Abstract
Osteoarthritis (OA) is a joint degenerative disease that has become one of the leading causes of disability in the world. It is estimated that OA affects 50 million adults in the U.S.A. Currently, there are no FDA-approved treatments that slow OA progression and its treatment is limited to pain management strategies and life style changes. Despite the discovery of several disease-modifying OA drugs (DMOADs) and promising results in pre-clinical studies, their clinical translation has been significantly limited because of poor intra-articular (IA) bioavailability and challenges in delivering these compounds to tissues of interest within the joint. Here, we review current OA treatments and their effectiveness at reducing joint pain, as well as novel targets for OA treatment and the challenges related to their clinical translation. Moreover, we discuss intra-articular (IA) drug delivery as a promising route of administration, describe its inherent challenges, and review recent advances in biomaterial-based IA drug delivery for OA treatment. Finally, we highlight the potential of tissue targeting in the development of effective IA drug delivery systems.
Keywords: Osteoarthritis, drug delivery, intra-articular, tissue-targeting
INTRODUCTION
Osteoarthritis (OA) is a joint degenerative disease characterized by cartilage loss, which leads to joint pain, swelling and stiffness. OA affected 303 million people in the world in 2017(1) and it was estimated that 30.8 million adults in the U.S.A. suffered from OA in 2011(2). OA prevalence in the U.S.A has increased over the last years(3) and it is estimated to affect around 50 million people in 2020.(4). In 2008, around 14 million people over 25 years old were affected by knee OA alone and around 50% of those cases required a total knee replacement(5). Annual medical care expenses associated with OA are approximately $185.5 billion dollars annually in the U.S.A.(6). In a country with rapidly aging population and high incidence of obesity, the prevalence of OA is expected to increase(7). A population-based study conducted in Sweden estimated that by 2032, around 30% of adults over 45 are expected to have consulted a physician for OA, and around half of those cases would be related to knee OA(3).
Despite the increasing prevalence of OA, no FDA-approved disease modifying OA drugs (DMOADs) exist(8) and its treatment is limited to pain management strategies and life style changes. Depending on the severity of the disease, OA patients require interventions ranging from weight management, physical therapy(9), dietary supplements(10) and systemic administration of anti-inflammatory and analgesic drugs(11),(12), and in more severe cases, intra-articular (IA) injections of hyaluronic acid (HA) (13) and total joint replacement(5). However, these treatment strategies present limited long-term benefits and do not prevent or slow OA progression(9),(14),(15).
A variety of promising DMOAD candidates have been investigated(16),(17). However, achieving appropriate IA bioavailability after systemic administration remains a major challenge (8). Intra-articular injection offers an attractive route of drug administration for OA treatment(8). Nevertheless, free drugs injected in the IA space are rapidly cleared, resulting in poor retention and insufficient drug concentrations in the tissues of interest(18). This challenges evidence the need for biomaterial-based drug delivery vehicles able to improve the drug bioavailability into the relevant tissues(8).
In the following sections, we discuss current understanding of OA pathophysiology as well as the effectiveness of current treatment strategies. Furthermore, a section summarizing novel OA targets and promising DMOAD candidates is presented. We also describe the advantages and unmet challenges of IA drug delivery and present recent advances on IA drug delivery systems.
OA PATHOPHYSIOLOGY
According to its cause, osteoarthritis can be classified in idiopathic and secondary OA. The former has its origin on non-traumatic conditions, where factors such as age and gender have been identified to play a role(19). It is estimated that by 2030, adults older than 65 years will account for around 50% of the total OA cases in the U.S.A(20). Additionally, the prevalence of OA in men over 60 years is 10%, whereas it is 13% in women, who additionally experience more severe symptoms(7).
Secondary OA can develop as a result of metabolic disorders, traumatic events or mechanical misalignment(9). In these cases, the etiology of OA is not fully understood, but it has been recently recognized that it is a multifactorial disease. Joint injury, abnormal joint development, metabolic disorders, obesity, age, biochemical reactions and inflammation have all been reported as possible OA causes(9),(19). Some research groups have suggested that these factors could elicit changes in joint biology, mechanics and structure leading to impaired joint remodeling and the associated progressive degenerative changes characteristic of OA(21).
OA affects the joint as a whole and induces articular cartilage degeneration, subchondral bone remodeling and osteophyte formation, ligament laxity, weakening of peri-articular muscles and joint swelling(9) (Fig. 1). The exact mechanisms involved in OA progression and the interplay between articular tissues remain under investigation(22). However, recent research has identified biological mechanisms and measured biomarker levels that have been used to partly recreate OA progression(8).
FIGURE 1.
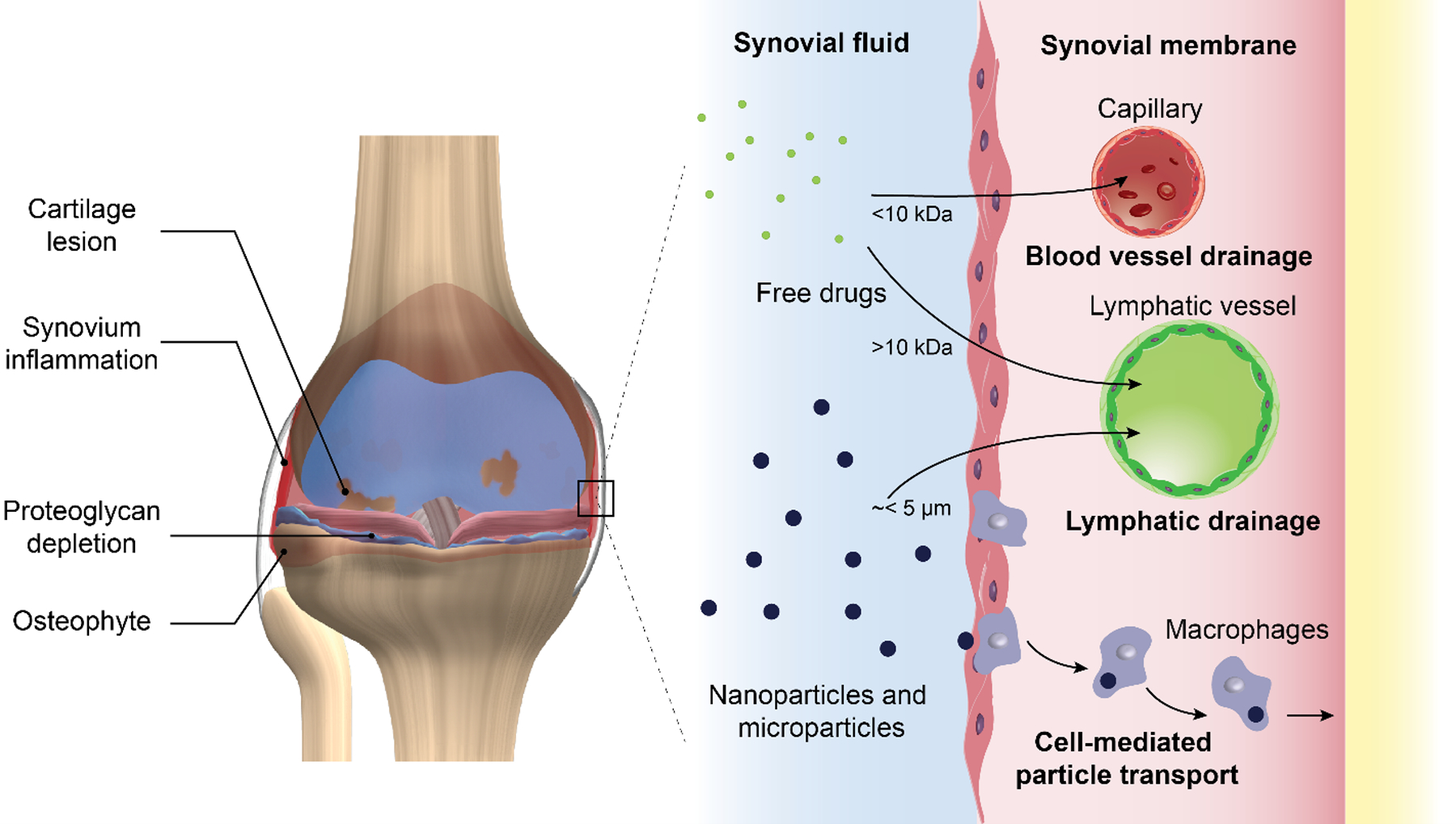
Schematic representation of an osteoarthritic knee presenting signs of cartilage degradation, bone remodeling and synovial membrane inflammation. Clearance mechanisms for free drugs and particulate drug delivery systems after IA injection. Molecules smaller than 10 kDa are eliminated from the joint space via blood vessels whereas larger molecules and particles in the nano-scale and up to few micros are eliminated via lymphatic drainage(8). Synovial macrophages also play an important role at eliminating particulate drug delivery systems via phagocytosis (23).
