Abstract
Cryptosporidium is a group of protistan parasites of a range of vertebrates including mammals and birds. Stimulated by previous work that revealed “zoonotic” Cryptosporidium meleagridis subtypes (i.e. IIIbA26G1R1b and IIIbA22G1R1c) in diarrhoeic children and domestic chickens in Wuhan city and environs in Hubei Province, China, here we explored whether zoonotic C. meleagridis subtypes might also occur in pet birds in Wuhan city. From 11 bird markets in this city, we collected 322 faecal samples from 48 species of birds (representing six taxonomic orders), isolated genomic DNA and then conducted PCR-based sequencing of genetic markers in the small subunit (SSU) of the nuclear ribosomal RNA and the 60 kDa glycoprotein (gp60) genes of Cryptosporidium. Using SSU, Cryptosporidium was detected in 55 (17%) of the 322 samples. Cryptosporidium avium, C. baileyi, C. meleagridis, C. muris and C. proventriculi were characterised in 18%, 47%, 11%, 2% and 20% of the 55 samples, respectively, and a novel Cryptosporidium galli-like taxon in one sample. Using gp60, only one subtype (IIIeA17G2R1) of C. meleagridis was identified, which had not been detected in a previous study of diarrhoeic children in Wuhan. However, IIIe subtypes have been found in both humans and birds around the world. The relatively high prevalence and genetic diversity of Cryptosporidium recorded here in pet birds raise awareness about possible reservoirs of zoonotic variants of Cryptosporidium in birds in Wuhan, and potentially, other provinces in China.
Keywords: Pet bird, Cryptosporidium, Zoonotic potential, PCR-based sequencing
Graphical abstract
Highlights
-
•
A total of 322 faecal samples from 48 species of pet birds were tested for Cryptosporidium.
-
•
Relatively high prevalence and genetic diversity of Cryptosporidium were found.
-
•
A novel Cryptosporidium galli-like genotype was recorded.
-
•
A Cryptosporidium meleagridis subtype with zoonotic potential was detected.
1. Introduction
Species of Cryptosporidium (Phylum Apicomplexa) infect vertebrates, including amphibians, fish, reptiles, birds and mammals (Santín, 2013). Currently, approximately 40 species and more than 70 genotypes are recognised (Zahedi et al., 2016; Holubová et al., 2019). Cryptosporidium species can cause intestinal or respiratory disease, called cryprosporidiosis (Bouzid et al., 2013). Cryptosporidiosis is a leading cause of diarrhoea and death in children (Striepen, 2013). Disease can be self-limiting in healthy hosts, but is life-threatening, particularly in young, old, or immuno-compromised individuals, such as those affected by HIV/AIDS (Bouzid et al., 2013).
The application of molecular epidemiological tools for the genetic identification and characterisation of Cryptosporidium (to the species, genotype and/or subtype levels) has improved our understanding of the distribution and transmission of cryptosporidiosis. Cryptosporidium species and genotypes vary in their host ranges, and some are recognised as zoonotic (Xiao & Feng, 2008; Feng et al., 2018; Khan et al., 2018), for example, with transmission occurring between mammalian species (sheep, cattle, dog or cat; Alves et al., 2003; Chalmers et al., 2005; Lucio-Forster et al., 2010) or bird species (Nakamura et al., 2009; da Silva et al., 2010; Qi et al., 2011; Li et al., 2016; da Cunha, 2018).
In a previous epidemiological survey of diarrhoeic children in Wuhan, we characterized molecular subtypes of C. meleagridis (IIIbA22G1R1c and IIIbA26G1R1b) by PCR-based sequencing of part of the gp60 gene (Wang et al., 2017) which matched those recorded in chickens in Hubei Province (Liao et al., 2018). The findings indicated that, in Wuhan and environs, chickens may contribute to the transmission of C. meleagridis to humans. It was also suggested that wild or other domestic birds (such as pets) might be involved in such transmission, warranting further investigation. In this study, we explore the occurrence of Cryptosporidium of pet birds sold at animal markets in Wuhan city in Hubei Province, China.
