Abstract
Background:
Biomarkers may soon be used to predict decline in older individuals. Extended follow-up studies are needed to determine the stability of such biomarker-based predictions.
Objective:
To examine the long-term performance of baseline cognitive, neuroimaging, and cerebrospinal fluid biomarker-assisted prognosis in patients with mild cognitive impairment.
Methods:
Established, biomarker-defined, cohorts of subjects with mild cognitive impairment were examined for progression to dementia. Subjects with a baseline volumetric MRI, lumbar puncture, and Rey Auditory Verbal Learning Test were included. Dementia-free survival time in each biomarker-defined risk group was determined with Kaplan-Meier survival curves. The influence of each risk factor or combination of factors on dementia-free survival was examined with Cox proportional hazard analyses.
Results:
185 subjects were followed longitudinally for a mean (SD) 4.3 (2.8) years. 59% of participants converted within the follow-up period and the median dementia-free survival time was 2.8 years. Each individual risk factor predicted conversion to dementia (HR 1.9–3.7). The joint presence of any two risk factors increased risk for conversion (HR 7.1–11.0), with the presence of medial temporal atrophy and memory impairment showing the greatest risk for decline. Concordant atrophy, memory impairment, and abnormal CSF amyloid and tau was associated with the highest risk for conversion (HR 15.1). The presence of medial temporal atrophy was associated with the shortest dementia-free survival time, both alone and in combination with memory impairment, abnormal CSF amyloid and tau, or both.
Conclusion:
These results suggest that baseline biomarker-assisted predictions of decline to dementia are stable over the long term, and that combinations of complementary biomarkers can improve the accuracy of these predictions.
Keywords: Mild Cognitive Impairment, Biomarkers, Prognosis, Dementia, Magnetic Resonance Imaging, Rey Auditory Verbal Learning Test, Cerebrospinal Fluid
INTRODUCTION
Biomarkers inform predictive prognosis in mild cognitive impairment (MCI) and are under consideration for wider use in clinical[1] and research[2,3] settings. Incorporation of available biomarkers into research and clinical frameworks requires long-term follow-up studies, which are rare, given the field’s rapid evolution and only recent development of such biomarkers.
Combining across biomarkers may allow biological staging in Alzheimer’s disease (AD). Longitudinal studies of unimpaired individuals demonstrated that amyloid positivity may appear years before clinically relevant decline[4] and does not predict decline as well alone as it does in combination with abnormal tau,[5,6] suggesting a benefit to evaluating multiple biomarkers. In MCI, the combination of medial temporal lobe atrophy, memory impairment, and abnormal amyloid and tau predicts near-term conversion to dementia.[7] Within amyloid positive individuals, hippocampal atrophy, alone[8] and in the presence of abnormal tau,[9] predicts progression to dementia.
It remains unknown how stable these biomarker-based predictions of progression from MCI to dementia are over the long term. The question of stability is vital to future clinical applications, such as how frequently biomarkers would need to be reassessed to maintain predictive power. The current study follows to dementia conversion or dropout an established cohort of MCI patients from the Alzheimer’s Disease Neuroimaging Initiative (ADNI)[10] that was characterized at baseline using cognitive, volumetric magnetic resonance imaging (vMRI), and cerebrospinal fluid (CSF) biomarker cutoffs. In this long-term follow-up investigation, we examined the performance of each baseline assessment of risk for decline, with attention to time-to-progression to dementia and stability of a negative biomarker result.
METHODS
ADNI
All data used in preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). In 2003, the ADNI began a large-scale, multi-site observational study of cognitively normal older adults and participants with MCI and AD that included the collection of clinical, neuroimaging, and other biomarker data.[10] Participants in ADNI are longitudinally followed and clinically evaluated at regular intervals, providing a well-characterized MCI cohort. Now that few ADNI phase 1 MCI subjects remain, this unique cohort can be evaluated across their entirety of observations to examine the stability of these biomarker-assisted predictions.
Subjects were limited to the original Heister et al. cohort, for which criteria have been described.[7] Briefly, these subjects were diagnosed as MCI at baseline, based on an MMSE score between 24 and 30, a CDR rating of 0.5, both a subjective memory complaint and an objective memory impairment, intact activities of daily living, and absence of dementia. Only the subset of subjects who completed a baseline vMRI, lumbar puncture, and Rey Auditory Verbal Learning Test (AVLT) were included. Clinical follow-up assessments occurred at 6-month intervals up to 2 years after baseline, and at 1-year intervals from year 2 onward, for a maximum of 15 visits over 11.5 years. Diagnosis of dementia at follow-up was determined by the study clinician.
Participants
For the present analysis, subjects from the original cohort of 192 were excluded if they did not complete at least one follow-up visit, leaving 185 individuals. For subjects who had a dementia diagnosis at their final follow-up visit, the date of conversion was calculated as the point halfway between the last visit at which the subject maintained an MCI diagnosis and the first visit at which the subject was diagnosed with dementia.
Standard Protocol Approvals, Registrations, and Patient Consents
The research protocol was approved by each local institutional review board and written informed consent was obtained from each participant or participant’s guardian.
MRI acquisition and analysis
For details on the original ADNI MRI acquisition protocol and processing pipeline, see Jack et al.[11] For each subject, the initial baseline MP-RAGE sequence was used for analysis. The baseline MRI visit occurred between August 26, 2005 and September 24, 2007. Raw DICOM files for each subject were downloaded from ADNI. NeuroQuant® software (version 1.4) was used for automated segmentation.[12] This process corrects for spatial distortion and intensity variation due to gradient nonlinearities and B1 field inhomogeneity and derives volumes of subcortical structures based on a probabilistic atlas. This processing pipeline received FDA 510K clearance for clinical use in measuring volumes of brain structures in MRI images[13] and thus matches that available in clinical practice.
