ABSTRACT
Reactivation of herpes simplex virus 1 (HSV-1) from latently infected neurons of the trigeminal ganglia (TG) leads to blinding recurrent herpetic disease in symptomatic (SYMP) individuals. Although the role of T cells in herpes immunity seen in asymptomatic (ASYMP) individuals is heavily explored, the role of B cells is less investigated. In the present study, we evaluated whether B cells are associated with protective immunity against recurrent ocular herpes. The frequencies of circulating HSV-specific memory B cells and of memory follicular helper T cells (CD4+ Tfh cells), which help B cells produce antibodies, were compared between HSV-1-infected SYMP and ASYMP individuals. The levels of IgG/IgA and neutralizing antibodies were compared in SYMP and ASYMP individuals. We found that (i) the ASYMP individuals had increased frequencies of HSV-specific CD19+CD27+ memory B cells, and (ii) high frequencies of HSV-specific switched IgG+CD19+CD27+ memory B cells detected in ASYMP individuals were directly proportional to high frequencies of CD45R0+CXCR5+CD4+ memory Tfh cells. However, no differences were detected in the level of HSV-specific IgG/IgA antibodies in SYMP and ASYMP individuals. Using the UV-B-induced HSV-1 reactivation mouse model, we found increased frequencies of HSV-specific antibody-secreting plasma HSV-1 gD+CD138+ B cells within the TG and circulation of ASYMP mice compared to those of SYMP mice. In contrast, no significant differences in the frequencies of B cells were found in the cornea, spleen, and bone-marrow. Our findings suggest that circulating antibody-producing HSV-specific memory B cells recruited locally to the TG may contribute to protection from symptomatic recurrent ocular herpes.
IMPORTANCE Reactivation of herpes simplex virus 1 (HSV-1) from latently infected neurons of the trigeminal ganglia (TG) leads to blinding recurrent herpetic disease in symptomatic (SYMP) individuals. Although the role of T cells in herpes immunity against blinding recurrent herpetic disease is heavily explored, the role of B cells is less investigated. In the present study, we found that in both asymptomatic (ASYMP) individuals and ASYMP mice, there were increased frequencies of HSV-specific memory B cells that were directly proportional to high frequencies of memory Tfh cells. Moreover, following UV-B-induced reactivation, we found increased frequencies of HSV-specific antibody-secreting plasma B cells within the TG and circulation of ASYMP mice compared to those of SYMP mice. Our findings suggest that circulating antibody-producing HSV-specific memory B cells recruited locally to the TG may contribute to protection from recurrent ocular herpes.
KEYWORDS: B memory cells, plasma cells, ocular herpes, asymptomatic herpes, virus-specific B cell
INTRODUCTION
With over one billion individuals worldwide currently infected with herpes simplex virus 1 (HSV-1), herpes remains one of the most prevalent viral eye infections (1–3). Ocular herpes is mainly caused by HSV-1, which infects the cornea and then establishes latency in sensory neurons of the trigeminal ganglia (TG). The ability of herpes to establish latency and its unique biological characteristics of using many evasion strategies to evade host immune system control allows it to reactivate from latency and replicate later in life (4). Sporadic spontaneous reactivation of HSV-1 from latently infected neurons leads to viral shedding in saliva and tears, which can ultimately cause symptomatic recurrent herpes stromal keratitis (HSK), a blinding corneal disease. Despite the availability of many intervention strategies, the global picture for ocular herpes continues to deteriorate (5). Current antiviral drug therapies (e.g., acyclovir and derivatives) do not eliminate the virus and reduce recurrent herpetic disease by only ∼45% (6, 7). The challenge in developing an effective herpes treatment or vaccine is to determine the immune correlates of protection (8–14). Profiling humoral immunity in asymptomatic (ASYMP) and symptomatic (SYMP) HSV-1-infected individuals will further help in the following ways: (i) understanding if SYMP individuals have dampened humoral immune response in natural infection, (ii) knowing immune correlates of protection that will help to understand vaccine efficacy, and (iii) standardizing methods to explore vaccine efficacy.
Although the role of CD4+ and CD8+ T cells against HSV-1 reactivation is heavily explored, the role of B cells is less investigated. Unlike T cell memory, which can be tissue resident, recent studies suggest a migratory role for memory B cells (MBCs) (15, 16). Memory B cells are potential antibody-secreting immune cells that differentiate following exposure to the virus. Following differentiation, MBCs remain in the peripheral circulation and secrete antibodies when they are reexposed to their cognate antigen (17). Studying MBCs in HSV-1-infected humans is not possible due to the low abundance of MBCs in peripheral blood. In fact, the low abundance of MBCs for any specific virus makes it challenging to study frequency, specificity, and breadth for the virus of interest. MBCs can be identified by their B cell receptor (BCR), a membrane-bound immunoglobulin (Ig) identical to the antibody that they secrete upon activation (18, 19). In this study, we explored the frequency of MBCs detected in the circulation of asymptomatic and symptomatic HSV-1-infected individuals by two different approaches, namely, antigen-specific flow cytometry and enzyme-linked immunosorbent spot assay (ELISPOT).
To understand the mechanisms that cause a differential humoral response between SYMP and ASYMP herpes individuals, we studied the levels of several B cell-associated ligands and cytokines like APRIL, BAFF, interleukin-21 (IL-21), IL-10, IL-17, and tumor necrosis factor beta (TNF-β) in serum. APRIL (a proliferation-inducing ligand) and BAFF (B cell-activating factor) are ligands that can be released by proteolytic cleavage to form active, soluble homotrimers (20–22). They are expressed by immune cells other than B cells, and their receptors BCMA (B cell maturation antigen) and TACI (TNFR homolog transmembrane activator and Ca2+ modulator and CAML interactor), are expressed exclusively by B cell lineage (23–25). IL-21, apart from its regulatory function plays a role in the maintenance of antigen-specific memory B cells (26), and IL-10 is known to be involved in the germinal center B cell responses (27).
Using the UV-B-induced HSV-1 reactivation mouse model, the frequencies of HSV-1-specific memory B and plasma cells were compared between SYMP and ASYMP mice by flow cytometry and ELISpot within cornea, TG, spleen, and bone marrow and circulation. We found a significant increase in the frequencies of HSV-specific antibody-secreting plasma B cells within the TG of ASYMP mice compared to that of SYMP mice. In contrast, no significant differences were found in the cornea, spleen, and bone marrow. Our findings suggest that circulating HSV-specific antibody-producing memory B cells recruited locally at the TG site could contribute to protection from symptomatic recurrent ocular herpes.
RESULTS
Increased frequency of HSV-1-specific circulating memory B cells in ASYMP HSV-1-infected individuals.