2. Materials and methods
Between August and December 2018, a total of 322 fresh faecal samples were obtained from pet birds of different breeds from 11 pet bird markets in Wuhan city, Hubei Province, China. Pet shop managers donated the samples for testing. The identification of bird species was performed using field guides to Australian and Chinese birds (Qian, 1995; MacKinnon, 2000; Slater et al., 2009). Single samples were collected from individual cages (containing 1–20 birds of a similar age). In total, 48 species, representing six orders of birds, were studied (Supplementary Table S1).
Genomic DNA was isolated from individual faecal samples using the PowerSoil DNA isolation kit (MoBio, Carlsbad, USA), according to the manufacturerʼs protocol, and frozen at −20°C. Then, aliquots (2 μl) of individual DNA samples were subjected to nested PCR-based amplification and sequencing, targeting a ∼830 bp fragment of the small subunit (SSU) rRNA gene (Xiao et al., 2001). For classification of C. meleagridis to the subtype level in samples test-positive for SSU, a ∼900–1100 bp fragment of the 60 kDa glycoprotein (gp60) gene was amplified by nested PCR (cf. Stensvold et al., 2014). Each PCR run included known positive, negative, and no-template controls. Individual PCR products were examined via 1.5% agarose gel electrophoresis (Liao et al., 2018). Following treatment with the enzyme ExoI plus FastAP thermosensitive alkaline phosphatase (ThermoFisher Scientific, USA), amplicons were subjected to direct, automated sequencing (BigDye Terminator v.3.1 chemistry, Applied Biosystems, USA) using the same internal primers (individually) as employed in nested PCR.
SSU and gp60 sequences obtained were aligned using the program MAFFT (Katoh et al., 2002), and alignments manually adjusted employing the program Mesquite v.3.61 (Maddison & Maddison, 2018). Sequences were compared with reference sequences available from GenBank (NCBI) using BLASTn. Separate phylogenetic analyses of the SSU (840 bp) and gp60 (869 bp) sequence alignments were conducted using the neighbour-joining (NJ) distance method (Saitou & Nei, 1987) in the program MEGA X v.10.1.8 (Stecher et al., 2020). Evolutionary distances were computed using the ‘number of differences’ method (Nei & Kumar, 2000), including ‘transitions and transversions’ for the nucleotide data. Rates of evolution among sites were considered uniform, and gaps were treated using pairwise deletion. Bootstrap replicates (n = 10,000) were performed, and bootstrap support (%) recorded. The outgroups used in the phylogenetic analyses of SSU and gp60 sequence data sets were Cryptosporidium molnari (GenBank: HM243547) and C. meleagridis subtype IIId (GenBank: DQ067570), respectively.
3. Results and discussion
From the 322 faecal DNA samples tested, pSSU was amplified from 55 (17%) of them. The 55 pSSU amplicons represented 14 bird species (i.e. crested myna, Indian myna, golden-crested myna, Java sparrow, spotted munia, Gouldian finch, zebra finch, Japanese white-eye, budgerigar, cockatiel, Fischerʼs lovebird, rosy-faced lovebird, chicken and pigeon) of four orders (Table 1). The overall prevalence of 17% is comparable or higher to findings for previous studies of wild and zoo birds (Ng et al., 2006; Nakamura et al., 2009; Qi et al., 2011; Nakamura & Meireles, 2015; Máca & Pavlásek, 2015; Reboredo-fernández et al., 2015; Helmy et al., 2017; Iijima et al., 2018).
Table 1.
