Abstract
Ehrlichia are small intracellular Gram-negative bacteria transmitted by ticks. These microorganisms cause ehrlichiosis, a complex of life-threatening emerging zoonoses and diseases of global veterinary relevance. The aim of this study was to investigate the presence of Ehrlichia in free-living Ixodes auritulus collected in Uruguay. Ticks were collected from vegetation in five localities from the southeast and northeast of the country between 2014 and 2017. Detection of Ehrlichia DNA was performed in pools of adults or nymphs grouped according to the collection site and date. A total of 1,548 I. auritulus ticks were collected in four of the five locations sampled. Fragments of three loci (16S rRNA, dsb and groEL) were obtained by PCR, and phylogenies inferred using Bayesian inference analysis for each gene independently. DNA of Ehrlichia spp. was found in 15 out of 42 tick pools. Based on the topology of the phylogenetic trees, our sequences represent two novel genotypes for the genus named as Ehrlichia sp. Serrana and Ehrlichia sp. Laguna Negra. Both genotypes were closely related to Ehrlichia sp. Magellanica, a species detected in Ixodes uriae and Magellanic penguins. Considering that all stages of I. auritulus and I. uriae are parasites of birds, their phylogenetic relationships, and common eco–epidemiological profiles, it is reasonable to state that these genotypes of Ehrlichia spp. may represent a natural group likely associated with birds. Our results constitute the first characterization of Ehrlichia spp. in Uruguay. Future studies on birds reported as hosts for I. auritulus are needed to further understand the epidemiological cycles of both Ehrlichia genotypes in the country. Finally, I. auritulus does not feed on humans, so the two Ehrlichia species reported herein might have no implications in human health.
Keywords: Anaplasmataceae, Ehrlichia, Novel genotypes, Phylogeny, Ixodes auritulus, Birds
Graphical abstract
Highlights
-
•
First molecular characterization of Ehrlichia in Uruguay.
-
•
Two novel genotypes of Ehrlichia were detected in Ixodes auritulus.
-
•
Evidence of a natural lineage of Ehrlichia associated with birds is reinforced.
1. Introduction
The order Rickettsiales (Alphaproteobacteria) includes obligate intracellular parasites that infect a variety of invertebrate and vertebrate hosts. Rickettsiales comprises the families Anaplasmataceae, Midichloriaceae and Rickettsiaceae (Montagna et al., 2013; Szokoli et al., 2016). The family Anaplasmataceae is currently divided into five established and two Candidatus genera: Anaplasma, Aegyptianella, Ehrlichia, Neorickettsia, Wolbachia, “Candidatus Neoehrlichia”, and “Candidatus Xenohaliotis” (Thomas et al., 2016).
Ehrlichia are small Gram-negative tick-transmitted bacteria that form microcolonies within membrane-bound cytoplasmic vacuoles called morulae (Popov et al., 1998). These bacteria are the agents of ehrlichiosis, a complex of life-threatening emerging zoonoses and diseases of veterinary importance worldwide (Esemu et al., 2011). Ehrlichia species differ in their target cells both in mammals (monocytes, neutrophils or endothelial cells) and ticks (salivary glands, intestinal epithelium, and hemolymph) (Brouqui & Matsumoto, 2007; Aguiar, 2017). In contrast to Rickettsia spp., there is no evidence of transovarial transmission in Ehrlichia spp. in ticks (Ismail & McBride, 2017). Instead, ticks become infected with Ehrlichia spp. while feeding on infected vertebrate reservoirs and the infection is perpetuated transstadially (i.e. larva-nymph-adult) (Ismail & McBride, 2017). Human and animal ehrlichiosis are caused worldwide by six Ehrlichia species, namely Ehrlichia canis, Ehrlichia chaffeensis, Ehrlichia muris, Ehrlichia ewingii, Ehrlichia ruminantium, and Ehrlichia minasensis (see Aguiar, 2017). In the Southern Cone of South America, E. canis, the etiological agent of canine monocytic ehrlichiosis, is the most commonly reported species of this genus (López et al., 2012; Lasta et al., 2013; Cicuttin et al., 2015).
Additionally, different strains closely related to E. chaffeensis were detected in Amblyomma parvum, Amblyomma tigrinum and in marsh deer (Blastocerus dichotomus) in Argentina (Tomassone et al., 2008; Cicuttin et al., 2017; Guillemi et al., 2019; Monje et al., 2019). Moreover, Ehrlichia sp. strain Cordoba was detected in A. tigrinum ticks (Cicuttin et al., 2017). Recently, also in Argentina, A. tigrinum, Amblyomma triste and Amblyomma neumanni were found positive after molecular screenings targeting this bacterium, and three novel strains (Ehrlichia sp. strain Iberá, Ehrlichia sp. strain Delta, and Ehrlichia sp. strain La Dormida) were identified (Cicuttin et al., 2020; Eberhardt et al., 2020; Fargnoli et al., 2020).
In Brazil, reports include the detection of Ehrlichia cf. chaffeensis in B. dichotomus (Sacchi et al., 2012), and some Ehrlichia spp. strains pending further molecular characterization were detected in wild carnivores (Widmer et al., 2011; Almeida et al., 2013) and a horse (Vieira et al., 2016). Cattle and Rhipicephalus microplus ticks maintain E. minasensis infections in Brazil (Cabezas-Cruz et al., 2016; Aguiar, 2017) and recently, Ehrlichia sp. was detected in Amblyomma sculptum in the Brazilian Pantanal (Muraro et al., 2021).