As OA advances, the articular cartilage experiences a continuous degeneration process characterized by partial surface lamina loss, chondrocyte hypertrophy and the appearance of cartilage fibrillations, calcified erosions and lesions(8). These morphological damages are accompanied by cartilage matrix compositional changes such as proteoglycan depletion and collagen type II cleavage(22). Furthermore, the activation of the nuclear factor NF-κB in hypertrophic chondrocytes, synovium macrophages and fibroblasts leads to the up-regulation of catabolic proteins including matrix metalloproteinases (MMPs), aggrecanases, cathepsins and A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)(4),(8),(22). Moreover, the expression of transforming growth factor beta (TGF-β) and vascular endothelial growth factor (VEGF) in chondrocytes, promotes blood vessel penetration into the hypertrophic cartilage and calcification(22). This unbalanced bone remodeling induces subchondral bone sclerosis, cysts and osteophyte formation, which result in severe pain(4),(22). Furthermore, the synovial membrane is affected by the infiltration of T lymphocytes, neutrophils and macrophages(22), which secrete pro-inflammatory mediators, cytokines and chemokines such as IL-1, IL-6, IL-15, TNF-α, nitric oxide and prostaglandins, which further exacerbate joint inflammation and cartilage degeneration(24). Additionally, synoviocyte secretion of synovial fluid components is impaired, leading to poor viscous lubrication and shock absorption capacity(8). In healthy patients, the hyaluronic acid concentration in the synovial fluid ranges from 2.5 to 4 mg/mL and has a molecular weight between 6300 and 7600 kDa; however, as a result of OA progression, its concentration and molecular weight decrease up to 1 – 2 mg/mL and 1600 – 3480 kDa, respectively(25).
Despite advances on elucidating the mechanisms involved in OA progression, there is still much investigation needed to fully comprehend OA pathophysiology. The lack of understanding of the underlying mechanisms of OA onset and development, in addition to difficulties in clinical trial design, as well as the need for more sensitive techniques to better detect changes related to OA progression(26), are all limitations that have hindered the development of appropriate disease-modifying OA drugs (DMOADs)(4),(8),(22).
CURRENT CLINICAL TREATMENTS FOR OSTEOARTHRITIS
Non-pharmacological management
Non-pharmacological approaches constitute the first line of treatment at early stages of OA progression and intend to reduce pain and improve joint functionality. The absence of mechanic loading increases cartilage degeneration(27), whereas excessive mechanic stimuli are also deleterious for joint health(9). Therefore, physical therapy are key components of non-pharmacological OA treatment. Exercises types recommended for OA patients at this stage include proprioception, stretching and resistance(9). In the case of overweighed patients, not only physical therapy is recommended, but an initial 10% weight loss is necessary in order to significantly reduce joint pain(28). Even though weight loss has been associated with a significant reduction in the risk of developing symptomatic knee OA in female patients with a body mass index (BMI) greater than 25 kg m−2, no effect of weight loss on OA was observed in women with BMI < 25 kg m−2 (28). These results suggest that weight management strategies may only be effective in overweighed populations. However, given the progressive character of this disease, and the inability of many obese patients to maintain a significant weight loss over time(28), patients often require pharmacological treatment.
An alternative to alleviate the pain is the use of dietary supplements, which account for US$25 billion annual sales(10). Approximately 70% of OA patients take oral supplements for pain management, with glucosamine and chondroitin sulfate being the most consumed compounds, accounting for a third of the oral supplements market value (US$872 million annual sales)(10). Despite the high sales volume, oral supplements have failed to induce clinically significant improvements in pain management in OA patients. Liu et al. in a meta-analysis study reviewed 69 randomized placebo-controlled clinical trials that evaluated the effects of 20 individual oral supplements for the treatment of hand, hip or knee OA. The results demonstrated that no supplements exhibited a clinically important effect on pain or physical function in the long term (>6 months). Between 4 and 6 months, only undenatured type II collagen and green-lipped mussel extract, a supplement rich in anti-inflammatory compounds such as omega-3, eicosapentaenoic acid and docosahexaenoic acid (DHA)](29), showed a significant clinical effect on pain reduction(10). Even though glucosamine and chondroitin sulfate are the most consumed dietary supplements among the OA population, according to Liu et al., these compounds only statistically improved pain scores at short term (<3 months), but their clinical effect is debatable(10). Additionally, clinical trials and meta-analysis studies have shown that the use of glucosamine and chondroitin sulfate in combination does not induce a relevant reduction in pain compared to placebo in most OA patients (30)–(32). Although glucosamine can be detected in the synovial fluid after oral administration(33), insufficient IA concentrations could be related to the poor outcomes seen in clinical trials. In fact, 90% of orally administered glucosamine is absorbed, but its concentration in plasma is significantly reduced due to the first-pass effect, leading to a bioavailability of 26–44%(33). On the other hand, oral delivery of chondroitin sulfate is challenging due to its high molecular weight (10 – 50 kDa)(33). Around 90% of orally administered chondroitin sulfate is absorbed as low molecular weight derivatives(34) and exhibits a plasma bioavailability of 5–15%(33). These challenges in the oral delivery of glucosamine and chondroitin sulfate may explain why these compounds have not induced a clinically relevant reduction of OA symptoms in several clinical trials.
Pharmacological management
Currently there are no approved DMOADs that reduce OA progression, thus treatment is limited to pain management and the regimen depends on the severity of the disease. Commonly used medications include cyclooxygenase inhibitors such as acetaminophen, systemic administration of opioids and non-steroidal anti-inflammatory drugs (NSAIDs). However, their prolonged use is limited due to their secondary effects on the hepatic, gastrointestinal, renal and cardiac systems, especially in the elderly population that often presents a wide range of comorbidities(8),(9),(35)–(37). Moreover, recent clinical studies have shown that acetaminophen is inferior to NSAIDs and not-superior than placebo for pain management in moderate and severe OA patients(27). The use of topical NSAIDs is a safer alternative, but their use has only been shown to be effective during the first two weeks of use(38). In the case of opioids, increasing awareness regarding their chronic use has limited their administration for long-term pain management. Also, studies have shown that opioids do not improve pain scores in OA patients compared to NSAIDs(39),(40).
In order to minimize adverse side effects associated with systemic administration of therapeutics and to improve drug’s bioavailability in the joint space, intra-articular (IA) injections raise an alternative that offers a more localized treatment. IA injection of corticoids has been shown to reduce pain scores and increase joint functionality due to their anti-inflammatory and immunosuppressive effects. Corticoids reduce pain and inflammation by decreasing IL-1 production, prostaglandins, leukotrienes and metalloproteinases(9),(41),(42). Several corticoids that have been FDA-approved for IA delivery as immediate release formulations include dexamethasone, beta-methasone, methylprednisolone, triamcinolone acetate and triamcinolone hexacetonide(9). However, their long-term efficacy is questionable primarily due to the short retention time. For example, the IA half-life time of cortisone and dexamethasone solutions are 1.5 h and 3.6 h respectively(43),(44). In an attempt to improve the IA retention of these molecules, crystalline drug suspensions have been used. However, around 10% of the patients experience crystal-induced “steroid flare”, characterized by an acute synovitis(45) which usually resolves within few days after injection (46).
Finally, hyaluronic acid (HA) is the only formulation currently approved for OA treatment as a lubricating agent(8),(35),(36),(47), which intends to restore healthy synovial fluid properties(9). Although clinical trials, systematic reviews and meta-analyses on the effects of HA injections on joint pain present confounding results, primarily due to a high variability in HA formulations, inappropriate blinding and small sample size, most evidence suggest that visco-supplementation may be a safe alternative to achieve clinically relevant pain reduction(13). A meta-analysis study that evaluated 19 clinical trial publications, with a total of 4,485 patients revealed that overall, HA injection significantly improved pain scores, but its clinical effect was only 29% of the minimal important difference (MID)(14). However, some evidence suggest that high molecular weight or cross-liked HA formulations are able to induce a clinically relevant reduction in knee pain(14),(25). In fact, the use of cross-linked HA formulations led to pain improvements closer to the MID (95%) whereas non-cross-linked formulations had a pain improvement of only 25%. However, if these studies are analyzed according to the clinical experimental design, double-blinded trials present a lower treatment effect (49% of MID) compared to studies with insufficient blinding (129% of MID)(14). Additionally, the use of HA injections did not have an important clinical effect on the Western Ontario and McMaster Universities Osteoarthritis (WOMAC) function or stiffness indexes(14). Consistent with other meta-analysis studies, the clinical effect of visco-supplementation using HA is unclear, primarily due to the lack of good quality, appropriately blinded studies(15).
NOVEL TARGETS FOR OA TREATMENT AND DRUG CANDIDATES
Considering that OA affects the joint as a whole, in addition to pain, pathways related to inflammation, cartilage catabolism and subchondral bone remodeling have become targets of interest to develop DMOADs. Regarding inflammation, inhibition of the nuclear factor NF-κB or individual downstream proteins (IL-1β, TNF-α, β-NGF, MMPs) has been investigated(16),(17). For example, a phase I and II clinical study for a small molecule NF-κB inhibitor, SAR113945, demonstrated drug tolerability but failed to show effectiveness 56 days after intra-articular administration(48). However, an analysis performed on a sub-population of the patients, who presented knee joint effusion at baseline demonstrated that IA injection of SAR113945 significantly reduced WOMAC scores of pain and physical function compared to placebo control(48).
