Abstract
Extremity arterial injuries account for up to 50% of all arterial traumas. The speed, accuracy, reproducibility, and close proximity of modern CT scanners to the trauma bay have led to the liberal use of CT angiography (CTA) when a limb is in ischemic jeopardy or is a potential source of life-threatening hemorrhage. The radiologist plays a critical role in the rapid communication of findings related to vessel transection and occlusion. Another role of CT that is often overlooked involves adding value to surgical planning. The following are some of the key questions addressed in this review: How does CTA help determine whether a limb is salvageable? How do concurrent multisystem injuries affect decision making? Which arterial injuries can be safely managed with observation alone? What damage control techniques are used to address compartment syndrome and hemorrhage? What options are available for definitive revascularization? Ideally, the radiologist should be familiar with the widely used Gustilo-Anderson open-fracture classification system, which was developed to prognosticate the likelihood of a functional limb salvage on the basis of soft-tissue and bone loss. When functional salvage is feasible or urgent hemorrhage control is required, communication with trauma surgeon colleagues is augmented by an understanding of the unique surgical, endovascular, and hybrid approaches available for each anatomic region of the upper and lower extremities. The radiologist should also be familiar with the common postoperative appearances of staged vascular, orthopedic, and plastic reconstructions for efficient clinically relevant reporting of potential down-range complications.
Online supplemental material is available for this article.
©RSNA, 2022
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Explain how CTA findings direct management of extremity arterial injuries in different anatomic regions of the upper and lower extremities.
■ Describe the utility of the Gustilo-Anderson open fracture classification system in prognosis and decision making.
■ Discuss the utility of CT and CTA in posttreatment surveillance and during staged reconstruction.
Introduction
The decision of functional limb salvage versus primary amputation after traumatic extremity injury is binary, but the factors involved are complex. CT and CT angiography (CTA) findings of trauma in the threatened or mangled extremity have specific management implications that dovetail with considerations extending beyond imaging. The initial therapeutic approach is individualized on the basis of the anatomic region; vascular, soft-tissue, and bone injury severity; expectations regarding functional outcome; and concurrent life-threatening intracavitary injuries. Clinically relevant postoperative CT and CTA interpretations require a basic understanding of the natural history of severe extremity trauma and the first principles of damage control, definitive vascular intervention, and reconstructive surgery. CT and CTA are performed for surveillance after open primary repair, graft placement, and angiographic stent placement; for planning staged reconstruction with myocutaneous flaps and bone grafts; and to evaluate complications after limb salvage or amputation. The aim of this article is to provide a comprehensive, pragmatic, and clinically oriented review of ways to augment the diagnostic radiologist’s role as consultant in the setting of threatened or mangled extremities.
Background and Epidemiologic Features
Extremity arterial injuries account for approximately 50% of all arterial traumas (1). A threatened extremity is in ischemic jeopardy owing to surgically important arterial injury (2). Approximately two-thirds of extremity arterial injuries involve the superficial femoral artery or brachial artery (3). Lower extremity vascular injuries from motor vehicle, industrial, and farm accidents are usually associated with greater degrees of soft-tissue damage (4). Amputation is three times more common following blunt rather than penetrating mechanisms and is unavoidable in up to 18% of vascular extremity injuries despite lengthy periods of staged reconstruction (5). Limb-threatening ischemia is of greatest concern after distal extremity injuries, whereas life-threatening exsanguination is more likely to result from proximal transections involving the axillosubclavian or iliofemoral vessels. This is related to a combination of vessel caliber and difficult surgical access to the noncompressible torso for proximal arterial control (4,6).
Basics of Extremity CTA: Indications, Utilization, and Common Findings
CTA reduces delays to extremity reperfusion and hemorrhage control (2,7). Historically, CTA has been a screening tool for patients without hard signs of hemorrhage or ischemia. Hard signs that have traditionally warranted immediate surgery or conventional angiography include external hemorrhage, expanding or pulsatile hematoma, bruit or thrill, pallor or cool extremity, and absence of a distal pulse or neurologic deficit such as loss of plantar sensation (4,8).
CTA is often performed following arterial pressure index (API) determination by using blood pressure cuff measurements. API is defined as the ratio of systolic blood pressure in an abnormal extremity to that in a normal extremity. Measurements lower than 0.9 are consistent with flow-limiting extremity vascular injury and warrant triage to CTA (9). Realistically, extensive bone, nerve, and soft-tissue injuries; hypovolemic shock; obesity; and peripheral vascular disease frequently confound API measurement and clinical assessment (1). Approximately 15% of vascular lesions, including non–flow-limiting pseudoaneurysms of inline arteries, which contribute directly to perfusion of the hand and foot, and transections of noninline arteries such as the profunda femoris, are clinically occult at presentation (10) (Fig 1).
Figure 1:
Pseudoaneurysm in a 60-year-old woman with a left subtrochanteric fracture caused by a fall and treated with open reduction and internal fixation. On hospital day 17, the patient’s proximal left lower extremity became swollen and tense. Coronal CT image coned down to the left inguinal region from lower extremity CTA shows a small pseudoaneurysm (arrow), measuring 4 mm in greatest cross-sectional diameter, arising from the left common femoral artery. Following hematoma evacuation, the pseudoaneurysm was managed conservatively with no subsequent thromboembolic complication.
CTA is now used for surgical planning in patients with some degree of shock and evidence of hard signs (1,11) and is warranted for proximity injuries (penetrating wound paths in the vicinity of major extremity vessels) (2). Currently, 85% of patients with multisystem trauma and shock undergo admission whole-body trauma CT at major level I trauma centers in the United States (12). Competing multisystem injuries occur in 30% of open extremity fractures (13). When performed as part of a whole-body trauma study, extremity CTA enables triage based on simultaneous consideration of extremity and intracavitary injuries (4,11). The feasibility and likelihood of successful limb salvage are weighed against the relative urgency of injuries in other body regions and the patient’s hemodynamic state.
Extremity CTA facilitates accurate and reproducible detection and characterization of arterial injuries (14). In a meta-analysis of 11 studies (15) examining the diagnostic performance of CTA for extremity arterial injury, pooled sensitivity and specificity values of 96.2% and 99.2%, respectively, were reported when non–diagnostic-quality examinations were excluded. Clinically significant injury can be confidently excluded when an artery is well enhanced, with no mural or perivascular abnormalities (16).
Routine evaluation of thin-section axial CT images is essential. Multiplanar reconstruction, maximum intensity projection (MIP) reconstruction, and volume-rendered (VR) images help illustrate the craniocaudal span of dissections and segmental occlusions with respect to osseous landmarks, and the level of reconstitution. Use of CTA road maps can potentially decrease image interpretation time and provide subjective benefits to surgeons and interventionalists who are planning surgical or angiographic interventions (7,14,15).
Important descriptors of injury include location and length, degree of stenosis (>50% of luminal caliber), and level of reconstitution (7). Following penetrating trauma, wound tracks or ballistic fragments within 5 mm of a neurovascular bundle raise suspicion for vascular injury (17).
The severity of arterial injury depends on the depth of mural involvement (Fig E1). CTA findings include active extravasation of contrast material (ie, active hemorrhage), pseudoaneurysm, abrupt luminal narrowing, intimal irregularity or flaps, nonopacification, and arteriovenous fistulas (7,16,18) (Fig E2). Active hemorrhage with lack of distal opacification is most consistent with complete transection, while continuous distal opacification signifies partial transection. Arterial occlusion, followed by pseudoaneurysm, is the most commonly reported injury (15). Dissections more often appear as semilunar luminal deformations and either eccentric stenosis or complete occlusions (16). Discrete nonocclusive dissection flaps and intimal injuries are uncommon (15).
Pseudoaneurysms are contained by adjacent soft tissue or adventitia and manifest as enhanced outpouchings from an adjacent artery with rounded margins, whereas active hemorrhage appears as an irregular contrast blush surrounded by hematoma or insinuating along disrupted fascia (7,16). Arteriovenous fistulas appear as early venous enhancement. Veins may be engorged, and a communicating channel is occasionally appreciated (7). Luminal narrowing is usually eccentric if it results from extrinsic compression by hematoma or bone fragments, nonocclusive thrombus, or dissection. Reactive vasospasm is typically smooth and concentric (14). Distinguishing between occlusion and severe spasm can be difficult in distal small arteries (14,19).
The extremity is not considered to be threatened and can be safely observed and managed nonsurgically if the arterial injuries are characterized by (a) small downstream-protruding intimal flaps, (b) pseudoaneurysms with minimal (less than approximately 5 mm in length or diameter) wall disruption (Fig 1) (6), (c) lack of transection (ie, no active hemorrhage or arteriovenous fistula), and (d) intact inline distal flow with a continuous contrast column through the distal runoff (1,10,20). Progression of approximately 10% of initially minor proximal traumatic arterial lesions to sizes larger than 5 mm in length or diameter at follow-up CTA may result in thromboembolization, requiring distal cutdown and thrombectomy in addition to primary lesion repair (21,22).
