Abstract
The pharmacokinetics and bioavailability of 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine (cyclic HPMPC) were examined at four doses in 22 patients with human immunodeficiency virus infection. Two groups of six patients received a single dose of cyclic HPMPC at 1.5 or 3.0 mg/kg of body weight by each of the oral and intravenous routes in a random order with a 2-week washout period between administrations. Additional patients received single intravenous doses of cyclic HPMPC at 5.0 mg/kg (n = 6) or 7.5 mg/kg (n = 4). Serial serum and urine samples were collected at intervals over 24 h after dosing. The concentrations of cyclic HPMPC and cidofovir in serum and urine samples were determined by validated reverse-phase ion-pairing high-performance liquid chromatography methods with derivatization and fluorescence detection. After intravenous administration of cyclic HPMPC, concentrations of cyclic HPMPC declined in a biexponential manner, with a mean ± standard deviation half-life of 1.09 ± 0.12 h (n = 22). The pharmacokinetics of cyclic HPMPC were independent of dose over the dose range of 1.5 to 7.5 mg/kg. The total clearance of cyclic HPMPC from serum and the volume of distribution of intravenous cyclic HPMPC were 198 ± 39.6 ml/h/kg and 338 ± 65.1 ml/kg, respectively (n = 22). The renal clearance of cyclic HPMPC (132 ± 27.3 ml/h/kg; n = 22) exceeded the creatinine clearance (86.2 ± 16.3 ml/h/kg), indicating active tubular secretion. The cyclic HPMPC excreted in urine in 24 h accounted for 71.3% ± 16.0% of the administered dose. Cidofovir was formed from cyclic HPMPC in vivo with a time to the maximum concentration in serum of 1.64 ± 0.23 h (n = 22). Cidofovir levels declined in an apparent monoexponential manner, with a mean terminal half-life of 3.98 ± 1.26 h (n = 22). The cidofovir excreted in urine in 24 h accounted for 9.40% ± 2.33% of the administered cyclic HPMPC dose. Exposure to cidofovir after intravenous administration of cyclic HPMPC was dose proportional and was 14.9% of that from an equivalent dose of cidofovir. The present study suggests that intravenous cyclic HPMPC also has a lower potential for nephrotoxicity in humans compared to that of intravenous cidofovir. The oral bioavailabilities of cyclic HPMPC were 1.76% ± 1.48% and 3.10% ± 1.16% with the administration of doses of 1.5 and 3.0 mg/kg, respectively (n = 6 per dose). The maximum concentrations of cyclic HPMPC in serum were 0.036 ± 0.021 and 0.082 ± 0.038 μg/ml after the oral administration of doses of 1.5 and 3.0 mg/kg, respectively. Cidofovir reached quantifiable levels in the serum of only one patient for each of the 1.5- and 3.0-mg/kg oral cyclic HPMPC doses.
Cidofovir is an acyclic nucleotide analog with broad-spectrum antiviral activity against herpesviruses (2). Cidofovir is currently approved for the systemic treatment of cytomegalovirus (CMV) retinitis in patients with AIDS (10, 12). The drug has also shown clinical efficacy against mucocutaneous acyclovir-resistant herpes simplex virus infection (11) and human papillomavirus infection (15) in immunocompromised patients. 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine (cyclic HPMPC) is a cyclic analog of cidofovir and has in vivo and in vitro antiviral activities similar to those of cidofovir (1). Cyclic HPMPC is selectively converted to cidofovir within MRC-5 cells in vitro (1). Intracellular cidofovir is phosphorylated to its active form (cidofovir diphosphate) by cellular enzymes (2). Intracellular levels of cidofovir diphosphate were similar after exposure of cells to either cidofovir or cyclic HPMPC (2), and the active metabolites were slowly cleared from the intracellular space. Cyclic HPMPC can therefore be regarded as an intracellular prodrug of cidofovir.
The dose-limiting toxicity of cidofovir in animals and humans is nephrotoxicity, characterized by effects on proximal tubular cells (18, 12). This toxicity is ameliorated by concomitant administration of probenecid, a known inhibitor of the active tubular secretion of acidic drug molecules. Preclinical pharmacokinetic studies with radiolabelled cidofovir in rats (5), rabbits (4), and monkeys (6) have demonstrated that the majority of the drug is distributed to the kidneys and is excreted unchanged in the urine within 24 h of intravenous administration. The renal clearance (CLR) of cidofovir in humans and animals exceeded the glomerular filtration rate, indicating net tubular secretion. Cidofovir appears to be transported into proximal tubular cells by a probenecid-sensitive anion transport system on the basolateral membrane (8). Very high concentrations of drug have been detected in the kidneys of animals given intravenous cidofovir (5, 6). As such, it was postulated that the nephrotoxicity of cidofovir may be directly related to its active tubular secretion in the kidney. Initial transport of cidofovir into proximal tubular cells across the basolateral membrane appears to be faster than its efflux into urine. As a result, cidofovir accumulates in kidney tissue, and the extent of accumulation is related to the severity of nephrotoxicity. In rabbits, concomitant oral probenecid administration decreased the initial concentration of cidofovir in the cortex of the kidney, while the levels in other tissues remained unaffected (4). Concomitant oral probenecid administration significantly decreased the nephrotoxicity of cidofovir in monkeys (9). The pharmacokinetics of intravenous cidofovir in patients with human immunodeficiency virus (HIV) infection (with or without asymptomatic CMV infection) have been reviewed previously (3, 12). Exposure to cidofovir was dose proportional, and the drug was excreted unchanged in the urine by a combination of filtration and tubular secretion. In the same clinical studies, concomitant administration of oral probenecid decreased the CLR of cidofovir by blocking tubular secretion.
