Abstract
Biologics which are given subcutaneously are usually injected at certain prementioned sites such as the upper arms, thighs, or any quadrant of the abdomen. In erythrodermic patients, these conventional sites are usually affected. In our series of six patients of psoriatic erythroderma, we selected unconventional apparently spared sites to inject secukinumab subcutaneously which returned similar results as compared to injecting at conventional sites as reported by other studies.
KEY WORDS: Biologics, erythrodermic psoriasis, secukinumab injected at unconventional site
Introduction
Secukinumab is a Food and Drug Administration (FDA)-approved biological targeting interleukin-17A (IL17A) cytokine used in the treatment of moderate-to-severe plaque psoriasis. Many clinical trials have been undertaken to establish its efficacy and safety in psoriasis and psoriatic arthritis. The results of phase 3 randomized placebo-controlled study by Bhat et al.[1] in the Indian population suggests the superior efficacy in comparison to placebo and Etanercept in treating moderate-to-severe plaque psoriasis along with good tolerability and no major adverse effects.
Moderate-to-severe psoriasis has a strong association with comorbidities and has a significant impact on the Dermatology Life Quality Index (DLQI). The commonest associations are arthritis and metabolic syndrome along with psychiatric comorbidity. This leads to an overall risk of increased morbidity and mortality in >30–40% of the cases having the association.
Erythrodermic psoriasis (EP) is a rare and severe variant of the disease, with an estimated prevalence among psoriatic patients ranging from 1 to 2.25%. Furthermore, psoriatic erythroderma is the most common cause of erythroderma, responsible for ~25% of all the cases.[2] The prognosis is unfavorable in EP associated with comorbidities, therefore, there is a need to aggressively manage these patients.
Among the biologics available, IL-17A inhibitor secukinumab is attributed to have the quickest lesional clearance and achieving the Psoriasis Area Severity Index (PASI) 100.[3,4,5,6,7,8] We are reporting six cases of EP with associated comorbidities showing remarkable response from the eastern part of India.
Case Report
All the patients had extensive psoriasis and were clinically diagnosed as erythroderma at the time of the first visit. They had a previous history of undergoing all sorts of treatment in the psoriasis armamentarium except biologicals. When the patients consulted us, the most common complaint of the patients was non-satisfactory previous treatments and/or outcomes [Table 1] and the disease was severely affecting their DLQI. The common expectation of all the patients from the biological treatment was a faster clearance and longer disease-free period.
Table 1.
Patient demographics
| Age (years) | Gender | Duration (years) | Reason for choosing biologicals |
|---|---|---|---|
| 40 | F | 10 | Cumulative dose of Methotrexate-12 g, debilitating arthritis and not responding to other treatments |
| 23 | M | 3 | Extensive psoriasis, pill burden, severe impact on DLQI leading to depression, not satisfied with treatment outcome |
| 35 | M | 7 | On combination therapy of methotrexate/acitretin along with phototherapy on and off, short-term remission and repeated flare-ups. |
| 40 | M | 5 | Not satisfied with treatment outcome and wanted faster clearance of lesions |
| 43 | F | 5 | Read about the option of biological treatment and was interested to try the same |
| 43 | M | 7 | Repeated acute episodes with recurrent flare-ups, not satisfied with treatment outcome of Cyclosporine |
The patients underwent extensive counseling sessions regarding the pre-treatment investigations, the expenses involved as well as the treatment outcomes and associated side effects, and most importantly having a realistic expectation from the proposed treatment.
All pre-requisite investigations were done. All the relevant lab parameters were within normal limits, except one patient, who had mild hypoalbuminemia and neutropenia. The other parameters like cardiovascular, respiratory, and thermoregulatory functions were within normal limits and no underlying sepsis was present.
