Abstract
Kisspeptin (KISS1) signaling in the hypothalamic-pituitary (H-P) axis plays an essential role in regulating gonadotropin secretion. KISS1 and KISS1 receptor (KISS1R) are also expressed in the ovary; however, the role of intraovarian KISS1 signaling remains unclear. Granulosa cell (GC)-specific expression of KISS1, and oocyte-specific expression of KISS1R indicate that GC-derived KISS1 may act on oocytes. Expression of KISS1 in GCs is induced by gonadotropins but it is absent in estrogen receptor β knockout (Erβnull) rat ovaries. We also observed that gonadotropin stimulation failed to induce maturation of Erβnull oocytes. Interestingly, KISS1 treatment of cumulus oocyte complexes (COCs) isolated from antral follicles promotes in vitro maturation of oocytes. Treatment of oocytes with KISS1 induced intracellular Ca2+ release, and increased activation of MAP kinase ERK1/2. KISS1 treatment also induced the expression of oocyte genes that are crucial for differentiation of GCs, and maturation of oocytes. Our findings suggest that ovarian KISS1-signaling plays an important role in gonadotropin induced follicle development and oocyte maturation.
Keywords: Kisspeptin, kisspeptin receptor, estrogen receptor β, gonadotropin, granulosa cells, oocyte maturation
1. Introduction
Estrogen receptor β (ERβ) is the predominant estrogen receptor in the ovary (Byers et al., 1997; Couse et al., 2004; Pelletier and El-Alfy, 2000; Słomczyńska et al., 2001) and disruption of ERβ signaling leads ovulatory defects (Asadi et al., 2013; Lang-Muritano et al., 2018). Failure of ovulation in Erβnull mice and rats is associated with defective follicle maturation and an attenuated preovulatory gonadotropin surge (Couse et al., 2005; Rumi et al., 2017). However, treatment with exogenous gonadotropins fails to induce ovulation, suggesting a primary ovarian defect due to the loss of ERβ (Rumi et al., 2017). To identify the ERβ-regulated genes that may affect ovarian follicle development, oocyte maturation, and ovulation, we performed RNA-sequencing analyses of gonadotropin stimulated granulosa cells (GCs) and oocytes (Chakravarthi et al., 2020; Chakravarthi et al., 2019; Khristi et al., 2018). We identified Kiss1 to be one of the most-differentially expressed gene in Erβnull GCs (Chakravarthi et al., 2020; Khristi et al., 2018). GCs showed a marked induction in Kiss1 expression in response to gonadotropin stimulation, which was absent in Erβnull rats (Chakravarthi et al., 2020; Chakravarthi et al., 2018; Khristi et al., 2018).
Hypothalamic KISS1 regulates gonadotropin secretion, which is essential for gonadal development, onset of puberty, and induction of ovulation (Javed et al., 2015; Moore et al., 2018). In recent studies, expression of KISS1 and KISS1R in the ovaries has been detected across species (Castellano et al., 2006; Gaytan et al., 2009; Shahed and Young, 2009; Terao et al., 2004). However, the physiological role of ovarian KISS1 signaling remains largely unknown. While the expression of Kiss1 is markedly higher in GCs, Kiss1r is detected predominantly in the oocytes, suggesting that GC-derived KISS1 may act on oocytes (Chakravarthi et al., 2018).
Bidirectional interactions between GCs and oocytes play vital roles in follicle development and ovulation (Craig et al., 2007; Emori and Sugiura, 2014; McNatty et al., 2003; Monniaux, 2016; Russell and Robker, 2007; Wigglesworth et al., 2013). While oocyte maturation is regulated by GC-derived factors, maturing oocytes contribute to GC differentiation and cumulus cell expansion (Craig et al., 2007; Emori and Sugiura, 2014; McNatty et al., 2003; Monniaux, 2016; Russell and Robker, 2007; Wigglesworth et al., 2013). KISS1 expressed in GCs represent the former group of signaling molecules. KISS1 expression in the ovary coincides with the preovulatory gonadotropin surge (Castellano et al., 2006) and intrafollicular KISS1 levels positively correlate with the stages of oocyte maturation (Taniguchi et al., 2017). Taken together, we hypothesize that GC-derived KISS1 may act on oocytes to effect oocyte maturation, and an attenuated expression of KISS1 in Erβnull GCs may cause failure of follicle development and oocyte maturation despite gonadotropin stimulation.
2. Materials and methods
2.1. Animal models
Wildtype and Erβnull Holtzman Sprague-Dawley (HSD) rats were studied for intraovarian kisspeptin signaling. Erβnull rat model was generated by targeted deletion of the exon 3 in the Erβ gene (Rumi et al., 2017). Exon 3 deletion caused a frameshift and null mutation (Rumi et al., 2017). Rats were screened for the presence of mutations by PCR using tail-tip DNA samples (RED extract-N-Amp Tissue PCR Kit, Sigma-Aldrich) as described previously (Rumi et al., 2014; Rumi et al., 2017). All procedures were performed in accordance with the protocols approved by the University of Kansas Medical Center Animal Care and Use Committee.
2.2. Gonadotropin treatment and ovulatory response
4-wk-old Erβnull and age-matched wildtype female rats were used to evaluate gonadotropin-induced follicular development. Synchronized follicular growth was performed by intraperitoneal injection of 30 IU pregnant mare serum gonadotropin (PMSG; BioVendor LLC, Asheville, NC). Forty-eight hours after the PMSG injection, 30 IU of human chorionic gonadotropin (hCG; BioVendor) was injected intraperitoneally. Rats were sacrificed prior to gonadotropin treatment (Basal), 48h after PMSG treatment (PMSG), 4h and 24h after hCG injection to PMSG treated rats and their ovaries were collected (Fig. 1B) as described previously (Chakravarthi et al., 2020; Chakravarthi et al., 2019; Chakravarthi et al., 2018). At 24 hours after the hCG injection, animals were sacrificed, oocytes were recovered from the oviduct, cumulus cells removed using hyaluronidase (Sigma-Aldrich), and oocytes counted. 8–12 week old Erβnull and age-matched wildtype female rats were sacrificed and oocytes were recovered from the oviduct, cumulus cells removed using hyaluronidase (Sigma-Aldrich), and oocytes counted (Rumi et al., 2017).