The injury mechanism also plays a role in determining whether an extremity is threatened. Closed diaphyseal long-bone fractures are associated with a 0.1% risk of arterial injury (10). Injuries requiring greater scrutiny include compound (ie, open) fractures, especially those involving severe fracture displacement, segmentation, comminution, or a “floating joint” (Fig 2), with which displaced diaphysis fractures proximal and distal to a joint render it highly unstable. Crush injury, shotgun blasts, and high-velocity or close-range gunshot wounds pose the greatest risk of vascular injury and amputation (23).
Figure 2:
Floating joint in two patients. (A) Three-dimensional (3D) VR image of the left lower extremity in a 39-year-old woman with left lower extremity deformities after a motor vehicle collision shows a floating joint flanked by a comminuted distal femur fracture (black arrow) and segmental fractures of the proximal tibia (white arrows) and fibula. (B) 3D VR image in a 21-year-old man after a motor vehicle collision with ejection shows a floating elbow flanked by a proximal humeral fracture (black arrow) and ulnar fracture (white arrow).
Amputation may be unavoidable for life-saving hemorrhage control if a tourniquet is ineffective at sites with a large soft-tissue envelope such as the thigh, for arterial injuries at the junctional zone with the noncompressible torso, if a hybrid suite with angiographic capabilities is not accessible for temporary balloon occlusion, or if two surgical teams are not available to address intracavitary and extremity hemorrhage simultaneously. An ischemic extremity is not an immediate threat to life, and intracavitary injuries take priority (4).
Basics of Extremity CTA: Protocol Considerations
Timing a study to achieve adequate distal arterial opacification is challenging, as modern CT gantries and tables can easily outpace the contrast material bolus (16,18,24). Specific protocols and acquisition parameters should be tailored to the specific scanner and according to whether CTA is performed alone or as part of a whole-body examination.
Fixed delays, bolus tracking, and test injections are used variably (18). Bolus tracking and test injections are considered to be more precise. However, Soto et al (25) describe relatively little (<10 second) variation in the scan timing between arteries at different levels of the upper and lower extremities and advocate using a fixed delay of 20–30 seconds in young healthy patients with normal cardiac output. Since patients may have decreased cardiac output owing to hemodynamic instability, we use bolus tracking with a region of interest in the descending thoracic aorta, particularly when CTA is performed as an extension of a whole-body CTA examination. The optimal trigger threshold is dependent on the manufacturer and scanning parameters.
Up to 8% of extremity trauma CTA examinations are nondiagnostic with use of contemporary scanners (15); this is frequently because of poor arterial opacification that results from early scan timing (18). Less commonly, venous opacification may interfere with interpretation if scanning is excessively delayed. Bolus timing and fixed delays can be adjusted ad hoc and the examination repeated by using a second contrast material bolus (26). In our experience, with use of a dual-source 128-section scanner (Somatom Force; Siemens Healthineers), the midcalf and forearm vessels can be rapidly re-imaged during the same bolus with minimal venous contamination when outpacing occurs (Fig 3) (16).
Figure 3:
Femur fracture in a pedestrian struck by a vehicle. (A) Sagittal oblique MIP CTA image obtained during the initial arterial phase shows nonopacification of the below-the-knee runoff (arrow). (B) Repeat sagittal MIP CTA image obtained at the technologist’s discretion during the same contrast material bolus administration after an additional 20 seconds shows continuous contrast columns in the trifurcation vessels (arrow) without venous contamination.
Although extremity CTA is typically performed in two phases for peripheral vascular disease, single arterial phase CTA is commonly acceptable for extremity trauma (15). Venous injuries occur with 20% of arterial injuries and are a modifier of amputation risk and need for fasciotomy (1). However, because conservative management is frequently feasible, additional phases rarely result in a change in management (27) and are not widely advocated.
Unlike conventional angiography, CTA offers the advantage of imaging both extremities simultaneously in a single, albeit static, acquisition. If only one leg is of interest, it still may be helpful to incorporate a wide–field-of-view axial series of both lower extremities in addition to coned-down and multiplanar reconstructions of the injured extremity. Comparison with an uninjured lower extremity may reveal delayed timing in potentially flow-limiting arterial injuries and lets the radiologist know whether distal nonenhancement is a function of injury or early scan timing (19). For the upper extremities, this may not be possible if the patient is unable to raise both arms in the “Superman” position owing to injury (7).
Loss of distal arterial enhancement can also result from advanced compartment syndrome or spasm (2) (Fig 4). Digital subtraction angiography performed with an intra-arterial vasodilator such as papaverine may be necessary to distinguish spasm from injury. Other pitfalls that limit diagnostic accuracy include beam hardening from hardware, debris, or ballistic fragments (26). Narrow collimation, smooth filters, iterative reconstruction, higher tube current, and higher peak voltage can reduce artifacts.
Figure 4:
Open right lower extremity fracture in a 53-year-old man involved in a motorcycle collision. (A) 3D VR CT image shows comminuted segmented tibial and fibular diaphysis fractures (arrows). (B) Corresponding 3D MIP CTA image of the right lower extremity shows narrowing of the posterior tibial (white arrows) and peroneal (black arrow) arteries and poor distal opacification. No vascular injury was seen at subsequently performed digital subtraction angiography. CTA findings are consistent with vasospasm, and the injury is classified as a Gustilo type IIIB open fracture.
Management and Complications
General Principles
Even in the absence of a surgically important vascular lesion, bone and soft-tissue injuries pose the risk for limb-threatening compartment syndrome. Surgical or endovascular management of arterial injuries combined with damage control techniques such as fasciotomy and rapid mechanical stabilization is the standard of care when limb salvage is favored (28) (Figs 5, 6). The principles for managing extremity injuries are summarized in Appendixes E1–E6.
Figure 5:
Crush injury to the distal left leg and clinical signs of compartment syndrome in a 39-year-old man. (A) 3D VR image shows a comminuted proximal tibial fracture (arrows). (B) Coned-down coronal MIP CTA image of the below-the-knee arterial runoff shows occlusion of the left anterior tibial artery (arrow) shortly beyond its origin. (C) Axial MIP CTA image of the lower legs shows enlarged edematous calf musculature (arrow) in the left lower extremity, compatible with clinically suspected compartment syndrome. Compartment pressures measured approximately 80 mm Hg. Four-compartment fasciotomy was performed.
Figure 6:
Damage control orthopedic interventions in a 27-year-old man with an open femur fracture after a motor vehicle collision. Sagittal CT image with wide window settings (A) and 3D surface-rendered image (B) of the left femur show defects and packing material from a prophylactic right-thigh fasciotomy (thick black arrows), and an external fixator (white arrows in A, thin black arrows in B) placed shortly after admission.
Definitive surgical techniques for restoring perfusion include (a) direct vascular repair (lateral suturing, vein patch graft placement for partial transections, or end-to-end anastomosis) (Fig 7) and (b) venous interposition or bypass with reverse autogenous vein grafts, typically with use of a valvulotomized contralateral saphenous vein (Fig 8) and less commonly with use of the cephalic vein for smaller-caliber brachial artery injuries (3,29).
Figure 7:
Stab wound to the right arm and displaced bicondylar intra-articular humeral fracture in a 38-year-old man. (A) 3D VR image of bicondylar humeral fracture. (B) Corresponding coronal oblique MIP CTA image shows segmental occlusion of the distal right brachial artery at the level of the bicipital aponeurosis due to thrombus (white arrow) below the takeoff of the deep brachial artery (black arrow). The length of injury was overestimated at CTA. A 1-cm segment of the brachial artery was débrided, and repair was performed with tension-free end-to-end anastomosis.
Figure 8:
Gunshot wound to the left chest with equal but diminished bilateral upper extremity pulses described at clinical examination in a 24-year-old man. (A) Coronal oblique MIP CTA image of the left upper extremity shows traumatic occlusion (arrows) of the left axillary and brachial arteries, flanking a short segment of reconstitution. (B) Postoperative CTA image of the left upper extremity shows patency of an end-to-end reverse saphenous vein interposition graft (arrows) used to address an avulsed axillary artery segment.
The length between the transition points of a normal artery and an abnormal artery can be difficult to determine for long nonopacified segments distal to a transection. However, this length, along with the zone of “real soft-tissue injury” (a penumbra of tissue at risk for necrosis that is substantially farther than the apparent injury on admission, according to the craniocaudal extent of the comminuted or segmented fracture and the injury mechanism), can be roughly estimated (30). Débridement of a devitalized vessel back to a normal artery is required for successful proximal and distal control with clamping, Fogarty balloon occlusion, and interposition graft placement (10). Proximal upper extremity and lower extremity arteries can be mobilized up to 2 cm for resection and primary anastomosis without sacrificing collateral vessels or causing excessive suture line tension. For defects longer than 2 cm, tension-free end-to-end anastomosis usually is not possible, and interposition grafts are necessary (Fig 8) (29,31).
Endovascular stents are increasingly being used for junctional zone injuries when surgical access is complex and time consuming (Fig 9). Nonrigid self-expanding stents mitigate further arterial injury. Covered stents are preferred for bleeding, and uncovered stents that preserve collateral perfusion are favored for dissections. Trans-stent coil placement may be warranted for large excluded pseudoaneurysms (6).
