Abstract
Background:
Psoriasis is a chronic inflammatory skin disease with an unknown pathogenesis. Recently, miR-31 have been shown to play an important role in psoriasis. Moreover, STAT3/p53 pathway has been used in tumor studies, but rarely in psoriasis studies.
Aims:
The present study aimed to investigate the role of STAT3/p53 pathway in psoriasis-like lesions in a mouse model of miR-31 overexpression.
Methods:
All mice (n = 44) were divided into four groups: normal mice treated with Vaseline® (NV; n = 10), normal mice treated with imiquimod (NI; n = 12), miR-31-overexpressing mice treated with Vaseline® (MV; n = 10), and miR-31-overexpressing mice treated with imiquimod (MI; n = 12). Then, we assayed the expression of STAT3 and p53.
Results:
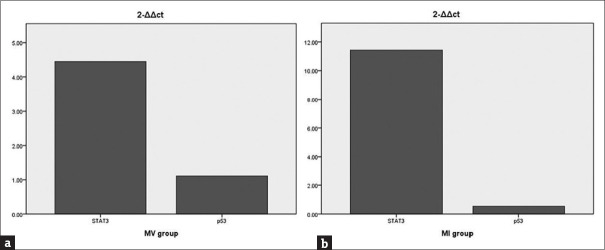
Our results showed that at the protein level (P < 0.01) and gene level (4.45 times), the expression of STAT3 in the MV group was higher than that in the NV group, and at the protein level (P < 0.01) and gene level (11.43 times), the expression of STAT3 in the MI group was higher than that in the NI group. At the protein level, the expression of p53 in MV group was higher than that in the NV group (P < 0.05), and the expression of p53 in MI group was higher than that in the NI group (P < 0.01).
Conclusions:
Our findings indicate that overexpression of miR-31 causes upregulation of STAT3, which further brings about upregulation of p53, and eventually leads to serious psoriasis skin lesion.
KEY WORDS: MicroRNA-31, mouse model, p53, psoriasis-like lesions, STAT3
Introduction
MicroRNAs (miRNAs or miRs), a type of non-coding, single-stranded RNAs, play an important role in the biology of cutaneous tissues, and have been shown to be potential biomarkers of immunopathological inflammation.[1,2] Borska et al.[2] reported that miR-31, a highly conserved miRNA, is involved not only in physiological processes but also in pathological processes. The activity of miR-31 is disrupted in inflammatory disorders including psoriasis.[2,3] In fact, psoriasis is characterized by a highly specific miR expression profile, with miR-31 overexpressed in the skin lesions of psoriasis patients.[4,5] Hence, miR-31 plays a significant role in psoriasis-like cutaneous lesions. However, the majority of research regarding miR-31 and psoriasis has relied on observational studies. Research describing the in vivo mechanisms of psoriasis and miR-31 activity has rarely been reported.
Signal transducer and activator of transcription 3 (STAT3), as a transcription factor, can regulate cytokines and growth factor,[6] and can cause cell proliferation.[7,8] P53, as tumor suppressor protein, can suppress tumor growth by controlling cell cycle and DNA replication.[9] Currently, the study of both focus largely on tumor. However, one of the key characteristics of psoriasis is excessive proliferation of cells, and STAT3 and p53 play a significant role in this regard.
Therefore, in this study, we aimed to investigate the action of miR-31/STAT3/p53 on psoriasis in vivo. We generated a mouse model of miR-31 overexpression. Then, we generated a mouse model of psoriasis-like cutaneous lesions by smearing imiquimod on the dorsal surface of the mice. Through the above models, we illustrated the relationship among mir-31, STAT3, and p53 in psoriasis-like cutaneous lesions. Our results indicate that miR-31 may promote the development of psoriasis-like cutaneous lesions through STAT3/p53 pathway.
Materials and Methods
Experimental animals
Forty-four female normal specific-pathogen-free (SPF)-grade FVB mice (6–8 weeks old) were obtained from Charles River (Beijing, China SCXK [jing], 2012-0001) and housed at the center for Experimental Animals at ShanXi Medical University. The mice were provided standard laboratory chow and clean water. They were housed in a room with constant humidity at 20–25°C.
A mouse model of miR-31 overexpression was generated using 22 mice as described below. All mice were divided into four groups: normal mice treated with Vaseline® (NV; n = 10), normal mice treated with imiquimod (NI; n = 12), miR-31-overexpressing mice treated with Vaseline® (MV; n = 10), and miR-31-overexpressing mice treated with imiquimod (MI; n = 12).