Hippocampal Occupancy (HOC), an estimate of medial temporal lobe atrophy, was calculated as the ratio of hippocampal volume to the sum of hippocampal and surrounding inferior lateral ventricle volume. Lower scores indicate an expansion of the inferior lateral ventricle as a function of tissue loss. Right and left occupancy scores were averaged for each individual. HOC was compared to hippocampal volume corrected for intracranial volume (HC % ICV), a commonly used measure of neurodegeneration. All imaging measures were normalized for age and sex.
CSF acquisition and analysis
Collection and processing of baseline CSF samples followed the standardized ADNI protocol, which has been described.[14] Amyloid-β1-42 (Aβ), total tau (t-tau), and phosphorylated tau (p-tau) were measured using a multiplex immunoassay.
Rey auditory-verbal learning test
Administration of the AVLT has been described.[15] Initially, the subject immediately recalls as many words as she can remember from a 15-word list over a series of 5 trials in which the same list is presented. Here, the sum of the scores from these 5 immediate learning trials of the AVLT was used to assess the degree of memory impairment.
Risk stratification
Subjects were stratified into risk and non-risk groups using the same cutoff thresholds established in Heister et al.[7] Briefly, vMRI thresholds were generated by identifying optimal separation of healthy and AD cohorts using an independent sample of subjects from ADNI.[7] Published threshold values for CSF measures[14] and for the sum of scores from the immediate learning trials of the AVLT[16] were used, each determined using ROC analyses to maximize the diagnostic accuracy between mild AD cases (mean MMSE 23.5 and 23.4, respectively) and normal controls.
Statistical analyses
Differences in subject demographics and clinical characteristics by conversion status were tested with Pearson’s chi-squared test with Yates’ continuity correction or Welch’s two sample t-test. Kaplan-Meier survival curves were used to determine dementia-free survival time. Cox proportional hazard analyses, controlling for age, were used to examine the influence of each risk factor or combination of factors on dementia-free survival. Hazard ratios (HR) were always calculated in comparison to the negative risk group. In order to further investigate the effect of neurodegeneration on survival, some analyses were repeated in a subset of subjects classified as prodromal AD,[2] defined as positive for CSF Aβ and p-tau. For subjects who did not complete an 11-year visit and did not convert to dementia over the course of follow-up, time to censoring was examined by risk group with Welch’s two sample t-test, corrected for multiple comparisons, to test for informative dropout. All analyses were done using R (version 3.3.3, https://www.r-project.org/). Significance was set to p < 0.05.
Data Availability
A request for access to data can be submitted and approved by the ADNI Data and Publications Committee (ida.loni.usc.edu/collaboration/access/appLicense.jsp).
RESULTS
Subject demographics and clinical characteristics, split by conversion status, are summarized in Table 1. In this study 185 MCI subjects were followed longitudinally for a mean (SD) 4.3 (2.8) years. 59% of participants converted to dementia within the follow-up period, with a median dementia-free survival time of 2.8 years (95% CI, 2.5–3.7). Subjects who did not convert to dementia were lost to follow-up at an average of 3.8 years. Sex was not a significant predictor of conversion when included as a covariate in the analysis for any single biomarker or biomarker combination. While the Cox proportional hazard analyses controlled for age, it was only a significant predictor of conversion in the HOC positive, CSF negative combination (HR 1.1, 95% CI, 1.0–1.2) and the CSF negative, AVLT positive combination (HR 1.1, 95% CI, 1.0–1.2). There was no significant difference in APOE ε4 allele carriers by conversion status. However, there was a significant relationship between APOE ε4 allele dose and age at conversion (Supplementary Figure 1). Of the subjects who did not complete their 11-year visit but retained an MCI diagnosis at their last completed clinical evaluation, the time to censoring was associated with risk factor group (FDR-corrected p <0.05), with subjects in the positive risk group for HOC, HC % ICV, t-tau, p-tau/Aβ ratio, p-tau, t-tau/Aβ ratio, or Aβ remaining in the study an average 1.6 years shorter than their negative risk counterparts.
Table 1.
Subject demographics and clinical characteristics split by conversion status. Reported as mean (SD) unless otherwise noted. p-value based on Pearson’s chi-squared test with Yates’ continuity correction or Welch’s two sample t-test.
| Stable (n=75) | Converted (n=110) | p-value | |
|---|---|---|---|
| Women, No. (%) | 24(32) | 39(35) | 0.74 |
| Age, y | 74.6(7.1) | 74.6(7.5) | 0.99 |
| Education, y | 15.5(3.0) | 15.8(3.0) | 0.58 |
| APOE ε4 allele carrier, No. (%) | 36(48) | 66(60) | 0.14 |
| MMSE | 27.2(1.7) | 26.7(1.8) | 0.07 |
| CDR-SB | 1.3(0.8) | 1.7(0.9) | 5.26×10−4 |
| ADAS-Cog 11 | 10.3(4.4) | 12.6(4.4) | 6.18×10−4 |
| AVLT | 33.5(9.5) | 28.2(7.1) | 7.17×10−5 |
| HOC | 0.70(0.10) | 0.65(0.11) | 6.94×10−4 |
| HC % ICV | 0.49(0.07) | 0.46(0.06) | 5.12×10−3 |
| CSF Aβ, pg/mL | 186.0(61.1) | 147.1(43.2) | 5.20×10−6 |
| CSF p-tau, pg/mL | 29.5(15.8) | 39.4(17.0) | 7.51×10−5 |
| CSF t-tau, pg/mL | 91.6(56.9) | 109.1(51.8) | 0.04 |
Abbreviations: MMSE, Mini-Mental State Examination; CDR-SB, Clinical Dementia Rating—Sum of Boxes; ADAS-Cog 11, Alzheimer’s Disease Assessment Scale—11-item Cognitive subscale; AVLT, sum of scores from the 5 immediate learning trials of the Rey Auditory Verbal Learning Test; HOC, hippocampal occupancy score; HC % ICV, hippocampal volume as a percent of intracranial volume.
