Abstract
Background:
Warts are a common dermatologic complaint with an increased incidence within the pediatric population. Warts are caused by multiple strains of the human papillomavirus (HPV). There is little research on how a patient's HPV immunization status affects the response to treatment of warts in pediatric patients.
Aims:
The purpose of this study is to investigate the relationship between HPV vaccination status and wart resolution.
Materials and Methods:
This is a retrospective chart review that investigates the relationship between response to routine treatment of warts and a subject's HPV vaccination status.
Results:
There was no significant relationship found between HPV vaccination status and resolution of warts (p = 0.797). However, there was a significant positive correlation between having the HPV vaccine and number of visits for the treatment of warts (r = 0.180, P = 0.024).
Conclusion:
This study did not show a significant correlation between HPV vaccination status and wart resolution, although it demonstrated a significant positive relationship between those immunized with the HPV vaccine and an increased number of treatment visits. Possible explanations for this unexpected correlation include the variation in HPV vaccine formulation, vaccination status, and frequency of office visits, since vaccinated patients are more likely to be compliant with office visits.
KEY WORDS: Cutaneous warts, filiform warts, flat warts, HPV vaccination, HPV, pediatrics, plantar warts, recalcitrant warts, verruca vulgaris
Introduction
Warts are a common dermatologic complaint with an increasing incidence among the pediatric patient population.[1,2] Human papillomaviruses (HPV) can cause a variety of wart morbidities such as verruca vulgaris (common warts), verruca plana (flat warts), palmoplantar warts, and external genital warts. In the pediatric population, warts are more common between the ages of 9 and 16.[3] Common warts on the hand and feet are primarily caused by HPV types 1, 2, 4, 27, and 57.[4] The majority of warts will spontaneously resolve in 1–4 years.[5] However, in some cases, warts will continue to enlarge in some locations (e.g., periungual), may be painful, and many times are associated with embarrassment for the patient.
Treatment of warts in the pediatric patient is challenging because there needs to be a balance among optimization of outcomes, comfort, and safety for patients. Many of the current treatments include topical compounds with salicylic acid, cantharidin, duct tape, retinoids, imiquimod, shave removal, cryotherapy, cimetidine, sinecatechins, immunotherapy with candida antigen, and pulsed dye laser.[6,7] Cryotherapy has been shown to resolve between 60% and 88% of warts, but require longer freezing cycles.[2] Despite these topical treatments, recurrence rates are high, where up to one-third of non-genital warts become recalcitrant.[8]
There are many research studies showing the use of the quadrivalent HPV vaccine, which protects against strains 6, 11, 16, and 18 to prevent genital warts in women; efficacy is high with a successful prophylactic rate in 90–100% of cases.[9] However, while many treatments exist for non-genital warts such as common and plantar warts, there is a lack of evidence surrounding prophylaxis for these warts aside from basic hygiene, especially in the pediatric population. There have been three published case reports that initially demonstrated a possible association between clearance of recalcitrant warts and intramuscular injection of the HPV vaccine. However, none of these reports could rule out the possibility of spontaneous clearance.[10,11,12] Nonetheless, these individual case reports sparked a great interest to investigate the role of the HPV vaccine in the treatment and prophylaxis of recalcitrant warts. The current theory surrounding the use of the HPV vaccine as a treatment for genital warts includes the well-known idea that vaccination leads to a general stimulation of the immune system. This activates both innate and adaptive immunity to destroy HPV-infected cells.
While previous studies have explored the role of the HPV vaccine as a treatment modality, this study aims to investigate the novel concept of a prophylactic role of the HPV vaccine for verruca vulgaris. With only a handful of studies evaluating the pediatric population, our study targets a larger cohort of pediatric patients. Here, we studied how a pediatric patient's HPV immunization status affects their response to routine treatment for common warts, palmoplantar warts, and recalcitrant warts—defined as warts that persist after three or more treatments.
