Abstract
Human epidermal melanocytes play a central role in sensing the environment and protecting the skin from the drastic effects of solar ultraviolet radiation and other environmental toxins or inflammatory agents. Melanocytes survive in the epidermis for decades, which subjects them to chronic environmental insults. Melanocytes have a poor self-renewal capacity; therefore, it is critical to ensure their survival with genomic stability. The function and survival of melanocytes is regulated by an elaborate network of paracrine factors synthesized mainly by epidermal keratinocytes and dermal fibroblasts. A symbiotic relationship exists between epidermal melanocytes and keratinocytes on the one hand, and between melanocytes and dermal fibroblasts on the other hand. Melanocytes protect epidermal keratinocytes and dermal fibroblasts from the damaging effects of solar radiation, and the latter cells synthesize biochemical mediators that maintain the homeostasis, and regulate the stress response of melanocytes. Disruption of the paracrine network results in pigmentary disorders, due to abnormal regulation of melanin synthesis, and compromise of melanocyte survival or genomic stability. This review provides an update of the current knowledge of keratinocyte- and fibroblast-derived paracrine factors and their contribution to melanocyte physiology, and how their abnormal production is involved in the pathogenesis of common pigmentary disorders.
Keywords: DNA damage response, melanocytes, paracrine factors, pigmentary disorders, solar UV
1 ∣. INTRODUCTION
By synthesizing the pigment melanin, human epidermal melanocytes regulate important cutaneous and extra-cutaneous physiological functions. Pigmentation is thought to be important for inhibiting the degradation of folic acid, which is critical for normal fetal neuronal development during pregnancy (Jablonski & Chaplin, 2000). Also, pigmentation is proposed to contribute to proper barrier function of skin to prevent transdermal water loss and invasion by pathogens (Man et al., 2014). Epidermal melanocytes play a major role in protecting the skin from the drastic effects of solar ultraviolet radiation (UV), mainly photocarcinogenesis and photoaging (Gilchrest et al., 1999; Gilchrest & Rogers, 1993). Sunburn can instigate melanocyte apoptosis, resulting in vitiligo, and sun exposure can cause hyperpigmentary disorders, such as melasma (Manga et al., 2016; Rajanala et al., 2019). Additionally, severe sunburns, particularly during the childhood or adolescence years, can initiate melanomagenesis (Green et al., 2011). Melanin synthesized by melanocytes serves as a physical barrier that reduces the penetration of solar UV rays through the epidermal layers (Kaidbey et al., 1979). Additionally, eumelanin, the dark brown pigment, is a scavenger of reactive oxygen species (ROS), which can cause oxidative damage to cellular DNA, proteins, and lipids (Bustamante et al., 1993; Meredith & Sarna, 2006). High constitutive melanin content and the ability to tan, as in skin phototypes III-VI, are associated with low risk for sun-induced non-melanoma skin cancers and melanoma and reduced photoaging (Halder & Bridgeman-Shah, 1995; Lopes et al., 2021). However, these phototypes are more prone to post-inflammatory hyperpigmentation (Davis et al., 2012). Unlike epidermal keratinocytes and dermal fibroblasts, melanocytes are unique in their low proliferation capacity and longevity for decades in the skin. Their long life span subjects melanocytes to chronic exposure to environmental stressors that can impact their function, survival, and/or genomic stability. Given the significance of melanocytes in normal skin physiology, it is highly critical to understand the mechanisms that maintain their homeostasis.
2 ∣. PARACRINE REGULATION OF MELANOCYTES BY KERATINOCYTES
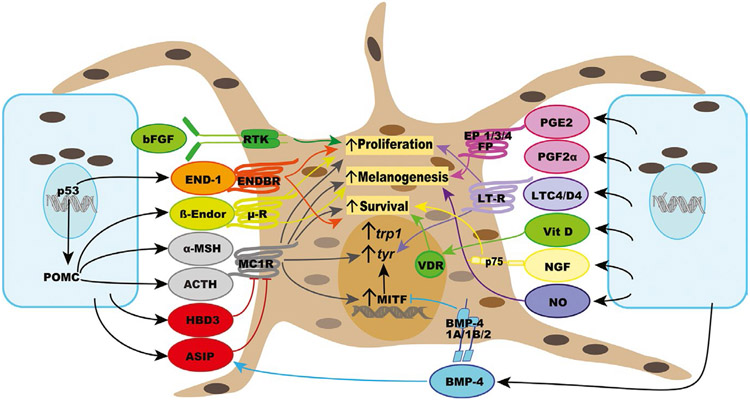
Melanocytes communicate physically with keratinocytes by the transfer of melanin-containing melanosomes, a mechanism that confers even skin pigmentation and photoprotection. Melanosomes in keratinocytes form supranuclear caps that protect genomic DNA from damage caused by impinging solar UV rays (Kobayashi et al., 1998). In turn, keratinocytes reciprocate by producing factors that regulate melanin synthesis, as well as survival, proliferation, and genomic stability of melanocytes (Figures 1 and 2). Early evidence for the role of keratinocyte-derived factors in regulating melanocytes was provided by the observation that media conditioned by cultured human keratinocytes stimulate the proliferation and melanogenesis of human melanocytes in vitro (Gordon et al., 1989). One of the first identified keratinocyte-derived paracrine factors for melanocytes was basic fibroblast growth factor (bFGF), which has a mitogenic effect on melanocytes via activating a specific tyrosinase kinase receptor and has been proposed to be “the natural growth factor for melanocytes in vivo” (Halaban et al., 1988; Pittelkow & Shipley, 1989). Basic FGF is associated with keratinocytes and is not secreted, and its synthesis is upregulated by UV radiation. Given the mitogenic effect of bFGF, it is commonly used in melanocyte growth media to promote the proliferation of melanocytes in vitro (Abdel-Malek et al., 1995; Swope et al., 1995).
FIGURE 1.
List of the major paracrine factors synthesized by keratinocytes, their receptors, and biological effects on melanocyte survival, proliferation, and melanogenesis
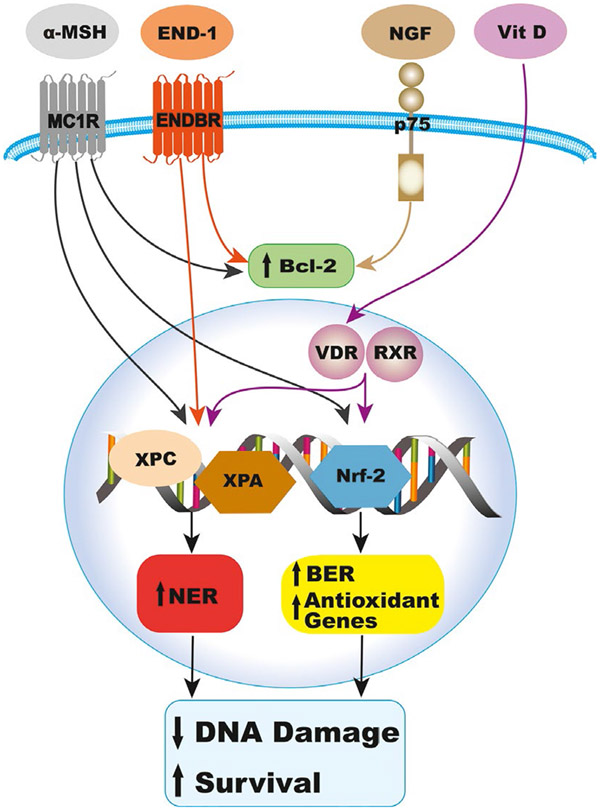
FIGURE 2.
Participation of α-MSH, End-1, NGF, and vitamin D in the DNA damage response to UV, which results in reduced DNA photoproducts by activating NER, oxidative DNA damage by upregulating BER and antioxidant genes, and apoptosis, by increasing Bcl2 levels, thereby maintaining melanocyte survival and genomic stability. BER, base excision repair; NER, nucleotide excision repair
The primary cytokine IL-1α indirectly regulates melanocytes by upregulating the synthesis of several paracrine factors by keratinocytes, such as α-melanocyte-stimulating hormone (α-melanocortin α-MSH) and endothelin-1 (End-1), and by fibroblasts, such as keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), and stem cell factor (SCF), as described below.
Exposure to UV increases the synthesis of the immune-inflammatory cytokine interleukin (IL)-1α, IL-6, and tumor necrosis factor (TNF)-α by keratinocytes (Chung et al., 1996; Kock et al., 1990). These cytokines inhibit melanogenesis of cultured human melanocytes (Swope et al., 1991). The balance between the levels and effects of these cytokines and those of the factors upregulated by IL-1α determines the final outcome on pigmentation.
α-Melanocyte-stimulating hormone has long been known as the physiological regulator of integumental pigmentation of many vertebrate species (Sawyer et al., 1983). Pro-opiomelanocortin (POMC), the precursor peptide of melanocortins and other bioactive peptides, including β-endorphin, is expressed by human keratinocytes, as well as melanocytes, and its gene expression is upregulated upon UV exposure by the transcription factor p53 (Bigliardi-Qi et al., 2004; Chakraborty et al., 1996; Corre et al., 2004; Cui et al., 2007; Wakamatsu et al., 1997). Given the abundance of keratinocytes relative to melanocytes in the epidermis, it is reasonable to assume that keratinocytes are the major source of POMC. The expression of POMC in epidermal cells is regulated by corticotropin-releasing hormone (CRH), which is expressed in keratinocytes and melanocytes (Slominski et al., 1995, 2006). The synthesis of CRH and expression of its receptors in melanocytes and other skin types suggest that an equivalent of the hypothalamic/pituitary/axis exists in the skin. Binding of CRH to CRH receptor-1 expressed on melanocytes increases cAMP levels (Slominski et al., 2005), which is expected to stimulate melanogenesis. However, a direct effect of CRH on melanogenesis has not been reported. Keratinocytes express the enzymes pro-convertases I and II that process POMC to its bioactive products, resulting in the synthesis and secretion of α-MSH and adrenocorticotropic hormone (ACTH), which are increased upon UV exposure and treatment with IL-1α (Chakraborty et al., 1996; Rousseau et al., 2007; Wakamatsu et al., 1997). Human melanocytes respond to both α-MSH and ACTH with stimulation of melanogenesis, dendrite formation, and proliferation (Abdel-Malek et al., 1995). Both peptides bind the melanocortin 1 receptor (MC1R), a Gs protein-coupled receptor, with the same affinity, and transcriptionally upregulate the expression of the MC1R (Abdel-Malek et al., 1995; Scott et al., 2002; Swope et al., 2012). The increase in dendrites in response to α-MSH and other melanogenic factors facilitates the transfer of mature melanosomes from melanocytes to keratinocytes, thereby increasing epidermal pigmentation. Rab proteins play important roles in melanosome biogenesis (Rab 7), trafficking of tyrosinase and TRP-1 (Rab3, 2, and 38), dendrite formation (Rab 21), and melanosome transport (Rab 27a) (Ohbayashi & Fukuda, 2012). The cAMP pathway, the main signaling pathway for MC1R, promotes melanosome trafficking and transfer via increasing Rab27a, Myosin Va, and Cdc42 (Lv et al., 2019). Treatment of human melanocytes with α-MSH enhances their adhesion, a potential mechanism to maintain the anchorage of melanocytes to the basement membrane in vivo (Scott et al., 1997). β-Endorphin is another POMC derivative synthesized by keratinocytes and stimulates melanocyte proliferation, dendricity, and melanogenesis, via activating the μ opiate receptor, a G0/Gi-coupled receptor (Bigliardi-Qi et al., 2004; Kauser et al., 2003). These results underscore the significance of POMC-derived bioactive peptides in regulating human pigmentation.
