Abstract
Xeroderma pigmentosum (XP) is an autosomal recessive genetic disease caused by a defect in the DNA repair system, exhibiting skin cancer on sun exposure. As it is an incurable disease, therapeutic strategies of this disease are critical. This review article takes an attempt to explore the current therapeutic advancements in XP. Different approaches including sun avoidance; surgical removal of cancerous lesions; laser and photodynamic therapy; use of retinoid, 5-fluorouracil, imiquimod, photolyase, and antioxidant; interferon therapy and gene therapy are chosen by doctors and patients to lessen the adverse effects of this disease. Among these options, sun avoidance, use of 5-fluorouracil and imiquimod, and interferon therapy are effective. However, some approaches including laser and photodynamic therapy, and the use of retinoids are effective against skin cancer having severe side effects. Furthermore, surgical removal of cancerous lesions and use of antioxidants are considered to be effective against this disease; however, efficacies of these are not experimentally determined. In addition, some approaches including oral vismodegib, immunotherapy, nicotinamide, acetohexamide, glimepiride-restricted diet are found to be effective to minimize the complications secondary to defects in the nucleotide excision repair (NER) system and also enhance the NER, which are under experimental level yet. Besides these, gene therapy, including the introduction of missing genes and genome edition, may be a promising approach to combat this disease, which is also not well established now. In the near future, these approaches may be effective tools to manage XP.
KEY WORDS: DNA repair, gene therapy, skin cancer, therapeutic strategies, xeroderma pigmentosum
Introduction
Xeroderma pigmentosum (XP), a rare genetic photosensitive disorder, exhibits increased susceptibility to skin cancers on the sun-exposed body sites of the patients. It affects people of all continents and all racial groups with autosomal recessive inheritance similarly affecting both males and females. According to an estimate made in the 1970s, in the USA 1 in 250,000 and in Japan 1 in 20,000 are affected with XP.[1] Another survey of Western Europe suggested that approximately 2.3 per million are affected by this disease.[2] Though research in rare genetic diseases such as XP is considered as a lesser priority compared to the common ones, the rare disease may be considered as human models for better understanding the physiology and mechanistic of the human body as like the role of rare genetic disorders tuberous sclerosis complex (TSC) in the understanding of the mammalian target of rapamycin complex 1 (mTORC1).[3] James E. Cleaver (1968) first reported that it is due to genetic abnormalities of the DNA repair process and XP cells were unable to repair DNA damage caused by ultraviolet radiation (UV).[4] Now XP is considered as one of the most serious photosensitive disorders in which patients are developing multiple skin cancers if not prevented by avoiding UV. XP patients experience idiopathic neurodegenerative complications reducing the quality of life (QOL). In accordance with Hong et al.,[5] the patients of XP have a 100% risk of skin cancer, and approximately two-thirds of them die of cancer before the age of 20. Therefore, early diagnosis and timely symptom management are very pivotal for the long-term survival of patients with XP.
Method
Different databases including Pubmed were accessed with different keywords such as “xeroderma pigmentosum,” “therapeutic strategies,” “skin cancer,” “DNA repair,” and “gene therapy.” Among the articles found in the databases, the articles focused on the treatment and management of xeroderma pigmentosum rather than only focusing on the molecular mechanism of XP and DNA repair mechanism were selected for this study. The level of evidence and grade of recommendation for each therapeutic option is assigned according to “Oxford center of evidence-based medicine (EBM) guidelines.”
Pathophysiology
Though UV irradiation has both UVA spectrum (320–400 nm) and the UVB spectrum (280–320 nm), UVB plays a more prominent role in causing XP.[6,7,8] UVB is considered as a causative factor of photoaging, immunosuppression,[9] and the formation of some photoproducts in DNA including cyclobutane pyrimidine dimers (CPDs),[6] which is thought to be responsible for the initiation of skin cancer.[8] Immunosuppression caused by UVB contributes to the formation of skin cancer by allowing the transformed cells to be replicated.[10,11] Nucleotide excision repair (NER) has two sub-categories: global genome nucleotide excision repair (GG-NER), which can globally repair lesions in the genome, and transcription-coupled nucleotide excision repair (TC-NER), which only repairs actively transcribed genes.[12] In the case of XP, eight different DNA repair genes including XPA, XPB (ERCC3), XPC, XPD (ERCC2), XPE (DDB2), XPF (ERCC4), XPG (ERCC5), and XPV (POLHI) have been implicated.[10] Among these, all are involved with nucleotide excision–related DNA repair (DNA damage recognition, DNA strand opening around the lesion, excision of the DNA lesion by endonucleases, synthesis of a new complementary DNA strand, and ligation), except XPV protein, exerting translesion DNA polymerase in the translesion synthesis system.[13] The link between UV exposure and neurological complications of XP patients is not clearly understood. However, it is considered that oxidative DNA damages that occur during normal metabolism in the central nervous system must be repaired by NER[14] [Figure 1]. In this circumstance, lesions persist and cause neuronal death.
Figure 1.
