Abstract
Sarcoptic mange caused by Sarcoptes scabiei has been present in the Swedish red fox (Vulpes vulpes) population since the 1970s. The disease has been described in other Swedish wildlife species, but not in the wild boar, Sus scrofa, until 2009. Single cases of sarcoptic mange have been diagnosed the last years in the expanding population of wild boar. This study aims to describe the histopathological lesions found on mangy wild boar and compare, by molecular methods, mites from wild boar cases with mites from mangy red foxes, raccoon dogs, and domestic pigs. Mangy wild boar with focal alopecia and clinical signs of pruritis were reported or submitted from various areas in southern Sweden to the National Veterinary Institute, Uppsala. The examined skin samples of wild boar infected with S. scabiei showed limited gross skin lesions, except for cases with severe exudative dermatitis. Histopathology of the affected wild boar skin samples showed an eosinophilic dermatitis with a variable hyperkeratosis and often low number of mites present. To study the relationship of S. scabiei mites isolated from different host species, a population genetics investigation was performed based on microsatellite markers. In total, 225 individual mites from eight individuals of four different host species; red fox (48 mites), wild boar (80 mites), domestic pig (48 mites) and raccoon dog (43 mites), were included in the study. In the phylogenetic analysis, all mites isolated from wild boar clustered together even though they originate from different geographical regions in Sweden. Mites from each individual host showed high similarity. The results indicate that wild boar mites differ from mites both from the red fox, raccoon dog, and domestic pig.
Keywords: Sarcoptes scabiei, Wildlife, Ectoparasite, Microsatellite, Sweden
Graphical abstract
Highlights
-
•
Detailed description of pathological findings and histological sections from wild boar diagnosed with sarcoptic mange.
-
•
Phylogenetic analysis of 225 Sarcoptes scabiei mites from 8 different hosts of 4 species using microsatellites.
-
•
Clustering of mites by host species as well as clustering of mites from carnivore hosts separate from Sus scrofa mites.
1. Introduction
Sarcoptic mange, caused by the arthropod Sarcoptes scabiei, is a condition that can affect several terrestrial mammals, including humans all over the world (Niedringhaus et al., 2019). There are over 300 million people globally affected by mange, and the condition accounts for significant financial losses in the livestock sector, particularly in pig production (Mounsey et al., 2010; Alasaad et al., 2011b). Presence of S. scabiei has been reported in a large variety of wild animals including the red fox (Vulpes vulpes), Pyrenean chamois (Rupicapra pyrenaica), and Iberian ibex (Capra pyrenaica) where endemic outbreaks occur with devastating mortality, e.g. the Scandinavian red fox population, which was severely affected in the mid-1980s, and the Iberian ibex in Spain, which has suffered substantial losses in the past decade (Borg, 1987; León-Vizcaíno et al., 1999) and recently in the Alpine chamois and Alpine ibex in the Eastern Alps (Turchetto et al., 2020). In Swedish wildlife, sarcoptic mange has, apart from in the red fox, also been diagnosed in the pine marten (Martes martes), Artic fox (Vulpes lagopus), Eurasian lynx (Lynx lynx), and wolf (Canis lupus) (Mörner, 1992; Ryser-Degiorgis et al., 2002; Mörner et al., 2005; Oleaga et al., 2008; Collins et al., 2010).
Sarcoptes infection is common in domestic pigs worldwide, and infection in wild boar populations has thus far been reported in several parts of the world, including Croatia, France, Italy, Spain and Switzerland (Mignone et al., 1995; Rajković-Janje et al., 2004; López-Olvera et al., 2006; Rasero et al., 2010; Wu et al., 2012). The wild boar is reported to be more resilient to parasites than other mammalian species (Rasero et al., 2010; Haas et al., 2018). Sweden has a fast-growing wild boar population with a hunting harvest of 120,000–150,000 individuals per year in the past years (Jägareförbundet, 2021). At the Swedish National Veterinary Institute (SVA), a systematic wildlife disease surveillance programme has been running with all received cases recorded since the late 1940ʼs. Within this programme, wild animals found dead or put down due to signs of disease are submitted and necropsied, as whole carcasses or as parts or tissue samples. In total, 94 wild boars have been examined in the period 2002–2012. Of these, 14% were diagnosed with sarcoptic mange.
The diagnostics of sarcoptic mange is mainly based on skin scrapings where mites are observed with the aid of microscopy. Other diagnostic techniques can be applied, such as serological ELISA tests, rubbing index and measurement of skin lesions (Jacobson et al., 2000). A further challenge in the study of the epidemiology of sarcoptic mange is that the morphology of mites is indistinguishable between different host species and geographical areas, although cross-infection attempts fail and there seems to be a strong host preference of these parasites (Arlian, 1989). However, a zoonotic potential has been indicated with transfer of disease from pigs to humans (Grahofer et al., 2018).
