Abstract
Volumetric muscle loss (VML) impacts skeletal muscles and causes damage to associated tissues such as blood vessels and other structural tissues. Despite progress in the VML field, current preclinical approaches are often ineffective at restoring muscle volume. Additional research is paramount to develop strategies that improve muscle mass and function, while restoring supporting tissues. We highlight mechanisms that govern normal muscle function that are also key players for VML, including intracellular calcium signaling/homeostasis, mitochondria signaling (calcium, reactiove oxidative species (ROS)/oxidative stress), and angiogenesis. We propose an integration of these processes within the context of emerging biomaterials that provide structural support for muscle regeneration. We posit that new biomarkers (i.e. myokines and lipid signaling mediators) may serve as sentinels of early muscle injury and regeneration. We conclude that as new ideas, approaches, and models come together, new treatments will emerge to allow the full rebuilding of skeletal muscles and functional recovery of skeletal muscles after VML.
Introduction
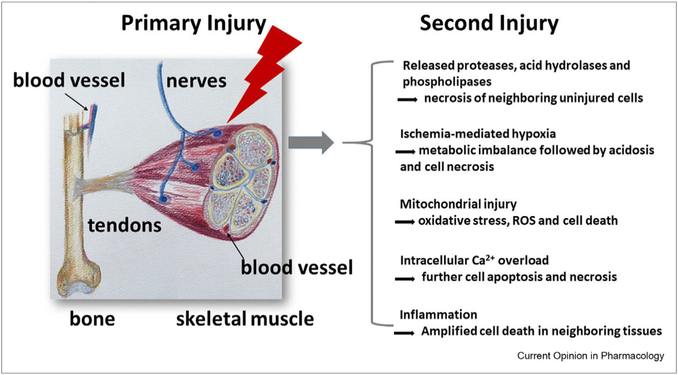
Volumetric muscle loss (VML) is the extensive damage and a quick loss of 20% or more of the total amount of muscle mass that results in significant and persistent impairment beyond the normal capacity of regeneration of the tissues. It typically occurs suddenly due to a traumatic or surgical event such as those experienced by soldiers wounded in combat missions or civilians injured in automobile accidents [1]. After acute trauma (primary injury), adjacent tissues are further damaged (secondary injury) by the mechanisms including enzymatic cellular death, ischemia/reperfusion injury, mitochondria injury and calcium ion (Ca2+) influx injury, and so on (Figure 1) [2]. In addition to alterations in muscle tissue function, the extensive muscle loss associated with this condition overwhelms the muscles’ endogenous ability to self-regenerate [3].
Figure 1.
Primary and secondary injuries during VML. In addition to skeletal muscle, nerves, tendons, bone, and blood vessels are all injured, impaired, or destroyed during initial injury, which further cause second injury in several ways. These pathological conditions in second injury have been observed in other muscle diseases.
Moreover, VML also involves damage to nerves, tendons, bone, and blood vessels, all injured, impaired, or destroyed during the primary and/or secondary injury (Figure 1). The cumulative result of this polytraumatic event is chronic inflammation, fibrosis, scar formation, and functional debilitation [3]. As such, the restoration of function requires the regeneration of both muscle and the supporting tissues (blood vessels, nerves, etc.). Tissue engineering using biomaterials is critical to provide scaffolds for regenerating muscle and other supporting tissues. Current treatment strategies largely depend on the type of injury (laceration with maintained tissue vs. a bomb blast with loss of tissues), and there is no effective treatment for extensive muscle loss [1].
Although we recognize VML as an acute catastrophic syndrome followed by long-term sequelae and the many advances in this field of research and practice, this review is not intended to summarize the recent advances of VML research. Rather, it takes a different angle to revisit VML by considering its links to other muscle diseases with the main objective of borrowing insights from these other pathological conditions that can be insightful for new therapeutics for VML. To this effect, we discuss key cellular processes that occur in these diseases that are most likely shared with VML, along with new biomaterial concepts and recently proposed biomarkers.
Significant muscle volume loss, sometimes extremely progressive, is also observed in many diseases, such as cachexia [4], sarcopenia (particularly during frailty syndrome) [5], and neuromuscular diseases [6,7]. Muscle loss, regardless due to VML or other muscle diseases, presents similar pathophysiological mechanisms and undergoes similar regeneration processes.
Although the current definition of VML does not comprise these other muscle losses, this review article revisits the pathophysiology and regeneration process of VML by embracing other muscle loss diseases as a new category of VML. Our second major goal is to go beyond the recognition of the more traditional VML biomarkers and propose new biomarkers in VML for damage and regeneration evaluation.
Pathophysiology in muscle loss and regeneration
After the initial trauma, the pathophysiologic response to traumatic injury can lead to secondary injury of otherwise uninjured cells by several causes (Figure 1, right panel) [2]. These factors resemble much of the underlying etiology for muscle loss revealed in human and animal studies, such that occurs with disuse [8], denervation [9,10], fasting [11], lack of gravity and unloading [12], aging (sarcopenia), cancer (cachexia), and neuromuscular diseases such as amyotrophic lateral sclerosis and spinal muscular atrophy [6,7].
Although the abovementioned five pathologic responses occur rather rapidly in traumatic muscle injury, these processes usually proceed in much slower pace in other disease conditions. However, there are cellular and signaling similarities, and in some cases, such as certain stages of cancer cachexia, unloading, denervation, and fasting, the maladaptation can proceed rapidly as well.