Figure 9:
Absent left upper extremity pulses in a 21-year-old man (same patient as in Fig 2B) at hospital admission after a motor vehicle collision with ejection. (A) Coronal oblique MIP CTA image of the left upper extremity shows long-segment occlusion of the left axillary artery (arrows). (B) Repeat CTA, performed after self-expanding covered stent placement, confirmed artery patency (arrow). On postoperative day 2, the patient developed decreased distal left upper extremity pulses. (C) Subsequently obtained CTA image shows a new segmental embolic occlusion of the brachial artery (arrow), which was treated with surgical cutdown and thrombectomy. The streak artifact is secondary to open reduction and internal fixation of the humerus and ulna.
Symptomatic complications warrant repeat CTA (22). Stent thromboses can be recanalized with catheter-directed thrombolysis or suction. Delayed open bypass is used when stent patency cannot be restored endovascularly (32). Additional uncommon endovascular complications include distal embolism (Fig 9C), endoleaks, incomplete lesion exclusion, migration, stent fracture, and stenosis from intimal hyperplasia (22). Transcatheter coil embolization or absorbable gelatin powder (Gelfoam; Pharmacia and Upjohn) can be used for active bleeding or pseudoaneurysms involving non-inline arteries in hemodynamically unstable patients (6,10,33).
Following stent placement, patients are treated with antiplatelet agents for a period ranging from 3 months to the entirety of life (22,32). The frequency or timing of surveillance CTA examinations is not well established (22). Some authors suggest two follow-up CTA examinations in the 1st year and yearly examinations thereafter (32).
Warm Ischemia, Compartment Syndrome, and Ischemia-Reperfusion Injury
Early reperfusion within 3–6 hours is critical for limb salvage (34). For dysvascular knee dislocations with occlusive popliteal artery injury, the amputation rates are 86% with delayed repair and 14% with early repair (35).
Compartment Syndrome.—Compartment syndrome is more common in young males who have more muscle mass and tighter compartments at baseline. Approximately half of acute extremity compartment syndromes result from tibial shaft fractures (see Fig 5C). Distal radius fractures are the second most common cause (36).
Compartment syndrome occurs in 59% of patients with the most severe class of compound fractures (grade IIIC) under the Gustilo classification system (described in “Gustilo-Anderson Classification and the Mangled Extremity” section) (5). CT is not formally used to determine the need for fasciotomy; however, CT findings of rhabdomyolysis, including focal or geographic areas of muscle hypoattenuation and enlarged edematous musculature, are suggestive of compartment syndrome (Fig 5C) (37). Peripherally enhancing intramuscular collections indicate progression to myonecrosis (37).
As time progresses, warm ischemia—that is, ischemic insult to tissues under normothermic conditions—initiates a cascade of microvascular changes that proceed from endothelial cell swelling to hemoconcentration, microcirculatory thrombosis, and cessation of blood flow in congested or occluded capillaries. Buildup of edema caused by the release of inflammatory mediators and compromised venous outflow worsens the compartment pressures. Elevated absolute pressure greater than 35 mm Hg compresses the microvasculature, further compromising perfusion in a self-propagating cycle of nerve, vascular, and muscle injury (34,38,39). The “no-reflow” phenomenon at the microvascular level can no longer be addressed with late repair of major axial vessels. Paradoxical ischemia-reperfusion injury ensues, as the no-longer-salvageable extremity releases lactic acid, potassium, inflammatory cytokines, reactive oxygen species, and myoglobin into the systemic circulation, potentially leading to multiorgan dysfunction syndrome (40).
Gustilo-Anderson Classification and the Mangled Extremity
The Gustilo-Anderson, or Gustilo, classification is the most widely adopted system for grading compound fractures because of its simplicity and clinical relevance. It is used to predict limb-threatening septic complications that require secondary amputation on the basis of the degree of soft-tissue injury. These complications include osteomyelitis and related deep infections, defined as chronic suppuration and abscess with a confirmed microbial agent (41–43). Type I, type II, and type IIIA injuries (Table) are compound segmental fractures that routinely heal without complication. Type IIIA injuries involve extensive lacerations greater than 10 cm in length with soft-tissue flaps but have an excellent prognosis without flap reconstruction since bone coverage is not compromised. Type IIIB and IIIC injuries are severe injuries with inadequate bone coverage and require flap reconstruction for limb salvage (41,44). Gunshot or farm injury–related open fractures are considered to be grossly contaminated and are included in the IIIA category, even if the soft-tissue defect is small.
Gustilo-Anderson Classification of Open Fractures
Gustilo type IIIB fractures have extensive bone loss and periosteal stripping with devitalized fragments, massive contamination, and poor soft-tissue coverage that requires free tissue transfer or rotational groin flaps. Type IIIC fractures have, in addition to the aforementioned features, arterial injuries that require reperfusion (41,43). Approximately 40% of severe Gustilo type III fractures have surgically important arterial injuries (45). Even though fracture of the femoral shaft is the most common long-bone fracture in patients with polytrauma, the femur is surrounded by the largest soft-tissue envelope of any long bone, affording considerable protection to soft tissue and vascular structures (40). The tibia is the most common anatomic region in patients with Gustilo type IIIB and IIIC fractures (41).
The term mangled extremity predates the Gustilo-Anderson classification and is defined as a severe open fracture with injury to at least three of the four major systems: integument and soft tissue, bone, nerves, and vessels (46). These injuries coincide with Gustilo type IIIB and IIIC categories, which are associated with high rates of nonunion and osteomyelitis, and represent 60% of all Gustilo fractures (42). Mangled extremity represents a wide spectrum of injuries, including subtotal traumatic amputation, defined as the separation of all critical neurovascular, musculotendinous, and bone structures and less than a one-quarter circumference soft-tissue bridge remaining, and traumatic amputation, defined as bridging by skin and subcutaneous tissue only (Fig 10) (4).
Figure 10:
Traumatic amputation in a 48-year-old male pedestrian who was struck by a vehicle. (A) MIP CTA image of the left lower extremity shows compound severely disorganized fractures of the femur and tibia, with bone protruding externally. There is no arterial enhancement beyond the proximal thigh (arrow). (B) Photograph of the left lower extremity shows extensive soft-tissue injury and traumatic amputation. The patient required formal primary above-the-knee amputation. Primary amputations are often performed one level (ie, one-third the length of a long bone) above the initially apparent level of injury owing to the expected penumbra of progressive soft-tissue necrosis.
Determining whether limb salvage will ultimately be unsuccessful or have a poor long-term result is challenging following hospital admission. The Lower Extremity Assessment Project study group showed that scoring systems based on clinical parameters such as the Mangled Extremity Severity Score have limited accuracy in predicting long-term functional outcomes after successful limb reconstruction (47). Risk modifiers favoring amputation include multisystem trauma, severe bacterial contamination, diabetes, obesity, and peripheral vascular disease. The level of injury also is important; below-the-knee amputations have considerably better functional outcomes than do above-the-knee amputations (4).
Mangled Gustilo type IIIB and IIIC extremities seen at CT and CTA with large circumferential soft-tissue defects, muscle loss involving two or more compartments, multilevel closed crush injury, segmental bone loss of more than 5 cm or one-third the length of a long bone, floating joint, and complete arterial occlusion with no runoff are indicative of poor outcome and favor primary amputation (Fig 10) (48,49).
Arterial injury provides immediate actionable information for general and vascular trauma surgeons who coordinate the overall care, communicate with the patient and family, and perform early vascular repairs and fasciotomies (4). However, early vascular repair does not guarantee a meaningful neuromuscular recovery. Patients and families may strongly favor an attempted salvage, even when the functional outcome and rehabilitation time would be better with an amputation and well-fitted prosthesis (4). Although major nerve injury resulting in a plantar-insensate non–weight-bearing foot is an indication for amputation, intractable hypovolemic shock is often considered the only absolute indication (4).
Severely mangled extremities can be salvaged by using contemporary microvascular tissue transfer techniques, even if functional salvage is not possible (48). In patients in whom neuromuscular recovery is likely, the rehabilitation time to full weight bearing can exceed 1–2 years (30,45). Other patients may require secondary amputation after repeated infections and graft failures exhaust the options for flap and bone revision (Fig 11). Emphasis on reconstruction and nerve graft placement is greater for the upper extremities since the psychosocial effect of losing a dominant extremity is devastating (2,46,49).
Figure 11:
Open left tibia fracture in a 56-year-old man with a lower extremity crush injury. The fracture was treated with an intramedullary nail and antibiotic spacer, and soft-tissue coverage was achieved with a latissimus dorsi free flap. Three months after presentation, the patient developed a fever and surgical site infection. Axial CT images show an enlarged edematous muscle flap (* in A), with a more caudal image (B) showing an intramuscular abscess (arrow) related to osteomyelitis with deep infection. Secondary below-the-knee amputation was performed.
Amputation
Amputation is typically performed at the lowest possible level that allows flap development for subsequent tension-free closure following débridement of nonviable tissue (50). Typically, tissue necrosis extends for up to one-third the length of a long bone above the initially apparent level of injury (30).