In Chile, a novel genotype, Ehrlichia sp. Magellanica, seems to represent a bird-associated lineage within the genus, since it was detected in Ixodes uriae collected on Magellanic penguins (Spheniscus magellanicus) (Muñoz-Leal et al., 2019).
While the occurrence of ehrlichiae in Uruguay is uncertain, Conti-Díaz (2001) referred to possible cases of ehrlichiosis in humans. Although the disease is listed as emergent in the country, infection in animals and vectors has not been investigated.
Ixodes auritulus is a cosmopolitan tick species distributed through the Afrotropical, Australasian, Nearctic, and Neotropical Zoogeographic Regions and all stages parasitize mainly birds (Nava et al., 2017; Guglielmone et al., 2020). The presence of Borrelia burgdorferi (sensu lato) was recently reported in I. auritulus from Argentina and Uruguay (Cicuttin et al., 2019; Carvalho et al., 2020); however, information about the presence of other pathogens is scarce.
As Ixodes spp. also can transtadially maintain bacteria of the family Anaplasmataceae, the aim of this study was to investigate the presence of Ehrlichia in free-living I. auritulus ticks collected in Uruguay.
2. Materials and methods
2.1. Study area
Fieldwork was conducted at five localities in Uruguay between 2014 and 2017. Three localities, Gruta de los Cuervos (−31.618888, −56.046389), Tacuarembó Department; Amarillo (−31.663611, −55.050555) and Lunarejo (−31.141388, −55.900277), Rivera Department, are located in the northeast region of the country, within the Uruguayan ecoregion Gondwanic Sedimentary Basin (Brazeiro et al., 2012). The other two localities, Reserva Natural Salus (−34.421111, −55.315000), Lavalleja Department, and Laguna Negra (−34.085833, −53.738055), Rocha Department, are in the southeast part of the country, and belong to the ecoregion Sierras del Este sensu Brazeiro et al., (2012).
2.2. Tick collection and identification
Questing ticks were collected from vegetation using the flagging method. The collection was carried out for a period of 2 h in the samplings in each visit to the sites. Ticks were picked up from the cloth at 5–10-m intervals and stored in plastic tubes with 95% ethanol. In the laboratory, each arthropod was identified using a stereomicroscope following the morphological keys for larval, nymph and adult stages by Kohls (1960), Webb et al. (1990), and Nava et al. (2017).
2.3. DNA extraction and detection of Ehrlichia spp.
Only free-living nymphs and adult ticks were used in this study. Since Ehrlichia spp. are not maintained by transovarial transmission, larvae were not analyzed.
Detection of Ehrlichia DNA was performed into pools of adult or nymphs (1–10 ticks per pool) separated according to site and collection date. Briefly, ticks were rinsed with distilled water to remove ethanol and bisected longitudinally using sterile scalpel blades and forceps. Finally, each pool was homogenized cutting thoroughly the ticks with dissecting scissors. DNA was extracted using the commercial kit GeneJET Genomic DNA Purification Kit (Thermo Scientific, Lithuania) following the manufacturer’s instructions. The concentration and purity of DNA was determined using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Molecular screening was carried out using primers EHR16SD/EHR16SR with PCR conditions described by Parola et al. (2000). This PCR enables detection of a 16S rRNA gene fragment of members of the family Anaplasmataceae including the genera Anaplasma, Ehrlichia, Neorickettsia and Wolbachia. Positive samples were subjected to four additional PCR protocols. Two overlapping fragments were obtained using primers fD1/EHR16SR and EHR16SD/Rp2 (Weisburg et al., 1991; Inokuma et al., 2001) to amplify nearly full-length sequence of the 16S rRNA gene. In addition, a semi-nested and nested PCRs targeting dsb (disulfide oxidoreductase) and groEL (60 kDa chaperonin) genes, respectively, were performed (Sumner et al., 1997; Lotric-Furlan et al., 1998; Nicholson et al., 1999; Doyle et al., 2005; Almeida et al., 2013). All primers used and fragment sizes are listed inTable 1. Distilled water and an Ehrlichia canis DNA-positive sample were included as negative and positive controls in all runs. Five microliters of PCR products were analyzed by electrophoresis into 1.5% agarose gels, stained with GoodView™ Nucleic Acid Stain (Beijing SBS Genetech Co., Ltd.), and examined under UV transillumination. Amplicons were purified using GeneJET PCR purification kit (Thermo Scientific, Lithuania) and sent for sequencing to Macrogen (Seoul, Korea). BLASTn analyses (www.ncbi.nlm.nih.gov/blast) were performed in order to infer closest identities with microorganisms available on GenBank database (Altschul et al., 1990), and to include these sequences into a phylogenetic analysis. Nucleotide identities of obtained sequences were calculated using the Sequence Identity and Similarity (SIAS) calculator (http://imed.med.ucm.es/Tools/sias.html).
Table 1.