All procedures in the study involving animals were performed in accordance with the Ethical standards of the Medical Ethics Committee of the Taiyuan City Central Hospital in Taiyuan, China.
Vector construction
In accordance with previously described methods,[10] attB1-miR31-attB2 was amplified via overlap polymerase chain reaction (PCR). The following primers were used: attB1 - Kozak - miR31,5'-GGGGACAAGTTTGTACAAAAA
AGCAGGCTGCCACCGAAAATGGCTGTATTCATCGTTG-3'; attB2-miR31, 5'-GGGGACCACTTTGTACAAGAAAGC
TGGGTGACAAGTCAGAGCAGGGTCAT-3'. Thereafter, using the gateway clone design, the BP reaction was carried out with the amplified attB1-miR31-attB2, and the BP products were transformed into E. coli Stbl3 for translation. Thereafter, the cells were incubated on LB agar overnight at 37°C. The plasmid pDown-miR31 was extracted after PCR amplification and the LR reaction was carried out using the mammalian expression vector PRP.Des2d for 3 h at 25°C. Reaction products were transformed into E. coli Stbl3 for translation. Positive plasmids were selected after PCR amplification. Finally, PRP.Ex2d-EF1A > miR31 was constructed as the final vector for miR-31 overexpression.
Generation of a mouse model of miR-31 overexpression
In accordance with previously described methods, oosperm were harvested from normal pregnant mice and micro-injected with the miR-31 overexpression vector. Oosperm containing the miR-31 overexpression vector were transferred into the oviduct of pseudopregnant mice to generate neonates with positive miR-31 overexpression. Four weeks after birth, approximately 1 cm of the tails of mouse pups was excised. Total DNA was extracted (TaKaRa MiniBEST Universal Genomic DNA Extraction Kit ver. 5.0 TaKaRa Bio, Japan) and amplified via PCR. The reaction system including 2 μL DNA, 1 μL primer, 25 μL premixTaq, 22 μL DEPC H2O.[10] Agarose gel electrophoresis was performed to identify vector-positive mice.
Generation of a mouse model of psoriasis-like skin lesions
Fur on the dorsal surface of all mice was shaved. Sixty milligrams of 5% imiquimod (Sichuan Med-Shine Pharmaceutical Co. Ltd., Sichuan, China) was smeared on the nude backsides of the mice once a day for 5 days. Changes in the skin were observed with the naked eye.
PASI score of mouse model of psoriasis-like skin lesions in the study
PASI score, as method of evaluating the severity of psoriasis, has been accepted internationally. According to PASI score criteria, erythema (E), incrassation (I), and scales (S) of dorsal skin of each mouse were scored. And then, we obtained a total score by adding the above three scores, and drew a trend line by average value of total score obtained in every day [Figure 1]. The scoring criteria were as follows: 0 (none), no significant erythema, incrassation, and scales; 1 (low grade), part of dorsal skin was injured or degree of injury was minor such as mild incrassation, scales, and light red skin lesion; 2 (moderate), the majority of dorsal skin was injured, evident incrassation, severe scales, and erythema; 3 (severe), the whole dorsal skin of mouse showed obvious incrassation, more severe scales, and erythema; 4 (very severe), very evident incrassation, extremely severe scales, and erythema.
Figure 1.
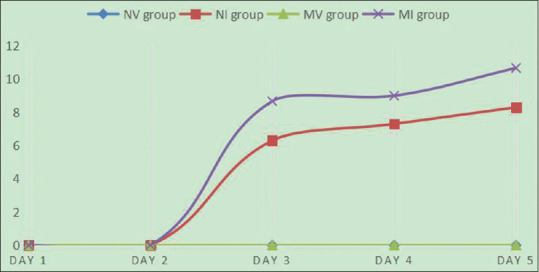
The line chart about PASI score of NV, NI, MV, and MI group
Immunohistochemistry
Dorsal skin specimens from all 44 mice were harvested and fixed in 4% paraformaldehyde. These specimens were then dehydrated and embedded in paraffin and cut into 4-μm-thick sections. Sections were dried at 65°C for 30 min, soaked twice independently in xylene for 15 min, then soaked twice independently in absolute ethanol, and later soaked in three consecutive ethanol concentrations (95%, 80%, and 70% ethanol) for 5 min each. Thereafter, sections were washed with PBS and incubated in 3% H2O2 for 15 min. Sections were blocked with normal goat serum for 30 min at 37°C and drained. Thereafter, primary antibodies were added (rabbit ployclonal anti-p53 antibody 1:100, ab131442, Abcam China and rabbit monoclonal anti-STAT3 antibody 1:200, ab68153, Abcam China) and the specimens were incubated at 4°C overnight under humid conditions. Biotinylated goat anti-rabbit secondary antibody was used with 3,3'-diaminobenzidine as the chromogen, and the chromogenic reaction was terminated with distilled water after brown particles were observed and staining intensity was moderate upon microscopic observation. Thereafter, samples were counterstained with hematoxylin and eosin (HE), dehydrated with ethanol, made transparent with two independent treatments with xylene, and sealed using resin. Sample staining was evaluated microscopically.