Cryptosporidium taxa molecularly identified in 55 of 322 faecal DNA samples from individual pet birds from markets in Wuhan city, China
| Bird species | Cryptosporidium species/taxon (number of samples) |
|---|---|
| Passeriformes | |
| Crested myna (Acridotheres cristatellus) | C. baileyi (2) |
| Indian myna (Acridotheres tristis) | C. baileyi (2) |
| Golden-crested myna (Ampeliceps coronatus) | C. baileyi (1) |
| Java sparrow (Lonchura oryzivora) | C. baileyi (1) |
| Spotted munia (Lonchura punctulata) | C. baileyi (1) |
| Gouldian finch (Erythrura gouldiae) | C. baileyi (2) |
| Zebra finch (Taeniopygia guttata) | C. baileyi (10) |
| Japanese white-eye (Zosterops japonicus) | C. galli-like (1) |
| Psittaciformes | |
| Budgerigar (Melopsittacus undulatus) | C. avium (8); C. baileyi (4); C. meleagridis (1) |
| Cockatiel (Nymphicus hollandicus) | C. avium (2); C. baileyi (1); C. meleagridis (2); C. proventriculi (7) |
| Fischerʼs lovebird (Agapornis fischeri) | C. baileyi (1); C. meleagridis (2); C. proventriculi (1) |
| Rosy-faced lovebird (Agapornis roseicollis) | C. proventriculi (3) |
| Galliformesx | |
| Chicken (Gallus gallus) | C. baileyi (1); C. meleagridis (1) |
| Columbiformes | |
| Pigeon (Columba livia) | C. muris (1) |
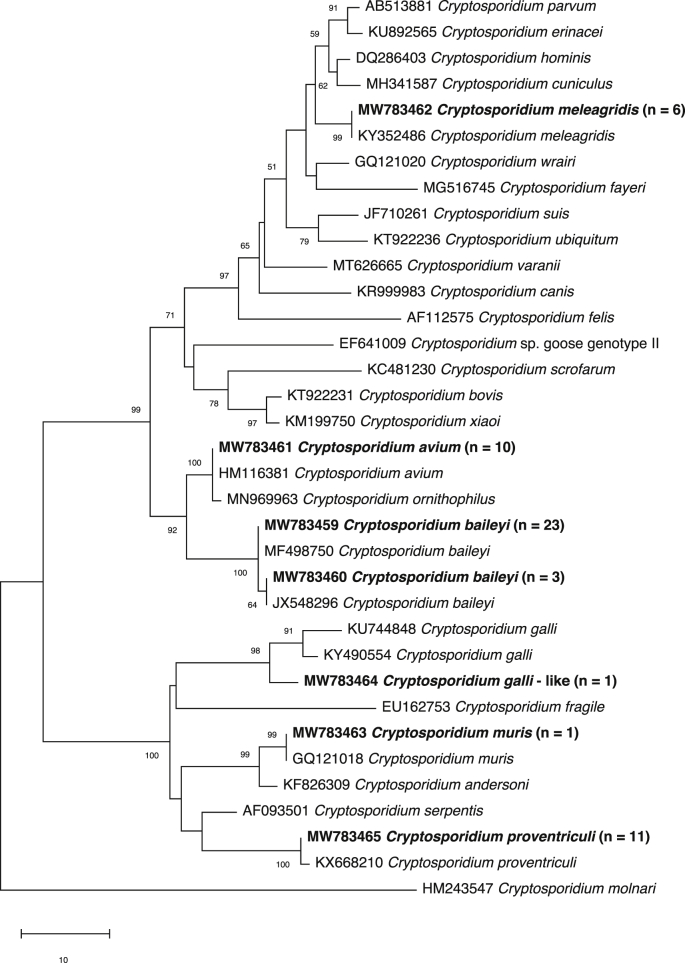
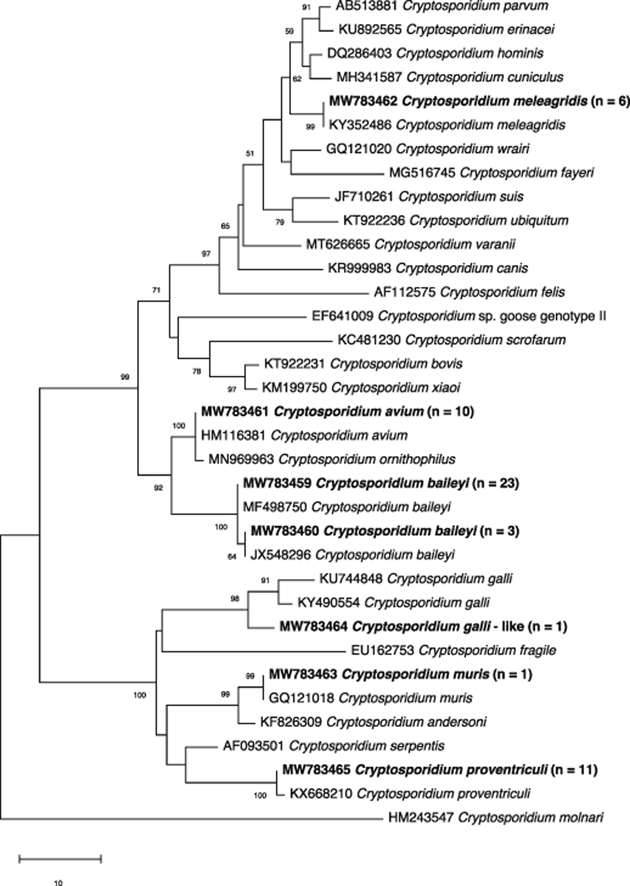
The pSSU sequences of the 55 amplicons were compared with reference sequences from GenBank (see Fig. 1). This comparison allowed us to identify seven distinct pSSU sequences representing six taxa (i.e. C. avium, C. baileyi, C. galli-like, C. meleagridis, C. muris and C. proventriculi; GenBank accession numbers MW783459-MW783465). The prevalences of these respective species were 3% (n = 10), 8% (n = 26), 0.3% (n = 1), 2% (n = 6), 0.3% (n = 1) and 3% (n = 11), with C. baileyi, C. proventriculi and C. avium being the predominant species (Table 1). The record of C. muris in pigeons is new, but may relate to pseudoparasitism (Xiao et al., 2004). Passeriforms were infected mostly with C. baileyi, whereas C. avium, C. meleagridis and C. proventriculi were detected mostly in psittaciforms (Table 1); similar findings have been reported previously (Ng et al., 2006; Nakamura et al., 2009; Sevá et al., 2011; Iijima et al., 2018).
Fig. 1.
