Abstract
The standard treatment of human visceral leishmaniasis involves the use of pentavalent antimony (SbV) compounds. In recent years increasing numbers of clinical failures of treatment with SbV have been reported, probably due to the development of parasite resistance to this compound. The mode of action and mechanisms of resistance to SbV have not been fully elucidated. In the present study an axenic amastigote culture was used to study the in vitro responses of Leishmania donovani to SbV. Susceptibility to both sodium stibogluconate and meglumine antimoniate was found to be stage specific. Amastigotes were 73 to 271 times more susceptible to SbV than were promastigotes. As opposed to SbV, trivalent antimony (SbIII) was similarly toxic to both developmental stages. When promastigotes were transformed to amastigotes, susceptibility to meglumine antimoniate developed after 4 to 5 days, upon the completion of differentiation. In contrast, with transformation from amastigotes to promastigotes, resistance to meglumine antimoniate was acquired rapidly, within 24 h, before the completion of differentiation. The culture of promastigotes at an acidic pH (5.5) or at an elevated temperature (37°C) alone did not lead to the appearance of SbV susceptibility, emphasizing the requirement of both these environmental factors for the development of SbV susceptibility. A previously isolated sodium stibogluconate (Pentostam)-resistant L. donovani mutant (Ld1S.20) is also resistant to meglumine antimoniate, indicating cross-resistance to SbV-containing compounds. In contrast, no cross-resistance was found with SbIII, suggesting a mechanism of SbV resistance different from that described in Leishmania tarentolae. These data show that L. donovani susceptibility to SbV is parasite intrinsic, stage specific, and macrophage independent.
Leishmania donovani is the major causative agent of visceral leishmaniasis. It exists either as the extracellular promastigote found in the alimentary tract of sandflies or as the obligatory intracellular amastigote found in the phagolysosomes of mammalian macrophages (7, 8). Historically, promastigotes have been readily cultured in cell-free media, while amastigotes were maintained either in animals or in macrophage cell lines. Thus, most of the in vitro studies on leishmanial cell biology as well as antileishmanial drug action or resistance have been performed with promastigotes. During the last few years, several laboratories have succeeded in culturing L. donovani amastigotes axenically (12, 13, 23, 30, 33). This technique allows the direct evaluation of cell biological and biochemical processes in the amastigote, which is devoid of the host macrophage.
The treatment of choice of human visceral leishmaniasis is the administration of a pentavalent antimony (SbV)-containing drug, sodium stibogluconate (Pentostam; The Wellcome Foundation Ltd., London, United Kingdom) or meglumine antimoniate (Glucantime; Rhone-Poulenc, Paris, France). Despite the extensive use of these compounds over the last decades, their mechanism of action remains unclear. Furthermore, clinical resistance to these drugs is being increasingly reported (3, 15, 16, 21, 25, 27).
On the basis of data showing that trivalent antimony (SbIII) is much more toxic to Leishmania than SbV (5, 9, 28), it has been hypothesized that SbV is accumulated by the macrophage and is reduced to SbIII in the phagolysosome, the site where the antileishmanial activity of antimony occurs (28). Recent studies have suggested that at least in the case of New World species, this hypothesis is not applicable because axenic amastigotes of Leishmania mexicana are as susceptible to SbV as intracellular amastigotes (6). An additional possibility is that SbV is directly and specifically toxic to amastigotes (14).
The host and parasite roles in these phenomena have yet to be defined. Cross-resistance between SbIII and SbV has been shown in nonpathogenic Leishmania tarentolae (11, 18), in which trypanothione plays a major role in parasite resistance to antimony. SbV is reduced to SbIII, which then forms complexes with trypanothione (10, 17, 26). However, this mechanism is apparently different in Leishmania spp. pathogenic to humans (24).
To date, Pentostam-based studies have only indirectly assessed the antileishmanial activity of SbV because of two problems: chlorocresol toxicity to both macrophage and parasite and the fact that amastigotes were studied in macrophage cell culture (21, 28, 29). In order to delineate the direct effect of SbV on both stages of L. donovani, the activities of sodium stibogluconate (without chlorocresol) and meglumine antimoniate were studied with an axenic culture system (30).
