Abstract
Cystic echinococcosis (CE), caused by the cestode Echinococcus granulosus (sensu lato), is a serious neglected zoonotic disease in many parts of the world, including Egypt. Thus far, the actual incidence of CE in the Egyptian population remains unknown. Infection with E. granulosus (s.l.) is common among stray dogs in rural and suburban areas owing to the spread of parasite eggs. Herein, we present an updated review of published data on the incidence of CE in humans and animals as well as the genotypes prevalent in Egypt. CE occurs in most parts of Egypt; however, available data are mostly from northern Egypt, particularly Cairo and Giza. In southern Egypt, the disease is likely to be underdiagnosed or underreported. A few risk factors were studied. In the Egyptian population, residency in rural areas, farming, and age were significant factors for acquiring CE. In livestock, age, sex and season have been associated with high prevalence of CE. Several genotypes have been identified among livestock (G1, G4, G5, G6 and G7) and humans (G1, G6 and G7). This literature review underscores the need for a precise national surveillance system to track CE distribution in humans and animals and design appropriate preventive and control strategies for this disease.
Keywords: Cystic echinococcosis, Echinococcus granulosus, Hydatid, Egypt, Livestock, Prevalence, Genotypes
Graphical abstract
Highlights
-
•
A review summarizing the epidemiological data for Echinococcus granulosus (s.l.) infections in Egypt.
-
•
Egypt is an endemic area for cystic echinococcosis in the human population and livestock.
-
•
Genotypes G1, G4, G5, G6 and G7 of E. granulosus (s.l.) were reported in Egypt.
-
•
A national surveillance system to track cystic echinococcosis in Egypt is needed.
1. Introduction
Cystic echinococcosis (CE) is a cosmopolitan zoonosis caused by the cestode Echinococcus granulosus (sensu lato) (Thompson, 2008). Dogs and other canids are definitive hosts as they harbour adult tapeworms in their intestine and shed parasite eggs into the faeces. The intermediate hosts, comprising a wide range of mammalian species including humans, can be infected by ingestion of eggs through contaminated food or water. Consequently, the larva hatched from the egg develops as a hydatid cyst in the internal organs of the intermediate host, most commonly in the liver and lungs. Once ingested by the definitive host, protoscoleces develop into metacestodes and eventually become adult worms in the host intestine. Humans act as intermediate hosts after accidental ingestion of eggs that develop into hydatid cysts, causing serious morbidity and mortality unless treated (Eckert & Deplazes, 2004; Thompson, 2008).
CE accounts for 285,500 disability-adjusted life years (DALYs) globally (Budke et al., 2006). Moreover, the average annual economic burden of CE is 3 billion US dollars. This is in part due to treatment costs in human cases alongside losses in the livestock industry because of poor productivity and performance, and condemnation of the infected organs in slaughterhouses (WHO, 2015). Echinococcosis has been listed among the 17 neglected tropical diseases targeted by the WHO for disease eradication or control (WHO, 2021).
CE is endemic in South America, Central Asia, Siberia, western China, Australia, Indian subcontinent, eastern part of the Mediterranean region, sub-Saharan Africa, and northern and eastern Africa, including Egypt (Sadjjadi, 2006; Brunetti et al., 2010; Zhang et al., 2015). Egypt is a North African country with a human population of more than 100 million. Currently, it is divided into 27 governorates, and Cairo (the capital of the country) is the largest city with a total population of more than 8 million. Approximately 57% of Egyptian residents live in rural areas, whereas 43% live in urbanised cities (World Population Review, 2019). The Egyptian economy is variable, where agriculture represents a key component. Egypt has a favourable climate and supports remarkable biodiversity (Ministry of State for Environmental Affairs, 2016), which provides a conducive environment for the growth of a wide range of pathogens including parasites. Echinococcus granulosus (s.l.) is one of the endemic parasites in Egypt (Abdel Aaty et al., 2012).
A previous study has provided evidence for CE infection in Egyptian mummies, wherein two hydatid cysts were identified by a palaeoparasitologist in the Manchester Museum collection: one cyst in the brain tissue from the detached head of a mummy and the other in a biopsy sample obtained from the lung tissue (Tapp, 1986). Several studies on CE in humans and animals have been conducted in Egypt. The earliest report of E. granulosus (s.l.) in Egypt dates back to 1938 where an investigation was conducted on dogs (Azim, 1938). Thereafter, hydatid cysts were recovered from slaughtered farm animals at Cairo abattoir in 1946 (El Kordy, 1946). The first reported case of CE in humans was detected among diseases of the chest in 1965 at Cairo using radiography (Abdel-Hakim, 1965).
Considering the great economic impact and public health significance of CE in Egypt, we summarised the available epidemiological data for CE in humans and animals from this country. We conducted a literature search in PubMed, Science Direct and Google Scholar using the following search terms: “Echinococcus”, “echinococcosis”, “hydatid disease”, “hydatid cyst”, “hydatidosis”, and “Egypt”. All available publications in English language, including original articles, abstracts, case series, case reports, and clinical trials relevant to echinococcosis in humans, dogs, rats and livestock, were included. Reviews, systematic reviews, books, duplicate articles, and studies conducted in countries other than Egypt were excluded.
2. Studies on cystic echinococcosis in Egypt
2.1. Human CE in the Egyptian population
In Egypt, there are no centralised data on the national prevalence of CE, and the true incidence remains unknown. Accordingly, CE is rarely considered for diagnosis during routine medical examination in rural health centres and hospitals. It appears that physicians in these institutions lack indispensable knowledge of the disease; therefore, cases with query diagnosis are frequently referred to specialised hospitals for conclusive diagnosis and surgical intervention (Kandeel et al., 2004).
In total, 43 articles were identified as relevant to human CE in Egypt (Table 1). Generally, no data are available regarding CE from South Sinai and Red Sea governorates. Most inhabitants of these governorates are Bedouins with pastoral livestock husbandry. In addition to the low hygiene measures and health care they receive, they commonly keep dogs for livestock protection during pastoralism, facilitating echinococcosis transmission (Abbas et al., 2020). Human CE occurs in most parts of Egypt; however, available data are mostly from northern Egypt, particularly Cairo and Giza. In southern Egypt, the disease is likely to be underdiagnosed or underreported. Generally, most published reports reveal that human CE is of low endemicity in Egypt (Table 1). The earliest report of patients presenting with pulmonary disorders in Cairo showed that 0.13% of 5314 patients had hydatid cysts in the lung as observed by radiography (Abdel-Hakim, 1965). Later, Botros et al. (1975) screened hospital patients presenting with pulmonary disorders using indirect hemagglutination (IHA), latex agglutination, and bentonite flocculation tests and found that 6.2% of 755 patients had antibodies against the echinococcal antigen (Botros et al., 1975).
Table 1.
Studies investigating human cystic echinococcosis in Egypt published during 1965–2021
| Governorate | Population | No. Examined | No. Positive (%) | Detection method | Organ infected | Reference |
|---|---|---|---|---|---|---|
| Matrouh | Hospital patients | 100 | 1 (1.0) | Nested PCR | Liver | Barghash & Darwish (2019) |
| Alexandria | Hospital patients | 53 | 6 (11.3) | Immunoelectrophoresis | na | Zawawy et al. (1995) |
| Alexandria | Hospital patients | 54 | Ultrasound, CT | Liver | El-Gendi et al. (2018) | |
| Dakahlia | Hospital patients | 20 | Serology, ultrasound, CT | Liver, spleen | Abu Zeid et al. (1998) | |
| Dakahlia | Hospital patients | 4 | Ultrasound, CT, IHA | Liver | Elshazly et al. (2009) | |
| Dakahlia | Hospital patients | 147 | Abdominal CT, ELISA, MRI | Liver | El Nakeeb et al. (2017) | |
| Dakahlia | Hospital patients | 70 | 36 (51.4) | Ultrasound, CT, ELISA | Liver | Abd El-Khalek et al. (2018) |
| Cairo | Hospital patients | 5314 | 7 (0.1) | Radiography | Lung | Abdel-Hakim (1965) |
| Cairo | Hospital patients | 130 | 2 (1.5) | Ultrasonography | Liver | Salama et al. (1988) |
| Cairo | Hospital patients | 45 | Ultrasonography, IHA, ELISA | Liver | Salama et al. (1995) | |
| Cairo | Hospital patients | 362 | Ultrasonography | Liver | Salama et al. (1998) | |
| Cairo | Hospital patients | 47 | EITB | Lung | Ramadan et al. (1999) | |
| Cairo | Hospital patients | 34 | Surgery, ELISA | na | Ramzy et al. (1999) | |
| Cairo | Slaughterhouse workers/Households | na | (1.6)/(1.1) | IHA, EITB, X-ray, ultrasound, CT | Liver, lung | Ramadan & el Damaty (2000) |
| Cairo | Hospital patients | 30 | Surgical, IHA, western blotting | na | Mahmoud & Abou Gamra (2004) | |
| Cairo | Human patients | 14 | MRI, ELISA | na | El-Arousy & Ismail (2005) | |
| Cairo | Hospital patient | 1 | Ultrasound, CT, MRI, IHA | Lumbar spine | Emara & Abd Elhameed (2007) | |
| Cairo | Hospital patients | 36 | 27 (75.0) | Histopathology, HCF, PCR | Liver, lung | Abd El Baki et al. (2009) |
| Cairo | Hospital patients | 33 | Ultrasound, surgery | Liver, spleen, kidney | El Kady et al. (2011) | |
| Cairo | Hospital patients | 11 | Ultrasound, CT, IHA | Liver | Abdelaal & Dabbousdl (2014) | |
| Cairo | Hospital patients | 54 | Ultrasound, CT, ELISA | Liver | Abdelraouf et al. (2015) | |
| Cairo | Hospital patient | 1 | Ultrasound | Liver | Barghash et al. (2017) | |
| Cairo | Hospital patients | 10 | Ultrasound, CT | Liver, lung | Ibrahim & Morsy (2020) | |
| Giza | Hospital patients | 60 | ELISA, ultrasound, CT | Liver | Elsebaie et al. (2006) | |
| Giza | Hospital patients | 32 | na | Liver | Abbas et al. (2006) | |
| Giza | Humans in suburban areas | 42 | 5 (11.9) | IHA | na | Abdel-Moein & Hamza (2016) |
| Cairo, Giza | Hospital patients | 187 | 3 (1.6) | Ultrasonography, IHA | na | Abdel Wahab et al. (1996) |
| Cairo, Giza | Hospital patients | 76 | Ultrasound, CT, MRI | Liver | Abdelraouf et al. (2016) | |
| Sharkia | Hospital patients | 2 | Parasitological examination of cysts | Vertebrae | Mazyad et al. (1999) | |
| Sharkia | Hospital patients | 103 | ELISA, CT, MRI, X-ray, endoscopy | Liver | Mansy et al. (2018) | |
| Gharbia | Hospital patients | 45 | X-Ray, CT, IHA, ELISA | Liver | Salama et al. (2014) | |
| Cairo, Giza, Menofia, Suez, Alexandria, North Sinai, Matrouh | Hospital patients (retrospective study) | 492,353 | 133 (0.03) | X-Ray, ultrasound, CT, MRI, IHA, ELISA | Liver, lung, spleen, brain, kidney, pancreas | Kandeel et al. (2004) |
| Qualyobia, Cairo, Benisuef, Sharkia, Giza, Damietta | Hospital patients | 41 | na | Liver, lung | Ibrahim et al. (2007) | |
| Sharkia, Elminia | Hospital patients | 300 | 3 (1.0) | ELISA | na | Hassan et al. (1996) |
| Qualyobia, Cairo, Giza, Beheira, Fayoum, Sharkia, Benisuef, Menofia | Hospital patients | 27 | Surgery, histopathology, HCF | Liver, lung | Alam-Eldin et al. (2015) | |
| Giza, Qualyobia | Children/Adults | 18/25 | X-Ray, ultrasonography, CT, IHA, ELISA | Liver | El-Ghareeb et al. (2016) | |
| Assiut | Hospital patients | 200 | 6 (3.0) | IHA | Liver | Ghanam et al. (2001) |
| Assiut, Aswan | Hospital patients | 100 | 5 (5.0) | IHA | Liver | Dyab et al. (2005) |
| Elminia, Assiut | Hospital patients | 45 | Ultrasonography, radiology, CT, surgical, ELISA | na | Gabr et al. (2011) | |
| na | Hospital patients | 755 | 47 (6.2) | IHA, bentonite flocculation, LAT | na | Botros et al. (1975) |
| na | Hospital patients | 2 | Surgical, histology, histopathology | Spinal cord | Mazyad et al. (1998) | |
| na | Hospital patients | 2 | Echocardiography, MRI | Heart | Mohsen et al. (2009) | |
| na | Hospital patient | 1 | Imaging, histopathology | Kidney | Gadelkareem et al. (2018) |
Abbreviations: IHA, indirect hemagglutination; LAT, latex agglutination; na, not available; ELISA, enzyme-linked immunosorbent assay; CT, computed tomography; EITB, enzyme-linked immunoelectrotransfer blot; MRI, magnetic resonance imaging; HCF, hydatid cyst fluid examination; PCR, polymerase chain reaction.
