ABSTRACT
Although HIV-specific CD8 T cells are effective in controlling HIV infection, they fail to clear infection even in the presence of antiretroviral therapy (ART) and cure strategies such as “shock-and-kill.” Little is known how ART is contributing to HIV-specific CD8 T cell function and the ability to clear HIV infection. Therefore, we first assessed the cytokine polyfunctionality and proliferation of CD8 T cells from ART-treated HIV+ individuals directly ex vivo and observed a decline in the multifunctional response as well as proliferation indices of these cells in individuals treated with integrase inhibitor (INSTI) based ART regimens compared to both protease inhibitor (PI) and nonnucleoside reverse transcriptase inhibitor (NNRTI) based regimens. We next cocultured CD8 T cells with different drugs individually and were able to observe reduced functional properties with significantly decreased ability of CD8 T cells to express IFN-γ, MIP1β and TNF-α only after treatment with INSTI-based regimens. Furthermore, previously activated and INSTI-treated CD8 T cells demonstrated reduced capacity to express perforin and granzyme B compared to PI and NNRTI treated cells. Unexpectedly, CD8 T cells treated with dolutegravir showed a similar killing ability 7 dpi compared to emtricitabine or rilpivirine treated cells. We next used a live cell imaging assay to determine the migratory capacity of CD8 T cells. Only INSTI-treated cells showed less migratory activity after SDF-1α stimulation compared to NRTI regimens. Our data show that the choice of ART can have a significant impact on CD8 T cell effector functions, but the importance for potential eradication attempts is unknown.
IMPORTANCE Integrase Strand Transfer Inhibitors (INSTI) are recommended by national and international guidelines as a key component of ART in the treatment of HIV infected patients. In particular, their efficacy, tolerability and low drug-drug interaction profile have made them to the preferred choice as part of the first-line regimen in treatment-naive individuals. Here, we demonstrate that the choice of ART can have a significant impact on function and metabolism of CD8 T cells. In summary, our study provides first evidence on a significant, negative impact on CD8 T cell effector functions in the presence of two INSTIs, dolutegravir and elvitegravir, which may contribute to the limited success of eradicating HIV-infected cells through “shock-and-kill” strategies. Although our findings are coherent with recent studies highlighting a possible role of dolutegravir in weight gain, further investigations are necessary to fully understand the impact of INSTI–based regimens on the health of the individual during antiretroviral therapy.
KEYWORDS: CD8, dolutegravir, antiretroviral agents, human immunodeficiency virus, immune response
INTRODUCTION
There is ample evidence that HIV-specific CD8 T cells play a critical role in the control of HIV viremia (1–3). However, despite their active and effective antiviral capacity, they are not able to fully eradicate HIV infection (4–6) and HIV persists in a small, dormant reservoir. With the development of antiretroviral therapy (ART), the face of HIV and AIDS has changed from an almost always deadly infection to a chronic manageable disease (7). Indeed, individuals living with HIV have a comparable life expectancy to HIV negative individuals when treated early and efficaciously (8, 9). However, even in the presence of highly effective antiretroviral therapy that suppresses HIV viremia to undetectable blood-levels and in the presence of an efficacious and functioning immune response, HIV can still not be eradicated (10). It is believed that HIV persists in a small pool of latently infected CD4 T cells. As HIV gene products are not expressed in these cells, HIV remains hidden for immune surveillance (5, 11–15). Therefore, new strategies have tried to overcome this issue by forcing latently infected cells to produce HIV proteins in the presence of antiretroviral therapies. These “shock-and-kill” strategies aim for the use of small molecule latency reversing agents (LRA) to activate virus transcription and viral protein production. In theory, latently infected cells are forced to produce viral proteins by which they become visible for the immune system (16). Interestingly, clinical trials have demonstrated that LRAs were able to effectively activate and increase HIV transcription from latently infected cells, but none of these studies have demonstrated a reduction in the pool of latently infected cells (17–19). Thus, it is surprising that HIV-specific CD8 T cells are not able to better eradicate HIV-infected cells in the presence of antiretroviral drugs (ARVs) even though these cells have been “made visible” for the immune system through LRAs. Nevertheless, previous studies have demonstrated that histone deacetylase inhibitor (HDACi), as part of LRAs, can impair the inhibitory functions of primary CD8 T cells (20, 21). This impairment is similar to HIV-specific CD8 T cells under ART treatment (6).
We therefore tested the impact of ARVs on the function of CD8 T cells to kill virally infected cells. We demonstrate that some ARVs, in particular two drugs from the integrase inhibitor class (INSTI), dolutegravir (DTG) and elvitegravir (EVG), had a significant impact on CD8 T cell functions. We were able to demonstrate that DTG significantly decreased the ability of CD8 T cells to proliferate, migrate, and express functional cytokines as well as cytolytic molecules, along with decreased respiratory activity. Our data show that the choice of ART can have a significant impact on CD8 T cell effector functions, which may have important implications for eradication strategies.
RESULTS
Reduced proliferation capacity of CD8 T cells in individuals treated with INSTI-based regimens ex vivo.
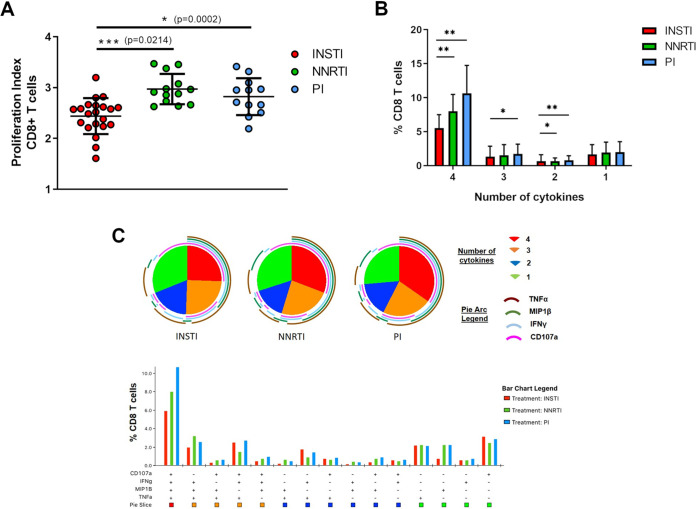
To understand the impact of ART on CD8 T cell function, we first assessed the ex vivo CD8 T cell proliferation in individuals that had been on antiretroviral therapy for more than 6 months and had fully suppressed viremia (HIV RNA detection <50 copies/ml). Using a CFSE proliferation assay, we assessed the ex vivo proliferative capacity of CD8 T cells from 46 HIV-treated individuals that either received an INSTI-, NNRTI- or PI-containing ART regimen. All individuals had similar CD4 and CD8 counts and had fully suppressed viremia for more than 6 months (Table S1A). PBMCs were stained with CFSE and cultured in the presence of the superantigen staphylococcus enterotoxin B (SEB) for 5 days. HIV infected individuals on long-term treatment have a reduction in the magnitude and breadth of their HIV-specific CD8 T cell response. Because viremia is one of the primary drivers of the magnitude of the HIV-specific T cell response, it was shown that the magnitude of HIV-specific CD8 T cell responses in untreated chronic infection is at least 2-fold greater than the magnitude of HIV-specific CD8 T cells from treated infection (22). Given the fact that these individuals were on stable ART treatment for at least 6 months, we detected only a weak and insufficient HIV-specific CD8 T cell response in ex vivo experiments using a Gag peptide pool as stimulus (Fig. S1). We therefore decided to use the superantigen SEB to evoke a strong and best possible response to be able to detect even slight differences between ART regimens. After 5 days of stimulation, we observed significant differences in the proliferative capacity of CD8 T cells depending on the ART treatment choice. CD8 T cells from individuals that either received NNRTI- or PI-based regimens showed similar ability to proliferate and had no significant differences in their proliferation index, which was characterized by the reduction in the signal intensity of CSFE for every division via flow cytometry (Fig. 1A and Fig. S1). Strikingly, the ability of CD8 T cells to proliferate in individuals receiving an INSTI-based ART regimen (2.4 ± 0.4) was significantly lower than the other two regimens, NNRTI (3.0 ± 0.3; P = 0.0002) and PI (2.8 ± 0.4; P = 0.0214). Thus, these ex vivo data suggest that INSTI-based regimens have a negative impact on CD8 T cell proliferation compared to NNRTI- and PI-based regimens.
FIG 1.