MATERIALS AND METHODS
Materials.
Meglumine antimoniate was a gift from Rhone-Poulenc. Sodium stibogluconate was a gift from The Wellcome Foundation Ltd. Ornithine, leupeptine, and E64 were obtained from Sigma Chemical Co. (St. Louis, Mo.). Medium 199 and fetal calf serum were obtained from Biological Industries, Inc. (Beit Haemek, Israel). All other chemicals were of analytical grade.
Parasites.
A cloned line of L. donovani 1SR (13, 30) and the Pentostam-resistant mutant L. donovani Ld1S.20 (14) were used.
Cell culture.
Promastigotes were grown at 26°C in medium 199 supplemented with 10% fetal calf serum. In vitro culture of amastigotes was performed as described by Saar et al. (30).
Transformation of amastigotes to promastigotes was performed by centrifugation of amastigotes (1,200 × g at room temperature for 10 min), suspension in promastigote medium, and incubation at 26°C. Under these conditions amastigotes differentiate to promastigotes within 48 h (30).
Assessment of effect of pH and temperature on Leishmania susceptibility to meglumine antimoniate.
The effect of acidic pH was determined by growing promastigotes at a pH of 5.5 and a temperature of 26°C and assessing their susceptibility to meglumine antimoniate, as described previously (14), periodically for up to 2 weeks. The effect of temperature was determined by growing promastigotes at a temperature of 37°C and a pH of 7.4 and assessing their susceptibility to meglumine antimoniate during a similar period.
Enzymatic assay of ODC activity.
Quantification of organisms was accomplished by measuring ornithine decarboxylase (ODC) activity and calculating relative cell density as described previously (14). In control experiments, the levels of ODC in wild-type (1SR) and mutant (Ld1S.20) L. donovani strains were compared and found to be the same in both promastigotes and axenic amastigotes.
RESULTS
The stage specificity of L. donovani susceptibility to SbV in the form of sodium stibogluconate was evaluated. Figure 1A shows the dose-response of L. donovani to sodium stibogluconate (free of the preservative chlorocresol). Amastigotes are susceptible (50% inhibitory concentration [IC50], 19.3 ± 2.3 μg of SbV/ml). For promastigotes, in contrast, the IC50 is 5,230 ± 980 μg of SbV/ml, and promastigotes still show a 27% relative cell density at an SbV concentration of 10 mg of SbV/ml. As shown in Fig. 1B, the dose-response of L. donovani to SbV in the form of meglumine antimoniate resembles the parasite’s response to sodium stibogluconate. Amastigotes are susceptible (IC50, 80 ± 18 μg of SbV/ml). For promastigotes, in contrast, the IC50 is 5,830 ± 1,000 μg of SbV/ml, and promastigotes still show a 38% relative cell density at an SbV concentration of 10 mg/ml.
FIG. 1.
Stage-specific susceptibility of L. donovani to SbV. Promastigotes (○) and amastigotes (•) were incubated in the presence of increasing concentrations of sodium stibogluconate (A) and meglumine antimoniate (B) for 48 h and were assayed for ODC activity as described in Materials and Methods. The results are expressed as means ± standard deviations (n = 6).
SbIII in the form of potassium antimonyl tartrate is more toxic than SbV to both promastigotes and amastigotes (28, 29). In order to elucidate the differential effects of antimony compounds (SbV and SbIII) on the two stages of L. donovani 1SR, the activity of SbIII was determined. As opposed to SbV, both axenic amastigotes and promastigotes are susceptible to low concentrations of SbIII (IC50s, of 3.6 ± 0.29 and 13.0 ± 2.4 μg of SbIII/ml, respectively, a 3.5-fold difference as opposed to the 73- and 271-fold differences for meglumine antimoniate and sodium stibogluconate, respectively, described above; Fig. 2). These findings further emphasize the marked stage specificity of SbV as opposed to that of SbIII.
FIG. 2.
Dose-response curve for L. donovani to SbIII. Promastigotes (○) and amastigotes (•) were incubated in the presence of increasing concentrations of potassium antimonyl tartrate for 48 h and were assayed for ODC activity as described in Materials and Methods. The results are expressed as means ± standard deviations (n = 3).