After primary injury, skeletal muscle immediately begins the regenerative process. Skeletal muscle regeneration occurs through a complex process loosely classified into four phases: the hemostasis phase, the destruction/inflammatory phase, the repair phase, and the remodeling phase. This process begins with the clotting of the wound, removal of damaged cells via several mechanisms, recruitment of muscle stem cells or progenitor/satellite cells, and rebalance of cell proliferation and differentiation. Activation of muscle stem cells or satellite cells then follows, along with the cell cycle exit, fusion of mononucleated myoblasts to regenerate myotubes and myofibers. However, achieving muscle regeneration with perfect functional restoration and minimal fibrosis or scaring is still a challenge for treating extensive VML. As such, examining general muscle loss and regeneration is important for mechanistic understanding of VML pathophysiology.
Ca2+ handling mechanism in muscle injury and regeneration
Most processes in both muscle injury and regeneration involve intracellular Ca2+ signaling [13–16]. As a ubiquitous second messenger, Ca2+ regulates many cellular processes, ranging from gene transcription to muscle contraction, from cell proliferation to apoptosis [15]. Ca2+ is especially important in maintaining the proper structure and function of skeletal muscle. Three major Ca2+ handling mechanisms control the homeostasis of Ca2+ in skeletal muscles; these include excitation–contraction coupling (EC-coupling) [17], excitation coupled Ca2+ entry [18], and store operated Ca2+ entry (SOCE) [19]. EC-coupling refers to coupling of external electrical excitation via activation of voltage-gated Ca2+ channels at the sarcolemma and transient explosive elevation of intracellular Ca2+ via opening of the ryanodine receptor/Ca2+ release channel on the sarcoplasmic reticulum (SR). When depolarization of skeletal muscle sustains, excitation coupled Ca2+ entry occurs and results in extracellular Ca2+ across the sarcolemma. Similarly, SOCE also mediates extracellular Ca2+ into muscle cells; however, its activation is triggered by reduction of the SR Ca2+ storage [15,17]. In this mechanism, the depletion of the SR stores due to activation of the ryanodine receptor or inositol trisphosphate receptor activates a Ca2+ sensor at the SR, leading to the opening of sarcolemma-located store-operated Ca2+ channels.
SOCE was originally identified in nonexcitable cells more than three decades ago, and its roles in skeletal muscle have been recognized much later [15,19]. It consists of two main components: stromal-interacting molecule 1 (STIM1) at the SR as a Ca2+ storage sensor and Orai or transient receptor potential cation channel subfamily C member 1 proteins at the sarcolemma as an SOCE Ca2+ channel. When there is a reduction in Ca2+ storage, STIM1 is activated and forms patches and induces the aggregation of Orai1, which further triggers the activation of SOCE, leading to the flow of extracellular Ca2+ into the cell. SOCE-mediated intracellular Ca2+ signaling is spatially temporally confined in the form of waves, spikes, or oscillations, and the latter often activates gene transcription. In particular, the frequency, amplitude, and duration of these intracellular Ca2+ oscillations compose the specific Ca2+ codes for cell proliferation, migration, and differentiation [20–25]. In skeletal muscles, Ca2+-dependent signaling cascades include the calcineurin-nuclear factor of activated T cells pathway, the Ca2+/calmodulin-dependent kinases–nuclear factor kappa B pathway, CaM-dependent kinases and phosphatases (calpain), and mitogen-activated protein kinases [26].
As mentioned earlier, an elevated intracellular Ca2+ level can cause muscle cell apoptosis in muscle diseases. The Ca2+ influx is usually mediated by SOCE [27]. Accumulating evidence shows that Ca2+ influx and its downstream signaling pathways are required during muscle regeneration, and the alterations in these pathways are associated with various muscle diseases. Therefore, it is plausible that the alteration of SOCE machinery genes, such as Orai1, STIM1, and TRPC, at different stages of muscle regeneration after VML may serve as new biomarkers to evaluate tissue repair.
Mitochondria as a sensor of intracellular Ca2+ profiles in muscle damage and regeneration
Despite variations in the initial cause of damage, evidence highlights apoptosis as a major factor contributing to muscle loss [10,28–30]. Mitochondria, taking up 10–15% volume of mammalian skeletal myofibers [31], not only serve as the energy provider, but are also intimately involved in apoptosis [30,32–34]. Excessive mitochondrial reactiove oxidative species (ROS) generation is revealed as an early event of the apoptotic process [35]. Although low level mitochondrial ROS serves as an essential signal regulating various physiological processes [36], excessive ROS production leads to oxidative stress, which is detrimental to cell function [37]. Indeed, prolonged muscle inactivity is implicated with excessive mitochondrial ROS production in myofibers [38–40], whereas mitochondria in contracting normal muscle do not show enhanced ROS production [41–43].
Although mitochondrial function in muscle is fine-tuned by intracellular Ca2+ signaling, mitochondria also have the ability to shape intracellular Ca2+ profiles in myofibers [44–47]. There is always a cross talk between mitochondrial Ca2+ and ROS signaling [48–50]. Cytosolic Ca2+ transients are spatiotemporally well-controlled Ca2+ release events from the SR in a myofiber responding to the motor nerve activation during EC-coupling. Skeletal muscle disuse induced by various pathological conditions including VML could partially or completely disrupt EC-coupling and eliminate cytosolic Ca2+ transients. What is the immediate response of mitochondria to the cessation of cytosolic Ca2+ transients in denervated myofibers? A study from Karam et al. [51] found that denervated myofibers lacking cytosolic Ca2+ transients exhibited frequent mitochondrial permeability transition (mPTP) opening and enhanced mitochondrial ROS production. Remarkably, restoration of cytosolic and subsequent mitochondrial Ca2+ transients by brief electric field stimulation immediately stopped mPTP opening and restored mitochondrial ROS production to the normal level within a minute. This suggests that dynamic mitochondrial Ca2+ transients induced during EC-coupling are vital to keep mPTP in a closed status in myofibers, which could be essential for optimal muscle repair and regeneration.