Compounds that inhibit cartilage catabolic activity have also been evaluated. For example, a phase II clinical trial demonstrated that recombinant human fibroblast growth factor 18 (Sprifermin)-treated patients presented a significant reduction in lateral femorotibial cartilage thickness and volume loss compared to placebo control (p<0.033 and p<0.014, respectively), when treated with 100 μg of Sprifermin. However, patients in all experimental groups, including placebo, exhibited improved symptoms as determined by the WOMAC index at 12 months, with less improvement for patients receiving 100 μg of Sprifermin compared to placebo (p<0.013)(49). Another promising molecule, kartogenin (KGN), has been shown to promote chondrogenic differentiation and reduction of OA progression in pre-clinical animal models(50)–(52). In fact, Kang et al., demonstrated that chondrocyte pellets treated with KGN present significantly higher expression of collagen type II and aggrecan compared to non-treated pellets (p<0.001)(50). Finally, DMOADs that affect subchondral bone such as the bone resorption inhibitor salmon calcitonin and the anti-osteoporotic agent strontium ranelate have been suggested to have promising effects on OA progression(8).
Another class of DMOAD candidates include senolytic agents and autophagy promoters. It has been observed in animal models of post-traumatic OA that senescent cells accumulate in the synovial membrane and the articular cartilage(53). Also, a reduced expression of autophagy regulators, which participate in protective mechanisms in healthy cartilage, has been observed in pathological human cartilage samples (54). Therefore, elimination of senescent cells (SnC), as well as re-activation of autophagy pathways have shown promising results at reducing OA progression in pre-clinical animal models. In fact, Jeon et al. used a transgenic mice model that allowed for selective elimination of senescent cells to demonstrate that removal of this cell population resulted in reduced cartilage degradation in a post-traumatic model of mice OA(53). These results were also confirmed using pharmacological elimination of SnC via IA administration of the senolytic molecule UBX0101(53). Moreover, Xia et al. demonstrated that IA delivery of cordycepin induce re-activation of autophagy markers in a mouse model of OA and significantly reduced joint degeneration compared to untreated joints(54).
Although OA pathogenesis does not seem to have an inflammatory origin, some researchers believe that synovial inflammation plays a key role on disease progression and have suggested the use of anti-rheumatic drugs as possible OA treatments(55),(56). In fact, anti-rheumatic drugs have shown promising results on in vitro models and animal studies, but their efficiency in clinical trials is still questionable(17). Persson et al., in a systematic review and meta-analysis study, evaluated placebo-controlled clinical trials that investigated the efficacy of FDA-approved anti-rheumatic drugs as possible OA treatments(56). In the study, small molecule drugs and biologics were investigated, including hydroxychloroquine, methotrexate, anakinra, adalimumab and etanercept. Results demonstrated that although these treatments induce a significant reduction in pain metrics, this effect is not clinically relevant(56).
INTRA-ARTICULAR DRUG DELIVERY STRATEGIES IN OA
Despite the encouraging advances in the discovery of DMOADs, the translation of these drugs into the clinic is limited given the challenging pharmacokinetics of the joints. Free small molecule drugs and even proteins injected in the joint space are rapidly cleared via lymphatic drainage and their retention time does not exceed few hours (Table 1). Also, most of these drugs have poor water solubility and require a delivery system in order to be administered via IA injections(8),(47),(57). Multiple intra-articular drug delivery vehicles including hydrogels, liposomes, nanoparticles and microparticles have been formulated and will be discussed in the following sections (Fig. 2).
Table 1.
Half-life of different molecules after intra-articular injection
| Molecule | Half-life (h) | Molecular Weight (Da) |
|---|---|---|
| Paracetamol(58) | 1.10 | 151 |
| Ibuprofen(59) | 2.20 | 206 |
| Naproxen(60) | 1.60 | 230 |
| Ketoprofen(60) | 1.90 | 254 |
| Diclofenac(58) | 5.20 | 296 |
| Cortisone(44) | 1.46 | 360 |
| Dexamethasone(43) | 3.60 | 392 |
| Methotrexate(61) | 2.90 | 454 |
| Hyaluronic acid(18) | 13.20 | 6,000 |
| IL-1Ra(62) | 23.04 | 65,400 |
| Bovine serum albumin(63) | 15.12 | 66,000 |
FIGURE 2.
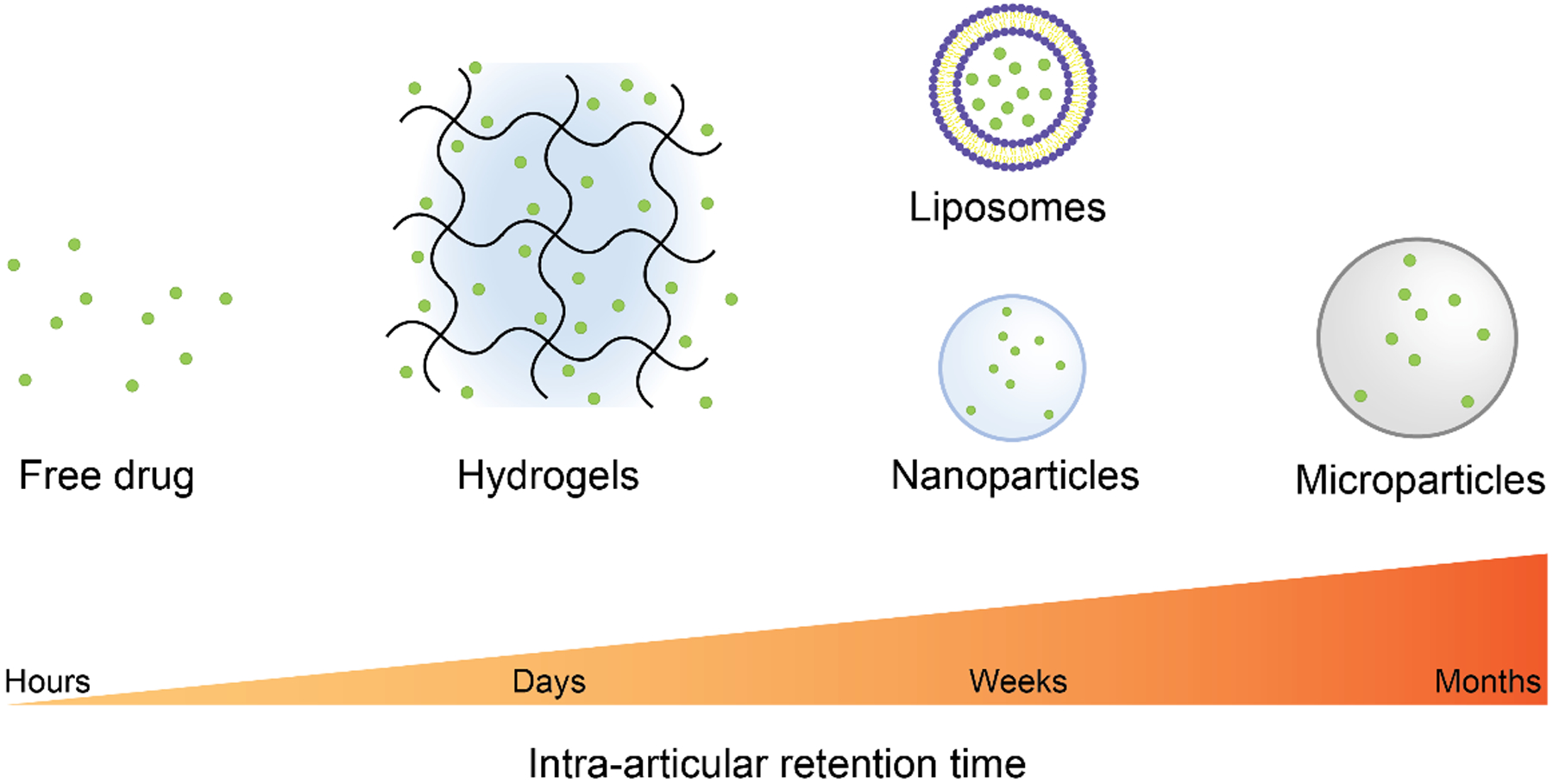
Drug delivery systems typically used for IA drug administration and their IA retention time. Free small molecule and macromolecule drugs are cleared from the joint space in few hours. The use of drug delivery vehicles increases drug IA retention time, typically in a size-dependent manner. Nano-scale vehicles such as nanoparticles and liposomes are generally retained up to a couple of weeks, whereas microparticles can be retained in the joint space up to a month. Hydrogels do not usually control the release rate of loaded drug molecules, thus present an IA retention time in the order of days.
Hydrogels
Various viscosupplementation products, such as lightly cross-linked HA hydrogel formulations (Synvisc-ONE®, EUFLEXXA®, Gel-One® and MonoVisc®)(13), represent an attractive alternative to use as drug delivery vehicles. Several research groups have shown that drug-loaded HA hydrogel formulations can be used to reduce the frequency of IA injections compared to free drug(64),(65). However, the retention of HA cross-linked formulations is still a concern. Yoshioka et al. demonstrated that the commercially available cross-linked HA formulation Gel-One® cannot be detected in the synovial fluid of rabbit knee joints after day 7, only 30% is retained in the synovial membrane at day 7 and 3.3% at day 28(66). In an attempt to improve hydrogel intra-articular retention, the use of synthetic hydrogels has been explored. For example, poly(caprolactone-co-lactide)-poly(ethylene glycol)-poly(caprolactone-co-lactide) (PCLA-PEG-PCLA) hydrogel, used to deliver celecoxib to horse knees, showed that the drug could be detected at day 28 in the synovial fluid, but more than 90% of it was cleared by day 7(67). Despite these advances, hydrogels serve as drug depots but are unable to control small molecule drug release rate because their mesh size is usually orders of magnitude larger than the loaded drugs(68),(69).