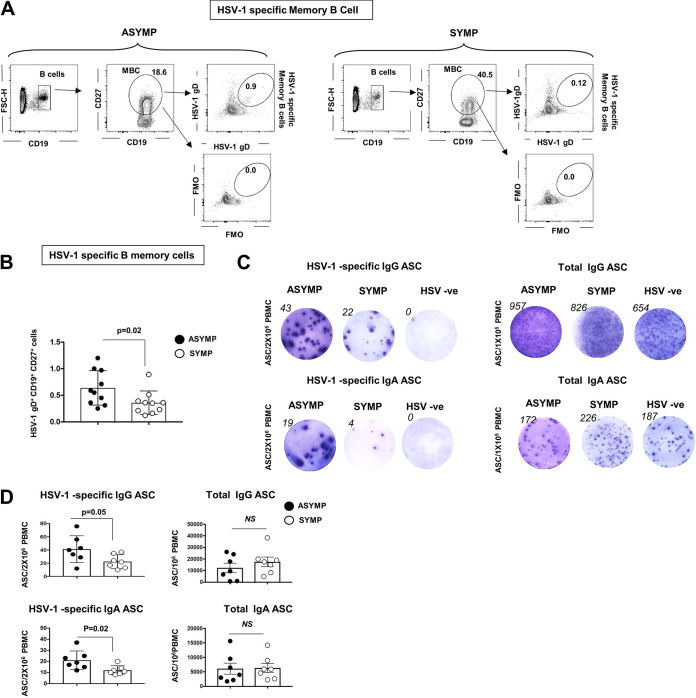
Asymptomatic and symptomatic HSV-1-infected individuals were recruited to the study to understand the role of B cells in herpes. We collected their peripheral blood samples. Peripheral blood mononuclear cells (PBMCs) from ASYMP and SYMP individuals were stained for total B cells (CD19+), memory B cells (CD19+CD27+), IgG memory B cells (IgG+CD19+CD27+), IgA memory B cells (IgA+CD19+CD27+), and IgM memory B cells (IgM+CD19+CD27+). ASYMP and SYMP individuals showed no differences in these general B cell profiles (see Fig. S1 in the supplemental material). We stained the ASYMP and SYMP individuals for HSV-1 glycoprotein D (gD) antigen-specific memory B cells. HSV-1-specific memory B cells were studied by both antigen-specific flow cytometry and enzyme-linked immunosorbent assay (ELISA). PBMCs ex vivo were stained for CD19+CD27+ B cells and analyzed for HSV-1 gD antigen binding cells. As expected, the percentage of herpes-specific memory B cells was very low in the peripheral blood. Memory B cells express the antibody on their surface. HSV-1 gD antigen was conjugated to Alexa Fluor 488 and A647 fluorophores. Cells binding to both the antigen-bound fluorophores were gated as HSV-1-specific cells. To confirm the gate, we performed florescence minus one (FMO) (Fig. 1A) and found an increased percentage of HSV-1 gD binding memory B cells in circulation of ASYMP individuals compared to that of SYMP herpes-positive subjects (0.639 ± 0.102% versus 0.358 ± 0.070%) (Fig. 1B). HSV-1- and HSV-2-negative individuals showed no detectable memory B cells compared to ASYMP individuals and SYMP herpes-positive subjects (data not shown). Single cells in the lymphogate were gated for B cells (CD19+ cells), memory B cells (CD19+CD27+), IgG memory B cells (IgG+CD19+CD27+), IgA memory B cells (IgA+CD19+CD27+), and IgM memory B cells (IgM+CD19+CD27+). FMO controls were run for each sample as controls.
FIG 1.
Circulating HSV-1 gD-specific memory B cell profile in asymptomatic and symptomatic herpes-infected individuals. PBMCs from asymptomatic and symptomatic HSV-1-infected individuals were stained for HSV-1 gD antigen-specific memory B cells. PBMCs were also treated with IL-2 and resiquimod for 5 days for polyclonal stimulation of memory B cells in plasma cells (antibody secreting cells [ASC]). The stimulated cells were then analyzed for HSV-1 gD-specific IgG/IgA ASC and total IgG/IgA ASC by ELISPOT. (A) FACS plot showing the gating strategy for HSV-1-specific memory B cells (CD19+CD27+ B cells) in PBMCs of asymptomatic (ASYMP) (Left) and symptomatic (SYMP) (Right) HSV-1-infected individuals. (B) Graph showing percentage of HSV-1-specific memory B cells (CD19+CD27+ B cells) in PBMCs of ASYMP and SYMP HSV-1-infected individuals. (C) Representative ELISPOT images for anti-HSV-1 IgG ASC (Left, top) and anti-HSV-1 IgA ASC (Left, bottom) from PBMCs of ASYMP and SYMP HSV-1-infected individuals and HSV-1 uninfected individuals (polyclonally stimulated for maturation of memory B cells to ASC). (D) Graph showing anti-HSV-1 IgG (Left, top) and IgA (Left, bottom) ASC; total IgG (Right, top) and total IgA (Right, bottom) ASC from PBMCs of ASYMP and SYMP HSV-1-infected individuals and HSV-1 uninfected individuals. Statistical analysis was done using Student's t test. NS, not significant.
PBMCs from ASYMP, SYMP HSV-1-infected individuals, and HSV-1-uninfected individuals were also treated with IL-2 and resiquimod for 5 days for polyclonal stimulation of memory B cells into plasma cells (antibody secreting cells [ASC]). Stimulated cells were further analyzed for HSV-1 gD-specific IgG/IgA ASC and total IgG/IgA ASC by ELISPOT. We detected an increase in HSV-1 gD-specific ASC (both IgG [41.36 ± 7.7 versus 22.57 ± 3.92] and IgA [21.14 ± 3.15 versus 12 ± 1.5]) in ASYMP compared to that in SYMP (Fig. 1C and D). The ELISPOT findings confirm the flow cytometry results that ASYMP individuals have increased circulating memory cells binding to HSV-1 gD compared to those of SYMP individuals. As expected, HSV-1- and HSV-2-negative individuals showed no detectable HSV-1 gD-specific IgG/IgA ASC compared to ASYMP and SYMP herpes-positive subjects (Fig. 1C). The total IgG/IgA wells were included as controls for each sample.
Circulating HSV-1-specific switched memory B cells positively correlates with memory Tfh.
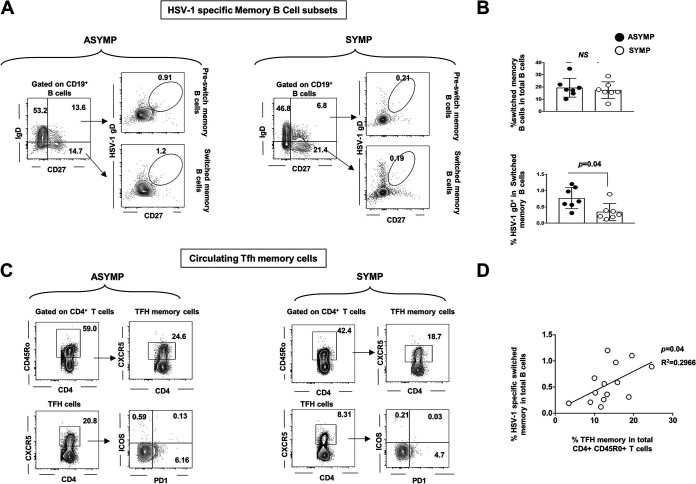
CD19+CD27+IgD− cells are known as “switched” memory B cells, which indicate B cell activation and development in germinal centers in lymph nodes or other secondary lymphoid tissues. PBMCs from asymptomatic and symptomatic HSV-1-infected individuals (not pooled) were stained for memory switched B cells (CD19+CD27+IgD−) and HSV-1 gD-specific memory switched B cells (Fig. 2A). We found a trend toward increased switched memory B cells in ASYMP (0.771 ± 0.12%) compared to those in SYMP (0.345 ± 0.09%) individuals (P = 0.04) (Fig. 2B), indicating that T cell-dependent memory B cells are diminished during SYMP herpes infection.
FIG 2.
