Abstract
Risk for smoking increases in a summative manner corresponding to the number of co-occurring vulnerabilities present (cumulative vulnerability). We examined whether cumulative vulnerabilities moderate response to reduced nicotine content cigarettes in a secondary analysis of results from 775 participants in three 12-week randomized clinical trials examining research cigarettes varying in nicotine content (0.4, 2.4, 15.8mg nicotine/g tobacco). Participants were categorized as having 0–1, 2–3, or ≥ 4 cumulative vulnerabilities. Vulnerabilities included: rural residence, current substance use disorder, current affective disorder, low educational attainment, poverty, unemployment, physical disability. The primary outcome was total cigarettes per day (CPD) during Week 12; secondary outcomes included CPD across weeks, toxin exposure, dependence severity, craving/withdrawal (17 dependent measures). Results were analyzed using repeated measures analysis of covariance and growth-curve modeling. Total CPD during Week 12 increased as cumulative-vulnerability increased (P=0.004), and decreased as nicotine content decreased (P<0.001), with no significant interaction of cumulative vulnerability and dose (P=0.67). Effects on other outcomes generally followed that same pattern. The only exception across the other outcomes was on Questionnaire-on-Smoking-Urges Factor-2 ratings for usual-brand cigarettes where cumulative vulnerability, dose, and time interacted (P=0.007), with craving at the 0.4 and 2.4 mg/g doses decreasing over time, but inconsistently across vulnerability categories. Overall, we saw little evidence that cumulative vulnerabilities moderate response to reduced nicotine content cigarettes suggesting that a policy reducing nicotine content in cigarettes to minimally addictive levels could benefit even highly vulnerable smokers including those residing in rural or other regions with overrepresentation of co-occurring vulnerabilities.
Keywords: cumulative vulnerability, vulnerable populations, cigarette smoking, reduced nicotine content cigarettes, toxin exposure, nicotine dependence
Rural America has been disproportionately impacted by chronic health conditions and premature death (Case & Deaton, 2015; Woolf & Schoomaker, 2019). There is little question that greater prevalence of cigarette smoking in rural compared to more metropolitan communities contributes to these disparities (Cepeda-Benito et al., 2018; Doogan et al., 2017; Nighbor et al., 2018; Roberts et al., 2016). While a complete explanation for these disparities remains to be provided, the overrepresentation of socioeconomic (e.g., disability, unemployment, lower educational attainment) and psychiatric (e.g., substance use disorders) vulnerabilities in rural communities is well recognized to be a contributor to greater smoking prevalence (Doogan et al., 2017). These vulnerabilities tend to co-occur, such that an appreciable portion of individuals facing one type of disadvantage (e.g., poverty) are challenged by an additional (e.g., poverty + low education) or multiple others (e.g., poverty + low education + affective disorder) (Gaalema et al., 2018; Higgins et al., 2016). Co-occurring vulnerabilities have been demonstrated to predict increasing risk for current smoking in an orderly, summative manner in studies of the U.S. population (Gaalema et al., 2018; Higgins et al., 2016), with those with greater cumulative vulnerabilities being at increased risk for smoking initiation, heavy smoking, greater dependence severity, and difficulties quitting (Levanthal et al., 2018). Policies that can successfully reduce cigarette smoking in populations facing multiple co-occurring vulnerabilities could substantially reduce the overall tobacco-attributable public-health burden and make great strides toward reducing disparities in life expectancy.
If policies initiated to reduce smoking prevalence are to benefit rural residents and other populations residing in regions with concentrated socioeconomic disadvantage (American Psychological Association, 2007), it is important that they impact those with multiple co-occurring vulnerabilities to smoking. One such regulatory policy currently under consideration by the U.S. Food and Drug Administration (FDA) would cap the maximal nicotine content in cigarettes at a minimally addictive level in order to reduce smoking prevalence by making it (a) less likely that adolescents or others who experiment with cigarettes will transition to chronic use and (b) easier for current smokers to quit should they choose to do so (Gottlieb & Zeller, 2017). Studies in the general population of smokers (Donny et al., 2015; Hatsukami et al., 2018) and among those from vulnerable populations with psychiatric conditions or socioeconomic disadvantage (Higgins et al. 2017; Higgins et al., 2020; Tidey et al., 2017; Tidey et al., 2019) demonstrate that reducing nicotine content in cigarettes to very low levels decreases the relative-reinforcing effects of smoking, smoking rate, toxin exposure, and nicotine-dependence severity, with minimal evidence of sustained compensatory smoking (i.e., smoking adjustments to sustain desired nicotine blood levels). The overarching aim of the present study is to examine whether a reduced nicotine content policy has potential to reduce smoking in populations with relatively high levels of cumulative vulnerability.
To address that aim, this secondary analysis uses data from three randomized clinical trials conducted with current daily smokers recruited based on meeting criteria for having (1) a current affective disorder, (2) opioid use disorder (OUD), or (3) socioeconomic disadvantage among women of reproductive age (Higgins et al., 2020). Rather than analyzing outcomes by the vulnerabilities for which participants were recruited into these trials, however, we collapsed across trials and categorized participants into three levels of cumulative-vulnerability severity based on the co-occurrence of 0–1, 2–3, or ≥ 4 of the following seven well-established vulnerabilities to cigarette smoking among U.S. adults: (1) rural residence, (2) a comorbid substance use disorder (SUD); (3) a current affective disorder; (4) low educational attainment; (5) poverty, (6) unemployment, and (7) physical disability (Creamer et al., 2019; Doogan, et al., 2017; Fu & Liu, 2019; Garrett et al., 2019; Higgins et al., 2016; Leventhal et al., 2019; Parker & Villanti, 2020; Tidey & Miller, 2015). Hereafter, we refer to these three categories as low, moderate, and high cumulative vulnerability. We focus on four outcome areas in evaluating the effects of reduced nicotine content cigarettes: cigarettes smoked per day (CPD), toxin exposure, dependence severity, and craving and withdrawal severity for a total of 17 dependent variables as several areas include multiple measures.
METHODS
Methods of the parent RCTs have been reported previously (Higgins et al., 2020) and are only briefly described here.
Participants
Participants in these multisite (Brown University, Johns Hopkins University, University of Vermont), RCTs were 775 adult, daily smokers who provided informed consent to participate in one of three parallel trials examining reduced nicotine content cigarettes in smokers from vulnerable populations. Study inclusion criteria common across the three trials were participants had to report daily smoking of ≥ five cigarettes for ≥ 1 year with limited current use of other tobacco products (< 10 days in past month), report no current illicit drug use other than marijuana, no intention to quit smoking within the next 30 days, and provide a breath CO sample > 8 ppm. Inclusion criteria specific to smokers with (a) affective disorders were males and females ages 18–70 years who met Mini-International-Neuropsychiatric-Interview (MINI) (Sheehan et al., 1998); (b) smokers with OUD were males and females ages 18–70 years who were currently receiving opioid-maintenance treatment and stable on their maintenance dose; (c) disadvantaged women of reproductive age were females only, ages 18–44 years, with highest academic degree being ≤ high school.
Procedures
Trial participants were assigned to one of three research cigarettes identical in appearance but varying in nicotine content: 0.4, 2.4 and 15.8 mg of nicotine per g tobacco, averaged across menthol/non-menthol cigarettes which differed slightly in nicotine content (Donny et al., 2015). The 15.8 mg/g dose is representative of nicotine levels in many commercial cigarettes and served as the control condition.
Participants completed a two-visit, one-week baseline. During 1st-baseline visit they received a free supply of usual-brand cigarettes for use during the subsequent week to establish baseline-smoking rate. The supply was 150% of self-reported smoking rate to accommodate increases or missed visits. Participants used an interactive-voice-response (IVR) system daily to report prior-day cigarette consumption, other tobacco/nicotine use, and nicotine-withdrawal symptoms. IVR adherence was compensated at $1.00/call plus $10.00 bonuses for 7-days consecutive calls.
Participants received first supply of study cigarettes at the 2nd-baseline session. They reported to clinic weekly for 12 weeks to return unused study cigarettes and be resupplied with study cigarettes. Participants received twice the number of cigarettes used during baseline to accommodate smoking-rate increases. At 2nd baseline and Week-2, −6, and −12 visits, first-void morning urine specimens, and blood/breath samples were collected to assess nicotine/toxin exposure.
