ABSTRACT
Background:
Platelet distribution width (PDW) and PDW-to-platelet ratio (PPR) have been proven to be good prognostic indicators for many diseases. However, their prognostic values in severe burns have not been reported.
Objective:
To investigate the early time course of PDW and PPR in severe burn patients and investigate their prognostic values.
Methods:
This is a 16-year, single-center retrospective study of 590 severe burn patients. The complete blood count parameters on day 1, day 3, and day 7 postburn, including PDW and PPR, were collected. Receiver operating characteristic curves (ROC) analysis, multiple logistic regression analysis and Kaplan–Meier survival analysis were performed to evaluate the prognostic values of PDW and PPR in severe burn patients.
Results:
According to 120-day follow-up records, 96 patients were nonsurvivors and 494 patients were survivors. ROC and area under the curve (AUC) analysis showed that, for predicting 120-day prognosis, the AUC of PDW (0.782) and PPR (0.816) on day 3 was the highest, followed by the AUC of PDW (0.764) and PPR (0.750) on day 7. The ROC–AUC of PPR (0.816) on day 3 was very close to that of the ABSI score (0.818). Multiple logistic regression analysis showed that the PDW (P = 0.033 and P = 0.009) and PPR (P = 0.052 and P = 0.046) on day 3 and day 7 were all significantly independently positively associated with 120-day mortality. Kaplan–Meier survival analysis showed that high PDW and PPR were both significantly associated with a high 120-day mortality rate on day 3 and day 7.
Conclusion:
PDW and PPR on day 3 and day 7 were independent risk factors for 120-day mortality in severe burn patients. These objective and readily available prognostic indicators may be more clinically favored.
Keywords: Burn, platelet, platelet distribution width, platelet distribution width-to-platelet ratio, prognosis
Abbreviations: ABSI score, abbreviated burn severity index score, AUC, area under the curve, CBC, complete blood count, CI, confidence intervals, G-CSF, granulocyte colony stimulating factor, IL-6, interleukin-6, MPV, mean platelet volume, NLR, neutrophil-to-lymphocyte ratio, ns, no significance, OR, odds ratio, PBI, prognostic burn index, PDW, platelet distribution width, PLR, platelet-to-lymphocyte ratio, PLT, platelet, PPR, PDW-to-PLT ratio, ROC, receiver operating characteristic curves, TBSA, total burn surface area, WBC, white blood cell
INTRODUCTION
Burn injuries are a serious pathophysiological change that may cause severe morbidity and significant mortality (1). The severity of illness at the time of admission seems to determine the patient's prognosis (2). Therefore, various burn severity scores, such as abbreviated burn severity index score (ABSI score) (3), revised Baux score (4), prognostic burn index (PBI) (5), still show a good ability to predict prognosis. However, these scores are relatively cumbersome and subjective. Due to the existence of individual differences, even if the patients with the same age, the same burn area and burn depth, their outcome may be different (6). In recent years, the improvement of burn care requires the establishment of new mortality prediction indicators and prediction models (6, 7). Simple, objective and readily available prognostic indicators may be more clinically favored (8–10).
Platelet distribution width (PDW) is a platelet (PLT) index that shows changes in PLT size and reflects the heterogeneity of PLT morphology (11). It is considered to be a mirror image of PLT function and PLT production rate (12). PLT volume heterogeneity occurs during its production process, and PDW is relatively increased, which indicates that the bone marrow produces PLT and quickly releases them into the circulation (13). Peripheral thrombocytopenia is the greatest motivation to induce PLT production in bone marrow (14). Therefore, PDW and peripheral PLT appear to be negatively correlated. The PDW-to-PLT ratio (PPR) seems more interesting and valuable. The prognostic values of PDW and PPR have been confirmed in a variety of disease states, except for severe burn injury (15–17). In the early stages of burn injury, PLT activation and increased aggregation lead to a large consumption of PLT, and fluid resuscitation leads to blood dilution, leading to a progressive decrease in circulating PLT (18, 19). The circulating PLT changed significantly after the burn injury, reaching the lowest point on the 3rd day postburn, reaching the highest peak on the 15th day post-burn, and gradually returning to normal on the 24th day postburn (19). Therefore, it seems interesting to understand the changes of the PDW (a marker of PLT activation) and its derivative index PPR with the time course of burn injury. In this study, we here in first investigated the early time course of PDW and PPR in patients with severe burns, and investigated their prognostic values.
METHODS
Patients
A retrospective study was conducted on 590 patients with severe burns admitted to Fujian Medical University Union Hospital between January 2005 and December 2020. All severe burn patients were clearly informed that their demographics and clinical and laboratory data could be used in research and signed informed consent forms. This study was approved by the Ethics Committee of Fujian Medical University Union Hospital, and all procedures were carried out in accordance with relevant guidelines and regulations. The inclusion criteria for patients were as follows:
-
(1)
age ≥18 years old;
-
(2)
thermal burn and total burn surface area (TBSA) ≥30%;
-
(3)
initial fluid resuscitation within 6 h after the burn injury;
-
(4)
admission to our hospital within 12 h after the burn injury;
-
(5)
more than 7 days of hospital stay.
Following patients were excluded:
-
(1)
patients with known pre-existing heart disease, kidney disease, malignancies, autoimmune disease, infection, blood transfusions or other diseases known to alter PLT, such as immune thrombocytopenia and hematological disease;
-
(2)
patients with multiple fractures, traumatic brain injury, visceral injury or other serious combined injury;
-
(3)
evidence of antiplatelet drug use such as aspirin, clopidogrel and ticagrinol within the preceding 2 months;
-
(4)
lost to the 120-day follow-up after burn injury;
-
(5)
incomplete clinical data and/or laboratory tests.