Correlation of HSV-1-specific memory B cell and memory T follicular helper T cells in herpes-infected individuals. PBMCs from asymptomatic and symptomatic HSV-1-infected individuals were stained for switched memory B cells (CD19+CD27+IgD−) and HSV-1 gD-specific switched memory B cells. (A) FACS plot showing gating strategy for HSV-1-specific switched memory B cells (CD19+CD27+ B cells) in PBMCs of ASYMP (Left) and SYMP (Right) HSV-1-infected individuals. (B) Graph showing percentage of HSV-1-specific switched memory B cells (CD19+CD27+IgD− B cells) in PBMC of ASYMP and SYMP HSV-1-infected individuals. (C) FACS plot showing gating strategy for T follicular helper cells (Tfh) (CD3+CD4+CXCR5+ T cells) and T follicular helper memory cells (Tfh memory) (CD3+CD4+CD45R0+CXCR5+ T cells) in PBMC of ASYMP (Left) and SYMP (Right) HSV-1-infected individuals. (B) Graph showing correlation of the percentage of HSV-1-specific switched memory B cells (CD19+CD27+IgD− B cells) and T follicular helper memory cells (Tfh memory) (CD3+CD4+CD45R0+CXCR5+ T cells) in PBMCs of HSV-1-infected individuals. Statistical analysis was done using Student's t test. NS, not significant.
Switched memory B cells are derived from naive B cells with T cell help in extrafollicular or germinal centers. Therefore, we explored the levels of circulating T follicular helper T memory cells (cTFH memory) that are found in the circulation of asymptomatic and symptomatic HSV-1-infected individuals by flow cytometry. ASYMP (n = 7) and SYMP (n = 7) individuals’ PBMCs were also stained for T follicular helper (Tfh) cells (CD3+CD4+CXCR5+ T cells) and Tfh memory cells (CD3+CD4+CD45R0+CXCR5+ T cells) (Fig. 2C). We detected a positive correlation between the percentage of HSV-1-specific switched memory B cells (CD19+CD27+IgD− B cells) and T follicular helper memory cells (Tfh memory) (CD3+CD4+CD45R0+CXCR5+ T cells) in PBMCs of HSV-1-infected individuals (P = 0.04). CD4 T cells (excluded for dead cells and doublets) were gated using the expression of CD3 and CD4 and then evaluated to identify Tfh cells (CD3+CD4+CXCR5+ T cells) and Tfh memory cells (CD3+CD4+CD45R0+CXCR5+ T cells).
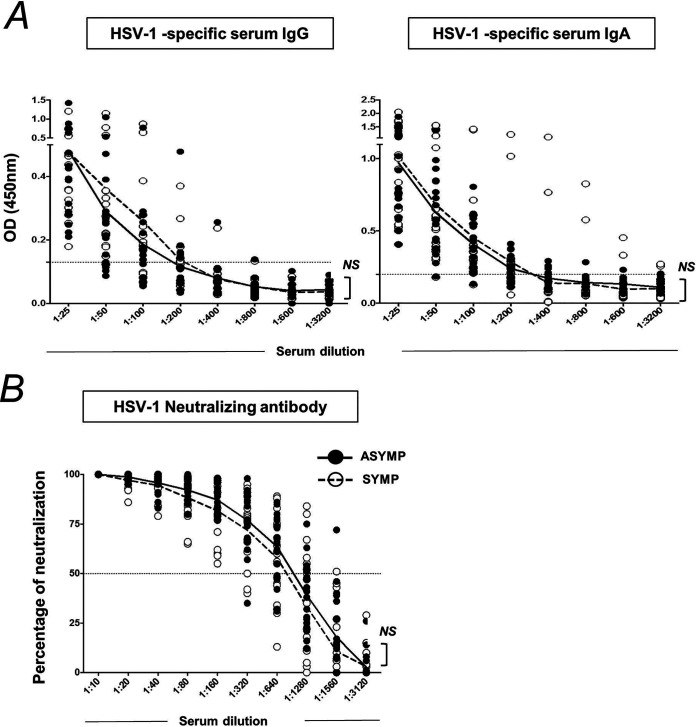
HSV-1 binding antibody and neutralizing antibody levels in plasma of ASYMP and SYMP herpes-infected individuals are similar.
Circulating binding/neutralizing antibodies (secreted by ASC/plasma cells) are the first-line of B cell defense that target the virus. Serum from asymptomatic and symptomatic HSV-1-infected individuals was used in the estimation of anti-HSV-1 gD antibodies by ELISA. No significant difference in the anti-HSV-1 IgG and IgA antibody levels in plasma of ASYMP and SYMP herpes-infected individuals was observed (Fig. 3C). Neutralizing antibody titers were completed by the plaque reduction assay. Similarly, the anti-HSV-1-neutralizing antibody titers of ASYMP (50% plaque reduction/neutralization titer [PRNT50] is 832) and SYMP (PRNT50 is 1,024) herpes individuals did not vary significantly. PRNT50 represents reciprocal serum dilution at which 50% virus neutralization was observed. Controls included wells without any serum.
FIG 3.
Anti-HSV-1 IgG and IgA antibody levels and neutralizing antibody titer in ASYMP and SYMP herpes-infected individuals. Serum samples from asymptomatic and symptomatic HSV-1-infected individuals were used for an estimation of anti-HSV-1 gD antibodies by ELISA. (A) Graphs showing levels of anti-HSV-1 IgG antibody level (Left) and anti-HSV-1 IgA antibody level (Left) in serum of ASYMP and SYMP herpes-infected individuals. (A) Graph showing anti-HSV-1 neutralizing antibody titer in serum of ASYMP and SYMP herpes-infected individuals. Statistical analysis was done using Student's t test. NS, not significant.
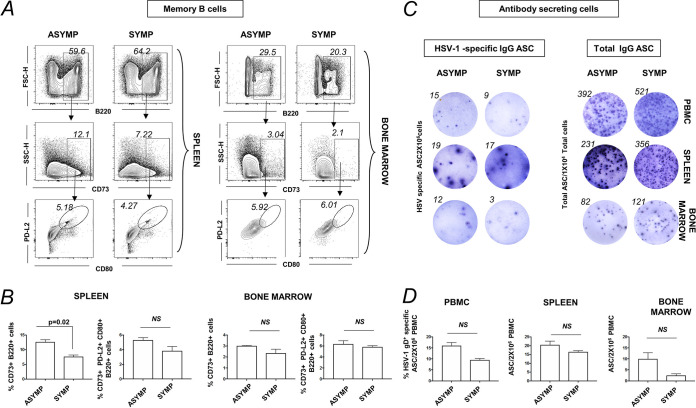
Increased memory B cells in spleen of ASYMP HSV-1-reactivated mice.
To further understand the role of circulating memory B cells in asymptomatic herpes, we used UV-B reactivation in the HSV-1-infected mouse model. Cornea of B6 mice were infected with HSV-1 McKrae (1 × 106 PFU/eye), and virus reactivation was provoked at day 35 postinfection (p.i.) in latently infected mice, using a 60-s corneal UV-B irradiation. At day 6 postreactivation, mice were categorized into ASYMP or SYMP depending on disease occurrence, euthanized, and immune cells from peripheral blood, spleen, and bone marrow were collected for flow cytometry staining of B memory cells. Memory B cells are B220+CD73+ B cells and their subsets B220+CD73+CD80+PD-L2+ B cells (Fig. 4A). The percentage of memory B cells B220+CD73+ in the spleen was observed to be higher in ASYMP (12.65 ± 0.55%) compared to SYMP (7.64 ± 0.42%), while no difference was observed in the memory B cell bone marrow in ASYMP and SYMP infected mice (3.01 ± 0.03% versus 2.35 ± 0.25%) (Fig. 4B).
FIG 4.