Smoking Vulnerabilities Included in Assessing Cumulative Vulnerabilities
The seven vulnerabilities examined were listed above. Each was defined dichotomously (yes-no): (1) rural residence based on a county-level classification from the Office of Management and Budget called the Rural Urban Continuum Codes, which classifies respondents as urban if they were from core counties that are part of an urbanized area with a population size > 10,000 or an outlying county with 25% or more of its labor force tied to a core county by commuting flows; respondents not residing in an urban area are classified as rural; (2) meets diagnostic criteria for a current SUD in addition to cigarette smoking (limited to OUD because presence of any other current SUD was an exclusion criterion in the parent study); (3) a current affective disorder as specified by MINI assessment (i.e., presence vs. absence of ≥ 1 current affective disorder); (4) low educational attainment based on participant report of maximal years of education completed (i.e., < 12 years or graduate-equivalence diploma; (5) current poverty based on participant-reported family annual income (i.e., < vs. ≥ U.S. federal poverty cut-point); (6) unemployment based on participant endorsement of “unemployed” from a list of employment-status options (i.e., unemployed vs. other; (7) physically disabled based on participant endorsement of “disabled” from a list of employment-status options (i.e., disabled vs. other).
Outcomes
We examined how study baseline smoking characteristics varied corresponding to the three cumulative-vulnerability categories. Analyses of trial outcomes included mean Total CPD (Study + Non-study CPD), Study CPD, and Non-study CPD. Total CPD during Week 12 was the primary outcome. Secondary outcomes included changes in Total, Study, and Non-study CPD across study weeks, toxin exposure including breath CO, urinary cotinine, urinary NNAL (marker of tobacco-specific n-nitrosamine exposure), nicotine-dependence severity based on Fagerström-Test-for-Nicotine-Dependence (FTND) total scores (minus item 4 on CPD) and Wisconsin-Inventory-of-Smoking-Dependence-Motives (Brief WISDM) total scores and primary- and secondary-dependence motives (PDM, SDM) subscale scores, Questionnaire-on-Smoking-Urges-Brief (QSU-Brief) Factor-1 and Factor-2 subscale scores for study and usual-brand cigarettes, and Minnesota-Tobacco-Withdrawal-Scale (MTWS) total scores and Desire-to-Smoke item. Moderation was inferred based on significant interactions of cumulative-vulnerability and dose (cigarette nicotine content) or cumulative-vulnerability, dose, and time (assessment week). Note that the definition of low educational attainment criterion is lower than the criterion used in the parent trial on disadvantaged women where high-school graduates were eligible. We did so to focus on the educational attainment level with greatest smoking risk in the U.S., which is < 12 years or GED (Substance Abuse and Mental Health Services Administration, 2016).
Data Analysis
Comparisons of baseline characteristics between cumulative-vulnerability categories were conducted using Chi-Square tests for categorical variables and Kruskal-Wallis tests for continuous variables. Analysis of covariance was used to examine the effects of cumulative-vulnerability category and dose on Total CPD during Week 12, adjusting for baseline values. Additional covariates included age, sex, and menthol-cigarette use, and parent-trial vulnerable population. Secondary outcomes were analyzed using linear-mixed models. Outcomes assessed weekly or biweekly used a growth-curve model, assuming an unstructured covariance matrix. Outcomes assessed less frequently were analyzed using repeated measures analysis of variance, assuming compound symmetry. Outcomes not normally distributed were transformed for analysis, with square-root transformation used for urine cotinine and log-10 transformation for NNAL. Initial models included all 2-way interactions of cumulative-vulnerability category, dose, and time, and the 3-way interactions of these factors, which were removed if not significant with alpha at P<.05. Significant effects were followed with pairwise comparisons using Bonferroni correction. Participants not completing the study were excluded in analyses-of-covariance models but included in linear-mixed models, with estimates of missing data produced using the maximum-likelihood method. Statistical analyses were conducted using SAS version 9.4 statistical analysis software (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Distribution of Vulnerabilities Across Cumulative-Vulnerability Categories
There were significant differences in how the three populations recruited for the parent trials were distributed across the cumulative-vulnerability categories (Table 1). Participants from the trials on affective disorders and disadvantaged women were distributed with most in the low (40.34% and 52.84%, respectively), intermediate percentages in the moderate (35.92% and 32.52%), and least in the high (20.86% and 16.04%) cumulative-vulnerability categories, while the distribution ran in the opposite direction among smokers from the trial on OUD, with a majority of participants in the high (63.10%), an intermediate percentage in the moderate (32.55%), and least in the low (6.82%) cumulative-vulnerability categories. Notably, there was representation from each parent trial in each cumulative-vulnerability category.
Table 1.
Participant Characteristics1
| Overall | Cumulative | Vulnerability | Categories | P-value | |
|---|---|---|---|---|---|
| n = 775 | low (n=176) | moderate (n=412) | high (n=187) | ||
| Population | < 0.0001 | ||||
| Affective Disorders | 258 (33.29) | 71 (40.34)a | 148 (35.92)b | 39 (20.86)c | |
| Opioid Use Dependent | 260 (33.55) | 12 (6.82)a | 130 (31.55)b | 118 (63.10)c | |
| Disadvantaged Women | 257 (33.16) | 93 (52.84)a | 134 (32.52)b | 30 (16.04)c | |
| Age (M ± SD) | 35.59 ± 11.05 | 33.19 ± 9.12a | 35.25 ± 11.21a | 38.58 ± 11.73b | < 0.0001 |
| Gender (% Female) | 551 (71.10) | 138 (78.41)a | 296 (71.84)a | 117 (62.57)b | 0.004 |
| Race/Ethnicity | 0.74 | ||||
| Non-Latino White | 630 (82.14) | 148 (84.57)a | 329 (80.84)a | 153 (82.70)a | |
| Non-Latino Black | 68 (8.87) | 11 (6.29)a | 39 (9.58)a | 18 (9.73)a | |
| Latino | 23 (3.00) | 6 (3.43)a | 14 (3.44)a | 3 (1.62)a | |
| Non-Latino Other or >1 race | 46 (6.00) | 10 (5.71)a | 25 (6.14)a | 11 (5.95)a | |
| Education | < 0.0001 | ||||
| 8th Grade or Less/Some High School | 102 (13.16) | 2 (1.14)a | 49 (11.89)b | 51 (27.27)c | |
| High School Graduate/Equivalent/Some college | 570 (73.55) | 132 (75.00)a | 310 (75.24)b | 128 (68.45)c | |
| 2-Year Associate’s Degree | 38 (4.90) | 10 (5.68)a | 24 (5.83)b | 4 (2.14)c | |
| College Graduate/4-Year Degree/Graduate Degree | 65 (8.39) | 32 (18.18)a | 29 (7.04)b | 4 (2.14)c | |
| Marital Status | 0.09 | ||||
| Married | 112 (14.45) | 29 (16.48)a | 66 (16.02)a | 17 (9.09)b | |
| Never married | 461 (59.48) | 107 (60.80)a | 243 (58.98)a | 111 (59.36)b | |
| Divorced or Separated/Widowed | 202 (26.06) | 40 (22.73)a | 103 (25.00)a | 59 (31.55)b | |
| Primary smoker of mentholated cigarettes | 351 (45.29) | 70 (39.77)a | 201 (48.79)b | 80 (42.78)a,b | 0.10 |
| Cigarettes smoked per day (M ± SD) | 17.79 ± 9.18 | 14.74 ± 6.78a | 17.39 ± 8.87b | 21.56 ± 10.49c | < 0.0001 |
| Urine Cotinine Level, ng/ml (M ± SD) | 4929.35 ± 3771.79 | 4329.94 ± 3193.98a | 4958.97 ± 3823.61a,b | 5430.12 ± 4088.13b | 0.03 |
| NMR (% with NMR >= 0.31) | 526 (73.06) | 115 (67.65)a | 279 (73.04)a,b | 132 (78.57)b | 0.07 |
| Breath CO level (M ± SD) | 18.02 ± 9.85 | 16.91 ± 8.98a | 17.69 ± 10.17a | 19.77 ± 9.74b | 0.002 |
| Age started smoking regularly (M ± SD) | 16.14 ± 3.97 | 16.73 ± 4.03a | 16.10 ± 3.63a | 15.66 ± 4.54b | 0.0002 |
| Fagerstrom Test for Cigarette Dependence (M ± SD) | 5.56 ± 2.35 | 4.72 ± 2.19a | 5.51 ± 2.35b | 6.45 ± 2.19c | < 0.0001 |
| Heaviness of Smoking Index (M ± SD) | 3.49 ± 1.54 | 2.96 ± 1.40a | 3.42 ± 1.58b | 4.13 ± 1.36c | < 0.0001 |
| Used Other Tobacco Products, Last 30 days | 117 (17.67) | 24 (14.63)a | 63 (18.16)a | 30 (19.87)a | 0.45 |
| Vulnerabilities | |||||
| Substance use | 260 (33.55) | 12 (6.82)a | 130 (31.55)b | 118 (63.10)c | < 0.0001 |
| Affective disorder | 394 (50.84) | 83 (47.16)a | 203 (49.27)a,b | 108 (57.75)b | 0.09 |
| Low education | 408 (52.65) | 25 (14.20)a | 221 (53.64)b | 162 (86.63)c | < 0.0001 |
| Unemployed | 230 (29.72) | 3 (1.70)a | 119 (28.95)b | 108 (57.75)c | < 0.0001 |
| Disabled | 116 (14.99) | 0 (0.00)a | 57 (13.87)b | 59 (31.55)c | < 0.0001 |
| Poverty | 405 (53.29) | 14 (8.14)a | 215 (53.48)b | 176 (94.62)c | < 0.0001 |
| Rural | 145 (18.71) | 15 (8.52)a | 84 (20.39)b | 46 (24.60)b | 0.0002 |
Unless otherwise indicated, data are expressed as number (percentage)
Measures not sharing a superscript letter differed significantly in post-hoc testing
NOTE: results that do not share superscript letters are significantly different from each other
The seven vulnerabilities that contributed to the cumulative-vulnerability categories were generally distributed in an inverted u-shaped manner with relatively lower percentages in the low and high categories and intermediate percentages in the moderate category (Table 1). The risk factor with the largest percentage of participants assigned to the high cumulative-vulnerability category was physical disability (50.86%), followed by unemployment (46.96%), SUD (45.38%), poverty (43.46), lower educational attainment (39.71%), rural residence (31.72%), and affective disorders (27.41%).