The MC1R is the major regulator of pigmentation, stimulating eumelanin synthesis upon activation by its agonists α-MSH and ACTH in human melanocytes, via increasing microphthalmia-associated transcription factor (MITF), the master transcriptional regulator in melanocytes, tyrosinase, and tyrosinase-related protein (TRP) 1 levels, and the activity of tyrosinase (Abdel-Malek et al., 1995; Hunt et al., 1994, 1995; Kadekaro et al., 2005). Eumelanin synthesis is inhibited when MC1R is bound by its physiological antagonist agouti signaling protein (ASIP) and human β-defensin 3 (HBD3) (Sakai et al., 1997; Suzuki et al., 1997; Swope et al., 2012). The latter is an antimicrobial peptide synthesized by keratinocytes, and mutation in HBD3 gene was reported to result in black coat color in dogs (Candille et al., 2007). ASIP is reported to be an inverse agonist, while HBD3 is a neutral agonist of MC1R (Nix et al., 2013; Siegrist et al., 1997). Overexpression of CBD103, the ortholog of HBD3 in mice mimicked the effect of α-MSH, resulting in a black coat color (Candille et al., 2007). When tested in vitro, CDB103 did not increase cAMP levels, and similarly, HBD3 alone had no effect on basal cAMP levels in cultured human melanocytes (Candille et al., 2007; Swope et al., 2012). Based on the in vivo effects of CBD103, it was suggested that it inhibits the binding of ASIP to MC1R, thereby maintaining a high basal level of MC1R signaling. When tested on cultured human melanocytes, ASIP competitively inhibited the binding of α-MSH to MC1R, and both ASIP and HBD3 antagonized the α-MSH-activated MC1R signaling via blocking the increase in cAMP (Suzuki et al., 1997; Swope et al., 2012), thereby inhibiting eumelanin synthesis, allowing only for the synthesis of pheomelanin. The apparent discrepancy between the results reported on the effects of HBD3 (Candille et al., 2007; Swope et al., 2012) could be explained by the conclusions of that HBD3 has no effect on the relative balance between active and inactive conformations of MC1R, but causes the melanocortin-binding pocket to be less accessible to either stimulatory or inhibitory ligand (Nix et al., 2013).
A third factor that antagonizes the effects of α-MSH is bone morphogenic protein-4 (BMP-4), a member of the tumor growth factor (TGF)-β superfamily (Yaar et al., 2006). Melanocytes express the BMP-4 receptors 1A, 1B, and 2, and respond directly to BMP-4 by inhibition of melanogenesis, evident as reduced levels of tyrosinase, TRP1, and MC1R (Park et al., 2009). Also, BMP-4 negatively regulates pigmentation indirectly by enhancing the expression of ASIP by fibroblasts and keratinocytes (Sharov et al., 2005). Irradiation of melanocytes with UV downregulates the expression of BMP-4 receptor 1B, thereby inhibiting the effect of BMP-4 and potentiating the melanogenic response to UV.
Endothelin-1 (End-1) is a potent keratinocyte-derived mitogen for human melanocytes, which binds the endothelin B receptor (ENDBR), a Gq-coupled receptor that activates protein kinase C (PKC) and calcium mobilization (Imokawa et al., 1992; Tada et al., 1998). Endothelin-1 (End-1) stimulates the migration of melanocytes, and during embryonic development, the expression of the related End-3 and ENDBR is critical for melanoblast migration from the neural crest and for their survival en route to the epidermis (Baynash et al., 1994; Puffenberger et al., 1994; Scott et al., 1997). Synthesis of End-1 is upregulated upon irradiation of keratinocytes with UV and by treatment of keratinocytes with IL-1α (Imokawa et al., 1992), and its gene is transcriptionally regulated by p53 (Hyter et al., 2013). Endothelin-1 interacts synergistically with α-MSH and bFGF to promote the proliferation of human melanocytes in vitro (Swope et al., 1995) and upregulates the expression of MC1R, to enhance, and/or maintain the response of melanocytes to α-MSH and ACTH (Swope et al., 2012).
The eicosanoid prostaglandins (PG) and leukotrienes (LT) are lipid signaling factors derived from the metabolism of arachidonic acid via the cyclooxygenase (COX) and lipoxygenase pathways, respectively. The main keratinocyte-derived prostaglandins are PGE2 and PGF2α, which are also synthesized by melanocytes, and their production is increased upon UV exposure (Scott et al., 2004, 2005). The expression of COX-2 seems to be upregulated by α-MSH, thereby increasing the production of prostaglandins (Kim et al., 2012). Human melanocytes express the PGE2 receptors EP1, EP3, and EP4, which couple to different G proteins, and respond to PGE2 with increased cAMP levels, and filopodia formation that drives melanosome transfer to keratinocytes (Scott et al., 2004; Starner et al., 2010). Human melanocytes also express the PGF2α receptor FP that is upregulated upon UV exposure and respond to PGF2α with increased melanogenesis and dendricity (Scott et al., 2005; Starner et al., 2010). These results provide evidence that PGE2 and PGF2α are inducible paracrine/autocrine regulators of melanocytes. Epidermal cells have the capacity to transform leukotriene (LT)A4 synthesized by myeloid cells, such as neutrophils, to LTC4 and LTD4, which are potent mitogens for melanocytes (Iversen et al., 1994; Morelli et al., 1989), and LTC4 increases dendricity and tyrosinase levels in melanocytes (Tomita et al., 1992).
Exposure to UV stimulates the production of nitric oxide (NO) in keratinocytes, as well as melanocytes, via increased constitutive NO synthase (Romero-Graillet et al., 1996, 1997). Treatment of melanocytes with NO donors stimulates melanogenesis and dendrite formation via activating cGMP production, while treatment with NO scavengers inhibits the melanogenic effect of UV. In human melanocytes, NO increases the expression of tyrosinase and the synthesis of eumelanin (Lassalle et al., 2003; Sasaki et al., 2000). In mouse and human melanoma cells, treatment with α-MSH was found to augment UV-induced NO levels, and treatment with NO was shown to stimulate melanogenesis (Tsatmali et al., 2000). These results suggest that the melanogenic effect of α-MSH is mediated at least in part by increased NO production by melanocytes. While the role of cAMP in stimulating melanogenesis is established, the potential role of cGMP needs to be further explored.
Keratinocyte-derived paracrine factors modulate the stress response of melanocytes, maintaining their survival, proper function, and genomic stability (Figure 2). Melanocytes co-cultured with keratinocytes or incorporated into reconstructed epidermis respond more avidly to UVB irradiation than melanocytes in mono-culture by robust increase in pigmentation (Duval et al., 2001). These results suggest that the tanning response to UVB is modulated by keratinocyte-derived factors. It is established that α-MSH, and by analogy ACTH, via activation of MC1R and its cAMP signaling pathway, modulates the DNA damage response (DDR) of melanocytes (Bohm et al., 2005; Kadekaro et al., 2010; Swope et al., 2014, 2020). α-MSH and ACTH are survival factors for melanocytes, as they reduce melanocyte apoptosis resulting from extensive UVB-induced DNA damage. Both α-MSH and ACTH activate Akt, which inhibits the pro-apoptotic protein Bad and thereby increasing the levels of the anti-apoptotic Bcl2 (Bohm et al., 2005; Kadekaro et al., 2010). The activation of the cAMP pathway by α-MSH promotes repair of UVB-induced DNA photoproducts by enhancing nucleotide excision repair (NER), via activating the DNA damage sensors ataxia-telangiectasia related (ATR) and ataxia-telangiectasia mutated (ATM), and promoting the chromatin localization of the NER proteins XPC, the DNA damage recognition protein, and XPA, the DNA damage verification protein, resulting in enhanced repair of cyclobutane pyrimidine dimers (CPD), the major form of DNA photoproducts (Swope et al., 2020). α-MSH also reduces ROS generation and the resulting oxidative stress (Kadekaro et al., 2012; Song et al., 2009). The antioxidant effects of α-MSH are mediated by the activation of the transcription factors nuclear factor erythroid 2-related factor 2 (Nrf2) and p53, major regulators of the antioxidant response, which upregulate the expression of target antioxidant genes (Kadekaro et al., 2012; Kokot et al., 2009). Nrf2, p53, and MITF activate base excision repair (BER), the main pathway for repair of oxidative DNA damage, with Nrf2 upregulating the expression of the BER enzyme 8-oxoguanine glycosylase (OGG1), p53 increasing the expression of OGG1 as well as the BER enzyme apurinic/apyrimidinic endonuclease 1 (APE1), and MITF upregulating the expression of APE1 (Hamann et al., 2011; Liu et al., 2009; Murray et al., 2018). The cAMP pathway is absolutely required for the melanogenic response (i.e., tanning response) to UVB (Im et al., 1998). Inability of melanocytes to respond to α-MSH, due to expression of loss-of-function MC1R, reduces their melanogenic response to UVB and compromises their DNA repair and antioxidant capacities, thereby increasing their vulnerability to malignant transformation to melanoma (Kadekaro et al., 2010; Kennedy et al., 2001; Palmer et al., 2000).
Endothelin-1 is another survival factor that enables melanocytes to overcome the stress response to UVB exposure. The activation of EDNBR had similar effects as MC1R on survival of UVB-irradiated melanocytes, mediated by activating the Akt pathway (Kadekaro et al., 2005). Endothelin-1 also enhances NER, by affecting the same targets as α-MSH (Swope et al., 2020). Therefore, by activating different receptors and signaling pathways, α-MSH and End-1 provide a double safety net to inhibit genomic instability and ensure survival of melanocytes exposed to UV. The neurotrophin nerve growth factor (NGF) synthesized by keratinocytes stimulates melanocyte migration and dendricity and inhibits UV-induced apoptosis by increasing the levels of the anti-apoptotic protein Bcl2 (Stefanato et al., 2003; Yaar et al., 1991; Zhai et al., 1996). The effects of NGF are mediated primarily by binding to p75 NGF receptor, a member of the TNF family/Fas/CD40 superfamily. Other paracrine factors that are involved in the tanning response, part of the DDR to UV, include PGE2 and PGF2α and NO, discussed above. However, the role of these factors in the DNA repair pathways is not yet known.
1,25(OH)2 vitamin D3 (D3), the active form of vitamin D3, is produced in the epidermis upon exposure to solar UVB (280–320 nm wavelength) by the photoisomerization of 7-dehydrocholesterol to cholecalciferol, and subsequent hydroxylation on positions 25 and 1 (Holick, 1994; Lehmann et al., 2001). Epidermal keratinocytes express 25-hydroxlyase (CYP27A1) and 1-α-hydroxylase (CYP27B1) and therefore have the capacity to synthesize the active 1,25(OH)2 vitamin D3 (Bikle et al., 1986; Lehmann et al., 2001). The effects of D3 are mediated by binding to the vitamin D receptor (VDR), which dimerizes with other nuclear receptors, mainly the retinoic acid receptor RXR (Christakos et al., 2016). Treatment with D3 has photoprotective effects on cultured human melanocytes and fibroblasts in vitro, and mouse skin in vivo, as depicted by enhanced repair of UV-induced CPD, the major form of DNA photoproducts (Dixon et al., 2005). In human keratinocytes, the expression of VDR is important for efficient NER, as it promotes the dissociation of XPC from DNA damage sites to allow subsequent enzymes to resume the repair of DNA photoproducts (Wong & Oh, 2021). Recently, it was reported that D3 has potent antioxidant effects on UV-irradiated keratinocytes by activating Nrf2 (Chaiprasongsuk et al., 2019). Additionally, D3 results in increased p53 levels and its transactivation in keratinocytes and melanocytes irradiated with UV (Chaiprasongsuk et al., 2019; Dixon et al., 2005). The antioxidant effect of D3 is supported by the findings that its analog tacalcitol reduces hydrogen peroxide-induced ROS and oxidative damage in melanocytes (Li et al., 2011), and enhances transactivation of Nrf2 in hydrogen peroxide-induced melanocytes (Tang et al., 2018). Further investigation of the antioxidant effects of D3 in melanocytes is warranted, given the negative impact on oxidative stress on melanocyte survival and genomic stability.
There is strong evidence for the role of VDR as a melanoma tumor suppressor. VDR expression is reported to be lost during melanoma progression (Brozyna et al., 2011; Slominski et al., 2017). Targeted deletion of VDR in mouse melanocytes in vivo results in increased levels of UV-induced CPD, possibly due to reduced NER (Chagani et al., 2016). Given the evidence that D3, via activation of VDR, reduces DNA damage and oxidative stress in epidermal cells, including melanocytes, further studies are needed to elucidate the efficacy of D3 in the prevention of photocarcinogenesis, particularly melanoma.