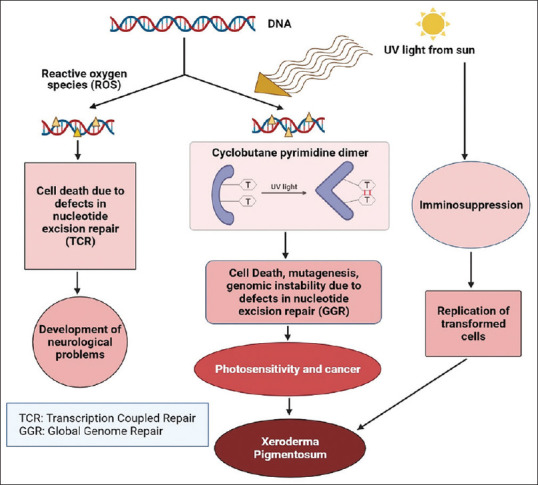
Pathophysiology of xeroderma pigmentosum
Therapeutic Approaches in XP
Xeroderma pigmentosum, an incurable disease, has some preventive actions and palliative therapeutics limiting skin cancer development and progression [Table 1]. Though the therapeutic strategies have roles in cancer prevention and progression, they cannot overcome the incapacity of the nucleotide excision repair system to remove DNA lesions in XP patients. The therapeutic approaches for XP can be discussed under two headings: current therapeutic approaches and futuristic therapeutic approaches.
Table 1.
Therapeutic approaches, their targets in XP, level of evidence, and grade of recommendation
| Approach | Target | References | *Level of evidence | Grade of recommendation |
|---|---|---|---|---|
| Current approaches | ||||
| 1. Sun avoidance | UVB spectrum of UV irradiation forms cyclobutane pyrimidine dimers (CPDs) and initiates skin cancer. It also causes immunosuppression which allows transformed cells to be replicated complicating the situation. Avoidance of sun provides protection of UV radiation delaying cancer manifestation in XP patients. | [6,8,11,15] | 1 | Strong evidence |
| 2. Tumor ablation, dermabrasion, and chemical peels | Surgical removal of cancerous lesions prevents the spread of cancer cells. Dermabrasion and chemical abrasion were used earlier as a prophylactic approach. | [20,21,22,23] | 3 | Weak evidence |
| 3. Laser and photodynamic therapy (PDT) | Laser treatments reduce the occurrence of nonmelanoma skin cancers and PTD uses photosensitizing agent that after light activation destroy cancer cells. | [5,23] | 3 | Weak evidence |
| 4. Retinoids | Prevents the formation of carcineous carcinoma in XP patient exerting their anticancer effect through their pro-extracellular matrix activity, the maintenance of stem cells (having mutagenic DNA lesion) and/or the inhibition of matrix degrading enzymes such as matrix metalloproteinases. | [24,25,26,27,28,29,30] | 1 | Strong evidence |
| 5. 5-Fluorouracil | Inhibits all production of thymidine triphosphate (TTP) from uracil, resulting cell death through apoptosis. | [31,32] | 3 | Weak evidence |
| 6. T4 endonuclease V | Repair UV-induced DNA lesions restoring normal level of undifferentiated sarcoma | [33] | 2 | Moderate evidence |
| 7. Imiquimod | Improves pigmentation alterations and defects in the skin texture | [34,35] | 4 | Theoretical evidence |
| 8. Photolyase | Involved in light-dependent DNA repair named photoreactivation. | [8] | 2 | Moderate evidence |
| 9. Antioxidants | Reduce oxidative stress, which is considered to cause neurological symptoms in XP patients. | [36] | 2 | Moderate evidence |
| 10. Interferon alpha | It is an immunomodulator and antiproliferative complex, effective against melanomas. | [37] | 4 | Theoretical evidence |
| Futuristic Approaches | ||||
| 11. Oral vismodegib | Inhibits hedgehog pathway | [21] | 4 | Theoretical evidence |
| 12. Immunotherapy | Blocks binding of PD-L1 and boosts immune response against cancer cell | [38,39] | 4 | Theoretical evidence |
| 13. Nicotinamide | Supposed to repress SIRT1 and rescues mitochondrial phenotype | [40,41,42,43] | 3 | Weak evidence |
| 14. Acetohexamide or glimepiride | Supposed to degrade MUTYH, a DNA glycosylase | [44,45] | 5 | Mechanism-based evidence |
| 15. Restricted diet | May increase resistance to stress induced by DNA-damage | [46] | 5 | Mechanism-based evidence |
| 11. Potentials of gene therapy | The transfer of missing/defective genetic materials and corrections by genome edition. | [47,48,49,50] | 5 | Mechanism-based evidence |
*The level of evidence for each therapeutic option are assigned according to “Oxford center of EBM guidelines.”[51]
Current therapeutic approaches
Sun avoidance
No outdoor activities during the day, use of sunglasses, a hat with neck protection, woolen clothes, and use of sunscreen with a high sun protection factor (SPF) provide protection from UV radiation delaying cancer manifestation in XP patients. It is better to cover the window with a screen absorbing UV radiation from the sun.[15] In the case of photoprotection, time-based photoprotection is effective where patients have to be conscious when out in the sun between 8 AM and 6 PM, particularly in the summer season.[16] Furthermore, UV light in an individual's home, school, or work environment can be measured with a light meter to identify high levels of environmental UV (e.g., halogen lamps). This UV meter can warn individuals about the unexpected exposure of environmental UV.[17] Artificial light including hospital theatre lights, metal halide lamps, and some fluorescent light should be avoided and if possible can be covered with UV-resistant film.[1] Garcia et al.[18] developed a mouse model to assess the in vivo efficacy of sun-protective formulations including not only sunscreen but also other therapeutic substances such as antioxidants or inhibitors of pathways with aberrant activation in XP cells constituting a valuable tool to evaluate the long-term effect of both chronic and acute UVB irradiation. As total avoidance of sun exposure causes vitamin D deficiency, and higher serum concentration of active vitamin D is associated with lower rates of some types of cancers, XP patients may be given an external source of vitamin D (50,000 IU once a month, per os).[19]
Tumor ablation, dermabrasion, and chemical peels