In recent years, extensive research has elucidated appropriate methods to study the molecular epidemiology of S. scabiei with some advancements made. Using microsatellite markers, Rasero et al. (2010) and Soglia et al. (2007) were able to distinguish between mites of both different host species and geographical regions, representing 15 and 10 wild host-derived populations, respectively, and more recently Moroni et al. (2021) used the same method to distinguish between mites from hare, rabbits and their main predators. A clear clustering was observed for carnivore-, herbivore- and omnivore-derived mites respectively. A clear distinction was also seen between mites from separate geographical populations of one host species.
With a low prevalence of sarcoptic mange in the Swedish population of domestic pigs, and the disease eradicated from most commercial herds or kept under control with routine medication in others (Jacobson et al., 2000), it is not clear if or how the wild boar could contract sarcoptic mange from farmed pigs. The fact that Sarcoptes mites only live for short periods of time off their host means that a close, often direct contact between different host species is necessary (Menzano et al., 2008; Mounsey et al., 2010). It is possible that the sporadic wild boar cases are due to contact with dead or debilitated mangy red foxes, since the Scandinavian red fox population has suffered from sarcoptic mange since the mid 1970ʼs (Borg, 1987). Cross-transmission of S. scabiei between different host species has been reported previously (Samuel, 1981; Lavin et al., 2000; Gakuya et al., 2011).
To establish the epidemiology of Sarcoptes infections, microsatellite genotyping can be used (Rasero et al., 2010; Alasaad et al., 2012). The possibility to determine the origin of infection is important for wildlife management but also to predict if wild boar could be a possible reservoir for spread of Sarcoptes to domestic pigs, questions also raised elsewhere (Haas et al., 2015).
This study aims to describe the histopathological lesions found on mangy wild boar and compare, by molecular methods, mites from wild boar cases with mites from mangy red foxes, raccoon dogs, and domestic pigs.
2. Materials and methods
2.1. Sarcoptes material
Retrospective records of whole carcasses or parts of wild boars sent in for examination to the National Veterinary Institute, Uppsala, between the years 2002–2012 were included in this study. Paraffin blocks with formalin-fixed and dehydrated skin samples from 12 wild boars diagnosed with sarcoptic mange and 5 wild boars with alopecia but not diagnosed with sarcoptic mange were obtained from the SVA archive. Fresh slides were made, stained with hematoxylin and eosin (HE) and examined under a light microscope.
Molecular analyses were carried out on mites collected from a total of eight host individuals representing four different mammalian species. Three individual wild boars shot at three different locations within the main distribution area, i.e. southern Sweden, two red foxes, one raccoon dog (Nyctereutes procyonoides), and two domestic pigs (both from the same mange-infested pig farm). Further details on the collection of mites for molecular analysis are listed in Table 1.
Table 1.
Collection data of the mites used for molecular analysis. All collected mites were identified as S. scabiei based on morphological characteristics
| Sample ID | Host species | Region | Year of collection | No. of mites |
|---|---|---|---|---|
| WB1 (2076/09) | Wild boar | Kronoberg | 2009 | 42 |
| WB2 (278/11) | Wild boar | Kronoberg | 2011 | 19 |
| WB3 (1293/10) | Wild boar | Kalmar | 2010 | 19 |
| DP1 | Domestic pig | Västmanland | 2010 | 16 |
| DP2 | Domestic pig | Västmanland | 2010 | 32 |
| FOX1 | Red fox | Not known | 2010 | 21 |
| FOX2 | Red fox | Not known | Before 2000 | 27 |
| RD1 | Racoon dog | Norrbotten | 2009 | 43 |
2.2. Mite isolation
The mites used for molecular analysis were isolated using either of two methods. For fresh material, a skin sample was placed in a Petri dish under a warm lamp overnight. The mites left the skin, moving towards the heat source, and could easily be collected from the inside of the lid of the Petri dish. For frozen material, the potassium hydroxide digestion technique was used (Alasaad et al., 2009b). Skin samples were digested with 10% KOH solution for 10–30 min. Samples were vortexed every 10 min to extract mites from the skin. Following extraction, tissue debris was removed by centrifugation at 3000× g for 3 min. The supernatant was poured into a Petri dish and single mites were collected.