Liposomes
Liposomes can provide controlled release rates of both lipophilic and water-soluble drugs. Also, compared to crystalline drug suspensions formed by hydrophobic drugs upon IA injection, liposomes are less inflammatory(70). Studies have shown that liposomes loaded with a model small molecule, such as the contrast agent iohexol, presented an IA half-life time of 134 h whereas the free molecule was not detected after 3 h(71). However, compared to other drug delivery vehicles like polymeric particles, liposomes have limited long term stability(72). Additionally, the elevated oxidative stress seen in OA joints(73) as well as the shear and compressive loads characteristic of the IA space can reduce liposomes stability and induce drug leakage or burst release(68),(72),(74).
Nanoparticles and microparticles
An alternative to overcome the mechanical instability of liposomes is the use of lipid or polymeric nanoparticles. These vehicles have been shown to be susceptible to microvascular and synovial macrophage-mediated drainage and can be retained in the joint space only for few weeks, depending on their size, charge and composition(8),(63),(75),(76). The use of larger particles that could better avoid lymphatic drainage and cell-mediated particles elimination (Fig. 1) is a potential strategy to achieve IA drug sustained release over longer periods of time. In fact, polycaprolactone (PCL) microparticles with an average size of 16 μm were found to remain in the joint space of rats for up to a month(77). Janssen et al. synthesized celecoxib-loaded polyester amide (PEA) microspheres with a mean particle size of 25 μm and were able to detect around 20% of the injected PEA 12 weeks after IA injection in Lewis rats(78). Additionally, the company Flexion Therapeutics recently received FDA approval to commercialize ZILRETTA®, an IA formulation of 45 μm triamcinolone acetonide-loaded poly(lactic-co-glycolic) acid PLGA microparticles for pain management in OA patients. The associated clinical trials revealed persistent pain relief until 3 months post-treatment(79),(80). All together, these studies show the potential of microparticles to provide a sufficient IA retention time able to ensure drug bioactivity during a relevant therapeutic window(8).
TARGETING FOR IA DRUG DELIVERY
A wide variety of DMOADs are being studied for the treatment of OA and can be classified according to their function as analgesic, anti-inflammatory, cartilage-protective, or bone resorption inhibitors(8). Depending on their function, these drugs act on specific biologic targets present in different tissues within the joint(47). Studies have shown that non-tissue specific delivery of these drugs may result in unwanted off-target effects. For example, the use of NSAIDs reduces proteoglycan secretion, thereby increasing cartilage degradation(81). Other groups have shown that nerve growth factor (NGF) blockade for pain relief induced rapid OA progression and osteonecrosis in a phase III clinical trial(82). Therefore, drugs that act on inflammatory and pain pathways should primarily target the synovium(47). Likewise, drugs that induce chondrogenesis should be preferentially delivered to the articular cartilage in order to prevent adverse effects on the surrounding tissues. In fact, IA injection of TGF-β1(83) and the chondrogenic molecule kartogenin(50),(51),(84)–(87), although beneficial for cartilage repair, increase synovium hyperplasia and induce the formation of cartilage-like tissues in ligaments and synovium(84).
Cartilage targeting
Cartilage extracellular matrix, primarily composed of collagen type II and sulfated glycosaminoglycans (GAGs), presents a small pore size (60–200 nm) and high negative charge, which difficult the penetration of molecules into this tissue(88),(89). Therefore, the size and charge of drug delivery vehicles play an important role on cartilage targeting and penetration. Drug delivery systems of diverse compositions, ranging from few nanometers up to 100 nm in diameter have been shown to penetrate the articular cartilage matrix(89)–(91). However, their retention is primarily controlled by their ability to bind to different components of this tissue.
One alternative to achieve cartilage targeting is to use ionic interactions between the negatively charged cartilage matrix and positively charged carriers(88). For example, Cook Sangar et al. recently developed a cysteine-dense peptide (CDP-11R) that due to its high surface positive charge is able to accumulate in mice cartilaginous tissues after IV administration and into human articular cartilage explants in vitro(92). Triamcinolone acetonide conjugated to CDP-11R peptide resulted in a dose-dependent reduction in rat paw inflammation after IV administration in a rat model of RA(92). Moreover, Geiger et al. used a positively charged, cartilage penetrating dendrimer to improve cartilage retention of insulin growth factor 1 (IGF-1), which resulted in significant cartilage protection and reduction of osteophyte formation compared to free IGF-1 in a rat model of OA(93). Yan et al. developed cationic peptidic nanoparticles for IA delivery of NF-κB siRNA able to penetrate into human OA articular cartilage explants and be retained in the chondrocyte lacunae for at least 2 weeks. Additionally, IA delivery of NF-κB siRNA-conjugated cationic nanoparticles resulted in reduction of cartilage lesion length, chondrocyte apoptosis and synovitis in a mouse model of OA(94). However, it is important to note that passive cartilage targeting based on electrostatic interactions is affected by the state of the disease. In fact, Vedadghavami et al. demonstrated that positively charged nano-carriers uptake and retention in articular cartilage explants with lower GAGs content were reduced due to a decrease in the cartilage net negative charge compared to healthy explants(90). Additionally, Brown et al. demonstrated that reduced GAGs content as well as the presence of synovial fluid significantly reduce PLGA NPs retention into articular cartilage explants compared to healthy tissue and saline, respectively(91).
Moreover, targeting cartilage ECM components such as collagen type II and aggrecan has gained attention as a promising strategy to target damaged areas of the articular cartilage (Fig. 3). In fact, monoclonal anti-type II collagen antibodies (MabCII) have been used in multiple drug delivery and diagnostics applications(95)–(97). For example, Cho et al. demonstrated that liposomes functionalized with a collagen type II monoclonal antibody are able to bind cartilage tissue proportionally to the severity of the disease in a mice model of OA after systemic administration(97). Moreover, Bedingfield et al. used MabCII-functionalized polymeric NPs for cartilage-specific MMP13 siRNA delivery. These vehicles significantly reduced MMP13 expression and protected articular cartilage as measured via OARSI scores in a mouse model of OA after IA injection, compared to NPs functionalized with a negative control antibody (98). Also, single-chain antibody variable fragment (scFv) specific to reactive oxygen species (ROS)-modified collagen II have been reported(95).
FIGURE 3.
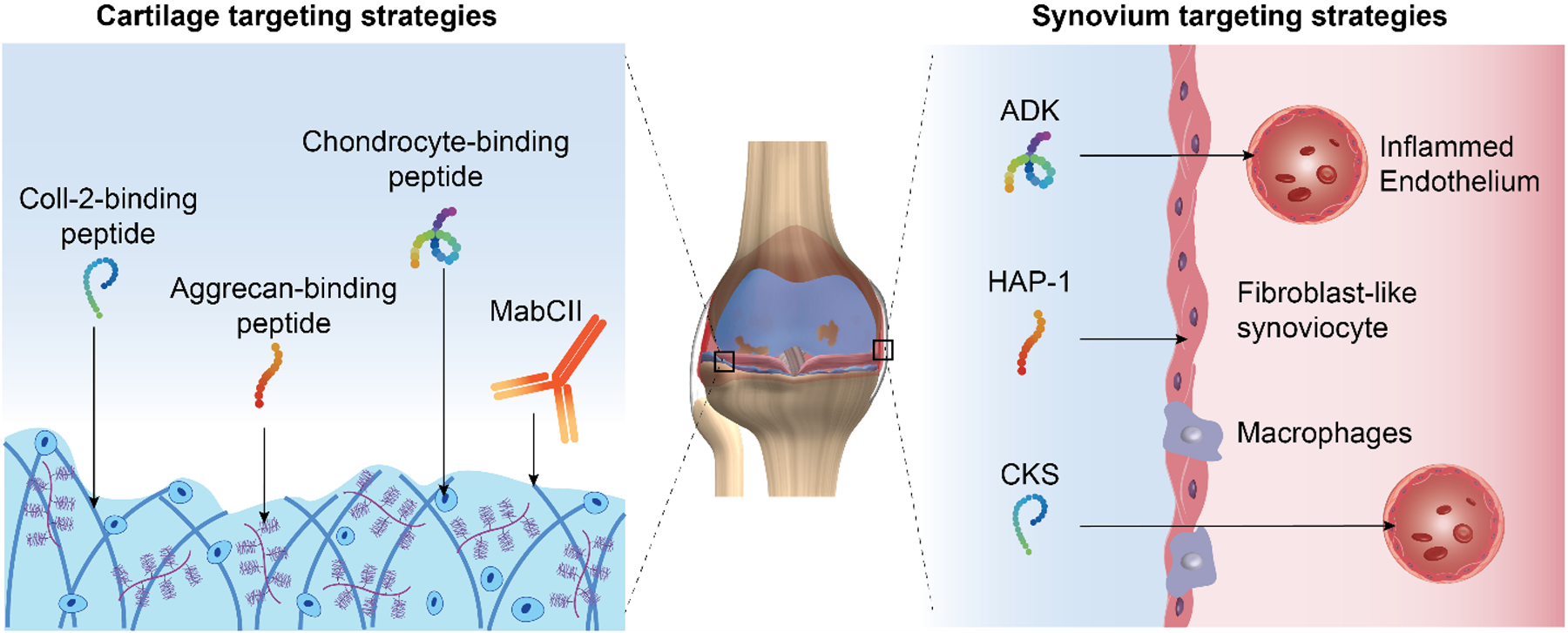
Active IA tissue targeting strategies. Targeting peptides binding articular chondrocytes(99) or cartilage ECM components, including collagen type II (Coll-2) (101) and aggrecan(100), have been reported. Anti-Coll-2 monoclonal antibodies (MabCII) have also been used for articular cartilage targeting(95)–(97). Synovial membrane targeting can be achieved by the use of inflamed synovial endothelium-binding peptides (CKS: CKSTHDRLC(105), ADK: CRNADKFPC(106)) or peptides targeting fibroblast-like synoviocytes (HAP-1: SFHQFARATLAS)(109).