PCR primers used to amplify the partial 16S rRNA, groEL, and dsb genes of Ehrlichia spp.
| Primer name | Targeted gene | Sequence | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| EHR16SDa | 16S rRNA | GGTACCYACAGAAGAAGTCC | 345 | Parola et al. (2000) |
| EHR16SRa | TAGCACTCATCGTTTACAGC | Parola et al. (2000) | ||
| fD1 | AGAGTTTGATCCTGGCTCAG | ∼1,500 | Weisburg et al. (1991) | |
| Rp2 | ACGGCTACCTTGTTACGACTT | Weisburg et al. (1991) | ||
| HS1a | groEL | AITGGGCTGGTAITGAAAT | ∼1,400 | Sumner et al. (1997); Nicholson et al. (1999) |
| HS6a | CCICCIGGIACIAIACCTTC | Sumner et al. (1997); Nicholson et al. (1999) | ||
| HS43 | ATWGCWAARGAAGCATAGTC | 1,297 | Lotric-Furlan et al. (1998) | |
| HSVR | CTCAACAGCAGCTCTAGTAGC | Lotric-Furlan et al. (1998) | ||
| Dsb-330 | dsb | GATGATGTTTGAAGATATSAAACAAAT | 401 | Doyle et al. (2005); Almeida et al. (2013) |
| Dsb-720b | CTATTTTACTTCTTAAAGTTGATAWATC | 349 | Doyle et al. (2005); Almeida et al. (2013) | |
| Dsb-380 | ATTTTTAGRGATTTTCCAATACTTGG | Doyle et al. (2005); Almeida et al. (2013) |
Primers used in the initial PCR screening.
Primer used in the first and second round.
2.4. Phylogenetic analyses
Independent alignments using obtained sequences for 16S rRNA, dsb and groEL loci were constructed with CLUSTAL W (Thompson et al., 1994) including homologue sequences downloaded from GenBank. Three phylogenetic trees were inferred using Bayesian inference analysis as implemented in MrBayes 3.2.5. (Huelsenbeck & Ronquist, 2001), using 1,000,000 generations. The general time reversible (GTR) model was chosen to run all the trees. Each tree was sampled every 100 generations, begun with random seeds and ran four times. The first 25% of the trees was discarded as “burn-in”, and the remaining subset of trees was used to calculate Bayesian posterior probabilities. Sequences of “Candidatus Neoehrlichia mikurensis” (EU810406; AB213021) and Ehrlichia ruminantium (AF308669) were used to root the phylogenetic trees.
3. Results
3.1. Tick collection
A total of 1,548 I. auritulus ticks (73 females, 185 nymphs and 1,290 larvae) were collected on vegetation at four of the five locations sampled (Table 2). Other tick species such as Amblyomma aureolatum, Haemaphysalis juxtakochi, and Ixodes fuscipes were also collected but not included in this study.
Table 2.
Data for Ixodes auritulus collected and detection of Ehrlichia spp.
| Collection site | Date | Stage | No. of ticks | Pools | Positive pools | Positive pools code | Ehrlichia genotype | GenBank accession numbers |
||
|---|---|---|---|---|---|---|---|---|---|---|
| 16S rRNA | dsb | groEL | ||||||||
| Gruta de los Cuervos (T) | August 2016 | Nymph | 1 | 1 | 0 | |||||
| December 2016 | Female | 1 | 1 | 0 | ||||||
| July 2017 | Nymph | 1 | 1 | 0 | ||||||
| Amarillo (Ri) | October 2016 | Female | 1 | 1 | 1 | S12IaH4a | Serrana | MW628647a | MW650903a | |
| Nymph | 1 | 1 | 0 | |||||||
| June 2017 | Female | 1 | 1 | 1 | S17IaH1a | Serrana | MW628649a | MW650901a | MW650909a | |
| Nymph | 16 | 3 | 0 | |||||||
| Larva | 6 | # | # | |||||||
| Lunarejo (Ri) | October 2016 | F-N-L | 0 | 0 | 0 | |||||
| December 2016 | F-N-L | 0 | 0 | 0 | ||||||
| April 2017 | F-N-L | 0 | 0 | 0 | ||||||
| July 2017 | F-N-L | 0 | 0 | 0 | ||||||
| Reserva Natural Salus (La) | May 2016 | Nymph | 48 | 5 | 1 | S10IaN9a | Serrana | MW650902a | MW650910a | |
| December 2016 | Female | 6 | 1 | 0 | ||||||
| Nymph | 5 | 1 | 0 | |||||||
| July 2017 | Nymph | 12 | 2 | 1 | S17IaN33a | Serrana | MW628650a | MW650904a | ||
| Larva | 440 | # | # | |||||||
| Laguna Negra (Ro) | March 2014 | Female | 4 | 1 | 1 | S5IaH4 | Serrana | |||
| Nymph | 9 | 1 | 0 | |||||||
| Larva | 4 | # | # | |||||||
| May 2014 | Nymph | 30 | 3 | 2 | S6IaN9; S6IaN11 | Serrana (2) | ||||
| Larva | 144 | # | # | |||||||
| November 2015 | Female | 4 | 1 | 1 | S6IaH12a | Laguna Negra | MW628646a | MW650906a | MW650908a | |
| August 2014 | Female | 6 | 1 | 0 | ||||||
| Nymph | 47 | 5 | 1 | S7IaN19 | Serrana | |||||
| Larva | 665 | # | # | |||||||
| November 2014 | Female | 50 | 10 | 5 | S8IaH27a; S8IaH28; S8IaH31; S8IaH33; S8IaH34 | Laguna Negra (1); Serrana (4) | MW650907a | |||
| Nymph | 16 | 2 | 1 | S8IaN38a | Serrana | MW628648a | MW650905a | |||
| Larva | 30 | # | # | |||||||
| Total | 1548 | 42 | 15 | |||||||
Abbreviations: T, Tacuarembó; Ri, Rivera; La, Lavalleja; Ro, Rocha; #, larvae are not included in pools; F, Female; N, Nymph; L, Larva.