Data collection
This is a 16-year retrospective study. Clinical data and laboratory data of severe burn patients who met the inclusion criteria were obtained from the Clinical Big Data Center of Fujian Medical University Union Hospital. The demographic and clinical data collected included age, sex, TBSA, percentage of full-thickness burns, ABSI score (20), presence of inhalation injury, length of stay, presence of sepsis as complications, and use of mechanical ventilation. The laboratory data collected are complete blood count (CBC) (CD600, Mindray) on admission (day 1), on the 3rd day after admission (day 3) and on the 7th day after admission (day 7), including white blood cell (WBC), neutrophils, lymphocyte, red blood cell (RBC), hemoglobin, PLT, mean platelet volume (MPV), PDW, and PDW-to-PLT ratio (PPR). The survival data of each patient were obtained through hospitalization records and 120-day follow-up records after burn injury. The last follow-up time was April 2021. The final enrolled patients were divided into survivors and non-survivors based on the outcome of the 120-day follow-up.
Statistical analysis
Continuous variables are expressed as the mean ± SD, and categorical variables are expressed as % (n). The comparison between the continuous variables of survivors and non-survivors was carried out by Student's t test, and the comparison of categorical variables was carried out by Fisher's exact test. To evaluate the independent effects of PDW and PPR on 120-day prognosis, a multivariate logistic regression analysis was performed. Statistically significant variables in univariate logistic regression analysis were included in multiple logistic regression analysis. The results are expressed as β, odds ratios (ORs) (95% CIs) and P values. Receiver operating characteristic (ROC) curves and the area under the curve (AUC) were generated to evaluate the prognostic values of PDW and PPR. The Uden index was used to describe the sensitivity and specificity of their truncations (Uden index = sensitivity + specificity − 1), and the optimal cut-off points were determined. The survival curves of PDW and PPR were designed by the Kaplan–Meier method and according to the results of multiple logistic regression. SPSS 21.0 software (SPSS, Chicago) was used to analyze all data. P < 0.05 was considered statistically significant.
RESULTS
Patient demographics
Between January 2005 and December 2020, a total of 699 patients met the inclusion criteria and their data were retrieved from the Clinical Big Data Center of Fujian Medical University Union Hospital. After screening by exclusion criteria, a total of 590 patients were enrolled in this study (Fig. 1). According to the records from the 120-day follow-up period, a total of 96 patients died from the initial burn injury, and 494 patients survived (Table 1). The demographic and clinical characteristics of the patients are shown in Table 1. In this study, the patient age was 45.8 ± 16.2 years, 72.4% (427) were male, the percent TBSA was 53.1 ± 19.6, the percentage of full-thickness burns was 19.2 ± 23, the ABSI score was 9.9 ± 2.4, and 37.5% (221) had inhalation injury. During the course of their hospital stay, 22.4% (132) needed mechanical ventilation, and 10.5% (62) developed sepsis. Compared with survivors, the above variables were significantly higher in non-survivors (Table 1).
Fig. 1.
CONSORT diagram describing enrollment in this study.
Table 1.
Demographics and clinical characteristics
| Variables | Non-survivors | Survivors | All patients | ∗ P |
| n | 96 | 494 | 590 | / |
| Gender, m (%) | 81.3% (78) | 70.6% (349) | 72.4% (427) | 0.034 |
| Age, years | 53.4 ± 16.1 | 44.3 ± 15.8 | 45.8 ± 16.2 | <0.001 |
| TBSA, % | 70.3 ± 23.2 | 49.8 ± 17.0 | 53.1 ± 19.6 | <0.001 |
| Percentage of full-thickness burns, % | 43.7 ± 31.6 | 14.5 ± 17.2 | 19.2 ± 23.0 | <0.001 |
| ABSI score | 12.4 ± 2.4 | 9.4 ± 2.1 | 9.9 ± 2.4 | <0.001 |
| Presence of inhalation injury, % | 71.9% (69) | 30.8% (152) | 37.5% (221) | <0.001 |
| Sepsis complications, % | 31.3% (30) | 6.5% (32) | 10.5% (62) | <0.001 |
| Mechanical ventilation, % | 57.3% (55) | 15.6% (77) | 22.4% (132) | <0.001 |
Values were presented as mean ± SD, or % (n).
P: non-survivors vs. survivors. ABSI score, abbreviated burn severity index score; TBSA, total burn surface area.
Laboratory data were grouped by the time points (day 1, day 3, and day 7) after burn injury, and compared between non-survivors and survivors (Table 2). Compared with the survivors, the PDW, MPV, and PPR of non-survivors on day 1, day 3 and day 7 were all significantly higher, while PLT was significantly lower. Compared with the survivors, the RBCs and hemoglobin of non-survivors were both significantly lower on day 3 and day 7, but there was no significant difference on day 1. Compared with survivors, lymphocytes of non-survivors had statistically significant differences only on day 3, while WBCs had differences only on day 1. Compared with the survivors, the neutrophils of non-survivors were both significantly higher on day 1 and day 7, but there was no significant difference on day 3.
Table 2.