2.3. Molecular analysis
Single mites were collected in 1.5 μl microtubes and DNA extraction was performed. In brief, 25 μl lysis reagent (25 mM NaOH, 0.2 mM disodium EDTA, pH = 12) was added and the mites were mechanically homogenized with a plastic pestle (Sigma-Aldrich). The solution was heat-treated at 95 °C for 20 min followed by ice water-bath for 2 min. Cell debris was removed by centrifugation at 6800× g for 2 min. The supernatant was removed, and the solution was neutralized with 25 μl neutralization buffer (40 mM Tris-HCl). Each sample was analyzed for 11 microsatellites (SARMS 15; SARMS 33; SARMS 34; SARMS 35; SARMS 36; SARMS 37; SARMS 38; SARMS 40; SARMS 41; SARMS 44; and SARMS 45) (Walton et al., 2004) amplified in a multiplex PCR. Amplification was performed in 15 μl reactions with 1 U HotStarTaq DNA polymerase and PCR buffer (Qiagen, Hilden, Germany), 2.0 mM MgCl2, 0.2 mM dNTPs, 0.1 μM SARMS15 oligos (forward and reverse), 0.035 μM SARMS 33, 0.04 μM SARMS 34, 0.06 μM SARMS 35, 40, 41, 44, 45, 0.1 μM SARMS 36, 0.05 μM SARMS 37, 38, and 3 μl template genomic DNA. Moreover, from each DNA preparation a short ribosomal DNA segment of the internal transcribed spacer 2 (ITS2) was sequenced. The segment was amplified in 15 μl PCR reactions using the primers RIB-18 and RIB-3 as reported by Zahler et al. (1999). The PCR reactions consisted of 0.5 U HotStarTaq DNA polymerase and PCR buffer (Qiagen), 2.0 mM MgCl2, 0.2 mM dNTPs, 0.5 μM of each oligonucleotide and 3 μl DNA template. The PCR products were directly prepared for sequencing reaction by adding 2 μl ExoSAP-IT (Affymetrix, Santa Clara, California, USA) to each reaction. Sequencing reaction was performed using BigDye Terminator sequencing kit 3.1 (Life Technologies, Carlsbad, California, USA) and analyzed on a Genetic Analyzer 3130xl.
2.4. Pylogenetic analysis
All markers were included for each individual mite in a phylogenetic analysis using Bionumerics 7.5 (Applied Maths, Sint-Martens-Latem, Belgium), with each marker as a categorical variable and default priority rules. The results are presented as a Minimum Spanning Tree.
3. Results
A total of 34 whole wild boar carcasses and 60 samples from wild boars were submitted to the SVA during the study period. Of these 94 cases, 13 were diagnosed with sarcoptic mange. Of the 13 diagnosed cases, 5 were whole carcasses and 8 were samples only. Samples of skin archived in paraffin blocks were available from 12 of these cases.
3.1. Gross lesions
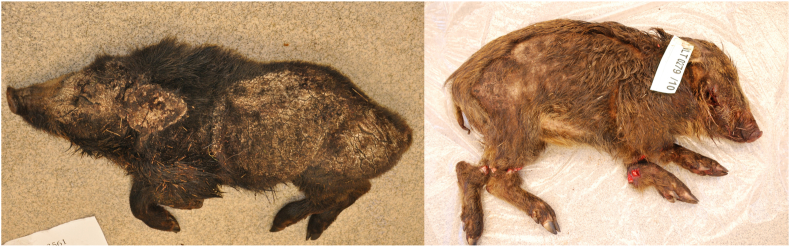
Mild to moderate alopecia, mild thickening of the dermis and superficial crusts with underlying exudate were the most common findings. The lesions were usually found on the ears, face, and dorsal and lateral aspects of the torso. Three cases showed severe changes with widespread alopecia and diffuse exudative dermatitis with thick crusts. These wild boars were emaciated with apparent body muscle wasting (Fig. 1).
Fig. 1.
Two juvenile wild boars, severely emaciated, with apparent body muscle wasting, widespread alopecia and diffuse exudative dermatitis with thick crusts, submitted to the National Veterinary Institute (SVA), Uppsala, Sweden.
3.2. Histopathology
The most common findings in sections from wild boars with sarcoptic mange were serocellular crusts, parakeratosis, moderate acanthosis with sparse subepidermal edema, and moderate superficial perivascular dermatitis, dominated by eosinophils, plasma cells, neutrophils, and fewer lymphocytes. In alopecic skin, the hair follicles were generally intact, with active hair buds, indicating regenerative processes. Occasionally, pustules with degenerate inflammatory cells and cellular debris, as well as bacteria, were seen. Globular parasites with spiny cuticle and articulated appendages were infrequently seen in the cornified hyperkeratotic epidermis, usually only as single parasites on one glass slide section. In Table 2, the gross and microscopic findings are listed and ranked 0–3, with zero indicating no visible change, and three indicating severe lesions.
Table 2.
Presentation of gross and microscopic findings in 17 wild boars and samples from wild boar sent to the National Veterinary Institute (SVA) during the years 2008–2013 and diagnoses with sarcoptic mange and/or alopecia
| Animal ID | Sarcoptic mange diagnosis at necropsy | Presence of mites | Hyperkeratosis/Parakeratosis | Crust formation | Acanthosis | Vasculitis | Eosinophils | Neutrophils | Plasma cells | Macrophages |
|---|---|---|---|---|---|---|---|---|---|---|
| 338/09 | Yes | 1 | 2 | 2 | 2 | 2 | 0 | 0 | 0 | 0 |
| 819/09 | Yes | 1 | 3 | 3 | 1 | 1 | 0 | 0 | 0 | 0 |
| 2076/09 | Yes | 1 | 2 | 2 | 2–3 | 2 | 2 | 2 | 1 | 1 |
| 279/10 | Yes | 2–3 | 3 | 3 | 2–3 | 2 | 1 | 1 | 1 | 0 |
| 624/10 | Yes | 1 | 2 | 2 | 2 | 2 | 2 | a | 0 | 0 |
| 1293/10 | Yes | 0 | 0 | 0 | 1 | 1b | 1 | 0 | 0 | 0 |
| 182/11 | Yes | 2–3 | 2 | 2–3 | 2 | 1–2 | 1 | 1 | 2 | 1 |
| 278/11 | Yes | 1 | 2 | 1–2 | 2 | 2 | 3 | 1 | 2 | 0 |
| 1200/11 | Yes | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 |
| 2405/11 | Yes | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 1 |
| 6052/11 | Yes | 0 | 2 | 3 | 2 | 3 | 2 | 1 | 1 | 0 |
| 480/13 | Yes | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 867/08 | No | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 143/10 | No | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 |
| 287/10 | No | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| 288/10 | No | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 |
| 181/11 | No | 0 | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 0 |
Note: The findings are listed and ranked 0–3, with 0 indicating no visible change/presence of mites, and 3 indicating severe lesions/heavy infestation of mites.