In a serological survey of CE using IHA, 3% (6/200) were seropositive among patients with acute and chronic hepatic disorders in Assiut Governorate (Ghanam et al., 2001). In another study, 5% (5/100) of patients in Assiut and Aswan governorates were seropositive (Dyab et al., 2005). Among the patients presenting with fever of unknown cause, CE was detected using ultrasonography in 6% (2/34) in Cairo (Salama et al., 1998) and 1.6% (3/187) in Cairo and Giza (Abdel Wahab et al., 1996). Among patients with intracranial or spinal cystic lesions, 9 out of 14 were seropositive for hydatidosis as detected using enzyme-linked immunosorbent assay (ELISA) (El-Arousy & Ismail, 2005). In slaughterhouse workers and households, antibodies against CE were detected in 1.6% and 1.1% of individuals, respectively, using IHA and enzyme-linked immunoelectrotransfer blot (EITB) in Cairo (Ramadan & el Damaty, 2000). In another serological examination of humans living in suburban areas of Giza Governorate with farming-related jobs, 11.9% of 42 individuals were positive for CE by IHA (Abdel-Moein & Hamza, 2016). In school children presenting with hepatomegaly, 3 out of 300 (1%) were positive for CE by ELISA (Hassan et al., 1996). Recently, 18 children tested seropositive for CE in Giza and Qualyobia using IHA and ELISA (El-Ghareeb et al., 2016).
Notably, in a retrospective study conducted over three years (1997–1999) in 14 Egyptian hospitals in Cairo, Giza, Menofia, Suez, Alexandria and North Sinai, 133 (0.03%) human CE cases were recorded among 492,353 patients (Kandeel et al., 2004). According to the study, the highest annual clinical incidence of CE was noted in Matrouh Governorate (1.34–2.60 per 100,000 individuals), followed by the Giza Governorate (0.80–1.16 per 100,000 individuals), and approximately one-third of the affected patients were aged ≤ 20 years. However, there were several limitations to the retrospective survey of Kandeel et al. (2004); accurate estimates of CE incidence in the whole country could not be obtained because the study did not cover all governorates. In addition, hospital surveys do not accurately reflect the true prevalence of CE infections because asymptomatic individuals do not visit hospitals. Furthermore, some of the patientsʼ corresponding data were unavailable in the hospital records (Kandeel et al., 2004).
An interesting study was conducted in Gharbia Governorate, wherein 45 serologically (IHA and ELISA) CE-positive individuals were investigated (Salama et al., 2014). In this study, the demographic data of the patients showed that the incidence of CE was significantly higher in males than in females, and residents of rural areas were more susceptible to CE infection than those of urban areas. The age group of 30–40 years was more susceptible to CE. Additionally, a higher incidence rate of CE was observed in farmers and housewives among the occupational groups. A higher incidence in housewives was associated with dog care and agriculture in the Nile Delta of Egypt. No further studies identifying the local risk factors associated with CE in humans have been conducted in Egypt; therefore, studies identifying transmission patterns of this disease represent unmet needs.
Regarding the organs involved, the liver and lungs were the most affected organs with hydatidosis (Table 1). An unusual presentation of the hydatid disease was described in two case reports, where a shepherd man and his wife from Sharkia Governorate harboured vertebral hydatid cysts that were confirmed by parasitological examination of the cyst (Mazyad et al., 1999). Additionally, spinal cord, lumbar spine, cardiac and renal hydatid cysts have been reported elsewhere (Mazyad et al., 1998; Emara & Abd Elhameed, 2007; Mohsen et al., 2009; Gadelkareem et al., 2018).
2.2. Studies on E. granulosus (s.l.) infection in dogs
In Egypt, a high number of stray dogs are thought to contribute to the transmission of E. granulosus infection in humans and animals because they have access to livestock offal and carcasses in rural regions (Amer et al., 2015; El-Dakhly et al., 2019). Home slaughtering of animals is a common practice in Egypt, where animals are slaughtered and consumed without any authorised inspection (Amer et al., 2015; El-Dakhly et al., 2019). Accordingly, infected stray dogs have access to livestock yards and participate in widespread environmental contamination with the parasite eggs (Amer et al., 2015). Moreover, since owned pet dogs in Egypt are mostly maintained for guarding property against intruders and wild animals, they are allowed to roam freely, which maintains continuous echinococcal transmission (Macpherson & Torgerson, 2013).
The first record of E. granulosus infection in stray dogs in Egypt was from Cairo, Alexandria, and Upper Egypt in 1938, where a prevalence of 4.4% (14/320) was revealed by dissection and examination of the intestinal contents of the dogs (Azim, 1938). Rubbish dump and residues from houses and markets were the main food of those dogs investigated. Adult worms of E. granulosus (s.l.) were reported in stray dogs with a prevalence rate of 3.9% (22/570) in Cairo through examination of intestinal contents using the sedimentation-decantation technique (Moch et al., 1974). In the latter study, a higher prevalence was noted in regions where abattoirs were located. In another survey, the recovery of intestinal helminths showed the presence of E. granulosus (s.l.) in 16% (8/50) of stray dogs in Cairo (Mazyad et al., 2007). El Shazly et al. (2007) found that the overall prevalence of E. granulosus (s.l.) infection was 5% (27/540) among stray dogs in Dakahlia. These authors reported a significantly higher prevalence in rural areas (6%) than in urban areas (3.2%), and a high infection rate was noted among young and male dogs although the difference was not significant. Further studies are warranted to clarify the current situation of E. granulosus infection in stray dogs in Egypt.
Owned dogs in Egypt are also a potential source of zoonotic intestinal parasites as reported in two coprological investigations of police dogs and housed dogs (exotic and mixed breeds) where a variety of enteric parasites were detected despite following hygienic measures and deworming (Ahmed et al., 2014; Ibrahim et al., 2016). Regarding E. granulosus infection, only two investigations were conducted in pet dogs; the prevalence of E. granulosus infection was 5.1% (6/117) in Cairo using coprological examination (direct smear and brine flotation methods) (Khaled et al., 1973) and 1.8% (9/500) in Giza (Haridy et al., 2008b).
2.3. Studies on CE in livestock animals
Various livestock animals in Egypt can serve as intermediate hosts for E. granulosus (s.l.) CE was first reported in intermediate hosts in 1964 in Cairo (El Kordy, 1946). In this study, the prevalence of CE was 31% in camels (68% of cysts were fertile), 2% in sheep (64% of cysts were fertile), 16% in buffaloes (51% of cysts were fertile) and 10% in cattle. Thereafter, a study on CE in livestock was conducted in 1980 in Cairo, wherein the prevalence rates were 8.0%, 4.5%, 0.3% and 0.3% in camels, pigs, cattle and sheep, respectively (Hamdy et al., 1980). Ten years later, a study was conducted in Sharkia Governorate where 100 camels were examined with a CE prevalence of 27% (El-Ridi et al., 1990).