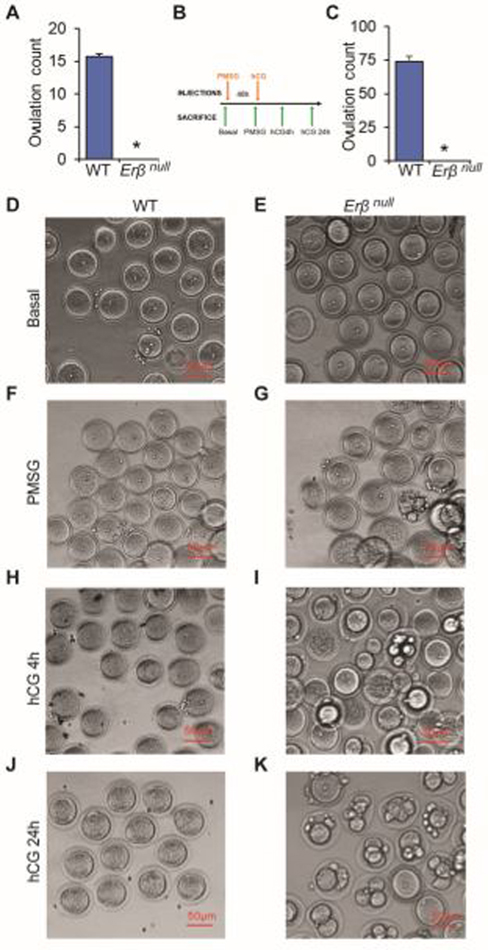
Fig. 1. Abnormal oocyte maturation in Erβnull rats.
8–12 wk-old Erβnull adult rats are infertile and they failed to ovulate either during natural estrus cycle (A) or induced by exogenous gonadotropins to 4 wk-old rats (C). Oocytes isolated from 4-wk-old wildtype (WT) and Erβnull rat ovaries at different stages of gonadotropin treatment were examined after removal of cumulus cells. Although unstimulated (Basal D and E) oocytes of both genotypes appeared similar, abnormal division and fragmentation was observed in Erβnull oocytes after PMSG 48h (F and G), 4h after hCG (H and I), and 24h after hCG (J and K) treatment. n = 6. *P ≤ 0.05.
2.2. Isolation of oocytes and GCs
Cumulus oocyte complexes (COCs) were isolated from the large antral follicles by needle puncture under microscopic observation as described previously (Chakravarthi et al., 2020; Chakravarthi et al., 2019; Chakravarthi et al., 2018). All cumulus cells were removed from the oocytes by mechanical pipetting followed by repeated washings into fresh media 199 using capillary suctions. GCs were collected by filtration of the remaining cells through 40μM cell strainers (Fisher Scientific).
2.3. RNA-sequencing of GCs and oocytes
Oocytes and GCs were collected from 4-wk-old wildtype and Erβnull rats with or without gonadotropin treatment. Total RNA was extracted using TRI Reagent (Millipore-Sigma) following the manufactures instructions. Approximately 500ng of total RNA was used for the RNA-seq library preparation. Libraries were prepared by using a TruSeq standard mRNA kit (Illumina) following manufacturer’s instructions. The cDNA libraries were sequenced at the Molecular Biology Core Laboratory of Mayo Clinic (Rochester, MN). RNA-Seq data were analyzed using CLC Genomics Workbench (Qiagen Bioinformatics). Gene expression values were reported as TPM (Transcripts per million base pairs). All the RNA seq data are available at SRA (SRX6376730, SRX6376729, SRX6376735, SRX6376752, SRX6376751, SRX6376757, SX6761695, SRX6761696, SRX6761697, SRX6761698, SRX6761699 and SRX67617) and the data articles are published in Data in Brief journal (Chakravarthi et al., 2020; Chakravarthi et al., 2019).
2.4. In vitro maturation of rat oocytes
COCs were collected from the antral follicles of 4-wk-old wildtype and Erβnull rats 48h after PMSG treatment. After washing three times in in vitro maturation media (BO-IVM, IVF Biosciences, UK) the COCs were placed in 20 μl drops of IVM medium in 35-mm culture dishes, covered with light weight mineral oil, and incubated at 37°C in a humidified CO2 incubator for an additional 24h (Kona et al., 2016; Praveen Chakravarthi et al., 2015). At the end of incubation, oocytes were denuded of the cumulus cells by repeated pipetting through a fine bore glass pipette and examined microscopically for extrusion of the polar body as indicative of oocyte maturation to MII stage (Kona et al., 2016; Praveen Chakravarthi et al., 2015).
2.5. Calcium release assay
4-wk-old wildtype rats were administered with PMSG, and 48h later COCs were isolated. COCs were incubated with 5μM Fluo-3AM (ThermoFisher Scientific) solution containing 20% Pluronic F-127 (ThermoFisher Scientific) for 30 min in a 37°C incubator. All cumulus cells were removed from the oocytes by pipetting followed by repeated washings into fresh media using capillary suction and oocytes were placed into HBSS drops containing 1nM of rat kisspeptin-10 (KP-10) (Tocris Bioscience, Bristol, UK). Oocytes were monitored under a fluorescent microscope for 3 min and images were recorded every 30 sec. Calcium efflux signal intensities were quantified by ImageJ analyses (National Institutes of Health, Bethesda, MD; https://imagej.nih.gov/ij/).
2.6. Protein extraction and western blotting
COCs were collected from 4-wk-old wildtype rats 48h after PMSG administration. Oocytes and GCs were purified as described in Section 2.2 and proteins were extracted in 1X SDS lysis buffer (62.5mMTris-HCl pH 6.8, 2% SDS, 42 mM dithiothreitol, 10% glycerol, and 0.01% bromophenol blue), containing protease and phosphatase inhibitors (Cell Signaling Technologies, Danvers, MA). In addition, oocytes were cultured with or without 1nM or 10nM KP-10. After treatment, oocytes were denuded mechanically, proteins were extracted in 1XSDS buffer. Oocyte or GC cell-lysates in 1XSDS buffer were sonicated to shear DNA and reduce viscosity, heat denatured, and separated on a 4–20% SDS-PAGE. Electrophoresed proteins were transferred from the gel to PVDF membranes, blocked with 5% skim milk in TBST (1XTBS buffer containing 0.1% Tween-20), and incubated overnight at 4°C with specific primary antibodies at the appropriate dilution in blocking solution (Supplementary Table 1). After removing the unbound primary antibody solution, membranes were washed with TBST, blocked, and incubated with peroxidase conjugated anti-mouse, or anti-rabbit secondary antibodies (Jackson Immunoresearch, West Grove, PA) at a dilution of 1:10,000 to 50,000, and the immunoreactivite signals were visualized with Luminata Crescendo HRP substrate (Millipore Sigma, Burlington, MA). Western blot signal intensities were quantified by ImageJ analyses.