Since all the six patients in our case series were erythrodermic, the conventional injection sites were more or less affected, sparing only a few areas such as the face, axillae, and groin folds, presenting a dilemma for us to choose an ideal non-conventional anatomical site for injecting secukinumab. Nonetheless, we proceeded in choosing the most disease spared site to inject, which has been mentioned in [Table 2]. PASI was documented at each visit and the important milestones as well as the associated comorbidities have been presented in Table 2 as well. The recommended dosing schedule was followed, i.e., 300 mg subcutaneously on days 0, 7, 14, 21, 28, and once monthly after that for another 5 months for a total of 10 injections[9] and followed up for another six months to look for flare-ups or relapse.
Table 2.
Injection site, comorbidities, and PASI scores
| Site of injection | Comorbidities | PASI | |||
|---|---|---|---|---|---|
|
| |||||
| Baseline | 8th week (6th inj) PASI improvement | 24th week (10th inj) PASI improvement | 52nd week of follow-up | ||
| Dorsum of right foot | Deforming psoriatic arthritis | 28 | 75 | 100 | Deformity persisted. No recurrence of psoriatic lesions was observed |
| Right outer arm | Depression | 32.4 | 90 | 100 | Lost to follow-up |
| Medial surface of right thigh | HTN, CKD | 24.6 | 90 | 100 | Lost to follow-up |
| Medial surface of left leg | Diabetes, HTN | 28 | 90 | 100 | PASI 100 maintained |
| Medial surface of right thigh | Diabetes, dyslipidemia | 22 | 90 | 100 | Recurrent plaques on shin were controlled with intralesional steroids and Vitamin D analogs. No relapse of EP was observed |
| Medial surface of left thigh | HTN, NAFLD | 20.8 | 90 | 100 | PASI 100 maintained |
Average baseline PASI: 25.97
Five patients achieved PASI 90 by the end of the first 4 weeks. One patient, who had achieved PASI 75 at 8 weeks also had deforming arthritis involving both the axial as well as peripheral joints and was suffering from unstable psoriasis for almost 4 years, experienced an immense improvement in her symptoms in terms of lesion clearance and joint improvement [Figures 1 and 2].
Figure 1.
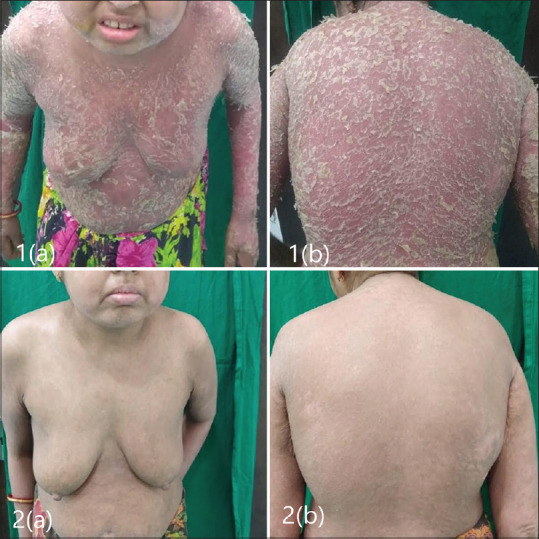
(1a and b): Baseline; (2a and b): At 28th week
Figure 2.
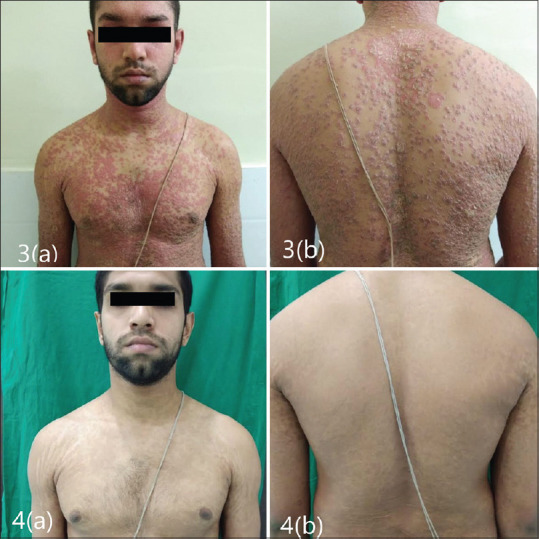
(3a and b): Baseline; (4a and b): At 28th week
The DLQI was recorded at each visit and a gradual but steady improvement was observed [Table 3].