Thus far, 42 studies have evaluated CE in herbivorous animals (camels, cattle, buffaloes, sheep, goats, pigs and donkeys) from 1946 to 2021 (Table 2). These studies were mostly conducted in the northern part of Egypt (Giza, Cairo and Dakahlia governorates) and only limited data are available from the southern areas (Benisuef, Assiut, Sohag, Qena, Aswan and Red Sea governorates). CE infection rates within the ranges of 2.5–44.0% in camels, 0–18.4% in cattle, 0–21.8% in buffaloes, 0–50.0% in sheep, 0–19.4% in goats, 0.2–18.9% in pigs, and 0.6–20.5% in donkeys, have been reported. The discrepancy in CE prevalence rates in livestock between studies could be attributed to differences in the husbandry system, origin of slaughtered animals, eradication of stray dogs, lack of breeder awareness of the domestic life-cycle of the parasite, and personal behaviour of abattoir workers and butchers in terms of proper disposal of infected offal (Abo-Aziza et al., 2019; El-Dakhly et al., 2019).
Table 2.
Epidemiological surveys of echinococcosis in livestock in Egypt published during 1938–2021
| Governorate | Host | No. Examined | No. Positive (%) | Organ infected | Reference |
|---|---|---|---|---|---|
| Matrouh | Camels | 1512 | 207 (13.7) | Lung, liver, GIT | Barghash & Darwish (2019) |
| Sheep | 867 | 236 (27.2) | |||
| Goats | 703 | 168 (23.9) | |||
| Donkeys | 192 | 17 (8.9) | |||
| Buffaloes | 98 | 0 (0) | |||
| Cattle | 1575 | 21 (1.3) | |||
| Dakahlia | Camels | na | (2.5) | na | Haridy et al. (2006) |
| Camels | 936 | 33 (3.5) | na | Abu-Elwafa et al. (2009) | |
| Buffaloes | 648 | 1 (0.2) | na | Abu-Elwafa & Al-Araby (2008) | |
| Buffaloes | 120 | 5 (4.2) | na | Abbas (2016) | |
| Buffaloes | 205 | 5 (2.4) | na | El-Alfy et al. (2017) | |
| Cattle | 2048 | 3 (0.2) | na | Abu-Elwafa & Al-Araby (2008) | |
| Cattle | 500 | 2 (0.4) | Liver, lung | Abbas et al. (2016) | |
| Cattle | 387 | 7 (1.8) | na | El-Alfy et al. (2017) | |
| Pigs | na | 0.7 | na | Haridy et al. (2006) | |
| Cattle, buffaloes | na | (6.4) | na | Haridy et al. (2006) | |
| Sheep | 133 | 0 (0) | na | Abu-Elwafa & Al-Araby (2008) | |
| Sheep | 151 | 1 (0.7) | na | Abu-Elwafa et al. (2009) | |
| Sheep | 347 | 0 (0) | na | El-Alfy et al. (2017) | |
| Sheep, goats | na | 0.3 | na | Haridy et al. (2006) | |
| Cairo | Camels | na | (31.0) | na | El Kordy (1946) |
| Camels | 1811 | (8.0) | na | Hamdy et al. (1980) | |
| Camels | na | (31.0) | Lung, liver | Rahman et al. (1992) | |
| Camels | 441 | 33 (7.5) | Lung, liver | Mousa et al. (2015) | |
| Camels | 125 | 55 (44.0) | Liver, lung | Kandil et al. (2019) | |
| Camels | 528 | 93 (17.6) | Liver, lung | El-Kattan et al. (2020) | |
| Camels | 180 | (18.9) | na | Mahdy et al. (2014) | |
| Buffaloes | na | (16.0) | na | El Kordy (1946) | |
| Buffaloes | na | (0) | Lung, liver | Rahman et al. (1992) | |
| Cattle | na | (10.0) | na | El Kordy (1946) | |
| Cattle | 1114 | (0.3) | na | Hamdy et al. (1980) | |
| Cattle | na | (0) | Lung, liver | Rahman et al. (1992) | |
| Cattle | 90 | (3.3) | na | Mahdy et al. (2014) | |
| Pigs | 1500 | (4.5) | na | Hamdy et al. (1980) | |
| Pigs | na | (4.6) | Lung, liver | Rahman et al. (1992) | |
| Donkeys | 120 | (14.2) | na | Mahdy et al. (2014) | |
| Sheep | na | (1.5) | na | El Kordy (1946) | |
| Sheep | 2200 | (0.3) | na | Hamdy et al. (1980) | |
| Sheep | na | (1.3) | Lung, liver | Rahman et al. (1992) | |
| Giza | Zoo donkeys | 160 | 17 (10.6) | na | Haridy et al. (2008a) |
| Zoo donkeys | 83 | 17 (20.5) | Liver | Al-Kappany et al. (2016) | |
| Zoo donkeys | 40 | 4 (10.0) | Liver | Desouky et al. (2017) | |
| Zoo donkeys | 103 | 16 (15.5) | na | Ahmed et al. (2018) | |
| Donkeys | 65 | 3 (4.6) | Liver | Ahmed et al. (2011) | |
| Sheep | 200 | 100 (50.0) | na | Bauomi et al. (2015) | |
| Sharkia | Camels | 100 | 27 (27.0) | na | El-Ridi et al. (1990) |
| Camels | 936 | 33 (3.5) | Lung, liver, spleen, heart | Gab-Allah & Saba (2010) | |
| Camels | 6416 | 234 (3.7) | Liver, lung | Ahmed et al. (2021) | |
| Cattle | 100 | 3 (3.0) | Lung, liver, spleen, heart | Gab-Allah & Saba (2010) | |
| Sheep | 2314 | 91 (3.9) | |||
| Goats | 2150 | 97 (4.5) | |||
| Menofia | Camels | 209 | 61 (29.2) | Lung | El-Bahy et al. (2019) |
| Camels | 670 | 123 (18.4) | Liver, lung | El-Meleh et al. (2019) | |
| Buffaloes | 456 | 8 (1.8) | Lung | El-Bahy et al. (2019) | |
| Buffaloes | 868 | 2 (0.2) | Liver, lung | El-Meleh et al. (2019) | |
| Cattle | 993 | 0 (0) | Lung | El-Bahy et al. (2019) | |
| Cattle | 3528 | 1 (0.0) | Liver, lung | El-Meleh et al. (2019) | |
| Sheep, goats | 258 | 0 (0) | Lung | El-Bahy et al. (2019) | |
| Sheep, goats | 787 | 0 (0) | Liver, lung | El-Meleh et al. (2019) | |
| Qualyobia | Camels | 556 | 54 (9.7) | na | Hassanin et al. (2013) |
| Cattle | 590 | 75 (12.7) | na | El-Madawy et al. (2011) | |
| Cattle | 598 | 11 (1.8) | na | Hassanin et al. (2013) | |
| Sheep | 660 | 52 (7.9) | na | El-Madawy et al. (2011) | |
| Buffaloes | 383 | 9 (2.3) | na | Hassanin et al. (2013) | |
| Sheep | 198 | 15 (7.6) | na | Hassanin et al. (2013) | |
| Goats | 95 | 7 (7.4) | na | Hassanin et al. (2013) | |
| Gharbia | Buffaloes | 19,089 | 0 (0) | na | Elmonir et al. (2015) |
| Cattle | 11,281 | 0 (0) | |||
| Sheep | 14,724 | 0 (0) | |||
| Cairo, Giza, Qualyobia | Sheep | 1000 | 4 (0.4) | na | Borai et al. (2013) |
| Cattle | 825 | 5 (0.6) | |||
| Buffaloes | 1470 | 3 (0.2) | |||
| Camels | 1360 | 44 (3.2) | |||
| Cairo, Giza, Alexandria, Kafr Elsheikh, Beheira, Assiut, Elminia | Sheep | 1355 | 331 (24.4) | Lung, liver, and GIT | Barghash et al. (2017) |
| Goats | 1322 | 256 (19.4) | |||
| Pigs | 137 | 26 (18.9) | |||
| Camels | 2212 | 414 (18.7) | |||
| Donkeys | 503 | 70 (13.9) | |||
| Cattle | 1619 | 37 (2.3) | |||
| Buffaloes | 722 | 3 (0.4) | |||
| Cairo, Giza, Benisuef | Sheep | 397 | 39 (9.8) | Lung, liver | Abo-Aziza et al. (2019) |
| Cattle | 401 | 74 (18.4) | |||
| Camels | 341 | 79 (23.2) | |||
| Buffaloes | 435 | 95 (21.8) | |||
| Cairo, Benisuef | Camel | 573 | 62 (10.8) | Liver, lung, spleen | El-Dakhly et al. (2019) |
| Sheep | 4300 | 33 (0.8) | |||
| Pigs | 1235 | 3 (0.2) | |||
| Benisuef | Zoo donkeys | 145 | 10 (6.9) | Liver, lung | Aboelhadid et al. (2013) |
| Elminia | Cattle/Buffaloes | 120/50 | 6 (5.0)/2 (4.0) | Lung, liver | Dyab et al. (2019) |
| Assiut | Camels | 200 | 12 (6.0) | Liver, lung, kidney | Dyab et al. (2017) |
| Sheep | 250 | (2.4) | na | Taher & Sayed (2011) | |
| Goats | 90 | 6 (6.7) | Liver | Abdelazeem et al. (2020) | |
| New valley | Sheep | 459 | 37 (8.1) | Liver, lung, kidney | Osman et al. (2014) |
| Goats | 528 | 29 (5.5) | |||
| Red Sea, Qena, Sohag, Aswan | Sheep | 820 | 58 (14.1) | Lung, liver, viscera | Omar et al. (2013) |
| Goats | 130 | 17 (13.1) | |||
| Camels | 240 | 12 (5.0) | |||
| Cattle | 2910 | 2 (0.1) | |||
| Buffaloes | 398 | 0 (0) | |||
| Aswan | Camels/Sheep | 2080/674 | 173 (8.3)/3 (0.54) | Lung, liver | Dyab et al. (2018) |
| Assiut and Aswan | Camels | 1395 | 107 (7.7) | na | Dyab et al. (2005) |
| na | Camels | 400,159 | (5.5) | Lung, liver | Haridy et al. (1998) |
| (6.1) | |||||
| (6.7) | |||||
| (8.2) | |||||
| (4.3) |
Abbreviations: na, not available; GIT, gastrointestinal tract.
Among all livestock, a higher prevalence rate was frequently detected in camels than in other animals. For instance, in Menofia and Cairo, prevalence rates of 17.6–44.0% have been reported in camel herds (El-Meleh et al., 2019; El-Bahy et al., 2019; Kandil et al., 2019; El-Kattan et al., 2020). This observation could be attributed to the importation of camels from countries bordering Egypt, such as Libya and Sudan where CE is endemic and the pastoral system of camels breeding along with nearby dogs exist (Omar et al., 2013; El-Dakhly et al., 2019; Abbas et al., 2020). Unfortunately, the demographic data and the associated risk factors for slaughtered camels are not available.