Laboratory parameters in severe burn non-survivors and survivors
| Day 1 | Day 3 | Day 7 | |||||||
| Variables | Non-survivor | Survivor | P | Non-survivor | Survivor | P | Non-survivor | Survivor | P |
| N | 96 | 494 | \ | 96 | 494 | \ | 96 | 494 | \ |
| WBC, ×109/L | 22.68 ± 9.40 | 18.33 ± 7.63 | <0.001 | 10.44 ± 4.97 | 10.66 ± 5.35 | ns | 13.24 ± 8.24 | 11.85 ± 5.34 | ns |
| Neutrophil, ×109/L | 19.58 ± 8.17 | 15.70 ± 7.11 | <0.001 | 8.69 ± 4.39 | 8.66 ± 4.82 | ns | 11.23 ± 7.40 | 9.56 ± 4.91 | 0.037 |
| Lymphocyte, ×109/L | 1.77 ± 1.85 | 1.44 ± 1.04 | ns | 0.96 ± 0.71 | 1.09 ± 0.54 | 0.038 | 1.14 ± 1.09 | 1.22 ± 0.57 | ns |
| RBC, ×1012/L | 4.78 ± 1.05 | 4.87 ± 0.87 | ns | 3.81 ± 0.80 | 4.09 ± 0.78 | 0.001 | 2.90 ± 0.58 | 3.48 ± 0.68 | <0.001 |
| Haemoglobin, g/L | 146.8 ± 33.6 | 148.3 ± 26.7 | ns | 117.6 ± 26.0 | 124.3 ± 24.0 | 0.014 | 89.4 ± 17.6 | 105.0 ± 20.5 | <0.001 |
| PLT, ×109/L | 141.7 ± 93.7 | 183.2 ± 86.0 | <0.001 | 70.1 ± 42.2 | 129.6 ± 79.0 | <0.001 | 138.7 ± 88.9 | 204.1 ± 99.1 | <0.001 |
| PDW, % | 16.67 ± 3.42 | 15.19 ± 3.21 | <0.001 | 18.97 ± 3.05 | 15.71 ± 3.40 | <0.001 | 17.23 ± 3.67 | 13.87 ± 3.07 | <0.001 |
| MPV, % | 11.73 ± 1.22 | 11.26 ± 1.50 | 0.001 | 11.85 ± 1.17 | 11.33 ± 1.48 | 0.001 | 12.14 ± 3.23 | 10.80 ± 1.30 | <0.001 |
| PPR, ratio | 0.201 ± 0.184 | 0.115 ± 0.115 | <0.001 | 0.407 ± 0.332 | 0.169 ± 0.128 | <0.001 | 0.293 ± 0.892 | 0.093 ± 0.092 | 0.031 |
Values were presented as mean ± SD. MPV, mean platelet volume; ns, no significance; PDW, platelet distribution width; PLT, platelet count; PPR, PDW-to-PLT ratio; RBC, red blood cell; WBC, white blood cell.
ROC analysis of various prognostic biomarkers for predicting 120-day mortality
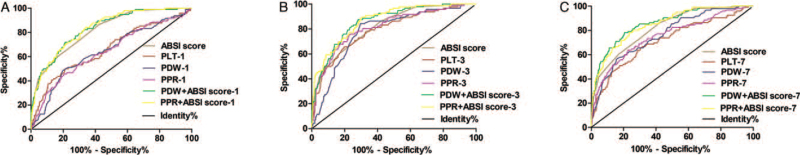
The ROC analysis results of PLT, PDW, PPR, and ABSI scores predicting the 120-day mortality of severe burn patients are shown in Table 3 and Figure 2. ROC analysis showed that the AUC of the ABSI score was 0.818 (95% CI: 0.774–0.862), and when the optimal cutoff value was 9.5, the sensitivity of predicting 120-day mortality was 86.5%, and the specificity was 57.7%. Compared with the AUC values of PLT, PDW, PPR, PDW + ABSI score and PPR + ABSI score on day 1 and day 7, the AUC values on day 3 were the highest, and were 0.792 (95% CI: 0.742–0.842), 0.782 (95% CI: 0.734–0.830), 0.816 (95% CI: 0.767–0.864), 0.861 (95% CI: 0.823–0.898), and 0.868 (95% CI: 0.832–0.905), respectively. When the optimal cutoff value of PLT was 74 × 109/L, the sensitivity was 80.7%, and the specificity was 64.9%. When the optimal cutoff value of PDW was 17.1%, the sensitivity was 84.4%, and the specificity was 70.2%. When the optimal cutoff value of PPR was 0.211, the sensitivity was 76.0%, and the specificity was 76.5%. When the optimal cutoff value of PDW + ABSI score was 0.122, the sensitivity was 87.5%, and the specificity was 71.9%. When the optimal cutoff value of PPR + ABSI score was 0.098, the sensitivity was 89.6%, and the specificity was 69.8%. On day 7, the AUC values of PLT, PDW, PPR, PDW + ABSI score and PPR + ABSI score were 0.724 (95% CI: 0.666–0.783), 0.764 (95% CI: 0.713–0.815), 0.750 (95% CI: 0.692–0.807), 0.858 (95% CI: 0.817–0.899), and 0.850 (95% CI: 0.809–0.891), respectively. When the optimal cutoff value of PLT was 143 × 109/L, the sensitivity was 72.5%, and the specificity was 63.9%. When the optimal cutoff value of PDW was 16.7%, the sensitivity was 56.3%, and the specificity was 84.4%. When the optimal cutoff value of PPR was 0.115, the sensitivity was 63.5%, and the specificity was 77.1%. When the optimal cutoff value of PDW + ABSI score was 0.170, the sensitivity was 78.1%, and the specificity was 79.6%. When the optimal cutoff value of PPR + ABSI score was 0.142, the sensitivity was 78.1%, and the specificity was 76.5%. It is worth noting that the AUC of PPR (0.816) on day 3 for predicting 120-day prognosis was very close to that of the ABSI score (0.818) (Table 3).
Table 3.
