Abstract
Objective:
To evaluate serum concentrations of polyfluoroalkyl substances (PFAS) in male firefighters and association with cardiometabolic markers.
Methods:
Serum PFAS were evaluated in 38 Arizona firefighters and 49 participants from the 2009–2010 National Health and Nutrition Examination Survey (NHANES). Cardiometabolic markers including carotid intima-medial thickness (CIMT) were measured in the firefighters.
Results:
Firefighters had elevated perfluorohexane sulfonic acid (PFHxS) and lower perfluorononanoic acid (PFNA) and perfluoroundecanoic acid (PFUA) compared to NHANES participants; for nine of the other 12 PFAS the values were not significantly different. There were significant negative associations among firefighters between perfluorodecanoic acid (PFDeA) and total cholesterol and PFUA and interleukin-6. PFAS concentrations were not associated with CIMT.
Conclusion:
PFHxS levels were elevated and PFNA and PFUA levels were decreased in firefighters compared to NHANES subjects. Serum PFAS concentrations were not associated with increased cardiometabolic risk measures in this population of firefighters.
Keywords: firefighters, cardiovascular, per- and polyfluoroalkyl substances (PFAS), National Health and Nutrition Examination Survey (NHANES), cardiometabolic, markers, carotid intima-media thickness (CIMT)
Introduction
Per- and poly-fluoroalkyl substances (PFAS) have been widely used since the 1950s in water and stain resistant coatings on fabrics, carpets, non-stick cookware, and take-out fast food containers, as well as some firefighting foams.1, 2 PFAS are persistent in the environment, are detectable in wildlife and humans worldwide, and can have an extended elimination half-life in humans (3–9 years).3 Human exposure to PFAS is predominantly through ingestion.4, 5, 6 To a lesser extent, exposure also occurs through inhalation and dermal contact.7, 8, 9 Ongoing biomonitoring since 2000 in the United States (US) has identified four PFAS chemicals, perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorohexane sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA), detectable in the serum of >95% of individuals aged 12 years or over.10, 11
Firefighters are occupationally exposed to PFAS when these chemicals are released from burning consumer products and structures including carpets, upholstered furniture, electronics, insulation material, and surface coatings.12, 13 Exposure to heat can enhance PFAS mobilization.14, 15, 16, 17 Firefighters have additional PFAS exposure through Class B aqueous film-forming foam (AFFF) products and potentially PFAS coated firefighting equipment.16, 18 Previous studies of predominantly male US firefighters have reported elevated PFHxS and perfluorodecanoic acid (PFDeA).19, 20 In California, US, women firefighters had higher serum levels of some PFAS, including PFHxS compared to office workers.21 Elevated PFOS and PFHxS serum concentrations have been observed among Australian firefighters, with a significant dose-response effect with years of exposure to AFFF.22 In Finnish firefighters, serum PFAS concentrations increased after three AFFF training sessions, with PFHxS and PFOS demonstrating the largest increases.23 PFOA has been documented in firefighter gear, and older AFFF contained PFAS that broke down to produce PFOA.20, 24
Adverse health effects observed in humans with PFAS exposure include immunotoxicity, cardiovascular disease (CVD), developmental and reproductive effects, and various cancers PFAS are also endocrine and metabolic disruptors.25, 26 Epidemiological evidence indicates a role of PFAS in disturbing lipid and liver metabolic pathways.27, 28 PFAS exposure may cause CVD through increased inflammation, atherosclerosis, and hypertension.29 PFAS serum levels have also been linked with increased carotid intima-media thickness (CIMT).30, 31
CVD is one of the most common causes of deaths in the US firefighters, accounting for almost 30% of all deaths.32, 33 However, the effects of PFAS on CVD and cardiometabolic profile specific to firefighters is currently not known. Given their potential for increased exposure, the objectives of the current study were to compare serum PFAS concentrations in firefighters to the US general population as measured in NHANES, and to evaluate for associations in firefighters between PFAS concentrations and cardiometabolic markers.
Methods
Firefighter selection
The study was approved by the University of Arizona Institutional Review Board (IRB) and written informed consent was obtained from all participants. A convenience sample of 38 male firefighters aged 49–54 from Phoenix and Tucson with greater than five years’ firefighting experience was selected from a larger research study evaluating cardiovascular risks including CIMT among 597 Phoenix and Tucson firefighters.
Socio-demographic characteristics and occupational history were collected using questionnaires (all data 2009), including information regarding age, race, ethnicity, working period as firefighters, and tobacco use. Weight was measured to the nearest 0.1 kilogram in street clothes. Height was measured without shoes. Body mass index (BMI) was calculated as weight/height2 (kg/m2). Seated systolic blood pressure (SBP, mmHg) and diastolic blood pressure (DBP, mmHg) were measured using a sphygmomanometer.