Phylogenetic relationships of Cryptosporidium taxa constructed using the neighbour-joining distance method, employing nucleotide sequence data from a portion of the small subunit of the nuclear ribosomal RNA gene (SSU). Cryptosporidium species or genotypes characterised in the present study are in bold-type. The GenBank accession number precedes the species designation; the number of samples of a particular species/genotype is indicated in parentheses. The scale-bar represents the number of substitutions per site. Cryptosporidium molnari (GenBank: HM243547) was used as an outgroup. Bootstrap support is indicated at the nodes
Phylogenetic analysis (Fig. 1) determined that all but one of the distinct pSSU sequences matched, with 100% identity, known sequences in GenBank for C. avium (HM116381), C. baileyi (KY352489 and JX548296), C. meleagridis (KY352486), C. muris (GQ121018) and C. proventriculi (HM116385). Additionally, the sequence from a Japanese white-eye was 99% identical (748 of 755 bp) to C. galli from an ibis in Australia (GenBank: MG516766). Here, we refer to it as C. galli-like, but the extent of sequence variation (7 bp) suggests that it could be a novel species; this proposal warrants further investigation using multiple genetic markers.
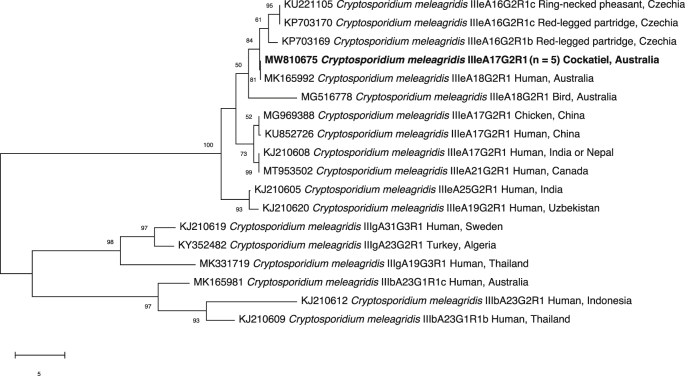
The five pSSU amplicons that were classified as C. meleagridis were further subtyped using gp60 primers (Stensvold et al., 2014). Sequence alignment and phylogenetic analysis of these five pgp60 sequences and reference sequences revealed a novel variant of the C. meleagridis IIIe subtype family (IIIeA17G2R1; GenBank accession no. MW810675) in Fischerʼs lovebirds (n = 2) and cockatiels (n = 3). This subtype (IIIeA17G2R1) has been detected previously in a farmed chicken in Hubei, China (GenBank: MG969388) and from humans in China (GenBank: KU852726) and India (or Nepal) (GenBank: KJ210608). Despite having a slightly different subtype, the present sequence (accession no. MW810675) is more closely related to a human sample from Australia, IIIeA18G2R1 (GenBank: MK165992) than to the other IIIeA17G2R1 subtypes (Fig. 2). Nevertheless, subtypes from group IIIe have been detected in both birds and humans (e.g. Stensvold et al., 2014; Máca & Pavlásek, 2015; Liao et al., 2018; Braima et al., 2019), including possible cases of transmission from poultry to immunosuppressed people (Wang et al., 2013). Although this subtype (IIIeA17G2R1) of C. meleagridis has not been detected previously in humans in Wuhan, other subtypes (IIIbA21G1R1, IIIbA22G1R1, IIIbA26G1R1) have been recorded previously in diarrhoeic children in this city (Wang et al., 2017).