ROC analysis of various prognostic biomarkers for predicting 120-day mortality
| Variables | AUC | 95% CI | Cut-off values | Sensitivity (%) | Specificity (%) |
| ABSI score | 0.818 | 0.774–0.862 | 9.5 score | 86.5 | 57.7 |
| Day 1 | |||||
| PLT | 0.655 | 0.591–0.719 | 112 × 109/L | 79.0 | 49.5 |
| PDW | 0.646 | 0.583–0.709 | 17.5% | 49.0 | 80.0 |
| PDW + ABSI score | 0.832 | 0.789–0.874 | 0.175 | 71.9 | 78.7 |
| PPR | 0.672 | 0.608–0.736 | 0.163 | 44.8 | 85.0 |
| PPR + ABSI score | 0.834 | 0.793–0.875 | 0.102 | 84.4 | 66.0 |
| Day 3 | |||||
| PLT | 0.792 | 0.742–0.842 | 74 × 109/L | 80.7 | 64.9 |
| PDW | 0.782 | 0.734–0.830 | 17.1% | 84.4 | 70.2 |
| PDW + ABSI score | 0.861 | 0.823–0.898 | 0.122 | 87.5 | 71.9 |
| PPR | 0.816 | 0.767–0.864 | 0.211 | 76.0 | 76.5 |
| PPR + ABSI score | 0.868 | 0.832–0.905 | 0.098 | 89.6 | 69.8 |
| Day 7 | |||||
| PLT | 0.724 | 0.666–0.783 | 143 × 109/L | 72.5 | 63.9 |
| PDW | 0.764 | 0.713–0.815 | 16.7% | 56.3 | 84.4 |
| PDW + ABSI score | 0.858 | 0.817–0.899 | 0.170 | 78.1 | 79.6 |
| PPR | 0.750 | 0.692–0.807 | 0.115 | 63.5 | 77.1 |
| PPR + ABSI score | 0.850 | 0.809–0.891 | 0.142 | 78.1 | 76.5 |
ABSI, abbreviated burn severity index; AUC, area under the curve; CI, confidence interval; PDW, platelet distribution width; PLT, platelet count; PPR, PDW-to-PLT ratio; ROC, receiver operating characteristic curve.
Fig. 2.
The ROC analysis of PLT, PDW, PPR, PDW + ABSI score, PPR + ABSI score and ABSI scores predicting the 120-day mortality of severe burn patients. ROC–AUC of ABSI score was 0.818 (95% CI: 0.774–0.862). (A) The AUC values of PLT, PDW, PPR, PDW + ABSI score and PPR + ABSI score on day 1 were 0.655 (95% CI: 0.591–0.719), 0.646 (95% CI: 0.583–0.709), 0.672 (95% CI: 0.608–0.737), 0.832 (95% CI: 0.789–0.874), and 0.834 (95% CI: 0.793–0.875), respectively. (B) The AUC values of PLT, PDW, PPR, PDW + ABSI score and PPR + ABSI score on day 3 were 0.792 (95% CI: 0.742–0.842), 0.782 (95% CI: 0.734–0.830), 0.816 (95% CI: 0.767–0.864), 0.861 (95% CI: 0.823–0.898), and 0.868 (95% CI: 0.832–0.905), respectively. (C) The AUC values of PLT, PDW, PPR, PDW + ABSI score and PPR + ABSI score on day 7 were 0.724 (95% CI: 0.666–0.783), 0.764 (95% CI: 0.713–0.815), 0.750 (95% CI: 0.692–0.807), 0.858 (95% CI: 0.817–0.899), and 0.850 (95% CI: 0.809–0.891), respectively. ABSI, abbreviated burn severity index; AUC, area under the curve; CI, confidence interval; PDW, platelet distribution width; PLT, platelet count; PPR, PDW-to-PLT ratio; ROC, receiver operating characteristic curve.
Survival analysis for predicting 120-day mortality in severe burn patients
Multiple logistic regression analysis results are shown in Table 4. Age (day 1, P < 0.001; day 3, P < 0.001; and day 7, P < 0.001), ABSI score (day 1, P < 0.001; day 3, P < 0.001; and day 7, P < 0.001), mechanical ventilation (day 1, P = 0.001; day 3, P = 0.010; and day 7, P = 0.008), sepsis (day 1, P = 0.002; day 3, P = 0.005; and day 7, P = 0.008), and hemoglobin (day 7, P = 0.016) were independent predictors of 120-day mortality according to multiple logistic regression analysis. The PDW on day 3 (P = 0.033) and day 7 (P = 0.009) was significantly independently positively associated with 120-day mortality. The PPR on day 3 (P = 0.052) was borderline independently positively associated with 120-day mortality. The PPR on day 7 (P = 0.046) was an independent variable of adverse outcomes in severe burn patients. Multiple logistic regression analysis showed that PLT and MPV were not associated with 120-day mortality on day 1, day 3 or day 7.
Table 4.