To promote soft-tissue envelope integrity for long-term orthotic use, the stump must have a pliable scar and should separate bone prominences from areas of maximal orthotic pressure while remaining sufficiently stable to support a weight-bearing prosthesis. Bone prominences are rounded, and myoplasty and myodesis are performed to attach antagonistic muscle groups to each other and to the periosteum, respectively. The resultant construction resists atrophy and loss of tissue bulk. Major arteries are ligated as distally as possible to prevent postoperative hematoma while preserving cutaneous circulation (50).
CTA Findings and Management Implications by Region
Following traumatic vascular occlusion of inline vessels, collateral circulation becomes responsible for distal extremity perfusion. The abundance of collateral flow is a major determinant of management and is highly dependent on the location of arterial injury (6).
Lower Extremity
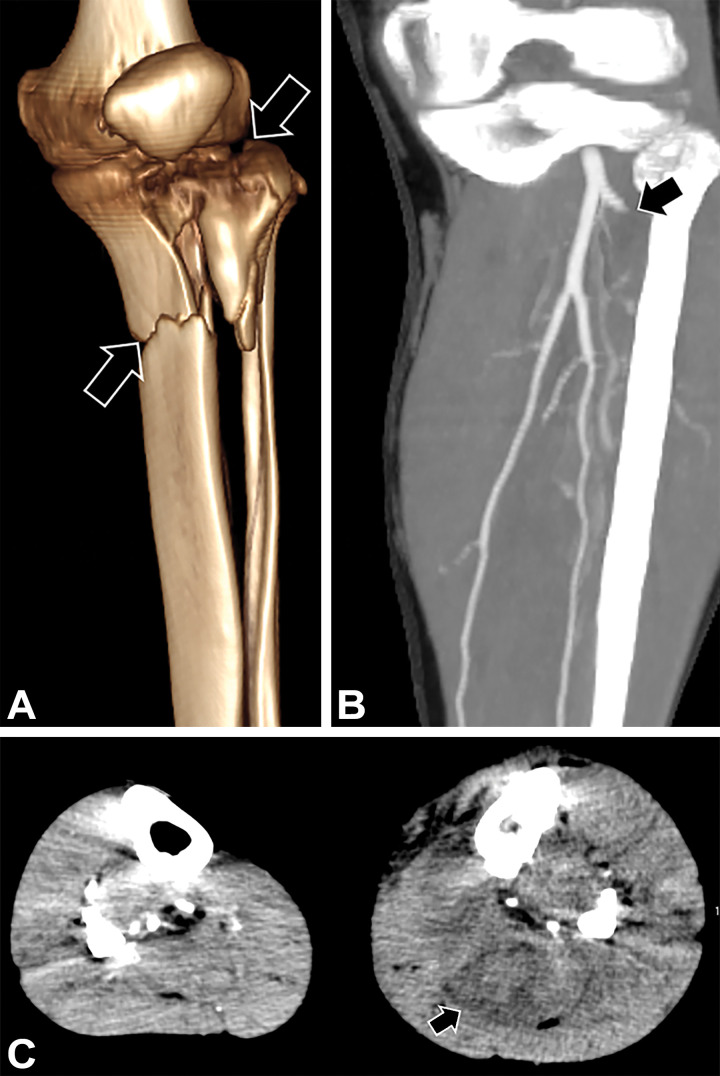
Iliac and femoral arterial injuries are associated with mortality from exsanguination, usually resulting from gunshot wounds (1,3,31). Knee dislocations and open comminuted tibial plateau fractures are prone to ischemia because of poor collateral circulation supporting the distal limb, with associated flow-limiting arterial injury in 16% and 9% of patients, respectively (10,51,52). Popliteal artery injuries account for one-fifth of lower extremity arterial injuries and pose the greatest threat to the limb since the popliteal artery is a true end artery with poor collateral supply (Fig 12) (1).
Figure 12:
Left knee dislocation, with no palpable distal left lower extremity pulses, in a 56-year-old woman after a fall. Sagittal left-knee CTA image shows long-segment occlusion of the popliteal artery (arrow). The limb was revascularized with saphenous vein interposition graft placement.
Widely displaced distal femoral metadiaphyseal or bicondylar fractures can injure the femoral-popliteal artery transition, which is tethered by the Hunter canal (53). Vascular compromise from complex, open pilon, ankle, hindfoot, and midfoot fractures is relatively uncommon (54).
Proximal Injuries
Proximal surgical control of iliac artery injuries is achieved with laparotomy, bowel reflection, and posterior peritoneal incision to expose the aortic bifurcation (55). Distal control involves dissecting the suprainguinal external iliac artery. Back bleeding from the internal inguinal artery can be prevented with ligation (31). Gross contamination, such as that from adjacent penetrating bowel injury, may necessitate extra-anatomic bypass (Fig 13).
Figure 13:
Remote history of gunshot wound to the pelvis, complicated by perigraft abscess from concurrent bowel injury, in a 36-year-old man. (A, B) MIP CTA images of the pelvis show a patent right aortofemoral bypass graft (arrow in A), which became occluded (arrow in B) 2 years later. (C, D) VR CTA image (C) and coronal oblique MIP CTA image (D) show multiple revisions, including polytetrafluoroethylene axillofemoral (black arrow in C) and then femorofemoral (arrow in D) bypass grafts, which were required owing to repeated occlusion over time. Popliteal artery occlusion (white arrow in C) after axillofemoral bypass required cutdown and thrombectomy. Although synthetic graft placement may be unavoidable in some patients, long-term patency rates are poor.
Endovascular stents allow earlier hemodynamic stabilization and revascularization of the iliofemoral vessels and are able to withstand orthopedic manipulation in these regions (56). Primary patency rates are high, with fewer procedural and postprocedural complications (eg, graft occlusions, pseudoaneurysms, and infection) compared with those from open surgical approaches (57).
Adjunctive temporary balloon occlusion is used to induce rapid hemostasis, particularly with mid–superficial femoral artery injuries, since external compression may be ineffective (6). Resuscitative endovascular balloon occlusion of the aorta (REBOA) increases central pressure and provides inflow control for suprainguinal arterial injuries in the noncompressible torso (Fig 14) (55). Greater than 90% of popliteal artery injuries are treated with tension-free interposition graft placement because division of geniculate arteries for popliteal artery mobilization compromises the limited collateral supply (58).
Figure 14:
Pelvic fractures and suspected arterial hemorrhage in a 24-year-old male cyclist in hemorrhagic shock after being struck by a car. (A) Sagittal MIP CTA image shows an inflated zone III resuscitative endovascular balloon occlusion of the aorta (arrow) between the level of the aortic bifurcation and the renal arteries. Arterial hemorrhage from the distal internal iliac artery branches was treated with coil embolization. (B) Supine abdominal radiograph shows the resuscitative endovascular balloon occlusion of the aorta in place. Radiopaque markers (arrows) denote the proximal and distal extents of the balloon.
Associated venous injuries are common and contribute to thrombophlebitis, chronic edema, and compartment syndrome. Iliac vein injuries are treated with lateral venorrhaphy when possible; however, prolonged shock, multisystem trauma, or gross contamination may necessitate ligation (31). The popliteal vein, which is largely responsible for lower-leg and foot drainage, is also repaired (1).
Distal Injuries
Below-the-knee arterial injury (BKAI), as compared with proximal arterial injuries, is associated with threefold lower mortality rates (59). However, BKAIs have worse functional outcomes and are associated with substantially higher amputation rates, especially when popliteal and tibial artery injuries co-occur (3,23,49).
Owing to small vessel caliber, endovascular repair does not have a defined role in the treatment of popliteal artery injury or BKAI. Surgical repair of BKAIs is technically challenging and time intensive and is performed as a last resort in fewer than one-quarter of patients with tibial arterial trauma (1,3). When a single midcalf artery is injured, it is often observed or ligated. This protocol is not risk free, because the posterior tibial artery provides perfusion to the tibial periosteum and traumatic occlusion increases the risk of nonunion and osteomyelitis in Gustilo type IIIC injuries (30,49). Anterior tibial artery injury and ligation also have the potential to increase the risk of limb loss due to reduced collateralization in the anterior compartment, with an increased likelihood of compartment syndrome and myonecrosis (3). Rarely, ligation of two below-the-knee vessels may be needed for hemorrhage control in hemodynamically unstable patients. Single-vessel BKAI is associated with an amputation rate as high as 18%. Amputations after two-vessel BKAI are needed in as many as 29% of patients when inline flow to the foot is maintained by one tibial vessel and in up to 40% of patients when perfusion is maintained by the peroneal artery alone (3,60). Three-vessel injuries require amputation in 50%–60% of patients (3,23).
If neither tibial artery is patent in an otherwise salvageable extremity, such as with three-vessel occlusion or inline flow through the peroneal artery only, bypass is usually necessary for limb salvage (Fig 15) (60). Early fasciotomies improve limb salvage and are used for 30%–40% of tibial arterial injuries (61).