Another pathological hallmark of long-term muscle inactivity is a steady-state elevation of cytosolic Ca2+ levels [52–55], leading to overload of mitochondrial matrix Ca2+ ([Ca2+]mito), inducing cell apoptosis through enhancing ROS production and increasing the release of proapoptotic proteins through mPTP opening [38]. Until recently, it remained elusive why an elevated cytosolic Ca2+ level is revealed in inactivated myofibers. A study from the Saez’s Research Laboratory discovered that de novo expression of connexin hemichannels in the sarcolemma of myofibers is the upstream cause of the enhanced membrane permeability that subsequently leads to elevated cytosolic Ca2+ levels [52,56,57]. Therefore, mitochondria act as sensors of spatiotemporal profiles of intracellular Ca2+ in myofibers. Loss of physiological mitochondrial Ca2+ influx, in combination with steady-state elevation of [Ca2+]mito, forms an important factor leading to further muscle loss in inactivated skeletal muscle in VML. It is possible that serum detection of ROS/reduction-oxidation (REDOX) along with mitochondrial DNA could lead to new directions to diagnose or evaluation of the efficacy of various treatments for VML.
Angiogenesis in muscle regeneration
After VML, the repair of thick, complex tissues, such as skeletal muscle, is challenged by the ability to create new blood vessels and restore the vascular supply [58], a prerequisite for tissue healing. Therapeutic approaches used to restore the loss of muscle mass and function include vascular grafts [59], synthetic and natural polymers [1], decellularized extracellular matrix [60], and bioactive glasses [61]. These therapeutic remedies often also attempt to regenerate the supporting tissues. For example, bioactive glass was effective at stimulating angiogenesis and the expression of genes related to muscle regeneration with in vitro experimentation, whereas the healing of VML corresponded to vascularization and the activation of satellite cells in in vivo studies [61]. Noteworthy with these findings is the fact that the bioactive glass was able to stimulate angiogenesis and muscle satellite cells in the absence of added stem cells or growth factors [61]. Our group found that this effect was also found with silicon oxynitride semiconductor biomaterials that release ionic Si. The release of ionic Si enhances the expression of antioxidants NRF2 and SOD1, myokines γ-aminobutyric acid (GABA) and NRTN, and genes MyoG and MyoD that stimulate cell viability under the aforementioned oxidative stress conditions [62].
Vascular constructs created with microvascular fragments from adipose tissue demonstrated angiogenic sprouting [63] and the capacity for perfusion in the newly created blood vessels [64]. Furthermore, the use of hydrogels containing microvascular fragments to treat VML augmented vascular density within the tibialis anterior muscle of rats versus the use of collagen gels devoid of cells or single-cell scaffolds [65]. In vitro analysis of constructs containing microvascular fragments and myoblasts elicited microvascular networks that were longer and more highly branched versus constructs containing only microvascular fragments [66], indicating the muscle itself participates in angiogenesis. Furthermore, two weeks after implantation into a large volumetric muscle defect created in the rat biceps femoris, a dense microvascular network capable of perfusion was observed in the vascularized collagen hydrogel seeded with myoblasts versus an empty construct [66]. However, total vascular volume within the muscle defect was similar between the vascularized hydrogels with and without myoblasts [66]. Although development of blood vessels capable of perfusion occurred under both circumstances, these constructs did not result in muscle regeneration 8 weeks after injury [66]. These preclinical experimental strategies aimed at restoring vascular development and function in concert with muscular regeneration and function are in their infancy but are promising. Given the heterogeneity of blood vessels, the various muscle fiber types, and issues associated with advancing age and/or disease, much work is needed in the development of these strategies to optimize recovery from VML in a wide range of patient populations. Strategies aimed at stimulating early angiogenesis should provide fertile ground for VML repair. In addition, advanced in vivo imaging techniques may assist, in the near future, in determining where tissues lack a sufficient vascular supply and are in need of angiogenic stimulation.
Biomaterials and tissue engineering approaches to enhance neuromuscular tissue regeneration
Because of the large volume depletion of muscle concomitant with neuromuscular and vasculature components, it is necessary to use tissue engineering approaches that provide structural support during VML regeneration. Tissue engineering using biomaterials (including natural approaches like decellularized extracellular matrix and synthetic polymers such as polycaprolactone, ply-L-lactic acid, and poly-l-glycolic acid) is needed to stabilize the resulting VML defects and offer a conductive path for revascularization coupled with neuromuscular regeneration. Biomaterials form the basis of tissue-engineered constructs and play critical roles during the processes of tissue regeneration. Each can be synthesized/fabricated in scaffolds, nanoparticles, or thin film structures.