To prevent the spread of cancer cells, XP patients are regularly followed by dermatologists having frequent surgical removal of cancerous lesions. As this surgical removal takes large margins around the lesion, XP patients frequently require plastic reconstructive surgery including grafts, where grafts are taken from nonsun-exposed areas including the abdomen and buttock.[20,21] Dermabrasion and chemical abrasion, used earlier as a prophylactic approach, have the advantage of promotion of constant-reepithelializations of the skin with cells that arise from those in the deeper layers of the epidermis and therefore less touched by UV light. The efficacy of these procedures is not evaluated and both the procedures are not presently used.[22,23]
Laser and photodynamic therapy (PDT)
Full-face laser resurfacing and PDT can be used as a preventive measure to be used in XP patients. Previous studies showed that laser treatments reduce the occurrence of nonmelanoma skin cancers.[23] PDT is less invasive, has fewer side effects, and does not damage the surrounding normal tissues, which uses a photosensitizing agent that after light activation destroys cancer cells. Though photofrin was previously used as a photosensitizer, due to its side effect, ALA is recommended to be used in XP patients. ALA, the precursor of the endogenous photosensitizer protoporphyrin IX, is used to treat squamous cell carcinoma (SCC) in XP patients with red and blue light (417–432 nm).[52,53] Hong et al.[5] treated the tumors of a 12-year-old girl with XP with carbondioxide laser therapy combined with PTD. However, one study conducted by Procianoy et al.[54] showed that treatment of SCCs on the eyelid, conjunctiva, and cornea with sodium salt hematoporphyrin derivative photosensitizer resulted in a higher incidence of SCC development, falling its effectiveness in controversy.
Retinoids
Retinoids, used as chemoprophylaxis agents to avoid the formation of carcinoid carcinoma in XP patients, may exert their anticancer effect through their pro-extracellular matrix activity, the maintenance of stem cells (having mutagenic DNA lesion), and/or the inhibition of matrix-degrading enzymes such as matrix metalloproteinases.[24,25,26] Though high doses (2 mg/kg) of oral daily intake during 2 years of a retinol derivative, isotretinoin, significantly decreased (60%) the occurrence of skin cancers in XP patients, following discontinuation of the treatment a burst of de novo cancerous lesions was observed.[10,27] Further, systemic treatment with 2 mg/kg/day of isotretinoin caused severe side effects including hypertriglyceridemia, hepatic dysfunction, teratogenicity, development of skeletal abnormalities, and mucocutaneous toxic effects. Its withdrawal also causes prompt reversal of prophylactic effects. For these reasons, retinoid treatment is now avoided.[27,28] Acitrein, a second-generation retinoid, has immunogenic anti-inflammatory effect, apoptosis inducing effect, and tumor promotion inhibitory effect. It is thought that acetrein antagonizes with different transcription factors and competes with retinoic acid for retinoic acid-binding protein. Further, it ensures normal keratinocyte differentiation in the epithelium. It also prevents the expression of proinflammatory cytokines and interferon.[29,30] It was recommended to prevent new neoplasm in a patient with multiple skin cancers. However, it also has similar side effects of isotretinoin.[17]
5-Fluorouracil
5-Flourouracil, a modified form of uracil, completely inhibits the production of thymidine triphosphate (TTP) from uracil by interacting with RNA molecules and interacting with the enzyme thymidylate synthase (TS), resulting in cell death through apoptosis. 5-Flourouracil can be used as an alternative treatment of surgical resection.[31,32]
T4 endonuclease V
Tanaka et al.[33] found that a bacteriophage T4 endonuclease V (T4N5) restored the normal level of undifferentiated sarcoma (UDS) in XP cells. Daniel Yorrosh (2001) showed that 1 mg/L of T4N5 conferred a 30% decrease of basal cell carcinoma (BCC) and a 70% decrease of actinic keratosis in XP patients, without any immune reactions. Topical applications of a liposome lotion containing bacteriophage T4 endonuclease V are reported useful in western countries.[55] However, it is not approved by the Food and Drug Administration (FDA).[17]
Imiquimod
Imiquimod, an immune response modifier, is found to be effective against skin malignancies and premalignancies.[56] Five percent topical imiquimod is found to be successful in (BCC). Its use also improves pigmentation alterations and defects in the skin texture. [34,35,57]
Photolyase
Photolyase, found in fish, reptiles, marsupials, and plants but not in humans, is involved in light-dependent DNA repair named photoreactivation.[8] In comparison with sites lacking light exposure, increased DNA repair and protection against immune suppression were shown by photolyase and light-treated areas.[58]
Antioxidants
Hayashi reported that neurological symptoms of XP are associated with oxidative stress.[36] However, the efficacy of antioxidants in this regard is not experimentally determined. Furthermore, a low dose of levodopa was found to be effective against respiratory disorders in XP patients.[59]
Interferon alpha
XP causes ocular abnormalities and an increased risk of developing neoplasms in sun-exposed areas of the skin, mucous membranes, and eyes. Interferon-α has been reported useful for melanoma.[37] A case study by Dirar et al.[60] showed that subconjunctival injections and topical mitomycin C (MMC) of PEG-IFN-α-2b were effective in the treatment of ocular surface squamous neoplasia (OSSN) in patients with XP.