In preclinical studies, cyclic HPMPC was subject to more extensive tubular secretion than cidofovir (5). The concentrations of radioactivity achieved in rat kidney were substantially lower after intravenous administration of [14C]cyclic HPMPC than after administration of [14C]cidofovir. In contrast, the concentrations in other tissues were similar, supporting the observation that these compounds have similar in vivo antiviral activities. In addition, cyclic HPMPC was significantly less nephrotoxic than cidofovir after repeated intravenous administration to rats, guinea pigs, and monkeys (8). These data suggest that more efficient tubular secretion of cyclic HPMPC leads to less accumulation of the drug in kidney cells and therefore decreased nephrotoxicity relative to that of cidofovir. The concentrations in tissues that do not possess an anion uptake mechanism are consequently similar for both drugs. As a result, cyclic HPMPC appears to have an improved therapeutic index compared to that of cidofovir, and this has led to its selection as a candidate for development as an antiviral agent. In addition, the oral bioavailability of cyclic HPMPC in the dog (22%) suggested that the drug may show sufficient absorption to support oral dosing in humans.
The present report describes the pharmacokinetics and oral bioavailability of cyclic HPMPC in a phase I study with HIV-infected patients. In addition, the significance of metabolic conversion of cyclic HPMPC to cidofovir was assessed by simultaneous analysis of both drugs in serum.
MATERIALS AND METHODS
Patients.
The clinical study was conducted with the informed consent of the patients and the approval of an institutional review board. Patients (16 males and 6 females) were selected on the basis of diagnosed HIV infection and normal renal, hepatic, hematologic, and coagulation functions. The mean patient age was 36.5 years (range, 26 to 50 years), and the median CD4 lymphocyte count was 329 cells per mm3 (range, 32 to 1,058 cells per mm3). Specific exclusion criteria included active serious infections other than HIV; evidence of gastrointestinal (GI) malabsorption syndrome, active GI disease, or drug or alcohol abuse; a history of CMV end-organ disease; or concomitant therapy with other investigational agents, potentially nephrotoxic agents (including aminoglycoside antibiotics, amphotericin B, cidofovir, diuretics, and foscarnet) or agents with significant effects on GI absorption. Concomitant therapy with antiretroviral drugs was permitted. Nine patients (41%) received zidovudine, six patients (27%) received lamivudine, three patients (14%) received stavudine, and one patient (5%) received zalcitabine. In addition, two patients (9%) received concomitant therapy with the HIV protease inhibitor indinavir (Crixivan).
Study design. (i) Drug administration.
An intravenous formulation of cyclic HPMPC was obtained from Gilead Sciences, Inc. (Foster City, Calif.). The formulation contained 75 mg of cyclic HPMPC per ml, on the basis of anhydrous material, in a sterile isotonic solution for parenteral administration. For intravenous administration studies, cyclic HPMPC in 100 ml of 0.9% (normal) saline was infused into a peripheral vein over a 1-h period. For oral administration studies, cyclic HPMPC was diluted to 30 ml with tap water and was administered orally, followed by oral administration of a further 100 ml of tap water. Cyclic HPMPC was administered in single doses to six patients at each of the doses of 1.5 and 3.0 mg/kg of body weight by both the intravenous route and the oral route in a random order with a 2-week washout period between doses. Each of these patients received a total of two doses. Patients receiving drug at the 1.5- or 3.0-mg/kg dose were fasted from midnight of the night prior to dosing until 4 h after dosing. Cyclic HPMPC was also administered to a third group of six patients at 5.0 mg/kg and to four additional patients at 7.5 mg/kg by the intravenous route only (no oral dose). Patients receiving 5.0 or 7.5 mg/kg were not fasted.
(ii) Sample collection.
Blood and urine samples were obtained from all patients after infusion or oral administration of cyclic HPMPC. Ten milliliters of blood (approximately 6 ml of serum) was withdrawn from each subject at 0 h (predosing), 0.5 h (midinfusion for the intravenous route), 1.0 h (end of infusion for the intravenous route), and 1.5, 2, 2.5, 3, 4, 6, 8, 12, and 24 h postinitiation of dosing. The blood was allowed to coagulate, and the serum was decanted, frozen, and stored at ≤−20°C until it was analyzed. Urine samples were obtained from all patients prior to dosing (predosing voiding) and over the periods 0 to 4, 4 to 8, 8 to 12, and 12 to 24 h postinitiation of dosing. The total volume of each collection was measured to the nearest 10 ml. Aliquots (10 ml) were frozen and were maintained at ≤−20°C until they were analyzed.
Determination of cyclic HPMPC and cidofovir concentrations in serum and urine.
The concentrations of cyclic HPMPC and cidofovir in clinical serum samples were determined simultaneously by a validated reverse-phase high-performance liquid chromatography method involving precolumn derivatization with phenacyl bromide and fluorescence detection (7). The method was linear over the range of 25 to 800 ng/ml for both compounds, and the limit of quantitation was 25 ng/ml. The interday precision was <7.8%. A similar method was applied to the simultaneous analysis of cyclic HPMPC and cidofovir in urine samples. The method with urine was linear over the range of 5 to 100 μg/ml for cyclic HPMPC and 0.5 to 10 μg/ml for cidofovir, and the limits of quantitation were 5 and 0.5 μg/ml for cyclic HPMPC and cidofovir, respectively. The interday precision was <7.9%.
