkaido | Dn-Ok1 | AB548647 | H_4 | A | Wicht et al. (2010b) | |
| Dn-Ok2 | AB548648 | H_25 | A | |||
| Dn-Ok3 | AB548649 | H_7 | B | |||
| Japan: Iwate | TD01–1 | LC511596 | H_20 | B | Jin et al. (unpublished) | |
| TD01–2 | LC511597 | H_21 | A | |||
| Cherry salmon (n = 16) | Japan: Hokkaido | Dnm1 | AB521686 | H_31 | A | Suzuki et al. (2010) |
| Japan: Iwate | Dnm2 | AB521687 | H_4 | A | ||
| Japan: Iwate | Dnm3 | AB521688 | H_26 | A | ||
| Japan: Iwate | Dnm4 | AB521689 | H_26 | A | ||
| Japan: Hokkaido | Dnm5 | AB521690 | H_4 | A | ||
| Japan: Hokkaido | Dnm6 | AB521691 | H_4 | A | ||
| Japan: Hokkaido | Dn-Om1 | AB548650 | H_4 | A | Wicht et al. (2010b) | |
| Japan: Niigata | Om7-2b | AB924498 | H_4 | A | Watanabe et al. (2014) | |
| Om33 | AB924500 | H_4 | A | |||
| Om38–1 | AB924505 | H_13 | A | |||
| Om38–2 | AB924506 | H_14 | B | |||
| Om38–5 | AB924503 | H_11 | A | |||
| Om38–7 | AB924504 | H_12 | A | |||
| Om38–9 | AB924499 | H_4 | A | |||
| Om38–10 | AB924501 | H_10 | A | |||
| Om38–12 | AB924502 | H_4 | A |
Isolates and haplotypes presented in bold originate from the same host individual.
All isolates originate from three cherry salmons (#7, #33, #38) that have returned to the Miomote River (Watanabe et al., 2014).
3. Results
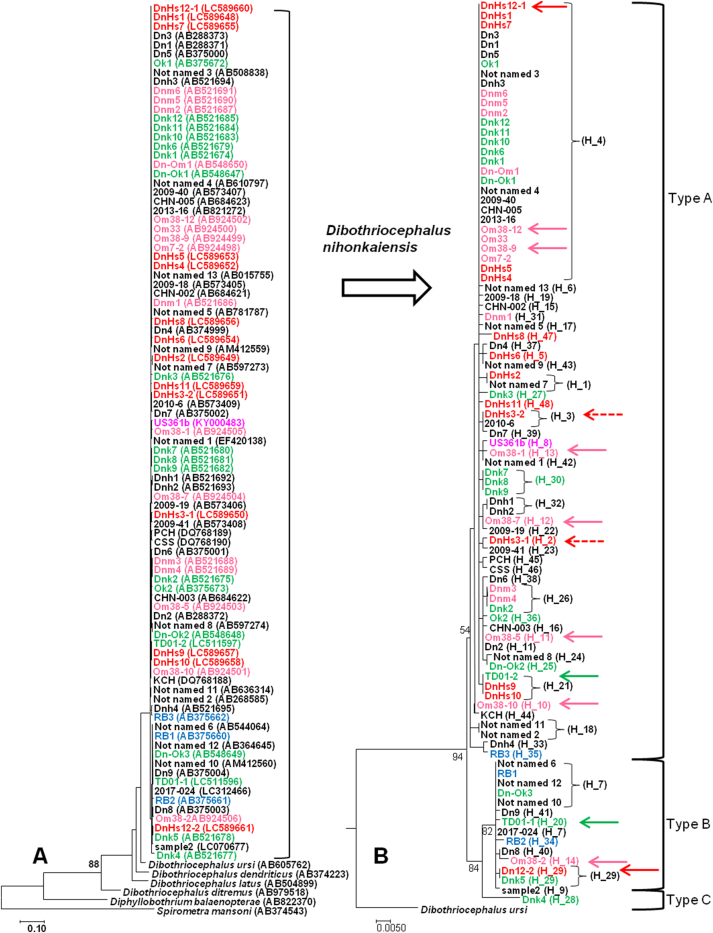
A partial cox1 fragment (666–711 nt) was sequenced for the 14 clinical isolates examined in this study. The phylogenetic tree inferred from the cox1 dataset is presented in Fig. 1. The 93 sequences of D. nihonkaiensis were well separated from the other species (Fig. 1A), the congeners D. ursi (AB605762), D. dendriticus (AB374223), D. latus (AB504899) and D. ditremus (AB979518), and D. balaenopterae (AB822370) and S. mansoni (AB374543) with genetic distances (Kimura-2-parameter model) of 0.049–0.062, 0.065–0.080, 0.072–0.080, 0.112–0.124, 0.155–0.163 and 0.189–0.199, respectively (Supplementary Table S2), and the values between D. nihonkaiensis and the other Dibothriocephalus species (D. dendriticus, D. latus and D. ditremus) correspond to the values previously reported by Wicht et al. (2010a). The sequences of D. nihonkaiensis formed three clades (Types A, B and C; Fig. 1B). The genetic distances within Type A and Type B were 0.000–0.014 and 0.000–0.006, respectively, and the values between Type A and Type B, Type A and Type C and Type B and Type C were 0.012–0.026, 0.018–0.026 and 0.017–0.021, respectively. These values correspond well to the intraspecific divergence values in D. nihonkaiensis based on partial cox1 sequences (Arizono et al., 2009a).