Biochemical Assays
Fasting blood samples were collected by venipuncture into vacutainer tubes (avoiding Teflon/PFAS coated collection equipment and containers). Biochemical assessment comprised of fasting insulin (FI), fasting blood glucose (FBG), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and triglycerides (TG). High sensitivity IL-6 (hsIL6) concentrations (R&D Systems, Minneapolis, MN) were measured in serum. Samples were assayed using enzyme-linked immunosorbent methodology and results were obtained using an automated microplate reader (BioTek ELx808, Winooski, VT). A four-parameter algorithm was deployed for best fit, determined by an associated automated software program (BioTek KC4, Winooski, VT). Testing was performed with standards and controls in duplicate for quality assurance purposes.
Serum PFAS levels were analyzed by the US Centers for Disease Control and Prevention (CDC) Analytical Toxicology Branch using HPLC isotopic dilution tandem mass spectrometry.34 Reference materials for the relevant matrix (serum) provided quality assurance for the performance of each analyte and stability of comparisons.11 A total of 12 congeners were analyzed including perfluorobutane sulfonic acid (PFBuS); perfluorodecanoic acid (PFDeA); perfluorododecanoic acid (PFDoA); perfluoroheptanoic acid (PFHpA); perfluorohexane sulfonic acid (PFHxS); perfluorononanoic acid (PFNA); perfluorooctane sulfonamide (PFOSA); 2-(N-ethyl-perfluorooctane sulfonamide) acetic acid (Et-PFOSA-AcOH); 2-(N-methyl-perfluorooctane sulfonamide) acetic acid (Me-PFOSA-AcOH); perfluorooctane sulfonic acid (PFOS); perfluorooctanoic acid (PFOA); and perfluoroundecanoic acid (PFUA). The level of quantification of all tested PFAS was 0.1 ng/ml except perfluorooctane sulfonic acid (PFOS) which was 0.2 ng/ml.
Carotid Intima-Media Thickness (CIMT)
CIMT was calculated from bilateral carotid ultrasound using an HD111XE unit and L9-L3 transducer (Phillips Medical Systems, Bothell, WA). Commercial software (Medical Imaging Applications, Coralville, IA) was used to measure CIMT at the posterior wall of the common carotid bilaterally using stored video files, as previously described.35
NHANES Analysis
Participants from NHANES 2009–2010 were restricted to men aged 49–54 years, comparable with the Arizona firefighters (serum collected 2009–2010, reported 2013). NHANES provides a nationally representative sample of the non-institutionalized US civilian population, using a stratified multistage probability sample, with oversampling of minorities.
Statistical analyses
Demographic differences between the firefighters and NHANES participants were assessed using t-tests for continuous measures and Chi-square tests for categorical variables. Cardiometabolic marker measurements, except for glucose and sICAM1, were log transformed to improve distributional characteristics. Measured PFAS concentrations from firefighters and NHANES participants were summarized using the geometric mean and its 95% confidence intervals. Two-sample t-tests for differences in geometric means between male firefighters and male NHANES participants were conducted. PFAS concentrations were log transformed to meet distributional assumptions of the t-test. Ethnicities from NHANES were mapped to those present in the firefighter data. Mexican Americans were classified as Hispanic and Non-Hispanic White as white. Other ethnicities were mapped to “others”.
Comparisons of multiple PFAS species between firefighters and NHANES participants used the Benjamini-Hochberg (BH) procedure to control false discovery rate. Correlations between the PFAS log concentrations were examined numerically and graphically using Pearson correlation. Linear models were used to evaluate the relationship between (log) PFAS concentrations, and inflammatory and cardiometabolic markers adjusting for ethnicity, smoking status, and scaled BMI (BMI was centered and scaled to have SD of 1). This analysis was not adjusted for multiple comparisons. Among firefighters, forest plots were used to display the relationship between PFAS analytes and cardiometabolic markers while displaying the uncertainty in the estimate. Associations between CIMT and PFAS log concentrations in the firefighters only were determined, adjusting for age, and cardiometabolic risk factors (family history of heart disease, smoking, exercise, serum concentration of HDL, Triglycerides, fasting blood sugar, and serum sICAM1 and hsIL6). There are no parallel data in NHANES regarding CIMT. All tests were two-sided and carried out at α=0.05 level of significance. Statistical analyses were performed using R (version 3.4.1).36
Results
Demographic data (Table 1) for male firefighters and NHANES indicated significant differences in proportions for race, smoking status, and mean glucose levels. The firefighters had a relatively high proportion of Caucasians compared to the NHANES group of comparable age. Cardiometabolic markers exhibited similar distributions in the two populations.