Provided the mechanical properties of the biomaterials can stabilize existing defects, stimulation of tissue regeneration then falls to the scaffold’s chemistry, ideally enhancing neurogenic, myogenic, and angiogenic biomarkers. Such enhancement must endure as the tissue recovers from initial increased concentration of ROS (e.g. H2O2 and O−2) and oxidative stress. Biopolymers usually have surface functional groups that offer cell guidance or stimulatory function to facilitate tissue growth. For example, three-dimensional (3D) printed poly-l-glycolic acid exhibited enhanced myogenic differentiation markers such as myosin heavy chain and myogenin [67]. From a fabrication standpoint, use of microfluidic-enhanced 3D bioprinting was able to align myoblast-laden hydrogels for functionally organized myofibers [68]. In an analogous aligned fiber fabrication study, basal lamina mimetic nanofibrous peptide networks enhanced myogenesis [69]. For neurogenesis and myogenesis, bioinspired 3D human neuromuscular junction development was enhanced in suspended hydrogel array [70]. Biopolymers such as gelatin and chitosan have also been used to increase the density of vascular tubules in human endothelial cells needed to form the basis for vascular supply to musculoskeletal (MSK) tissues. Modification of these materials using nanoparticles and multidomain peptides has been shown to augment these 3D constructs for tissue regeneration. Nanoparticles also play a key role in stimulating cellular activity. For example, gold and gold–silver alloy nanoparticles enhance the myogenic differentiation of myoblasts through the p38 mitogen-activated protein kinase signaling pathway and promote in vivo skeletal muscle regeneration [71]. Nanosilicate biomaterials that release ionic silicon (Si) have also exhibited proangiogenic responses in human primary endothelial cells under normal and toxic oxidative stress conditions. Zinc oxide nanoparticles promote the formation of myogenic differentiation into myotubes in mouse myoblasts [72]. The mechanical stimulation of adhesion receptors using light-responsive nanoparticle actuators enhanced myogenesis [72]. Furthermore, surface modification of biopolymers with multidomain peptides offers added functionality to promote adhesion and growth of cells and offers self-assembly as nanofibers that deliver bioactive molecules and support tissue regeneration [73]. For example, targeted axonal import peptides delivered functional proteins for motor neurons after peripheral administration [74]. These biomarkers represent the cascade of events that result in vascular and neuromuscular tissue regeneration. Reflecting on the abovementioned rationale, several key choices can be made to arrive at a series of biomaterials that can be used to regenerate lost neuromuscular tissue. Furthermore, additional modifications could include the addition of specific growth factors with time-released functions or mRNA encapsulated nanoparticles that would target a very specific protein or pathway.
New biomarkers in VML regeneration
In the previous sections of this article, we brought together the fundamental and new potential roles in VML of intracellular Ca2+, mitochondria, angiogenesis, and targeted biomaterials by attempting to draw cellular and signaling similarities from other pathologies and the fundamental process of muscle and adjacent tissues that are damaged in VML.
As emphasized in our introduction, our goal is not minimizing as an entity by itself but to view in a broader context, to offer perhaps new insights from a different vantage view.
In the last decade, our understanding of endocrinology and hormonal regulation and tissue-to-tissuc communication has completely changed [75–77]. Adipose tissue, bones, skeletal muscles, and even tendons are all now considered secretory tissues. New animal models have been created to study these biochemical bone-muscle interactions.
Much progress also occurred in the area of analytical chemistry with the identification of several molecules broadly involved in tissue repair and regeneration, some specifically involved in muscle repair and regeneration via bone–muscle cross talk. Our group, studying bone-muscle biochemical cross talk, coined the term osteokine [78] to indicate specific hormone-like factors secreted by bone cells acting in muscle cells to modulate their function. Among some of the osteokines we are studying, PGE2 is a lipid-signaling mediator that enhances myogenic differentiation [79] and proliferation [80] of muscle cells, whereas fibroblast growth factor 9 reduces myogenic differentiation, pointing perhaps to the yin-yang of this process; in that, some factors might counteract or balance the action of other factors leading to optimal musculoskeletal health. We also developed a quick, accurate, and sensitive screening method for the precise isolation and quantification of aminobutyric acid isomers and enantiomers [62]. This test allowed us to quantify for the first time the levels in different media and substrates of the isomers of [β-aminoisobutyric acid (D- and L-BAIBA) as well as GABA, and so on [62]. BAIBA is secreted from muscle and converts white fat into brown fat and seems very important to protect muscles and individuals against insulin resistance [81]. We showed in mice that BAIBA acted as a boneprotective factor against the loss of bone induced by unloading. This appears to be mediated by the ability of BAIBA to protect osteocytes against cell death induced by ROS [82]. Using our recently developed method, we studied the serum of ~200 women with low and high bone mineral density, yielding correlations with bone mineral density and osteoporotic fracture. In serum, GABA and (R)-3-aminoisobutyric acid (D-BAIBA) have positive associations with physical activity in young lean women, and they might serve as early biomarkers for diagnosis and treatment of osteoporosis [62].
We are not suggesting that these are the only secreted factors with therapeutic potential for VML. In fact, there are many of other candidates, certainly other growth factors, and regulators of muscle stem cell proliferation and myoblast and myotube formation, along with specific genes and factors for the other tissues.
Notwithstanding, based on the new theory of bone-muscle cross talk, we propose that myokines and osteokines might be a set of convenient and attractive biomarkers for VML muscle regeneration. These new set of biomarkers along with the aforementioned processes/biomarkers (angiogenesis, calcium, and mitochondria) and the refinement of smart design of biomaterials could very well lead to a new way of treating VML in the near future.