2.7. Preparation cDNA and RT-qPCR analyses
COCs from wildtype oocytes were treated with 1nM KP-10 for 30 min. Cumulus cells were removed by repeated pipetting, oocytes were denuded, and total RNA was extracted from the control and KP-10 treated oocytes using TRI Reagents (Sigma-Aldrich). 1000 ng of total RNA from each sample was used for the preparation of cDNAs using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA). RT-qPCR amplification of cDNAs was carried out in a 10μl reaction mixture containing Applied Biosystems Power SYBR Green PCR Master Mix (Thermo Fisher Scientific). Amplification and fluorescence detection of RT-qPCR were carried out on Applied Biosystems Quant Studio Flex 7 Real Time PCR System (Thermo Fisher Scientific). The ΔΔCT method was used for relative quantification of target mRNA expression level normalized to Rn18s (18S rRNA). A list of qPCR primers is shown in Supplementary Table 2.
2.8. Statistical analyses
Ovulation counting was performed on 6 individual rats of the same genotype at each time point. Each RNA-sequencing library was prepared using pooled RNA samples form three individual wildtype or Erβnull rats and each group of RNA-sequencing consisted of three libraries. Gene expression analyses were performed on at least 6 individual rats. The experimental results are expressed as mean ± SE. Statistical comparisons between two means were determined with Student’s t-test while comparisons among multiple means were evaluated with ANOVA and the significance of mean differences was determined by Duncan post hoc test, with P ≤ 0.05 considered a significant level of difference. All statistical calculations were done with SPSS 22 (IBM, Armonk, NY).
3. Results
3.1. ERβ is essential for oocyte maturation
8–12 wk old adult Erβnull rats failed to ovulate during a natural estrous cycle (Fig. 1A). Administration of exogenous gonadotropins (Fig. 1B) also failed to induce ovulation in 4-wk-old Erβnull rats (Fig. 1C). We observed that Erβnull oocytes isolated from the antral follicles prior to gonadotropin stimulation appeared similar to those isolated from wildtype follicles (Fig. 1D and E). However, gonadotropin treatment resulted in an abnormal maturation of the Erβnull oocytes (Fig. 1F–K). Development of abnormal and fragmented nuclei in Erβnull oocytes became more evident after hCG treatment (Fig. 1H–K).
3.2. Kiss1 is expressed in GCs and Kiss1r in oocytes
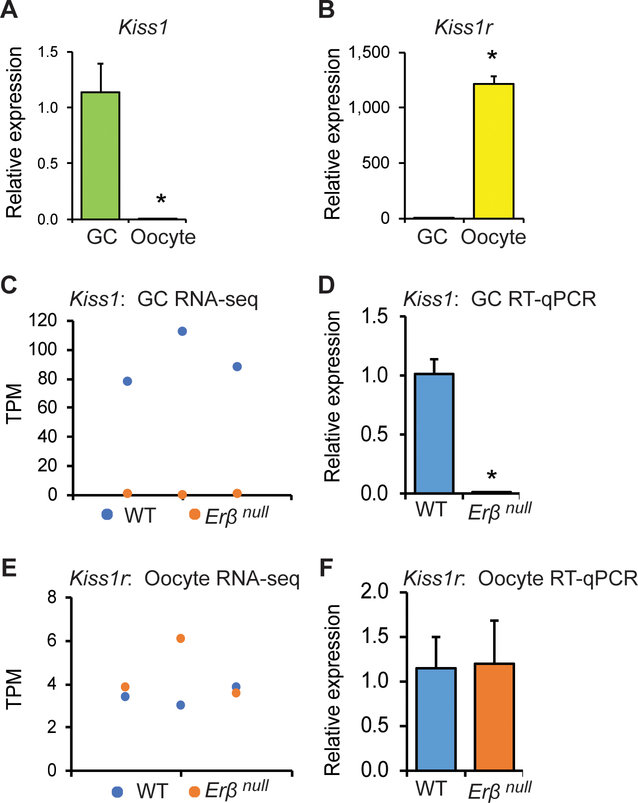
We observed a prominent expression of Kiss1 in gonadotropin treated GCs (Fig. 2A) while Kiss1r in oocytes (Fig. 2B). RNA-sequencing revealed a high level of Kiss1 transcripts in wildtype GCs, but the expression was severely reduced or absent in Erβnull GCs (Fig. 2C). These findings were validated by qRT-PCR analyses (Fig. 2D). In contrast to Kiss1, Kiss1r expression did not show any significant difference between the wildtype or Erβnull oocytes (Fig. 2E and F).
Fig. 2. Kiss1 and Kiss1r expression in rat ovary.
Oocytes and granulosa cells (GCs) were isolated from the ovaries collected 4h after hCG administration to PMSG treated 4-wk-old rats. RT-qPCR analyses showed that expression of Kiss1 was remarkably higher in (GCs) (A), whereas Kiss1r in the oocytes (B). RNA sequencing analyses showed a significantly higher expression of Kiss1 in wildtype (WT) GCs compared to Erβnull GCs (C), but a similar level of Kiss1r expression in both WT and Erβnull oocytes (E). RT-qPCR data from GCs and oocytes further confirmed the results of RNA-sequencing (D and F). RNA-sequencing data are presented in triplicates. RT-qPCR data are represented as mean ± SE. n = 6. *P ≤ 0.05.
3.3. KISS1 treatment induces in vitro maturation of oocytes
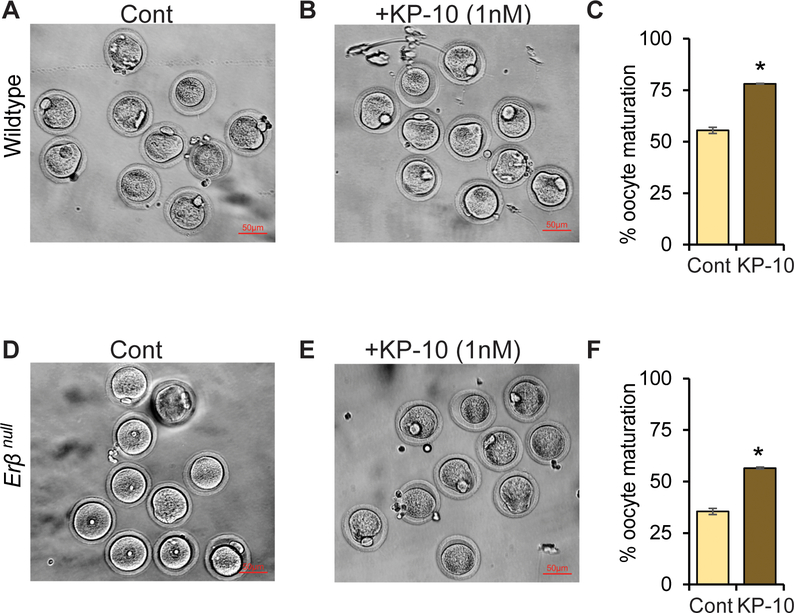
We observed that Erβnull oocytes exhibit defects in in vitro maturation. While approximately 55% of wildtype oocytes mature to MII stage (Fig. 3A), less than 35% of Erβnull oocytes undergo in vitro maturation (Fig. 3D) when intact COCs were used. Remarkably, treatment with rat kisspeptin 10 (KP-10) increased the rate of oocyte maturation in both wildtype (Fig. 3B and C) and Erβnull oocytes (Fig. 3E and F) when intact COCs were used. However, both wildtype and Erβnull denuded oocytes showed a very low efficiency of in vitro maturation with or without KP-10 treatment (Supplementary Fig. 1).