Sequences of positive pools used for phylogenetic tree construction.
3.2. Detection of Ehrlichia DNA in ticks
Ticks were processed and analyzed in 42 pools (17 containing females and 25 containing nymphs). Of these, 15 (9 containing females and 6 containing nymphs) were positive for DNA of Ehrlichia spp. Geographically, Ehrlichia spp. DNA was detected in Amarillo (two pools of females), Reserva Natural Salus (two pools of nymphs) and Laguna Negra (seven pools of females and two of nymphs) (Table 2).
3.3. Phylogenetic analyses and nucleotide comparisons of sequences
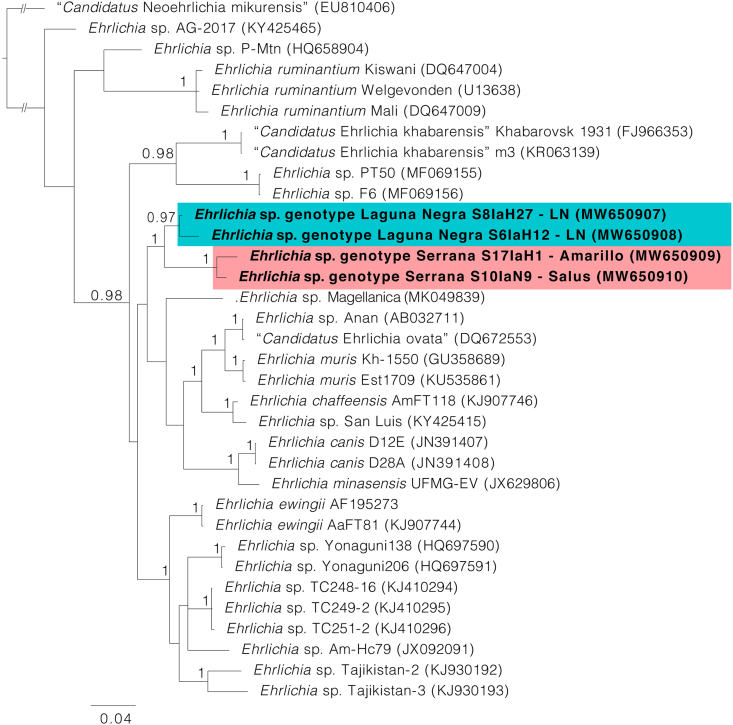
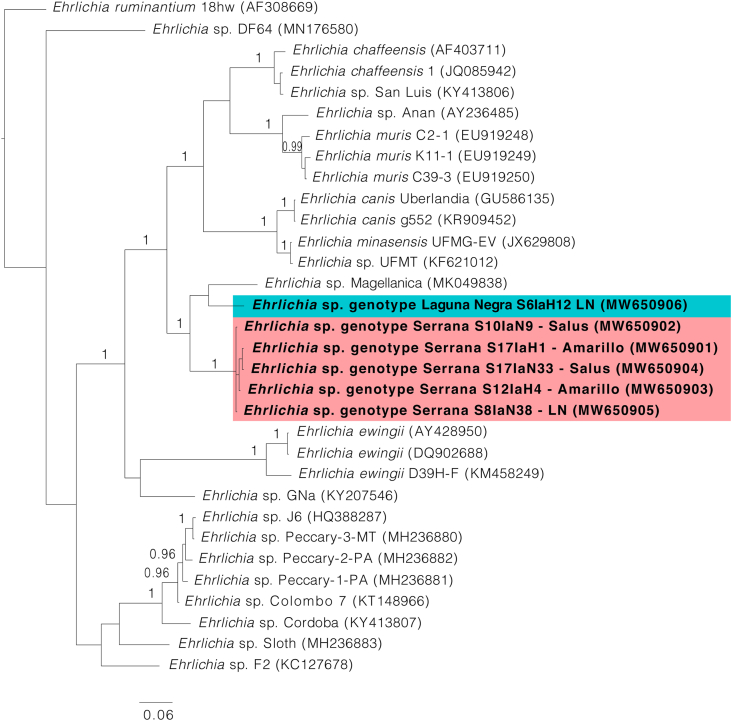
Trees inferred by Bayesian analysis were constructed to define the phylogenetic position of the generated sequences. With the exception of the groEL tree, our sequences clustered into a monophyletic group including Ehrlichia sp. Magellanica from Chile (Fig. 1, Fig. 2, Fig. 3). However, sequences for all three loci were obtained from only two pools (S6laH12 and S17laH1) (Table 2). Based on the topology of the phylogenetic trees, two genotypes of Ehrlichia with different haplotypes appear in all processed pools; these are referred to as Ehrlichia sp. genotype Serrana and Ehrlichia sp. genotype Laguna Negra. The genotype Serrana was identified in 13 pools (86.7%), and the genotype Laguna Negra in 2 (13.3%) positive pools of ticks. The sequences generated in this study were deposited in the GenBank database and the accession numbers are listed in Table 2. The percent nucleotide identities among 16S rDNA, dsb and groEL sequences between Ehrlichia sp. genotype Serrana, Ehrlichia sp. genotype Laguna Negra and the most similar sequence available on GenBank, Ehrlichia sp. Magellanica (MK049840, MK049838, MK049839), are shown in Table 3, Table 4, Table 5.
Fig. 1.