CD8 T cells from individuals treated with INSTI-based regimens showed reduced proliferation capacity and functionality ex vivo. (A) Assessment of the ex vivo proliferation capacity of CD8 T cells via Flow Cytometry. The Proliferation Index was calculated through the FlowJo’s Proliferation platform (V10) to quantify the CSFE proliferation assay. Division was characterized by the reduction in the signal intensity of CSFE. The proliferation assay showed significantly decreased proliferation of CD8 T cells of ART-treated HIV+ individuals receiving INSTI-containing regimen (red circles, n = 21) compared to NNRTI (green circles, n = 13) and PI (blue circles, n = 12) containing regimens. Bar chart shows the mean value ± SD. Statistical significance was assessed by one-way ANOVA, nonparametric test with Kruskal-Wallis multiple-comparison test. (B) and (C) Functional characterization of the co-expression of CD107a, IFN-γ, MIP1β, and TNF-α on CD8 T cells from ART-treated HIV+ individuals was carried out following SEB stimulation of PBMCs using multicolor flow cytometry analysis. CD8 T cell responses after stimulation were calculated by subtracting the negative control for every sample individually. In the following, Boolean-gate analysis was performed showing the frequency of CD8 T cells expressing CD107a, IFN-γ, MIP1β and TNF-α, in HIV+ patients with different treatment regimens (INSTI-containing regimen red bar n = 13; NNRTI- containing regimen green bar n = 13; PI-containing regimen blue bar, n = 10). (B) Bar graph represents mean values ± SD of CD8 T cells which co-expressed different combination of cytokines simultaneously (4, 3 or 2 cytokines) or just expressed one single cytokine. Significant less CD8 T cells from of ART-treated HIV+ individuals receiving INSTI-containing regimen expressed 4 cytokines simultaneously, compared to CD8 T cells from individuals receiving NNRTI- and PI-based regimens. Mann-Whitney nonparametric statistical tests were applied for comparison, (*P < 0.05; **P < 0.01). (C) SPICE-generated graphical output. Boolean-gated frequencies within FlowJo were formatted and exported into SPICE. SPICE analysis showing a shift in the polyfunctionality profile of CD8 T cells from individuals receiving INSTI-containing regimen toward a less multifunctional response compared to individuals receiving NNRTI- and PI-based regimens. The pie charts represent the average frequencies of cytokine-expressing CD8 T cells expressing every possible combination (pie-legend: green to red) of the four cytokines analyzed. The size of the pie segment correlates to the frequency of the particular population. The arcs around the circumference indicate the particular cytokine expressed by the proportion of cells that lie under the arc. The bar graph beneath the pie segment shows frequencies of CD8 T cells with individual combinations of cytokines and represents an extension of Fig. 1B.
Reduced ability of CD8 T cells to express cytokines in individuals treated with INSTI-based regimens ex vivo.
Given the impaired proliferation of CD8 T cells in individuals receiving INSTI-based ART regimens, we next assessed whether ART regimens also have an impact on CD8 T cell polyfunctionality. We used PBMCs from 36 ART-treated individuals with fully suppressed viremia (Table S1B) that either received an INSTI-, NNRTI- or PI-containing ART regimen and stimulated the cells with SEB for 6 h. We then analyzed the functionality of CD8 T cells via multicolor flow cytometry using a polyfunctionality panel consisting of four functional markers: CD107a, IFN-γ, MIP1β and TNF-α. CD8 T cells from all individuals responded well to SEB stimulation (Fig. S2), however, we observed a striking perturbation in the composition of the responses depending on the ART regimen each individual had received as their treatment. While NNRTI- and PI-based regimens had a comparatively similar polyfunctionality profile with the majority of CD8 T cells showing the ability to simultaneously express 4 cytokines followed by a 3 functional response upon superantigen stimulation, we found a significantly lower multifunctional response in individuals receiving an INSTI-based ART regimen (Fig. 1B and C). Despite a full suppression of HIV viremia in all individuals, we observed that significantly less CD8 T cells from these individuals had the ability to express 4 cytokines simultaneously, compared to CD8 T cells from individuals receiving NNRTI- (P < 0.01) and PI-based regimens (P < 0.01). Similarly, there was a significant reduction in the three-functional CD8 T cell response (P < 0.05) in comparison to individuals that received a PI-based ART regimen. It is important to stress that it was particularly striking as all assays were done ex vivo with no supplementation of ARVs or other potential confounding factors. Taken together, our data demonstrate significant differences in the multifunctional response of CD8 T cells ex vivo, where individuals who received an INSTI-based ART regimen showed a reduced ability to simultaneously express multiple cytokines compared to individuals on PI- or NNRTI based regimens.
Negative impact of dolutegravir treatment on CD8 T cell mobility.
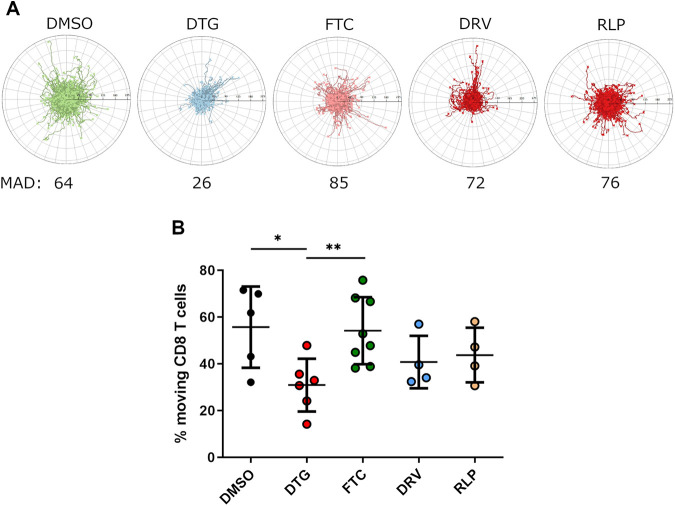
Given the perturbed function and proliferation of CD8 T cells in individuals receiving an INSTI-based regimen, we next aimed at a broader understanding of the impact of INSTI on CD8 T cell function and performed time-lapse video microscopy on CD8 T cells to assess their motility in vitro. For this purpose, we used a live-cell imaging assay to investigate the effect of ARVs on the migratory capacity of CD8 T cells as described previously for neutrophils (23, 24). CD8 T cells from HIV negative individuals (n = 8) were isolated, treated with each ARV individually for 24 h and placed on an ICAM-1 coated plate. The migration of individual CD8 T cells was tracked after CXCL12α (SDF-1α) stimulation with an auto tracking software that provides trajectory plots and further migration parameters such as percentage of moving cells. DMSO-treated CD8 T cells responded well to the chemotactic cytokine and showed an equally distributed migration pattern over time with an average accumulated distance of 64 µm (Fig. 2A) as well as a 55% migration induction of the total seeded CD8 T cells (Fig. 2B). Treatment with an exemplary substance for each ART class (NRTI: FTC, PI: DRV and NNRTI: RLP) did not significantly change the migration pattern or activity of CD8 T cells compared to the DMSO-treated control. However, we observed a significantly impaired migration pattern of CD8 T cells treated with the INSTI DTG. Interestingly, overall, we observed that INSTI-treated cells showed less migration over time and had a significantly decreased percentage of moving cells (30.9 ± 11.3), compared to DMSO (55.7 ± 17.3; P < 0.05) and NRTI FTC (54.1 ± 14.3; P < 0.01) Taken together, the data suggest that DTG interferes with the capability of CD8 T cells to migrate.
FIG 2.
Assessment of the mobility of CD8 T cells treated with different ARTs individually. Isolated CD8 T cells from HIV-negative individuals (n = 8) were cultured in the presence of DMSO or different ART regimens at bio-active plasma concentrations for 1 day and afterwards stimulated with SDF-1α. Migration of cells were recorded via video microscopy for a period of 3 h with one image taken every 15 s. (A) The panel shows the exemplary trajectory plots (track origins set to 0.0) for the respective conditions generated using the ACAS autotracking tool. The scale bar indicates the distance between the ticks of the trajectory plots. The mean accumulated distance (MAD) is shown in µm. (B) Overall reduced percentage of moving CD8 T cells which were treated with DTG (red circles, n = 6) compared to DMSO (black circles, n = 5), FTC (green circles, n = 8), DRV (blue circles, n = 4), and RLP (yellow circles, n = 4). Chart shows the mean value ± SD. Mann-Whitney nonparametric statistical tests were applied for comparison, (*P < 0.05; **P < 0.01).
Dolutegravir and elvitegravir have a reductive effect on the cytokine expression profile.