Futuristic therapeutic approaches
Oral vismodegib
Oral vismodegib, which has been approved by the FDA for the treatment of BCC, is an inhibitor of the hedgehog pathway. Though the treatment may be effective for some XP patients, no studies on the efficacy of this drug in XP patients have been published. Additionally, it is a teratogen having adverse effects on the embryo.[17]
Immunotherapy
Lehmann and Fassihi (2020) found that though immunotherapy with anti-PD1 immunotherapy (pembrolizumab) was not recommended in the case of angiosarcoma, it might be successful in one XP-C patient with advanced metastatic cancer arising from an angiosarcoma.[38] Further studies are required to confirm its efficacy in other patients.
Nicotinamide
Though still now xeroderma patients are not treated with nicotinamide, it is thought to increase the efficiency of NER. Chen et al.[40] showed that an intake of 500 mg nicotinamide twice daily for 1 year by dermatology patients reduced the rate of incidences of non-melanoma skin cancer, BCC, and SCC as opposed to the dermatology patients who took placebos. Furthermore, Vélez-Cruz et al.[41] (2013) demonstrated that after UV radiation damage, the nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, sirtuin 1 (SIRT1) actively repressed transcription in XPD/Cockayne syndrome (CS) cells. Pretreatment of XPD/CS cells with nicotinamide showed rapid recovery of repressed gene expression, where nicotinamide served as a repressor of SIRT1. Apart from this, XPA-deficient cells showed pathological mitochondria and reduced mitophagy due to downregulation of UCP2, downstream of SIRT1 signaling. Nicotinamide mononucleotide (NMN) or nicotinamide riboside (NR), derived from nicotinamide, positively regulates SIRT1 and rescues mitochondrial phenotype.[42,43]
Acetohexamide or glimepiride
Acetohexamide or glimepiride, sulfonylurea used for treating diabetes, have been found to target the molecular abnormalities that occurred as a secondary consequence of NER defect.[44] It is demonstrated by Mazouzi et al.[45] that acetohexamide improved resistance of HAP1 cells with a frameshift mutation in XPA (ΔXPA) to UVR damage, as indicated by enhanced postexposure viability and enhanced CPD clearance by promoting degradation of MUTYH, a DNA glycosylase, involved in the base excision repair pathway. However, the process through which MUTYH exerts deleterious effects on NER-deficient cells is not clear. It is presumed that the production of single-stranded breaks after pyrimidine dimers formation or possibly inhibitory MUTYH binding to CPD stymies other enzymes from repairing the lesion.
Restricted diet
Vermeij et al.[46] indicated a correlation of caloric restriction with reduced neurological decline. They found that caloric restriction of a progeroid mouse model deficient in the nucleotide excision repair gene Ercc1 (Ercc1Δ/-), the binding partner for XPF, tripled the lifespan of these mice and delayed several aspects of premature aging. XPG-/- mice also exhibited similar trends. It is thought by the authors that dietary restriction may increase resistance to stress induced by DNA damage, raise antioxidant defenses, and alter metabolic signaling to induce a shift from the production of pro- to anti-inflammatory cytokines. The authors also recommended that this dietary restriction could be applied to diseases such as xeroderma pigmentosum.
Potentials of gene therapy
For many years, gene therapy has been considered as an alternative approach to treat genetic disorders, still lacking curative therapy. There are two main approaches: the transfer of genetic materials and genome editing.[47] Like many other recessive genetic diseases, XP has also been considered as a good target disease toward gene therapy approaches. Several technical approaches have been considered to correct and restore the effects of defective genes.