Bayesian phylogenetic analysis inferred for partial fragments of the 16S rRNA gene. Bayesian posterior probabilities > 0.95 are indicated above or below the nodes. The positions of Ehrlichia sp. genotype Serrana and Ehrlichia sp. genotype Laguna Negra are highlighted within red and blue boxes, respectively. The scale-bar indicates the number of substitutions per nucleotide position. GenBank accession numbers are in parentheses
Fig. 2.
Bayesian phylogenetic analysis inferred for partial fragments of the groEL gene. Bayesian posterior probabilities > 0.95 are indicated above or below the nodes. The positions of Ehrlichia sp. genotype Serrana and Ehrlichia sp. genotype Laguna Negra are highlighted within red and blue boxes, respectively. The scale-bar indicates the number of substitutions per nucleotide position. GenBank accession numbers are in parentheses
Fig. 3.
Bayesian phylogenetic analysis inferred for partial fragments of the dsb gene. Bayesian posterior probabilities > 0.95 are indicated above or below the nodes. The positions of Ehrlichia sp. genotype Serrana and Ehrlichia sp. genotype Laguna Negra are highlighted within red and blue boxes, respectively. The scale-bar indicates the number of substitutions per nucleotide position. GenBank accession numbers are in parentheses
Table 3.
Pairwise comparison matrix for 16S rDNA sequences of Ehrlichia sp. Serrana, Ehrlichia sp. Laguna Negra, and Ehrlichia sp. Magellanica
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|
| 1 | Ehrlichia sp. Laguna Negra S6IaH12 (LN) | – | |||||
| 2 | Ehrlichia sp. Serrana S8IaN38 (LN) | 99.79 | – | ||||
| 3 | Ehrlichia sp. Serrana S12IaH4 (Amaril) | 99.78 | 100 | – | |||
| 4 | Ehrlichia sp. Serrana S17IaH1 (Amarillo) | 99.68 | 99.89 | 99.89 | – | ||
| 5 | Ehrlichia sp. Serrana S17IaN33 (Salus) | 99.78 | 100 | 100 | 99.89 | – | |
| 6 | Ehrlichia sp. Magellanica (MK049840) | 97.12 | 97.12 | 90.8 | 92.24 | 92.52 | – |
Table 4.
Pairwise comparison matrix for dsb sequences of Ehrlichia sp. Serrana, Ehrlichia sp. Laguna Negra, and Ehrlichia sp. Magellanica
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Ehrlichia sp. Laguna Negra S6IaH12 (LN) | – | ||||||
| 2 | Ehrlichia sp. Serrana S8IaN38 (LN) | 88.47 | – | |||||
| 3 | Ehrlichia sp. Serrana S10IaN9 (Salus) | 88.47 | 100 | – | ||||
| 4 | Ehrlichia sp. Serrana S12IaH4 (Amarillo) | 88.47 | 100 | 100 | – | |||
| 5 | Ehrlichia sp. Serrana S17IaH1 (Amarillo) | 88.16 | 99.68 | 99.68 | 99.68 | – | ||
| 6 | Ehrlichia sp. Serrana S17IaN33 (Salus) | 88.16 | 99.68 | 99.68 | 99.68 | 100 | – | |
| 7 | Ehrlichia sp. Magellanica (MK049838) | 88.78 | 87.22 | 87.22 | 87.22 | 86.91 | 86.91 | – |
Table 5.
Pairwise comparison matrix for groEL sequences of Ehrlichia sp. Serrana, Ehrlichia sp. Laguna Negra, and Ehrlichia sp. Magellanica
| 1 | 2 | 3 | 4 | 5 | ||
|---|---|---|---|---|---|---|
| 1 | Ehrlichia sp. Laguna Negra S6IaH12 (LN) | – | ||||
| 2 | Ehrlichia sp. Laguna Negra S8IaH27 (LN) | 99.78 | – | |||
| 3 | Ehrlichia sp. Serrana S10IaN9 (Salus) | 96.05 | 95.83 | – | ||
| 4 | Ehrlichia sp. Serrana S17IaH1 (Amarillo) | 95.61 | 95.39 | 98.02 | – | |
| 5 | Ehrlichia sp. Magellanica (MK049839) | 92.85 | 92.51 | 92.17 | 91.83 | – |
4. Discussion
In the present study, we performed molecular screening of Ehrlichia spp. in I. auritulus and revealed the occurrence of two genotypes of Ehrlichia provisionally named Serrana and Laguna Negra. Genetic comparisons conducted for the three studied genes are consistent to consider them as separate organisms. This assumption was confirmed by the topologies of phylogenetic trees since Serrana and Laguna Negra genotypes formed well-defined and separated clades within the genus Ehrlichia. Interestingly, Ehrlichia sp. Magellanica seems to correspond to a closely related species, because it grouped with both genotypes in all phylogenies. In addition, Ehrlichia sp. Magellanica and both genotypes herein characterized were similar when performing nucleotide pairwise comparisons, and all three were detected in ticks that parasitize birds.
In the Southern Cone of South America, the majority of the Ehrlichia spp. detected in ticks have been found in ticks from the genera Amblyomma and Rhipicephalus. Currently, in this region of the American continent, species such as E. canis and E. minasensis are of medical and veterinary concern (Aguiar, 2017). A remarkable exception is Ehrlichia sp. Magellanica that was detected in I. uriae in Southern Chile (Muñoz-Leal et al., 2019).
Considering the phylogenetic relationships between these genotypes and their common eco–epidemiological profiles, it is reasonable to state that these species may correspond to a natural lineage associated with birds.