More recently, the use of phage display technology has resulted in the discovery of tissue-specific peptides, which compared to larger proteins such as monoclonal antibodies, are easier to manufacture, less immunogenic, smaller in size and more stable(72). Using this technology, Yanbin et al. discovered a cartilage affinity peptide (CAP: DWRVIIPPRPSA) able to specifically bind to rabbit chondrocytes and human chondrocytes isolated from a patient with OA. Compared to the scrambled peptide, conjugation of the CAP peptide to 50 nm polyethylenimine nanoparticles, a classical and efficient non-viral vector for gene therapy, resulted in particle binding and internalization into chondrocytes in vitro and 48 h after IA injection into rabbit knee joints(99). Later, Cheung et al., discovered two peptide sequences (RLDPTSYLRTFW and HDSQLEALIKFM) via phage display able to preferentially bind aggrecan in vitro. However, no scrambled control peptides were used and the ability of these sequences to bind cartilage in vivo was not assessed(100). These aggrecan-binding and CAP peptides have not yet been used by other research groups for intra-articular drug delivery or diagnostics applications.
Rothenfluh et al. reported the ligand WYRGRL, a collagen type II α1-targeting peptide. Functionalization of poly(propylene sulphide nanoparticles and subsequent IA injection in mice knees resulted in a 72-fold increase in cartilage-targeting ability compared to nanoparticles functionalized with the scrambled control(101). In contrast to other reported peptides, this sequence has been successfully used in pre-clinical models for diagnostics and drug delivery applications(11),(89),(101)–(104). In fact, the conjugation of this peptide to magnetic resonance imaging (MRI) contrast agents has allowed in vivo localization of cartilage hypertrophic changes in a rat model of OA(102). Other researchers have coupled this ligand to near infra-red probes for in vivo imaging and detection of age-related decrease in collagen type II in mice(104). Furthermore, conjugation of this peptide to dexamethasone has proven to increase its retention into bovine articular cartilage explants and decreased the glycosaminoglycan depletion in an in vitro model of OA(11).
Synovial membrane targeting
Although drug delivery into the articular cartilage has been recognized as a key and very challenging aspect in the field, targeted delivery into the synovial membrane has gained interest as well. Originally, synovium targeting emerged as a strategy to minimize the secondary effects of systemic administration of NSAIDs and other anti-inflammatory therapeutics (Fig. 3). Two peptides that bind to inflamed synovial vasculature(105),(106) have been discovered via phage display and have shown promising targeting results after systemic administration in small animal models of rheumatoid arthritis(107),(108). The first peptide was discovered by screening the ability of peptides administered intravenously (IV) to specifically bind to the vasculature of human synovium grafted into immunodeficient mice(105). The resulting peptide (CKS: CKSTHDRLC) was later used by Wythe et al. to formulate a fusion protein formed by the anti-inflammatory cytokine IL-4 and the synovium-targeting peptide, and demonstrated that this construct elicited a biological response specifically into human synovium grafts implanted into immunodeficient mice compared to the scrambled control(107). To date, this peptide has not been used in pre-clinical models of OA. Another group reported the discovery of a peptide (ADK: CRNADKFPC) able to bind to inflamed synovial vasculature and showed that IV administration of ADK peptide in a rat model of adjuvant arthritis resulted in reduced inflammation scores, decreased T-cell trafficking and angiogenesis inhibition(106). Additionally systemic administration of ADK-functionalized liposomes loaded with the immunomodulatory cytokine IL-27 resulted in in vivo targeting of arthritic joints and significant reduction in rat paw inflammation compared to non-targeting liposomes or free IL-27 in a rat model of rheumatoid arthritis(108). Despite these results, the ADK peptide is also able to bind to inflamed skin(106) and the control scrambled sequence has not been characterized.
A different approach for synovium targeting was proposed by Mi et al., who discovered a peptide (HAP-1: SFHQFARATLAS) that directly binds to synoviocytes(109). HAP-1-functionalized liposomes loaded with prednisone(110) or an anti-inflammatory NF-κB-blocking peptide(111) showed promising results in terms of liposome localization into the arthritic joints and the reduction of rat paw inflammation after IV injection in a rat model of rheumatoid arthritis. Considering that synovium endothelium is not directly exposed to synovial fluid but synoviocytes are, the most promising strategy to target the synovial lining after IA injection could be HAP-1 peptide.
Multi-target therapy
Current understanding of OA pathology indicates that it is a complex, multi-factorial disease, which suggest that multi-target treatment may be a promising strategy to address the diverse mechanisms involved in OA progression. Although this idea has gained interest in the community, only few studies have explored the concept of multi-target therapy. One of the most investigated approaches is the use of dual-function lubricating drug-loaded nanoparticles(112). Fan et al. developed HA nano-micelles containing the inti-inflammatory molecule, curcumin. These nano-micelles exhibited low friction coefficient and reduced paw inflammation by 30% in a rat model of rheumatoid arthritis(113). Other researchers have focused on dual drug delivery to achieve multi-target therapies for OA treatment. Kang et al. developed chitosan-based thermoresponsive nanoparticles for independent delivery of kartogenin, a potent chondrogenic molecule, and diclofenac for pain and inflammation management(114). These particles induced chondrogenic differentiation of mesenchymal stem cells in vitro, slowed OA progression and reduced the concentration of cyclooxygenase-2 in serum and synovial fluid in a rat model of post-traumatic OA compared to a solution of free drugs. However, the effect of combinatorial treatment compared to mono-therapy was not evaluated(114). Moreover, Stone et al. demonstrated that IA combinatorial gene therapy using viral vectors expressing IL-1 receptor antagonist and lubricin induced the expression of anabolic and cartilage matrix genes, decreased the expression of catabolic and inflammatory mediators and provided significant cartilage protection compared to mono-therapy(115). Despite the advances in the development of combinatorial therapies, the use of tissue-specific drug delivery vehicles for multi-target treatment of OA is yet to be explored.
CONCLUSIONS AND FUTURE PERSPECTIVES
Current OA treatment strategies do not address the underlying joint degenerative processes and are ineffective at managing long-term pain. The lack of approved DMOADs has not only resulted in poor quality of life for OA patients, but has also made this disease a major cause of disability worldwide. Significant advances on elucidating OA etiology have moved the field forward in terms of developing promising DMOADs. However, much research is still needed in this regard. In addition to developing better DMOADs, there is an unmet need to design appropriate IA drug delivery vehicles that are able to increase drugs’ IA retention time and directly release these molecules into the tissues of interest. Different biomaterials have been proposed in order to overcome the limitations related to IA drug administration including hydrogels, nanoparticles, liposomes and microparticles. To date, there is only one FDA-approved drug formulation that utilizes a biomaterial-based drug delivery system for IA injection in OA patients, which consist of triamcinolone acetonide-loaded PLGA microparticles(80). Although microparticles generally present longer IA retention compared to other biomaterial-based formulations, extensive research on the use of different drug delivery vehicles, especially at a clinical level is still needed. Additionally, considering the complex nature of the disease, multi-target treatment strategies could represent a promising alternative to address the diverse underlying joint degenerative processes occurring in OA. In this regard, not only the development of appropriate IA drug delivery vehicles is imperative, but also the use of tissue-targeting strategies is essential. Future research on combinatorial drug delivery systems for the administration of therapeutic molecules with different IA tissue targets is still needed and could significantly contribute to the development of effective strategies for OA treatment.
ACKNOWLEDGEMENTS
The authors acknowledge Laura Daniela Mancipe Castro for her support in the artwork production. This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01AR062920 and S10OD016264 and the Department of Defense PRMRP Grant (PR171379).