Table 1:
Descriptive characteristics of Male Arizona firefighters and Male NHANES 2009–10 participants.
| Characteristics, mean (SD) | Males | ||
|---|---|---|---|
| Arizona Firefighters Age 49–54 |
NHANES 2009–10 Age 49–54 |
P-value | |
| n | 38 | 49 | |
| Age, years | 51.38 (1.53) | 51.40 (1.83) | 1.00 |
| Ethnicity, n (%) | <.001 | ||
| White | 29 (76.30) | 45 (41.70) | |
| Hispanic | 5 (13.20) | 21 (19.4) | |
| Others | 2 (5.30) | 42 (38.9) | |
| Unknown | 2 (5.30) | 0 (0.00) | |
| Weight, kg | 93.20 (17.60) | 88.00 (21.30) | 0.230 |
| Height, cm | 177.30 (10.30) | 173.90 (9.00) | 0.110 |
| BMI, kg/m2 | 29.80 (6.00) | 28.90 (6.10) | 0.510 |
| Waist circumference, cm | 98.00 (11.20) | 101.2 (15.50) | 0.280 |
| Ever smoked, yes (%) | 7 (18.90) | 26 (53.10) | <.001 |
| HDL, mg/dL | 49.60 (14.50) | 48.80 (18.60) | 0.820 |
| LDL, mg/dL | 114.90 (43.50) | 128.50 (40.20) | 0.150 |
| Triglycerides, mg/dL | 145.90 (92.30) | 147.60 (81.0) | 0.930 |
| Total Cholesterol, mg/dL | 199.80 (44.70) | 205.60 (43.20) | 0.540 |
| Fasting Blood Glucose, mg/dL | 92.30 (8.10) | 100.90 (22.60) | 0.030 |
| sICAM1, ng/mL | 191.50 (45.40) | Not available | NA |
| hsIL6, pg/mL | 1.50 (0.83) | Not available | NA |
Comparison of the PFAS concentrations between the male firefighters and NHANES participants (Table 2) using geometric mean and corresponding confidence intervals showed significantly elevated PFHxS and significantly lower PFNA and PFUA among firefighters. For nine of the other 12 PFAS the values were not significantly different. Among firefighters, the highest serum PFAS concentration correlations (Figure 1) were between PFDeA and PFNA (r=0.68) followed by PFDeA and PFOS (r=0.57) and PFNA and PFOS (r=0.56). Among the NHANES participants, the highest correlations were between PFDeA and PFUA (r=0.71), followed by PFHxS and PFOS (r=0.66) and PFDeA and PFNA (r=0.65).
Table 2:
Distribution of PFAS in Male Arizona firefighters and Male NHANES 2009–10 participants.
| PFAS, ng/ml | Arizona Fire Fighters Age 49–54 (n = 38) |
NHANES 2009–2010 Age 49–54 (n = 49) |
|---|---|---|
| Geometric Mean (95% CI) | Geometric Mean (95% CI) | |
| Et-PFOSA-AcOH | 0.07 (0.07, 0.08) | 0.07 (0.07, 0.07) |
| Me-PFOSA-AcOH2 | 0.21 (0.17, 0.26) | 0.19 (0.15, 0.24) |
| PFBuS | 0.07 (0.07, 0.07) | 0.07 (0.07, 0.07) |
| PFDeA | 0.25 (0.22, 0.29) | 0.29 (0.25, 0.35) |
| PFDoA | 0.07 (0.07, 0.07) | 0.07 (0.07, 0.07) |
| PFHpA | 0.07 (0.07, 0.08) | 0.08 (0.07, 0.09) |
| PFHxS* | 3.07 (2.66, 3.55) | 2.05 (1.72, 2.44) |
| PFNA* | 0.93 (0.81, 1.06) | 1.43 (1.23, 1.66) |
| PFOA | 3.33 (2.89, 3.84) | 3.47 (2.98, 4.05) |
| PFOS | 13.36 (11.64, 15.34) | 11.21 (9.55, 13.15) |
| PFOSA | 0.07 (0.07, 0.07) | 0.07 (0.07, 0.07) |
| PFUA* | 0.12 (0.10, 0.14) | 0.19 (0.15, 0.24) |
PFAS log concentrations significantly different across the two groups using a two sample t-test are bolded and have an asterisk (*).