Some Ehrlichia species are considered pathogenic for humans and domestic animals, and currently mammals are the only group of vertebrates demonstrated to sustain infections in nature (Rar & Golovljova, 2011; Gofton et al., 2018). However, molecular evidence for a group of ehrlichiae that may infect Magellanic penguins (S. magellanicus) was reported in Chile (Muñoz-Leal et al., 2019), and sequences of 16S rRNA gene related to E. chaffeensis were retrieved from a song thrush (Turdus philomelos) in Hungary (Hornok et al., 2020). In this study, we detected Ehrlichia DNA in I. auritulus, a tick that feeds chiefly on birds (Nava et al., 2017; Guglielmone et al., 2020), reinforcing the hypothesis that avian hosts should be evaluated as competent hosts for bacteria of this genus.
Finally, our results constitute the first characterization of Ehrlichia spp. in Uruguay. Future studies on birds reported as hosts for I. auritulus are needed to gain epidemiological data on both Ehrlichia genotypes in the country. Since I. auritulus does not parasitize humans, these putatively novel Ehrlichia species are probably not involved in human ehrlichiosis.
CRediT author statement
María L. Félix: Conceptualization, Data Curation, Methodology, Formal Analysis, Investigation, Project Administration, Writing - Original Draft, Writing - Review & Editing. Sebastián Muñoz-Leal: Conceptualization, Formal Analysis, Methodology, Writing - Original Draft, Writing - Review & Editing. Luis A. Carvalho: Formal Analysis, Investigation, Methodology, Writing - Review & Editing. Diego Queirolo: Investigation, Methodology, Writing - Review & Editing. Susana Remesar Alonso: Investigation, Methodology, Writing - Review & Editing. Santiago Nava: Formal Analysis, Writing - Original Draft, Writing - Review. María T. Armúa-Fernández: Conceptualization, Data Curation, Methodology, Formal Analysis, Investigation, Writing - Original Draft, Writing - Review & Editing. José M. Venzal: Conceptualization, Data Curation, Methodology, Resources, Formal Analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Methodology, Formal Analysis, Investigation, Writing - Original Draft, Writing - Review & Editing. All authors read and approved the final manuscript.
Funding
This research was partly supported by the Comisión Sectorial de Investigación Científica (Programa Iniciación a la Investigación 2017 - Project ID 160) for the financial support to MLF.
Declaration of competing interests
The authors declare that they have no competing interests.
Acknowledgements
We would like to thank Gustavo de Souza and Fernando Ramos (Colonia Don Bosco, Laguna Negra, Rocha); Eduardo Méndez, Alejandro Rodríguez and Andrés de Mello (Reserva Natural Salus, Lavalleja); Hugo Pereda and Ricardo Palle (Gruta de los Cuervos, Tacuarembó); Daniel Casalás, Winderson Rodriguez da Cunha, and Lorena Ojeda (Amarillo, Rivera), for their collaborations during the fieldwork.
References
- Aguiar D.M. In: Emerging and Re-emerging infectious diseases of livestock. Springer, Cham, Switzerland. Bayry J., editor. 2017. Ehrlichiosis; pp. 365–375. [Google Scholar]
- Almeida A., Souza T., Marcili A., Labruna M. Novel Ehrlichia and Hepatozoon agents infecting the crab-esting fox (Cerdocyon thous) in southeastern Brazil. J. Med. Entomol. 2013;50:640–646. doi: 10.1603/me12272. [DOI] [PubMed] [Google Scholar]
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Brazeiro A., Panario D., Soutullo A., Gutierrez O., Segura A., Mai P. Clasificación y delimitación de las eco-regiones de Uruguay. Informe Técnico. Convenio MGAP/PPR–Facultad de Ciencias/Vida Silvestre/Sociedad Zoológica del Uruguay/CIEDUR. 2012 http://vidasilvestre.org.uy/wp-content/uploads/2012/05/Ecorregiones.pdf March 2021. [Google Scholar]
- Brouqui P., Matsumoto K. In: Rickettsial diseases. Raoult D., Parola P., editors. Informa; New York: 2007. Bacteriology and phylogeny of Anaplasmataceae; pp. 179–198. [Google Scholar]
- Cabezas-Cruz A., Zweygarth E., Vancová M., Broniszewska M., Grubhoffer L., Passos L., et al. Ehrlichia minasensis sp. nov., isolated from the tick Rhipicephalus microplus. Int. J. Syst. Evol. Microbiol. 2016;66:1423–1430. doi: 10.1099/ijsem.0.000895. [DOI] [PubMed] [Google Scholar]
- Carvalho L.A., Maya L., Armua-Fernandez M.T., Félix M.L., Bazzano V., Barbieri A.M., et al. Borreliaburgdorferi sensu lato infecting Ixodes auritulus ticks in Uruguay. Exp. Appl. Acarol. 2020;80:109–125. doi: 10.1007/s10493-019-00435-8. [DOI] [PubMed] [Google Scholar]