Multivariate logistic regression analysis of risk factors associated with 120-day mortality
| Day 1 | Day 3 | Day 7 | |||||||
| Variables | β | OR (95% CI) | P | β | OR (95% CI) | P | β | OR (95% CI) | P |
| Age, years | 0.043 | 1.044 (1.024–1.064) | <0.001 | 0.038 | 1.038 (1.019–1.059) | <0.001 | 0.046 | 1.047 (1.025–1.069) | <0.001 |
| ABSI score | 0.283 | 1.328 (1.154–1.528) | <0.001 | 0.289 | 1.335 (1.151–1.548) | <0.001 | 0.289 | 1.335 (1.148–1.554) | <0.001 |
| Inhalation injury, % | 0.594 | 1.812 (0.958–3.428) | 0.068 | 0.651 | 1.918 (0.986–3.733) | 0.055 | 0.538 | 1.713 (0.869–3.377) | ns |
| Mechanical ventilation, % | 1.077 | 2.937 (1.531–5.634) | 0.001 | 0.887 | 2.429 (1.233–4.786) | 0.010 | 0.914 | 2.495 (1.270–4.901) | 0.008 |
| Sepsis, % | 1.129 | 3.093 (1.512–6.327) | 0.002 | 1.062 | 2.893 (1.387–6.034) | 0.005 | 0.982 | 2.671 (1.295–5.506) | 0.008 |
| WBC, ×109/L | −0.107 | 0.899 (0.571–1.416) | ns | –0.564 | 0.569 (0.244–1.328) | ns | −0.034 | 0.967 (0.719–1.301) | ns |
| Neutrophils, ×109/L | 0.157 | 1.170 (0.720–1.901) | ns | 0.623 | 1.864 (0.761–4.562) | ns | 0.104 | 1.110 (0.807–1.525) | ns |
| Lymphocytes, ×109/L | 0.131 | 1.140 (0.677–1.922) | ns | 0.528 | 1.695 (0.547–5.249) | ns | 0.034 | 1.035 (0.629–1.703) | ns |
| Haemoglobin, g/L | −0.001 | 0.999 (0.989–1.009) | ns | 0.008 | 1.008 (0.995–1.021) | ns | −0.019 | 0.981 (0.966–0.996) | 0.016 |
| MPV, % | 0.088 | 1.092 (0.881–1.352) | ns | –0.066 | 0.936 (0.748–1.170) | ns | 0.218 | 1.244 (0.992–1.559) | 0.058 |
| PLT, ×109/L | −0.002 | 0.998 (0.994–1.003) | ns | –0.005 | 0.995 (0.987–1.003) | ns | 0.000 | 1.000 (0.996–1.004) | ns |
| PDW, % | 0.020 | 1.020 (0.914–1.139) | ns | 0.118 | 1.125 (1.010–1.254) | 0.033 | 0.138 | 1.148 (1.034–1.274) | 0.009 |
| PPR, ratio | 0.647 | 1.910 (0.156–23.371) | ns | 2.048 | 7.754 (0.978–61.446) | 0.052 | 2.815 | 16.690 (1.050–265.194) | 0.046 |
P-values were shown for variables with P < 0.10. ABSI score, abbreviated burn severity index score; CI, confidence intervals; MPV, mean platelet volume; ns, no significance; OR, odd ratio; PDW, platelet distribution width; PLT, platelet count; PPR, PDW-to-PLT ratio; WBC, white blood cell.
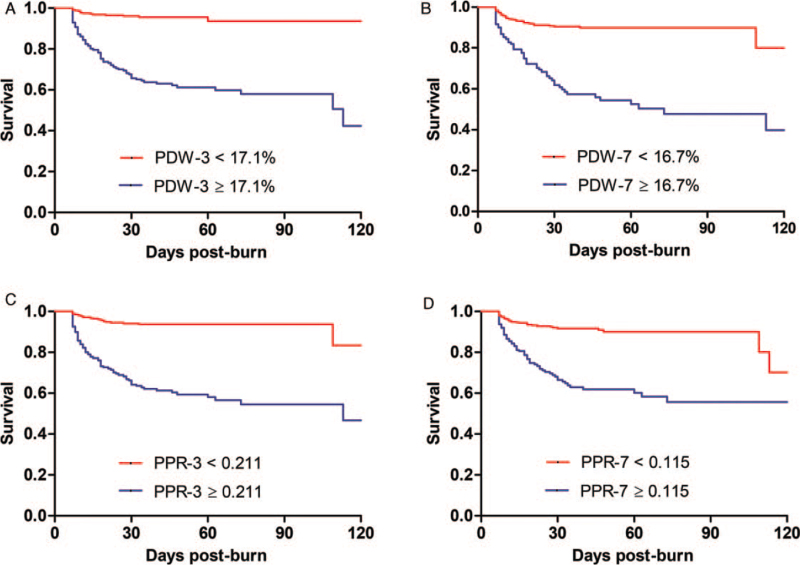
All patients were divided into two groups based on the optimal cutoff values of PDW and PPR as the cutoff points. Patients whose values were equal to or greater than the cutoff values were defined as the high group, and patients whose values were less than the cutoff values were defined as the low group. Kaplan–Meier survival curves were generated to compare the survival rates of the two groups. The results are shown in Figure 3. The high PDW group had a lower survival rate than the low PDW group (P < 0.001). The high PPR group had a lower survival rate than the low PPR group (P < 0.001). On day 3, the mortality rate in the high PDW group was 35.5%, while in the low PDW group, it was 4.1%, and the mortality rate in the high PPR group was 38.6%, while in the low PPR group, it was 5.7%. On day 7, the mortality rate in the high PDW group was 41.2%, while in the low PDW group, it was 9.2%, and the mortality rate in the high PPR group was 35.1%, while in the low PPR group, it was 8.4%.
Fig. 3.
(A) Kaplan–Meier survival curves for PDW higher or lower than 17.1% at day 3; (B) Kaplan–Meier survival curves for PDW higher or lower than 16.7% at day 7; (C) Kaplan–Meier survival curves for PPR higher or lower than 0.211 at day 3; (D) Kaplan–Meier survival curves for PPR higher or lower than 0.115 at day 7. PDW, platelet distribution width; PPR, PDW-to-PLT ratio.