Regarding sociodemographic characteristics not treated as vulnerabilities in the analysis, mean age increased and the percentage of women decreased significantly as number of cumulative vulnerabilities increased, while race/ethnicity and marital status did not vary significantly by cumulative-disadvantage category (Table 1).
Distribution of Smoking Characteristics Across Cumulative-Vulnerability Categories
Baseline mean age of initiating daily smoking decreased significantly while mean CPD, breath CO, urine-cotinine levels, and nicotine-dependence severity all increased significantly corresponding to increasing cumulative vulnerabilities (Table 1). Use of mentholated cigarettes, nicotine-metabolite ratio, and use of tobacco products other than cigarettes did not vary significantly by cumulative-vulnerability category.
Trial Outcomes
CPD
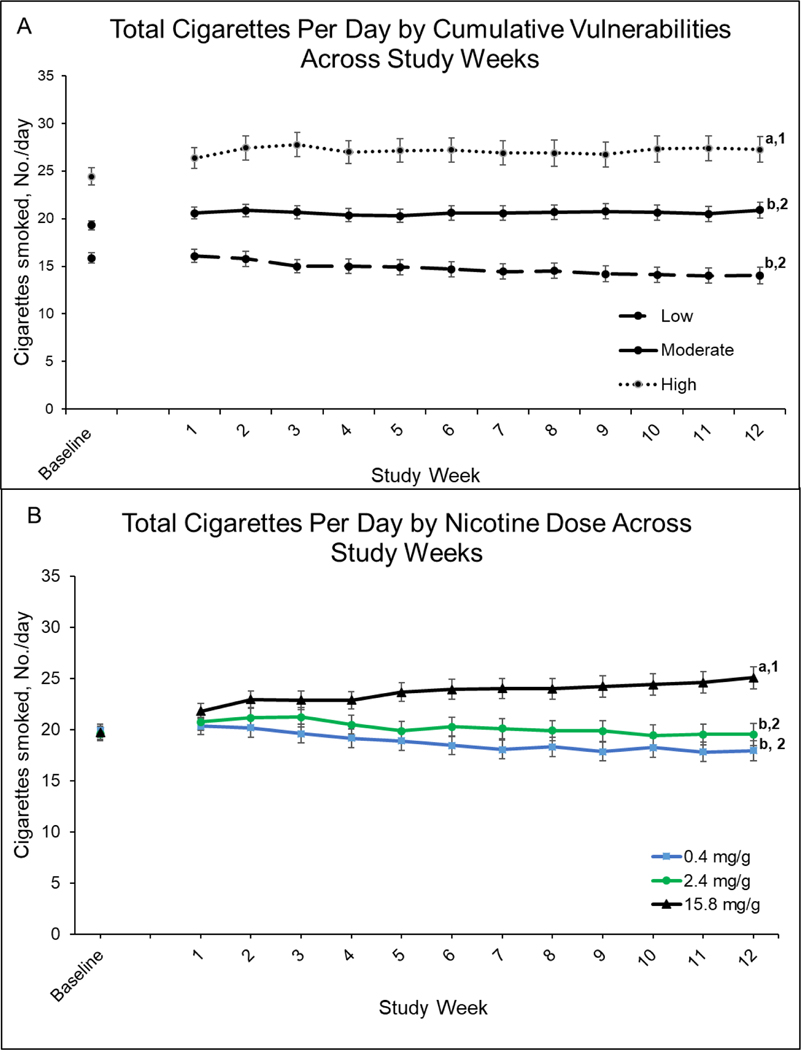
There were significant main effects of cumulative vulnerability (F[2,609]=6.16, P=0.002) and dose (F[2,609]=22.85, P<0.001) on total CPD at Week 12 (Figure 1, upper and lower panels for cumulative vulnerability and dose effects, respectively), but no significant interaction (F[4,609]=0.96, P=0.43). Mean CPD collapsed across doses was lower among those in the low compared to moderate and high cumulative-vulnerability categories (Ps=0.02 and 0.001, respectively), with no significant difference between the moderate and high categories (P=0.36). Smoking rates at the 0.4 and 2.4 mg doses collapsed across cumulative-vulnerability categories were each significantly below those at 15.8 mg/g dose (Ps<0.001) but did not differ from each other (P=0.11) (see Week 12, Figure 1, lower panel).
Figure 1.
Total Cigarettes Per Day by Cumulative Vulnerabilities and Nicotine Dose Across Study Week
Upper panel (A) shows mean number of total cigarettes (Study + Non-study) smoked per day (CPD) for each of the three cumulative-vulnerability categories (low, moderate, high) collapsed across nicotine-content cigarette doses (0.4, 2.4, and 15.8 mg/g) by study week. Data points are arithmetic means ± SEM. Data points not sharing a superscript letter differed significantly in post-hoc testing and in slope of linear trends. Lower panel (B) shows mean number of Total cigarettes (Study + Non-study) smoked per day (CPD) for each of the three nicotine-content-cigarette doses collapsed across cumulative-vulnerabilities categories (low, moderate, high) by study weeks. Data points are arithmetic means ± SEM. Data points not sharing a superscript letter differed in post-hoc testing at Week 12; data points not sharing a superscript number differed in linear slopes over time.
When examining effects on total CPD across the 12-week study period, cumulative vulnerability interacted significantly with time (F[2,7450]=3.17, P=0.04) (see results across repeated assessments in Figure 1, upper panel), but not dose (F[4,753]=0.60, P=0.66) nor dose and time (F[4,7446]=0.81, P=0.52). Collapsed across cigarette doses, there is a significant decreasing linear slope across weeks in the low category (−0.20, 95%CI: −0.34 to 0.05, P<0.001) but not in the moderate (−0.00, 95%CI: −0.10 to 0.09, P=0.97) or high (0.05, 95%CI: −0.09 to 0.18, P=0.52) cumulative-vulnerability categories (Figure 2, upper panel). Effects of cigarette dose interacted significantly with time (F[2,7450]=28.30, P<0.001). Collapsed across vulnerability categories, the negative linear slopes over time seen at the 0.4 (−0.28, 95%CI: −0.39, −0.17, P<0.001) and 2.4 mg/g (−0.12, 95%CI: −0.24 to 0.003, P=0.04) doses differed significantly (Ps<0.001) from the increasing slope seen at the 15.8 mg/g dose (0.30, 95%CI: 0.19 to 0.42, P<0.001). The 0.4 and 2.4 mg/g doses did not differ significantly from each other (P=0.40).