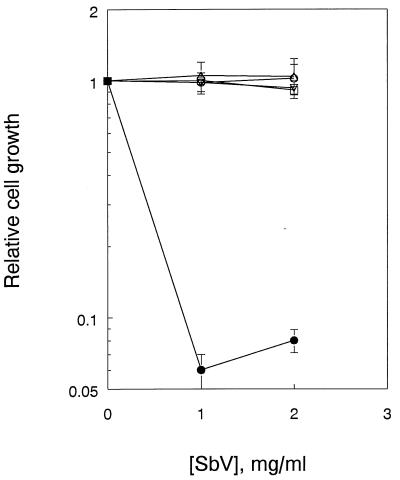
In order to show that extrinsic conditions of the promastigote growth medium do not affect SbV activity, the independent effects of pH and temperature on the susceptibility of promastigotes to SbV were evaluated. Figure 3 shows that the resistance of promastigotes to SbV remains unchanged over a period of up to 2 weeks when they are subjected to changes of either pH or temperature alone. These data imply that extracellular reduction of SbV to SbIII under the experimental conditions described does not occur. Together with the data in Fig. 1, it seems that SbV possesses intrinsic antileishmanial activity which is stage specific.
FIG. 3.
Dose-response curve for L. donovani promastigotes and meglumine antimoniate: effects of pH and temperature over time. Promastigotes were maintained at 26°C and pH 7.4 (■), at 37°C and pH 7.4 (•), or at 26°C and pH 5.5 (○). After ∼2 weeks, the parasites were incubated and were assayed for ODC activity.
In order to show that SbV resistance is common to both commercial SbV preparations (sodium stibogluconate [Pentostam] and meglumine antimoniate [Glucantime]), the activity of SbV against L. donovani Ld1S.20, a Pentostam-resistant mutant of L. donovani 1SR, was assessed. Table 1 shows that this mutant is resistant to both sodium stibogluconate and meglumine antimoniate. Compared to the wild type, amastigotes of L. donovani Ld1S.20 are 64 and 19 times more resistant to SbV in the form of sodium stibogluconate and meglumine antimoniate, respectively.
TABLE 1.
IC50 of SbIII and SbV for L. donovani 1SR (wild type) and Ld1S.20a
| Compound | IC50 (μg/ml)
|
|||
|---|---|---|---|---|
| 1SR
|
Ld1S.20
|
|||
| Promastigotes | Amastigotes | Promastigotes | Amastigotes | |
| Meglumine antimoniate (SbV) | 5,830 ± 1,000 | 80 ± 18 | 6,000 ± 960 | 1,500 ± 210 |
| Sodium stibogluconate (SbV) | 5,230 ± 980 | 19.3 ± 2.3 | 7,800 ± 880 | 1,230 ± 150 |
| Potassium antimonyl tartrate (SbIII) | 13.0 ± 2.4 | 3.6 ± 0.29 | 9.7 ± 1.7 | 7.3 ± 1.45 |
Arsenite (As)-resistant mutants of L. tarentolae show cross-resistance to SbIII and SbV (5, 11). Furthermore, it was recently shown that sodium stibogluconate-resistant mutants of this species show cross-resistance to SbV, SbIII, and As (18). Given the previously mentioned difference in the mechanism of resistance between nonpathogenic L. tarentolae and pathogenic Leishmania species (L. major, L. donovani, and L. mexicana), the possibility of cross-resistance between SbV and SbIII was investigated in Pentostam-resistant L. donovani mutant Ld1S.20 (14). As shown in Table 1, both promastigotes and amastigotes of wild-type and Ld1S.20 L. donovani strains were equally susceptible to SbIII but not to SbV. This indicates that in L. donovani, resistance to SbV can occur by a mechanism that is not related to SbIII susceptibility.
The axenic amastigote culture system also allows the evaluation of changes in parasite susceptibility to SbV during the process of differentiation. Relative cell density was measured every 24 h for 6 to 7 days beginning with the induction of differentiation. In order to ensure good discrimination between susceptibility and resistance, concentrations of meglumine antimoniate highly toxic to amastigotes but essentially nontoxic to promastigotes were chosen, 1,000 and 2,000 μg of SbV/ml were used because even 1,000 μg of SbV/ml is a concentration well above the IC90 for amastigotes (Fig. 1).