Fig. 3. Role of KISS1 in oocyte maturation.
48h after PMSG treatment, cumulus oocyte complex (COCs) were isolated from 4-wk-old wildtype and Erβnull rats and cultured in BO-IVM in presence or absence of 1nM of rat kisspeptin 10 (KP-10) for 20h. Approximately 55% of wildtype (A and C) and only 35% of Erβnull oocytes achieved meiotic maturation (MII) in BO-IVM culture (control) (D and F). Remarkably, addition of KP-10 to BO-IVM increased the in vitro maturation to 78% of wildtype (B and C) and 56% of Erβnull oocytes (E and F). Each treatment group contained at least 25 oocytes at each time point and repeated three times. Cont, Control.
3.4. KISS1 signaling in rat oocytes
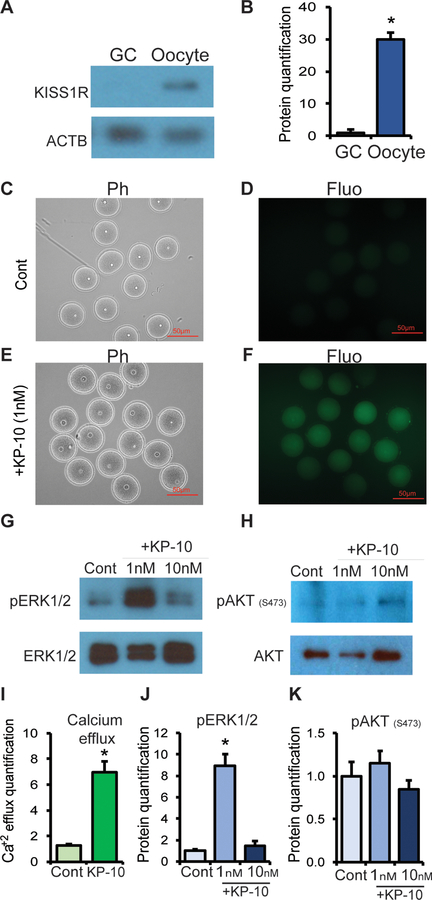
Western blot analyses detected the expression of KISS1R in oocytes but not in GCs (Fig. 4A). Treatment with 1nM KP-10 induced a prominent intracellular Ca2+ release in wildtype (Fig. 4C–F, I) as well as Erβnull oocytes (Supplementary Fig. 2). KP-10 treatment also activated the ERK1/2 in oocytes (Fig. 4G, J), but not the AKT (Fig. 4H, K). While 1nM KP-10 induced marked activation of ERK1/2, an increased concentration failed to show such an effect (Fig. 4G, J).
Fig. 4. Role of KISS1 in inducing calcium efflux and ERK signaling.
The expression of KISS1R was significantly higher in oocytes compared to granulosa cells (GCs) as shown by western blot analysis (A, B). Oocytes from PMSG treated rats were examined for calcium efflux using a Fluo-3 assay kit. Calcium efflux increased after the treatment with kisspeptin 10 (KP-10) (F, I) compared to control (D). 1nM of KP-10 also markedly elevated ERK1/2 phosphorylation in oocytes (G, J) but not AKT (H, K). Ph, Phase contrast; Fluo, Fluorescence microscopy; Cont, Control. Calcium efflux from 15 oocytes and Western blot data from three different samples were quantified by ImageJ analyses. Data represent the mean ± SE. *P < 0.05, n ≥ 3.
3.5. KP-10 treatment induces genes involved in oocyte maturation
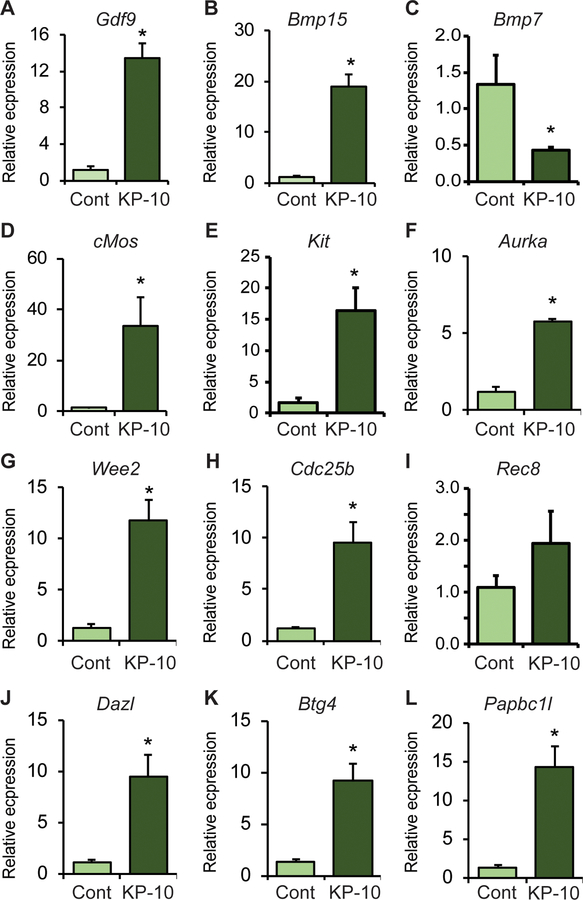
Expression of genes known to be involved in regulating oocyte maturation were assessed by RT-qPCR analyses (Fig. 5). KP-10 treatment did not result in any morphological changes in the oocytes and the germinal vesicles remained intact (Supplementary Fig. 3). KP-10 treatment upregulated the expression of Gdf9 and Bmp15, (Fig. 5 A and B), whereas it downregulated the expression of Bmp7 (Fig. 5 C). Expression of genes involved in protein kinase activation required for oocyte maturation including cMos, Kit and Aurka (Fig. 5 D–F) were also increased by KP-10 stimulation of oocytes. Similarly, KP-10 treatment increased the expression of genes involved in meiosis including Wee2, Cdc25b and Rec8 (Fig. 5 G–I). RNA binding proteins like Dazl, Btg4 and Papbc1l, which are known to induce mRNA stability and selective activation of genes were also upregulated with KP-10 treatment (Fig. 5 J–L). KP-10 induced the upregulation of Bmp15, and cMos in Erβnull oocytes was lower than that of wildtype oocytes (Supplementary Fig. 4 B, D) but it was higher in case of Kit expression (Supplementary Fig. 4 E). Remarkably, the basal level of all KP-10 induced genes (Fig. 5) in Erβnull oocytes were significantly higher prior to kisspeptin treatment (Supplementary Fig. 4 A–L) and KP-10 had only a minimal effect on the remaining genes (Supplementary Fig. 4A, F–L). While the expression of Bmp7 was downregulated in wildtype oocytes, it was upregulated in Erβnull oocytes (Supplementary Fig. 4 C).