At first, replication-defective recombinant Moloney murine leukemia virus (MMLV) retroviruses were used to transduce XP genes (XPC, XPD) into patient's fibroblasts, and it was found that these fibroblasts retained survival and NER capacity in UV-irradiated cultures.[48] After that, Arnaudeau-Bergard et al.[61] demonstrated an efficient genetic correction of XP-C keratinocytes. Later, in 2004, Marchetto et al.[62] used recombinant adenovirus (AdXPA) as a vehicle for delivering human XPA cDNA to an XPA -/- mice to correct XP phenotype. These XPA-/- mice showed recovery from the defective DNA repair and did not develop tumors after UV irradiation. Warrick et al. developed a retrovirus-based strategy to transduce the wild-type XPC gene into clonogenic human primary XP-C keratinocytes.[63] The absence of adverse effects including oncogenic activation and clonal expansion indicated the integration of genes in isolated keratinocyte stem cells. In addition, the transduced keratinocytes showed full NER capacity and normal features of epidermal differentiation in organotypic skin cultures and in a preclinical murine model of human skin regeneration in vivo.
Genome editing has become an effective approach to target a DNA sequence bearing a specific germinal DNA mutation to be replaced by its correct version. In CHO-K1 and MRC5 immortalized cells derivatives, I-CreI endonucleases were designed to cleave different regions of the XPC gene successfully. However, there was a problem of methylation of CpG dinucleotides in the downstream region.[49,50] Therefore, it may be an interesting approach where downstream CpG dinucleitides are absent. Until now, CRISPR-Cas9 technology is successful in correcting mutations in the DYSTROPHIN gene and the cystic fibrosis transmembrane conductance regulator.[64] It is believed that in the near future CRISP-Cas9 will certainly be possible to correct XPC mutations.[65]
Conclusion
As with other genetic diseases, there is no functional clinical treatment of XP at present. The management includes light avoidance and treatment of tumors. Among these management strategies, some have both efficacy and side effects. Thus, the choice of management approaches is crucial. Here, the physicians and the patients should consider the fact that the benefit from the management approach should outweigh the side effects of the approach. The therapeutic interventions that focus on the complications that originated from NER deficit and enhance the efficiency of NER, can be considered to be potential to enhance the quality of life. As a lot of XP DNA excision repair genes have been successfully cloned, and the understanding of the pathogenesis of XP has greatly advanced, gene therapy may be an effective treatment strategy. However, as the skin is the largest organ of the human body, its complete transplantation following genetic correction may not be possible. The ongoing effort of genome editing might be an effective treatment approach in the future. Meanwhile, early diagnosis and preventive measures to reduce the adverse effect of this disease have to be executed carefully to prolong and improve the lives of the affected patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lehmann AR, McGibbon D, Stefanini M. Xeroderma pigmentosum. Orphanet J Rare Dis. 2011;6:70. doi: 10.1186/1750-1172-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleijer WJ, Laugel V, Berneburg M, Nardo T, Fawcett H, Gratchev A, et al. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7:744–50. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Dodd KM, Dunlop EA. Tuberous sclerosis—A model for tumour growth. Semin Cell Dev Biol. 2016;52:3–11. doi: 10.1016/j.semcdb.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218:652–6. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- 5.Hong C, Qing-Qi Y, Chao M, Dong-Xu Z, Yi-Xia W, Ping S, et al. Photodynamic therapy in the treatment of xeroderma pigmentosum: A case report. Photodiagnosis Photodyn Ther. 2020:30. doi: 10.1016/j.pdpdt.2020.101761. doi: 10.1016/j.pdpdt. 2020.101761. [DOI] [PubMed] [Google Scholar]
- 6.Yarosh D, Klei J, Kibitel J, Alas L, O’Connor A, Cummings B, et al. Enzyme therapy of xeroderma pigmentosum: Safety and efficacy of T4N5 liposome lotion containing a prokaryotic DNA repair enzyme. Photodermatol Photoimmunol Photomed. 1996;12:122–30. doi: 10.1111/j.1600-0781.1996.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 7.Copeland NE, Hanke CW, Michalak JA. The molecular basis of xeroderma pigmentosum. Dermatol Surg. 1997;23:447–55. doi: 10.1111/j.1524-4725.1997.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 8.Stege H. Effect of xenogenic repair enzymes on photoimmunology and photocarcinogenesis. J Photochem Photobiol B Biol. 2001;65:105–8. doi: 10.1016/s1011-1344(01)00246-9. [DOI] [PubMed] [Google Scholar]