Materials and Methods
This retrospective chart review investigates the relationship between response to routine treatment of common warts (recalcitrant and non-recalcitrant) and a subject's HPV vaccination status. About 2000 patient charts between 2017 and 2018 were reviewed from the University of Rochester's Pediatric Dermatology Clinic. Subjects were between the ages of 9 and 18 and met the study inclusion criteria—being English speaking and having an EPIC ICD 9 and 10 diagnosis of verruca vulgaris or plantar warts (extragenital). Subjects who did not have an apparent confirmed HPV vaccination status within EPIC and those who were lost to follow-up were excluded. Subjects were not contacted regarding confirmation of their HPV vaccine status. The HPV-9 Gardasil vaccine as well as Gardasil quadrivalent vaccine were studied in this chart review. About 182 patients were found to carry an ICD 9 and 10 code for verruca vulgaris or plantar warts. After reviewing these charts with the exclusion criteria, the sample size was determined to be 151 subjects. Recalcitrant warts are caused by an inflammatory process in response to the HPV infection that has persisted despite three or more office-based treatments, such as liquid nitrogen cryotherapy and chemical topical therapies.[10]
The primary outcome measure was wart resolution, which was defined as complete disappearance of warts. In order to assess for complete disappearance, follow-up visits with the dermatology clinic were reviewed. For patients that had dermatologic follow-up, resolution was clearly indicated in the patient's visit note. A patient was considered not resolved if the patient was noted to have continued warts with adequate follow-up. Patients were considered lost to follow-up if the last note by dermatologist instructed the patient to return to clinic, yet there were no more patient visits. Secondary outcomes such as number of treatment visits, type of treatment, and number of warts were also collected.
Statistical analysis was performed using SPSS Software (IBM SPSS
Statistics 25, SPSS Science, Chicago, IL, USA). To establish data normality, data was reviewed by creating a histogram and a Q-Q plot. It was expected that the data would not be normally distributed, due to a small sample size, so it was transformed into a logarithmic plot. To assess the correlation of various patient factors with the resolution of recalcitrant warts, Pearson's correlation analysis was performed, setting the significance level at P = 0.05. Additionally, a histogram and a scatter plot were inspected. Demographic data based on our study cohort are displayed in Table 1.
Table 1.
Summary statistics
| HPV vaccine + | HPV vaccine - | Total Population | |
|---|---|---|---|
| Sample Size | 73 | 78 | 151 |
| Sex | |||
| Male | 32 (44%) | 24 (31%) | 56 (37%) |
| Female | 41 (56%) | 54 (69%) | 95 (63%) |
| Age (years) (9-18) | 13.5 | 13.1 | 13.3, P=0.9174 |
| Recalcitrant | |||
| Yes | 11 (15%) | 25 (32%) | 52 (34%) |
| No | 62 (85%) | 53 (68%) | 99 (66%) |
| Resolution | |||
| Yes | 33 (45%) | 36 (46%) | 69 (46%) |
| No | 40 (55%) | 42 (54%) | 82 (54%) |
| Cryotherapy | |||
| Yes | 72 (99%) | 76 (97%) | 148 (98%) |
| No | 1 (1%) | 2 (3%) | 3 (2%) |
| Candida | |||
| Yes | 20 (27%) | 11 (14%) | 31 (21%) |
| No | 53 (73%) | 67 (86%) | 120 (79%) |
| Other* | |||
| Yes | 15 (21%) | 9 (12%) | 24 (16%) |
| No | 58 (79%) | 69 (88%) | 127 (84%) |
| Required >1 Tx Type | 25 (34%) | 17 (22%) | 42 (28%) |
| Required 1 Tx Type | 48 (66%) | 61 (78%) | 109 (72%) |
*Cantharidin, compound wart formulation, imiquimod, 5-FU
Results
In our study, 27 patients who received the HPV vaccine and 24 patients without the HPV vaccine developed recalcitrant warts. Also, 46 patients who received the HPV vaccine did not develop recalcitrant warts, and 53 patients who did not receive the HPV vaccine did not develop recalcitrant warts.