Fig. 2.
Phylogenetic relationships of Cryptosporidium meleagridis constructed using the neighbour-joining distance method, employing nucleotide sequence data from fragment of the 60 kDa glycoprotein (gp60) gene. Cryptosporidium meleagridis sequence generated in the present study is in bold-type. The GenBank accession number precedes the species designation; the number of samples of a particular species/genotype is indicated in parentheses. The scale-bar represents the number of substitutions per site. Cryptosporidium meleagridis subtype IIIb (GenBank: KJ210609) was used as the outgroup. Bootstrap support is indicated at the nodes
In conclusion, we investigated here the presence and genetic identity of Cryptosporidium in birds (48 species of 6 orders) in 11 pet markets in Wuhan, Hubei Province. The prevalence (17%) and genetic diversity in species established here and the detection of some taxa, such as C. meleagridis subtype IIIe, that might be zoonotic emphasize the need to undertake more detailed investigations in humans and animals in Wuhan and other provinces in China, in order to infer zoonotic transmission patterns of cryptosporidiosis.
Funding
This project was supported by Huazhong Agricultural University Scientific & Technological Self Innovation Foundation (program no. 2015RC005).
Ethical approval
The study was approved (permit no. HZAUBI-2018-001) by the Animal Management and Ethics Committee of the Huazhong Agricultural University, China.
CRediT author statement
Cong Liao: Investigation, Original draft preparation. Tao Wang: Conceptualisation, Validation, Writing - Reviewing and Editing. Min Hu: Investigation, Writing - Reviewing and Editing. Anson V. Koehler: Visualisation, Software, Validation, Writing-Reviewing and Editing. Robin B. Gasser: Conceptualisation, Supervision, Writing - Reviewing and Editing.
Data availability
The newly generated sequences were deposited in the GenBank database under the accession numbers MW783459-MW783465 (pSSU) and MW810675 (pgp60).
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This project was supported by Huazhong Agricultural University Scientific & Technological Self Innovation Foundation (Program no. 2015RC005). We thank the pet market managers for donating faecal samples from birds.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2021.100025.
Contributor Information
Tao Wang, Email: tao.wang1@unimelb.edu.au.
Robin B. Gasser, Email: robinbg@unimelb.edu.au.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Supplementary Table S1. Occurrence of Cryptosporidium taxa in faecal samples of birds in pet markets in Wuhan city, Hubei Province, China.