- 9.Norgauer J, Idzko M, Panther E. Xeroderma pigmentosum. Eur J Dermatol. 2003;13:4–9. [PubMed] [Google Scholar]
- 10.Kraemer KH. Sunlight and skin cancer: Another link revealed. Proc Natl Acad Sci USA. 1997;94:11–4. doi: 10.1073/pnas.94.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streilein JW, Taylor JR, Vincek V. Relationship between ultraviolet radiation–induced immunosuppression and carcinogenesis. J Invest Dermatol. 1994;103:107S–11S. doi: 10.1111/1523-1747.ep12399400. [DOI] [PubMed] [Google Scholar]
- 12.Marteijn JA, Lans H, Vermeulen W, Hoeijmakers JH. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol. 2014;15:465–81. doi: 10.1038/nrm3822. [DOI] [PubMed] [Google Scholar]
- 13.Moriwaki S, Kanda F, Hayashi M, Yamashita D, Sakai Y, Nishigori C. Xeroderma pigmentosum clinical practice guidelines revision committee. Xeroderma pigmentosum clinical practice guidelines. J Dermatol. 2017;44:1087–96. doi: 10.1111/1346-8138.13907. [DOI] [PubMed] [Google Scholar]
- 14.Brooks PJ. The 8, 5'-cyclopurine-2'-deoxynucleosides: Candidate neurodegenerative DNA lesions in xeroderma pigmentosum, and unique probes of transcription and nucleotide excision repair. DNA Repair (Amst) 2008;7:1168–79. doi: 10.1016/j.dnarep.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emmert S, Ueda T, Zumsteg U, Weber P, Khan SG, Oh KS, et al. Strict sun protection results in minimal skin changes in a patient with xeroderma pigmentosum and a novel c.2009delG mutation in XPD (ERCC2) Exp Dermatol. 2009;18:64–8. doi: 10.1111/j.1600-0625.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xeroderma pigmentosum, National diagnosis and treatment protocol for a rare disease. 2007; Communications Department, 2 avenue du Stade de France - F 93218 Saint-Denis La Plaine CEDEX [Google Scholar]
- 17.Kraemer KH, DiGiovanna JJ. Xeroderma pigmentosum. 2003 Jun 20 [Updated 2016 Sep 29] In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, et al., editors. GeneReviews® [Internet] Seattle (WA): University of Washington, Seattle; 1993-2021. [PubMed] [Google Scholar]
- 18.García M, Llames S, García E, Meana A, Cuadrado N, Recasens M, et al. In vivo assessment of acute UVB responses in normal and Xeroderma Pigmentosum (XP-C) skin-humanized mouse models. Am J Pathol. 2010;177:865–72. doi: 10.2353/ajpath.2010.091096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reichrath J. Sunlight, skin cancer and vitamin D: What are the conclusions of recent findings that protection against solar ultraviolet (UV) radiation causes 25-hydroxy vitamin D deficiency in solid organ-transplant recipients, xeroderma pigmentosum, and other risk groups? J Steroid Biochem Mol Biol. 2007;103:664–7. doi: 10.1016/j.jsbmb.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 20.Ashall G, Quaba AA, Hackett MEJ. Facial resurfacing in xeroderma pigmentosum: Are we spoiling the ship for a ha'p'orth of tar? Br J Plast Surg. 1987;40:610–3. doi: 10.1016/0007-1226(87)90156-1. [DOI] [PubMed] [Google Scholar]
- 21.Tayeb T, Laure B, Sury F, Lorette G, Goga D. Facial resurfacing with split-thickness skin grafts in xeroderma pigmentosum variant. J Cranio-Maxillofacial Surg. 2011;39:496–8. doi: 10.1016/j.jcms.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Nelson BR, Fader DJ, Gillard M, Baker SR, Johnson TM. The role of dermabrasion and chemical peels in the treatment of patients with xeroderma pigmentosum. J Am Acad Dermatol. 1995;32:623–6. doi: 10.1016/0190-9622(95)90348-8. [DOI] [PubMed] [Google Scholar]
- 23.Iyer S, Friedli A, Bowes L, Kricorian G, Fitzpatrick RE. Full face laser resurfacing: Therapy and prophylaxis for actinic keratoses and non-melanoma skin cancer. Lasers Surg Med. 2004;34:1149. doi: 10.1002/lsm.20012. [DOI] [PubMed] [Google Scholar]
- 24.Khillan JS. Vitamin A/retinol and maintenance of pluripotency of stem cells. Nutrients. 2014;6:1209–22. doi: 10.3390/nu6031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niles RM. Recent advances in the use of vitamin A (retinoids) in the prevention and treatment of cancer. Nutrition. 2000;16:1084–9. doi: 10.1016/s0899-9007(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 26.Burrage PS, Huntington JT, Sporn MB, Brinckerhoff CE. Regulation of matrix metalloproteinase gene expression by a retinoid X receptor-specific ligand. Arthritis Rheum. 2007;56:892–904. doi: 10.1002/art.22417. [DOI] [PubMed] [Google Scholar]