Pharmacokinetics and statistical analysis. (i) Pharmacokinetic calculations.
The pharmacokinetic parameters for cyclic HPMPC administered intravenously were assessed by application of the nonlinear curve-fitting software package PCNONLIN (16) by using standard noncompartmental methods. The parameters estimated by PCNONLIN included the maximum concentration of cidofovir in serum (Cmax), the time to Cmax (Tmax), the area under the serum concentration-versus-time curve (AUC) up to the time of the last quantifiable concentration (AUC0–tlast), the value of AUC extrapolated to infinity (AUC0–∞), the slope of the terminal elimination phase estimated by linear regression of the log of concentrations (kel), the half-life of the terminal elimination phase (0.693/kel), the area under the first moment of the serum concentration-versus-time curve extrapolated to infinity, and the mean residence time. The Cmax and Tmax values were confirmed by visual inspection. A minimum of the last three datum points were used in the projection of the terminal phase, based on the maximum value of the correlation coefficient. Additional parameters were calculated manually. The total clearance from serum (CL) was calculated as dose/AUC0–∞. The steady-state volume of distribution was calculated as the mean residence time × CL. The volume of distribution based on area was calculated as CL/kel. For the oral route, percent bioavailability was calculated as 100 × (AUC0–∞oral/AUC0–∞intravenous). In the absence of sufficient quantifiable concentrations of cyclic HPMPC for the accurate determination of a terminal phase, percent bioavailability was calculated as 100 × (AUC0–tlast,oral/AUC0–tlast,intravenous). The cumulative amount of cyclic HPMPC excreted at the end of each urine collection period (U0–t), was calculated as the sum of the amounts excreted in all previous collection periods. The cumulative percentage of the dose excreted at the end of each collection period was calculated as 100 × (U0–t/dose). When not directly available, the concentration of cidofovir in serum at the end of the 24-h urine collection period (C24) was calculated by extrapolation of the concentrations in serum as Clast × e[−kel × (24 − tlast)]. The AUC up to the end of the urine collection period (AUC0–24) was calculated as AUC0–∞ − (C24/kel). The CLR of cidofovir (in milliliters per hour per kilogram) after intravenous administration was calculated as (U0–24 × 1,000)/(AUC0–24 × Wt), where Wt is the body weight of the patient (in kilograms). Baseline creatinine clearance values were determined by direct measurement of creatinine levels in urine. For cidofovir determined in serum after intravenous administration of cyclic HPMPC, the observed AUC was expressed as a percentage of the total observed AUC (i.e., the sum of AUC values for cyclic HPMPC and cidofovir expressed in units of microgram equivalents of cyclic HPMPC · hour per milliliter). In addition, the observed AUC of cidofovir was expressed as a percentage of the anticipated AUC if all of the cyclic HPMPC dose was converted to cidofovir (analogous to the bioavailability of a prodrug). For example, a 1.5-mg/kg dose of cyclic HPMPC (molecular weight = 261) is equivalent to a 1.60-mg/kg dose of cidofovir (molecular weight = 279). On the basis of historical data for intravenous cidofovir without probenecid (3), a 3.0-mg/kg dose of intravenous cidofovir would produce an AUC of 10.0 μg · h/ml. Therefore, the AUC anticipated for an intravenous dose of 1.60 mg of cidofovir per kg is calculated as 10.0 × (1.60/3.0) = 5.33 μg · h/ml). The observed cidofovir AUC after intravenous administration of 1.5 mg of cyclic HPMPC per kg was then expressed as a percentage of this value.
(ii) Statistical analysis.
Statistical comparisons between CLR and baseline creatinine clearance values determined for the same patients were performed by a paired t test. The dose proportionality of intravenous cyclic HPMPC was evaluated by comparison of dose-normalized AUC and Cmax values by an unpaired t test. The effects of dose on CL, CLR, etc., were assessed by unpaired t tests. A P value of <0.05 was considered significant.
Protein binding.
Binding of cyclic HPMPC to plasma or serum proteins was evaluated over the concentration range of 0.25 to 25.0 μg/ml by using 14C-labelled cyclic HPMPC in pooled normal human plasma or serum. Duplicate samples were incubated at 37°C for 20 min and were centrifuged through Ultrafree 10,000-molecular-weight-cutoff filters (Millipore, Bedford, Mass.) in a heated (approximately 32°C) centrifuge. The results were corrected for nonspecific binding by comparison with the recovery from buffer. Binding of cyclic HPMPC to protein was negligible (<0.5%) over the entire concentration range.
RESULTS
Intravenous cyclic HPMPC.