3 ∣. PARACRINE REGULATION OF MELANOCYTES BY FIBROBLASTS
Fibroblasts are mesenchymal cells that constitute the major cellular component of the dermis, and are involved in producing extracellular matrix (ECM) components, as well as various factors that regulate epidermal melanocytes (Figure 3). Evidence for the regulation of melanocytes by fibroblast-derived factors was first provided by the demonstration that fibroblast-conditioned medium and ECM markedly increase human melanocyte proliferation and stimulate tyrosinase activity in vitro (Buffey et al., 1994; Imokawa et al., 1998). These findings sparked interest in identifying the nature of fibroblast-derived paracrine factors and their effects on melanocytes.
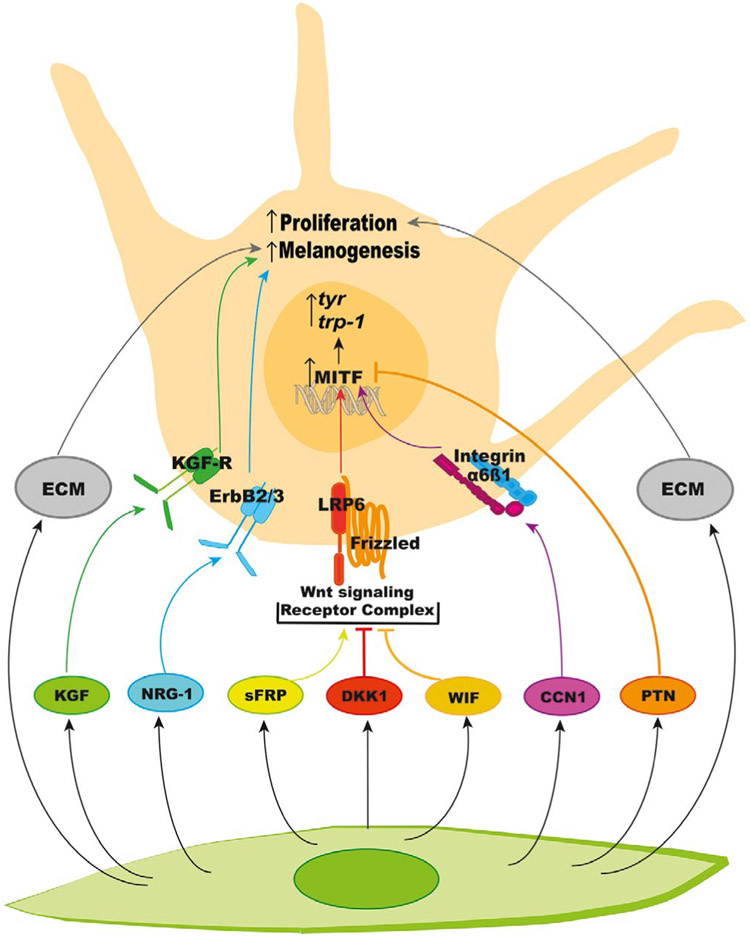
FIGURE 3.
List of the known fibroblast-derived paracrine factors, and the biological effects of these factors via their receptors and of ECM on melanocyte proliferation and melanogenesis. ECM, extracellular matrix
The most apparent physiological effect of fibroblasts on melanocytes is in palmoplantar skin, which is significantly less pigmented and thicker than other anatomical locations. The key regulator of this reduced pigmentation is Dickkopf-related protein 1 (DKK1), which is secreted by palmoplantar fibroblasts and inhibits differentiation, growth, and melanogenesis of melanocytes (Yamaguchi et al., 2004). DKK1 is a secreted antagonist of Wnt signaling and acts on Frizzled-lipoprotein receptor-related protein 6 (LRP6) receptor complex. The resulting inhibition of the canonical downstream (ß-catenin/GSK3ß signaling is associated with downregulation of proteins involved in melanogenesis including MITF, dopachrome tautomerase (DCT; TRP-2), and tyrosinase (Yamaguchi et al., 2004, 2007). Additionally, Wnt inhibitory factor (WIF-1), another secreted Wnt modulating protein, was reported to have a similar inhibitory effect on melanogenesis (Kim et al., 2013). However, another report showed overexpression of WIF-1 in primary human melanocytes stimulates melanogenesis by the activation of ß-catenin pathway (Park et al., 2014). Further confirmation of the effects of WIF-1 on melanocytes is needed to define its role in regulation of melanogenesis. Secreted frizzled-related protein 2 (sFRP2), another WNT modulating protein, proved to activate ß-catenin signaling in human melanocytes and stimulate melanogenesis by upregulating MITF and tyrosinase (Kim et al., 2016). These effects were observed in melanocytes overexpressing sFRP2 or co-cultured with fibroblasts overexpressing sFRP2. The melanogenic effect of sFRP2 was further confirmed by treatment of human skin ex vivo with recombinant sFRP2. Expression of sFRP2 was significantly increased in skin acutely exposed to UV, further suggesting paracrine effects of WNT signaling and its participation in regulating melanogenesis (Kim et al., 2016).
Another fibroblast-secreted protein, neuregulin-1 (NRG-1), is highly expressed in dark skin, as compared to light skin (Choi et al., 2010). The levels of NRG-1 correlate directly with expression of its receptor ErbB3 on melanocytes, suggesting that NRG-1/ErbB3 signaling is involved in determining constitutive skin pigmentation. NRG-1 also acts on ErbB4 receptor, which, contrary to ErbB3, is expressed at higher levels in light skin melanocytes, as compared to their dark counterparts; the significance of ErbB4 on pigmentation remains unknown. NRG1 stimulates melanogenesis in cultured human melanocytes and 3-D skin model and increases melanocyte proliferation through PI3K/Akt signaling (Choi et al., 2010). Further investigation is warranted to determine the regulation of NRG-1 synthesis by UV and its participation in the DDR of melanocytes to UV exposure.
Keratinocyte growth factor is synthesized and secreted by fibroblasts, and its synthesis is upregulated by UV exposure via stimulation of IL-1 production by keratinocytes. Besides regulating the proliferation of keratinocytes, KGF stimulates the transfer of melanosomes from melanocytes by enhancing phagocytosis by keratinocytes. The latter process is more prominent in lighter skin due to higher expression of KGF receptor (Cardinali et al., 2008). In addition, KGF stimulates melanogenesis directly in cultured melanocytes by upregulating tyrosinase mRNA levels, as well as in human skin explants (Chen et al., 2010). The effect of KGF on pigmentation was attributed to its synergistic interaction with other paracrine factors including bFGF, End-1, and IL-1, and with activators of the cAMP pathway (Chen et al., 2010; Hirobe et al., 2013).
Another growth factor produced by dermal fibroblasts is pleiotrophin (PTN), also called heparin-binding growth factor, which is involved in various biological functions including, but not limited to, cell growth and differentiation, angiogenesis, and tumorigenesis (Choudhuri et al., 1997; Gonzalez-Castillo et al., 2014). In human skin, PTN is expressed mainly in fibroblasts and to a lesser extent in melanocytes (Choi et al., 2015; Zhang et al., 2013). The PTN receptors anaplastic lymphoma kinase (ALK) and receptor tyrosine phosphatase β/ζ are expressed in melanocytes, as well as keratinocytes and fibroblasts (Choi et al., 2015). PTN was found to inhibit melanogenesis in human melanocytes by promoting MITF degradation via ERK1/2 activation, effects that are mediated by receptor tyrosine phosphatase β/ζ. Reduction in pigmentation was also observed in ex vivo treated with recombinant PTN. Irradiation of fibroblasts with UV results in downregulation of PTN, which should reduce its suppressive effect of melanogenesis, allowing for the tanning response to UV (Choi et al., 2015).
CCN1, also called Cyr61 or cysteine-rich 61, is a secreted ECM protein from fibroblasts that belong to CCN protein family, coded for by six different genes. CCN1 is involved primarily in maintaining collagen homeostasis and was recently reported to stimulate melanogenesis in melanocytes by binding to integrin α6ß1 receptor and activating p38 and ERK1/2 MAPK signaling (Xu et al., 2018). Exposure to solar UV upregulates CCN1 and CCN2 in fibroblasts through transcription factor AP-1, but downregulates the expression of the remaining CCN genes (Quan et al., 2009, 2010), suggesting that CCNs modulate the melanogenic response of melanocytes to solar UV.
4 ∣. KERATINOCYTE- AND FIBROBLAST-DERIVED PARACRINE FACTORS
Other important paracrine factors for melanocytes derived from both fibroblasts and keratinocytes include HGF, SCF, and semaphorin 7a (sema7a) (Table 1). HGF, also known as scatter factor, is highly expressed by skin fibroblasts and is a potent mitogen for melanocytes (Czyz, 2018; Matsumoto et al., 1991). The effects of HGF are mediated by binding to c-Met receptor expressed on melanocytes, which, similar to other tyrosine kinase receptors, activates MAPK and PI3K/Akt signaling to modulate CREB activity upstream of MITF and affects melanocyte proliferation, motility, and survival. The presence of HGF, in addition to other melanocyte growth factors in melanocyte culture medium, significantly increases dendrite number and length and increases tyrosinase activity and melanin content of cultured human melanocytes (Halaban et al., 1992; Hirobe et al., 2013). Upregulation of HGF synthesis by fibroblasts is caused by UV-induced secretion of IL-1α by keratinocytes, and not by direct irradiation of cultured fibroblasts (Mildner et al., 2007), indicating the role of keratinocytes in regulating HGF production by fibroblasts.
TABLE 1.
Common paracrine factors derived from both keratinocytes and fibroblasts
| Paracrine factor | Melanocyte receptors | Signaling pathway | Effect on melanocyte |
|---|---|---|---|
| HGF | c-Met | MAPK/PI3K-Akt | Induction of proliferation, motility, and survival |
| SCF | c-kit | MAPK/PI3K-Akt | Increased proliferation and survival |
| Sema7a | Plexin C1 | LIMKII | Inhibition of cell attachment and dendricity |
| ß1-integrin | MAPK/FAK | Increased cell attachment and dendricity | |
| Sema4D | Plexin B1 | Shp2 non-tyrosine phosphatase | Inhibition of c-Met signaling |
| Increased growth and survival |
Stem cell factor is a cytokine synthesized by fibroblasts and keratinocytes and is important for melanocyte survival (Grabbe et al., 1994). It is constitutively secreted by fibroblasts and binds to c-kit receptor tyrosine kinase on melanocytes to activate ERK and PI3K/Akt pathways (Hachiya et al., 2001). There is also a membrane-bound form of SCF that is highly expressed in keratinocytes. As in the case of HGF, production of SCF by fibroblasts is increased upon stimulation by keratinocyte-derived IL-1β (Shin et al., 2012). Treatment of human skin grafted onto mice with SCF increases melanocyte density in the epidermis and melanocyte proliferation in vitro (Cario-Andre et al., 2006; Grichnik et al., 1998). Conversely, injection of adult human skin xenografts with SCF- or c-kit neutralizing antibody results in loss of melanocytes and decreased expression of TRP-1 and the melanosome protein Pmel 17, suggesting the significance of SCF in the maintenance of melanocyte survival in adult skin and regulation of melanogenesis (Grichnik et al., 1998).
Semaphorin (Sema) family is a class of secreted and membrane-bound proteins that functions in neuronal pathfinding and axonal development (Alto & Terman, 2017). Skin fibroblasts and keratinocytes express sema7a, which stimulates melanocyte dendricity (Scott et al., 2008). Sema7a is a ligand for Plexin C1 and (ß1-integrin receptors expressed in melanocytes. Upon UVB exposure, Sema7a is strongly expressed in fibroblasts and is associated with increased attachment, spreading, and dendricity, which are mediated by binding to integrins. Binding to Plexin C1 receptor inhibits these effects, suggesting opposing roles of these two receptors. Another Sema family member, Sema 4D, is expressed by keratinocytes and fibroblasts and acts on Plexin B1 receptor in melanocytes (Soong et al., 2012). The activated Plexin B1 inhibits HGF-dependent c-Met signaling, thereby reducing the decrease in E-cadherin by c-Met, mainly through Shp2, a non-receptor tyrosine phosphatase (Soong & Scott, 2013). Sema 4D is a protective paracrine factor for human melanocytes, as it inhibits UVB-induced apoptosis and stimulates their proliferation. UVB irradiation reduces the expression of Plexin B1, which is expected to increase c-Met signaling. These results suggest modulation of melanocyte adhesion and survival by Semaphorins.