With regards to demographics, the average age of the study population was 13.3 years for our overall study population with a female predominance (95:56, females:males). A count of 148 of the 151 subjects, or 98% of our study population, received cryotherapy for wart resolution; 31 or 21% of patients received Candida injections for wart resolution. Finally, 42 or 28% of our patients required more than one treatment type for wart treatment and resolution.
Overall, there was no significant relationship found between HPV vaccination status and resolution of warts (p = 0.797). However, there was a significant positive correlation between HPV vaccination status and the number of visits required for the treatment of verruca vulgaris (r = 0.180, P = 0.024). Stratification by HPV vaccination status did not yield any other significant correlation. Furthermore, none of the other statistics analyzed were significant, such as gender and the effect on wart resolution, and number of warts compared to the number of treatment visits (r = 0.170, P = 0.137 for number of warts compared to number of treatment visits). Other non-significant statistics are shown in Table 2. The amount of warts the patients in this study had before seeking treatment can be seen in Figure 1. The number of treatment visits stratified by HPV vaccination status can be seen in Figure 2.
Table 2.
Two-tailed significance of the relationship between selected variables
| Variables | Pearson coefficient or Phi | P | Number of cases |
|---|---|---|---|
| HPV vaccine status vs. Number of Treatment visits | 0.180- Pearson | 0.024* | 158 |
| HPV Vaccine and eventual resolution of warts | -0.021- Phi | 0.797 | 158 |
| HPV Vaccine status and Presence of recalcitrant warts | 0.052- Phi | 0.524 | 158 |
| HPV Vaccine Status vs. need for Candida Injection | 0.166- Phi | 0.037* | 158 |
| Treatment with Candida Antigen and resolution of warts | -0.064- Phi | 0.583 | 73 |
| Female HPV vaccination status and resolution of warts | 0.380- Phi | 0.380 | 94 |
| Females positive for HPV vaccination and the number of visits to wart resolution | 0.215- Pearson | 0.068 | 73 |
| Females positive for HPV vaccination and Presence of Recalcitrant Warts | 0.013-Phi | 0.899 | 94 |
| Male HPV vaccination status and resolution of warts | -0.175- Phi | 0.196 | 56 |
| Males positive for HPV vaccination and Presence of Recalcitrant Warts | 0.198- Phi | 0.139 | 56 |
| Number of warts and number of treatments to resolution | 0.170- Pearson | 0.137 | 78 |
| Height and resolution of warts | 0.052- Pearson | 0.517 | 157 |
| Cryotherapy and resolution | -0.162- Phi | 0.042* | 158 |
Figure 1.

This figure shows the frequency of the amount of warts in our patient population. The majority of our patients had minimal warts (1–2 warts) before seeking treatment
Figure 2.
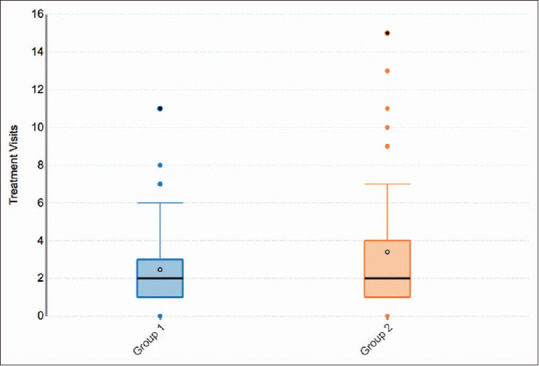
This figure illustrates the number of treatment visits stratified by HPV vaccination status. As seen in this chart, there is an association between those who received the HPV vaccine (Group 2) and increased treatment visits, which was statistically significant when compared to the group who did not receive the vaccine (Group 1) (r = 0.180, P = 0.024). The maximum treatment visits for those who received the HPV vaccine is 15 visits, whereas the maximum for those who did not receive the vaccine is 11 visits
Discussion
This study investigated the relationship between HPV vaccination status and the response to standard treatment of extragenital warts (recalcitrant and non-recalcitrant). Contrary to what was expected based on the role of HPV in the pathogenesis of verruca vulgaris, this study did not show a significant correlation between HPV vaccination status and wart resolution. Furthermore, there was a positive correlation between receiving the HPV vaccine and requiring more treatment visits to eliminate the warts. Cryotherapy was shown to be significantly associated with wart resolution, which is expected given that cryotherapy is one of the most widely used first-line treatments for verruca vulgaris.[13] Cryotherapy works by inducing tissue damage through cold temperature via disruption of the capillaries supplying the wart in addition to promoting osmotic cellular injury, resulting in ischemia and damages to the wart.[14] Given that this study focused on routine treatment and did not randomize therapeutic modalities, secondary treatment regimens were used infrequently and expectedly shown to not significantly improve wart resolution. We were unable to stratify by therapeutic modality as subjects were treated with a multitude of therapeutic strategies, which is often standard in verruca vulgaris treatment when the first-line treatment fails.