Memory B cell profile in PBMCs, spleen, and bone marrow of ASYMP and SYMP HSV-1-reactivated mice. For this experiment, the corneas of B6 mice were infected with HSV-1 McKrae (1 × 106 PFU/eye) by scarification, and virus reactivation was provoked at day 35 p.i. in latently infected mice using 60 s corneal UV-B irradiation. At day 6 postreactivation, mice were categorized into ASYMP or SYMP depending on disease occurrence. ASYMP and SYMP mice were euthanized and immune cells from peripheral blood, spleen, and bone marrow were collected for flow cytometry staining for memory B cells. (A) FACS plot showing representative plots for memory B cells (B220+CD73+ B cells) and the subsets (B220+CD80+PD-L2+ B cells) in spleen (Left) and bone marrow (Right) of ASYMP and SYMP infected mice. (B) Graph showing percentage of memory B cells (B220+CD73+ B cells) and the subsets (B220+CD73+CD80+PD-L2+ B cells) in spleen (left) and bone marrow (Right) of ASYMP and SYMP infected mice. (C) Representative ELISPOT images for anti-HSV-1 IgG ASC (Left) and total IgG ASC (Right) from PBMCs, spleen, and bone marrow from ASYMP and SYMP infected mice (polyclonally stimulated with mouse polyclonal B cell activator (IL-2 and resiquimod) for maturation of memory B cells to ASC). Graph showing anti-HSV-1 IgG (Left) and total IgG (Right) ASC from PBMCs of ASYMP, SYMP HSV-1-infected mice. Statistical analysis was done using Student's t test. NS, not significant.
Immune cells from the peripheral blood, spleen, and bone marrow were stimulated with mouse polyclonal B cell activator (IL-2 plus resiquimod) for maturation of memory B cells to ASC (using human B-Poly-S from ImmunoSpot, OH, USA). The stimulated cells were incubated in ELISPOT plates to enumerate anti-HSV-1 IgG ASC and total IgG ASC (from PBMCs, spleen, and bone marrow). Anti-HSV-1 IgG ASC from PBMCs, spleen, and bone marrow (BM) depicted an increased trend in ASYMP compared to that in SYMP individuals. The results from the mouse reactivation model confirm the results from humans showing an increased memory B cell profile in ASYMP herpes. However, we did not find any differences in the memory B cell frequency in the cornea of infected ASYMP mice compared to SYMP mice (data not shown).
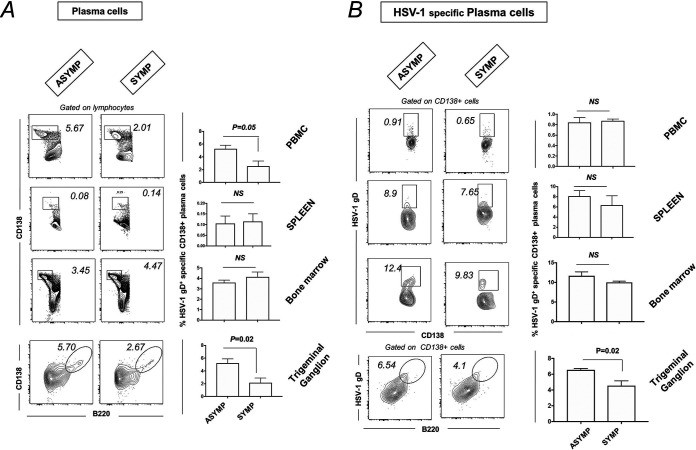
Increased plasma cells in TG of ASYMP mice compared to SYMP mice.
ASYMP and SYMP mice were euthanized, and immune cells from peripheral blood, spleen, BM, and TG cells were stained for plasma cells (CD138+ B cells) by flow cytometry. There was an increase in the percentage of plasma B cells (CD138+ B cells) in TG of ASYMP (5.23 ± 0.47%) compared to that of SYMP (2.15 ± 0.50%) mice (Fig. 5A). ASYMP mice had increased HSV-1-specific plasma B cells (HSV-1 gD+ CD138+ B cells) (6.53 ± 0.15% versus 4.54 ± 0.44%) compared to those of SYMP mice (Fig. 5B).
FIG 5.
Plasma cell profile in PBMC, spleen, bone marrow, and TG of ASYMP and SYMP HSV-1-infected mice. For this experiment, the corneas of B6 mice (n = 6) were infected with HSV-1 McKrae (1 × 106 PFU/eye) by scarification, and virus reactivation was provoked at day 35 p.i. in latently infected mice using 60 s corneal UV-B irradiation. At day 6 postreactivation, mice were categorized into ASYMP or SYMP depending on disease occurrence. ASYMP and SYMP mice were euthanized, and immune cells from peripheral blood, spleen, and bone marrow were collected for flow cytometry staining for plasma cells. (A) Representative plots for plasma B cells (CD138+ B cells) in PBMC, spleen, BM, and TG (Right) of ASYMP and SYMP mice is shown. Graph showing percentage of plasma B cells (CD138+ B cells) in PBMC, spleen, PBMC, and TG (Right) of ASYMP and SYMP mice. (B) Representative FACS plot showing HSV-1-specific plasma B cells (HSV-1 gD+CD138+ B cells) in PBMC, spleen, PBMC, and TG (Left) of ASYMP and SYMP mice. Graph showing percentage of HSV-1-specific plasma B cells (HSV-1 gD+CD138+ B cells) in PBMC, spleen, PBMC, and TG (Right) of ASYMP and SYMP mice.
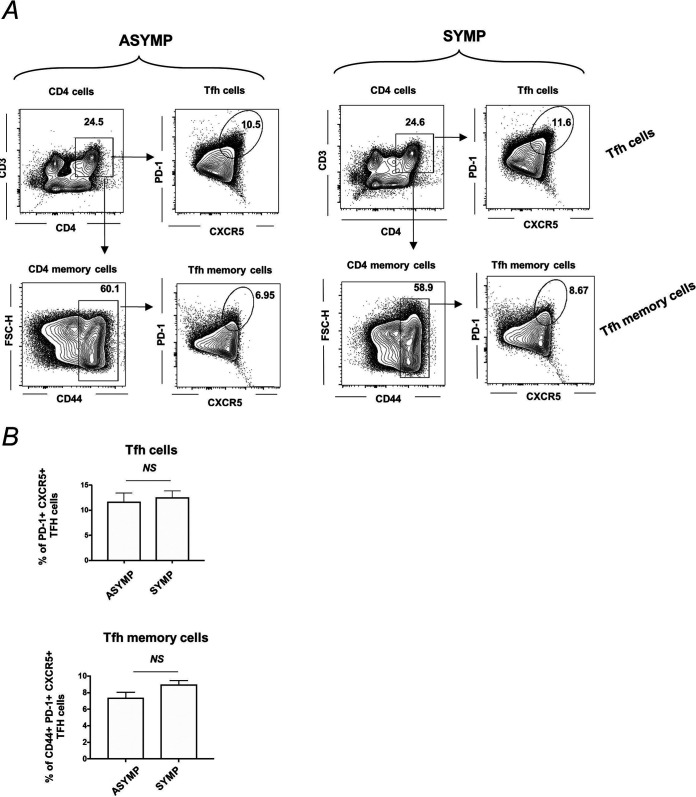
Tfh and Tfh memory cell profile in spleen of ASYMP and SYMP HSV-1-infected mice.