5 ∣. ABNORMAL PARACRINE FACTOR PRODUCTION AND PIGMENTARY DISORDERS
Dysregulation of production of paracrine factors such as α-MSH, NO, SCF, KGF, HGF, End-1, TNF-α, sFRPs, DKK1, and eicosanoids has been implicated in multiple common pigmentary disorders, including melasma, solar lentigines, post-inflammatory hyperpigmentation, and vitiligo. Melasma is a common acquired hyperpigmentation characterized by irregularly shaped variably brown patches on sun-exposed areas of the face, neck, and upper extremities. Endocrine signaling and paracrine signaling are thought to contribute to the pathogenesis of melasma. By immunohistochemistry and immunofluorescence, increased levels of α-MSH are observed in the epidermis of melasma lesions when compared to perilesional normal-appearing skin (Espósito et al., 2018; Im et al., 2002; Miot et al., 2010). Increased inducible NO synthase is also observed in the basal keratinocytes of melasma lesions, and it is likely that increased NO contributes to the persistent hyperpigmentation of lesional skin. (Jo et al., 2009). Also, there is increased expression of SCF (Byun et al., 2016; Kang et al., 2006), KGF (Hasegawa et al., 2015), and NGF (Byun et al., 2016) in skin affected by melasma. Additionally, Wnt signaling is altered in melasma via upregulation of sFRP2, which was one of the most upregulated genes in lesional skin (Kang et al., 2011; Kim et al., 2016), and decreased expression of WIF-1 (Kim et al., 2013). In melasma, the contribution of paracrine factors might be potentiated by the disruption of the dermal-epidermal junction, which might allow for greater diffusion of signaling molecules from the dermis to the epidermis (Lee et al., 2012; Torres-Álvarez et al., 2011).
Similarly, paracrine factors are involved in the formation of solar lentigines, where both KGF and sFRP2 are upregulated (Hasegawa et al., 2015; Kim et al., 2016). In addition, earlier immunohistochemical studies showed increased expression of End-1, SCF, and HGF in the epidermis of affected skin (Hattori et al., 2004; Kadono et al., 2001; Kovacs et al., 2010). Both KGF and SCF are necessary for the initiation of solar lentigines. In early-stage solar lentigines, characterized by lower levels of melanin accumulation as well as shorter and less complex rete ridges, the expression of KGF and SCF is increased, compared to both normal skin and more advanced lesions (Lin et al., 2010). The importance of KGF in the induction of solar lentigines is further supported by in vivo experiments in which application of topical KGF and IL-1α results in the formation of hyperpigmented patches with elongated rete ridges in swine and human facial skin grafted onto immunodeficient mice (Chen et al., 2010). Finally, disruption of heparan sulfate at the dermal-epidermal junction may enhance diffusion of cytokines between the dermis and epidermis of solar lentigines, which may further contribute to their progression (Iriyama et al., 2011).
Solar lentigines are commonly known as aging spots, and factors synthesized by aged fibroblasts may contribute to the development of these hyperpigmented lesions. Fibroblasts from old skin and photo-aged or senescent fibroblasts have a greater melanogenic activity on melanocytes than young fibroblasts, due to higher expression and release of fibroblast-derived cytokines including HGF, SCF, and KGF (Imokawa et al., 1998; Kovacs et al., 2010). Reconstructed skin model established with the same keratinocytes and melanocytes and either young or aged fibroblast showed that aged fibroblasts result in greater pigmentation (Duval et al., 2014). Aged fibroblasts synthesize lower levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) than their young counterpart. Moreover, recently it was found that UV-irradiated senescent fibroblasts express high level of stress-responsive cytokine GDF15, which activates ß-catenin signaling to induce MITF expression, suggesting the role of fibroblasts on age-associated pigmentation (Kim et al., 2020). Stimulation of melanogenesis by GDF-15 was confirmed using cultured human melanocytes, human skin explants ex vivo, and reconstructed skin.
Post-inflammatory hyperpigmentation is a common acquired pigmentary disorder that occurs following inflammatory skin conditions or cutaneous injury, and disproportionately affects Black and Hispanic patients (Davis et al., 2012). The inflammatory mediators PGE2 and LTB4 are upregulated in skin affected by atopic dermatitis (Fogh et al., 1989) and psoriasis (Brain et al., 1984; Hammarström et al., 1975), suggesting that inhibition of the COX and lipoxygenase pathways might be efficacious in the prevention and/or treatment of post-inflammatory hyperpigmentation.
Vitiligo is a common depigmentary autoimmune disease, with a complex etiology, and aberrant production of paracrine factors may contribute to the loss of functional melanocytes in lesional skin (Abdel-Malek et al., 2020). Levels of these signaling molecules appear to vary among patients, and there are conflicting reports about the alterations in SCF, End-1, HGF, GM-CSF, and bFGF levels in vitiligo skin, which makes it difficult to determine conclusively their role in vitiligo pathogenesis (Kitamura et al., 2004; Lee et al., 2005; Moretti et al., 2002, 2009). Modulation of the Wnt signaling pathway has been implicated in the pathogenesis of vitiligo, since high levels of the Wnt inhibitor DKK1 are found in lesional skin compared with non-lesional skin of vitiligo patients (Oh et al., 2012). The expression of α-MSH is reduced in skin of vitiligo patients, but it is not clear if it is different in lesional versus perilesional skin (Graham et al., 1999; Thody et al., 1983). Treatment with the potent α-MSH analog NDP-α-MSH, known also as afamelanotide, enhances the effects of narrowband UVB on repigmentation of skin of vitiligo patients (Grimes et al., 2013; Lim et al., 2015). The clinical efficacy of this melanocortin analog supports the role of α-MSH in promoting the migration, survival, proliferation, and differentiation of melanocyte precursors, which are essential for repigmentation of vitiligo skin.
The aberrant cutaneous microenvironment in vitiligo skin upregulates the production by keratinocytes of a number of cytokines and chemokines that enhances the recruitment of CD8+ T cells, resulting in autoimmune destruction of melanocytes. TNF-α is detected at higher levels in lesional vitiligo epidermis compared with perilesional and healthy control skin by immunohistochemistry, ELISA, in situ hybridization, and quantitative PCR (Birol et al., 2006; Moretti et al., 2002, 2009; Seif El Nasr et al., 2013), as well as IL-6, IL-8, and IL-18 (Cui et al., 2019; Mitra et al., 2017). Additionally, genetic polymorphisms in the promoter region of TNF-α may be a risk factor for vitiligo (Laddha et al., 2012). Keratinocytes in vitiligo skin also produce high levels of the chemokines CXCL 9, 10, and 16 (Cui et al., 2019; Rashighi et al., 2014). These results connect aberrant production of paracrine factors with the autoimmune response that instigates melanocyte death in vitiligo.
6 ∣. CONTRIBUTION OF ABERRANT PRODUCTION OF PARACRINE AND AUTOCRINE FACTORS TO MELANOMA PROGRESSION
While the survival effects of keratinocyte- and fibroblast-derived paracrine factors protect normal melanocytes from genotoxic effects of environmental factors, mainly solar UV, their aberrant synthesis can have detrimental effects on melanoma cells, promoting their survival, proliferation, and resistance to targeted therapies. As described above, End-1 is known to be a survival factor for normal melanocytes that reduce UV-induced DNA damage by enhancing NER (Kadekaro et al., 2005; Swope et al., 2020; von Koschembahr et al., 2015). However, aberrant autocrine expression of End-1 by melanoma tumor cells is associated with resistance to MAPK inhibitors (Smith et al., 2017). Melanoma tumor cells from patients treated with BRAF inhibitor express high levels of End-1 and ENDBR, and conditioned media from melanoma tumor cells treated with BRAF inhibitor increase the tolerance of naïve melanoma cells to BRAF inhibitor. Melanoma tumor cells are heterogeneous in the levels of Mitf and the receptor tyrosine kinase AXL, and treatment with MAPK inhibitors increases the expression of both Mitf and AXL. Endothelin-1 supports the survival of Mitf-high melanoma cells via activating the ENDBR and that of AXL-high cells via activating the ENDAR. These results ascribe a role of End-1 as an autocrine factor for melanoma cells, involved in acquisition of drug resistance to BRAF inhibitors and thereby increasing tumor progression and invasiveness. In addition to End-1, autocrine production of HGF is implicated in melanoma development and acquisition of metastatic phenotype (Czyz, 2018), and overexpression of factors, such as bFGF and SCF in vitro in skin explants, led to melanoma initiation (Haass & Herlyn, 2005). Additionally, NRG1 is expressed at high levels in melanoma tumor cells, as compared to normal melanocytes, and stimulates tumor growth in an autocrine manner (Zhang et al., 2012).
Fibroblast represents the major constituents of the stroma associated with tumors. With age, the secretome of fibroblasts is altered in a manner that is conducive to melanoma drug resistance and invasiveness. This can explain why elderly melanoma patients usually have a poorer prognosis than young patients (Kaur et al., 2016). The senescence-associated secretory phenotype (SASP), composed of proinflammatory cytokines, chemokines, growth factors, and proteases, promotes tumor cell progression and invasion. Conditioned media from cultured aged fibroblasts, but not from young fibroblasts, significantly increase the invasiveness melanoma tumor cells and their resistance to BRAF inhibitor (Kaur et al., 2016). These effects are attributed to increased production of secreted frizzled-related protein 2 (sFRP2), which acted as a WNT antagonist, and decreased β-catenin and MITF, and the MITF target APE1, the base excision repair gene, rendering melanoma cells less responsive to oxidative damage and to BRAF inhibitors. sFRP2 increases angiogenesis, thereby melanoma metastasis. It is paradoxical that the above effects of sFRP2 on melanoma cells seem to be in contrast to its effects on normal melanocytes, where it acted as a WNT agonist, which increased the expression of β-catenin and MITF (Kim et al., 2016).
Aged fibroblasts also have altered lipid secretome and increase the secretion of neutral lipids, mainly ceramides (Alicea et al., 2020). Exposure of melanoma cells to aged fibroblast lipid secretome upregulates the expression of the fatty acid transporter FATP2, and blocking this transporter reverses the age-related resistance to BRAF/MEK inhibitors, and increases the survival of melanoma tumor-bearing mice.
There is unequivocal evidence that paracrine factors synthesized by epidermal keratinocytes and dermal fibroblasts play essential roles in maintaining the normal physiology of melanocytes and modulating their DDR to solar UV. To ensure normal melanocyte physiology, these factors can have redundant effects, despite activating different signaling pathways. This is exemplified by the ability of the keratinocyte-derived End-1 to compensate for loss of responsiveness to α-MSH in melanocytes expressing loss-of-function MC1R variants, by activating DNA repair and survival pathways in order to mitigate the genotoxic and apoptotic effects of UV (Swope et al., 2020). Paracrine factors can also interact synergistically, as in the case of bFGF, End-1, and α-MSH, which synergistically stimulate the proliferation of melanocytes in vitro. Some paracrine factors can modulate the response of melanocytes to other factors by regulating the expression of their receptors, exemplified by upregulation of MC1R expression by End-1 (Swope et al., 2012) and upregulation of c-Met by bFGF (Czyz, 2018).
Dysregulation of synthesis of paracrine factors leads to pigmentary abnormalities, jeopardizing the normal function, and/or survival of melanocytes. The autocrine synthesis of certain growth factors by melanoma cells supports their autonomous growth (Czyz, 2018; Zhang et al., 2012) and confers drug resistance (Smith et al., 2017). Altered production of paracrine factors in the melanoma tumor microenvironment contributes to tumor progression and reduces response to targeted therapy. Identifying the nature and regulation of production of paracrine factors and their mechanisms of action can potentially translate to the development of novel therapies for prevention and/or management of various pigmentary disorders, including melanoma.