Figure 1:
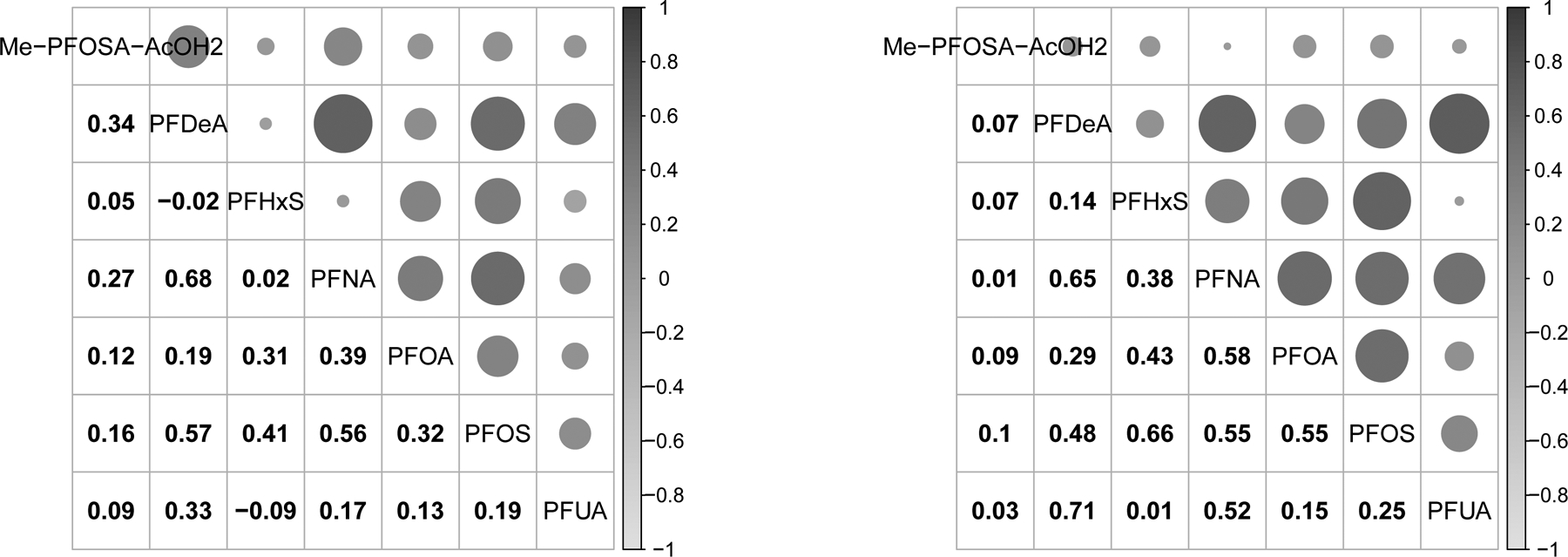
Pearson correlation between the different PFAS in male Arizona firefighters (left panel) and Male NHANES 2009–10 participants (right panel).
Forest plots (Figure 2) from the regression of cardiometabolic markers on PFAS log concentrations indicated significant negative associations among male firefighters between PFDeA and total cholesterol and PFUA and hsIL6. Exposure to PFAS among firefighters showed no association with measured atherosclerosis (CIMT) when adjusted for age, and cardiometabolic risk factors. As expected, BMI was positively associated with hsIL6 and glucose among the firefighters. BMI and waist circumference were highly correlated; therefore, BMI was adjusted in analysis and no additional adjustment for waist circumference was performed.
Figure 2:

Forest plot showing change in the cardiometabolic markers, including CIMT (points estimate and 95% confidence interval) for a unit change in the PFAS (log) concentration in male Arizona firefighters and Male NHANES 2009–10 participants. All cardiometabolic markers except blood glucose and sICAM1 are log transformed.
Discussion
Male Arizona firefighters had significantly higher PFHxS concentrations than the NHANES comparison group, and significantly lower levels of PFNA and PFUA. PFAS are known ingredients in Class B AFFFs.18, 37 These chemicals were used extensively at aircraft rescue and firefighting (ARFF) facilities based on federal requirements (14 CFR 139.317), and also used outside airports for fighting flammable liquid fires since 1970.38 Although not currently being sold, legacy AFFFs with higher toxicity longer-chain PFAS including PFOS and PFHxS are stockpiled for continuing use.18, 39, 40 Possible exposure routes are ingestion from hand-to-mouth transfer, direct skin contact with foam and inhalation of aerosolized AFFF.41 In addition, skin contact could also possibly occur with the actual foam such as when protective gear becomes soaked with foam.24 PFAS have been added to personal protective gear to make it oil, and stain resistant.42 Unfortunately records of AFFF use for the firefighters participating in the study were not available.