Fig. 5. KP-10 treatment regulated genes involved in inducing oocyte maturation.
48h after PMSG treatment, cumulus oocyte complex (COCs) were isolated from 4-wk-old wildtype female rats and treated with 1nM of rat kisspeptin 10 (KP-10) for 30 min. KP-10 treatment upregulated the mRNA levels of Gdf9, Bmp15, but downregulated Bmp7 (A-C). It also upregulated the mRNA levels of protein kinases cMos, Kit, and Aurka (D-F), as well as Wee2 and Cdc25b (G and H) important for meiotic maturation. Moreover, KP-10 treatment upregulated the mRNA levels of RNA binding proteins Dazl, Btg4, and Papbc1l (J-L) required for selective mRNA stability during oocyte maturation. RT-qPCR data represent the mean ± SE. *P < 0.05, n = 6.
4. Discussion
We have studied the role of ovarian derived KISS1 using an Erβnull mutant rat model that fails to induce Kiss1 gene expression in GCs in response to gonadotropins (Chakravarthi et al., 2020; Chakravarthi et al., 2018; Khristi et al., 2018) and suffer from defective oocyte maturation. We observed abnormal fragmentations of Erβnull oocytes matured in vivo following gonadotropin administration (Fig. 1G, I, K). Nuclear fragmentation increased after the administration of hCG, which coincides with GV breakdown and progression of meiosis I. Lack of ERβ or dysregulation of ERβ regulated factors might lead to an aberrant LHCGR signaling, which causes such oocyte fragmentation. Kisspeptin may be an important ERβ-regulated factor related to this phenotype.
Intraovarian Kiss1 gene expression is induced by the preovulatory gonadotropin surge that initiates the final steps of follicle development and oocyte maturation (Castellano et al., 2006). We also detected a dramatic upregulation of Kiss1 gene expression in GCs following the administration of hCG to PMSG primed rats (Chakravarthi et al., 2020; Chakravarthi et al., 2018; Khristi et al., 2018). The expression pattern of Kiss1 and Kiss1r (Chakravarthi et al., 2018) suggest that GC-derived KISS1 may act on the KISS1R in oocytes to regulate oocyte maturation (Fig. 6). An absence of Kiss1 gene induction in Erβnull GCs may be linked to the defective oocyte maturation in Erβnull rats in response to gonadotropin treatment.
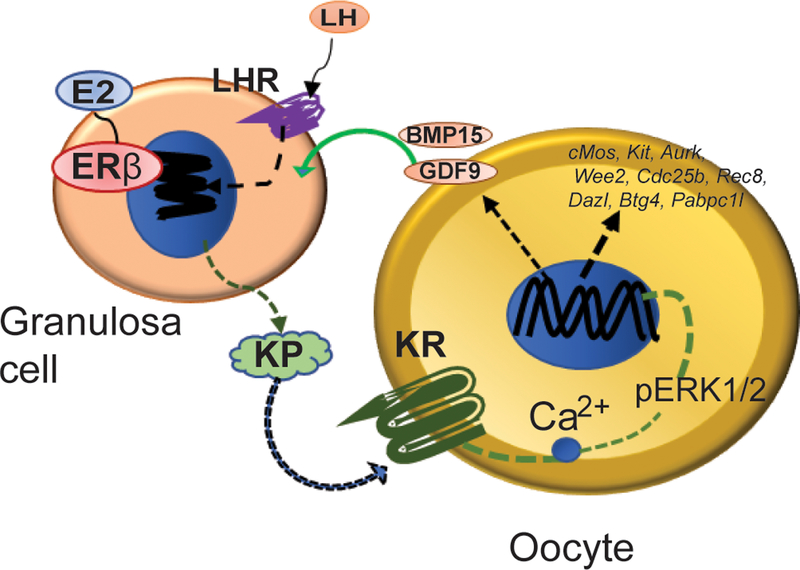
Fig. 6. Intraovarian role of ovarian derived KISS1.
Based on our findings, we conclude that the preovulatory LH surge induces the expression of KISS1 (KP) by granulosa cells (GCs), which is ERβ-dependent. GC-derived KP acts on the KISS1R (KR) expressed in oocytes to increase Ca2+ release, ERK phosphorylation, and modulation of genes, which are important for inducing oocyte maturation.
We observed that treatment of oocytes with a low dose (1nM) of KP-10 improved in vitro maturation of rat oocytes. Previous studies on pig (Saadeldin et al., 2012) and sheep (Byri et al., 2017) oocytes also support our findings. Although Erβnull oocytes showed a reduced rate of in vitro maturation, both of wildtype and Erβnull oocytes responded to rat KP-10 treatment showing a similar improvement. This can be explained by similar levels of Kiss1r expression in wildtype and Erβnull oocytes (Fig. 2E, F) and we would assert normal intracellular signaling induced by KP-10.