The highest prevalence rates of CE infection in cattle (18.4% of 401) and buffaloes (21.8% of 435) were recorded in Cairo, Giza and Benisuef (Abo-Aziza et al., 2019). Additionally, the highest prevalence rate (50.0% of 200) in sheep was reported in Giza (Bauomi et al., 2015), whereas that in goats (23.9% of 703) originated from Matrouh Governorate (Barghash et al., 2019). One reason for this observation is that northern Egypt has a greater livestock and dog density and favourable temperatures, supporting the survival of E. granulosus eggs and leading to a high risk of CE infection (UNDP, 2013; FAO, 2017). However, studies investigating the effect of dog/livestock populations and different environmental and climatic factors (e.g. temperature, humidity and rainfall) on the distribution of CE in humans or livestock in Egypt are lacking.
In donkeys slaughtered at Giza Zoo, a relatively high prevalence ranging from 17.0% to 20.0% was recorded (Haridy et al., 2008a; Al-Kappany et al., 2016; Ahmed et al., 2018). These donkeys were native breeds reared in Egyptian villages before being transferred to the Giza Zoo for feeding wild animals, and they were likely to be infected through contaminated pastures with parasite eggs. Practices such as feeding carnivores and wild animals in the Zoo on donkeys and improper disposal of donkeys’ carcasses into water canals or cultivated land facilitate the access to dogs and spread of E. granulosus (s.l.) infection in Egypt (Aboelhadid et al., 2013).
Pigs are not popular in Egypt owing to religious considerations. Most pigs are raised in farm breeding systems in Cairo, Qalyobiya and Dakahlia governorates rather than at a small scale which reduces pig exposure to infected dogs (El-Dakhly et al., 2019). Previous investigations have shown a low prevalence of CE in pigs in Cairo and Dakahlia except for the report by Barghash et al. (2017) who recorded a relatively high prevalence (18.9% of 137) of CE in Cairo. However, no data on the pig breeding system and the associated risk factors for acquiring CE are available.
Twenty-two studies investigated hydatid cyst localisation in different organs of livestock and demonstrated that most cysts were located in the lungs and liver (Table 2). The presence of fertile cysts varied between 0% and 100% in slaughtered animals; for instance, high fertility rates of 73.4% (Mousa et al., 2015) and 32.8% (Kandil et al., 2019) were recorded in camels slaughtered in Cairo Governorate and a fertility rate of 41.2% (Al-Kappany et al., 2016) was recorded in Zoo donkeys in Giza Governorate. A recent survey performed in Matrouh Governorate recorded high cyst fertility rates of 70.1%, 33.1%, 37.5% and 17.6% in camels, sheep, goats and donkeys, respectively (Barghash & Darwish, 2019).
2.4. Studies on E. granulosus infection in wildlife
Assessment of the potential of wild animals as transmitters of E. granulosus (s.l.) is important for carrying out control measures. Interestingly, a recent investigation of rats from rural and suburban areas of Giza and Cairo governorates using IHA revealed that antibodies against E. granulosus was detected in 36% (18 out of 50) of Rattus norvegicus rats (Abdel-Moein & Hamza, 2016). Hydatid cysts were identified in the liver of three rats using histopathology (one rat) and duplex polymerase chain reaction (PCR; two rats). Genotyping of these cysts showed that they were genotype G6. It is generally recognised that rodents do not serve as an intermediate host for E. granulosus (s.l.). A previous study investigated the role of rodents (Clethrionomys gapperi, Microtus pennsylvanicus and Peromyscus leucopus) in the epidemiological life-cycle of E. granulosus and showed that none of the examined species were susceptible to both experimental and natural infection with E. granulosus (Gibbs, 1957). Unfortunately, the histopathological findings of the earlier report did not clearly prove that the infection was due to the hydatid cyst, and the sequence data were not provided; thus, validation of the findings could not be performed.
3. Risk factors associated with CE in Egypt
In endemic areas worldwide, a high prevalence of CE is associated with pastoral communities, poor water resources, abundance of stray dogs, frequency of dog-human contact, poor hygienic conditions of abattoirs, and improper disposal of carcasses (Pawłowski et al., 2001; Njoroge et al., 2002; Budke et al., 2005; Wang et al., 2014). In the Egyptian population, residency in rural areas, occupation (farmers and housewives), and age (30–40 years) were significant factors for acquiring CE (Salama et al., 2014). Likewise, high prevalence rates were recorded in the age group of less than 40 years in Iran, Lebanon and Turkey (Akalin et al., 2014; Mahmoudi et al., 2019; Joanny et al., 2021).
In a systematic analysis of the global risk factors for hydatid disease in livestock, several factors including locations, host species, age, sex, seasonal variations, and environmental factors were associated with the diseases burden (Otero-Abad & Torgerson, 2013). In Egyptian livestock, age, sex and season have been associated with high prevalence of CE in camels and sheep (Dyab et al., 2018a; Ahmed et al., 2021). Female camels, for example, were more susceptible to CE infection than male camels possibly because of the slaughtering of female camels at an older age, as they are maintained for reproduction (Dyab et al., 2018a). In addition, older camels exhibited a higher prevalence of CE than younger camels presumably because camels are frequently slaughtered at an advanced age, resulting in a higher exposure rate to the parasites (Dyab et al., 2018a; Ahmed et al., 2021). Moreover, a significantly higher infection rate of CE was observed in sheep in the autumn season (Dyab et al., 2018b), whereas in camels the higher rate was observed in the winter season (Ahmed et al., 2021). Seasonal variation in the prevalence of CE in livestock was also observed in Saudi Arabia (Ibrahim et al., 2008; Ibrahim, 2010).
4. Genotypes/strains of E. granulosus (s.l.) in Egypt
Based on the phenotypes and molecular data, E. granulosus (s.l.) was divided into five species: E. granulosus (sensu stricto) (sheep strain, genotypes G1-G3); Echinococcus equinus (horse strain, genotype G4); Echinococcus ortleppi (cattle strain, genotype G5); Echinococcus canadensis (camel strain, genotype G6; pig strain, genotype G7; cervid strains, genotypes G8 and G10); and Echinococcus felidis (lion strain, no genotype assigned) (Nakao et al., 2013a, 2013b; Alvarez Rojas et al., 2014; Romig et al., 2015).
Across Africa, several genotypes and species/strains of E. granulosus (s.l.) are distributed. For instance, genotypes G6/G7 (E. canadensis) accounted for 60.3% and 97.4% of the total isolates from North and West Africa, respectively. Genotypes G1 and G3 (E. granulosus (s.s.)) accounted for 74.45% of isolates from East Africa, whereas genotype G5 (E. ortleppi) was recorded in 81.3% of isolates from South Africa (Ohiolei et al., 2020).
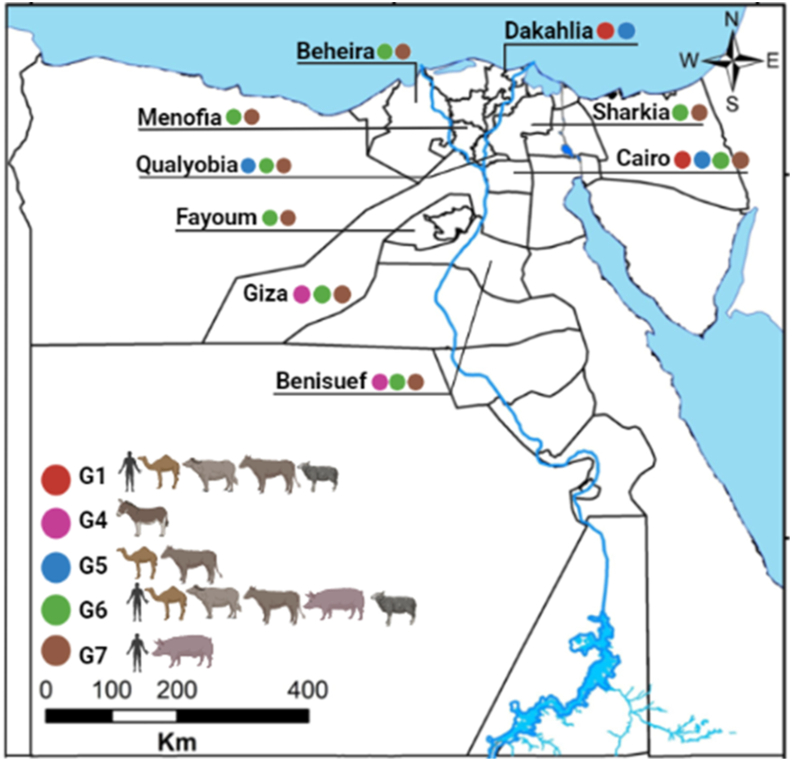
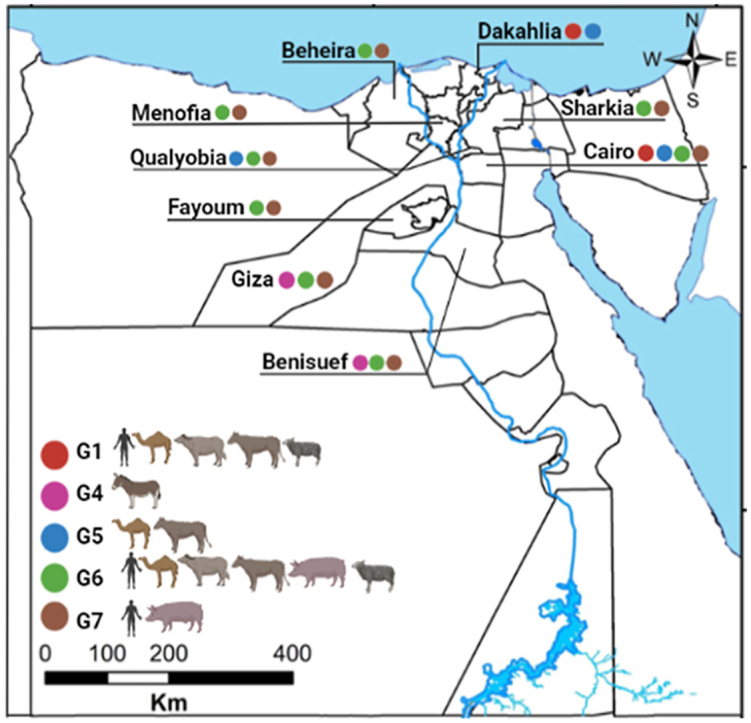
In Egypt, a considerable number of molecular studies have been performed to characterise hydatid cysts from several intermediate hosts (Table 3) by PCR amplification and sequencing of mitochondrial markers (cox1, 12S rRNA and nad1), nuclear marker (actin II), or internal transcribed spacer 1 (ITS1) sequences. The identified genotypes and strains of E. granulosus (s.l.) include G1 (E. granulosus (s.s.) from camel, sheep, buffalo and cattle), G4 (from donkey), G5 (from camel and cattle), G6 (from camel, pig, sheep, buffalo, cattle and Norway rat), and G7 (from pig) (Fig. 1) (Abd El Baki et al., 2009; Abdel Aaty et al., 2012; Aboelhadid et al., 2013; Khalifa et al., 2014; Alam-Eldin et al., 2015; Amer et al., 2015; Abbas, 2016; Abbas et al., 2016; Abdel Aziz & Meghanawy, 2016; Abdel-Moein & Hamza, 2016; Desouky et al., 2017; Mousa et al., 2020). Given that the majority of camels slaughtered at abattoirs in Cairo and Qualyobia governorates are imported from Sudan where G5 infections have been reported in camels (Omer et al., 2010; Ibrahim et al., 2011; Ahmed et al., 2013), it is likely to identify the same genotype in Egypt.