REFERENCES
- 1.Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthr Cartil [Internet]. 2020. [cited 2020 Apr 9];28:242–8. Available from: 10.1016/j.joca.2020.01.002 [DOI] [PubMed] [Google Scholar]
- 2.Cisternas MG, Murphy L, Sacks JJ, Solomon DH, Pasta DJ, Helmick CG. Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey. Arthritis Care Res. 2016;68:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turkiewicz A, Petersson IF, Björk J, Hawker G, Dahlberg LE, Lohmander LS, Englund M. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthr Cartil. 2014;22:1826–32. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, Im H-J. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, Suter LG, Losina E, Katz J. The number of persons with symptomatic knee osteoarthritis in the United States: Impact of race/ethnicity, age, sex, and obesity HHS Public Access. Arthritis Care Res. 2016;68:1743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotlarz H, Gunnarsson CL, Fang H, Rizzo J a. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum. 2009;60:3546–53. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Jordan JM. Epidemiology of osteoarthritis [Internet]. Clin Geriatr Med NIH Public Access; 2010. [cited 2020 Jul 14]. Available from: /pmc/articles/PMC2920533/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maudens P, Jordan O, Allémann E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov Today. 2018;23:1761–75. [DOI] [PubMed] [Google Scholar]
- 9.Mora JC, Przkora R, Cruz-Almeida Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J Pain Res. 2018;11:2189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Machado GC, Eyles JP, Ravi V, Hunter DJ. Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis. Br J Sport Med. 2018;52:167–75. [DOI] [PubMed] [Google Scholar]
- 11.Formica FA, Barreto G, Zenobi-Wong M. Cartilage-targeting dexamethasone prodrugs increase the efficacy of dexamethasone. J Control Release. 2019;295:118–29. [DOI] [PubMed] [Google Scholar]
- 12.Janssen M, Timur UT, Woike N, Welting TJM, Draaisma G, Gijbels M, van Rhijn LW, Mihov G, Thies J, Emans PJ. Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J Control Release. 2016;244:30–40. [DOI] [PubMed] [Google Scholar]
- 13.Johal H, Devji T, Schemitsch EH, Bhandari M. Viscosupplementation in knee osteoarthritis: Evidence revisited. JBJS Rev. 2016;4:1–11. [DOI] [PubMed] [Google Scholar]
- 14.Jevsevar David, MD, MBA, Donnelly Patrick, MA,Brown Gregory A., MD, PhD, and Deborah S. Cummins P. Viscosupplementation for osteoarthritis of the knee: A Systematic Review of the Evidence. J Bone Jt Surg. 2015;97:2047–60. [DOI] [PubMed] [Google Scholar]
- 15.Antie WS Rutjes, Peter Jiini, Bruno R. da Costa, Sven Trelle, Eveline Niiesch SR, Background: Viscosupplementation for osteoarthritis of the knee. N Engl J Med. 2015;372:2569–70. [Google Scholar]
- 16.Goldring MB, Berenbaum F. Emerging Targets in Osteoarthritis Therapy. Curr Opin Pharmacol. 2015;22:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat Rev Rheumatol. 2013;9:400–10. [DOI] [PubMed] [Google Scholar]
- 18.Brown T, Laurent U, Fraser. Turnover of hyaluronan in synovial joints: elimination of labelled hyaluronan from the knee joint of the rabbit. Exp Physiol. 1991;76:125–34. [DOI] [PubMed] [Google Scholar]
- 19.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24–33. [DOI] [PubMed] [Google Scholar]
- 20.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–9. [DOI] [PubMed] [Google Scholar]
- 21.Andriacchi TP, Favre J, Erhart-Hledik JC, Chu CR. A Systems View of Risk Factors for Knee Osteoarthritis Reveals Insights into the Pathogenesis of the Disease. Ann Biomed Eng. 2015;43:376–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horisawa E, Kubota K, Tuboi I, Sato K, Yamamoto H, Takeuchi H, Kawashima Y. Size-Dependency of DL-Lactide/ Glycolide Copolymer Particulates for Intra-Articular Delivery System on Phagocytosis in Rat Synovium. Pharma. 2002;19. [DOI] [PubMed] [Google Scholar]
- 24.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. [DOI] [PubMed] [Google Scholar]
- 25.Pontes-Quero GM, García-Fernández L, Aguilar MR, San Román J, Pérez Cano J, Vázquez-Lasa B. Active viscosupplements for osteoarthritis treatment. Semin Arthritis Rheum. 2019;49:171–83. [DOI] [PubMed] [Google Scholar]
- 26.Qvist P, Bay-Jensen A-C, Christiansen C, Dam EB, Pastoureau P, Karsdal MA. The disease modifying osteoarthritis drug (DMOAD): Is it in the horizon? Pharmacol Res. 2008;58:1–7. [DOI] [PubMed] [Google Scholar]
- 27.Esser S, Bailey A. Effects of Exercise and Physical Activity on Knee Osteoarthritis. Curr Pain Headache Rep. 2011;15:423–30. [DOI] [PubMed] [Google Scholar]
- 28.Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: Evidence, hypotheses and horizons - a scoping review. Obes Rev [Internet]. Blackwell Publishing Ltd; 2014. [cited 2020 Jul 15];15:578–86. Available from: /pmc/articles/PMC4238740/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brien S, Prescott P, Coghlan B, Bashir N, Lewith G. Review Systematic review of the nutritional supplement Perna Canaliculus (green-lipped mussel) in the treatment of osteoarthritis. Q J Med [Internet]. 2008. [cited 2020 Jul 15];101:167–79. Available from: https://academic.oup.com/qjmed/article-abstract/101/3/167/1520706 [DOI] [PubMed] [Google Scholar]
- 30.Wandel S, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ, Reichenbach S, Trelle S. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: Network meta-analysis. Br Med J. 2010;341:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clegg DO, Reda DJ, Harris CL, Klein MA, O’dell JR, Hooper MM, Bradley JD, Bingham CO, Weisman MH, Jackson CG, Lane NE, Cush JJ, Moreland LW, Schumacher HR, Oddis CV, Wolfe F, Molitor JA, Yocum DE, Schnitzer TJ, Furst DE, Sawitzke AD, Shi H, Brandt KD, Moskowitz RW, Williams HJ, Veterans H. Glucosamine, Chondroitin Sulfate, and the Two in Combination for Painful Knee Osteoarthritis. N Engl J Med. 2006;354:795–808. [DOI] [PubMed] [Google Scholar]
- 32.Sawitzke AD, Shi H, Finco MF, Dunlop DD, Bingham CO, Harris CL, Singer NG, Bradley JD, Silver D, Jackson CG, Lane NE, Oddis CV., Wolfe F, Lisse J, Furst DE, Reda DJ, Moskowitz RW, Williams HJ, Clegg DO. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: A report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 2008;58:3183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.AGIBA AM. Nutraceutical Formulations Containing Glucosamine and Chondroitin Sulphate in the Treatment of Osteoarthritis: Emphasis on Clinical Efficacy and Formulation Challenges. Int J Curr Pharm Res. 2017;9:1. [Google Scholar]
- 34.Henrotin Y, Mathy M, Sanchez C, Lambert C. Chondroitin sulfate in the treatment of osteoarthritis: From in vitro studies to clinical recommendations [Internet]. Ther Adv Musculoskelet Dis. SAGE Publications; 2010. [cited 2020 Jul 16]. Available from: /pmc/articles/PMC3383492/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence. Osteoarthritis: Care and management in adults. National Institute for Health and Care Excellence; 2014. [PubMed] [Google Scholar]
- 36.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, Hawker G a., Henrotin Y, Hunter DJ, Kawaguchi H, Kwoh K, Lohmander S, Rannou F, Roos EM, Underwood M. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil. 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- 37.Pelletier JP, Martel-Pelletier J, Rannou F, Cooper C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum [Internet]. W.B. Saunders; 2016. [cited 2020 Jul 15];45:S22–7. Available from: 10.1016/j.semarthrit.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 38.Lin J, Zhang W, Jones A, Doherty M. Primary care Efficacy of topical non-steroidal anti-inflammatory drugs in the treatment of osteoarthritis: meta-analysis of randomised controlled trials. Br Med J. 2004;329:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review S.R. Osteoarthr Cartil. 2016;24:962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, Kroenke K, Bair MJ, Noorbaloochi S. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain the SPACE randomized clinical trial. J Am Med Assoc. 2018;319:872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richards MM, Maxwell JS, Weng L, Angelos MG, Golzarian J. Intra-articular Treatment of Knee Osteoarthritis: from Anti-inflammatories to Products of Regenerative Medicine. Physician Sport Med. 2016;44:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soma LR, Uboh CE, Liu Y, Li X, Robinson MA, Boston RC, Colahan PT. Pharmacokinetics of dexamethasone following intra-articular, intravenous, intramuscular, and oral administration in horses and its effects on endogenous hydrocortisone. J Vet Pharmacol Ther. 2013;36:181–91. [DOI] [PubMed] [Google Scholar]