Spleen cells were collected for flow cytometry staining of T follicular helper (Tfh) (CD3+CD4+CXCR5+PD-1+ cells) and T follicular helper memory (Tfh memory cells) (CD3+CD4+CD44+CXCR5+PD-1+ cells) cells. There was no difference detected in the percentages of Tfh cells (top) and Tfh memory cells (bottom) in spleen of ASYMP (Tfh, 11.7 ± 1.2%; Tfh memory, 7.45 ± 0.45%) and SYMP (Tfh, 12.57 ± 0.88%; Tfh memory, 8.99 ± 0.32%) infected mice (Fig. 6A and B).
FIG 6.
T follicular helper (Tfh) and T follicular helper memory (Tfh memory) cell profile in spleen of ASYMP and SYMP HSV-1-infected mice. B6 mice (n = 6) were infected with HSV-1 McKrae (1 × 106 PFU/eye) by scarification, and at day 35 p.i., reactivation was done by 60 s corneal UV-B irradiation. Mice were categorized as ASYMP/SYMP and euthanized at day 6 postreactivation. Spleen cells were collected for flow cytometry staining of Tfh (CD3+CD4+CXCR5+PD-1+ cells) and Tfh memory (CD3+CD4+CD44+CXCR5+PD-1+ cells). (A) Representative FACS plots for Tfh cells (CD3+CD4+CXCR5+PD-1+ cells) are shown in the top panel, and Tfh memory cells (CD3+CD4+CD44+CXCR5+PD-1+ cells) are shown in bottom panels for ASYMP (Left) and SYMP (Right) infected mice. (A) Graph showing percentage of Tfh cells (top) and Tfh memory cells (bottom) in spleen of ASYMP and SYMP infected mice is shown.
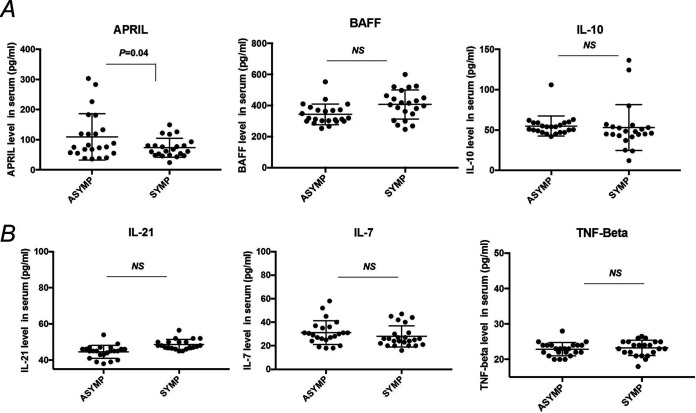
Increased APRIL (B cell proliferation-inducing ligand) in asymptomatic versus symptomatic herpes individuals.
Asymptomatic and symptomatic patients’ serum samples (heat-inactivated at 56°C for 30 min) were assayed for cytokines involved in B cell development APRIL (a B cell proliferation-inducing ligand), BAFF (B cell activating factor), IL-10, IL-21, IL-7, and TNF-β (B cell development cytokine) by Luminex. We found increased APRIL levels in serum of ASYMP herpes individuals (109.4 ± 16.03 pg/mL) compared to those of SYMP herpes individuals (73.5 ± 6.7 pg/mL). The levels of other B cell-associated cytokines such as BAFF, IL-10, IL-21, IL-7, and TNF-β did not vary significantly between asymptomatic and symptomatic herpes individuals (Fig. 7).
FIG 7.
B cell ligand and cytokine level in serum of asymptomatic and symptomatic herpes. Asymptomatic (n = 20) and symptomatic (n = 20) patient serum samples were assayed for cytokines involved in B cell development. Graph showing serum level of APRIL (B cell proliferation inducing ligand), BAFF (B cell-activating factor), and IL-10 (B regulatory cell cytokine) (A) as well as IL-21 (involved in expansion and differentiation of plasma cells), IL-7 (B cell development cytokine), and TNF-β (B cell development cytokine) by Luminex. Statistical analysis was done using Student's t test. NS, not significant.
DISCUSSION
The development of an effective therapy to alleviate symptoms of recurrent herpes stromal keratitis is dependent on our understanding of immune response to herpes infection. In the current study, we are examining the role of B cell-mediated immunity in the human response to HSV-1 reactivation. Anti-herpes antibodies are reported to not be protective during primary herpes infection (28–30). Reports indicate that high titers of herpes-neutralizing antibodies in the blood of symptomatic herpes is neither associated with the frequency of symptomatic herpes (31, 32) nor protection after vaccination (30, 33). Thus, the general understanding is that although anti-herpes antibodies are neutralizing or binding, they are not protective (34, 35). Another theory states that certain HSV-specific antibodies may function protectively in tissue near the site of viral release. However, circulating antibody levels do not reflect this tissue-based event (36). Cell-to-cell spread of the virus is sufficient for propagation and development of symptomatic reactivation even in the presence of effective neutralizing antibodies. Therefore, the concentration of such antibodies is below the threshold of efficacy (37). Recent evidence suggests a more migratory role of B cells to the site of herpes reactivation in skin (16).
Memory B cells can survive for long periods and can induce faster and stronger humoral responses when they reencounter the same antigen (34), in contrast to plasma cells, which provide the first line of protection against infection but do not respond to the second infection because of low expression of membrane-bound Ig (35). There is evidence that indicates that human memory B cells reside mostly in the spleen, and some memory B cells recirculate in the blood. In the present study, we evaluated whether and if memory B cell response is associated with protection in HSV-1 infection, a question that is not yet fully understood. The frequencies of memory B cells and a counterpart of follicular helper T cells (Tfh) found in circulation of asymptomatic and symptomatic HSV-1-infected individuals were studied by flow cytometry. The memory B cells can respond to reinfection with rapid formation of extrafollicular foci, thereby broadening the oligo-clonal repertoire of germ line-encoded B cells causing a broadening of the antiviral B cell repertoires (38). Our results show that asymptomatic HSV-1-infected individuals do not only have an increased frequency of circulating memory B cells specific to HSV-1 gD antigen but also an increased HSV-1-specific memory B cell functional response as estimated by ELISPOT, and the levels of circulating HSV-1-specific memory B cells were directly proportional to the levels of circulating follicular helper CD4 T cells. We also examined if there were any differences in B cell development-associated cytokines and ligands (APRIL, BAFF, IL-10, IL-21, TNF-β) in the serum samples of ASYMP and SYMP herpes individuals. We found that APRIL, released by myeloid and stromal cells, which can enhance the longevity of the humoral immune response, was increased in the serum of asymptomatic herpes individuals.
Currently, there is no effective cure or vaccine against HSV infection. HSV vaccines from the past focused on subunit formulations designed to elicit neutralizing antibodies targeting the envelope glycoprotein D (gD). Passive antibody transfer and sequential infection experiments demonstrated “original antigenic suppression,” a phenomenon in which antibodies suppress memory responses to the priming antigenic site. While these vaccines elicited high levels of neutralizing antibodies in animals and humans, they failed to protect against HSV-2 infections in clinical trials. Our observation showed that in spite of increased memory B cells in circulation, the herpes antibody levels remained the same between ASYMP and SYMP individuals. Thus, we wanted to explore whether HSV-1-specific memory B cells found in circulation would possibly have any role or effect that is confined locally at the site of reactivation in asymptomatic herpes using a UV-B-induced HSV-1 reactivation mouse model. Although the spleen and tonsil are the major reservoirs for antigen-specific human memory B cells, they appear to be dispensable for preserving immunological memory following a reencounter with the antigen (39). There was a trend toward an increased memory B cell response but not the antibody-secreting plasma cells in PBMCs, spleen, and bone-marrow of asymptomatic compared to symptomatic HSV-1-reactivated mice. In another recent study, investigators have shown that maternal antibodies were found at fetal trigeminal ganglia conferring complete protection for newborn mice against HSV infection. In our study, we detected an increase in HSV-1-specific plasma B cells in the TG of asymptomatic mice compared to symptomatic mice. Thus, the discordance between HSV-specific memory B cells and antibody levels in circulation in human natural infection can be explained by a virus-driven memory B cell recruitment mechanism that leads to antibody production at the site of reactivation (TG) using mouse model. Our findings suggest that circulating HSV-specific antibody-producing memory B cells recruited locally at the TG site could contribute to protection from symptomatic recurrent ocular herpes.