Table 3.
Genetic diversity of Echinococcus granulosus in Egypt in humans and animals
| Genotype | No. of isolates | Host | Gene marker | Governorate | Reference |
|---|---|---|---|---|---|
| G1 | 2 | Buffaloes | cox1 | Dakahlia | Abbas (2016) |
| G1 | 1 | Cattle | cox1 | Dakahlia | Abbas et al. (2016) |
| G5 | 1 | ||||
| G6 | 47 | Camels | 12S rRNA | Cairo | Abdel Aaty et al. (2012) |
| G6 | 6 | Pigs | |||
| G6 | 30 | Human | |||
| G1 | 1 | Human | |||
| G6 | 26 | Camels | cox1, nad1, ITS1 | Cairo | Amer et al. (2015) |
| G1 | 1 | Camels | |||
| G5 | 1 | Camels | |||
| G6 | 3 | Sheep | |||
| G1 | 4 | Sheep | |||
| G1 | 2 | Buffaloes | |||
| G6 | 5 | Human | nad1 | Qualyobia | Khalifa et al. (2014) |
| G6 | 20 | Camels | |||
| G6 | 49 | Camels | cox1, nad1 | Qualyobia | Abdel Aziz & Meghanawy (2016) |
| G5 | 6 | ||||
| G6 | 40 | Camels | Qualyobia, Cairo, Giza, Beheira, Fayoum, Sharkia, Benisuef, Menofia | Alam-Eldin et al. (2015) | |
| G6, G7 | 5 | Pigs | |||
| G6, G7 | 26 | Human | |||
| G4 | 10 | Donkeys | cox1, nad1 | Benisuef | Aboelhadid et al. (2013) |
| G6 | 2 | Norway rats | na | Giza | Abdel-Moein & Hamza (2016) |
| G1 | na | Human | na | na | Abd El Baki et al. (2009) |
| G4 | 4 | Donkeys | nad1 | Giza | Desouky et al. (2017) |
| G4 | 3 | Donkeys | ITS1 | na | Mousa et al. (2020) |
| G6 | 6 | Camels | ITS1 | na | Mousa et al. (2020) |
| G1 | 1 | Cattle | ITS1 | na | Mousa et al. (2020) |
| G1 | 1 | Sheep | ITS1 | na | Mousa et al. (2020) |
Abbreviation: na, not available.
Fig. 1.
Map of Egypt demonstrating the geographical distribution of Echinococcus granulosus (s.l.) genotypes isolated from different intermediate hosts in Egyptian governorates. A colored bullet indicates a specific genotype. The map was downloaded from http://www.gadm.org and modified by ArcGis 10.5 software.
Only three genotypes (G1, G6 and G7) have been identified from the Egyptian population (Abd El Baki et al., 2009; Abdel Aaty et al., 2012; Khalifa et al., 2014; Alam-Eldin et al., 2015). G6 was the dominant genotype among human isolates with a frequency of 58.1% followed by G7 (40.3%) and G1 (1.6%) (Table 3). Since the number of samples analysed so far was low, it is possible that other genotypes such as G5 may exist in humans. These genotyping studies were performed in only seven of the 27 governorates. A larger study analysing a number of human isolates from multiple geographical areas is needed to clarify the epidemiology of CE in the Egyptian population.
5. Conclusion and recommendations
Although there are numerous published articles on CE in animals and humans from Egypt, there is no statistically valid nationwide survey, and no central laboratory or researcher group is actively investigating CE in the Egyptian population and livestock. The present review suggests that CE is endemic and neglected by health authorities and thus is likely to be underestimated in many regions of Egypt. Given that precise epidemiological surveys with a representative sample size are lacking and that most data are from case reports, the actual prevalence of CE is not clear in humans. Nevertheless, variable infection rates in livestock animals suggest an urgent need to carry out a comprehensive, well-structured study to evaluate CE burden in humans and to identify risk factors for acquiring E. granulosus (s.l.) infection in different geographical areas. Additionally, molecular characterization of the parasites from diverse intermediate hosts, including humans, through a large-scale study, is needed to critically assess their role in the epidemiology of CE in Egypt.
Funding
This research was funded by JSPS KAKENHI grant number 20K06402.
CRediT author statement
Abdelbaset Eweda Abdelbaset: conceptualization, formal analysis, investigation, data curation, writing–original draft. Kinpei Yagi: writing - review & editing, funding acquisition. Nariaki Nonaka: resources, writing - review & editing. Ryo Nakao: conceptualization, writing - review & editing, supervision, funding acquisition. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Dr Ahmed Abdelhalim, Department of Geology, Assiut University, Egypt for providing the map.
References
- Abbas I. Molecular and epidemiological updates on cystic echinococcosis infecting water buffaloes from Egypt. Vet. World. 2016;9:1355–1363. doi: 10.14202/vetworld.2016.1355-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas I.E.A., Al-Kappany Y.M., Al-Araby M.A. Prevalence and molecular characterization of hydatid cyst isolates from cattle in Egypt. Asian J. Anim. Vet. Adv. 2016;11:794–804. doi: 10.3923/ajava.2016.794.804. [DOI] [Google Scholar]
- Abbas M., Nafeh A.I., Youssef Y.F., Nasr M.M., Radwan H.S. Conservative versus radical surgery for treatment of uncomplicated hepatic hydatid cysts. J. Egypt. Soc. Parasitol. 2006;36:559–576. [PubMed] [Google Scholar]
- Abbas I.E., Villena I., Dubey J.P. A review on toxoplasmosis in humans and animals from Egypt. Parasitology. 2020;147:135–159. doi: 10.1017/S0031182019001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El Baki M.H., El Missiry A.M.G., Abd El Aaty H.E.M., Mohamad A.A., Aminou H.A.R. Detection of G1 genotype of human cystic echinococcosis in Egypt. J. Egypt. Soc. Parasitol. 2009;39:711–721. [PubMed] [Google Scholar]
- Abd El-Khalek A.M., El-Sayed N.T.M., Elalfy H., Besheer T., Farid K., ElAgezy M., et al. Percutaneous US-guided combined ethanol and tetracycline injection for treating symptomatic simple and hydatid hepatic cysts. Egypt. J. Radiol. Nucl. Med. 2018;49:797–803. doi: 10.1016/j.ejrnm.2018.04.005. [DOI] [Google Scholar]
- Abdel Aaty H.E., Abdel-Hameed D.M., Alam-Eldin Y.H., El-Shennawy S.F., Aminou H.A., Makled S.S., et al. Molecular genotyping of Echinococcus granulosus in animal and human isolates from Egypt. Acta Trop. 2012;121:125–128. doi: 10.1016/j.actatropica.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Abdel Aziz A.R., Meghanawy R.A. El. Molecular characterization of hydatid cyst from Egyptian one humped camels (Camelus dromedaries) PSM Vet. Res. 2016;1:13–16. [Google Scholar]
- Abdel Wahab M.F., Younis T.A., Fahmy I.A., el Gindy I.M. Parasitic infections presenting as prolonged fevers. J. Egypt. Soc. Parasitol. 1996;26:509–516. [PubMed] [Google Scholar]
- Abdelaal A., Dabbousdl H. Hydatid disease of the liver: laparoscopic approach, initial result in Egypt. J. Egypt. Soc. Parasitol. 2014;44:619–625. [PubMed] [Google Scholar]
- Abdelazeem A., Dyab A., Mohamed Y. Helminthic infection in digestive system of goats in slaughterhouse, Manfalout, Assiut governorate, Egypt. J. Egypt. Soc. Parasitol. 2020;50:2090–2549. [Google Scholar]
- Abdel-Hakim M. Parasitic lung disease. Dis. Chest. 1965;48:580–583. doi: 10.1378/chest.48.6.580. [DOI] [Google Scholar]
- Abdel-Moein K.A., Hamza D.A. Norway rat (Rattus norvegicus) as a potential reservoir for Echinococcus granulosus: A public health implication. Acta Parasitol. 2016;61:815–819. doi: 10.1515/ap-2016-0113. [DOI] [PubMed] [Google Scholar]
- Abdelraouf A., El-Aal A.A., Shoeib E.Y., Attia S.S., Hanafy N., Hassani M., et al. Clinical and serological outcomes with different surgical approaches for human hepatic hydatidosis. Rev. Soc. Bras. Med. Trop. 2015;48:587–593. doi: 10.1590/0037-8682-0223-2015. [DOI] [PubMed] [Google Scholar]
- Abo-Aziza F.A.M., Oda S.S., Aboelsoued D., Farag T.K., Almuzaini A.M. Variabilities of hydatidosis in domestic animals slaughtered at Cairo and Giza abattoirs, Egypt. Vet. World. 2019;12:998–1007. doi: 10.14202/vetworld.2019.998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboelhadid S.M., El-Dakhly K.M., Yanai T., Fukushi H., Hassanin K.M. Molecular characterization of Echinococcus granulosus in Egyptian donkeys. Vet. Parasitol. 2013;193:292–296. doi: 10.1016/j.vetpar.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Abu Zeid M., El-Eibiedy G., Abu-El-Einien A., Gad El-Hak N., Abd El-Wahab M., Azzat F. Surgical treatment of hepatic hydatid cysts. Hepatogastroenterology. 1998;445:1802–1806. [PubMed] [Google Scholar]
- Abu-Elwafa S., Al-Araby M. Prevalence of tissue parasites among slaughtered animals in Dakahlia province. Mansoura Vet. Med. J. 2008;10:79–91. [Google Scholar]