Table 3.
DLQI
| DLQI score | ||
|---|---|---|
|
| ||
| Baseline | On the day of 6th injection | On the day of 10th injection |
| 17 | 10 | 3 |
| 14 | 5 | 1 |
| 14 | 4 | 1 |
| 13 | 2 | 0 |
| 10 | 5 | 0 |
| 11 | 3 | 0 |
In the patient with psoriatic arthritis, in spite of achieving PASI100, there was a residual deformity and associated pain in the joints even after the 10th injection of secukinumab which continued to affect her DLQI.
Visual analog scoring (VAS) was also done at each visit and patients were highly satisfied with the treatment received [Table 4].
Table 4.
VAS
| VAS (patient satisfaction) | ||
|---|---|---|
|
| ||
| Baseline | On the day of 6th injection | On the day of 10th injection |
| 10 | 2 | 1 |
| 9 | 0 | 0 |
| 9 | 0 | 0 |
| 8 | 0 | 0 |
| 8 | 0 | 0 |
| 8 | 0 | 0 |
None of the patients experienced any untoward adverse effects. No flare-ups were observed in any of the patients.
Discussion
The management of psoriasis with arthritis, associated with metabolic syndrome in extensive Body Surface Area (BSA) involvement is a challenge addressing multiple aspects like the choice of medication, cost of therapy, patient compliance, psychological component, DLQI, drug safety, and remission period in the long run.
Numerous studies have highlighted the efficacy and safety of secukinumab.[3] A study by Mateu-Puchades et al.[10] on the efficacy and safety of secukinumab in EP showed high effectiveness and safety with the reduction in PASI (90) and DLQI (15 points) at week 16.
The Indian study, a randomized placebo-controlled phase 3 study by Bhat et al.,[1] investigated the efficacy, Investigator's Global Assessment (IGA), and reduction in PASI with secukinumab 300 mg as well as 150 mg in comparison with Etanercept and placebo'; 61 and 55.9% of the patients in the secukinumab 300 and 150 mg groups, respectively, were able to achieve PASI 75 as compared to 20% in the Etanercept group and 7.1% in the placebo group. About 43.9 and 20.6% the patients in the secukinumab 300 and 150 mg, respectively, were able to achieve Investigator's Global Assessment (IGA) 0 or 1 response at week 12 versus 13.3% in the Etanercept and 2.4% in the placebo groups. The PASI 90 response was seen in 41.5 and 20.6% in the secukinumab 300 and 150 mg groups versus 10 and 0% in the Etanercept and placebo groups. The authors concluded that secukinumab was highly efficacious and well-tolerated in the Indian population.
In a retrospective observational study, Neema et al.[11] reported 75% of the patients to have achieved PASI 75 at 4 weeks. Furthermore, 85, 65, and 10% achieved PASI 75, 90, and 100, respectively, at 12 weeks. At the end of 52 weeks, 90, 50, and 35% were able to maintain a PASI of 75, 90, and 100, respectively. This is indicative of the quick response of psoriasis to the treatment with secukinumab. Similarly, in our patients, each patient showed a quick response and achieved PASI 100 at the 24th week, while also maintaining the same disease clearance over a longer period.
All our six patients with EP had more than 90% BSA involvement with an average baseline PASI of 25.97, average baseline DLQI of 13.17, and were able to complete the full therapeutic schedule with remarkable response to secukinumab and no significant side effects. There was a sustained remission and the patients were immensely satisfied.