Figure 15:
Multiple orthopedic injuries, including open fractures of the left lower extremity and absent distal pulses, in a 27-year-old man involved in a motorcycle collision. (A) 3D VR image of the left lower extremity shows Gustilo type IIIC distal tibia fractures (bracket) with an external fixator in place. There is severe comminution and segmental bone loss of the distal tibia, and antibiotic beads (circle) are placed in areas of extensive soft-tissue loss. The patient had a two-vessel BKAI, with perfusion from the peroneal artery only (not shown). (B) Sagittal MIP CTA image shows occlusion of the anterior (white arrow) and posterior (black arrow) tibial arteries. A reverse saphenous vein graft used for posterior tibial artery bypass was thrombosed on postoperative day 2. Below-the-knee amputation was performed secondary to progressive limb ischemia.
Upper Extremities
Proximal Injuries.— Upper extremity arterial injuries are usually caused by penetrating trauma and most frequently involve the brachial artery (29,62). Brachial artery injuries also result from blunt humeral shaft and supracondylar fractures (6,31). Axillosubclavian artery injuries are less common because the sternum can shield these vessels from projectiles (Fig 16) (29); however, they are associated with high mortality from exsanguination (22,29,31). Morbidity after timely repair of an axillosubclavian artery injury is typically related to profound permanent neurologic deficits from primary brachial plexus injury rather than ischemia (29,63).
Figure 16:
Severe right upper extremity deformity in a 39-year-old man after a motorcycle collision. (A) MIP CTA image through the right axilla shows transection of the right subclavian artery with active contrast material extravasation (arrow), no distal opacification, and a large right axillary and chest wall hematoma. Owing to the extent of vascular and soft-tissue injury, severe hemodynamic instability, and complete avulsion of the brachial plexus found at exploration, right transhumeral amputation was performed. Trapdoor thoracotomy facilitated arterial and venous ligation, performed as a lifesaving procedure to prevent exsanguination. The patient’s postoperative course was complicated by a widening area of muscle necrosis and repeated soft-tissue infection, ultimately necessitating amputation at the level of the shoulder girdle. (B) VR chest CT image 3 months later shows a left pectoralis major and rectus abdominis rotational flap (arrows) placed for chest wall soft-tissue coverage.
In terms of surgical techniques, open approaches to subclavian arterial injuries are technically challenging (Fig 16). The subclavian artery is divided anatomically into thirds, with the management of arterial injury based on these subdivisions. In hemodynamically stable patients, proximal control of the innominate and first (prescalene) portion of the right subclavian artery is achieved with upper median sternotomy. The proximal left subclavian artery is approached with left posterolateral thoracotomy (31). Distal control is achieved with access to the second (scalene) and third (postscalene) subclavian segments through a supraclavicular incision (31).
Medial clavicle resection and upper median sternotomy can be combined to perform a trapdoor thoracotomy for improved proximal and distal surgical access and control (29). In hemodynamically unstable patients, bilateral anterolateral “clamshell” thoracotomy provides wide access to the great vessels and other vital thoracic organs (31).
The axillary arteries extend from the lateral first rib to the lower teres major margin and are approached via an infraclavicular incision that can be extended distally into the deltopectoral groove (29). The presence of a large hematoma or pseudoaneurysm in the axillary region is an indirect marker of brachial plexus injury (Fig 16). Management is controversial, as early evacuation of the hematoma compressing the brachial plexus can improve functional outcome and decrease chronic pain, but exploration can cause or worsen brachial plexus palsy (29).
Associated axillosubclavian venous injuries are common (Fig 17). Axillary and subclavian vein ligation results in high rates of thrombophlebitis and chronic edema. Axillary vein injuries are often managed with close observation because of the aforementioned risks associated with exploration. Subclavian vein repair may be attempted in hemodynamically stable patients. The brachial and more peripheral forearm veins can be safely ligated, as edema is rare (29).
Figure 17:
Large expanding hematoma in a 29-year-old man with multiple left upper extremity gunshot wounds. (A) Axial MIP CTA image of the chest shows a left axillosubclavian pseudoaneurysm (black arrow) with arteriovenous fistula, as evidenced by early venous filling (white arrow). Multiple adjacent ballistic fragments are present. (B) Subsequent digital subtraction angiogram shows successful endovascular stent graft repair (arrow).
Subclavian artery ligation may be well tolerated, if necessary, as a lifesaving hemostatic intervention owing to the excellent collateral circulation about the shoulder girdle (29). Temporary angiographic balloon occlusion of large proximal vessels is preferred if a hybrid surgical-angiographic suite is available (Fig 18) (22).
Figure 18:
Compound severely comminuted left humerus fracture and absent ipsilateral radial pulse in a 35-year-old man after a gunshot wound to the left chest. (A) Coronal MIP CTA image shows a long-segment occlusion extending from the axillary artery to the brachial artery (arrows), with faint distal reconstitution. (B) Digital subtraction angiogram of the left axilla shows the use of angiographic balloon occlusion (arrow) for proximal control, which facilitated reverse saphenous vein interposition graft placement.
For brachial artery injuries with hemodynamic instability, brachial artery ligation may be unavoidable (64). The degree of ischemia after brachial artery injuries or ligation depends on whether the injury occurred above or below the deep brachial artery, which is a critical source of collateral blood supply arising from the brachial artery just distal to the teres major. Ligation proximal to the deep brachial artery origin results in a twofold increase in major amputation, defined as amputation proximal to the wrist joint (29,65). Extra-anatomic bypass grafts are often used in this region if there is extensive soft-tissue loss (1). Brachial artery injuries at the bicipital aponeurosis caused by supracondylar humeral fracture should be promptly repaired in hemodynamically stable patients (Fig 7) to prevent Volkmann ischemic contracture, which is characterized by forearm flexor necrosis and often necessitates major amputation (2,29).
With regard to endovascular approaches, endovascular axillosubclavian artery stent placement is increasingly being used to reduce blood loss and ischemia time (Fig 9) (22,66,67). Combined femoral and brachial catheter-directed approaches facilitate rendezvous access for difficult-to-cross transections (22,68). Primary and assisted long-term patency rates are high (68). Cerebral infarction from vertebral artery occlusion after stent deployment is a rare complication (22), but it is important to note whether the ipsilateral vertebral artery is dominant or nondominant. To minimize the risk of this complication, a contralateral nondominant vertebral artery should exhibit obvious continuity with the basilar artery.
Distal Injuries.— The brachial artery divides into the radial artery and larger ulnar artery several centimeters below the antecubital fossa. Radial and ulnar fractures have a strong association with arterial injury (Fig 19) (45). The ulnar artery terminates in the superficial palmar arch to provide most of the blood supply to the fingers, and the radial artery continues as the deep palmar arch, supplying the thumb and the thenar side of the index finger (29). CTA acts as an imaging analog of the Allen test; an isolated injury to the radial or ulnar artery may be tolerated if the uninjured artery provides adequate medial and lateral hand perfusion. With an isolated radial or ulnar artery injury, the injured artery can be quickly ligated in unstable patients (69).
Figure 19:
Open right forearm injury in a 32-year-old man who was dragged behind a tractor trailer. (A) 3D surface-rendered CT image of the left elbow shows large soft-tissue defects (arrow) involving the elbow and forearm. (B, C) CTA image (B), as well as follow-up diagnostic angiogram (C), of the forearm and hand after external fixator placement show segmental occlusion of the ulnar artery (black arrow) with a patent radial artery (white arrow). Vascular repair was not required since the superficial and deep palmar arches were opacified (Gustilo type IIIB injury). The fracture was treated with open reduction and internal fixation and placement of a latissimus dorsi free flap (anastomosed to the intact brachial artery) with a split-thickness skin graft.
Among upper extremity vascular traumas, radial artery injuries have the best outcomes overall (1,2,70). If both radial and ulnar arteries are injured, microvascular repair of the dominant ulnar artery is preferred (1,29). Flexor compartment fasciotomy is performed for severe forearm injuries (29). Major residual functional impairment after salvage is common owing to the close apposition of neurovascular structures to muscle-tendon units (45).
Postoperative Imaging
In patients with threatened or mangled extremities, damage control methods include rapid interventions for hemorrhage control and restoration of blood flow. The term damage control orthopedics refers to the early use of fasciotomies, and rapid temporary reduction and stabilization with splint placement, traction, or external fixation to protect the vascular repair and ensure a tension-free anastomosis (28,34). The sequence of vascular repair versus traction is debated because of the potential for an increasing warm ischemia time. Practice patterns depend in part on the availability of a 24-hour in-house orthopedic service (4,71).
Vascular damage control procedures include surgical placement of temporary synthetic (polytetrafluoroethylene) interposition grafts as a bridge to primary vascular repair (1,34). Polytetrafluoroethylene grafts are not a viable long-term solution for small-caliber vessels and have a high risk of thrombosis when used in open contaminated wounds (Fig 13) (29,72). Synthetic grafts may be the only definitive option if a long extra-anatomic conduit is needed or for patients who lack venous donor sites, such as those with end-stage renal disease.
Interpretation of CT findings is challenging following fasciotomies, vascular bypass grafting, myocutaneous flap reconstruction, partial-thickness skin grafting, multiple revision surgeries, and use of external fixator devices (which cause beam-hardening artifact). Basic knowledge of the principles, timing, and potential complications of staged reconstruction is helpful.