Fig. 1.
A Phylogenetic relationships of the 93 Dibothriocephalus nihonkaiensis isolates from humans, bears, and Pacific salmon with congeners (Dibothriocephalus ursi, D. dendriticus, D. latus and D. ditremus) and related species (Diphyllobothrium balaenopterae and Spirometra mansoni), based on partial cox1 sequences (666 bp). B Expanded phylogenetic tree of the Dibothriocephalus nihonkaiensis isolates from the present (14 in red) and previous (40 in black) clinical cases, bears (3 in blue), pink salmon (1 in purple), chum salmon (19 in green) and cherry salmon (16 in pink). The two isolates from cases #3 and #12 are indicated with red dashed and solid arrows, respectively. Similarly in both clinical isolates, the two isolates (TD01-1, 2) from a chum salmon and the seven isolates (Om38-1, Om38-2, Om38-5, Om38-7, Om38-9, Om38-10 and Om38-12) from a cherry salmon are indicated by green and pink arrows, respectively.
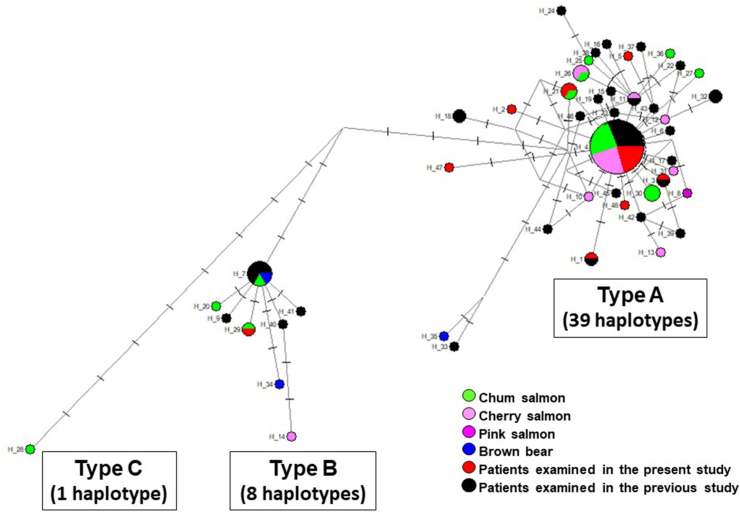
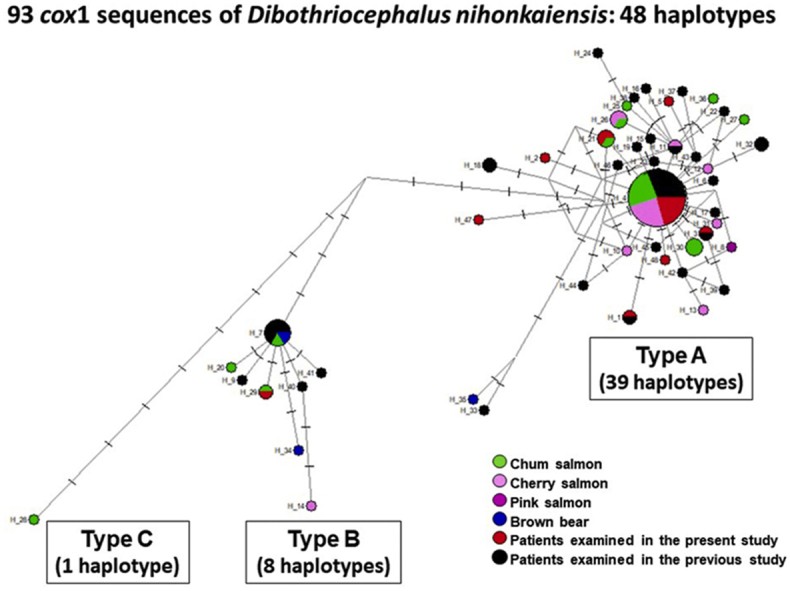
A total of 48 haplotypes were identified among the 93 partial cox1 sequences in the dataset studied (Fig. 2, Table 1). These haplotypes fell into three haplotype groups corresponding to Types A, B and C identified in the phylogenetic analysis (Fig. 2). Type A and Type B both had a central haplotype with a high frequency of appearance. Type A haplogroup was composed of 39 haplotypes among 78 sequences and these haplotypes formed a diffuse network. Type B haplogroup was composed of 8 haplotypes among 14 sequences, and Type C haplogroup was represented by a single haplotype with at least 11 nucleotide differences from Type B populations. A total of 33 haplotypes were identified among 54 sequences from patients. Three sequences for isolates from bears represented 3 haplotypes; 19 sequences for isolates from the chum salmon (Onchorhynchus keta) represented 11 haplotypes; 16 sequences for isolates from the cherry salmon (Onchorhynchus masou) represented 8 haplotypes; and one sequence