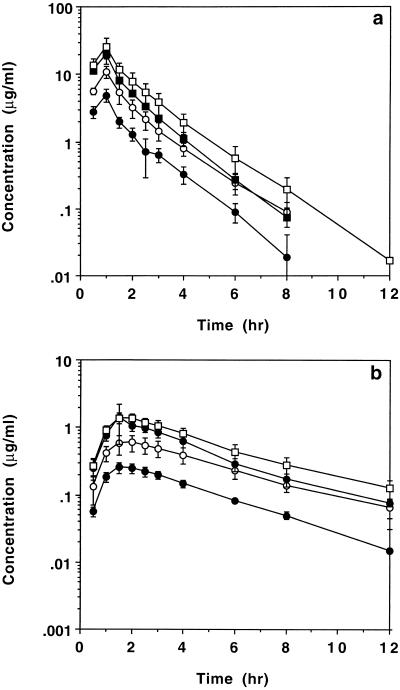
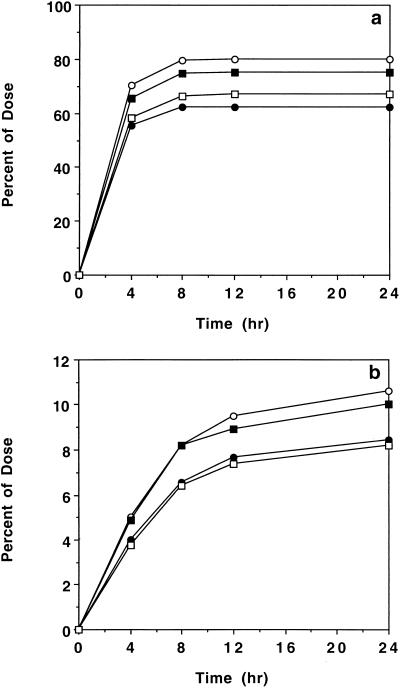
Figure 1a compares the mean ± standard deviation (SD) concentrations of cyclic HPMPC in serum after intravenous administration to HIV-infected patients at four doses (1.5, 3.0, 5.0, and 7.5 mg/kg). For samples with concentrations below the limit of quantitation, a value of zero was used in the calculation of mean data. The corresponding appearance of cyclic HPMPC in urine is indicated in Fig. 2a. After intravenous administration, the concentrations of cyclic HPMPC in serum declined in an apparent biexponential manner, with a terminal half-life of 1.09 ± 0.12 h (n = 22). Table 1 summarizes the noncompartmental pharmacokinetic parameters for intravenous cyclic HPMPC over the dose range of 1.5 to 7.5 mg/kg and the overall mean parameters for 22 patients given cyclic HPMPC by the intravenous route. The overall mean ± SD urinary recovery of unchanged cyclic HPMPC after an intravenous dose was 71.3% ± 16.0% (n = 22). The overall mean CL of the drug (198 ± 39.6 ml/h/kg; n = 22) exceeded the CLR (132 ± 27.3 ml/h/kg; n = 22), which in turn was significantly higher than the baseline creatinine clearance determined for the same patients prior to cyclic HPMPC administration (86.2 ± 16.3 ml/h/kg; n = 22). A fraction of the administered cyclic HPMPC dose was converted to cidofovir after intravenous infusion. Figure 1b presents the resulting pharmacokinetic data for the concentrations of cidofovir in the serum of patients after intravenous administration of cyclic HPMPC. The corresponding appearance of cidofovir in urine is indicated in Fig. 2b. Cidofovir was formed from cyclic HPMPC in vivo, with a Tmax of 1.64 ± 0.23 h (n = 22). Table 2 presents the pharmacokinetic parameters for cidofovir in serum derived from intravenous administration of cyclic HPMPC. The Cmax of cidofovir was proportional to the cyclic HPMPC dose on the basis of a statistical comparison of dose-normalized values. Cidofovir levels declined in an apparent monoexponential manner, with a mean terminal half-life of 3.98 ± 1.26 h (n = 22). The cidofovir excreted in urine in 24 h accounted for 9.40% ± 2.33% of the administered intravenous cyclic HPMPC dose. The nonrenal clearance of cyclic HPMPC (calculated as CL − CLR) was apparently greater at the lowest dose (1.5 mg/kg). However, urinary recovery at this dose (62.2% ± 22.1%) showed high variability, and there were no significant differences in either the CLR values or the CL values for the four dose cohorts.
FIG. 1.
Effect of dose on mean ± SD concentrations of cyclic HPMPC (a) and cidofovir (b) in serum after intravenous infusion of cyclic HPMPC to HIV-infected patients. •, 1.5 mg/kg (n = 6); ○, 3.0 mg/kg (n = 6); ■, 5.0 mg/kg (n = 6); □, 7.5 mg/kg (n = 4).
FIG. 2.
Cumulative urinary excretion (mean ± SD) of cyclic HPMPC (a) and cidofovir (b) after intravenous administration of cyclic HPMPC to HIV-infected patients. •, 1.5 mg/kg (n = 6); ○, 3.0 mg;kg (n = 6); ■, 5.0 mg/kg (n = 6); □, 7.5 mg/kg (n = 4).
TABLE 1.