A limited number of previous studies have evaluated PFAS levels in firefighters. In a 2011 study of serum PFAS concentrations among California firefighters, most measured PFAS were comparable or lower than the US population, with the exception of perfluorodecanoic acid (PFDA) concentrations, which were three times higher in firefighters compared with NHANES and other published studies in firefighters.20 PFDA was not measured in our study. Mid-Ohio valley firefighters had higher mean serum PFHxS compared to the general Ohio population in 2005.19 This suggests that the firefighters had an exposure source different from or greater in magnitude than the community’s exposure through polluted drinking water as reported for this region. In a 2010 Finnish study of firefighters exposed to AFFF, elevation in serum PFHxS and PFNA were observed within two weeks of exposure to foam.24 Among Australian firefighters in 2013, serum PFOS and PFHxS were positively associated with years of employment including AFFF use, with the highest levels of PFOS and PFHxS elevated an order of magnitude compared to the general population in Australia.41 The average serum PFHxS concentration in the current study was approximately 50% higher in firefighters compared to the general US population, a smaller percent difference than observed in the Australian firefighters who had serum PFHxS concentrations 10 to 15 times higher than general population.41
More recent studies have also documented increased PFAS concentrations in firefighters compared with the general population. In a 2019 pilot study, firefighters in Southwest Ohio had 18% to 74% elevated serum concentrations of PFOA, PFOS, and PFHxS than the US general population.43 Further, as compared with the US general population, an elevated risk of diastolic blood pressure was noted in firefighters and PFOS was significantly correlated with diastolic blood pressure.43
In a separate study of individuals living near the former Pease Air Force Base, New Hampshire, in 2015, serum PFOS and PFHxS were elevated in adults and children compared with the NHANES population.38 The drinking water at Pease was contaminated with multiple PFAS, particularly PFOS and PFHxS, following AFFF use which contaminated groundwater serving three supply wells. Firefighters in this exposed group had elevated PFAS as compared to other adults in the same exposed community; PFHxS, PFOS and PFOA were 48%, 33% and 11% higher, respectively, in firefighters. Among all PFAS serum levels assessed, PFHxS was the most strongly associated with contaminated water consumption, although PFOS and PFOA serum levels were also elevated in persons who drank larger volume of contaminated water.
Exposures and adverse health effects of PFHxS have not been studied to the same extent as with PFOS or PFOA. However, animal toxicology models show a range of adverse health outcomes.44 PFHxS is utilized in a wide range of consumer products as to make fluoropolymers, fire-suppression foam, and stain- and water protective coatings for carpets, papers, and textiles. Although as with other major PFAS, PFHxS serum levels have been declining in the serum of the US population, PFHxS exposure is anticipated to persist for many years predominantly associated with stain resistant coating on consumer products such as carpeting.8, 10, 45 Among the commonly studied PFAS, PFHxS has a particularly long half-life and dwell time in the body long after exposure has ceased.46
There are a limited number of human studies regarding PFHxS health impacts. In a cross-sectional Canadian Health Measures Survey (Cycle 1 2007–2009), serum PFHxS had significant positive associations with total cholesterol (TC), low-density lipoprotein cholesterol (LDL), total cholesterol/high density lipoprotein cholesterol ratio (TC/HDL) and non-HDL cholesterol.47 However, there was no significant relation between PFHxS, PFOS, and PFOA with glycemic biomarkers or metabolic syndrome.47 Elevated serum concentrations of PFOA and PFHxS were positively associated with thyroid hormones in a US population,.48 and another cross-sectional analysis found a positive association between PFHxS and total T4.49 In a cross sectional study among the US general population, a positive association between PFAS exposure and earlier menopause was reported, which was strongest for PFHxS.50
Firefighter serum PFOS concentrations in the current study were also elevated, although not significantly higher than the NHANES comparison group. Epidemiological evidence links PFOS exposure and cardiovascular risk including elevated cholesterol, hypertension, and liver disease.38 Other systemic effects include hyperuricemia, thyroid dysfunction, immune function abnormality, adverse reproductive and developmental effects lower bone mineral density, and higher risk of osteoporosis and osteoarthritis.51 There is suggestive evidence that PFOS may also cause prostate, bladder and colorectal cancer.51 Based on its toxicity, bioaccumulation, and persistence in the environment, the Stockholm Convention on Persistent Organic Pollutants added PFOS to Annex B in 2009, restricting its production and use.52
Our study found negative associations between PFDeA and total cholesterol. Generally in population studies PFAS were associated with higher total cholesterol, higher low-density lipoprotein (LDL), and elevated triglycerides indicating a less favorable lipid profile with elevated PFAS exposure.53 In a prospective Boston-area pre-birth cohort (1999–2010), higher mid-childhood quartiles of plasma PFDeA, PFOS, and PFOA concentrations were associated with higher TC and LDL-C among girls.54 However, in this same cohort higher plasma PFOS, PFOA, and PFDeA concentrations were also associated with higher HDL-C and/or lower TG among boys and girls combined. More research will help improve our understanding of the possible role of PFDeA on lipids, and other health effects.