KISS1R protein is expressed in rat oocytes and responds to KISS1 treatment. Intracellular Ca2+ release immediately after KP-10 treatment further verifies the presence of KISS1R and indicates specific KISS1/KISS1R signaling. KISS1R is a G-protein coupled receptor that signals through intracellular Ca2+ release. KISS1R is a seven-transmembrane domain, Gq/11-coupled receptor. Upon binding of kisspeptin, the intracellular portion of KISS1R phosphorylates Gq/11. The α-subunit of Gq/11 activates PLC, which cleaves PIP2 into IP3 and DAG. IP3 promotes intracellular Ca2+ release from the endoplasmic reticulum, while DAG activates a signaling cascade by phosphorylating PKC. PKC activation induces the phosphorylation of MAP kinases, such as ERK1/2 and p38 (Hu et al., 2017; Wahab et al., 2016). Activation of MAPK kinase ERK1/2 following kisspeptin treatment has been reported with KISS1/KISS1R signaling in hypothalamic neurons (d’Anglemont de Tassigny and Colledge, 2010) and we have confirmed that this signaling pathway is maintained in oocytes. Conversely, we did not observe activation of AKT by KP-10 in oocytes, but this is a typical response to KISS1/KISS1R signaling in the hypothalamic neurons (d’Anglemont de Tassigny and Colledge, 2010). In oocytes, Ca2+ stored in the endoplasmic reticulum and generates a Ca2+ efflux that is important for signaling and inducing oocyte maturation (Kline, 2000; Stricker et al., 1998; Tosti, 2006; Wang and Machaty, 2013). Ca2+ efflux has also been reported to induce RAF/MEK/ERK pathway through activation of RAS (Atherfold et al., 1999; Li et al., 2005). Furthermore, ERK1/2 activation has also been found essential for oocyte maturation (Choi et al., 1996). We observed that KP-10 induced Ca2+ efflux in oocytes, which resulted in an upregulation of ERK1/2 activation. Thus KP-10 induction of both Ca2+ efflux and ERK1/2 activation may be responsible for the enhanced oocyte maturation that was observed in the current study.
To elucidate the mechanisms downstream of Ca2+ signaling and ERK1/2 activation, we investigated transcript levels of genes that play important roles in oocyte maturation. We observed that KP-10 treatment upregulated the expression of Gdf9, Bmp15, cMos, Kit, Aurka, Wee2, Cdc25b, Dazl, Btg4, and Papbc1l. BMP15 and GDF9 are known to promote cumulus expansion and regulate oocyte maturation (Chang et al., 2016; McNatty et al., 2005a; McNatty et al., 2005b; Persani et al., 2014). ERK/MAPK pathway has been shown to induce the expression of Bmp15 and Gdf9 which are essential for growth and differentiation of GCs during oocyte maturation (Mottershead et al., 2012; Reader et al., 2011). KP-10 treatment reduced the expression of BMP7 which has been shown to inhibit oocyte maturation (Yang et al., 2018). cMOS has been reported to be involved in the spindle formation, activation of maturation promoting factor, and oocyte maturation (Araki et al., 1996; Chang et al., 2016; Choi et al., 1996; Hashiba et al., 2001). KIT/KITL signaling also enhances the oocyte maturation in in vitro by overcoming the inhibitory effect of NPPC signaling (Medvedev et al., 2011; Ye et al., 2009). AURKA is a serine /threonine kinase that accumulates on microtubule organizing centers and is involved in the resumption of meiosis (Saskova et al., 2008). WEE2 is involved in the inhibitory phosphorylation of CDC2 that drives the exit from metaphase II (MII), promoting oocyte maturation (Oh et al., 2011). CDC25 is a dual specificity phosphatase that activates cyclin dependent kinases and regulates meiotic maturation of oocytes (Lincoln et al., 2002). Dazl encodes an RNA binding protein that plays an important role in spindle assembly, MI-MII transition and oocyte maturation (Chen et al., 2011). PABPC, a poly A binding protein that increases stability of selective mRNAs, promotes mRNA translation and improves oocyte maturation (Lowther and Mehlmann, 2015). In addition, BTG4 is involved in selective degradation of oocyte mRNA that is required for maternal-zygotic transition (Yu et al., 2016). We speculate that these KP-10 induced changes in gene expression play important role in promoting oocyte maturation.
We also expected that the mRNA transcripts involved in oocyte maturation would be lower in Erβnull oocytes compared to wildtype oocytes prior to kisspeptin treatment and KP-10 would rescue these reduced levels. However, we did not observe that (Supplemental Figure 4). The reason for that was not lack of gene induction by KP-10, rather it was premature (inappropriate) induction with PMSG in absence of ERβ. We speculate that lack ERβ resulted in an abnormal response to PMSG treatment of Erβnull oocytes, and they failed to express Kiss1 in response to hCG treatment. But the PMSG treatment of Erβnull oocytes also led to abnormal/ inappropriate upregulation of oocyte specific genes and KP-10 treatment failed to upregulate the genes further. An explanation can be requirement of ERβ in addition to kisspeptin in the upregulation of oocyte specific genes for maturation. The mechanism can also be impacted by whether the genes are really induced by increased expression of new mRNA or relative degradation of other mRNAs, which are not required for oocyte maturation.
During the final stage of oocyte maturation, it is transcriptionally silent. However, we examined the expression of genes in antral follicles 48h after PMSG injection followed by 30 min of kisspeptin treatment, when GV breakdown does not occur (Supplementary Figure 3). During this early phase of oocyte maturation, still there can be some changes in the mRNA expression as reported (Clift and Schuh, 2013). Alternatively, this might be contributed (at least in part) by selective degradation of mRNAs, which are unimportant for oocyte maturation that makes the relative values of important mRNAs like Gdf9 and Bmp15 higher than that of prior to kisspeptin treatment.
It is well established that KISS1 regulation of oocyte maturation and induction of ovulation is mediated indirectly through gonadotropin secretion from the hypothalamic-pituitary (H-P) axis. Our findings indicate that the role for KISS1 in regulating female fertility can be explained beyond the HP-axis to intrafollicular expression of KISS1 and signaling, which plays an important role in regulating oocyte maturation.
Supplementary Material
Acknowledgements:
This study was supported in part by the grant supports from KUMC SOM, COBRE and K-INBRE.
Grants support:
This study was supported in part by the grant supports from KUMC SOM, COBRE and K-INBRE.
Footnotes
Disclosure: The authors do not have any conflict of interest.