From a pathophysiologic view, it was hypothesized that the HPV vaccination series would create enough systemic immunogenicity to augment the verruca vulgaris treatment response measured by an increase in wart resolution. This study demonstrated the opposite of what was hypothesized, suggesting that the HPV vaccine may have no role in increasing responsiveness of routine wart treatment. The latency from receiving the HPV vaccine to wart appearance was not successfully investigated in this study given the challenges of manual data extraction, but could be an interesting, follow-up investigation in a prospective, observational study. Further research must be conducted to examine whether our study's conclusions are reproducible as the theoretical benefit of the HPV vaccine in the treatment of warts seems plausible given the well-understood pathophysiology of HPV's role in verruca vulgaris. However, there are various limitations that can explain our study's results and the discrepancy between the immunology and in vivo data.
Vaccination status and likelihood to attend office visits
Widespread recommendation for HPV vaccination is a fairly recent guideline, and the HPV vaccination series was not recommended for females until 2006 and recommended for males until 2011.[15] In a nationally representative sampling of 1,219 pediatricians and family medicine practitioners, it was found that <15% of physicians recommended the vaccines to eligible male patients at least 75% of the time.[16] A primary healthcare provider's influence on patients and their parents has a great impact on whether or not a patient chooses to receive the HPV vaccine. Patients who opt to receive vaccines often have a stronger tendency to follow-up with medical visits. This predisposition may act as a potential confounder, where those who received the HPV vaccine were more likely to receive in-office wart treatments due to their inherent propensity to visit the dermatologist rather than an effect of the HPV vaccine itself. This inherent bias between treatment groups may explain why patients with the HPV vaccine had an increased number of treatment visits without a positive association with wart resolution.
HPV vaccine formulations and varying immunization schedules
The novelty of the HPV vaccine has led to several formulation changes with the expansion of strain coverage and immunization scheduling alterations. The immunization schedule recommendation is based on age, where a two-dose series (0, 6–12 months) is recommended for children aged 9–15 years and a three-dose series (0, 1–2, 6 months) is recommended for children who are above the age of 15.[4] Iversen et al.[17] conducted a wide-scale open-trial RCT in 52 ambulatory sites across 12 countries to compare the immunogenic response of the two-dose vaccine in males and females aged 9–14 years compared to a three-dose series in females aged 16–26 years. Their results were variable, where immunogenicity—measured by antibody ratios—was found to be equivalent between the two series, but the quantitative antibody response appeared to be stronger in the two-dose series compared to the three-dose series. This study brings into question the varying efficacies of the different series, and how this may impact the overall systemic immunogenicity and effect on wart resolution. Our study did not stratify based on vaccination regimen and therefore could not assess this possible confounding effect.
Conclusion
While this study had the potential to elucidate predisposing factors that may impact the response of routine treatment for warts given our larger sample size compared to previous studies, no significant difference between HPV vaccine status and resolution of verruca vulgaris treated with routine therapies in either recalcitrant or non-recalcitrant groups was found. This was limited by numerous confounding factors such as inherent discordance to seek medical care between HPV vaccinated and non-vaccinated populations, and the difference in HPV vaccine regimens.