Each individual risk factor predicted conversion to dementia, with HRs ranging from 1.9 to 3.7 (Table 2). Figure 1 shows Kaplan-Meier survival curves for subjects stratified into positive and negative risk groups based on individual vMRI and CSF measures and AVLT scores. Hippocampal occupancy (HR 3.7) and the t-tau/Aβ ratio (HR 3.6) outperformed hippocampal volume as a percent of ICV (HR 2.4) and any other CSF measure (HR 1.9-3.5), and were therefore the vMRI and CSF measure, respectively, used in joint risk analyses.
Table 2.
Results of Cox proportional hazards regressions controlling for age. Each hazard ratio is reported relative to the negative risk group. Asterisks indicate p-values that survive Bonferroni correction.
| Individual Risk Factors | No. positive (%) | HR (95% CI) | p-value |
|---|---|---|---|
| HOC | 83 (45) | 3.7 (2.5–5.5) | 1.82×10−10 * |
| t-tau/Aβ ratio | 130 (70) | 3.6 (2.2–6.1) | 9.83×10−7 * |
| Aβ | 139 (75) | 3.5 (2.0–6.1) | 1.63×10−5 * |
| p-tau/Aβ ratio | 145 (78) | 3.3 (1.9–5.9) | 4.03×10−5 * |
| AVLT | 129 (70) | 3.1 (1.9–5.1) | 3.17×10−6 * |
| p-tau | 132 (71) | 2.9 (1.7–4.7) | 3.08×10−5 * |
| HC % ICV | 109 (59) | 2.4 (1.6–3.6) | 2.19×10−5 * |
| t-tau | 81 (44) | 1.9 (1.3–2.8) | 1.15×10−3 * |
| Risk Factor Combinations | No. (%) | HR (95% CI) | p-value |
| HOC, AVLT, & t-tau/Aβ ratio Positive | 54 (29) | 15.1 (5.1–44.7) | 8.98×10−7 * |
| HOC, AVLT, & t-tau/Aβ ratio Negative | 18 (10) | ||
| HOC & AVLT | |||
| HOC & AVLT Positive | 64 (35) | 11.0 (5.3–22.8) | 1×10−10 * |
| HOC positive, AVLT negative | 19 (10) | 6.2 (2.2–17.2) | 4.83×10−4 * |
| HOC negative, AVLT positive | 65 (35) | 3.4 (1.7–7.0) | 6.9×10−4 * |
| HOC & AVLT Negative | 37 (20) | ||
| HOC & t-tau/Aβ ratio | |||
| HOC & CSF Positive | 65 (35) | 10.0 (4.7–21.0) | 1.47×10−9 * |
| HOC Positive, CSF Negative | 18 (10) | 7.6 (2.4–23.6) | 4.78×10−4 * |
| HOC Negative, CSF Positive | 65 (35) | 4.3 (2.0–9.2) | 1.3×10−4 * |
| HOC & CSF Negative | 37 (20) | ||
| t-tau/Aβ ratio & AVLT | |||
| CSF & AVLT Positive | 100 (54) | 7.1 (3.2–15.7) | 1.46×10−6 * |
| CSF Positive, AVLT Negative | 30 (16) | 2.6 (1.0–6.7) | 0.04 |
| CSF Negative, AVLT Positive | 29 (16) | 1.9 (0.7–4.8) | 0.21 |
| CSF & AVLT Negative | 26 (14) | ||
Abbreviations: AVLT, sum of scores from the 5 immediate learning trials of the Rey Auditory Verbal Learning Test; HOC, hippocampal occupancy score; HC % ICV, hippocampal volume as a percent of intracranial volume; HR, hazard ratio; CI, confidence interval.
Figure 1. Kaplan-Meier survival curves for each individual risk factor.