With regards to the current pandemic, there is a clinical benefit targeting IL17A signaling and synergistic inflammatory cytokine IL6 as well to manage the Coronavirus disease (COVID-19) patients, particularly those presenting with cytokine storm. As IL17 has been found to play a role in the COVID-19 patients with pneumonia, secukinumab has an additional benefit when used in the psoriasis treatment.[12]
The novelty of our study is that in spite of non-conventional sites of administering the injection, the response to treatment was as efficacious and safe as compared to the conventional sites. This aspect has not been documented to the best of our knowledge. In conclusion, we suggest that the absence of an uninvolved conventional injection site should not deter the treating dermatologist from using this molecule and should proceed to treat by selecting an apparently spared unconventional site. We also suggest that the treating dermatologists may use the same technique using other biologicals in such types of patients and report their observations, emphasizing the fact that the mode of administration of the drug should not be restricted to the conventional sites.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bhat RM, Leelavathy B, Aradhya SS, Gopal MG, Pratap D, Mubashir M, et al. Secukinumab efficacy and safety in Indian patients with moderate-to-severe plaque psoriasis: Subanalysis from FIXTURE, a randomized, placebo-controlled, phase 3 study. Indian Dermatol Online J. 2017;8:16–24. doi: 10.4103/2229-5178.198765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh RK, Lee KM, Ucmak D, Brodsky M, Atanelov Z, Farahnik B, et al. Erythrodermic psoriasis: Pathophysiology and current treatment perspectives. Psoriasis (Auckl) 2016;6:93–104. doi: 10.2147/PTT.S101232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CEM, Papp K, et al. Secukinumab in plaque psoriasis − Results of two phase 3 trials. N Engl J Med. 2014;371:326–38. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 4.Ohtsuki M, Morita A, Abe M, Takahashi H, Seko N, Karpov A, et al. Secukinumab efficacy and safety in Japanese patients with moderate-to-severe plaque psoriasis: Subanalysis from ERASURE, a randomized, placebo-controlled, phase 3 study. J Dermatol. 2014;41:1039–46. doi: 10.1111/1346-8138.12668. [DOI] [PubMed] [Google Scholar]
- 5.Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment- as-needed versus fixed-interval maintenance regimen for moderate-to-severe plaque psoriasis: A randomized, double-blind, noninferiority trial (SCULPTURE) J Am Acad Dermatol. 2015;73:27–36.e1. doi: 10.1016/j.jaad.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Thaçi D, Humeniuk J, Frambach Y, Bissonette R, Goodman JJ, Shevade S, et al. Secukinumab in psoriasis: Randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE) Br J Dermatol. 2015;173:777–87. doi: 10.1111/bjd.13814. [DOI] [PubMed] [Google Scholar]
- 7.Paul C, Lacour JP, Tedremets L, Kreutzer K, Jazayeri S, Adams S, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: A randomized, controlled trial (JUNCTURE) J Eur Acad Dermatol Venereol. 2015;29:1082–90. doi: 10.1111/jdv.12751. [DOI] [PubMed] [Google Scholar]
- 8.Blauvelt A, Prinz JC, Gottlieb AB, Kingo K, Sofen H, Ruer-Mulard M, et al. Secukinumab administration by pre-filled syringe: Efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE) Br J Dermatol. 2015;172:484–93. doi: 10.1111/bjd.13348. [DOI] [PubMed] [Google Scholar]
- 9.COSENTYX (secukinumab) [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2016. [Google Scholar]
- 10.Mateu-Puchades A, Santos-Alarcón S, Martorell-Calatayud A, Pujol-Marco C, Sánchez-Carazo JL. Erythrodermic psoriasis and secukinumab: Our clinical experience. Dermatol Ther. 2018;31:e12607. doi: 10.1111/dth.12607. [DOI] [PubMed] [Google Scholar]
- 11.Neema S, Radhakrishnan S, Singh S, Vasudevan B, Chatterjee M. Real-life efficacy and safety of secukinumab: A single-center, retrospective observational study with 52-week follow-up. Indian J Drugs Dermatol. 2019;5:14–8. [Google Scholar]
- 12.Shibabaw T. Inflammatory cytokine: IL-17A signaling pathway in patients present with COVID-19 and current treatment strategy. J Inflamm Res. 2020;13:673–80. doi: 10.2147/JIR.S278335. [DOI] [PMC free article] [PubMed] [Google Scholar]