Intracutaneous pustules.
Focally around an attached tick, here is also severe eosinophilic infiltrate.
3.3. Microsatellite analysis
Eleven microsatellite markers alongside ITS2 were analyzed from 225 individual mites. These originated from 8 host individuals representing four host species. Little variation was identified in the ITS2 and no fixed differences could be found among the populations (data not shown) indicating this molecular marker is not useful for distinguishing among populations of Sarcoptes mites. For the other 11 loci, 55 alleles were detected. The number of alleles ranged from 3 to 7 for the different loci. Of the 55 alleles, 8 were private (present in only one population); most of these were detected in the wild boar populations (Table 3). Few differences were observed among mites collected from the same animal, although all populations showed variation at some markers. Only two private alleles, one each for marker SARMS 15 and SARMS 35, were present in only one individual mite. For both domestic pig and wild boar, several alleles were present only in mites from the specific host (14 in pig and 15 in wild boar). No alleles were specific for fox or raccoon dog. However, if results from wild canines were combined, eight alleles were specific for these two host species (Table 3). Only six alleles were found in both porcine and canine hosts.
Table 3.
Number of alleles present in mites from the different hosts, domestic pigs (DP), wild boar (WB), red fox (FOX) and raccoon dog (RD). Total number of alleles for the 11 loci is 55
| Unique to individual | Unique to population | Unique to host | |
|---|---|---|---|
| DP1 | 2 | ||
| DP2 | |||
| WB1 | 1 | 1 | |
| WB2 | |||
| WB3 | 3 | ||
| FOX1 | 1 | ||
| FOX2 | 1 | ||
| RD1 | 1 | ||
| DP all | 14 | ||
| WB all | 15 | ||
| FOX all | |||
| RD | |||
| Canine | 8 | ||
| Porcine | 2 |
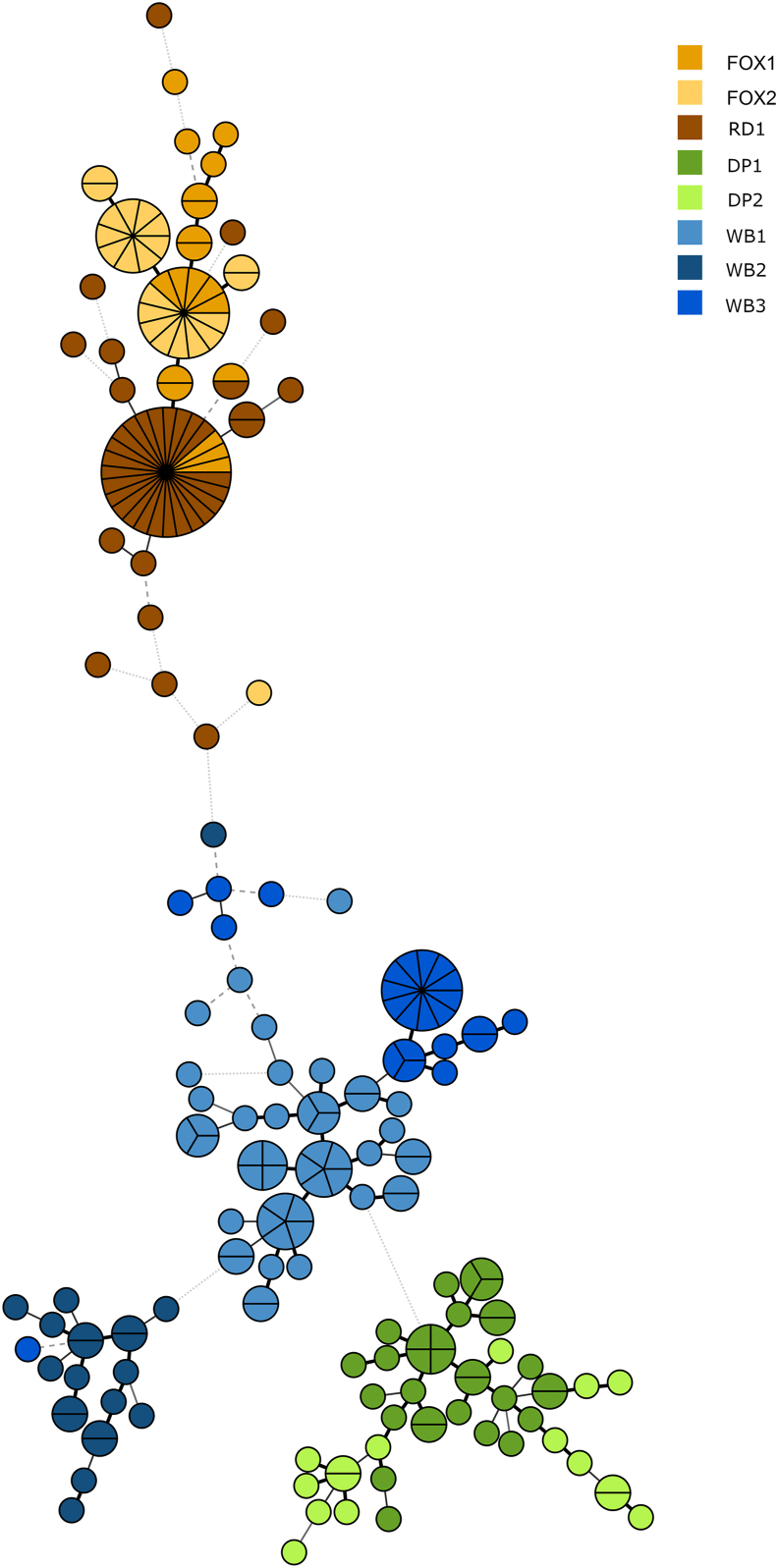
The Minimum Spanning Tree based on the microsatellite analysis of individual mites from the eight hosts revealed a clustering into groups representing the populations. With a few exceptions of mites from wild canine hosts grouping tight together (FOX and RD in Fig. 2), most individuals grouped with the other mites from the same host species. The phylogenetic analysis of relationships between populations (Fig. 2) shows that mites from domestic pigs grouped in one cluster close to but not together with wild boar mites, whereas fox and raccoon dog mites formed one clade. Within the clade with the three wild boar samples, single mites from one host are seen within distinct clusters of mites from another host. These wild boars were collected from different geographical regions, over a three-year period while the two fox samples where sampled ten years apart and in regions far from where the raccoon dog was collected.