DISCUSSION
The circulating PLT changes with the time course of burn injury (21). It decreased to the lowest level on the third day after burn injury, and then increased gradually (19). The main reason for this change may be related to the increase of platelet activation and aggregation after burn injury, resulting in a large amount of PLT consumption, and a large amount of fluid resuscitation leading to hemodilution (18, 22). It has been found (23, 24) that thrombocytopenia in the early stage of burns was an independent risk factor for the adverse outcomes of patients with severe burns. However, due to the existence of clinical interfering factors such as exogenous PLT supplementation and blood dilution, the accuracy of PLT in predicting the prognosis of severe burns is questionable. Platelet index, such as PDW and PPR (a derivative indicator of CBC), could be used together with PLT to assess the severity of various diseases, rather than relying solely on PLT (15, 25–27). In this study, we here in first found that PDW and PPR at 3 and 7 days postburn were independent risk factors for 120-day mortality in severe burn patients. The specific mechanism is not yet very clear. A previous study (28) has proved that PDW was a quantification of platelet heterogeneity caused by the heterogeneity of bone marrow megakaryocytes. The progressive decrease in circulating PLT in the early stages of severe burns is well known (19, 21). Circulating thrombocytopenia positive feedback induces heterogeneous proliferation of bone marrow megakaryocytes, and the young PLT produced become larger and more active (14). During the activation process, the shape of the PLT changes from a biconcave disc to a spherical shape, and obvious pseudopodia are formed, which leads to the increase of MPV and PDW during the PLT activation process (29). The more severe the burn injury, the more obvious the decrease in circulating PLT (24), the more obvious the heterogeneous proliferation of bone marrow megakaryocytes, and the higher the PDW value. After severe burns, a large number of inflammatory cytokines were produced and released into the circulation, and the more severe the burn injury was, the more inflammatory cytokines such as interleukin-6 (IL-6) and granulocyte colony stimulating factor (G-CSF) were released (7). Studies (30, 31) have shown that inflammatory cytokines, including IL-6 and G-CSF, could stimulate bone marrow megakaryocytes to increase PLT and increase PDW. Another study (32) showed that high PDW value indicated a wide range of PLT volume, which was caused by swelling, destruction and immaturity. In other words, the higher the PDW value, the more obvious the damage and immaturity of PLT. The above literature evidences may support our experimental results that PDW and PPR were independent risk factors for adverse outcomes in severe burn patients.
In this study, ROC analysis showed that the AUC of PPR (0.816) on day 3 predicted 120-day mortality was very close to the AUC of ABSI score (0.818). The ABSI score and PBI was generally recognized as a good predictor of severe burn mortality (33). However, their calculation process are relatively complex and less objective compared with the basic CBC parameters and derived indicators. An ideal biomarker must be easy to measure, repeatable and sensitive to changes in disease activity (8). Simple and easily available biomarkers are more popular and accepted by clinicians (34). PPR, the ratio of PDW to PLT, is derived from CBC parameters (35). PPR was a simple and cheap parameter, which could be evaluated in routine clinical practice with minimal additional cost, and could help clinicians quickly identify high-risk patients (26, 29, 36, 37). Our results once again confirmed the prognostic value of PPR. On day 3, the AUC of PPR for predicting 120-day mortality was 0.816, and when the optimal cut-off value was 0.211 ratio, the sensitivity was 76.0%, and the specificity was 76.5%. At present, there are few other simple and easily available biomarkers that can predict the prognosis of severe burns. A recent study (38) found that initial elevated lactate levels were a factor of poor prognosis of severe burns, however, the global clearance of lactate in the first 24 h, unlike what occurred in other injuries, did not correlate with mortality. Ding X B et al. (39) found that the ROC-AUC of serum lactate at 48 h post-admission to predict death of 127 patients was 0.811, which was very close to the ROC-AUC of PPR (0.816) on day 3 in our study. Le Q et al. (34) reported that red blood cell distribution width (RDW)-to-PLT ratio (RPR) was found to be an important prognostic indicator for severe burns, but not RDW or PLT. They found that the AUC of RPR for adverse outcome prediction on day 3 were 0.712, while 0.750 on day 7. Angulo M et al. (35) found that neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and RPR in the early stage of severe burns could identify patients with increased mortality. In our study, the AUC of PPR for predicting 120-day mortality was 0.816 on day 3, while 0.750 on day 7. PPR seems to be superior to PRP in predicting adverse outcomes of severe burns. A retrospective analysis (23) of 280 patients with TBSA ≥ 20% found that early thrombopenia and lymphopenia were independent risk factors for 60-day mortality. It is puzzling that this result was not reproduced in our study. This may be related to the inconsistent inclusion criteria of the two studies. In summary, better prognostic markers are indeed much needed in the field of severe burn injury, our study would be helpful to expand more upon other diagnostic and prognostic indicators of poor outcomes after severe burns.
Combining multiple independent risk factors to establish a risk prediction model has been proved to significantly improve the prognostic value (40, 41). There is an urgent need for novel predictive models to improve and personalize burn outcomes (7). In our study, the ability of PPR or PDW combined with ABSI score to predict the adverse outcomes of severe burns was higher than that of ABSI score alone, especially in improving the specificity of prediction. Therefore, we believe that the combined application of prognostic makers that have been identified such as ABSI score, PBI, lactate, NLR, PLR, PLT, PPR, PDW, and PRP for predicting the prognosis of severe burns may be more valuable and instructive. It is worthy of further study.
The PDW and PPR can be evaluated in routine clinical practice with minimal additional cost, and can help clinicians quickly identify high-risk burn patients (36). Since the PDW and PPR levels in non-survivors were significantly higher than those in survivors in our study, it seems feasible and attractive to improve burn survival rate by reducing PDW and PPR levels. Since the sharp decrease of peripheral platelets would lead to the increase of PDW and PPR levels (14, 29), it may be effective to reduce the PDW and PPR levels by supplementing exogenous platelets, which needs to be verified by large-scale prospective clinical studies.
There are several limitations to our research. First, this is a single center study. Although the sample size is not too small, the results may not be extrapolated. There is an urgent need for multi center and larger sample size studies to verify. Second, this is a case retrospective study, which has its own inherent limitations and can not make causal inference. It is urgent to carry out relevant prospective research. Third, other risk factors, such as race and economic status, were not recorded. Four, the daily fluctuations of PDW and PPR were not studied. The study of daily fluctuations may lead to more comprehensive conclusions.
CONCLUSION
PDW and PPR on day 3 and day 7 were independent risk factors for 120-day mortality in severe burn patients. These objective and readily available prognostic indicators may be more clinically favored.
Footnotes
JCL and GHW: These authors contributed equally to this work.