for isolate from the pink salmon (Onchorhynchus gorbuscha) represented a different type from other haplotypes. Haplotype H_4 was dominant (29 out of 93 sequenced isolates, 31%). Among these haplotypes, several haplotypes originated from the same host (Table 1, isolates and their haplotypes in bold). Namely, 4 haplotypes originated from two patients examined in the present study (H_2 and H_3 from patient #3; and H_4 and H_29 from patient #12), 2 haplotypes (H_20 and H_21) were from one chum salmon (both sequences are published on GenBank only) and 6 haplotypes (H_4, H_10, H_11, H_12, H_13 and H_14) were from one cherry salmon; these sequences were published by Watanabe et al. (2014), but their differences and haplotypes have not been identified.
Fig. 2.
Haplotype network for Dibothriocephalus nihonkaiensis constructed based on partial cox1 sequences. A total 93 sequences were analyzed falling into three types (A, B and C). The size of each circle corresponds to the frequency of the relevant haplotype. The minimum size of the node indicates one individual. Punctuations on branches indicate the number of mutated positions. The circles are colour-coded based on host species (green, chum salmon; pink, cherry salmon; purple, pink salmon; blue, brown bears; red, patients examined in the present study; black, patients examined in previous studies).
Table 2 shows the indices of genetic diversity in D. nihonkaiensis populations examined in the present study. Haplotype diversity for all populations was high (0.825–0.898), but the nucleotide diversity was low (0.00226–0.00692). Tajimaʼs D values were negative in both types (A, B) and all populations with statistically significant values for Type A and all populations, suggesting that D. nihonkaiensis population is genetically diverse and rapidly expanding.
Table 2.
Population genetic analysis using partial cox1 sequences of Dibothriocephalus nihonkaiensis
| Population | n | h | Hd | π | Tajimaʼs D | P-value |
|---|---|---|---|---|---|---|
| A | 78 | 39 | 0.860 | 0.00339 | −2.23538 | 0.01 |
| B | 14 | 8 | 0.825 | 0.00226 | −1.51416 | 0.10 |
| A, B, C | 93 | 48 | 0.898 | 0.00692 | −1.84475 | 0.05 |
Abbreviations: n, number of samples; h, number of haplotypes; Hd, haplotype diversity; π, nucleotide diversity.