Fig. 2.
Minimum Spanning Tree of isolates of Sarcoptes scabiei (n = 225) from eight different host individuals of Swedish wild boar (WB), domestic pig (DP), red fox (FOX) and racoon dog (RD). The tree shows the relationship, based on microsatellite genotyping, of individual mites isolated from different hosts. Created in Bionumerics 7.5. The strength of the lines from dotted grey to thick black as well as the distance between the circles represent relatedness.
4. Discussion
Sarcoptic mange appears to be endemic in the Swedish wild boar population. A continuous influx of mangy wild boars and reports of observed hairless individuals to the wildlife disease surveillance programme at the SVA supports this as well as a recent serological study by Haas et al. (2018) on the presence of S. scabei antibodies in samples from five European countries. Two out of the three sampling locations in Sweden included in the study had a seroprevalence of 10.3% while no positive samples were identified in the third (Haas et al., 2018).
The first confirmed Swedish case of sarcoptic mange in free-ranging wild boar that can be traced in the SVA records was diagnosed in 2009. Before this, only single cases from penned wild boar in game farms have been diagnosed with sarcoptic mange. Photographs of free-ranging wild boar with alopecic skin areas have been submitted occasionally with the suspicion of mange, but without any tissue samples, the diagnosis could not be confirmed. The Swedish wild boar population has grown rapidly during the last decade, doubling in number every third year. This increase in population density might explain the increased submission of sarcoptic mange cases to the SVA, as the wild boar hunting harvest in the 10-year study period increased from 20,000 to 100,000 harvested animals yearly (Jägareförbundet, 2021). In Sweden, sarcoptic mange has previously been reported in the red fox, artic fox, lynx, wolf, raccoon dogs, martens and in domestic animals such as dogs, cats and domestic pigs (Bornstein et al., 2006). The red fox population suffered severe decline from a sarcoptic mange outbreak starting in the late 1980ʼs and still present throughout the country, except for the island of Gotland (Mörner, 1992). Domestic pig farms, which have not been sanitized for mange, generally treat animals regularly with the antiparasitic drug ivermectin to control clinical signs of mange, but these farms may harbor subclinical mange to some extent (Jacobson et al., 2000). This has led to the hypotheses that wild boar mange may originate from another sympatric species or alternatively, that these mites are specific for wild boar.