This work was supported by the Fujian Burn Medical Center ([2017]171), and Fujian Provincial Key Laboratory of Burn and Trauma, China.
Author contributions: Jian-Chang Lin, Guo-Hua Wu and Xiao-Dong Chen: study design, data collection, data analysis, data interpretation, literature search, generation of figures, writing of the manuscript; Zhao-Hong Chen: study design, writing of the manuscript; Jian-Jun Zheng: data analysis, data interpretation, literature search.
Ethics approval and consent to participate: This study was approved by the ethics committee of Fujian Medical University Union Hospital, and informed consent was obtained from each participant or their family representatives. All patients whose case reports were presented provided consent for participation.
Consent for publication: All patients whose case reports were presented provided consent for publication.
Author agreement: All authors have reviewed and approved the final version of the manuscript. The article is an original work that has not been previously published and is not under consideration for publication elsewhere.
All authors declare that there are no conflicts of interest.
Availability of data and materials: All data from the present study will be shared upon reasonable request (email address: alaspring@163.com).
REFERENCES
- 1.Brusselaers N, Monstrey S, Vogelaers D, Hoste E, Blot S. Severe burn injury in Europe: a systematic review of the incidence, etiology, morbidity, and mortality. Crit Care 14 (5):R188, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Féry-Lemonnier E, Landais P, Loirat P, Kleinknecht D, Brivet F. Evaluation of severity scoring systems in ICUs – translation, conversion and definition ambiguities as a source of inter-observer variability in Apache II, SAPS and OSF. Intensive Care Med 21 (4):356–360, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Tsurumi A, Que YA, Yan S, Tompkins RG, Rahme LG, Ryan CM. Do standard burn mortality formulae work on a population of severely burned children and adults? Burns 41 (5):935–945, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Dokter J, Meijs J, Oen Irma MMH, van Baar ME, van der Vlies CH, Boxma H. External validation of the revised Baux score for the prediction of mortality in patients with acute burn injury. J Trauma Acute Care Surg 76 (3):840–845, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Tagami T, Matsui H, Fushimi K, Yasunaga H. Validation of the prognostic burn index: a nationwide retrospective study. Burns 41 (6):1169–1175, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Duke JM, Randall SM, Wood FM, Boyd JH, Fear MW. Burns and long-term infectious disease morbidity: a population-based study. Burns 43 (2):273–281, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Jeschke MG, Gauglitz GG, Finnerty CC, Kraft R, Mlcak RP, Herndon DN. Survivors versus nonsurvivors postburn: differences in inflammatory and hypermetabolic trajectories. Ann Surg 259 (4):814–823, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisetsky DS. Anti-DNA antibodies – quintessential biomarkers of SLE. Nat Rev Rheumatol 12 (2):102–110, 2016. [DOI] [PubMed] [Google Scholar]
- 9.Fogagnolo A, Taccone FS, Benetto G, Franchi F, Scolletta S, Cotoia A, Kozhevnikova I, Volta CA, Spadaro S. Platelet morphological indices on Intensive Care Unit admission predict mortality in septic but not in non-septic patients. Minerva Anestesiol 87 (2):184–192, 2021. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Lee Y, Cho YS, Sohn YJ, Hyun JH, Ahn SM, Lee WJ, Seong H, Kim JH, Ahn JY, et al. A modified simple scoring system using the red blood cell distribution width, delta neutrophil index, and mean platelet volume-to-platelet count to predict 28-day mortality in patients with sepsis. J Intensive Care Med 36 (8):873–878, 2021. [DOI] [PubMed] [Google Scholar]
- 11.Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia 14 (1):28–32, 2010. [PMC free article] [PubMed] [Google Scholar]
- 12.Khaled SAA, NasrEldin E, Makarem YS, Mahmoud HFF. Value of platelet distribution width and mean platelet volume in disease activity score of rheumatoid arthritis. J Inflamm Res 13:595–606, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sachdev R, Tiwari AK, Goel S, Raina V, Sethi M. Establishing biological reference intervals for novel platelet parameters (immature platelet fraction, high immature platelet fraction, platelet distribution width, platelet large cell ratio, platelet-X, plateletcrit, and platelet distribution width) and their correlations among each other. Indian J Pathol Microbiol 57 (2):231–235, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Branehög I, Kutti J, Ridell B, Swolin B, Weinfeld A. The relation of thrombokinetics to bone marrow megakaryocytes in idiopathic thrombocytopenic purpura (ITP). Blood 45 (4):551–562, 1975. [PubMed] [Google Scholar]
- 15.Yuan J, Cai J, Zhao P, Zhao N, Hong RH, Ding J, Yang J, Fan QL, Zhu J, Zhou XJ, et al. Association between low-density lipoprotein cholesterol and platelet distribution width in acute ischemic stroke. Front Neurol 12:631227, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia W, Chen W, Tu J, Ni C, Meng K. Prognostic value and clinicopathologic features of platelet distribution width in cancer: a meta-analysis. Med Sci Monit 24:7130–7136, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gialluisi A, Izzi B, Di CA, Cerletti C, Donati MB, de Gaetano G, Iacoviello L. Revisiting the link between platelets and depression through genetic epidemiology: new insights from platelet distribution width. Haematologica 105 (5):e246–e248, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupia E, Bosco O, Mariano F, Dondi AE, Goffi A, Spatola T, Cuccurullo A, Tizzani P, Brondino G, Stella M, et al. Elevated thrombopoietin in plasma of burned patients without and with sepsis enhances platelet activation. J Thromb Haemost 7 (6):1000–1008, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Marck RE, Montagne HL, Tuinebreijer WE, Breederveld RS. Time course of thrombocytes in burn patients and its predictive value for outcome. Burns 39 (4):714–722, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Tobiasen J, Hiebert J, Edlich R. The abbreviated burn severity index. Ann Emerg Med 11 (5):260–262, 1982. [DOI] [PubMed] [Google Scholar]