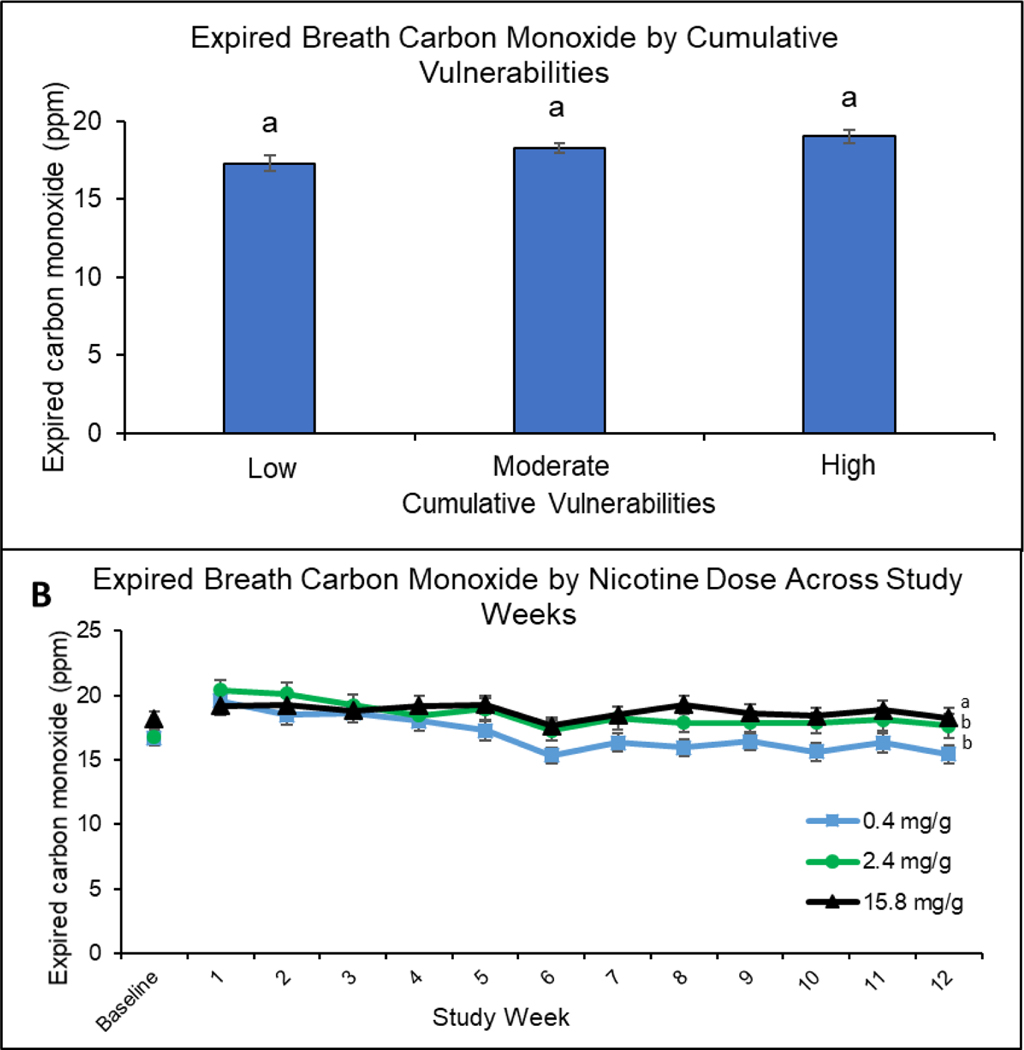
Figure 2.
Expired Breath Carbon Monoxide by Cumulative Vulnerabilities and Nicotine Dose Across Study Week
Upper panel (A) shows mean expired breath carbon monoxide (CO) values for each of the three cumulative vulnerability categories (low, moderate, high) collapsed across study weeks and nicotine-content-cigarette doses. Data points are means + SEM. Data points not sharing a superscript letter differed significantly in post-hoc testing. Lower panel (B) shows expired breath carbon monoxide (CO) values for each of the three nicotine-content-cigarette doses (0.4, 2.4, and 15.8 mg/g) at baseline and across study weeks. Data points are means + SEM. Data points not sharing a superscript letter differed in slope of linear trends across the 12-week study period.
Effects of cumulative vulnerability and dose on study and Non-study CPD were consistent with those reported above for Total CPD (not shown). There were significant main effects of cumulative vulnerability (Fs[2,757]≥ 4.47, Ps=0.01), but no significant interactions of cumulative vulnerability with dose (Fs[4,753]≤0.70, Ps≥0.59), time (Fs[2,7447]≤2.39, Ps≥0.09), or dose and time (Fs[4,7447]≤0.82, Ps>0.51). Effects of cigarette dose interacted significantly with time (Fs[2,7452]≥3.67, Ps≤0.03).
Biomarkers of Toxin Exposure
There was no significant main effect of cumulative vulnerability on breath CO (F[2,738]=1.70, P=.18) nor interactions of cumulative vulnerability with dose (F[4,738]=.61, P=0.65), time (F[2,7082]=1.18, P=0.31), or dose and time (F[4,7082]=0.77, P=0.55) (Figure 2, upper panel). Effects of cigarette dose on breath CO interacted with time across the 12-week study period (F[2,7088]=10.48, P<0.001) (Figure 2, lower panel), with steeper negative linear slopes over time at the 0.4 (−0.31, 95%CI: −0.40 to −0.23) and 2.4 mg/g doses (−0.25, 95%CI: −0.34 to −0.16) than 15.8 mg/g (−0.05, 95%CI: −0.13 to 0.04) (P<0.001, P=0.004, respectively); the 0.4 and 2.4 mg/g doses did not differ from each other (P=0.93).
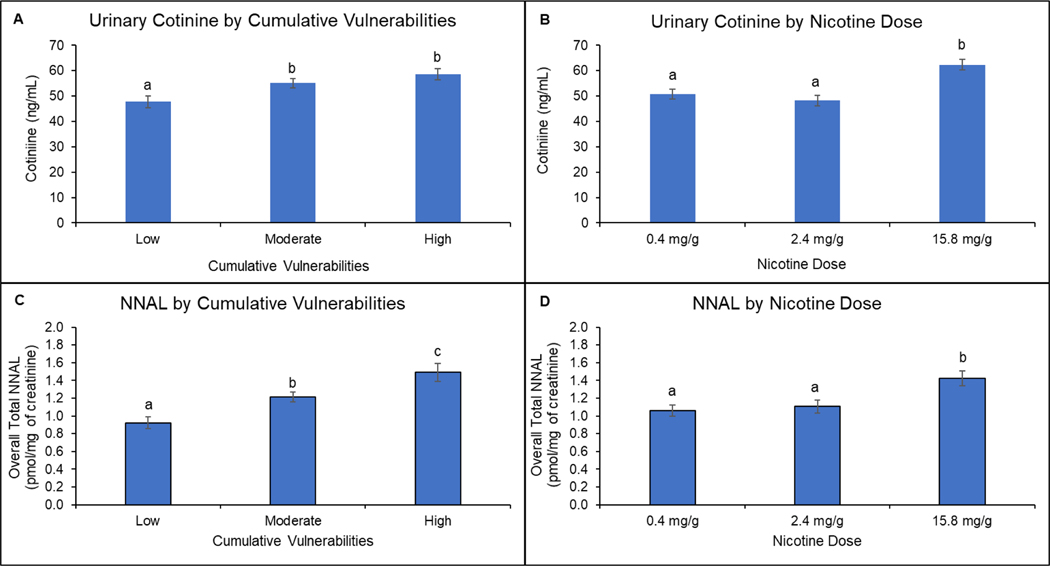
There were significant main effects of cumulative vulnerability on urine cotinine and NNAL (Fs[2,652]≥9.52, Ps<0.001), but no significant interactions of cumulative vulnerability with dose (Fs[4,648]≤1.34, Ps≥0.25), time (Fs[2,569]<1.68, Ps≥0.19), or dose and time (Fs[4,569]≤0.49, Ps≥0.74) for either outcome (Figure 3, upper and lower left panels). Collapsed across doses, levels in the low cumulative-vulnerability category were less than those in the moderate (Ps≤0.003) and high (Ps<0.001) categories in analyses for both cotinine and NNAL; levels in the moderate and high categories did not differ significantly in analyses on cotinine (P=0.32) but did in NNAL analyses (P=0.03). Cigarette dose also had a significant main effect on urine cotinine and NNAL (Fs[2,655]=26.21, P<0.001) (Figure 3, upper and lower right panels). Collapsed across cumulative-vulnerability categories, urine cotinine and NNAL levels at the 0.4 and 2.4 mg/g doses were below levels at 15.8 mg/g (Ps<0.001) and levels at the 0.4 and 2.4 mg/g doses did not differ from each other (Ps≥0.75).