Microsatellites have been shown to be useful to obtain a better resolution in studying the temporal stability, skin-scale genetic structure, origin of infection, geographical and host-species separation in S. scabiei (Rasero et al., 2010; Alasaad et al. 2011a, 2012; Rentería-Solís et al., 2014; Cardells et al., 2021). In the present study, we have also been able to investigate the origin of S. scabiei infection in wild boar in Sweden. The results of the present study further support the earlier findings that few loci and low sample sizes are sufficient to identify strong population differentiation between host species as suggested by Alasaad et al. (2012). With these results, a further investigation with more host species and a larger geographical spread would be favored in order to investigate if differences between geographically separated populations occur and if other species are involved in the spread and persistence of S. scabiei in Swedish wildlife.
The 225 studied mites all clustered according to their original host population, and the mite populations from the same host species clustered together. The only exception was that two red fox samples and the raccoon dog sample formed one compact cluster. With the two fox samples being collected more than 10 years apart and from different geographical regions, these results are in line with previous findings (Alasaad et al., 2011a). Our results suggest that the mites are rather host-specific or that the studied host habitats do not overlap. However, to better pinpoint the origin of infection, we would suggest expanding the study with mites from additional hosts such as lynx, wolf, domestic dogs and humans.
The low variability of the 450 bp long fragment of the ribosomal DNA ITS2 region sequenced in this study agrees with previous studies suggesting that this region is unsuitable for population genetic studies for S. scabiei (Alasaad et al., 2009a). In conclusion, the present study indicates that S. scabei mites from clinically mangy wild boar are host-specific and differ from mites from both domestic pig samples, red foxes, and raccoon dogs, although a closer relationship is observed between the samples from wild and domestic pigs. This suggests that the wild boar carry a host-specific sarcoptic mite populations, well adapted to its host. It is also possible that S. scabei is widespread in the wild boar population with most carriers showing no symptoms although harboring mites, possibly in their ear canals, as seen in farms with subclinically infected domestic pigs (Bornstein, 2004). The spread of the mites within the wild boar population would be possible due to the social behavior and an increasing population size of the host. Serological surveys of wild boars in five European countries indicate that sarcoptic mange can be present in local wild boar populations without any clinical cases reported (Haas et al., 2018).
5. Conclusions
The present study indicates that using microsatellites and a limited number of mites from each host could be a useful method for further epidemiological studies of sarcoptic mange in Swedish wildlife and at the interface between wildlife and domestic pigs and dogs. When outbreaks of mange occur in domestic pigs, it is important to determine how the biosecurity of the farm has been compromised to prevent failure of eradication efforts, or risk of reoccurring outbreaks.
Funding
This project was funded by the Swedish Environmental Protection Agency.
Ethical approval
Not applicable.
CRediT author statement
KT and MA designed and performed the microsatellite analysis and the phylogenetic analysis. AS and EÅ designed and performed the pathological examinations and the gathering of stored journal data as well as the histopathological examinations of stored samples. AS and KT drafted the first manuscript. AS, EÅ and KT finalized the manuscript and were involved in the revision process. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We wish to thank Dr Robert Söderlund for help with analyses in Bionumerics, hunters submitting samples within the framework of the wildlife disease surveillance programme and to all personnel working with analysis and necropsies of these samples at the National Veterinary Institute (SVA), Department of Pathology and Wildlife. Further, we wish to thank Lauren Davies for proof-reading the manuscript as well as the Swedish Environmental Protection Agency for funding.
References