MATERIALS AND METHODS
Human study population and PBMC isolation.
Twenty-two volunteers (10 ASYMP, 10 SYMP, and 2 HSV-1 and HSV-2 negative) were enrolled in the study (Table 1). Peripheral blood was collected, and PBMCs (peripheral blood mononuclear cells) were isolated using the Ficoll-Paque density gradient. The cells were then washed in phosphate-buffered saline (PBS) and resuspended in complete culture medium consisting of RPMI 1640 medium containing 10% fetal bovine serum (FBS) (Bio-Products, Woodland, CA) supplemented with 1× penicillin/l-glutamine/streptomycin, 1× sodium pyruvate, and 1× nonessential amino acids. The blood plasma was stored at −20°C for further evaluation of herpes-specific antibodies. HSV-1 infection was confirmed using a commercially available kit that detects anti-gG antigen of HSV-1 (HerpeSelect1 IgG ELISA; Focus Diagnostics, Cypress, CA).
TABLE 1.
Cohorts of HSV-1-seropositive symptomatic and asymptomatic subjects enrolled in the study
| Subject-level characteristic | All subjects (n = 22) |
|---|---|
| Gender (no. [%]) | |
| Female | 14 (64) |
| Male | 8 (36) |
| Race (no. [%]) | |
| White | 12 (55) |
| Nonwhite | 10 (45) |
| Age (yr) (median [range]) | 40 (23–67) |
| HSV status (no. [%]) | |
| HSV-1 positive | 10 (45) |
| HSV-2 positive | 10 (45) |
| HSV-1 and HSV-2 negative | 2 (9) |
| Herpes disease status (no. [%]) | |
| ASYMP | 10 (50) |
| SYMP | 10 (50) |
Flow cytometry.
PBMCs of HSV-1-infected individuals were stained ex vivo with the following flow antibodies listed below. Additionally, HSV-1 gD antigen (Virusys Corporation, Taneytown, MD) was coupled using fluorescein isothiocyanate (FITC) or AP lightning kit (Novus Biologicals, Littleton, CO) to detect HSV-1-specific B memory cells ex vivo (40). ASYMP and SYMP mice were euthanized, and immune cells from the peripheral blood, spleen, bone marrow, TG, and cornea were collected for flow cytometry staining for memory B cells and T follicular helper (Tfh) cells. Harvested TG was digested with collagenase III (5 mg/mL) in RPMI 1640 containing 10% FBS, 1% antibiotic/antimycotic, and gentamicin at 37° C. TG and cornea were dissociated with a 3-mL syringe-plunger head in the presence of medium. Cell suspensions were passed through a 40-μm filter before staining. Single cell suspensions were labeled with the following fluorochrome-conjugated monoclonal antibodies (MAbs): anti-mouse B220, CD73, PD-L2, CD80, CD45(A20), CD3(145-2C11), CD4(GK1.5), CXCR5, CD44, CD62L, ICOS, and PD-1 (BD Biosciences, San Jose, CA). For surface staining, MAbs were added against various cell markers for a total of 1 × 106 cells in PBS containing 1% FBS and 0.1% sodium azide (fluorescence-activated cell sorter [FACS] buffer) and left for 45 min at 4°C. For intracellular/intranuclear staining, cells were first treated with Cytofix/Cytoperm (BD Biosciences) for 30 min. Upon washing with Perm/Wash buffer, MAbs were added to the cells and incubated for 45 min on ice in the dark, washed with Perm/TFFACS buffer, and fixed in PBS containing 2% paraformaldehyde. Labeled cells were suspended in 1% bovine serum albumin (BSA) in PBS and analyzed using the BD Fortessa flow cytometer. Intracellular staining was performed to detect HSV-1-specific plasma cells.
HSV-1 gD-specific ASC ELISPOT assay.
PBMCs from human or immune cells were stimulated (2 to 4 million cells/mL) in B cell medium containing human or mouse polyclonal B cell activator (Immunospot) for 5 days. Both CTL human B-Poly-S and CTL mouse B-Poly-S are stock solutions containing resiquimod and either recombinant human IL-2 or recombinant mouse IL-2, respectively, used for the polyclonal expansion of memory B cells. This will activate the memory B cells to ASC. Cells were then washed in RPMI medium and plated in specified cell numbers in ELISPOT membrane plates coated with either HSV-1 gD antigen (Virusys) (1 ng/well) or IgG/IgA capture antibody (ImmunoSpot Basic ELIPOT kits). The ASC-secreting cells were detected 48 h after the of addition of cells to the ELISPOT plates.
HSV-1-specific IgA/IgG ELISA.
Serum or plasma was isolated from blood by centrifugation for 10 min at 800 × g. The heat-inactivated HSV-1 McKrae strain was used for coating the ELISA plates (Nunc Immunosorbent). The affinity of binding of antigen-specific antibodies to the HSV-1 McKrae strain was measured by ELISA plates coated overnight at 4°C with 104 PFU/well heat-inactivated HSV-1. Heat-inactivated human or mouse serum (56°C for 1 h) was then incubated at a specified dilution overnight at 4°C. HSV-1-specific IgG/IgA were then detected using human or mouse IgG/IgA detection antibodies conjugated to horseradish peroxidase (HRP). Subsequently, the TMB (3,3′,5,5′-tetramethylbenzidine) substrate was added to stop the reaction before reading at 450 nm using the ELISA plate reader (iMark microplate reader; Bio-Rad).
Viral plaque neutralization assay.
Neutralizing antibody titers were determined by incubating 100 PFU of HSV-1 strain McKrae with serial dilutions of serum starting at 1:40 for 1 h at 37°C. The endpoint neutralization titer was determined by the plaque assay on rabbit skin (RS) cells and was calculated as the serum dilution that reduced the number of plaques by 50% compared with PBS controls.
Virus propagation and titration.
For virus propagation, RS cells (ATCC, Manassas, VA) were grown in minimum essential medium Eagle with Earl’s salts and l-glutamine (Corning, Manassas, VA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. The HSV-1 laboratory strain McKrae was propagated in RS cells and purified by ultracentrifugation in a sucrose gradient and titrated by the plaque assay.
Mice and infection.
All animals were handled with care according to the guidelines of the American Association for Laboratory Animal Science (AALAS). For primary herpes infection, 6- to 8-week-old male and female B6 mice were purchased from Jackson Laboratory. The mice were anaesthetized with xylazine (6.6 mg/kg) and ketamine (100 mg/kg) prior to infection. Both corneas in each mouse was briefly scarified with a 25-gauge needle, tear film blotted, and 1 × 106 PFU/eye of HSV-1 (strain McKrae) in 2 μL of sterile PBS were inoculated into the cornea. Corneal infection in all of the infected mice was confirmed by the viral plaque assay in tear swabs. Virus shedding in tear swabs was collected at day 2, 7, and 7 postinfection (p.i.). At day 35 p.i., eyes were reactivated by exposure to UV-B radiation for 1 min, and at day 6 postreactivation, mice were categorized into ASYMP or SYMP depending on disease occurrence. Mice were scored at day 6 post-UV-B reactivation for pathological symptoms of stromal keratitis (scored as follows: 0, no disease; 1, cloudiness, some iris detail visible; 2, iris detail obscured; 3, cornea totally opaque; and 4, cornea perforation). Mice with a disease score of 0 or 1 were grouped as ASYMP, while mice with a disease score between 2 and 4 were grouped as SYMP. ASYMP and SYMP mice were euthanized, and immune cells from peripheral blood, spleen, bone marrow, and TG were collected for flow cytometry staining for memory B cells and Tfh cells.