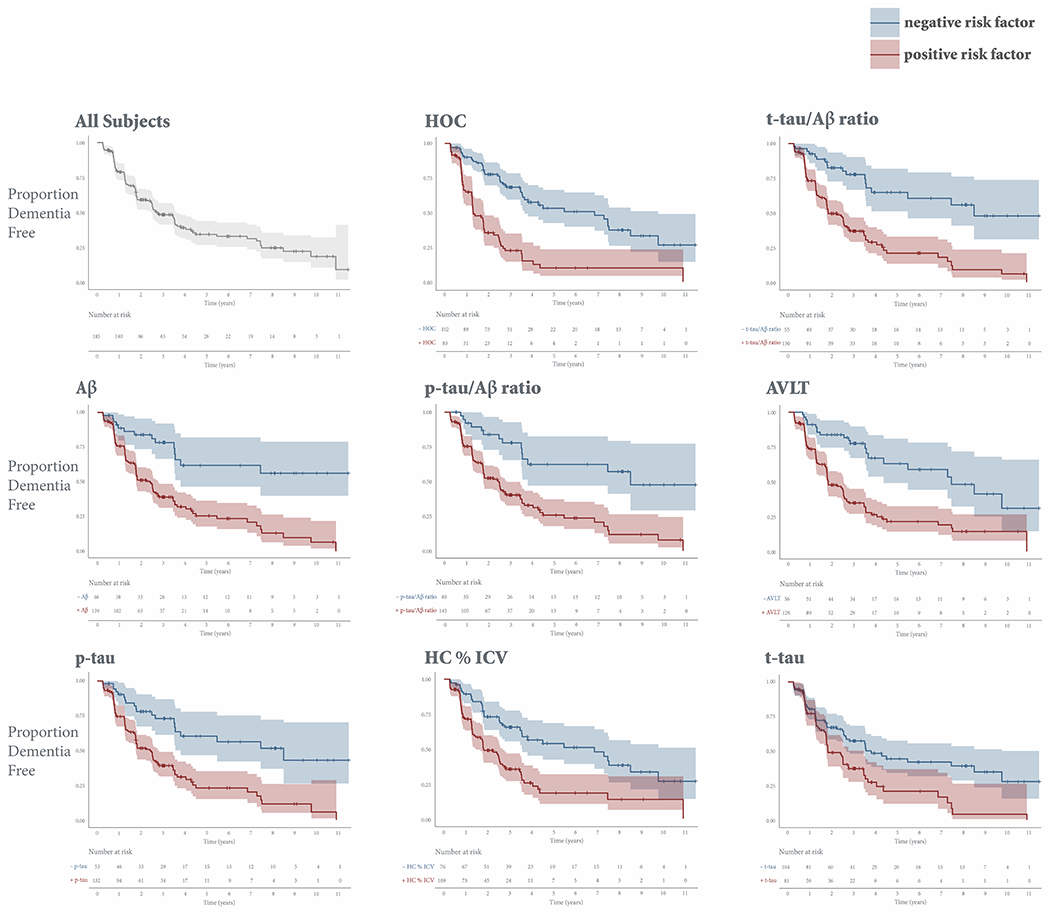
Kaplan-Meier survival curves, which estimate the probability that a subject will remain dementia free at a given time, are displayed for the entire cohort and stratified by positive (red) or negative (blue) risk for each individual risk factor. The y-axis shows the proportion of stable subjects. The x-axis shows time in years. Vertical drops indicate conversion. Tick marks indicate censoring. Shading represents 95% confidence intervals. Only subjects who have not yet converted or dropped out are considered at risk at a given time point.
Abbreviations: AVLT, sum of scores from the 5 immediate learning trials of the Rey Auditory Verbal Learning Test; HOC, hippocampal occupancy score; HC % ICV, hippocampal volume as a percent of intracranial volume.
The joint presence of any two risk factors increased risk for conversion (HR 7.1-11.0), with the presence of both medial temporal lobe atrophy and memory impairment on the AVLT showing the greatest risk for decline. Figure 2 shows Kaplan-Meier survival curves for subjects stratified into positive and negative risk groups based on combinations of joint HOC, t-tau/Aβ ratio, and AVLT risk.
Figure 2. Kaplan-Meier survival curves stratified by risk factor combinations.
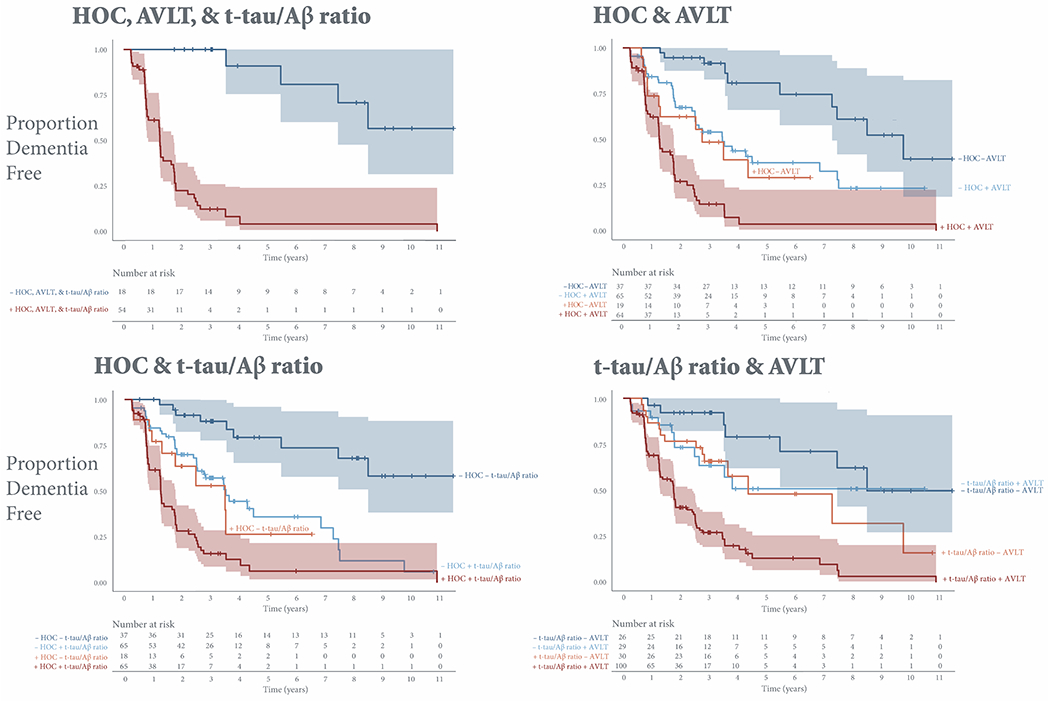
Kaplan-Meier survival curves estimate the probability that a subject will remain dementia free at a given time. The y-axis shows the proportion of stable subjects. The x-axis shows time in years. Vertical drops indicate conversion. Tick marks indicate censoring. Shading represents 95% confidence intervals. Only subjects who have not yet converted or dropped out are considered at risk at a given time point.