Reverse transcription quantitative PCR (RT-qPCR)
We also assessed the expression of STAT3 and p53 by RT-qPCR. Dorsal skin specimens were harvested from all 44 mice and total RNA was extracted from skin specimens using Trizol (Invitrogen, Karlsruhe, Germany). After reverse transcription by reverse transcription kit (TaKaRa Bio Japan), qPCR amplification was performed using a reaction system including 5 μL TB Green Premix Ex Taq II, 0.2 μL ROX Reference Dye II (TaKaRa Bio, Japan), 1 μL cDNA, 0.2 μL of upstream and downstream primers each, and 3.4 μL ddH2O. β-actin was used as the reference gene. The primers for qPCR are listed in Table 1.
Table 1.
primers used for qPCR
| Target | Primer sequence | Annealing temperature(°C) | Products size (bp) |
|---|---|---|---|
| STAT3 | F: 5’- CAATACCATTGACCTGCCGAT-3’ | 45 | 109 |
| R: 5’- GAGCGACTCAAACTGCCCT-3’ | |||
| P53 | F: 5’- TCACAGCGTCTGTTGACATTT-3’ | 45 | 210 |
| R: 5’- ACCAAGCTCATTACCCTGACA-3’ |
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM, USA). After validating the homogeneity of variance, a paired t-test was performed to analyze the results of immunohistochemistry. A P value <0.05 was considered a statistically significant difference. In addition, we used the 2-△△ct method to analyze RT-qPCR data.
Results
Validation of the mouse model of miR-31 overexpression
Four weeks after birth, approximately 1 cm of the tails of mouse pups was excised and evaluated for vector expression [Figure 2]. The size of primers for miR-31 was 212 bp. After detection, 22 mice that contained the expression vector were used for building mice model.
Figure 2.
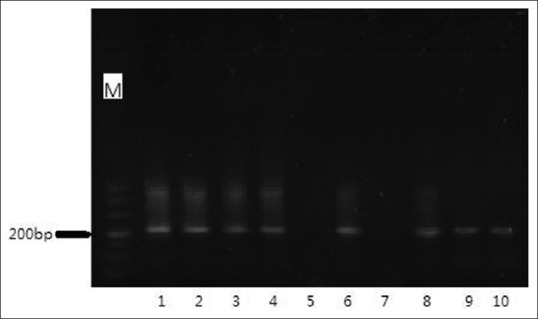
Agarose gel electrophoresis of miR-31 products amplified by PCR. Except band 5 and 7, all lanes indicated positive appropriate transformation of mice with the miR-31 expression vector
Validation of the mouse model of psoriasis-like skin lesion
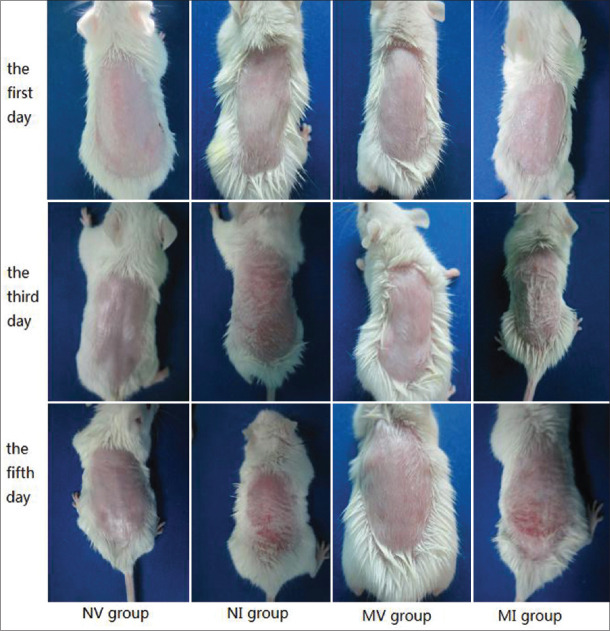
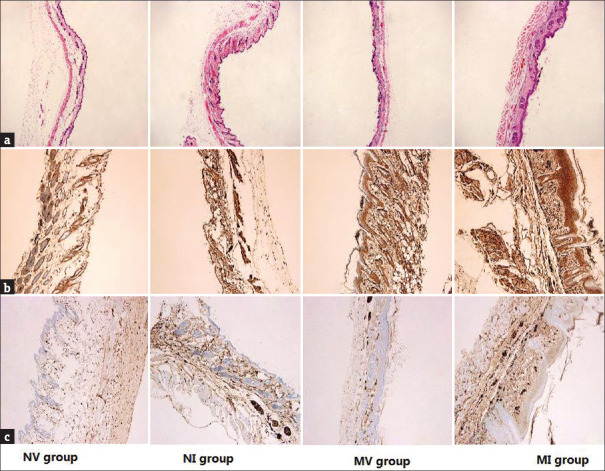
Changes in the dorsal skin of mice, such as cutaneous incrassation, coarseness, and development of scales of psoriasis-like lesions, were first observed on the third day (approximately) and peaked on the fifth day [Figure 3]. Pathological dorsal skin sections of mice were evaluated using an inverted microscope. Changes such as dermal incrassation, vascular proliferation, microabscess, and so on were observed [Figure 4a].
Figure 3.
Clinical manifestation of four groups treated with vaseline or imiquimod on the first day, the third day, and the fifth day
Figure 4.
(a) Hematoxylin and eosin (HE) staining of pathological skin sections of the four groups (magnification × 400). (b and c) Results of immunohistochemistry of STAT3 and p53 in the NV, NI, MV, and MI groups, respectively. Brown color indicates positive staining (magnification × 400)