Quantification of infectious virus.
Tears were collected from both eyes using a Dacron swab (type 1; Spectrum Laboratories, Los Angeles, CA) on days 3, 5, and 7 p.i. Individual swabs were transferred to a 2-mL sterile cryogenic vial containing 1 mL culture medium and stored at −80°C until further use. The HSV-1 titers in tear samples were determined by standard plaque assays on RS cells as previously described (41). Eye swabs (tears) were analyzed for viral titers using the plaque assay. RS cells were grown to 70% confluence in 24-well plates. Infected monolayers were incubated at 37°C for 1 h and rocked every 15 min for viral adsorption and then overlaid with medium containing carboxymethyl cellulose. After 48 h of incubation at 37°C, cells were fixed and stained with crystal violet viral plaques and counted under a light microscope. Positive control assays used previously titrated laboratory stocks of McKrae.
B cell development by Luminex.
Asymptomatic and symptomatic patient serum samples (heat-inactivated at 56°C for 30 min) were assayed for cytokines involved in B cell development, namely, APRIL, BAFF, IL-10, IL-21, IL-7, and TNF-β using the Luminex kit according to the manufacturer’s instructions (R&D systems). Samples were assayed using the Luminex assay system (Magpix).
Statistical analyses.
Data for each assay were compared by analysis of variance (ANOVA) and Student's t test using GraphPad Prism version 5 (La Jolla, CA). Differences between the groups were identified by ANOVA and multiple comparison procedures as we previously described (42). Data are expressed as the mean ± standard deviation (SD). Results were considered statistically significant at a P value of <0.05.
ACKNOWLEDGMENTS
This work is supported by Public Health Service Research R01 grants EY026103, EY019896, and EY024618 from the National Eye Institute (NEI) and R21 grant AI110902 from National Institute of Allergy and Infectious Diseases (NIAID) (to L.B.), and in part by the Discovery Center for Eye Research (DCER) and the Research to Prevent Blindness (RPB) grant. This work is supported by a grant from Trefoil Therapeutics, Inc. and by Public Health Service research grant EY14900 from the National Eye Institute and AI150091, AI143348, AI147499, AI143326, AI138764, and AI124911 from the National Institute of Allergy and Infectious Diseases (NIAID) to L.B.
The authors have declared that no conflict of interest exists.
Footnotes
Supplemental material is available online only.
Contributor Information
Lbachir BenMohamed, Email: lbenmoha@uci.edu.
Jae U. Jung, Lerner Research Institute, Cleveland Clinic
REFERENCES
- 1.Duan R, Xu Y, Zheng L, Yao Y. 2020. Research progress on etiologic diagnosis of ocular viral diseases. Zhejiang Da Xue Xue Bao Yi Xue Ban 49:644–650. (In Chinese.) 10.3785/j.issn.1008-9292.2020.07.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erdem E, Harbiyeli İİ, Öztürk G, Oruz O, Açıkalın A, Yağmur M, Ersöz R, Yarkın F. 2020. Atypical herpes simplex keratitis: frequency, clinical presentations and treatment results. Int Ophthalmol 40:659–665. 10.1007/s10792-019-01226-1. [DOI] [PubMed] [Google Scholar]
- 3.Khieu C, Kongyai N, Pathanapitoon K, Van Der Eijk AA, Rothova A. 2020. Causes of hypertensive anterior uveitis in Thailand. Ocul Immunol Inflamm 28:559–565. 10.1080/09273948.2019.1678651. [DOI] [PubMed] [Google Scholar]
- 4.Agelidis AM, Shukla D. 2015. Cell entry mechanisms of HSV: what we have learned in recent years. Future Virol 10:1145–1154. 10.2217/fvl.15.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liesegang TJ. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13. 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Herpetic Eye Disease Study Group. 1998. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med 339:300–306. 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 7.Kalke K, Lehtinen J, Gnjatovic J, Lund LM, Nyman MC, Paavilainen H, Orpana J, Lasanen T, Frejborg F, Levanova AA, Vuorinen T, Poranen MM, Hukkanen V. 2020. Herpes simplex virus type 1 clinical isolates respond to UL29-targeted siRNA swarm treatment independent of their acyclovir sensitivity. Viruses 12:1434. 10.3390/v12121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulon PG, Roy S, Prakash S, Srivastava R, Dhanushkodi N, Salazar S, Amezquita C, Nguyen L, Vahed H, Nguyen AM, Warsi WR, Ye C, Carlos-Cruz EA, Mai UT, BenMohamed L. 2020. Upregulation of multiple CD8+ T cell exhaustion pathways is associated with recurrent ocular herpes simplex virus type 1 infection. J Immunol 205:454–468. 10.4049/jimmunol.2000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhanushkodi NR, Srivastava R, Prakash S, Roy S, Coulon PA, Vahed H, Nguyen AM, Salazar S, Nguyen L, Amezquita C, Ye C, Nguyen V, BenMohamed L. 2020. High frequency of gamma interferon-producing PLZFloRORgammatlo invariant natural killer 1 cells infiltrating herpes simplex virus 1-infected corneas is associated with asymptomatic ocular herpesvirus infection. J Virol 94:e00140-20. 10.1128/JVI.00140-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava R, Coulon PA, Prakash S, Dhanushkodi NR, Roy S, Nguyen AM, Alomari NI, Mai UT, Amezquita C, Ye C, Maillere B, BenMohamed L. 2020. Human epitopes identified from herpes simplex virus tegument protein VP11/12 (UL46) recall multifunctional effector memory CD4+ TEM cells in asymptomatic individuals and protect from ocular herpes infection and disease in “humanized” HLA-DR transgenic mice. J Virol 94:e01991-19. 10.1128/JVI.01991-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy S, Coulon PG, Prakash S, Srivastava R, Geertsema R, Dhanushkodi N, Lam C, Nguyen V, Gorospe E, Nguyen AM, Salazar S, Alomari NI, Warsi WR, BenMohamed L. 2019. Blockade of PD-1 and LAG-3 immune checkpoints combined with vaccination restores the function of antiviral tissue-resident CD8+ TRM cells and reduces ocular herpes simplex infection and disease in HLA transgenic rabbits. J Virol 93:e00827-19. 10.1128/JVI.00827-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vahed H, Agrawal A, Srivastava R, Prakash S, Coulon PA, Roy S, BenMohamed L. 2019. Unique type I interferon, expansion/survival cytokines, and JAK/STAT gene signatures of multifunctional herpes simplex virus-specific effector memory CD8+ TEM cells are associated with asymptomatic herpes in humans. J Virol 93:e01882-18. 10.1128/JVI.01882-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srivastava R, Coulon PG, Roy S, Chilukuri S, Garg S, BenMohamed L. 2018. Phenotypic and functional signatures of herpes simplex virus-specific effector memory CD73+CD45RAhighCCR7lowCD8+ TEMRA and CD73+CD45RAlowCCR7lowCD8+ TEM cells are associated with asymptomatic ocular herpes. J Immunol 201:2315–2330. 10.4049/jimmunol.1800725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan AA, Srivastava R, Vahed H, Roy S, Walia SS, Kim GJ, Fouladi MA, Yamada T, Ly VT, Lam C, Lou A, Nguyen V, Boldbaatar U, Geertsema R, Fraser NW, BenMohamed L. 2018. Human asymptomatic epitope peptide/CXCL10-based prime/pull vaccine induces herpes simplex virus-specific gamma interferon-positive CD107+ CD8+ T cells that infiltrate the corneas and trigeminal ganglia of humanized HLA transgenic rabbits and protect against ocular herpes challenge. J Virol 92:e00535-18. 