4. Discussion
The global analysis of the genetic variation of cox1 gene revealed that the isolates identified as Type A and Type B in the previous studies by Arizono et al. (2009a) and Suzuki et al. (2010) exhibit similar clustering in the present analysis.
However, the genetic divergence between the isolate Dnk4 and Type A, or between the isolate Dnk4 and Type B was similar to that between Type A and Type B (Supplementary Table S2), and the isolate Dnk4 formed a separate clade from Type B with statistical support (Fig. 1B). Moreover, the isolate Dnk4 was clearly different from Type A and Type B haplogroups in the haplotype network (Fig. 2). Therefore, in the present study, the isolate Dnk4 originating from a chum salmon landed at Hokkaido was identified as a new Type C (or haplotype group C). To the best of our knowledge, no haplotype network analysis has been carried out for D. nihonkaiensis. The present analysis demonstrates the usefulness for detecting sequence variation among D. nihonkaiensis population. Two haplotypes (H_33 and H_35) were slightly more mutated than the other haplotypes in Type A (Fig. 2). Since the bootstrap value of Type A was low in the phylogenetic analysis (Fig. 1B), further analysis of the isolates closely related to both haplotypes is required to determine whether these two haplotypes belong to a new type.
There has been no report about co-infection with genetically different D. nihonkaiensis in humans and salmon, and the present study is the first confirmation for this phenomenon in the definitive and second intermediate hosts. As in the present clinical cases, genetic analysis of the tapeworms made it possible to clearly identify multiple infections. Especially, in the case of excretion of one scolex and two strobilae, it is also effective for subsequent treatment and follow-up care to distinguish whether the strobilae are derived from the same specimen.
Species of Pacific salmon, the second intermediate hosts of D. nihonkaiensis, migrate widely across the northern Pacific including the Okhotsk and the Bering seas, which is an endemic area of this cestode, until returning to their respective rivers where they were born. It remains unclear where the Pacific salmon are becoming infected with this cestode. The genetic separation of D. nihonkaiensis populations might be related to regional differences in endemic areas, although no molecular evidence about this has been shown yet. There would be at least two ecological populations if the theory about brackish-water origin of D. nihonkaiensis is correct (Muratov, 1992; Kuchta et al., 2015, 2017): one of the Asian coastal areas and one of the North American coastal areas. In the present analysis, one isolate (Table 1, US361b) from a wild pink salmon captured at south-central Alaska (Kuchta et al., 2017) was included in Type A. Moreover, two isolates (Table 1, Not named 9 and 10) from two Swiss clinical cases suspected to be caused by eating wild Pacific salmon imported from Canada or North America (Wicht et al., 2007) were also included in Type A, suggesting that D. nihonkaiensis population does not exhibit regional genetic differences based on the cox1 sequence analysis.
Further comparative haplotype analysis of the isolates from definitive and second intermediate hosts collected in Pacific and Atlantic areas may be necessary to clarify the ecology of D. nihonkaiensis.
5. Conclusion
The present updated assessment of the genetic diversity of D. nihonkaiensis populations by phylogenetic and haplotype network analyses targeting the partial cox1 gene showed the presence of three genetic lineages in this cestode species. The present study also provides the first published evidence of co-infection with genetically different D. nihonkaiensis isolates in the same definitive or second intermediate host individual.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (C) (20K05902) from the Japan Society for the Promotion of Sciences (JSPS).
CRediT author statement
Niichiro Abe: Conceptualisation, Methodology, Validation, Formal Analysis, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualisation, Supervision, Investigation. Takashi Baba: Methodology, Formal Analysis, Data Curation, Writing - Review & Editing. Yoshitaka Nakamura: Methodology, Investigation, Data Curation, Writing - Review & Editing. Shintaro Murakami: Methodology, Investigation, Data Curation, Writing - Review & Editing. All authors read and approved the final manuscript.
Data availability
The sequences generated in the present study were deposited in DNA Data Bank of Japan (DDBJ) under the accession numbers LC589648-LC589661.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Dr Takeo Watanabe for his valuable information related to the origin of the D. nihonkaiensis isolates in the cherry salmon (Onchorhynchus masou), which were presented in their original manuscript (Watanabe et al., 2014).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2021.100042.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Table S1. Information of the 12 patients infected with Dibothriocephalus nihonkaiensis infection examined in this study during 2007–2020.