- Abu-Elwafa S., Al-Araby M., Abbas I. Metacestodes among sheep slaughtered at Mansoura abattoir, Dakahlia province, Egypt. Mansoura Vet. Med. J. 2009;11:21–33. [Google Scholar]
- Ahmed B., Desouky E., Abd-Elghany A., Gouda A.A. Prevalence of cystic echinococcosis in Egyptian donkeys. Egypt. Vet. Med. Soc. Parasitol. J. 2018;14:115–120. doi: 10.21608/evmspj.2018.35326. [DOI] [Google Scholar]
- Ahmed M.E., Eltom K.H., Musa N.O., Ali I.A., Elamin F.M., Grobusch M.P., et al. First report on circulation of Echinococcus ortleppi in the one-humped camel (Camelus dromedarius ), Sudan. BMC Vet. Res. 2013;9 doi: 10.1186/1746-6148-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W.M., Mousa W.M., Aboelhadid S.M., Tawfik M.M. Prevalence of zoonotic and other gastrointestinal parasites in police and house dogs in Alexandria, Egypt. Vet. World. 2014;7:275–280. doi: 10.14202/vetworld.2014.275-280. [DOI] [Google Scholar]
- Ahmed A.B., Ras R., Mahmoud A.F., El-Ghazaly E., Widmer G., Dahshan H., Elsohaby I. Prevalence and bacterial isolation from hydatid cysts in dromedary camels (Camelus dromedarius) slaughtered at Sharkia abattoirs, Egypt. J. Parasit. Dis. 2021;45:236–243. doi: 10.1007/s12639-020-01300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kappany Y., Awadin W., Abd El-Wahed O., Elharery A. Parasitological and pathological studies on hepatic hydatidosis in donkeys from Egypt. Egypt. Vet. Med. Soc. Parasitol. J. 2016;12:25–34. doi: 10.21608/evmspj.2016.37004. [DOI] [Google Scholar]
- Akalin S., Kutlu S.S., Caylak S.D., Onal O., Kaya S., Bozkurt A.I. Seroprevalence of human cystic echinococcosis and risk factors in animal breeders in rural communities in Denizli, Turkey. J. Infect. Develop. Ctries. 2014;8:1188–1194. doi: 10.3855/jidc.4343. [DOI] [PubMed] [Google Scholar]
- Alam-Eldin Y., Abdel Aaty H., Ahmed M. Molecular characterization of cystic echinococcosis: First record of G7 in Egypt and G1 in Yemen. Acta Parasitol. 2015;60:662–665. doi: 10.1515/ap-2015-0094. [DOI] [PubMed] [Google Scholar]
- Alvarez Rojas C.A., Romig T., Lightowlers M.W. Echinococcus granulosus sensu lato genotypes infecting humans - review of current knowledge. Int. J. Parasitol. 2014;44:9–18. doi: 10.1016/j.ijpara.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Amer S., Helal I.B., Kamau E., Feng Y., Xiao L. Molecular characterization of Echinococcus granulosus sensu lato from farm animals in Egypt. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim M.A. On the intestinal helminths of dogs in Egypt. J. Egypt. Med. Assoc. 1938;21:118–122. [Google Scholar]
- Barghash S., Darwish A. Epidemiology and genetic variants within Echinococcus granulosus identified based on ITS-1 ribosomal DNA in north-west Egypt. J. Anim. Vet. Adv. 2019;18:187–194. doi: 10.36478/javaa.2019.187.194. [DOI] [Google Scholar]
- Barghash S., El-sayed R., Shaimaa A., Ahmes M. Prevalence and molecular identification of Echinococcus granulosus in humans and slaughtered animals in Egypt. Eur. J. Biomed. Pharm. Sci. 2017;4:34–42. [Google Scholar]
- Bauomi I.R., El-Amir A.M., Fahmy A.M., Zalat R.S., Diab T.M. Evaluation of purified 27.5 kDa protoscolex antigen-based ELISA for the detection of circulating antigens and antibodies in sheep and human hydatidosis. J. Helminthol. 2015;89:577–583. doi: 10.1017/S0022149X14000479. [DOI] [PubMed] [Google Scholar]
- Borai M.G.E., Nagi A.R.A., Gab-Allah M.S., Baset A., El-Mashad I., Moustafa S.A. Comparative pathological studies on parasitic affections of liver in farm animals. Benha Vet. Med. J. 2013;25:284–295. [Google Scholar]
- Botros B.A.M., Moch R.W., Barsoum I.S., Mahmoud A.H., S Fahmi M.S.E.-L. Echinococcosis in Egypt: Evaluation of the indirect haemagglutination and latex agglutination tests for echinococcal serologic surveys. J. Trop. Med. Hyg. 1975;76:243–247. [PubMed] [Google Scholar]
- Brunetti E., Kern P., Vuitton D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Budke C.M., Deplazes P., Torgerson P.R. Global socioeconomic impact of cystic echinococcosis. Emerg. Infect. Dis. 2006;12:296–303. doi: 10.3201/eid.1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budke C.M., Jiamin Q., Craig P.S., Torgerson P.R. Modeling the transmission of Echinococcus granulosus and Echinococcus multilocularis in dogs for a high endemic region of the Tibetan plateau. Int. J. Parasitol. 2005;35:163–170. doi: 10.1016/j.ijpara.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Desouky A.Y., Helmy N.M., Sorour S.S., Amer M.M. Prevalence and molecular studies on Echinococcus equinus isolated from necropsied donkeys. Iraqi J. Vet. Sci. 2017;31:101–106. doi: 10.33899/ijvs.2017.145605. [DOI] [Google Scholar]
- Dyab A.K., Ahmed H.A., Hefnawy Y.A., Abdel Aziz A.R., Gomaa M.M. Prevalence of tissue parasites in cattle and buffaloes slaughtered in El-Minia governorate abattoirs, Egypt. PSM Vet. Res. 2019;4:49–58. [Google Scholar]
- Dyab K.A., Hassanein R., Hussein A.A.A., Metwally S.E., Gaad H.M. Hydatidosis among man and animals in Assiut and Aswan governorates. J. Egypt. Soc. Parasitol. 2005;35:157–166. [PubMed] [Google Scholar]
- Dyab A.K., Marghany M.E., Othman R.A., Ahmed M.A., Abd-Ella O.H. Hydatidosis of camels and sheep slaughtered in Aswan governorate, southern Egypt. Russ. J. Parasitol. 2018;12:33–41. doi: 10.31016/1998-8435-2018-12-3-33-41. [DOI] [Google Scholar]
- Dyab A.K., Marghany M.E., Othman R.A., Ahmed M.A., Abd-Ella O.H. Hydatidosis of camels and sheep slaughtered in Aswan Governorate, southern Egypt. Russ. J. Parasitol. 2018;12:33–41. doi: 10.31016/1998-8435-2018-12-3-33-41. [DOI] [Google Scholar]
- Dyab A., Mohamed G., Abdella O. Seroprevalence of hydatidosis in camels of Assuit province, Egypt. Madridge J. Vaccines. 2017;1:5–8. doi: 10.18689/mjv.2017-101. [DOI] [Google Scholar]
- Eckert J., Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kady N., Ramzy I., Hanan H., Haleem A., El-Bahnasawy M. Echoguided pair technique in diagnosis and treatment of abdominal hydatid cystic disease in Egyptian patients: clnical and ultrasonographic follow up. J. Egypt. Soc. Parasitol. 2011;41:527–542. [PubMed] [Google Scholar]
- El Kordy M.I. On the incidence of hydatid disease in domestic animals in Egypt. J. Egypt. Med. Assoc. 1946;29:265–279. [Google Scholar]
- El Nakeeb A., Salem A., El Sorogy M., Mahdy Y., Abd Ellatif M., Moneer A., et al. Cystobiliary communication in hepatic hydatid cyst: Predictors and outcome. Turkish J. Gastroenterol. 2017;28:125–130. doi: 10.5152/tjg.2017.17553. [DOI] [PubMed] [Google Scholar]
- El Shazly A.M., Awad S.E., Nagaty I.M., Morsy T.A. Echinococcosis in dogs in urban and rural areas in Dakahlia Governorate, Egypt. J. Egypt. Soc. Parasitol. 2007;37:483–492. [PubMed] [Google Scholar]
- el Zawawy L.A., el Nassery S.F., al Azzouni M.Z., Abou el Naga I.F., el Temsahi M.M., Awadalla H.N. A study on patients with eosinophilia of suspected parasitic origin. J. Egypt. Soc. Parasitol. 1995;25:245–255. [PubMed] [Google Scholar]
- El-Alfy E., Al-kappany Y., Abuelwafa S. Parasitological and pathological studies on tissue parasites among slaughtered animals in Dakahlia province, Egypt. Egypt. Vet. Med. Soc. Parasitol. J. 2017;13:78–98. doi: 10.21608/evmspj.2017.37734. [DOI] [Google Scholar]
- El-Arousy M., Ismail S.A. Cerebrospinal echinococcosis: Serodiagnosis using different hydatid cyst fluid antigens. J. Egypt. Soc. Parasitol. 2005;35:193–204. [PubMed] [Google Scholar]
- El-Bahy N., El-Bagory A.-E.-R., AbouLaila M., Elkhatam A., Mady H. Prevalence of Sarcocystis fusiformis and hydatid cyst among different ruminants at Menofia Governorate, Egypt. J. Curr. Vet. Res. 2019;1:1–10. doi: 10.21608/jcvr.2019.10307.1001. [DOI] [Google Scholar]
- El-Dakhly K.M., Arafa W.M., El-Nahass E.S.N., Shokier K.A.M., Noaman A.F. The current prevalence and diversity of cystic echinococcosis in slaughtered animals in Egypt. J. Parasit. Dis. 2019;43:711–717. doi: 10.1007/s12639-019-01151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gendi A.M., El-Shafei M., Bedewy E. The role of prophylactic endoscopic sphincterotomy for prevention of postoperative bile leak in hydatid liver disease: A randomized controlled study. J. Laparoendosc. Adv. Surg. Tech. 2018;28:990–996. doi: 10.1089/lap.2017.0674. [DOI] [PubMed] [Google Scholar]