- 27.Kraemer KH, DiGiovanna JJ, Moshell AN, Tarone RE, Peck GL. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N Engl J Med. 1988;318:1633–7. doi: 10.1056/NEJM198806233182501. [DOI] [PubMed] [Google Scholar]
- 28.Jones E, Korzenko A, Kriegel D. Oral isotretinoin in the treatment and prevention of cutaneous squamous cell carcinoma. J Drugs Dermato. 2004;3:498–502. [PubMed] [Google Scholar]
- 29.Ighani A, Partridge ACR, Shear NH, Lynde C, Gulliver WP, Sibbald C, et al. Comparison of management guidelines for moderate-to-severe plaque psoriasis: A review of phototherapy, systemic therapies, and biologic agents. J Cutan Med Surg. 2019;23:204–21. doi: 10.1177/1203475418814234. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Zhang X, Zhang W, Peng C, Zhu W, Chen X. Polymorphisms of SLCO1B1 rs4149056 and SLC22A1 rs2282143 are associated with responsiveness to acitretin in psoriasis patients. Sci Rep. 2018;8:13182. doi: 10.1038/s41598-018-31352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamouda B, Jamila Z, Najet R, Slim T, Rafiaa N, Noureddine B, et al. Topical 5-fluorouracil to treat multiple or unresectable facial squamous cell carcinomas in Xeroderma Pigmentosum. J Am Acad Dermatol. 2001;44:1054. doi: 10.1067/mjd.2001.113476. [DOI] [PubMed] [Google Scholar]
- 32.Lambert WC, Lambert MW. Development of effective skin cancer treatment and prevention in xeroderma pigmentosum. Photochem Photobiol. 2015;91:475–83. doi: 10.1111/php.12385. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Sekiguchi M, Okada Y. Restoration of ultraviolet induced unscheduled DNA synthesis of xeroderma pigmentosum cells by the concomitant treatment with bacteriophage T4 endonuclease V and HVJ (Sendai virus) Proc Natl Acad Sci USA. 1975;72:4071–5. doi: 10.1073/pnas.72.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malhotra AK, Gupta S, Khaitan BK, Verma KK. Multiple basal cell carcinomas in xeroderma pigmentosum treated with imiquimod 5% cream. Pediatr Dermatol. 2008;25:488–91. doi: 10.1111/j.1525-1470.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang J-Q, Chen X-Y, Engle MY, Wang JY. Multiple facial basal cell carcinomas in xeroderma pigmentosum treated with topical imiquimod 5% cream. Dermatol Ther. 2015;28:243–7. doi: 10.1111/dth.12217. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi M. Roles of oxidative stress in xeroderma pigmentosum. Adv Exp Med Biol. 2008;637:120–7. doi: 10.1007/978-0-387-09599-8_13. [DOI] [PubMed] [Google Scholar]
- 37.Turner ML, Moshell AN, Corbett DW, Stern JB, Roth MJ, DiGiovanna J, et al. Clearing of melanoma in situ with intralesional interferon alfa in a patient with xeroderma pigmentosum. Arch Dermatol. 1994;130:1491–4. [PubMed] [Google Scholar]
- 38.Lehmann AR, Fassihi H. Molecular analysis directs the prognosis, management and treatment of patients with xeroderma pigmentosum. DNA Repair. 2020;93:1–5. doi: 10.1016/j.dnarep.2020.102907. [DOI] [PubMed] [Google Scholar]
- 39.Kumar P, Saini S, Prabhakar BS. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin Cancer Biol. 2020;64:29–35. doi: 10.1016/j.semcancer.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Chen AC, Martin AJ, Choy B, Fernández-Peñas P, Dalziell RA, McKenzie CA, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618–26. doi: 10.1056/NEJMoa1506197. [DOI] [PubMed] [Google Scholar]
- 41.Velez-Cruz R, Zadorin AS, Coin F, Egly JM. Sirt1 suppresses RNA synthesis after UV irradiation in combined xeroderma pigmentosum group D/Cockayne syndrome (XP-D/CS) cells. Proc Natl Acad Sci USA. 2013;110:E212–20. doi: 10.1073/pnas.1213076110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, et al. Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 2014;157:882–96. doi: 10.1016/j.cell.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai SI, Guarente L. It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech Dis. 2016;2:16017. doi: 10.1038/npjamd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weon JL, Glass DA. Novel therapeutic approaches to xeroderma pigmentosum. Br J Dermatol. 2019;181:249–55. doi: 10.1111/bjd.17253. [DOI] [PubMed] [Google Scholar]
- 45.Mazouzi A, Battistini F, Moser SC, Ferreira da Silva J, Wiedner M, Owusu M, et al. Repair of UV-induced DNA damage independent of nucleotide excision repair is masked by MUTYH. Mol Cell. 2017;68:797–807.e7. doi: 10.1016/j.molcel.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Vermeij WP, Dolle ME, Reiling E, Jaarsma D, Payan-Gomez C, Bombardieri CR, et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537:427–31. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verma IM, Weitzman MD. Gene therapy: Twenty-first century medicine. Annu Rev Biochem. 2005;74:711–38. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- 48.Otto AI, Riou L, Marionnet C, Mori T, Sarasin A, Magnaldo T, et al. Differential behaviors toward ultraviolet A and B radiation of fibroblasts and keratinocytes from normal and DNA-repair-deficient patients. Cancer Res. 1999;59:1212–8. [PubMed] [Google Scholar]