6. References
- Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, and Sato E. 1996. Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biology of reproduction. 55:1315–1324. [DOI] [PubMed] [Google Scholar]
- Asadi M, Ghafouri-Fard S, Zare-Abdollahi D, Ebrahim-Habibi A, and Matin N. 2013. Estrogen receptor mutation in a girl with primary amenorrhea. Clinical genetics. 83:497–498. [DOI] [PubMed] [Google Scholar]
- Atherfold PA, Norris MS, Robinson PJ, Gelfand EW, and Franklin RA. 1999. Calcium-induced ERK activation in human T lymphocytes. Molecular immunology. 36:543–549. [DOI] [PubMed] [Google Scholar]
- Byers M, Kuiper GG, Gustafsson JA, and Park-Sarge OK. 1997. Estrogen receptor-beta mRNA expression in rat ovary: down-regulation by gonadotropins. Molecular endocrinology (Baltimore, Md.). 11:172–182. [DOI] [PubMed] [Google Scholar]
- Byri P, Gangineni A, Reddy KR, and Raghavender KBP. 2017. Effect of kisspeptin on in vitro maturation of sheep oocytes. Veterinary world. 10:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, Dieguez C, Aguilar E, Sanchez-Criado JE, Pellicer A, Pinilla L, Gaytan F, and Tena-Sempere M. 2006. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology. 147:4852–4862. [DOI] [PubMed] [Google Scholar]
- Chakravarthi VP, Ghosh S, Dai E, Pathak D, and Rumi MAK. 2020. Transcriptome datasets of ESR2-regulated genes in rat granulosa cells during gonadotropin-induced follicle maturation. Data in brief. 30:105405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthi VP, Ghosh S, Roy R, Dai E, Pathak D, and Rumi MAK. 2019. Transcriptome datasets of gonadotropin-induced ESR2-regulated genes in rat oocytes. Data in brief. 27:104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthi VP, Khristi V, Ghosh S, Yerrathota S, Dai E, Roby KF, Wolfe MW, and Rumi MAK. 2018. ESR2 Is Essential for Gonadotropin-Induced Kiss1 Expression in Granulosa Cells. Endocrinology. 159:3860–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HM, Qiao J, and Leung PC. 2016. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Human reproduction update. 23:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Melton C, Suh N, Oh JS, Horner K, Xie F, Sette C, Blelloch R, and Conti M. 2011. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes & development. 25:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, and Vande Woude GF. 1996. The Mos/mitogen-activated protein kinase (MAPK) pathway regulates the size and degradation of the first polar body in maturing mouse oocytes. Proceedings of the National Academy of Sciences of the United States of America. 93:7032–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift D, and Schuh M. 2013. Restarting life: fertilization and the transition from meiosis to mitosis. Nat Rev Mol Cell Biol. 14:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, and Korach KS. 2005. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 146:3247–3262. [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Sanford R, Nyska A, Nilson JH, and Korach KS. 2004. Formation of cystic ovarian follicles associated with elevated luteinizing hormone requires estrogen receptor-beta. Endocrinology. 145:4693–4702. [DOI] [PubMed] [Google Scholar]
- Craig J, Orisaka M, Wang H, Orisaka S, Thompson W, Zhu C, Kotsuji F, and Tsang BK. 2007. Gonadotropin and intra-ovarian signals regulating follicle development and atresia: the delicate balance between life and death. Frontiers in bioscience : a journal and virtual library. 12:3628–3639. [DOI] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, and Colledge WH. 2010. The role of kisspeptin signaling in reproduction. Physiology (Bethesda, Md.). 25:207–217. [DOI] [PubMed] [Google Scholar]
- Emori C, and Sugiura K. 2014. Role of oocyte-derived paracrine factors in follicular development. Animal science journal = Nihon chikusan Gakkaiho. 85:627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan F, Gaytan M, Castellano JM, Romero M, Roa J, Aparicio B, Garrido N, Sanchez-Criado JE, Millar RP, Pellicer A, Fraser HM, and Tena-Sempere M. 2009. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. American journal of physiology. Endocrinology and metabolism. 296:E520–531. [DOI] [PubMed] [Google Scholar]
- Hashiba Y, Asada Y, Heikinheimo O, Lanzendorf SE, and Mizutani S. 2001. Microinjection of antisense c-mos oligonucleotides prevents the progression of meiosis in human and hamster oocytes. Fertility and sterility. 76:143–147. [DOI] [PubMed] [Google Scholar]
- Hu KL, Zhao H, Chang HM, Yu Y, and Qiao J. 2017. Kisspeptin/Kisspeptin Receptor System in the Ovary. Frontiers in endocrinology. 8:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed Z, Qamar U, and Sathyapalan T. 2015. The role of kisspeptin signalling in the hypothalamic-pituitary-gonadal axis--current perspective. Endokrynologia Polska. 66:534–547. [DOI] [PubMed] [Google Scholar]
- Khristi V, Chakravarthi VP, Singh P, Ghosh S, Pramanik A, Ratri A, Borosha S, Roby KF, Wolfe MW, and Rumi MAK. 2018. ESR2 regulates granulosa cell genes essential for follicle maturation and ovulation. Molecular and cellular endocrinology. 474:214–226. [DOI] [PubMed] [Google Scholar]
- Kline D 2000. Attributes and dynamics of the endoplasmic reticulum in mammalian eggs. Current topics in developmental biology. 50:125–154. [DOI] [PubMed] [Google Scholar]
- Kona SS, Praveen Chakravarthi V., Siva Kumar A.V., Srividya D, Padmaja K, and Rao VH. 2016. Quantitative expression patterns of GDF9 and BMP15 genes in sheep ovarian follicles grown in vivo or cultured in vitro. Theriogenology. 85:315–322. [DOI] [PubMed] [Google Scholar]
- Lang-Muritano M, Sproll P, Wyss S, Kolly A, Hurlimann R, Konrad D, and Biason-Lauber A. 2018. Early-Onset Complete Ovarian Failure and Lack of Puberty in a Woman With Mutated Estrogen Receptor beta (ESR2). The Journal of clinical endocrinology and metabolism. 103:3748–3756. [DOI] [PubMed] [Google Scholar]
- Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma WY, Dong Z, Pike HM, Brown RE, and Reed JC. 2005. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Molecular biology of the cell. 16:4437–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, Tessarollo L, and Donovan PJ. 2002. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nature genetics. 30:446–449. [DOI] [PubMed] [Google Scholar]