Abbreviations: AVLT, sum of scores from the 5 immediate learning trials of the Rey Auditory Verbal Learning Test; HOC, hippocampal occupancy score.
Concordant HOC, AVLT, and t-tau/Aβ ratio risk was associated with the highest risk for conversion to dementia (HR 15.1) (Figure 2). Subjects with concordant positive risk on all three factors (n= 54) converted to dementia at a median 1.3 (95% CI, 0.9–1.8) years (Figure 3). The 13% of concordant positive subjects who did not convert were lost to follow-up at a mean (SD) 1.7 (1.3) years, and their subsequent cognitive status is therefore unknown.
Figure 3. Median dementia-free survival time stratified by individual risk factors and risk factor combinations.
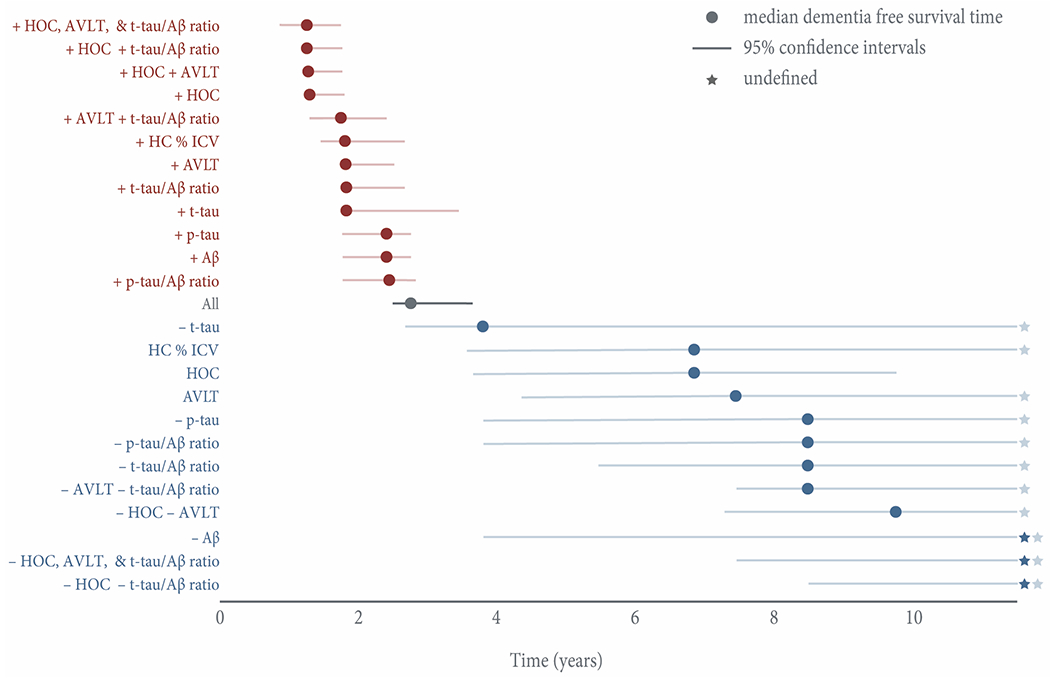
Positive risk factors are displayed in red; negative risk factors are displayed in blue. All combinations represent concordant positive or negative risk. The x-axis shows time in years. Error bars represent 95% confidence intervals. Due to high survival, three groups (– Aβ; –HOC, AVLT, and t-tau/Aβ ratio; – HOC – t-tau/Aβ ratio) never reached a median dementia-free survival time, and in several groups the upper limit of the 95% confidence interval was undefined past the last timepoint of 11.5 years. Stars represent such undefined estimates past the study completion.
Abbreviations: AVLT, sum of scores from the 5 immediate learning trials of the Rey Auditory Verbal Learning Test; HOC, hippocampal occupancy score; HC % ICV, hippocampal volume as a percent of intracranial volume.
As was previously reported,[7] the presence of medial temporal lobe atrophy was associated with the shortest median dementia-free survival time, both alone (1.3 [95% CI, 1.3–1.8] years) and in combination with AVLT impairment (1.3 [95% CI, 1.2–1.8] years), abnormal CSF (1.3 [95% CI, 1.2–1.8] years), or both (1.3 [95% CI, 0.9–1.8] years). When limited to those with prodromal AD, atrophy remained predictive of conversion (HR 2.5 [95% CI, 1.6–3.8]), shifting median dementia-free survival time over 2 years (1.3 [95% CI, 1.2–1.8] years HOC+, 3.5 [95% CI, 2.5–7.3] years HOC–).
Conversely, the presence of two negative biomarkers at baseline was associated with long-term stability. Individuals testing negative for AVLT and either CSF t-tau/Aβ ratio or medial temporal lobe atrophy risk retained their MCI diagnosis for a median 8.5 (95% LCI 7.5) or 9.8 (95% LCI 7.3) years, respectively. Due to the high survival of subjects with joint negative HOC and t-tau/Aβ ratio risk and concordant negative HOC, AVLT, and t-tau/Aβ subjects, the median time to conversion is undefined greater than the last the last study timepoint (11.5 years). 78% of subjects with concordant negative HOC, AVLT, and t-tau/Aβ ratio risk (n = 18) remained stable over the follow-up period. The mean (SD) time to conversion in the four concordant negative subjects who received diagnoses of dementia was 6.2 (2.2) years. Two of these subjects remained amyloid negative on florbetapir (AV-45) scans acquired an average 4.5 years after baseline, suggesting a dementing amyloid negative illness.