Supplementary Table S2. Genetic distance values (Kimura 2-parameter model) among 93 Dibothriocephalus nihonkaiensis isolates and their related species.
References
- Arizono N., Shedko M., Yamada M., Uchikawa R., Tegoshi T., Takeda K., et al. Mitochondrial DNA divergence in populations of the tapeworm Diphyllobothrium nihonkaiense and its phylogenetic relationship with Diphyllobothrium klebanovskii. Parasitol. Int. 2009;58:22–28. doi: 10.1016/j.parint.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Arizono N., Yamada M., Nakamura-Uchiyama F., Ohnishi K. Diphyllobothriasis associated with eating raw Pacific salmon. Emerg. Infect. Dis. 2009;15:866–870. doi: 10.3201/eid1506.090132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier B., Belaz S., Degeilh B., Gangneux J.-P., Rober-Gangneux F. Dibothriocephalus nihonkaiensis: an emerging foodborne parasite in Brittany (France)? Parasit. Vectors. 2019;12 doi: 10.1186/s13071-019-3516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y.C., Chen S.H., Yamasaki H., Chen J.X., Lu Y., Zhang Y.N., et al. Four human cases of Diphyllobothrium nihonkaiense (Eucestoda: Diphyllobothriidae) in China with a brief review of Chinese cases. Kor. J. Parasitol. 2017;55:319–325. doi: 10.3347/kjp.2017.55.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Ai L., Zhang Y., Chen J., Zhang W., Li Y., et al. Molecular detection of Diphyllobothrium nihonkaiense in humans, China. Emerg. Infect. Dis. 2014;20:315–318. doi: 10.3201/eid2002.121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T., Tamura D., Sato Y., Ichihashi K., Matsuoka H., Yamasaki H. Two pediatric cases of Diphyllobothrium nihonkaiense infection in summer (July-August) 2010. Pediatr. Int. 2012;54:163–165. doi: 10.1111/j.1442-200X.2011.03529.x. [DOI] [PubMed] [Google Scholar]
- Kuchta R., Oros M., Ferguson J., Scholz T. Diphyllobothrium nihonkaiense tapeworm larvae in salmon from North America. Emerg. Infect. Dis. 2017;23:351–353. doi: 10.3201/eid2302.161026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchta R., Scholz T., Brabec J., Narduzzi-Wicht B. In: Biology of foodborne parasites. Section III. Important foodborne helminths. Xiao L., Ryan U., Feng F., editors. CRC Press; Boca Raton: 2015. Diphyllobothrium, diplogonoporus and Spirometra; pp. 299–326. [Google Scholar]
- Kim K.H., Jeon H.K., Kang S., Sultana T., Kim G.J., Eom K., et al. Characterization of the complete mitochondrial genome of Diphyllobothrium nihonkaiense (Diphyllobothriidae: cestoda), and development of molecular markers for differentiating fish tapeworms. Mol. Cell. 2007;43:899–973. [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis ver. 7.0 for larger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyadera H., Kokaze A., Kuramochi T., Kita K., Machinami R., Noya O., et al. Phylogenetic identification of Sparganum proliferum as a pseudophyllidean cestode by the sequence analyses on mitochondrial COI and nuclear sdhB. Parasitol. Int. 2001;50:93–104. doi: 10.1016/S1383-5769(01)00071-X. [DOI] [PubMed] [Google Scholar]
- Muratov I.V. A new type of diphyllobothriasis foci in the Far East. Med. Parazitol. (Moscow) 1992;61:25–27. [PubMed] [Google Scholar]
- Nakao M., Abmed D., Yamasaki H., Ito A. Mitochondrial genomes of the human broad tapeworms Diphyllobothrium latum and Diphyllobothrium nihonkaiense (Cestoda: Diphyllobothriidae) Parasitol. Res. 2007;101:233–236. doi: 10.1007/s00436-006-0433-3. [DOI] [PubMed] [Google Scholar]
- Ohta K., Yamazaki S., Takai T., Honjo Y., Yoshii S., Kageyama F., et al. A case of Diphyllobothrium nihonkaiense infection probably caused by eating raw trout. J. Jpn. Soc. Intern. Med. 2011;100:3336–3338. doi: 10.2169/naika.100.3336. [DOI] [PubMed] [Google Scholar]