ACKNOWLEDGEMENTS
Part of this work has been supported in part by 1l01BX003668 VA Merit Award and NIEHS P30ES006096 for ZAM. We wish to thank the collaborators, as well as students, fellows, and laboratory staff in the Abdel-Malek laboratory, particularly Viki Swope, DVM, and Renny Starner, BS, who contributed to the results discussed in this article. We apologize from colleagues if we inadvertently missed citing their contributions to the topic of this review.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Abdel-Malek ZA, Jordan C, Ho T, Upadhyay PR, Fleischer A, & Hamzavi I (2020). The enigma and challenges of vitiligo pathophysiology and treatment. Pigment Cell & Melanoma Research, 33, 778–787. 10.1111/pcmr.12878 [DOI] [PubMed] [Google Scholar]
- Abdel-Malek Z, Swope VB, Suzuki I, Akcali C, Harriger MD, Boyce ST, Urabe K, & Hearing VJ (1995). Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proceedings of the National Academy of Sciences, 92, 1789–1793. 10.1073/pnas.92.5.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alicea GM, Rebecca VW, Goldman AR, Fane ME, Douglass SM, Behera R, Webster MR, Kugel CH, Ecker BL, Caino MC, Kossenkov AV, Tang H-Y, Frederick DT, Flaherty KT, Xu X, Liu Q, Gabrilovich DI, Herlyn M, Blair IA … Weeraratna AT (2020). Changes in Aged Fibroblast Lipid Metabolism Induce Age-Dependent Melanoma Cell Resistance to Targeted Therapy via the Fatty Acid Transporter FATP2. Cancer Discovery, 10, 1282–1295. 10.1158/2159-8290.CD-20-0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alto LT, & Terman JR (2017). Semaphorins and their signaling mechanisms. Methods in Molecular Biology, 1493, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, & Yanagisawa M (1994). Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell, 79, 1277–1285. 10.1016/0092-8674(94)90018-3 [DOI] [PubMed] [Google Scholar]
- Bigliardi-Qi M, Sumanovski LT, Buchner S, Rufli T, & Bigliardi PL (2004). Mu-opiate receptor and Beta-endorphin expression in nerve endings and keratinocytes in human skin. Dermatology, 209, 183–189. 10.1159/000079887 [DOI] [PubMed] [Google Scholar]
- Bikle DD, Nemanic MK, Whitney JO, & Elias PW (1986). Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry, 25, 1545–1548. 10.1021/bi00355a013 [DOI] [PubMed] [Google Scholar]
- Birol A, Kisa U, Kurtipek GS, Kara F, Kocak M, Erkek E, Caglayan O (2006). Increased tumor necrosis factor alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the lesional skin of patients with nonsegmental vitiligo. International Journal of Dermatology, 45, 992–993. [DOI] [PubMed] [Google Scholar]
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, & Schwarz A (2005). alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. Journal of Biological Chemistry, 280, 5795–5802. [DOI] [PubMed] [Google Scholar]
- Brain S, Camp R, Dowd P, Black AK, & Greaves M (1984). The release of leukotriene B4-like material in biologically active amounts from the lesional skin of patients with psoriasis. The Journal of Investigative Dermatology, 83, 70–73. 10.1111/1523-1747.ep12261712 [DOI] [PubMed] [Google Scholar]
- Brozyna AA, Jozwicki W, Janjetovic Z, & Slominski AT (2011). Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Human Pathology, 42, 618–631. 10.1016/j.humpath.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffey JA, Messenger AG, Taylor M, Ashcroft AT, Westgate GE, & Macneil S (1994). Extracellular matrix derived from hair and skin fibroblasts stimulates human skin melanocyte tyrosinase activity. British Journal of Dermatology, 131, 836–842. 10.1111/j.1365-2133.1994.tb08586.x [DOI] [PubMed] [Google Scholar]
- Bustamante J, Bredeston L, Malanga G, & Mordoh J (1993). Role of melanin as a scavenger of active oxygen species. Pigment Cell Research, 6, 348–353. 10.1111/j.1600-0749.1993.tb00612.x [DOI] [PubMed] [Google Scholar]
- Byun JW, Park IS, Choi GS, & Shin J (2016). Role of fibroblast-derived factors in the pathogenesis of melasma. Clinical and Experimental Dermatology, 41, 601–609. 10.1111/ced.12874 [DOI] [PubMed] [Google Scholar]
- Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, Kerns JA, Schmutz SM, Millhauser GL, & Barsh GS (2007). A -defensin mutation causes black coat color in domestic dogs. Science, 318, 1418–1423. 10.1126/science.1147880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali G, Bolasco G, Aspite N, Lucania G, Lotti LV, Torrisi MR, & Picardo M (2008). Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. The Journal of Investigative Dermatology, 128, 558–567. 10.1038/sj.jid.5701063 [DOI] [PubMed] [Google Scholar]
- Cario-Andre M, Pain C, Gauthier Y, Casoli V, & Taieb A (2006). In vivo and in vitro evidence of dermal fibroblasts influence on human epidermal pigmentation. Pigment Cell Research, 19, 434–442. 10.1111/j.1600-0749.2006.00326.x [DOI] [PubMed] [Google Scholar]
- Chagani S, Kyryachenko S, Yamamoto Y, Kato S, Ganguli-Indra G, & Indra AK (2016). In vivo role of vitamin D receptor signaling in UVB-induced DNA damage and melanocyte homeostasis. The Journal of Investigative Dermatology, 136, 2108–2111. [DOI] [PubMed] [Google Scholar]
- Chaiprasongsuk A, Janjetovic Z, Kim T-K, Jarrett SG, D'Orazio JA, Holick MF, Tang EKY, Tuckey RC, Panich U, Li W, & Slominski AT (2019). Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biology, 24, 101206. 10.1016/j.redox.2019.101206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, & Ichihashi M (1996). Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: Regulation by ultraviolet B. Biochimica et Biophysica Acta, 1313, 130–138. 10.1016/0167-4889(96)00063-8 [DOI] [PubMed] [Google Scholar]
- Chen N, Hu Y, Li WH, Eisinger M, Seiberg M, & Lin CB (2010). The role of keratinocyte growth factor in melanogenesis: A possible mechanism for the initiation of solar lentigines. Experimental Dermatology, 19, 865–872. 10.1111/j.1600-0625.2009.00957.x [DOI] [PubMed] [Google Scholar]
- Choi WJ, Kim M, Park JY, Park TJ, & Kang HY(2015). Pleiotrophin inhibits melanogenesis via Erk1/2-MITF signaling in normal human melanocytes. Pigment Cell & Melanoma Research, 28, 51–60. [DOI] [PubMed] [Google Scholar]
- Choi W, Wolber R, Gerwat W, Mann T, Batzer J, Smuda C, Liu H, Kolbe L, & Hearing VJ (2010). The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. Journal of Cell Science, 123, 3102–3111. 10.1242/jcs.064774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri R, Zhang HT, Donnini S, Ziche M, & Bicknell R (1997). An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Research, 57, 1814–1819. [PubMed] [Google Scholar]
- Christakos S, Dhawan P, Verstuyf A, Verlinden L, & Carmeliet G (2016). Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiological Reviews, 96, 365–408. 10.1152/physrev.00014.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Youn SH, Koh WS, Eun HC, Cho KH, Park KC, & Youn JI (1996). Ultraviolet B irradiation-enhanced interleukin (IL)-6 production and mRNA expression are mediated by IL-1 alpha in cultured human keratinocytes. The Journal of Investigative Dermatology, 106, 715–720. [DOI] [PubMed] [Google Scholar]
- Corre S, Primot A, Sviderskaya E, Bennett DC, Vaulont S, Goding CR, & Galibert MD (2004). UV-induced expression of key component of the tanning process, the POMC and MC1R genes, is dependent on the p-38-activated upstream stimulating factor-1 (USF-1). Journal of Biological Chemistry, 279, 51226–51233. 10.1074/jbc.M409768200 [DOI] [PubMed] [Google Scholar]
- Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, & Fisher D (2007). Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell, 128, 853–864. 10.1016/j.cell.2006.12.045 [DOI] [PubMed] [Google Scholar]
- Cui T, Zhang W, Li S, Chen X, Chang Y, Yi X, Kang P, Yang Y, Chen J, Liu L, Jian Z, Li K, Wang G, Gao T, Song P, & Li C (2019). Oxidative stress-induced HMGB1 release from melanocytes: A Paracrine mechanism underlying the cutaneous inflammation in Vitiligo. The Journal of Investigative Dermatology, 139, 2174–2184.e4. [DOI] [PubMed] [Google Scholar]
- Czyz M (2018). HGF/c-MET Signaling in Melanocytes and Melanoma. International Journal of Molecular Sciences, 19. 10.3390/ijms19123844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SA, Narahari S, Feldman SR, Huang W, Pichardo-Geisinger RO, & Mcmichael AJ (2012). Top dermatologic conditions in patients of color: An analysis of nationally representative data. Journal of Drugs in Dermatology, 11, 466–473. [PubMed] [Google Scholar]
- Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, Posner GH, Ishizuka S, Halliday GM, Reeve VE, & Mason RS (2005). Skin cancer prevention: A possible role of 1,25dihydroxyvitamin D3 and its analogs. Journal of Steroid Biochemistry and Molecular Biology, 97, 137–143. 10.1016/j.jsbmb.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Duval C, Cohen C, Chagnoleau C, Flouret V, Bourreau E, & Bernerd, (2014). Key regulatory role of dermal fibroblasts in pigmentation as demonstrated using a reconstructed skin model: Impact of photo-aging. PLoS One, 9, e114182. 10.1371/journal.pone.0114182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval C, Regnier M, & Schmidt R (2001). Distinct melanogenic response of human melanocytes in mono-culture, in co-culture with keratinocytes and in reconstructed epidermis, to UV exposure. Pigment Cell Research, 14, 348–355. 10.1034/j.1600-0749.2001.140506.x [DOI] [PubMed] [Google Scholar]
- Espósito ACC, Brianezi G, De Souza NP, Miot LDB, Marques MEA, & Miot HA (2018). Exploring pathways for sustained melanogenesis in facial melasma: An immunofluorescence study. International Journal of Cosmetic Science, 40, 420–424. 10.1111/ics.12468 [DOI] [PubMed] [Google Scholar]
- Fogh K, Herlin T, & Kragballe K (1989). Eicosanoids in skin of patients with atopic dermatitis: Prostaglandin E2 and leukotriene B4 are present in biologically active concentrations. The Journal of Allergy and Clinical Immunology, 83, 450–455. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, Eller MS, Geller AC, & Yaar M (1999). The pathogenesis of melanoma induced by ultraviolet radiation. New England Journal of Medicine, 340, 1341–1348. 10.1056/NEJM199904293401707 [DOI] [PubMed] [Google Scholar]
- Gilchrest BA, & Rogers GS (1993). Photoaging. In Lim HW, & Soter NA (Eds.), Clinical photomedicine (pp. 95–111). Marcel Dekker, Inc. [Google Scholar]
- Gonzalez-Castillo C, Ortuno-Sahagun D, Guzman-Brambila C, Pallas M, & Rojas-Mayorquin AE(2014). Pleiotrophin as a central nervous system neuromodulator, evidences from the hippocampus. Frontiers in Cellular Neuroscience, 8, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PR, Mansur CP, & Gilchrest BA (1989). Regulation of human melanocyte growth, dendricity, and melanization by keratinocyte derived factors. Journal of Investigative Dermatology, 92, 565–572. 10.1111/1523-1747.ep12709595 [DOI] [PubMed] [Google Scholar]
- Grabbe J, Welker P, Dippel E, & Czarnetzki BM (1994). Stem cell factor, a novel cutaneous growth factor for mast cells and melanocytes. Archives of Dermatological Research, 287, 78–84. 10.1007/BF00370723 [DOI] [PubMed] [Google Scholar]
- Graham A, Westerhof W, & Thody AJ (1999). The expression of alpha-MSH by melanocytes is reduced in vitiligo. Annals of the New York Academy of Sciences, 885, 470–473. [DOI] [PubMed] [Google Scholar]