Figure 3.
Biomarkers of Exposure as a Function of Cumulative Vulnerabilities and Nicotine Dose
Upper left panel (A) shows mean total urinary cotinine values in each of the three cumulative vulnerability categories (low, moderate, high) collapsed across the three nicotine-content-cigarette doses (0.4, 2.4, and 15.8 mg/g). Upper right panel (B) shows mean total urinary cotinine values for each of the three nicotine content doses collapsed across the three cumulative-vulnerability categories. Data points are geometric means ± SEM. Data points not sharing a superscript letter differed significantly in post-hoc testing. Lower left panel (C) shows mean total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) values in each of the three cumulative vulnerability categories (low, moderate, high) collapsed across the three nicotine-content-cigarette doses. Lower right panel (D) shoes mean total NNAL values for each of the three study cigarette doses collapsed across the three cumulative-vulnerability categories. Data points are geometric means ± SEM. Data points not sharing a superscript letter differed significantly in post-hoc testing.
Dependence Severity
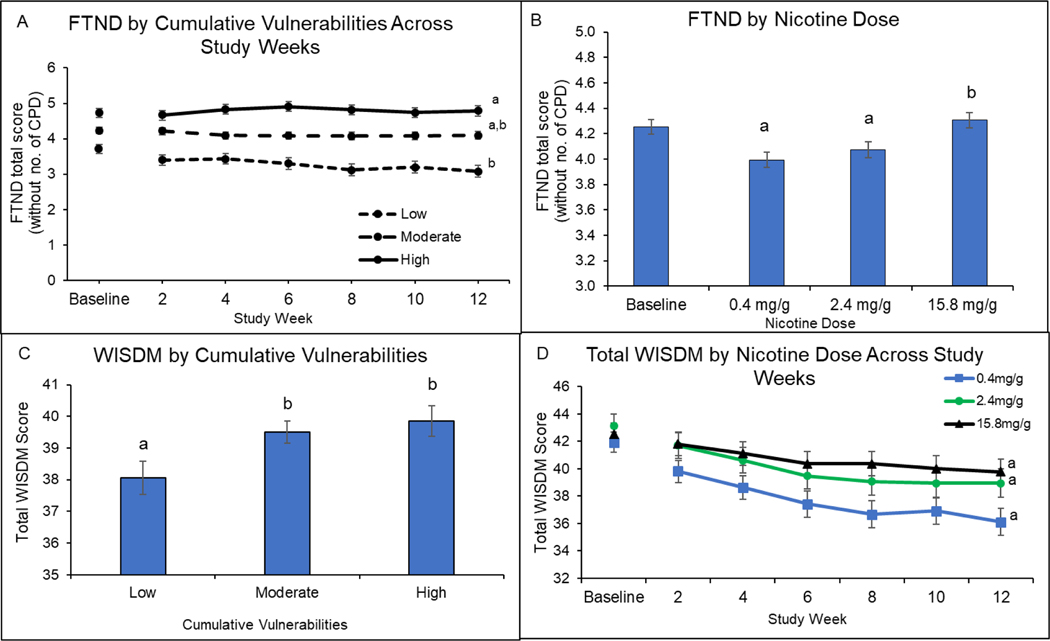
There was a significant interaction of cumulative vulnerability and time on FTND total scores (F[2,3157]=5.15, P=0.006) (Figure 4, upper left panel) but not cumulative vulnerability and dose (F[4,717]=0.02, P = 0.99) or cumulative vulnerability, dose, and time (F[4,3151]=1.47, P =0.20). Collapsed across doses, there was a steeper linear slope over time in scores in the low cumulative-vulnerability category (−0.03, 95%CI: −0.04 to −0.01) than in the high (0.01, 95% CI: −0.01 to 0.02) (P=0.004) but not the moderate category (−0.01, 95%CI: −0.02 to −0.00) (P=0.33); there was no significant difference between slopes in the moderate and high categories (P=0.09). Cigarette dose had a significant main effect on FTND total scores (F[2,717]=6.94, P=0.001) (Figure 4, upper right panel). Collapsed across vulnerability categories and time, scores at the 0.4 and 2.4 mg/g doses were each significantly below those at 15.8 mg/g (P=0.01 and P<0.001, respectively) but did not differ from each other (P=0.99).
Figure 4.
Nicotine Dependence by Cumulative Vulnerabilities and Nicotine Dose
Upper left panel (A) shows Fagerström Test for Nicotine Dependence (FTND) total scores (minus item 4) by cumulative-vulnerability categories (low, moderate, high) across study weeks collapsing across cigarette-nicotine-content dose (0.4, 2.4, and 15.8 mg/g). Data points are least-square means ± SEM. Data points not sharing a superscript letter differed significantly in post-hoc testing. Upper right panel (B) shows FTND total scores (minus item 4) by cigarette-nicotine-content dose collapsing across cumulative-vulnerability categories. Data points are arithmetic means ± SEM. Data points not sharing a superscript letter differed significantly in post-hoc testing. Lower left panel (C) shows Wisconsin Inventory of Smoking Dependence Motives (WISDM) total scores by cumulative-vulnerability categories collapsing across cigarette-nicotine-content dose. Data points are least-square means ± SEM. Data points not sharing a superscript letter differed significantly in post-hoc testing. Lower right panel (D) shows WISDM total scores by cigarette-nicotine-content dose at bi-weekly assessments collapsing across cumulative-vulnerability categories. Data points are arithmetic means ± SEM. Doses not sharing a superscript differed in slope of linear trends.
Cumulative vulnerability had a significant main effect on WISDM total scores (F[2,721]=4.04, P=0.02) (Figure 4, lower left panel), but there were no significant interactions of cumulative vulnerability with dose (F[4,717]=1.10, P=0.36), time (F[2,3151]=0.30, P=0.74), or dose and time (F[4,3151]=1.86, P=0.12). Collapsing across doses, mean scores in the low vulnerability category fell below scores in the moderate and high categories (Ps<0.05); the moderate and high categories did not differ (P=.99). Effects of Cigarette dose on WISDM total scores interacted with time (F[2,3151]=3.35, P=0.04) (Figure 4, lower right panel); linear slopes of the 0.4 mg/g dose approached although did not reach a significant difference with 15.8 mg/g after Bonferroni correction (P=0.06); the 0.4 and 2.4 mg/g doses did not differ (P=0.99) nor did the 2.4 and 15.8mg/g doses (P=0.21).
There was no significant main effect of cumulative vulnerability nor interactions of cumulative vulnerability with dose (F[4,717]=0.60, P=0.66), time (F[2,3150]=2.38, P=09), or dose and time (F[4,3150]=1.83, P=0.12) on WISDM PDM subscale scores (not shown). Cigarette dose had a significant main effect (F[2,721]=3.47, P=0.03) with mean scores at the 0.4 and 2.4 mg/g doses falling significantly below those at 15.8 mg/g (P<0.001 and P=0.003, respectively) (not shown).
There were no significant main effects of cumulative vulnerability or dose, nor interactions of those two factors with each other or time on the WISDM SDM subscale (Ps>0.15) (not shown).