- El-Ghareeb A., Waked N., Al-Feky H. Clinical and parasitological studies on pulmonary and hepatic hydatid cysts in hospitalized children and adults. J. Egypt. Soc. Parasitol. 2016;46:9–18. doi: 10.12816/0026145. [DOI] [PubMed] [Google Scholar]
- El-Kattan A.,M., Abdel-Ra’ouf A., Yousef R., AbouElnga T., Mousa W. Sensitivity and specificity of indirect enzyme-linked immunosorbent assay (ELISA) for diagnosis of hydatidosis in dromedary camels using hydatid cyst fluid antigens. J. Vet. Med. Res. 2020;27:66–75. doi: 10.21608/jvmr.2020.69696. [DOI] [Google Scholar]
- El-Madawy R., Khalifa N., Afify S. Epidemiological and molecular studies of hydatid cyst in slaughtered cattle and sheep in Toukh, Egypt. Benha Vet. Med. J. 2011;1:95–101. [Google Scholar]
- El-Meleh G., El-Meghanawy R., Sabike I., Hassan M. Parasitic affections of edible offals of slaughtered animals at El-Shohada abattoir, Menofia Governorate, Egypt. Benha Vet. Med. J. 2019;36:117–128. doi: 10.21608/bvmj.2019.13933.1031. [DOI] [Google Scholar]
- Elmonir W., Mousa W., Sultan K. The prevalence of some parasitic zoonoses in different slaughtered animal species at abattoir in the Mid-Delta of Egypt; with special reference to its economic implications. Alexandria J. Vet. Sci. 2015;47:97. doi: 10.5455/ajvs.204290. [DOI] [Google Scholar]
- El-Ridi A., Habeeb Y., Nada S., Nada M. Echinococcosis in camels as revealed by indirect haemagglutination test in Belbas. J. Egypt. Soc. Parasitol. 1990;20:95–98. [PubMed] [Google Scholar]
- Elsebaie S.B., El-Sebae M.M.A, Esmat M.E., Nasr M.M., Kamel M.M. Modified endocystectomy versus pericystectomy in echinococcus granulosus liver cysts: a randomized controlled study, and the role of specific anti-hydatid IgG in detection of early recurrence. J. Egypt. Soc. Parasitol. 2006;36:993–1006. [PubMed] [Google Scholar]
- Elshazly A.M., Azab M.S., ElBeshbishi S.N., Elsheikha H.M. Hepatic hydatid disease: Four case reports. Cases J. 2009;2:1–4. doi: 10.1186/1757-1626-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara K., Abd Elhameed D. Hydatid disease of the lumbar spine: Combined surgical and medical treatment - a case report. Am. J. Orthopsychiatry (Belle Mead NJ) 2007;36:E12–E14. [PubMed] [Google Scholar]
- FAO . Food and Agriculture Organization; Rome: 2017. Africa sustainable livestock 2050 - Egypt.https://www.fao.org/home/en/ [Google Scholar]
- Gab-Allah H., Saba S. Incidance of hydatid cysts in slaughtered animals and their relation to public health at Sharkia province. Egypt. J. Agric. Res. 2010;88:285–290. [Google Scholar]
- Gabr N., Shatat M., Ramadan M. Studies on diagnosis of hydatid disease in Minia and Assiut governorates. Al-Azhar Assiut Med. J. 2011;9:70–81. [Google Scholar]
- Gadelkareem R.A., Elqady A.A., Abd-Elshafy S.K., Imam H., Abolella H.A. Isolated renal hydatid cyst misdiagnosed and operated as a cystic renal tumor. Med. Princ. Pract.: Int. J. Kuwait Univ. Health Sci. Cent. 2018;27:297–300. doi: 10.1159/000488878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanam M.E., Shataat M.A., Monib M. el S., Hassan A.A., Younis A.I. Evaluation of the role of some parasitic infections as a cause of acute and chronic hepatic diseases. J. Egypt. Soc. Parasitol. 2001;31:37–42. [PubMed] [Google Scholar]
- Gibbs H.C. On the role of rodents in the epidemiology of hydatid disease in the Mackenzie River Basin. Can. J. Comp. Med. Vet. Sci. 1957;21:287–289. [PMC free article] [PubMed] [Google Scholar]
- Hamdy I.I., Mikhail E.G., Soliman A.A., Hamed H.H. A study on hydatidosis in some animals in Egypt. J. Egypt. Soc. Parasitol. 1980;10:43–51. [Google Scholar]
- Haridy F.M., Abdel Gawad A.G.E., Ibrahim B.B., Hassan A.A., El-Sherbi G.T., El Shazly A.M., et al. Zoonotic hydatidosis in donkeys: Post mortum examination in the Zoo, Giza, Egypt. J. Egypt. Soc. Parasitol. 2008;38:305–312. [PubMed] [Google Scholar]
- Haridy F., Badawiya B., Elshazly A., Awad S., Sultan D., El-Sherbini G., et al. Hydatidosis granulosus in Egyptian slaughtered animals in the years 2000-2005. J. Egypt. Soc. Parasitol. 2006;36:1087–1100. [PubMed] [Google Scholar]
- Haridy F.M., Holw S.A.A., Hassan A.A., Morsy T.A. Cystic hydatidosis: A zoonotic silent health problem. J. Egypt. Soc. Parasitol. 2008;38:635–644. [PubMed] [Google Scholar]
- Haridy F.M., Ibrahim B.B., Morsy T.A. Studies on hydatidosis in slaughtered camels in Egypt. J. Egypt. Soc. Parasitol. 1998;28:673–681. [PubMed] [Google Scholar]
- Hassan M.M., Farghaly A.M., Gaber N.S., Nageeb H.F., Hegab M.H., Galal N. Parasitic causes of hepatomegaly in children. J. Egypt. Soc. Parasitol. 1996;26:177–189. [PubMed] [Google Scholar]
- Hassanin F., Shaltout F., Afifi M. Parasitic affections in edible offal. Benha Vet. Med. J. 2013;25:46–55. [Google Scholar]
- Ibrahim M.M. Study of cystic echinococcosis in slaughtered animals in Al Baha region, Saudi Arabia: Interaction between some biotic and abiotic factors. Acta Trop. 2010;113:26–33. doi: 10.1016/J.ACTATROPICA.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Ibrahim M.M., Ahmad M., Ghamdi A.L., Gahmdi M.S. Al. Helminths community of veterinary importance of livestock in relation to some ecological and biological factors. Türk. Parazitol. Derg. 2008;32:42–47. [PubMed] [Google Scholar]
- Ibrahim M.A., Gihan K.A., Aboelhadid S.M., Abdel-Rahim M.M. Role of pet dogs in transmitting zoonotic intestinal parasites in Egypt. Asian J. Anim. Vet. Adv. 2016;11:341–349. doi: 10.3923/ajava.2016.341.349. [DOI] [Google Scholar]
- Ibrahim B.B., Haridy F.M., Hegazi M.M., Morsy T.A. Human hydatidosis granulosus in greater Cairo, Egypt: with general review. J. Egypt. Soc. Parasitol. 2007;37:681–688. [PubMed] [Google Scholar]
- Ibrahim E., Morsy A. Liver hydatid in young age treated per-cutaneous by using Puncture-Aspiration-Injection-Reaspiration (PAIR) technique. J. Egypt. Soc. Parasitol. 2020;50:431–438. doi: 10.21608/jesp.2020.113085. [DOI] [Google Scholar]
- Ibrahim K., Thomas R., Peter K., Omer R.A. A molecular survey on cystic echinococcosis in Sinnar area, Blue Nile State (Sudan) Chin. Med. J. (Engl). 2011;124:2829–2833. doi: 10.3760/CMA.J.ISSN.0366-6999.2011.18.006. [DOI] [PubMed] [Google Scholar]
- Joanny G., Cappai M.G., Nonnis F., Tamponi C., Dessì G., Mehmood N., et al. Human cystic echinococcosis in Lebanon: A retrospective study and molecular epidemiology. Acta Parasitol. 2021 doi: 10.1007/S11686-021-00453-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeel A., Ahmed E.S., Helmy H., Setouhy M. El, Craig P.S., Ramzy R.M.R. A retrospective hospital study of human cystic echinococcosis in Egypt. East. Mediterr. Health J. 2004;10:349–357. [PubMed] [Google Scholar]
- Kandil O.M., Hassan N.M.F., Sedky D., Beshir Ata E. Studies on the specific immunodiagnosis of cystic echinococcosis in camels using enzyme-linked immunosorbent assay. Bulg. J. Vet. Med. 2019;22:305–313. doi: 10.15547/bjvm.2136. [DOI] [Google Scholar]
- Khaled M.L.M., Khalil H.M., Rifaat M.A. Incidence of Toxocara canis infection among pet dogs in Cairo. Ain Shams Med. J. 1973;24:273–275. [Google Scholar]
- Khalifa N., Khater H., Fahmy H., Radwan M., Afify J. Genotyping and phylogenetic analysis of cystic echinococcosis isolated from camels and humans in Egypt. Am. J. Epidemiol. Infect. Dis. 2014;2:74–82. doi: 10.12691/ajeid-2-3-2. [DOI] [Google Scholar]
- Macpherson C.N.L., Torgerson P.R. In: Dogs, zoonoses and public health. 2nd ed. Macpherson C.N.L., Meslin F.X., Wandeler A.I., editors. CABI; Wallingford, UK: 2013. Dogs and cestode zoonoses; pp. 127–152. [DOI] [Google Scholar]
- Mahdy O.A., Abdel-Maogood S.Z., Abdel-Wahab A.M., El-Bahy M.M. Molecular characterization of Echinococcus granulosus cysts isolated from some animals in Egypt. Glob. Vet. 2014;12:594–598. [Google Scholar]
- Mahmoud M.S.E., Abou Gamra M.M.M. Alkaline phosphatase from Echinococcus granulosus metacestodes for immunodiagnosis of human cystic echinococcosis. J. Egypt. Soc. Parasitol. 2004;34:865–879. [PubMed] [Google Scholar]