- 44.Peterson Ralph E., Robert L. Black JJB. Disposition of intra-articularly injected cortisone and hydrocortisone. Arthritis Rheum. 1959;2:433–9. [DOI] [PubMed] [Google Scholar]
- 45.Berger RG, Yount WJ. Immediate “steroid flare” from intraarticular triamcinolone hexacetonide injection: Case report and review of the literature. Arthritis Rheum [Internet]. 2010. [cited 2020 Jul 16];33:1284–6. Available from: http://doi.wiley.com/10.1002/art.1780330833 [DOI] [PubMed] [Google Scholar]
- 46.Butoescu N, Jordan O, Doelker E. Intra-articular drug delivery systems for the treatment of rheumatic diseases: A review of the factors influencing their performance. Eur J Pharm Biopharm [Internet]. 2009. [cited 2017 Dec 18];73:205–18. Available from: https://ac.els-cdn.com/S0939641109001945/1-s2.0-S0939641109001945-main.pdf?_tid=e5c0cde4-e41f-11e7-8b49-00000aab0f02&acdnat=1513621311_75a0a515e0de3692e83d1ab7333ea5f2 [DOI] [PubMed] [Google Scholar]
- 47.Geiger Brett C., Grodzinsky Alan J. PTH. Designing Drug Delivery Systems for Articular Jointst. Chem Eng Prog. 2018;114:46–51. [Google Scholar]
- 48.Grothe K, Flechsenhar K, Paehler T, Ritzeler O, Beninga J, Saas J, Herrmann M, Rudolphi K. IκB kinase inhibition as a potential treatment of osteoarthritis – results of a clinical proof-of-concept study. Osteoarthr Cartil. 2017;25:46–52. [DOI] [PubMed] [Google Scholar]
- 49.Lohmander LS, Hellot S, Dreher D, Krantz EFW, Kruger DS, Guermazi A, Eckstein F. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: A randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014;66:1820–31. [DOI] [PubMed] [Google Scholar]
- 50.Kang ML, Ko J-Y, Kim JE, Im G-I. Intra-articular delivery of kartogenin-conjugated chitosan nano/ microparticles for cartilage regeneration. Biomaterials. 2014;35:9984–94. [DOI] [PubMed] [Google Scholar]
- 51.Mohan G, Magnitsky S, Melkus G, Subburaj K, Kazakia G, Burghardt AJ, Dang A, Lane NE, Majumdar S. Kartogenin treatment prevented joint degeneration in a rodent model of osteoarthritis: A pilot study. J Orthop Res. 2016;34:1780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ono Y, Ishizuka S, Knudson CB, Knudson W. Chondroprotective Effect of Kartogenin on CD44-Mediated Functions in Articular Cartilage and Chondrocytes. Cartilage. 2014;5:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hee Jeon O, Kim C, Laberge R-M, Demaria M, Rathod S, Vasserot AP, Wook Chung J, Hun Kim D, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia C, Chen P, Mei S, Ning L, Lei C, Wang J, Zhang J, Ma J, Fan S. Photo-crosslinked HAMA hydrogel with cordycepin encapsulated chitosan microspheres for osteoarthritis treatment [Internet]. Oncotarget 2017. Available from: www.impactjournals.com/oncotarget/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Persson MSM, Sarmanova A, Doherty M, Zhang W. Meta-analysis Conventional and biologic disease-modifying anti-rheumatic drugs for osteoarthritis: a meta-analysis of randomized controlled trials. Rheumatology. 2018;57:1830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kavanaugh TE, Werfel TA, Cho H, Hasty KA, Duvall CL. Particle Based Technologies for Osteoarthritis Detection and Therapy. Drug Deliv Transl Res. 2016;6:132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Owen S, Francis H, Roberts M. Disappearance kinetics of solutes from synovial fluid after intra‐ articular injection. Br J Clin Pharmacol. 1994;38:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elmquist WF, Chan RJS KKH. Synovial mean transit time of diclofenac and other nonsteroidal antiinflammatory drugs. Pharm Res. 1994;11:1689–97. [DOI] [PubMed] [Google Scholar]
- 60.Simkin PA, Wu MP, Foster DM. Articular Pharmacokinetics of Protein-Bound Antirheumatic Agents. Clin Pharmacokinet. 1993;25:342–50. [DOI] [PubMed] [Google Scholar]
- 61.Wigginton SM, Chu BCF, Weisman MH, Howell SB. Methotrexate pharmacokinetics after intraarticular injection in patients with rheumatoid arthritis. Arthritis Rheum. 1980;23:119–22. [DOI] [PubMed] [Google Scholar]
- 62.Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, García AJ. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials. 2012;33:7665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh A, Agarwal R, Diaz-Ruiz CA, Willett NJ, Wang P, Andrew Lee L, Wang Q, Guldberg RE, García AJ. Nano-engineered particles for enhanced intra-articular retention and delivery of proteins. Adv Heal Mater. 2014;3:1562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmieri B, Rottigni V, Iannitti T. Preliminary study of highly cross-linked hyaluronic acid-based combination therapy for management of knee osteoarthritis-related pain. Drug Des Devel Ther. 2013;7:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan Woong Park, Kyung Wan Ma, Sun Woo Jang, Miwon Son MJK Comparison of Piroxicam Pharmacokinetics and Anti-Inflammatory Effect in Rats after intra-Articular and Intramuscular Administration. Biomol Ther (Seoul). 2014;22:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshioka K, Yasuda Y, Kisukeda T, Nodera R, Tanaka Y, Miyamoto K. Pharmacological effects of novel cross-linked hyaluronate, Gel-200, in experimental animal models of osteoarthritis and human cell lines. Osteoarthr Cartil. 2014;22:879–87. [DOI] [PubMed] [Google Scholar]
- 67.Petit A, Redout EM, Van De Lest CH, De Grauw JC, Müller B, Meyboom R, Van Midwoud P, Vermonden T, Hennink WE, Ren E Van Weeren P. Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PEG-PCLA triblock copolymers in horses. Biomaterials. 2015;53:426–36. [DOI] [PubMed] [Google Scholar]
- 68.Samad A, Sultana Y, Aqil M, Sultana Y, Aqil M. Liposomal Drug Delivery Systems: An Update Review. Curr Drug Deliv. 2007;4:297–305. [DOI] [PubMed] [Google Scholar]
- 69.Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia X. Hyaluronic Acid-Based Hydrogels: from a Natural Polysaccharide to Complex Networks. Soft Matter. 2012;8:3280–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Woo Joo S, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edwards SHR, Cake MA, Spoelstra G, Read RA. Biodistribution and Clearance of Intra-articular Liposomes in a Large Animal Model Using a Radiographic Marker. J Liposome Res. 2007;17:249–61. [DOI] [PubMed] [Google Scholar]
- 72.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeter polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;29:1883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paździor M, Kiełczykowska M, Kurzepa J, Luchowska-kocot D, Kocot J, Musik I. The oxidative stress in knee osteoarthritis patients. An attempt of evaluation of possible compensatory effects occurring in the disease development. Med [Internet]. MDPI AG; 2019. [cited 2020 Jul 16];55. Available from: /pmc/articles/PMC6572222/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Natsume T, Yoshimoto M. Membrane Permeability and Stability of Liposomes Suspended in Shear Flow. J Dispers Sci Technol [Internet]. 2013. [cited 2020 Jul 16];34:1557–62. Available from: https://www.tandfonline.com/action/journalInformation?journalCode=ldis20 [Google Scholar]
- 75.Pradal J, Maudens P, Gabay C, Seemayer CA, Jordan O, Allémann E. Effect of particle size on the biodistribution of nano- and microparticles following intra-articular injection in mice. Int J Pharm. 2016;498:119–29. [DOI] [PubMed] [Google Scholar]
- 76.Morgen M, Tung D, Boras B, Miller W, Malfait A-M, Tortorella M, Morgen M, Miller W, Tung D, Boras B, Malfait A-M, Tortorella M. Nanoparticles for Improved Local Retention after Intra-Articular Injection into the Knee Joint. Pharm Res. 2013;30:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arunkumar P, Indulekha S, Vijayalakshmi S, Srivastava R. Synthesis, characterizations, in vitro and in vivo evaluation of Etoricoxib-loaded Poly (Caprolactone) microparticles-a potential Intra-articular drug delivery system for the treatment of Osteoarthritis. J Biomater Sci. 2016;27:303–16. [DOI] [PubMed] [Google Scholar]
- 78.Janssen Maarten, Timur Ufuk Tan, Woike Nina, Welting Tim J M, Draaisma Guy, Gijbels Marion, van Rhijn Lodewijk W, Mihov George, PJE Jens Thies. Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J Control Release. 2016;244:30–40. [DOI] [PubMed] [Google Scholar]
- 79.Flexion Therapeutics. ZILRETTA® (triamcinolone acetonide extended-release injectable suspension) [Internet]. [cited 2019 Jan 18]. Available from: https://flexiontherapeutics.com/our-product/
- 80.Conaghan PG, Hunter DJ, Cohen SB, Kraus VB, Berenbaum F, Lieberman JR, Jones DG, Spitzer AI, Jevsevar DS, Katz NP, Burgess DJ, Lufkin J, Johnson JR, Bodick N. Effects of a Single Intra-Articular Injection of a Microsphere Formulation of Triamcinolone Acetonide on Knee Osteoarthritis Pain. J Bone Jt Surg. 2018;100:666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suh J-K, Muzzonigro TS, Fu FH. Injury and Repair of Articular Cartilage: Related Scientific Issues. Oper Tech Orthop. 1997;7:270–8. [Google Scholar]