Questionnaire-on-Smoking-Urges (QSU)-Brief Ratings for Study and Usual-brand Cigarettes
There were significant main effects of cumulative vulnerability on QSU-Brief Factor-1 and Factor-2 ratings for Study cigarettes (Fs[2,742] ≥17.85, Ps<0.001) (Table 2, Sections A & B), but no significant interactions of cumulative vulnerability with dose (Fs[4,738]≤1.21, Ps≥0.31), time (Fs[2,3168]≤1.44, Ps≥0.24), or dose-by-time (Fs[4,3168]≤1.84, Ps≥0.12). Collapsed across doses, craving ratings increased in an orderly manner as cumulative vulnerability increased (Ps ≤0.004). Effects of cigarette dose interacted significantly with time on QSU-Brief Factor-1 and Factor-2 ratings for Study cigarettes (Fs[2,3174]≥8.33, P<0.001) (Table 2, Sections A & B), with linear slopes over time at the 0.4 mg/g and 2.4 mg/g doses differing from 15.8 mg/g (Ps≤0.02) but not each other (Ps≥.79).
Table 2.
Questionnaire on Smoking Urges (QSU)–brief Ratings
| Main Effect | Weeks | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | 11 | ||
| Panel A: QSU Factor-1 Study Cigarettes | |||||||
| Cum. Vul. | |||||||
| low | 2.7 ± 0.13a | -- | -- | -- | -- | -- | -- |
| moderate | 3.24 ± 0.08b | -- | -- | -- | -- | -- | -- |
| high | 3.72 ± 0.17c | -- | -- | -- | -- | -- | -- |
| Dose | |||||||
| 0.4 mg/g | -- | 3.10 ± 0.11 | 3.02 ± 0.11 | 2.83 ± 0.11 | 2.89 ± 0.12 | 2.74 ± 0.11 | 2.75 ± 0.12a |
| 2.4 mg/g | -- | 3.39 ± 0.12 | 3.26 ± 0.12 | 3.27 ± 0.13 | 3.27 ± 0.13 | 3.12 ± 0.14 | 3.01 ± 0.14a |
| 15.8 mg/g | -- | 3.42 ± 0.12 | 3.52 ± 0.12 | 3.51 ± 0.12 | 3.56 ± 0.12 | 3.60 ± 0.13 | 3.53 ± 0.13b |
| Panel B: QSU Factor-2 Study Cigarettes | |||||||
| Cum. Vul. | |||||||
| low | 1.72 ± 0.10a | -- | -- | -- | -- | -- | -- |
| moderate | 2.10 ± 0.07b | -- | -- | -- | -- | -- | -- |
| high | 2.55 ± 0.09c | -- | -- | -- | -- | -- | -- |
| Dose | |||||||
| 0.4 mg/g | -- | 2.14 ± 0.09 | 2.11 ± 0.09 | 2.00 ± 0.09 | 2.05 ± 0.09 | 1.96 ± 0.09 | 1.95 ± 0.10a |
| 2.4 mg/g | -- | 2.21 ± 0.10 | 2.13 ± 0.09 | 2.13 ± 0.10 | 2.15 ± 0.11 | 2.11 ± 0.11 | 2.12 ± 0.11a |
| 15.8 mg/g | -- | 2.10 ± 0.08 | 2.21 ± 0.09 | 2.23 ± 0.10 | 2.24 ± 0.10 | 2.30 ± 0.11 | 2.30 ± 0.11b |
| Panel C: QSU Factor-1 Usual-brand Cigarettes | |||||||
| Cum. Vul. | |||||||
| low | 3.71 ± 0.11a | -- | -- | -- | -- | -- | -- |
| moderate | 3.93 ± 0.70a | -- | -- | -- | -- | -- | -- |
| high | 3.86 ± 0.10a | -- | -- | -- | -- | -- | -- |
| Dose | |||||||
| 0.4 mg/g | -- | 4.18 ± 0.11 | 3.96 ± 0.12 | 3.90 ± 0.12 | 3.71 ± 0.13 | 3.67 ± 0.13 | 3.59 ± 0.13a |
| 2.4 mg/g | -- | 4.27 ± 0.13 | 4.11 ± 0.13 | 4.01 ± 0.14 | 4.05 ± 0.14 | 3.84 ± 0.14 | 3.79 ± 0.14a |
| 15.8 mg/g | -- | 3.72 ± 0.18 | 3.77 ± 0.12 | 3.70 ± 0.13 | 3.72 ± 0.13 | 3.70 ± 0.13 | 3.69 ± 0.13b |
There was no significant main effect of cumulative vulnerability (F[2,741]=1.59, P=0.21) nor interaction of cumulative vulnerability with dose (F[4,737]=0.46, P=0.76) time (F[2,3168]=1.32, P=0.27) or dose and time (F[4,3168]=1.31, P=0.27) on QSU-Brief Factor 1 ratings of Usual-brand cigarettes (Table 2, Section C). Effects of cigarette dose interacted with time (F[2,3174]=5.71, P<0.01), with linear slopes at the 0.4 and 2.4 mg/g doses differing significantly from 15 mg/g (Ps≤0.04) but not each other (P=0.99).
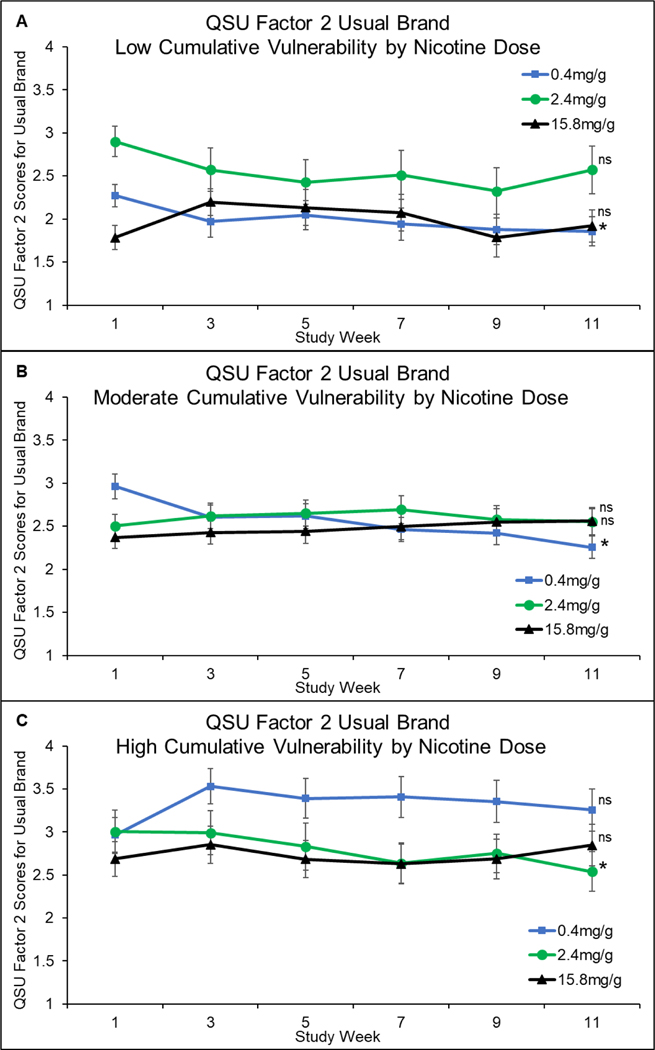
Effects of cumulative vulnerability on QSU-Brief Factor-2 ratings of Usual-brand cigarettes, interacted significantly with dose, and time (F[4,3168]=3.52, P=0.007) (Figure 5). This three-way interaction was due to variability in whether craving at the 0.4 or 2.4 mg/g doses showed significant negative linear slopes across time and within which vulnerability category they did so. The 0.4 mg/g dose did so within the low (−0.03, 95%CI: −0.07 to −0.001, P=0.04) and moderate (−0.05, 95%CI: −0.07 to −0.02, P<0.001) categories, but not the high category (0.004, 95%CI: −0.03 to 0.04, P=0.82). The 2.4 mg/dose did so within the high category (−0.04, 95%CI: −0.08 to −0.004, P=0.03) but not low (−0.04, 95% CI: −0.08 to 0.001, P=0.06) or moderate (0.01, 95% CI: −0.01 to 0.04, P=0.35). There were no significant linear slope changes at the 15.8 mg/g dose in any cumulative-vulnerability category: −0.01 (95%CI: −0.05 to 0.02, P=0.54), 0.02 (95%CI: −0.001 to 0.04, P=0.06), and −0.01 (95%CI: −0.04 to 0.03, P=0.71) within the low, moderate, and high cumulative-vulnerability categories, respectively.