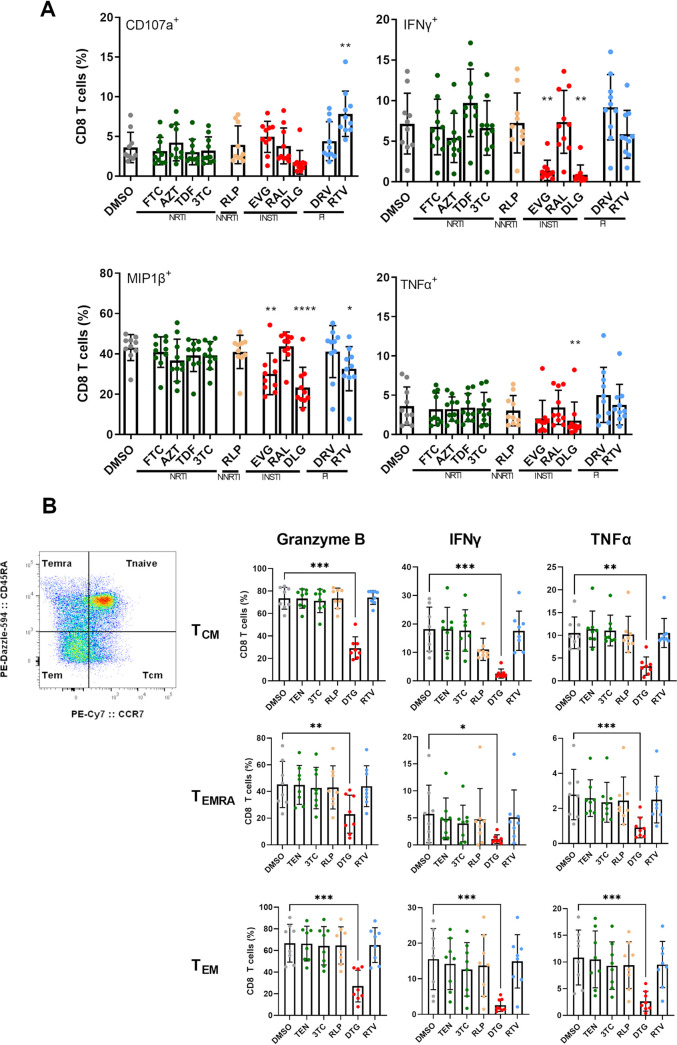
Given the perturbation in variable functions of INSTI on CD8 T cells, we next wanted to dissect the impact of individual antiretroviral medications and not the whole ART regimen, often consisting of two or more drugs, on these cells. We therefore enriched CD8 T cells from HIV-negative individuals (n = 10) and cocultured them in the presence of individual ARVs at previously described bioactive plasma concentrations for 3 days (Table S2). We then analyzed the multifunctional profile of CD8 T cells via multicolor flow cytometry using a 4-marker functional panel after SEB stimulation. Similar frequencies of CD8 T cells stimulated in the presence of DMSO expressed CD107a, IFN-γ, MIP1β and TNF-α compared to a treatment with NRTI-, NNRTI- or PI-based drugs. There was no significant difference in the expression profile for these cytokines, except for ritonavir (RTV), which showed increased frequencies of CD8 T cells expressing CD107a compared to DMSO. However, stimulated CD8 T cells in the presence of INSTI-based regimens showed significant differences in the ability to express IFN-γ, MIP1β and TNF-α. Cells stimulated in the presence of both INSTIs DTG and elvitegravir (EVG) but not raltegravir (RAL) demonstrated a significant decrease in the overall immune responses (Fig. 3A and Fig. S3). In particular, CD8 T cells treated with DTG showed reduced functional properties with significantly decreased ability to express IFN-γ (P < 0.01), MIP1β (P < 0.001) and TNF-α (P < 0.01) compared to cells treated with DMSO. Interestingly, this was also the case when we analyzed the cytokine expression of different memory CD8 T cell subsets. Significantly less CD8 T cells treated with DTG showed expression of granzyme B, IFN-γ and TNF-α of CD8 central memory (TCM), CD8 terminally differentiated effector memory (TEMRA), and CD8 effector memory (TEM) T cells compared to cells treated with DMSO, respectively (Fig. 3B). Thus, we found that DTG and EVG had a modulatory and reductive effect on the cytokine expression profile of CD8 T cells.
FIG 3.
Assessment of CD8 T cell cytokine expression profile after stimulation with SEB in the presence of different ARTs at bio-active plasma concentrations. (A) PBMCs from HIV-negative individuals (n = 10) were stained with fluorochrome-conjugated Abs specific for CD4, CD8, CD107a, IFN-γ, MIP1β and TNF-α and were analyzed by multicolor flow cytometry. Overall reduced functionality was observed in the presence of DTG and EVG for CD107a and significant for IFN-γ, MIP1β and TNF-α. Bar charts show the mean value ± SD. Statistical significance was assessed by one-way ANOVA, nonparametric test with Friedman multiple-comparison test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). (B) Assessment of the cytokine expression profile of different CD8 T cell subsets after stimulation with SEB in the presence of different ARTs at bio-active plasma concentrations. PBMCs from HIV-negative individuals (n = 8) were stained with fluorochrome-conjugated Abs specific for CD8, CCR7, CD45RA, IFN-γ, TNF-α, and Granzyme-B and were analyzed by multicolor flow cytometry. To delineate memory CD8 T cell subsets (TCM, central memory cells; TEMRA, terminally differentiated effector memory cells and TEM, effector memory cells), gates for CCR7 and CD45RA were adjusted due to fluorescence minus one (FMO) control. Overall reduced functionality was observed in the presence of DTG. Bar charts show the mean value ± SD. Statistical significance was assessed by one-way ANOVA, nonparametric test with Friedman multiple-comparison test (*P < 0.05; **P < 0.01; ***P < 0.001).
Dolutegravir has a negative impact on expression of cytolytic molecules.
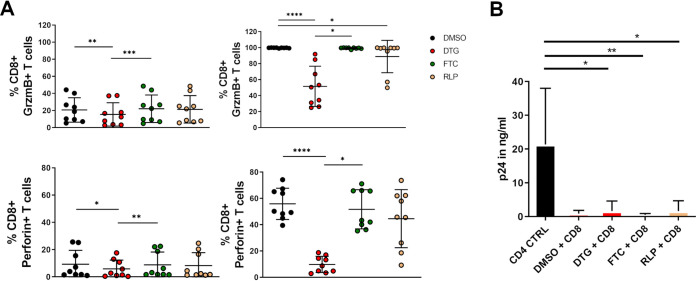
We next hypothesized that INSTI may impair the ability of CD8 T cells to kill virally infected cells as their function and mobility are reduced. We therefore activated CD8 T cells from HIV-negative individuals with SEB or left them untouched in the presence of different ARVs and subsequently measured their expression of perforin and granzyme B via multicolor flow cytometry. Interestingly, already of resting and not activated CD8 T cells we could observe a significant effect of DTG on the expression of perforin and granzyme B after 3 days of treatment compared to the DMSO control and FTC treated CD8 T cells (Fig. 4A). These observations were even more strikingly in previously activated CD8 T cells, we found that the frequency of CD8 T cells with the ability to express both cytolytic molecules, granzyme b and perforin, to be significantly decreased after treatment with DTG (51.6 ± 25.1, 9.8 ± 6.2, respectively) compared to the DMSO control (99.7 ± 0.3; P < 0.0001; 55.9 ± 12.0; P < 0.0001, respectively) and FTC (99.3 ± 0.9; P < 0.05; 51.7 ± 15.0; P < 0.05, respectively) treated cells. We next wanted to find out if CD8 T cells in the presence of DTG are less efficient in killing infected CD4 T cells. We therefore investigated how ARVs affect the inhibitory capacity of CD8 T cells in the presence or absence of the respective drugs using a viral inhibition assay. We isolated CD8 T cells from HIV-infected treatment-naive individuals, stimulated them with SEB and treated them with the respective ARVs for 3 days. Autologous treatment-naive CD4 T cells from the same individuals were then infected with a NVP-resistant virus and cocultured with CD8 T cells in the presence of NVP. It is important to note that the cocultures were performed just in the presence of NVP and no other ARVs. Furthermore, only CD8 T cells had been previously exposed to other individual ARVs. Unexpectedly, CD8 T cells treated with DTG showed a similar killing ability 7 days postinfection compared to FTC or RLP treated cells (Fig. 4B) and we found no reduction despite the impact of INSTI on T cell function. Overall, our data suggested that DTG has a negative impact on the ability of CD8 T cells to express granzyme B and perforin but showed no reduction in the capability to kill infected CD4 T cells.
FIG 4.
Assessment of the impact of individual ARTs on CD8 T cell cytolytic activity. (A) DTG effects the new synthesis of cytolytic molecules. Isolated CD8 T cells from HIV-negative individuals (n = 9) were treated with different ART regiments at bio-active plasma concentrations for 3 days. Afterwards, cells were stimulated with 5 µg/ml SEB for 6 h and analyzed via flow cytometry. A significant reduction in the ability to express both cytolytic molecules perforin and granzyme B were observed in cells which were treated with DTG (red circles) compared to DMSO (black circles), FTC (green circles) and RLP (yellow circles) treated cells only in activated CD8 T cells. Charts show the mean value ± SD. Statistical significance was assessed by one-way ANOVA, nonparametric test with Friedman multiple-comparison test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001). (B) CD8 T cells from HIV infected treatment-naive individuals (n = 6) were isolated, stimulated with 3 µg/ml SEB and incubated in the presence of DMSO or different ART regimens at bio-active plasma concentrations for 3 days. Afterwards, CD8 T cells were cocultured at a ratio of 1:2 (effector: target ratio) with previously nevirapine-resistant HIV infected autologous CD4 T cells. At day 7 postinfection, an HIV-1 Gag p24 Quantikine ELISA was performed to analyze the amount of p24 (in ng/ml) in the supernatant. No impact of any ART drug on CD8 T cells’ ability to kill virally infected cells was overserved. Statistical significance was assessed by one-way ANOVA, nonparametric test with Friedman multiple-comparison test (*P < 0.05; **P < 0.01).