Overexpression of miR-31 facilitated upregulation of STAT3 and p53
Our results showed no statistical differences in the expression of STAT3 at the protein level and gene level between the NV and NI groups, implying that the expression of STAT3 was not different in psoriatic skin lesions and non-lesion skin of wild type mice. Additionally, at the protein level (P < 0.01) and gene level (4.45 times), the expression of STAT3 in the MV group was higher than that in the NV group [Figures 4b and 5a], and at the protein level (P < 0.01) and gene level (11.43 times), the expression of STAT3 in the MI group was higher than that in the NI group [Figures 4b and 5b], indicating that STAT3 was upregulated in psoriatic skin lesions and non-lesion skin of miR-31-overexpressing mice. Further indicated overexpression of miR-31 facilitates the upregulation of STAT3.
Figure 5.
(a) Compared to NV group, the expression of STAT3 and p53 in the MV group. (b) Compared to NI group, the expression of above targets in the MI group
As for p53, there was no statistical difference at gene level [Figure 5]. However, at the protein level, the expression of p53 in MV group was higher than that in the NV group (P < 0.05), and the expression of p53 in MI group was higher than that in the NI group (P < 0.01) [Figure 4c], indicating that p53 (at protein level) was upregulated in psoriatic skin lesions and non-lesion skin of miR-31-overexpressing mice.
Discussion
Psoriasis is a chronic inflammatory cutaneous disease manifesting excess proliferation of keratinocyte, hyperkeratinization, and neoangiogenesis.[11] In recent years, numerous studies have shown that miRs play an important role in cutaneous diseases. In particular, miR-31 is a highly conserved miR involved in the development of psoriasis. Shi et al.[12] reported that under normal conditions, miR-31 does not play a crucial role in the epidermis and its expression is moderate; however, during inflammation, miR-31 is upregulated. Moreover, Li D et al.[3] reported that miR-31 is dysregulated in patients with psoriasis. Some scholars have shown that miR-31 is overexpressed in the cutaneous lesions of patients with psoriasis.[5,13] Moreover, STAT3 and p53 are closely related to cell proliferation, which is one of the typical traits of psoriasis. However, the study of STAT3/p53 pathway is common in tumor and little research on STAT3/p53 pathway in psoriasis is reported. Therefore, to further investigate the role of miR-31 and STAT3/p53 pathway in psoriatic cutaneous lesions, we generated a mouse model of miR-31 overexpression and a mouse model of psoriasis-like skin lesion and investigated the expression of STAT3 and p53.