References
- Alves M., Xiao L., Sulaiman I., Lal A.A., Matos O., Antunes F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 2003;41:2744–2747. doi: 10.1128/JCM.41.6.2744-2747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzid M., Hunter P.R., Chalmers R.M., Tyler K.M. Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 2013;26:115–134. doi: 10.1128/CMR.00076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braima K., Zahedi A., Oskam C., Reid S., Pingault N., Xiao L., Ryan U. Retrospective analysis of Cryptosporidium species in Western Australian human populations (2015–2018), and emergence of the C. hominis IfA12G1R5 subtype. Infect. Genet. Evol. 2019;73:306–313. doi: 10.1016/j.meegid.2019.05.018. [DOI] [PubMed] [Google Scholar]
- Chalmers R.M., Ferguson C., Caccio S., Gasser R.B., Abs E.L.O.Y.G., Heijnen L., Xiao L., Elwin K., Hadfield S., Sinclair M., Stevens M. Direct comparison of selected methods for genetic categorisation of Cryptosporidium parvum and Cryptosporidium hominis species. Int. J. Parasitol. 2005;35:397–410. doi: 10.1016/j.ijpara.2005.01.001. [DOI] [PubMed] [Google Scholar]
- da Cunha M.J.R., Cury M.C., Santín M. Molecular characterization of Cryptosporidium spp. in poultry from Brazil. Res. Vet. Sci. 2018;118:331–335. doi: 10.1016/j.rvsc.2018.03.010. [DOI] [PubMed] [Google Scholar]
- da Silva D.C., Homem C.G., Nakamura A.A., Teixeira W.F., Perri S.H., Meireles M.V. Physical, epidemiological, and molecular evaluation of infection by Cryptosporidium galli in Passeriformes. Parasitol. Res. 2010;107:271–277. doi: 10.1007/s00436-010-1858-2. [DOI] [PubMed] [Google Scholar]
- Feng Y., Ryan U.M., Xiao L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018;34:997–1011. doi: 10.1016/j.pt.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Helmy Y.A., Krücken J., Abdelwhab E.M., von Samson-Himmelstjerna G., Hafez H.M. Molecular diagnosis and characterization of Cryptosporidium spp. in turkeys and chickens in Germany reveals evidence for previously undetected parasite species. Plos One. 2017;12 doi: 10.1371/journal.pone.0177150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holubová N., Zikmundová V., Limpouchová Z., Sak B., Konečný R., Hlásková L., Rajský D., Kopacz Z., McEvoy J., Kváč M. Cryptosporidium proventriculi sp. n. (Apicomplexa: Cryptosporidiidae) in psittaciforme birds. Eur. J. Protistol. 2019;69:70–87. doi: 10.1016/j.ejop.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Iijima Y., Itoh N., Phrompraphai T., Ito Y., Kimura Y., Kameshima S. Molecular prevalence of Cryptosporidium spp. among companion birds kept in pet shops in Japan. Korean J. Parasitol. 2018;56:281–285. doi: 10.3347/kjp.2018.56.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., Misawa K., Kuma K., Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Shaik J.S., Grigg M.E. Genomics and molecular epidemiology of Cryptosporidium species. Acta Tropica. 2018;184:1–14. doi: 10.1016/j.actatropica.2017.10.023. [DOI] [PubMed] [Google Scholar]
- Liao C., Wang T., Koehler A.V., Fan Y., Hu M., Gasser R.B. Molecular investigation of Cryptosporidium in farmed chickens in Hubei Province, China, identifies ‘zoonotic’ subtypes of C. meleagridis. Parasit. Vectors. 2018;11:484. doi: 10.1186/s13071-018-3056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Li L., Tao W., Jiang Y., Wan Q., Lin Y., Li W. Molecular investigation of Cryptosporidium in small caged pets in northeast China: Host specificity and zoonotic implications. Parasitol. Res. 2016;115:2905–2911. doi: 10.1007/s00436-016-5076-4. [DOI] [PubMed] [Google Scholar]
- Lucio-Forster A., Griffiths J.K., Cama V.A., Xiao L., Bowman D.D. Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol. 2010;26:174–179. doi: 10.1016/j.pt.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Máca O., Pavlásek I. First finding of spontaneous infections with Cryptosporidium baileyi and C. meleagridis in the red-legged partridge Alectoris rufa from an aviary in the Czech Republic. Vet. Parasitol. 2015;209:164–168. doi: 10.1016/j.vetpar.2015.03.003. [DOI] [PubMed] [Google Scholar]
- MacKinnon J. Oxford University Press; Oxford, UK: 2000. A FieldGuide to theBirds of China. [Google Scholar]
- Maddison W., Maddison D.R. 2018. Mesquite: A modular system for evolutionary analysis. Version 3.40.https://mesquiteproject.org/ [Google Scholar]