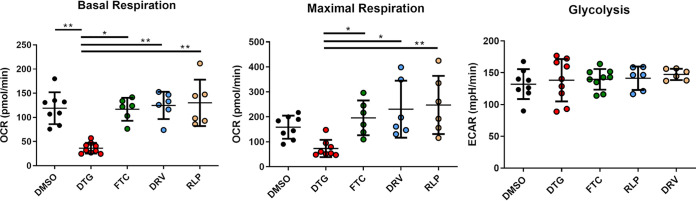
Dolutegravir decreased mitochondrial respiration in CD8 T cells.
We next wanted to understand the underlying factors associated with the perturbation and reduced activity of CD8 T cells. Given the reduced T cell function in the presence of DTG, we hypothesized that a change in the metabolic profile of the CD8 T cells could play a major role. It has been reported that cellular metabolism regulates T cell function and differentiation on a fundamental level and differences in the bioenergetic profile of CD4 T cells, which were treated with INSTI have previously been described (25, 26). We therefore isolated CD8 T cells from HIV-negative donors and treated them with individual ARVs to dissect the impact on CD8 T cell metabolic activity. We chose to determine differences in two major metabolic pathways – oxidative phosphorylation and glycolysis. Mitochondrial respiration was assessed by measuring the oxygen consumption rate (OCR) and aerobic glycolysis by measuring the acidification of the medium (extracellular acidification rate–ECAR) using an extracellular flux analyzer (Seahorse XFe). As expected, we could observe no significant impact on the metabolic activity in cells treated with the non-INSTI regiments FTC, RLP, and DRV compared to cells treated with DMSO (Fig. 5). However, DTG caused a significant decrease in basal respiration compared to DMSO, NRTI (FTC), NNRTI (RLP) and PI (DRV) treated CD8 T cells (P < 0.01, P < 0.05, P < 0.01, P < 0.01, respectively) and in maximal respiration (P < 0.05, P < 0.05; P < 0.01, respectively). Interestingly, DTG had no impact on cellular glycolysis, suggesting that the inference of DTG specifically occurs in the mitochondrial respiration pathway. Nevertheless, the observed impact was not due to potential cellular cytotoxicity demonstrating stable viability over the same period of time (Fig. S4). Additionally, we wanted to know if different amounts of FCS in the media and the available concentrations of free drug could change the observed decrease in the metabolic activity. Therefore, cells were incubated in R10 media with different amounts of FCS (10%, 20%, 50%), activated with SEB and treated with DTG at bio-active plasma concentration or DMSO for 3 days (Fig. S5). Comparison between the different amounts of FCS shows no significant difference in the impact of DTG on the metabolic activity.
FIG 5.
Assessment of the impact of individual ARTs on CD8 T cell metabolism. Basal and maximal respiration of CD8 T cells from healthy individuals (n = 9), which were exposed to different ARTs at bio-active plasma concentrations for 3 days and analyzed by using a Seahorse XFe Extracellular Flux Analyzer. Significant reduction in the oxygen consumption rate (OCR) was observed for CD8 T cells treated with DTG (red circles, n = 9) compared to DMSO control (black circles, n = 8), FTC (green circles, n = 6), DRV (blue circles, n = 6) and RLP (yellow circles, n = 6). No differences in glycolysis were observed. Charts show the mean value ± SD. Statistical significance was assessed by one-way ANOVA, nonparametric test with Kruskal-Wallis multiple-comparison test. (*P < 0.05; **P < 0.01).
Collectively, our data suggest that treatment with the newer INSTIs (DTG and EVG), had a significant negative impact on major CD8 T cell functions like cytokine expression, proliferation, T cell mobility, the new synthesis of effector molecules as well as on the respiratory activity.
DISCUSSION
HIV persists dormant in a small pool of infected cells. As these cells do not produce viral gene products, they remain invisible to immune surveillance. However, even in the presence of activation as well as forced production of viral proteins, HIV-specific CD8 T cells are unable to reduce the pool of latently infected cells (27). While the reasons for this are most likely multifactorial, we assessed in the present study the impact of ARVs on the function, proliferation, metabolism, migratory properties and inhibitory activity of CD8 T cells. We observed that some ARVs, in particular two drugs from the INSTI class (DTG and EVG), had a significant impact on major CD8 T cell functions. We demonstrated that DTG, as part of first line ART regimen, significantly decreased the ability of CD8 T cells to proliferate, migrate and express functional cytokines. In addition, we showed a reduction in the CD8 T cell respiratory activity and the new synthesis of cytolytic molecules, which could affect current eradication strategies.
HIV infection induces a robust and durable CD8 T cell response. In chronic HIV infected individuals, the HIV-specific CD8 T cell response is highly dynamic and CD8 T cells exert selection pressure on HIV with the emergence of various escape mutations (28–30). Virus escape results in an ongoing shift in the HIV-specific CD8 T cell response pattern, with new CD8 T cell responses constantly emerging (31). However, HIV infection is also characterized by a progressive loss of CD8 T cell immune functions, specifically the loss of the capacity to simultaneously produce antiviral cytokines and release lytic molecules following antigenic stimulation (1, 2). This increasing loss of function in CD8 T cells is associated with the upregulation of immune checkpoint markers characterizing a dysfunctional phenotype of these critical effector cells. Initiation of ART has been linked to a partial restoration of CD8 T cell function, including a restored functional and less dysfunctional phenotype (32).
However, despite a partially improved function, HIV-specific CD8 T cells on ARV show signs of overall dysfunction in comparison to HIV uninfected individuals. Studies have demonstrated a heightened immunosenescence under ART compared to HIV-negative individuals. Moreover, multiple studies have reported that HIV-specific CD8 T cells from individuals durably suppressed on ART do not exhibit the same breadth of function as HIV-specific CD8 T cells from HIV elite controllers, including a reduced proliferative capacity as well as cytokine and lytic molecule production following stimulation with HIV antigens (6, 33). Even more importantly, CD8 T cells from ART-suppressed individuals have a reduced capacity to eliminate both, productively and latently infected CD4 T cells compared with CD8 T cells from elite controllers (21, 34). One of the striking findings is that “shock-and-kill” strategies were in fact able to activate and increase HIV transcription from latency but several clinical trials showed so far only limited or no clearance of the reactivated cells (17–19). However, McBrien et al. demonstrated that complete depletion of CD8 T cells in SIV-infected rhesus marques in combination with ART and a IL-15 superagonist as LRA can achieve a sufficient latency-reversing effect in vivo and in vitro. This effect was absence in the presence of CD8 T cells (35). Although it is surprising that LRAs approaches in clinical trials have no impact on the HIV latent reservoir pool, which indicating a lack of the cytolytic ability of CD8 T cells, findings suggest that CD8 T cells can have an impact on viral replication during latency reversal.
In our study, we assessed the impact of ARV classes on the function of CD8 T cells. We demonstrated in both ex vivo and in vitro experiments that a treatment with INSTIs decreased CD8 T cells’ ability to proliferate and express effector cytokines. In particular, in our ex vivo experiments we were able to demonstrate that individuals on an INSTI-based ART regimen had a measurable reduction of their multifunctional CD8 T cell response after using SEB stimulation as a nonphysiological stimulus but surrogate for optimal T cell receptor–triggered stimulation. We could show that treatment with INSTI-regimens, in particular DTG and EVG, negatively impact the cytokine expression of CD8 T cells. In addition, DTG caused disrupted mitochondrial function of CD8 T cells. The decreased mitochondrial capacity may cause an overall reduction in the CD8 T cell activity, which can lead to the possibility that less T cells respond to SEB stimulation. However, this does not change the overall observation of reduced T cell responses. While CD8 T cell polyfunctionality has been linked to the inhibitory capacity of CD8 T cells, we were also able to demonstrate that the ART choice affected the production of cytolytic molecules, T cell proliferation and migratory capacity of CD8 T cells. Clonal expansion of activated T cells, the ability to migrate from tissues into lymph nodes, or the migration of memory T cells to the site where their target antigen is present are important for protective immunity (36–38). Impaired proliferation or migration capacity after receiving an INSTI-based regimen can diminish inflammatory responses. Nevertheless, we could not detect any impact of DTG on the ability of CD8 T cells to reduce Gag-expression in HIV-treatment naive CD4 T cells in vitro, despite the reduced expression of granzyme B and perforin after SEB stimulation. Although in vitro viral inhibition assays can be limited in terms of physiological relevance, these unexpected findings were particularly puzzling and warrants further investigation. In particular, it may suggest that the reduction of inhibitory capacities only happens after several rounds of killing. Or on the contrary that cytokine expressions may be impacted by DTG but not the ability to kill. In addition, studies have shown, that the slower induced Fas ligand-driven killing mechanism can play a role in the control of virus infection (39–41).