After reperfusion, often with extra-anatomic graft placement, the goals of severe open fracture treatment are prevention of infection, immobilization to promote fracture healing, and restoration of extremity function (13). Fracture stabilization with an external fixator is performed within 24 hours (Fig 6) (40). Serial débridement of contaminated devascularized tissue and devitalized bone fragments is performed during the first 48–72 hours until the marginal soft tissue is viable and can receive a flap (30,45). Flap coverage for bone and nerve graft placement is performed between 48 hours and 3 weeks, depending on the injury severity (Fig 20) (4,30,40). Defect fractures are filled with antibiotic beads at the time of flap reconstruction to prevent deep infection (Fig 15) (73). Bone graft placement and plate and screw reconstruction of articular fractures are usually delayed for several weeks after flap coverage to allow the edema to subside (30,40,74).
Figure 20:
Injuries in a 21-year-old man involved in a motor vehicle collision with ejection (same patient as in Figs 2B and 9). (A) Axial admission CTA image through the calf shows degloving involving the posterior and medial compartments (arrows) and an anterior compartment muscle tear (*). (B, C) Axial CT performed 6 weeks later to evaluate a draining wound shows a left vastus lateralis free flap (arrow in B) placed on hospital day 9, and an abscess and sinus track communicating with the skin surface (arrow in C) more distally. Multiple revision surgeries were performed for recurrent infection, and the limb was ultimately salvaged.
External fixation allows access for wound management after grossly contaminated exposed fractures and provides rigidity for bone and soft-tissue healing (45). CT is often performed with external fixators in place before definitive plate-and-screw fixation. Surgical times can be decreased with improved understanding of the relationships between articular fragments and selection of optimal incisions. Entrapped tendinous or neurovascular structures also can be identified (75,76).
CTA is sometimes used to plan anastomosis of myocutaneous free-tissue transfers or pedicled fibular grafts (30). The latissimus dorsi muscle is a common donor site because of its long vascular pedicle and large bulk (30). The posterior tibial and peroneal arteries are common recipient vessels for free myocutaneous flaps and fibular grafts, respectively (Fig 20). A contralateral free vascularized fibular graft with a peroneal artery pedicle provides optimal healing for large (>5–8 cm) defects, while cancellous grafts are used for smaller defects (73).
Vascular graft thrombosis is a common and feared complication of limb salvage. Autogenous vein grafts become occluded as a result of infection or excessive anastomotic tension in 10%–20% of patients (77). CTA may be performed to evaluate the patency or blowout of vascular grafts. Contrast-enhanced CT may reveal infection at the incision site, wound breakdown, myonecrosis, and osteomyelitis with deep infection at successive stages of reconstruction (73).
Absent callus formation over a 3-month period in patients with long-standing fractures (persisting for at least 9 months) is consistent with nonunion (78). Nonunion occurs with infection in up to 50% of patients with Gustilo type IIIC fractures (49). CT is not definitive for osteomyelitis, with limited sensitivity and specificity: 47% and 60%, respectively. However, lucency around hardware, bone sclerosis or mottling, periosteal reaction, soft-tissue edema, and abscesses or sinus tracks require further workup (Fig 20) (79). Bone regrafting is frequently required for Gustilo type IIIB and IIIC injuries (41). Approximately 22% of patients with tibial fractures ultimately require secondary below-the-knee amputation for osteomyelitis (Fig 11) (80).
After amputation, CT scans obtained to evaluate stump complications should be scrutinized for any evidence of surgical dehiscence, ulceration, infection, postoperative hematoma, persistent bone fragments, heterotopic ossification, excessive scar formation, and/or vascular patency (Fig 21).
Figure 21:
Amputation stump complications in two patients. (A) Lower extremity crush injury in a 56-year-old man who required secondary amputation following osteomyelitis, deep infection, and latissimus dorsi free flap failure (same patient as in Fig 11). Coronal CT image obtained to assess stump pain 3 months after below-the-knee amputation shows heterotopic ossification (arrow), which was subsequently excised. (B) Right Gustilo type IIIC open proximal tibial fracture in a 47-year-old man after a motor vehicle collision who required above-the-knee amputation owing to progressive necrosis. The patient developed exuberant heterotopic ossification, which was resected. Contrast-enhanced CT image of the right femur 1 month later shows enhancing fluid and gas collections compatible with abscesses from a superinfected hematoma at the resection bed (arrows).
Conclusion
Understanding how the type, extent, and location of arterial injury and the degree of concurrent soft-tissue and skeletal injuries seen at admission extremity CT and CTA influence anatomy-specific management approaches facilitates informed discussion of management options with surgical colleagues regarding patients with threatened or mangled extremities. Management approaches include observation; attempted salvage with damage control or definitive surgical and endovascular approaches followed by staged reconstruction; and primary or secondary amputation. In addition, familiarity with the common postoperative CT and CTA appearances and complications of extremity artery injuries can augment efficient, clinically salient radiology reporting.
Presented as an education exhibit at the 2020 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors, editor, and reviewers have disclosed no relevant relationships.
D.D. supported by the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health (K08 EB027141-01A1).
Abbreviations:
- BKAI
- below-the-knee arterial injury
- CTA
- CT angiography
- MIP
- maximum intensity projection
- 3D
- three dimensional
- VR
- volume rendered
References
- 1. Franz RW , Shah KJ , Halaharvi D , Franz ET , Hartman JF , Wright ML . A 5-year review of management of lower extremity arterial injuries at an urban level I trauma center . J Vasc Surg 2011. ; 53 ( 6 ): 1604 – 1610 . [DOI] [PubMed] [Google Scholar]
- 2. Cikrit DF , Dalsing MC , Bryant BJ , Lalka SG , Sawchuk AP , Schulz JE . An experience with upper-extremity vascular trauma . Am J Surg 1990. ; 160 ( 2 ): 229 – 233 . [DOI] [PubMed] [Google Scholar]
- 3. Scalea JR , Crawford R , Scurci S , et al . Below-the-knee arterial injury: the type of vessel may be more important than the number of vessels injured . J Trauma Acute Care Surg 2014. ; 77 ( 6 ): 920 – 925 . [DOI] [PubMed] [Google Scholar]
- 4. Scalea TM , DuBose J , Moore EE , et al . Western Trauma Association critical decisions in trauma: management of the mangled extremity . J Trauma Acute Care Surg 2012. ; 72 ( 1 ): 86 – 93 . [DOI] [PubMed] [Google Scholar]
- 5. Rozycki GS , Tremblay LN , Feliciano DV , McClelland WB . Blunt vascular trauma in the extremity: diagnosis, management, and outcome . J Trauma 2003. ; 55 ( 5 ): 814 – 824 . [DOI] [PubMed] [Google Scholar]
- 6. Doody O , Given MF , Lyon SM . Extremities: indications and techniques for treatment of extremity vascular injuries . Injury 2008. ; 39 ( 11 ): 1295 – 1303 . [DOI] [PubMed] [Google Scholar]
- 7. Fritz J , Efron DT , Fishman EK . Multidetector CT and three-dimensional CT angiography of upper extremity arterial injury . Emerg Radiol 2015. ; 22 ( 3 ): 269 – 282 . [DOI] [PubMed] [Google Scholar]
- 8. Frykberg ER , Dennis JW , Bishop K , Laneve L , Alexander RH . The reliability of physical examination in the evaluation of penetrating extremity trauma for vascular injury: results at one year . J Trauma 1991. ; 31 ( 4 ): 502 – 511 . [DOI] [PubMed] [Google Scholar]