Our results showed that the expression of STAT3 was no different in psoriatic skin lesions and non-lesion skin in wild type mice; however, it was upregulated in psoriatic skin lesions and non-lesion skin in miR-31-overexpressing mice. These findings indicate that overexpression of miR-31 facilitates the upregulation of STAT3. Moreover, miR-31 expression is also inhibited by suppression of STAT3.[14] Therefore, there is synergetic relationship between the two. Further study discloses that STK40, as target gene of miR-31, can negatively regulate NF-kB, and then NF-kB can activate STAT3. Hence, when miR-31 is overexpressed, its target gene, expression of STK40 is downregulated. The low expression of STK40 facilitates activation of NF-kB. Afterwards, STAT3 is activated by activation of NF-kB. Therefore, miR-31 overexpression eventually leads to upregulation of STAT3.[14] STAT3 is related to the activation of pro-inflammatory factors.[15] STAT3 is also involved in cellular proliferation and apoptosis, and it is significantly overexpressed in psoriatic skin lesions.[8,16] In our study, whether at gene level or at protein level, whether in psoriatic skin lesions or in non-lesion skin, the results show STAT3 is upregulated while in miR-31 overexpression.
Activated STAT3 can cause upregulation of p53 by binding promoter of p53 or tumorigenic products of activated STAT3.[17,18] However, at present, there is no evidence to show miR-31 overexpression could result in upregulation of p53. Hence, in the study, upregulation of p53 is likely to be caused by activated STAT3. Upregulation of p53 is closely associated with keratinocyte hyperproliferation. Tanja Batinac et al.[19,20] reported that there is a positive correlation between p53 expression and keratinocyte hyperproliferation; it has also been shown that p53 plays an important role during psoriatic hyperproliferation.
In conclusion, in the mouse models, miR-31 overexpression causes upregulation of STAT3, which further brings about upregulation of p53. High expression of STAT3 and p53 causes hyperproliferation of cells, especially that of keratinocyte. Keratinocyte hyperproliferation is one of the typical traits of psoriasis. Moreover, judging from the PASI score, the severity of skin lesion of miR-31-overexpressing mice (MI group) is higher than that of skin lesion of normal mice (NI group), indicating that in the two groups having psoriatic lesion, the group of miR-31 overexpression may cause more serious cutaneous lesion by STAT3/p53 pathway. miR-31/STAT3/p53 pathway has been used in tumor studies, but rarely in psoriasis studies. Therefore, it may be a new way to treat psoriasis.
Financial support and sponsorship
This work was supported by the National Nature Science Foundation of China (No. 81602768 and 81773336).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Kaiming Zhang offered ideas. Qiang Wang conducted the experiments and drafted the manuscript. Xincheng Zhao, Chunfang Wang, Xiao Li, and Guohua Yin assisted to finish the experiments. Junqin Li and Ruixia Hou analyzed the data.
References
- 1.Wu T, Chen G. miRNAs participate in MS pathological processes and it's therapeutic response. Mediators Inflamm. 2016;2016:4578230. doi: 10.1155/2016/4578230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borska L, Andrys C, Chmelarova M, Kovarikova H, Krejsek J, Hamakova K, et al. Roles of miR-31 and endothelin-1 in psoriasis vulgaris: Pathophysiological functions and potential biomarkers. Physiol Res. 2017;66:987–92. doi: 10.33549/physiolres.933615. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Li XI, Wang A, Meisgen F, Pivarcsi A, Sonkoly E, et al. MicroRNA-31 promotes skin wound healing by enhancing keratinocyte proliferation and migration. J Invest Dermatol. 2015;135:1676–85. doi: 10.1038/jid.2015.48. [DOI] [PubMed] [Google Scholar]
- 4.Xu N, Meisgen F, Butler LM, Han G, Wang XJ, Söderberg-Nauclér C, et al. MicroRNA-31 is overexpressed in psoriasis and modulates inflammatory cytokine and chemokine production in keratinocytes via targeting serine/threonine kinase 40. J Immunol. 2013;190:678–88. doi: 10.4049/jimmunol.1202695. [DOI] [PubMed] [Google Scholar]