While the lack of reduction of the viral reservoir by “shock-and-kill” strategies is most likely multifactorial, our data suggest that the choice of ARVs may have an impact on the success of such strategies. Indeed, previous studies have already highlighted several explanations for the failure of LRAs to reduce the viral reservoir. It is believed that the B cell follicle is an immune privileged site restricting HIV-specific CD8 T cells to enter. Thus, activation with LRAs, especially of the HDACi, may only have a limited impact as activated virus-producing cells in the follicles may not be reached by virus-specific CD8 T cells (15, 42). The lack of accessibility of effector cells due to compartmentalization in primary lymphoid organs could prevent effective killing even after successful activation of latently infected cells. Additionally, studies showed that LRAs also may have a cytotoxic effect on immune cells. It has been demonstrated that the HDAC inhibitors, romidepsin and panobinostat, have highly cytotoxic effects on CD8 T cells (43, 44). While both factors are a very likely explanation, our data indicate an additional layer as a potential reason for failed shock and kill attempts suggesting that the choice of INSTI before shock and kill therapies may not be the best for the observed effects. In addition, INSTI as well as NRTI/NNRTI block HIV entry into the genome and therefore presentation of any viral gene products. In contrast, a double PI regimen strategy during shock and kill trials may boost the production of new viral RNA as well as the expression of uncleaved viral protein precursors. In this way, LRAs are able to activate viral transcription in the absence of fully viral assembly and latently infected CD4 T cells can be recognized by effector cells. A strategy that should be considered in the future.
We were not able to elucidate the precise mechanism of action of INSTI on CD8 T cell activity. Nevertheless, we were able to demonstrate a significantly impaired mitochondrial respiration of CD8 T cells after exposure to the integrase inhibitor DTG. Moreover, we previously published that some ARVs, in particular two drugs from the INSTI class (DTG and EVG) also had a significant impact on the CD4 T cell respiratory activity. We demonstrated that these INSTIs interfere with the electron transport chain of mitochondria, severely impairing the respiratory capacity of CD4 T cells. Additionally, we were able to link increased production of reactive oxygen species (ROS) and higher content of mitochondrial DNA to a treatment with INSTI. These changes led to a skewing of CD4 T cell immune response toward a reduced and monofunctional CD4 T cell response (26). It has been reported that INSTI treatment, especially DTG- and EVG-based regimens, are linked to specific side effects like weight gain and metabolic disorders (45). Recent studies highlighted a possible role of DTG in body fat composition changes in individuals who switched from efavirenz-based to DTG-based regimens (46). In addition, Moure et al. reported elevated levels of the antidiabetic hormone fibroblast growth factor 21 (FGF21) in human hepatocytes after treatment with EVG. Higher levels of FGF21 are generally associated with insulin resistance and glycemia (47). Although we did not see any impact on cellular glycolysis, the reported side effects of an INSTI treatment points to a deteriorated and disrupted mitochondrial function. Especially significantly increased weight gain after switching to DTG-based regimens can be linked to impaired reduced oxygen consumption in obese individuals (48, 49). Interestingly, we could not see any reductive impact of the third drug from the INSTI class, RAL, on the cytokine expression profile of CD8 T cells. Although further investigations are necessary to understand this behavior, we recently could also show that RAL had no significant impact on the CD4 T cell polyfunctionality or respiratory activity (26). Additionally, a previously published study showed a stronger increase in body weight among Asian people treated with DTG-regimens compared to regimens containing RAL (50). Our data suggest a substantial interference of DTG with CD8 T cell metabolism, which could be explained by possible interference of DTG (but not RAL) in the electronic transport chain and can be linked to weight gain (51, 52).
In summary, our study provides evidence on a significant, negative impact on major CD8 T cell functions in the presence of two INSTIs, DTG and EVG, showing decreased proliferation capability, polyfunctionality, reduced synthesis of effector molecules, migratory potential and on the respiratory activity of CD8 T cells. Further investigations are necessary to fully understand the mechanism of action involved in this observation, and their potential serious for long-term toxicity.
MATERIALS AND METHODS
Study design.
Subset of HIV infected treatment-naive (n = 6) and HIV infected treatment-experienced participants (n = 48) were recruited from the outpatient HIV and STD clinic at the University Hospital Essen (HPSTD-Ambulanz, University Hospital Essen, Essen, Germany) and from the clinic for Internal Medicine, Infectious Diseases Department (University Hospital Bonn, Germany). It should be noted that the large majority of HIV infected participants were middle-aged men. Different HIV uninfected specimens (n = 57) were obtained from the blood donation center University Hospital Essen (Essen, Germany). None of the funding sources were involved in the design or carry-out of the study. The study was approved by IRB at the University Duisburg-Essen (No. 17-7846-BO). Informed consent was obtained from all participants involved in the study.
Isolation of peripheral blood mononuclear cells (PBMC).
EDTA-supplemented blood samples were obtained from HIV-negative individuals, HIV-positive treated and treatment-naive individuals and blood mononuclear cells were isolated by standard density gradient protocol and cryopreserved as previously described (53).
Antiretroviral drugs.
To assess the impact of antiretroviral medications, whole PBMCs or isolated CD8 T cells were incubated in the presence of different ART regimens (depending on the experiment) – emtricitabine (FTC), zidovudine (AZT), tenofovir-disoproxil (TDF), lamivudine (3TC), rilpivirine (RLP), raltegravir (RAL), dolutegravir (DTG), elvitegravir (EVG), darunavir ethanolate (DRV), ritonavir (RTV) (Selleckchem) at bio-active plasma concentration. The concentration of each antiretroviral medication is described in Table S2. The percentage of DMSO (0.1%) was identical across all drug conditions and DMSO controls.
Assessment of ex vivo proliferation by flow cytometry.
Cryopreserved PBMCs from HIV infected, ART-treated individuals were thawed in R10 media (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS; Sigma-Aldrich), 2 mM l-glutamine (Sigma-Aldrich), 100 U/ml penicillin (Sigma-Aldrich), 100 µg/ml streptomycin (Sigma-Aldrich) and 10 mM HEPES (Sigma-Aldrich)) and allowed to rest overnight at 37°C, 5% CO2. Afterwards, approximately 3 million PBMC from each individual were washed with PBS and stained with 1.25 µM the cell division tracker carboxyfluorescein succinimidyl ester (CFSE; Biolegend) in 1 ml PBS at 37°C, 5% CO2 for 20 min. Subsequently, the staining was quenched by adding 5 times the original staining volume of R10 media. Cells from each individual were centrifuged, resuspended in R10 media and transferred in a 24-well polystyrene plate (Starlab) at concentration of 1 million/ml. Cells were stimulated with 5 µg/ml of the staphylococcus entrotoxin B (SEB; Sigma-Aldrich), 1 µg/ml HIV-1 PTE Gag Peptide Pool (AIDS Reagent) in the presence of co-stimulatory molecule CD28/CD49d (BD Bioscience) or left unstimulated as a control at 37°C, 5% CO2 for 5 days. On the 5th day, cells were washed with PBS and stained with the viability dye Zombie Aqua (Biolegend) for 30 min. After washing with staining buffer (PBS supplemented with 2% FCS), cells were stained extracellularly for 20 min with fluorescently conjugated antibodies: anti-CD3+APC-Cy7 (clone UCHT1; Biolegend), anti-CD4-BV421 (clone RPA-T4; Biolegend), anti-CD8-AF647 (clone C8/144B; Biolegend). Data were collected at FACS Celesta (BD Bioscience). PBMCs were gated on living CD3+CD8+ T cells and proliferation was analyzed via the proliferation tool of FlowJo Software version 10.0.7 (TreeStar). The Proliferation Index is the total number of divisions divided by the number of CD8 T cells that went into division. Division was characterized by the reduction of the signal intensity of CSFE.
Assessment of ex vivo polyfunctionality by flow cytometry.
Cryopreserved PBMCs from HIV infected, ART-treated individuals were thawed and rested overnight as described above. Cells were then stimulated with 5 µg/ml SEB and incubated in the presence of co-stimulatory molecule CD28/CD49d (BD Bioscience) together with anti-CD107a-PE/Dazzle 594 (clone H4A3; Biolegend) at 37°C, 5% CO2 for 6 h. An unstimulated condition served as control. After 1 h, Golgi Stop and Golgi Plug (BD Bioscience) were added and cells were incubated for additional 5 h. Cells were then washed with phosphate-buffered saline (PBS; Gibco, Life Technologies) and stained with viability dye Zombie Aqua (Biolegend). After washing with staining buffer (PBS supplemented with 2% FCS), cells were stained extracellularly for 20 min with the fluorescently conjugated antibody anti-CD8-PE-Cy7 (clone RPA-T8; BD Bioscience). After washing with staining buffer, CytoFix/CytoPerm Solution (BD Bioscience) was added and cells were incubated for 20 min at 4°C. Cells were then washed with 1× Perm/Wash Buffer (BD Bioscience) and stained intracellularly with fluorescently conjugated antibodies: anti-CD3-APC-Cy7 (clone UCHT1; Biolegend), anti-CD4-AF700 (clone RPA-T4; Biolegend), anti-IFN-γ-APC (clone B27; Biolegend), anti-TNF-α-BV421 (clone Mab11; Biolegend), anti-MIP1β-PE (clone D21-1351; BD Bioscience) for 30 min at 4°C. PBMCs were gated on living CD3+CD8+ T cells with the corresponding expression of CD107a, IFN-γ, TNF-α or MIP1β. Data were collected at FACS Celesta (BD Bioscience) and analyzed with the FlowJo Software version 10.0.7 (TreeStar). Based on size and granularity, lymphocytes were discriminated using forward and side scatter. Subsequently, lymphocytes were further divided into living CD3 and CD8 T cells. Gates for the individual cytokines were chosen due to the unstimulated control for every donor separately. The frequency of responding cells was calculated by subtracting negative control from the stimulated sample. Boolean gating on living CD3+CD8+ T cells allowed to analyze polyfunctionality via SPICE Data Analysis.