- 9. Peng PD , Spain DA , Tataria M , Hellinger JC , Rubin GD , Brundage SI . CT angiography effectively evaluates extremity vascular trauma . Am Surg 2008. ; 74 ( 2 ): 103 – 107 . [DOI] [PubMed] [Google Scholar]
- 10. Halvorson JJ , Anz A , Langfitt M , et al . Vascular injury associated with extremity trauma: initial diagnosis and management . J Am Acad Orthop Surg 2011. ; 19 ( 8 ): 495 – 504 . [DOI] [PubMed] [Google Scholar]
- 11. Dreizin D , Munera F . Blunt polytrauma: evaluation with 64-section whole-body CT angiography . RadioGraphics 2012. ; 32 ( 3 ): 609 – 631 . [DOI] [PubMed] [Google Scholar]
- 12. Costantini TW , Coimbra R , Holcomb JB , et al . Current management of hemorrhage from severe pelvic fractures: results of an American Association for the Surgery of Trauma multi-institutional trial . J Trauma Acute Care Surg 2016. ; 80 ( 5 ): 717 – 723 ; discussion 723–725 . [DOI] [PubMed] [Google Scholar]
- 13. Fochtmann A , Binder H , Rettl G , et al . Third degree open fractures and traumatic sub-/total amputations of the upper extremity: outcome and relevance of the mangled extremity severity score . Orthop Traumatol Surg Res 2016. ; 102 ( 6 ): 785 – 790 . [DOI] [PubMed] [Google Scholar]
- 14. Rieger M , Mallouhi A , Tauscher T , Lutz M , Jaschke WR . Traumatic arterial injuries of the extremities: initial evaluation with MDCT angiography . AJR Am J Roentgenol 2006. ; 186 ( 3 ): 656 – 664 . [DOI] [PubMed] [Google Scholar]
- 15. Jens S , Kerstens MK , Legemate DA , Reekers JA , Bipat S , Koelemay MJ . Diagnostic performance of computed tomography angiography in peripheral arterial injury due to trauma: a systematic review and meta-analysis . Eur J Vasc Endovasc Surg 2013. ; 46 ( 3 ): 329 – 337 . [DOI] [PubMed] [Google Scholar]
- 16. Gakhal MS , Sartip KA . CT angiography signs of lower extremity vascular trauma . AJR Am J Roentgenol 2009. ; 193 ( 1 ): W49 – W57 . [DOI] [PubMed] [Google Scholar]
- 17. Pieroni S , Foster BR , Anderson SW , Kertesz JL , Rhea JT , Soto JA . Use of 64-row multidetector CT angiography in blunt and penetrating trauma of the upper and lower extremities . RadioGraphics 2009. ; 29 ( 3 ): 863 – 876 . [DOI] [PubMed] [Google Scholar]
- 18. Miller-Thomas MM , West OC , Cohen AM . Diagnosing traumatic arterial injury in the extremities with CT angiography: pearls and pitfalls . RadioGraphics 2005. ; 25 ( Suppl 1 ): S133 – S142 . [DOI] [PubMed] [Google Scholar]
- 19. Uyeda JW , Anderson SW , Sakai O , Soto JA . CT angiography in trauma . Radiol Clin North Am 2010. ; 48 ( 2 ): 423 – 438 , ix – x . [DOI] [PubMed] [Google Scholar]
- 20. Stain SC , Yellin AE , Weaver FA , Pentecost MJ . Selective management of nonocclusive arterial injuries . Arch Surg 1989. ; 124 ( 10 ): 1136 – 1140 ; discussion 1140–1141 . [DOI] [PubMed] [Google Scholar]
- 21. Frykberg ER . Advances in the diagnosis and treatment of extremity vascular trauma . Surg Clin North Am 1995. ; 75 ( 2 ): 207 – 223 . [DOI] [PubMed] [Google Scholar]
- 22. DuBose JJ , Rajani R , Gilani R , et al . Endovascular management of axillo-subclavian arterial injury: a review of published experience . Injury 2012. ; 43 ( 11 ): 1785 – 1792 . [DOI] [PubMed] [Google Scholar]
- 23. Keeley SB , Snyder WH 3rd , Weigelt JA . Arterial injuries below the knee: fifty-one patients with 82 injuries . J Trauma 1983. ; 23 ( 4 ): 285 – 292 . [PubMed] [Google Scholar]
- 24. Hoshino T , Ichikawa K , Hara T , et al . Optimization of scan timing for aortic computed tomographic angiography using the test bolus injection technique . Acta Radiol 2016. ; 57 ( 7 ): 829 – 836 . [DOI] [PubMed] [Google Scholar]
- 25. Soto JA , Múnera F , Cardoso N , Guarín O , Medina S . Diagnostic performance of helical CT angiography in trauma to large arteries of the extremities . J Comput Assist Tomogr 1999. ; 23 ( 2 ): 188 – 196 . [DOI] [PubMed] [Google Scholar]
- 26. Colip CG , Gorantla V , LeBedis CA , Soto JA , Anderson SW . Extremity CTA for penetrating trauma: 10-year experience using a 64-detector row CT scanner . Emerg Radiol 2017. ; 24 ( 3 ): 223 – 232 . [DOI] [PubMed] [Google Scholar]
- 27. Masi Z , Gussman K , Hazelton JP , Gefen R . Evaluation of the diagnostic value of a venous phase in CT angiography of the extremities in the setting of trauma: is vein imaging in vain? Emerg Radiol 2017. ; 24 ( 4 ): 335 – 340 . [DOI] [PubMed] [Google Scholar]
- 28. Taeger G , Ruchholtz S , Waydhas C , Lewan U , Schmidt B , Nast-Kolb D . Damage control orthopedics in patients with multiple injuries is effective, time saving, and safe . J Trauma 2005. ; 59 ( 2 ): 409 – 416 ; discussion 417 . [DOI] [PubMed] [Google Scholar]
- 29. McCready RA . Upper-extremity vascular injuries . Surg Clin North Am 1988. ; 68 ( 4 ): 725 – 740 . [DOI] [PubMed] [Google Scholar]
- 30. Yaremchuk MJ , Brumback RJ , Manson PN , Burgess AR , Poka A , Weiland AJ . Acute and definitive management of traumatic osteocutaneous defects of the lower extremity . Plast Reconstr Surg 1987. ; 80 ( 1 ): 1 – 14 . [DOI] [PubMed] [Google Scholar]
- 31. Hoyt DB , Coimbra R , Potenza BM , Rappold JF . Anatomic exposures for vascular injuries . Surg Clin North Am 2001. ; 81 ( 6 ): 1299 – 1330 , xii . [DOI] [PubMed] [Google Scholar]
- 32. Piffaretti G , Tozzi M , Lomazzi C , et al . Endovascular treatment for traumatic injuries of the peripheral arteries following blunt trauma . Injury 2007. ; 38 ( 9 ): 1091 – 1097 . [DOI] [PubMed] [Google Scholar]
- 33. Dreizin D , Munera F . Multidetector CT for penetrating torso trauma: state of the art . Radiology 2015. ; 277 ( 2 ): 338 – 355 . [DOI] [PubMed] [Google Scholar]
- 34. Percival TJ , Rasmussen TE . Reperfusion strategies in the management of extremity vascular injury with ischaemia . Br J Surg 2011. ; 99 ( Suppl 1 ): 66 – 74 . [DOI] [PubMed] [Google Scholar]
- 35. Green NE , Allen BL . Vascular injuries associated with dislocation of the knee . J Bone Joint Surg Am 1977. ; 59 ( 2 ): 236 – 239 . [PubMed] [Google Scholar]
- 36. McQueen MM , Gaston P , Court-Brown CM . Acute compartment syndrome: who is at risk? J Bone Joint Surg Br 2000. ; 82 ( 2 ): 200 – 203 . [PubMed] [Google Scholar]
- 37. Lu CH , Tsang YM , Yu CW , Wu MZ , Hsu CY , Shih TTF . Rhabdomyolysis: magnetic resonance imaging and computed tomography findings . J Comput Assist Tomogr 2007. ; 31 ( 3 ): 368 – 374 . [DOI] [PubMed] [Google Scholar]
- 38. Menger MD , Rücker M , Vollmar B . Capillary dysfunction in striated muscle ischemia/reperfusion: on the mechanisms of capillary “no-reflow.” . Shock 1997. ; 8 ( 1 ): 2 – 7 . [PubMed] [Google Scholar]
- 39. Gillani S , Cao J , Suzuki T , Hak DJ . The effect of ischemia reperfusion injury on skeletal muscle . Injury 2012. ; 43 ( 6 ): 670 – 675 . [DOI] [PubMed] [Google Scholar]
- 40. Hildebrand F , Giannoudis P , Kretteck C , Pape HC . Damage control: extremities . Injury 2004. ; 35 ( 7 ): 678 – 689 . [DOI] [PubMed] [Google Scholar]
- 41. Gustilo RB , Mendoza RM , Williams DN . Problems in the management of type III (severe) open fractures: a new classification of type III open fractures . J Trauma 1984. ; 24 ( 8 ): 742 – 746 . [DOI] [PubMed] [Google Scholar]
- 42. Kim PH , Leopold SS . In brief: Gustilo-Anderson classification . Clin Orthop Relat Res 2012. ; 470 ( 11 ): 3270 – 3274 . [Published correction appears in Clin Orthop Relat Res 2019;477(10):2388.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gustilo RB , Anderson JT . Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses . J Bone Joint Surg Am 1976. ; 58 ( 4 ): 453 – 458 . [PubMed] [Google Scholar]
- 44. Court-Brown CM , Rimmer S , Prakash U , McQueen MM . The epidemiology of open long bone fractures . Injury 1998. ; 29 ( 7 ): 529 – 534 . [DOI] [PubMed] [Google Scholar]
- 45. Levin LS , Goldner RD , Urbaniak JR , Nunley JA , Hardaker WT Jr . Management of severe musculoskeletal injuries of the upper extremity . J Orthop Trauma 1990. ; 4 ( 4 ): 432 – 440 . [PubMed] [Google Scholar]