Pharmacokinetic parameters for cyclic HPMPC following intravenous administration to HIV-infected patientsa
| Dose (mg/kg) | No. of subjects | Wt (kg) | Cmax (μg/ml) | AUC0–∞ (μg · h/ml) | CL (ml/h/kg) | CLR (ml/h/kg) | CLCR (ml/h/kg) | Varea (ml/kg) | MRT (h) | VSS (ml/kg) | t1/2β (h) | % Dose excreted in 24 h | % Dose excreted as cidofovir |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 | 6 | 84.6 (9.56) | 4.90 (1.03) | 7.11 (1.39) | 219 (50.2) | 113 (31.9) | 85.2 (25.8) | 337 (69.9) | 1.69 (0.11) | 368 (73.3) | 1.07 (0.08) | 62.2 (22.1) | 8.43 (1.84) |
| 3.0 | 6 | 78.7 (18.7) | 11.0 (2.33) | 16.6 (3.42) | 187 (33.7) | 145 (25.5) | 76.7 (9.10) | 315 (64.1) | 1.78 (0.16) | 331 (55.8) | 1.17 (0.125) | 79.8 (18.0) | 10.6 (3.45) |
| 5.0 | 6 | 78.1 (9.09) | 18.5 (5.48) | 27.3 (3.64) | 186 (27.0) | 138 (15.0) | 91.3 (9.62) | 264 (45.6) | 1.62 (0.113) | 301 (53.5) | 0.981 (0.074) | 74.9 (4.86) | 10.0 (1.59) |
| 7.5 | 4 | 71.4 (7.54) | 25.6 (8.75) | 39.7 (12.1) | 200 (46.6) | 133 (29.8) | 94.6 (11.5) | 326 (55.2) | 1.80 (0.100) | 357 (75.6) | 1.15 (0.138) | 66.9 (6.62) | 8.19 (1.06) |
| Mean | 22 | 78.8 (12.4) | 198 (39.6) | 132 (27.3) | 86.2 (16.3) | 309 (62.8) | 1.72 (0.123) | 338 (65.1) | 1.09 (0.123) | 71.3 (16.0) | 9.40 (2.33) |
CLCR, creatinine clearance; Varea, volume of distribution based on area; MRT, mean residence time; VSS, volume of distribution at steady state; t1/2β, elimination half-life; the other abbreviations are defined in the text. Values are given as means (SDs).
TABLE 2.
Pharmacokinetic parameters for cidofovir formed in vivo following intravenous administration of cyclic HPMPC to HIV-infected patientsa
| Cyclic HPMPC dose (mg/kg) | Tmax (h) | Cmax (μg/ml) | β (h−1) | t1/2β (h) | AUC0–tlast (μg · h/ml) | AUC0–∞ (μg · h/ml) | AUMC0–∞ (μg · h2/ml) | MRT (h) | % Dose excreted in 24 h | % of total AUC | % of i.v. CDV AUCb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 | 1.58 (0.20) | 0.253 (0.045) | 0.234 (0.069) | 3.21 (1.02) | 1.11 (0.19) | 1.30 (0.15) | 6.75 (1.74) | 5.16 (1.13) | 8.43 (1.84) | 16.0 (3.81) | 12.2 (1.44) |
| 3.0 | 1.75 (0.27) | 0.617 (0.170) | 0.205 (0.049) | 3.66 (1.40) | 3.23 (1.07) | 3.50 (1.10) | 20.4 (10.8) | 5.64 (0.99) | 10.6 (3.45) | 17.3 (3.10) | 16.4 (5.14) |
| 5.0 | 1.58 (0.204) | 1.41 (0.821) | 0.174 (0.063) | 4.37 (1.29) | 5.54 (1.19) | 5.80 (1.19) | 32.7 (8.85) | 5.63 (0.982) | 10.0 (1.59) | 17.6 (3.59) | 16.3 (3.33) |
| 7.5 | 1.63 (0.250) | 1.40 (0.231) | 0.137 (0.005) | 5.06 (0.200) | 7.49 (1.68) | 7.81 (1.74) | 51.8 (12.4) | 6.62 (0.25) | 8.19 (1.06) | 16.7 (2.34) | 14.6 (3.26) |
| Mean | 1.64 (0.228) | 3.98 (1.26) | 5.68 (1.01) | 9.40 (2.33) | 16.9 (3.17) | 14.9 (3.77) |
β, elimination rate constant; t1/2β, elimination half-life; AUMC0–∞, area under the first moment of the concentration-time curve from time zero to infinity; MRT, mean residence time; the other abbreviations are defined in the text. Values are means (SDs).
Based on historical data for intravenous (i.v.) cidofovir (CDV) without probenecid (7).
Oral cyclic HPMPC.
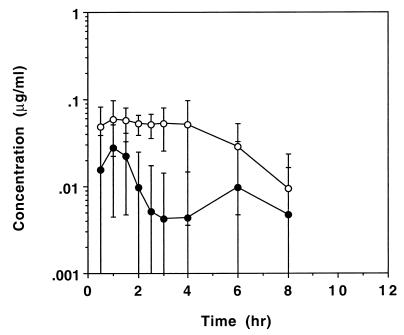
Figure 3 shows the mean ± SD cyclic HPMPC serum concentration-versus-time profiles after oral administration of cyclic HPMPC at two doses (1.5 and 3.0 mg/kg). Cyclic HPMPC reached quantifiable levels in five of six patients at the 1.5-mg/kg dose and all patients at the 3.0-mg/kg dose. Cidofovir was formed from cyclic HPMPC after oral administration; cidofovir reached quantifiable levels in the serum of only one patient for each of the 1.5 and 3.0 mg/kg cyclic HPMPC doses. Table 3 summarizes the mean (SD) pharmacokinetic parameters for cyclic HPMPC and cidofovir in patients given oral cyclic HPMPC at doses of 1.5 and 3.0 mg/kg. The Cmax values for oral cyclic HPMPC were 0.036 ± 0.021 and 0.082 ± 0.038 μg/ml for doses of 1.5 and 3.0 mg/kg, respectively. The corresponding Tmax values for oral cyclic HPMPC were 1.3 ± 1.0 and 2.0 ± 1.3 h. The oral bioavailabilities of cyclic HPMPC was 1.76% ± 1.48% and 3.10% ± 1.16% for doses of 1.5 and 3.0 mg/kg, respectively (n = 6 per dose). Cyclic HPMPC reached quantifiable levels in the urine of only one patient after oral administration at the 1.5-mg/kg dose. Cyclic HPMPC recovered in urine within 24 h of oral administration accounted for 1.1% (n = 1) and 1.48% ± 0.69% (n = 6) of the dose for the 1.5 and 3.0 mg/kg doses, respectively. Cidofovir reached quantifiable levels in the urine of only two patients after oral administration of cyclic HPMPC at the 1.5-mg/kg dose. The cidofovir recovered in urine within 24 h of oral administration accounted for 0.14% (n = 2) and 0.61% ± 0.38% (n = 6) of the dose for the 1.5- and 3.0-mg/kg cyclic HPMPC doses.