Figure 4 demonstrates that when promastigotes are transformed to amastigotes, the development of SbV susceptibility occurs within 4 to 5 days. Previous studies have shown that 4 to 5 days is required for the completion of differentiation of promastigotes to amastigotes (1, 2, 22, 30). These results indicate that the development of SbV susceptibility parallels the differentiation process.
FIG. 4.
Development of L. donovani susceptibility to meglumine antimoniate during in vitro transformation of promastigotes to amastigotes. Promastigotes were transformed to amastigotes as follows: mid-logarithmic-phase promastigotes were transferred to 37°C for 24 h and then shifted to pH 5.5 as described in Materials and Methods. Meglumine antimoniate dose-response measurements were performed daily over 5 additional days: 1 day at 37°C and pH 7.4 (•) and 1 day (▴), 2 days (⧫), 3 days (■), 4 days (▾), and 5 days (✚) at 37°C and pH 5.5. The results are expressed as means ± standard deviations (n = 3).
In the process of transformation from amastigotes back to promastigotes, previous studies have shown that more than 24 and up to 48 h is required for full parasite differentiation (30). Figure 5 shows that resistance to SbV was acquired within 24 h of the onset of transformation, prior to the completion of full differentiation from amastigotes to promastigotes.
FIG. 5.
Loss of L. donovani susceptibility to meglumine antimoniate during in vitro transformation of amastigotes to promastigotes. Amastigotes (•) were transformed to promastigotes as follows: amastigotes were centrifuged, suspended in promastigote medium, and incubated at 26°C and pH 7.4 as described in Materials and Methods. Meglumine antimoniate dose-response measurements were performed daily for 5 additional days: day 1 (○), day 2 (▵), day 3 (□), and day 5 (▿). The results are expressed as means ± standard deviations (n = 3).
DISCUSSION
Axenic amastigote and promastigote culture systems were used to evaluate the direct effects of SbV on the two developmental forms of L. donovani. Susceptibility to SbV is stage specific. Promastigotes are relatively resistant (meglumine antimoniate IC50, 5,830 μg of SbV/ml; sodium stibogluconate IC50, 5,230 μg of SbV/ml), although at high concentrations they are somewhat susceptible (27 and 38% growth with 10 mg of SbV/ml as sodium stibogluconate and meglumine antimoniate, respectively). In contrast, amastigotes are highly susceptible to both forms of SbV, with the meglumine antimoniate IC50 being 73 times lower (80 μg SbV/ml) and the sodium stibogluconate IC50 being 271 times lower (19.3 μg SbV/ml) for amastigotes than for promastigotes.
In agreement with previous data (12, 28) and as opposed to SbV, SbIII is highly toxic to both amastigotes and promastigotes. For both forms of the pathogenic species L. donovani, SbIII IC50s are similar, with only a 3- to 4-fold difference between the IC50s for promastigotes and amastigotes, whereas the difference for SbV is 73- to 271-fold, indicating that the activity of SbIII is not stage specific. Because these data are derived from experiments with axenic cultures, the difference in activities between SbV and SbIII is clearly macrophage independent. Furthermore, the possibility that SbV (either as sodium stibogluconate or as meglumine antimoniate) is reduced to SbIII by the growth medium has been ruled out. Therefore, we conclude that SbV enters the parasite cells and subsequently, either directly or after intracellular reduction to SbIII, exerts its antileishmanial effect.
To date, a direct effect of SbV on amastigotes has been shown, but it has not been fully characterized. For example, in L. mexicana, axenic amastigotes are 370 times more susceptible than promastigotes (6). It has been hypothesized that in vivo, SbV activity is indirect and results from macrophage-dependent reduction of minimally toxic SbV to highly toxic SbIII (4, 29). If this, in fact, occurs, it is probably not due to the spontaneous reduction of SbV in the acidic environment of the lysosome but might be via an enzymatic reaction. The data in Fig. 3 show that SbV exerts an effect on axenic amastigotes of L. donovani which is macrophage independent and exclude the possibility of spontaneous reduction since neither acidic pH nor elevated temperature alone resulted in increased toxicity of SbV to promastigotes. Hence, SbV is specifically toxic to Leishmania amastigotes in axenic culture. The possibility that enzymatic reduction of SbV to SbIII occurs in vivo by the macrophage cannot be excluded.