- 46. Durham RM , Mistry BM , Mazuski JE , Shapiro M , Jacobs D . Outcome and utility of scoring systems in the management of the mangled extremity . Am J Surg 1996. ; 172 ( 5 ): 569 – 573 ; discussion 573–574 . [DOI] [PubMed] [Google Scholar]
- 47. Ly TV , Travison TG , Castillo RC , Bosse MJ , MacKenzie EJ ; LEAP Study Group . Ability of lower-extremity injury severity scores to predict functional outcome after limb salvage . J Bone Joint Surg Am 2008. ; 90 ( 8 ): 1738 – 1743 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Russell WL , Sailors DM , Whittle TB , Fisher DF Jr , Burns RP . Limb salvage versus traumatic amputation: a decision based on a seven-part predictive index . Ann Surg 1991. ; 213 ( 5 ): 473 – 480 ; discussion 480–481 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Soni A , Tzafetta K , Knight S , Giannoudis PV . Gustilo IIIC fractures in the lower limb: our 15-year experience . J Bone Joint Surg Br 2012. ; 94 ( 5 ): 698 – 703 . [DOI] [PubMed] [Google Scholar]
- 50. Noblet T , Lineham B , Wiper J , Harwood P . Amputation in trauma: how to achieve good result from lower extremity amputation irrespective of the level . Curr Trauma Rep 2019. ; 5 ( 1 ): 69 – 78 . [Google Scholar]
- 51. Caudle RJ , Stern PJ . Severe open fractures of the tibia . J Bone Joint Surg Am 1987. ; 69 ( 6 ): 801 – 807 . [PubMed] [Google Scholar]
- 52. Cone JB . Vascular injury associated with fracture-dislocations of the lower extremity . Clin Orthop Relat Res 1989. ;( 243 ): 30 – 35 . [PubMed] [Google Scholar]
- 53. Levy BA , Zlowodzki MP , Graves M , Cole PA . Screening for extermity arterial injury with the arterial pressure index . Am J Emerg Med 2005. ; 23 ( 5 ): 689 – 695 . [DOI] [PubMed] [Google Scholar]
- 54. Wynes J , Kirksey L . Assessing vascular status and risk of latent ischemia with ankle fracture: a case report and algorithm for treatment . J Foot Ankle Surg 2014. ; 53 ( 3 ): 353 – 355 . [DOI] [PubMed] [Google Scholar]
- 55. Poon H , Morrison JJ , Clasper JC , Midwinter MJ , Jansen JO . Use and complications of operative control of arterial inflow in combat casualties with traumatic lower-extremity amputations caused by improvised explosive devices . J Trauma Acute Care Surg 2013. ; 75 ( 2 suppl 2 ): S233 – S237 . [DOI] [PubMed] [Google Scholar]
- 56. Simmons JD , Walker WB , Gunter Iii JW , Ahmed N . Role of endovascular grafts in combined vascular and skeletal injuries of the lower extremity: a preliminary report . Arch Trauma Res 2013. ; 2 ( 1 ): 40 – 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. White R , Krajcer Z , Johnson M , Williams D , Bacharach M , O’Malley E . Results of a multicenter trial for the treatment of traumatic vascular injury with a covered stent . J Trauma 2006. ; 60 ( 6 ): 1189 – 1195 ; discussion 1195–1196 . [DOI] [PubMed] [Google Scholar]
- 58. Frykberg ER . Popliteal vascular injuries . Surg Clin North Am 2002. ; 82 ( 1 ): 67 – 89 . [DOI] [PubMed] [Google Scholar]
- 59. Kauvar DS , Sarfati MR , Kraiss LW . National trauma databank analysis of mortality and limb loss in isolated lower extremity vascular trauma . J Vasc Surg 2011. ; 53 ( 6 ): 1598 – 1603 . [DOI] [PubMed] [Google Scholar]
- 60. Burkhardt GE , Cox M , Clouse WD , et al . Outcomes of selective tibial artery repair following combat-related extremity injury . J Vasc Surg 2010. ; 52 ( 1 ): 91 – 96 . [DOI] [PubMed] [Google Scholar]
- 61. Farber A , Tan TW , Hamburg NM , et al . Early fasciotomy in patients with extremity vascular injury is associated with decreased risk of adverse limb outcomes: a review of the National Trauma Data Bank . Injury 2012. ; 43 ( 9 ): 1486 – 1491 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zellweger R , Hess F , Nicol A , Omoshoro-Jones J , Kahn D , Navsaria P . An analysis of 124 surgically managed brachial artery injuries . Am J Surg 2004. ; 188 ( 3 ): 240 – 245 . [DOI] [PubMed] [Google Scholar]
- 63. Diamond S , Gaspard D , Katz S . Vascular injuries to the extremities in a suburban trauma center . Am Surg 2003. ; 69 ( 10 ): 848 – 851 . [PubMed] [Google Scholar]
- 64. Andreev A , Kavrakov T , Karakolev J , Penkov P . Management of acute arterial trauma of the upper extremity . Eur J Vasc Surg 1992. ; 6 ( 6 ): 593 – 598 . [DOI] [PubMed] [Google Scholar]
- 65. DeBakey ME , Simeone FA . Battle injuries of the arteries in World War II: an analysis of 2,471 cases . Ann Surg 1946. ; 123 ( 4 ): 534 – 579 . [PMC free article] [PubMed] [Google Scholar]
- 66. Xenos ES , Freeman M , Stevens S , Cassada D , Pacanowski J , Goldman M . Covered stents for injuries of subclavian and axillary arteries . J Vasc Surg 2003. ; 38 ( 3 ): 451 – 454 . [DOI] [PubMed] [Google Scholar]
- 67. Carrick MM , Morrison CA , Pham HQ , et al . Modern management of traumatic subclavian artery injuries: a single institution’s experience in the evolution of endovascular repair . Am J Surg 2010. ; 199 ( 1 ): 28 – 34 . [DOI] [PubMed] [Google Scholar]
- 68. Carrafiello G , Laganà D , Mangini M , et al . Percutaneous treatment of traumatic upper-extremity arterial injuries: a single-center experience . J Vasc Interv Radiol 2011. ; 22 ( 1 ): 34 – 39 . [Published correction appears in J Vasc Interv Radiol 2016;27(12):1940.] [DOI] [PubMed] [Google Scholar]
- 69. Sitzmann JV , Ernst CB . Management of arm arterial injuries . Surgery 1984. ; 96 ( 5 ): 895 – 901 . [PubMed] [Google Scholar]
- 70. Manord JD , Garrard CL , Kline DG , Sternbergh WC 3rd , Money SR . Management of severe proximal vascular and neural injury of the upper extremity . J Vasc Surg 1998. ; 27 ( 1 ): 43 – 47 ; discussion 48–49 . [DOI] [PubMed] [Google Scholar]
- 71. Fowler J , Macintyre N , Rehman S , Gaughan JP , Leslie S . The importance of surgical sequence in the treatment of lower extremity injuries with concomitant vascular injury: a meta-analysis . Injury 2009. ; 40 ( 1 ): 72 – 76 . [DOI] [PubMed] [Google Scholar]
- 72. Felician DV , Mattox KL , Graham JM , Bitondo CG . Five-year experience with PTFE grafts in vascular wounds . J Trauma 1985. ; 25 ( 1 ): 71 – 82 . [DOI] [PubMed] [Google Scholar]
- 73. Christian EP , Bosse MJ , Robb G . Reconstruction of large diaphyseal defects, without free fibular transfer, in grade-IIIB tibial fractures . J Bone Joint Surg Am 1989. ; 71 ( 7 ): 994 – 1004 . [PubMed] [Google Scholar]
- 74. Marino JT , Ziran BH . Use of solid and cancellous autologous bone graft for fractures and nonunions . Orthop Clin North Am 2010. ; 41 ( 1 ): 15 – 26 . [DOI] [PubMed] [Google Scholar]
- 75. Liporace FA , Yoon RS . Decisions and staging leading to definitive open management of pilon fractures: where have we come from and where are we now? J Orthop Trauma 2012. ; 26 ( 8 ): 488 – 498 . [DOI] [PubMed] [Google Scholar]
- 76. Tornetta P 3rd , Gorup J . Axial computed tomography of pilon fractures . Clin Orthop Relat Res 1996. ; 323 ( 323 ): 273 – 276 . [DOI] [PubMed] [Google Scholar]
- 77. Morrison JJ , McMahon J , DuBose JJ , Scalea TM , Lawson JH , Rasmussen TE . Clinical implementation of the Humacyte human acellular vessel: implications for military and civilian trauma care . J Trauma Acute Care Surg 2019. ; 87 ( 1S Suppl 1 ): S44 – S47 . [DOI] [PubMed] [Google Scholar]
- 78. Cunningham BP , Brazina S , Morshed S , Miclau T 3rd . Fracture healing: a review of clinical, imaging and laboratory diagnostic options . Injury 2017. ; 48 ( suppl 1 ): S69 – S75 . [DOI] [PubMed] [Google Scholar]
- 79. Govaert GA , IJpma FF , McNally M , McNally E , Reininga IH , Glaudemans AW . Accuracy of diagnostic imaging modalities for peripheral post-traumatic osteomyelitis: a systematic review of the recent literature . Eur J Nucl Med Mol Imaging 2017. ; 44 ( 8 ): 1393 – 1407 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chung KC , Shauver MJ , Saddawi-Konefka D , Haase SC . A decision analysis of amputation versus reconstruction for severe open tibial fracture from the physician and patient perspectives . Ann Plast Surg 2011. ; 66 ( 2 ): 185 – 191 . [DOI] [PMC free article] [PubMed] [Google Scholar]