- Green AC, Wallingford SC, & Mcbride P (2011). Childhood exposure to ultraviolet radiation and harmful skin effects: Epidemiological evidence. Progress in Biophysics and Molecular Biology, 107, 349–355. 10.1016/j.pbiomolbio.2011.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichnik JM, Burch JA, Burchette J, & Shea CR (1998). The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. The Journal of Investigative Dermatology, 111, 233–238. 10.1046/j.1523-1747.1998.00272.x [DOI] [PubMed] [Google Scholar]
- Grimes PE, Hamzavi I, Lebwohl M, Ortonne JP, & Lim HW (2013). The efficacy of afamelanotide and narrowband UV-B phototherapy for repigmentation of vitiligo. JAMA Dermatology, 149, 68–73. 10.1001/2013.jamadermatol.386 [DOI] [PubMed] [Google Scholar]
- Haass NK, & Herlyn M (2005). Normal human melanocyte homeostasis as a paradigm for understanding melanoma. The Journal of Investigative Dermatology Symposium Proceedings, 10, 153–163. 10.1111/j.1087-0024.2005.200407.x [DOI] [PubMed] [Google Scholar]
- Hachiya A, Kobayashi A, Ohuchi A, Takema Y, & Imokawa G (2001). The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. The Journal of Investigative Dermatology, 116, 578–586. 10.1046/j.1523-1747.2001.01290.x [DOI] [PubMed] [Google Scholar]
- Halaban R, Kwon BS, Ghosh S, Delli-Bovi P, & Baird A (1988). bFGF is an autocrine growth factor for human melanomas. Oncogene Research, 3, 177–186. [PubMed] [Google Scholar]
- Halaban R, Rubin JS, Funasaka Y, Cobb M, Boulton T, Faletto D, Rosen E, Chan A, Yoko K, & White W (1992). Met and hepatocyte growth factor/scatter factor signal transduction in normal melanocytes and melanoma cells. Oncogene, 7, 2195–2206. [PubMed] [Google Scholar]
- Halder RM, & Bridgeman-Shah S (1995). Skin cancer in African Americans. Cancer, 75, 667–673. [DOI] [PubMed] [Google Scholar]
- Hamann I, Konig C, Richter C, Jahnke G, & Hartwig A (2011). Impact of cadmium on hOGG1 and APE1 as a function of the cellular p53 status. Mutation Research, 736, 56–63. [DOI] [PubMed] [Google Scholar]
- Hammarström S, Hamberg M, Samuelsson B, Duell EA, Stawiski M, & Voorhees JJ (1975). Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proceedings of the National Academy of Sciences of the United States of America, 72, 5130–5134. 10.1073/pnas.72.12.5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Fujiwara R, Sato K, Shin J, Kim SJ, Kim M, & Kang HY (2015). Possible Involvement of Keratinocyte Growth Factor in the Persistence of Hyperpigmentation in both Human Facial Solar Lentigines and Melasma. Annals of Dermatology, 27, 626–629. 10.5021/ad.2015.27.5.626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori H, Kawashima M, Ichikawa Y, & Imokawa G (2004). The epidermal stem cell factor is over-expressed in lentigo senilis: Implication for the mechanism of hyperpigmentation. The Journal of Investigative Dermatology, 122, 1256–1265. 10.1111/j.0022-202X.2004.22503.x [DOI] [PubMed] [Google Scholar]
- Hirobe T, Hasegawa K, Furuya R, Fujiwara R, & Sato K (2013). Effects of fibroblast-derived factors on the proliferation and differentiation of human melanocytes in culture. Journal of Dermatological Science, 71, 45–57. 10.1016/j.jdermsci.2013.03.012 [DOI] [PubMed] [Google Scholar]
- Holick MF (1994). McCollum Award Lecture, 1994: Vitamin D–new horizons for the 21st century. American Journal of Clinical Nutrition, 60, 619–630. 10.1093/ajcn/60.4.619 [DOI] [PubMed] [Google Scholar]
- Hunt G, Donatien PD, Lunec J, Todd C, Kyne S, & Thody AJ (1994). Cultured human melanocytes respond to MSH peptides and ACTH. Pigment Cell Research, 7, 217–221. 10.1111/j.1600-0749.1994.tb00052.x [DOI] [PubMed] [Google Scholar]
- Hunt G, Kyne S, Wakamatsu K, Ito S, & Thody AJ (1995). Nle4DPhe7 alpha-melanocyte-stimulating hormone increases the eumelanin:phaeomelanin ratio in cultured human melanocytes. The Journal of Investigative Dermatology, 104, 83–85. [DOI] [PubMed] [Google Scholar]
- Hyter S, Coleman DJ, Ganguli-Indra G, Merrill GF, Ma S, Yanagisawa M, & Indra AK (2013). Endothelin-1 is a transcriptional target of p53 in epidermal keratinocytes and regulates ultraviolet-induced melanocyte homeostasis. Pigment Cell & Melanoma Research, 26, 247–258. 10.1111/pcmr.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S, Kim J, On WY, & Kang WH (2002). Increased expression of alpha-melanocyte-stimulating hormone in the lesional skin of melasma. The British Journal of Dermatology, 146, 165–167. [DOI] [PubMed] [Google Scholar]
- Im S, Moro O, Peng F, Medrano EE, Cornelius J, Babcock G, Nordlund JJ, & Abdel-Malek ZA (1998). Activation of the cyclic AMP pathway by alpha-melanotropin mediates the response of human melanocytes to ultraviolet B radiation. Cancer Research, 58, 47–54. [PubMed] [Google Scholar]
- Imokawa G, Yada Y, & Miyagishi M (1992). Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. Journal of Biological Chemistry, 267, 24675–24680. 10.1016/S0021-9258(18)35817-4 [DOI] [PubMed] [Google Scholar]
- Imokawa G, Yada Y, Morisaki N, & Kimura M (1998). Biological characterization of human fibroblast-derived mitogenic factors for human melanocytes. The Biochemical Journal, 330(Pt 3), 1235–1239. 10.1042/bj3301235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriyama S, Ono T, Aoki H, & Amano S (2011). Hyperpigmentation in human solar lentigo is promoted by heparanase-induced loss of heparan sulfate chains at the dermal-epidermal junction. Journal of Dermatological Science, 64, 223–228. 10.1016/j.jdermsci.2011.09.007 [DOI] [PubMed] [Google Scholar]
- Iversen L, Kristensen P, Gron B, Ziboh VA, & Kragballe K (1994). Human epidermis transforms exogenous leukotriene A4 into peptide leukotrienes: Possible role in transcellular metabolism. Archives of Dermatological Research, 286, 261–266. 10.1007/BF00387598 [DOI] [PubMed] [Google Scholar]
- Jablonski NG, & Chaplin G (2000). The evolution of human skin coloration. Journal of Human Evolution, 39, 57–106. 10.1006/jhev.2000.0403 [DOI] [PubMed] [Google Scholar]
- Jo HY, Kim CK, Suh IB, Ryu SW, Ha KS, Kwon YG, & Kim YM (2009). Co-localization of inducible nitric oxide synthase and phosphorylated Akt in the lesional skins of patients with melasma. The Journal of Dermatology, 36, 10–16. 10.1111/j.1346-8138.2008.00579.x [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Chen J, Yang J, Chen S, Jameson J, Swope VB, Cheng T, Kadakia M, & Abdel-Malek Z (2012). Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Molecular Cancer Research, 10, 778–786. 10.1158/1541-7786.MCR-11-0436 [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H, Terzieva S, Hauser J, Kobayashi N, Schwemberger S, Cornelius J, Babcock G, Shertzer HG, Scott G, & Abdel-Malek ZA (2005). alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Research, 65, 4292–4299. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Leachman S, Kavanagh RJ, Swope V, Cassidy P, Supp D, Sartor M, Schwemberger S, Babcock G, Wakamatsu K, Ito S, Koshoffer A, Boissy RE, Manga P, Sturm RA, & Abdel-Malek ZA (2010). Melanocortin 1 receptor genotype: An important determinant of the damage response of melanocytes to ultraviolet radiation. The FASEB Journal, 24, 3850–3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadono S, Manaka I, Kawashima M, Kobayashi T, & Imokawa G(2001). The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. Journal of Investigative Dermatology, 116, 571–577. 10.1046/j.1523-1747.2001.01296.x [DOI] [PubMed] [Google Scholar]
- Kaidbey KH, Poh Agin P, Sayre RM, & Kligman AM (1979). Photoprotection by melanin - A comparison of black and Caucasian skin. Journal of the American Academy of Dermatology, 1, 249–260. 10.1016/S0190-9622(79)70018-1 [DOI] [PubMed] [Google Scholar]
- Kang HY, Hwang JS, Lee JY, Ahn JH, Kim JY, Lee ES, & Kang WH (2006). The dermal stem cell factor and c-kit are over-expressed in melasma. The British Journal of Dermatology, 154, 1094–1099. 10.1111/j.1365-2133.2006.07179.x [DOI] [PubMed] [Google Scholar]
- Kang HY, Suzuki I, Lee DJ, Ha J, Reiniche P, Aubert J, Deret S, Zugaj D, Voegel JJ, & Ortonne JP (2011). Transcriptional profiling shows altered expression of wnt pathway- and lipid metabolism-related genes as well as melanogenesis-related genes in melasma. The Journal of Investigative Dermatology, 131, 1692–1700. 10.1038/jid.2011.109 [DOI] [PubMed] [Google Scholar]
- Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, Kugel CH, Dang VM, Appleton J, O’Connell MP, Cheng P, Valiga AA, Morissette R, McDonnell NB, Ferrucci L, Kossenkov AV, Meeth K, Tang H-Y, Yin X, Wood WH, … Weeraratna AT (2016). sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature, 532, 250–254. 10.1038/nature17392 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kauser S, Schallreuter KU, Thody AJ, Gummer C, & Tobin DJ (2003). Regulation of human epidermal melanocyte biology by beta-endorphin. The Journal of Investigative Dermatology, 120, 1073–1080. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Ter Huurne J, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, & Bouwes Bavinck JN (2001). Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. Journal of Investigative Dermatology, 117, 294–300. 10.1046/j.0022-202x.2001.01421.x [DOI] [PubMed] [Google Scholar]
- Kim JY, Lee TR, & Lee AY (2013). Reduced WIF-1 expression stimulates skin hyperpigmentation in patients with melasma. The Journal of Investigative Dermatology, 133, 191–200. 10.1038/jid.2012.270 [DOI] [PubMed] [Google Scholar]
- Kim JY, Shin JY, Kim MR, Hann SK, & Oh SH (2012). siRNA-mediated knock-down of COX-2 in melanocytes suppresses melanogenesis. Experimental Dermatology, 21, 420–425. 10.1111/j.1600-0625.2012.01483.x [DOI] [PubMed] [Google Scholar]
- Kim M, Han JH, Kim JH, Park TJ, & Kang HY (2016). Secreted frizzled-related protein 2 (sFRP2) functions as a melanogenic stimulator; the role of sFRP2 in UV-induced hyperpigmentary disorders. The Journal of Investigative Dermatology, 136, 236–244. 10.1038/JID.2015.365 [DOI] [PubMed] [Google Scholar]
- Kim Y, Kang B, Kim JC, Park TJ, & Kang HY (2020). Senescent fibroblast-derived GDF15 induces skin pigmentation. The Journal of Investigative Dermatology, 140(12), 2478–2486.e4. 10.1016/j.jid.2020.04.016 [DOI] [PubMed] [Google Scholar]
- Kitamura R, Tsukamoto K, Harada K, Shimizu A, Shimada S, Kobayashi T, & Imokawa G (2004). Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: Role of SCF/KIT protein interactions and the downstream effector, MITF-M. The Journal of Pathology, 202, 463–475. 10.1002/path.1538 [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Nakagawa A, Muramatsu T, Yamashina Y, Shirai T, Hashimoto MW, Ishigaki Y, Ohnishi T, & Mori T (1998). Supranuclear melanin caps reduce ultraviolet induced DNA photoproducts in human epidermis. The Journal of Investigative Dermatology, 110, 806–810. 10.1046/j.1523-1747.1998.00178.x [DOI] [PubMed] [Google Scholar]
- Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, & Luger TA (1990). Human keratinocytes are a source for tumor necrosis factor α: Evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. Journal of Experimental Medicine, 172, 1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokot A, Metze D, Mouchet N, Galibert MD, Schiller M, Luger TA, & Bohm M (2009). Alpha-melanocyte-stimulating hormone counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin. Endocrinology, 150, 3197–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs D, Cardinali G, Aspite N, Cota C, Luzi F, Bellei B, Briganti S, Amantea A, Torrisi MR, & Picardo M (2010). Role of fibroblast-derived growth factors in regulating hyperpigmentation of solar lentigo. British Journal of Dermatology, 163, 1020–1027. 10.1111/j.1365-2133.2010.09946.x [DOI] [PubMed] [Google Scholar]