Our study found negative associations between PFUA and interleukin-6. Interleukin-6 is an inflammatory cytokine produced in response to infection but also involved in regulation of metabolic, and regenerative processes.55 PFAS exposure may downregulate immune system and antibody response.56, 57 Overall, there is scientific support that PFAS exposure is associated with deficient immune function however, in our study the negative association of PFUA with interleukin-6 may be a novel finding and could be substantiated with further research.
The current study was limited by the relatively small number of firefighters analyzed and their geographic area, including only Phoenix and Tucson firefighters as compared to the national distribution of participants from NHANES. No information is available to determine if any of the firefighters had worked at an airport fire station and no information is available on PFAS levels in their drinking water. Given that the blood samples were collected in 2009 and both occupational and other environmental exposures may have changed, the study findings may not be representative of current firefighter exposures. PFAS serum concentrations in the US population measured by NHANES have declined by more than 70% over the recent years due to the phase out of PFOA, PFOS, and PFHxS.11 However, the concentration of PFNA has increased.10, 58
Conclusions
Serum concentrations of PFHxS were higher in this study of Arizona firefighters than in the general US population, whereas PFNA and PFUA were lower. PFAS concentrations were not associated with measures of increased cardiometabolic risk in this study population.
Acknowledgments:
The contribution of study participants is gratefully acknowledged.
Funding Sources:
The Southwest Environmental Health Sciences Center (SWEHSC) through National Institute of Environmental Health Sciences grant P30 ES006694 and the Federal Emergency Management Agency grant EMW-2007-FP-01499
Footnotes
Conflict of Interest: none declared
Ethical Considerations & Disclosure(s) (e.g., IRB information, consent process, if applicable).
The study was approved by the University of Arizona Institutional Review Board (IRB) and written informed consent was obtained from all participants.
References
- 1.Giesy JP and Kannan K, Global distribution of perfluorooctane sulfonate in wildlife. Environmental science & technology, 2001. 35(7): p. 1339–1342. [DOI] [PubMed] [Google Scholar]
- 2.Fromme H, et al. , Perfluorinated compounds--exposure assessment for the general population in Western countries. Int J Hyg Environ Health, 2009. 212(3): p. 239–70. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, et al. , A Never-Ending Story of Per-and Polyfluoroalkyl Substances (PFASs)? 2017, ACS Publications. [DOI] [PubMed] [Google Scholar]
- 4.Tittlemier SA, Pepper K, and Edwards L, Concentrations of perfluorooctanesulfonamides in Canadian total diet study composite food samples collected between 1992 and 2004. J Agric Food Chem, 2006. 54(21): p. 8385–9. [DOI] [PubMed] [Google Scholar]
- 5.Trudel D, et al. , Estimating consumer exposure to PFOS and PFOA. Risk Anal, 2008. 28(2): p. 251–69. [DOI] [PubMed] [Google Scholar]
- 6.Zhang T, et al. , Perfluorinated compounds in human blood, water, edible freshwater fish, and seafood in China: daily intake and regional differences in human exposures. J Agric Food Chem, 2011. 59(20): p. 11168–76. [DOI] [PubMed] [Google Scholar]
- 7.Stahl T, Mattern D, and Brunn H, Toxicology of perfluorinated compounds. Environmental Sciences Europe, 2011. 23(1): p. 38. [Google Scholar]
- 8.Beesoon S, et al. , Exceptionally high serum concentrations of perfluorohexanesulfonate in a Canadian family are linked to home carpet treatment applications. Environ Sci Technol, 2012. 46(23): p. 12960–7. [DOI] [PubMed] [Google Scholar]
- 9.Franko J, et al. , Dermal penetration potential of perfluorooctanoic acid (PFOA) in human and mouse skin. J Toxicol Environ Health A, 2012. 75(1): p. 50–62. [DOI] [PubMed] [Google Scholar]
- 10.Kato K, et al. , Trends in Exposure to Polyfluoroalkyl Chemicals in the US Population: 1999–2008. Environ Sci Technol, 2011. 45(19): p. 8037–8045. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (January 2019). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/ https://www.cdc.gov/biomonitoring/PFAS_BiomonitoringSummary.html [Accessed 24 July 2020]. 2019. [Google Scholar]
- 12.Prevedouros K, et al. , Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol, 2006. 40(1): p. 32–44. [DOI] [PubMed] [Google Scholar]
- 13.Lau C, et al. , Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci, 2007. 99(2): p. 366–94. [DOI] [PubMed] [Google Scholar]