DISCUSSION
In this longitudinal investigation, we demonstrate the stability of baseline cognitive, biofluid, and neuroimaging biomarker-assisted predictive prognosis in MCI. While each individual risk factor predicted conversion to dementia, individuals with three risk factors were at higher risk of conversion than individuals with only one or two risk factors. Subjects with concordant positive risk on all three factors (n= 54) converted to dementia at a median 1.3 (95% CI, 0.9–1.8) years, while subjects with concordant negative risk had such high survival that they retained their MCI diagnoses for a median time that is undefined greater than the last study timepoint (11.5 years). These findings align with the mounting body of evidence suggesting the predictive prognostic benefit of assessing multiple complementary biomarkers, which may provide nonoverlapping information about disease progression.
Subjects with medial temporal lobe atrophy showed the greatest hazard of converting to dementia and, as previously demonstrated, those with medial temporal lobe atrophy remained dementia free for the shortest amount of time[7]. Neurodegeneration—as measured by atrophy in MRI—better correlates with clinical impairment and progression from MCI to dementia than does amyloid plaque burden.[7,17] Neither neurodegeneration nor progression to dementia is AD specific. Nevertheless, atrophy on MRI provides a useful indication that the underlying disease process is neurodegenerative and is the most effective predictor of near-term clinical progression. Even within subjects with prodromal AD, time to conversion shifted over 2 years with the amount of baseline atrophy.
Within the amyloid positive MCI subjects in this cohort (n= 139), 31% did not convert to dementia over the follow-up period, a mean (SD) 3.2 (2.0) years after baseline. Converging evidence suggests the pathologic changes underlying AD occur continuously over a long period. Reports indicate 10-65%[14,18–20] of healthy older adults are amyloid positive, a figure that increases with age from 10-18% of those in their 60s to 40-65% of those in their 80s. If the goal is to identify individuals at greatest risk for cognitive decline, amyloid status alone fails to provide the clinician guidance regarding near-term outcomes, and, in fact, in this slowly developing disease, may lead to misattribution of the current complaint to AD with possible distraction from concurrent treatable etiologies.
International Working Group 2 (IWG-2) research diagnostic criteria consider the combination of elevated tau and low Aβ in CSF to be in-vivo evidence of AD pathology,[21] noting the improved predictive and discriminative accuracy of such a combination over CSF Aβ alone. In normal controls followed longitudinally, only those with both low amyloid and elevated tau had significantly greater cognitive decline than those without either.[5] This aligns with converging evidence that amyloid is necessary but not sufficient for AD dementia, and that both Aβ-associated neurodegeneration[22] and clinical decline[23] occur only in the presence of tau. Here, we find the ratio of t-tau to Aβ predicts risk for decline better than any other CSF measure.
In patients with progressive or persistent unexplained MCI, amyloid PET positivity is said to increase the certainty that this impairment represents early AD.[24] However, the patient’s MCI may not be solely caused by or even related to this amyloid positivity, and, hence, even in the setting of a positive amyloid test and cognitive impairment, it remains imperative to screen for presence of disease mimics and retain vigilance for concurrent depression, sleep apnea, polypharmacy (e.g. opiate or anticholinergic medications), and other frequent causes of cognitive impairment in older adults. Conversely, amyloid negative status was the strongest single predictor of stability in this cohort, with the caveat that, in the broader context of clinical practice, a significant proportion of individuals destined for dementia are amyloid negative.[25–27]
Despite interest in identifying underlying pathology, the question of highest clinical relevance in current practice is whether or not a patient will progress and decline. In the current study, eight of the twelve amyloid negative subjects with medial temporal lobe atrophy at baseline converted to dementia at a mean (SD) 1.6 (1.3) years. To ensure the amyloid negative status of these subjects was not merely a consequence of our CSF cutoff selection, we used Cohen’s kappa to quantify the agreement between positive and negative classifications for CSF and PET measures, taking agreement by chance into account. For subjects who eventually underwent an AV-45 scan (n=52, mean (SD) 4.6 (0.7) years after baseline), there was excellent agreement between PET categorization (SUVRs calculated using a whole cerebellum reference region, cutoff 1.11)[28] and baseline Aβ CSF risk group (Cohen’s kappa = 0.92). Consistent with the results from Heister et al.,[7] subjects with this biomarker profile, which has come to be labeled suspected non-Alzheimer’s pathophysiology (SNAP),[29] showed risk for decline that warrants close monitoring. In clinical trials, focus may be placed on the earliest changes, such as amyloid deposition, rather than those most proximal to cognitive decline. While the approach may change when disease modifying therapies are available, identification of subgroups likely to progress in the near term provides valuable information in clinical decision-making. Biomarker-informed prognostic information, distinct from amyloid status, may be particularly valuable to individuals in prodromal stages of dementing amyloid negative illnesses, who might otherwise have little information about their likelihood of progression, and it may also provide clinicians with improved risk-benefit analysis for patients when treatments become available. In moving toward incorporating precision medicine techniques, additional information such as genetic background might be incorporated to better understand how outcomes differ in these biomarker-defined groups.