- Mahmoudi S., Mamishi S., Banar M., Pourakbari B., Keshavarz H. Epidemiology of echinococcosis in Iran: A systematic review and meta-analysis. BMC Infect. Dis. 2019;19:929. doi: 10.1186/S12879-019-4458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansy W., Mohamed M., Saber S. Outcomes of radical surgical management in liver hydatid cysts: 7 years center experience. Mini-invasive Surg. 2018;2:36. doi: 10.20517/2574-1225.2018.48. [DOI] [Google Scholar]
- Mazyad S., Mahmoud L., Hegazy M. Echinococcosis granulosus in stray dogs and Echino-IHAT in the hunters in Cairo, Egypt. J. Egypt. Soc. Parasitol. 2007;37:523–532. [PubMed] [Google Scholar]
- Mazyad M.A., Morsy T.A., Habib K.S. Vertebral unilocular hydatidosis in a shepherd and his wife. J. Egypt. Soc. Parasitol. 1999;29:547–550. [PubMed] [Google Scholar]
- Mazyad M.A., Mostafa M.M., Morsy T.A. Spinal cord hydatid cysts in Egypt. J. Egypt. Soc. Parasitol. 1998;28:655–658. [PubMed] [Google Scholar]
- Ministry of State for Environmental Affairs, E.E.A.A. CBD strategy and action plan - Egypt. 2016. Egyptian biodiversity strategy and action plan (2015–2030)https://www.cbd.int/ [Google Scholar]
- Moch R.W., Fairchild D.G., Botros B.A.M., Barsoum I.S. Echinococcosis in Egypt: II. Prevalence of canine infection in the Cairo area. J. Trop. Med. Hyg. 1974;77:163–164. [PubMed] [Google Scholar]
- Mohsen T., El Beharry N., Maree T., Akl E.S. Cardiac echinococcosis of the interventricular septum in early childhood: Report of two cases. J. Thorac. Cardiovasc. Surg. 2009;137:e14–e16. doi: 10.1016/j.jtcvs.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Mousa W.M., Abdel-Wahab A.M., El-Gameel Sohila M., Mahdy O.A. Genetic characterization of hydatid cysts of different intermediate hosts. Helminthologia. 2020;57:185–195. doi: 10.2478/helm-2020-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa W., Mahdy O., Abdel-Wahab A., El-Gameel O. Epidemiological and serological studies on cystic echinococcosis among camels in Egypt. J. Parasitol. Res. 2015;105:212–218. [Google Scholar]
- Nakao M., Lavikainen A., Yanagida T., Ito A. Phylogenetic systematics of the genus Echinococcus (cestoda: Taeniidae) Int. J. Parasitol. 2013;43:1017–1029. doi: 10.1016/j.ijpara.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Nakao M., Yanagida T., Konyaev S., Lavikainen A., Odnokurtsev V.A., Zaikov V.A., et al. Mitochondrial phylogeny of the genus Echinococcus (Cestoda: Taeniidae) with emphasis on relationships among Echinococcus canadensis genotypes. Parasitology. 2013;140:1625–1636. doi: 10.1017/S0031182013000565. [DOI] [PubMed] [Google Scholar]
- Njoroge E.M., Mbithi P.M.F., Gathuma J.M., Wachira T.M., Gathura P.B., Magambo J.K., et al. A study of cystic echinococcosis in slaughter animals in three selected areas of northern Turkana, Kenya. Vet. Parasitol. 2002;104:85–91. doi: 10.1016/S0304-4017(01)00614-8. [DOI] [PubMed] [Google Scholar]
- Ohiolei J.A., Li L., Ebhodaghe F., Yan H.B., Isaac C., Bo X.W., et al. Prevalence and distribution of Echinococcus spp. in wild and domestic animals across Africa: A systematic review and meta-analysis. Transbound. Emerg. Dis. 2020;67:2345–2364. doi: 10.1111/tbed.13571. [DOI] [PubMed] [Google Scholar]
- Omar M., Sultan K., Haridy M., Omran A. Prevalence of cystic echinococcosis in slaughtered ruminants in different abattoirs, upper Egypt. Am. J. Anim. Vet. Sci. 2013;8:117–121. doi: 10.3844/ajavsp.2013.117.121. [DOI] [Google Scholar]
- Omer R.A., Dinkel A., Romig T., Mackenstedt U., Elnahas A.A., Aradaib I.E., et al. A molecular survey of cystic echinococcosis in Sudan. Vet. Parasitol. 2010;169:340–346. doi: 10.1016/J.VETPAR.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Osman F., Mohamad M., Gadee H. The prevalence and biochemical characters of hydatid cyst in sheep and goats slaughtered at El-Karhga, New-Valley Governorate, Egypt. Sky J. Agric. Res. 2014;3:17–24. [Google Scholar]
- Otero-Abad B., Torgerson P.R. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/JOURNAL.PNTD.0002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawłowski Z.S., Eckert J., Vuitton D.A., Ammann R.W., Kern P., Craig P.S., et al. WHO/OIE manual on echinococcosis in humans and animals: A public health problem of global concern. World Health Organization; Geneva: 2001. Echinococcosis in humans: Clinical aspects, diagnosis and treatment.https://apps.who.int/iris/handle/10665/42427 [Google Scholar]
- Rahman M.S., Sokkar S.M., Dahab S. Comparative studies on hydatidosis in farm animals in Egypt. Dtsch. Tierarztl. Wochenschr. 1992;99:438–440. [PubMed] [Google Scholar]
- Ramadan N.I., Abel Aaty H.E., Mahmoud M.S., El Nori A. An enzyme-linked immunoelectrotransfer blot assay for diagnosis of human cystic echinococcosis. J. Egypt. Soc. Parasitol. 1999;29:849–857. [PubMed] [Google Scholar]
- Ramadan N.I., el Damaty S.I. A preliminary screening study on human cystic echinococcosis in Cairo slaughter house personnel. J. Egypt. Soc. Parasitol. 2000;30:329–339. [PubMed] [Google Scholar]
- Ramzy R.M.R., Helmy H., Zayyat E.A., Rifaat M.M.A., Hameed D.M., Abdel-Baki M.H. An enzyme-linked immunosorbent assay for detection of IgG antibodies specific to human cystic echinococcosis in Egypt. Trop. Med. Int. Health. 1999;4:616–620. doi: 10.1046/j.1365-3156.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- Romig T., Ebi D., Wassermann M. Taxonomy and molecular epidemiology of Echinococcus granulosus sensu lato. Vet. Parasitol. 2015;213:76–84. doi: 10.1016/j.vetpar.2015.07.035. [DOI] [PubMed] [Google Scholar]
- Sadjjadi S.M. Present situation of echinococcosis in the Middle East and Arabic North Africa. Parasitol. Int. 2006;55:S197–S202. doi: 10.1016/j.parint.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Salama H., Abdel-Wahab M.F., Strickland G.T. Diagnosis and treatment of hepatic hydatid cysts with the aid of echo-guided percutaneous cyst puncture. Clin. Infect. Dis. 1995;21:1372–1376. doi: 10.1093/clinids/21.6.1372. [DOI] [PubMed] [Google Scholar]
- Salama H., Ahmed N., El Deeb N., Ahmed R. Hepatic hydatid cysts: Sonographic follow-up after percutaneous sonographically guided aspiration. J. Clin. Ultrasound. 1998;26:455–460. doi: 10.1002/(SICI)1097-0096(199811/12)26:9<455::AID-JCU4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Salama A.A., Othman A.A., Zayed H.A. Cystic echinococcosis in the middle region of the Nile Delta, Egypt: Clinical and radiological characteristics. Egypt. J. Radiol. Nucl. Med. 2014;45:641–649. doi: 10.1016/j.ejrnm.2014.05.004. [DOI] [Google Scholar]
- Taher G.A., Sayed G.M. Some studies on parasitic liver affections of sheep in Assiut governorate. Assiut Vet. Med. J. 2011;57:343–359. [Google Scholar]
- Tapp E. Proceedings of the 1979 and 1984 symposia, Manchester University Press. Manchester University Press; Manchester: 1986. Histology and histopathology of the Manchester mummies; pp. 347–350.https://documents.manchester.ac.uk/display.aspx?DocID=42994 [Google Scholar]
- Thompson R.C.A. The taxonomy, phylogeny and transmission of Echinococcus. Exp. Parasitol. 2008;119:439–446. doi: 10.1016/j.exppara.2008.04.016. [DOI] [PubMed] [Google Scholar]
- UNDP . 2013. Potential impacts of climate change on the Egyptian economy.https://www.eg.undp.org/content/egypt/en/home/library/environment_energy/publication_1.html [Google Scholar]
- Wang Q., Huang Y., Huang L., Yu W., He W., Zhong B., et al. Review of risk factors for human echinococcosis prevalence on the qinghai-tibet plateau, China: A prospective for control options. Infect. Dis. Poverty. 2014;3:3. doi: 10.1186/2049-9957-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2015. WHO estimates of the global burden of foodborne diseases: Foodborne diseases burden epidemiology reference group 2007-2015.https://www.who.int/publications/i/item/9789241565165 [Google Scholar]
- WHO . World Health Organization; Geneva: 2021. Echinococcosis.https://www.who.int/news-room/fact-sheets/detail/echinococcosis [Google Scholar]
- World Population Review Egypt population. 2019. http://www.worldpopulationreview.com/countries/egypt-population
- Zhang W., Zhang Z., Wu W., Shi B., Li J., Zhou X., et al. Epidemiology and control of echinococcosis in central Asia, with particular reference to the People’s Republic of China. Acta Trop. 2015;141:235–243. doi: 10.1016/j.actatropica.2014.03.014. [DOI] [PubMed] [Google Scholar]