FIG. 3.
Effect of dose on mean ± SD concentrations of cyclic HPMPC in serum after oral administration of cyclic HPMPC to HIV-infected patients. •, 1.5 mg/kg (n = 6); ○, 3.0 mg/kg (n = 6).
TABLE 3.
Pharmacokinetic parameters for cyclic HPMPC and cidofovir following oral administration of cyclic HPMPC to HIV-infected patientsa
| Cyclic HPMPC dose (mg/kg) | Species | Tmax (h) | Cmax (μg/ml) | t1/2β (h) | AUC0–tlast (μg · h/ml) | AUC0–∞ (μg · h/ml) | % Total oral AUC | % Dose excreted in 24 h | Bioavail-ability (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1.5 | Cyclic HPMPC | 1.3 (1.0)b | 0.036 (0.021) | 2.02 (0.706)c | 0.068 (0.090) | 0.188 (0.099)c | 96.5 (7.86) | 1.1d | 1.76 (1.48) |
| Cidofovir | 12d | 0.008 (0.020) | NDe | 0.004 (0.010) | ND | 2.93 (7.18) | 0.14f | 0.04 (0.10) | |
| 3.0 | Cyclic HPMPC | 2.0 (1.27) | 0.082 (0.038) | 4.36 (2.88) | 0.292 (0.125) | 0.497 (0.166) | 95.2 (11.9) | 1.48 (0.69) | 3.10 (1.16) |
| Cidofovir | 6d | 0.006 (0.014) | ND | 0.043 (0.106) | ND | 9.80 (24.0) | 0.61 (0.38) | 0.75 (1.85) |
t1/2β, elimination half-life. The other abbreviations are defined in the text. Values are means (SDs).
Data for five patients. The concentrations in the remaining patient did not exceed the limit of quantitation (<0.025 μg/ml).
Data for four patients. There were insufficient datum points with quantifiable levels in the remaining patients to calculate a terminal phase.
Data for one patient. The concentrations in the remaining patients did not exceed the limit of quantitation (<0.025 μg/ml in serum or <5 μg/ml in urine).
ND, not determined due to insufficient datum points above the limit of quantitation (<0.025 μg/ml).
Data for two patients. Concentrations in the remaining patients did not exceed the limit of quantitation (<0.025 μg/ml in serum or <0.5 μg/ml in urine).
DISCUSSION
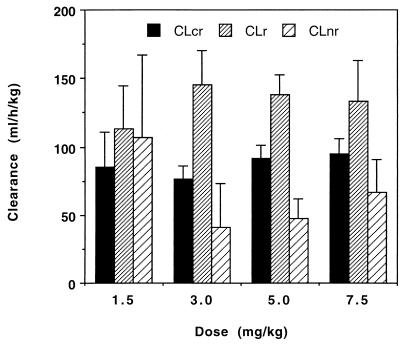
The pharmacokinetics of intravenous cyclic HPMPC were independent of dose over the range of 1.5 to 7.5 mg/kg. The observed Cmax and AUC for cyclic HPMPC were dose proportional on the basis of a comparison of dose-normalized values. After intravenous administration, cyclic HPMPC was cleared by a combination of renal and nonrenal mechanisms. Approximately 70% of the intravenous dose was recovered unchanged in the urine in 24 h. The CLR of unchanged cyclic HPMPC (132 ± 27 ml/h/kg; n = 22) was significantly higher (P < 0.001, paired t test) than the baseline creatinine clearance for the same patients (86.2 ± 16.3 ml/h/kg; n = 12), indicating that active tubular secretion played a significant role in the clearance of intact cyclic HPMPC. This is consistent with observations in studies with animals. Furthermore, the CLR of intact cyclic HPMPC was very similar to that of cidofovir itself (129 ± 42 ml/h/kg; n = 25) (3, 12). The apparent nonrenal clearance of cyclic HPMPC (CL − CLR) was approximately 66 ml/h/kg and was presumably the result of conversion to cidofovir (Fig. 4). Cidofovir was formed rapidly in vivo after the intravenous administration of cyclic HPMPC, achieving a Tmax of 1.64 ± 0.23 h (n = 22). No other metabolites of cyclic HPMPC were observed in human serum or urine. Once formed, cidofovir levels declined in an apparent monoexponential manner, with a mean terminal half-life of 3.98 ± 1.26 h (n = 22). This rate is slower than the rate of clearance of cyclic HPMPC and presumably reflects the slower rate of efflux of cidofovir from cells. The relatively short half-life of cidofovir after intravenous administration of cyclic HPMPC probably does not reflect the true duration of action of the drug, since the antiviral effect is dependent on the concentrations of the active phosphorylated metabolites of cidofovir present within the cell. The limitations of the current analytical methods may preclude observation of a more prolonged terminal elimination phase representing the efflux of cidofovir from cells. Such a prolonged phase has been observed in preclinical studies with radiolabelled drug (6).