T cell migration assay.
CD8 T cells were isolated from 7 ml EDTA-supplemented HIV-negative blood via magnetic negative separation using the MACSxpress Whole Blood CD8 T Cell isolation kit (Miltenyi Biotec). Residual erythrocytes were removed afterwards with an additional magnetic separation step using the MACSxpress Erythrocyte Depletion kit (Miltenyi Biotec). Next, isolated CD8 T cells were washed with 10 ml sterile PBS, resuspended in sterile hematopoietic progenitor growth medium (HPGM; Lonza) supplemented with 0.3× sterile Serum Replacement 3 (Sigma-Aldrich), 1× sterile Non-Essential Amino Acids Solution (Thermo Fisher) as well as 1 mM sodium pyruvate (Thermo Scientific) and counted using a Cellometer Auto T4 (Nexcelom Bioscience). Cells were transferred to an ICAM-1-coated (5 µg/ml per well; R&D Systems) hydrophobic, optical imaging, tissue-culture treated 384 µ-well plate (Corning) at a density of 2,500 cells per well. To assess the impact of antiretroviral medications, CD8 T cells were incubated in the presence of DMSO or different ART regimens- emtricitabine (FTC), dolutegravir (DTG), darunavir ethanolate (DRV) and rilpivirine (RLP) (Selleckchem) at previously described bio-active plasma concentrations at 37°C, 5% CO2 for 1 day. After 24 h, cells were stimulated with PBS (control) or 500 ng/ml SDF-1α (Peprotech) and imaged on an AxioObserver.Z1 (Carl Zeiss) with a motorized stage, ×20 magnification and a rate of one frame/15 s for 3 h at 37°C with 0% CO2 atmosphere. Generated Multi-TIFF files were converted to *.mov files. With these files, automated segmentation and tracking were performed using the Automated Cellular Analysis System (ACAS, MetaVí Labs). The parameter presented in the manuscript is the percentage of moving cells upon stimulation.
Assessment of T cell cytokine expression profile.
Cryopreserved PBMCs isolated from HIV-negative individuals were thawed and rested overnight as described above. Cells were transferred in a 24-well polystyrene plate (Starlab) at concentration of 1 million/ml and stimulated with 200 ng/ml SEB (Sigma-Aldrich) (to cause continuous low T cell activation) in the presence of different ART regiments at previously described bio-active plasma concentrations at 37°C, 5% CO2 for 3 days. On day 3, to evoke a strong and best possible response, cells were re-stimulated with 5 µg/ml SEB, and incubated in the presence of co-stimulatory molecule CD28/CD49d (BD Bioscience) together with anti-CD107a-PE-Cy5 (clone H4A3; BD Bioscience) for 6 h. After 1 h, Golgi Stop and Golgi Plug (BD Bioscience) were added and cells were incubated for additional 5 h. Cells were then washed with PBS and stained with viability dye Zombie Aqua (Biolegend). After washing with staining buffer (PBS supplemented with 2% FCS), cells were stained extracellularly for 20 min with the fluorescently conjugated antibody anti-CD8-APC-Cy7 (clone RPA-T8; Biolegend). After washing with staining buffer (PBS supplemented with 2% FCS), CytoFix/CytoPerm Solution (BD Bioscience) was added and cells were incubated for 20 min at 4°C. Cells were then washed with 1× Perm/Wash Buffer (BD Bioscience) and stained intracellularly with fluorescently conjugated antibodies: anti-CD3-PacificBlue (clone UCHT1; Biolegend), anti-CD4-BV421 (clone RPA-P4; Biolegend), anti-MIP1β-PE (clone D21-1351; BD Bioscience), anti-TNF-α-AF700 (clone Mab11; Biolegend), anti-IFN-γ-PE-Cy7 (cloneB27; Biolegend) for 30 min at 4°C. To analyze the ability of cytokine expression of different CD8 T cell subsets (TCM, central memory cells; TEMRA, terminally differentiated effector memory cells and TEM, effector memory cells) cells were stained extracellularly for 20 min with the fluorescently conjugated antibodies: anti-CD27-AF700 (clone O323; Biolegend), anti-CD45RA-PE-Dazzle-594 (clone HI100; Biolegend), anti-CD95-AF647 (clone Dx2; Biolegend), anti-CCR7-PE-Cy7 (clone 3D12; BD Bioscience) and anti-CD8-FITC (clone RPA-T8; Biolegend). After washing and fixation as previously described, cells were stained intracellularly with fluorescently conjugated antibodies: anti-CD3-APC-Cy7 (clone UCHT1; Biolegend), anti-CD4-BV510 (clone RPA-T4; Biolegend), anti-TNF-α-BV605 (clone MAb11; Biolegend), anti-IFN-γ-PE (clone B27; Biolegend) and anti-Granzyme-B-BV421 (clone GB11; BD Bioscience). Data were collected and PBMCs were analyzed with FlowJo (Version 10.1). Based on size and granularity, lymphocytes were discriminated using forward and side scatter. Subsequently, lymphocytes were further divided into living CD3 and CD8 T cells. To delineate memory CD8 T cell subsets, gates for CCR7 and CD45RA were adjusted due to fluorescence minus one (FMO) controls. Gates for the individual cytokines (TNF-α and IFN-γ) were chosen due to the unstimulated control for every HIV-negative donor separately. The frequency of responding cells was calculated by subtracting negative control from the stimulated sample.
Assessment of T cell cytolytic phenotype.
Cryopreserved PBMCs isolated from HIV-negative individuals were thawed and rested overnight as described above. Cells were transferred to a 24-well polystyrene plate (Starlab) at concentration of 1 million/ml per well and either stimulated with 200 ng/ml SEB (Sigma-Aldrich) (to cause continuous low T cell activation) or left unstimulated in the presence of different ART regiments at bio-active plasma concentrations at 37°C, 5% CO2 for 3 days. On day 3, to evoke a strong and best possible response, cells were re-stimulated with 5 µg/ml SEB, and incubated in the presence of co-stimulatory molecule CD28/CD49d (BD Bioscience) together with anti-CD107a-PE-Dazzle 594 (clone H4A3; Biolegend) for 6 h. After 1 h, Golgi Stop and Golgi Plug (BD Bioscience) were added, and cells were incubated for additional 5 h. Cells were then washed with PBS and stained with viability dye Zombie Aqua (Biolegend). After washing with staining buffer (PBS supplemented with 2% FCS), cells were stained extracellularly for 20 min with the fluorescently conjugated antibody anti-CD8-AF647 (clone C8/144B; Biolegend). After washing with staining buffer (PBS supplemented with 2% FCS), CytoFix/CytoPerm Solution (BD Bioscience) was added and cells were incubated for 20 min at 4°C. Cells were then washed with 1× Perm/Wash Buffer (BD Bioscience) and stained intracellularly with fluorescently conjugated antibodies: anti-CD3-APC-Cy7 (clone UCHT1; Biolegend), anti-CD4-AF700 (clone RPA-P4; Biolegend), anti-Granzyme-B-BV421 (clone GB11; BD Bioscience), anti-Perforin-PE-Cy7 (clone B-D48; Biolegend) for 30 min at 4°C. Data were collected at FACSCelesta (BD Bioscience) and analyzed with FlowJo (Version 10.1). Based on size and granularity, lymphocytes were discriminated using forward and side scatter. Subsequently, lymphocytes were gated on living CD3+CD8+Granzyme-B+ or Perforin+ T cells.
Viral inhibition assay.
Cryopreserved PBMCs isolated from HIV infected treatment-naive individuals were thawed and rested overnight as described above. CD8 T cells were subsequently isolated by magnetic positive separation using CD8 T cell Multisort isolation kit (Miltenyi Biotec) and CD4 T cells were isolated by magnetic negative separation using CD4 T Cell isolation kit (Miltenyi Biotec). After isolation, CD4 T cells and CD8 T cells were resuspended in R10 media supplemented with IL-2 (50 U/ml; eBioscience) and transferred to a 48-well polystyrene plate (Starlab) at concentration of 500.000/ml per well. Additionally, CD8 T cells were stimulated with 3 µg/ml SEB (Sigma-Aldrich) and incubated in the presence of DMSO or different ART regimens- emtricitabine (FTC), dolutegravir (DTG) and rilpivirine (RLP) (Selleckchem) at bio-active plasma concentration at 37°C, 5% CO2 for 3 days. On day 3, CD4 T cells were spinoculated at 1200 × g for 1 h with HIV nevirapine-resistant containing supernatant (NIH AIDS Reagents, Bethesda, USA) and subsequently incubated at 37°C, 5% CO2 for 2 h. Afterwards, the virus was washed off the cells. At the same time, CD8 T cells were washed 6 times with sterile PBS to ensure the ART medication was washed off and afterwards cells were cocultured in R10 media supplemented with IL-2 (50 U/ml) and nevirapine (Selleckchem) in a ratio of 1:2 (effector: target ratio) with the nevirapine-resistant virus infected autologous CD4 T cells. At day 3 and 7 postinfection, an HIV-1 Gag p24 Quantikine ELISA (R&D Systems) was performed according to the manufacturer′s instructions. The ELISA plate was measured with a NanoQuant Infinite M2000 plate reader (Tecan).