Roberts et al. (28) showed stage-specific differences in the accumulation of SbV in the form of sodium stibogluconate in Leishmania panamensis. In order to reach the IC50, promastigotes needed to be exposed to a concentration ∼90 times greater than that to which amastigotes were exposed. This difference was required to achieve equivalent intracellular concentrations of SbV at the IC50. These data, as well as those presented here, suggest that differential SbV accumulation is a major factor in the determination of amastigote and promastigote susceptibility to SbV.
The kinetics of the development of SbV susceptibility was evaluated during the process of parasite differentiation. As shown both in vivo (7, 22) and in vitro (23, 30), full differentiation from promastigotes to amastigotes occurs over a period of 4 to 5 days. In axenic culture, the full differentiation of promastigotes to amastigotes requires changing both pH and temperature (1, 2, 12, 19, 23, 30, 31). When promastigotes are grown at pH 5.5 and 26°C or pH 7.4 and 37°C, parasite susceptibility to SbV does not develop. Only the combination of elevated temperature and acidic pH will result in full parasite differentiation, and only fully differentiated axenic amastigotes are susceptible to SbV.
Not all stage-specific functions of L. donovani require both pH and temperature changes. For example, the expression of proline transport systems is regulated by extracellular pH (32). Also, the expression of amastigote-specific heat shock protein 100 is regulated by temperature (20). It seems, therefore, that the phenotypic expression of SbV susceptibility can serve as a marker of complete parasite differentiation in vitro.
As shown in vitro, full differentiation from amastigotes to promastigotes occurs over a period of about 48 h (30). When axenic amastigotes are exposed to growth conditions that induce their differentiation to promastigotes, the loss of SbV susceptibility occurs by 24 h and thus is not dependent on completion of the transformation process.
A previously isolated Pentostam-resistant L. donovani mutant (Ld1S.20) is resistant to chlorocresol and, by inference, sodium stibogluconate (14). Current data show that this mutant is resistant to both sodium stibogluconate and meglumine antimoniate, indicating that resistance is related to the SbV component of both formulations. The results obtained with this mutant, isolated from promastigotes grown continuously in Pentostam (14), together with the data presented in Fig. 1, suggest that promastigotes are somewhat susceptible to SbV. These data also support the idea that L. donovani Ld1S.20 is resistant to both SbV and chlorocresol. Furthermore, no cross-resistance was observed between SbIII and SbV since promastigotes and amastigotes of both the wild-type strain 1SR and the mutant strain Ld1S.20 were similarly susceptible to SbIII.
Previous studies have shown that in nonpathogenic L. tarentolae, cross-resistance between SbIII and SbV exists (11, 18). However, this is not necessarily the case for L. donovani, in which both promastigotes and amastigotes of L. donovani Ld1S.20 remain highly susceptible to low concentrations of SbIII, despite their resistance to SbV. This further supports the hypothesis that mechanistic differences in drug susceptibility and resistance exist between pathogenic and nonpathogenic Leishmania strains. It is therefore possible that SbV and SbIII act differently on L. donovani than on nonpathogenic Leishmania, and furthermore, significant differences in parasite susceptibility and resistance patterns (quantitative if not qualitative) may exist between different species of pathogenic Leishmania, e.g., L. donovani and L. mexicana. Therefore, the relationship between the transport of SbV and SbIII in L. donovani must be further delineated.
ACKNOWLEDGMENT
This work was supported by grant 3668 from the Chief Scientist, Ministry of Health, Jerusalem, Israel.