- Lowther KM, and Mehlmann LM. 2015. Embryonic Poly(A)-Binding Protein Is Required During Early Stages of Mouse Oocyte Development for Chromatin Organization, Transcriptional Silencing, and Meiotic Competence. Biology of reproduction. 93:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, Western A, Meerasahib MF, Mottershead DG, Groome NP, Ritvos O, and Laitinen MP. 2005a. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function. Reproduction (Cambridge, England). 129:473–480. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, Western A, Meerasahib MF, Mottershead DG, Groome NP, Ritvos O, and Laitinen MP. 2005b. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction (Cambridge, England). 129:481–487. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Wilson T, Galloway SM, Davis GH, Hudson NL, Moeller CL, Cranfield M, Reader KL, Laitinen MP, Groome NP, Sawyer HR, and Ritvos O. 2003. Oocyte-derived growth factors and ovulation rate in sheep. Reproduction (Cambridge, England) Supplement. 61:339–351. [PubMed] [Google Scholar]
- Medvedev S, Pan H, and Schultz RM. 2011. Absence of MSY2 in mouse oocytes perturbs oocyte growth and maturation, RNA stability, and the transcriptome. Biology of reproduction. 85:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monniaux D 2016. Driving folliculogenesis by the oocyte-somatic cell dialog: Lessons from genetic models. Theriogenology. 86:41–53. [DOI] [PubMed] [Google Scholar]
- Moore AM, Coolen LM, Porter DT, Goodman RL, and Lehman MN. 2018. KNDy Cells Revisited. Endocrinology. 159:3219–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottershead DG, Ritter LJ, and Gilchrist RB. 2012. Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15. Molecular human reproduction. 18:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JS, Susor A, and Conti M. 2011. Protein tyrosine kinase Wee1B is essential for metaphase II exit in mouse oocytes. Science (New York, N.Y.). 332:462–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier G, and El-Alfy M. 2000. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. The Journal of clinical endocrinology and metabolism. 85:4835–4840. [DOI] [PubMed] [Google Scholar]
- Persani L, Rossetti R, Di Pasquale E, Cacciatore C, and Fabre S. 2014. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Human reproduction update. 20:869–883. [DOI] [PubMed] [Google Scholar]
- Praveen Chakravarthi V., Kona SS, Siva Kumar AV, Bhaskar M, and Rao VH. 2015. Quantitative expression of antiapoptotic and proapoptotic genes in sheep ovarian follicles grown in vivo or cultured in vitro. Theriogenology. 83:590–595. [DOI] [PubMed] [Google Scholar]
- Reader KL, Heath DA, Lun S, McIntosh CJ, Western AH, Littlejohn RP, McNatty KP, and Juengel JL. 2011. Signalling pathways involved in the cooperative effects of ovine and murine GDF9+BMP15-stimulated thymidine uptake by rat granulosa cells. Reproduction (Cambridge, England). 142:123–131. [DOI] [PubMed] [Google Scholar]
- Rumi MA, Dhakal P, Kubota K, Chakraborty D, Lei T, Larson MA, Wolfe MW, Roby KF, Vivian JL, and Soares MJ. 2014. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 155:1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumi MAK, Singh P, Roby KF, Zhao X, Iqbal K, Ratri A, Lei T, Cui W, Borosha S, Dhakal P, Kubota K, Chakraborty D, Vivian JL, Wolfe MW, and Soares MJ. 2017. Defining the Role of Estrogen Receptor beta in the Regulation of Female Fertility. Endocrinology. 158:2330–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DL, and Robker RL. 2007. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Human reproduction update. 13:289–312. [DOI] [PubMed] [Google Scholar]
- Saadeldin IM, Koo OJ, Kang JT, Kwon DK, Park SJ, Kim SJ, Moon JH, Oh HJ, Jang G, and Lee BC. 2012. Paradoxical effects of kisspeptin: it enhances oocyte in vitro maturation but has an adverse impact on hatched blastocysts during in vitro culture. Reproduction, fertility, and development. 24:656–668. [DOI] [PubMed] [Google Scholar]
- Saskova A, Solc P, Baran V, Kubelka M, Schultz RM, and Motlik J. 2008. Aurora kinase A controls meiosis I progression in mouse oocytes. Cell cycle (Georgetown, Tex.). 7:2368–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahed A, and Young KA. 2009. Differential ovarian expression of KiSS-1 and GPR-54 during the estrous cycle and photoperiod induced recrudescence in Siberian hamsters (Phodopus sungorus). Molecular reproduction and development. 76:444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Słomczyńska M, Duda M, and Galas J. 2001. Estrogen receptor alpha and beta expression in the porcine ovary. Folia histochemica et cytobiologica. 39:137–138. [PubMed] [Google Scholar]
- Stricker SA, Silva R, and Smythe T. 1998. Calcium and endoplasmic reticulum dynamics during oocyte maturation and fertilization in the marine worm Cerebratulus lacteus. Developmental biology. 203:305–322. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Kuwahara A, Tachibana A, Yano Y, Yano K, Yamamoto Y, Yamasaki M, Iwasa T, Hinokio K, Matsuzaki T, and Irahara M. 2017. Intra-follicular kisspeptin levels are related to oocyte maturation and gonadal hormones in patients who are undergoing assisted reproductive technology. Reproductive medicine and biology. 16:380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terao Y, Kumano S, Takatsu Y, Hattori M, Nishimura A, Ohtaki T, and Shintani Y. 2004. Expression of KiSS-1, a metastasis suppressor gene, in trophoblast giant cells of the rat placenta. Biochimica et biophysica acta. 1678:102–110. [DOI] [PubMed] [Google Scholar]
- Tosti E 2006. Calcium ion currents mediating oocyte maturation events. Reproductive biology and endocrinology : RB&E. 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab F, Atika B, Shahab M, and Behr R. 2016. Kisspeptin signalling in the physiology and pathophysiology of the urogenital system. Nature reviews. Urology. 13:21–32. [DOI] [PubMed] [Google Scholar]
- Wang C, and Machaty Z. 2013. Calcium influx in mammalian eggs. Reproduction (Cambridge, England). 145:R97–r105. [DOI] [PubMed] [Google Scholar]
- Wigglesworth K, Lee KB, O’Brien MJ, Peng J, Matzuk MM, and Eppig JJ. 2013. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proceedings of the National Academy of Sciences of the United States of America. 110:E3723–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shu L, Jiang Q, Huang H, and Ye H. 2018. Does the bone morphogenetic protein 7 inhibit oocyte maturation by autocrine/paracrine in mud crab? General and comparative endocrinology. 266:119–125. [DOI] [PubMed] [Google Scholar]
- Ye Y, Kawamura K, Sasaki M, Kawamura N, Groenen P, Gelpke MD, Rauch R, Hsueh AJ, and Tanaka T. 2009. Kit ligand promotes first polar body extrusion of mouse preovulatory oocytes. Reproductive biology and endocrinology : RB&E. 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Ji SY, Sha QQ, Dang Y, Zhou JJ, Zhang YL, Liu Y, Wang ZW, Hu B, Sun QY, Sun SC, Tang F, and Fan HY. 2016. BTG4 is a meiotic cell cycle-coupled maternal-zygotic-transition licensing factor in oocytes. Nature structural & molecular biology. 23:387–394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.