Concluding remarks and future perspectives
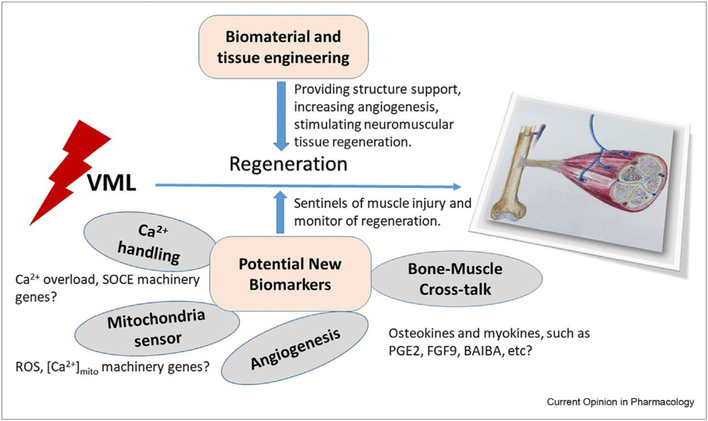
This is an exciting time for VML research as recent advances in research methodologies, technologies, and complementary markers of muscle regeneration provide an unprecedented opportunity to develop more robust VML regeneration tracking and tissue engineering strategies to repair multiple tissues (i.e. muscle, neuron, and blood vessel) for all patients with VML. The loss of associated tissues (e.g. blood vessels) with VML needs also to be considered when developing strategies for muscle regeneration. In addition, a holistic approach where the process of muscle regeneration is globally viewed, and its key check points used as biomarkers of the regeneration itself, could bring new hope to those affected with VML (Figure 2). The addition of other specific biomarkers such as myokines and lipid signaling mediators as early and long-term biomarkers of muscle injury, muscle remodeling, muscle repair, and regeneration will lead to new therapies.
Figure 2.
Potential new biomarkers in VML tissue regeneration. During VML tissue regeneration, effective biomaterial and tissue engineering is essential for reconstruction of the complex functioning system, including skeletal muscles, bone, blood vessels, and nerves. New biomarkers can serve as sentinels of muscle injury and monitor the regeneration. Our understanding of cross talk between bone and muscle and the pathophysiological conditions in other muscle diseases will shed light to identify new biomarkers in VML.
Acknowledgements
The authors are thankful for the generous support from the George W and Hazel M. Jay and Evanston Research Endowments and the UTA College of Nursing & Health Innovation Bone-Muscle Research Center (https://bonemusclecenter.uta.edu/; https://www.uta.edu/conhi/research/bmrc/index.php). M.B. was supported by NIH Grants: National Institutes of Aging (NIA) 2-PO1AG039355; NIA-R01AG056504, NIA-R01AG060341, National Institutes of Diabetes, Digestive, and Kidney Diseases Kidney (NIDDK)-ROIDKI 19066 to M.B. and National Institutes of Neurological Disorders and Stroke (NINDS) 2-R01NS105621 to J.Z./M.B. J.Z. was supported by Grants from Muscular Dystrophy Association (MDA-4351), NIH AR057404, NIH R01HL138570, DOD AL170061, and the ALS Association (16-IIP-288). R.P. was supported by Grants NSF 1710948, AHA 16IRG27550003, NIH CoBRE P20 GM11312S, INBRE P20 GM103446, R15AR062882. Z.P. was supported by NIH Grants R01CA185055 and S100D02S230.
Footnotes
Conflict of interest statement
Kerrie Downing, Rhonda Prisby, Venu Varanasi, Jingsong Zhou, and Zui Pan have no conflicts to declare. Marco Brotto is the partner founding member of Bioform Sciences, LLC, the makers of MusQuLexx®, a topical muscle cream for muscle pain and inflammation.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Grasman JM, Zayas MJ, Page RL, Pins GD: Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater 2015, 25:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merrick MA: Secondary injury after musculoskeletal trauma: a review and update. J Athl Train 2002, 37:209–217. [PMC free article] [PubMed] [Google Scholar]
- 3.Shayan M, Huang NF: Pre-clinical cell therapeutic approaches for repair of volumetric muscle loss. Bioengineering (Basel) 2020, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tisdale MJ: Cancer cachexia. Curr Opin Gastroenterol 2010, 26: 146–151. [DOI] [PubMed] [Google Scholar]
- 5.Dupont-Versteegden EE: Apoptosis in muscle atrophy: relevance to sarcopenia. Exp Gerontol 2005, 40:473–481. [DOI] [PubMed] [Google Scholar]
- 6.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD: Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 2004, 185:232–240. [DOI] [PubMed] [Google Scholar]
- 7.Monani UR: Spinal muscular atrophy: a deficiency in a ubiquitous protein; a motor neuron-specific disease. Neuron 2005, 48:885–895. [DOI] [PubMed] [Google Scholar]
- 8.Jackman RW, Kandarian SC: The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 2004, 287: C834–C843. [DOI] [PubMed] [Google Scholar]
- 9.Hoellwarth U, Christian Hofer D: Muscle biopsies show that fes of denervated muscles reverses human muscle degeneration from permanent spinal motoneuron lesion. J Rehabil Res Dev 2005:1. [DOI] [PubMed] [Google Scholar]
- 10.Adhihetty PJ, O’Leary MF, Chabi B, Wicks KL, Hood DA: Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 2007, 102:1143–1151. [DOI] [PubMed] [Google Scholar]
- 11.Qiu J, Fang Q, Xu T, Wu C, Xu L, Wang L, Yang X, Yu S, Zhang Q, Ding F: Mechanistic role of reactive oxygen species and therapeutic potential of antioxidants in denervation-or fasting-induced skeletal muscle atrophy. Front Physiol 2018, 9:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitts RH, Riley DR, Widrick JJ: Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol 2001, 204:3201–3208. [DOI] [PubMed] [Google Scholar]
- 13.Filip S, Mokry J, Forostyak O, Dayanithi G: The extracellular matrix and ca(2+) signaling mechanisms. Physiol Res 2019, 68:161–170. [DOI] [PubMed] [Google Scholar]
- 14.Avila G: Disturbed ca(2+) homeostasis in muscle-wasting disorders. Adv Exp Med Biol 2018, 1088:307–326. [DOI] [PubMed] [Google Scholar]
- 15. Pan Z, Brotto M, Ma J: Store-operated ca2+ entry in muscle physiology and diseases. BMB Rep 2014, 47:69–79. * This his review article summarized the important roles of SOCE and intracellular Ca2+ signals in muscle normal physiology and pathophysiology