- 82.Hochberg MC, Tive LA, Abramson SB, Vignon E, Verburg KM, West CR, Smith MD, Hungerford DS. When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol. 2016;68:382–91. [DOI] [PubMed] [Google Scholar]
- 83.Bakker AC, Van De Loo FAJ, Van Beuningen HM, Sime P, Van Lent PLEM, Van Der Kraan PM, Richards CD, Van Den Berg WB. Overexpression of active TGF-beta-1 in the murine knee joint: evidence for synovial-layer-dependent chondro-osteophyte formation. Osteoarthr Cartil. 2001;9:128–36. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J, Wang JHC. Kartogenin induces cartilage-like tissue formation in tendon-bone junction. Bone Res. 2014;2:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi D, Xu X, Ye Y, Song K, Cheng Y, Di J, Hu Q, Li J, Ju H, Jiang Q, Gu Z. Photo-Cross-Linked Scaffold with Kartogenin- Encapsulated Nanoparticles for Cartilage Regeneration. ACS Nano. 2016;10:1292–9. [DOI] [PubMed] [Google Scholar]
- 86.Kang M-L, Jeong S-Y, Im G-I. Hyaluronic Acid Hydrogel Functionalized with Self-Assembled Micelles of Amphiphilic PEGylated Kartogenin for the Treatment of Osteoarthritis. Tissue Eng Part A. 2017;23:630–9. [DOI] [PubMed] [Google Scholar]
- 87.Li X, Ding J, Zhang Z, Yang M, Yu J, Wang J, Chang F, Chen X. Kartogenin-Incorporated Thermogel Supports Stem Cells for Significant Cartilage Regeneration. ACS Appl Mater Interfaces. 2016;8:5148–59. [DOI] [PubMed] [Google Scholar]
- 88.Bajpayee AG, Grodzinsky AJ. Cartilage-targeting drug delivery: can electrostatic interactions help? [Internet]. Nat Publ Gr. 2017. Available from: www.nature.com/nrrheum [DOI] [PubMed] [Google Scholar]
- 89.Brown SB, Wang L, Jungels RR, Sharma B. Effects of cartilage-targeting moieties on nanoparticle biodistribution in healthy and osteoarthritic joints. Acta Biomater. 2020;101:469–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vedadghavami A, Wagner EK, Mehta S, He T, Zhang C, Bajpayee AG. Cartilage Penetrating Cationic Peptide Carriers for Applications in Drug Delivery to Avascular Negatively Charged Tissues. Acta Biomater. 2019;93:258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown S, Pistiner J, Adjei IM, Sharma B, Crayton Pruitt J. Nanoparticle Properties for Delivery to Cartilage: The Implications of Disease State, Synovial Fluid, and Off-Target Uptake. Mol Pharm [Internet]. 2019. [cited 2020 Jul 17];16:469–79. Available from: https://pubs.acs.org/sharingguidelines [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cook Sangar ML, Girard EJ, Hopping G, Yin C, Pakiam F, Brusniak M-Y, Nguyen E, Ruff R, Gewe MM, Byrnes-Blake K, Nairn NW, Miller DM, Mehlin C, Strand AD, Mhyre AJ, Correnti CE, Strong RK, Simon JA, Olson JM. A potent peptide-steroid conjugate accumulates in cartilage and reverses arthritis without evidence of systemic corticosteroid exposure. Sci Transl Med [Internet]. 2020. [cited 2020 Jul 17];12:1041. Available from: http://stm.sciencemag.org/ [DOI] [PubMed] [Google Scholar]
- 93.Geiger BC, Wang S, Padera RF, Grodzinsky AJ, Hammond PT. N A N O M E D I C I N E Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci Transl Med [Internet]. 2018. [cited 2020 Jul 17];10. Available from: http://stm.sciencemag.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan H, Duan X, Pan H, Holguin N, Rai MF, Akk A, Springer LE, Wickline SA, Sandell LJ, Pham CTN. Suppression of NF-κB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. PNAS [Internet]. 2016. [cited 2020 Jul 17]; Available from: www.pnas.org/cgi/doi/10.1073/pnas.1608245113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hughes C, Faurholm B, Dell’Accio F, Manzo A, Seed M, Eltawil N, Marrelli A, Gould D, Subang C, Al-Kashi A, De Bari C, Winyard P, Chernajovsky Y, Nissim A. Human single-chain variable fragment that specifically targets arthritic cartilage. Arthritis Rheum. 2010;62:1007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho H, Pinkhassik E, David V, Stuart JM, Hasty KA. Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model. Nanomedicine Nanotechnology, Biol Med. 2015;11:939–46. [DOI] [PubMed] [Google Scholar]
- 97.Cho Hongsik, Kim Byoung Ju, Sang-Hyug Park, Hasty Karen A HM B. Noninvasive visualization of early osteoarthritic cartilage using targeted nanosomes in a destabilization of the medial meniscus mouse model. Int J Nanomedicine. 2018;13:1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bedingfield Sean K, Yu Fang, Liu Danielle D., Jackson Meredith A., Himmel Lauren E., Cho Hongsik, Colazo Juan M., Crofford Leslie J., Hasty Karen A. D CL. Matrix-targeted Nanoparticles for MMP13 RNA Interference Blocks Post-Traumatic Osteoarthritis. 2020;1–54. [Google Scholar]
- 99.Pi Y, Zhang X, Shi J, Zhu J, Chen W, Zhang C, Gao W, Zhou C, Ao Y. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials. 2011;32:6324–32. [DOI] [PubMed] [Google Scholar]
- 100.Cheung CSF, Lui JC, Baron J. Identification of Chondrocyte-Binding Peptides by Phage Display. J Orthop Res. 2013;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rothenfluh DA, Bermudez H, O ‘neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7:248–54. [DOI] [PubMed] [Google Scholar]
- 102.Hu H-Y, Lim N-H, Juretschke H-P, Ding-Pfennigdorff D, Florian P, Kohlmann M, Kandira A, Peter Von Kries J, Saas J, Rudolphi KA, Wendt KU, Nagase H, Plettenburg O, Nazare M, Schultz C. In vivo visualization of osteoarthritic hypertrophic lesions. Chem Sci. 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen H, Qin Z, Zhao J, He Y, Ren E, Zhu Y, Liu G, Mao C, Zheng L. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials. Elsevier Ltd; 2019;225. [DOI] [PubMed] [Google Scholar]
- 104.Yi W, Zhou H, Li A, Yuan Y, Guo Y, Li P, Qi B, Xiao Y, Yu A, Hu X. A NIR-II fluorescent probe for articular cartilage degeneration imaging and osteoarthritis detection. Biomater Sci. 2019;7:1043. [DOI] [PubMed] [Google Scholar]
- 105.Lee L, Buckley C, Blades MC, Panayi G, George AJT. Identification of Synovium-Specific Homing Peptides by In Vivo Phage Display Selection 2002;46:2109–20. [DOI] [PubMed] [Google Scholar]
- 106.Yang Y-H, Rajaiah R, Ruoslahti E, Moudgil KD. Peptides targeting inflamed synovial vasculature attenuate autoimmune arthritis. Proc Natl Acad Sci U S A. 2011;108:12857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wythe SE, Dicara D, Taher TEI, Finucane CM, Jones R, Bombardieri M, Man YKS, Nissim A, Mather SJ, Chernajovsky Y, Pitzalis C. Targeted delivery of cytokine therapy to rheumatoid tissue by a synovial targeting peptide. Ann Rheum Dis. 2013;72:129–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meka RR, Venkatesha SH, Moudgil KD. Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis. J Control Release. Elsevier B.V.; 2018;286:279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mi Z, Lu X, Mai JC, Ng BG, Wang G, Lechman ER, Watkins SC, Rabinowich H, Robbins PD. Identification of a synovial fibroblast-specific protein transduction domain for delivery of apoptotic agents to hyperplastic synovium. Mol Ther. The American Society of Gene Therapy; 2003;8:295–305. [DOI] [PubMed] [Google Scholar]
- 110.Vanniasinghe AS, Manolios N, Schibeci S, Lakhiani C, Kamali-Sarvestani E, Sharma R, Kumar V, Moghaddam M, Ali M, Bender V. Targeting fibroblast-like synovial cells at sites of inflammation with peptide targeted liposomes results in inhibition of experimental arthritis. Clin Immunol. Elsevier Inc.; 2014;151:43–54. [DOI] [PubMed] [Google Scholar]
- 111.You C, Zu J, Liu X, Kong P, Song C, Wei R, Zhou C, Wang Y, Yan J. Synovial fibroblast-targeting liposomes encapsulating an NF-κB-blocking peptide ameliorates zymosan-induced synovial inflammation. J Cell Mol Med. 2018;22:2449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ji X, Zhang H. Current Strategies for the Treatment of Early Stage Osteoarthritis. Front Mech Eng [Internet]. Frontiers Media SA; 2019. [cited 2020 Jul 17];5:57. Available from: www.frontiersin.org [Google Scholar]
- 113.Fan Z, Li J, Liu J, Jiao H, Liu B. Anti-Inflammation and Joint Lubrication Dual Effects of a Novel Hyaluronic Acid/Curcumin Nanomicelle Improve the Efficacy of Rheumatoid Arthritis Therapy. ACS Appl Mater Interfaces [Internet]. 2018. [cited 2020 Jul 17];10:23595–23604. Available from: www.acsami.org [DOI] [PubMed] [Google Scholar]
- 114.Kang ML, Kim JE, Im G Il. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater Elsevier Ltd; 2016;39:65–78. [DOI] [PubMed] [Google Scholar]
- 115.Stone A, Grol MW, Ruan ZC Merry, Dawson B, Chen Y, Jiang M-M, Song I-W, Jayaram P, Cela R, Gannon F, Lee BHL. Combinatorial Prg4 and Il-1ra Gene Therapy Protects Against Hyperalgesia and Cartilage Degeneration in Post-Traumatic Osteoarthritis. Hum Gene Ther [Internet]. 2019. [cited 2020 Jul 17];30:225–35. Available from: www.liebertpub.com [DOI] [PMC free article] [PubMed] [Google Scholar]