FIG. 4.
Effect of dose on CLR and nonrenal clearance (CLnr) of cyclic HPMPC after intravenous administration to HIV-infected patients; data are compared to baseline creatinine clearance (CLcr) data for the same patients.
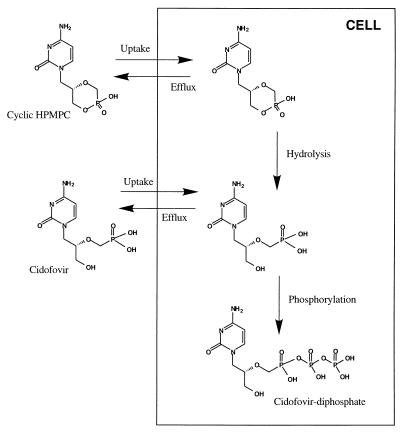
Cidofovir excreted in urine in 24 h accounted for 9.40% ± 2.33% of the intravenously administered cyclic HPMPC dose. The extent of conversion of the cyclic HPMPC to cidofovir was independent of dose. The observed AUC of cidofovir after intravenous administration of cyclic HPMPC accounted for 16.9% ± 3.17% of the total observed AUC. These data suggest that the intracellular conversion of cyclic HPMPC to cidofovir was not saturated over the dose range examined. Since cyclic HPMPC is essentially stable in human plasma and blood, the conversion of cyclic HPMPC to cidofovir appears to be the result of intracellular enzymatic activity. It has been shown that the cytosolic enzyme cyclic CMP phosphodiesterase is responsible for the hydrolysis of cyclic HPMPC in cultured cells (13). The antiviral activity of cyclic HPMPC is thus governed by a complex kinetic scheme that involves uptake of cyclic HPMPC into cells, intracellular hydrolysis of cyclic HPMPC, intracellular phosphorylation of cidofovir, and efflux of cyclic HPMPC and cidofovir from cells (Fig. 5).
FIG. 5.
Proposed kinetic scheme for intracellular conversion of cyclic HPMPC to cidofovir in vivo.
The steady-state volume of distribution of cyclic HPMPC was approximately 338 ± 65.1 ml/kg. This volume of distribution is somewhat less than that observed for cidofovir (490 ml/kg) (3), which is consistent with data for rats (5), and possibly reflects the fact that cyclic HPMPC accumulates to a lesser degree in kidney tissue.
When compared to historical data for intravenous cidofovir without probenecid (3), an intravenous dose of cyclic HPMPC provides an exposure of cidofovir equivalent to 14.9% ± 3.77% of the equivalent cidofovir dose. The antiviral activity of cyclic HPMPC has been shown to be equivalent to that of cidofovir, whereas cyclic HPMPC is 10- to 20-fold less nephrotoxic than cidofovir in animals (8). The reduced nephrotoxicity of cyclic HPMPC appears to be the result of decreased exposure to cidofovir. In addition, intravenous administration of cidofovir in clinical studies without concomitant probenecid led to dose-related nephrotoxicity (10, 12), while no significant renal adverse events were observed in the present study following intravenous administration of cyclic HPMPC at similar doses. These observations suggest that intravenous administration of cyclic HPMPC would lead to a greatly reduced potential for nephrotoxicity compared to that from administration of cidofovir.
The oral bioavailability of cyclic HPMPC was low, consistent with observations from studies with animals (5, 6). The maximum levels of cyclic HPMPC in serum after oral dosing (0.036 ± 0.021 and 0.082 ± 0.038 μg/ml for doses of 1.5 and 3.0 mg/kg, respectively) were approximately sevenfold lower than those after a 1-h intravenous infusion. After oral administration of cyclic HPMPC, cidofovir reached quantifiable levels in the serum of only one patient for each of the 1.5 and 3.0-mg/kg oral doses. These data indicate that oral administration of cyclic HPMPC is unlikely to achieve sufficient exposure to the active drug. As a result, a number of lipophilic prodrugs of cyclic HPMPC are being investigated and show greatly improved bioavailability compared to that of cyclic HPMPC (14, 17).
In summary, the pharmacokinetics of intravenous cyclic HPMPC in HIV-infected patients were reproducible and dose independent. The level of systemic exposure to the drug was proportional to the intravenous dose. The drug was cleared by a combination of renal (filtration and secretion) and nonrenal mechanisms. The decreased systemic exposure to cidofovir after intravenous administration of cyclic HPMPC supports observations from toxicology studies with animals and suggests that the cyclic prodrug would demonstrate a decreased potential for nephrotoxicity compared to that of intravenous cidofovir.
ACKNOWLEDGMENTS
We gratefully acknowledge the staff of Harris Laboratories, Lincoln, Nebr., for technical assistance.