- Laddha NC, Dwivedi M, & Begum R(2012). Increased tumor necrosis factor (TNF)-α and its promoter polymorphisms correlate with disease progression and higher susceptibility towards vitiligo. PLoS One, 7, e52298. 10.1371/journal.pone.0052298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassalle MW, Igarashi S, Sasaki M, Wakamatsu K, Ito S, & Horikoshi T (2003). Effects of melanogenesis-inducing nitric oxide and histamine on the production of eumelanin and pheomelanin in cultured human melanocytes. Pigment Cell Research, 16, 81–84. 10.1034/j.1600-0749.2003.00004.x [DOI] [PubMed] [Google Scholar]
- Lee AY, Kim NH, Choi WI, & Youm YH (2005). Less keratinocyte-derived factors related to more keratinocyte apoptosis in depigmented than normally pigmented suction-blistered epidermis may cause passive melanocyte death in vitiligo. The Journal of Investigative Dermatology, 124, 976–983. 10.1111/j.0022-202X.2005.23667.x [DOI] [PubMed] [Google Scholar]
- Lee DJ, Park KC, Ortonne JP, & Kang HY (2012). Pendulous melanocytes: A characteristic feature of melasma and how it may occur. The British Journal of Dermatology, 166, 684–686. 10.1111/j.1365-2133.2011.10648.x [DOI] [PubMed] [Google Scholar]
- Lehmann B, Genehr T, Knuschke P, Pietzsch J, & Meurer M (2001). UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. The Journal of Investigative Dermatology, 117, 1179–1185. [DOI] [PubMed] [Google Scholar]
- Li Z, Musich PR, Serrano MA, Dong Z, & Zou Y (2011). XPA-mediated regulation of global nucleotide excision repair by ATR Is p53-dependent and occurs primarily in S-phase. PLoS One, 6, e28326. 10.1371/journal.pone.0028326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW, Grimes PE, Agbai O, Hamzavi I, Henderson M, Haddican M, Linkner RV, & Lebwohl M (2015). Afamelanotide and narrowband UV-B phototherapy for the treatment of vitiligo: A randomized multicenter trial. JAMA Dermatology, 151, 42–50. 10.1001/jamadermatol.2014.1875 [DOI] [PubMed] [Google Scholar]
- Lin CB, Hu Y, Rossetti D, Chen N, David C, Slominski A, … Seiberg M (2010). Immuno-histochemical evaluation of solar lentigines: The association of KGF/KGFR and other factors with lesion development. Journal of Dermatological Science, 59, 91–97. 10.1016/j.jdermsci.2010.06.006 [DOI] [PubMed] [Google Scholar]
- Liu F, Fu Y, & Meyskens FL Jr (2009). MiTF regulates cellular response to reactive oxygen species through transcriptional regulation of APE-1/Ref-1. The Journal of Investigative Dermatology, 129, 422–431. 10.1038/jid.2008.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes F, Sleiman MG, Sebastian K, Bogucka R, Jacobs EA, & Adamson AS (2021). UV exposure and the risk of cutaneous melanoma in skin of color: A systematic review. JAMA Dermatology, 157, 213–219. [DOI] [PubMed] [Google Scholar]
- Lv J, Fu Y, Gao R, Li J, Kang M, Song G, & Yun C (2019). Diazepam enhances melanogenesis, melanocyte dendricity and melanosome transport via the PBR/cAMP/PKA pathway. The International Journal of Biochemistry & Cell Biology, 116, 105620. 10.1016/j.biocel.2019.105620 [DOI] [PubMed] [Google Scholar]
- Man M-Q, Lin T-K, Santiago JL, Celli A, Zhong L, Huang Z-M, Roelandt T, Hupe M, Sundberg JP, Silva KA, Crumrine D, Martin-Ezquerra G, Trullas C, Sun R, Wakefield JS, Wei ML, Feingold KR, Mauro TM, & Elias PM (2014). Basis for enhanced barrier function of pigmented skin. The Journal of Investigative Dermatology, 134, 2399–2407. 10.1038/jid.2014.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manga P, Elbuluk N, & Orlow SJ (2016). Recent advances in understanding vitiligo. F1000Research, 5. 10.12688/f1000research.8976.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Tajima H, & Nakamura T (1991). Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochemical and Biophysical Research Communications, 176, 45–51. 10.1016/0006-291X(91)90887-D [DOI] [PubMed] [Google Scholar]
- Meredith P, & Sarna T (2006). The physical and chemical properties of eumelanin. Pigment Cell Research, 19, 572–594. 10.1111/j.1600-0749.2006.00345.x [DOI] [PubMed] [Google Scholar]
- Mildner M, Mlitz V, Gruber F, Wojta J, & Tschachler E (2007). Hepatocyte growth factor establishes autocrine and paracrine feedback loops for the protection of skin cells after UV irradiation. The Journal of Investigative Dermatology, 127, 2637–2644. 10.1038/sj.jid.5700938 [DOI] [PubMed] [Google Scholar]
- Miot LD, Miot HA, Polettini J, Silva MG, & Marques ME (2010). Morphologic changes and the expression of alpha-melanocyte stimulating hormone and melanocortin-1 receptor in melasma lesions: A comparative study. The American Journal of Dermatopathology, 32, 676–682. 10.1097/DAD.0b013e3181cd4396 [DOI] [PubMed] [Google Scholar]
- Mitra S, De Sarkar S, Pradhan A, Pati AK, Pradhan R, Mondal D, Sen S, Ghosh A, Chatterjee S, & Chatterjee M (2017). Levels of oxidative damage and proinflammatory cytokines are enhanced in patients with active vitiligo. Free Radical Research, 51, 986–994. 10.1080/10715762.2017.1402303 [DOI] [PubMed] [Google Scholar]
- Morelli JG, Yohn JJ, Lyons MB, Murphy RC, & Norris DA (1989). Leukotrienes C4 and D4 as potent mitogens for cultured human melanocytes. Journal of Investigative Dermatology, 93, 719–722. [DOI] [PubMed] [Google Scholar]
- Moretti S, Fabbri P, Baroni G, Berti S, Bani D, Berti E, Nassini R, Lotti T, & Massi D (2009). Keratinocyte dysfunction in vitiligo epidermis: Cytokine microenvironment and correlation to keratinocyte apoptosis. Histology and Histopathology, 24, 849–857. [DOI] [PubMed] [Google Scholar]
- Moretti S, Spallanzani A, Amato L, Hautmann G, Gallerani I, Fabiani M, & Fabbri P (2002). New insights into the pathogenesis of vitiligo: Imbalance of epidermal cytokines at sites of lesions. Pigment Cell Research, 15, 87–92. 10.1034/j.1600-0749.2002.1o049.x [DOI] [PubMed] [Google Scholar]
- Murray D, Mirzayans R, & Mcbride WH (2018). Defenses against pro-oxidant forces - Maintenance of cellular and genomic integrity and longevity. Radiation Research, 190, 331–349. 10.1667/RR15101.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nix MA, Kaelin CB, Ta T, Weis A, Morton GJ, Barsh GS, & Millhauser GL (2013). Molecular and functional analysis of human beta-defensin 3 action at melanocortin receptors. Chemistry & Biology, 20, 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Kim JY, Kim MR, Do JE, Shin JY, & Hann SK (2012). DKK1 is highly expressed in the dermis of vitiligo lesion: Is there association between DKK1 and vitiligo? Journal of Dermatological Science, 66, 163–165. 10.1016/j.jdermsci.2012.01.010 [DOI] [PubMed] [Google Scholar]
- Ohbayashi N, & Fukuda M (2012). Role of Rab family GTPases and their effectors in melanosomal logistics. Journal of Biochemistry, 151, 343–351. 10.1093/jb/mvs009 [DOI] [PubMed] [Google Scholar]
- Palmer JS, Duffy DL, Box NF, Aitken JF, O'Gorman LE, Green AC, Hayward NK, Martin NG, & Sturm RA (2000). Melanocortin-1 receptor polymorphisms and risk of melanoma: Is the association explained solely by pigmentation phenotype? American Journal of Human Genetics, 66, 176–186. 10.1086/302711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HY, Wu C, Yaar M, Stachur CM, Kosmadaki M, & Gilchrest BA (2009). Role of BMP-4 and Its signaling pathways in cultured human melanocytes. International Journal of Cell Biology, 2009, 750482. 10.1155/2009/750482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Kim M, Kim H, Park SY, Park KC, Ortonne JP, & Kang HY (2014). Wnt inhibitory factor (WIF)-1 promotes melanogenesis in normal human melanocytes. Pigment Cell & Melanoma Research, 27, 72–81. 10.1111/pcmr.12168 [DOI] [PubMed] [Google Scholar]
- Pittelkow MR, & Shipley GD (1989). Serum-free culture of normal human melanocytes: Growth kinetics and growth factor requirements. Journal of Cellular Physiology, 140, 565–576. 10.1002/jcp.1041400323 [DOI] [PubMed] [Google Scholar]
- Puffenberger EG, Hosoda K, Washington SS, Nakao K, Dewit D, Yanagisawa M, & Chakravarti A (1994). A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell, 79, 1257–1266. 10.1016/0092-8674(94)90016-7 [DOI] [PubMed] [Google Scholar]
- Quan T, Qin Z, Xu Y, He T, Kang S, Voorhees JJ, & Fisher GJ (2010). Ultraviolet irradiation induces CYR61/CCN1, a mediator of collagen homeostasis, through activation of transcription factor AP-1 in human skin fibroblasts. The Journal of Investigative Dermatology, 130, 1697–1706. 10.1038/jid.2010.29 [DOI] [PubMed] [Google Scholar]
- Quan T, Shin S, Qin Z, & Fisher GJ (2009). Expression of CCN family of genes in human skin in vivo and alterations by solar-simulated ultraviolet irradiation. Journal of Cell Communication and Signaling Al, 3, 19–23. 10.1007/s12079-009-0044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanala S, Maymone MBC, & Vashi NA (2019). Melasma pathogenesis: A review of the latest research, pathological findings, and investigational therapies. Dermatology Online Journal, 25(10), 13030/qt47b7r28c. [PubMed] [Google Scholar]
- Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su M-W, Zhou Y, Deng A, Hunter CA, Luster AD, & Harris JE, (2014). CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Science Translational Medicine, 6, 223ra23. 10.1126/scitranslmed.3007811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Graillet C, Aberdam E, Biagoli N, Massabni W, Ortonne JP, & Ballotti R (1996). Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes. Journal of Biological Chemistry, 271, 28052–28056. 10.1074/jbc.271.45.28052 [DOI] [PubMed] [Google Scholar]
- Romero-Graillet C, Aberdam E, Clement M, Ortonne JP, & Ballotti R (1997). Nitric oxide produced by ultraviolet-irradiated keratinocytes stimulates melanogenesis. Journal of Clinical Investigation, 99, 635–642. 10.1172/JCI119206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau K, Kauser S, Pritchard LE, Warhurst A, Oliver RL, Slominski A, Wei ET, Thody AJ, Tobin DJ, & White A (2007). Proopiomelanocortin (POMC), the ACTH/melanocortin precursor, is secreted by human epidermal keratinocytes and melanocytes and stimulates melanogenesis. The FASEB Journal, 21, 1844–1856. 10.1096/fj.06-7398com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai C, Ollmann M, Kobayashi T, Abdel-Malek Z, Muller J, Vieira WD, Imokawa G, Barsh GS, & Hearing VJ (1997). Modulation of murine melanocyte function in vitro by agouti signal protein. EMBO Journal, 16, 3544–3552. 10.1093/emboj/16.12.3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Horikoshi T, Uchiwa H, & Miyachi Y (2000). Up-regulation of tyrosinase gene by nitric oxide in human melanocytes. Pigment Cell Research, 13, 248–252. [DOI] [PubMed] [Google Scholar]
- Sawyer TK, Hruby VJ, Hadley ME, & Engel MH (1983). α-Melanocyte stimulating hormone: Chemical nature and mechanism of action. American Zoologist, 23, 529–540. [Google Scholar]
- Scott G, Cassidy L, & Abdel-Malek Z (1997). α-Melanocyte-stimulating hormone and endothelin-1 have opposing effects on melanocyte adhesion, migration, and pp125FAK phosphorylation. Experimental Cell Research, 237, 19–28. [DOI] [PubMed] [Google Scholar]