- Alasaad S., Oleaga A., Casais R., Rossi L., Min A.M., Soriguer R.C., Gortazar C. Temporal stability in the genetic structure of Sarcoptes scabiei under the host-taxon law: empirical evidences from wildlife-derived Sarcoptes mite in Asturias, Spain. Parasit. Vectors. 2011;4:151. doi: 10.1186/1756-3305-4-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasaad S., Schuster R.K., Gakuya F., Theneyan M., Jowers M.J., Maione S., et al. Applicability of molecular markers to determine parasitic infection origins in the animal trade, a case study from Sarcoptes mites in wildebeest. Forensic Sci. Med. Pathol. 2012;8:280–284. doi: 10.1007/s12024-011-9268-z. [DOI] [PubMed] [Google Scholar]
- Alasaad S., Soglia D., Spalenza V., Maione S., Soriguer R.C., Pérez J.M., et al. Is ITS-2 rDNA suitable marker for genetic characterization of Sarcoptes mites from different wild animals in different geographic areas? Vet. Parasitol. 2009;159:181–185. doi: 10.1016/j.vetpar.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Alasaad S., Soglia D., Maione S., Sartore S., Soriguer R.C., Pérez J.M., et al. Effectiveness of the postponed isolation (post-frozen isolation) method for PCR-quality Sarcoptes mite gDNA. Exp. Appl. Acarol. 2009;47:173–178. doi: 10.1007/s10493-008-9196-0. [DOI] [PubMed] [Google Scholar]
- Alasaad S., Walton S., Rossi L., Bornstein S., Abu-Madi M., Soriguer R.C., et al. Sarcoptes-world molecular Network (Sarcoptes-WMN), integrating research on scabies. Int. J. Infect. Dis. 2011;15:e294–e297. doi: 10.1016/j.ijid.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Arlian L.G. Biology, host relations, and epidemiology of Sarcoptes scabiei. Annu. Rev. Entomol. 1989;34:139–161. doi: 10.1146/annurev.en.34.010189.001035. [DOI] [PubMed] [Google Scholar]
- Borg K. A review of wildlife diseases from Scandinavia. J. Wildl. Dis. 1987;23:527–533. doi: 10.7589/0090-3558-23.4.527. [DOI] [PubMed] [Google Scholar]
- Bornstein S. Sarcoptic mange in pigs. Pig News Inf. 2004;25:11N–24N. [Google Scholar]
- Bornstein S., Frössling J.,, Näslund K.,, Zakrisson G., Mörner T. Evaluation of a serological test (indirect ELISA) for the diagnosis of sarcoptic mange in red foxes (Vulpes vulpes) Vet. Dermatol. 2006;17:411–416. doi: 10.1111/j.1365-3164.2006.00548.x. [DOI] [PubMed] [Google Scholar]
- Cardells J., Lizana V., Martí-Marco A., Lavín S., Velarde R., Rossi L., Moroni B. First description of sarcoptic mange in an Iberian hare (Lepus granatensis) Curr. Res. Parasitol. Vector Borne Dis. 2021;1:100021. doi: 10.1016/j.crpvbd.2021.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R., Wessels M.E., Wood R., Couper D., Swift A. Sarcoptic mange in badgers in the UK. Vet. Rec. 2010;167:668. doi: 10.1136/vr.c5672. [DOI] [PubMed] [Google Scholar]
- Gakuya F., Rossi L., Ombui J., Maingi N., Muchemi G., Ogara W., Soriguer R.C., Alasaad S. The curse of the prey, Sarcoptes mite molecular analysis reveals potential prey-to-predator parasitic infestation in wild animals from Masai Mara, Kenya. Parasit. Vectors. 2011;4:193. doi: 10.1186/1756-3305-4-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahofer A., Bannoehr J., Nathues H., Roosje P. Sarcoptes infestation in two miniature pigs with zoonotic transmission - a case report. BMC Vet. Res. 2018;14 doi: 10.1186/s12917-018-1420-5/. https://doi.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C., Origgi F.C., Akdesir E., Batista Linhares M., Giovannini S., Mavrot F., et al. First detection of sarcoptic mange in free-ranging wild boar (Sus scrofa) in Switzerland. Schweiz. Arch. Tierheilkd. 2015;157:269–275. doi: 10.17236/sat00020. [DOI] [PubMed] [Google Scholar]
- Haas C., Origgi F.C., Rossi S., López-Olvera J.R., Rossi L., Castillo-Contreras R., et al. Serological survey in wild boar (Sus scrofa) in Switzerland and other European countries: Sarcoptes scabiei may be more widely distributed than previously thought. BMC Vet. Res. 2018;14:117. doi: 10.1186/s12917-018-1430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M., Bornstein S., Palmer E., Wallgren P. Elimination of Sarcoptes scabiei in pig herds by single or double administrations of an avermectin. Acta Vet. Scand. 2000;41:227–235. doi: 10.1186/BF03549631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jägareförbundet . Swedish Association for Hunting and Wildlife Management Jägareförbundet; Sweden: 2021. Swedish Association for Hunting and Wildlife Management.https://jagareforbundet.se/vilt/vildsvinsbarometern/ [Google Scholar]
- Lavin S., Ruiz-Bascaran M., Marco I., Fondevila M.D., Ramis A.J. Experimental infection of chamois (Rupicapra pyrenaica parva) with Sarcoptes scabiei derived from naturally infected goats. J. Vet. Med. B. 2000;47:693–699. doi: 10.1046/j.1439-0450.2000.00406.x. [DOI] [PubMed] [Google Scholar]
- León-Vizcaíno L., Ruíz de Ybáñez M.R., Cubero M.J., Ortíz J.M., Espinosa J., Pérez L., et al. Sarcoptic mange in Spanish ibex from Spain. J. Wildl. Dis. 1999;35:647–659. doi: 10.7589/0090-3558-35.4.647/. https://doi.org. [DOI] [PubMed] [Google Scholar]