- 16.Zhao X, Yamazaki D, Kakizawa S, Pan Z, Takeshima H, Ma J: Molecular architecture of ca2+ signaling control in muscle and heart cells. Channels 2011, 5:391–396. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Pan Z: Junctional membrane structure and store operated calcium entry in muscle cells. Front Biosci: J Virt Libr 2003, 8:d242–d255. [DOI] [PubMed] [Google Scholar]
- 18.Cherednichenko G, Hurne AM, Fessenden JD, Lee EH, Allen PD, Beam KG, Pessah IN: Conformational activation of ca2+ entry by depolarization of skeletal myotubes. Proc Natl Acad Sci USA 2004, 101:15793–15798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, Takeshima H, Cheng H, Ma J: Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol 2002, 4: 379–383. ** This paper provided evidence to show that MG29, an important triad junction structure protein regulates SOCE. It also revealed that dysfunction of SOCE contributes to muscle fatigue
- 20.Berridge MJ, Bootman MD, Roderick HL: Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 2003, 4:517–529. [DOI] [PubMed] [Google Scholar]
- 21.Berridge MJ, Lipp P, Bootman MD: The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000, 1: 11–21. [DOI] [PubMed] [Google Scholar]
- 22.Hajnoczky G, Davies E, Madesh M: Calcium signaling and apoptosis. Biochem Biophys Res Commun 2003, 304:445–454. [DOI] [PubMed] [Google Scholar]
- 23.Lipskaia L, Lompre AM: Alteration in temporal kinetics of ca2+ signaling and control of growth and proliferation. Biol Cell 2004, 96:55–68. [DOI] [PubMed] [Google Scholar]
- 24.Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhaes PJ, Di Virgilio F, Pozzan T: Calcium and apoptosis: facts and hypotheses. Oncogene 2003, 22:8619–8627. [DOI] [PubMed] [Google Scholar]
- 25.Bell N, Hann V, Redfern CP, Cheek TR: Store-operated ca(2+) entry in proliferating and retinoic acid-differentiated n- and stype neuroblastoma cells. Biochim Biophys Acta 2013, 1833: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu MK, Levin JB, Hamilton AM, Borodinsky LN: Calcium signaling in skeletal muscle development, maintenance and regeneration. Cell Calcium 2016, 59:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Weisleder N, Collet C, Zhou J, Chu Y, Hirata Y, Zhao X, Pan Z, Brotto M, Cheng H, Ma J: Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol 2005, 7:525–530. [DOI] [PubMed] [Google Scholar]
- 28.Borisov AB, Carlson BM: Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec: Offl PubI Am Assoc Anatomists 2000, 258:305–318. [DOI] [PubMed] [Google Scholar]
- 29.Tews D: Apoptosis and muscle fibre loss in neuromuscular disorders. Neuromuscul Disord 2002, 12:613–622. [DOI] [PubMed] [Google Scholar]
- 30.Siu PM, Alway SE: Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol 2005, 565:309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenberg BR: Quantitative ultrastructure of mammalian skeletal muscle. Compr Physiol 2010:73–112. [Google Scholar]
- 32.Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA: Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 2008, 7:2–12. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Youle RJ: The role of mitochondria in apoptosis. Annu Rev Genet 2009, 43:95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA: Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol 2005, 289:C994–C1001. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez MG, Troiano L, Moretti L, Nasi M, Pinti M, Salvioli S, Dobrucki J, Cossarizza A: Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ 2002, 13:449–455. [PubMed] [Google Scholar]
- 36.D'Autreaux B, Toledano MB: Ros as signalling molecules: mechanisms that generate specificity in ros homeostasis. Nat Rev Mol Cell Biol 2007, 8:813–824. [DOI] [PubMed] [Google Scholar]
- 37.Zorov DB, Juhaszova M, Sollott SJ: Mitochondrial reactive oxygen species (ros) and ros-induced ros release. Physiol Rev 2014, 94:909–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA: Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab 2012, 303:E31–E39. * A comprehensive review on mitochondrial dysfunction in disused muscle atrophy.
- 39.Xiao Y, Karam C, Yi J, Zhang L, Li X, Yoon D, Wang H, Dhakal K, Ramlow P, Yu T: Ros-related mitochondrial dysfunction in skeletal muscle of an als mouse model during the disease progression. Pharmacol Res 2018, 138:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H: Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ros production. Am J Physiol Regul Integr Comp Physiol 2007, 293:R1159–R1168. [DOI] [PubMed] [Google Scholar]
- 41.Henriquez-Olguin C, Knudsen JR, Raun SH, Li Z, Dalbram E, Treebak JT, Sylow L, Holmdahl R, Richter EA, Jaimovich E: Cytosolic ros production by nadph oxidase 2 regulates muscle glucose uptake during exercise. Nat Commun 2019, 10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ: Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase (s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxidants Redox Signal 2013, 18:603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powers SK, Radak Z, Ji LL: Exercise-induced oxidative stress: past, present and future. J Physiol 2016, 594:5081–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kavanagh NI, Ainscow EK, Brand MD: Calcium regulation of oxidative phosphorylation in rat skeletal muscle mitochondria. Biochim Biophys Acta 2000, 1457:57–70. [DOI] [PubMed] [Google Scholar]
- 45. Yi J, Ma C, Li Y, Weisleder N, Rios E, Ma J, Zhou J: Mitochondrial calcium uptake regulates rapid calcium transients in skeletal muscle during excitation-contraction (e-c) coupling. J Biol Chem 2011, 286:32436–32443. * Quantification of mitochondrial Ca2+ uptake during EC-coupling in skeletal muscle.