- Scott G, Jacobs S, Leopardi S, Anthony FA, Learn D, Malaviya R, & Pentland A (2005). Effects of PGF2alpha on human melanocytes and regulation of the FP receptor by ultraviolet radiation. Experimental Cell Research, 304, 407–416. [DOI] [PubMed] [Google Scholar]
- Scott G, Leopardi S, Printup S, Malhi N, Seiberg M, & Lapoint R (2004). Proteinase-activated receptor-2 stimulates prostaglandin production in keratinocytes: Analysis of prostaglandin receptors on human melanocytes and effects of PGE2 and PGF2α on melanocyte dendricity. The Journal of Investigative Dermatology, 122, 1214–1224. [DOI] [PubMed] [Google Scholar]
- Scott GA, McClelland LA, & Fricke AF (2008). Semaphorin 7a promotes spreading and dendricity in human melanocytes through beta1-integrins. The Journal of Investigative Dermatology, 128, 151–161. [DOI] [PubMed] [Google Scholar]
- Scott MC, Suzuki I, & Abdel-Malek ZA (2002). Regulation of the human melanocortin 1 receptor expression in epidermal melanocytes by paracrine and endocrine factors and by ultraviolet radiation. Pigment Cell Research, 15, 433–439. 10.1034/j.1600-0749.2002.02051.x [DOI] [PubMed] [Google Scholar]
- Seif El Nasr H, Shaker OG, Fawzi MM, & El-Hanafi G (2013). Basic fibroblast growth factor and tumour necrosis factor alpha in vitiligo and other hypopigmented disorders: Suggestive possible therapeutic targets. Journal of the European Academy of Dermatology and Venereology, 27, 103–108. 10.1111/j.1468-3083.2011.04368.x [DOI] [PubMed] [Google Scholar]
- Sharov AA, Fessing M, Atoyan R, Sharova TY, Haskell-Luevano C, Weiner L, Funa K, Brissette JL, Gilchrest BA, & Botchkarev VA (2005). Bone morphogenetic protein (BMP) signaling controls hair pigmentation by means of cross-talk with the melanocortin receptor-1 pathway. Proceedings of the National Academy of Sciences, 102, 93–98. 10.1073/pnas.0408455102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Kim JH, & Kim EK(2012). Repeated exposure of human fibroblasts to UVR induces secretion of stem cell factor and senescence. Journal of the European Academy of Dermatology and Venereology, 26, 1577–1580. [DOI] [PubMed] [Google Scholar]
- Siegrist W, Drozdz R, Cotti R, Willard DH, Wilkison WO, & Eberle AN (1997). Interactions of à-melanotropin and agouti on B16 melanoma cells: Evidence for inverse agonism of agouti. Journal of Receptor and Signal Transduction Research, 17, 75–98. [DOI] [PubMed] [Google Scholar]
- Slominski AT, Brozyna AA, Zmijewski MA, Jozwicki W, Jetten AM, Mason RS, Tuckey RC, & Elmets CA (2017). Vitamin D signaling and melanoma: Role of vitamin D and its receptors in melanoma progression and management. Laboratory Investigation, 97, 706–724. 10.1038/labinvest.2017.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Hwang J, Chakraborty A, Mazurkiewicz JE, & Mihm M (1995). Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS Letters, 374, 113–116. 10.1016/0014-5793(95)01090-2 [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, & Wortsman J (2005). CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. American Journal of Physiology-Endocrinology and Metabolism, 288, E701–E706. 10.1152/ajpendo.00519.2004 [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, & Tobin DJ (2006). Corticotropin releasing hormone and the skin. Frontiers in Bioscience, 11, 2230–2248. 10.2741/1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MP, Rowling EJ, Miskolczi Z, Ferguson J, Spoerri L, Haass NK, Sloss O, McEntegart S, Arozarena I, Kriegsheim A, Rodriguez J, Brunton H, Kmarashev J, Levesque MP, Dummer R, Frederick DT, Andrews MC, Cooper ZA, Flaherty KT, … Wellbrock C (2017). Targeting endothelin receptor signalling overcomes heterogeneity driven therapy failure. EMBO Molecular Medicine, 9, 1011–1029. 10.15252/emmm.201607156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Mosby N, Yang J, Xu A, Abdel-Malek Z, & Kadekaro AL (2009). alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell & Melanoma Research, 22, 809–818. [DOI] [PubMed] [Google Scholar]
- Soong J, Chen Y, Shustef EM, & Scott GA (2012). Sema4D, the ligand for Plexin B1, suppresses c-Met activation and migration and promotes melanocyte survival and growth. The Journal of Investigative Dermatology, 132, 1230–1238. 10.1038/jid.2011.414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong J, & Scott G (2013). Plexin B1 inhibits MET through direct association and regulates Shp2 expression in melanocytes. Journal of Cell Science, 126, 688–695. 10.1242/jcs.119487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starner RJ, McClelland L, Abdel-Malek Z, Fricke A, & Scott G (2010). PGE(2) is a UVR-inducible autocrine factor for human melanocytes that stimulates tyrosinase activation. Experimental Dermatology, 19, 682–684. 10.1111/j.1600-0625.2010.01074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanato CM, Yaar M, Bhawan J, Phillips TJ, Kosmadaki MG, Botchkarev V, & Gilchrest BA (2003). Modulations of nerve growth factor and Bcl-2 in ultraviolet-irradiated human epidermis. Journal of Cutaneous Pathology, 30, 351–357. 10.1034/j.1600-0560.2003.00065.x [DOI] [PubMed] [Google Scholar]
- Suzuki I, Tada A, Ollmann MM, Barsh GS, Im S, Lamoreux ML, Hearing VJ, Nordlund JJ, & Abdel-Malek ZA (1997). Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to alpha-melanotropin. The Journal of Investigative Dermatology, 108, 838–842. [DOI] [PubMed] [Google Scholar]
- Swope VB, Abdel-Malek ZA, Kassem L, & Nordlund JJ (1991). Interleukins 1α and 6 and tumor necrosis factor-α are paracrine inhibitors of human melanocyte proliferation and melanogenesis. Journal of Investigative Dermatology, 96, 180–185. [DOI] [PubMed] [Google Scholar]
- Swope V, Alexander C, Starner R, Schwemberger S, Babcock G, & Abdel-Malek ZA (2014). Significance of the melanocortin 1 receptor in the DNA damage response of human melanocytes to ultraviolet radiation. Pigment Cell& Melanoma Research, 27, 601–610. 10.1111/pcmr.12252 [DOI] [PubMed] [Google Scholar]
- Swope VB, Jameson JA, Mcfarland KL, Supp DM, Miller WE, Mcgraw DW, Patel MA, Nix MA, Millhauser GL, Babcock GF, Abdel-Malek ZA (2012). Defining MC1R regulation in human melanocytes by its agonist alpha-melanocortin and antagonists agouti signaling protein and beta-defensin 3. The Journal of Investigative Dermatology, 132, 2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swope VB, Medrano EE, Smalara D, & Abdel-Malek ZA (1995). Long-term proliferation of human melanocytes is supported by the physiologic mitogens alpha-melanotropin, endothelin-1, and basic fibroblast growth factor. Experimental Cell Research, 217, 453–459. [DOI] [PubMed] [Google Scholar]
- Swope VB, Starner RJ, Rauck C, & Abdel-Malek ZA (2020). Endothelin-1 and alpha-melanocortin have redundant effects on global genome repair in UV-irradiated human melanocytes despite distinct signaling pathways. Pigment Cell Melanoma Res, 33, 293–304. [DOI] [PubMed] [Google Scholar]
- Tada A, Suzuki I, Im S, Davis MB, Cornelius J, Babcock G, Nordlund JJ, & Abdel-Malek ZA (1998). Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth & Differentiation, 9, 575–584. [PubMed] [Google Scholar]
- Tang L, Fang W, Lin J, Li J, Wu W, & Xu J (2018). Vitamin D protects human melanocytes against oxidative damage by activation of Wnt/beta-catenin signaling. Laboratory Investigation, 98, 1527–1537. [DOI] [PubMed] [Google Scholar]
- Thody AJ, Ridley K, Penny RJ, Chalmers R, Fisher C, & Shuster S (1983). MSH peptides are present in mammalian skin. Peptides, 4, 813–816. 10.1016/0196-9781(83)90072-4 [DOI] [PubMed] [Google Scholar]
- Tomita Y, Maeda K, & Tagami H (1992). Melanocyte-stimulating properties of arachidonic acid metabolites: Possible role in postinflammatory pigmentation. Pigment Cell Research, 5, 357–361. 10.1111/j.1600-0749.1992.tb00562.x [DOI] [PubMed] [Google Scholar]
- Torres-Álvarez B, Mesa-Garza IG, Castanedo-Cázares JP, Fuentes-Ahumada C, Oros-Ovalle C, Navarrete-Solis J, & Moncada B (2011). Histochemical and immunohistochemical study in melasma: Evidence of damage in the basal membrane. The American Journal of Dermatopathology, 33, 291–295. [DOI] [PubMed] [Google Scholar]
- Tsatmali M, Graham A, Szatkowski D, Ancans J, Manning P, Mcneil CJ, Graham AM, Thody AJ (2000). α-Melanocyte-stimulating hormone modulates nitric oxide production in melanocytes. Journal of Investigative Dermatology, 114, 520–526. [DOI] [PubMed] [Google Scholar]
- Von Koschembahr AM, Swope VB, Starner RJ, & Abdel-Malek ZA (2015). Endothelin-1 protects human melanocytes from UV-induced DNA damage by activating JNK and p38 signaling pathways. Experimental Dermatology, 24, 269–274. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Graham A, Cook D, & Thody AJ (1997). Characterisation of ACTH peptides in human skin and their activation of the melanocortin-1 receptor. Pigment Cell Research, 10, 288–297. 10.1111/j.1600-0749.1997.tb00688.x [DOI] [PubMed] [Google Scholar]
- Wong CT, & Oh DH (2021). Vitamin D receptor promotes global nucleotide excision repair by facilitating XPC dissociation from damaged DNA. The Journal of Investigative Dermatology. 10.1016/j.jid.2020.11.033. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Xu Z, Chen L, Jiang M, Wang Q, Zhang C, & Xiang LF (2018). CCN1/Cyr61 Stimulates Melanogenesis through Integrin alpha-6beta1, p38 MAPK, and ERK1/2 Signaling Pathways in Human Epidermal Melanocytes. The Journal of Investigative Dermatology, 138, 1825–1833. [DOI] [PubMed] [Google Scholar]
- Yaar M, Grossman K, Eller M, & Gilchrest BA (1991). Evidence for nerve growth factor-mediated paracrine effects in human epidermis. Journal of Cell Biology, 115, 821–828. 10.1083/jcb.115.3.821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Wu C, Park HY, Panova I, Schutz G, & Gilchrest BA (2006). Bone morphogenetic protein-4, a novel modulator of melanogenesis. Journal of Biological Chemistry, 281, 25307–25314. 10.1074/jbc.M600580200 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Itami S, Watabe H, Yasumoto K, Abdel-Malek ZA, Kubo T, Rouzaud F, Tanemura A, Yoshikawa K, & Hearing VJ (2004). Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. Journal of Cell Biology, 165, 275–285. 10.1083/jcb.200311122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Passeron T, Watabe H, Yasumoto K, Rouzaud F, Hoashi T, & Hearing VJ (2007). The effects of dickkopf 1 on gene expression and Wnt signaling by melanocytes: Mechanisms underlying its suppression of melanocyte function and proliferation. The Journal of Investigative Dermatology, 127, 1217–1225. 10.1038/sj.jid.5700629 [DOI] [PubMed] [Google Scholar]
- Zhai S, Yaar M, Doyle S, & Gilchrest B (1996). Nerve growth factor rescues pigment cells from ultaviolet-induced apoptosis by upregulating BCL-2 levels. Experimental Cell Research, 224, 335–343. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wong P, Zhang L, Jacobs B, Borden EC, Aster JC, & Bedogni B (2012). A Notch1-neuregulin1 autocrine signaling loop contributes to melanoma growth. Oncogene, 31, 4609–4618. 10.1038/onc.2011.606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Tao K, Huang W, Tian Y, & Liu X (2013). Elevated expression of pleiotrophin in human hypertrophic scars. Journal of Molecular Histology, 44, 91–96. 10.1007/s10735-012-9453-8 [DOI] [PubMed] [Google Scholar]