- Cicuttin G.L., De Salvo M.N., Díaz Pérez P., Silva D., Félix M.L., Venzal J.M., Nava S. A novel Ehrlichia strain (Rickettsiales: Anaplasmataceae) detected in Amblyomma triste (Acari: Ixodidae), a tick species of public health importance in the Southern Cone of America. Pathog. Glob. Health. 2020;114:318–322. doi: 10.1080/20477724.2020.1795579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicuttin G.L., De Salvo M.N., Nava S. Two novel Ehrlichia strains detected in Amblyomma tigrinum ticks associated to dogs in peri-urban areas of Argentina. Comp. Immunol. Microbiol. Infect. Dis. 2017;53:40–44. doi: 10.1016/j.cimid.2017.07.001. [DOI] [PubMed] [Google Scholar]
- Cicuttin G.L., De Salvo M.N., Venzal J.M., Nava S. Borrelia spp. in ticks and birds from a protected urban area in Buenos Aires city, Argentina. Ticks Tick Borne Dis. 2019;10:101282. doi: 10.1016/j.ttbdis.2019.101282. [DOI] [PubMed] [Google Scholar]
- Cicuttin G.L., Tarragona E.L., De Salvo M.N., Mangold A.J., Nava S. Infection with Ehrlichia canis and Anaplasma platys (Rickettsiales: Anaplasmataceae) in two lineages of Rhipicephalus sanguineus sensu lato (Acari: Ixodidae) from Argentina. Ticks Tick Borne Dis. 2015;6:724–729. doi: 10.1016/j.ttbdis.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Conti-Díaz I.A. Enfermedades emergentes y reemergentes en. Uruguay. Rev. Med. Urug. 2001;17:180–199. [Google Scholar]
- Doyle C.K., Labruna M.B., Breitschwerdt E.B., Tang Y., Corstvet R.E., Hegarty B.C., et al. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time PCR of the dsb Gene. J. Mol. Diag. 2005;7:504–510. doi: 10.1016/S1525-1578(10)60581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt A.T., Fernandez C., Fargnoli L., Beldomenico P.M., Monje L.D. A putative novel strain of Ehrlichia infecting Amblyomma tigrinum associated with Pampas fox (Lycalopex gymnocercus) in Esteros del Iberá ecoregion, Argentina. Ticks Tick Borne Dis. 2020;11:101318. doi: 10.1016/j.ttbdis.2019.101318. [DOI] [PubMed] [Google Scholar]
- Esemu S.N., Ndip L.M., Ndip R.N. Ehrlichia species, probable emerging human pathogens in sub-Saharan Africa: Environmental exacerbation. Rev. Environ. Health. 2011;26:269–279. doi: 10.1515/reveh.2011.034. [DOI] [PubMed] [Google Scholar]
- Fargnoli L., Fernandez C., Monje L.D. Novel Ehrlichia strain infecting cattle tick Amblyomma neumanni, Argentina, 2018. Emerg. Infect. Dis. 2020;26:1027–1030. doi: 10.3201/eid2605.190940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gofton A.W., Loh S.M., Barbosa A.D., Paparini A., Gillett A., Macgregor J., et al. A novel Ehrlichia species in blood and Ixodes ornithorhynchi ticks from platypuses (Ornithorhynchus anatinus) in Queensland and Tasmania, Australia. Ticks Tick Borne Dis. 2018;9:435–442. doi: 10.1016/j.ttbdis.2017.12.011. [DOI] [PubMed] [Google Scholar]
- Guglielmone A.A., Petney T.N., Robbins R.G. Ixodidae (Acari: Ixodoidea): Descriptions and redescriptions of all known species from 1758 to December 31, 2019. Zootaxa. 2020;4871:1–322. doi: 10.11646/zootaxa.4871.1.1. [DOI] [PubMed] [Google Scholar]
- Guillemi E.C., Orozco M.M., Argibay H.D., Farber M.D. Evidence of Ehrlichia chaffeensis in Argentina through molecular detection in marsh deer (Blastocerus dichotomus) Int. J. Parasitol. Parasites Wildl. 2019;8:45–49. doi: 10.1016/j.ijppaw.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornok S., Boldogh S.A., Takács N., Juhász A., Kontschán J., Földi D., et al. Anaplasmataceae closely related to Ehrlichia chaffeensis and Neorickettsia helminthoeca from birds in Central Europe, Hungary. Antonie van Leeuwenhoek. 2020;113:1067–1073. doi: 10.1007/s10482-020-01415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Inokuma H., Parola P., Raoult D., Brouqui P. Molecular survey of Ehrlichia infection in ticks from animals in Yamaguchi Prefecture Japan. Vet. Parasitol. 2001;99:335–339. doi: 10.1016/s0304-4017(01)00470-8. [DOI] [PubMed] [Google Scholar]
- Ismail N., McBride J.W. Tick-borne emerging infections: Ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2017;37:317–340. doi: 10.1016/j.cll.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Kohls G.M. Records and new synonymy of New World Haemaphysalis ticks, with descriptions of the nymph and larva of H. juxtakochi Cooley. J. Parasitol. 1960;46:355–361. [PubMed] [Google Scholar]
- Lasta C.S., Santos A.P.D., Messick J.B., Oliveira S.T., Biondo A.W., Vieira R.F.C., et al. Molecular detection of Ehrlichia canis and Anaplasma platys in dogs in southern Brazil. Rev. Bras. Parasitol. Vet. 2013;22:360–366. doi: 10.1590/S1984-29612013000300007. [DOI] [PubMed] [Google Scholar]
- López J., Abarca K., Mundaca M.I., Caballero C., Valiente-Echeverría F. Identificación molecular de Ehrlichia canis en un canino de la ciudad de Arica, Chile. Rev. Chil. Infect. 2012;29:527–530. doi: 10.4067/S0716-10182012000600008. [DOI] [PubMed] [Google Scholar]
- Lotric-Furlan S., Petrovec M., Zupanc T.A., Nicholson W.L., Sumner J.W., Childs J.E., Strle F. Human granulocytic ehrlichiosis in Europe: Clinical and laboratory findings for four patients from Slovenia. Clin. Infect. Dis. 1998;27:424–428. doi: 10.1086/514683. [DOI] [PubMed] [Google Scholar]