10.1128/JVI.00535-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh JE, Iijima N, Song E, Lu P, Klein J, Jiang R, Kleinstein SH, Iwasaki A. 2019. Migrant memory B cells secrete luminal antibody in the vagina. Nature 571:122–126. 10.1038/s41586-019-1285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford ES, Sholukh AM, Boytz R, Carmack SS, Klock A, Phasouk K, Shao D, Rossenkhan R, Edlefsen PT, Peng T, Johnston C, Wald A, Zhu J, Corey L. 2021. B cells, antibody-secreting cells, and virus-specific antibodies respond to herpes simplex virus 2 reactivation in skin. J Clin Invest 131:e142088. 10.1172/JCI142088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyski ZL, Messer WB. 2019. Approaches to interrogating the human memory B-cell and memory-derived antibody repertoire following dengue virus infection. Front Immunol 10:1276. 10.3389/fimmu.2019.01276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akkaya M, Kwak K, Pierce SK. 2020. B cell memory: building two walls of protection against pathogens. Nat Rev Immunol 20:229–238. 10.1038/s41577-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palm AE, Henry C. 2019. Remembrance of things past: long-term B cell memory after infection and vaccination. Front Immunol 10:1787. 10.3389/fimmu.2019.01787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu HB, Hu WH, Johnson H. 1999. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol 65:680–683. 10.1002/jlb.65.5.680. [DOI] [PubMed] [Google Scholar]
- 21.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. 1999. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science 285:260–263. 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 22.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. 1999. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 189:1747–1756. 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madry C, Laabi Y, Callebaut I, Roussel J, Hatzoglou A, Le Coniat M, Mornon JP, Berger R, Tsapis A. 1998. The characterization of murine BCMA gene defines it as a new member of the tumor necrosis factor receptor superfamily. Int Immunol 10:1693–1702. 10.1093/intimm/10.11.1693. [DOI] [PubMed] [Google Scholar]
- 24.Naismith JH, Sprang SR. 1998. Modularity in the TNF-receptor family. Trends Biochem Sci 23:74–79. 10.1016/s0968-0004(97)01164-x. [DOI] [PubMed] [Google Scholar]
- 25.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH. 2000. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 404:995–999. 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 26.Franke F, Kirchenbaum GA, Kuerten S, Lehmann PV. 2020. IL-21 in conjunction with anti-CD40 and IL-4 constitutes a potent polyclonal B cell stimulator for monitoring antigen-specific memory B cells. Cells 9:433. 10.3390/cells9020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guthmiller JJ, Graham AC, Zander RA, Pope RL, Butler NS. 2017. Cutting edge: IL-10 is essential for the generation of germinal center B cell responses and anti-plasmodium humoral immunity. J Immunol 198:617–622. 10.4049/jimmunol.1601762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daheshia M, Deshpande S, Chun S, Kuklin NA, Rouse BT. 1999. Resistance to herpetic stromal keratitis in immunized B-cell-deficient mice. Virology 257:168–176. 10.1006/viro.1999.9613. [DOI] [PubMed] [Google Scholar]
- 29.Deshpande SP, Zheng M, Daheshia M, Rouse BT. 2000. Pathogenesis of herpes simplex virus-induced ocular immunoinflammatory lesions in B-cell-deficient mice. J Virol 74:3517–3524. 10.1128/jvi.74.8.3517-3524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flechtner JB, Long D, Larson S, Clemens V, Baccari A, Kien L, Chan J, Skoberne M, Brudner M, Hetherington S. 2016. Immune responses elicited by the GEN-003 candidate HSV-2 therapeutic vaccine in a randomized controlled dose-ranging phase 1/2a trial. Vaccine 34:5314–5320. 10.1016/j.vaccine.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Mertz GJ, Schmidt O, Jourden JL, Guinan ME, Remington ML, Fahnlander A, Winter C, Holmes KK, Corey L. 1985. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis 12:33–39. 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Cairns TM, Huang ZY, Whitbeck JC, Ponce de Leon M, Lou H, Wald A, Krummenacher C, Eisenberg RJ, Cohen GH. 2014. Dissection of the antibody response against herpes simplex virus glycoproteins in naturally infected humans. J Virol 88:12612–12622. 10.1128/JVI.01930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernstein DI, Wald A, Warren T, Fife K, Tyring S, Lee P, Van Wagoner N, Magaret A, Flechtner JB, Tasker S, Chan J, Morris A, Hetherington S. 2017. Therapeutic vaccine for genital herpes simplex virus-2 infection: findings from a randomized trial. J Infect Dis 215:856–864. 10.1093/infdis/jix004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan C, Baron S, Dianzani F, Barber J, Stanton GJ. 1983. Ocular herpes simplex virus infection is diminished by depletion of B lymphocytes. J Immunol 131:1554–1557. [PubMed] [Google Scholar]
- 35.Burn C, Ramsey N, Garforth SJ, Almo S, Jacobs WR, Jr, Herold BC. 2018. A herpes simplex virus (HSV)-2 single-cycle candidate vaccine deleted in glycoprotein D protects male mice from lethal skin challenge with clinical isolates of HSV-1 and HSV-2. J Infect Dis 217:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Patel CD, Manivanh R, North B, Backes IM, Posner DA, Gilli F, Pachner AR, Nguyen LN, Leib DA. 2017. Maternal antiviral immunoglobulin accumulates in neural tissue of neonates to prevent HSV neurological disease. mBio 8:e00678-17. 10.1128/mBio.00678-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Criscuolo E, Castelli M, Diotti RA, Amato V, Burioni R, Clementi M, Ambrosi A, Mancini N, Clementi N. 2019. Cell-to-cell spread blocking activity is extremely limited in the sera of herpes simplex virus 1 (HSV-1)- and HSV-2-infected subjects. J Virol 93:e00070-19. 10.1128/JVI.00070-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baumgarth N. 2013. How specific is too specific? B-cell responses to viral infections reveal the importance of breadth over depth. Immunol Rev 255:82–94. 10.1111/imr.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giesecke C, Frolich D, Reiter K, Mei HE, Wirries I, Kuhly R, Killig M, Glatzer T, Stolzel K, Perka C, Lipsky PE, Dorner T. 2014. Tissue distribution and dependence of responsiveness of human antigen-specific memory B cells. J Immunol 192:3091–3100. 10.4049/jimmunol.1302783. [DOI] [PubMed] [Google Scholar]
- 40.Boonyaratanakornkit J, Taylor JJ. 2019. Techniques to study antigen-specific B cell responses. Front Immunol 10:1694. 10.3389/fimmu.2019.01694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. 2005. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine 23:873–883. 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. 2009. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2:129–143. 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 . Download jvi.02057-21-s0001.pdf, PDF file, 0.2 MB (163.7KB, pdf)