- López-Olvera J.R., Höfle U., Vicente J., Fernández-de-Mera I.G., Gortázar C. Effects of parasitic helminths and ivermectin treatment on clinical parameters in the European wild boar (Sus scrofa) Parasitol. Res. 2006;98:582–587. doi: 10.1007/s00436-005-0099-2. [DOI] [PubMed] [Google Scholar]
- Menzano A., Rambozzi L., Molinar Min A.R., Meneguz P.G., Rossi L. Description and epidemiological implications of S. scabiei infection in roe deer (Capreolus capreolus) originating from chamois (Rupicapra rupicapra) Eur. J. Wildl. Res. 2008;54:757–761. https://10.1007/s10344-008-0195-6 [Google Scholar]
- Mignone W., Poggi M., Pistone G.C., Caramelli M., Bollo E., Biolatti B. Pathology of wild boar (Sus scrofa) in Liguria, Italy, between 1989 and 1992. J. Mountain Ecol. 1995;3:85–87. [Google Scholar]
- Moroni B., Angelone S., Pérez J.M., Molinar Min A.R., Pasquetti M., Tizzani P., et al. Sarcoptic mange in wild ruminants in Spain, solving the epidemiological enigma using microsatellite markers. Parasit. Vectors. 2021;14:171. doi: 10.1186/s13071-021-04673-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörner T. Sarcoptic mange in Swedish wildlife. Rev. Sci. Tech. 1992;11:1115–1121. [PubMed] [Google Scholar]
- Mörner T., Eriksson H., Bröjer C., Nilsson K., Uhlhorn H., Ågren E., et al. Disease and mortality in free-ranging brown bear (Ursus arctos), gray wolf (Canis lupus), and wolverine (Gulo gulo) in Sweden. J. Wildl. Dis. 2005;41:298–303. doi: 10.7589/0090-3558-41.2.298. [DOI] [PubMed] [Google Scholar]
- Mounsey K., Ho M.-F., Kelly A., Willis C., Pasay C., Kemp D.J., et al. A tractable experimental model for study of human and animal scabies. PLoS Negl. Trop. Dis. 2010;4 doi: 10.1371/journal.pntd.0000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedringhaus K.D., Brown J.D., Sweeley K.M., Yabsley M.J. A review of sarcoptic mange in North American wildlife. Int. J. Parasitol. Parasites Wildl. 2019;9:285–297. doi: 10.1016/j.ijppaw.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleaga A., Balseiro A., Gortázar C. Sarcoptic mange in two roe deer (Capreolus capreolus) from northern Spain. Eur. J. Wildl. Res. 2008;54:134–137. doi: 10.1007/s10344-007-0105-3. [DOI] [Google Scholar]
- Rajković-Janje R., Manojlović L., Gojmerac T. In-feed 0.6% ivermectin formulation for treatment of wild boar in the Moslavina hunting ground in Croatia. Eur. J. Wildl. Res. 2004;50:41–43. doi: 10.1007/s10344-003-0033-9. [DOI] [Google Scholar]
- Rasero R., Rossi L., Soglia D., Maione S., Sacchi P., Rambozzi L., et al. Host taxon-derived Sarcoptes mite in European wild animals revealed by microsatellite markers. Biol. Conserv. 2010;143:1269–1277. doi: 10.1016/j.biocon.2010.03.001. [DOI] [Google Scholar]
- Rentería-Solís Z., Min A.M., Alasaad S., Müller K., Michler F.-U., Schmäschke R., et al. Genetic epidemiology and pathology of raccoon-derived Sarcoptes mites from urban areas of Germany. Med. Vet. Entomol. 2014;28:98–103. doi: 10.1111/mve.12079. [DOI] [PubMed] [Google Scholar]
- Ryser-Degiorgis M.-P., Ryser A., Bacciarini L.N., Angst C., Gottstein B., Janovsky M., Breitenmoser U. Notoedric and sarcoptic mange in free-ranging Lynx from Switzerland. J. Wildl. Dis. 2002;38:228–232. doi: 10.1016/j.vetimm.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Samuel W.M. Attempted experimental transfer of sarcoptic mange (Sarcoptes scabiei, Acarina, Sarcoptidae) among red fox, coyote, wolf and dog. J. Wildl. Dis. 1981;17:343–347. doi: 10.7589/0090-3558-17.3.343. [DOI] [PubMed] [Google Scholar]
- Soglia D., Rasero R., Rossi L., Sartore S., Sacchi P., Maione S. Microsatellites as markers for comparison among different populations of Sarcoptes scabiei. Ital. J. Anim. Sci. 2007;6(Supp. 1):214–216. https://10.4081/ijas.2007.1s.214 [Google Scholar]
- Turchetto S., Obber F., Rossi L., DAmelio S., Cavallero S., Poli A., et al. Sarcoptic mange in wild Caprinae of the Alps: Could pathology help in filling the gaps in knowledge? Front. Vet. Sci. 2020;7:193. doi: 10.3389/fvets.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton S.F., Dougall A., Pizzutto S., Holt D., Taplin D., Arlian L.G., et al. Genetic epidemiology of Sarcoptes scabiei (Acari: Sarcoptidae) in northern Australia. Int. J. Parasitol. 2004;34:839–849. doi: 10.1016/j.ijpara.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wu N., Abril C., Thomann A., Grosclaude E., Doherr M.G., Boujon P., Ryser-Degiorgis M.P. Risk factors for contacts between wild boar and outdoor pigs in Switzerland and investigations on potential Brucella suis spill-over. BMC Vet. Res. 2012;8:116. doi: 10.1186/1746-6148-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler M., Essig A., Gothe R., Rinder H. Molecular analyses suggest monospecificity of the genus Sarcoptes (Acari: Sarcoptidae) Int. J. Parasitol. 1999;29:759–766. doi: 10.1016/S0020-7519(99)00034-X. [DOI] [PubMed] [Google Scholar]