- Monje L.D., Fernandez C., Percara A. Detection of Ehrlichia sp. strain San Luis and ‘Candidatus Rickettsia andeanae’ in Amblyomma parvum ticks. Ticks Tick Borne Dis. 2019;10:111–114. doi: 10.1016/j.ttbdis.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Montagna M., Sassera D., Epis S., Bazzocchi C., Vannini C., Lo N., et al. “Candidatus Midichloriaceae” fam. nov. (Rickettsiales), an ecologically widespread clade of intracellular alphaproteobacteria. Appl. Environ. Microbiol. 2013;79:3241–3248. doi: 10.1128/AEM.03971-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Leal S., Clemes Y.S., Lopes M.G., Acosta I.C.L., Serpa M.C.A., Mayorga L.F.S.P., et al. Novel Ehrlichia sp. detected in Magellanic penguins (Sphenicus magellanicus) and in the seabird tick Ixodes uriae from Magdalena Island, southern Chile. Ticks Tick Borne Dis. 2019;10:101256. doi: 10.1016/j.ttbdis.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Muraro L.S., Nogueira M.F., Borges A.M.C.M., Souza A.O., Vieira T.S.W.J., Aguiar D.M. Detection of Ehrlichia sp. in Amblyomma sculptum parasitizing horses from Brazilian Pantanal wetland. Ticks Tick Borne Dis. 2021;12:101658. doi: 10.1016/j.ttbdis.2021.101658. [DOI] [PubMed] [Google Scholar]
- Nava S., Venzal J.M., González-Acuña D., Martins T.F., Guglielmone A.A. Elsevier Academic Press; London, San Diego, Cambridge, Oxford: 2017. Ticks of the Southern Cone of America. [Google Scholar]
- Nicholson W.L., Castro M.B., Kramer V.L., Sumner J.W., Childs J.E. Dusky-footed wood rats (Neotoma fuscipes) as reservoirs of granulocytic ehrlichiae (Rickettsiales: Ehrlichieae) in northern California. J. Clin. Microbiol. 1999;37:3323–3327. doi: 10.1128/jcm.37.10.3323-3327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P., Roux V., Camicas J.L., Baradji I., Brouqui P., Raoult D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 2000;94:707–708. doi: 10.1016/s0035-9203(00)90243-8. [DOI] [PubMed] [Google Scholar]
- Popov V.L., Han V.C., Chen S.M., Dumler J.S., Feng H.M., Andreadis T.G., et al. Ultrastructural differentiation of the genogroups in the genus Ehrlichia. J. Med. Microbiol. 1998;47:235–251. doi: 10.1099/00222615-47-3-235. [DOI] [PubMed] [Google Scholar]
- Rar V., Golovljova I. Anaplasma, Ehrlichia, and ‘Candidatus Neoehrlichia’ bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect. Genet. Evol. 2011;11:1842–1861. doi: 10.1016/j.meegid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Sacchi A.B.V., Duarte J.M.B., André M.R., Machado R.Z. Prevalence and molecular characterization of Anaplasmataceae agents in free-ranging Brazilian marsh deer (Blastocerus dichotomus) Comp. Immunol. Microbiol. Infect. Dis. 2012;35:325–334. doi: 10.1016/j.cimid.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Sumner J.W., Nicholson W.L., Massung R.F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szokoli F., Sabaneyeva E., Castelli M., Krenek S., Schrallhammer M., Soares C.A., et al. “Candidatus Fokinia solitaria”, a novel “stand-alone” symbiotic lineage of Midichloriaceae (Rickettsiales) PLoS One. 2016;11 doi: 10.1371/journal.pone.0145743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S., Alexander W., Gilligan J., Rikihisa Y. In: Rickettsiales. Biology, molecular biology, epidemiology, and vaccine development. Thomas S., editor. Springer International Publishing AG; Switzerland: 2016. Chapter 1. The importance of Rickettsiales infections; pp. 3–21. [Google Scholar]
- Thompson J.D., Higgins D., Gibson T.J. CLUSTALW: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassone L., Nuñez P., Gurtler R., Ceballos L.A., Orozco M.A., Kitron U.D., Farber M. Molecular detection of Ehrlichia chaffeensis in Amblyomma parvum ticks, Argentina. Emerg. Infect. Dis. 2008;14:1953–1955. doi: 10.3201/eid1412.080781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira T.S., Vieira R.F., Krawczak F.S., Soares H.S., Guimarães A.M., Barros-Filho I.R., et al. Ehrlichia sp. infection in carthorses of low-income owners: Southern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2016;48:1–5. doi: 10.1016/j.cimid.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Webb J.P., Jr., Bennett S.G., Challet G.L. The larval ticks of the genus Ixodes Latreille (Acari: Ixodidae) of California. Bull. Soc. Vector Ecol. 1990;15:73–124. [Google Scholar]
- Weisburg W.G., Bams S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173:679–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer C.E., Azevedo F.C., Almeida A.P., Ferreira F., Labruna M.B. Tickborne bacteria in free-living jaguars (Panthera onca) in Pantanal, Brazil. Vector Borne Zoonotic Dis. 2011;11:1001–1005. doi: 10.1089/vbz.2011.0619. [DOI] [PubMed] [Google Scholar]