- 14.Ellis DA, et al. , Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature, 2001. 412(6844): p. 321–4. [DOI] [PubMed] [Google Scholar]
- 15.Ellis DA, et al. , The use of 19F NMR and mass spectrometry for the elucidation of novel fluorinated acids and atmospheric fluoroacid precursors evolved in the thermolysis of fluoropolymers. Analyst, 2003. 128(6): p. 756–64. [DOI] [PubMed] [Google Scholar]
- 16.OECD, Organisation for Economic Co-operation and Development (OECD). Environment Directorate. 2002. “Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and its Salts.” www.oecd.org/chemicalsafety/risk-assessment/2382880.pdf. 2002. [Google Scholar]
- 17.Williams SJ, Baker BB, and Lee KP, Formation of acute pulmonary toxicants following thermal degradation of perfluorinated polymers: evidence for a critical atmospheric reaction. Food Chem Toxicol, 1987. 25(2): p. 177–85. [DOI] [PubMed] [Google Scholar]
- 18.Place BJ and Field JA, Identification of novel fluorochemicals in aqueous film-forming foams used by the US military. Environ Sci Technol, 2012. 46(13): p. 7120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin C, et al. , Perfluoroalkyl Acids Including Perfluorooctane Sulfonate and Perfluorohexane Sulfonate in Firefighters. Journal of Occupational and Environmental Medicine, 2011. 53(3): p. 324–328. [DOI] [PubMed] [Google Scholar]
- 20.Dobraca D, et al. , Biomonitoring in California firefighters: metals and perfluorinated chemicals. J Occup Environ Med, 2015. 57(1): p. 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trowbridge J, et al. , Exposure to perfluoroalkyl substances in a cohort of women firefighters and office workers in San Francisco. Environmental science & technology, 2020. 54(6): p. 3363–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotander A, et al. , Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). Environment International, 2015. 82: p. 28–34. [DOI] [PubMed] [Google Scholar]
- 23.Laitinen JA, et al. , Firefighters’ exposure to perfluoroalkyl acids and 2-butoxyethanol present in firefighting foams. Toxicology Letters, 2014. 231(2): p. 227–232. [DOI] [PubMed] [Google Scholar]
- 24.Laitinen JA, et al. , Firefighters’ exposure to perfluoroalkyl acids and 2-butoxyethanol present in firefighting foams. Toxicol Lett, 2014. 231(2): p. 227–32. [DOI] [PubMed] [Google Scholar]
- 25.Heindel JJ, et al. , Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol, 2017. 68: p. 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gore A, et al. , Executive summary to EDC-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocrine reviews, 2015. 36(6): p. 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiger SD, et al. , The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere, 2014. 98: p. 78–83. [DOI] [PubMed] [Google Scholar]
- 28.Nelson JW, Hatch EE, and Webster TF, Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general US population. Environmental health perspectives, 2010. 118(2): p. 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian Y, et al. , Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: role in endothelial permeability. J Toxicol Environ Health A, 2010. 73(12): p. 819–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lind PM, et al. , Changes in plasma levels of perfluoroalkyl substances (PFASs) are related to increase in carotid intima-media thickness over 10 years - a longitudinal study. Environ Health, 2018. 17(1): p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CY, et al. , Association between levels of serum perfluorooctane sulfate and carotid artery intima-media thickness in adolescents and young adults. Int J Cardiol, 2013. 168(4): p. 3309–16. [DOI] [PubMed] [Google Scholar]
- 32.Soteriades ES, et al. , Cardiovascular disease in US firefighters: a systematic review. Cardiol Rev, 2011. 19(4): p. 202–15. [DOI] [PubMed] [Google Scholar]
- 33.Daniels RD, et al. , Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup Environ Med, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato K, et al. , Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A, 2011. 1218(15): p. 2133–7. [DOI] [PubMed] [Google Scholar]
- 35.Burgess JL, et al. , Risk factors for subclinical atherosclerosis in firefighters. J Occup Environ Med, 2012. 54(3): p. 328–35. [DOI] [PubMed] [Google Scholar]
- 36.Team, R.C., R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Accessed 27 July 2020] 2020. [Google Scholar]
- 37.OECD/UNEP Global PFC Group. Synthesis Paper on per- and polyfluorinated chemicals (PFCs). 2013. 12/April/13]; Available from: http://www.oecd.org/env/ehs/risk-management/PFC_FINAL-Web.pdf.