REFERENCES
- 1.Bates P A. Complete developmental cycle of Leishmania mexicana in axenic culture. Parasitology. 1994;108:1–9. doi: 10.1017/s0031182000078458. [DOI] [PubMed] [Google Scholar]
- 2.Bates P A, Robertson C D, Tetley L, Coombs G H. Axenic cultivation and characterization of Leishmania mexicana amastigote-like forms. Parasitology. 1992;105:193–202. doi: 10.1017/s0031182000074102. [DOI] [PubMed] [Google Scholar]
- 3.Berman J D, Chulay J D, Hendricks L D, Oster C N. Susceptibility of clinically sensitive and resistant Leishmania to pentavalent antimony in vitro. Am J Trop Med Hyg. 1982;31:459–465. doi: 10.4269/ajtmh.1982.31.459. [DOI] [PubMed] [Google Scholar]
- 4.Berman J D, Waddell D, Hanson B D. Biochemical mechanisms of the antileishmanial activity of sodium stibogluconate. Antimicrob Agents Chemother. 1985;27:916–920. doi: 10.1128/aac.27.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borst P, Ouellette M. New mechanisms of drug resistance in parasitic protozoa. Annu Rev Microbiol. 1995;49:427–460. doi: 10.1146/annurev.mi.49.100195.002235. [DOI] [PubMed] [Google Scholar]
- 6.Callahan H L, Portal A C, Devereaux R, Grogl M. An axenic amastigote system for drug screening. Antimicrob Agents Chemother. 1997;41:818–822. doi: 10.1128/aac.41.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang K P, Dwyer D M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vivo. Science. 1976;193:678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- 8.Chang K P, Fong D, Bray R S. Biology of Leishmania and leishmaniasis. In: Chang K P, Bray D, editors. Leishmaniasis. Amsterdam, The Netherlands: Elsevier; 1985. pp. 1–30. [Google Scholar]
- 9.Detke S, Katakura K, Chang K P. DNA amplification in arsenite-resistant Leishmania. Exp Cell Res. 1989;180:161–170. doi: 10.1016/0014-4827(89)90220-6. [DOI] [PubMed] [Google Scholar]
- 10.Dey S, Ouellette M, Lightbody J, Papadopoulou B, Rosen B P. An ATP-dependent As(III)-glutathione transport system in membrane vesicles of Leishmania tarentolae. Proc Natl Acad Sci USA. 1996;93:2192–2197. doi: 10.1073/pnas.93.5.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dey S, Papadopoulou B, Roy G, Grondin K, Dou D, Rosen B P, Ouellette M, Haimeur A. High level arsenite resistance in Leishmania tarentolae is mediated by active extrusion system. Mol Biochem Parasitol. 1994;67:49–57. doi: 10.1016/0166-6851(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 12.Doyle P S, Engel J C, Pimenta P F, Da Silva P P, Dwyer D M. Leishmania donovani: long-term culture of axenic amastigotes at 37°C. Exp Parasitol. 1991;73:326–334. doi: 10.1016/0014-4894(91)90104-5. [DOI] [PubMed] [Google Scholar]
- 13.Dwyer D M. Leishmania donovani: surface membrane carbohydrate of promastigotes. Exp Parasitol. 1977;41:341–358. doi: 10.1016/0014-4894(77)90107-2. [DOI] [PubMed] [Google Scholar]
- 14.Ephros M, Waldman E, Zilberstein D. Pentostam induces resistance to antimony and preservative chlorocresol in Leishmania donovani promastigotes and axenically grown amastigotes. Antimicrob Agents Chemother. 1997;41:1064–1068. doi: 10.1128/aac.41.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, Faugere B, Dumon H. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob Agents Chemother. 1997;41:827–830. doi: 10.1128/aac.41.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grogl M, Thomason T N, Franke E D. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg. 1992;47:117–126. doi: 10.4269/ajtmh.1992.47.117. [DOI] [PubMed] [Google Scholar]