Metabolic assay.
Cryopreserved PBMCs isolated from HIV negative individuals were thawed, rested overnight and CD8 T cells were subsequently isolated by magnetic separation as described above. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were determined using a Seahorse XFe Extracellular Flux Analyzer according to manufacturer´s protocol (54). Briefly, cells were plated on poly-d-lysine coated 96-well polystyrene Seahorse plate (Agilent) at density of 200,000 cells per well. Cells were equilibrated in nonbuffered DMEM medium supplemented with 2 mM glutamine, 2 mM sodium pyruvate and 10 mM glucose (Agilent) for 45 min at 37°C with 0% CO2 atmosphere prior to the experiment. Metabolic assays were performed using the following concentrations of subsequently injected compounds: oligomycin 1 µM, mitochondrial uncoupler p-trifluormethoxy carbonyl cyanice phenol hydrazine (FCCP) 1 µM, Antimycine A and rotenone (both 0.5 µM; Agilent). At each time interval, the OCR and ECAR values were measured as indicators mitochondrial respiration and glycolysis, respectively.
Statistical analysis.
Prism (GraphPad Software) was used for statistical analysis. Data are presented as mean ± SD. Given donor variance and the sample sizes, data were not normally distributed and the appropriate nonparametric tests were used. Statistical significance is indicated in all figures by the following annotations: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
ACKNOWLEDGMENTS
We thank all the subjects who participated in our studies.
Footnotes
Supplemental material is available online only.
Contributor Information
Hendrik Streeck, Email: hstreeck@uni-bonn.de.
Guido Silvestri, Emory University.
REFERENCES
- 1.Trautmann L. 2016. Kill: boosting HIV-specific immune responses. Curr Opin HIV AIDS 11:409–416. 10.1097/COH.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath SL, Sabbaj S, Bansal A, Kilby JM, Goepfert PA. 2011. CD8 T-cell proliferative capacity is compromised in primary HIV-1 infection. J Acquir Immune Defic Syndr 56:213–221. 10.1097/QAI.0b013e3181ff2aba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Streeck H, Lu R, Beckwith N, Milazzo M, Liu M, Routy JP, Little S, Jessen H, Kelleher AD, Hecht F, Sekaly RP, Alter G, Heckerman D, Carrington M, Rosenberg ES, Altfeld M. 2014. Emergence of individual HIV-specific CD8 T cell responses during primary HIV-1 infection can determine long-term disease outcome. J Virol 88:12793–12801. 10.1128/JVI.02016-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones RB, Walker BD. 2016. HIV-specific CD8+ T cells and HIV eradication. J Clin Invest 126:455–463. 10.1172/JCI80566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reuter MA, Del Rio Estrada PM, Buggert M, Petrovas C, Ferrando-Martinez S, Nguyen S, Sada Japp A, Ablanedo-Terrazas Y, Rivero-Arrieta A, Kuri-Cervantes L, Gunzelman HM, Gostick E, Price DA, Koup RA, Naji A, Canaday DH, Reyes-Teran G, Betts MR. 2017. HIV-specific CD8(+) T cells exhibit reduced and differentially regulated cytolytic activity in lymphoid tissue. Cell Rep 21:3458–3470. 10.1016/j.celrep.2017.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren JA, Clutton G, Goonetilleke N. 2019. Harnessing CD8(+) T cells under HIV antiretroviral therapy. Front Immunol 10:291. 10.3389/fimmu.2019.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arts EJ, Hazuda DJ. 2012. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med 2:a007161. 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789. 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wandeler G, Johnson LF, Egger M. 2016. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS 11:492–500. 10.1097/COH.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun TW, Moir S, Fauci AS. 2015. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat Immunol 16:584–589. 10.1038/ni.3152. [DOI] [PubMed] [Google Scholar]
- 11.Vanhamel J, Bruggemans A, Debyser Z. 2019. Establishment of latent HIV-1 reservoirs: what do we really know? J Virus Erad 5:3–9. 10.1016/S2055-6640(20)30275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, Deeks SG, Luciw PA, Chipman JG, Beilman GJ, Hoskuldsson T, Khoruts A, Anderson J, Deleage C, Jasurda J, Schmidt TE, Hafertepe M, Callisto SP, Pearson H, Reimann T, Schuster J, Schoephoerster J, Southern P, Perkey K, Shang L, Wietgrefe SW, Fletcher CV, Lifson JD, Douek DC, McCune JM, Haase AT, Schacker TW. 2017. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med 23:1271–1276. 10.1038/nm.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deleage C, Wietgrefe SW, Del Prete G, Morcock DR, Hao XP, Piatak M, Jr, Bess J, Anderson JL, Perkey KE, Reilly C, McCune JM, Haase AT, Lifson JD, Schacker TW, Estes JD. 2016. Defining HIV and SIV reservoirs in lymphoid tissues. Pathog Immun 1:68–106. 10.20411/pai.v1i1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buggert M, Nguyen S, McLane LM, Steblyanko M, Anikeeva N, Paquin-Proulx D, Del Rio Estrada PM, Ablanedo-Terrazas Y, Noyan K, Reuter MA, Demers K, Sandberg JK, Eller MA, Streeck H, Jansson M, Nowak P, Sonnerborg A, Canaday DH, Naji A, Wherry EJ, Robb ML, Deeks SG, Reyes-Teran G, Sykulev Y, Karlsson AC, Betts MR. 2018. Limited immune surveillance in lymphoid tissue by cytolytic CD4+ T cells during health and HIV disease. PLoS Pathog 14:e1006973. 10.1371/journal.ppat.1006973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Bronnimann MP, Skinner PJ, Connick E. 2018. The B-cell follicle in HIV infection: barrier to a cure. Front Immunol 9:20. 10.3389/fimmu.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim Y, Anderson JL, Lewin SR. 2018. Getting the “kill” into “shock and kill”: strategies to eliminate latent HIV. Cell Host Microbe 23:14–26. 10.1016/j.chom.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G, Roney J, Watson J, Prince MH, Hoy JF, Chomont N, Fromentin R, Procopio FA, Zeidan J, Palmer S, Odevall L, Johnstone RW, Martin BP, Sinclair E, Deeks SG, Hazuda DJ, Cameron PU, Sekaly RP, Lewin SR. 2014. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 10:e1004473. 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Archin NM, Kirchherr JL, Sung JA, Clutton G, Sholtis K, Xu Y, Allard B, Stuelke E, Kashuba AD, Kuruc JD, Eron J, Gay CL, Goonetilleke N, Margolis DM. 2017. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Invest 127:3126–3135. 10.1172/JCI92684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock G, Moron-Lopez S, Kopycinski J, Puertas MC, Giannoulatou E, Rose A, Salgado M, Hayton EJ, Crook A, Morgan C, Angus B, Chen F, Yang H, Martinez-Picado J, Hanke T, Dorrell L. 2017. Evaluation of the immunogenicity and impact on the latent HIV-1 reservoir of a conserved region vaccine, MVA.HIVconsv, in antiretroviral therapy-treated subjects. J Int AIDS Soc 20:21171. 10.7448/IAS.20.1.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mota TM, McCann CD, Danesh A, Huang SH, Magat DB, Ren Y, Leyre L, Bui TD, Rohwetter TM, Kovacs CM, Benko E, MacLaren L, Wimpelberg A, Cannon CM, Hardy WD, Safrit JT, Jones RB. 2020. Integrated assessment of viral transcription, antigen presentation, and CD8(+) t cell function reveals multiple limitations of class I-selective histone deacetylase inhibitors during HIV-1 latency reversal. J Virol 94. 10.1128/JVI.01845-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker-Sperling VE, Pohlmeyer CW, Tarwater PM, Blankson JN. 2016. The effect of latency reversal agents on primary CD8+ T cells: implications for shock and kill strategies for human immunodeficiency virus eradication. EBioMedicine 8:217–229. 10.1016/j.ebiom.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Addo MM, Yu XG, Rathod A, Cohen D, Eldridge RL, Strick D, Johnston MN, Corcoran C, Wurcel AG, Fitzpatrick CA, Feeney ME, Rodriguez WR, Basgoz N, Draenert R, Stone DR, Brander C, Goulder PJ, Rosenberg ES, Altfeld M, Walker BD. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol 77:2081–2092. 10.1128/jvi.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuster M, Moeller M, Bornemann L, Bessen C, Sobczak C, Schmitz S, Witjes L, Kruithoff K, Kohn C, Just O, Kundgen A, Pundt N, Pelzer B, Ampe C, Van Troys M, Nusch A, Haas R, Germing U, Martens L, Jockel KH, Gunzer M. 2018. Surveillance of myelodysplastic syndrome via migration analyses of blood neutrophils: a potential prognostic tool. J Immunol 201:3546–3557. 10.4049/jimmunol.1801071. [DOI] [PubMed] [Google Scholar]