- 21.Cato LD, Wearn CM, Bishop JRB, Stone MJ, Harrison P, Moiemen N. Platelet count: a predictor of sepsis and mortality in severe burns. Burns 44 (2):288–297, 2018. [DOI] [PubMed] [Google Scholar]
- 22.Levin GY, Egorihina MN. The role of fibrinogen in aggregation of platelets in burn injury. Burns 36 (6):806–810, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Osuka A, Ishihara T, Shimizu K, Shintani A, Ogura H, Ueyama M. Natural kinetics of blood cells following major burn: impact of early decreases in white blood cells and platelets as prognostic markers of mortality. Burns 45 (8):1901–1907, 2019. [DOI] [PubMed] [Google Scholar]
- 24.Sen S, Hsei L, Tran N, Romanowski K, Palmieri T, Greenhalgh D, Cho K. Early clinical complete blood count changes in severe burn injuries. Burns 45 (1):97–102, 2019. [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Varma R. Role of platelet distribution width (PDW) and plateletcrit in the assessment of nonthrombocytopenic preeclampsia and eclampsia. J Obstet Gynaecol India 68 (4):289–293, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo E, Zhang C, Guo L, Song K, Wang G, Duan C, Yang X, Yuan Z, Guo J, Sun J, et al. Prognostic value of platelet distribution width and mean platelet volume in patients with laryngeal cancer. Future Oncol 17 (9):1025–1037, 2021. [DOI] [PubMed] [Google Scholar]
- 27.Golwala ZM, Shah H, Gupta N, Sreenivas V, Puliyel JM. Mean platelet volume (MPV), platelet distribution width (PDW), platelet count and plateletcrit (PCT) as predictors of in-hospital paediatric mortality: a case–control study. Afr Health Sci 16 (2):356–362, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulus JM. Recent advances in the story of megakaryocyte physiology. Pathol Biol (Paris) 29 (3):133–135, 1981. [PubMed] [Google Scholar]
- 29.Budak YU, Polat M, Huysal K. The use of platelet indices, plateletcrit, mean platelet volume and platelet distribution width in emergency non-traumatic abdominal surgery: a systematic review. Biochem Med 26 (2):178–193, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AS, Hong Y, de Belder A, Beacon H, Beeso J, Sherwood R, Edmonds M, Martin JF, Erusalimsky JD. Megakaryocyte ploidy and platelet changes in human diabetes and atherosclerosis. Arterioscler Thromb Vasc Biol 17 (4):802–807, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Kaushansky K. Growth factors and hematopoietic cell fate. A new feature: controversies in hematology. Blood 92 (2):345–354, 1998. [PubMed] [Google Scholar]
- 32.Ren ZJ, Ren PW, Yang B, Liao J, Liu SZ, Lu DL, Wei X, Liu LR, Dong Q. Mean platelet volume, platelet distribution width and platelet count in erectile dysfunction: a systematic review and meta-analysis. Andrologia 49 (10):e12777, 2017. [DOI] [PubMed] [Google Scholar]
- 33.Woods JFC, Quinlan CS, Shelley OP. Predicting mortality in severe burns-what is the score? Evaluation and comparison of 4 mortality prediction scores in an Irish population. Plast Reconstr Surg Glob Open 4 (1):e606, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu L, Chen C, Li SJ, Wang C, Guo F, Peszel A, Liu S, Wang F, Sun YX, Wang YJ, et al. Prognostic values of red blood cell distribution width, platelet count, and red cell distribution width-to-platelet ratio for severe burn injury. Sci Rep 7 (1):13720, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angulo M, Moreno L, Aramendi I, Dos SG, Cabrera J, Burghi G. Complete blood count and derived indices: evolution pattern and prognostic value in adult burned patients. J Burn Care Res 41 (6):1260–1266, 2020. [DOI] [PubMed] [Google Scholar]
- 36.Qin L, Li JY, Huang WJ, Zhang ML, Wang RT, Shen W. Higher platelet distribution width is associated with unfavorable prognosis in ovarian cancer. Cancer Biomark 28 (3):365–370, 2020. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Wang H, Huang C, Meng Z, Zhang W, Li Y, Yu X, Du X, Liu M, Sun J, et al. Association between platelet distribution width and serum uric acid in Chinese population. Biofactors 45 (3):326–334, 2019. [DOI] [PubMed] [Google Scholar]
- 38.Herrero De LE, Sanchez-Sanchez M, Cachafeiro FL, Agrifoglio RA, Martínez MJ, Flores CE, Millan EP, García-de-Lorenzo A. Lactate and lactate clearance in critically burned patients: usefulness and limitations as a resuscitation guide and as a prognostic factor. Burns 46 (8):1839–1847, 2020. [DOI] [PubMed] [Google Scholar]
- 39.Ding XB, Chen J, Yang YT, Peng X, Yan H, Peng YZ. [Retrospective cohort study on the correlation between high value of lactic acid and risk of death in 127 patients with extensive burn during shock stage]. Zhonghua Shao Shang Za Zhi 35 (5):326–332, 2019. [DOI] [PubMed] [Google Scholar]
- 40.Tran NK, Albahra S, Pham TN, Holmes JH, Greenhalgh D, Palmieri TL, Wajda J, Rashidi HH. Novel application of an automated-machine learning development tool for predicting burn sepsis: proof of concept. Sci Rep 10 (1):12354, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karakaya E, Akdur A, Aydoğan C, Türk E, Sayin CB, Ayvazoğlu SE, Yücebaş SC, Alshalabi O, Haberal M. A model for acute kidney injury in severe burn patients. Burns 2021. [DOI] [PubMed] [Google Scholar]