- 38.ATSDR., Feasibility assessment for epidemiological studies at Pease International Tradesport, Portsmouth, New Hampshire. US Department of Health and Human Services. https://www.atsdr.cdc.gov/pfas/docs/pease/pease-feasibility-assessment-november-2017-508.pdf [Accessed 27 July 2020]. 2017. [Google Scholar]
- 39.Lindstrom AB, Strynar MJ, and Libelo EL, Polyfluorinated compounds: past, present, and future. Environ Sci Technol, 2011. 45(19): p. 7954–61. [DOI] [PubMed] [Google Scholar]
- 40.Moody CA, et al. , Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in groundwater at a fire-training area at Wurtsmith Air Force Base, Michigan, USA. Journal of Environmental Monitoring, 2003. 5(2): p. 341–345. [DOI] [PubMed] [Google Scholar]
- 41.Rotander A, et al. , Elevated levels of PFOS and PFHxS in firefighters exposed to aqueous film forming foam (AFFF). Environ Int, 2015. 82: p. 28–34. [DOI] [PubMed] [Google Scholar]
- 42.Peaslee GF, et al. , Another Pathway for Firefighter Exposure to Per-and Polyfluoroalkyl Substances: Firefighter Textiles. Environmental Science & Technology Letters, 2020. [Google Scholar]
- 43.Leary DB, et al. , Perfluoroalkyl Substances and Metabolic Syndrome in Firefighters: A Pilot Study. Journal of Occupational and Environmental Medicine, 2020. 62(1): p. 52–57. [DOI] [PubMed] [Google Scholar]
- 44.Ramhøj L, et al. , Perfluorohexane Sulfonate (PFHxS) and a Mixture of Endocrine Disrupters Reduce Thyroxine Levels and Cause Antiandrogenic Effects in Rat. Toxicological Sciences, 2018. 163(2): p. 579–591. [DOI] [PubMed] [Google Scholar]
- 45.Barzen-Hanson KA, et al. , Discovery of 40 classes of per-and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environmental science & technology, 2017. 51(4): p. 2047–2057. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, et al. , Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occupational and environmental medicine, 2018. 75(1): p. 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fisher M, et al. , Do perfluoroalkyl substances affect metabolic function and plasma lipids?--Analysis of the 2007–2009, Canadian Health Measures Survey (CHMS) Cycle 1. Environ Res, 2013. 121: p. 95–103. [DOI] [PubMed] [Google Scholar]
- 48.Wen LL, et al. , Association between serum perfluorinated chemicals and thyroid function in U.S. adults: the National Health and Nutrition Examination Survey 2007–2010. J Clin Endocrinol Metab, 2013. 98(9): p. E1456–64. [DOI] [PubMed] [Google Scholar]
- 49.Jain RB, Association between thyroid profile and perfluoroalkyl acids: data from NHNAES 2007–2008. Environ Res, 2013. 126: p. 51–9. [DOI] [PubMed] [Google Scholar]
- 50.Taylor KW, et al. , Polyfluoroalkyl chemicals and menopause among women 20–65 years of age (NHANES). Environ Health Perspect, 2014. 122(2): p. 145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.EPA, Health Effects Support Document for Perfluorooctane Sulfonate (PFOS).EPA 822-R-16–002. www.epa.gov/ground-water-anddrinking-water/supporting-documents-drinkingwater-health-advisories-pfoa-and-pfos. 2016.
- 52.Stockholm C, What Are POPs?chm.pops.int/TheConvention/ThePOPs/tabid/673/Default.aspx. 2016.
- 53.Kirk M, et al. , The PFAS Health Study: Systematic Literature Review. Canberra: The Australian National University, 2018. [Google Scholar]
- 54.Mora AM, et al. , Early life exposure to per-and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environment international, 2018. 111: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheller J, et al. , The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 2011. 1813(5): p. 878–888. [DOI] [PubMed] [Google Scholar]
- 56.ATSDR., Agency for Toxic Substances and Disease Registry (ATSDR). 2018. Toxicological profile for Perfluoroalkyls. (Draft for Public Comment). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=1117&tid=237#bookmark01 [Accessed 24 July 2020]. 2018. [Google Scholar]
- 57.Grandjean P, et al. , Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. Journal of immunotoxicology, 2017. 14(1): p. 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang M, Park J-S, and Petreas M, Temporal changes in the levels of perfluorinated compounds in California women’s serum over the past 50 years. Environmental science & technology, 2011. 45(17): p. 7510–7516. [DOI] [PubMed] [Google Scholar]