Figure 5.
Questionnaire of Smoking Urges Factor 2 Scores for Usual Brand Cigarettes by Cumulative Vulnerabilities
Mean (±SEM) Questionnaire-on-Smoking-Urges (QSU) Factor-2 scores across repeated assessments during the 12-week study for each of the three dose conditions among participants in the low (upper panel), moderate (middle panel), and high (lower panel) cumulative-vulnerability categories. Data points are arithmetic means ± SEM. Doses not sharing a superscript differed in slope of linear trends.
MNWS Desire to Smoke and Total Score Ratings for Study Cigarettes
There was no significant main effect of cumulative vulnerability (F[2,741]=1.68, P=0.19) nor interaction of cumulative vulnerability with dose (F[4,737]=0.78, P=0.54), time (F[2,3168]=1.61, P=0.20), or dose and time (F[4,3168]=1.20, P=0.31) on Desire-to-Smoke ratings (not shown). Effects of cigarette dose interacted with time (F[2,3174]=4.51, P=0.01) (not shown), with the negative linear slope over time being significantly steeper at the 0.4 mg/g dose (−0.06, 95%CI: −0.07 to −0.04) compared to 15.8 mg/g (−0.03, 95%CI: −0.04 to −0.01) (P=0.009). The linear slope at the 2.4 mg/g dose (−0.04, 95%CI: −0.05 to −0.02) did not differ significantly from 0.4 mg/g (P=0.21) or 15.8 mg/g (P=0.90). There were no significant main effects of cumulative vulnerability, dose, nor interactions of those factors with each other or time on MNWS Total Scores.
DISCUSSION
The overarching aim of the present study was to examine whether response to reduced nicotine content cigarettes was moderated by the presence of multiple co-occurring vulnerabilities to smoking. In addressing that question we replicated prior findings from U.S. national samples demonstrating that severity of a broad range of smoking characteristics increases in an orderly manner as the cumulative number of co-occurring vulnerabilities to smoking increase (Gaalema et al., 2018; Higgins et al., 2016; Leventhal et al., 2018) and extended them to a sample consisting exclusively of smokers recruited from vulnerable populations. Indeed, the significant positive associations between cumulative vulnerability and severity of smoking characteristics in the present study were seen across a broad range of baseline smoking characteristics, including age at initiation of daily smoking, CPD, toxin exposure, and dependence severity as well as 12 of the 17 trial outcomes. In combination, the prior and present studies on cumulative vulnerability provide a compelling body of empirical evidence on the fundamental role of cumulative vulnerability in accounting for individual differences in smoking risk and severity. Indeed, the overarching purpose of the present study was to examine whether cumulative vulnerability would significantly moderate how participants respond to reducing the nicotine content of cigarettes to minimally addictive levels. We saw no evidence of significant moderation across 16 of the 17 dependent measures examined including the primary outcome (total CPD during Week 12).
One potential explanation for this consistency of response to reduced nicotine content cigarettes across the different levels of cumulative vulnerability is the shared role that nicotine reinforcement plays in promoting and sustaining daily smoking across different levels of severity. For example, we previously examined whether preference for smoking cigarettes varying in nicotine content differed by nicotine-dependence severity using a concurrent-choice procedure in a double-blind, multi-site study with daily smokers recruited from the same vulnerable populations as in the present study (Higgins et al., 2018). Participants reliably chose to smoke higher over lower nicotine content cigarettes independent of whether they were categorized as low, moderate, or high nicotine-dependence severity using the Heaviness of Smoking Index (Higgins et al., 2018). Said differently, preference for higher over lower nicotine content cigarettes did not differentiate between differing levels of dependence severity. As such, perhaps it is not surprising that smokers who share that preference for higher over lower nicotine content cigarettes would also show a qualitatively similar response to reductions in nicotine content.
The one instance where cumulative vulnerability did interact with the effects of reduced nicotine content cigarettes in the present study was on QSU-Brief Factor 2 ratings of Usual-brand cigarettes. In that one instance there was variability across the three-cumulative vulnerability categories regarding which of the two reduced nicotine content cigarettes decreased craving for usual-brand cigarettes across the 12-week study period, with the 0.4 mg/g dose but not the 2.4 mg/g dose doing so in the low- and moderate-severity vulnerability categories and the reverse in the high-severity category. Three-way interactions are often difficult to interpret and this one is no exception. At a minimum, this observation suggests that a policy that reduces the nicotine content of commercial cigarettes to minimally addictive levels may leave some smokers with ongoing craving for the negative reinforcing effects of their Usual-brand cigarettes, and that this outcome may vary by cumulative vulnerability in combination with the nicotine-content level that is adopted as the standard. We anticipate that smokers with the highest level of cumulative vulnerability, and associated dependence severity, may be most impacted considering their greater smoking volume and possible physical dependence and withdrawal. If that was the case here, it was not sufficiently robust to be detected in the MNWS. Nevertheless, regarding possible withdrawal-like effects when transitioning to reduced nicotine content cigarettes, it is important to note that there is empirical evidence that they can be managed effectively through supplemental use of nicotine replacement therapy (NRT) (e.g., AhnAllen et al., 2015; Keith et al., 2017; McClernon et al., 2016).
Successful reduction of smoking prevalence over time has been disproportionately concentrated among populations without socioeconomic or health-related disadvantages or from non-rural regions (e.g., Drope et al., 2018). Reducing trends in smoking prevalence in recent years have been far less substantial among those with 1 or 2 vulnerabilities, and the prevalence of smoking has remained static amongst those with 3+ vulnerabilities (Leventhal et al., 2019). These concerning trends suggest that existing tobacco control and regulatory policies are disproportionately less effective for populations facing cumulative vulnerabilities, resulting in a widening of health disparities for these groups. The current findings provide no evidence that the potentially powerful policy of reducing nicotine content in cigarettes that is under consideration at the federal level would disproportionately benefit only the non-vulnerable. Hence, there is no reason to believe that this policy would further widen disparities, and instead might serve to reduce smoking and associated disparities in populations facing multiple vulnerabilities.
While we believe the results of the present study are encouraging, some limitations merit mention. First, study participants in the parent trials were not recruited to be nationally representative and thus interactions between cumulative vulnerability involving characteristics not represented in the present sample may be revealed in the context of a national policy. Second, as we noted regarding the parent trials (Higgins et al., 2020), the goal of a reduced nicotine content policy is to eventually eliminate use of combusted cigarettes. Ideally, current smokers would eventually quit, but we recognize that some may be unable or unwilling to completely discontinue nicotine use. Thus when we note that the evidence from the current study suggests that a reduced nicotine policy has potential to benefit even highly vulnerable smokers, we mean that in the framework articulated in FDA’s Nicotine Focused Framework (Gottlieb & Zeller, 2017) wherein the policy would be implemented in a context in which, for example, non-combusted sources of nicotine (e.g., NRT, other smoking-cessation medications, e-cigarettes, heat-not-burn products) would be readily available to facilitate cessation or to function as substitutes for combusted cigarettes.
Overall, we believe the current study provides additional strong empirical evidence that a national policy that reduces the nicotine content of cigarettes to minimally addictive levels has the potential to benefit smokers from highly vulnerable populations including smokers residing in rural or other regions with overrepresentation of co-occurring vulnerabilities to smoking.
ACKNOWLEDGEMENTS
This project was supported by Tobacco Centers of Regulatory Science (TCORS) awards U54DA036114 from the National Institute on Drug Abuse and Food and Drug Administration (FDA) and U54CA180908 from the National Cancer Institute and FDA. Preparation of the report was also supported in part by a Centers of Biomedical Research Excellence award P20GM103644 from the National Institute on General Medical Sciences. The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Food and Drug Administration. Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
DISCLOSURES:
Stephen T. Higgins, nothing to disclose.
Michael DeSarno, nothing to disclose.
Diann E. Gaalema, nothing to disclose.
Adam M. Levanthal, PhD, nothing to disclose
Danielle R. Davis, nothing to disclose.
Joanna M. Streck, nothing to disclose.
Roxanne Harfmann, nothing to disclose.
Catherine Markesich, nothing to disclose.
Eva Orr, nothing to disclose.
Stacey C. Sigmon, nothing to disclose.
Sarah H. Heil, nothing to disclose.