- Nakamura A.A., Meireles M.V. Cryptosporidium infections in birds - a review. Rev. Bras. Parasitol. Vet. 2015;24:253–267. doi: 10.1590/S1984-29612015063. [DOI] [PubMed] [Google Scholar]
- Nakamura A.A., Simões D.C., Antunes R.G., da Silva D.C., Meireles M.V. Molecular characterization of Cryptosporidium spp. from fecal samples of birds kept in captivity in Brazil. Vet. Parasitol. 2009;166:47–51. doi: 10.1016/j.vetpar.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Nei M., Kumar S. Oxford University Press; Oxford, UK: 2000. Molecular Evolution and Phylogenetics. [Google Scholar]
- Ng J., Pavlasek I., Ryan U. Identification of novel genotypes from avian hosts. Appl. Environ. Microbiol. 2006;72:7548–7553. doi: 10.1128/AEM.01352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y. Henan Science and Technology Press; Henan, China: 1995. Atlas of Birds of China. [Google Scholar]
- Qi M., Wang R., Ning C., Li X., Zhang L., Jian F., Sun Y., Xiao L. Cryptosporidium spp. in pet birds: Genetic diversity and potential public health significance. Exp. Parasitol. 2011;128:336–340. doi: 10.1016/j.exppara.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Reboredo-Fernández A., Ares-Mazás E., Cacciò S.M., Gómez-Couso H. Occurrence of Giardia and Cryptosporidium in wild birds in Galicia (Northwest Spain) Parasitology. 2015;142:917–925. doi: 10.1017/S0031182015000049. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Santín M. Clinical and subclinical infections with Cryptosporidium in animals. N. Z. Vet. J. 2013;61:1–10. doi: 10.1080/00480169.2012.731681. [DOI] [PubMed] [Google Scholar]
- Sevá A.P., Funada M.R., Richtzenhain L., Guimarães M.B., Souza Sde O., Allegretti L., Sinhorini J.A., Duarte V.V., Soares R.M. Genotyping of Cryptosporidium spp. from free-living wild birds from Brazil. Vet. Parasitol. 2011;175:27–32. doi: 10.1016/j.vetpar.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Slater P., Slater P., Slater R. 2nd ed. New Holland Publishers; Sydney, Australia: 2009. The Slater Field Guide to Australian Birds. [Google Scholar]
- Stecher G., Tamura K., Kumar S. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 2020;37:1237–1239. doi: 10.1093/molbev/msz312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold C.R., Beser J., Axén C., Lebbad M. High applicability of a novel method for gp60-based subtyping of Cryptosporidium meleagridis. J. Clin. Microbiol. 2014;52:2311–2319. doi: 10.1128/JCM.00598-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striepen B. Parasitic infections: Time to tackle cryptosporidiosis. Nature. 2013;503:189–191. doi: 10.1038/503189a. [DOI] [PubMed] [Google Scholar]
- Wang T., Fan Y., Koehler A.V., Ma G., Li T., Hu M., Gasser R.B. First survey of Cryptosporidium, Giardia and Enterocytozoon in diarrhoeic children from Wuhan, China. Infect. Genet. Evol. 2017;51:127–131. doi: 10.1016/j.meegid.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang H., Zhao X., Zhang L., Zhang G., Guo M., Liu L., Feng Y., Xiao L. Zoonotic Cryptosporidium species and Enterocytozoon bieneusi genotypes in HIV-positive patients on antiretroviral therapy. J. Clin. Microbiol. 2013;51:557–563. doi: 10.1128/JCM.02758-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Fayer R., Ryan U., Upton S.J. Cryptosporidium taxonomy: Recent advances and implications for public health. Clin. Microbiol. Rev. 2004;17:72–97. doi: 10.1128/CMR.17.1.72-97.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Feng Y. Zoonotic cryptosporidiosis. FEMS Immunol. Med. Microbiol. 2008;52:309–323. doi: 10.1111/j.1574-695X.2008.00377.x. [DOI] [PubMed] [Google Scholar]
- Xiao L., Singh A., Limor J., Graczyk T.K., Gradus S., Lal A. Molecular characterization of Cryptosporidium oocysts in samples of raw surface water and wastewater. Appl. Environ. Microbiol. 2001;67:1097–1101. doi: 10.1128/AEM.67.3.1097-1101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi A., Paparini A., Jian F., Robertson I., Ryan U. Public health significance of zoonotic Cryptosporidium species in wildlife: Critical insights into better drinking water management. Int. J. Parasitol. Parasites Wildl. 2016;5:88–109. doi: 10.1016/j.ijppaw.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Occurrence of Cryptosporidium taxa in faecal samples of birds in pet markets in Wuhan city, Hubei Province, China.
Data Availability Statement
The newly generated sequences were deposited in the GenBank database under the accession numbers MW783459-MW783465 (pSSU) and MW810675 (pgp60).