- 46.Zhou J, Yi J, Royer L, Launikonis BS, Gonzalez A, Garcia J, Rios E: A probable role of dihydropyridine receptors in repression of ca2+ sparks demonstrated in cultured mammalian muscle. Am J Physiol Cell Physiol 2006, 290: C539–C553. [DOI] [PubMed] [Google Scholar]
- 47.Zhou J, Dhakal K, Yi J: Mitochondrial ca(2+) uptake in skeletal muscle health and disease. Sci China Life Sci 2016, 59: 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feissner RF, Skalska J, Gaum WE, Sheu SS: Crosstalk signaling between mitochondrial ca2+ and ros. Front Biosci 2009, 14:1197–1218. * An insightful review on the crosstalk between mitochondrial Ca2+ and ROS signaling.
- 49.Zhou J, Li A, Li X, Yi J: Dysregulated mitochondrial ca(2+) and ros signaling in skeletal muscle of als mouse model. Arch Biochem Biophys 2019, 663:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li A, Yi J, Li X, Zhou J: Physiological ca(2+) transients versus pathological steady-state ca(2+) elevation, who flips the ros coin in skeletal muscle mitochondria. Front Physiol 2020, 11: 595800. *A review on potential mechanisms underlying the response of mPTP to intracellular Ca2+ spatiotemporal profiles in skeletal muscle myofibers.
- 51. Karam C, Yi J, Xiao Y, Dhakal K, Zhang L, Li X, Manno C, Xu J, Li K, Cheng H, Ma J, et al. : Absence of physiological ca2+ transients is an initial trigger for mitochondrial dysfunction in skeletal muscle following denervation. Skeletal Muscle 2017, 7:6. ** Evidence suggested that physiological mitochondrial Ca2+ transients are vital to keep mPTP function of skeletal muscle in check.
- 52.Cisterna BA, Vargas AA, Puebla C, Saez JC: Connexin hemichannels explain the ionic imbalance and lead to atrophy in denervated skeletal muscles. Biochim Biophys Acta 2016, 1862:2168–2176. [DOI] [PubMed] [Google Scholar]
- 53.Ingalls CP, Warren GL, Armstrong RB: Intracellular ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J Appl Physiol 1999, 87:386–390. [DOI] [PubMed] [Google Scholar]
- 54.Picken JR, Kirby AC: Denervated frog skeletal muscle: calcium content and kinetics of exchange. Exp Neurol 1976, 53:64–70. [DOI] [PubMed] [Google Scholar]
- 55.Tischler ME, Rosenberg S, Satarug S, Henriksen EJ, Kirby CR, Tome M, Chase P: Different mechanisms of increased proteolysis in atrophy induced by denervation or unweighting of rat soleus muscle. Metab Clin Exp 1990, 39:756–763. [DOI] [PubMed] [Google Scholar]
- 56. Cea LA, Cisterna BA, Puebla C, Frank M, Figueroa XF, Cardozo C, Willecke K, Latorre R, Saez JC: De novo expression of connexin hemichannels in denervated fast skeletal muscles leads to atrophy. Proc Natl Acad Sci USA 2013, 110: 16229–16234. ** Evidence suggested that the de novo expression of connexin hemichannels on the sarcolemma as a main cause of elevated intracellular Ca2+ level revealed in the disused skeletal muscle.
- 57.Cisterna BA, Vargas AA, Puebla C, Fernandez P, Escamilla R, Lagos CF, Matus MF, Vilos C, Cea LA, Barnafi E, Gaete H, et al. Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation. Nat Commun 2020, 11:1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Juhas M, Engelmayr GC Jr, Fontanella AN, Palmer GM, Bursae N: Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc Natl Acad Sci USA 2014, 111:5508–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koffler J, Kaufman-Francis K, Shandalov Y, Egozi D, Pavlov DA, Landesberg A, Levenberg S: Improved vascular organization enhances functional integration of engineered skeletal muscle grafts. Proc Natl Acad Sci USA 2011, 108: 14789–14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garg K, Ward CL, Rathbone CR, Corona BT: Transplantation of devitalized muscle scaffolds is insufficient for appreciable de novo muscle fiber regeneration after volumetric muscle loss injury. Cell Tissue Res 2014, 358:857–873. [DOI] [PubMed] [Google Scholar]
- 61. Jia W, Hu H, Li A, Deng H, Hogue CL, Mauro JC, Zhang C, Fu Q: Glass-activated regeneration of volumetric muscle loss. Acta Biomater 2020, 103:306–317. * In this study, the authors utilized bioactive glasses to regenerate volumetric muscle loss. The use of these glasses for muscle regeneration did not require growth factors or stem cells. Ions released from the bioactive glasses stimulated angiogenesis, as well as the release of muscle-related growth factors, which aided in regeneration.
- 62.Wang Z, Bian L, Mo C, Shen H, Zhao LJ, Su KJ, Kukula M, Lee JT, Armstrong DW, Recker R, Lappe J, et al. :. Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis. Commun Biol 2020, 3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Laschke MW, Menger MD: Adipose tissue-derived microvascular fragments: natural vascularization units for regenerative medicine. Trends Biotechnol 2015, 33:442–448. * This article is an overview of the importance of vascularization in regenerative medicine and how microvascular fragments are used for angiogenesis, the development of microvascular networks and the generation of pre-vascularized tissue constructs.