REFERENCES
- 1.Bischofberger N, Hitchcock M J M, Chen M S, Cundy K C, Barkhimer D, Kent K M, Lacy S A, Lee W A, Li Z-H, Louie M, Mendel D B, Smee D S, Smith J L. Cyclic HPMPC: an intracellular prodrug for HPMPC with improved therapeutic index in vivo. Antimicrob Agents Chemother. 1994;38:2387–2391. doi: 10.1128/aac.38.10.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronson J J, Ferrara L M, Hitchcock M J M, Ho H-T, Woods K L, Ghazzouli I, Kern E R, Soike K F, Martin J C. (S)-1-(3-Hydroxy-2-(phosphonylmethoxy)propyl) cytosine (HPMPC): a potent antiherpesvirus agent. In: Lopez C, et al., editors. Immunobiology and prophylaxis of human herpesvirus infections. New York, N.Y: Plenum Press; 1990. pp. 277–283. [DOI] [PubMed] [Google Scholar]
- 3.Cundy K C, Petty B G, Flaherty J, Fisher P E, Polis M A, Wachsman M, Lietman P S, Lalezari J P, Hitchcock M J M, Jaffe H S. Clinical pharmacokinetics of cidofovir in HIV-infected patients. Antimicrob Agents Chemother. 1995;39:1247–1252. doi: 10.1128/aac.39.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cundy K C, Li Z-H, Lee W A. Effect of probenecid on the distribution, metabolism, and excretion of cidofovir in rabbits. Drug Metab Dispos. 1995;24:315–321. [PubMed] [Google Scholar]
- 5.Cundy K C, Bidgood A M, Lynch G, Shaw J-P, Griffin L, Lee W A. Pharmacokinetics, bioavailability, metabolism, and tissue distribution of cidofovir (HPMPC) and cyclic HPMPC in rats. Drug Metab Dispos. 1996;24:745–752. [PubMed] [Google Scholar]
- 6.Cundy K C, Li Z-H, Hitchcock M J M, Lee W A. Pharmacokinetics of cidofovir in monkeys: evidence for a prolonged elimination phase representing phosphorylated drug. Drug Metab Dispos. 1996;24:738–744. [PubMed] [Google Scholar]
- 7.Eisenberg E J, Cundy K C. HPLC determination of cytosine containing compounds by precolumn fluorescence derivatization with phenacyl bromide: application to antiviral nucleosides and nucleotides. J Chromatogr Biomed Appl. 1996;679:119–127. doi: 10.1016/0378-4347(95)00585-4. [DOI] [PubMed] [Google Scholar]
- 8.Hitchcock M J M, Lacy S A, Lindsey J R, Kern E R. The cyclic congener of cidofovir has reduced nephrotoxicity in three species. Antivir Res. 1995;26:A358. [Google Scholar]
- 9.Lacy S A, Hitchcock M J M, Lee W A, Cundy K C. Effect of oral probenecid on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys. Toxicol Sci. 1998;44:97–106. doi: 10.1006/toxs.1998.2481. [DOI] [PubMed] [Google Scholar]
- 10.Lalezari J, Holland G, Stagg R, Ives D, Kramer F, Kuppermann B, Kemper C, Youle M, Lewis R, Weinberg D, Johnson M, Northfelt D, Drew W L, Jaffe H S. Program and addendum of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. A randomized controlled study of cidofovir (CDV) for relapsing cytomegalovirus retinitis (CMV-R) in patients with AIDS, abstr. LB-9; p. 9. [Google Scholar]
- 11.Lalezari J P, Schacker T, Feinberg J, Gathe J, Lee S, Cheung T, Kramer F, Kessler H, Corey L, Drew W L, Boggs J, McGuire B, Jaffe H S, Safrin S. A randomized, double-blind, placebo-controlled trial of cidofovir gel for the treatment of acyclovir-unresponsive mucocutaneous herpes simplex virus infection in patients with AIDS. J Infect Dis. 1997;176:892–898. doi: 10.1086/516542. [DOI] [PubMed] [Google Scholar]
- 12.Lalezari J P, Stagg R J, Kuppermann B D, Holland G N, Kramer F, Ives D V, Youle M, Robinson M R, Drew W L, Jaffe H S. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. Ann Intern Med. 1997;126:257–263. doi: 10.7326/0003-4819-126-4-199702150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Mendel D B, Cihlar T, Moon K, Chen M S. Conversion of 1-[((S)-2-hydroxy-2-oxo-1,4,2-dioxaphosphorinan-5-yl)methyl]cytosine to cidofovir by an intracellular cyclic CMP phosphodiesterase. Antimicrob Agents Chemother. 1997;41:641–646. doi: 10.1128/aac.41.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw J-P, Oliyai R, Jones R J, Bidgood A M, Lee W A, Cundy K C. Proceedings of the Fourth International Meeting of the International Society for the Study of Xenobiotics. Cabin John, Md: International Society for the Study of Xenobiotics; 1995. Pharmacokinetics and metabolism of cyclic HPMPC and selected prodrugs in Sprague-Dawley rats and beagle dogs, abstr. 328. [Google Scholar]
- 15.Snoeck R, Van Ranst M, Andrei G, De Clercq E, De Wit S, Poncin M, Clumeck N. Treatment of anogenital papillomavirus infections with an acyclic nucleoside phosphonate analogue. N Engl J Med. 1995;333:943–944. doi: 10.1056/NEJM199510053331418. [DOI] [PubMed] [Google Scholar]
- 16.Statistical Consultants Inc. PCNONLIN®, version 4.2. Software for the statistical analysis of nonlinear models on micros. Lexington, Ky: Statistical Consultants Inc.; 1993. [Google Scholar]
- 17.Sueoka C M, Arimilli M, Jones R, Lee W A, Cundy K C, Shaw J-P. Program and abstracts of the 5th Annual Meeting of the International Society for the Study of Xenobiotics. Cabin John, Md: International Society for the Study of Xenobiotics; 1996. Salicylate ester prodrugs of cyclic HPMPC. II. Species differences in metabolism in vitro. [Google Scholar]