Structural imaging is already used in clinical assessment to rule out potentially treatable etiologies and, with small modifications to protocols, images can be collected that allow for fully-automated segmentation.[12,17] Implementing volumetric MRI in clinical practice allows for the assessment of AD-predominant structural changes, such as medial temporal lobe atrophy, and should improve predictive prognosis for patients with MCI. Algorithms can be trained to identify AD patterns of brain atrophy in non-demented subjects that correlate with poorer cognitive performance and aid in predictive prognosis.[30–32] Such patterns, perhaps difficult for most community radiologists to consistently identify, could be built into algorithms to allow standard imaging devices to assist in their detection. Their refinement can leverage the wealth of clinical informatics now available, allowing the algorithm to gain enduring experience that extends well beyond any individual clinician.
One important limitation to this study is that the highly selected amnestic MCI cohort in ADNI does not represent the variety of underlying pathologies seen in clinical practice. Yet, biomarker-assisted predictive prognosis might be expected to yield better performance in cohorts matching clinical practice, where greater heterogeneity of MCI is seen. Further, 97% of the converters in this cohort received a clinical diagnosis of probable AD, but without neuropathological confirmation it is unknown what mixture of underlying pathologies is truly present in these subjects as heterogeneity is common, even within amnestic MCI cohorts.[25] In our sample, time to censoring is biased by a few, disproportionately biomarker negative subjects who continue ADNI visits for a decade or longer without converting to dementia. Nevertheless, time to censoring is not significantly different between risk groups before year 10. As subjects in biomarker positive risk groups are more likely to convert to dementia and were observed for a shorter period of time, our results represent a conservative estimate in these latest timepoints. There is also a need for diversity in cohorts to generalize these findings beyond the well-educated subjects of European ancestry who make up both this cohort and the ones upon which the cutoffs used here were initially established. Further, while there is a need to establish cut-points in certain cases, such as to determine eligibility in clinical trials, it is also clear that AD is a continuum and potentially relevant information is lost when biomarkers are reduced to a binary classification of positive or negative.
The current study demonstrates that predictive prognosis in MCI is more accurate when supplemented by an assessment of baseline cognitive, biofluid, and neuroimaging biomarkers and supports incorporating multiple complementary biomarkers in future clinical and research frameworks. Our data demonstrate the long-term stability of such baseline biomarker-assisted predictive prognosis in MCI.
Supplementary Material
Supplementary Figure 1. APOE is related to age at conversion. There was a significant relationship between age at conversion and APOE ε4 allele dose (p=.02). However, APOE ε4 allele dose had no effect on the time to dropout in cases of dropout without conversion (p=.29). The y-axis shows the age at event. The x-axis shows ε4 allele dose.
ACKNOWLEDGMENTS
This study was supported by grant P50-AG005131 from the National Institutes of Health (University of California, San Diego Alzheimer’s Disease Research Center). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE STATEMENT
Dr. Brewer has served on advisory boards for Elan, Bristol-Myers Squibb, Avanir, Novartis, Genentech, and Eli Lilly and holds stock options in CorTechs Labs, Inc. and Human Longevity, Inc. Mss. Spencer and Jennings have no conflict of interest to report.
REFERENCES
- [1].Mallik A, Drzezga A, Minoshima S (2017) Clinical Amyloid Imaging. Semin Nucl Med 47, 31–43. [DOI] [PubMed] [Google Scholar]
- [2].Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein S, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo J, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jack CR, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B (2016) A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Donohue MC, Sperling RA, Petersen R, Sun C-K, Weiner MW, Aisen PS, Alzheimer’s Disease Neuroimaging Initiative (2017) Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA 317, 2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Soldan A, Pettigrew C, Cai Q, Wang M-CC, Moghekar AR, O’Brien RJ, Selnes OA, Albert MS, BIOCARD Team (2016) Hypothetical Preclinical Alzheimer Disease Groups and Longitudinal Cognitive Change. JAMA Neurol 73, 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, Cairns NJ, Morris JC, Holtzman DM, Fagan AM (2013) Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol 12, 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Heister D, Brewer J, Magda S, Blennow K, McEvoy L, Alzheimer’s Disease Neuroimaging Initiative (2011) Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology 77, 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jack CR, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, Bernstein MA, Gunter JL, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, aw L, Trojanowski JQ, Knopman DS (2010) Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 133, 3336–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van Rossum I, Vos S, Burns L, Knol D, Scheltens P, Soininen H, Wahlund L-O, Hampel H, Tsolaki M, Minthon L, L’Italien G, van der Flier W, Teunissen C, Blennow K, Barkhof F, Rueckert D, Wolz R, Verhey F, Visser P (2012) Injury markers predict time to dementia in subjects with MCI and amyloid pathology. Neurology 79, 1809–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Toga AW, Beckett L (2005) The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin N Am 15, 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jack CR, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, Whitwell JL, Ward C, Dale A, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW, Alzheimer’s Disease Neuroimaging Initiative (2008) The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J Magn Reson Imaging 27, 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brewer J (2009) Fully-automated volumetric MRI with normative ranges: translation to clinical practice. Behav Neurol 21, 21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brewer JB, Magda S, Airriess C, Smith ME (2009) Fully-Automated Quantification of Regional Brain Volumes for Improved Detection of Focal Atrophy in Alzheimer Disease. AJNR Am J Neuroradiol 30, 578–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shaw L, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee V, Trojanowski JQ, Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rey A (1964) L’examen clinique en psychologie, Presses Universitaires de France, Paris. [Google Scholar]