- 5.Wang M-J, Xu Y-Y, Huang R-Y, Chen X-M, Chen H-M, Han L, et al. Role of an imbalanced miRNAs axis in pathogenesis of psoriasis: Novel perspectives based on review of the literature. Oncotarget. 2017;8:5498–507. doi: 10.18632/oncotarget.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.You L, Wang Z, Li H, Shou J, Jing Z, Xie J, et al. The role of STAT3 in autophagy. Autophagy. 2015;11:729–39. doi: 10.1080/15548627.2015.1017192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabarek B, Krzaczynski J, Strzalka-Mrozik B, Wcisło-Dziadecka D, Gola J. The influence of ustekinumab on expression of STAT1, STAT3, STAT4, SOCS2, and IL17 in patients with psoriasis and in a control. Dermatol Ther. 2019;32:e13029. doi: 10.1111/dth.13029. [DOI] [PubMed] [Google Scholar]
- 8.Farag AGA, Samaka R, Elshafey EN, Shehata WA, El Sherbiny EG, Hammam MA. Immunohistochemical study of janus kinase 1/signal transducer and activator of transcription 3 in psoriasis vulgaris. Clin Cosmet Investig Dermatol. 2019;12:497–508. doi: 10.2147/CCID.S202835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanapathipillai M. Treating p53 mutant aggregation-associated cancer. Cancers (Basel) 2018;6:154. doi: 10.3390/cancers10060154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu M, Wang C, Li X, Tian F, Zhang Y, Li P, et al. Establishment of a miR-31 transgenic mouse and its expression in tissues and organs. Acta Laboratorium Anim Sci Sin. 2018;26:1–7. [Google Scholar]
- 11.Okan G, Yıldız Z, Gökdemir G, Yorulmaz E, Vural P, Doğru-Abbasoğlu S, et al. G-231A and G+70c polymorphisms of endothelin receptor type-A gene could affect the psoriasis area and severity index score and endothelin 1 level. Indian J Dermatol. 2015;60:211. doi: 10.4103/0019-5154.152561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi J, Ma X, Su Y, Song Y, Tian Y, Yuan S, et al. MiR-31 mediates inflammatory signaling to promote re-epithelialization during skin wound healing. J Invest Dermatol. 2018;138:2253–63. doi: 10.1016/j.jid.2018.03.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepicheva NA, Song JL. Function and regulation of microRNA-31 in development and disease. Mol Reprod Dev. 2016;83:654–74. doi: 10.1002/mrd.22678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishigami K, Nosho K, Koide H, Kanno S, Mitsuhashi K, Igarashi H, et al. MicroRNA-31 reflects IL-6 expression in cancer tissue and is related with poor prognosis in bile duct cancer. Carcinogenesis. 2018;39:1127–34. doi: 10.1093/carcin/bgy075. [DOI] [PubMed] [Google Scholar]
- 15.Rao R, Nagarkatti P, Nagarkatti M. Role of miRNA in the regulation of inflammatory genes in staphylococcal enterotoxin B-induced acute inflammatory lung injury and mortality. Toxicol Sci. 2015;144:284–97. doi: 10.1093/toxsci/kfu315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabarek B, Krzaczyński J, Strzałka-Mrozik B, Wcisło-Dziadecka D, Gola J. The influence of ustekinumab on expression of STAT1, STAT3, STAT4, SOCS2, and IL17 in patients with psoriasis and in a control. Dermatol Ther. 2019;32:e13029. doi: 10.1111/dth.13029. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Lv J, Liu J, Liang X, Jin X, Xie J, et al. STAT3/p53 pathway activation disrupts IFN-β-induced dormancy in tumor-repopulating cells. J Clin Invest. 2018;128:1057–73. doi: 10.1172/JCI96329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Tang H, Jin X, Jia G, Hsieh JT. p53 regulates Stat3 phosphorylation and DNA binding activity in human prostate cancer cells expressing constitutively active Stat3. Oncogene. 2002;21:3082–8. doi: 10.1038/sj.onc.1205426. [DOI] [PubMed] [Google Scholar]
- 19.Batinac T, Zamolo G, Hadzisejdić I, Zauhar G, Brumini G, Ruzić A, et al. Expression of Bcl-2 family proteins in psoriasis. Croat Med J. 2007;48:319–26. [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SA, Ryu YW, Kwon JI, Choe MS, Jung JW, Cho JW. Differential expression of cyclin D1, Ki-67, pRb, and p53 in psoriatic skin lesions and normal skin. Mol Med Rep. 2018;17:735–42. doi: 10.3892/mmr.2017.8015. [DOI] [PMC free article] [PubMed] [Google Scholar]