- Scholz T., Kuchta R., Brabec J. Broad tapeworms (Diphyllobothriidae), parasites of wildlife and humans: recent progress and future challenges. Int. J. Parasitol. Parasites Wildl. 2019;9:359–369. doi: 10.1016/j.ijppaw.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J., Murata R., Sadamasu K., Araki J. Detection and identification of Diphyllobothrium nihonkaiense plerocercoids from wild Pacific salmon (Oncorhynchus spp.) in Japan. J. Helminthol. 2010;84:434–440. doi: 10.1017/S0022149x1000155. [DOI] [PubMed] [Google Scholar]
- Waeschenbach A., Brabec J., Scholz T., Littlewood D.T.J., Kuchta R. The catholic taste of broad tapeworms - multiple routes to human infection. Int. J. Parasitol. 2017;47:831–843. doi: 10.1016/j.ijpara.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Sawada M., Yanagida T., Ogawa K. Investigation of the infection with Diphyllobothrium nihonkaiense plerocercoids and Metagonimus metacercariae in freshwater salmonids cultured in Japan. Fish Pathol. 2014;49:198–201. doi: 10.3147/jsfp.49.198. [DOI] [Google Scholar]
- Wicht B., de Marval F., Peduzi R. Diphyllobothrium nihonkaiense (Yamane et al., 1986) in Switzerland: first molecular evidence and case reports. Parasitol. Int. 2007;56:195–199. doi: 10.1016/j.parint.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Wicht B., Ruggeri-Bernardi N., Yanagida T., Nakao M., Peduzzi R., Ito A. Inter- and intra-specific characterization of tapeworms of the genus Diphyllobothrium (Cestoda: dphyllobothriidea) from Switzerland, using nuclear and mitochondrial DNA targets. Parasitol. Int. 2010;59:35–39. doi: 10.1016/j.parint.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Wicht B., Yanagida T., Scholz T., Ito A., Garcia H.H., Brabec J. Multiplex PCR for differential identification of broad tapeworms (Cestoda: Diphyllobothrium) infecting humans. J. Clin. Microbiol. 2010;48:3111–3116. doi: 10.1016/j.parint.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane Y., Shiwaku K. Progress of medical parasitology in Japan. vol. 8. Meguro Parasitological Museum; Tokyo: 2003. Diphyllobothrium nihonkaiense and other marine-origin cestodes; pp. 245–259. [Google Scholar]
- Yamasaki H., Kuramochi T. A case of Diphyllobothrium nihonkaiense infection possibly linked to salmon consumption in New Zealand. Parasitol. Res. 2009;105:583–586. doi: 10.1007/s00436-009-1468-z. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Matsuoka H., Kanai T., Nakao M., Ito A. Anomalous segmentation of Diphyllobothrium nihonkaiense. Parasitol. Int. 2010;59:268–270. doi: 10.1016/j.parint.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Yera H., Estran C., Delaunay P., Gari-Toussaint M., Dupouy-Camet J., Marty P. Putative Diphyllobothrium nihonkaiense acquired from a Pacific salmon (Onchorhynchus keta) eaten in France: genomic identification and case report. Parasitol. Int. 2006;55:45–49. doi: 10.1016/j.parint.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Yera H., Nicoulaun J., Dupouy-Camet J. Use of nuclear and mitochondrial DNA PCR and sequencing for molecular identification of Diphyllobothrium isolates potentially infective for humans. Parasite. 2008;15:402–407. doi: 10.1051/parasite/2008153402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Information of the 12 patients infected with Dibothriocephalus nihonkaiensis infection examined in this study during 2007–2020.
Supplementary Table S2. Genetic distance values (Kimura 2-parameter model) among 93 Dibothriocephalus nihonkaiensis isolates and their related species.
Data Availability Statement
The sequences generated in the present study were deposited in DNA Data Bank of Japan (DDBJ) under the accession numbers LC589648-LC589661.