- 17.Grondin K, Haimeur A, Mukhopadhyay R, Rosen B P, Ouellette M. Co-amplification of the gamma-glutamylcysteine synthetase gene gsh1 and of the ABC transporter gene pgpA in arsenite-resistant Leishmania tarentolae. EMBO J. 1997;16:3057–3065. doi: 10.1093/emboj/16.11.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haimeur A, Ouellette M. Gene amplification in Leishmania tarentolae selected for resistance to sodium stibogluconate. Antimicrob Agents Chemother. 1998;42:1689–1694. doi: 10.1128/aac.42.7.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodgkinson V H, Soong L, Duboise S M, McMahon-Pratt D. Leishmania amazonensis: cultivation and characterization of axenic amastigote-like organisms. Exp Parasitol. 1996;83:94–105. doi: 10.1006/expr.1996.0053. [DOI] [PubMed] [Google Scholar]
- 20.Hubel A, Brandau S, Dresel A, Clos J. A member of the ClpB family of stress proteins is expressed during heat shock in Leishmania spp. Mol Biochem Parasitol. 1995;70:107–118. doi: 10.1016/0166-6851(95)00012-p. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim M E, Hag-Ali M, El-Hassan A M, Theander I G, Kharazmi A. Leishmania resistant to sodium stibogluconate: drug-associated macrophage-dependent killing. Parasitol Res. 1994;80:569–574. doi: 10.1007/BF00933004. [DOI] [PubMed] [Google Scholar]
- 22.Jaffe C L, Rachamim N. Amastigote stage-specific monoclonal antibodies against Leishmania major. Infect Immun. 1989;57:3770–3777. doi: 10.1128/iai.57.12.3770-3777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi M, Dwyer D M, Nakhasi H L. Cloning and characterization of expressed genes from in vitro- grown ‘amastigotes’ of Leishmania donovani. Mol Biochem Parasitol. 1993;58:345–354. doi: 10.1016/0166-6851(93)90057-5. [DOI] [PubMed] [Google Scholar]
- 24.Legare D, Papadopoulou B, Roy G, Mukhopadhyay R, Haimeur A, Dey S, Grondin K, Brochu C, Rosen B P, Ouellette M. Efflux systems and increased trypanothione levels in arsenite-resistant Leishmania. Exp Parasitol. 1997;87:275–282. doi: 10.1006/expr.1997.4222. [DOI] [PubMed] [Google Scholar]
- 25.Mebrahtu Y, Lawyer P, Githure J, Were J B, Muigai R, Hendricks L, Leeuwenburg J, Koech D, Roberts C. Visceral leishmaniasis unresponsive to Pentostam caused by Leishmania tropica in Kenya. Am J Trop Med Hyg. 1989;41:289–294. doi: 10.4269/ajtmh.1989.41.289. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhyay R, Dey S, Xu N, Gage D, Lightbody J, Ouellette M, Rosen B P. Trypanothione overproduction and resistance to antimonials and arsenicals in Leishmania. Proc Natl Acad Sci USA. 1996;93:10383–10387. doi: 10.1073/pnas.93.19.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olliaro P L, Bryceson A D M. Practical progress and new drugs for changing patterns of leishmaniasis. Parasitol Today. 1993;9:323–328. doi: 10.1016/0169-4758(93)90231-4. [DOI] [PubMed] [Google Scholar]
- 28.Roberts W L, Berman J D, Rainey P M. In vitro antileishmanial properties of tri- and pentavalent antimonial preparations. Antimicrob Agents Chemother. 1995;39:1234–1239. doi: 10.1128/aac.39.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts W L, Rainey P M. Antileishmanial activity of sodium stibogluconate fractions. Antimicrob Agents Chemother. 1993;37:1842–1846. doi: 10.1128/aac.37.9.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saar Y, Asamoa R, Waldman E, Mazareb S, Amin-Spector S, Plumblee J, Turco S J, Zilberstein D. Characterization of developmentally-regulated activities in axenic amastigotes of Leishmania donovani. Mol Biochem Parasitol. 1998;95:9–20. doi: 10.1016/s0166-6851(98)00062-0. [DOI] [PubMed] [Google Scholar]
- 31.Shapira M, McEwen G, Jaffe C L. Temperature effect in molecular processes which lead to stage differentiation in Leishmania. EMBO J. 1988;7:2895–2901. doi: 10.1002/j.1460-2075.1988.tb03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zilberstein D, Gepstein A. Regulation of L-proline transport in Leishmania donovani by extracellular pH. Mol Biochem Parasitol. 1993;61:197–206. doi: 10.1016/0166-6851(93)90066-7. [DOI] [PubMed] [Google Scholar]
- 33.Zilberstein D, Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]