- 64. Shepherd BR, Chen HY, Smith CM, Gruionu G, Williams SK, Hoying JB: Rapid perfusion and network remodeling in a microvascular construct after implantation. Arterioscler Thromb Vase Biol 2004, 24:898–904. * In this study, the authors isolated micro vessel fragments with intact endothelial and perivascular cells and implanted these fragments into severe combine immunodeficient mice. Vessel inosculation with the host circulation begin rapidly, i.e., within 1 day. Over 28 days, mature functional microvascular beds developed. When human-derived micro vessels were implanted, similar results were obtained. This model provides a means of reproducing vascularization, angiogenesis, inosculation and network remodeling.
- 65. Pilia M, McDaniel JS, Guda T, Chen XK, Rhoads RP, Allen RE, Corona BT, Rathbone CR: Transplantation and perfusion of microvascular fragments in a rodent model of volumetric muscle loss injury. Eur Cell Mater 2014, 28:23. discussion 23–14. ** In this study, the authors created muscle defects in a rodent model and replaced the defect with collagen constructs containing microvascular fragments, adipose tissue derived stem cells or collagen only. One to two weeks following surgery, vessel density was highest and vessel perfusion occurred sooner in the muscle that received the construct plus the micro vessel fragments.
- 66.Li MT, Ruehle MA, Stevens HY, Servies N, Willett NJ, Karthikeyakannan S, Warren GL, Guldberg RE, Krishnan L: (*) skeletal myoblast-seeded vascularized tissue scaffolds in the treatment of a large volumetric muscle defect in the rat biceps femoris muscle. Tissue Eng 2017, 23:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen H, Zhong J, Wang J, Huang R, Qiao X, Wang H, Tan Z: Enhanced growth and differentiation of myoblast cells grown on e-jet 3d printed platforms. Int J Nanomed 2019,14:937–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Costantini M, Testa S, Mozetic P, Barbetta A, Fuoco C, Fornetti E, Tamiro F, Bernardini S, Jaroszewicz J, Swieszkowski W, Trombetta M, et al. : Microfluidic-enhanced 3d bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131:98–110. ** This article shows the important role of the viscoelasticity of materials and their impact on aligned myofiber induced myogenesis that translate from in vitro model into in vivo model.
- 69.Yasa IC, Gunduz N, Kilinc M, Guler MO, Tekinay AB: Basal lamina mimetic nanofibrous peptide networks for skeletal myogenesis. Sci Rep 2015, 5:16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Awad K, Ahuja N, Fiedler M, Peper S, Wang Z, Aswath P, Brotto M, Varanasi V: Ionic silicon protects oxidative damage and promotes skeletal muscle cell regeneration. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ge J, Liu K, Niu W, Chen M, Wang M, Xue Y, Gao C, Ma PX, Lei B: Gold and gold-silver alloy nanoparticles enhance the myogenic differentiation of myoblasts through p38 mapk signaling pathway and promote in vivo skeletal muscle regeneration. Biomaterials 2018, 175:19–29. [DOI] [PubMed] [Google Scholar]
- 72.Ramalingam V, Hwang I: Zinc oxide nanoparticles promoting the formation of myogenic differentiation into myotubes in mouse myoblast c2c12 cells. J Ind Eng Chem 2020, 83: 315–322. [Google Scholar]
- 73. Moore AN, Hartgerink JD: Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Accounts Chem Res 2017, 50:714–722. ** This article shows the ability of surface chemistry alterations that can greatly impact myogenic differentiation on a biomaterial surface.
- 74.Sellers DL, Bergen JM, Johnson RN, Back H, Ravits JM, Horner PJ, Pun SH: Targeted axonal import (taxi) peptide delivers functional proteins into spinal cord motor neurons after peripheral administration. Proc Natl Acad Sci USA 2016, 113:2514–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li F, Li Y, Duan Y, Hu CA, Tang Y, Yin Y: Myokines and adipokines: involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev 2017, 33:73–82. [DOI] [PubMed] [Google Scholar]
- 76.Leal LG, Lopes MA, Batista ML Jr: Physical exercise-induced myokines and muscle-adipose tissue crosstalk: a review of current knowledge and the implications for health and metabolic diseases. Front Physiol 2018, 9:1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gomarasca M, Banfi G, Lombardi G: Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem 2020, 94:155–218. [DOI] [PubMed] [Google Scholar]
- 78. Isaacson J, Brotto M: Physiology of mechanotransduction: how do muscle and bone "talk" to one another? Clin Rev Bone Miner Metabol 2014, 12:77–85. ** This review provides insights in a biochemical interaction between bone-muscle cross talk and their interactions with other tissues and the global impact of these multi-tissue interactions on chronic diseases.
- 79.Mo C, Romero-Suarez S, Bonewald L, Johnson M, Brotto M: Prostaglandin e2: from clinical applications to its potential role in bone-muscle crosstalk and myogenic differentiatior Recent Pat Biotechnol 2012, 6:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huang J, Wang K, Shiflett LA, Brotto L, Bonewald LF, Wacker MJ, Dallas SL, Brotto M: Fibroblast growth factor 9 (fgf9) inhibits myogenic differentiation of c2c12 and human muscle cells. Cell Cycle 2019, 18:3562–3580. * This study provided evidence to support that FGF9 modulate myogenesis via a complex signaling mechanism.
- 81.Jung TW, Hwang HJ, Hong HC, Yoo HJ, Baik SH, Choi KM: Baiba attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an ampk-ppardelte dependent pathway in mice. Diabetologia 2015, 58:2096–2105 [DOI] [PubMed] [Google Scholar]
- 82.Kitase Y, Vallejo JA, Gutheil W, Vemula H, Jahn K, Yi J, Zhou ,, Brotto M, Bonewald LF: Beta-aminoisobutyric acid, l-baiba, is muscle-derived osteocyte survival factor. Cell Rep 2018, 22 1531–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]