Jennifer W. Tidey, nothing to disclose.
Dustin Lee, nothing to disclose.
John R. Hughes, received consulting and speaking fees from several companies that develop or market pharmacologic and behavioral treatments for smoking cessation or harm reduction and from several nonprofit organizations that promote tobacco control and consulting for Swedish Match (without payment).
Janice Y. Bunn, nothing to disclose.
Clinicaltrials.gov identifiers: NCT02232737, NCT02250664, NCT02250534.
REFERENCES
- American Psychological Association, Task Force on Socioeconomic Status. (2007). Report of the APA Task Force on Socioeconomic Status. Washington, DC: American Psychological Association. https://www.researchgate.net/publication/275100689_Report_of_the_APA_Task_Force_on_Socioeconomic_Status. Accessed January 22, 2021. [Google Scholar]
- AhnAllen CG, Bidwell LC, Tidey JW. 2015. Cognitive effects of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine Tob Res. 17:510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case A, Deaton A. 2015. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci. 112(49):15078–83. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer MR, Wang TW, Babb S, Cullen KA, Day H, Willis G, Jamal A, Neff L. 2019. Tobacco Product Use and Cessation Indicators Among Adults - United States, 2018. MMWR Morb Mortal Wkly Rep. 15;68(45):1013–1019. doi: 10.15585/mmwr.mm6845a2.PMID: 31725711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, et al. 2015. Randomized trial of reduced-nicotine standards for cigarettes. N Engl J Med. 373(14):1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doogan NJ, Roberts ME, Wewers ME, Stanton CA, Keith DR, Gaalema DE, Kurti AN, Redner R, Cepeda-Benito A, Bunn JY, Lopez AA, Higgins ST. 2017. A growing geographic disparity: Rural and urban cigarette smoking trends in the United States. Prev Med. 2017. 104:79–85. doi: 10.1016/j.ypmed.2017.03.011. Epub 2017 Mar 16.PMID: 28315761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drope J, Liber AC, Cahn Z, Stoklosa M, Kennedy R, Douglas CE, Henson R. and Drope J. 2018. Who’s still smoking? Disparities in adult cigarette smoking prevalence in the United States. CA: A Cancer Journal for Clinicians, 68: 106–115. [DOI] [PubMed] [Google Scholar]
- Fu W, Liu F. 2019. Unemployment insurance and cigarette smoking. J Health Econ. 63:34–51. doi: 10.1016/j.jhealeco.2018.10.004. Epub 2018 Nov 3.PMID: 30453224 [DOI] [PubMed] [Google Scholar]
- Garrett BE, Martell BN, Caraballo RS, King BA. 2019. Socioeconomic Differences in Cigarette Smoking Among Sociodemographic Groups. Prev Chronic Dis. 16:E74. doi: 10.5888/pcd16.180553.PMID: 31198164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Zeller M. 2017. A nicotine-focused framework for public health. NEJM. 377:1111–1114. Doi: 10.1056/NEJMp1707409. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Luo X, Jensen JA, al’Absi M, Allen SS, Carmella SG, Chen M, Cinciripini PM, Denlinger-Apte R, Drobes DJ, Koopmeiners JS, Lane T, Le CT, Leischow S, Luo K, McClernon FJ, Murphy SE, Paiano V, Robinson JD, Severson H, Sipe C, Strasser AA, Strayer LG, Tang MK, Vandrey R. 2018. Effect of immediate vs gradual reduction in nicotine content of cigarettes on biomarkers of smoke exposure a randomized clinical trial. JAMA. 320: 880–891. doi: 10.1001/jama.2018.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Kurti AN, Redner R, White TJ, Keith DR, Gaalema DE, Sprague BL, Stanton CA, Roberts ME, Doogan NJ, Priest JS. 2016. Co-occurring risk factors for current cigarette smoking in a U.S. nationally representative sample. Prev Med. 2016 Nov;92:110–117. doi: 10.1016/j.ypmed.2016.02.025. Epub 2016 Feb 21.PMID: 26902875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Sigmon SC, Tidey JW, Gaalema DE, Hughes JR, Stitzer ML, Durand H, Bunn JY, Priest JS, Arger CA, Miller ME, Bergeria CL, Davis DR, Streck JM, Reed DD, Skelly JM, Tursi L. 2017. Addiction potential of reduced nicotine content cigarettes in populations with psychiatric disorders and other vulnerabilities to tobacco addiction. JAMA Psychiatry. 74:1056–1064. doi: 10.1001/jamapsychiatry.2017.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Bergeria CL, Davis DR, Streck JM, Villanti AC, Hughes JR, Hughes JR, Tidey JW, Heil SH, Gaalema DE, Stitzer ML, Priest JS, Skelly JM, Reed DD, Bunn JY, Tromblee MA, Arger CA, Miller ME. 2018. Response to reduced nicotine content cigarettes among smokers differing in tobacco dependence severity. Prev Med. 117:15–23. Doi: 10.1016/j.ypmed.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Tidey JW, Sigmon SC, Heil SH, Gaalema DE, Lee D, Hughes JR, Villanti AC, Bunn JY, Davis DR, Bergeria CL, Streck JM, Parker MA, Miller ME, DeSarno M, Priest JS, Cioe P, MacLeod D, Barrows A, Markesich C, Harfmann RF. 2020. Changes in cigarette consumption with reduced nicotine content cigarettes among smokers with psychiatric conditions or socioeconomic disadvantage: 3 Randomized Clinical Trials. JAMA Netw Open. 3(10):e2019311. doi: 10.1001/jamanetworkopen.2020.19311.PMID: 33079196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DR, Kurti AN, Davis DR, Zvorsky IA, Higgins ST. 2017. A review of the effects of very low nicotine content cigarettes on behavioral and cognitive performance. Prev Med. 104: 100–116. Doi: 10.1016/j/ypmed.2017.06.016. PMID: 28647546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Bello MS, Galstyan E, Higgins ST, Barrington-Trimis JL. 2019. Association of Cumulative Socioeconomic and Health-Related Disadvantage With Disparities in Smoking Prevalence in the United States, 2008 to 2017. JAMA Intern Med. 179(6):777–785. doi: 10.1001/jamainternmed.2019.0192.PMID: 31009023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Froeliger B, Rose JE, Kozink RV, Addicott MA, Sweitzer MM, Westman EC, Van Wert DM. 2016. The effects of nicotine and non-nicotine smoking factors on working memory and associated brain function. Addict Biol. 21:954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Villanti AC. 2020. Relationship between Comorbid Drug Use Disorders, Affective Disorders, and Current Smoking. Subst Use Misuse. 1–8. doi: 10.1080/10826084.2020.1840591. Online ahead of print. PMID: 33143491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. 1998. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 59 Suppl 20: 22–33. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2016 National Survey on Drug Use and Health: Detailed Tables. pdf icon[PDF–56.2 KB]external icon Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality, 2017. [accessed 2020 Dec 26]. [Google Scholar]
- Tidey JW, Miller ME. 2015. Smoking cessation and reduction in people with chronic mental illness. BMJ. 351:h4065. doi: 10.1136/bmj.h4065.PMID: 26391240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Pacek LR, Koopmeiners JS, Vandrey R, Nardone N, Drobes DJ, Benowitz NL, Dermody SS, Lemieux A, Denlinger RL, Cassidy R, al’Absi M, Hatsukami DK, Donny EC. Effects of 6-week use of reduced-nicotine content cigarettes in smokers with and without elevated depressive symptoms. Nicotine Tob Res. 2017. Jan;19(1):59–67. doi: 10.1093/ntr/ntw199. Epub 2016 Aug 3. PMID: 27613885; PMCID: PMC5157715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Colby SM, Denlinger-Apte RL, Goodwin C, Cioe PA, Cassidy RN, Swift RM, Lindgren BR, Rubin N, Murphy SE, Hecht SS, Hatsukami DK, Donny EC. 2019. Effects of six-week use of very low nicotine content cigarettes in smokers with serious mental illness. Nicotine Tob Res. 21(Suppl 1):S38–S45. doi: 10.1093/ntr/ntz133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf SH, Schoomaker H. 2019. Life expectancy and mortality rates in the United States, 1959–2017. JAMA. 322(20): 1996–2016. Doi: 10.1001/jama.2019.16932. [DOI] [PMC free article] [PubMed] [Google Scholar]