Even though warts are not life-threatening, the discomfort and morbidity, both physical and mental from self-esteem issues as a child, can be severe. Research into how HPV vaccination affects the treatment response in verruca vulgaris can potentially guide clinicians' decisions regarding additional indications for HPV vaccination along with immunization scheduling recommendations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bruggink SC, Eekhof JA, Egberts PF, van Blijswijk SC, Assendelft WJ, Gussekloo J. Natural course of cutaneous warts among primary schoolchildren: A prospective cohort study. Ann Fam Med. 2013;11:437–41. doi: 10.1370/afm.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipke MM. An armamentarium of wart treatments. Clin Med Res. 2006;4:273–93. doi: 10.3121/cmr.4.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuwabara AM, Rainer BM, Basdag H, Cohen BA. Children with warts: A retrospective study in an outpatient setting. Pediatr Dermatol. 2015;32:679–83. doi: 10.1111/pde.12584. [DOI] [PubMed] [Google Scholar]
- 4.“Human Papillomavirus (HPV) Infection - 2015 STD Treatment Guidelines.” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 4 June 2015. Available from: www.cdc.gov/std/tg2015/hpv.htm .
- 5.Abeck D, Fölster-Holst R. Quadrivalent human papillomavirus vaccination: A promising treatment for recalcitrant cutaneous warts in children. Acta Derm Venereol. 2015;95:1017–9. doi: 10.2340/00015555-2111. [DOI] [PubMed] [Google Scholar]
- 6.Boull C, Groth D. Update: Treatment of cutaneous viral warts in children. Pediatr Dermatol. 2011;28:217–29. doi: 10.1111/j.1525-1470.2010.01378.x. [DOI] [PubMed] [Google Scholar]
- 7.Fox PA, Tung MY. Human papillomavirus: Burden of illness and treatment cost considerations. Am J Clin Dermatol. 2005;6:365–81. doi: 10.2165/00128071-200506060-00004. [DOI] [PubMed] [Google Scholar]
- 8.Leung L. Recalcitrant nongenital warts. Aust Fam Physician. 2011;40:40–2. [PubMed] [Google Scholar]
- 9.Yanofsky VR, Patel RV, Goldenberg G. Genital warts: A comprehensive review. J Clin Aesthet Dermatol. 2012;5:25–36. [PMC free article] [PubMed] [Google Scholar]
- 10.Landis MN, Lookingbill DP, Sluzevich JC. Recalcitrant plantar warts treated with recombinant quadrivalent human papillomavirus vaccine. J Am Acad Dermatol. 2012;67:e73–4. doi: 10.1016/j.jaad.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (Types 6, 11, 16, and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475–7. doi: 10.1001/archdermatol.2010.71. [DOI] [PubMed] [Google Scholar]
- 12.Kost Y, Zhu TH, Blasiak RC. Clearance of recalcitrant warts in a pediatric patient following administration of the nine-valent human papillomavirus vaccine. Pediatr Dermatol. 2020;37:748–9. doi: 10.1111/pde.14150. [DOI] [PubMed] [Google Scholar]
- 13.SUMMARY OF EVIDENCE. Cryotherapy Systems for Wart Removal: A Review of the Clinical Effectiveness, Cost-Effectiveness, and Guidelines. U.S. National Library of Medicine, 12 June 2014. [PubMed] [Google Scholar]
- 14.Prohaska J, Jan AH. StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. Cryotherapy. [Updated 2020 Aug 23] Available from: https://www.ncbi.nlm.nih.gov/books/NBK482319/ [Google Scholar]
- 15.Meites E, Kempe A, Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination- Updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–8. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 16.Malo TL, Giuliano AR, Kahn JA, Zimet GD, Lee JH, Zhao X, et al. Physicians' human papillomavirus vaccine recommendations in the context of permissive guidelines for male patients: A national study. Cancer Epidemiol Biomarkers Prev. 2014;23:2126–35. doi: 10.1158/1055-9965.EPI-14-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–21. doi: 10.1001/jama.2016.17615. [DOI] [PubMed] [Google Scholar]