- [16].Landau S, Harvey D, Madison C, Reiman E, Foster N, Aisen P, Petersen R, Shaw L, Trojanowski J, Jack C, Weiner M, Jagust W, Alzheimer’s Disease Neuroimaging Initiative (2010) Comparing predictors of conversion and decline in mild cognitive impairment. Neurology 75, 230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McEvoy LK, Brewer JB (2012) Biomarkers for the clinical evaluation of the cognitively impaired elderly: amyloid is not enough. Imaging Med 4, 343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL (2010) Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 31, 1275–83. [DOI] [PubMed] [Google Scholar]
- [19].Mormino EC (2014) The Relevance of Beta-Amyloid on Markers of Alzheimer’s Disease in Clinically Normal Individuals and Factors That Influence These Associations. Neuropsychol Rev 24, 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Group A, Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, van Berckel BN, Bibeau K, Blennow K, Brooks DJ, van Buchem MA, Camus V, Cavedo E, Chen K, Chetelat G, Cohen AD, Drzezga A, Engelborghs S, Fagan AM, Fladby T, Fleisher AS, van der Flier WM, Ford L, Förster S, Fortea J, Foskett N, Frederiksen KS, Freund-Levi Y, Frisoni GB, Froelich L, Gabryelewicz T, Gill KD, Gkatzima O, Gómez-Tortosa E, Gordon MF, Grimmer T, Hampel H, Hausner L, Hellwig S, Herukka S-KK, Hildebrandt H, Ishihara L, Ivanoiu A, Jagust WJ, Johannsen P, Kandimalla R, Kapaki E, Klimkowicz-Mrowiec A, Klunk WE, Köhler S, Koglin N, Kornhuber J, Kramberger MG, Laere K, Landau SM, Lee DY, de Leon M, Lisetti V, Lleó A, Madsen K, Maier W, Marcusson J, Mattsson N, de Mendonça A, Meulenbroek O, Meyer PT, Mintun MA, Mok V, Molinuevo JL, Møllergård HM, Morris JC, Mroczko B, der Mussele S, Na DL, Newberg A, Nordberg A, Nordlund A, Novak GP, Paraskevas GP, Parnetti L, Perera G, Peters O, Popp J, Prabhakar S, Rabinovici GD, Ramakers IH, Rami L, de Oliveira C, Rinne JO, Rodrigue KM (2015) Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313, 1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo J, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert M-O, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza LC, Vellas B, Visser PJ, Schneider L, Stern Y, Scheltens P, Cummings JL (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 13, 614–629. [DOI] [PubMed] [Google Scholar]
- [22].Desikan RS, McEvoy LK, Thompson WK, Holland D, Roddey J, Blennow K, Aisen PS, Brewer JB, Hyman BT, Dale AM, Alzheimer’s Disease Neuroimaging Initiative (2011) Amyloid-β associated volume loss occurs only in the presence of phospho-tau. Ann Neurol 70, 657–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Desikan RS, McEvoy LK, Thompson WK, Holland D, Brewer JB, Aisen PS, Sperling RA, Dale A, Alzheimer’s Disease Neuroimaging Initiative (2012) Amyloid-β–Associated Clinical Decline Occurs Only in the Presence of Elevated P-tau. Arch Neurol 69, 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, Karlawish JH, Rowe CC, Carrillo MC, Hartley DM, Hedrick S, Pappas V, Thies WH, Alzheimer’s Association, Society of Nuclear Medicine and Molecular Imaging, Amyloid Imaging Taskforce (2013) Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement 9, e-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, Tangalos EG, Boeve BF, Knopman DS, Braak H, Petersen RC (2006) Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol 63, 674–81. [DOI] [PubMed] [Google Scholar]
- [26].Abner EL, Kryscio RJ, Schmitt FA, Fardo DW, Moga DC, Ighodaro ET, Jicha GA, Yu L, Dodge HH, Xiong C, Woltjer RL, Schneider JA, Cairns NJ, Bennett DA, Nelson PT (2017) Outcomes after diagnosis of mild cognitive impairment in a large autopsy series. Ann Neurol 81, 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Petersen RC, Aisen P, Boeve BF, Geda YE, Ivnik RJ, Knopman DS, Mielke M, Pankratz VS, Roberts R, Rocca WA, Weigand S, Weiner M, Wiste H, Jack CR (2013) Mild cognitive impairment due to Alzheimer disease in the community. Ann Neurol 74, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, Weiner MW, Jagust WJ, Initiative A (2012) Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol 72, 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jack CR, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Rocca WA, Boeve BF, Petersen RC (2012) An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol 71, 765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McEvoy LK, Fennema-Notestine C, Roddey J, Hagler DJ, Holland D, Karow DS, Pung CJ, Brewer JB, Dale A, Alzheimer’s Disease Neuroimaging Initiative (2009) Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology 251, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].McEvoy LK, Holland D, Hagler DJ, Fennema-Notestine C, Brewer JB, Dale A, Alzheimer’s Disease Neuroimaging Initiative (2011) Mild cognitive impairment: baseline and longitudinal structural MR imaging measures improve predictive prognosis. Radiology 259, 834–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Davatzikos C, Xu F, An Y, Fan Y, Resnick SM (2009) Longitudinal progression of Alzheimer’s-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain 132, 2026–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. APOE is related to age at conversion. There was a significant relationship between age at conversion and APOE ε4 allele dose (p=.02). However, APOE ε4 allele dose had no effect on the time to dropout in cases of dropout without conversion (p=.29). The y-axis shows the age at event. The x-axis shows ε4 allele dose.
Data Availability Statement
A request for access to data can be submitted and approved by the ADNI Data and Publications Committee (ida.loni.usc.edu/collaboration/access/appLicense.jsp).