- 24.Bornemann L, Schuster M, Schmitz S, Sobczak C, Bessen C, Merz SF, Jöckel KH, Haverkamp T, Gunzer M, Göthert JR. 2020. Defective migration and dysmorphology of neutrophil granulocytes in atypical chronic myeloid leukemia treated with ruxolitinib. BMC Cancer 20:650. 10.1186/s12885-020-07130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce EL. 2010. Metabolism in T cell activation and differentiation. Curr Opin Immunol 22:314–320. 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korencak M, Byrne M, Richter E, Schultz BT, Juszczak P, Ake JA, Ganesan A, Okulicz JF, Robb ML, de Los Reyes B, Winning S, Fandrey J, Burgess TH, Esser S, Michael NL, Agan BK, Streeck H. 2019. Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight 4. 10.1172/jci.insight.126675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang SH, Ren Y, Thomas AS, Chan D, Mueller S, Ward AR, Patel S, Bollard CM, Cruz CR, Karandish S, Truong R, Macedo AB, Bosque A, Kovacs C, Benko E, Piechocka-Trocha A, Wong H, Jeng E, Nixon DF, Ho YC, Siliciano RF, Walker BD, Jones RB. 2018. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J Clin Invest 128:876–889. 10.1172/JCI97555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen TM, Altfeld M, Geer SC, Kalife ET, Moore C, O'Sullivan KM, Desouza I, Feeney ME, Eldridge RL, Maier EL, Kaufmann DE, Lahaie MP, Reyor L, Tanzi G, Johnston MN, Brander C, Draenert R, Rockstroh JK, Jessen H, Rosenberg ES, Mallal SA, Walker BD. 2005. Selective escape from CD8+ T-cell responses represents a major driving force of human immunodeficiency virus type 1 (HIV-1) sequence diversity and reveals constraints on HIV-1 evolution. J Virol 79:13239–13249. 10.1128/JVI.79.21.13239-13249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iglesias MC, Almeida JR, Fastenackels S, van Bockel DJ, Hashimoto M, Venturi V, Gostick E, Urrutia A, Wooldridge L, Clement M, Gras S, Wilmann PG, Autran B, Moris A, Rossjohn J, Davenport MP, Takiguchi M, Brander C, Douek DC, Kelleher AD, Price DA, Appay V. 2011. Escape from highly effective public CD8+ T-cell clonotypes by HIV. Blood 118:2138–2149. 10.1182/blood-2011-01-328781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arcia D, Acevedo-Saenz L, Rugeles MT, Velilla PA. 2017. Role of CD8(+) T cells in the selection of HIV-1 immune escape mutations. Viral Immunol 30:3–12. 10.1089/vim.2016.0095. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Zhao Y, Peng Y, Han Z, Liu G, Qin L, Liu S, Sun H, Wu H, Dong T, Zhang Y. 2016. Multiple T-cell responses are associated with better control of acute HIV-1 infection: an observational study. Medicine (Baltimore, MD) 95:e4429. 10.1097/MD.0000000000004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, Brumme CJ, Rosenberg ES, Alter G, Allen TM, Walker BD, Altfeld M. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med 5:e100. 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owen RE, Heitman JW, Hirschkorn DF, Lanteri MC, Biswas HH, Martin JN, Krone MR, Deeks SG, Norris PJ. 2010. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. Aids 24:1095–1105. 10.1097/QAD.0b013e3283377a1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clutton GT, Jones RB. 2018. Diverse impacts of HIV latency-reversing agents on CD8+ T-cell function: implications for HIV cure. Front Immunol 9:1452. 10.3389/fimmu.2018.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McBrien JB, Wong AKH, White E, Carnathan DG, Lee JH, Safrit JT, Vanderford TH, Paiardini M, Chahroudi A, Silvestri G. 2020. Combination of CD8β depletion and Interleukin-15 superagonist N-803 induces virus reactivation in simian-human immunodeficiency virus-infected, long-term ART-treated rhesus macaques. J Virol 94. 10.1128/JVI.00755-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer A, Zhang Y, Perelson AS, Wingreen NS. 2019. Regulation of T cell expansion by antigen presentation dynamics. Proc Natl Acad Sci U S A 116:5914–5919. 10.1073/pnas.1812800116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampton HR, Chtanova T. 2019. Lymphatic migration of immune cells. Front Immunol 10:1168. 10.3389/fimmu.2019.01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Lakkis FG. 2015. Memory T cell migration. Front Immunol 6:504. 10.3389/fimmu.2015.00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shrestha B, Diamond MS. 2007. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J Virol 81:11749–11757. 10.1128/JVI.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassin D, Garber OG, Meiraz A, Schiffenbauer YS, Berke G. 2011. Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology 133:190–196. 10.1111/j.1365-2567.2011.03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malyshkina A, Littwitz-Salomon E, Sutter K, Zelinskyy G, Windmann S, Schimmer S, Paschen A, Streeck H, Hasenkrug KJ, Dittmer U. 2017. Fas Ligand-mediated cytotoxicity of CD4+ T cells during chronic retrovirus infection. Sci Rep 7:7785. 10.1038/s41598-017-08578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M, Jr, Estes JD, Lifson JD, Picker LJ. 2015. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 21:132–139. 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao M, De Crignis E, Rokx C, Verbon A, van Gelder T, Mahmoudi T, Katsikis PD, Mueller YM. 2019. T cell toxicity of HIV latency reversing agents. Pharmacol Res 139:524–534. 10.1016/j.phrs.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Olesen R, Vigano S, Rasmussen TA, Sogaard OS, Ouyang Z, Buzon M, Bashirova A, Carrington M, Palmer S, Brinkmann CR, Yu XG, Ostergaard L, Tolstrup M, Lichterfeld M. 2015. Innate immune activity correlates with CD4 T cell-associated HIV-1 DNA decline during latency-reversing treatment with Panobinostat. J Virol 89:10176–10189. 10.1128/JVI.01484-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolakowska A, Maresca AF, Collins IJ, Cailhol J. 2019. update on adverse effects of HIV integrase inhibitors. Curr Treat Options Infect Dis 11:372–387. 10.1007/s40506-019-00203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norwood J, Turner M, Bofill C, Rebeiro P, Shepherd B, Bebawy S, Hulgan T, Raffanti S, Haas DW, Sterling TR, Koethe JR. 2017. brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 76:527–531. 10.1097/QAI.0000000000001525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moure R, Domingo P, Villarroya J, Gasa L, Gallego-Escuredo JM, Quesada-López T, Morón-Ros S, Maroto AF, Mateo GM, Domingo JC, Villarroya F, Giralt M. 2018. Reciprocal effects of antiretroviral drugs used to treat HIV Infection on the fibroblast growth factor 21/β-Klotho system. Antimicrob Agents Chemother 62. 10.1128/AAC.00029-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bournat JC, Brown CW. 2010. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes 17:446–452. 10.1097/MED.0b013e32833c3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ritov VB, Menshikova EV, Azuma K, Wood R, Toledo FG, Goodpaster BH, Ruderman NB, Kelley DE. 2010. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 298:E49–58. 10.1152/ajpendo.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ando N, Nishijima T, Mizushima D, Inaba Y, Kawasaki Y, Kikuchi Y, Oka S, Gatanaga H. 2021. Long-term weight gain after initiating combination antiretroviral therapy in treatment-naïve Asian people living with human immunodeficiency virus. Int J Infect Dis 110:21–28. 10.1016/j.ijid.2021.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Schöttl T, Kappler L, Fromme T, Klingenspor M. 2015. Limited OXPHOS capacity in white adipocytes is a hallmark of obesity in laboratory mice irrespective of the glucose tolerance status. Mol Metab 4:631–642. 10.1016/j.molmet.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchner DA, Yazbek SN, Solinas P, Burrage LC, Morgan MG, Hoppel CL, Nadeau JH. 2011. Increased mitochondrial oxidative phosphorylation in the liver is associated with obesity and insulin resistance. Obesity (Silver Spring) 19:917–924. 10.1038/oby.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Streeck H, Lichterfeld M, Alter G, Meier A, Teigen N, Yassine-Diab B, Sidhu HK, Little S, Kelleher A, Routy JP, Rosenberg ES, Sekaly RP, Walker BD, Altfeld M. 2007. Recognition of a defined region within p24 gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J Virol 81:7725–7731. 10.1128/JVI.00708-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelletier M, Billingham LK, Ramaswamy M, Siegel RM. 2014. Extracellular flux analysis to monitor glycolytic rates and mitochondrial oxygen consumption. Methods Enzymol 542:125–149. 10.1016/B978-0-12-416618-9.00007-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Materials and Methods; Fig. S1 to S5; Tables S1 and S2<br>. Download jvi.01730-21-s0001.pdf, PDF file, 1.6 MB (1.7MB, pdf)