- 49.Arnould S, Perez C, Cabaniols J-P, Smith J, Gouble A, Grizot S, et al. Engineered I-CreI derivatives cleaving sequences from the human XPC gene can induce highly efficient gene correction in mammalian cells. J Mol Biol. 2007;371:49–65. doi: 10.1016/j.jmb.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 50.Redondo P, Prieto J, Muñoz IG, Alibés A, Stricher F, Serrano L, et al. Molecular basis of Xeroderma Pigmentosum group cDNA recognition by engineered meganucleases. Nature. 2008;456:107–11. doi: 10.1038/nature07343. [DOI] [PubMed] [Google Scholar]
- 51.Howick J, Chalmers I, James Lind Library. Glasziou P, Greenhalgh T, Heneghan C, Liberati A, et al. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine [Google Scholar]
- 52.Zeitouni NC, Shieh S, Oseroff AR. Laser and photodynamic therapy in the management of cutaneous malignancies. Clin Dermatol. 2001;19:328–38. doi: 10.1016/s0738-081x(01)00170-5. [DOI] [PubMed] [Google Scholar]
- 53.Larson DM, Cunningham BB. Photodynamic therapy in a teenage girl with xeroderma pigmentosum type C. Pediatr Dermatol. 2012;29:373–4. doi: 10.1111/j.1525-1470.2011.01657.x. [DOI] [PubMed] [Google Scholar]
- 54.Procianoy F, Cruz AAV, Baccega A, Ferraz V, Chahud F. Aggravation of eyelid and conjunctival malignancies following photodynamic therapy in DeSanctis-Cacchione syndrome. Ophthal Plast Reconstr Surg. 2006;22:498–9. doi: 10.1097/01.iop.0000246600.80517.d3. [DOI] [PubMed] [Google Scholar]
- 55.Yarosh D, Klein J, O’Connor A, Slim T, Rafiaa N, Noureddine B, et al. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in Xeroderma Pigmentosum: A randomised study. Lancet. 2001;357:926–9. doi: 10.1016/s0140-6736(00)04214-8. [DOI] [PubMed] [Google Scholar]
- 56.Lacarrubba F, Potenza MC, Gurgone S, Micali G. Successful treatment and management of large superficial basal cell carcinomas with topical imiquimod 5% cream: A case series and review. J Dermatolog Treat. 2011;22:353–8. doi: 10.3109/09546634.2010.548503. [DOI] [PubMed] [Google Scholar]
- 57.Greig A, Aloni K, Orchard G, Craythorne E, Fassihi H. Treatment of multiple facial basal cell carcinomas in a child with xeroderma pigmentosum complementation group C with Mohs micrographic surgery. Br J Dermatol. 2021;184:e4. doi: 10.1111/bjd.19323. [DOI] [PubMed] [Google Scholar]
- 58.Yarosh DB, O’Connor A, Alas L, Potten C, Wolf P. Photoprotection by topical DNA repair enzymes: Molecular correlates of clinical studies. Photochem Photobiol. 1999;69:136–40. [PubMed] [Google Scholar]
- 59.Miyata R, Sasaki T, Hayashi M, Araki S, Shimohira M, Kohyama J. Low-dose levodopa is effective for laryngeal dystonia in xeroderma pigmentosum group A. Brain Dev. 2010;32:685–7. doi: 10.1016/j.braindev.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 60.Dirar QS, Musalem HM, Al-Hazzaa SAF, Zoba AAA, Almalki AA. Effect of pegylated interferon and mitomycin C on ocular surface squamous neoplasia in xeroderma pigmentosum: A case series? Am J Case Rep. 2020;21:e921301. doi: 10.12659/AJCR.921301. doi: 10.12659/AJCR.921301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arnaudeau-Bégard C, Brellier F, Chevallier-Lagente O, Hoeijmakers J, Bernerd F, Sarasin A, et al. Genetic correction of DNA repair-deficient/cancer-prone xeroderma pigmentosum group C keratinocytes. Hum Gene Ther. 2003;14:983–96. doi: 10.1089/104303403766682241. [DOI] [PubMed] [Google Scholar]
- 62.Marchetto MCN, Muotri AR, Burns DK, Hoeijmakers J, Bernerd F, Sarasin A, et al. Gene transduction in skin cells: Preventing cancer in Xeroderma Pigmentosum mice. Proc Natl Acad Sci USA. 2004;101:17759–64. doi: 10.1073/pnas.0406304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warrick E, Garcia M, Chagnoleau C, Chevallier O, Bergoglio V, Sartori D, et al. Preclinical corrective gene transfer in xeroderma pigmentosum human skin stem cells. Mol Ther. 2012;20:798–807. doi: 10.1038/mt.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwank G, Koo B-K, Sasselli V, Dekkers JF, Heo I, Demircan T, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–8. doi: 10.1016/j.stem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Goncalves-Maia M, Magnaldo T. Genetic therapy of xeroderma pigmentosum: Analysis of strategies and translation. Expert Opin Orphan Drugs. 2017;5:5–17. [Google Scholar]


